Abstract
Protein targets in autoimmune disease vary in location, originating within cells as in SLE, or found on cell surfaces or in extracellular spaces. The term “autoantigenesis” is first defined here as the changes that arise in self-proteins as they break self tolerance and trigger autoimmune B and/or T cell responses. As illustrated in many studies, between 50 and 90% of the proteins in the human body acquire posttranslational modification. In some cases, it may be that these modifications are necessary for the biological functions of proteins of the cells in which they reside or as extracellular mediators. Summarized herein, it is clear that some posttranslational modifications can create new self-antigens by altering immunologic processing and presentation. While many protein modifications exist, we will focus on those created, amplified, or altered in the context of inflammation or other immune system responses. Finally, we will address how posttranslational modifications in self-antigens may affect the analyses of B and T cell specificity, current diagnostic techniques, and/or the development of immunotherapies for autoimmune diseases.
Keywords: posttranslational modifications, autoimmunity, tolerance
Posttranslational modification of amino acids and proteins
Classical biochemistry tells us twenty amino acids make up most proteins in nature. Closer examination, however, reveals a number that far exceeds those twenty original structures. Indeed, when posttranslational modifications (PTMs) are considered, more than 140 unique amino acids compose protein [1]. For simplicity, this review will refer to PTMs as any protein in which certain amino acids within that protein have been covalently modified. PTMs can arise either by enzymatic modification, as is the case of N-linked glycosylation or phosphorylation, or can occur spontaneously, as is the case in the deamidation of asparagine to aspartic acid or isoaspartic acid.
PTMs alter many aspects of protein chemistry, including primary and tertiary structure, biological (and/or enzymatic) functions, and proteolytic degradation that may be critical in generating immunogenic or tolerogenic self-peptides. Eventually, the accumulation of these modifications can induce disease in the host (Figure 1). There are a host of factors that influence the ability and rate of PTMs that occur in a given protein. For example, flanking residues around a targeted amino acid greatly influence its ability to be modified. In the case of phosphorylation of serine or threonine residues, a specific motif is required [–Arg-X-Y- (Z)-Ser-(Thr)] while sequence-regulated conformations can also affect the accessibility of the modifying enzymes. The location of a modifying enzyme is also a consideration, since enzymes that mediate modifications are compartmentalized in such areas as intracellular organelles, the endoplasmic reticulum, or in extracellular spaces. Finally, changes within the protein itself, such as proteolytic cleavages and previous modifications, will affect if and how a particular residue is modified [2].
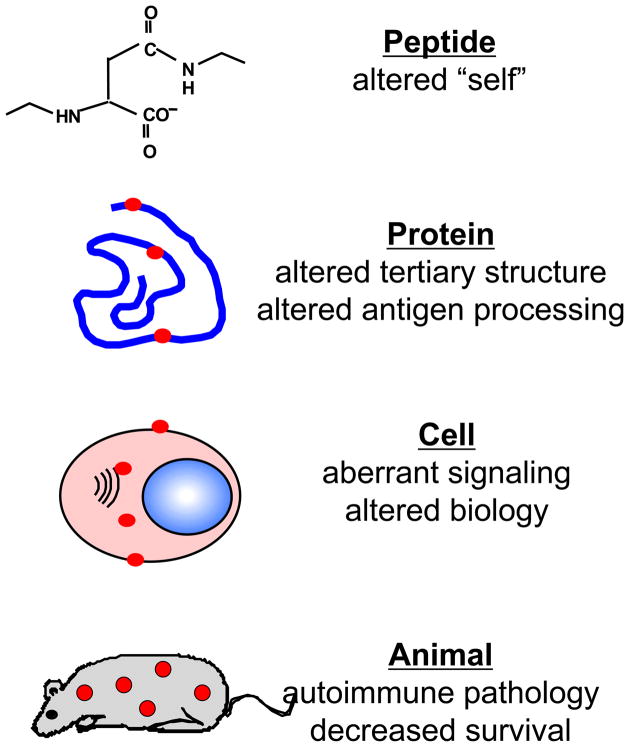
Figure 1.
