Abstract
Background
The Cox-Maze procedure (CMP) has achieved high success rates in the therapy of atrial fibrillation (AF) while becoming progressively less invasive. This report evaluates our experience with the CMP in the treatment of lone AF over two decades and compares the original cut-and-sew CMP-III to the ablation-assisted CMP-IV, which uses bipolar radiofrequency and cryoenergy to create the original lesion pattern.
Methods and Results
Data were collected prospectively on 212 consecutive patients (mean age: 53.5±10.4, 78% males), who underwent a stand-alone CMP from 1992 through 2010. Median duration of preoperative AF was 6 (IQR 2.9–11.5) years, with 48% paroxysmal and 52% persistent or longstanding persistent AF. Univariate analysis with preoperative and perioperative variables used as covariates for the CMP-III (n=112) and the CMP-IV (n=100) was performed. Overall, 30-day mortality was 1.4% with no intraoperative deaths. Freedom from AF was 93% and freedom from AF off antiarrhythmics was 82% at a mean follow-up time of 3.6 ± 3.1 years. Freedom from symptomatic AF at 10 years was 85%. Only one late stroke occurred with 80% of patients being off anticoagulation. The less invasive CMP-IV had significantly shorter cross-clamp times (41±13 vs. 92±26 minutes, p<0.001) while achieving high success rates with 90% freedom from AF and 84% freedom from AF off antiarrhythmics at 2 years.
Conclusions
The CMP, while simplified and shortened by alternative energy sources, has excellent results even with improved follow-up and stricter definition of failure.
Keywords: ablation, arrhythmia (Heart Rhythm Disorders), atrial fibrillation, surgery, tachyarrhythmias
Atrial fibrillation (AF) is the most common sustained arrhythmia worldwide with an expected increase in our aging population.1 In addition to the significant morbidity and mortality secondary to hemodynamic compromise and tachycardia-induced cardiomyopathy in some patients, stroke remains the most feared complication.2 AF accounts for about 25% of strokes in patients older than 80 years and increases a person’s risk of stroke by 5-fold.3 The limitations of pharmacological therapy with failure rates as high as 60% have led to the development and proliferation of interventional approaches in the treatment of AF, including catheter ablation and surgery.4–7
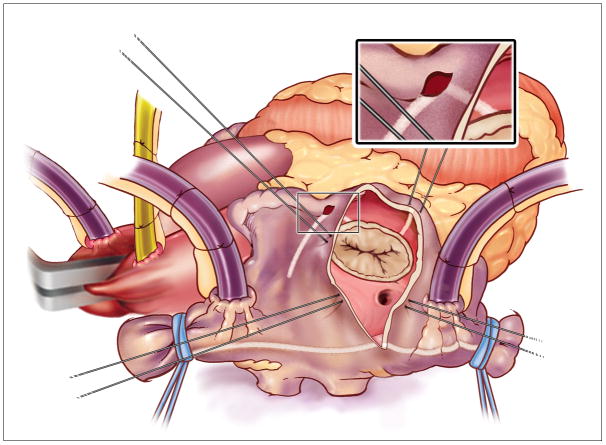
In 1987, Dr. Cox introduced the maze procedure (CMP) for the surgical treatment of AF at our institution. His surgical approach was designed to block the multiple macroreentrant circuits which were the putative cause of AF.7,8 The final iteration of his cut-and-sew technique termed the CMP-III, proved to be highly efficacious with 97% freedom from symptomatic AF and became the gold standard for the surgical therapy of AF for more than a decade (Figure 1).9,10 While early follow-up was excellent and included 24-h Holter monitoring only few patients had electrocardiograms or prolonged monitoring at long-term follow-up.5,11 The endpoint was generally self-reported freedom from symptomatic AF. Moreover, this procedure was not widely adopted because of its complexity and invasiveness.
Figure 1.
The original cut and sew Cox-Maze Procedure III.
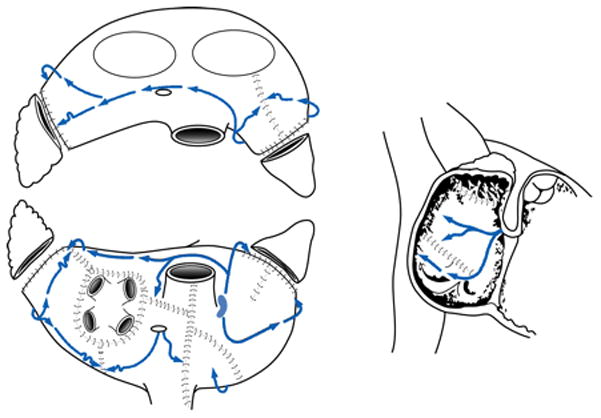
The development of alternative energy sources has enabled surgeons to create lines of ablation to replace most incisions of the original CMP-III which shortened and simplified the procedure.12,13 In our Laboratory, bipolar radiofrequency energy was found to be able to create reliable transmural lines of ablation while minimizing the risk of collateral damage to the surrounding tissue.14–16 In 2002, our institution introduced a new iteration termed the CMP-IV, which used bipolar radiofrequency and cryoenergy to replace most of the original incisions.13 While we initially performed only a single inferior connecting lesion between the ablations isolating the right and left pulmonary veins (PVs), we implemented the final version of the CMP-IV two years later, in which the entire posterior left atrium was isolated by adding a superior connecting line, termed the box lesion-set (Figure 2 and 3).17 This resembled closely the original cut-and-sew lesion-set of the CMP-III. In this recent group of patients, a stricter follow-up regime was implemented with all patients having electrocardiograms or 24-h Holter monitoring at 3, 6, and 12 months and annually thereafter. Also the definition of success as outlined in recent guidelines was applied.5,11
Figure 2.
