Abstract
Coronary vessel development depends on a subpopulation of epicardial cells that undergo epithelial to mesenchymal transformation (EMT) and invade the subepicardial space and myocardium. These cells form the smooth muscle of the vessels and fibroblasts, but the mechanisms that regulate these processes are poorly understood. Mice lacking the Type III Transforming Growth Factor β Receptor (TGFβR3) die by E14.5 due to failed coronary vessel development accompanied by reduced epicardial cell invasion. BMP2 signals via TGFβR3 emphasizing the importance of determining the relative contributions of the canonical BMP signaling pathway and TGFβR3-dependent signaling to BMP2 responsiveness. Here we examined the role of TGFβR3 in BMP2 signaling in epicardial cells. Whereas TGFβ induced loss of epithelial character and smooth muscle differentiation, BMP2 induced an ALK3-dependent loss of epithelial character and modestly inhibited TGFβ-stimulated differentiation. Tgfbr3−/− cells respond to BMP2 indicating that TGFβR3 is not required. However, Tgfbr3−/− cells show decreased invasion in response to BMP2 and overexpression of TGFβR3 in Tgfbr3−/− cells rescued invasion. Invasion was dependent on ALK5, ALK2, ALK3, and Smad4. Expression of TGFβR3 lacking the 3 C-terminal amino acids required to interact with the scaffolding protein GIPC (GAIP-interacting protein, C terminus) did not rescue. Knockdown of GIPC in Tgfbr3+/+ or Tgfbr3−/− cells rescued with TGFβR3 decreased BMP2-stimulated invasion confirming a requirement for TGFβR3/GIPC interaction. Our results reveal the relative roles of TGFβR3-dependent and TGFβR3-independent signaling in the actions of BMP2 on epicardial cell behavior and demonstrate the critical role of TGFβR3 in mediating BMP2-stimulated invasion.
Keywords: epithelial to mesenchymal transformation, transforming growth factor beta, bone morphogenic protein, epicardium, invasion, differentiation, coronary vessels
1.0 Introduction
Transforming Growth Factor β (TGFβ) plays a critical role in regulating epithelial to mesenchymal transformation (EMT), an important process in the regulation of both embryonic development and disease progression [1–5]. The regulation of EMT by members of the TGFβ family is a major component of heart valve [6, 7] and coronary vessel [8, 9] development. Formation of the coronary vessels begins when cells of the proepicardium attach to the heart to form the epicardium [8, 10–12]. A fraction of the cells of the epithelial epicardium undergo EMT and the resulting mesenchymal cells populate the subepicardial space with some cells invading further into the myocardium [13]. Mesenchymal cells in the subepicardial space and myocardium contribute to several lineages that include cardiac fibroblasts and vascular smooth muscle [14, 15].
Despite the recognition of the importance of TGFβ in regulating EMT, the mechanism of TGFβ signaling is not well understood. The roles of the serine-threonine kinase containing Type I (TGFβR1 or ALK5) and Type II (TGFβR2) receptors in TGFβ signaling are well described [16, 17], but the contributions of the Type III receptor (TGFβR3) are poorly understood. However a significant role for TGFβR3 is demonstrated by the observation that targeting of Tgfbr3 in mice causes embryonic lethality due to failed coronary vessel development [18] associated with dysregulated epicardial cell invasion [19]. TGFβR3 binds multiple members of the TGFβ family. In addition to binding TGFβ1 and TGFβ3, TGFβR3 is required for the high affinity binding of TGFβ2 [20]. TGFβR3 has also been identified as the inhibin receptor [21] and binds BMP2 [22].
Studies of epicardial cells have shown that TGFβ stimulates the loss of epithelial cell character and smooth muscle differentiation [23]. Although loss of epithelial character and smooth muscle differentiation does not require TGFβR3, TGFβ-mediated epicardial cell invasion was shown to be dependent on specific cytoplasmic residues of TGFβR3 and the interaction of these residues with the scaffolding protein GIPC [19]. TGFβ-stimulated epicardial cell invasion also requires TGFβR3 to access the Par6/Smurf1/RhoA pathway which is necessary for cell invasion [24]. The role of TGFβR3 in BMP2 signaling is less well described. BMP2 binds TGFβR3 and is required for endothelial cell transformation in vitro [22]. In endothelial cells, both TGFβ and BMP2 share a common, TGFβR3-dependent pathway to signal transformation that includes activation of the Par6/Smurf1/RhoA pathway [25, 26] and a requirement for specific cytoplasmic residues of TGFβR3 and the interaction of these residues with the scaffolding protein GIPC [27]. In epicardial cells, BMP2 is known to induce invasion in vitro that is dependent on the Par6/Smurf1/RhoA pathway [24].
Here we show that TGFβR3 is required for BMP2-stimulated epicardial cell invasion in vitro although TGFβR3 is not required for BMP2-stimulated loss of epithelial character as measured by the redistribution of ZO1. BMP2-stimulated invasion was shown to require specific cytoplasmic residues in TGFβR3 that are known to interact with the scaffolding protein GIPC. Deletion of these residues, or the targeting of GIPC, demonstrated a requirement for each in BMP2-stimulated invasion. These data suggest that loss of BMP2 responsiveness, as well as the previously recognized loss of TGFβ responsiveness, may underlie the epicardial defects associated with failed coronary vessel development in Tgfbr3−/− mice [18].
2.0 Methods
2.1 Immortalized Epicardial Explant Culture
Immortalized epicardial cell lines from Tgfbr3+/+ and Tgfbr3−/− mice were generated as described previously [23]. To sustain the cell’s immortalized state, they were grown at 33°C in immorto media: 10% fetal bovine serum (FBS), 100U/ml Penicillin/Streptomycin (P/S), 1X Insulin-Transferrin-Selenium (ITS; 1 μg/ml insulin, 5.5×10−4 μg/ml transferrin, 0.677 μg/ml selenium), and 10U/ml interferon γ (INFγ). For growth factor addition, cells were transferred to standard DMEM media (5% FBS and 100U/ml P/S) and cultured at 37°C for 24 hours prior to growth factor addition. Growth factors (TGFβ1, TGFβ2, or BMP2) or small molecule inhibitors were added to the cell medium and assayed after 24, 48, or 72 hours. Multiple immortalized epicardial cell lines (E11.5) were generated from Tgfbr3+/+ and Tgfbr3−/− littermate pairs and used in experiments.
2.2 Growth Factors and Inhibitors
Reagents were obtained from the following sources: TGFβ1, TGFβ2, and BMP2 were purchased from R&D Systems; SB431542, from Sigma-Aldrich. DMH1 was a generous gift from Dr. Charles Hong.
2.3 Immunohistochemistry
Tgfbr3+/+ and Tgfbr3−/− epicardial cells (E11.5) were plated in 4-well collagen coated chamber slides (BD Biosciences) at a density of 50,000 cells per well. Cells for ZO-1 staining were fixed in 70% methanol for 10 min at room temperature, then blocked with 2% bovine serum albumin in PBS for 1 hr and incubated with diluted primary antibody (ZO-1, 2 μg/ml) overnight at 4°C. For SM22α (Abcam) staining, cells were fixed with 2% paraformaldehyde (PFA) for 30 min at room temperature and permeabilized with PBS and 0.1% Triton X-100 for 5 min. Cells for SM22α staining were blocked with 5% horse serum, and incubated with primary antibody (SM22α, 1:200) overnight at 4°C. Primary antibody detection was with goat anti-rabbit cy3 (ZO-1) or donkey anti-goat cy3 (SM22α) secondary antibody (1:800; Jackson ImmunoResearch). Nuclei were stained with 4′, 6-diamidino-2-phenylinodole (DAPI; Sigma). Photomicrographs were captured with Nikon Eclipse E800 microscope and SPOT imaging software.
2.4 qRT-PCR
To determine changes in gene expression, we used qRT-PCR as previously described [19]. Total RNA was isolated using the TRIzol reagent (Invitrogen). cDNA was generated from 1 μg total RNA using oligo-dT primers and Superscript III polymerase (Invitrogen). Primer pairs are shown in the table below. Real-time PCR analysis was done with iQ SYBR green supermix (Bio-Rad) in the Bio-Rad iCycler for 40 cycles. The expression levels are calculated using the ΔΔCT method. The threshold cycle (CT) represents the PCR cycle at which an increase of the reporter fluorescence above the baseline is first detected. The fold change in expression levels, R, is calculated as follows: R=2− Δ ΔCT (where R = 2 (ΔCT treated-ΔCT control)) to normalize the abundance of all transcripts to the level of GAPDH RNA expression.
