Abstract
The phenotype of B cells responsible for the production of anti-pneumococcal polysaccharide antibody has been unclear. Although individuals that respond poorly to the 23-valent pneumococcal polysaccharide(s) (PPS) vaccine, Pneumovax®, such as children<2 years, the asplenic and a subset of Common Variable Immunodeficiency (CVID) patients are profoundly deficient or lack IgM memory cells (CD27+IgM+), they are also deficient in the switched memory (CD27+ IgM−) compartment. Direct characterization of PPS-specific B cells has not been performed. In this study we labeled PPS14 and PPS23F with fluorescent markers. Fluorescent labeled PPSs were used in FACSAria flow cytometry to characterize the phenotype of PPS-specific B cells obtained from 18 young adults pre- and post-immunization with Pneumovax®. The labeled PPS were capable of inhibiting binding of antibody to the native PPS. Similarly, the native PPS were able to inhibit binding of PPS-specific B cells in a flow cytometric assay demonstrating specificity and functionality. Phenotypic analysis of unselected B cells, pre- and post-immunization demonstrated a predominance of naïve CD27−IgM+ cells accounting for 61.4% of B cells. Likewise, the PPS-specific B cells obtained pre-immunization consisted primarily of naïve, CD27− B cells, 55.4–63.8%. In contrast, the PPS-specific B cells obtained post-immunization were predominantly IgM memory cells displaying the CD27+IgM+, 54.2% for PPS14 and 66% for PPS23F, significantly higher than both unselected B cells and PPS-specific B cells. There was no significant difference in switched memory B cell populations (CD27+IgM−) between groups. These results suggest a dominant role of IgM memory cells in the immune response to pneumococcal polysaccharides.
Introduction
Streptococcus pneumoniae is a major cause of morbidity and mortality in young children, elderly adults, and immune compromised hosts. There are currently two types of vaccines that offer protection against pneumococcal disease: conjugate vaccines for children under 2 years age and a 23-valent pneumococcal polysaccharide vaccine (PPV23) for protection in adults (1). Both vaccines elicit serotype specific opsonic antibodies, which are necessary for protection (2, 3). The phenotype of the B lymphocyte population responsible for the immune response to the purified pneumococcal vaccine (Pneumovax®) has been controversial. The debate centers primarily on the surface antigens expressed by the responding B lymphocytes. Recently, it has been suggested that peripheral blood CD27+ IgM+ or IgM memory B lymphocytes are recirculating splenic marginal zone (MZ) B lymphocytes (4, 5). These lymphocytes are believed to recognize TI-2 antigens such as pneumococcal polysaccharide by virtue of a pre-diversified surface IgM and respond immediately without T cell help (6, 7). This view treats CD27+ IgM+ B lymphocytes as innate immune cells in the first line of defense (8–10).
In support of this concept, it has been shown that persons with decreased or absent IgM memory B lymphocytes such as the splenectomized, infants under 2 years of age, elderly, HIV infected, and a subgroup of common variable immunodeficiency patients, all respond poorly to polysaccharide vaccines and are highly susceptible to infections with encapsulated organisms (5–7, 11–13). It is however unlikely that IgM memory B lymphocytes are exclusively responsible for anti-polysaccharide antibody production as switched memory B lymphocytes (IgM−CD27+) secrete anti-PPS antibody following in vitro stimulation (14). Furthermore, sequence analysis of anti-PPS antibodies, 5 days post-vaccination, demonstrate a predominance of IgG and IgA antibodies, derived from switched memory cells that have undergone somatic hypermutation (15–17).
Moreover, IgM and switched memory B cells likely play important roles in the immune response to PPV. Although several studies have demonstrated that loss of IgM and/or switched memory B cells in the HIV-negative and HIV-infected populations, they did not focus on the PPS-specific cells (7, 13, 18). We have established a technique to identify PPS-specific B lymphocytes, enabling us to characterize the phenotype of PPS-specific B lymphocytes.
In this study we have identified PPS specific B lymphocytes using fluorescently labeled polysaccharides and analyzed the phenotype of these polysaccharide-specific B cells by flow cytometry. The results of our study demonstrates a significant increased representation of IgM memory B cells in the polysaccharide-specific B cell fraction compared to the unselected B cell fraction, providing direct evidence of the importance of IgM memory cells in the response to pneumococcal polysaccharides.
Materials and Methods
Human volunteers
Twenty two pneumococcal polysaccharide vaccine-naïve healthy volunteers between the ages of 18–30 years (mean=24) participated in the University of Toledo IRB committee approved study (IRB # 105137). Each individual was questioned about medications, previous illness and present health. In addition, Hepatitis B, Hepatitis C, HTLV 1 &2, and HIV screening was performed. Each individual was explained about study design and protocol in detail. Informed consent was obtained from all participants. Volunteers were immunized with the 23-valent pneumococcal polysaccharide vaccine (Pneumovax® 23, Merck). Blood samples were collected pre-vaccination, day six, and four –six weeks post-vaccination. Lymphocytes were obtained for flow cytometric analysis at day 0 and day 6 post-vaccination. Serum samples obtained at day 0 and between 4–6 weeks were used to measure serum antibody responses and opsonophagocytic activity.
Labeling of polysaccharide 14 and 23F with fluorescent dye
Conjugation of PPS-14 to Cascade Blue Ethylenediamine (CB) (Invitogen Cat # C-621); or PPS-23F to 5 - (4,6 - Dichlorotriazinyl)aminofluorescein (DTAF) (Sigma Fluka Cat # 36565) was carried out as follows. Ten mg of PPS-14 or 23F (10 mg/ml in 0.1 M Borate Buffer, pH 9.0) was incubated with 1.0 mg of Cascade Blue or 1.0 mg of 5-DTAF respectively for 2.5 hours at 4°C. The mixture was dialyzed against PBS for 24 hours at 4°C with 4 changes of PBS (MW cutoff = 8kDa). Approximately 10μl of 5M Sodium cyanoborohydride was added to the dialysate and the samples were mixed for another 30 minutes at 4°C in the dark. The samples were again dialyzed against PBS for 24 hours at 4°C with 4 changes of PBS. Finally samples were subjected to chromatography on a Sephadex G-25 column (1cm Diameter × 17cm height) at 4°C in the dark. Fractions (150μl each) containing PPS-14 or PPS- 23F complexed with Cascade Blue or DTAF respectively were pooled and stored at −20°C.
