Abstract
Objective
While biomedical risks contribute to poor pregnancy and neonatal outcomes in African American (AA) populations, behavioral and psychosocial risks (BPSR) may also play a part. Among low income AA women with psychosocial risks, this report addresses the impacts on pregnancy and neonatal outcomes of an integrated education and counseling intervention to reduce BPSR, as well as the contributions of other psychosocial and biomedical risks.
Methods
Subjects were low income AA women ≥18 years living in the Washington, DC, metropolitan area and seeking prenatal care. Subjects (n=1044) were screened for active smoking, environmental tobacco smoke exposure (ETSE), depression, or intimate partner violence (IPV) and then randomized to intervention (IG) or usual care (UCG) groups. Data were collected prenatally, at delivery, and postpartum by maternal report and medical record abstraction. Multiple imputation methodology was used to estimate missing variables. Rates of pregnancy outcomes (miscarriage, live birth, perinatal death), preterm labor, Caesarean section, sexually transmitted infection (STI) during pregnancy, preterm birth (<37 weeks), low birth weight (<2,500 grams), very low birth weight (<1,500 grams), small for gestational age, neonatal intensive care unit (NICU) admission, and >2 days of hospitalization were compared between IG and UCG. Logistic regression models were created to predict outcomes based on biomedical risk factors and the four psychosocial risks (smoking, ETSE, depression, and IPV) targeted by the intervention.
Results
Rates of adverse pregnancy and neonatal outcomes were high and did not differ significantly between IG and UCG. In adjusted analysis, STI during the current pregnancy was associated with IPV (OR=1.41, 95% CI 1.04-1.91). Outcomes such as preterm labor/caesarian section in pregnancy and preterm birth, low birth weight, small for gestational age, NICU admissions and >2 day hospitalization of the infants were associated with biomedical risk factors including preexisting hypertension and diabetes, previous preterm birth (PTB), and late initiation of prenatal care, but they were not significantly associated with active smoking, ETSE, depression, or IPV.
Conclusions
Neither the intervention to reduce BPSR nor the psychosocial factors significantly contributed to the pregnancy and neonatal outcomes. This study confirms that biomedical factors significantly contribute to adverse outcomes in low income AA women. Biomedical factors outweighed psychosocial factors in contributing to adverse pregnancy and neonatal outcomes in this high-risk population. Early identification and management of hypertension, diabetes and previous PTB in low income AA women may reduce health disparities in birth outcomes.
Introduction
Infant mortality rates (IMR) in the US have substantially decreased in the past decade, but significant disparities continue between African Americans (AA) and Whites, particularly for AAs living in poverty [1,2]. While the IMR was 15.1 per 1000 live births among AAs residing in the District of Columbia (DC) in 2000, the rate among Whites in DC was so low (1.3 per 1000) that it was considered unstable [3]. The Eunice Kennedy Shriver NICHD-DC Initiative to Reduce Infant Mortality in Minority Populations (DCI), a community-based cooperative research network of six institutions in Washington, DC, was created to address the high rate of infant mortality in the District. The phase of the study covered in this report was initiated in 2001. For the year 2000, the IMR among AAs in DC (15.1 per 1000 live births) was approximately two and a half times that of the US as a whole (5.7 per1000 live births) [3].
The major contributors to infant mortality are disorders related to short gestation, preterm birth (PTB), and low birth weight (LBW) [4], accounting for more than 20% of deaths in AA infants. This increased risk for PTB and LBW among AA infants is well known but not well understood. Biomedical factors alone do not appear to account fully for the disparities in PTB and LBW. Evidence from the literature suggests that behavioral and psychosocial risks (BPSR) may impact both maternal and infant outcomes [5].
Cigarette smoking during pregnancy adversely impacts many reproductive health outcomes, including LBW, intrauterine growth restriction, small-for-gestational-age (SGA), PTB, and stillbirth. Smoking cessation can reduce the risk of these adverse health outcomes [6]. Environmental tobacco smoke exposure (ETSE) lowers birth weight and may be associated with other negative health outcomes [7].
Studies have shown an association between depression and reproductive outcomes. Orr and colleagues found that high levels of depressive symptoms are significantly associated with PTB and/or LBW in low-income AA women [8]. Depression may be a mediator for other negative health behaviors such as use of tobacco, drugs, or alcohol [9].
Between 3.9% and 8.3% of pregnant women experience intimate partner violence (IPV) leading to adverse pregnancy outcomes for themselves or their unborn infants [10]. Consequences of IPV include miscarriage, LBW, PTB, and fetal injury [11,12].
In summary, previous research confirms the adverse effects of smoking, ETSE, depression, and IPV on pregnancy and neonatal outcomes. However, a woman may present with multiple BPSR [13]. Consequently, intervening on only a single risk factor may be unsuccessful because other risks may continue as barriers to the desired change or continue to impact the pregnancy outcomes. Low-income populations may be inconsistently screened and have difficulty accessing mental health and behavioral interventions in primary care [14]. Co-location of psychosocial and behavioral interventions in primary care settings may increase patient access compared to service provision at separate facilities [15]. Earlier DC Healthy Outcomes of Pregnancy Expectations (DC-HOPE) reports describe the impact of an integrated education and counseling intervention on reducing smoking, ETSE, depression and IPV during pregnancy [16] and postpartum [17]. This report addresses the impact of the intervention on pregnancy and neonatal outcomes, as well as the contribution of other existing psychosocial and biomedical risks.
