Abstract
Background
Deep brain stimulation (DBS) of the subthalamic nucleus is an accepted therapy for advanced Parkinson’s disease (PD). In animal models, pharmacologic ablation and stimulation of the subthalamic nucleus have resulted in clinical improvement and, in some cases, improved survival of dopaminergic neurons. DBS has not been studied in the early stages of PD, but early application should be explored to evaluate safety, efficacy, and the potential to alter disease progression.
Methods
We are conducting a prospective, randomized, single-blind clinical trial of optimal drug therapy (ODT) compared to medication plus DBS (ODT + DBS) in subjects with Hoehn & Yahr Stage II idiopathic PD who are without motor fluctuations or dementia. We report here subject screening, enrollment, baseline characteristics, and adverse events.
Results
30 subjects (average age 60 ± 6.9 years, average duration of medicine 2.1 ± 1.3 years, average UPDRS-III scores 14.9 on medication and 27.0 off medication) are enrolled in the ongoing study. Twelve of 15 subjects randomized to DBS experienced perioperative adverse events, the majority of which were related to the procedure or device and resolved without sequelae. Frequently reported adverse events included wound healing problems, headache, edema, and confusion.
Conclusion
This report demonstrates that subjects with early stage PD can be successfully recruited, consented and retained in a long term clinical trial of DBS. Our ongoing pilot investigation will provide important preliminary safety and tolerability data concerning the application of DBS in early stage PD.
Keywords: Parkinson’s disease, Subthalamic Nucleus, Deep Brain Stimulation
Introduction
Parkinson’s disease (PD) is one of the most common movement disorders, affecting nearly one million Americans and millions more worldwide. It is typified by the cardinal features of tremor, rigidity, bradykinesia, and postural instability. These characteristics are treatable with medication in early stages, but the disease continues on a progressive course, causing significant disability secondary to both motor and non-motor symptoms. In the advancing stages, when motor fluctuations are severe or medications do not adequately control symptoms, deep brain stimulation (DBS) of the subthalamic nuclei (STN) may be applied [1]. While DBS has been approved in the United States for tremor since 1997 and for Parkinson’s disease since 2002 [2, 3], the mechanism of action remains unclear. The efficacy of DBS with regard to motor function, quality of life, and medication reduction, lessening financial burden, is established [4–8]. These benefits persist long-term [9].
Despite these encouraging results, DBS has not been studied in the earliest stages of PD. Case reports have indicated favorable effects of both unilateral STN stimulation in mild unilateral disease and also bilateral STN stimulation for motor fluctuations developing after a shorter course of illness [10, 11]. A study of bilateral STN DBS in mid-stage disease patients suffering from motor fluctuations after 5–10 years of illness demonstrated improved quality of life, decreased parkinsonism severity in the “off” state, and a reduction in medication-induced motor complications [12].
The underlying problem in PD is a loss of the dopaminergic neurons in multiple areas including the substantia nigra pars compacta (SNpc). The etiology is likely a complex interplay between genetic and environmental factors. Multiple mechanisms have been proposed, including oxidative stress, mitochondrial dysfunction, inflammation, and excitotoxicity [13, 14]. With regard to the latter, it has been posited that dopamine deficiency in PD leads to disinhibition and overactivity of the STN, which may then exacerbate damage of the target nucleus, the SNpc [15]. This hypothesis has led to multiple studies evaluating the effect of a reduction of STN activity on dopaminergic neurons. In animal models of PD, pharmacologic ablation and high frequency stimulation of the STN have allowed for clinical improvement and in some cases, improved survival of dopamine neurons [16–19]. Collectively, these results suggest a potential protective effect of altered STN output. Although neuroprotection has not been proven in human clinical trials, long-term follow-up of advanced PD patients who have undergone STN DBS has revealed minimal deterioration in motor symptoms, which is inconsistent with the expected decline of a progressive neurodegenerative illness [20].
The search for a neuroprotective therapy continues, and multiple medication trials have not yet provided an answer [21, 22]. A modality that might slow, halt, or even reverse disease progression could theoretically reduce disability, extend employability, improve quality of life, or prolong independence. The advantage of STN DBS in late-stage PD patients is clear. The suggestions that younger age and shorter duration of illness possibly predict greater improvement with DBS [23] and that applying DBS early might slow progression [24] led to our undertaking of a randomized, single-blind clinical trial of optimal drug therapy (ODT) compared to medication plus DBS (ODT + DBS) in the early stages of disease (Clinical Trials.Gov Identifier NCT 00282152). This pilot trial will provide preliminary information concerning safety and tolerability and data necessary to design a multicenter trial to test potential disease modifying effects of DBS.