Fluorescent labeling of polysaccharide-specific hybridoma cells and human B lymphocytes
Mouse hybridoma cells with specificity for pneumococcal polysaccharides 14 and 23F, used as positive control were a gift from Pfizer (Pearl River, N.Y.). Hybridoma cells with specificity for PPS14 and/or PPS23 were stained by incubation with 10μg/ml fluorescently labeled pneumococcal polysaccharides 14-Cascade Blue and 23F-DTAF. PPS14 and PPS23-positive B lymphocytes were identified using the following fluorescently labeled antibodies: anti-CD19-PE, anti-CD27-PerCP-Cy5.5, anti-IgM-APC, anti-IgD (Alexa Fluor 700) (BD Pharmingen), and fluorescently labeled PPS 14-Cascade Blue and 23F-DTAF (BioCentra LLC, Sugar Land, TX).
PBMC were collected from immunized volunteers at day zero and 6 days post-vaccination. Buffy coats were harvested and mixed 1:1 with PBS 2mM EDTA and layered onto lymphocyte separation medium (Cellgro®) followed by centrifugation. Cells were then resuspended in RBC lysis buffer followed by addition of 10ml PBS 2mM EDTA 0.1% BSA. Cells were counted, centrifuged, and resuspended to 1×108 cells/ml. Before staining, cells were absorbed with 10μg/ml of cell wall polysaccharide (CPS) (Statens Serum Institut, 3459; MiraVista Diagnostics, Indianapolis, IN) and PPS 22F (ATCC) for 20 minutes; this step has been shown to reduce non-specific binding in ELISA (19). For inhibition flow experiments listed concentrations of homologous pneumococcal polysaccharide were included during the absorption step. Cells were then labeled with 10μg/ml of labeled polysaccharide, either 14-CB or 23F-DTAF, and previously mentioned markers per manufacturer's instructions. Cells were washed and resuspended in PBS and analyzed with three laser FACSAria using FACSDiva software (BD Biosciences). FCS files were further analyzed using FlowJo software (TreeStar, Ashland, OR).
Pneumococcal Polysacchardie ELISA
ELISA was performed to examine the anti-PPS specific human antibodies in all volunteers. The pneumococcal polysaccharide ELISA is a modification of the WHO assay (20). Briefly, 5 μg/ml of pneumococcal polysaccharide, either 14 or 23F, were absorbed onto Nunc Maxisorp microtiter plates (Nunc Roskilde, Denmark) at 37°C overnight. Plates were then washed with PBS + 0.1%Tween-20 (PBST). Sera was diluted 1/200 in PBST then adsorbed with CPS (10 μg/ml) and 22F (10 μg/ml) for 30min at room temperature. After absorption sera were serially diluted onto the plates and incubated at 37°C for 2hrs; the standard serum 89-SF was used as a positive control. Plates were washed and bound antibody was detected using HRP-conjugated anti-human Ig(H+L) monoclonal antibody (Southern Biotech) diluted 1/3000 in 1% BSA PBST and incubated at 37°C for 1hr. After washing, plates were developed by using an OPD substrate and the O.D. was read at a wavelength of 490nm.
Inhibition ELISA
The inhibition ELISA is similar to the 22F absorption ELISA as reported by Concepcion and Frasch (19). Briefly, 10μg/ml of purified pneumococcal polysaccharides, either 14 or 23F, were adsorbed onto Nunc Maxisorp (Nunc Roskilde, Denmark) high-binding microtiter plates at 37°C overnight. The plates were blocked with 1% bovine serum albumin (BSA) in PBS/0.1% Tween-20 (PBST) for 2hrs at 37°C. Supernatants were absorbed with 10ug/ml CPS (Statens Serum Institut, 3459; MiraVista Diagnostics, Indianapolis, IN) and 22F (ATCC) for 30 min at room temperature. Furthermore, supernatants were blocked for 30 min at room temperature with increasing concentrations of homologous fluorescently labeled polysaccharide, either 14-cascade blue or 23F-DTAF, to show inhibition. For negative controls the previously described CPS and 22F absorbed samples were blocked with heterologous polysaccharide 23F-DTAF for polysaccharide 14 and 14-CB for 23F. Supernatants were added to the ELISA plates and incubated at 37°C for 1hr. The standard serum 89-SF was used as a positive control. Bound antibody was detected using HRP-conjugated anti-human Ig(H+L) monoclonal antibody (Southern Biotech) diluted 1/3000 in 1% BSA PBST and incubated at 37°C for 1hr. Plates were developed by using OPD substrate, stopped with H2SO4 and O.D. was read at a wavelength of 490nm.
Opsonophagocytic assay
Opsonophagocytic assay was performed as previously described (21, 22) to determine functional vaccine response to pneumococcal polysaccharides 14 and 23F. Briefly, S. pneumoniae, serotypes 14 and 23F were incubated with serial diluted heat inactivated pre-vaccination and post-vaccination sera. Newborn rabbit serum (Pel-Freez, Brown Deer, WI) was added as a source of complement. Differentiated HL-60 cells were added at an effector/target ratio of 400:1. All sera were tested in duplicate. The opsonophagocytic titer was determined as the reciprocal of the dilution with 50% killing when compared to serum free controls and analyzed using the Opsotiter1 software program from the University of Alabama at Birmingham.
Statistical analysis
Geometric mean concentration of IgG, IgM and IgA and Flow cell numbers, specific to PPS14 and 23F were calculated for each group. Correlation between two groups was examined using Pearson's correlation coefficient. Comparison between two group values was performed using unpaired t test. P values less than 0.05 were considered to be significant.
