Abstract
Neutrophil transmigration requires the localization of neutrophils to endothelial cell junctions, where receptor-ligand interactions and the action of serine proteases promote leukocyte diapedesis. NB1 (CD177) is a neutrophil-expressed surface molecule that has been reported to bind proteinase 3 (PR3), a serine protease released from activated neutrophils. PR3 has demonstrated proteolytic activity on a number of substrates, including extracellular matrix proteins, although its role in neutrophil transmigration is unknown. Recently, NB1 has been shown to be a heterophilic binding partner for the endothelial cell junctional protein, PECAM-1. Disrupting the interaction between NB1 and PECAM-1 significantly inhibits neutrophil transendothelial cell migration on endothelial cell monolayers. Because NB1 interacts with endothelial cell PECAM-1 at cell junctions where transmigration occurs, we considered that NB1-PR3 interactions may play a role in aiding neutrophil diapedesis. Blocking antibodies targeting the heterophilic binding domain of PECAM-1 significantly inhibited transmigration of NB1-positive neutrophils through IL-1β-stimulated endothelial cell monolayers. PR3 expression and activity were significantly increased on NB1-positive neutrophils following transmigration, while neutrophils lacking NB1 demonstrated no increase in PR3. Finally, using selective serine protease inhibitors, we determined that PR3 activity facilitated transmigration of NB1-positive neutrophils under both static and flow conditions. These data demonstrate that PR3 contributes in the selective recruitment of the NB1-positive neutrophil population.
Keywords: PECAM-1, CD31, transmigration, serine protease, endothelial cells
Introduction
Emigration of neutrophils from the bloodstream into tissue is a critical event in the immune response. A key player in this process is PECAM-1, a molecule expressed on both leukocytes and endothelial cells. PECAM-1 is a 130 kDa type I transmembrane glycoprotein composed of six extracellular immunoglobulin (Ig)-like homology domains, a 19-residue transmembrane domain and a 118 residue cytoplasmic tail (1). Endothelial cells express approximately 1–2 × 106 copies/cell (2) and homophilic trans-interactions between PECAM-1 Ig domains 1 and 2 localize this molecule to endothelial cell junctions (3–6) where it plays an important role in maintaining vascular integrity (7,8). Neutrophils and monocytes express approximately 50,000 copies of PECAM-1 on their surface, and antibodies against Ig-domain 1 and 2 of leukocyte or endothelial cell PECAM-1 have been shown to significantly inhibit leukocyte transmigration in vitro (9) and in vivo (10).
In addition to PECAM-1 Ig domain 1/2-mediated homophilic interactions, monoclonal antibodies against endothelial cell PECAM-1 Ig-domain 6 have also been shown to inhibit leukocyte transmigration (11), suggesting the existence of a heterophilic binding partner for PECAM-1. Several leukocyte receptors, including αvβ3 (12) and CD38 (13), have been proposed as putative heterophilic binding partners for endothelial cell PECAM-1, however their biological significance has never been demonstrated. Recently, NB1 (CD177) has been identified as a high-affinity heterophilic binding partner for endothelial cell PECAM-1 (14). NB1 is a 55 kDa GPI-coupled receptor that is expressed on a subpopulation of neutrophils (30–70%), with approximately 13–70,000 copies per positive cell (15). Interestingly, about 3% of the human population does not express NB1 on any of their neutrophils. This has been reported to be due to the introduction of a stop codon causing early termination of the NB1 protein (16,17). NB1 was first characterized in 1971 as a target of maternal antibodies in neonatal allo-immune neutropenia (18), however, the function of NB1 remained unknown until recently. NB1 interacts with PECAM-1 in a heterophilic manner involving Ig domain 6 of PECAM-1 and a still-to-be identified region of the NB1 molecule. Based on dissociation constants, heterophilic interaction between NB1 and PECAM-1 is approximately 15 times stronger than PECAM-1 homophilic interactions (14,19). Furthermore, blocking antibodies against NB1 significantly inhibit neutrophil transmigration across endothelial monolayers (14) and NB1 has been shown to promote PECAM-1 phosphorylation (20). The mechanism by which PECAM-1/NB1 interactions contribute to neutrophil transendothelial migration, however, is not known.
An interesting characteristic of NB1 is its ability to associate with proteinase 3 (PR3), a serine protease stored in neutrophil azurophil, secretory and specific granules. Following neutrophil activation, PR3 is released into the extracellular environment, after which it rebinds to the neutrophil surface through a specific interaction with NB1 (21). PR3 was first characterized as an elastin-degrading protease (22) and it is the antigenic target in the auto-immune disease, Wegener’s Granulomatosis (23,24). Besides elastin, PR3 can also digest other substrates, including proteoglycans, IgG (25), vWF (26) fibronectin, laminin, vitronectin, and collagen type IV (27). The proteolytic activity of PR3 is normally held in check by circulating α1-antitrypsin (28), however in diseases where α1-antitrypsin expression is reduced or absent, significant neutrophil-mediated pulmonary damage and vascular inflammation has been reported (29). Despite these observations, the biological role of NB1-associated PR3 on the neutrophil surface is unknown. However, since NB1 interacts with PECAM-1 at endothelial cell- cell junctions, it seems reasonable to examine whether PR3 may contribute to neutrophil transmigration.
In this study, we report that PR3 expression and activity are significantly increased on transmigrating neutrophils. Disrupting PR3 activity or blocking NB1-PECAM-1 interactions dramatically inhibits neutrophil transmigration under both static and flow conditions. In addition, neutrophils expressing NB1 and PR3 appear to be selectively recruited for transmigration on IL-1β-stimulated endothelial cells.
Materials and Methods
Cells and Reagents
Primary isolated human umbilical vein endothelial cells (HUVEC) were maintained in RPMI (Invitrogen) with 10% FBS, 2 mM L-glutamine and 500 µg/ml gentamycin. Cells were used between passages 3–4. The NB1 mAb 7D8 was kindly provided by Dr. D. Stroncek, while the antibodies against NB1 (MEM166) and PR3 (PR3G-2) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). PECAM-1 blocking antibody 1.2 was produced and characterized by our laboratory, and has been previously described (30). Fab fragments of PECAM-1 antibodies were generated using the Fab generation kit from Pierce Biotechnology, following the manufacturer’s instructions (Rockford, IL). The protease inhibitors elafin and AEBSF was purchased from AnaSpec (San Jose, CA) and Roche (Mannheim, Germany) respectively. TNFα, IL-1β, and IL-8 were purchased from PeproTech (Rocky Hill, NJ). Lipopolysaccharide (LPS) and N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP) were purchased from Sigma (St. Louis, MO).
Serine protease activity detection
To detect serine protease activity, Förster resonance energy transfer (FRET) was used. A fluorophore and a quencher dye were coupled to the N- and C-terminal ends of a peptide substrate highly selective for PR3. On intact peptides, the emission energy of the fluorophore was captured by the quencher. Following cleavage of the substrate the quencher is not longer able to absorb the fluorescent energy of the fluorophore and this increase in fluorescence was measured. The FRET coupled peptide for PR3 was not available commercially and was synthesized by our protein core facility as previously described (31). The peptide sequence used (VADCADQ) was reported to be cleaved by PR3 but not by other neutrophil elastases (NE, CG) (32). Following previously published methods, the FRET fluorophore (ortho-aminobenzoic acid, Abz) and quencher (nitro-tyrosine) were coupled to the N- and C-terminal ends of the PR3 peptide substrate (32,33). The substrate (final concentration 20 µM) was then incubated at 37°C with culture supernatants (150 µl) or isolated neutrophils (2×105) in a 96 well plate. In studies using transwells, the PR3 activity detected in the culture media was corrected for the volume of neutrophils isolated. After 45 minutes the plate was read in a Perkin-Elmer 1420 Victor 2 fluorescent plate reader at λex = 320 nm and λem = 420 nm. The background fluorescence from the FRET substrate control was later subtracted out. The specificity of this substrate for PR3 over NE and CG was confirmed by our laboratory (data not shown).