| Gene | Sense Primer (5′ → 3′) | Anti-Sense Primer (5′ → 3′) |
|---|---|---|
| GAPDH | ATGACAATGAATACGGCTACAG | TCTCTTGCTCAGTGTCCTTG |
| SmαA | GAGAAGCCCAGCCAGTCG | CTCTTGCTCTGGGCTTCA |
| Sm22α | AGCCAGTGAAGGTGCCTGAGAAC | TGCCCAAAGCCATTAGAGTCCTC |
| Calponin | GAAGGCAGGAACATCATTGGACTG | CTCAAAGATCTGCCGCTTGGTGCC |
| Snai1 | GTGAAGAGATACCAGTGCCAG | AAGATGCCAGCGAGGATG |
| SKIL | GAAATGCACCTGTGACTCAAC | TTCATCTTGGAGTTCCTGCC |
| FXYD5 | TCCTACATTGAACATCCACTGG | GACAACTGCCTACACTTCCC |
| Flk1 | TGCGGGCTCCTGACTACACTA | TTCCCAAATGCTCCACCAACTCTG |
| Tie2 | TTGGATTGTCACGAGGTCAAGAAG | CAATACACCATAGGACCAGACATCAC |
| VE Cadherin | CAGCACTTCAGGCAAAAACA | TTCTGGTTTTCTGGCAGCTT |
| Cx43 | TTCCTTTGACTTCAGCCTCC | CGTGGAGTAGGCTTGGAC |
| Nkx2.5 | AAGTGCTCTCCTGCTTTCC | CGTCTCGGCTTTGTCCAG |
| cTnT | AGGAGCTGATTTCCCTCAAAG | TTTCCTTCTCCCGCTCATTG |
| GIPC1 | TTGGATTGTCACGAGGTCAAGAAG | CAATACACCATAGGACCAGACATCAC |
| ALK2 | AGAGGGTCGATATTTGGGC | AACTTGGGTCATTGGGAAC |
| ALK3 | ACCATTTCCAGCCCTACA | TCACTGGGCACCATGTT |
| ALK5 | CCTTCTGATCCATCGGTTGA | CCATTGGCATACCAGCAT |
| Smad4 | CGTGGCAGGGAACATC | GCCCTTCACAAAGCTCAT |
2.5 Measurement of lacZ Staining and Activity
Immortalized E11.5 Sm22α-lacZ::Immorto epicardial cells were assayed as previously described [23] with the Galacto-Light Plus system (Applied Biosystems). Luminescence was quantified on a Turner 20/20 Luminometer.
2.6 Western Blot
Tgfbr3+/+ epicardial cells were incubated with vehicle, TGFβ1, TGFβ2, or BMP2 for 72 h prior to Western blot analysis. Cells were lysed and diluted in NP-40 lysis buffer (20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 20mM NaF). Total cellular lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a ployvinylidene diflouride (PVDF) membrane. After blocking in 5% non-fat milk, membranes were incubated with primary antibody (SM22α (abcam – ab10135 goat polyclonal to SM22 alpha); 0.5 μg/mL) diluted in 1% blocking solution for 1hr at 4°C. After 3 washes for 10 min each in TBST, the blot was incubated with the secondary antibody (abcam – ab6885 donkey polyclonal to goat IgG – H&L (HRP conjugated) for one hour. Beta tubulin (sigma T7816; 1:10000) was used as a loading control. Following 3 additional washes, detection was performed using ECL Western lightning chemiluminescent reagent (Perkin Elmer).
2.7 Transfections and Adenovirus Infections
Plasmids
Cells were plated at a density of 50,000 per well of a 6-well plate in immorto media without pen/strep and allowed to adhere overnight at 33°C. The cells were transfected the following day with 2 μg of pAdTrackCMV vector alone or expressing constitutively active (ca) ALK2, caALK3, or caALK5 and 3 μl of X-treme gene DNA transfection reagent (Roche). The media was changed 24 h after transfection to 5% FBS/DMEM and cells were incubated at 37°C. 48 h after transfection, cells were fixed for immunohistochemistry, harvested for qRT-PCR analysis, or used for invasion assays.
siRNA
Cells were plated at a density of 200,000 per well of a 6-well plate in immorto media without pen/strep and allowed to adhere overnight at 33°C. The following day the cells were transfected with 2 μg siRNA (Ambion) and 8 μl Xtreme siRNA transfection reagent (Roche). The media was changed to 5% FBS/DMEM 16 h after transfection and cells were incubated at 37°C. 24 h later cells were harvested for qRT-PCR analysis to confirm knockdown, or used directly for invasion assays. Where overexpression of TGFβR3 was done in conjunction with knockdown of GIPC1, cells were transfected with siRNA, then infected with adenovirus 24 h after, and plated for invasion assays 24 h after that.
| siRNA | Sense (5′ → 3′) | Anti-Sense (5′ → 3′) |
|---|---|---|
| Scramble | CUCCTUGTCAATUUACCGCTT | AAGCGGTAAATTGACAAGGAG |
| ALK2-a | GAGACGGAAUUGUACAACATT | UGUUGUACAAUUCCGUCUCCC |
| ALK2-b | GAGGCAUGAAAAUAUCUUATT | UAAGAUAUUUUCAUGCCUCAA |
| ALK3-a | GGCUCGUCGUUGUAUUACATT | UGUAAUACAACGACGAGCCAT |
| ALK3-b | GGCUGACAUCUAUAGCUUUTT | AAAGCUAUAGAUGUCAGCCAT |
| ALK5-a | CAAACGCGCUGACAUCUAUTT | AUAGAUGUCAGCGCGUUUGAA |
| ALK5-b | GAGUAGGCACUAAAAGGUATT | UACCUUUUAGUGCCUACUCTG |
| GIPC1-a | GCAGUGUGAUUGACCACAUTT | AUGUGGUCAAUCACACUGCCT |
| GIPC1-b | AGGACAAAAGGAACCCGGATT | UCCGGGUUCCUUUUGUCCUTT |
| Smad4-a | CGGCGAUUGUGCAUUCUCATT | UGAGAAUGCACAAUCGCCGGA |
| Smad4-b | GGAGAAGGUUUUGUAUAAATT | UUUAUACAAAACCUUCUCCCT |
Adenovirus
Adenoviruses were generated using the pAdEasy system [28]. Viruses were tittered by performing serial dilutions of the concentrated virus and counting the number of GFP-expressing HEK293 cells after 18–24 h. The following adenoviruses co-expressing GFP were used: full length TGFβR3 (FL), TGFβR3 lacking the cytoplasmic domain (CYTO), TGFβR3 lacking the last 3 amino acids (Δ3), Smad1, and Smad3. Cells were plated at a density of 200,000 per well in immorto media and allowed to adhere overnight at 33°C. The following day, virus was added directly to the cells at a final concentration of 108 PFU/ml. 24 h later, the cells were plated for invasion as described below.
2.8 Invasion Assay
Invasion assays were performed using a modified Boyden chamber assay as previously described [19]. Briefly, cells were fluorescently labeled with CalceinAM (BD Biosciences) and plated at 12,000 cells per well in 0.5% FBS/DMEM on collagen gels [29] in the top chamber. Cells were allowed to adhere overnight at 37°C. 24 h later, vehicle, growth factors, or growth factors plus inhibitors were added in 20% FBS/DMEM to the bottom chamber. In addition, cells also received inhibitor in the top well in 0.5% FBS/DMEM. After 24 h, the top insert was removed and placed in 0.25% Trypsin/2.21 mM-EDTA in HBSS (CellGro). Cells that invaded the collagen and crossed the membrane detached from the membrane into the trypsin containing plate, which was then read using SpectraMax 96-well plate reader (Ex: 485, Em: 538, Cutoff: 530; sensitivity: 30).
2.9 Proliferation Assay
Proliferation was scored using a MTS assay as previously described [24], which relies on the in vivo reduction of MTS tetrazolium to a colored formazan product by NADPH in metabolically active cells. The product formed is read at 490 nm and is directly proportional to the number of living cells in culture. Briefly, cells were plated at a density of 5,000 cells/well in 100 μl of 10 % FBS/DMEM in triplicate in a 96 well plate and incubated at 37°C. After 48 h, 20 μl of substrate (Promega: Cell Titer 96 Aqueous Solution) was added to each well and incubated for 30 min at 37°C. Absorbance of the colorimetric reaction was read at 490 nm using SpectraMax 96-well plate reader.
2.10 Statistical Analysis
Paired students t-test was performed to establish significance. Data are presented as the mean of three experiments ± SEM for one littermate pair, unless otherwise specified. P-values of <0.05 were considered significant.