Results
Donor antibody response to vaccination
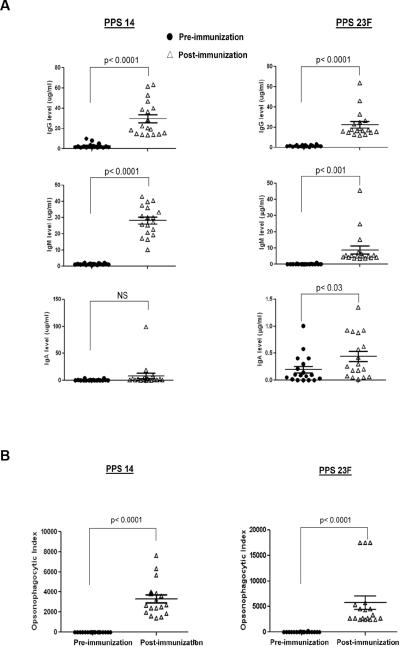
To confirm pneumococcal polysaccharide specific immune response to vaccination we obtained pre-vaccination sera, day zero, and post-vaccination sera, day 28–42, from healthy young adults and measured antibody responses to serotypes 14 and 23F. Antibody concentrations were determined following absorption with PS22F and cell wall polysaccharide (CPS) as previous studies have demonstrated that absence of absorption with 22F and CPS results in an over estimation of polysaccharide-specific antibody concentration (19, 21). Post-vaccination, donors had a significant increase in concentration of PPS14 specific IgG from 2.96±2.5 μg/ml to 29.78±17.07 μg/ml (p<0.0001) and IgM from 1.37±0.49 μg/ml to 28.17±9.26 μg/ml (p<0.0001). Although post-vaccination PPS14 specific IgA titers were higher than pre-vaccination titers, but no significant difference was found (p=0.08). Similarly, post-vaccination responses to PPS23F were significantly increased compared to pre-vaccination sera, IgG from 1.54±0.71 μg/ml to 21.54±13.09 μg/ml (p<0.0001) and IgM from 0.17±0.22 μg/ml to 8.86±10.57 μg/ml (p<0.0001). In our sample population the greatest increase in pre- to post-vaccination concentration was for the isotype IgG followed by IgM for both PPS14 and PPS23F. There was a positive correlation between pre- and post-vaccination PPS14-specific IgM (r2 = 0.88; p <0.0001) and pre- and post-vaccination PPS23F-specific IgM (r2 = 0.98, p<0.0001) and IgG (r2 = 0.79, p<0.0001) and IgA (r2 = 0.81; p < 0.001). In summary all donors displayed an increase in serotype specific antibody response in immunoglobulin isotypes IgG, IgM, and IgA with the exception of donor 4 whose IgA levels remained undetectable after vaccination. This data confirms that the young healthy donors used in this study responded to Pneumovax23®. Donor antibody responses are summarized in Figure 1A.
Figure 1.
Serum antibody response and opsonophagocytic activity. Healthy young volunteers (n=18) were immunized with Pneumovax®. Serum samples were obtained pre- and 4–6 weeks post-immunization. Serum samples were tested for PPS14 and PPS23F specific IgG, IgA, IgM (A) and opsonophagocytic activity (B). Serum antibody levels are expressed as μg/mL and opsonophagocytic activity is expressed as opsonophagocytic index.
Functional antibody response
The functional or opsonophagocytic response of serum antibody obtained pre-vaccination (day 0) and 28–42 days post-vaccination against both serotype 14 and 23F PPS was determined for all donors (Figure 1B). Data is reported as the opsonophagocytic index (OPI) or reciprocal of the antibody dilution required to obtain 50% opsonophagocytic killing by differentiated HL-60 cells. For all donors, post-vaccination sera had a significant increase in OPI against serotype 14 and serotype 23F when compared to prevaccination sera. Positive correlations were found between post-vaccination IgG and post-vaccination OPI for both PPS14 and PPS23F (r2 = 0.8, p<0.0001; r2 = 0.86, p<0.0001 respectively). Moreover, the sample population displayed a functional immune response after vaccination with Pneumovax23®.
Labeled polysaccharide maintains native epitope(s)
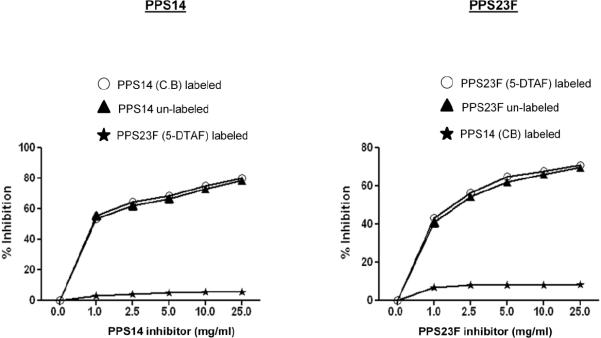
We evaluated the ability of fluorescently labeled pneumococcal polysaccharide to maintain the native epitope(s) of unlabeled polysaccharide. To this purpose, we performed an ELISA using increasing concentrations of fluorescently labeled PPS to inhibit binding of the polyclonal control serum 89-SF to wells coated with native unlabeled PPS14 or 23F. Unlabeled homologous polysaccharide was used as a positive control and unlabeled heterologous PPS was used as a negative control. The ability of native pneumococcal polysaccharide and fluorescently labeled pneumococcal polysaccharide to inhibit 89-SF binding to homologous polysaccharides were similar as shown in Figure 2. At 1μg/ml both cascade blue labeled PPS14 and unlabeled PPS14 were able to inhibit 89-SF binding by greater than 50%. Incremental addition of inhibitory PPS, up to 25μg/ml, further increased inhibition of 89-SF binding for both native and cascade blue labeled PPS14. Likewise, DTAF labeled PPS23F was able to inhibit 89-SF binding comparable to the native unlabeled PPS23F. Both labeled PPS14 and PPS23F displayed the same trend and similar magnitude of inhibitory affect as their unlabeled homologous counterparts, while minimal inhibition was seen in control wells where heterologous polysaccharide was used.
Figure 2.
Inhibition ELISA with fluorescent-labeled PPS14 and PPS23F. Various amounts of labeled PPS14 or PPS23F were pre-incubated with a 1:1000 dilution of standard serum 89SF. Percent inhibition was calculated compared to uninhibited samples.
