Abstract
The current study examined developmental changes in fear learning and generalization in 40 healthy 8–13 year-olds using an aversive conditioning paradigm adapted from Lau and colleagues (2008). In this task, the conditioned stimuli (CS+/CS−) are two neutral female faces, and the unconditioned stimulus is a fearful, screaming face. The second phase of the study also included a generalization stimulus (GS): a 50% blend of the CS+/− faces. The eye-blink startle reflex was utilized to measure defensive responding. Patterns of fear learning and generalization were qualified by child age. Older children demonstrated greater fear learning (i.e., larger startle during CS+ than CS−) than younger children. In addition, older children exhibited the typical pattern of generalization observed in adults, whereas younger children did not. Finally, fear learning also related to contingency awareness; only children who correctly identified the CS+ demonstrated fear-potentiated startle to the CS+. Clinical implications and future directions are discussed.
Keywords: fear learning, fear generalization, aversive conditioning, startle reflex, development
Introduction
Fear conditioning is the process whereby a neutral stimulus acquires the capacity to elicit a fear response when it is paired with an aversive unconditioned stimulus (UCS). Abnormal fear conditioning has been central to theories of anxiety disorder pathophysiology (for a review: see Mineka & Oehlberg, 2008). However, most of this research has been conducted with adults, and less is known about fear learning in children and adolescents.
The transition from childhood to early adolescence is of particular interest because it is a high-risk period for both anxiety and mood disorders (Costello, Egger, & Angold, 2005). Anxious children who fail to develop more complex fear learning capacities (e.g., extinction, discrimination) may face heightened risk for anxiety disorders that persist into adulthood (Britton, Lissek, Grillon, Norcross, & Pine, 2010). Further, evidence from animal research suggests that whereas pre-adolescent rodents show capacities to manifest relatively simple forms of fear conditioning, more complex aspects of fear learning continue to develop well into adulthood (Kim & Richardson, 2010; Rudy, 1993). In research with humans, however, few studies to date have examined developmental changes in fear learning from late childhood to early adolescence. Thus, there is a clear need to understand how fear learning normally develops over childhood and adolescence. Work in this area could provide insight into the relationship between deficits in conditioning and anxiety disorders while also informing the integration of basic and clinical work on development.
Despite the importance of such developmental work, it has been difficult to extend the aversive conditioning research in animals and adult anxiety disorders to children and adolescents. Direct extension requires a paradigm where meaningful levels of learned fear can be produced in an ethically responsible manner. Most fear conditioning research in adults has used electrical shock as a UCS, which may not be feasible with children. Instead, paradigms with children and adolescents have utilized a variety of unconditioned stimuli that are more appropriate for youth, including a loud tone (Craske et al., 2008; Liberman, Lipp, Spence, & March, 2006), metal scraping on a slate (Neumann, Waters, Westbury, & Henry, 2008; Waters, Henry, & Neumann, 2009), or an air blast to the larynx either presented alone (Borelli, Sbarra, Crowley, & Mayes, 2011; Grillon, Dierker, & Merikangas, 1998; Reeb-Sutherland et al., 2009) or paired with a loud scream and fearful face (Schmitz, Merikangas, Swendsen, Cui, Heaton, & Grillon, 2011). In addition to a variety of UCS, fear learning studies in children have also varied in the dependent measure of fear learning utilized: Craske et al. (2008), Liberman et al. (2006), Neumann et al. (2008) and Waters et al. (2009) used skin conductance to index fear learning, whereas Grillon et al. (1998), Reeb-Sutherland et al. (2009), Borelli et al. (2011), and Schmitz et al. (2011) used the startle reflex.
In general, findings are inconsistent across paradigms, and the degree of conditioning within many of the paradigms is limited. For instance, skin conductance studies have produced mixed results, with some studies finding evidence of fear conditioning in all skin conductance response (SCR) interval windows (Neumann et al., 2008; Waters et al., 2009), whereas others have not (Craske et al., 2008; Liberman et al., 2006). In addition, there have been inconsistencies related to contingency awareness (i.e., the UCS-CS relationship). In Waters et al. (2009), only 55% of non-anxious children reported learning fear contingencies, which is lower than any other fear learning study. Moreover, Craske et al. (2008) found that SCRs were not sensitive to contingency awareness. That is, children who correctly identified the CS+ had similar SCRs to children who were not aware of the task contingencies. However, previous fear learning studies using the startle reflex in adults have found that the startle response tracks contingency awareness. Moreover, research on contingency awareness appears to have important implications for understanding the role of anxiety in fear learning (Grillon, 2002a; 2002b).
One of the most likely explanations for the inconsistent findings across studies in youth could be the potency of the unconditioned stimuli. A weak UCS is known to produce relatively low levels of fear learning and more variable results across studies, as compared to a strong UCS. In fact, data in adolescents suggest that increasing the potency of the UCS does produce stronger, more reliable results. For example, Grillon and colleagues had used an air-puff UCS in an initial series of studies with children and adolescents. Schmitz et al. (2011) then adapted this air-puff UCS by adding a fearful face paired with a shrieking female scream. This modification did in fact augment the aversiveness of the air-puff UCS. More importantly, however, the addition of the scream-face pair to the air-puff UCS produced a fear learning pattern of findings in adolescents that appeared more consistent with findings in adults, relative to earlier studies in adolescents that relied on a less aversive UCS.