3.0 Results
3.1 BMP2 induces loss of epithelial character but not smooth muscle differentiation
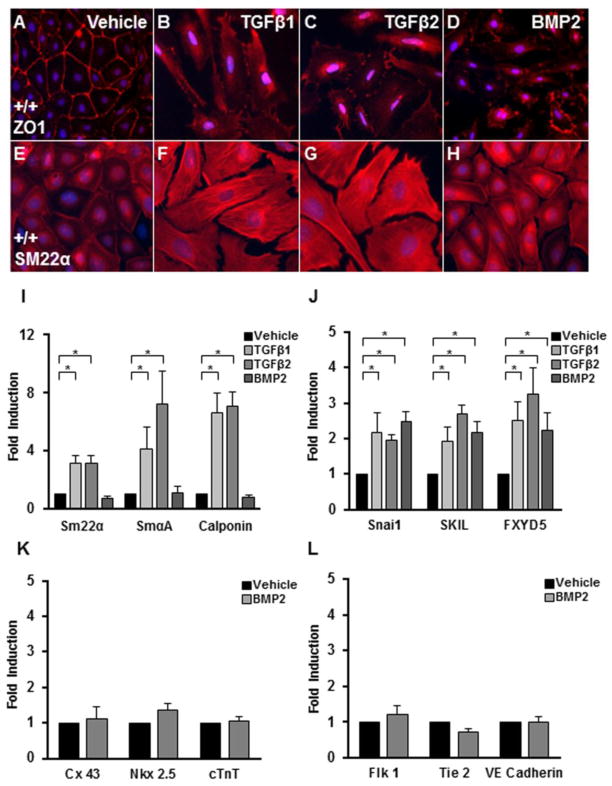
We have previously shown that TGFβ1 or TGFβ2 induces loss of epithelial character and smooth muscle differentiation in epicardial cells [23]. The loss of TGFβR3 does not alter these responses to ligand but does result in decreased invasion in response to ligand [19]. Since TGFβR3 also binds BMP2 [22], and BMP2 has been reported to induce the migration of proepicardial cells [30] we characterized BMP2 responsiveness in Tgfbr3+/+ epicardial cells. The addition of 5 nM BMP2 induced redistribution of ZO-1 (Fig. 1A,D) but failed to increase SM22α (Fig. 1 E,H) while the addition of 250 pM TGFβ1 or TGFβ2 both induced redistribution of ZO1 and increased SM22α (Fig. 1A–C & E–G). We examined the expression by qRT-PCR of additional markers associated with smooth muscle differentiation and the loss of epithelial character to confirm the effects of BMP2. To confirm that BMP2 does not induce smooth muscle differentiation, the smooth muscle markers SMα-actin and calponin, in addition to SM22α, were examined. TGFβ1 or TGFβ2 but not BMP2 significantly induced the expression of SM22α, SMα-actin, and calponin (Fig. 1I). To confirm the loss of epithelial cell character we examined the expression of Snai1 [31–33], SKIL [34, 35], and FXYD5 [36, 37]. The expression of each of these markers was increased by 24 hours after the addition of BMP2 (Fig. 1J). These data demonstrate that although BMP2 induced loss of epithelial cell character, unlike TGFβ1 or TGFβ2, BMP2 does not induce smooth muscle differentiation. Given that epicardial cells have been suggested to contribute to other lineages in the heart [38–42], we examined the endothelial markers Flk 1, Tie 2, and VE Cadherin as well as the myocardial markers Nkx2.5, Connexin 43, and cardiac troponin T(cTnT) by qRT-PCR. The addition of BMP2 did not alter the expression of any markers associated with the endothelial or myocardial lineages (Fig. 1K,L). These data demonstrate that BMP2 induced loss of epithelial cell character but not smooth muscle, endothelial, or myocardial differentiation.
Fig. 1. TGFβ and BMP2 have distinct effects on epicardial cells.
Immortalized epicardial cells (E11.5) were incubated with vehicle, 250 pM TGFβ1, 250 pM TGFβ2, or 5000 pM BMP2 for 24 or 72 h. A–H: Immunohistochemistry: epicardial cells were stained with an epithelial marker, zonula occludens-1 (ZO-1), or smooth muscle marker, smooth muscle 22 alpha (SM22α). Cells incubated for 72 h with vehicle formed a compact monolayer and displayed a round epithelial phenotype (A). Cells incubated with TGFβ1 (B), TGFβ2 (C) or BMP2 (D) for 72 h are elongated and separated from one another and display a redistribution of ZO-1 expression. Cells incubated with vehicle (E) lacked SM22α expression. Cells incubated with TGFβ1 (F), or TGFβ2 (G) are elongated and separated from one another and express SM22α in organized fibers consistent with a smooth muscle phenotype. Cells incubated with BMP2 (H) are elongate with no up-regulation of SM22α expression (compared to vehicle). (n=3, results shown for one littermate pair) I–L: qRT-PCR: TGFβ1 and TGFβ2, but not BMP2, increase the expression of SM22α, SMαA, and calponin after 72 h (I). TGFβ1, TGFβ2, and BMP2 induce expression of mesenchymal markers (Snail, SKIL, and FXYD5) after 24 h (J). BMP2 did not induce expression of endothelial (K) or myocardial (L) markers after 72 h. Columns, median fold change obtained from 5 separate experiments; bars, SEM. *=P<0.05.
3.2 BMP2 can partially inhibit smooth muscle differentiation
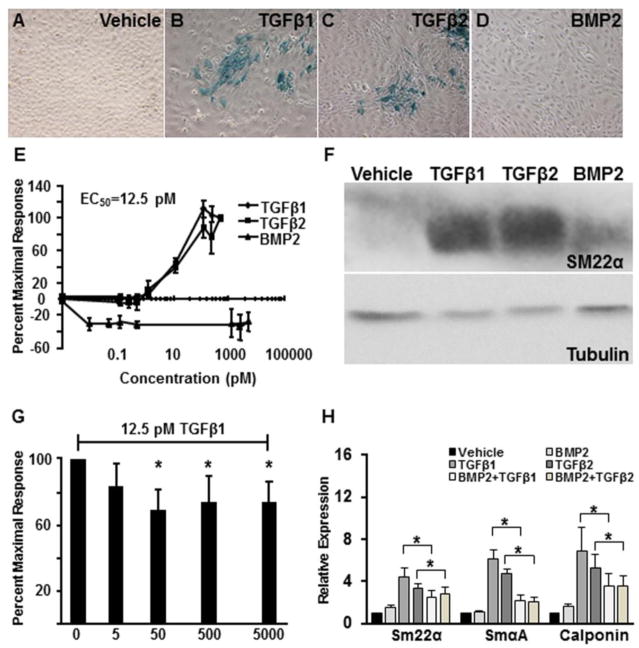
To further elucidate the actions of BMP2, we next took advantage of Tgfbr3+/+ epicardial cells isolated from SM22α-lacZ mice. The addition of TGFβ1 or TGFβ2, but not BMP2, induces lacZ expression (Fig. 2A–D) with an EC50 of 12.5 pM (Fig. 2E). BMP2 at concentrations from 0.1 to 5000 pM failed to induce lacZ expression (Fig. 2E). Similarly, Western blot analysis revealed significantly increased expression of SM22α protein in response to 250 pM TGFβ1 or TGFβ2 but not 5000 pM BMP2 (Fig. 2F). The concentration response data suggested that BMP2 inhibits SM22α expression. To address this question, cells with the SM22α-lacZ transgene were preincubated with either vehicle, 5, 50, 500, or 5000 pM BMP2. After 1 hour 12.5 pM TGFβ1 or TGFβ2 was added and the cells assayed after an additional 72 hours of incubation. Cells preincubated with concentrations of BMP2 of 50 pM and greater showed an approximate 35% decrease in lacZ expression (Fig. 2G). The inhibition of TGFβ-mediated smooth muscle differentiation by BMP2 was confirmed when the smooth muscle markers SMα-actin and calponin as well as SM22α were examined by qRT-PCR. In Tgfbr3+/+ cells, BMP2 significantly inhibited the expression of SM22α, SMα-actin, and calponin in response to TGFβ1 (45%, 64%, and 48% respectively) or TGFβ2 (28%, 57%, 34% respectively) (Fig. 2H). These data demonstrate that BMP2 can partially inhibit TGFβ-mediated smooth muscle differentiation in epicardial cells.
Fig. 2. TGFβ1 or TGFβ2, but not BMP2, induces smooth muscle 22 alpha (SM22α) expression.
SM22α-lacZ::Immortalized epicardial cells (E11.5) were incubated with vehicle, TGFβ1 (250 pM), TGFβ2 (250 pM), or BMP2 (5000 pM) for 72 h and assayed for lacZ activity. A–D: Cells incubated with vehicle retained an epithelial phenotype and did not express lacZ (A). Cells incubated with TGFβ1 (B) and TGFβ2 (C) elongated and expressed lacZ. Cells incubated with BMP2 (D) are elongate but did not express lacZ. (n=3, results shown for one littermate pair) E: Dose response: cells were incubated with vehicle or the following concentrations of ligand: TGFβ1 or TGFβ2 - 0.125, 0.25, 0.5, 1.25, 12.5, 125, 250, and 500 pM; BMP2 - 0.01, 0.05, 0.125, 0.25, 0.5, 1250, 2500, and 5000 pM. Cells incubated with increasing concentrations of TGFβ1 (squares) or TGFβ2 (triangles) show an increase in lacZ expression. There was no up-regulation of lacZ expression at any concentration of BMP2 (diamonds). (n=3) F: Immortalized epicardial cells (E11.5) were incubated with vehicle, 250 pM TGFβ1, 250 pM TGFβ2, or 5000 pM BMP2 for 72 h prior to harvest, SDS-PAGE, and immunoblot analysis for SM22α. TGFβ1 and TGFβ2 increased SM22α expression while BMP2, did not. β-tubulin was used as a loading control. Chicken gizzard was used as a positive control (not shown). (n=3, results shown for one littermate pair) G: Immortalized epicardial cells (E11.5) carrying the SM22α-lacZ transgene were preincubated with either vehicle, 5, 50, 500, or 5000 pM BMP2. After I h 12.5 pM TGFβ1 or TGFβ2 was added and the cells assayed after an additional 72 h of incubation. Cells preincubated with concentrations of BMP2 of 50 pM and greater showed an approximate 35% decrease in lacZ expression (n=3, *=p<0.05) (G). Immortalized epicardial cells (E11.5) were preincubated with either vehicle or BMP2 (5000 pM) for 1 h followed by incubation with either vehicle, TGFβ1 (250pM), TGFβ2 (250pM), BMP2 (5000pM), TGFβ1+BMP2, or TGFβ2+BMP2 for 72 h prior to qRT-PCR. Pre and Co-incubation of BMP2 significantly inhibited the expression of SM22α, SMα-actin, and calponin in response to TGFβ1 (45 %, 64 %, and 48 % respectively) or TGFβ2 (28 %, 57 %, 34 % respectively). Columns, median fold change obtained from 5 separate experiments; bars, SE. *=p< 0.05.