Neutrophil isolation
Neutrophils were isolated as previously described (34). Briefly, blood from healthy, consenting adult donors was collected in vacutainer tubes (BD Bioscience, Franklin Lakes, NJ) using 2 mM EDTA as an anti-coagulant. Whole blood was layered over a ficoll-histopaque gradient (Sigma) and centrifuged for 30 minutes at 1000 ×g. Neutrophils were isolated from the buffy coat layer and the volume brought to 10 ml with PBSA (Dulbecco’s PBS without calcium or magnesium with 0.1% BSA). Cells were then washed twice with PBSA at 200 ×g for 10 minutes before being quantified.
Transwell assays
HUVEC were cultured (1×105 cells/ml) on Corning CoStar 6.5 mm transwells with 3 µm pore sizes (Sigma). Transwells were first coated with 50 µg/ml fibronectin (Sigma, St. Louis, MO) before HUVEC were cultured on the inserts overnight. HUVEC were stimulated four hours with IL-1β (1 ng/ml) or TNFα (100 ng/ml) and in some experiments, PECAM-1 blocking Fab (PECAM-1 1.2, 10 µg/ml) was added to the endothelial cell monolayer for 10 minutes before the experiment to disrupt the heterophilic binding site on PECAM-1. In other experiments, neutrophils were pre-incubated with the serine protease inhibitors AEBSF (10 µM) or elafin (2 µM) for 10 minutes before being added to the transwells. Neutrophils were loaded into the top chamber (1×106) and allowed to transmigrate for 60 minutes at 37°C. At the end of the experiment, neutrophils were collected from the upper and lower chambers. The bottom of the insert was washed twice to collect any transmigrated neutrophils still adherent to the insert. Neutrophils were then quantified using an Animal Blood Counter (Scil Animal Care Company; Gurnee, IL).
Flow cytometry
Neutrophils analyzed for flow cytometry were either collected from the upper or lower chambers of the transwell assay or isolated from whole blood (1×105) as described previously. Neutrophils collected from whole blood were stimulated with TNFα (1 U/ml), fMLP (50 nM), LPS (1 µg/ml) or IL-8 (100 ng/ml) for 30 minutes at 37°C. Before staining, neutrophils were incubated with FcR blocking reagent (Miltenyi Biotec; Auburn, CA) for 10 minutes, and then washed with PBSA. Fluorescently labeled monoclonal antibodies to NB1 (7D8) were prepared using an antibody labeling kit from Molecular Probes (Carlsbad, CA). Staining was quantified using a LSRII flow cytometer (BD Biosciences) and offline analysis was done using Flow Jo software (Ashland, OR).
Flow adhesion and transmigration assay
Neutrophil adhesion and transmigration were observed under flow conditions using the VenaFlux in vitro flow assay (Cellix, Dublin, Ireland). Endothelial cells were stimulated with IL-1β (1 ng/ml) for 4 hours before being transferred to Vena8 EC microfluidic chambers coated with 50 µg/ml fibronectin. After two hours the microfluidic chambers were observed on a Zeiss Axio Observer A1 (Thornwood, NY) using a Hamamatsu Orca R2 camera (Bridgewater, NJ). Neutrophils for flow adhesion experiments were resuspended in RPMI with calcium and magnesium then perfused for 5 minutes at 1 dyne/cm2 (140 s−1), followed by a 5 minute wash with RPMI containing no neutrophils. Adhesion and transmigration of neutrophils was observed over 10 minutes and data analyzed offline using DucoCell software (Cellix). Neutrophils were characterized as rolling if their velocities were greater than 0.4 µm s−1.
Statistical analysis
Results, where applicable, are expressed as mean ± SEM. Statistical analysis was performed on GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA) and significance was determined using ANOVA and the Bonferroni post hoc test.
Results
Neutrophil surface expression of PR3 requires NB1
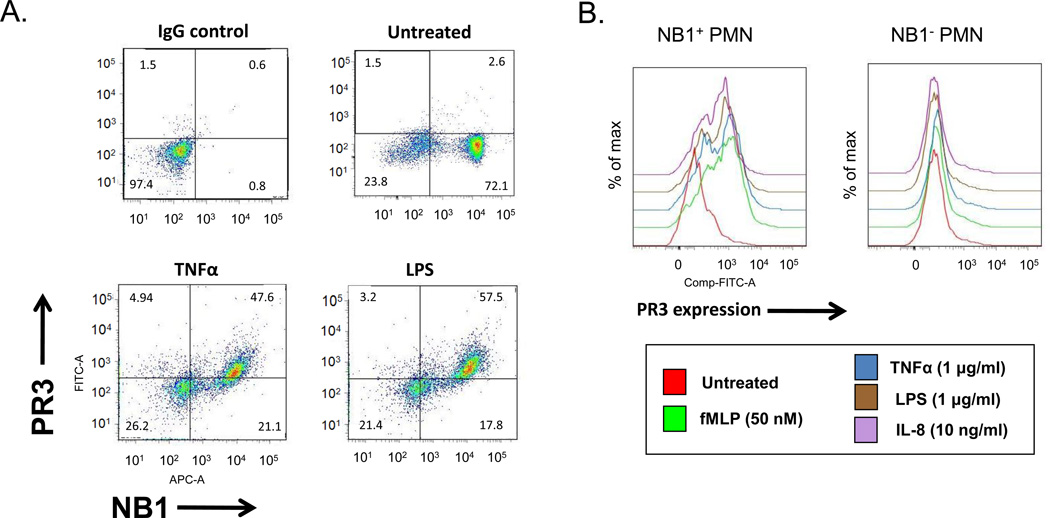
Neutrophils express PR3 in neutrophil azurophil, secretory and specific granules, the contents of which are secreted into the extracellular environment following neutrophil activation. Previous studies have shown that PR3 is capable of forming a complex with NB1 on the neutrophil surface (21). To confirm that NB1 is required for PR3 surface expression, we examined neutrophils from NB1-positive and NB-null individuals both before and after stimulation with a variety of agonists. As shown in Figure 1A, NB1 was present on 70–80% of unstimulated neutrophils of a typical normal individual. PR3 was not present on the surface of either the NB1-positive or NB1-negative resting neutrophil populations. Following stimulation with TNFα or LPS, only the NB1-positive population of cells became PR3-positive. Neutrophils from an individual genetically-deficient in NB1 (NB1-null) failed to capture PR3 using a variety of stimuli (Figure 1B). Taken together, these data demonstrate that NB1 is required for PR3 presentation on the neutrophil surface.
Figure 1. NB1-positive neutrophils express PR3 following activation.
Neutrophils (1 × 105) isolated from NB1-positive or -negative donors were treated with TNFα (10 µg/ml), IL-8 (10 ng/ml), LPS (1 µg/ml), or fMLP (50 nM) for 30 minutes or left untreated. (A) Scatter plot showing NB1-positive and NB1-null neutrophils stimulated with TNFα or LPS and stained with antibodies for NB1 and PR3. Note that PR3 is expressed only on the NB1-positive neutrophil population. (B) Histogram showing PR3 surface expression NB1-positive but not NB1-null neutrophils after stimulation with various agonists. Representative of four experiments.