Thus, findings from Schmitz et al. (2011), coupled with other recent studies (Lau et al., 2008; Lau et al., 2011), suggest that a fearful face paired with a shrieking scream represents a more powerful UCS than other unconditioned stimuli employed in research with children and adolescents. This novel fear learning approach uses neutral female faces as conditioned danger cues (CS+) and conditioned safety cues (CS−). The UCS is the CS+ female with a fearful facial expression paired with an aversive female scream. Preliminary self-report data from Lau et al. (2008) demonstrated that the CS+ was significantly more fear-provoking than the CS− and induced high levels of avoidance among children and adolescents. Further, in a follow-up study using this paradigm, Lau and colleagues (2011) found comparable fear learning patterns, as measured by the galvanic skin response (GSR), in adolescents and adults. However, no published studies have utilized this novel UCS paradigm to compare levels of fear learning in children and adolescents using fear-potentiated startle (FPS) – arguably the most reliable measure of fear learning in animals and humans (Grillon, Ameli, Woods, Merikangas, & Davis, 1991).
Fear-potentiated startle (FPS), an increased eye-blink reflex when an organism is in a fearful state (Lang, Davis, & Ohman, 2000; Lissek et al., 2005), is an ideal measure for indexing fear learning because it has a non-zero baseline, is valence-specific (Bradley, Cuthbert, & Lang, 1990), has well-documented neurobiological substrates (Hitchcock & Davis, 1986; Phelps et al., 2001), and manifests in both human and nonhuman animals (Grillon et al., 1991). However, most previous studies of fear learning in children have used skin conductance, and the choice of skin conductance as a dependent measure of fear learning could provide another explanation for the inconsistent findings across, and within, studies. Skin conductance is a nonspecific measure of arousal; SCR increases when an organism is orienting to novel stimuli and is sensitive to arousal but not valence (i.e., similar responses for both pleasant and unpleasant stimuli; Cook & Turpin, 1997). In addition, SCR habituates more rapidly over the course of a task than the startle reflex (Bradley, Lang, & Cuthbert, 1993; Codispoti, Ferrari, & Bradley, 2006). Further, some studies suggest that SCR may be more closely linked to anticipatory arousal rather than fear responding (Bechara, Tranel, Damasio, & Damasio, 1996; Critchley, Elliott, Mathias, & Dolan, 2000). Notably, one study, using both the startle reflex and skin conductance, suggests that the startle reflex indexes fear learning when skin conductance may not (e.g., Grillon, 2004). In sum, the startle reflex specifically assesses defensive mobilization and is an ideal measure to index aversive conditioning. Although it has been used reliably in both animal and adult human studies of fear learning (see review: Lang et al., 2000), it has not been widely used in developmental research, particularly with humans.
A handful of studies (Borelli et al., 2011; Grillon et al., 1998; Reeb-Sutherland et al., 2009; Schmitz et al., 2011) have used the startle reflex to examine fear learning or fear expression in adolescents. However, only two studies (Borelli et al., 2011; Schmitz et al., 2011) have used the startle reflex to examine fear expression in children. Borelli et al. (2011) studied fear learning in children ages 7 to 12 using an air-puff UCS. Although startle magnitude differences in low and high depression groups were discussed, the authors did not report whether fear-potentiated startle (FPS: greater startle magnitude during CS+ compared to during CS−) was observed in the study. Therefore, a shortcoming of Borelli et al. (2011) is the absence of the key fear learning results, which makes it difficult to use this study to inform the current project.
In the second startle study in children, Schmitz et al. (2011) examined cued and un-cued fear using a UCS that was an air blast to the larynx combined with the fearful female face and scream UCS developed by Lau et al. (2008). Notably, although Schmitz et al. (2011) used the UCS developed by Lau et al. (2008), the full fear learning paradigm that included the face CSs was not employed: rather, the CSs in Schmitz et al. (2011) were geometric shapes. Moreover, subjects were explicitly told about the CS-UCS contingencies, as is done classically in research on threat-related fear expression (i.e., instructed fear); participants did not learn these contingencies through experience, as is done classically in research on fear learning through Pavlovian fear conditioning. Fear potentiation was evident in the sample when the UCS was cued. However, FPS did not differ as a function of child age. In sum, there has only been one study to date that has reported FPS startle in children, but that study did not examine age-related differences in fear learning (Schmitz et al., 2011).
Building upon this previous research, the first set of goals for the current study were: (1) to examine the feasibility of using the full screaming female UCS paradigm with the startle reflex to measure fear learning in children and young adolescents (referred to collectively as children), (2) to assess developmental changes in fear learning from ages 8 to 13, and (3) to examine fear-potentiated startle as a function of contingency awareness. First, given the promising studies with the screaming female UCS (Lau et al., 2008; Lau et al., 2011; Schmitz et al., 2011), we hypothesized that this paradigm would result in reliable FPS in the current sample. Second, based on data from animal models, suggesting that fear conditioning improves with age (Kim & Richardson, 2010; Rudy, 1993), we predicted that older compared to younger children would better learn to discriminate the CS+ from the CS−. Finally, based on previous work by Grillon (2002a; 2002b), we hypothesized that only children who could accurately identify the CS+ at the end of task (i.e., via self-report) would exhibit normal FPS.
