Abstract
1,25-dihydroxyvitamin D (1,25D), through association with the nuclear vitamin D receptor (VDR), exerts control over a novel endocrine axis consisting of the bone-derived hormone FGF23, and the kidney-expressed klotho, CYP27B1, and CYP24A1 genes, which together prevent hyperphosphatemia/ectopic calcification and govern the levels of 1,25D to maintain bone mineral integrity while promoting optimal function of other vital tissues. When occupied by 1,25D, VDR interacts with RXR to form a heterodimer that binds to VDREs in the region of genes directly controlled by 1,25D (e.g., FGF23, klotho, Npt2c, CYP27B1 and CYP24A1). By recruiting complexes of comodulators, activated VDR initiates a series of events that induces or represses the transcription of genes encoding proteins such as: the osteocyte-derived hormone, FGF23; the renal anti-senescence factor and protein co-receptor for FGF23, klotho; other mediators of phosphate transport including Npt2a/c; and vitamin D hormone metabolic enzymes, CYP27B1 and CYP24A1. The mechanism whereby osteocytes are triggered to release FGF23 is yet to be fully defined, but 1,25D, phosphate, and leptin appear to play major roles. The kidney responds to FGF23 to elicit CYP24A1-catalyzed detoxification of the 1,25D hormone while also repressing both Npt2a/c to mediate phosphate elimination and CYP27B1 to limit de novo 1,25D synthesis. Comprehension of these skeletal and renal actions of 1,25D should facilitate the development of novel mimetics to prevent ectopic calcification, chronic renal and vascular disease, and promote healthful aging.
Keywords: 1,25-dihydroxyvitamin D hormone; vitamin D receptor; retinoid X receptor; phosphate homeostasis; X-linked hypophosphatemic rickets; autosomal dominant hypophosphatemic rickets; tumor-induced osteomalacia; tumoral calcinosis
1 Introduction
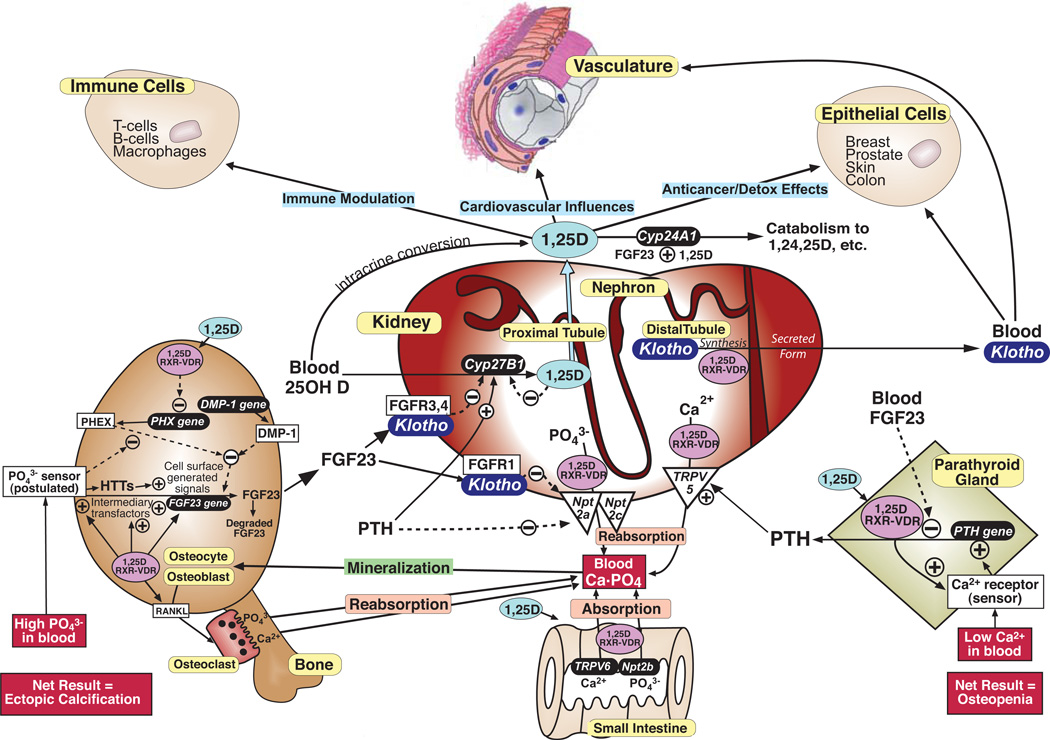
During the last decade, a new understanding of the homeostatic control of phosphate has emerged, emanating originally from the characterization of unsolved familial hypo- or hyperphosphatemic disorders which we now know are caused by deranged levels of bone-derived FGF23 [1]. In short, FGF23 has materialized as a novel chronic phosphate regulator. Several groups [2–4] have shown that 1,25D induces the release of FGF23 from bone, specifically from osteocytic cells of the osteoblastic lineage (Fig. 1). Also uncovered was the FGF23 coreceptor, α-klotho (termed "klotho" herein), a bona fide longevity factor expressed primarily in the renal distal tubule [5, 6] (Fig. 1). The dominant role of FGF23 is the elimination of phosphate at the kidney [7], acting similarly to PTH by inhibiting Npt2a/c-facilitated reabsorption of phosphate. However, unlike PTH, which induces CYP27B1 under conditions of hypocalcemia, FGF23 represses CYP27B1 in hyperphosphatemic states [7]. Therefore the second major action of FGF23 at the kidney is to feedback repress vitamin D bioactivation (Fig. 1). Concomitantly, FGF23 induces renal CYP24A1 to further lower 1,25D via degradation [8] (Fig 1). The 1,25D/VDR-FGF23-klotho-CYP24A1-phosphate system has surfaced as being equally significant to the well characterized PTH-CYP27B1-1,25D/VDR-calcium axis [9]. The current review addresses the 1,25D/VDR-FGF23-klotho-CYP24A1 axis in its ability not only to control phosphate and preclude ectopic calcification, but also to feedback govern vitamin D bioactivation and catabolism to prevent hypervitaminosis D. Moreover, we present the novel hypothesis that this metered bone-kidney axis is crucial for healthful aging by reducing risk of chronic diseases. Bone is an omnipresent source of FGF23, and the kidney is a nexus of control that produces both klotho and 1,25D, ultimately benefiting not only mineral metabolism, but perhaps also the vasculature, the immune system, and epithelial cells prone to fatal cancers [10] (Fig. 1). Thus, with respect to the chronic diseases of aging, it is now becoming clear that the kidneys represent endocrine organs for healthful aging, and we contend that klotho is a third renal hormone after erythropoietin and 1,25D. These concepts emphasize the importance of renal health during aging, unveiling the kidney as a focal point for the prevention of chronic diseases (Fig. 1).
Fig. 1.
Bone, kidney and parathyroid comprise an endocrine trio for the regulation of phosphate and calcium metabolism to prevent osteopenia/osteoporosis and ectopic calcification. Renal hormones 1,25D (shaded in light blue) and klotho (shaded in dark blue) may reach beyond bone mineral homeostasis to delay other chronic disorders of aging besides osteoporosis, such as cardiovascular disease, epithelial cell cancers and autoimmune disease. HTTs (shown in the osteoblast/osteocyte) are hyperphosphatemia transducing transfactors, as discussed in the text and in the legend to Fig. 5.
2 Fibroblast growth factor 23
2.1 Actions and pathophysiology
FGF23 is a recently recognized phosphaturic peptide hormone which functions acutely in concert with PTH, and chronically when PTH is suppressed by calcium and 1,25D. In fact, FGF23 directly represses PTH [11] (Fig. 1) to abolish the activation of CYP27B1 by PTH, while at the same time appropriating from PTH the role of phosphate elimination. Like PTH, FGF23 inhibits renal Npt2a and Npt2c to elicit phosphaturia [7] (Fig. 1). Yet oppositely to PTH, FGF23 is upregulated by 1,25D in osteocytes [1, 2, 4], its major cell of endocrine production by bone. As illustrated in Fig. 1 (lower left), hyperphosphatemia enhances osteocytic FGF23 production independently of 1,25D, rendering FGF23 the perfect phosphaturic counter-1,25D hormone because it inhibits renal phosphate reabsorption, and 1,25D biosynthesis via inhibition of CYP27B1, while enhancing 1,25D degradation by inducing CYP24A1 in all tissues (Fig. 1). In this fashion, FGF23 allows osteocytes to communicate with the kidney to govern circulating 1,25D levels as well as circulating phosphate, thereby preventing excess 1,25D function and hyperphosphatemia. FGF23 signals via renal FGFR1/klotho coreceptors to promulgate phosphaturia [12] and via renal FGFR3,4/klotho [13] to repress CYP27B1 [14] and induce CYP24A1 [7, 12] (Fig. 1). In the case of FGF23 control of phosphate, signal transduction occurs through phospho-ERK and induction of the early response gene Egr-1 [15], whereas signaling to regulate circulating 1,25D levels is transduced through phospholipase Cγ and PKC [16].