Labeled polysaccharides bind homologous and not heterologous anti-PPS hybridoma cells
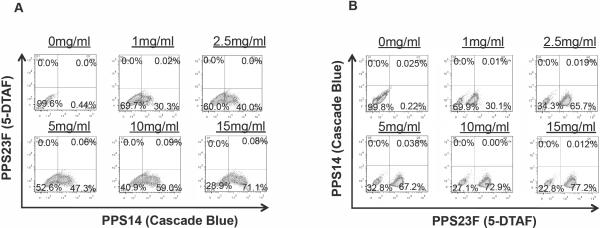
To further confirm functionality and specificity of the labeled polysaccharides, hybridoma cells with specificity for PPS14 (α14g2b) and for PPS23F (α23F) were incubated with PPS14-CB and with PPS23F-DTAF and subjected to flow cytometry. The results of these studies demonstrated that hybridoma cells α23F with specificity for PPS23F, uniquely bound PPS23F-DTAF and failed to bind PPS14-CB in a concentration dependent manner as shown in Figure 3. A sub-population of small likely non-secreting cells consistently failed to bind PPS23F-DTAF. Conversely, hybridoma cells α14g2b with specificity for PPS14, uniquely bound PPS14-CB and failed to bind PPS23F-DTAF, demonstrating both specificity and functionality of the labeled PPSs.
Figure 3.
Specific staining of hybridoma cells. Hybridoma cells with specificity for PPS14 (α14g2b) (A) or PPS23F (α23F) (B) were incubated with various amounts of PPS23F-DTAF and PPS14-CB, and subjected to flow cytometry. PPS14 hybridoma cells specifically bound PPS14-CB in a dose dependent manner and failed to stain with PPS23F-DTAF. PPS23F hybridoma cells specifically bound PPS23F-DTAF in a dose dependent manner and failed to stain with PPS14-CB. Fifty thousand events were recorded.
Fluorescently labeled polysaccharides recognize PPS-specific B lymphocytes
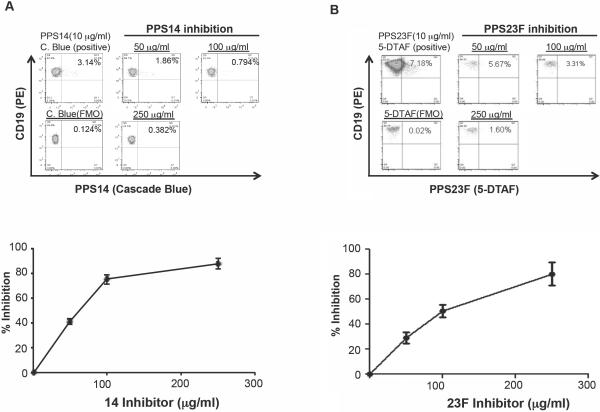
To demonstrate the specificity of binding of the fluorescently labeled PPS, an inhibition assay was performed. This was accomplished by pre-treating lymphocytes isolated 6 days post-vaccination, with increasing concentrations of homologous unlabeled polysaccharide before addition of fluorescently labeled polysaccharide. Inhibition of fluorescent polysaccharide binding was 87.8% at 250 ug/ml inhibitory PPS for PPS14-CB and greater than 80% for PPS23F-DTAF. Inhibition occurred in a concentration dependent manner as shown in Figure 4.
Figure 4.
Inhibition of binding fluorescently labeled PPS. Lymphocytes isolated 7 days post-vaccination were pre-treated with increasing concentrations of homologous unlabeled polysaccharide before addition of fluorescently labeled polysaccharide A=PPS14 and B=PPS23F. Fifty thousand events were recorded. Percent inhibition of binding to fluorescently labeled PPS was determined by comparison to the uninhibited cells.
Phenotypic analysis of polysaccharide specific B lymphocytes
To determine the phenotype of B lymphocytes that respond to vaccination with Pneumovax23®, 18 healthy young donors were immunized. Pre-vaccination (n=9) and 7 days post-vaccination (n=18) circulating PBMC were isolated, labeled and subjected to Flow cytometry analyses by using the following fluorescently labeled antibodies/antigen: CD19, CD27, IgM, IgD, PPS14 and PPS23F. The phenotype of the pre- and post-vaccination specific B cells was compared to the phenotype of unselected B cells. CD19+ B lymphocytes were subdivided into four categories: naïve (CD27− IgM+), CD27− IgM− class switched, IgM memory (CD27+ IgM+), and class switched memory (CD27+ IgM−) B cells.
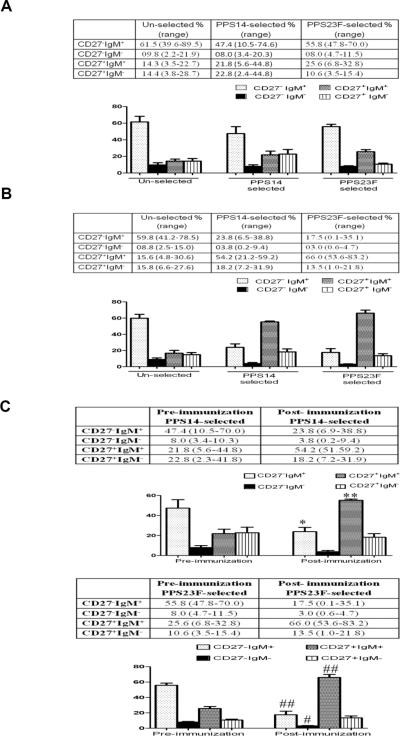
Analysis of the unselected B cell populations obtained pre-vaccination showed that a large proportion, 71.2% (41.8–97.2%), of these B cell were naïve or CD27−. The majority (61.4%) of the CD27− B cells expressed the CD27−IgM+ phenotype, while a minority, (9.8%) of total B cells were CD27−IgM−. The memory or CD27+ B cell population represented 28.7% of the B cells with 14.3% expressing the IgM memory phenotype (CD27+IgM+) and 14.4% were classic switched memory B cells (CD27+ IgM−). Analysis of the post-immunization unselected B cell population did not differ significantly from pre-immunization values (Figure 5A and Fig 5B).
Figure 5.