NB1-PECAM-1 interactions and PR3 activity are required for neutrophil transmigration
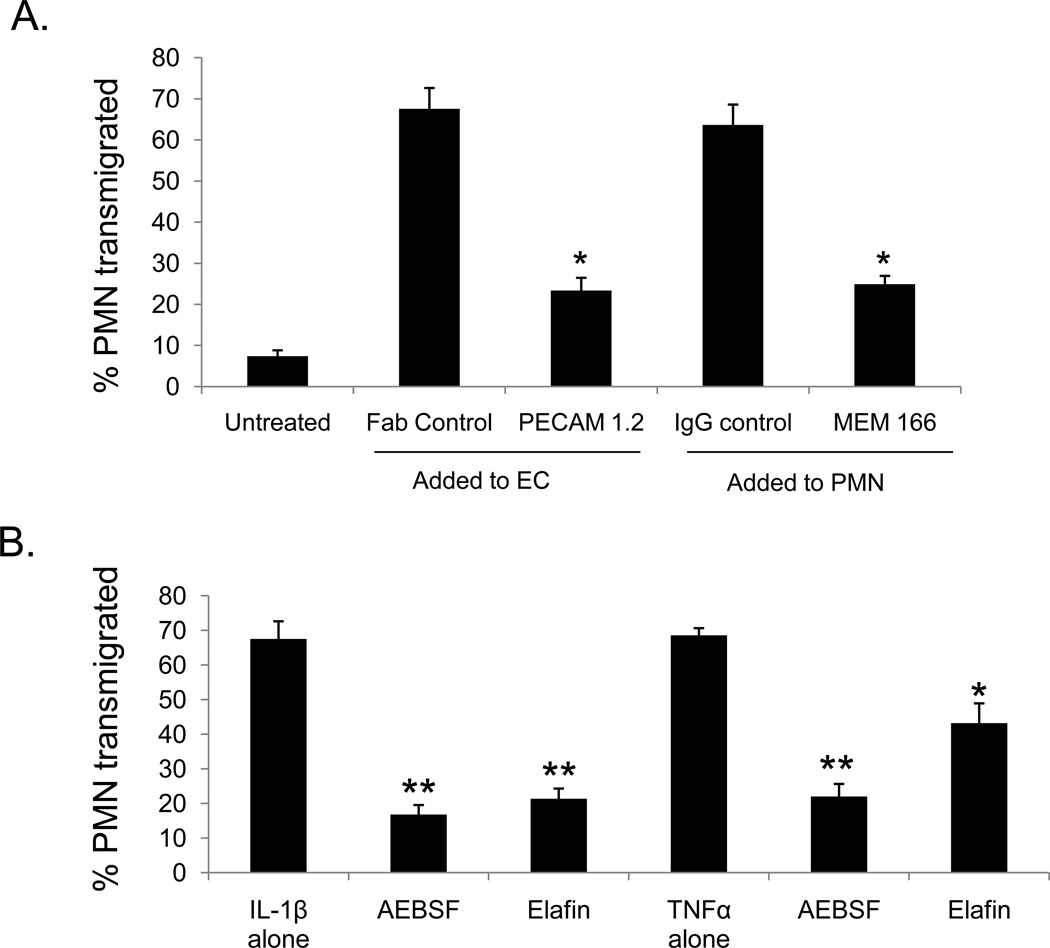
Although it is well-established that neutrophil transmigration involves PECAM-1 (9,35), recent studies have demonstrated that heterophilic interactions between endothelial cell PECAM-1 and neutrophil expressed NB1 also play a role (14). To examine whether NB1-associated PR3 might also play a role in neutrophil transmigration, neutrophils were pre-incubated with blocking antibodies to NB1 (MEM 166), inhibitors of serine protease activity (AEBSF, elafin), or blocking antibodies specific for the NB1-binding region on endothelial cell PECAM-1 and then added to monolayers of IL-1β-stimulated endothelial cells. As shown in Figure 2A, blocking antibodies specific for either NB1 or PECAM-1 were able to significantly inhibit neutrophil transmigration. Neutrophil transmigration was also inhibited by elafin and AEBSF (Figure 2B). These data demonstrate that both NB1 and PR3 play significant roles in neutrophil transmigration, and that the enzymatic activity of PR3 also contributes to this process.
Figure 2. NB1 and PR3 both contribute to neutrophil transmigration.
HUVEC cultured on transwells were stimulated with IL-1β (1 ng/ml) or TNFα (100 ng/ml) for four hours. For some treatments neutrophils were pre-incubated with Fc blocking antibodies followed by a 10 minute incubation with either NB1 blocking (MEM166; 10 µg/ml) or control antibodies. In other experiments, endothelial cells were incubated with antibodies against the heterophilic binding domain of PECAM-1 (Fab 1.2, 10 µg/ml) or control Fab. After 1 hour neutrophils were collected from the bottom chamber and quantified. (A) Antibodies against neutrophil NB1 (MEM 166) or PECAM-1 Ig domain 6 (PECAM-1 1.2) significantly inhibit neutrophil transmigration in response to IL-1β * p<0.01 compared to control antibody. (B) Neutrophils were pre-incubated with the PR3 inhibitor elafin (2 µM) or the pan-serine protease inhibitor AEBSF (10 µM before being added to IL-1β or TNFα stimulated HUVEC. Note that AEBSF and elafin each inhibit neutrophil transmigration on both IL-1β or TNFα stimulated HUVEC. **p<0.01, *p<0.05 compared to control. These data represent the SEM± of four separate experiments.
Neutrophil transmigration promotes expression of active PR3
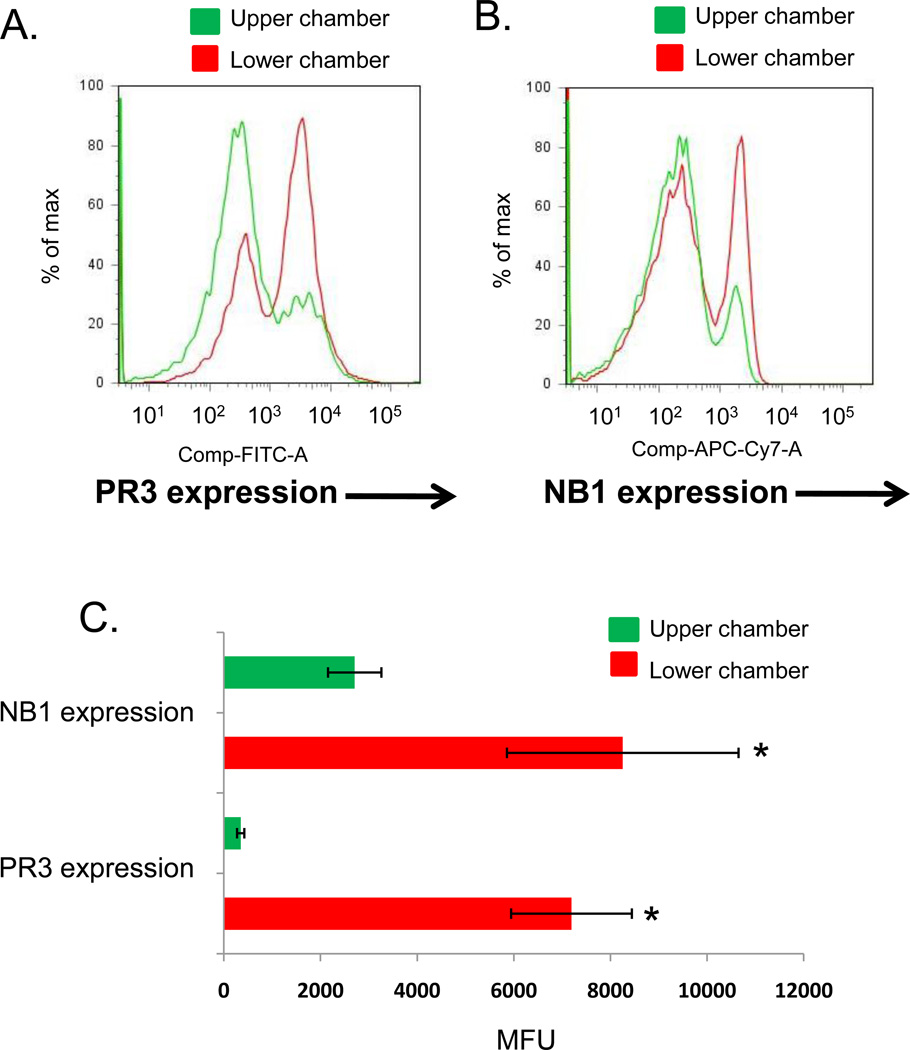
To determine whether PR3 expression and activity are increased on the surface of transmigrating neutrophils, neutrophils from an NB1-positive individual were allowed to transmigrate through IL-1β-stimulated HUVEC cultured on a transwell membrane. Neutrophils were then collected from the upper and lower chambers of the transwell and analyzed by flow cytometry for surface expression of NB1 and PR3. As shown in Figure 3, cells collected in the bottom chamber displayed significantly increased surface expression of PR3 following neutrophil transmigration. NB1-positive neutrophils also transmigrated more efficiently than did NB1-negative neutrophils from the same individual, as reported previously (14).