3.3 TGFβR3 is not required for loss of epithelial character to TGFβ1, TGFβ2, or BMP2
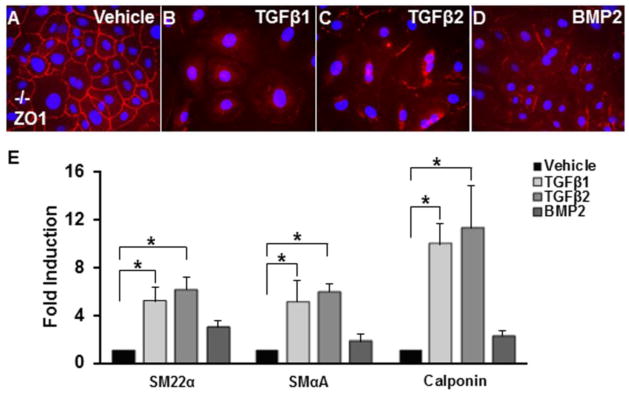
Given that TGFβR3 binds TGFβ1, TGFβ2, and BMP2 we used Tgfbr3−/− epicardial cells to determine if TGFβR3 was required for the actions of these ligands. Immunostaining revealed that Tgfbr3−/− cells have abundant ZO-1 at the cell borders (Fig. 3A). The addition of 250 pM TGFβ1 or TGFβ2 resulted in a significant loss of ZO-1 from cell borders (Fig. 3A–C) and expression of the smooth muscle markers SM22α, SMα-actin, and calponin (Fig. 3E) as previously reported [19]. However, the addition of 5 nM BMP2 to Tgfbr3−/− cells resulted in the loss of ZO-1 from cell borders (Fig. 3D) without induction of SM22α, SMα-actin, or calponin (Fig. 3F). These data demonstrate that TGFβR3 is not required for the BMP2-stimulated loss of epithelial character in epicardial cells.
Fig. 3. Tgfbr3−/− epicardial cells loose epithelial character in response to BMP2.
Tgfbr3−/− immortalized epicardial cells (E11.5) were incubated with vehicle, 250 pM TGFβ1, 250 pM TGFβ2, or 5000 pM BMP2 for 72h. A–D: Immunohistochemistry: Tgfbr3−/− cells were stained with an epithelial marker, zonula occludens-1 (ZO-1). Cells incubated with vehicle express ZO-1 along the cell-cell borders consistent with an epithelial phenotype (A). Cells incubated with TGFβ1 (B), TGFβ2 (C) or BMP2 (D) are elongated and have decreased cell-cell contacts and redistributed ZO-1 expression. (n=3, results shown for one littermate pair). TGFβ1 and TGFβ2, but not BMP2, increase the expression of SM22α, SMαA, and calponin after 72 h as shown by qRT-PCR analysis (E). Columns, median fold change obtained from 5 separate experiments; bars, SEM. *=p<0.05.
3.4 BMP2 signals loss of epithelial character via ALK3
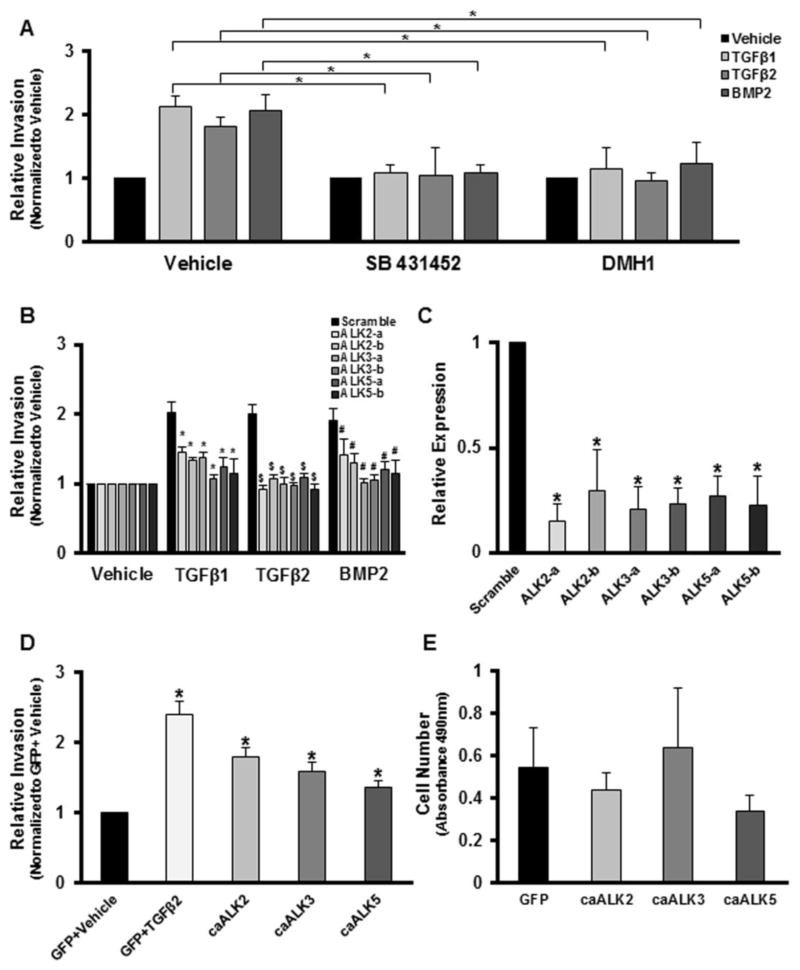
The data above suggests that TGFβ and BMP2 are signaling through their respective canonical pathways via ALK signaling [43] to mediate the loss of epithelial character. Since BMP2 may signal through several ALK’s, including ALK2, ALK3, or ALK6, we next addressed the relative role of each of these ALKs. Analysis of ALK expression in epicardial cells by RT-PCR revealed that ALKs 1–5 are abundantly expressed, ALK6 is barely detectable, and 7 is absent (Fig. 4A). RNAseq analysis confirmed abundant expression of ALKs 1–5 and the lack of ALK6 and ALK7 expression (data not shown). We have previously shown that both the loss of epithelial character and smooth muscle differentiation in response to 250 pM TGFβ1 or TGFβ2 in epicardial cells requires ALK5 kinase activity [23]. Here we sought to determine the ALKs required for the effects of BMP2 on epicardial cells. Consistent with prior results [23], the addition of 2.5 μM of the ALK5 kinase inhibitor SB431542 blocked the actions of TGFβ1 or TGFβ2 in Tgfbr3+/+ cells (Fig. 4D, F). However, SB431542 had no effect on the actions of BMP2 (Fig. 4H). DMH1 is a recently identified highly selective and potent BMP inhibitor with selectivity for ALK2 and ALK3 [44]. The addition of 2 μM DMH1 blocked BMP2 induced loss of epithelial character, but not the actions of TGFβ1 or TGFβ2 in Tgfbr3+/+ cells. Cells co-incubated with DMH1 and TGFβ1 or TGFβ2 following pre-incubation with DMH1 showed redistribution of ZO-1 staining characteristic of the loss of epithelial character (Fig. 4E, G) while cells co-incubated with DMH1 and BMP2 following pre-incubation with DMH1 retained ZO-1 at cell borders (Fig. 4I).
Fig. 4. Distinct ALK activity is required for the effects of TGFβ and BMP2.
RT-PCR analysis confirmed expression of ALK1, ALK2, ALK3, ALK4, and ALK5 in Tgfbr3+/+ and Tgfbr3−/− epicardial cells. (n=3, data shown for one littermate pair). Immortalized Tgfbr3+/+ epicardial cells (E11.5) were incubated with vehicle, 250 pM TGFβ1, 250 pM TGFβ2, or 5000 pM BMP2 in the presence or absence of 2.5 μM of the ALK5 kinase inhibitor SB431542 or 2 μM DMH1 (ALK2/3 kinase inhibitor) for 72 h prior to fixation. AH: Immunohistochemistry: Cells were stained with an epithelial marker, zonula occludens-1 (ZO-1). A–D: Cells incubated with vehicle and ALK5 inhibitor formed a compact monolayer and displayed an epithelial phenotype (A). Cells incubated with TGFβ1 (D), or TGFβ2 (F) in the presence of ALK5 inhibitor retained zonula occludens-1 (ZO-1) expression at cell-cell borders consistent with an epithelial phenotype. Cells incubated with BMP2 (H) in the presence of ALK5 inhibitor are elongated and separated from one another with a loss or decrease of ZO-1 expression. E–H: Cells incubated with vehicle and ALK2/3 inhibitor express ZO-1 along the cell-cell borders consistent with an epithelial phenotype (C). Cells incubated with TGFβ1 (E), or TGFβ2 (G), in the presence of ALK2/3 inhibitor are elongated and have decreased cell-cell contacts and ZO-1 expression. BMP2 and ALK2/3 inhibitor incubated cells (I) retained the cell-cell contacts and ZO-1 expression. (n=3, data shown for one littermate pair).