B cell phenotypes. The phenotype of B lymphocytes that respond to vaccination with Pneumovax23® was determined by flow cytometry. Pre-vaccination (n=9) and seven days post-vaccination (n=18) circulating PBMC were isolated and labeled for analysis using the following fluorescently labeled antibodies/antigen: CD19, CD27, IgM, IgD, PPS14 and PPS23F. The phenotype of pre-vaccination unselected B cells were compared to PPS-specific B cells (A), the phenotype of post-vaccination unselected B cells were compared to PPS-specific B cells (B) and pre-immunization PPS-specific B cells were compared to post-immunization PPS-specific B cells (C). In each sample 75,000 events were recorded. *p<0.0001 **p=0.0002 #p=0.037 ##p=0.006
In the pre-immunization samples, a small percentage of B cells, 0.56±0.34%, stained with fluorescently labeled PPS (Table 1). The PPS14 and PPS23F-specific B cells in this population demonstrated a predominance of CD27− B cells with a total of 55.4% (14–80.3%) for PPS14 and 63.8% (55.8–81.5%) for PPS23F. The CD27−IgM+ phenotype represented the majority of these cells with 47.4% for PPS14 and 55.8% for PPS23F of the total B cell population. The CD27+ population represented a total of 44.6% (8.1–86.6) and 36.2% (10.3–48.2) of the PPS14 and PPS23F-specific B cells with the IgM memory component (CD27+IgM+) representing 21.8% of the total PPS14-specific B cells and 25.6% of the PPS23F-specific B cells. The remainder of the B cell population consisted of switched memory B cells (CD27+IgM−), accounting for 22.8% of the PPS14-specific B cells and 10.6% of the PPS23F-specific B cells (Figure 5A).
Table 1.
The percentage of CD19+ B cells stained with fluorescently labeled PPS14 and PPS23F in peripheral blood samples obtained pre- and 7 days post-immunization.
| PPS-14 specific % | PPS-23F specific % | |
|---|---|---|
| Pre-immunization | 0.56 (± 0.13) | 0.53 (± 0.13) |
| Post-immunization | 2.75 (± 0.56) | 2.08 (± 0.41) |
In the 7 day post-immunization samples, the percentage of PPS fluorescently labeled B cells increased significantly to 2.75±1.48% for PPS14 and 2.08±1.17% for PPS23F (Table 1). In sharp contrast to both the unselected and pre-immunization PPS-selected B cell populations, the minority of post-immunization PPS-specific B cell populations consisted of naïve CD27− B cells, 27.6% for PPS14 and 20.5% for PPS23F. The naïve B cell population consisted primarily of CD27−IgM+ B cells, 23.8% and 17.5% for PPS14 and PPS23F respectively. A small percentage, 3.8% and 3% of B cells were naïve, class switched CD27−IgM− B cells. The majority of the PPS-selected B cells were memory B cells (CD27+) accounting for 72–79% of the total B cell population (Figures 5B and 6). Moreover, the IgM memory population, CD27+IgM+, was significantly overrepresented compared to both unselected and pre-immunization PPS-specific B cells, representing 54.2% of the PPS14-specific B cells and 66% of the PPS23F-specifc B cells (Fig. 5B and 5C). In contrast, there was no significant difference in switched memory (CD27+IgM−) population between post-immunization PPS14 and PPS23F-specific B cells and the unselected or pre-immunization PPS-selected populations, accounting for 18.2% and 13.5% respectively. Furthermore, there was a strong correlation between post-immunization IgM antibody concentration and post-immunization IgM memory B cell percentage for both PPS14 (r2=0.87) and PPS23F (r2=0.88). In contrast, the correlation between post-immunization IgG antibody concentration and post-immunization switched memory B cell percentage was much lower, r2=0.56 for PPS14 and r2=0.51 for PPS23F.
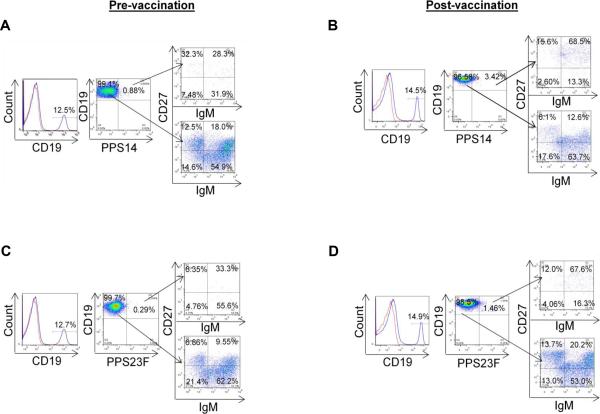
Figure.6.
Phenotype analyses of B cells in the human peripheral blood. Healthy donor PBMC sample was stained with Abs to CD19, CD27, IgM, and fluorescent labeled PPS14 (A and B) and 23F (C and D). CD19+ B cells (shown in histogram, dotted line=isotype control) were gated on PPS23F or PPS14. PPS14 or 23F specific B cells (CD19+PPS14+, CD19+PPS23F+) and PPS14 or 23F negative B cells (CD19+PPS14−, CD19+PPS23F−) cells were separated into CD27+IgM+, CD27+IgM−, CD27−IgM+ and CD27−IgM-. In each sample 75,000 events were recorded. Representative data of FACS analyses; (A and C) pre-vaccination, (B and D) post-vaccination of PPS14 (A and B) or PPS23F (C and D).
Discussion
The goal of this study was to characterize the phenotype of B cells responding to Pneumovax23® vaccination. We specifically chose pneumococcal polysaccharides 14 and 23F as they are the most common disease causing serotypes found in adult high risk groups such as the elderly and HIV infected (23–25).
To assess the immune competency of our volunteers we studied pre- and post-vaccination polysaccharide specific immunoglobulin concentration and opsonophagocytic assays. All individuals responded to vaccination displaying a significant increase in anti-polysaccharide antibody concentration, specifically IgG, with a concomitant significant increase in opsonophagocytic activity. Within our test group there was variability in pre- and post-vaccination PPS specific antibody concentration. Variability in antibody concentrations occurred between individuals and between serotypes within individuals. Therefore, a high response to one PPS present in the multivalent vaccine did not necessarily correlate with a high response to other PPS included in the vaccine. This data is consistent with previous studies investigating the pre- and post-vaccination anti-PPS antibody response in young healthy individuals to all serotypes included in Pneumovax23® (26). Moreover, all volunteers demonstrated a two-fold or higher increase in pre- versus post-immunization antibody concentrations. A positive correlation between pre- and post-immunization titers was noted and between post-immunization IgG and opsonophagocytic index. Although not the main focus of our study these basic immunological assays demonstrate the immune competency of our study group.