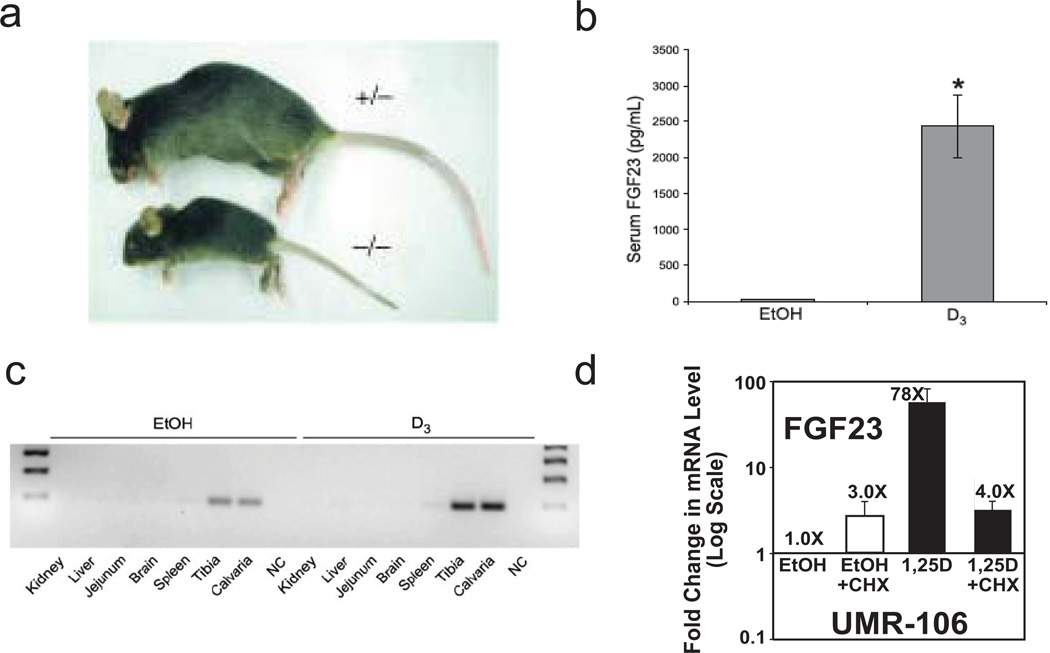
The physiologic roles of FGF23 are highlighted by the phenotype of the FGF23 knockout mouse [8], for which the two dominant characteristics are hyperphosphatemia and ectopic calcification. FGF23 null mice (Fig. 2a) possess markedly elevated 1,25D in blood, and also have a shortened lifespan/premature aging, arteriosclerosis, osteoporosis, muscle atrophy, skin atrophy, hearing loss, and chronic obstructive pulmonary disease, with complications from the latter usually having fatal consequences [8]. Thus, FGF23, a gene induced by 1,25D, could be considered a longevity gene [10]. However, double knockouts of FGF23 with either VDR [17] or CYP27B1 [18] essentially rescue the FGF23 null phenotype in mice, indicating that the capability of FGF23 to function as a counter-regulatory hormone to 1,25D is key to its health and longevity benefits. The endocrine "Rosetta Stone" that allows the body to keep vitamin D in check is FGF23 and its signaling.
Fig. 2.
Phenotypic biological functions of FGF23 and regulation by 1,25D. (a) Ablation of FGF23 elicits growth retardation and a senescent phenotype [8]. (b) 1,25D injection (6 µg/kg body weight once per day for 2 days) generates circulating FGF23 in the mouse, in vivo. (c) The origin of FGF23 is bone, measured as FGF23 mRNA enhancement by 1,25D (24 hours after the second injection) in mouse tibia and calvaria. (d) Striking induction of FGF23 mRNA by 1,25D in UMR-106 osteocyte-like cells of the osteoblastic lineage is partially sensitive to inhibition by cycloheximide (10 µM CHX was added 30 min prior to the 1,25D), indicating secondary as well as primary regulation of transcription.
Notably, FGF23 regulation is complex and multifactorial. For instance, PHEX, the gene encoding an endoproteinase which is loss-of-function mutated in X-linked hypophosphatemic rickets (XLH), acts to repress FGF23 expression [19] (Fig. 1), and excess FGF23 causes the hypophosphatemic pathology in three disorders: 1) XLH; 2) tumor-induced osteomalacia, due to ectopic FGF23 secretion [20]; and 3) autosomal dominant hypophosphatemic rickets (ADHR) [20], wherein FGF23 is altered to preclude proteolytic inactivation. Conversely, loss of function mutations in the FGF23 gene elicit severe hyperphosphatemia and the clinical syndrome of tumoral calcinosis [1].
2.2 Mechanism of control by 1,25D
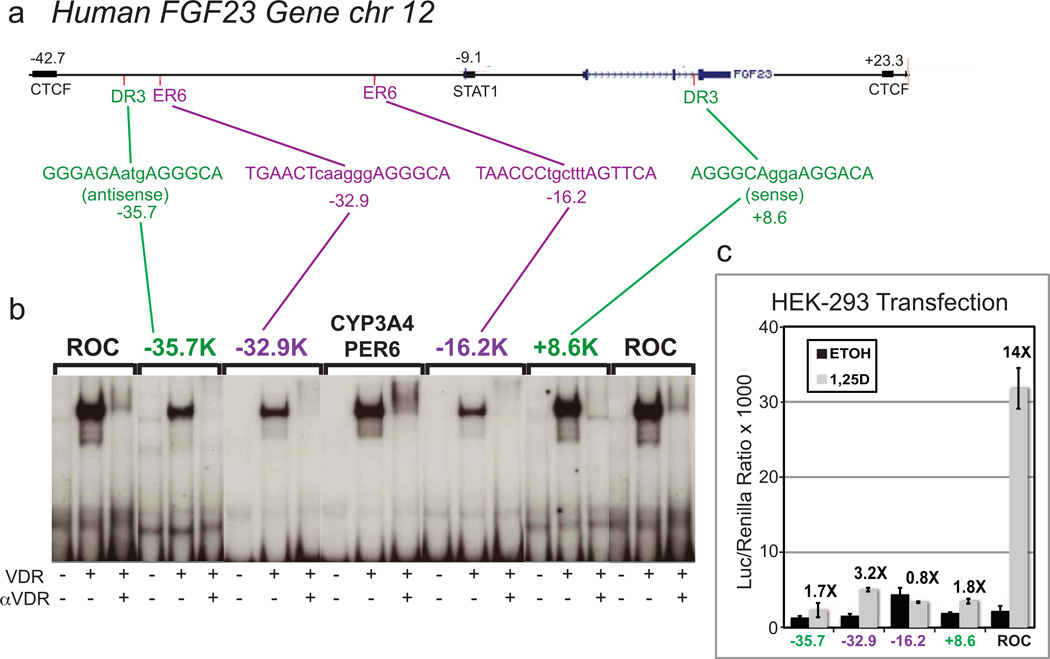
That FGF23 production is highly dependent on 1,25D is proven by the extremely low levels of circulating FGF23 in VDR null mice [21], and the dramatic 80-fold increase in serum FGF23 (Fig. 2b), almost entirely from bone (Fig. 2c), when normal mice are treated with 1,25D [2]. Moreover, FGF23 mRNA is upregulated 78-fold by 1,25D (100 nM for 24 hours) as shown in Fig. 2d in rat UMR-106 osteocyte-like cells of the osteoblast lineage. Generally, 1,25D-signaled increases in steady-state mRNA levels are the result of primary transcriptional activation mediated by liganded VDR in association with its RXR heterodimeric partner via vitamin D responsive elements (VDREs) present in or in the vicinity of the gene in question. Interestingly, with respect to the mechanism for control of FGF23 by 1,25D, we observed that induction of FGF23 by 1,25D is partially cycloheximide-sensitive (Fig. 2d), suggesting either that part of the effect is a secondary one elicited via an intermediary transcription factor, or that VDR requires a rapidly turning over auxiliary factor. However, even in the presence of cycloheximide, there is a significant four-fold increase in the induction of FGF23 mRNA by 1,25D, suggesting that at least part of the dramatic 78-fold upregulation of FGF23 in osteocyte-like cells is mediated by a primary action of 1,25D-VDR/RXR. Consequently, we examined, in silico, the human FGF23 gene located on chromosome 12 for the presence of candidate VDREs. Putative VDREs were not detected in the proximal promoter, but two VDREs of the DR3 type are located at −35.7 kb and +8.6 kb, and two of the ER6 type are located at −32.9 kb and −16.2 kb (Fig. 3a). In choosing an interval for in silico searches for candidate VDREs, we have assumed that transcription factors generally act on genes that are contained within the same CCCTC-binding factor (CTCF) site boundaries, denoted "insulators" [22]. The above listed VDREs are therefore all contained within the boundaries of insulators ZHAO12486 and ZHAO12487 [23].