Methods
Participants were recruited at six prenatal care clinics in Washington, DC from July 2001 to October 2003 and followed until July 2004. IRB approval was secured from participating institutions and informed consent obtained from participants. Pregnant AA women ages 18 years and above and less than 29 weeks gestation were asked to complete a brief computerized screening interview (Audio-Computer Assisted Self-Interview: A-CASI) to assess their eligibility and status with respect to the four psychosocial risk factors: active smoking, ETSE, depression, and IPV [18].
The A-CASI screening batteries were drawn from previously validated screening measures. Criteria for smoking risk included having smoked at least a puff of a cigarette within the 6 months before or since becoming pregnant, or any ETSE during pregnancy, as determined by items adapted from the Smoke-Free Families (SFF) core screening [19]. Depressive symptoms were screened on A-CASI using the Beck Depression Inventory (BDI)-FastScreen for Medical Patients, a reduced version of the BDI [20], selected for its 7-item brevity and applicability to patients in primary medical care. The recall period for the BDI-FastScreen was the past two weeks. IPV was identified by the Abuse Assessment Screen (AAS) [21] if a woman reported being the victim of physical or sexual abuse in the previous year or reported fear of her current partner. Scores on the A-CASI determined a woman's risk eligibility for the study and which risk components would be addressed should she be randomized to intervention.
Demographically eligible women reporting smoking, ETSE, depression, or IPV were invited to participate in the study. Consenting participants who completed a baseline interview were randomized to the intervention (IG) or usual care (UCG) group using a site- and risk-specific block randomization design. A target sample size of 1050 participants was selected in order to allow detection of a 10 to 20% reduction in the psychosocial risk factors, which was the primary outcome of the study. Testing the effect of the intervention on adverse pregnancy and neonatal outcomes was a pre-specified secondary objective. A full discussion of recruitment and retention procedures of the study can be found in a previous report [22].
The intervention, specific to the psychosocial and behavioral risks targeted, was designed to be delivered in prenatal care clinics. To address smoking and ETSE, elements from the successful Smoking Cessation or Reduction in Pregnancy Program Treatment (SCRIPT) trial [23], the transtheoretical model of behavior change, and the “pathways to change” self-help manual [24] were incorporated. To address depression, a group cognitive–behavioral therapy treatment developed by Miranda and Munoz [25] was adapted for individual delivery. A single-visit intervention developed by Parker et al. [26] for women experiencing IPV was modified to provide ongoing guidance throughout pregnancy.
Intervention sessions were provided at each routine prenatal care visit and were designed to last 35 to 55 minutes depending on the number of risks being addressed. Eight prenatal sessions were required to deliver the complete intervention. However, a minimum of 4 sessions was deemed “adequate” on the basis of the amount of material that could be covered in 4 sessions. Individualized counseling sessions were tailored to the specific risks reported by each woman. Session content could include identifying smoking triggers, developing strategies for mood management, and conducting a danger assessment depending on individual risks. In each session, the pregnancy advisors and participants developed a plan for “homework” to reinforce the intervention in the woman's real-life circumstances. Women in need of other social services were provided with referrals to specific resources. A single counselor was assigned to each clinic to provide consistency for participating women. A full description of the intervention service delivery strategy has been reported [27].
The impact of the intervention on pregnancy outcomes and reducing the four risk factors was assessed via telephone interviews, biomarker assessments, and medical record abstractions. Telephone interviews were conducted at baseline, during the second and third trimesters, and postpartum. Measures in the baseline and follow-up battery included smoking and ETSE abstinence items from the Smoke-Free Families (SFF) core questionnaires [19]. The 20-item Hopkins Symptom Checklist-Depression Scale (HSCL-D) [28, 29] and the Conflict Tactics Scale (CTS) [30, 31] were selected to assess baseline and follow-up levels of depression symptoms and IPV more fully than could be done with the brief A-CASI screening tools. Saliva cotinine samples were collected paralleling the telephone interviews. Data on medical risk factors and pregnancy and neonatal outcomes were abstracted from maternal and infant medical records.
Pregnancy outcomes selected for analysis were pregnancy result (miscarriage, live birth, perinatal death), preterm labor, Caesarean section (C-section), and sexually transmitted infection (STI) during pregnancy. Selected neonatal outcomes were PTB (<37 weeks), LBW (<2,500 grams), VLBW (<1,500 grams), SGA, neonatal intensive care unit (NICU) admission, and >2 days of hospitalization. In order to conduct an intent-to-treat (ITT) analysis, which requires that outcomes be known for all subjects, multiple imputation (MI) was used to estimate missing data, including pregnancy and infant outcomes. Levels of missing data for each outcome ranged from 12% for pregnancy result to 17% for >2 days of hospitalization. MI was accomplished using IVEware imputation and variance estimation software [32]. Continuous variables, including gestational age at birth and birth weight, were imputed as categorical variables (prematurity and low birth weight, respectively).
Statistical analysis was performed using SAS version 9.1.3 (SAS Institute Inc., Cary, NC). Standard statistical methods were applied to each of five imputed data sets and the results were combined (using the MIANALYZE procedure) to produce parameter estimates that accounted for both between- and within-imputation variance. Women were analyzed according to their care group assignment at baseline, regardless of actual participation in the intervention.
Bivariate analyses of the associations between assignment to IG or UCG, and demographic characteristics, reproductive and medical history variables, and pregnancy and neonatal outcomes were conducted. SAS's GLM and GENMOD procedures were used for continuous and categorical variables, respectively.