Figure 3. Neutrophil transmigration increases surface expression of PR3.
HUVEC (1×105) were cultured on porous transwell inserts (3 µm) coated with fibronectin (50 µg/ml). HUVEC were stimulated 4 hours with IL-1β (1 ng/ml) and then neutrophils (1×106 cells) were added to the upper chamber of the transwell. After 1 hour, neutrophils were collected from the upper and lower chambers and analyzed for PR3 and NB1 cell surface expression by flow cytometry. (A) Transmigration of NB1-positive neutrophils dramatically increased their cell surface expression of PR3. (B) NB1-positive cells were selectively recruited during neutrophil transmigration. (C) Neutrophil transmigration resulted in a significant increase in the cell surface expression of NB1 and PR3. Data represent the SEM± of four separate experiments, *p<0.01 compared to upper chamber.
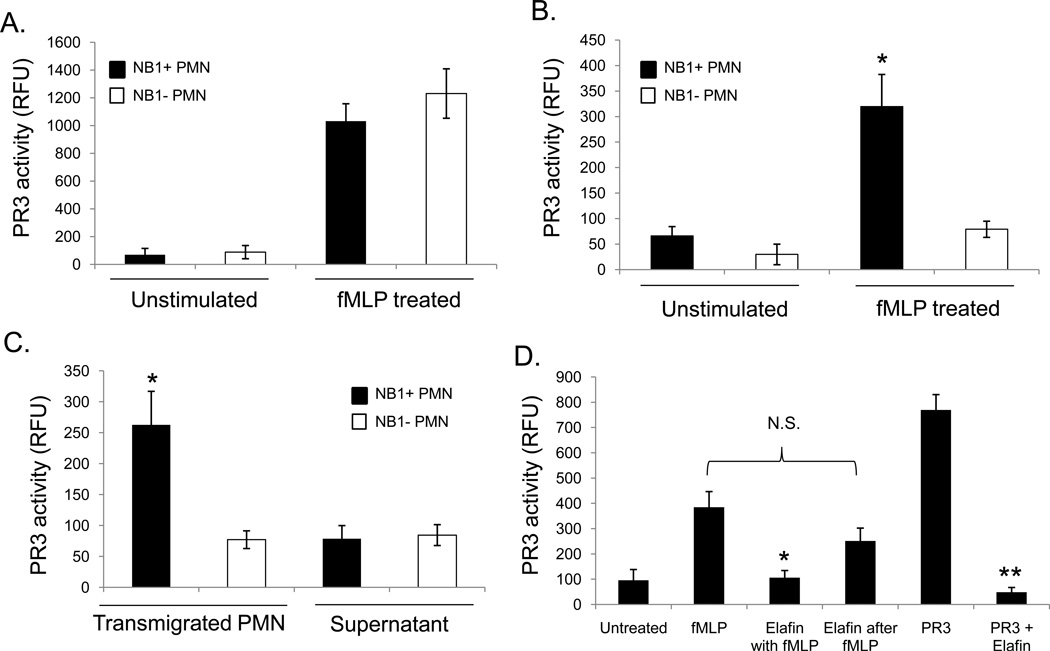
To determine whether PR3 activity was preserved on the surface of transmigrated cells, we employed a FRET peptide substrate specific for PR3 protease activity. As shown in Figure 4A, culture media from fMLP-stimulated neutrophils derived from an NB1-positive and NB1-null individual contained similar levels of PR3 activity. Only NB1-positive neutrophils, however, displayed significant PR3 activity on their surface (Figure 4B). Following neutrophil transmigration on transwell-cultured endothelial cells, we likewise found that only NB1 positive cells had significant levels of PR3 activity on their cell surface (Figure 4C). Interestingly, nearly all the serine protease activity was restricted to the neutrophil surface, as culture supernatants collected from the bottom chambers had significantly less PR3 activity.
Figure 4. Transmigrating neutrophils express catalytically active PR3.
The enzymatic activity of PR3 was determined by FRET using a peptide substrate coupled to a fluorophore (Abz) and a quencher (nitrotyrosine). Neutrophils (1×106) were stimulated with fMLP (50 nM) to promote PR3 secretion. The supernatant or isolated neutrophils were then tested for PR3 enzymatic activity which was expressed in relative fluorescence units (RFU). (A) PR3 activity was detected in the supernatants of both NB1-positive and NB1-null individuals following fMLP stimulation. SEM± of four separate experiments SEM± of four separate experiments (B) PR3 was only detected on the surface of NB1-positive neutrophils; *p<0.01 compared to NB1- cells. (C) NB1-positive and NB1-null neutrophils (1×106) were allowed to transmigrate 60 minutes on IL-1β stimulated HUVEC cultured on transwell inserts. Neutrophils and culture media from the bottom chamber were then examined for PR3 activity. The activity of PR3 was restricted to the surface of NB1-positive neutrophils; *p<0.01 compared to NB1-null cells or supernatants. SEM± of four separate experiments. (D) Neutrophils (1 × 106) were stimulated with fMLP (50 nM, 30 min) in the presence or absence of elafin (2 µM). In some experiments the neutrophils were first incubated for 30 minutes with fMLP before being treated with elafin for an additional 30 minutes. Neutrophils were then isolated and examined for PR3 activity. *p<0.05 compared to NB1-null cells or supernatants, **p<0.01 compared to PR3 alone. SEM± of four separate experiments. (PR3 concentration 1x 10−7 M)
PR3 activity could be significantly inhibited by elafin when added prior to fMLP stimulation (Figure 4D). However, if neutrophils were stimulated with fMLP for 30 minutes prior to the addition of elafin, PR3 was protected from inactivation. These data suggest that association with NB1 protects PR3 from proteolytic inactivation.