The right atrial lesion set of the Cox-Maze Procedure IV.
Figure 3.
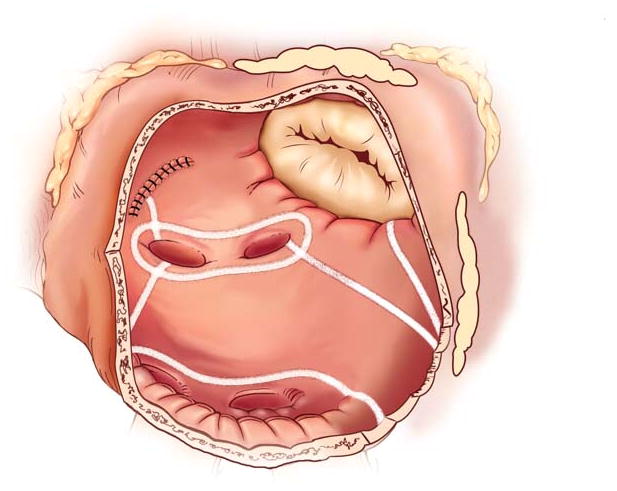
The left atrial lesion set of the Cox-Maze Procedure IV.
While we have previously reported excellent results with the CMP, the majority of these patients underwent concomitant cardiac surgery procedures.9,10,18,19 Since 1992, our institution performed a stand-alone CMP in 212 patients, reflecting the largest series in literature. This report evaluates our experience in the surgical treatment of lone AF over two decades and compares the outcome of the original cut-and-sew CMP-III to the ablation-assisted CMP-IV.
Methods
From April 1992 through October 2010, 212 consecutive patients underwent a stand-alone CMP for the surgical treatment of AF at our institution. AF was defined as paroxysmal, persistent or longstanding persistent per recent guidelines.5,11 In the first decade, the CMP-III was performed in 112 patients using the cut-and-sew technique. Since 2002, 100 patients received the CMP-IV, which was performed with a bipolar radiofrequency clamp and cryoprobes. In 72% of patients, the AtriCure Isolator, Isolator I, II, and Synergy series (AtriCure, Inc., Cincinnati, OH) were used. In 28% of patients, the irrigated Medtronic Cardioblate BP and LP (Medtronic, Inc., Minneapolis, MN) were applied. Algorithms measuring tissue conductance or impedance were used to determine the time of ablation and to estimate transmurality. Linear and bell-shaped cryoprobes (AtriCure, Inc., Cincinnati, OH) were used and cooled to −60°C for 2–3 minutes by nitrous oxide for all ablations.
This study was approved by the Washington University School of Medicine Institutional Review Board. Informed consent and permission for release of information were obtained from all patients.
Surgical technique of the CMP-III
The surgical technique of the CMP-III has been previously described (Figure 1).20 All patients underwent a median sternotomy and cardiopulmonary bypass with bicaval cannulation. The right atrial (RA) incisions were performed on the beating heart and included excision of the RA appendage, a free wall incision, a linear incision from the superior to the inferior vena cava and a perpendicular incision to the tricuspid annulus. A second incision to the tricuspid annulus was performed from the RA appendage. At the tricuspid annulus, a 3 mm cryoprobe (CCS200, Frigitronics, Inc., Trumbull, CT) was applied.
The left atrial (LA) lesion-set was performed under cardioplegic arrest. The LA appendage was amputated and a standard left atriotomy was performed. The remaining incisions included an encirclement of the PVs with extension to the mitral annulus and an atrial septal incision. A cryoprobe was used between the appendage amputation site and the two ends of the incision encircling the PVs, as well as over the coronary sinus and at the mitral valve annulus.
Surgical technique of the CMP-IV
The CMP-IV was performed using cardiopulmonary bypass with bicaval cannulation as described previously.13,17,21 Patient underwent either a median sternotomy (n=79) or a right minithoracotomy (n=21).21 All patients underwent intraoperative transesophageal echocardiography and cardioversion was performed if needed after the presence of a LA appendage clot was excluded. Electrical isolation was documented by pacing from each PV to confirm exit block. In all patients in whom pacing could be performed (93%), ablation was continued until exit block was documented from each PV. We routinely applied the bipolar radiofrequency clamp two to three times and then tested for exit block. This was successful 98% of the time. In patients undergoing a right minithoracotomy, pacing was performed only from the right PVs.