Fig. 3.
Characterization of putative remote VDREs in the human FGF23 gene. (a) In silico identification of four candidate VDREs in the human FGF23 gene. (b) Capacity of each VDRE to bind VDR in an electrophoretic mobility shift assay. Positive control VDREs are the CYP3A4 proximal ER6 (PER6) and a rat osteocalcin DR3 element (ROC). VDR = probes were incubated with a lysate of COS-7 cells transfected with a VDR overexpression plasmid; α-VDR = assay contained anti-VDR antibody 9A7γ. (c) Transcriptional enhancer activity in a luciferase reporter gene assay (2 copies of each VDRE) in transfected human embryonic kidney cells (HEK-293). The most potent VDRE (TGAACTcaagggAGGGCA) is an ER6 located at −32.9kb.
These putative FGF23 VDREs were tested by electrophoretic mobility shift assays (EMSA) and shown to bind VDR to varying degrees (Fig. 3b). Insertion upstream of a luciferase reporter plasmid conferred 1,25D responsiveness using three (−35.7, −32.9, +8.6 kb) of the four candidate VDREs in the human FGF23 gene (Fig. 3c). These three positive VDREs correspond to the most effective elements in the gel mobility shift assay, and possess moderate transcriptional activity, suggesting that these three elements, in their proper context, could explain in part the striking induction of FGF23 by 1,25D. With respect to other species, Liu et al. have reported a VDRE at −1156 bp in the proximal promoter of the rat FGF23 gene [4] and we have observed five candidate VDREs downstream of the rat FGF23 gene at +88 to +119 kb (data not shown). Recent data from the J.W. Pike group, summarized in a companion review in this issue, indicate that VDREs positioned far upstream as well as downstream of the promoter are highly significant in VDR-mediated gene control [24].
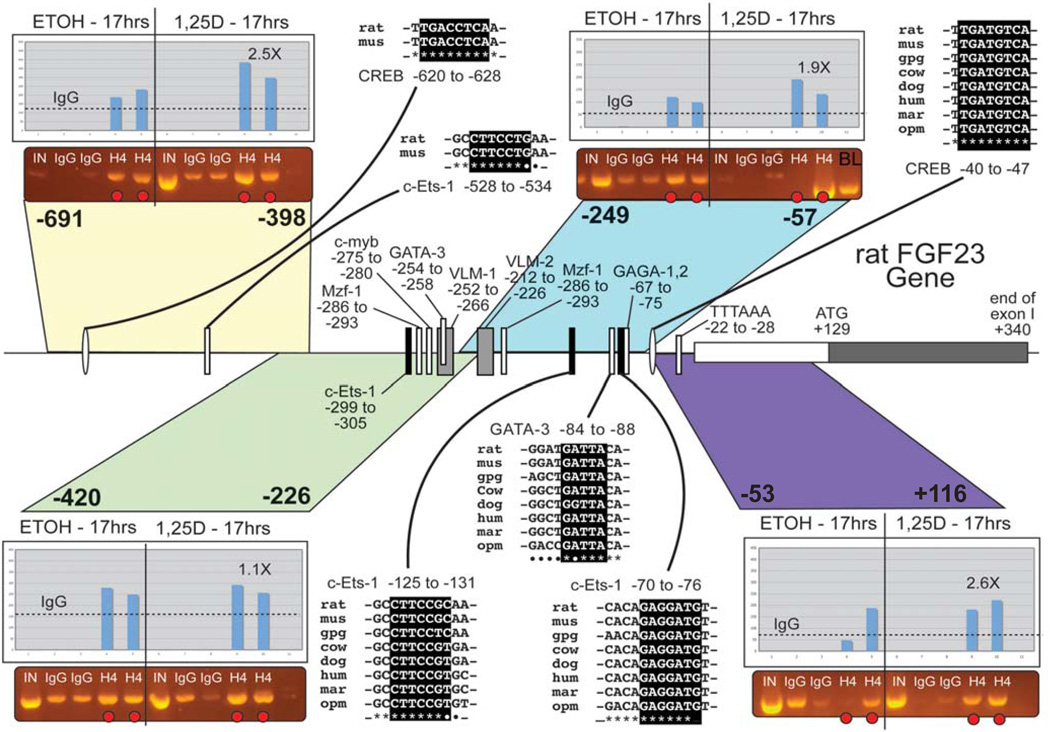
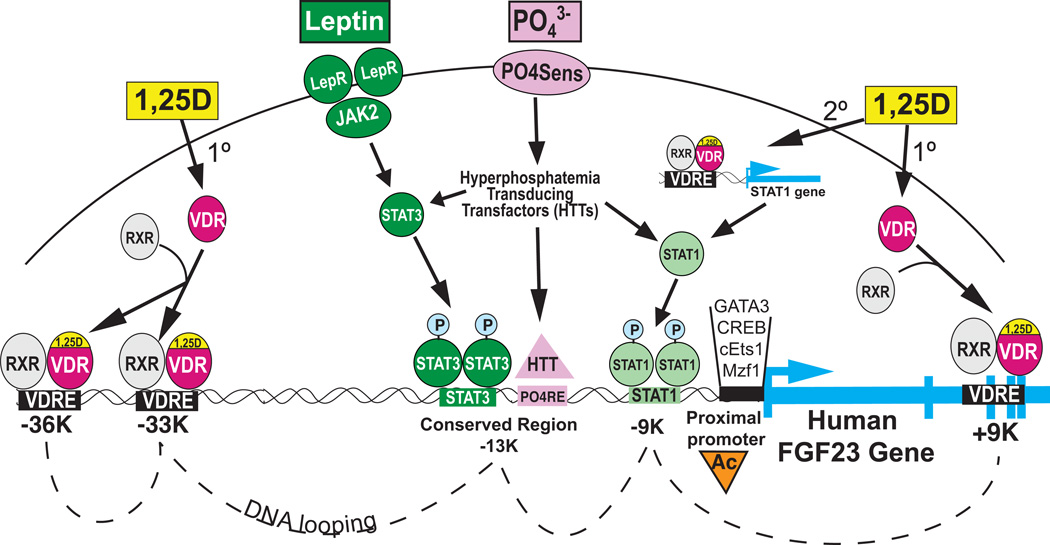
Although part of the effect of 1,25D on FGF23 mRNA levels may be through VDREs, and a significant activation of FGF23 transcription occurs rapidly (4 hours) after 1,25D treatment [2], results from the R. Bouillon group reveal that a paracrine mediator derived from chondrocytes may be required for osteoblasts to respond fully to 1,25D when inducing FGF23 [25]. Also, as discussed above, the induction of FGF23 by 1,25D is partially blocked by cycloheximide treatment of the cells (Fig. 2d). Thus, there is a good possibility that a 1,25D-induced secondary factor controls the FGF23 promoter. We find that the first 300 bp of the FGF23 promoter contain transcription factor (TF) binding sites that are highly conserved among mammals, including two c-Ets-1 binding sites, one CREB site and one GATA-3 site (Fig. 4), along with selected other conserved TF binding sites. ChIP assays using antisera against acetylated histone H4 in rat UMR-106 cells revealed that the regions of the rat FGF23 proximal promoter from: −53 to +114 (purple in Fig. 4; CREB), −249 to −57 (c-Ets-1 & GATA-3), and −691 to −398, showed an enrichment in acetylated H4 after treatment for 17 hrs with 10 nM 1,25D (Fig. 4, insets). The more distal −691 to −398 region is not well conserved among mammals but does contain two TF binding sites (CREB and c-Ets-1) that are conserved between mouse and rat (Fig. 4, upper left). Moreover, real-time PCR experiments using mRNA from UMR-106 cells reveal that the rat c-Ets-1 factor is upregulated 2-fold after 24 hr of 1,25D treatment [26]. In addition, another TF, STAT-1, is upregulated 2.4-fold by 1,25D in UMR-106 cells [26]. Therefore, as depicted schematically in Figure 5, 1,25D appears to act in a primary fashion through VDREs in the FGF23 gene located at approximately −36 kb, −33 kb, and +9 kb, as well as secondarily through 1,25D-induced transcription factors (GATA3, CREB, cEts1, and STAT1) that activate the proximal promoter collectively to induce the FGF23 gene.