PR3 and NB1 contribute to neutrophil transmigration under flow conditions
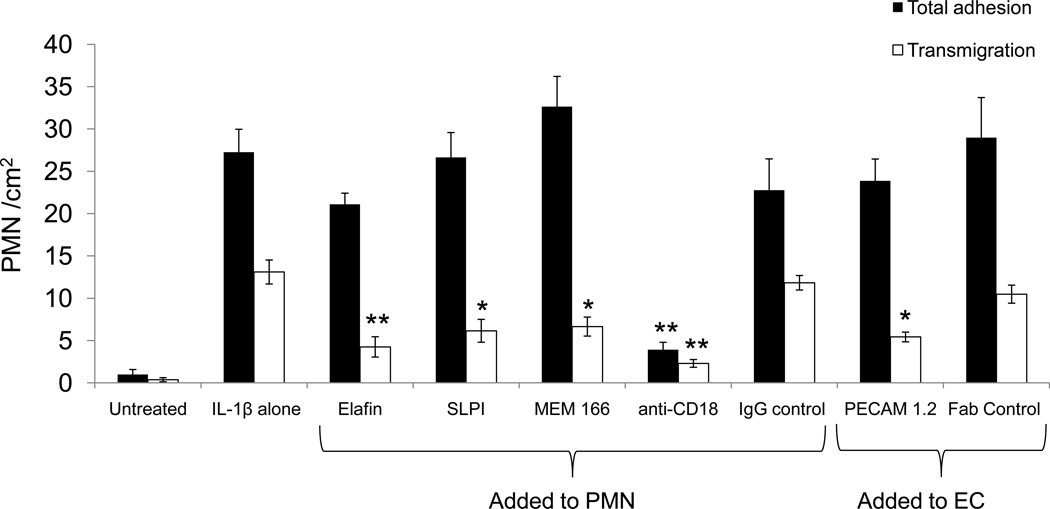
To determine whether PR3 or NB1 play a role in neutrophil adhesion and transmigration under physiological flow conditions, endothelial cells were treated with IL-1β for four hours, transferred to microfluidic chambers, and neutrophils were then perfused over the endothelial cell surface at 1 dyne/cm2 (140 sec−1) for 5 minutes followed by a 5 minute washout. Before perfusion, endothelial cells were treated with blocking antibodies specific for the heterophilic NB1-binding region of PECAM-1, or for NB1 (MEM166), or with selective serine protease inhibitors (elafin, SLP1). As shown in Figure 5, disrupting NB1-PECAM-1 interactions with mAb PECAM-1 1.2 did not disrupt leukocyte adhesion per se to the endothelial surface. We also did not observe any differences in rolling velocity in the presence of blocking antibodies to NB1 or PECAM-1 compared to our controls (data not shown). Likewise, total neutrophil adhesion and cell rolling was not inhibited using serine protease inhibitors, although function blocking antibodies against the β2-integrin CD18 did block adhesion as expected (36,37). In contrast, neutrophil transmigration was significantly inhibited by the addition of blocking antibodies to NB1 or PECAM-1. Likewise, inhibition of serine protease activity with the PR3-selective inhibitor elafin also blocked neutrophil transmigration. SLP1, a serine protease inhibitor specific for neutrophil elastase and cathepsin G also inhibited neutrophil transmigration. These data demonstrate that PR3 plays an important role in transmigration, though other neutrophil serine proteases also contribute to this process.
Figure 5. NB1 and PR3 contribute to neutrophil transmigration under flow conditions.
HUVEC were treated with IL-1β (1 ng/ml) for 4 hours and then transferred to Vena8 EC+ flow chambers. Neutrophils were perfused over the endothelial cell monolayers (140 sec−1) for 5 minutes then washed for an additional 5 minutes. Neutrophil adhesion and transmigration were observed and later quantified offline. In some experiments, neutrophils were pre-treated for 10 minutes with inhibitors of PR3 (elafin, 2 µM) or CG/NE (SLP1, 10 µM) or antibodies against NB1 (MEM 166, 10 µg/ml) or CD18 (10 µg/ml). In other experiments the endothelial cells were treated with a blocking Fab to PECAM-1 (1.2, 10 µg/ml). Black bars indicate total neutrophil adhesion and white bars signify neutrophil transmigration. Note that inhibiting NB1 binding to PECAM-1 or using protease inhibitors does not affect neutrophil adhesion. In contrast, both NB1 and neutrophil serine protease activity are required for neutrophil transmigration. **p<0.01 compared to IL-1β alone. SEM± of four separate experiments.
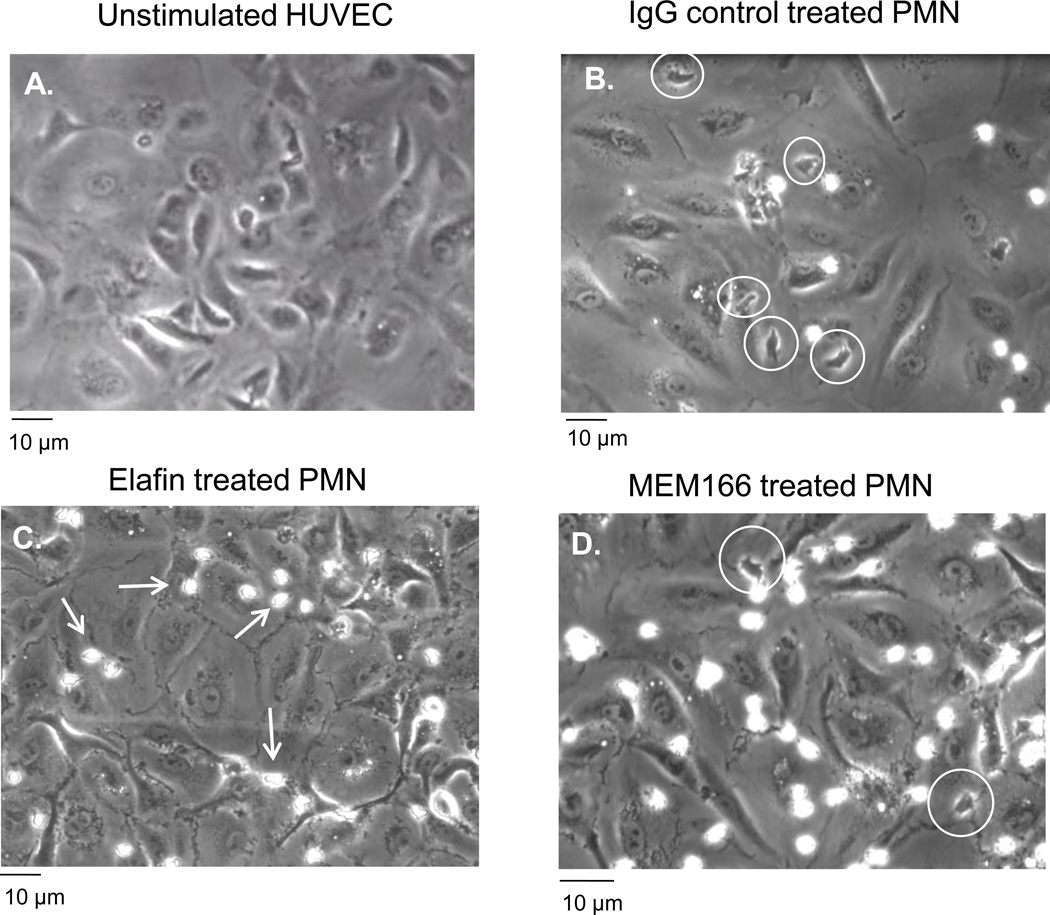
Visualizing neutrophil interactions with endothelial monolayers, elafin-treated neutrophils were found near endothelial cell junctions; however, these neutrophils were unable to transmigrate (Figure 6C). When we measured crawling velocity of neutrophils on the luminal side of the endothelial cells we did not observe any significant decrease in the total number of crawling neutrophils or crawling speed compared to untreated cells (data not shown). Therefore, blocking PR3 activity does not inhibit the ability of neutrophils to crawl toward endothelial cell borders where transmigration occurs. In contrast, blocking antibodies against NB1 dramatically inhibited the ability of neutrophils to reach endothelial cell junctions (Figure 6D). Therefore it appears that PR3 and NB1 contribute to neutrophil transmigration by complementary but distinct mechanisms.
Figure 6. Differential disruption of neutrophil transmigration by NB1 and PR3 under flow conditions.
Neutrophils were perfused over IL-1β-stimulated endothelial cells for 10 minutes and adhesion and transmigration were recorded for offline analysis. (A) Unstimulated HUVEC demonstrated no neutrophil adhesion or transmigration. (B) Significant numbers of transmigrated neutrophils were observed (phase-dark cells indicated by white circles) on IL-1β stimulated HUVEC treated with an IgG control antibody. (C) Pre-treatment of neutrophils with elafin (2 µM, 10 min) did not affect adhesion, but neutrophil transmigration was arrested at the cell junctions (phase-bright cells indicated by white arrows). (D) Blocking antibodies against NB1 (MEM166) significantly reduced neutrophil transmigration (white circles) but total neutrophil adhesion was not inhibited. Representative of four separate experiments.