We next addressed the potential roles of ALK2, ALK3, and ALK5 by expressing constitutively active (ca) forms in epicardial cells. All 3 caALKs induced loss of epithelial character as determined by the redistribution of ZO-1 (Fig. 5A–E). Cells expressing caALKs were generally elongated and found to lie in a plane beneath the uninfected epithelial cells. However examination of smooth muscle markers by qRT-PCR reveals that only caALK5 and caALK2 induce smooth muscle differentiation while caALK3 does not (Fig. 5F). None of the caALK’s tested induced endothelial markers (Fig. 5G). Taken together, these data are consistent with ALK3 activation downstream of BMP2 being responsible for the loss of epithelial character in epicardial cells.
Fig. 5. ALKs are sufficient to induce loss of epithelial character or smooth muscle differentiation in epicardial cells.
Tgfbr3+/+ immortalized epicardial cells (E11.5) were transfected with plasmid encoding green fluorescent protein (GFP) alone or GFP and constitutively active (ca) ALK2, caALK3, and caALK5. A–D: Immunohistochemistry: Cells were stained with an epithelial marker, zonula occludens-1 (ZO-1). GFP alone expressing cells form an epithelial sheet and express ZO-1 at cell-cell borders (A). Cells expressing caALK2 (B), caALK3 (C), or caALK5 (D) appear elongated, separated from their neighbor, and have discontinuous ZO-1 expression. (n=3, results shown for one littermate pair) E: Quantification of the percent of cells that loose epithelial character after transfection with plasmids. Data are plotted as the mean percent ± SD of three independent experiments (*=p<0.05) F–G: qRT-PCR analysis of cells transfected with plasmids. Cells expressing caALK2 and caALK5, but not caALK3, undergo smooth muscle differentiation as shown by induction of SM22α, SMαA, and Calponin (F). Cells transfected with plasmids do not induce endothelial marker expression (G). Columns, mean fold change obtained from 5 separate experiments; bars, SD. *=p<0.05.
3.5 TGFβR3 and GIPC interaction is required for BMP2 induced invasion
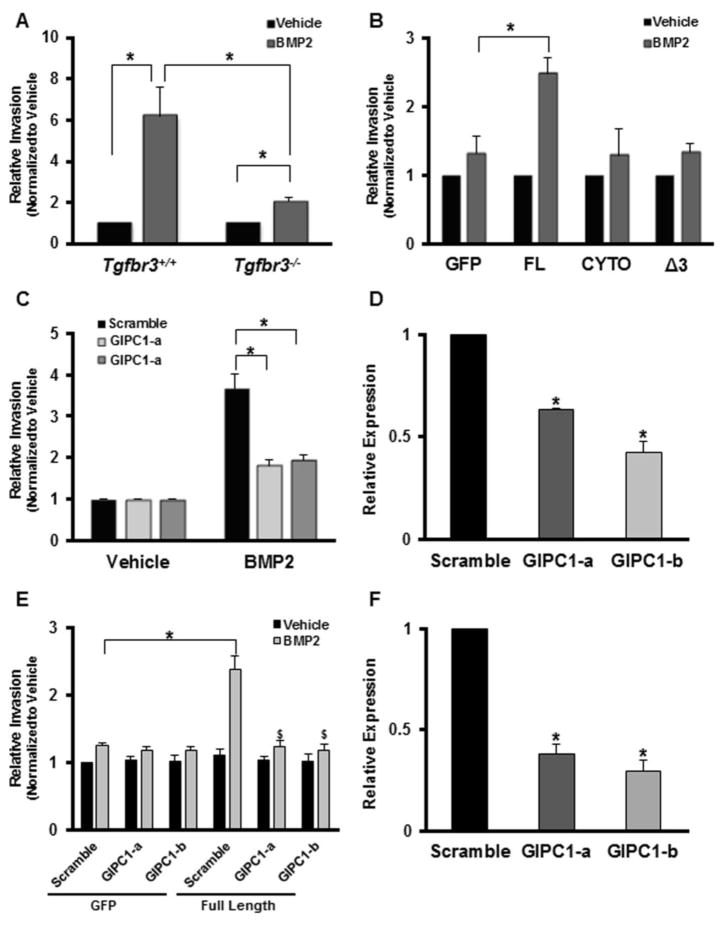
We recently reported that TGFβR3 is required for TGFβ-stimulated epicardial cell invasion in vitro and in vivo [19] and that BMP2 can access the Par6/Smurf1/RhoA pathway to mediate invasion [24]. Here we report that BMP2-mediated epicardial cell invasion requires TGFβR3 and interaction with GIPC. BMP2 stimulates the invasion of Tgfbr3+/+ epicardial cells while Tgfbr3−/− epicardial cells display decreased invasion in response to BMP2 (Fig. 6A). Overexpression of TGFβR3 (TGFβR3-FL) in Tgfbr3−/− epicardial cells rescues invasion in response to BMP2 (Fig. 6B). Consistent with results obtained for TGFβ [19], receptor constructs lacking the entire cytoplasmic domain (TGFβR3-CYTO) or the 3 C-terminal amino acids required for GIPC binding (TGFβR3-Δ3) failed to rescue invasion in Tgfbr3−/− cells (Fig. 6B). Targeting of GIPC by siRNA revealed that GIPC was required for BMP2-stimulated cell invasion in Tgfbr3+/+ cells (Fig. 6C) and in Tgfbr3−/− cells rescued with TGFβR3-FL (Fig. 6D). Knockdown of GIPC is confirmed in Fig. 6D and F, respectively. These data demonstrate that BMP2-stimulated invasion requires TGFβR3 interaction with GIPC.
Fig. 6. TGFβR3 interaction with GIPC is required for BMP2 induced epicardial cell invasion.
Quantification of invasion using a modified boyden chamber assay of Tgfbr3+/+ and Tgfbr3−/− immortalized epicardial cells (E11.5, littermate pairs). (A) Tgfbr3−/− epicardial cells have a reduced ability to invade in response to 5000 pM BMP2 when compared to Tgfbr3+/+ epicardial cells. (n=3, replicates of 6, *=p<0.05). (B) Tgfbr3−/− epicardial cells were infected with adenovirus co-expressing GFP and either FL, CYTO, or Δ3 receptor and incubated with 5000 pM BMP2. Cells expressing the full length receptor were restored in their ability to invade. (n=3, replicates of 6, *=p<0.05). (C) Tgfbr3+/+ epicardial cells were transfected with control siRNA or siRNA to GIPC1 and incubated with 5000 pM BMP2. Knockdown of GIPC results in reduced invasion potential. (n=3, replicates of 6, *=p<0.05). (D) Relative expression levels following transfection with siRNA targeting GIPC1 (n=3; *=p<0.05 [FL+Scramble+BMP2] vs. [GFP+Scramble+BMP2] $=p<0.05 [FL+Scramble+BMP2] vs. [FL+GIPCsiRNA+BMP2]). (E) Tgfbr3−/− cells infected with adenovirus expressing GFP or FL receptor, transfected with siRNA to GIPC1, and incubated with vehicle or 5000 pM BMP2. GIPC knockdown in the cells inhibited rescue of invasion by FL receptor. (n=3, replicates of 6, *=p<0.05). (F) Relative expression levels following transfection with siRNA targeting GIPC1 (n=3; *=p<0.05).
3.6 BMP2 induced invasion requires ALK activation and is Smad dependent
We have reported that TGFβR3-dependent invasion of endocardial cells requires ALK2, ALK3, and ALK5 while ALK4 and ALK6 are dispensable [25, 27]. To address the role of ALK signaling in TGFβR3-dependent invasion of epicardial cells we determined invasion in Tgfbr3+/+ cells incubated with 2.5 μM of the ALK5 kinase inhibitor SB431542 or 2 μM of the BMP inhibitor DMH1. Both inhibitors blocked invasion in response to 250 pM TGFβ1 or TGFβ2 or 5 nM BMP2 (Fig. 7A) consistent with our results in endocardial cells where multiple ALKs are required for invasion [25] but distinct from that seen for the loss of epithelial character in epicardial cells reported here. As a second, independent method to address the role of ALKs in mediating TGFβR3-dependent invasion we used siRNA to target ALK2, ALK3, or ALK5 in Tgfbr3+/+ cells. The targeting of either of these ALKs inhibited invasion to all ligands (Fig. 7B) which is compatible with our results using small molecule inhibitors. Knockdown was confirmed for each of 2 independent constructs used to target each ALK (Fig. 7C). However, the use of constitutively active forms of ALK2, ALK3, and ALK5 revealed that each was sufficient to increase epicardial cell invasion (Fig. 7D) without any effects on proliferation (Fig. 7E). These data suggest that although each of these constitutively active ALK’s is sufficient for invasion, the activity of the native forms of these receptors is coordinately regulated and interdependent when stimulated by ligand. This conclusion is also consistent with the ability of small molecule inhibitors of ALK5 (SB431452) or ALK2, ALK3 (DMH1) kinase activity to block invasion of Tgfbr3+/+ epicardial cells (Fig. 7A).