Logistic regression models were created to predict pregnancy and neonatal outcomes based on smoking, ETSE, depression and IPV at baseline. Other known demographic and medical risk factors considered as possible covariates were: maternal age, education, marital status, employment status, Medicaid enrollment and WIC, drug and alcohol use at baseline, previous PTB, previous miscarriage/stillbirth, previous live birth, gestational age at baseline, early prenatal care initiation, diabetes, and hypertension. Backward selection of demographic and medical control variables was conducted to create parsimonious models that excluded covariates not significantly associated with the outcomes. The four behavioral risk factors and demographic and medical predictors that were significant at the p<0.05 level were retained in the final models. The LOGISTIC procedure in SAS was used.
Results
A total of 4213 women were invited to participate in the A-CASI screenings. Of these, 649 refused and 651 never completed screening to determine their eligibility. As Figure 1 shows, 2913 women were screened and 1398 were eligible for participation. A total of 1070 women provided baseline data and consented to participate. The remaining 328 women either refused to complete the baseline interview (n=17), refused to provide consent (n=207), were excluded because attempts to re-contact were unsuccessful (n=70), were no longer pregnant (n=24), or were excluded for other reasons (n=10). Of the 1070 women who completed the baseline interview, these analyses include the 1025 women who were AA, had singleton pregnancies, and were still pregnant at the time of the baseline interview. Five hundred and ten (510) women were randomized to IG and 515 to UCG. The overall retention rate was 93% [22].
Figure 1. Profile of Project DC-HOPE Randomized Controlled Trial.
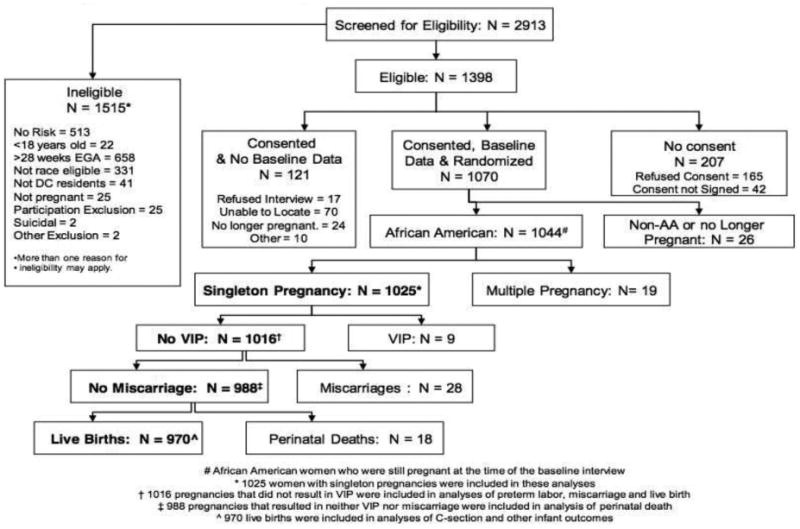
Randomization successfully balanced maternal characteristics between the two care groups (Table 1). There were no significant differences in the baseline characteristics of women randomized to IG versus UCG. Most women were single, had a high school education or less, and were receiving Medicaid. Two-thirds had previous live births. There was a high rate of previous pregnancy loss (33.8%). Six point three percent (6.3%) of women had gestational diabetes and 4.2% had preexisting diabetes; 3.7% had gestational hypertension and 6.9% had preexisting hypertension. At baseline, 18.8% of participants reported active smoking, 44.3% depression, and 31.9% IPV. ETSE was high (72.5%).
Table 1. Demographic Characteristics by Care Group.
| Variables | Intervention (n=510) |
Usual Care (n=515) |
Total (N=1025) |
|||
|---|---|---|---|---|---|---|
| Demographic | Mean | SE | Mean | SE | Mean | SE |
| Maternal age | 24.4 | 0.2 | 24.7 | 0.2 | 24.6 | 0.2 |
| (n) | % | (n) | % | (n) | % | |
| Single | 388 | 76.1 | 394 | 76.5 | 783 | 76.3 |
| Maternal education <High School | 153 | 30.0 | 152 | 29.5 | 305 | 29.8 |
| Medicaid | 404 | 79.1 | 395 | 76.7 | 799 | 77.9 |
| Reproductive | ||||||
| First trimester PNC initiation | 312 | 61.2 | 304 | 59.1 | 617 | 60.1 |
| Number of PNC visits (<4) | 69 | 13.6 | 86 | 16.7 | 155 | 15.1 |
| Previous premature delivery | 73 | 14.4 | 68 | 13.2 | 141 | 13.8 |
| Previous pregnancy loss | 170 | 33.4 | 176 | 34.1 | 346 | 33.8 |
| Short Inter pregnancy Interval | 80 | 18.3 | 89 | 20.6 | 168 | 19.4 |
| Primiparous | 172 | 33.7 | 159 | 31.0 | 331 | 32.3 |
| Medical Conditions | ||||||
| Diabetes, preexisting | 23 | 4.5 | 20 | 4.0 | 43 | 4.2 |
| Diabetes, gestational | 30 | 5.9 | 35 | 6.7 | 65 | 6.3 |
| Hypertension, preexisting | 37 | 7.2 | 33 | 6.5 | 70 | 6.9 |
| Hypertension, gestational | 16 | 3.1 | 22 | 4.3 | 38 | 3.7 |
| Psychosocial Risk at A-CASI | ||||||
| Depression | 176 | 34.5 | 189 | 36.8 | 365 | 35.6 |
| IPV | 101 | 19.8 | 110 | 21.4 | 211 | 20.6 |
| Smoking | 256 | 50.2 | 235 | 45.6 | 491 | 47.9 |
| ETSE | 424 | 83.0 | 435 | 84.4 | 858 | 83.7 |
| Psychosocial Risk at Baseline | ||||||
| Depression | 224 | 43.9 | 230 | 44.7 | 454 | 44.3 |
| IPV | 164 | 32.1 | 164 | 31.8 | 327 | 31.9 |
| Smoking | 102 | 20.1 | 91 | 17.6 | 193 | 18.8 |
| ETSE | 364 | 71.3 | 379 | 73.6 | 743 | 72.5 |
| Drug Use | 66 | 12.9 | 54 | 10.4 | 119 | 11.7 |
| Alcohol Use | 105 | 20.7 | 109 | 21.2 | 215 | 21.0 |
PNC, Prenatal care; IPV, Intimate partner violence; A-CASI, Audio-computer assisted self-interview; ETSE, Environmental tobacco smoke exposure.