Fig. 4.
The proximal promoter of the rat FGF23 gene. The first exon and 691 bp of 5' flank are shown along with conserved transcription factor binding sites as indicated. ChIP assays (see insets) were performed on colored segments using antisera to acetylated histone H4 (Millipore Corp.) and were quantified by densitometry (see bar graphs). Species key: mus = mouse; gpg = guinea pig (Cavia porcellus); mar = marmoset (Callithrix jacchus); opm = opossum (Monodelphis domestica).
Fig. 5.
Model for concerted control of FGF23 gene expression by 1,25D, phosphate and leptin. Liganded VDR associates with distal and downstream VDREs and induces STAT1, cEts1, etc., intermediary transcription factors for secondary regulation of FGF23 transcription. Leptin signals through JAK2 to generate phospho-STAT3 binding to its responsive element in a conserved region centered at approximately −13 kb relative to the start site of FGF23 transcription. Hyperphosphatemia, detected by the hypothetical PO4 sensor, elicits a cascade of hyperphosphatemia transducing transfactors (HTTs) which are postulated to both amplify STAT1/3 signaling and terminate at a putative PO4 responsive element (PO4RE) in the conserved region at −13kb. Phosphate is hypothesized to be the central regulator, with the net result being histone acetylation (Ac) at the proximal promoter, which facilitates the assembly of multiple transcriptional activation complexes on the conserved elements shown in Fig. 4, likely amplified by DNA looping.
2.3 Control by phosphate, leptin, and other mediators
FGF23 synthesis is also governed by high phosphate [27], possibly via an undiscovered transcription factor (analogous to signaling through Gq by the calcium sensing receptor in the parathyroid and other tissues) to induce the FGF23 gene, with the provocative possibility that these two independent secretagogues for FGF23 converge via VDR and another transactivator at a locus of regulation, possibly involving DNA looping, in the FGF23 gene. Additional factors that play a specific role in transduction of the phosphate signal are termed "hyperphosphatemia transducing transfactor(s)" (HTTs) (Fig. 5). The targeting of these factors will be of great interest to those attempting to modulate FGF23 in patients such as those in renal failure who may benefit from reduced FGF23 secretion [28, 29]. Finally, identification of these factors will also increase our comprehension of the control of FGF23 and we may for the first time be able to integrate the 1,25D and phosphate arms of FGF23 regulation with other known osteocyte players such as PHEX and DMP1 (Fig. 1), as well as renal phosphate transporters, Npt2a/c. Indeed, Demay et al. [30] have recently shown, by ablation of renal phosphate transporter Npt2a, that phosphate is the central regulator of the FGF23 gene, capable of prevailing over vitamin D because 1,25D fails to induce FGF23 when hypophosphatemia and elevated 1,25D occur concurrently.
Strikingly, leptin has been shown to stimulate FGF23 expression in the Ob mouse, as well as in rat calvarial outgrowth cultures via its STAT3 transcriptional mediator [31] (Fig. 5), and the mouse FGF23 gene is replete with occupied phospho-STAT3 dimer sites as determined by ChIP-Seq [32]. Thus, at least three known triggers in the osteocyte, namely 1,25D, phosphate, and leptin, control FGF23 expression (Fig. 5), and potential VDR accessory transcription factors may be the key to the integrated regulation of FGF23 by these three secretagogues.
There may also exist crosstalk between leptin and 1,25D and/or phosphate signaling, either in the cytoplasm via networking of phosphorylation pathways, or at the level of the FGF23 gene through overlapping or looped out responsive elements for leptin (STAT3), vitamin D (VDRE), and phosphate (termed PO4RE) (Fig. 5). As shown in Fig. 5, phosphate may be the pivotal modulator of FGF23 expression, with fine-tuning by 1,25D and leptin signaling likely being dependent upon looping out of DNA to bring the PO4RE(s) near occupied VDREs and STAT elements.
FGF23 regulation is complex and multifactorial, including the suppressive proteins PHEX and dentin matrix acidic phosphoprotein 1 (DMP-1) (Fig. 1). 1,25D represses PHEX expression in UMR-106 osteocyte-like cells, which is in accordance with the induction of FGF23 in that the PHEX suppressor is attenuated to permit maximal induction of FGF23 by 1,25D. It is conceivable that the mechanism of FGF23 induction by 1,25D is, in part or entirely, a consequence of PHEX repression, yet the PHEX substrate which ultimately regulates FGF23 transcription is not known. Although DMP-1 is apparently not a PHEX substrate, loss of function mutations in DMP1 cause a phenotype identical to XLH, with excess FGF23 producing hypophosphatemia. This suggests that DMP1, like PHEX, normally represses FGF23 expression in osteocytes; although this is inconsistent with the observation [33] that 1,25D induces DMP1 in UMR-106 cells. Fascinatingly, it has been shown recently [34] that PHEX and DMP1 regulate FGF23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling, intimating that PHEX and DMP-1 regulate FGF23 expression by impacting an autocrine loop in the osteocyte whereby FGF23 governs its own synthesis.
3 Klotho and fibroblast growth factor receptors
3.1 Forms, actions and pathophysiology
Klotho is the only reported single gene mutation that leads to a premature aging phenotype in the mouse [5], and a recessive inactivating mutation in the human klotho gene elicits a phenotype of severe tumoral calcinosis [35]. Klotho- and FGF23-null mice have identical hyperphosphatemic phenotypes of short life-span/premature aging, ectopic calcification, arteriosclerosis, osteoporosis, muscle atrophy, skin atrophy, and hearing loss [5, 8]. Klotho is a known co-receptor for FGF23, whereas β-klotho is a coreceptor for other FGF hormones. Klotho exists in multiple forms [36], a full-length (130 kDa) transmembrane co-receptor with a minimal cytoplasmic region of 11 amino acids and two extracellular domains with homology to glycosyl hydrolases, at least three proteolyzed forms that are shed into the circulation [37], and finally a hypothetical 80 kDa secreted form produced by alternative splicing in exon 3 that generates a protein species possessing a portion of the extracellular domain containing one of the glycosyl hydrolase domains [38]. Because of the potential significance of these forms in klotho biology, much remains to be learned about how their expression is regulated. Recently, Thurston et al. have demonstrated that TNFα and γ-interferon are suppressors of renal klotho expression [39]; the FGF23 ligand is also a putative repressor of klotho expression [40]. With the exception of a preliminary report by Tsujikawa et al. [41] who treated mice with various dietary and pharmacologic regimens, including vitamin D, inducers of klotho are poorly characterized. However, as detailed below, we observe that 1,25D significantly induces klotho mRNA expression at the cellular and molecular level in human and mouse renal cell lines.