The multiple effects of posttranslational modifications. The effects of posttranslational modifications can be seen on multiple levels. Posttranslationally modified peptides become sources of altered self, and when in the context of proteins, can affect the tertiary structure of a protein and how it is processing by antigen processing cells. When significant amounts of certain posttranslational modifications accumulate in cells, they also have the potential to alter how the cell functions (i.e., proliferation rates, cell signaling). Finally, the culmination of all of these individual effects is the onset of autoimmune pathology in whole organisms and ultimately decreased survival.
Posttranslationally modified proteins are crucial for a wide variety of cellular events, from cell signaling to DNA replication. However, recent studies have suggested that there are times when the presence or absence of posttranslational alterations in self-proteins can profoundly affect antigen recognition in immune functions.
Posttranslational modifications in the generation of autoimmunity
Models of T and B cell selection and maturation indicate that lymphocytes reacting too strongly to self-peptides presented in the thymus and bone marrow are deleted within the respective organ. Studies demonstrating that a wide variety of peripheral antigens are expressed by medullary thymic epithelial cells is evidence that central tolerance develops to antigens expressed in organs [3]. However, the same modifications to proteins that arise in the periphery may not occur in an identical manner in the thymus and thus these proteins never tolerize developing thymocytes (Figure 2). Consequently, when PTMs arise during various cellular responses, such as inflammation, these modified self-antigens can be taken up and processed by APC, that then present the modified antigens to autoreactive T and B cells. These cells in turn can infiltrate into host tissue where the modified antigen then serves to amplify the autoimmune responses, eventually leading to autoimmune pathology (Figure 2).
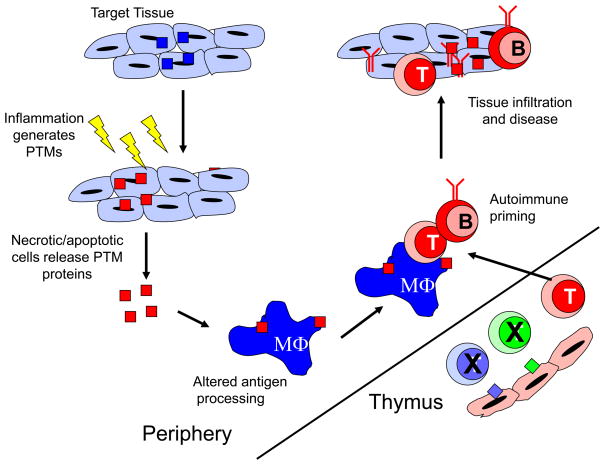
Figure 2.
Posttranslationally modified proteins initiate autoimmune responses. Posttranslational modifications can occur in self-proteins during cellular stress, such as inflammation, ageing or apoptosis. These PTM self-proteins are then released from necrotic or apoptotic cells, where they are phagocytosed by antigen presenting cells (APC), such as macrophages. Once inside the APC, the presence of these modifications can alter how proteases cleave the self-antigen, thereby generating new epitopes. These modified peptides are then presented in the context of MHC class II molecules to T and B cells that have escaped the thymus or bone marrow during negative selection because the modified peptide is not present in those organs. Finally, autoreactive T cells and B cells infiltrate host tissue and induce autoimmune pathology.
The number of autoimmune diseases associated with posttranslationally modified autoantigens continually increases (Table 1). Psoriasis, Goodpasture’s disease, antiphospholipid syndrome and juvenile idiopathic arthritis all have autoantigens described as being posttranslationally modified [4–6]. In juvenile idiopathic arthritis, antibodies against the nuclear phosphoprotein DEK are present in immune complexes isolated from the synovial fluid of patients. Analysis of DEK from these complexes revealed a high number of acetylated lysine residues. Patient synovial fluid reacts to highly acetylated synthetic DEK. However, the inhibition of DEK acetylation reduces the reactivity of patient synovial fluid to that seen with healthy controls [6].
Table 1.