In addition to fear acquisition, the current paradigm also afforded an opportunity to examine the development of a more subtle fear learning process: fear generalization. A recent review (Lissek et al., 2005) suggests that conditioning-related deficits in individuals with anxiety disorders might manifest most clearly on tests of fear generalization, the process by which stimuli that are perceptually similar to the conditioned danger cue (CS+) produce some degree of conditioned fear response (LeDoux, 1998). Thus, stimuli that are safe, but perceptually like the CS+, can activate fear in a parametric fashion based on the degree of perceptual similarity to the CS+. In laboratory paradigms examining generalization of FPS in adults, participants’ reports of perceived risk and their FPS increased from the most perceptually dissimilar to the most perceptually similar stimuli, being greatest to the CS+ itself (Hajcak et al., 2009; Lissek et al., 2008). Moreover, this generalization might occur more strongly in patients than in healthy subjects; preliminary data indicating overgeneralization in adults with panic disorder support this view (Lissek et al., 2010).
However, no studies to date have examined fear generalization in children. Therefore, the final aim of this study was to extend previous research on fear conditioning in children by examining fear generalization. To this end, the current study adapted previous shock-based fear generalization startle paradigms used in adult research (Hajcak et al., 2009; Lissek et al., 2008) to be more appropriate for use with children. Specifically, the current study used FPS to examine fear generalization to a face that is perceptually similar to the CS+ (i.e., GS: generalization stimulus). Again, our hypotheses were informed by data from animal models of fear learning. This research suggests that relatively complex aspects of fear learning, such as generalization to the conditioning context, increase with age as the hippocampal formation and prefrontal cortex mature (Kim & Richardson, 2010; Rudy, 1993). These aspects of fear learning require the organism to learn how to classify stimuli along a continuum of safety and danger. This form of learning is more complex than basic fear conditioning procedures, where organisms learn only to classify one or two, relatively easy-to-distinguish stimuli as either “safe” or “dangerous”. Thus, insofar as fear generalization is a more sophisticated form of fear learning, and there is recent evidence to suggest that complex aspects of fear learning increase from adolescence into adulthood (Lau et al. 2011), it stands to reason that older compared to younger children would better discriminate between the CS+, GS, and CS−. That is, we hypothesized that older children would demonstrate the linear pattern of fear generalization observed in adults (Hajcak et al., 2009; Lissek et al., 2008) and thus manifest more developed generalization compared to younger children.
In sum, the second set of aims for the current study were to examine the feasibility of using the novel screaming female UCS in a startle paradigm to measure fear generalization in children, and to assess developmental changes in fear generalization from ages 8 to 13. The current study is novel in its use of a new UCS (i.e., screaming female) in a startle paradigm, examination of fear generalization in children, and focus on development of fear learning and generalization from middle childhood to early adolescence.
Methods
Participants
Participants were recruited from the community via a commercial mailing list for a larger study on early adolescent emotionality. The original sample consisted of 68 children. Ten participants stopped the startle task before it was complete, when they reported being too afraid of the experimental stimuli to continue. Thus, even in healthy youth, these procedures were too aversive for 15% of participants to continue, a rate of discontinuation that is generally higher than in other fear conditioning studies in children. Further, 10 of the remaining 58 participants were excluded from further analyses for having no measurable startle response on more than 2/3 of startle trials in any one condition. Finally, eight participants were excluded from the analyses due to disproportionate artifacts in the baseline period (i.e., 50 ms before the onset of the startle probe) on more than half of all startle trials.
The final sample reported here consisted of 40 children (15 female) aged 8 to 13 years old (M = 10.83 years, SD = 1.62) recruited from the community in suburban New York. The majority (85.0%) were Caucasian. These 40 participants were not significantly distinct in demographic features or clinical symptoms from the 18 children who were excluded from analyses (all ps > .13). In addition, the participants who completed the task were not significantly distinct from the 10 participants who stopped the task (all ps > .123), with one exception; participants who stopped the startle task were more likely to be female than participants who completed the task (stopped task: 80% female; completed task: 36.2% female; χ2 [1, N = 68] = 6.69, p = .010, Φ = .31).
Stimuli and Presentation
The fear conditioning stimuli used in this study were based on Lau and colleagues (2008): participants were presented with pictures of two neutral female faces (NimStim: 01F, 03F; Tottenham et al., 2009), one serving as the CS+ and the other as the CS−. The UCS was a fearful female face (same actress as the CS+) presented for three seconds along with an aversive scream for one second. On reinforced CS+ trials, the neutral CS+ face was replaced by the fearful face, and this transition was accompanied by a loud female scream. The CS− and GS were never paired with the UCS.
Acquisition
In the acquisition phase, participants viewed eight CS+ faces (75% UCS reinforcement) and eight CS− faces. The female actresses used as the CS+ and CS− were counterbalanced across participants. All neutral facial stimuli were presented for six seconds. Startle probes were presented on six of the eight CS+ and six of the eight CS− trials, randomly between 3.5 and 4.5 seconds after picture onset. Startle probes were not presented on the remaining trials in order to decrease startle predictability. For the CS+, startled and reinforced trials were not always the same; that is, the presence of a startle probe did not predict the UCS. In addition, four intertrial interval startle probes were presented to reduce startle predictability and were used as a baseline for deriving fear-potentiated startle measures (i.e., ITI-corrected data). Inter-trial intervals ranged from 9 to 11 seconds. Fear-potentiated startle (FPS) was calculated as the difference between ITI-adjusted startle magnitudes elicited during the CS+ compared to during the CS−.
Generalization
The fear generalization phase was identical to the fear acquisition phase except that it also included the generalization stimulus (GS): a 50% blend of the CS+ and CS− faces created using morphing software. In the generalization phase, eight CS+, eight CS−, and eight GS trials were displayed with startle probes presented on six of each trial type. Again, CS− and GS trials were never reinforced. Generalization was operationalized as the difference between ITI-adjusted startle magnitudes during the GS compared to the CS− (i.e., increased startle during the GS relative to the CS−).