Patients were cooled to 34°C and the RA lesion-set was performed on the beating heart (Figure 2). A single incision was usually made in the RA free wall, but recently a three purse-string approach has been adopted to eliminate this incision in patients undergoing a minithoracotomy. All ablations were performed with the bipolar radiofrequency clamp except for two endocardial ablation lines to the tricuspid annulus, which were performed with a linear cryoprobe. The LA lesion-set was performed under cardioplegic arrest (Figure 3). The LA appendage was amputated and the bipolar clamp was used to create an ablation line from this site into one of the left PVs. A small left atriotomy was performed and the remainder of the ablation lines was completed with the bipolar clamp. Cryoablation was used to connect the isthmus ablation line to the mitral annulus. In patients undergoing a right minithoracotomy, cryoablation was more extensively applied to isolate the left PVs and to complete the posterior LA isolation. The LA appendage was oversewn from the inside.21
Postoperative Care and Follow-up
Following surgery, antiarrhythmic drugs were begun as soon as the patient restored a normal sinus rhythm. Warfarin was also initiated in all patients, unless contraindicated. If patients developed early tachyarrhythmias they were started on antiarrhythmic drugs and then electrically cardioverted if needed. If patients were in sinus rhythm, antiarrhythmic drugs were discontinued at two months. The patients then underwent prolonged monitoring and an echocardiogram at three months. Warfarin was discontinued if patients were free of atrial tachyarrhythmias and an echocardiogram ruled out atrial stasis or thrombus.
In patients who underwent the CMP-III between 1992 and 2001, the clinical profiles and perioperative outcomes were collected prospectively. Follow-up was conducted by office visits at 6 months and included a history, physical exam, 24-h Holter monitoring, transthoracic echocardiogram and a dynamic MRI. The first 69 patients also underwent an electrophysiological study at this time. Since AF could not be induced in one single patient this follow-up regime was discontinued. Long-term follow-up consisted of a retrospective cross-sectional analysis performed in 2001. This included a mailed questionnaire or telephone interview, and contact to either the cardiologist or the primary care physician to evaluate recurrence of AF.
In patients who received the CMP-IV since 2002, the clinical demographics and postoperative outcome variables were collected prospectively in a longitudinal database. Follow-up was conducted by office visits at 3, 6, and 12 months and annually thereafter. At each visit a history, physical examination, and electrocardiogram were obtained. Since 2006, when new follow-up guidelines were established, 24-hour Holter monitoring or pacemaker interrogation was obtained in 95% (62/65) of patients. Late recurrence was defined as any episode of ATAs including AF, atrial flutter or atrial tachycardia, which lasted longer than 30 seconds. Any patient requiring an interventional procedure after three months postoperatively was deemed a permanent failure. Patients were only considered to be a success if they were both free of AF and free of antiarrhythmic drugs (class I or III).
Data Analysis
Continuous data were checked for normality using the Shapiro-Wilk statistic. Normally distributed continuous data were expressed as: mean ± standard deviation and 95% confidence interval (95% CI), non-normally distributed data were expressed as: median and interquartile range (IQR Q1-Q3), and outcome percentages are expressed as the percentage and 95% CI. Categorical data were expressed as absolute numbers and proportions. Freedom from AF recurrence was calculated by using Kaplan-Meier analysis; Clinical profiles were compared using the χ2 test or the Fisher exact test. The unpaired t-test was used to compare normally distributed data and the Mann-Whitney U test was used for non-normal data. All data analyses were performed with the SPSS system for statistics (SPSS 11.0 for Windows, SPSS, Inc., Chicago, IL).
Results
Patient Demographics
In the entire series of stand-alone CMP, patients (n=212) had a mean age of 53.5±10.4 (95% CI 52.0–54.8) years with 78% males. The median duration of preoperative AF was 6.0 (IQR 2.9–11.5) years with 48% paroxysmal and 52% persistent or longstanding persistent AF. Thirteen percent of patients were in NYHA class III or IV. Transient ischemic attacks or stroke was the reason for surgical referral in 14% of cases. Overall, 20% of patients had failed previous catheter ablation.
CMP-III
Patient’s characteristics are shown in Table 1. Reasons for surgical referral were documented cerebral vascular accidents in 17%, intolerance of medication in 4% and development of clinical symptoms of the arrhythmia in 79%. Two patients had failed previous catheter ablation.
Table 1.
Patient Demographics
| CMP III (n=112) | CMP IV (n=100) | p - value | |
|---|---|---|---|
| Mean Age (years) | 51±10 (95% CI 20–77) | 56±10 (95% CI28–75) | 0.002* |
| Male gender | 80% | 76% | 0.548 |
| Median AF duration (years) | 7.0 (IQR 3.2–13) | 7.4±6.76 (IQR 2.4–10.0) | 0.039 |
| Paroxysmal AF | 63% | 31% | <0.001 |
| NYHA class III or IV | 5% | 22% | <0.001 |
| Failed previous catheter ablation | 2% | 40% | <0.001 |
CMP, Cox-Maze Procedure; NYHA, New York Heart Association; AF, Atrial Fibrillation; 95% CI, 95% confidence interval; IQR, interquartile range.
CMP-IV
Patients who received the CMP-IV were significantly older than in the CMP-III cohort (p=0.002). There was also a significant higher incidence of congestive heart failure NYHA class III or IV (p<0.001). This reflects our expanding indications for the CMP into higher-risk patients in the recent era. The incidence of paroxysmal AF significantly decreased in the CMP-IV cohort (p<0.001). The median duration of preoperative AF decreased from 7 (IQR 3.2–13) to 6 (IQR 2.4–10) years (p=0.039). The mean LA diameter measured by echocardiography was 4.7±1.1 cm. The reasons for surgical referral were patients with symptomatic AF who had failed medical therapy or catheter ablation (88%) and the occurrence of transient ischemic attacks or stroke (12%). Overall, 40% (40/100) of patients had failed a mean of 2.6±1.3 (range: 1–6) previous catheter ablations. This was significantly higher than in the CPM-III cohort (p<0.001), since catheter ablation has increased over the last decade. The indication for surgery in this subgroup was stroke in 13% (n=5) and clinical symptoms in 87% (n=35). The 60 patients who had not undergone prior catheter ablation were referred because their treating physicians or the patient preferred a surgical approach, or because they were felt to be poor candidates for catheter ablation.