Fig. 7. ALK activity is required and sufficient for TGFβ and BMP2 induced invasion.
Invasion was analyzed as described in Fig. 6. (A) Tgfbr3+/+ immortalized epicardial cells (E11.5) were incubated with either vehicle, 2.5 μM of the ALK5 kinase inhibitor SB431542, or 2 μM DMH1 (ALK2/3 kinase inhibitor) and scored for invasion in response to vehicle, 250 pM TGFβ1, 250 pM TGFβ2, or 5000 pM BMP2. Inhibition of ALK2/3 or ALK5 activity results in the cells inability to invade in response to all ligands. (n=3, replicates of 6, *=p<0.05). (B) Tgfbr3+/+ immortalized epicardial cells (E11.5) were transfected with control siRNA or siRNA to ALK2, ALK3, or ALK5 and incubated with vehicle, 250 pM TGFβ1, 250 pM TGFβ2, or 5000 pM BMP2. Knockdown of any ALKs resulted in a decrease in invasion potential in response to all ligands. (n=3, replicates of 6, *=p<0.05 [Scramble+TGFβ1] vs. [ALKsiRNA+TGFβ1] $=p<0.05 [Scramble+TGFβ2] vs. [ALKsiRNA+TGFβ2] #=p<0.05 [Scramble+BMP2] vs. [ALKsiRNA+BMP2]). (C) Relative expression levels following transfection with siRNA targeting ALK2, ALK3, and ALK5 (n=3; *p=<0.05). (D) Tgfbr3−/− epicardial cells were transfected with plasmids expressing either green fluorescent protein alone (GFP) or GFP and constitutively active (ca) ALK2. caALK3, and caALK5 and scored for invasion. Overexpression of all constitutively active receptors was sufficient to rescue invasion. (n=3, replicates of 6, *=p<0.05). (E) Quantification of cell number using MTS assay in Tgfbr3+/+ cells transfected with plasmids coding for GFP, GFP + caALK2, GFP + caALK3, and GFP + caALK5. (n=3)
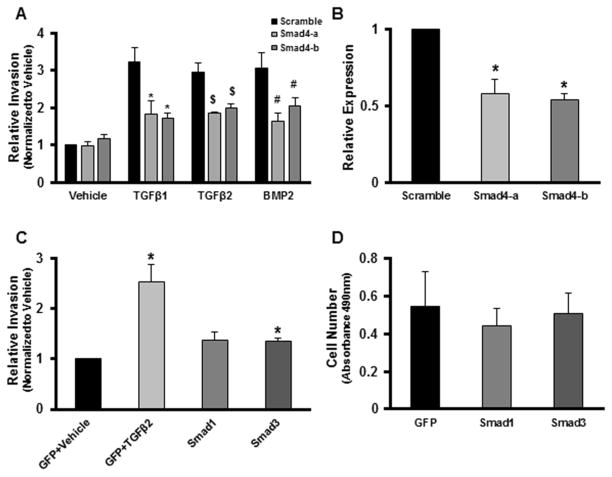
ALK activation results in stimulation of the Smad signaling pathway [43]. ALK5 activates Smad2 and 3 while ALK2 or ALK3 activates Smad1, 5, and 8. Each of these activated Smads binds to Smad4 and the complex is translocated to the nucleus to alter gene transcription [43]. To determine if Smads are required for epicardial cell invasion, we took advantage of the described role of Smad4 and used siRNA targeted to Smad4 to prevent the translocation of all Smads. Targeting of Smad4 significantly inhibited invasion in response to 5nM BMP2 demonstrating that invasion requires Smad signaling (Fig. 8A). Knockdown of Smad4 was confirmed for each of 2 independent siRNA constructs (Fig. 8B). We next investigated whether Smad activation is sufficient to induce EMT in epicardial cells. Since overexpression of Smads results in constitutive activity [25, 45] we chose to overexpress representative Smads downstream of specific ALKs, Smad3 (downstream of ALK5) or Smad1 (downstream of ALK2, ALK3), in Tgfbr3+/+ epicardial cells and score for invasion. Overexpression of Smad1 resulted in a marginally significant increase in epicardial cell invasion while Smad3 overexpression had no effect on invasion (Fig. 8C). Neither Smad altered proliferation (Fig. 8D). Overexpression of Smad1 or Smad3 in Tgfbr3−/− cells failed to alter invasion (data not shown). These data demonstrate that Smad activation alone is not sufficient to induce epicardial cell invasion.
Fig. 8. Smads are required, but not sufficient for invasion of epicardial cells.
Invasion was analyzed as described in Fig. 6. (A) Tgfbr3+/+ immortalized epicardial cells (E11.5) were transfected with control siRNA or siRNA to Smad4 and incubated with vehicle, 250 pM TGFβ1, 250 pM TGFβ2, or 5000 pM BMP2. Knockdown of Smad4 resulted in a decrease in invasion potential in response to all ligands. (n=3, replicates of 6, *=p<0.05 [Scramble+TGFβ1] vs. [Smad4siRNA+TGFβ1] $=p<0.05 [Scramble+TGFβ2] vs. [Smad4siRNA+TGFβ2] #=p<0.05 [Scramble+BMP2] vs. [Smad4siRNA+BMP2]).). (B) Relative expression levels following transfection with siRNA targeting Smad4 (n=3; *p=<0.05). (C) Tgfbr3−/− epicardial cells were infected with adenovirus expressing either GFP alone, GFP + Smad1, or GFP + Smad3 and scored for invasion. Overexpression of Smad1 was not sufficient to rescue invasion, whereas overexpression of Smad3 was barely significantly different from control infected cells. (n=3, replicates of 6, *=p<0.05). (D) Quantification of cell number using MTS assay in Tgfbr3+/+ cells infected with adenovirus expressing either GFP alone, GFP + Smad1, or GFP + Smad3. (n=3)
4.0 Discussion
Our experiments reveal that BMP2 induces the loss of epithelial character in epicardial cells but, unlike TGFβ, BMP2 does not induce smooth muscle differentiation of epicardial cells. The use of small molecule inhibitors and constitutively active ALKs suggest that BMP2-stimulates loss of epicardial cell character via activation of ALK3 while TGFβ-stimulated loss of epithelial character and smooth muscle differentiation requires ALK5. These actions of BMP2 or TGFβ do not require TGFβR3. However, BMP2- or TGFβ-stimulated invasion of epicardial cells does require TGFβR3 (Fig. 9). These results and the observation that Tgfbr3−/− embryos have failed coronary vessel development [18] associated with decreased invasion of epicardial cells into the subepicardial space and myocardium [19] suggests that the loss of BMP2 responsiveness, as well as TGFβ responsiveness, may underlie the defects seen in Tgfbr3−/− mice. Recently BMP2 has been identified as an important signal in directing the migration of proepicardial cells to the myocardium [30]. Analysis of Tgfbr3−/− mice did not reveal any defects in proepicardial cell migration to the myocardium [18] suggesting that proepicardial cell migration is independent of TGFβR3. However, our observation that BMP2 at least partially inhibits TGFβ-stimulated smooth muscle differentiation suggests that this may be a mechanism by which BMP2 attenuates responsiveness to TGFβ-induced differentiation so that cells may remain motile while interacting with the myocardium.
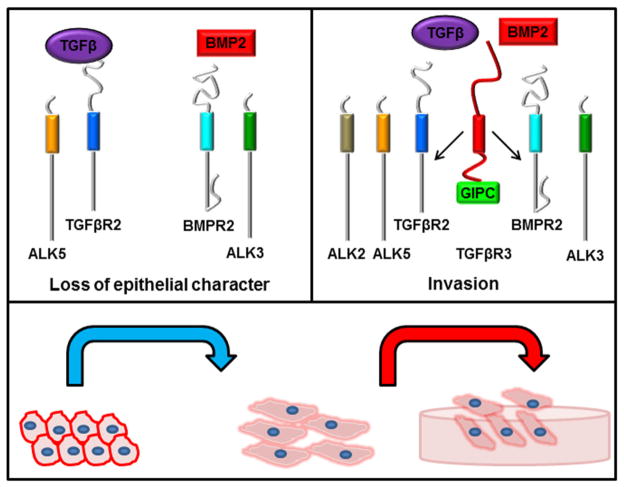
Fig. 9. Canonical and non-canonical BMP2 signaling regulates epicardial cell behavior.
The loss of epithelial character in response to TGFβ or BMP2 is mediated by canonical signaling via ALK5 or ALK3, respectively. Both TGFβ- and BMP2-stimulated invasion requires TGFβR3 and contribution from ALK5, ALK2, and ALK3 signaling. We propose that TGFβR3, through interaction with GIPC, is required for the coordinate activation of these ALKs in order to mediate invasion.