The specific subset(s) of B lymphocytes responsible for immune response to polysaccharide antigens remains to be elucidated. Studies have implicated the role of IgM memory cells, by some postulated to be splenic marginal zone B cells, in response to polysaccharide antigens (5, 7, 9, 27). The nature of the B cell phenotype responsible for the production of anti-polysaccharide antibodies, remains to be defined.
We used fluorescently labeled PPS in conjunction with flow cytometry for identification and analysis of PPS-specific B lymphocytes. Identification of antigen specific B lymphocytes using fluorescently labeled antigen has several advantages over previously used methods. Direct labeling of the polysaccharides allows for polysaccharide-specific B cell phenotype analysis while minimizing potential cross reactivity to linking agents used by indirect labeling methods. Overall this results in lower background binding and more accurate phenotype analysis. We have demonstrated the ability to identify pneumococcal polysaccharide specific B lymphocytes using fluorescently labeled polysaccharide in conjunction with flow cytometry. The specificity of our labeled PPS is supported by their ability to inhibit binding of the control sera 89-SF to homologous unlabeled polysaccharide in ELISA and the ability to bind to PPS14 or PPS23F-specific monoclonal cell lines. Likewise, binding of the labeled PPS to post-vaccination peripheral blood B lymphocytes in flow cytometry can be inhibited with addition of homologous unlabeled polysaccharide.
Flow cytometric analysis of unselected CD19+ B cells showed a predominance of naïve CD27−IgM+ B cells, as previously described in the peripheral blood of healthy adults (28–30). The CD27+ memory B cell population constituted about 30% of total B cells with an equal distribution between IgM memory and switched memory B cells. We found no significant difference in phenotype distribution between pre- and post-immunization unselected samples. This is not surprising as the PPS-specific B cells represented a small fraction, between 2–2.75%, of the total post-immunization B cell population insufficient to cause a significant shift in overall phenotype distribution. Analysis of the pre-immunization polysaccharide-specific B cells, 0.5% of the total B cell population, resulted in a phenotypic pattern very similar to that of unselected B cells with a predominance of naïve CD27−IgM+ B cells. There was however an increased percentage of CD27+IgM+ cells in the PPS-specific B cells although this was only significant for PPS23F. In contrast, in the 7 day post-immunization PPS-specific B cells, there was a highly significant shift in cell surface expression. The majority of post-immunization PPS14 and PPS23F-specific B cells expressed the IgM memory, CD27+IgM+, phenotype with a concomitant decrease in naïve, CD27−IgM+, expression when compared to the unselected B cells and to the pre-immunization PPS-specific B cells. It should be mentioned that we found a strong correlation between the post-immunization PPS-specific IgM and the number of PPS-specific IgM memory B cells. Notably, there was no significant change in the CD27+IgM− or switched memory population post-immunization and it correlated poorly with the IgG antibody concentration.
Isolation of antigen-specific B cells is known to be a challenging enterprise due to their limited presence in the B cell population. Overall, antigen selected B cells represent <1–2% (31–33) of total B cells, depending on technique and timing of isolation, and in line with our findings. Moreover, although a variety of techniques have been used, all have limitations in yield and purity. Both yield and purity can affect phenotype analysis performed in our studies. We employed direct fluorescent labeling of PPS in conjunction with flow cytometry/sorting. The PPS-selected population was inhibited 80–87% by unlabeled PPS, suggesting that eighty to 87%, depending on serotype, of our PPS-labeled B cells were indeed PPS-specific. In accordance, the remaining 13–20% of our PPS-labeled cells were not PPS-specific, false-positive, thus likely expressing the unselected cell phenotype, i.e. 14.3–15.6% CD27+IgM+, a much lower percentage than the PPS-positive B cells. Moreover, the presence of the false-positive PPS-labeled population therefore likely diminished, not increased, virtual expression of the CD27+IgM+ phenotype in our PPS-specific population. As both unselected and PPS-selected populations expressed a similar percentage of CD27+IgM−, it is unlikely that the contaminating or false-positive PPS-labeled population changed the percentage of switched memory B cells in the PPS-specific population.
These data suggest that a large portion of the PPS-responding B cells are IgM memory cells and this concept is supported by several experimental and clinical findings. First, both asplenic individuals and children younger than two years of age have an undetectable or severely reduced number of IgM memory cells, are at increased risk of pneumococcal infection and respond poorly to polysaccharide vaccines (5–7, 11–13). Second, the percentage of IgM memory cells in CVID patients correlates with incidence of encapsulated bacterial infection (13). In further support of these findings, Kruetzmann et al established a significant correlation between serum anti-polysaccharide serotype 3, 9 and 22 IgM antibody concentration and the number of IgM memory B cells in the peripheral blood of children. Several recent studies have focused on memory B lymphocyte sub-populations and immune response to pneumococcal polysaccharide vaccination particularly in HIV-positive individuals (13, 18). These studies analyzed the peripheral blood B lymphocyte population in HIV-positive individuals and correlated the results with either quantitative IgG or IgM antibody response to PPV. Moreover, both studies demonstrated a significant decrease in the switched memory B cell population in the HIV-infected. However, there was no correlation found between the number of switched memory B cells and anti-PPS IgG antibody concentration. In another study, Hart et al (13) described a highly significant loss of IgM memory B cells in HIV-positive individuals. The loss of B memory cells correlated with the decrease in the anti-PPS IgM antibody response. It should be mentioned that in these studies, as well as others, B lymphocyte populations were not selected for PPS-specificity complicating the interpretation of the data. The results of the present study however, support the hypothesis that IgM memory B cells play an important, if not crucial role in the immune response to pneumococcal polysaccharides.