Perioperative Results
The 30-day mortality of the entire series was 1.4% (n=3) with no intraoperative deaths. The mean aortic cross-clamp time was 68±33 minutes. Median length of stay (LOS) at the Intensive Care Unite (ICU) was 2 days (IQR 1.0–3.0); median length of hospital stay was 8 days (IQR 6.7–11). There were 2 (0.9%) early strokes within 30 days after surgery and 8% of patients required postoperative pacemaker implantation.
CMP-III
The 30-day mortality was 1.8% (Table 2). One patient died of multisystem organ failure, one death was caused by acute respiratory failure. The median aortic cross-clamp time was 90 (95% CI 73.5–105) minutes. The median LOS at the ICU was 2 (1–3.5) days the median length of hospital stay was 9 (7–12.2) days. The major complication rate (Table 2) was 10%, which was significantly higher than in the CMP-IV cohort (p=0.004). Early postoperative ATAs were documented in 34% (n=38) of patients. Nine patients (8%) required postoperative pacemaker implantation due to chronotropic incompetence (n=6) or slow junctional rhythm (n=3).
Table 2.
Perioperative Variables
| CMP III (n=112) | CMP IV (n=100) | p - value | |
|---|---|---|---|
| Median CPB time (min.) | 163 (IQR 145–183) | 129 (IQR 113–150) | <0.001 |
| Mean CCT (min.) | 90 (95% CI 73.5- 105) | 39 (95% CI 33.2–46.7) | <0.001 |
| 30-day mortality | 2 (2%) | 1 (1%) | 0.625 |
| Early ATAs | 38 (34%) | 40 (40%) | 0.732 |
| Pacemaker Implantation (≤ 90 days) | 9 (8%) | 7 (7%) | 0.776 |
| Major Complication Rate | 11 (10%) | 1 (1%) | 0.003 |
| Reoperation for Bleeding | 3 (3%) | 0 | |
| Early Stroke (≤ 30 days) | 1 (1%) | 1 (1%) | |
| Renal failure | 2 (2%) | 0 | |
| Mediastinitis | 1 (1%) | 0 | |
| Intraaortic Balloon Pump | 4 (4%) | 0 |
CMP, Cox-Maze Procedure; CPB, Cardiopulmonary Bypass; CCT, Aortic Cross-Clamp Time; ATAs, Atrial Tachyarrhythmias; 95% CI, 95% confidence interval; IQR, interquartile range.
CMP-IV
The 30-day mortality was 1% (n=1). There were no intraoperative deaths. The only mortality occurred in a woman who suffered a pulmonary embolism on the day of discharge, despite being fully anticoagulated. The median aortic cross-clamp time was 39 (IQR 33–46.7) minutes, which was significantly shorter than in the CMP-III group (p<0.001). Seventy-eight percent of patients (n=78) received a complete box lesion-set isolating the entire posterior LA. The median LOS at the ICU was 1 (IQR 1–3) days and the median length of hospital stay was 7 (IQR 6–9.5) days. A single stroke (1%) was the only major perioperative complication. Early postoperative ATAs were documented in 40% of patients. Seven patients (7%) required a postoperative pacemaker implantation for chronotropic incompetence (n=2) or slow junctional rhythm (n=5).
Late Follow-Up
In the entire series, freedom from AF was 93% and freedom from AF off antiarrhythmics was 82% at last follow up with a median follow-up time of 2.3 (IQR 0.9–6.3) years (Table 3). The Kaplan-Meier estimate for freedom from AF at 10 years was 85% (95% CI 70–92); Figure 4). There was no significant difference in late success rate for patients with paroxysmal AF, 96%(95% CI 86–98) versus persistent or longstanding persistent AF (91%,(95% CI 81–95) p=0.094). Late recurrence occurred at a median time of 1.2 (IQR 0.9–2.1) years postoperatively. There was one late stroke (0.5%) with 80% of patients being off anticoagulation therapy at last follow up. The late mortality (>30 days after surgery) was 1.4%.
Table 3.
Late Follow-Up
| CMP III (n=112) | CMP IV (n=100) | CMP III + IV (n = 212) | |
|---|---|---|---|
| Median follow-up (years) | 5.9 (IQR 2.5–7.8) | 1.0 (IQR 0.74–1.9) | 2.2 (IQR 0.9–6.2) |
| Freedom from AF | 96% (95% CI 86–98) | 90% (95% CI 81–95) | 93% (95% CI 87–96) |
| Freedom from AF off antiarrhythmics | 83% (95% CI 68–88) | 82% (95% CI 71–89) | 82% (95% CI 75–87) |
| Freedom from Warfarin | 86% (95% CI 75–92) | 74% (95% CI 62–83) | 80% (95% CI 72–86) |
| Late Stroke (> 30 days) | 1 (0.8%) | 0 | 1 (0.4%) |
CMP, Cox-Maze Procedure; AF, Atrial Fibrillation; 95% CI, 95% confidence interval; IQR, interquartile range.
Figure 4.