This manuscript describes the trial planning, participant enrollment, screening, and baseline characteristics, initial medication washout experience, and the DBS implantation procedure in those randomized to receive it.
Methods
This clinical trial was approved by the Vanderbilt University Institutional Review Board (040797). An investigational device exemption was obtained from the FDA (G050016) for the use of the DBS device. Participants were recruited from the Movement Disorders Clinic at Vanderbilt University Medical Center (VUMC) and through public radio and print advertisements. Subjects aged 50–75 years old with idiopathic PD who had taken medication for a period of more than six months but less than four years were eligible. Their medication response had to be stable and without evidence of motor fluctuations or dyskinesias. Those with dementia, major psychiatric disease, previous brain injury or operative intervention, or contraindication to DBS were excluded.
The ethical concerns of offering surgery with its inherent risks to early stage PD patients were raised during trial preparation. In response, biomedical ethicists at VUMC designed an expanded four-phase informed consent process. The procedure involved distribution of the informed consent document to an interested individual followed by three separate meetings with the principal investigator and study coordinator, neurosurgeon, and biomedical ethicist. During these appointments, the rationale and risks of the study were described, the DBS procedure was detailed, and the subject’s comprehension of the study was probed. Patients were then given an informational handout and a voluntary questionnaire exploring their understanding of the trial and potential risks, benefits and motivating factors of enrollment. Patients could agree to join no sooner than seventy-two hours later. An appointment to discuss informed consent was then held with the biomedical ethicist, study coordinator and a neurologist other than the principal investigator. Immediately following that appointment, the ethicist, study coordinator, and potential participant engaged in detailed review of the informed consent document, with several key portions read aloud by the study coordinator, which the potential subject also read on his or her own copy of the consent document. Throughout, potential subjects were encouraged to ask questions.
To achieve an appreciation of actual risks for the pilot study described here, each potential subject needed to understand the probabilities for any surgical complications, including intracranial hemorrhage, as well as other postoperative complications such as infection or electrode malfunction. In addition, they needed to appreciate burdens created by the study design that included that included five separate inpatient evaluations for each subject at 6-month intervals over a span of 2 years during which considerable symptom burden was anticipated because each evaluation included a “washout” of both medication and DBS for a period of 8 days. Both the number and duration of inpatient evaluations highlighted the need to discuss vulnerability of individual subjects and the feasibility of long-term retention.
These risks and implications were explicitly discussed with each potential participant during each initial conversation with a neurologist, a neurosurgeon, and a biomedical ethicist. The potential participant’s understanding, including his or her own motivation for therapeutic benefit, was evaluated during the discussion with the biomedical ethicist. Clear understanding meant that potential research subjects demonstrated enough knowledge to comprehend the goals of the study and to accurately assess and discuss the risks to their health and quality of life, the possible benefits, and any available alternatives. For this study, potential participants needed to understand explicitly that the identifiable potential benefits would be for other PD patients in the future.
All of these components were then reviewed during the appointment with the biomedical ethicist, study coordinator, and a neurologist other than the principal investigator. If a potential participant did not understand a particular component of the study, or had questions specific to his or her own circumstances and medical condition, these were identified and answered before moving to the next step of reviewing the informed consent document. If necessary, the review of the informed consent document was rescheduled in order to provide sufficient time to get accurate information to answer questions, and if needed, to allow the potential participant to incorporate new information into his or her decision about whether to enroll in the study.
Once informed consent was provided, subjects underwent a detailed screening procedure to ensure their appropriateness for the study. This process entailed a complete history and physical examination, psychiatric consultation, neuropsychiatric evaluation, and review of a recent brain MRI by the study neurosurgeon. Hoehn and Yahr scale and Unified Parkinson’s Disease Rating Scale, part III (UPDRS-III) ratings were performed in the “off” state, after medications had been discontinued for thirty-six hours. UPDRS-III was then repeated in the “on” state, just after the patient had taken his or her usual dose of medication. To proceed, patients had to be Hoehn and Yahr stage II and demonstrate at least a 30% improvement in the UPDRS-III between the “off” and “on” states [25].