As stated previously, one could argue that IgM memory B cells are not solely responsible for the antibody response to pneumococcal polysaccharide antigens. First, the elderly, asplenic and CVID patients not only have reduced or absent IgM memory cells but also significantly reduced numbers of switched memory B cells (6, 7). Second, switched memory (CD27+IgM−) B cells secrete higher levels of anti-pneumococcal polysaccharide antibody than IgM memory B cells (CD27+IgM+) following in vitro stimulation (14). Third, sequence analysis of anti-pneumococcal polysaccharide antibodies, obtained 5 days post-immunization, are predominately IgG and IgA isotypes (15–17, 34). Fourth, recent studies performed in SCID mice transplanted with human lymphocyte subsets demonstrated that switched memory B cells produced an IgG anti-polysaccharide response following vaccination with PPS (35). In support of this finding, Wardemann et al reported that both switched memory B cells (CD27+ IgG+) and naïve B cells expressed Ig with specificity for T-independent antigens (36, 37). In the present study, we did not find a significant quantitative difference in the switched memory B cell populations in the unselected versus selected pre- and post-immunization samples. All samples consisted of approximately 10–20% CD27+IgM− B cells. It should be mentioned however that the peripheral blood samples were obtained early, i.e. 7 days post-vaccination, at which time the highest number of antibody secreting cells (ASC) are found in the peripheral circulation (32, 38) while serum antibody response was evaluated at 4–6 weeks. The number of ASC diminish rapidly thereafter, significantly compromising analysis of PPS-specific B cells at later time points. Moens et al (35) demonstrated that mice reconstituted with purified CD27+IgM+ B cells produced both an anti-PPS IgM and IgG response following immunization. They hypothesized that immunization with PPS induced an isotype switch from IgM memory cells to IgG producing plasma cells. We examined the B cell phenotype in a small (n=4) number of individuals 4 to 6 weeks post-vaccination. These preliminary studies indicated that the number of PPS-specific B cells was similar to pre-immunization values, at a mere 0.5% of total B cells. Moreover, the phenotype of the PPS-specific B cells isolated at 4–6 weeks was not significantly different than the pre-immunization PPS-specific B cells. These data strongly suggest that the antibody secreting B cells are no longer present in the peripheral circulation at 4 to 6 weeks post-immunization. Extensive analysis of the phenotype of the PPS-specific B cells present in the peripheral blood at 4 to 6 weeks would probably result in an invalid analysis of the antibody secreting PPS-specific B cell population, likely present elsewhere in the B cell compartment, such as the spleen or bone marrow, at more distant time points. Thus a large number of questions remain to be elucidated.
We are presently expanding our studies to include a 2, 3 and 4 week time point post-vaccination for analysis of PPS-specific B cell phenotype. In addition, immune response and B cell phenotype following Pneumovax® will be compared to those generated following conjugate vaccination. Finally, although beyond the scope of the present studies, it will be interesting to determine cytokine profiles at various time points post-vaccination. Recent studies have demonstrated that immunization with PPS induced secretion of IL-6 and TNF-α from macrophages attributed to the presence of TLR2 and TLR4 ligands in Pneumovax® and thought to be responsible for the IgG component of the immune response to this T-independent type 2 antigen (39).
Acknowledgements
We thank Dr. P. Fernsten for his constructive review and discussions and Pfizer Inc. for their generous gift of hybridoma cells. We would like to thank all of the volunteers who participated in this study.
This work was supported by National Institutes of Health grants RO1A081558 and RO1AG015978 to M.A.J.W.
Abbreviations used in this article
- PPS
pneumococcal polysaccharide
- CVID
Common Variable Immunodeficiency
- PPV
23-valent pneumococcal polysaccharide vaccine
- MZ
marginal zone
- DTAF
5 - (4,6 - Dichlorotriazinyl)aminofluorescein
- CB
cascade blue
- HIV
human immunodeficiency virus
Footnotes
Disclosures The authors have no financial conflicts of interest.
References
- 1.Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children - Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59:258–261. [PubMed] [Google Scholar]
- 2.Musher DM, Chapman AJ, Goree A, Jonsson S, Briles D, Baughn RE. Natural and vaccine-related immunity to Streptococcus pneumoniae. J Infect Dis. 1986;154:245–256. doi: 10.1093/infdis/154.2.245. [DOI] [PubMed] [Google Scholar]
- 3.Vidarsson G, Sigurdardottir ST, Gudnason T, Kjartansson S, Kristinsson KG, Ingolfsdottir G, Jonsson S, Valdimarsson H, Schiffman G, Schneerson R, Jonsdottir I. Isotypes and opsonophagocytosis of pneumococcus type 6B antibodies elicited in infants and adults by an experimental pneumococcus type 6B-tetanus toxoid vaccine. Infect Immun. 1998;66:2866–2870. doi: 10.1128/iai.66.6.2866-2870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002;195:771–780. doi: 10.1084/jem.20011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, Tchernia G, Steiniger B, Staudt LM, Casanova JL, Reynaud CA, Weill JC. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Y, Yamazaki T, Okubo Y, Uehara Y, Sugane K, Agematsu K. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J Immunol. 2005;175:3262–3267. doi: 10.4049/jimmunol.175.5.3262. [DOI] [PubMed] [Google Scholar]
- 7.Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, Berner R, Peters A, Boehm T, Plebani A, Quinti I, Carsetti R. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillai S, Cariappa A, Moran ST. Positive selection and lineage commitment during peripheral B-lymphocyte development. Immunol Rev. 2004;197:206–218. doi: 10.1111/j.0105-2896.2003.097.x. [DOI] [PubMed] [Google Scholar]
- 9.Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu Rev Immunol. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- 10.Weller S, Reynaud CA, Weill JC. Vaccination against encapsulated bacteria in humans: paradoxes. Trends Immunol. 2005;26:85–89. doi: 10.1016/j.it.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. Immaturity of the human splenic marginal zone in infancy. Possible contribution to the deficient infant immune response. J Immunol. 1989;143:3200–3206. [PubMed] [Google Scholar]
- 12.Zandvoort A, Timens W. The dual function of the splenic marginal zone: essential for initiation of anti-TI-2 responses but also vital in the general first-line defense against blood-borne antigens. Clin Exp Immunol. 2002;130:4–11. doi: 10.1046/j.1365-2249.2002.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart M, Steel A, Clark SA, Moyle G, Nelson M, Henderson DC, Wilson R, Gotch F, Gazzard B, Kelleher P. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J Immunol. 2007;178:8212–8220. doi: 10.4049/jimmunol.178.12.8212. [DOI] [PubMed] [Google Scholar]
- 14.Takizawa M, Sugane K, Agematsu K. Role of tonsillar IgD+CD27+ memory B cells in humoral immunity against pneumococcal infection. Hum Immunol. 2006;67:966–975. doi: 10.1016/j.humimm.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Kolibab K, Smithson SL, Rabquer B, Khuder S, Westerink MA. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults: analysis of the variable heavy chain repertoire. Infect Immun. 2005;73:7465–7476. doi: 10.1128/IAI.73.11.7465-7476.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas AH, Moulton KD, Tang VR, Reason DC. Combinatorial library cloning of human antibodies to Streptococcus pneumoniae capsular polysaccharides: variable region primary structures and evidence for somatic mutation of Fab fragments specific for capsular serotypes 6B, 14, and 23F. Infect Immun. 2001;69:853–864. doi: 10.1128/IAI.69.2.853-864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Lottenbach KR, Barenkamp SJ, Reason DC. Somatic hypermutation and diverse immunoglobulin gene usage in the human antibody response to the capsular polysaccharide of Streptococcus pneumoniae Type 6B. Infect Immun. 2004;72:3505–3514. doi: 10.1128/IAI.72.6.3505-3514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Orsogna LJ, Krueger RG, McKinnon EJ, French MA. Circulating memory B-cell subpopulations are affected differently by HIV infection and antiretroviral therapy. Aids. 2007;21:1747–1752. doi: 10.1097/QAD.0b013e32828642c7. [DOI] [PubMed] [Google Scholar]
- 19.Concepcion NF, Frasch CE. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 2001;8:266–272. doi: 10.1128/CDLI.8.2.266-272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nahm MG, D. Training manual for enzyme linked immunosorbent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG (PnPg ELISA) 2002 http://www.vaccine.uab.edu.