Three pseudorandom trial orders were constructed and randomized between participants; the first CS+ was always reinforced in both the acquisition and generalization phases of all orders. Images were displayed using Presentation software (Neurobehavioral Systems, Inc; Albany, CA) to control timing and presentation of stimuli. All images were presented in gray scale on a 19-inch monitor set to a resolution of 506 × 618 pixels. Stimuli viewing distance was 23 inches and each stimulus occupied approximately 4 inches vertically and 2.5 inches horizontally of the screen.
Auditory startle probes, consisting of 50 msec, 95 dB bursts of white noise with near instantaneous rise time, were presented binaurally through sound-attenuating headphones. Startle probes were produced with a noise/tone generator (Contact Precision Instruments; Cambridge, MA). The UCS scream, experienced at approximately 80dB, was presented for one second through two Dell computer speakers approximately 25 inches from the participant.
Procedure
The project was approved by Stony Brook University’s Institutional Review Board and informed consent/assent was obtained from both parent and child prior to the experiment. All participants performed multiple tasks during the experiment. The order of the tasks was counterbalanced across participants and the results of other tasks will be reported elsewhere (e.g., Bress, Smith, Foti, Klein, & Hajcak, in press). For the startle task, parents and children were told that the procedure involved exposure to neutral and fear-provoking stimuli. Subjects were specifically told about the possibility of becoming frightened and that they could discontinue participation at any time.
Each participant was tested individually in a sound-attenuated and dimly lit chamber where electrodes and headphones were positioned. The task began with a habituation phase to reduce extreme startle responses in the first few trials. During the habituation phase, participants viewed four female faces with either neutral or fearful expressions while startle probes were presented over the headphones – none of these stimuli were the CS+, CS−, or GS. Before the actual experimental task began, participants were presented with the UCS scream through speakers, as well as the CS+ (neutral only) and CS− stimuli; children rated each stimulus on a 9-point scale from 1 = Not at all scary to 9 = Very scary (5 = Somewhat scary).
Participants were then given the following instructions, “When I begin the task, you will see pictures of two women on the screen. Please watch the pictures while they are on the computer screen. While you are watching the pictures, one of the women may change to look scared and you will hear the scream through the speakers. If you pay attention, you may be able to predict when the scream happens. Also, you will continue to hear the noises in the headphones every once in a while. Just ignore these sounds and watch the pictures.” Participants then completed the acquisition and generalization phases with a short (15 second) break between the two phases. At the end of the startle task, participants were again asked to rate how scary they found the scream, CS+ face, and CS− face on the 9-point scale. On the same 9-point scale, participants also rated how scary they found the GS face (i.e., generalization stimulus). Finally, to determine contingency awareness, participants were asked if they noticed that the scream followed a certain picture. Then, they viewed a slide containing the CS+, CS−, and GS and were asked to identify which face was followed by the scream. Participants who correctly chose the CS+ as predicting the scream were labeled “learners” because they understood the CS-UCS relationship. Conversely, participants who chose either the CS− or GS face were labeled “non-learners.”
Following the startle task, participants watched a short (three minute) youtube.com video of funny cats to serve as a positive mood induction before finishing the study. Participants who stopped the startle task also completed the final positive mood induction. Children and their parents were debriefed before leaving and no participants reported being distressed at the end of the study. Participants were compensated for their time.
Physiological Data Recording, Reduction, and Analysis
Startle-elicited EMG activity was collected in accordance with current guidelines (see Blumenthal et al., 2005). Two electrodes, 4 mm diameter Ag-AgCl filled with electrode gel (TD-40; Mansfield R and D), were positioned beneath the left eye over the orbicularis oculi muscle approximately 25 mm apart, and a third electrode was placed on the forehead to serve as an isolated ground. EMG activity was recorded using a PSYLAB Stand Alone Monitor (SAM) Unit and an attached BioAmplifier system (Contact Precision Instruments; Cambridge, MA), and was sampled at 1000 Hz and filtered between 30 and 500 Hz. EMG responses were rectified in a window 200 milliseconds long, beginning 50 milliseconds before the onset of the startle probe. A 6-point running average was applied to the rectified data to smooth out sharp peaks. Raw startle magnitude was expressed as the difference between the average of the EMG in the 50 ms window prior to the startle probe and the maximum in the 150 ms post-probe window. Each participant’s data was examined on a trial-by-trial basis. Trials with no perceptible eye-blink response were scored as zero and included in the overall averages; trials with excessive baseline artifacts were excluded. Raw startle magnitudes were converted to ITI-corrected scores in order to reduce between-subject variability1. ITI-corrected scores were created by subtracting each individual’s average ITI startle magnitude from their other startle magnitude averages in that phase of the task (e.g., acquisition CS+ startle magnitude minus acquisition ITI startle magnitude; generalization CS+ startle magnitude minus generalization ITI startle magnitude).
Repeated measures ANOVA with the Greenhouse-Geisser correction applied were used to statistically evaluate startle responding during the acquisition (CS+, CS−) and generalization (CS+, GS, CS−) phases. Significant results were followed by paired-samples t-tests; the Benjamini–Hochberg procedure was used to control the false discovery rate for multiple comparisons (Benjamini & Hochberg, 1995). Startle magnitude difference scores were computed to examine the relationship between startle response and age. Specifically, FPS was defined in terms of the difference between CS+ and CS− in both the acquisition and generalization phases. Fear generalization was defined as the difference between the GS and CS− in the generalization phase. Finally, repeated measures ANOVA with the Greenhouse-Geisser correction applied were used to statistically evaluate startle magnitude differences between children who correctly identified the CS+ (learners) and children who were not able to identify the CS+ (non-learners) at the end of the fear conditioning task. Significant results were followed by paired-samples t-tests. Again, the Benjamini–Hochberg procedure used to control the false discovery rate for multiple comparisons (Benjamini & Hochberg, 1995).