Data are mean ± standard error or n (%).
Among the 1025 singleton pregnancies included in these analyses were 28 miscarriages, 18 perinatal deaths, 9 voluntary interruptions of pregnancy (VIP), and 970 live births with neonatal outcomes (Figure 1). Analyses of the preterm labor, miscarriage and live birth outcomes excluded VIP (N=1016). Analysis of perinatal death excluded VIP and miscarriages (N=988). Analyses of C-section and all other infant outcomes included only live births (N=970).
There were no statistically significant differences between women assigned to IG or UCG with respect to pregnancy and neonatal outcomes (Table 2). Pregnancy outcomes included STI during pregnancy (32.4%), preterm labor (19.9%), C-section delivery (28.4%), non-live birth (4.5%) and miscarriage (2.8%). Fourteen point seven percent (14.7%) of neonates were preterm, 13.6% were LBW, 2.2% were VLBW and 15.1% were SGA. Thirty-nine and one half percent (39.5%) of infants were hospitalized for longer than 2 days, and 13.9% were admitted to NICU. Perinatal death rate was 1.8/1000 live births.
Table 2. Medical Outcomes by Care Group.
| Denominator for Intervention + Usual Care* | Intervention | Usual Care | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n=510) | % | (n=515) | % | (n=1025) | % | |||||
| Pregnancy Outcomes | ||||||||||
| STI | 1025 | 161 | 31.6 | 171 | 33.1 | 332 | 32.4 | |||
| Preterm Labor | 1016 | 97 | 19.1 | 105 | 20.6 | 202 | 19.9 | |||
| C-Section | 970 | 140 | 28.7 | 136 | 28.1 | 275 | 28.4 | |||
| Live Births | 1016 | 488 | 96.3 | 482 | 94.7 | 970 | 95.5 | |||
| Neonatal Outcomes | ||||||||||
| Miscarriage | 1016 | 9 | 1.9 | 19 | 3.7 | 28 | 2.8 | |||
| Perinatal Death | 988 | 9 | 1.9 | 8 | 1.7 | 18 | 1.8 | |||
| PTB (< 37 wks) | 970 | 71 | 14.5 | 72 | 15.0 | 143 | 14.7 | |||
| Very PTB (<33 wks) | 970 | 11 | 2.3 | 18 | 3.7 | 29 | 3.0 | |||
| LBW (<2500g) | 970 | 62 | 12.8 | 70 | 14.6 | 132 | 13.6 | |||
| VLBW (<1500g) | 970 | 8 | 1.6 | 14 | 2.9 | 21 | 2.2 | |||
| SGA | 970 | 78 | 16.0 | 68 | 14.2 | 147 | 15.1 | |||
| NICU admission | 970 | 60 | 12.2 | 75 | 15.5 | 134 | 13.9 | |||
| 2 Day Hospitalization | 970 | 191 | 39.2 | 192 | 39.7 | 383 | 39.5 | |||
STI, Sexually transmitted infections; C-section Caesarean section; PTB, Preterm birth; VPTB, Very preterm birth; LBW, low birth weight; VLBW, Very low birth weight; SGA Small for gestational age; NICU, Neonatal intensive care unit.
1016 excludes voluntary interruptions of pregnancy (VIP); 988 excludes VIP and miscarriages; 970 includes only live births.
Average gestational age at birth in pre-imputation data was 38.6 2.2 weeks (N=818). Average birth weight in pre-imputation data was 3154.9 594.4 grams (N=817).
Numbers for Intervention and Usual Care groups may not add up to Total because decimal values resulting from multiple imputation have been rounded to whole numbers.
There were no significant differences between the care groups.
Of the 510 women randomized to the intervention group, 258 (51%) attended four or more intervention sessions during pregnancy and 126 women (25%) did not attend any prenatal sessions.
Table 3 shows adjusted odds ratios from logistic regression models predicting pregnancy outcomes based on psychosocial and biomedical risks. IPV (OR=1.41; 95% Confidence Interval (95%CI): 1.04-1.91) and low maternal education (less than high school) (OR=1.65; 95%CI: 1.21-2.26) were predictive of STI during the pregnancy. For other pregnancy outcomes, only biomedical factors were significant predictors. Preterm labor was predicted by previous pregnancy loss (OR=1.57, 95%CI: 1.13-2.19), previous PTB (OR=1.76; 95%CI: 1.12-2.77), and preexisting hypertension (OR=1.90; 95%CI: 1.01-3.59). The odds of C-section were increased with preexisting diabetes (OR=2.39; 95%CI: 1.22-4.68).