- 21.Kolibab K, Smithson SL, Shriner AK, Khuder S, Romero-Steiner S, Carlone GM, Westerink MA. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults. I. Antibody concentrations, avidity and functional activity. Immun Ageing. 2005;2:10. doi: 10.1186/1742-4933-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, Keyserling HL, Carlone GM. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–422. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feikin DR, Klugman KP. Historical changes in pneumococcal serogroup distribution: implications for the era of pneumococcal conjugate vaccines. Clin Infect Dis. 2002;35:547–555. doi: 10.1086/341896. [DOI] [PubMed] [Google Scholar]
- 24.Janoff EN, Breiman RF, Daley CL, Hopewell PC. Pneumococcal disease during HIV infection. Epidemiologic, clinical and immunologic perspectives. Ann Intern Med. 1992;117:314–324. doi: 10.7326/0003-4819-117-4-314. [DOI] [PubMed] [Google Scholar]
- 25.Kupronis BA, Richards CL, Whitney CG. Invasive pneumococcal disease in older adults residing in long-term care facilities and in the community. J Am Geriatr Soc. 2003;51:1520–1525. doi: 10.1046/j.1532-5415.2003.51501.x. [DOI] [PubMed] [Google Scholar]
- 26.Go ES, Ballas ZK. Anti-pneumococcal antibody response in normal subjects: a meta-analysis. J Allergy Clin Immunol. 1996;98:205–215. doi: 10.1016/s0091-6749(96)70244-0. [DOI] [PubMed] [Google Scholar]
- 27.Park S, Nahm MH. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infect Immun. 2011;79:314–320. doi: 10.1128/IAI.00768-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pneumococcal vaccines: WHO position paper. Weekly Epidemiological Record. 1999;74:177–183. [PubMed] [Google Scholar]
- 29.Carsetti R, Rosado MM, Donnanno S, Guazzi V, Soresina A, Meini A, Plebani A, Aiuti F, Quinti I. The loss of IgM memory B cells correlates with clinical disease in common variable immunodeficiency. J Allergy Clin Immunol. 2005;115:412–417. doi: 10.1016/j.jaci.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 30.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irsch J, Hunzelmann N, Tesch H, Merk H, Maggi E, Ruffilli A, Radbruch A. Isolation and characterization of allergen-binding cells from normal and allergic donors. Immunotechnology. 1995;1:115–125. doi: 10.1016/1380-2933(95)00012-7. [DOI] [PubMed] [Google Scholar]
- 32.Kehrl JH, Fauci AS. Activation of human B lymphocytes after immunization with pneumococcal polysaccharides. J Clin Invest. 1983;71:1032–1040. doi: 10.1172/JCI110830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oshiba A, Renz H, Yata J, Gelfand EW. Isolation and characterization of human antigen-specific B lymphocytes. Clin immunology Immunopath. 1994;72:342–349. doi: 10.1006/clin.1994.1151. [DOI] [PubMed] [Google Scholar]
- 34.Zhou J, Lottenbach KR, Barenkamp SJ, Lucas AH, Reason DC. Recurrent variable region gene usage and somatic mutation in the human antibody response to the capsular polysaccharide of Streptococcus pneumoniae type 23F. Infect Immun. 2002;70:4083–4091. doi: 10.1128/IAI.70.8.4083-4091.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moens L, Wuyts M, Meyts I, De Boeck K, Bossuyt X. Human memory B lymphocyte subsets fulfill distinct roles in the anti-polysaccharide and anti-protein immune response. J Immunol. 2008;181:5306–5312. doi: 10.4049/jimmunol.181.8.5306. [DOI] [PubMed] [Google Scholar]
- 36.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuiji M, Yurasov S, Velinzon K, Thomas S, Nussenzweig MC, Wardemann H. A checkpoint for autoreactivity in human IgM+ memory B cell development. J Exp Med. 2006;203:393–400. doi: 10.1084/jem.20052033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heilmann C, Pedersen FK. Quantitation of blood lymphocytes secreting antibodies to pneumococcal polysaccharides after in vivo antigenic stimulation. Scand J Immunol. 1986;23:189–194. doi: 10.1111/j.1365-3083.1986.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 39.Sen G, Khan AQ, Chen Q, Snapper CM. In vivo humoral immune responses to isolated pneumococcal polysaccharides are dependent on the presence of associated TLR ligands. J Immunol. 2005;175:3084–3091. doi: 10.4049/jimmunol.175.5.3084. [DOI] [PubMed] [Google Scholar]