Discussion
Neutrophil transendothelial cell migration is a critical event in the inflammatory cascade. A major player in this process is endothelial cell PECAM-1, which can interact with neutrophils through both homophilic and heterophilic interactions. The strongest association is the heterophilic interaction between endothelial cell PECAM-1 and neutrophil NB1, which plays an important role in neutrophil transmigration. The most interesting part of this interaction may be the association of NB1 with the serine protease PR3. In this study we have demonstrated that PR3 activity also plays a critical role in neutrophil transmigration and this requires the presence of NB1. We also found that neutrophils expressing NB1 and PR3 on their surface are selectively recruited, and that the efficiency of transmigration correlates with increased PR3 activity.
PR3 is unique from other neutrophil serine proteases in that it is highly expressed on the surface of activated neutrophils via its interaction with NB1. The association of PR3 with NB1 requires 6 hydrophobic residues on PR3 (Phe-165, Phe-166, Ile-217, Trp-218, Leu-223, Phe-224) that are not found on neutrophil elastase or cathepsin G, nor are these residues found in mouse or gibbon PR3 (38). Because NB1 is a heterophilic binding partner for PECAM-1, NB1 is therefore likely to present PR3 to endothelial cell junctional PECAM-1. We found that following neutrophil transmigration, catalytically-active PR3 concentrated on the neutrophil surface, rather than in the surrounding media. This is significant since we also observed that the activity of PR3 on the neutrophil surface is protected from inhibition by elafin. This suggests that NB1 may play an important role in protecting PR3 from inactivation, and may allow NB1-positive neutrophils to exert a greater pro-inflammatory potential, even after transmigration has occurred. The mechanism by which NB1 protects PR3 from inactivation, while still maintaining its proteolytic activity, is not known. One possibility is that inhibitors like elafin may be sterically hindered and unable to interact with NB1-associated PR3. In contrast, a small peptide substrate like our FRET peptide may still be accessible for cleavage by PR3.
It is well-established that neutrophil serine proteases are essential contributors for transmigration (39–50). Many studies have focused on the role of neutrophil elastase, a molecule closely related to PR3. Neutrophil elastase can degrade matrix proteins and is found at the leading edge of migrating neutrophils (51–53). Transmigrating leukocytes lacking neutrophil elastase, however, still induce the focal loss of junctional proteins (54) and neutrophil elastase knockout mice have no deficiency in neutrophil transmigration (48). In vivo mouse models have demonstrated, however, that neutrophil transmigration is significantly disrupted by inhibition of serine protease activity (48). This suggests that other serine proteases, such as PR3, must play a role. One limitation to our study is our inability to specifically inhibit PR3. Elafin can selectively inhibit PR3 protease activity, but it can also inhibit neutrophil elastase. Protease inhibition experiments, (Figures 2 and 5) support a role for PR3 in transmigration, in addition to the selective transmigration of PR3-expressing neutrophils (Figure 3) and increased PR3 activity on those cells (Figure 4C). Therefore it appears likely that the ability of elafin to inhibit transmigration is largely due to its effects on PR3 and not neutrophil elastase. This being stated, we cannot fully discount the contribution of neutrophil elastase and cathepsin G in neutrophil transmigration, since the selective inhibitor of these proteases, SLPI, was also able to inhibit transmigration. This suggests that neutrophil transmigration involves some redundancy on the part of the serine proteases which may participate. Unfortunately there is not currently a commercially available inhibitor specific for PR3. Therefore this will be an area of continued investigation as more specific inhibitors of neutrophil proteases become available.
At present, it is not known what molecular mechanisms are involved in PR3-mediated neutrophil transmigration. Extracellular matrix proteins such as fibronectin, laminin, vitronectin, and collagen type IV are all substrates for PR3 (27). PR3 may also be able to degrade junctional proteins (e.g. VE-cadherin, occludins). Neutrophil-released progranulin, a molecule with anti-inflammatory properties, has been shown to be inactivated by PR3 (55). In the absence of neutrophil PR3, the presence of progranulin can inhibit neutrophil transmigration. A recent report by Zen et al., showed that PR3 can cleave CD11b and this may be important in promoting neutrophil release from endothelial cell adhesion proteins during transmigration (56). A final target for PR3 may be protease-activated receptors expressed on endothelial cells. PR3 has been shown to activate both PAR-1 and PAR-2, although how this might affect transmigration is unclear (57,57). In future studies we plan to investigate the molecules that are targeted by PR3, and their contribution to neutrophil transmigration.
While the majority of individuals express NB1 on the surface of their neutrophils, approximately 3% of the population does not. This absence is thought to be due to the introduction of a stop codon resulting in early termination of the NB1 protein during translation (17). Our data demonstrates that neutrophils of NB1-null individuals capture little to no PR3 on their surface. We also found that NB1 and PR3 are critical for neutrophil transmigration, therefore we would expect that neutrophils from NB1-null individuals would have impaired transmigration compared to normal individuals. Interestingly, we did not find a transmigration defect for these individuals compared to neutrophils from NB1-positive subjects (data not shown). Therefore it appears that a compensatory mechanism may exist in NB1-null individuals that corrects for the absence of NB1. Future studies will be directed toward investigating alternative mechanisms of endothelial transmigration for neutrophils congenitally deficient in NB1.
In conclusion, the present investigation has demonstrated that PR3 plays an important role in neutrophil transmigration under both static and flow conditions. This requires PR3 enzymatic activity and interactions with NB1, a molecule that localizes PR3 to endothelial cell junctions via its heterophilic interaction with PECAM-1. Furthermore, we have found that NB1-positive neutrophils are selectively recruited to IL-1β activated endothelial cell monolayers, suggesting that under specific inflammatory conditions, tissues may accumulate high levels of neutrophil-expressed PR3. These findings open the possibility that neutrophil recruitment during inflammatory responses may be more regulated than we currently believe, and that NB1 and PR3 may be novel targets for disrupting neutrophil recruitment during inflammation.
Acknowledgments
We would like to thank Trudy Holyst in our protein chemistry core for preparing the FRET peptide substrate used in this study. We also want to thank Dr. Brian Curtis, Director of the Platelet and Neutrophil Immunology Lab, for providing us with neutrophils from NB1-null patients.