Table 3. Adjusted Odds Ratios for Pregnancy Outcomes.
| STI (Model N=1025) | Preterm Labor (Model N= 1016) | C-Section (Model N=970) | Live Births (Model N=1016) | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI |
| Psychosocial Risk at BL | ||||||||
| Depression | 1.03 | (0.78–1.38) | 1.21 | (0.83–1.75) | 1.07 | (0.78–1.46) | 0.99 | (0.50–1.98) |
| IPV | 1.41* | (1.04–1.91) | 1.03 | (0.72–1.47) | 0.91 | (0.64–1.30) | 1.43 | (0.65–3.15) |
| Smoking | 0.71 | (0.48–1.04) | 1.10 | (0.70–1.72) | 1.09 | (0.74–1.60) | 0.72 | (0.34–1.54) |
| ETSE | 1.05 | (0.76–1.46) | 0.82 | (0.56–1.19) | 0.83 | (0.59–1.17) | 0.68 | (0.30–1.57) |
| Demographics | ||||||||
| Education (<HS vs. HS+) | 1.65† | (1. 21–2.26) | ||||||
| Reproductive | ||||||||
| Previous miscarriage or stillbirth | 1.57† | (1.13–2.19) | ||||||
| Previous PTB | 1.76* | (1.12–2.77) | ||||||
| Medical Condition | ||||||||
| Diabetes, preexisting | 2.39* | (1.22–4.68) | ||||||
| Hypertension, preexisting | 1.90* | (1.01–3.59) | ||||||
IPV, Intimate partner violence; ETSE, Environmental tobacco smoke exposure; HS High School; C-section, Caesarean section; PTB, Preterm birth; CI, Confidence interval.
= p<.05
= <.01
< .001
Covariates screened for significance in the models were: maternal age, education, marital status, and employment status; receipt of Medicaid and WIC; illicit drug and alcohol use at baseline; previous live birth, premature delivery, miscarriage or stillbirth; early PNC initiation; preexisting and gestational diabetes and hypertension. Only the four risk factors targeted by the intervention (smoking, ETSE, depression, and IPV), and other variables that were significant at the p<0.05 level after backwards selection of covariates, were retained in the final models.
Table 4 shows the results of logistic regression models predicting neonatal outcomes based on BPSR. Baseline smoking, ETSE, depression, and IPV were not significant predictors of neonatal outcomes. Biomedical risks were more likely to be associated with poor neonatal outcomes. The odds of PTB were higher for women with previous PTB (OR=2.44; 95%CI: 1.52-3.93) and preexisting diabetes (OR=2.37; 95%CI: 1.04-5.41). LBW was increased by previous PTB (OR=1.76; 95%CI: 1.07-2.89) and gestational hypertension (OR=2.37; 95%CI: 1.08-5.20). The odds of SGA were reduced by early prenatal care initiation (OR=0.68; 95%CI: 0.47-0.99) yet increased by gestational hypertension (OR=2.41; 95%CI: 1.02-5.71). NICU admission was predicted by preexisting diabetes (OR=3.30; 95%CI: 1.50-7.26). Hospital stay >2 days was increased with primiparous delivery (OR= 1.38; 95%CI: 1.02-1.87), and preexisting diabetes (OR= 2.64; 95%CI: 1.19-5.87).
Tabel 4. Adjusted Odds Ratios for Neonatal Outcomes.
| PTB (<37wk) (Model N=970) | LBW (<2500) (Model N=970) | SGA (Model N=970) | NICU Admissions (Model N=970) | >2 day Hospitalization (Model N=970) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Psychosocial Risk at Baseline | ||||||||||
| Depression | 1.22 | (0.8-1.88) | 1.41 | (0.94-2.10) | 1.00 | (0.67-1.49) | 1.31 | (0.86-1.99) | 1.21 | (0.90-1.64) |
| IPV | 1.33 | (0.8-2.00) | 1.26 | (0.80-1.99) | 0.87 | (0.55-1.37) | 1.13 | (0.71-1.80) | 1.08 | (0.78-1.50) |
| Smoking | 1.24 | (0.7-2.10) | 1.31 | (0.77-2.24) | 1.26 | (0.80-1.98) | 0.87 | (0.52-1.46) | 1.20 | (0.83-1.72) |
| ETSE | 0.75 | (0.4-1.16) | 0.79 | (0.52-1.20) | 1.02 | (0.62-1.69) | 0.77 | (0.49-1.22) | 0.93 | (0.66-1.30) |
| Reproductive | ||||||||||
| Previous PTB | 2.44‡ | (1.52-3.93) | 1.76* | (1.07-2.89) | ||||||
| Primiparous | 1.38* | (1.02-1.87) | ||||||||
| Early PNC initiation | 0.68* | (0.47-0.99) | ||||||||
| Medical Conditions | ||||||||||
| Diabetes, preexisting | 2.37* | (1.04-5.41) | 3.30† | (1.50-7.26) | 2.64* | (1.19-5.87) | ||||
| Hypertension, gestational | 2.37‡ | (1.08-5.20) | 2.41‡ | (1.02-5.71) | ||||||
OR, odds ratio; CI, confidence interval; LBW, low birth weight; SGA, Small for gestational age; NICU, Neonatal intensive care unit.
Infants of mothers who smoked at baseline had an average birth weight of 3042.3 578.4 grams (n=147) compared to 3179.6 595.5 grams (n=670) for infants born to non-smokers in pre-imputation data.
= p<.05
= <.01
< .001
Covariates screened for significance in the models were: maternal age, education, marital status, and employment status; receipt of Medicaid and WIC; illicit drug and alcohol use at baseline; previous live birth, premature delivery, miscarriage or stillbirth; early PNC initiation; preexisting and gestational diabetes and hypertension. Only the four risk factors targeted by the intervention (smoking, ETSE, depression, and IPV), and other variables that were significant at the p<0.05 level after backwards selection of covariates, were retained in the final models.