Those who met the above criteria underwent a baseline evaluation, which consisted of an eight-day inpatient assessment. For two weeks prior, patients kept a diary categorizing their waking hours into thirty minute increments of “on,” “off,” or “on with dyskinesias.” Day zero was defined as the date of admission. On day one, subjects took their usual PD medications. In the “on” state, they were scored on the Hoehn and Yahr scale, Schwab and England scale, and complete UPDRS. UPDRS-III was videotaped. Patients were also timed in the performance of dressing, finger tapping, and the stand, walk, sit tests. At 1600 on day one, all PD medication was stopped. This medication washout duration was selected to balance the desire to measure an approximation of the untreated state versus what is reasonable and not overly burdensome to request of study participants [26]. On each subsequent day, UPDRS-III was measured and pulse and blood pressure were recorded in the supine, seated, and standing positions. On day eight, the same assessments completed on day one were repeated and the UPDRS-III was again videotaped.
At the conclusion of the hospitalization, subjects were equally randomized to either ODT or ODT+DBS. Bone markers were placed and CT and MRI imaging of the brain were performed for surgical planning and construction of the customized miniframe STarFix™ platform (FHC, Inc., Bowdoin, ME). Patients then presented, awake and off medication for at least thirty-six hours, for implantation of bilateral DBS leads (Model 3389; Medtronic, Inc.; Minneapolis, MN). The VUMC DBS protocol executed for advanced PD patients was followed.
To begin, a microdrive was employed to pass four microelectrodes bilaterally in simultaneous trajectories two millimeters apart from a central microelectrode. With the aid of a neurophysiologist, firing patterns of the STN and neighboring structures were distinguished. Microstimulation identified the most favorable tract, taking into account beneficial effects (per patient report of symptomatic improvement and examination by the principal investigator), adverse reactions, and the largest margin between these two. DBS leads were positioned and macrostimulation verified ideal localization. A postoperative CT scan of the brain confirmed lead placement. Within two weeks of lead insertion, subjects returned for attachment of electrodes (Model 7482A-S1; Medtronic, Inc.; Minneapolis, MN) and placement of bilateral implantable pulse generators (IPG) (Model 7426 Soletra Neurostimulator; Medtronic, Inc.; Minneapolis, MN).
Initial programming of the stimulator occurred approximately four weeks after surgery. Medication was discontinued thirty-six hours prior to this procedure. All four contacts on each lead were explored for side effects and beneficial response. The best contact was chosen as the negative and the case served as positive in a monopolar formation. Final settings were a pulse width of 60 microseconds, rate of 130 Hertz, and amplitude in the range of 0.8 to 1.0 Volts. At least two subsequent visits were held to increase the voltage and reduce medications as necessary.
All study participants remained under the care of the referring neurologist. There were no constraints on medication selection, dosing or adjustment. This allowed for the best medical treatment to be individually adapted and is consistent with methods in other trials of ODT vs. DBS [4,13]. DBS voltage titration by the patient’s neurologist was also allowed, although this was exercised in only one case.
Repeat inpatient assessments identical to the baseline evaluation are performed every six months for a total of two years post-randomization. During these washout periods, medication is held and stimulation is discontinued in those with DBS. A full neuropsychological battery of tests is administered at baseline, twelve months, and twenty-four months.
A data safety and monitoring board (DSMB) was impaneled prior to study initiation to create terms for safety monitoring and criteria for suspension of enrollment, protocol amendment, or study closure. The board is comprised of a neurologist, neurosurgeon, internist, and statistician who are otherwise independent of the trial. They meet every six months to discuss findings and prepare a confidential report of adverse events and comparison of disease progression, gauged by changes in individual UPDRS-III scores, between the study groups. The primary endpoint is a four-point increase (worsening) in an individual UPDRS-III off medication. This safety measure was selected to ensure that the DBS group did not experience an unexpected decline resulting from STN stimulation.
Results
Subject enrollment was completed between August 2006 and April 2009 with 329 interested individuals. Sixty-five attended the informational sessions of the expanded informed consent process and thirty-seven ultimately provided consent. Seven of these subjects did not advance past screening. One withdrew prior to the baseline evaluation and six others failed due to dementia (1), uncontrolled psychiatric disease (1), Hoehn and Yahr stage greater than II (3), and abnormal brain MRI (1).
A total of thirty participants were thus divided equally among treatment factions. A full listing of patient attributes is provided in Table I. All patients were Caucasian. Twenty-seven were male and three female. The average age was sixty years. In response to the voluntary questionnaire, 30% expressed that “helping others” was a primary motivation for study involvement. At the time of enrollment, length of medication use was just over two years in both sections. The mean UPDRS-III scores were nearly identical; they were fairly low, indicating only mild motor dysfunction. There were no significant differences between the groups with regard to length of medication use, total medication dosage, measured in levodopa equivalents [5], total UPDRS or part III UPDRS scores.
Table 1.