3.2 Upregulation by 1,25D and curcumin
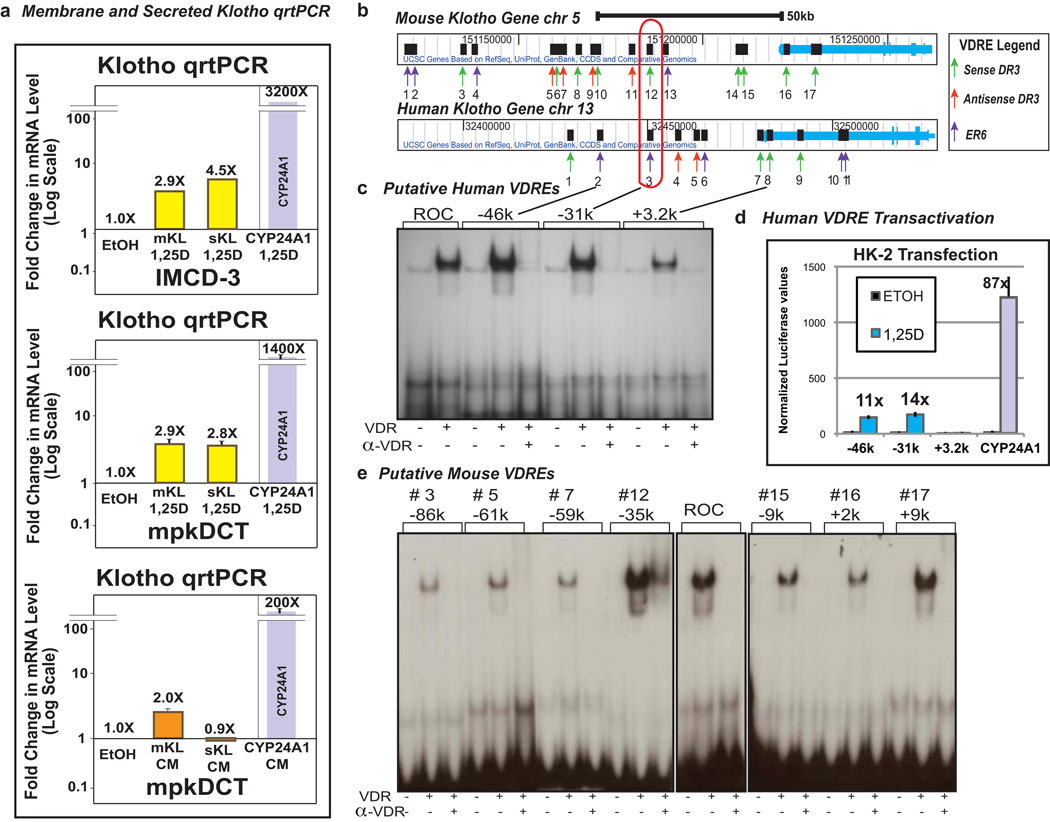
We carried out real time PCR utilizing RNA isolated from mouse (IMCD-3 and mpkDCT) cells as described previously [42] with primers designed to capture both alternatively spliced mRNAs for the membrane and secreted forms of klotho. The results (Fig. 6a) indicate that 1,25D treatment (100 nM for 24 hours) induces klotho mRNA expression in the mouse distal tubule cell line (mpkDCT) as well as in the inner medullary collecting duct cells (IMCD-3). This induction is evident for both the membrane and secreted forms of klotho mRNA, suggesting that 1,25D may be capable of both amplifying FGF responsiveness and eliciting secretion of circulating klotho hormone. These data complement our previous demonstration of klotho mRNA induction by 1,25D in human proximal kidney (HK-2) cells [10]. Interestingly, curcumin, an alternative VDR ligand [43], selectively upregulates membrane klotho mRNA in mpkDCT cells, indicating that distinct VDR ligands can differentially modulate the membrane and secreted forms of klotho. These data lead to the hypothesis that designer vitamin D analogs could promote the healthful aging benefits of systemic klotho without accentuating FGF23 action to perhaps elicit hypophosphatemia.
Fig. 6.
VDR-mediated regulation of mouse and human klotho genes. The mouse klotho gene is induced by 1,25D in a cell line derived from mouse intermedullary collecting duct (IMCD)-3 (a, top panel) as well as in a mouse distal convoluted tubule cell line (mpkDCT) (a, center panel); curcumin (50 µM, 24 hours), an alternative VDR ligand, also induces klotho in mpkDCT cells but the effect is selective for membrane klotho (mKL; a, lower panel) whereas 1,25D significantly upregulates both klotho mRNA spliceforms in both IMCD-3 and mpkDCT cells (a, upper and middle panels). Human klotho VDREs (b) at −46kb (DR3 = GGTTCGtagAGTTCA) and −31kb (DR3 = AGTTCAagaAGTTCA) display VDR electrophoretic mobility shift (c) and transactivity (d). Mouse klotho VDREs, especially those at −35k and +9k (#12 and #17, respectively) exhibit strong VDR electrophoretic mobility shift activity (e). A rat osteocalcin DR3 element (ROC) serves as the positive control for the EMSAs in c and e.
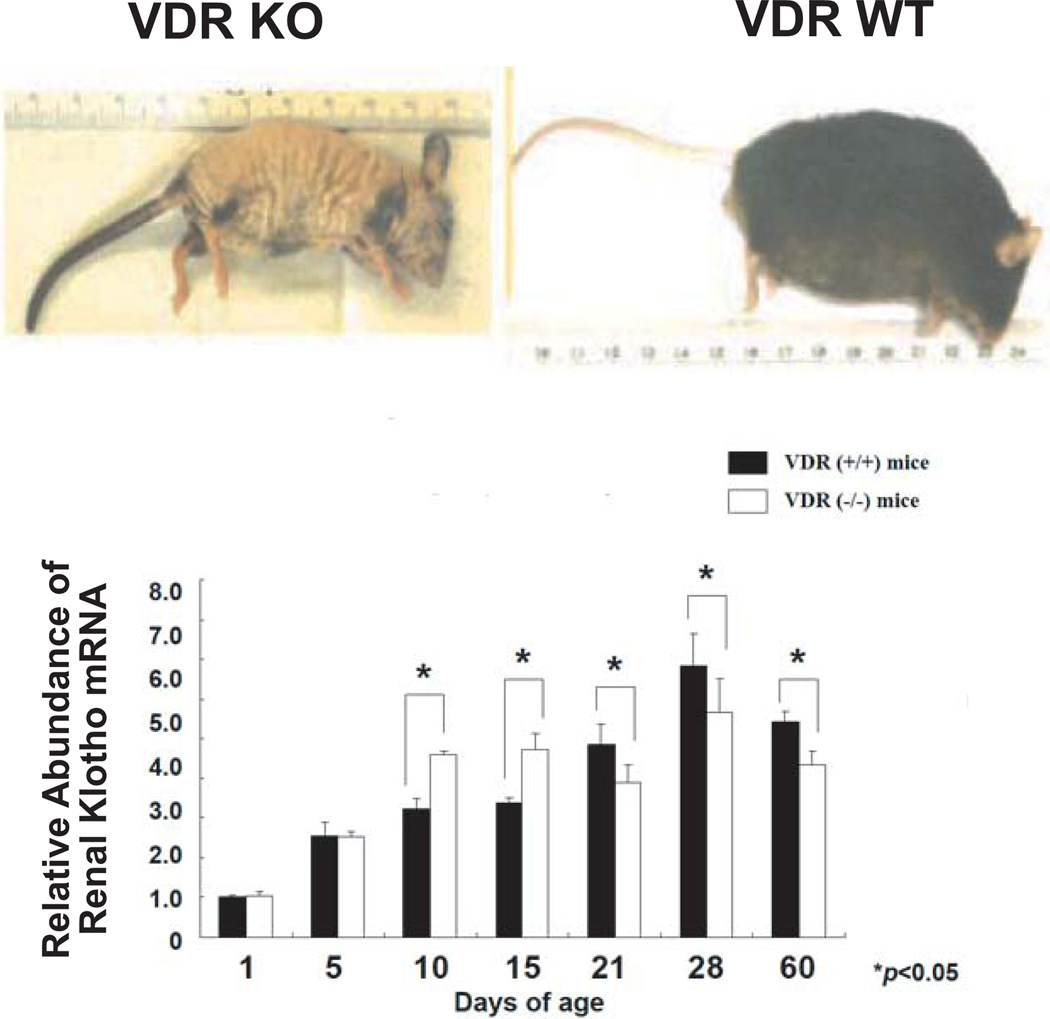
A bioinformatic analysis of both the human and mouse klotho genes reveals 11 candidate VDREs in the human gene and 17 putative VDREs in the mouse gene (Fig. 6b). Electrophoretic mobility shift assays and transcriptional assays in transfected renal cells were performed with VDRE-luciferase reporter plasmids. EMSA of eleven candidate VDREs in the human klotho gene reveals that three of these (−46 kb, −31 kb and +3.2 kb) display an ability to bind VDR/RXR which is abrogated by the 9A7 anti-VDR mAb. Two of these VDREs (−46 kb and −31 kb) are more potent in this assay than the established rat osteocalcin VDRE (Fig. 6c). Interestingly, the remote location of these two VDREs in relation to the transcriptional start site of klotho, yet residing between two insulators, is consistent with the new paradigm of VDR/RXR action in which the chromatin conformation loops out DNA to bring distant enhancers together adjacent to the RNA Pol II docking site [44]. Finally, we analyzed the functional activity of candidate VDREs at −46 kb, −31 kb and +3.2 kb in transfected HK-2 renal cells and observed striking (>10-fold) 1,25D responsiveness of VDREs corresponding to sequences at −46 kb and −31 kb, but not +3.2 kb, in the context of synthetic VDRE-luciferase reporter constructs (Fig. 6d). A similar analysis of 17 candidate mouse VDREs, of which only one shows a degree of positional (but not sequence) conservation with a human VDRE (Fig. 6b, red outline), revealed only two mouse VDREs at −35 kb and +9 kb displayed gel shift activity comparable to that of rat osteocalcin VDRE (Fig. 6e). However, only the mouse klotho VDRE located at −35 kb displays transactivation ability (data not shown). Thus, it appears that 1,25D-liganded VDR-RXR induces klotho expression by binding to functional VDREs in the range of 31 to 46 kb 5' of the transcriptional start site of both the human and mouse klotho genes. Regarding the relevance of liganded VDR in maintaining klotho expression, the data in Fig. 7 suggest that klotho mRNA displays an age-related dependence on VDR, since lower levels of klotho expression were observed in older VDR KO mice kidneys. The decline in klotho mRNA in VDR KO vs. WT littermates after 21 days is statistically significant (Fig. 7), but modest in magnitude; nevertheless, assuming the protein reflects the mRNA concentrations, a modest increase (even only 25%) within this span of life could still make a significant impact in protecting aging tissues such as the vascular system. Keisala and colleagues have reported that renal klotho mRNA expression is lower in VDR null mice compared to their normal littermates, but the difference is not statistically significant as it is for depressed hepatic FGF23 mRNA expression in VDR KO mice [45]. Thus, FGF23 appears to be more dramatically dependent on VDR activation in liver than is klotho on VDR activation in distal renal tubule.