Results
Self-Report Ratings
Children’s ratings of the startle task stimuli are presented in Table 1. Both before and after the task, children rated the CS+ and CS− face as ‘not very scary’ (i.e., approximately 2 on the 9-point scale) and the scream as ‘somewhat scary’ (i.e., approximately 4 on the 9-point scale). There were no overall differences between the CS+ and CS− (F[1, 39] = 0.11, p = .737, ηp2 = .003), and no overall differences between pre- and post-task ratings (F[1, 39] = 0.01, p = .938, ηp2 = 0). However, there was a trend toward a significant interaction of CS (CS+, CS−) ratings from pre- to post-task (F[1, 39] = 3.62, p = .064, ηp2 = .085); CS+ fearfulness ratings generally increased from pre- to post-task whereas CS− fearfulness ratings decreased from pre- to post-task. After the task, children rated the GS face as ‘not very scary’ (i.e., 1.8). Age was not significantly associated with (1) the difference in ratings between the CS+ and CS− at the end of the task (r[40] = −.15, p = .356), (2) ratings of the CS+ (r[40] = .11, p = .504) from pre- to post-task, or (3) ratings of the CS− from pre to post task (r[40] = .04, p = .790).
Table 1.
Self-Report Ratings of Startle Task Stimuli (N = 40).
| Stimuli1 | Pre Task Ratings M (SD) |
Post Task Rating M (SD) |
|---|---|---|
| CS+ | 1.93 (1.21)a | 2.15 (1.55)a |
| CS− | 2.10 (1.30)a | 1.90 (1.37)a |
| Scream | 4.13 (1.87)b | 3.65 (2.58)b |
| GS | —— | 1.80 (1.07)a |
Self-report ratings on a scale from 1 = Not at all Scary to 9 = Very Scary.
Superscripts with different letters indicate significant differences between ratings at p < .05.
In regard to contingency awareness, most children (80%) were able to correctly identify the CS+ at the end of the fear conditioning task (i.e., learners; n = 32; M age = 10.72, SD = 1.65). However, the other 20% of children were not able to correctly identify the CS+ (i.e., non-learners; n = 8; M age = 11.25, SD = 1.49); six incorrectly chose the CS− and two chose the GS as being paired with the scream. Learners and non-learners did not differ with respect to age (t[38] = 0.83, p = .413, d = 0.27).
Startle Results
A repeated measures ANOVA was first conducted with ITI-corrected data to examine the pattern of startle responding during the acquisition and generalization phases in the overall sample (see Figure 1). There was a significant effect of stimulus type (CS+, CS−) in the acquisition phase, F(1, 39) = 23.30, p < .001, ηp2 = .374. That is, participants exhibited fear learning, as evidenced by greater startle magnitude elicited during the CS+ than the CS−.
Figure 1.
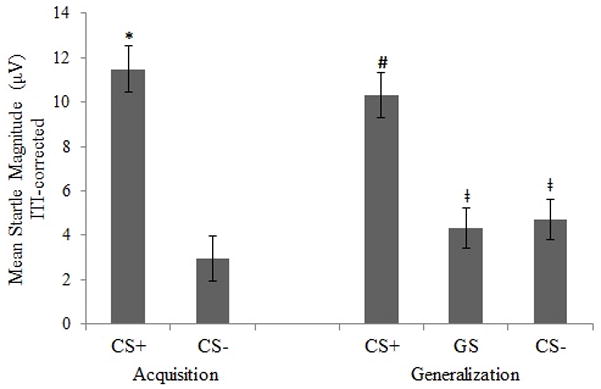
Startle during the generalization phase also varied by stimulus type (CS+, GS, CS−), F(2, 78) = 12.83, p < .001, ηp2 = .247. Follow-up paired-samples t-tests revealed that startle magnitude was greater during the CS+ compared to both the CS− (t[39] = 4.19, p < .001, d = 1.03) and the GS (t[39] = 4.12, p < .001, d = 1.10). However, startle magnitude during the GS and CS− did not differ from one another (t[39] = −.33, p = .732, d = 0.08), indicating a lack of generalization to the GS.