Autoimmune diseases in which posttranslationally modified autoantigens have been identified.
| Disease | Autoantigen | Modifications |
|---|---|---|
| Multiple Sclerosis/EAE | αB-crystallin | Phosphorylation |
| MBP | Citrullination, Acetylation | |
| MOG | Malondialdehyde | |
|
| ||
| Collagen-induced arthritis | Type II collagen | Glycosylation, Hydroxylation |
|
| ||
| Rheumatoid arthritis | Fillagrin | Citrullination, Carbamylation |
| Fibrin | Citrullination | |
| Vimentin | Citrullination | |
| Collagen | Citrullination | |
| α-Enolase | Citrullination | |
|
| ||
| Juvenile idiopathic arthritis | DEK | Acetylation |
|
| ||
| Systemic lupus erythematosus | Multiple | Phosphorylation |
| snRNP D, H2B | Deamidation | |
| U1-70K | Caspase cleavage | |
| La/SSB | Phosphorylation | |
| SmD1, SmD2 | Phosphorylation, Methylation | |
| Self-proteins | Lipid peroxidation | |
|
| ||
| Type 1 diabetes | Insulin chain A | Oxidation |
|
| ||
| Psoriasis | Pso27 | Endoprotease cleavage |
|
| ||
| Goodpasteur’s disease | Collage IV | Sulfilimine bond |
|
| ||
| Celiac disease | Transglutaminase | Deamidation |
|
| ||
| Atherlosclerosis | LDL | Peroxidation |
|
| ||
| Antiphospholipid Syndrome | β2 glycoprotein 1 | Oxidation |
One autoimmune disease that has conspicuously little evidence of how modified autoantigens contribute to pathogenesis is type 1diabetes (T1D). Recently, however, there has been newfound interest in examining the role of posttranslational modifications in T1D. Stadinski and colleagues have shown that chromogranin A is a new autoantigen in T1D, and that the peptide WE14 from chromogranin A stimulates diabetogenic CD4 T cell clones [7]. Further studies of WE14 have shown that its antigenicity is greatly altered when treated with transglutaminase, an enzyme that is known to mediate several posttranslational modifications [8]. The natural form of the antigen in beta cell extracts is far more potent than an unmodified synthetic WE14 peptide [7], further suggesting that this peptide may be posttranslationally modified. In addition, our laboratory has evidence indicating that prolyl-4-hydroxylase beta (P4Hb) is posttranslationally modified with a carbonyl group in murine pancreatic islets. Both T1D patient serum and diabetic NOD mouse serum have antibodies to P4Hb (Connolly and Mamula, unpublished results).
The types of modifications that can occur within a particular autoimmune disease also continue to increase, as one autoimmune disease can have multiple modifications associated with distinct autoantigens (Table 1). For example, citrullinated filaggrin has been known to be an autoantigen in rheumatoid arthritis for some time now, such that it is a component of diagnostic assays for the disease [9]. Recently, though, another modification of filaggrin, carbamylation (the nonenzymatic conversion of lysine to homocitrulline), has been described [10]. Carbamylated lysine residues in filaggrin trigger a primary immune response, complete with CD4+ T cell proliferation, and can induce arthritis in murine models of arthritis. Rheumatoid arthritis patients with erosive arthritis have increased levels of homocitrulline in their blood and synovial fluid. This modified autoantigen is critical to the development of anticitrulline autoantibodies in RA [10].
Just as the presence of a posttranslationally modified self-antigen affects immune recognition, the lack of a normally occurring posttranslational modification within a self-antigen can induce autoimmunity. For example, SLE patient sera contains autoantibodies to both phosphorylated and non-phosphorylated SR proteins (pre-mRNA splicing factors) [11]. Similarly, mice lacking the gene for α-mannosidase II, which regulates hybrid to complex branching patterns of extracellular asparagine (N)-linked oligosaccharides chains (N-glycans), develop a lupus-like nephritis characterized by autoantibodies to histones, Sm snRNP (small nuclear ribonuclear protein) antigen, and DNA [12]. It is thought that the lack of complex N-glycan branching may affect self-antigen recognition, although the exact mechanism behind how the absence of complex N-glycan branching leads to autoimmunity remains to be elucidated.