Fig. 7.
VDR KO mice (upper left), compared to wild-type mice (upper right) [66], display a statistically significant trend toward lower renal expression of klotho mRNA at ≥21 days of age versus WT controls. However, this decline is preceded by increased klotho mRNA in younger VDR KO mice. Thus, klotho may be VDR-dependent only in aging mice.
3.3 Cardiovascular benefits
Both klotho and VDR knockout mice display increased cardiovascular complications [5, 46]. Klotho knockout mice show ectopic vascular calcification in large and small vessels and endothelial dysfunction, the latter of which can be restored in a parabiosis model, suggesting reversibility by circulating klotho [47, 48]. Similarly, VDR null mice demonstrate elevated cardiovascular risk factors including increased blood pressure and left ventricular hypertrophy (LVH) and fibrosis [46]. Recently, klotho gene transfer to an adult spontaneously hypertensive rat (SHR) model was reported to result in a prevention of aging-related increases in blood pressure and reductions in aortic markers of oxidative stress [49]. In addition, a link between this anti-aging gene and the renin-angiotensin system has been suggested based on recent studies demonstrating angiotensin II-induced downregulation of klotho mRNA [49]. Furthermore, klotho gene transfer has been shown to mitigate angiotensin II-induced renal damage [49].
4 Phosphate homeostasis, bone disease and aging
1,25D is primarily a calcemic hormone through its actions to stimulate intestinal calcium absorption, bone calcium resorption, and renal calcium reabsorption. Although 1,25D enhances intestinal phosphate absorption via the induction of Npt2b [50], because phosphate is abundant in the diet and constitutively absorbed by the small intestine, the phosphate absorption effect of 1,25D may not be as physiologically important as the profound effect of 1,25D to trigger calcium transport. Although 1,25D is thought to provoke phosphate reabsorption at the kidney, it has been difficult to show that this effect is physiologically relevant, perhaps because of the weak induction of renal Npt2a by 1,25D [51]. 1,25D does induce Npt2c [9, 52], however, ablation of this gene in mice does not cause hypophosphatemia [53] despite the fact that a loss of function mutation in human Npt2c does elicit hypophosphatemia [54]. Thus, there may be a species difference between the impact of Npt2c on phosphate homeostasis, and data from mice suggest that Npt2c may be more important as a controller of calcium metabolism through control of FGF23 levels [53]. Therefore, the dominant regulators of renal phosphate reclamation appear to be PTH and FGF23, with 1,25D playing little or no role at the kidney but instead regulating PTH at the parathyroid glands and FGF23 in osteocytes. Based on the above, it is likely that hypophosphatemic rickets and osteomalacia occur only in situations of excess FGF23 action, unlike hypocalcemic rickets and osteomalacia which transpire with either vitamin D or calcium insufficiency.
Chronic hyperphosphatemia, such as occurs in FGF23 and klotho knockout mice, apparently through aberrantly high 1,25D levels, seems to promote senescence. Interestingly, mice models with hypervitaminosis D [45], as well as vitamin D receptor null mice, also undergo premature aging. Thus, in pharmacologic doses or pathological excess, 1,25D generates a phenocopy of the FGF23 ablated mouse, including ectopic calcification, skin atrophy, osteoporosis, vascular disease and emphysema, while the physiologic activities of optimal 1,25D provide for healthful aging and prolong life by lowering the risk of chronic disorders of old age.
Phosphate is abundant in a normal diet and is a fundamental biologic component of not only mineralized bone, but essential biomolecules such as DNA, RNA, phospholipids, phosphoproteins, ATP, metabolic intermediates, etc. Yet phosphate excess may act as a pro-senescence factor independently of 1,25D. For example, an excess of phosphate in the blood can lead to ectopic calcification and arteriosclerosis, COPD, chronic kidney disease, loss of hearing, etc. Fortunately, FGF23 and klotho are designed to eliminate systemic phosphate and perhaps promote healthspan. Klotho, as discussed above, appears to have systemic anti-aging properties independent of its phosphaturic actions, perhaps through its glycosyl hydrolase enzymatic activity [55]. By analogy, the kidney also releases renin, a hormone with proteolytic enzyme activity. Conversely, although FGF23 is anti-aging at the kidney by eliciting phosphate elimination and detoxifying 1,25D, its "off-target" actions could actually be pro-aging in terms of coronary artery disease, and it is possible that these off-target FGF23 pathologies are opposed by secreted klotho [1].
Therefore, the conceptual insights summarized in the present review not only provide greater fundamental understanding of vitamin D and phosphate homeostasis, but may also enhance our comprehension of the pathophysiology of aging disorders (e.g., osteoporotic fractures, muscle weakness, atherosclerosis, ectopic calcification, and hearing loss). Perhaps the prevention and treatment of diseases of aging could not only include statins, a low fat diet and exercise, to reverse the pathology of atheromas, obesity, and Type II diabetes, but would also feature enhanced levels of vitamin D and klotho, to prevent hypertension, improve the underlying cellular infrastructure of the vasculature, preclude ectopic calcification, and maintain a fracture-free skeleton through remodeling. These clinical benefits may be realized through the actions of the FGF23/klotho/1,25D/phosphate axis which are analogous to the healthful effects of antagonizing the "sister" FGF19/FXR/bile acid axis to enhance cholesterol clearance, and the activation of the FGF21/PPARα/polyunsaturated fatty acids axis to stimulate degradation and impede synthesis of saturated fatty acids and cholesterol. Strikingly, there is evidence [56] that the FGF19, FGF21, and FGF23 sibling axes network to achieve optimal lipid, carbohydrate and mineral homeostasis. Finally, the α-klotho co-receptor/mediator of FGF23 effects appears also to function with FGF19 and FGF21, perhaps explaining the broad chronic disease spectrum of the α-klotho null mouse [5, 56].