Discussion
While the BPSR were significantly reduced by the intervention in this RCT [16], we did not detect an impact on pregnancy and neonatal outcomes. Greater numbers of adverse outcomes occurred in the UCG compared to the IG, but the differences were not statistically significant. Only STI was associated with one of the psychosocial risks targeted by the intervention (IPV). For most pregnancy and neonatal outcomes biomedical risks had the most impact, particularly diabetes, hypertension and previous PTB.
This report focuses on the relationship of BPSR risk factors to pregnancy and neonatal outcomes, and the ability of an integrated behavioral intervention to improve these outcomes in a large sample of African American women. The study differed from most prior clinical trials in testing the efficacy of an integrated behavioral intervention provided within the prenatal care setting and designed to reduce specific BPSR, thus improving pregnancy and neonatal outcomes.
Our findings have four implications for understanding the relationship between the targeted risks and pregnancy and neonatal outcomes and for designing future studies in this area:
First, positive outcomes occurred more frequently among the intervention participants, although differences in pregnancy and neonatal outcomes in the IG and UCG were not statistically significant. Although the intervention was effective in resolving participants'; BPSR risks, as reported previously [16], the lack of significant impact on pregnancy and neonatal outcomes may have been due to the intent to power the study for risk reduction rather than the improvement of pregnancy and neonatal outcomes. Findings of previous studies of prenatal behavioral interventions to improve pregnancy outcomes have been inconsistent. Success in reducing PTB for AA intervention participants by providing education and support has been reported [33, 34], but there was no reduction in LBW. Similar to DC-HOPE, Klerman et al. [35] found that AA women in the intervention group reduced behavioral risks such as smoking, but without impact on pregnancy outcomes. Differences in methodology and sample sizes make comparing these studies with the DC-HOPE intervention difficult.
Second, this study failed to find an effect of active smoking on pregnancy outcomes other than a slight reduction in birthweight. This fact may relate to a dose-response gradient [36]. The definition of active smoking risk in DC-HOPE included women with low smoking frequency that may have fallen below the dose necessary for significant impact on pregnancy. With regard to the effect of ETSE on PTB and SGA, our findings concur with those from the meta-analysis of Leonardi-Bee et al. [7] that did not demonstrate an effect of ETSE on gestational age at birth or SGA.
Third, DC-HOPE failed to find an impact of depression on pregnancy or neonatal outcomes. This may have been due to the present study defining depression risk by self-reported symptoms rather than diagnosis of major depressive disorder. Including women with milder degrees of symptoms may have diluted the effects of depression on pregnancy outcomes. Other studies that found significant contributions to PTB or LBW for smoking, depression, or IPV usually considered populations screened for one of these risks individually. Given that these risks often overlap, other studies that demonstrate the contribution of any of those single risks may not have controlled for the presence of other risks or cumulative risks that may have contributed to demonstrated effects.
Fourth, DC-HOPE found the prevalence of medical conditions, including hypertension, diabetes, and rates of both gestational and preexisting conditions, similar to previous reports for AA populations [37, 38]. Consistent with our results here, other studies also report medical factors to outweigh psychosocial and behavioral risks for low birthweight and preterm delivery in African American women [39].
The DC-HOPE study had a number of limitations. Similar to Klerman et al. [35], this trial found positive trends for the effect of the intervention on pregnancy outcomes but had inadequate sample size to detect statistically significant differences. Twelve to 17 percent of the data for each outcome were missing and were estimated with MI. Between-imputation variation reflected the uncertainty inherent in predicting unknown values, but might have limited our ability to detect differences between groups.
A feature of several successful programs was the inclusion of case management, in which a social services worker directly assisted clients in accessing needed community resources. The emphasis in DC-HOPE was on empowering women to access resources, rather than rely on a case manager. Whether including case management would have enhanced outcomes was beyond the scope of DC-HOPE but is an interesting design for future study. Additionally, life stresses and limited social support in low income AA women may continue to impact their health, despite reduction in some specific risks.
In the DC-HOPE study, inconsistent participant attendance at intervention sessions may also have reduced any impacts on pregnancy and neonatal outcomes. Despite co-location of intervention sessions with PNC, 25% of women failed to attend any prenatal intervention sessions. Typically within primary care settings, less than 50% percent of women with mental health problems pursue recommended mental health services when the services are not co-located within their primary care settings [40]. In contrast to patients seeking care in mental health settings, patients in primary care may not acknowledge psychosocial or behavioral problems and may not want or expect intervention [41]. In such cases, acceptance of the problem and motivation for treatment may be difficult to achieve. The complicated lives of these women also resulted in inconsistent prenatal care attendance, making it difficult to evaluate the relative contribution of intervention response versus prenatal care intensity.
Many potential psychosocial/behavioral factors affecting pregnancy outcomes were not addressed by this intervention, including unmet economic needs, low levels of education, and associated behavioral challenges including alcohol and drug use. The focus on low-income, urban AA women in this study may mean that findings cannot be generalized to other populations of pregnant women, but may apply only to urban, low income, pregnant AA women with BPSR. Because willingness to participate in intervention sessions was variable, broader supports may be needed to assure that women consistently attend prenatal care visits and take advantage of ameliorative interventions made available to them in prenatal care settings.
In this study, AA women with multiple BPSR experienced high rates of adverse pregnancy and neonatal outcomes. Efforts to understand adverse birth outcomes of AAs should focus on risks throughout their lives rather than only those occurring during pregnancy. The high rates of adverse outcomes in our low-income population suggest that poverty may contribute to adverse outcomes through mechanisms such as intergenerational health disadvantages or cumulative stress. Biomedical risks increase adverse birth outcomes in this low income AA population. Continued efforts to manage hypertension and diabetes should be addressed in this population to reduce adverse outcomes. In addition to prenatal care, low income AA women may need a variety of support services, outside of the prenatal setting, to improve outcomes. To explore the complex interactions of many of these key factors, large national collaborative studies would likely be needed.