Baseline Characteristics of Patients.
| Characteristic | ODT (n=15) | ODT+DBS (n=15) |
|---|---|---|
| Gender | ||
| Male | 13 | 14 |
| Female | 2 | 1 |
| Age (yrs) | ||
| Mean | 60 ± 7.0 | 60 ± 6.8 |
| Range | 51 – 69 | 52–74 |
| Baseline Medicine Use | ||
| Mean Duration (yrs) | 2.1 ± 1.1 | 2.2 ± 1.4 |
| Mean L-dopa equivalents (mg/day)† | 569 ± 389 | 451 ± 304 |
| Baseline UPDRS Score | ||
| Mean Total | 36 ± 15 | 39 ± 14 |
| Mean UPDRS-III | 15 ± 7.6 | 15 ± 8.5 |
100 mg of Levodopa with a dopa-decarboxylase inhibitor = 130 mg of controlled-release Levodopa preparations = 83 mg of Levodopa with dopa-decarboxylase and COMT inhibitors = 1 mg of Pergolide, Pramipexole, or Lisuride = 3 mg of Ropinirole 3.
All subjects tolerated the seven-day washout period without major side effects and without evidence of neuroleptic malignant syndrome. Pre-randomization adverse events are displayed in Table II. There were a total of fourteen complaints among nine patients, most of which were impermanent; only minor gastrointestinal disturbances continued beyond randomization.
Table II.
Adverse events from Enrollment until Randomization; All study patients (n=14)
| Related to Study | Transient | Ongoing |
|---|---|---|
| Insomnia | 3 | 0 |
| Myalgias | 1 | 0 |
| Not Related to Study | ||
| Constipation/Diarrhea | 0 | 1 |
| Foot Pain/Edema | 1 | 0 |
| Nausea | 2 | 1 |
| Cold Symptoms | 4 | 0 |
| Weight Gain | 1 | 0 |
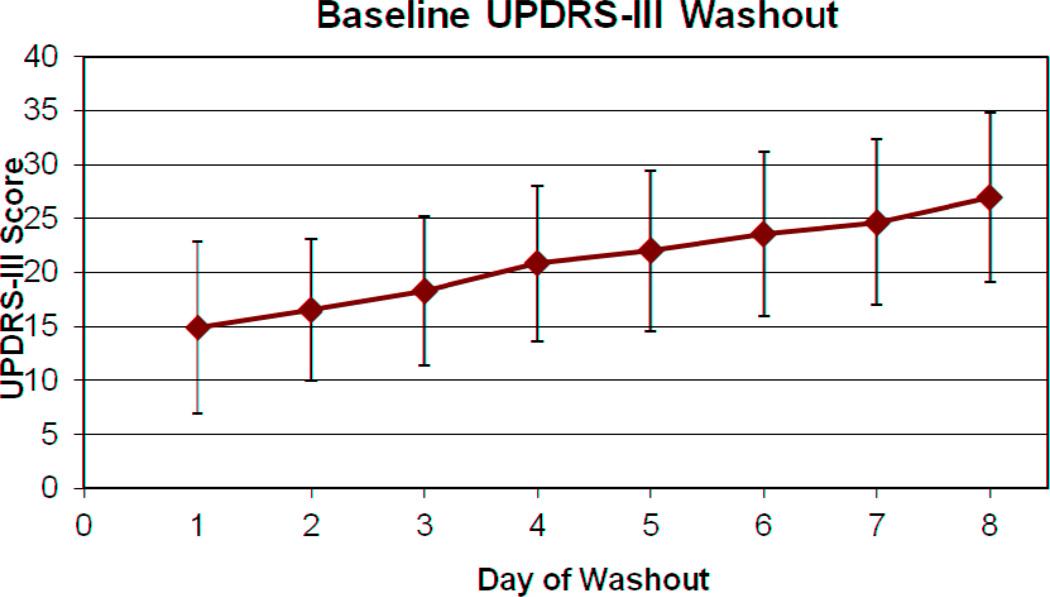
Figure I demonstrates the gradual change in average UPDRS-III scores over the washout period. The final UPDRS-III was twelve-points greater, an increase from 14.92 at admission to 26.97 on the last day of hospitalization. The scores were similar between groups (27.1±7.2 in ODT and 26.8±8.7 in ODT+DBS).
Figure 1. Mean UPDRS-III scores during Medication Washout.
Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at VUMC 29. REDCap is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
The DBS procedure was well received among the fifteen participants randomized to surgical intervention. Table III lists detailed adverse events among these patients for the period from lead implantation through hospital discharge from IPG placement [27]. A total of fifty-six incidences were reported among twelve patients (80%); most were short-term and related to the procedure or device. Two procedures were aborted in the operating room: one with concern for unsterile equipment and the other for failure, during intraoperative test stimulation, to identify an optimal target that produced the expected symptomatic benefit. Both subjects later returned to the operating room and were successfully implanted.
Table III.
Perioperative (Stage I to Stage III) Adverse Events; ODT+DBS patients (n=56)
| Type of Adverse Event | Transient | Ongoing |
|---|---|---|
| Related to Procedure or Device | ||
| Wound healing problems | 10 | 0 |
| Erythema | 2 | 0 |
| Edema | 2 | 0 |
| Pain | 2 | 0 |
| Drainage | 2 | 0 |
| Tingling | 0 | 1 |
| Tenderness | 1 | 0 |
| Headache | 5 | 0 |
| Edema | 4 | 0 |
| Scalp | 2 | 0 |
| Facial | 2 | 0 |
| Confusion | 4 | 0 |
| Imbalance | 3 | 0 |
| Drowsiness | 2 | 0 |
| Nausea | 2 | 0 |
| Vomiting | 2 | 0 |
| Expressive aphasia | 2 | 0 |
| Neck problems | 2 | 0 |
| Pain | 1 | 0 |
| Stiffness | 1 | 0 |
| Throat problems | 2 | 0 |
| Pain | 1 | 0 |
| Edema | 1 | 0 |
| Aborted procedure | 2 | 0 |
| Hematoma | 1 | 0 |
| Dysphagia | 1 | 0 |
| Intracranial edema | 1 | 0 |
| Basal ganglia infarct | 0 | 1 |
| Extremity weakness | 1 | 0 |
| Hallucination | 1 | 0 |
| Urinary retention | 1 | 0 |
| Constipation | 0 | 1 |
| Rigidity | 1 | 0 |
| Divergent Gaze | 1 | 0 |
| Apnea | 1 | 0 |
| Related to Study | ||
| Syncope | 1 | 0 |
| Not Related to Study | ||
| Incidental CT imaging sinus findings |
0 | 4 |
| Paresthesias | 1 | 0 |
| Fever | 1 | 0 |
| Chest soreness | 1 | 0 |
Temporary neurocognitive disturbances of confusion, hallucinations, and drowsiness were frequently reported. There were no psychiatric issues or cerebral hemorrhages. One patient suffered a symptomatic basal ganglia infarct. Minor issues regarding wound healing were raised but there were no infectious complications or hardware malfunctions. In summary, serious adverse events resulting in lasting sequelae were uncommon. Our complication rate is less than or equal to complication rates reported in numerous other surgical studies [23, 28–30].
To date, twenty-eight patients (fourteen in each category) have completed all mandatory follow-up visits. Unfortunately one individual in the ODT group withdrew from the study due to family and work-related circumstances.
Discussion
The three main goals of this study were to: prove that early PD patients would enroll in and complete a surgical trial, gain information about medication washout in early stages of disease, and, most importantly, to gather preliminary data on the safety and tolerability of DBS in early PD.
During study planning, we debated the principles of offering surgery to patients who might respond to conventional medical therapy for years. In the same vein, the feasibility of the trial, whether this patient group would be willing to participate in such a time-consuming and potentially risky undertaking, was questioned. Our ability to recruit a sufficient number of patients coupled with the questionnaire results and the trial completion rate thus far indicate that early PD patients will, in fact, participate and comply with the obligations of a long-term trial without misplaced expectations or false impressions. This supports the practicability of effective and ethical recruitment for a multicenter clinical trial to evaluate safety, efficacy, and impact on disease progression of DBS in earlier stages PD that have yet been examined.
Ascertaining an adequate time period for medication washout that would not be overly burdensome to study participants was difficult. The ELLDOPA trial included a two week washout period of levodopa and reported little deterioration after one week [31]. A small cohort study showed steady decline over one week in patients who had a stable response to levodopa [32]. Another investigation asserted a return to baseline at an average of 6.8 days and 6.2 days following withdrawal of controlled release levodopa/carbidopa and ropinirole, respectively [33]. Modeling data have estimated the mean half-life of levodopa, calculated by a loss of clinical benefit, as 7.9 days [34]. Further complicating this matter are unknown interactions between PD medications that could potentially prolong the symptomatic response. As evidenced by these varied study results, the issue has not been resolved and a universally accepted time period for washout has not been agreed upon. In our study, a one-week washout period was chosen and carried out without significant adverse effects other than the expected worsening of Parkinsonism. Medications were abruptly withdrawn without evidence of neuroleptic malignant syndrome.