An examination of the T and B cell responses to these modified self-antigens reveals several interesting points. First, the B cell responses to various posttranslationally modified self-antigens tends to be promiscuous in that antibodies bind both the modified and unmodified forms of the self-protein. The ability of antibodies to bind both modified and unmodified forms of the same protein is due to the identity of flanking amino acid sequences of both forms. Second, the T cell responses to these modifications tend to be specific and directed only towards the modified self-antigen (not cross-reactive). As one example, the acetylation of the N-terminal peptide of myelin basic protein (MBP Ac1-11) is required for the development of experimental autoimmune encephalomyelitis (EAE), the murine model of human multiple sclerosis. Encephalitogenic T cell clones specific for MBP Ac1-11 induce EAE, while the non-acetylated peptide fails to stimulate T cells or induce EAE [13]. Autoantibodies can be elicited with an isoaspartyl-modified form (isoAsp) of self-antigen in murine models of system lupus erythematosus (SLE). SLE is characterized by both T and B cell responses to a variety of intracellular molecules, most notably nucleosomes and snRNPs. Mice immunized with an isoAsp containing-peptide of the Sm D protein develop T cell responses to the isoAsp D peptide, but not to the normal form of the peptide. In contrast, mice immunized with the isoAsp D peptide develop autoantibodies not only to the isoAsp form, but also bind the normal D peptide as well as other autoantigens [14]. Both of these examples demonstrate that autoimmunity can be elicited to some alterations that occur in self-peptides while immune tolerance is maintained to the unmodified self-protein.
How are posttranslational modifications generated?
Posttranslational modifications can occur spontaneously, as with isoAsp generation [15], or carbamylation [10], or as the result of an ordered enzymatic process as described earlier. Certain cellular processes, such as aging, disease, inflammation, and trauma, are known to increase the frequency of posttranslational modifications. Aged cellular proteins, especially those with relatively long half-lives in vivo, are prone to posttranslational modifications. αB-crystallin, a major lens protein, has increased amounts of isoAsp [16] and phosphorylated residues with aging [17]. It is believed that some of these modifications, such as isoAsp formation, may mark aged proteins for degradation [18].
Numerous cellular stresses also induce the posttranslational modification of cellular proteins. Mitogen or antigen stimulation increases the amount of isoAsp in T and B cells [14], while reactive oxygen species generated during infection are known to modify proteins [19]. All of these modifications may be relevant since apoptosis induces a number of posttranslational modifications, such as phosphorylation [20], transglutamination, ubiquitination [21], and citrullination [22]. Apoptotic cells have been considered a source of autoantigens in the induction of SLE (reviewed in refs. [23] and [24]). Even though apoptotic cells remain intact, certain antigens that are common targets of autoantibody responses in SLE patients localize to surface blebs of apoptotic cells [25] and mice immunized with apoptotic Jurkat cells develop antibodies to multiple autoantigens and autoantigen complexes associated with SLE [26]. The observation that SLE patients develop antibodies specific to phosphorylated self-proteins supports the premise that apoptotic cells may be an important source of these autoantigens [27]. Likewise, the dephosphorylation of normally phosphorylated proteins during apoptosis may also influence autoantibody responses. Fas-L induced apoptosis in Jurkat cells also leads to the dephosphorylation of ribosomal P proteins and this lack of phosphorylation may expose self-epitopes to the immune system, thus triggering autoantibody production [28].
Overall, neo self-antigens resulting from these processes could make their way into the extracellular milieu where they can be taken up by antigen presenting cells and presented to T cells. In this way, an immune response could be generated to a protein that is otherwise ignored by the immune system.
Tolerance and posttranslational modifications
It is clear from the literature that there is often a lack of immune tolerance to various posttranslationally modified self-antigens. One explanation for the lack of tolerance is that these PTM self-antigens are not present in the thymus during T cell development and the modifications may arise in the periphery due to different biochemical conditions (pH, for example). T cells not exposed to peptide PTMs may be allowed to escape into the periphery. Once in the periphery, the modified form is now viewed as “foreign”. Moreover, upon initial stimulus by modified peptides, the response may then be amplified to other sites on the protein or particle (so called “intramolecular epitope spreading”).