Invasion stimulated by BMP2 is dependent upon the presence of the cytoplasmic domain of TGFβR3, specifically the 3 C-terminal amino acids that are required for interaction with the scaffolding protein GIPC. The finding that siRNAs targeting GIPC inhibit invasion stimulated by BMP2 in Tgfbr3+/+ cells, and that siRNA to GIPC blocks rescue by TGFβR3 in Tgfbr3−/− cells, confirms that interaction of GIPC with TGFβR3 is critical to invasion. We have previously shown that TGFβ-stimulated, TGFβR3-mediated invasion of epicardial cells [19], as well as TGFβ-stimulated, TGFβR3-mediated endocardial cell transformation [27], is dependent on GIPC interaction with TGFβR3. However, the role of TGFβR3/GIPC in regulating cell behavior is not clearly understood. In cancer cell lines the regulation of proliferation, migration, and adhesion is regulated by TGFβR3 [46]. In contrast to results in endocardial cell transformation [25, 27, 47] or epicardial cell invasion described here that implicates TGFβR3 in mediating invasion, TGFβR3 has been shown to inhibit cell proliferation, migration, and adhesion in myeloma cells [48] and suppress breast cancer progression through GIPC-mediated inhibition of TGFβ signaling [49]. TGFβR3 expression is reported to be decreased in human breast, ovarian, pancreatic, prostate, and non-small lung cell cancers [50–54]. Although TGFβR3 regulation of Cdc42 has been shown to regulate cancer cell motility [55], Cdc42 does not play a role in regulating endocardial cell [26] or epicardial cell migration or invasion [24]. Overall these data demonstrate that TGFβR3 and GIPC have critical roles in development and disease but also underscore our lack of understanding of the mechanisms that regulate context- and cell-specific responses.
Our finding that several ALKs are required for TGFβR3-dependent epicardial cell invasion is consistent with prior findings for TGFβR3-dependent endocardial cell transformation where ALK2, ALK3, and ALK5 are all required [27]. Epicardial cell invasion also requires ALK2, ALK3, and ALK5. However, whereas ALK2, ALK3, and ALK5 are sufficient for invasion in epicardial cells, in endocardial cells ALK2 and ALK3 are sufficient for transformation and ALK5 is not [56, 57]. A requirement for ALK5 in endocardial and epicardial cells is consistent with the described role of ALK5 in activating the Par6/Smurf1/RhoA pathway that aids in the maintenance of epithelial cell polarity [58]. In addition, ALK5 is also required for the full activation of ALK1 downstream of endoglin in endothelial cells [59]. Endoglin, although unable to bind TGFβ directly, functions as a co-receptor for TGFβ in the presence of TGFβR2 [60]. The cytoplasmic domain of endoglin is identical to that of TGFβR3 [20, 61] and endoglin has been shown to regulate the activation of ALK5 and ALK1 to regulate endothelial cell proliferation and migration [62, 63]. ALK1 downstream of endoglin is not fully active without ALK5 activity [59] demonstrating the interdependence of ALK activity in response to ligand. Therefore, although several lines of evidence support roles for ALK5 in regulating transformation or cell invasion, the mechanisms that underlie the lack of sufficiency in endocardial cells or sufficiency in epicardial cells is not understood. In both endocardial [25, 64] and epicardial cells TGFβR3-dependent invasion is Smad-dependent. In endocardial cells this dependency has been shown both by the targeting of the common mediator Smad, Smad4 [25, 64], and using siRNA to target individual receptor Smads [25], Smads1, 2, 3, and 5, each of which were shown to be required for endocardial cell transformation. The requirement of multiple Smads that are known to be downstream of several ALKs is consistent with the finding that numerous ALKs are also required. However, the overexpression of Smad1 or Smad3 in endocardial cells, Smads primarily associated with the BMP or TGFβ pathways respectively, failed to induce transformation. Consistent with these data, experiments reported here demonstrate that Smad overexpression is not sufficient to drive epicardial cell invasion. Taken together these data suggest that cell transformation or invasion requires the coordinated activation of ALKs and Smads associated with both the canonical TGFβ and BMP signaling pathways, and this coordinated activation is dependent on TGFβR3 (Fig. 9)
Recent findings have revealed that BMP2 can signal via TGFβR3 which has highlighted the importance of determining the relative contributions of the canonical BMP signaling pathway and TGFβR3-dependent signaling in mediating BMP2 responsiveness. Our results reveal the relative roles of TGFβR3-dependent and TGFβR3-independent signaling in the actions of BMP2 on epicardial cell behavior. TGFβR3 is required for BMP2-stimulated epicardial cell invasion in vitro which suggests that the decreased epicardial cell invasion seen in Tgfbr3−/− mice [18] may be at least partially due to loss of BMP2 responsiveness.
Highlights.
BMP2 signals the ALK3-dependent loss of epithelial character in epicardial cells
BMP2-stimulated invasion is dependent on TGFβR3 interaction with GIPC
Invasion also requires activation of ALK2, ALK3, ALK5, and Smad4
Acknowledgments
The authors thank members of the Barnett laboratory for helpful discussions and comments and George Davis for adenoviral constructs. Overall project support by National Institutes of Health, HL085708. N.S.S. and J.D.L. were supported by supplements to HL085708. J.A.A. was supported by R25 HL96223 short term training for minority students. J.V.B. acknowledges the support of the Vanderbilt-Ingram Cancer Center.
Abbreviations
- ALK
Activin Receptor-Like Kinases
- BMP
Bone Morphogenetic Protein
- Cdc42
Cell division control protein 42 homolog
- EMT
Epithelial-Mesenchymal Transformation
- GFP
Green Fluorescent Protein
- GIPC
GAIP-interacting protein, C terminus
- Par6
Par-6 partitioning defective 6 homolog gamma
- RhoA
Ras homolog gene family member A
- Smurf1
Smad ubiquitination regulatory factor1
- TGFβ
Transforming Growth Factor Beta
- TGFβR1
Type I TGFβ receptor
- TGFβR2
Type II TGFβ receptor
- TGFβR3
Type III TGFβ receptor
- TGFβR3-FL
Type III TGFβ receptor-full length
- TGFβR3-CYTO
Type III TGFβ receptor-lacking the entire cytoplasmic domain
- TGFβR3-Δ3
Type III TGFβ receptor-lacking the 3 C-terminal amino acids
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Cynthia R. Hill, Email: cynthia.r.allison@vanderbilt.edu.
Nora S. Sanchez, Email: nora.s.sanchez@vanderbilt.edu.
Joseph D. Love, Email: joey.love@vanderbilt.edu.
Julian A. Arrieta, Email: julian.a.arrieta@vanderbilt.edu.
Charles C. Hong, Email: charles.c.hong@vanderbilt.edu.
Christopher B. Brown, Email: chris.brown@vanderbilt.edu.
Anita F. Austin, Email: aaustin@mmc.edu.
Joey V. Barnett, Email: joey.barnett@vanderbilt.edu.