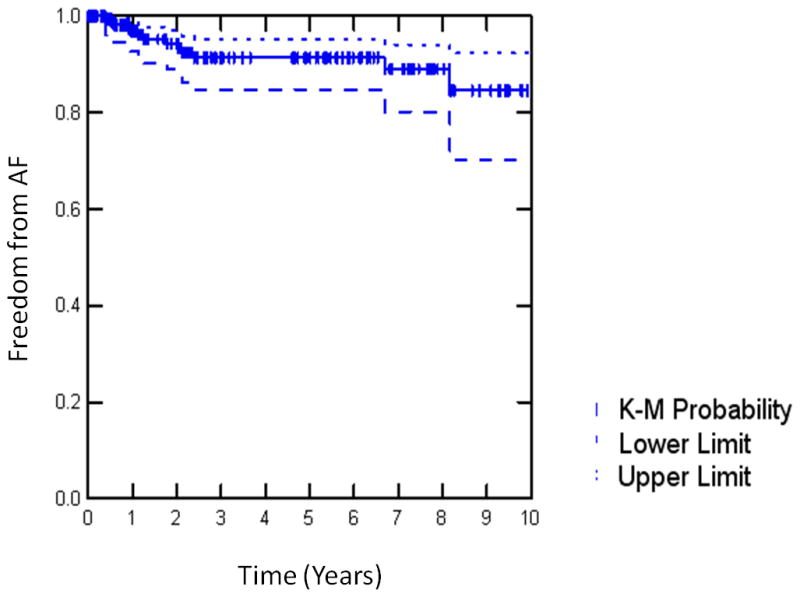
Kaplan-Meier (K-M) Analysis of freedom from atrial fibrillation (AF) for the Cox- Maze-Procedure (III+IV). Pts, patients.
CMP-III
The median follow-up was 5.9 (IQR 2.5–7.8) years and was 88% complete. The freedom from symptomatic AF was 95% (95% CI 86–98) at last follow-up with a freedom from AF off antiarrhythmic drugs of 83% (95% CI 68–88). There was no significant difference in late success rates for patients with paroxysmal AF (96%) compared to persistent or longstanding persistent AF (93%, p=0.556). One late stroke (0.9%) occurred in a patient with restored sinus rhythm who was not anticoagulated. Eighty-six percent of patients were free of anticoagulation therapy with Warfarin. There were 3 late deaths (2.6%) with all patients being free from AF.
CMP-IV
The median follow-up was 1.0 (IQR 0.7–2.0) years. No patient was lost to follow-up. Two-year follow-up was available on 50% of patients. The freedom from AF was 94% (95% CI 85–98), 93% (95% CI 65–99), 90% (95% CI 78–96), and 90% (95% CI 68–99) at 3, 6, 12 and 24 months, respectively. The freedom from both, AF and antiarrhythmic drugs were 72% (95% CI 60–81), 82% (95% CI 54–95), 82% (95% CI 68–90) and 84% (95% CI 58–95) at 3, 6, 12 and 24, respectively. In patients receiving a complete box lesion-set (n=78), freedom from AF was 96% (95% CI 87–99) and freedom from AF off antiarrhythmic drugs was 86% (95% CI 74–93) after 1 year. This compares to patients receiving a non-box ablation-set (n=22) with a freedom from AF of 77% (95% CI 49–93) and a freedom from AF off antiarrhythmic drugs of 46% (95% CI 21–69) (p=0.004 and p<0.001, respectively). In 40 patients who had failed previous catheter ablation, the postoperative freedom from AF was 92% 95% CI (76–98) at 3, 6 and 12 months, respectively. The freedom from both, AF and antiarrhythmic drugs was 72% (95% CI 53–86), 86% (95% CI 67–95) and 84% (95% CI 68–95) at 3, 6, and 12 months, respectively. There was no significant difference in success rate off antiarrhythmic drugs for patients with paroxysmal AF (68%) versus persistent or longstanding persistent AF (72%, p=0.886). There were no late strokes. At 12 months, 74% (95% CI 62–83) of patients were free of anticoagulation therapy with warfarin. The recurrent arrhythmias were atrial fibrillation (80%), atrial flutter (10%) and atrial tachycardia (10%). Four patients reconverted to sinus rhythm after AF was documented previously at follow-up.