A lack of tolerance to modified self-antigens may be due to antigen processing differences between modified and unmodified self-antigens. The PTM of an amino acid residue critical for recognition and cleavage by certain proteases has the potential to influence the antigenic peptides generated or the rate in which they are generated (Figure 3). For example, the lack of N-glycosylation of the neuronal glutamate receptor subunit 3 in Rasmussens’ encephalitis, a severe form of pediatric epilepsy, exposes a granzyme B cleavage site, thus creating a new autoantigen [29]. In the case of isoAsp containing peptides, most proteases and peptidases do not recognize the β-peptide linkage connecting isoAsp residues to its neighbor on the carboxyl side [30]. Moss and coworkers demonstrated that the spontaneous deamidation of asparagine residues in a model protein (tetanus toxin C fragment) inhibits the processing by asparagine endopeptidase (AEP), resulting in decreased antigen presentation [31]. Altered enzyme recognition also changes the repertoire of peptides generated during antigen processing. An isoaspartylated form of cytochrome c peptide is processed differently by cathepsin D compared to the aspartyl form of the same peptide. These processing differences are similar for the isoapsartylated form of whole cytochrome c [32]. Additionally, the presence of citrulline in peptides of MBP increases the rate of its digestion with cathepsin D, an enzyme involved in MHC class II antigen processing, compared to peptides without the citrulline residue [33].
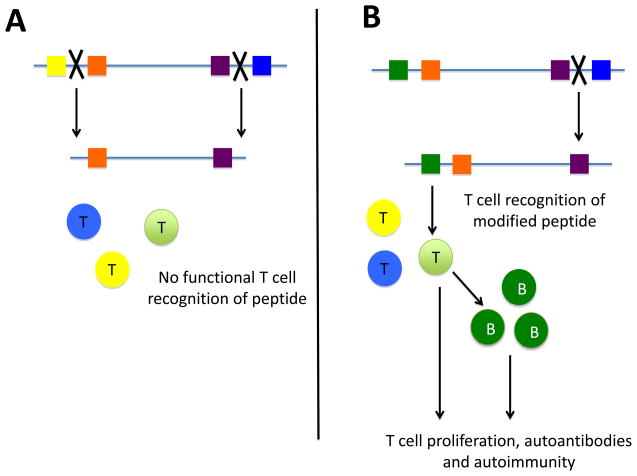
Figure 3.
Antigen processing altered by the posttranslational modification of self-antigen. (A) Native forms of self-antigens are cleaved by proteases (as represented by the X) into distinct peptides. These peptides are not recognized by the immune system since functional T cells specific for the peptides generated do not exist in the normal immune repertoire due to mechanisms such as clonal deletion and anergy. (B) However, proteases involved in antigen processing may not be able to cleave after a modified amino acid, thereby creating a peptide in which no immune tolerance has developed. T cells in the periphery recognize this peptide, and provide T cell help to autoreactive B cells which then produce autoantibodies and promote disease.
Modified self-proteins and peptides derived therein, may change how a peptide interacts with MHC molecules. Studies performed on phosphorylated αB-crystallin peptides and isoAsp containing snSNP D peptides bind with the same affinity to their respective MHC class II molecules as the unmodified peptide [14,17]. Moreover, in the case with the αB-crystallin, the modified residue extends out of the peptide-binding groove, explaining why there is little cross-reactivity between TCR recognition of modified and unmodified peptides [17].
In contrast, the lack of posttranslational modifications can also lead to a lack of tolerance to self-proteins, as evidenced in the mice lacking the N-glycosylation enzyme, N-acetylglucosaminyltransferase V (Mgat5). Upon stimulation, Mgat5 −/− T cells have increased TCR recruitment as compared to wild-type T cells and reduced requirement for CD28 costimulation [34]. It appears that Mgat5−/− T cells bypass the CD28 costimulatory pathway.