In summary, we hypothesize that 1,25D is an anti-aging/wellness hormone through its ability to induce klotho, a bona fide anti-senescence principle, and assert that optimum levels of 25D manifest as anti-aging. However, as shown by Keisala et al. [45], both the VDR KO and vitamin D toxic animals display similar pro-aging phenotypes. This situation of dichotomy with respect to 1,25D action is analogous to the actions of the only other known toxic fat soluble vitamin, namely vitamin A and its active retinoic acid metabolite. In physiologic quantities, retinoids mediate epithelial cell differentiation and barrier formation, embryonic development, etc., yet pharmacologic excesses of vitamin A and retinoic acid yield epithelial pathologies such as gastroenteritis and exfoliation, and embryopathy, respectively. Whereas no feedback control exists in the vitamin A system, the FGF23 endocrine system allows the body to keep vitamin D in check by inducing CYP24A1 in conjunction with 1,25D (Fig. 1). Thus, we hypothesize that CYP24A1 represents a second anti-aging gene, along with klotho, that is expressed in response to the vitamin D hormone. Moreover, we propose that CYP24A1, the catabolic enzyme that prevents vitamin D toxicity and ectopic calcification, is also induced by FGF23, a unique mechanism for control of steroid hormone degradation in an endocrine system. Additionally, we postulate (above) that the 1,25D-VDR/FGF23/klotho/CYP24A1 system is analogous to "sister" paradigms [57] of bile acids-FXR/FGF15,19 and polyunsaturated fatty acids-PPARα/FGF21 that protect the vasculature via cholesterol catabolism (i.e., CYP7A1) and fatty acid clearance (i.e., β-oxidation), respectively, with the 1,25D anti-aging axis limiting inflammation and calcification.
5 Bone and kidney in an endocrine/phosphate metabolism duet
The environmental cues that activate the novel endocrine duo of bone and kidney are exposure of the skin to solar radiation and/or dietary intake of vitamin D, as well as the nutritional availability of calcium and phosphate. Thus, the skin and the small intestine represent the "receivers" of micronutrient and mineral input that is required for optimal health and bone mineral density. The endocrine axes are initiated when sensors of calcium on the parathyroid gland and of phosphate on the osteocyte in bone read the prevailing levels of their respective ions and secrete peptide hormones with actions focused on the kidney. One of the actions of PTH at the kidney is to stimulate 1,25D production, which in turn serves as an agent for PTH to promote intestinal calcium absorption and close the endocrine loop triggered by low calcium availability. PTH also elicits acute phosphaturia to counter any excess phosphate absorption caused by 1,25D. Independently, the osteocyte likely perceives phosphate concentrations to elaborate FGF23 in situations of hyperphosphatemia. FGF23 causes chronic phosphaturia to prevent ectopic calcification and maintain phosphate homeostasis. In addition, FGF23 functions as a three-pronged attenuator of 1,25D action, namely by: a) inhibition of PTH secretion, b) repression of renal CYP27B1, and c) induction of renal CYP24A1. This multi-faceted action of FGF23 as a counter regulator of 1,25D results in dramatic attenuation of circulating levels of the 1,25D hormone. Notably, 1,25D is a significant secretagogue for FGF23 at the osteocyte, and therefore the ability of FGF23 to diminish 1,25D constitutes a classic counter-regulatory feedback loop between the two hormones in bone and kidney, respectively. As a result of this endocrine duet, bone mineralization is maintained at a normal level and calcium and phosphate are optimally available for their myriad of other functions in biochemistry and physiology.
Besides bone and mineral, 1,25D, FGF23, and klotho are candidates for modulating the aging process and determining healthspan. For instance, low or very high levels of circulating 25(OH)D are associated with divergent age-related diseases including frailty [58, 59], prostate [60] and pancreatic [61] cancers, and cardiovascular disease [62, 63] suggesting that optimal vitamin D levels promote healthful aging. Similarly, the absence of FGF23 [8] or its excess, for example in end-stage renal disease, leads to premature mortality [29] from heart disease, suggesting again that only optimal levels of FGF23 provide longevity through lowering the risk of chronic disease. Finally, hyperphosphatemia appears to be an independent risk factor for senescence [64, 65] despite the crucial presence of phosphate in virtually all cellular biomolecules. In conclusion, it is the ability of 1,25D, FGF23, and klotho to tightly control phosphate metabolism, as well as to regulate each other and interface with calcium metabolism, that facilitates longevity free of chronic disease.
Acknowledgments
We thank Dr. Shigeaki Kato (University of Tokyo), Dr. Ken-ichi Miyamoto and Dr. Hiroko Segawa (University of Tokushima Graduate School) for sharing the data on klotho expression in VDR knockout mice, and Andrew Hopper IV for performing the experiment shown in Fig. 4. Supported by National Institutes of Health grants to M. R. H.
References
- 1.Bergwitz C, Juppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 2010;61:91–104. doi: 10.1146/annurev.med.051308.111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolek OI, Hines ER, Jones MD, Lesueur LK, Lipko MA, Kiela PR, et al. 1{alpha},25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005;289(6):G1036–G1042. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- 3.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, et al. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280(4):2543–2549. doi: 10.1074/jbc.M408903200. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17(5):1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 5.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 6.Kiela PR, Ghishan FK. Recent advances in the renal-skeletal-gut axis that controls phosphate homeostasis. Lab Invest. 2009;89(1):7–14. doi: 10.1038/labinvest.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 8.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113(4):561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haussler MR, Haussler CA, Bartik L, Whitfield GK, Hsieh JC, Slater S, et al. Vitamin D receptor: molecular signaling and actions of nutritional ligands in disease prevention. Nutr Rev. 2008;66(10 Suppl 2):S98–S112. doi: 10.1111/j.1753-4887.2008.00093.x. [DOI] [PubMed] [Google Scholar]
- 10.Haussler MR, Haussler CA, Whitfield GK, Hsieh JC, Thompson PD, Barthel TK, et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the "Fountain of Youth" to mediate healthful aging. J Steroid Biochem Mol Biol. 2010;121:88–97. doi: 10.1016/j.jsbmb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117(12):4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5(11):611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattineni J, Twombley K, Goetz R, Mohammadi M, Baum M. Regulation of serum 1,25(OH)2Vitamin D3 levels by fibroblast growth factor 23 is mediated by FGF receptors 3 and 4. Am J Physiol Renal Physiol. 2011;301(2):F371–F377. doi: 10.1152/ajprenal.00740.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293(5):F1577–F1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda T, Kanomata K, Nojima J, Urakawa I, Suzawa T, Imada M, et al. FGF23 induces expression of two isoforms of NAB2, which are corepressors of Egr-1. Biochem Biophys Res Commun. 2007;353(1):147–151. doi: 10.1016/j.bbrc.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16(2):139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Hesse M, Frohlich LF, Zeitz U, Lanske B, Erben RG. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 2007;26(2):75–84. doi: 10.1016/j.matbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Renkema KY, Alexander RT, Bindels RJ, Hoenderop JG. Calcium and phosphate homeostasis: concerted interplay of new regulators. Ann Med. 2008;40(2):82–91. doi: 10.1080/07853890701689645. [DOI] [PubMed] [Google Scholar]
- 19.Yuan B, Takaiwa M, Clemens TL, Feng JQ, Kumar R, Rowe PS, et al. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J Clin Invest. 2008;118(2):722–734. doi: 10.1172/JCI32702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukumoto S. Fibroblast growth factor (FGF) 23 works as a phosphate-regulating hormone and is involved in the pathogenesis of several disorders of phosphate metabolism. Rinsho Byori. 2007;55(6):555–559. [PubMed] [Google Scholar]