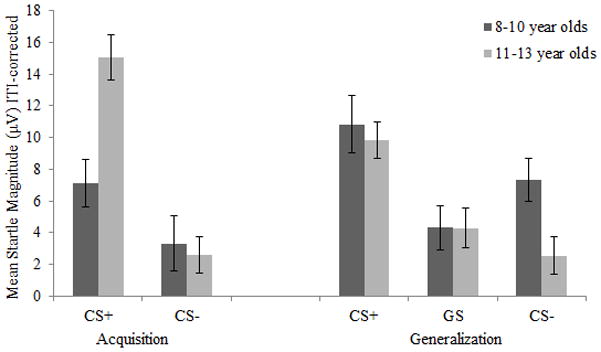
Next, in order to examine the relationship between startle response and child age, difference scores were computed with the ITI-corrected data (i.e., acquisition CS+ minus acquisition CS− [FPS], generalization CS+ minus generalization CS− [FPS], generalization GS minus generalization CS− [fear generalization]). There was a significant positive relationship between child age and FPS (i.e., the difference between CS+ and CS−) in the acquisition phase (r[40] = .36, p = .024), indicating that FPS increased with age. In addition, there was also a significant correlation between child age and fear generalization (i.e., the difference between GS and CS− in the generalization phase), r(40) = .47, p = .004, reflecting larger startle magnitude during the GS compared to the CS− among older children. Taken together, older children exhibited greater discrimination of fearful stimuli. That is, older children were both more able to discriminate dangerous from safe stimuli in the acquisition phase and to discriminate between the three stimuli in the generalization phase where they exhibited a linear pattern of fear generalization, increasing from CS− to GS to CS+2. To illustrate the effect of age on fear learning and generalization, Figure 2 presents startle data based on a median split of child age (i.e., 8–10 year olds and 11–13 year olds separately). All other correlations between child age and startle response (including overall ITI startle in the acquisition and generalization phases) were nonsignificant (ps ranged from .116 to .564).
Figure 2.
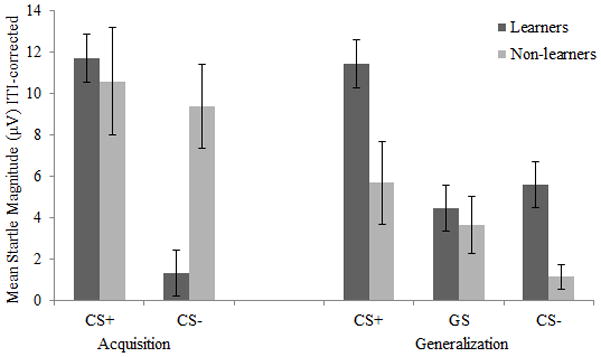
Finally, we examined differences between children who correctly identified the CS+ at the end of the task (learners) and those who did not (non-learners). In the acquisition phase, there was a significant group (learner, non-learner) by stimulus type (CS+, CS−) interaction, F(1, 38) = 4.68, p = .037, ηp2 = .110. Follow-up paired-samples t-tests revealed that learners demonstrated significantly larger startle potentiation during the CS+ compared to the CS− (t[31] = 5.67, p < .001, d = 1.80), but non-learners did not exhibit a difference between the CS+ and the CS− (t[7] = .29, p = .784, d = 0.23; see Figure 3 Acquisition phase). However, a similar group by stimulus type interaction was not found in the generalization phase of the experiment, F(1, 38) = 1.20, p = .305, ηp2 = .031 (see Figure 3 Generalization phase).
Figure 3.
Discussion
The current study examined the feasibility of using a novel UCS startle paradigm to examine developmental changes in fear learning and fear generalization in a sample of children and adolescents (referred to collectively as children) aged 8 to 13. Based on physiological and self-report data, results demonstrated that, overall, children were able to differentiate the CS+ from the CS− in both the fear acquisition and generalization phases. In addition, from pre- to post-task, there was a trend toward the CS+ being rated as ‘more scary’ and the CS− as ‘less scary’. However, startle differences between the CS+ and CS− were more robust than the self-report ratings.
Interestingly, the overall pattern of fear learning and generalization was qualified by child age. In the acquisition phase, older children demonstrated greater fear-potentiated startle (FPS) than younger children (i.e., age was positively related to FPS). Further, in the generalization phase, the difference between the GS and CS− also correlated with child age. However, the introduction of a GS appeared to impact the response to the CS− differentially in older compared to younger children. When a GS was included, older children displayed a linear fear generalization pattern (i.e., increasing from CS− to GS to CS+), as has been reported in studies of fear generalization among adults (Hajcak et al., 2009; Lissek et al. 2008; 2010). In contrast, younger children did not exhibit this adult-like, linear fear generalization pattern and instead displayed larger responses to the CS− compared to the GS. Taken together, these results indicate that older children were better able to discriminate danger from safety cues. Specifically, older children both conditioned more robustly and were more likely than younger children to discriminate between gradations of the CS+ in the generalization phase (i.e., increasing from CS−to GS to CS+).
As mentioned above, older children’s pattern of fear generalization closely resembled the pattern found in three shock-based FPS fear generalization studies in adults (Hajcak et al., 2009; Lissek et al. 2008; 2010). This linear pattern demonstrates that older children are more able to discriminate between gradations of fear stimuli. These results are consistent with recent findings in rodents, where data increasingly show that subtle forms of fear learning, such as fear discrimination and extinction, continue to develop from weaning-age into adulthood (Kim & Richardson, 2010; Rudy, 1993).
Although the startle findings did not parallel children’s self-report ratings of fear, the pattern of startle results did correspond with children’s self-report ratings of contingency awareness. Consistent with previous adult studies (Grillon, 2002a; 2002b), only children who correctly identified the CS+ exhibited normative FPS. In contrast, non-learners (i.e., children who could not correctly identify the CS+) exhibited similar defensive responding to both threat (CS+) and safety cues (CS−). Previous research from Grillon and colleagues (Grillon, 2002a; 2002b; Grillon et al., 2004) suggests that the inability to learn CS-UCS contingencies is related to anxiety. Future research is needed with clinical samples to examine whether the ability to learn fear contingencies is related to anxiety disorders in childhood.
In contrast to the fear acquisition results, significant differences in fear generalization based on contingency awareness were not evident. The lack of group differences in the generalization phase could be due to the small sample size in the non-learning group (8 children), which limited power to detect effects. Inspection of the startle patterns in the generalization phase suggests that the introduction of a generalization stimulus may differentially impact fear responding to the CS+ and CS− as a function of contingency awareness. Future studies should consider examining how contingency awareness impacts both children’s fear learning and generalization using larger samples.