Reference List
- 1.Newman PJ, Berndt MC, Gorski J, White GC, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- 2.Newman PJ. The role of PECAM-1 in vascular cell biology. Ann. N. Y. Acad. Sci. 1994;714:165–174. doi: 10.1111/j.1749-6632.1994.tb12041.x. [DOI] [PubMed] [Google Scholar]
- 3.Sun QH, DeLisser HM, Zukowski MM, Paddock C, Albelda SM, Newman PJ. Individually distinct Ig homology domains in PECAM-1 regulate homophilic binding and modulate receptor affinity. J. Biol. Chem. 1996;271:11090–11098. doi: 10.1074/jbc.271.19.11090. [DOI] [PubMed] [Google Scholar]
- 4.Newton JP, Buckley CD, Jones EY, Simmons DL. Residues on both faces of the first immunoglobulin fold contribute to homophilic binding sites of PECAM-1/CD31. J. Biol. Chem. 1997;272:20555–20563. doi: 10.1074/jbc.272.33.20555. [DOI] [PubMed] [Google Scholar]
- 5.Sun J, Paddock C, Shubert J, Zhang HB, Amin K, Newman PJ, Albelda SM. Contributions of the extracellular and cytoplasmic domains of platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) in regulating cell-cell localization. J. Cell Sci. 2000;113(Pt 8):1459–1469. doi: 10.1242/jcs.113.8.1459. [DOI] [PubMed] [Google Scholar]
- 6.Bergom C, Paddock C, Gao C, Holyst T, Newman DK, Newman PJ. An alternatively spliced isoform of PECAM-1 is expressed at high levels in human and murine tissues, and suggests a novel role for the C-terminus of PECAM-1 in cytoprotective signaling. J. Cell Sci. 2008;121:1235–1242. doi: 10.1242/jcs.025163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrero E, Ferrero ME, Pardi R, Zocchi MR. The platelet endothelial cell adhesion molecule-1 (PECAM1) contributes to endothelial barrier function. FEBS Lett. 1995;374:323–326. doi: 10.1016/0014-5793(95)01110-z. [DOI] [PubMed] [Google Scholar]
- 8.Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J. Clin. Invest. 2002;109:383–392. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J. Exp. Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakada MT, Amin K, Christofidou-Solomidou M, O'Brien CD, Sun J, Gurubhagavatula I, Heavner GA, Taylor AH, Paddock C, Sun QH, Zehnder JL, Newman PJ, Albelda SM, DeLisser HM. Antibodies against the first Ig-like domain of human platelet endothelial cell adhesion molecule-1 (PECAM-1) that inhibit PECAM-1-dependent homophilic adhesion block in vivo neutrophil recruitment. J. Immunol. 2000;164:452–462. doi: 10.4049/jimmunol.164.1.452. [DOI] [PubMed] [Google Scholar]
- 11.Liao F, Huynh HK, Eiroa A, Greene T, Polizzi E, Muller WA. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J. Exp. Med. 1995;182:1337–1343. doi: 10.1084/jem.182.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong CW, Wiedle G, Ballestrem C, Wehrle-Haller B, Etteldorf S, Bruckner M, Engelhardt B, Gisler RH, Imhof BA. PECAM-1/CD31 trans-homophilic binding at the intercellular junctions is independent of its cytoplasmic domain; evidence for heterophilic interaction with integrin alphavbeta3 in Cis. Mol. Biol. Cell. 2000;11:3109–3121. doi: 10.1091/mbc.11.9.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deaglio S, Morra M, Mallone R, Ausiello CM, Prager E, Garbarino G, Dianzani U, Stockinger H, Malavasi F. Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J. Immunol. 1998;160:395–402. [PubMed] [Google Scholar]
- 14.Sachs UJ, Andrei-Selmer CL, Maniar A, Weiss T, Paddock C, Orlova VV, Choi EY, Newman PJ, Preissner KT, Chavakis T, Santoso S. The neutrophil-specific antigen CD177 is a counter-receptor for platelet endothelial cell adhesion molecule-1 (CD31) J. Biol. Chem. 2007;282:23603–23612. doi: 10.1074/jbc.M701120200. [DOI] [PubMed] [Google Scholar]
- 15.Gohring K, Wolff J, Doppl W, Schmidt KL, Fenchel K, Pralle H, Sibelius U, Bux J. Neutrophil CD177 (NB1 gp, HNA-2a) expression is increased in severe bacterial infections and polycythaemia vera. Br. J. Haematol. 2004;126:252–254. doi: 10.1111/j.1365-2141.2004.05027.x. [DOI] [PubMed] [Google Scholar]
- 16.Kissel K, Santoso S, Hofmann C, Stroncek D, Bux J. Molecular basis of the neutrophil glycoprotein NB1 (CD177) involved in the pathogenesis of immune neutropenias and transfusion reactions. Eur. J. Immunol. 2001;31:1301–1309. doi: 10.1002/1521-4141(200105)31:5<1301::AID-IMMU1301>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 17.Kissel K, Scheffler S, Kerowgan M, Bux J. Molecular basis of NB1 (HNA-2a, CD177) deficiency. Blood. 2002;99:4231–4233. doi: 10.1182/blood.v99.11.4231. [DOI] [PubMed] [Google Scholar]
- 18.Lalezari P, Murphy GB, Allen FH., Jr NB1, a new neutrophil-specific antigen involved in the pathogenesis of neonatal neutropenia. J. Clin. Invest. 1971;50:1108–1115. doi: 10.1172/JCI106582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newton JP, Hunter AP, Simmons DL, Buckley CD, Harvey DJ. CD31 (PECAM-1) exists as a dimer and is heavily N-glycosylated. Biochem. Biophys. Res. Commun. 1999;261:283–291. doi: 10.1006/bbrc.1999.1018. [DOI] [PubMed] [Google Scholar]
- 20.Bayat B, Werth S, Sachs UJ, Newman DK, Newman PJ, Santoso S. Neutrophil transmigration mediated by the neutrophil-specific antigen CD177 is influenced by the endothelial S536N dimorphism of platelet endothelial cell adhesion molecule-1. J. Immunol. 2010;184:3889–3896. doi: 10.4049/jimmunol.0903136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von VS, Tunnemann G, Eulenberg C, Wellner M, Cristina CM, Luft FC, Kettritz R. NB1 mediates surface expression of the ANCA antigen proteinase 3 on human neutrophils. Blood. 2007;109:4487–4493. doi: 10.1182/blood-2006-10-055327. [DOI] [PubMed] [Google Scholar]
- 22.Kao RC, Wehner NG, Skubitz KM, Gray BH, Hoidal JR. Proteinase 3. A distinct human polymorphonuclear leukocyte proteinase that produces emphysema in hamsters. J. Clin. Invest. 1988;82:1963–1973. doi: 10.1172/JCI113816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludemann J, Utecht B, Gross WL. Anti-neutrophil cytoplasm antibodies in Wegener's granulomatosis recognize an elastinolytic enzyme. J. Exp. Med. 1990;171:357–362. doi: 10.1084/jem.171.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludemann J, Utecht B, Gross WL. Anti-cytoplasmic antibodies in Wegener's granulomatosis are directed against proteinase 3. Adv. Exp. Med. Biol. 1991;297:141–150. doi: 10.1007/978-1-4899-3629-5_12. [DOI] [PubMed] [Google Scholar]
- 25.Dolman KM, Jager A, Sonnenberg A, von dem Borne AE, Goldschmeding R. Proteolysis of classic anti-neutrophil cytoplasmic autoantibodies (C-ANCA) by neutrophil proteinase 3. Clin. Exp. Immunol. 1995;101:8–12. doi: 10.1111/j.1365-2249.1995.tb02269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raife TJ, Cao W, Atkinson BS, Bedell B, Montgomery RR, Lentz SR, Johnson GF, Zheng XL. Leukocyte proteases cleave von Willebrand factor at or near the ADAMTS13 cleavage site. Blood. 2009;114:1666–1674. doi: 10.1182/blood-2009-01-195461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao NV, Wehner NG, Marshall BC, Gray WR, Gray BH, Hoidal JR. Characterization of proteinase-3 (PR-3), a neutrophil serine proteinase. Structural and functional properties. J. Biol. Chem. 1991;266:9540–9548. [PubMed] [Google Scholar]
- 28.Ying QL, Simon SR. Elastolysis by proteinase 3 and its inhibition by alpha(1)-proteinase inhibitor: a mechanism for the incomplete inhibition of ongoing elastolysis. Am. J. Respir. Cell Mol. Biol. 2002;26:356–361. doi: 10.1165/ajrcmb.26.3.4704. [DOI] [PubMed] [Google Scholar]