Discussion
The CMP has been the gold standard in the treatment of AF with the highest late success rates of any single-interventional procedure.10,22,23 This surgical approach was developed at our institution and has gone through various iterations to improve and simplify the procedure.7,19,24,25 The original CMP-III was empirically designed to interrupt the macroreentrant circuits in both atria which were thought to be responsible for AF.7,26,27 However, it is now known that there are multiple mechanisms responsible for AFand this complex arrhythmia is still not thoroughly understood in many patients.28–30
With the anticipated goal to preserve the high success rates of the CMP-III and to decrease invasiveness, the CMP-IV was designed to simplify the operation by using bipolar radiofrequency energy to replace most of the traditional incisions. This energy source was chosen after extensive investigation in our laboratory that demonstrated its ability to reliably create discrete and transmural lesions.15,16 By achieving complete lines of ablations in a matter of seconds it overcame the major limitations of other energy sources. Furthermore, the focused application of energy within the jaws of the clamp minimized the risk of collateral damage to surrounding tissue that had been reported for unipolar energy sources.31 Because invasiveness is a major concern, the ability to reduce cross-clamp time and enable a minimally invasive approach made the CMP-IV more attractive to patients with lone AF.21
This report of 212 consecutive patients undergoing a CMP for lone AF over almost 20 years demonstrated excellent long-term success rates with 93% freedom from AF and 82% freedom from AF off antiarrhythmic medication. Only one late stroke occurred over a total of 763 patient-years of follow-up, with 80% of patients being free from anticoagulation therapy with Warfarin. Considering the side effects of Warfarin, including the higher risk of anticoagulation-associated intracranial hemorrhage, this is important in improving quality of life.32 However, in few patients other indications for anticoagulation therapy were present or developed despite restored sinus rhythm. The technical complexity of the CMP-III kept it from a wide adoption, while its invasiveness made catheter ablation the preferred choice of treatment for most patients with drug-refractory, symptomatic lone AF. Based on isolating the PV (PVI), the results of catheter ablation have been variable with single-procedure success rates between 16 – 84%.6,11,33,34 A recent report from Haïssaguerre’s group, who pioneered PVI, reported a single-procedure success rate as low as 29% after 5 years.35 Certain patient subgroups have done particular poorly, such as patients with longstanding persistent AF and large atria.36,37 A recent review suggested a success rate for a single-procedure ranging from 22% to 45% in patients with persistent or longstanding persistent AF.11
Our experience with the CMP defines the long-term results with this procedure. The CMP-III had excellent freedom from symptomatic AF at 10 years. The less invasive CMP-IV has showed significantly shorter operating times and lower complication rates while resulting in equivalent early freedom from AF, despite more rigid definitions of success and improved follow-up. At the present time, the cut-and-sew CMP-III is no longer performed at our institution. The results of this study confirm the efficacy of the CMP-III lesion set. Moreover, the CMP was equally effective for paroxysmal and longstanding persistent AF. It was also very effective in patients who had failed previous catheter ablation. These results can be achieved with minimal operative risk. Our data would suggest that more patients should be referred for the CMP, particularly symptomatic patients who have either failed a catheter ablation or belong to a subgroup who have poor results with catheter ablation.
The need of pacemaker remains a problem following the Cox-Maze procedure. Although the CMP-IV lesion set might cause a sinus node dysfunction, it is not the only possible mechanism. The majority of patients requiring pacemakers presented with preexisting sick sinus syndrome. Moreover, AF is known to induce sinus node dysfunction. Although sinus node recovery time seems to normalize after termination of AF, the time course of reversing this electrical remodeling is variable, and the risk for pacemaker implantation can not be eliminated completely. It is possible that eliminating right atrial ablations would decrease the need for postoperative pacemaker implantation, however, this also would likely result in a lower cure rate. There are several limitations to this report. While follow-up in the historical series was longer and showed a freedom from symptomatic AF and antiarrhythmics of 83%, few of these patients had electrocardiographic or Holter monitoring at 12 or 24 months. With constantly improving follow-up, recent guideline requirements have been met since 2006.5,11,38 The lack of electrocardiographic or Holter follow-up likely resulted in an underestimation of the long-term failure rate in the CMP-III group. However, the recent CMP-IV cohort has been well monitored with 24-hour Holter or pacemaker interrogation. This cohort reflects the current standard of surgical treatment and shows excellent success rates that compare favorably to our CMP-III experience. This was particularly true for patients who had an isolation of the entire posterior LA. Our success rate off antiarrhythmic drugs at one year in this group was 86%. Moreover, 69% of patients presented preoperatively with persistent or longstanding persistent AF and 40% had previously failed catheter ablation, reflecting a more difficult cohort for successful treatment than the CMP-III group. However, a comparison of the two groups remains difficult. A Kaplan-Meier analysis was most suitable to present the results of the entire series because of the difference in follow-up. However, AF is a dynamic endpoint and reconversion to sinus rhythm after an episode of AF was still shown as permanent failure. To take this into account, we presented the recent CMP-IV results as % of freedom from AF at various time points. In a previous study, patient-specific variables were adjusted by a propensity analysis and we showed similar results with the CMP-III and -IV.19 Finally, the mechanisms for AF recurrence were not well defined. The question remains unanswered whether our failure rate was due to technical difficulties, untreated atrial pathology or a focal mechanism of the recurrent ATAs.
This report gives a benchmark for the excellent long-term success rate of a stand-alone CMP and will provide a useful comparison for the myriad of procedures that are presently performed surgically, including left atrial lesion-sets and PVI, as well as for new and less invasive approaches currently under development.
Acknowledgments
Funding Sources: Funded in part from the NIH Grants 5RO1 HL032257, RO1 HL085113, and T32 HL07776
Footnotes
Conflict of Interest Disclosures: Dr. Damiano is a consultant for AtriCure and Medtronic and has received research grants from AtriCure, Medtronic, and Estech. Dr. Schuessler receives research support from AtriCure, Estech, and Cardialen and serves on the scientific advisory board of Cardialen. Dr. Cox has a financial relationship with SentreHEART and CorMatrix. Dr. Maniar is a consultant for nContact Surgery and Estech. Ms. Bailey is a consultant for AtriCure.