PTMs in diagnostics and immunotherapy
Most investigations defining T and/or B cell epitopes in autoimmune disease rely on the synthesis of peptides or on recombinant proteins [35]. However, these approaches and technologies do consider the presence of PTM proteins/peptides. T and B cell responses to certain antigenic self-peptides may be completely missed if appropriate PTMs are not incorporated. Similarly, the correct diagnosis of autoimmune disease depends significantly on PTM proteins or peptides. One particular example is the second generation cyclic citrullinated peptide (CCP2) currently used to detect anti-citrullinated peptide/protein antibodies in the diagnosis of rheumatoid arthritis [9]. The presence of anti-CCP antibodies is a predictor of the development of RA, since anti-CCP antibodies develop prior to disease onset. Moreover, the presence of anti-CCP antibodies in RA patients tends to be predictive of a more erosive form of the disease.
Another important PTM in diagnostics is the major autoantigen of antiphospholipid syndrome, β2 glycoprotein 1 (β2GPI). Antiphospholipid syndrome (APS) is an autoimmune condition noted by vascular thrombosis of the arterial and/or venous systems. It is demonstrated that APS patients have higher levels of total and oxidized β2GP1 as compared to healthy and disease state controls [36]. It is thought that the risk of thrombosis can be correlated with the levels of oxidized β2GP1, and thus be an indication for prophylactic treatment during periods of elevated thrombosis risk.
Conclusions
We have attempted to define several parameters in which protein modifications alter many functions of proteins; in their biological functions, their processing and presentation by the immune system, and in the practical diagnostics of disease. As described here, posttranslational modification of self-antigens is one important explanation of how immune tolerance is lost to self-antigens. Clearly, we have yet to fully define the scope of PTMs that alter the course of health and disease. However, being wary of their existence will bring us closer to defining important parameters of PTMs in the induction and perpetuation of disease. Finally, the pathways that control PTMs may become targets of immunotherapeutic strategies to alter the states of immune tolerance versus autoimmunity.
Highlights.
Protein modifications are relevant to inflammation, the onset and development of autoimmune disease, and the diagnostics of autoimmune disease. Clear examples of specific posttranslational modifications are observed in both human disease and murine models. This review will detail how protein modifications may alter protein biology, processing and presentation of autoantigens, and the selection and development of the immune repertoire.
Acknowledgments
Sources of support: Studies from authors’ laboratory supported by NIH grants AI36529 and AI48120; the Alliance for Lupus Research, The Heimbold Foundation (to MJM) and by AR057155 (to HAD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* Of special interest
** Of outstanding interest
- 1.Uy R, Wold F. Posttranslational covalent modifications of proteins. Science. 1977;198:890–896. doi: 10.1126/science.337487. [DOI] [PubMed] [Google Scholar]
- 2.Wold F. In vivo chemical modification of proteins (post-translational modification) Annu Rev Biochem. 1981;50:783–814. doi: 10.1146/annurev.bi.50.070181.004031. [DOI] [PubMed] [Google Scholar]
- 3.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nature Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 4.Iversen OJ, Lysvand H, Hagen L. The autoantigen Pso p27: a post-translational modification of SCCA molecules. Autoimmunity. 2011;44:229–234. doi: 10.3109/08916934.2010.530628. [DOI] [PubMed] [Google Scholar]