- 21.Yu X, Sabbagh Y, Davis SI, Demay MB, White KE. Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone. 2005;36(6):971–977. doi: 10.1016/j.bone.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Kim TH, Abdullaev ZK, Smith AD, Ching KA, Loukinov DI, Green RD, et al. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell. 2007;128(6):1231–1245. doi: 10.1016/j.cell.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Pike JW, Meyer MB, Martowicz ML, Bishop KA, Lee SM, Nerenz RD, et al. Emerging regulatory paradigms for control of gene expression by 1,25-dihydroxyvitamin D(3) J Steroid Biochem Mol Biol. 2010;121:130–135. doi: 10.1016/j.jsbmb.2010.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masuyama R, Stockmans I, Torrekens S, Van Looveren R, Maes C, Carmeliet P, et al. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest. 2006;116(12):3150–3159. doi: 10.1172/JCI29463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh A, Gurevich M, Mathern D, Kaczmarska MJ, Haussler CA, Whitfield GK, et al. Identification of cEts1 and STAT1 as potential primary targets in the secondary gene regulation of the human fibroblast growth factor-23 gene by 1,25-dihydroxyvitamin D3. J Bone Miner Res. 2007;22:S402. [Google Scholar]
- 27.Ito M, Sakai Y, Furumoto M, Segawa H, Haito S, Yamanaka S, et al. Vitamin D and phosphate regulate fibroblast growth factor-23 in K-562 cells. Am J Physiol Endocrinol Metab. 2005;288(6):E1101–E1109. doi: 10.1152/ajpendo.00502.2004. [DOI] [PubMed] [Google Scholar]
- 28.Juppner H, Wolf M, Salusky IB. FGF-23: More than a regulator of renal phosphate handling? J Bone Miner Res. 2010;25(10):2091–2097. doi: 10.1002/jbmr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukumoto S. FGF23: Phosphate Metabolism and Beyond. IBMS BoneKEy. 2010;7(8):268–278. [Google Scholar]
- 30.Miedlich SU, Zhu ED, Sabbagh Y, Demay MB. The receptor-dependent actions of 1,25-dihydroxyvitamin D are required for normal growth plate maturation in NPt2a knockout mice. Endocrinology. 2010;151(10):4607–4612. doi: 10.1210/en.2010-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuji K, Maeda T, Kawane T, Matsunuma A, Horiuchi N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J Bone Miner Res. 2010;25(8):1711–1723. doi: 10.1002/jbmr.65. [DOI] [PubMed] [Google Scholar]
- 32.Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32(5):605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrow EG, Davis SI, Ward LM, Summers LJ, Bubbear JS, Keen R, et al. Molecular analysis of DMP1 mutants causing autosomal recessive hypophosphatemic rickets. Bone. 2009;44(2):287–294. doi: 10.1016/j.bone.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin A, Liu S, David V, Li H, Karydis A, Feng JQ, et al. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011;25(8):2551–2562. doi: 10.1096/fj.10-177816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117(9):2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuro-o M. Klotho. Pflugers Arch. 2010;459(2):333–343. doi: 10.1007/s00424-009-0722-7. [DOI] [PubMed] [Google Scholar]
- 37.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A. 2007;104(50):19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242(3):626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 39.Thurston RD, Larmonier CB, Majewski PM, Ramalingam R, Midura-Kiela M, Laubitz D, et al. Tumor necrosis factor and interferon-gamma down-regulate Klotho in mice with colitis. Gastroenterology. 2010;138(4):1384–1394. doi: 10.1053/j.gastro.2009.12.002. 94 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118(12):3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17(12):2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 42.Barthel TK, Mathern DR, Whitfield GK, Haussler CA, Hopper HAt, Hsieh JC, et al. 1,25-Dihydroxyvitamin D3/VDR-mediated induction of FGF23 as well as transcriptional control of other bone anabolic and catabolic genes that orchestrate the regulation of phosphate and calcium mineral metabolism. J Steroid Biochem Mol Biol. 2007;103(3–5):381–388. doi: 10.1016/j.jsbmb.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 43.Bartik L, Whitfield GK, Kaczmarska M, Lowmiller CL, Moffet EW, Furmick JK, et al. Curcumin: a novel nutritionally derived ligand of the vitamin D receptor with implications for colon cancer chemoprevention. J Nutr Biochem. 2010;21:1153–1161. doi: 10.1016/j.jnutbio.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bishop KA, Meyer MB, Pike JW. A novel distal enhancer mediates cytokine induction of mouse RANKl gene expression. Mol Endocrinol. 2009;23(12):2095–2110. doi: 10.1210/me.2009-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keisala T, Minasyan A, Lou YR, Zou J, Kalueff AV, Pyykko I, et al. Premature aging in vitamin D receptor mutant mice. J Steroid Biochem Mol Biol. 2009;115(3–5):91–97. doi: 10.1016/j.jsbmb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagai R, Saito Y, Ohyama Y, Aizawa H, Suga T, Nakamura T, et al. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci. 2000;57(5):738–746. doi: 10.1007/s000180050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248(2):324–329. doi: 10.1006/bbrc.1998.8943. [DOI] [PubMed] [Google Scholar]
- 49.Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, et al. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension. 2002;39(4):838–843. doi: 10.1161/01.hyp.0000013734.33441.ea. [DOI] [PubMed] [Google Scholar]
- 50.Katai K, Miyamoto K, Kishida S, Segawa H, Nii T, Tanaka H, et al. Regulation of intestinal Na+-dependent phosphate co-transporters by a low-phosphate diet and 1,25-dihydroxyvitamin D3. Biochem J. 1999;343(Pt 3):705–712. [PMC free article] [PubMed] [Google Scholar]
- 51.Taketani Y, Segawa H, Chikamori M, Morita K, Tanaka K, Kido S, et al. Regulation of type II renal Na+- dependent inorganic phosphate transporters by 1,25-dihydroxyvitamin D3. Identification of a vitamin D-responsive element in the human NAPi-3 gene. J Biol Chem. 1998;273(23):14575–14581. doi: 10.1074/jbc.273.23.14575. [DOI] [PubMed] [Google Scholar]
- 52.Masuda M, Yamamoto H, Kozai M, Tanaka S, Ishiguro M, Takei Y, et al. Regulation of renal sodium-dependent phosphate co-transporter genes (Npt2a and Npt2c) by all-trans-retinoic acid and its receptors. Biochem J. 2010;429(3):583–592. doi: 10.1042/BJ20100484. [DOI] [PubMed] [Google Scholar]
- 53.Segawa H, Onitsuka A, Kuwahata M, Hanabusa E, Furutani J, Kaneko I, et al. Type IIc sodium-dependent phosphate transporter regulates calcium metabolism. J Am Soc Nephrol. 2009;20(1):104–113. doi: 10.1681/ASN.2008020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, et al. Hereditary Hypophosphatemic Rickets with Hypercalciuria Is Caused by Mutations in the Sodium-Phosphate Cotransporter Gene SLC34A3. Am J Hum Genet. 2006;78(2):193–201. doi: 10.1086/499410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL. Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol. 2009;76(1):38–46. doi: 10.1124/mol.109.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu X, Li Y. Role of FGF19 induced FGFR4 activation in the regulation of glucose homeostasis. Aging (Albany NY) 2009;1(12):1023–1027. doi: 10.18632/aging.100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurosu H, Kuro-o M. The Klotho gene family and the endocrine fibroblast growth factors. Curr Opin Nephrol Hypertens. 2008;17(4):368–372. doi: 10.1097/MNH.0b013e3282ffd994. [DOI] [PubMed] [Google Scholar]
- 58.Shardell M, Hicks GE, Miller RR, Kritchevsky S, Andersen D, Bandinelli S, et al. Association of low vitamin D levels with the frailty syndrome in men and women. J Gerontol A Biol Sci Med Sci. 2009;64(1):69–75. doi: 10.1093/gerona/gln007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ensrud KE, Ewing SK, Fredman L, Hochberg MC, Cauley JA, Hillier TA, et al. Circulating 25-hydroxyvitamin D levels and frailty status in older women. J Clin Endocrinol Metab. 2010;95(12):5266–5273. doi: 10.1210/jc.2010-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tuohimaa P, Tenkanen L, Ahonen M, Lumme S, Jellum E, Hallmans G, et al. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries. Int J Cancer. 2004;108(1):104–108. doi: 10.1002/ijc.11375. [DOI] [PubMed] [Google Scholar]
- 61.Manson JE, Mayne ST, Clinton SK. Vitamin D and prevention of cancer--ready for prime time? N Engl J Med. 2011;364(15):1385–1387. doi: 10.1056/NEJMp1102022. [DOI] [PubMed] [Google Scholar]
- 62.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, Detrano R, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96(6):1755–1760. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 64.Ohnishi M, Razzaque MS. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 2010;24(9):3562–3571. doi: 10.1096/fj.09-152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takemura A, Iijima K, Ota H, Son BK, Ito Y, Ogawa S, et al. Sirtuin 1 Retards Hyperphosphatemia-Induced Calcification of Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.110.216739. [DOI] [PubMed] [Google Scholar]
- 66.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16(4):391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]