- 29.Ranes J, Stoller JK. A review of alpha-1 antitrypsin deficiency. Semin. Respir. Crit Care Med. 2005;26:154–166. doi: 10.1055/s-2005-869536. [DOI] [PubMed] [Google Scholar]
- 30.Yan HC, Pilewski JM, Zhang Q, DeLisser HM, Romer L, Albelda SM. Localization of multiple functional domains on human PECAM-1 (CD31) by monoclonal antibody epitope mapping. Cell Adhes. Commun. 1995;3:45–66. doi: 10.3109/15419069509081277. [DOI] [PubMed] [Google Scholar]
- 31.Korkmaz B, Attucci S, Juliano MA, Kalupov T, Jourdan ML, Juliano L, Gauthier F. Measuring elastase, proteinase 3 and cathepsin G activities at the surface of human neutrophils with fluorescence resonance energy transfer substrates. Nat. Protoc. 2008;3:991–1000. doi: 10.1038/nprot.2008.63. [DOI] [PubMed] [Google Scholar]
- 32.Korkmaz B, Attucci S, Moreau T, Godat E, Juliano L, Gauthier F. Design and use of highly specific substrates of neutrophil elastase and proteinase 3. Am. J. Respir. Cell Mol. Biol. 2004;30:801–807. doi: 10.1165/rcmb.2003-0139OC. [DOI] [PubMed] [Google Scholar]
- 33.Korkmaz B, Attucci S, Juliano MA, Kalupov T, Jourdan ML, Juliano L, Gauthier F. Measuring elastase, proteinase 3 and cathepsin G activities at the surface of human neutrophils with fluorescence resonance energy transfer substrates. Nat. Protoc. 2008;3:991–1000. doi: 10.1038/nprot.2008.63. [DOI] [PubMed] [Google Scholar]
- 34.Kuckleburg CJ, Yates CM, Kalia N, Zhao Y, Nash GB, Watson SP, Rainger GE. Endothelial cell-borne platelet bridges selectively recruit monocytes in human and mouse models of vascular inflammation. Cardiovasc. Res. 2011;91:134–141. doi: 10.1093/cvr/cvr040. [DOI] [PubMed] [Google Scholar]
- 35.Vaporciyan AA, DeLisser HM, Yan HC, Mendiguren II, Thom SR, Jones ML, Ward PA, Albelda SM. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science. 1993;262:1580–1582. doi: 10.1126/science.8248808. [DOI] [PubMed] [Google Scholar]
- 36.Tonnesen MG, Anderson DC, Springer TA, Knedler A, Avdi N, Henson PM. Adherence of neutrophils to cultured human microvascular endothelial cells. Stimulation by chemotactic peptides and lipid mediators and dependence upon the Mac-1, LFA-1, p150,95 glycoprotein family. J. Clin. Invest. 1989;83:637–646. doi: 10.1172/JCI113928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 38.Korkmaz B, Kuhl A, Bayat B, Santoso S, Jenne DE. A hydrophobic patch on proteinase 3, the target of autoantibodies in Wegener granulomatosis, mediates membrane binding via NB1 receptors. J. Biol. Chem. 2008;283:35976–35982. doi: 10.1074/jbc.M806754200. [DOI] [PubMed] [Google Scholar]
- 39.Carden D, Xiao F, Moak C, Willis BH, Robinson-Jackson S, Alexander S. Neutrophil elastase promotes lung microvascular injury and proteolysis of endothelial cadherins. Am. J. Physiol. 1998;275:H385–H392. doi: 10.1152/ajpheart.1998.275.2.H385. [DOI] [PubMed] [Google Scholar]
- 40.Carden DL, Korthuis RJ. Protease inhibition attenuates microvascular dysfunction in postischemic skeletal muscle. Am. J. Physiol. 1996;271:H1947–H1952. doi: 10.1152/ajpheart.1996.271.5.H1947. [DOI] [PubMed] [Google Scholar]
- 41.Cepinskas G, Noseworthy R, Kvietys PR. Transendothelial neutrophil migration. Role of neutrophil-derived proteases and relationship to transendothelial protein movement. Circ. Res. 1997;81:618–626. doi: 10.1161/01.res.81.4.618. [DOI] [PubMed] [Google Scholar]
- 42.Korthuis RJ, Carden DL, Kvietys PR, Shepro D, Fuseler J. Phalloidin attenuates postischemic neutrophil infiltration and increased microvascular permeability. J. Appl. Physiol. 1991;71:1261–1269. doi: 10.1152/jappl.1991.71.4.1261. [DOI] [PubMed] [Google Scholar]
- 43.Kvietys PR, Granger DN. Endothelial cell monolayers as a tool for studying microvascular pathophysiology. Am. J. Physiol. 1997;273:G1189–G1199. doi: 10.1152/ajpgi.1997.273.6.G1189. [DOI] [PubMed] [Google Scholar]
- 44.Lewis RE, Granger HJ. Diapedesis and the permeability of venous microvessels to protein macromolecules: the impact of leukotriene B4 (LTB4) Microvasc. Res. 1988;35:27–47. doi: 10.1016/0026-2862(88)90048-9. [DOI] [PubMed] [Google Scholar]
- 45.Weiss SJ. Tissue destruction by neutrophils. N. Engl. J. Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 46.Woodman RC, Reinhardt PH, Kanwar S, Johnston FL, Kubes P. Effects of human neutrophil elastase (HNE) on neutrophil function in vitro and in inflamed microvessels. Blood. 1993;82:2188–2195. [PubMed] [Google Scholar]
- 47.Zimmerman BJ, Granger DN. Reperfusion-induced leukocyte infiltration: role of elastase. Am. J. Physiol. 1990;259:H390–H394. doi: 10.1152/ajpheart.1990.259.2.H390. [DOI] [PubMed] [Google Scholar]
- 48.Young RE, Voisin MB, Wang S, Dangerfield J, Nourshargh S. Role of neutrophil elastase in LTB4-induced neutrophil transmigration in vivo assessed with a specific inhibitor and neutrophil elastase deficient mice. Br. J. Pharmacol. 2007;151:628–637. doi: 10.1038/sj.bjp.0707267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young RE, Thompson RD, Larbi KY, La M, Roberts CE, Shapiro SD, Perretti M, Nourshargh S. Neutrophil elastase (NE)-deficient mice demonstrate a nonredundant role for NE in neutrophil migration, generation of proinflammatory mediators, and phagocytosis in response to zymosan particles in vivo. J. Immunol. 2004;172:4493–4502. doi: 10.4049/jimmunol.172.7.4493. [DOI] [PubMed] [Google Scholar]
- 50.Cepinskas G, Sandig M, Kvietys PR. PAF-induced elastase-dependent neutrophil transendothelial migration is associated with the mobilization of elastase to the neutrophil surface and localization to the migrating front. J. Cell Sci. 1999;112(Pt 12):1937–1945. doi: 10.1242/jcs.112.12.1937. [DOI] [PubMed] [Google Scholar]
- 51.Cepinskas G, Noseworthy R, Kvietys PR. Transendothelial neutrophil migration. Role of neutrophil-derived proteases and relationship to transendothelial protein movement. Circ. Res. 1997;81:618–626. doi: 10.1161/01.res.81.4.618. [DOI] [PubMed] [Google Scholar]
- 52.Cepinskas G, Sandig M, Kvietys PR. PAF-induced elastase-dependent neutrophil transendothelial migration is associated with the mobilization of elastase to the neutrophil surface and localization to the migrating front. J. Cell Sci. 1999;112(Pt 12):1937–1945. doi: 10.1242/jcs.112.12.1937. [DOI] [PubMed] [Google Scholar]
- 53.Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J. Exp. Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allport JR, Muller WA, Luscinskas FW. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J. Cell Biol. 2000;148:203–216. doi: 10.1083/jcb.148.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kessenbrock K, Frohlich L, Sixt M, Lammermann T, Pfister H, Bateman A, Belaaouaj A, Ring J, Ollert M, Fassler R, Jenne DE. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. J. Clin. Invest. 2008;118:2438–2447. doi: 10.1172/JCI34694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zen K, Guo YL, Li LM, Bian Z, Zhang CY, Liu Y. Cleavage of the CD11b extracellular domain by the leukocyte serprocidins is critical for neutrophil detachment during chemotaxis. Blood. 2011;117:4885–4894. doi: 10.1182/blood-2010-05-287722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steppich BA, Seitz I, Busch G, Stein A, Ott I. Modulation of tissue factor and tissue factor pathway inhibitor-1 by neutrophil proteases. Thromb. Haemost. 2008;100:1068–1075. [PubMed] [Google Scholar]