- 5.Pedchenko V, Vanacore R, Hudson B. Goodpasture’s disease: molecular architecture of the autoantigen provides clues to etiology and pathogenesis. Curr Opin Nephrol Hypertens. 2011;20:290–296. doi: 10.1097/MNH.0b013e328344ff20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mor-Vaknin N, Kappes F, Dick AE, Legendre M, Damoc C, Teitz-Tennenbaum S, Kwok R, Ferrando-May E, Adams BS, Markovitz DM. DEK in the synovium of patients with juvenile idiopathic arthritis: characterization of DEK antibodies and posttranslational modification of the DEK autoantigen. Arthritis Rheum. 2011;63:556–567. doi: 10.1002/art.30138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Piganelli JD, Barbour G, Bradley B, Crawford F, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–231. doi: 10.1038/ni.1844. This paper is the first to report that chromogranin A is an autoantigen in type 1 diabetes and a ChgA peptide, WE14, is identified as the ligand for three diabetogenic CD4 T cell clones. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haskins K, Cooke A. CD4 T cells and their antigens in the pathogenesis of autoimmune diabetes. Curr Opin Immunol. 2011;23:1–7. doi: 10.1016/j.coi.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Wiik AS, van Venrooij WJ, Pruijn GJ. All you wanted to know about anti-CCP but were afraid to ask. Autoimmun Rev. 2010;10:90–93. doi: 10.1016/j.autrev.2010.08.009. A review on the background and significance of anti-cyclic citrullinated peptides (CCP) in the diagnosis of rheumatoid arthritis. [DOI] [PubMed] [Google Scholar]
- 10*.Mydel P, Wang Z, Brisslert M, Hellvard A, Dahlberg LE, Hazen SL, Bokarewa M. Carbamylation-dependent activation of T cells: a novel mechanism in the pathogenesis of autoimmune arthritis. J Immunol. 184:6882–6890. doi: 10.4049/jimmunol.1000075. Carbamylation of the rheumatoid arthritis autoantigen filaggrin is critical for the development of anti-citrullinated antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neugebauer KM, Merrill JT, Wener MH, Lahita RG, Roth MB. SR proteins are autoantigens in patients with systemic lupus erythematosus: importance of phosphepitopes. Arthritis Rheum. 2000;43:1768–1778. doi: 10.1002/1529-0131(200008)43:8<1768::AID-ANR13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Chui D, Sellakumar G, Green RS, Sutton-Smith M, McQuistan T, Marek KW, Morris HR, Dell A, Marth JD. Genetic remodeling of protein glycosylation in vivo induces autoimmune disease. Proc Natl Acad Sci USA. 2001;98:1142–1147. doi: 10.1073/pnas.98.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamvil SS, Mitchell DJ, Moore AC, Kitamura K, Steinman L, Rothbard JB. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986;324:258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- 14.Mamula MJ, Gee RJ, Elliot JI, Sette A, Southwood S, Jones P, Blier PR. Isoaspartyl post-translational modification triggers autoimmune responses to self-proteins. J Biol Chem. 1999;274:22321–22327. doi: 10.1074/jbc.274.32.22321. [DOI] [PubMed] [Google Scholar]
- 15.Najbauer J, Orpiszewski J, Aswad DW. Molecular aging of tubulin: accumulation of isoaspartyl sites in vivo. Biochem. 1996;35:5183–5190. doi: 10.1021/bi953063g. [DOI] [PubMed] [Google Scholar]
- 16.Takemoto LJ. Degradation of aspartyl and asparaginyl residues of lens proteins in vivo. In: Aswad DW, editor. Deamidation and isoaspartate formation in peptides and proteins. CRC Press; 1995. pp. 157–165. [Google Scholar]
- 17.van Stipdonk MJB, Willems AA, Amour S, Persoon-Deen C, Travers PJ, Boog JP, van Noort JD. T cells discriminate between differentially phosphorylated forms of αB-crystallin, a major central nervous system myelin antigen. Int Immunol. 1998;10:943–950. doi: 10.1093/intimm/10.7.943. [DOI] [PubMed] [Google Scholar]
- 18.Tarcsa E, Szymanska G, Lecker S, O’Connor CM, Goldberg AL. Ca2+-free calmodulin and calmodulin damaged by in vitro aging are selectively degraded by 26 S proteasome without ubiquitination. J Biol Chem. 2000;275:20295–20301. doi: 10.1074/jbc.M001555200. [DOI] [PubMed] [Google Scholar]