References
- 1.Wendt MK, Tian M, Schiemann WP. Deconstructing the mechanisms and consequences of TGF-beta-induced EMT during cancer progression. Cell Tissue Res. 2011 doi: 10.1007/s00441-011-1199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur HM, Bamforth SD. TGFbeta signaling and congenital heart disease: Insights from mouse studies. Birth Defects Res A Clin Mol Teratol. 2011;91(6):423–34. doi: 10.1002/bdra.20794. [DOI] [PubMed] [Google Scholar]
- 3.Hawinkels LJ, Ten Dijke P. Exploring anti-TGF-beta therapies in cancer and fibrosis. Growth Factors. 2011;29(4):140–52. doi: 10.3109/08977194.2011.595411. [DOI] [PubMed] [Google Scholar]
- 4.Azhar M, et al. Transforming growth factor beta in cardiovascular development and function. Cytokine Growth Factor Rev. 2003;14(5):391–407. doi: 10.1016/s1359-6101(03)00044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santibanez JF, Quintanilla M, Bernabeu C. TGF-beta/TGF-beta receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2011;121(6):233–51. doi: 10.1042/CS20110086. [DOI] [PubMed] [Google Scholar]
- 6.DeLaughter DM, et al. What chick and mouse models have taught us about the role of the endocardium in congenital heart disease. Birth Defects Res A Clin Mol Teratol. 2011;91(6):511–25. doi: 10.1002/bdra.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnett JV, Desgrosellier JS. Early events in valvulogenesis: a signaling perspective. Birth Defects Res C Embryo Today. 2003;69(1):58–72. doi: 10.1002/bdrc.10006. [DOI] [PubMed] [Google Scholar]
- 8.Olivey HE, Compton LA, Barnett JV. Coronary vessel development: the epicardium delivers. Trends Cardiovasc Med. 2004;14(6):247–51. doi: 10.1016/j.tcm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Olivey HE, Svensson EC. Epicardial-myocardial signaling directing coronary vasculogenesis. Circ Res. 2010;106(5):818–32. doi: 10.1161/CIRCRESAHA.109.209197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manner J. Experimental study on the formation of the epicardium in chick embryos. Anat Embryol (Berl) 1993;187(3):281–9. doi: 10.1007/BF00195766. [DOI] [PubMed] [Google Scholar]
- 11.Tomanek RJ. Formation of the coronary vasculature during development. Angiogenesis. 2005;8(3):273–84. doi: 10.1007/s10456-005-9014-9. [DOI] [PubMed] [Google Scholar]
- 12.Viragh S, Challice CE. The origin of the epicardium and the embryonic myocardial circulation in the mouse. Anat Rec. 1981;201(1):157–68. doi: 10.1002/ar.1092010117. [DOI] [PubMed] [Google Scholar]
- 13.Dettman RW, et al. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol. 1998;193(2):169–81. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- 14.Mikawa T, et al. Clonal analysis of cardiac morphogenesis in the chicken embryo using a replication-defective retrovirus: I. Formation of the ventricular myocardium. Dev Dyn. 1992;193(1):11–23. doi: 10.1002/aja.1001930104. [DOI] [PubMed] [Google Scholar]
- 15.Poelmann RE, et al. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ Res. 1993;73(3):559–68. doi: 10.1161/01.res.73.3.559. [DOI] [PubMed] [Google Scholar]
- 16.Pardali E, Goumans MJ, ten Dijke P. Signaling by members of the TGF-beta family in vascular morphogenesis and disease. Trends Cell Biol. 2010;20(9):556–67. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580(12):2811–20. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Compton LA, et al. Coronary vessel development is dependent on the type III transforming growth factor beta receptor. Circ Res. 2007;101(8):784–91. doi: 10.1161/CIRCRESAHA.107.152082. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez NS, et al. The cytoplasmic domain of TGFbetaR3 through its interaction with the scaffolding protein, GIPC, directs epicardial cell behavior. Dev Biol. 2011;358(2):331–43. doi: 10.1016/j.ydbio.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Casillas F, et al. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991;67(4):785–95. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- 21.Wiater E, et al. Identification of distinct inhibin and transforming growth factor beta-binding sites on betaglycan: functional separation of betaglycan co-receptor actions. J Biol Chem. 2006;281(25):17011–22. doi: 10.1074/jbc.M601459200. [DOI] [PubMed] [Google Scholar]
- 22.Kirkbride KC, et al. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J Biol Chem. 2008;283(12):7628–37. doi: 10.1074/jbc.M704883200. [DOI] [PubMed] [Google Scholar]
- 23.Austin AF, et al. Primary and immortalized mouse epicardial cells undergo differentiation in response to TGFbeta. Dev Dyn. 2008;237(2):366–76. doi: 10.1002/dvdy.21421. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez NS, Barnett JV. TGFbeta and BMP-2 regulate epicardial cell invasion via TGFbetaR3 activation of the Par6/Smurf1/RhoA pathway. Cell Signal. 2011 doi: 10.1016/j.cellsig.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Townsend TA, et al. BMP-2 and TGFbeta2 shared pathways regulate endocardial cell transformation. Cells Tissues Organs. 2011;194(1):1–12. doi: 10.1159/000322035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Townsend TA, et al. Transforming growth factor-beta-stimulated endocardial cell transformation is dependent on Par6c regulation of RhoA. J Biol Chem. 2008;283(20):13834–41. doi: 10.1074/jbc.M710607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Townsend TA, et al. Endocardial cell epithelial-mesenchymal transformation requires Type III TGFbeta receptor interaction with GIPC. Cell Signal. 2012;24(1):247–56. doi: 10.1016/j.cellsig.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He TC, et al. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95(5):2509–14. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craig EA, et al. TGFbeta2-mediated production of hyaluronan is important for the induction of epicardial cell differentiation and invasion. Exp Cell Res. 2010;316(20):3397–405. doi: 10.1016/j.yexcr.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishii Y, et al. BMP signals promote proepicardial protrusion necessary for recruitment of coronary vessel and epicardial progenitors to the heart. Dev Cell. 2010;19(2):307–16. doi: 10.1016/j.devcel.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Batlle E, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2(2):84–9. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 32.Cano A, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 33.Carver EA, et al. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol. 2001;21(23):8184–8. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed JA, et al. SKI pathways inducing progression of human melanoma. Cancer Metastasis Rev. 2005;24(2):265–72. doi: 10.1007/s10555-005-1576-x. [DOI] [PubMed] [Google Scholar]
- 35.Cohen SB, et al. Heterodimers of the SnoN and Ski oncoproteins form preferentially over homodimers and are more potent transforming agents. Nucleic Acids Res. 1999;27(4):1006–14. doi: 10.1093/nar/27.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimamura T, et al. Dysadherin expression facilitates cell motility and metastatic potential of human pancreatic cancer cells. Cancer Res. 2004;64(19):6989–95. doi: 10.1158/0008-5472.CAN-04-1166. [DOI] [PubMed] [Google Scholar]
- 37.Ino Y, et al. Dysadherin, a cancer-associated cell membrane glycoprotein, down-regulates E-cadherin and promotes metastasis. Proc Natl Acad Sci U S A. 2002;99(1):365–70. doi: 10.1073/pnas.012425299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai CL, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454(7200):104–8. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christoffels VM, et al. Tbx18 and the fate of epicardial progenitors. Nature. 2009;458(7240):E8–9. doi: 10.1038/nature07916. discussion E9–10. [DOI] [PubMed] [Google Scholar]
- 40.Gittenberger-de Groot AC, et al. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res. 1998;82(10):1043–52. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 41.Grieskamp T, et al. Notch signaling regulates smooth muscle differentiation of epicardium-derived cells. Circ Res. 2011;108(7):813–23. doi: 10.1161/CIRCRESAHA.110.228809. [DOI] [PubMed] [Google Scholar]
- 42.Lie-Venema H, et al. Periostin expression by epicardium-derived cells is involved in the development of the atrioventricular valves and fibrous heart skeleton. Differentiation. 2008;76(7):809–19. doi: 10.1111/j.1432-0436.2007.00262.x. [DOI] [PubMed] [Google Scholar]
- 43.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 44.Hao J, et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5(2):245–53. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edlund S, et al. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13(3):902–14. doi: 10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gatza CE, Oh SY, Blobe GC. Roles for the type III TGF-beta receptor in human cancer. Cell Signal. 2010;22(8):1163–74. doi: 10.1016/j.cellsig.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown CB, et al. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science. 1999;283(5410):2080–2. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- 48.Lambert KE, et al. The type III transforming growth factor-beta receptor inhibits proliferation, migration, and adhesion in human myeloma cells. Mol Biol Cell. 2011;22(9):1463–72. doi: 10.1091/mbc.E10-11-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JD, et al. The type III TGF-beta receptor suppresses breast cancer progression through GIPC-mediated inhibition of TGF-beta signaling. Carcinogenesis. 2010;31(2):175–83. doi: 10.1093/carcin/bgp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong M, et al. The type III TGF-beta receptor suppresses breast cancer progression. J Clin Invest. 2007;117(1):206–17. doi: 10.1172/JCI29293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hempel N, et al. Loss of betaglycan expression in ovarian cancer: role in motility and invasion. Cancer Res. 2007;67(11):5231–8. doi: 10.1158/0008-5472.CAN-07-0035. [DOI] [PubMed] [Google Scholar]
- 52.Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta. 2008;1782(4):197–228. doi: 10.1016/j.bbadis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Turley RS, et al. The type III transforming growth factor-beta receptor as a novel tumor suppressor gene in prostate cancer. Cancer Res. 2007;67(3):1090–8. doi: 10.1158/0008-5472.CAN-06-3117. [DOI] [PubMed] [Google Scholar]
- 54.Finger EC, et al. Endocytosis of the type III transforming growth factor-beta (TGF-beta) receptor through the clathrin-independent/lipid raft pathway regulates TGF-beta signaling and receptor down-regulation. J Biol Chem. 2008;283(50):34808–18. doi: 10.1074/jbc.M804741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mythreye K, Blobe GC. The type III TGFbeta receptor regulates directional migration: new tricks for an old dog. Cell Cycle. 2009;8(19):3069–70. doi: 10.4161/cc.8.19.9419. [DOI] [PubMed] [Google Scholar]
- 56.Desgrosellier JS, et al. Activin receptor-like kinase 2 and Smad6 regulate epithelial-mesenchymal transformation during cardiac valve formation. Dev Biol. 2005;280(1):201–10. doi: 10.1016/j.ydbio.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 57.Okagawa H, Markwald RR, Sugi Y. Functional BMP receptor in endocardial cells is required in atrioventricular cushion mesenchymal cell formation in chick. Dev Biol. 2007;306(1):179–92. doi: 10.1016/j.ydbio.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ozdamar B, et al. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307(5715):1603–9. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- 59.Goumans MJ, et al. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21(7):1743–53. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheifetz S, et al. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267(27):19027–30. [PubMed] [Google Scholar]
- 61.Wang XF, et al. Expression cloning and characterization of the TGF-beta type III receptor. Cell. 1991;67(4):797–805. doi: 10.1016/0092-8674(91)90074-9. [DOI] [PubMed] [Google Scholar]
- 62.Lebrin F, et al. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 2004;23(20):4018–28. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goumans MJ, et al. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell. 2003;12(4):817–28. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 64.Moskowitz IP, et al. Transcription factor genes Smad4 and Gata4 cooperatively regulate cardiac valve development. [corrected] Proc Natl Acad Sci U S A. 2011;108(10):4006–11. doi: 10.1073/pnas.1019025108. [DOI] [PMC free article] [PubMed] [Google Scholar]