Acknowledgments
We thank the field work staff, the interviewers, and the data management staff. Also, we thank the participants, who welcomed us into their lives in hopes of helping themselves and their children.
This study was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center on Minority Health and Health Disparities, National Institutes of Health (3U18HD030445, 3U18HD030447, 5U18HD31206, 3U18HD031919, and 5U18HD036104).
References
- 1.MacDorman MF, Callaghan WM, Mathews TJ, Hoyert DL, Kochanek KD. Trends in preterm-related infant mortality by race and ethnicity, United States, 1999-2004. International Jouranl of Health Services. 2007;37(4):635–41. doi: 10.2190/HS.37.4.c. [DOI] [PubMed] [Google Scholar]
- 2.Paul DA, Mackley A, Locke RG, Stefano JL, Kroelinger C. State infant mortality: an ecologic study to determine modifiable risks and adjusted infant mortality rates. Maternal Child Health Journal. 2009;13(3):343–8. doi: 10.1007/s10995-008-0358-9. [DOI] [PubMed] [Google Scholar]
- 3.SCHSA. SCHSA State Center for Health Statistics Administration: Briefing paper on the 2000 infant mortality rate for the District of Columbia. 2002. [Google Scholar]
- 4.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2005 period linked birth/infant death data set. National Vital Statistics Report. 2008;57(2):1–32. [PubMed] [Google Scholar]
- 5.Nothnagle M, Marchi K, Egerter S, Braveman P. Risk factors for late or no prenatal care following Medicaid expansions in California. Maternal Child Health Journal. 2000;4(4):251–9. doi: 10.1023/a:1026647722295. [DOI] [PubMed] [Google Scholar]
- 6.Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine & Tobacco Research. 2004;6 2:S125–40. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 7.Leonardi-Bee J, Smyth A, Britton J, Coleman T. Environmental tobacco smoke and fetal health: systematic review and meta-analysis. Archives of Disease in Childhood Fetal and Neonatal Edition. 2008;93(5):F351–61. doi: 10.1136/adc.2007.133553. [DOI] [PubMed] [Google Scholar]
- 8.Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. American Journal of Epidemiology. 2002;156(9):797–802. doi: 10.1093/aje/kwf131. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Patten SB. Prospective study of frequent heavy alcohol use and the risk of major depression in the Canadian general population. Depression and Anxiety Journal. 2002;15(1):42–5. doi: 10.1002/da.1084. [DOI] [PubMed] [Google Scholar]
- 10.Chambliss LR. Intimate partner violence and its implication for pregnancy. Clinical Obstetrics and Gynecology. 2008;51(2):385–97. doi: 10.1097/GRF.0b013e31816f29ce. [DOI] [PubMed] [Google Scholar]
- 11.Sharps PW, Laughon K, Giangrande SK. Intimate partner violence and the childbearing year: maternal and infant health consequences. Trauma, Violence & Abuse. 2007;8(2):105–16. doi: 10.1177/1524838007302594. [DOI] [PubMed] [Google Scholar]
- 12.Silverman JG, Decker MR, Reed E, Raj A. Intimate partner violence victimization prior to and during pregnancy among women residing in 26 U.S. states: associations with maternal and neonatal health. American Journal of Obstetrics & Gynecology. 2006;195(1):140–8. doi: 10.1016/j.ajog.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 13.Jun HJ, Rich-Edwards JW, Boynton-Jarrett R, Wright RJ. Intimate partner violence and cigarette smoking: association between smoking risk and psychological abuse with and without co-occurrence of physical and sexual abuse. American Journal of Public Health. 2008;98(3):527–35. doi: 10.2105/AJPH.2003.037663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alegria M, Chatterji P, Wells K, Cao Z, Chen CN, Takeuchi D, Jackson J, Meng XL. Disparity in depression treatment among racial and ethnic minority populations in the United States. Psychiatr Services. 2008;59(11):1264–72. doi: 10.1176/appi.ps.59.11.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pomerantz A, Cole BH, Watts BV, Weeks WB. Improving efficiency and access to mental health care: combining integrated care and advanced access. General Hospital Psychiatry. 2008;30(6):546–51. doi: 10.1016/j.genhosppsych.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Joseph JG, El-Mohandes AA, Kiely M, El-Khorazaty MN, Gantz MG, Johnson AA, Katz KS, Blake SM, Rossi MW, Subramanian S. Reducing psychosocial and behavioral pregnancy risk factors: results of a randomized clinical trial among high-risk pregnant African American women. American Journal of Public Health. 2009;99(6):1053–61. doi: 10.2105/AJPH.2007.131425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Mohandes AA, Kiely M, Joseph JG, Subramanian S, Johnson AA, Blake SM, Gantz MG, El-Khorazaty MN. An intervention to improve postpartum outcomes in African-American mothers: a randomized controlled trial. Obstetrics & Gynecology. 2008;112(3):611–20. doi: 10.1097/AOG.0b013e3181834b10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thornberry JS. Acceptance, communication mode and use of audio computer-assisted self interview using touchscreen to identify risk factors among pregnant minority women. RTIPRESS-D-09-0016R1, RTI, International; Research Triangle Park, NC: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melvin CL, Tucker P. Measurement and definition for smoking cessation intervention research: the smoke-free families experience. Smoke-Free Families Common Evaluation Measures for Pregnancy and Smoking Cessation Projects Working Group. Tobacco Control. 2000;9 3:III87–90. doi: 10.1136/tc.9.suppl_3.iii87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck A, Steer R, Brown G. BDI - Fast Screen for Medical Patients Manual. Psychological Corporation, Harcourt Assessment, Inc.; San Antonio, TX: 2000. [Google Scholar]