Taken together, findings from the current study add to the growing literature on fear learning in childhood and adolescence in a number of ways. This study is the first to examine fear learning using an adapted version of the screaming female UCS paradigm (developed by Lau et al. [2008]) with the startle reflex. In line with previous startle studies in children and adolescents (Grillon et al., 1998; Reeb-Sutherland et al., 2009; Schmitz et al., 2011), the current study found evidence of fear learning in children using the startle reflex and a new UCS. Second, this is the first study, to our knowledge, to study fear generalization in children and adolescents, and moreover, is novel in its developmental examination of fear learning and generalization across children ages 8 to 13. Results suggest that there are important developmental changes in fear learning from middle childhood to early adolescence that warrant further investigation. Finally, similar to adult findings (Grillon, 2002a; 2002b), the startle reflex, unlike skin conductance (Craske et al., 2008), is sensitive to contingency awareness in children and adolescence. Taken together, results from the current study provide preliminary evidence for fear learning in children aged 8–13 and fear generalization in older children using a new UCS startle paradigm. However, the current report represents the first study of its kind in children, and replication across a wider age range (into adulthood) is certainly necessary.
Although there are key strengths of the current study, several limitations to this research deserve discussion, and highlight areas for future research. First, although children could correctly identify the CS+ and exhibited fear-potentiated startle, they did not report the CS+ to be much scarier than the CS− over the course of the task. A previous study using this paradigm with children found robust self-reported differences in fear in response to the CS+ compared to the CS− (Lau et al., 2008). Inconsistencies between physiological responding and perception of emotional experience have also been found in previous studies with children using other paradigms (Quas, Hong, Alkon, & Boyce, 2000; Stroud et al., 2009; Waters et al., 2009). In the current study, this discrepancy could be attributed to timing of the self-report fear ratings (i.e., after learning in Lau et al., 2008, but only after both learning and generalization in the current study). In addition, this difference could be due to variations in the question used to obtain fear ratings (i.e., how scary is the picture [current study] vs. how afraid the subjects were when viewing the pictures [Lau et al., 2008]). Further, in other studies with adults using this paradigm, we found robust differences between CS+ and CS− fear ratings when we have used a more restricted range (i.e., 0–3 instead of 0–9). Finally, there could have been a social desirability bias in self-report ratings (i.e., children may want to seem fearless). Future studies could obtain multiple self-report ratings, including items that might be less susceptible to participant biases.
A second limitation is that the interpretation of fear generalization results is complicated. Fear generalization was not observed in the overall sample; however, child age was positively related to increased differentiation between the GS and CS−. Indeed, the pattern of findings among older children suggested a generalization gradient similar to what has been found in adults (Hajcak et al., 2009; Lissek et al., 2008; 2010). However, it appeared that the introduction of the GS may have actually increased the response to the CS− among younger children. There are two potential conclusions from these results. The first is that older children exhibit more adult-like patterns of fear generalization, whereas younger children do not. Instead, the introduction of the GS may alter response to the CS− among younger participants who are less able to discriminate stimuli. The second possibility is that the current generalization paradigm needs improvement in order to be utilized across childhood and adolescence. For instance, the current study included only one generalization stimulus (to limit the number of startle probes presented and prevent habituation). However, future studies may want to include multiple generalization stimuli in order to assess a wider range of fear generalization, similar to Hajcak et al. (2009) and Lissek et al. (2008).
As discussed above, a third limitation is that the group of children who did not learn the CS-UCS contingency was small and therefore it was unclear, in the generalization phase specifically, whether the lack of significant differentiation between the conditioned and generalization stimuli was due to low power. Future studies should seek to replicate this design in larger samples. In addition, it is possible that certain modifications to the UCS paradigm (e.g., using only the UCS scream without the face, or using a different UCS face) could make the fear learning task more challenging thereby increasing the size of the non-learning group. Although future studies might consider modifications in order to examine the impact of contingency awareness on fear generalization, it is also important to attend to the potency of any alternative unconditioned stimuli.
Finally, this study examined the efficacy of a new UCS fear-potentiated startle paradigm in an unselected sample of children and adolescents. A logical next step is to replicate this study in a clinical sample to assess whether fear learning and generalization in this paradigm is sensitive to individual differences in anxiety.
Acknowledgments
This study was supported, in part, by NIMH RO1 MH069942 (DNK) and NIH F31 MH086197 (CRG).
Footnotes
Main analyses reported in the Results section were also significant when T-scored data was used.
Greater learning was not related to more generalization; that is, there was not a significant correlation between FPS (CS+ minus CS−) in the acquisition phase and generalization (GS minus CS−) in the generalization phase (r[40] = .16, p = .320).
Contributor Information
Catherine R. Glenn, Email: cassie.glenn@gmail.com.
Daniel N. Klein, Email: daniel.klein@stonybrook.edu.
Shmuel Lissek, Email: smlissek@umn.edu.
Jennifer C. Britton, Email: brittonjen@mail.nih.gov.
Daniel S. Pine, Email: pined@mail.nih.gov.
Greg Hajcak, Email: greg.hajcak@stonybrook.edu.