References
- 1.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: The framingham heart study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 2.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics--2006 update: A report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 4.Marcus GM, Sung RJ. Antiarrhythmic agents in facilitating electrical cardioversion of atrial fibrillation and promoting maintenance of sinus rhythm. Cardiology. 2001;95:1–8. doi: 10.1159/000047335. [DOI] [PubMed] [Google Scholar]
- 5.Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NA, 3rd, Page RL, Ezekowitz MD, Slotwiner DJ, Jackman WM, Stevenson WG, Tracy CM, Fuster V, Ryden LE, Cannom DS, Le Heuzey JY, Crijns HJ, Olsson SB, Prystowsky EN, Halperin JL, Tamargo JL, Kay GN, Jacobs AK, Anderson JL, Albert N, Hochman JS, Buller CE, Kushner FG, Creager MA, Ohman EM, Ettinger SM, Guyton RA, Tarkington LG, Yancy CW. 2011 accf/aha/hrs focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2011;123:104–123. [Google Scholar]
- 6.Oral H, Knight BP, Tada H, Ozaydin M, Chugh A, Hassan S, Scharf C, Lai SW, Greenstein R, Pelosi F, Jr, Strickberger SA, Morady F. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105:1077–1081. doi: 10.1161/hc0902.104712. [DOI] [PubMed] [Google Scholar]
- 7.Cox JL, Schuessler RB, D'Agostino HJ, Jr, Stone CM, Chang BC, Cain ME, Corr PB, Boineau JP. The surgical treatment of atrial fibrillation. Iii. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991;101:569–583. [PubMed] [Google Scholar]
- 8.Cox JL, Boineau JP, Schuessler RB, Ferguson TB, Jr, Cain ME, Lindsay BD, Corr PB, Kater KM, Lappas DG. Successful surgical treatment of atrial fibrillation. Review and clinical update. JAMA. 1991;266:1976–1980. [PubMed] [Google Scholar]
- 9.Cox JL, Schuessler RB, Lappas DG, Boineau JP. An 8 1/2-year clinical experience with surgery for atrial fibrillation. Ann Surg. 1996;224:267–273. doi: 10.1097/00000658-199609000-00003. discussion 273–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad SM, Maniar HS, Camillo CJ, Schuessler RB, Boineau JP, Sundt TM, 3rd, Cox JL, Damiano RJ., Jr The cox maze iii procedure for atrial fibrillation: Long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–1828. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 11.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. Hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for personnel, policy, procedures and follow-up. A report of the heart rhythm society (hrs) task force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Khargi K, Hutten BA, Lemke B, Deneke T. Surgical treatment of atrial fibrillation; a systematic review. Eur J Cardiothorac Surg. 2005;27:258–265. doi: 10.1016/j.ejcts.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Gaynor SL, Diodato MD, Prasad SM, Ishii Y, Schuessler RB, Bailey MS, Damiano NR, Bloch JB, Moon MR, Damiano RJ., Jr A prospective, single-center clinical trial of a modified cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128:535–542. doi: 10.1016/j.jtcvs.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 14.Prasad SM, Maniar HS, Schuessler RB, Damiano RJ., Jr Chronic transmural atrial ablation by using bipolar radiofrequency energy on the beating heart. J Thorac Cardiovasc Surg. 2002;124:708–713. doi: 10.1067/mtc.2002.125057. [DOI] [PubMed] [Google Scholar]
- 15.Prasad SM, Maniar HS, Diodato MD, Schuessler RB, Damiano RJ., Jr Physiological consequences of bipolar radiofrequency energy on the atria and pulmonary veins: A chronic animal study. Ann Thorac Surg. 2003;76:836–841. doi: 10.1016/s0003-4975(03)00716-1. discussion 841–832. [DOI] [PubMed] [Google Scholar]
- 16.Gaynor SL, Ishii Y, Diodato MD, Prasad SM, Barnett KM, Damiano NR, Byrd GD, Wickline SA, Schuessler RB, Damiano RJ., Jr Successful performance of cox-maze procedure on beating heart using bipolar radiofrequency ablation: A feasibility study in animals. Ann Thorac Surg. 2004;78:1671–1677. doi: 10.1016/j.athoracsur.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 17.Voeller RK, Bailey MS, Zierer A, Lall SC, Sakamoto S, Aubuchon K, Lawton JS, Moazami N, Huddleston CB, Munfakh NA, Moon MR, Schuessler RB, Damiano RJ., Jr Isolating the entire posterior left atrium improves surgical outcomes after the cox maze procedure. J Thorac Cardiovasc Surg. 2008;135:870–877. doi: 10.1016/j.jtcvs.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 18.Damiano RJ, Jr, Gaynor SL, Bailey M, Prasad S, Cox JL, Boineau JP, Schuessler RP. The long-term outcome of patients with coronary disease and atrial fibrillation undergoing the cox maze procedure. J Thorac Cardiovasc Surg. 2003;126:2016–2021. doi: 10.1016/j.jtcvs.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Lall SC, Melby SJ, Voeller RK, Zierer A, Bailey MS, Guthrie TJ, Moon MR, Moazami N, Lawton JS, Damiano RJ., Jr The effect of ablation technology on surgical outcomes after the cox-maze procedure: A propensity analysis. J Thorac Cardiovasc Surg. 2007;133:389–396. doi: 10.1016/j.jtcvs.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Cox JL, Jaquiss RD, Schuessler RB, Boineau JP. Modification of the maze procedure for atrial flutter and atrial fibrillation. Ii. Surgical technique of the maze iii procedure. J Thorac Cardiovasc Surg. 1995;110:485–495. doi: 10.1016/S0022-5223(95)70245-8. [DOI] [PubMed] [Google Scholar]