- 21.McFarlane J, Parker B, Soeken K, Bullock L. Assessing for abuse during pregnancy. Severity and frequency of injuries and associated entry into prenatal care. JAMA. 1992;267(23):3176–8. doi: 10.1001/jama.267.23.3176. [DOI] [PubMed] [Google Scholar]
- 22.El-Khorazaty MN, Johnson AA, Kiely M, El-Mohandes AA, Subramanian S, Laryea HA, Murray KB, Thornberry JS, Joseph JG. Recruitment and retention of low-income minority women in a behavioral intervention to reduce smoking, depression, and intimate partner violence during pregnancy. BMC Public Health. 2007;7:233. doi: 10.1186/1471-2458-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Windsor RA. Counselling smokers in Medicaid maternity care: the SCRIPT project. Tobacco Control. 2000;91 1:I62. doi: 10.1136/tc.9.suppl_1.i62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall SM, Munoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. Journal of Consulting and Clinical Psychology. 1994;62:141–6. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]
- 25.Miranda J, Munoz R. Intervention for minor depression in primary care patients. Psychosomatic Medicine. 1994;56:136–41. doi: 10.1097/00006842-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Parker B, McFarlane J, Soeken K, Silva C, Reel S. Testing an intervention to prevent further abuse to pregnant women. Research in Nursing & Health. 1999;22:59–66. doi: 10.1002/(sici)1098-240x(199902)22:1<59::aid-nur7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 27.Katz KS, Blake SM, Milligan RA, Sharps PW, White DB, Rodan MF, Rossi M, Murray KB. The design, implementation and acceptability of an integrated intervention to address multiple behavioral and psychosocial risk factors among pregnant African American women. BMC Pregnancy Childbirth. 2008;8:22. doi: 10.1186/1471-2393-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behavioral Science. 1974;19(1):1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- 29.Lipman RS, Covi L, Shapiro AK. The Hopkins Symptom Checklist (HSCL)--factors derived from the HSCL-90. Jouranl of Affective Disorders. 1979;1(1):9–24. doi: 10.1016/0165-0327(79)90021-1. [DOI] [PubMed] [Google Scholar]
- 30.Straus M. Manual for the Conflict Tactics Scales. Family Research Laboratory, University of New Hampshire; Durham, NH: 1995. [Google Scholar]
- 31.Straus M, Hamby SL, Boney-McCoy S, Sugarman DB. The Revised Conflict Tactics Scale (CTS2): development and preliminary psychometric data. Journal of Family Issues. 1996;17:283–316. [Google Scholar]
- 32.Raghunathan T, Solenberger P, VanHoewyk J. IVEware: imputation and variance estimation software: user guide 2002 [Google Scholar]
- 33.Ickovics JR, Kershaw TS, Westdahl C, Magriples U, Massey Z, Reynolds H, Rising SS. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstetrics & Gynecology. 2007;110(2 Pt 1):330–9. doi: 10.1097/01.AOG.0000275284.24298.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willis WO, Eder CH, Lindsay SP, Chavez G, Shelton ST. Lower rates of low birthweight and preterm births in the California Black Infant Health Program. Journal of the National Medical Association. 2004;96(3):315–24. [PMC free article] [PubMed] [Google Scholar]
- 35.Klerman LV, Ramey SL, Goldenberg RL, Marbury S, Hou J, Cliver SP. A randomized trial of augmented prenatal care for multiple-risk, Medicaid-eligible African American women. American Journal of Public Health. 2001;91(1):105–11. doi: 10.2105/ajph.91.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savitz DA, Dole N, Terry JW, Jr, Zhou H, Thorp JM., Jr Smoking and pregnancy outcome among African-American and white women in central North Carolina. Epidemiology. 2001;12(6):636–42. doi: 10.1097/00001648-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Getahun D, Nath C, Ananth CV, Chavez MR, Smulian JC. Gestational diabetes in the United States: temporal trends 1989 through 2004. American Journal of Obstetrics and Gynecology. 2008;198(5):525–e1-5. doi: 10.1016/j.ajog.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka M, Jaamaa G, Kaiser M, Hills E, Soim A, Zhu M, Shcherbatykh IY, Samelson R, Bell E, Zdeb M, McNutt LA. Racial disparity in hypertensive disorders of pregnancy in New York State: a 10-year longitudinal population-based study. American Journal of Public Health. 2007;97(1):163–70. doi: 10.2105/AJPH.2005.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldenberg RL, Cliver SP, Mulvihill FX, Hickey CA, Hoffman HJ, Klerman LV, Johnson MJ. Medical, psychosocial, and behavioral risk factors do not explain the increased risk for low birth weight among black women. American Journal of Obstetrics & Gynecology. 1996;175(5):1317–24. doi: 10.1016/s0002-9378(96)70048-0. [DOI] [PubMed] [Google Scholar]
- 40.Dew MA, Dunn LO, Bromet EJ, Schulberg HC. Factors affecting help-seeking during depression in a community sample. Journal of Affective Disorders. 1988;14(3):223–234. doi: 10.1016/0165-0327(88)90038-9. [DOI] [PubMed] [Google Scholar]
- 41.Klinkman MS. Competing demands in psychosocial care: a model for the identification and treatment of depressive disorders in primary care. General Hospital Psychiatry. 1997;19(2):98–111. doi: 10.1016/s0163-8343(96)00145-4. [DOI] [PubMed] [Google Scholar]