Study procedures were endured well and standard methods of DBS implantation for advanced PD patients were applied. Operative recordings and stimulation successfully identified target nuclei and guided lead placement. The early PD patients were more adept than the advanced population at cooperating during surgery and articulating stimulation effects. This is seemingly secondary to less disability and a higher tolerance for the “off” medication state. There were few perioperative adverse events; importantly, no hemorrhages or infectious complications occurred. These results are comparable or better than those for advanced PD patients currently receiving DBS [23, 28–30].
There are a few limitations of this study. One is that neither the participants nor the principal investigator were blinded. Once all subjects complete the trial, however, a group of movement disorder neurologists will evaluate all UPDRS-III videotapes in a single-blinded fashion. Secondly, there was no placebo group. This is consistent with most similar studies, though, in which sham surgery is rarely done. Third, patients were allowed any combination of PD medications at study entry and throughout the trial. Thus, an untreated baseline was not obtained and a standardized regimen of medication was not employed. We must therefore take into account that medication interactions could have interfered with washout UPDRS-III scores. Still, this would not affect our ability to evaluate safety and tolerability of therapy and medication washout.
This small, randomized investigation provides valuable information concerning the preliminary safety and tolerability of DBS in early PD and demonstrates that those in the earliest stages of PD can be successfully recruited, consented, and retained in a long-term clinical trial of DBS. If present, the length of time that will be necessary to demonstrate a neuroprotective effect will be dependent upon the number of patients enrolled in the trial, the magnitude of the neuroprotective effect provided by the therapy, and the sensitivity of the measure used for the primary endpoint. We estimate that approximately 300 patients participating in a similarly designed clinical trial would need to be followed for at least 2 years. The final results of this pilot study will provide data necessary to design a large scale multicenter clinical trial to determine if the application of DBS in early stage PD can modify disease progression as demonstrated by reduced disability, improved quality of life, extended employment, or prolonged independence.
Acknowledgements
The clinical trial from which this case is reported is funded by Medtronic, Inc., by Vanderbilt CTSA grant 1 UL1 RR024975 from the NCRR-NIH, and by private donations. Medtronic representatives did not take part in data collection, management, analysis, or interpretation of the data or in preparation, review, or approval of the manuscript.
Appendix
Financial Disclosures
Dr. Charles has received personal compensation and Vanderbilt University has received grants to support research from Medtronic in excess of $10,000. Dr. Charles has also received funding from Allergan for speaking and consulting services as well as research grants.
Dr. Dolhun reports no disclosures.
Ms. Gill reports no disclosures.
Dr. Davis has received personal compensation and Vanderbilt University has received grants to support research from Medtronic in excess of $10,000. He also served as a consultant for Allergan, Novartis, and UCB. He has also received payment for lectures from Allergan and TEVA as well as payment for development of educational presentations from UCB and TEVA.
Dr. Bliton reports no disclosures.
Dr. Tramontana reports no disclosures.
Dr. Salomon reports no disclosures.
Dr. Wang reports no disclosures.
Dr. Hedera has received payment for lectures from Lundbeck.
Dr. Phibbs reports no disclosures.
Dr. Neimat has received personal compensation and Vanderbilt University has received grants to support research from Medtronic in excess of $10,000. Dr. Neimat has also received payment for lectures from Medtronic and FHC, Inc. He has also received reimbursement for travel/accommodations/meeting expenses apart from consultancy.
Dr. Konrad has received personal compensation and Vanderbilt University has received grants to support research from Medtronic in excess of $10,000. Dr. Konrad also serves as a consultant for Medtronic and FHC, Inc.
Author Roles
Charles: Conception and design; acquisition of data; interpretation of data; critical revision of the manuscript; obtaining funding; supervision. Dr. Charles has full access to the study data and takes full responsibility for the integrity and accuracy of data analysis.
Dolhun : Acquisition of data ; interpretation of data ; drafting of the manuscript ; critical revision of the manuscript.
Gill: Acquisition of data; interpretation of data; drafting of the manuscript; critical revision of the manuscript; administrative or technical support.
Davis: Conception and design; critical revision of the manuscript; administrative, technical or material support.
Bliton: Conception and design; critical revision of the manuscript; administrative, technical or material support.
Tramontanta: Conception and design; acquisition of data; interpretation of data; critical revision of the manuscript; administrative, technical or material support.
Salomon: Conception and design; acquisition of data; interpretation of data; critical revision of the manuscript; administrative, technical or material support.