- 19.Leib SL, Kim YS, Chow LL, Sheldon RA, Tauber MG. Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J Clin Invest. 1996;98:2632–2639. doi: 10.1172/JCI119084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rathmell JC, Thompson CB. The central effectors of cell death in the immune system. Annu Rev Immunol. 1999;17:781–828. doi: 10.1146/annurev.immunol.17.1.781. [DOI] [PubMed] [Google Scholar]
- 21.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 22.Asaga H, Yamada M, Senshu T. Selective deimination of vimentin in calcium ionophor-induced apoptosis of mouse peritoneal macrophages. Biochem Biophys Res Commun. 1998;243:641–646. doi: 10.1006/bbrc.1998.8148. [DOI] [PubMed] [Google Scholar]
- 23.Andrade F, Casciola-Rosen L, Rosen A. Apoptosis in systemic lupus erythematosus. Clinical implications. Rheum Dis Clin North Am. 2000;26:215, 227, v. doi: 10.1016/s0889-857x(05)70136-8. [DOI] [PubMed] [Google Scholar]
- 24.Utz PJ, Gensler TJ, Anderson P. Death, autoantigen modifications, and tolerance. Arthritis Res. 2000;2:101–114. doi: 10.1186/ar75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gensler TJ, Hottelet M, Zhang C, Schlossman S, Anderson P, Utz PJ. Monoclonal antibodies derived from BALB/C mice immunized with apoptotic Jurkat T cells recognize known autoantigens. J Autoimmun. 2001;16:59–69. doi: 10.1006/jaut.2000.0464. [DOI] [PubMed] [Google Scholar]
- 27.Utz PJ, Hottelet M, Schur PH, Anderson P. Proteins phosphorylated during stress-induced apoptosis are common targets for autoantibody production in patients with systemic lupus erythematosus. J Exp Med. 1997;185:843–854. doi: 10.1084/jem.185.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zampieri S, Degen W, Ghirardello A, Doria A, Venrooij WJv. Dephosphorylation of autoantigenic ribosomal P proteins during Fas-L induced apoptosis: a possible trigger for the development of the autoimmune response in patients with systemic lupus erythematosus. Ann Rheum Dis. 2001;60:72–76. doi: 10.1136/ard.60.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gahring LC, Carlson NG, Meyer EL, Rogers SW. Granzyme B proteolysis of a neruonal glutamate receptor generates an autoantigen and is modulated by glycosylation. J Immunol. 2001;166:1433–1438. doi: 10.4049/jimmunol.166.3.1433. [DOI] [PubMed] [Google Scholar]
- 30.Johnson BA, Aswad DW. Fragmentation of isoaspartyl peptides and proteins by carboxypeptidase Y: release of isoaspartyl dipeptides as a result of internal and external cleavage. Biochemistry. 1990;29:4373–4380. doi: 10.1021/bi00470a017. [DOI] [PubMed] [Google Scholar]
- 31.Moss CX, Matthews SP, Lamont DJ, Watts C. Asparagine deamidation perturbs antigen presentation on class II major histocompatibility complex molecules. J Biol Chem. 2005;280:18498–18503. doi: 10.1074/jbc.M501241200. [DOI] [PubMed] [Google Scholar]
- 32.Doyle HA, Gee RJ, Mamula MJ. Altered immunogenicity of isoaspartate containing proteins. Autoimmunity. 2007;40:131–137. doi: 10.1080/08916930601165180. [DOI] [PubMed] [Google Scholar]
- 33.Pritzker LB, Joshi S, Gowan JJ, Harauz G, Moscarello MA. Deimination of myelin basic protein. 1. Effect of deimination of arginyl residues of myelin basic protein on its structure and susceptibility to digestion by cathepsin D. Biochem. 2000;39:5374–5381. doi: 10.1021/bi9925569. [DOI] [PubMed] [Google Scholar]
- 34.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 35.Sahin U, Tureci O, Schmitt H, Cochlovius B, Hohannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci USA. 1995;92:11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Ioannou Y, Zhang JY, Qi M, Gao L, Qi JC, Yu DM, Lau H, Sturgess AD, Vlachoyiannopoulos PG, Moutsopoulos HM, et al. Novel assays of thrombogenic pathogenicity in the antiphospholipid syndrome based on the detection of molecular oxidative modification of the major autoantigen beta2-glycoprotein I. Arthritis Rheum. 2011;63:2774–2782. doi: 10.1002/art.30383. This paper descibes how levels of oxidized beta-2 glycoprotein I are elevated in patients with antiphospholipid syndrome, and alludes to how correlating these levels with disease would be of diagnostic value in treating thrombosis. [DOI] [PMC free article] [PubMed] [Google Scholar]