- 21.Lee AM, Clark K, Bailey MS, Aziz A, Schuessler RB, Damiano RJ. A minimally invasive cox-maze procedure: Operative technique and results. Innovations (Phila) 2010;5:281–286. doi: 10.1097/IMI.0b013e3181ee3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaff HV, Dearani JA, Daly RC, Orszulak TA, Danielson GK. Cox-maze procedure for atrial fibrillation: Mayo clinic experience. Semin Thorac Cardiovasc Surg. 2000;12:30–37. doi: 10.1016/s1043-0679(00)70014-1. [DOI] [PubMed] [Google Scholar]
- 23.Millar RC, Arcidi JM, Jr, Alison PJ. The maze iii procedure for atrial fibrillation: Should the indications be expanded? Ann Thorac Surg. 2000;70:1580–1586. doi: 10.1016/s0003-4975(00)01707-0. [DOI] [PubMed] [Google Scholar]
- 24.Melby SJ, Kaiser SP, Bailey MS, Zierer A, Voeller RK, Lall SC, Munfakh N, Moon MR, Damiano RJ., Jr Surgical treatment of atrial fibrillation with bipolar radiofrequency ablation: Mid-term results in one hundred consecutive patients. J Cardiovasc Surg (Torino) 2006;47:705–710. [PubMed] [Google Scholar]
- 25.Damiano RJ, Jr, Voeller RK. Biatrial lesion sets. J Interv Card Electrophysiol. 2007;20:95–99. doi: 10.1007/s10840-007-9178-x. [DOI] [PubMed] [Google Scholar]
- 26.Cox JL, Schuessler RB, Boineau JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg. 1991;101:402–405. [PubMed] [Google Scholar]
- 27.Cox JL, Canavan TE, Schuessler RB, Cain ME, Lindsay BD, Stone C, Smith PK, Corr PB, Boineau JP. The surgical treatment of atrial fibrillation. Ii. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg. 1991;101:406–426. [PubMed] [Google Scholar]
- 28.Schuessler RB, Kay MW, Melby SJ, Branham BH, Boineau JP, Damiano RJ., Jr Spatial and temporal stability of the dominant frequency of activation in human atrial fibrillation. J Electrocardiol. 2006;39:S7–12. doi: 10.1016/j.jelectrocard.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Sahadevan J, Ryu K, Peltz L, Khrestian CM, Stewart RW, Markowitz AH, Waldo AL. Epicardial mapping of chronic atrial fibrillation in patients: Preliminary observations. Circulation. 2004;110:3293–3299. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- 30.Nattel S, Shiroshita-Takeshita A, Brundel BJ, Rivard L. Mechanisms of atrial fibrillation: Lessons from animal models. Prog Cardiovasc Dis. 2005;48:9–28. doi: 10.1016/j.pcad.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Doll N, Borger MA, Fabricius A, Stephan S, Gummert J, Mohr FW, Hauss J, Kottkamp H, Hindricks G. Esophageal perforation during left atrial radiofrequency ablation: Is the risk too high? J Thorac Cardiovasc Surg. 2003;125:836–842. doi: 10.1067/mtc.2003.165. [DOI] [PubMed] [Google Scholar]
- 32.Mant J, Hobbs FD, Fletcher K, Roalfe A, Fitzmaurice D, Lip GY, Murray E. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the birmingham atrial fibrillation treatment of the aged study, bafta): A randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 33.Gerstenfeld EP, Guerra P, Sparks PB, Hattori K, Lesh MD. Clinical outcome after radiofrequency catheter ablation of focal atrial fibrillation triggers. J Cardiovasc Electrophysiol. 2001;12:900–908. doi: 10.1046/j.1540-8167.2001.00900.x. [DOI] [PubMed] [Google Scholar]
- 34.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 35.Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, Lellouche N, Knecht S, Wright M, Nault I, Miyazaki S, Scavee C, Clementy J, Haissaguerre M, Jais P. Catheter ablation for atrial fibrillation: Are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–166. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 36.Cheema A, Vasamreddy CR, Dalal D, Marine JE, Dong J, Henrikson CA, Spragg D, Cheng A, Nazarian S, Sinha S, Halperin H, Berger R, Calkins H. Long-term single procedure efficacy of catheter ablation of atrial fibrillation. J Interv Card Electrophysiol. 2006;15:145–155. doi: 10.1007/s10840-006-9005-9. [DOI] [PubMed] [Google Scholar]
- 37.Lo LW, Tai CT, Lin YJ, Chang SL, Udyavar AR, Hu YF, Ueng KC, Tsai WC, Tuan TC, Chang CJ, Kao T, Tsao HM, Wongcharoen W, Higa S, Chen SA. Predicting factors for atrial fibrillation acute termination during catheter ablation procedures: Implications for catheter ablation strategy and long-term outcome. Heart Rhythm. 2009;6:311–318. doi: 10.1016/j.hrthm.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Shemin RJ, Cox JL, Gillinov AM, Blackstone EH, Bridges CR. Guidelines for reporting data and outcomes for the surgical treatment of atrial fibrillation. Ann Thorac Surg. 2007;83:1225–1230. doi: 10.1016/j.athoracsur.2006.11.094. [DOI] [PubMed] [Google Scholar]