Wang: Conception and design; interpretation of data; statistical analysis; critical revision of the manuscript.
Hedera: Conception and design; acquisition of data; interpretation of data; critical revision of the manuscript; administrative, technical or material support.
Phibbs: Conception and design; acquisition of data; interpretation of data; critical revision of the manuscript; administrative, technical or material support.
Neimat: Conception and design; critical revision of the manuscript; administrative, technical or material support; supervision.
Konrad: Conception and design; critical revision of the manuscript; administrative, technical or material support; supervision.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olanow CW. The scientific basis for the current treatment of Parkinson's disease. Annu Rev Med. 2004;55:41–60. doi: 10.1146/annurev.med.55.091902.104422. [DOI] [PubMed] [Google Scholar]
- 2.FDA. [updated 2011 Jul 29; cited 2011 Aug 11];Premarket approval for Medtronic Activa tremor control system. 1997 Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=8621.
- 3.Schulz D. Medtronic Activa Parkinson’s control therapy. Rockville, MD: Food and Drug Administration; 2002. [cited 2011 Aug 11]. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf/p960009S7a.pdf. [Google Scholar]
- 4.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. Jama. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 6.Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurol. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- 7.Lozano AM, Mahant N. Deep brain stimulation surgery for Parkinson's disease: mechanisms and consequences. Parkinsonism Relat Disord. 2004;10(Suppl 1):S49–S57. doi: 10.1016/j.parkreldis.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Charles PD, Padaliya BB, Newman WJ, Gill CE, Covington CD, Fang JY, et al. Deep brain stimulation of the subthalamic nucleus reduces antiparkinsonian medication costs. Parkinsonism Relat Disord. 2004;10:475–479. doi: 10.1016/j.parkreldis.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003;349:1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 10.Shichi T, Okiyama R, Yokochi F, Taniguchi M, Takahashi H, Hamada I. Unilateral subthalamic stimulation for early-stage Parkinson's disease. No To Shinkei. 2005;57:495–498. [PubMed] [Google Scholar]
- 11.Mesnage V, Houeto JL, Welter ML, Agid Y, Pidoux B, Dormont D, et al. Parkinson's disease: neurosurgery at an earlier stage? J Neurol Neurosurg Psychiatry. 2002;73:778–779. doi: 10.1136/jnnp.73.6.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schupbach WM, Maltete D, Houeto JL, du Montcel ST, Mallet L, Welter ML, et al. Neurosurgery at an earlier stage of Parkinson disease: a randomized, controlled trial. Neurology. 2007;68:267–271. doi: 10.1212/01.wnl.0000250253.03919.fb. [DOI] [PubMed] [Google Scholar]
- 13.Schapira AH, Olanow CW. Neuroprotection in Parkinson disease: mysteries, myths, and misconceptions. Jama. 2004;291:358–364. doi: 10.1001/jama.291.3.358. [DOI] [PubMed] [Google Scholar]
- 14.Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease. Neurology. 2009;72:S1–S136. doi: 10.1212/WNL.0b013e3181a1d44c. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez MC, Obeso JA, Olanow CW. Subthalamic nucleus-mediated excitotoxicity in Parkinson's disease: a target for neuroprotection. Ann Neurol. 1998;44:S175–S188. doi: 10.1002/ana.410440726. [DOI] [PubMed] [Google Scholar]
- 16.Piallat B, Benazzouz A, Benabid AL. Subthalamic nucleus lesion in rats prevents dopaminergic nigral neuron degeneration after striatal 6-OHDA injection: behavioural and immunohistochemical studies. Eur J Neurosci. 1996;8:1408–1414. doi: 10.1111/j.1460-9568.1996.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakao N, Nakai E, Nakai K, Itakura T. Ablation of the subthalamic nucleus supports the survival of nigral dopaminergic neurons after nigrostriatal lesions induced by the mitochondrial toxin 3-nitropropionic acid. Ann Neurol. 1999;45:640–651. [PubMed] [Google Scholar]