References
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Borelli JL, Sbarra DA, Crowley MJ, Mayes LC. Mood symptoms and emotional responsiveness to threat in school-aged children. Journal of Clinical Child and Adolescent Psychology. 2011;40:220–232. doi: 10.1080/15374416.2011.546047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Startle reflex modification: Emotion or attention? Psychophysiology. 1990;27:513–522. doi: 10.1111/j.1469-8986.1990.tb01966.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ, Cuthbert BN. Emotion, novelty, and the startle reflex: Habituation in humans. Behavioral Neuroscience. 1993;107:970–980. doi: 10.1037//0735-7044.107.6.970. [DOI] [PubMed] [Google Scholar]
- Bress JN, Smith E, Foti D, Klein DN, Hajcak G. Biological Psychology. Neural response to reward and depressive symptoms in late childhood to early adolescence. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS. Development of anxiety: The role of threat appraisal and fear learning. Depression and Anxiety. 2010;0:1–13. doi: 10.1002/da.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetitive picture processing: Autonomic and clinical correlates. Brain Research. 2006;1068:213–220. doi: 10.1016/j.brainres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Cook EW, Turpin G. Differentiating orienting, startle, and defense response: The role of affect and its implications for psychopathology. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and orienting: Sensory and motivational processes. Mahwah, NJ: Erlbaum; 1997. pp. 137–164. [Google Scholar]
- Costello EJ, Egger HL, Angold A. The developmental epidemiology of anxiety disorders: Phenomenology, prevalence, and comorbidity. Child and Adolescent Psychiatric Clinics of North America. 2005;14(4):631–648. doi: 10.1016/j.chc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Craske MG, Waters AM, Bergman RL, Naliboff B, Lipp OV, Negoro H, Ornitz EM. Is aversive learning a marker of risk for anxiety disorder in children? Behaviour Research and Therapy. 2008;46:954–967. doi: 10.1016/j.brat.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. Journal of Neuroscience. 2000;20:3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C. Associative learning deficits increase symptoms of anxiety in humans. Biological Psychiatry. 2002a;51:851–858. doi: 10.1016/s0006-3223(01)01370-1. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biological Psychiatry. 2002b;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. Fear-potentiated startle in humans: Effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28:588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious response to predictable and unpredictable aversive events. Behavioral Neuroscience. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Fear-potentiated startle in adolescent offspring of parents with anxiety disorders. Biological Psychiatry. 1998;44:990–997. doi: 10.1016/s0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Castille C, Olvet DM, Dunning JP, Roohi J, Hatchwell E. Genetic variation in brain-derived neurotrophic factor and human fear conditioning. Genes, Brain and Behavior. 2009;8:80–85. doi: 10.1111/j.1601-183X.2008.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behavioral Neuroscience. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. New findings on extinction of conditioned fear early in development: Theoretical and clinical implications. Biological Psychiatry. 2010;67:297–303. doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M, Ohman A. Fear and anxiety: Animal models and human cognitive psychophysiology. Journal of Affective Disorders. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, Grillon C, Leibenluft E, Lissek S, Norcross M, Shiffrin N, Pine DS. Distinct neural signatures of threat learning in adolescents and adults. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4500–4505. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JYF, Lissek S, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, Jenness J, Ernst M, Grillon C, Pine DS. Fear conditioning in adolescents with anxiety disorders: Results from a novel experimental paradigm. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:94–102. doi: 10.1097/chi.0b01e31815a5f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. Fear and the brain: Where have we been, and where are we going? Biological Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Liberman LC, Lipp OV, Spence SH, March S. Evidence for retarded extinction of aversive learning in anxious children. Behaviour Research and Therapy. 2006;44:1491–1502. doi: 10.1016/j.brat.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, Grillon C. Generalization of conditioned fear-potentiated startle in humans: Experimental validation and clinical relevance. Behaviour Research and Therapy. 2008;46:678–687. doi: 10.1016/j.brat.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: A meta-analysis. Behaviour Research and Therapy. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin SJ, Heller RE, Luckenbaugh D, Geraci M, Pine DS, Grillon C. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. American Journal of Psychiatry. 2010;167:47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychologica. 2008;127:567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Neumann DL, Waters AM, Westbury HR, Henry J. The use of an unpleasant sound conditional stimulus in an aversive conditioning procedure with 8- to 11-year-old children. Biological Psychology. 2008;79:337–342. doi: 10.1016/j.biopsycho.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Quas JA, Hong M, Alkon A, Boyce WT. Dissociations between psychobiologic reactivity and emotional expression in children. Developmental Psychobiology. 2000;37:153–175. doi: 10.1002/1098-2302(200011)37:3<153::aid-dev4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Reeb-Sutherland BC, Helfinstein SM, Degnan KA, Perez-Edgar K, Henderson HA, Lissek S, Chronis-Tuscano A, Grillon C, Pine DS, Fox NA. Startle response in behaviorally inhibited adolescents with a lifetime occurrence of anxiety disorders. Journal of the Academy of Child and Adolescent Psychiatry. 2009;48:610–617. doi: 10.1097/CHI.0b013e31819f70fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behavioral Neuroscience. 1993;107:887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Merikangas K, Swendsen H, Cui L, Heaton L, Grillon C. Measuring anxious responses to predictable and unpredictable threat in children and adolescents. Journal of Experimental Child Psychology. 2011;110:159–170. doi: 10.1016/j.jecp.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Henry J, Neumann DL. Aversive pavlovian conditioning in childhood anxiety disorders: Impaired response inhibition and resistance to extinction. Journal of Abnormal Psychology. 2009;118:311–321. doi: 10.1037/a0015635. [DOI] [PubMed] [Google Scholar]