- 18.Maesawa S, Kaneoke Y, Kajita Y, Usui N, Misawa N, Nakayama A, et al. Long-term stimulation of the subthalamic nucleus in hemiparkinsonian rats: neuroprotection of dopaminergic neurons. J Neurosurg. 2004;100:679–687. doi: 10.3171/jns.2004.100.4.0679. [DOI] [PubMed] [Google Scholar]
- 19.Temel Y, Visser-Vandewalle V, Kaplan S, Kozan R, Daemen MA, Blokland A, et al. Protection of nigral cell death by bilateral subthalamic nucleus stimulation. Brain Res. 2006;1120:100–105. doi: 10.1016/j.brainres.2006.08.082. [DOI] [PubMed] [Google Scholar]
- 20.Visser-Vandewalle V, van der Linden C, Temel Y, Celik H, Ackermans L, Spincemaille G, et al. Long-term effects of bilateral subthalamic nucleus stimulation in advanced Parkinson disease: a four year follow-up study. Parkinsonism Relat Disord. 2005;11:157–165. doi: 10.1016/j.parkreldis.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Effects of tocopherol and deprenyl on the progression of disability in early Parkinson's disease. The Parkinson Study Group. N Engl J Med. 1993;328:176–183. doi: 10.1056/NEJM199301213280305. [DOI] [PubMed] [Google Scholar]
- 22.Olanow CW, Rascol O, Hauser R, Feigin PD, Jankovic J, Lang A, et al. A double-blind, delayed-start trial of rasagiline in Parkinson's disease. N Engl J Med. 2009;361:1268–1278. doi: 10.1056/NEJMoa0809335. [DOI] [PubMed] [Google Scholar]
- 23.Pahwa R, Factor SA, Lyons KE, Ondo WG, Gronseth G, Bronte-Stewart H, et al. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:983–995. doi: 10.1212/01.wnl.0000215250.82576.87. [DOI] [PubMed] [Google Scholar]
- 24.Charles PD, Gill CE, Davis TL, Konrad PE, Benabid AL. Is deep brain stimulation neuroprotective if applied early in the course of PD? Nat Clin Pract Neurol. 2008;4:424–426. doi: 10.1038/ncpneuro0848. [DOI] [PubMed] [Google Scholar]
- 25.Lang AE, Widner H. Deep brain stimulation for Parkinson's disease: patient selection and evaluation. Mov Disord. 2002;17(Suppl 3):S94–S101. doi: 10.1002/mds.10149. [DOI] [PubMed] [Google Scholar]
- 26.Hauser RA, Holford NHG. Quantitative Description of Loss of Clinical Benefit Following Withdrawal of Levodopa-Carbidopa and Bromocriptine in Early Parkinson’s Disease. Movement Disorders. 2002;5(17):961–968. doi: 10.1002/mds.10226. [DOI] [PubMed] [Google Scholar]
- 27.Tabbal SD, Revilla FJ, Mink JW, et al. Safety and efficacy of subthalamic nucleus deep brain stimulation performed with limited intraopeerative mapping for treatment of Parkinson’s disease. Neurosurgery. 2007;61(Suppl 3):119–127. doi: 10.1227/01.neu.0000289725.97211.51. [DOI] [PubMed] [Google Scholar]
- 28.Lyons KE, Wilkinson SB, Overman J, Pahwa R. Surgical and hardware complications of subthalamic stimulation: a series of 160 procedures. Neurology. 2004;63:612–616. doi: 10.1212/01.wnl.0000134650.91974.1a. [DOI] [PubMed] [Google Scholar]
- 29.Pahwa R, Wilkinson SB, Overman J, Lyons KE. Bilateral subthalamic stimulation in patients with Parkinson disease: long-term follow up. J Neurosurg. 2003;99:71–77. doi: 10.3171/jns.2003.99.1.0071. [DOI] [PubMed] [Google Scholar]
- 30.Wider C, Pollo C, Bloch J, Burkhard PR, Vingerhoets FJ. Long-term outcome of 50 consecutive Parkinson's disease patients treated with subthalamic deep brain stimulation. Parkinsonism Relat Disord. 2008;14:114–119. doi: 10.1016/j.parkreldis.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 32.Ogasahara S, Nishikawa Y, Takahashi M, Wada K, Nakamura Y, Yorifuji S, et al. Dopamine metabolism in the central nervous system after discontinuation of L-dopa therapy in patients with Parkinson disease. J Neurol Sci. 1984;66:151–163. doi: 10.1016/0022-510x(84)90003-0. [DOI] [PubMed] [Google Scholar]
- 33.Barbato L, Stocchi F, Monge A, Vacca L, Ruggieri S, Nordera G, et al. The long-duration action of levodopa may be due to a postsynaptic effect. Clin Neuropharmacol. 1997;20:394–401. doi: 10.1097/00002826-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Hauser RA, Holford NH. Quantitative description of loss of clinical benefit following withdrawal of levodopa-carbidopa and bromocriptine in early Parkinson's disease. Mov Disord. 2002;17:961–968. doi: 10.1002/mds.10226. [DOI] [PubMed] [Google Scholar]