Abstract
Neurofibromas, schwannomas and malignant peripheral nerve sheath tumors (MPNSTs) all arise from the Schwann cell lineage. Despite their common origin, these tumor types have distinct pathologies and clinical behaviors; a growing body of evidence indicates that they also arise via distinct pathogenic mechanisms. Identification of the genes that are mutated in genetic diseases characterized by the development of either neurofibromas and MPNSTs [neurofibromatosis type 1 (NF1)] or schwannomas [neurofibromatosis type 2 (NF2), schwannomatosis and Carney complex type 1] has greatly advanced our understanding of these mechanisms. The development of genetically engineered mice with ablation of NF1, NF2, SMARCB1/INI1 or PRKAR1A has confirmed the key role these genes play in peripheral nerve sheath tumorigenesis. Establishing the functions of the NF1, NF2, SMARCB1/INI1 and PRKAR1A gene products has led to the identification of key cytoplasmic signaling pathways promoting Schwann cell neoplasia and identified new therapeutic targets. Analyses of human neoplasms and genetically engineered mouse models have established that interactions with other tumor suppressors such as TP53 and CDKN2A promote neurofibroma-MPNST progression and indicate that intratumoral interactions between neoplastic and non-neoplastic cell types play an essential role in peripheral nerve sheath tumorigenesis. Recent advances have also provided new insights into the identity of the neural crest-derived populations that give rise to different types of peripheral nerve sheath tumors. Based on these findings, we now have an initial outline of the molecular mechanisms driving the pathogenesis of neurofibromas, MPNSTs and schwannomas. However, this improved understanding in turn raises a host of intriguing new questions.
Keywords: Neurofibromatosis, Schwannoma, Tumor suppressor mutations, Genetically engineered mouse tumor models, Tumor microenvironment, Aberrant growth factor signaling
Introduction
Peripheral nerve sheath tumors [neurofibromas, malignant peripheral nerve sheath tumors (MPNSTs) and schwannomas] are relatively common lesions, representing 8.9% of the nervous system tumors resected in the United States between 2004 and 2006 [27]. Schwann cells, the myelinating glia of the peripheral nervous system (PNS), are the source of the neoplastic cells within each of these tumor types. However, despite their common origin, the pathology and clinical behavior of neurofibromas, MPNSTs and schwannomas is quite distinct. It has also become apparent that the pathogenesis of these three tumor types differs in several key features including: 1) mutations affecting distinct sets of cancer driver genes, 2) distinct intercellular interactions in the tumor microenvironment and 3) origin from different Schwann cell and/or neural crest-derived cellular populations. Information gained from studies of human tumors and cleverly designed genetically engineered mouse (GEM) models has been used to construct an initial scheme detailing the steps involved in the development and progression of Schwann cell neoplasms. Intriguingly, though, each answer gleaned from these studies has led to further questions. As a result, we now appreciate that the development of Schwann cell neoplasms is far more complicated than we initially imagined.
Below, I will review our current understanding of the molecular mechanisms responsible for the development of peripheral nerve sheath tumors. In large part, the mechanisms involved in the pathogenesis of neurofibromas and MPNSTs are distinct from those promoting the development of schwannomas. Consequently, I will consider these groups of tumors separately. Our understanding of the events involved in the molecular pathogenesis of neurofibromas and MPNSTs owes a great deal to the identification of the gene that is mutated in neurofibromatosis type 1 (NF1; OMIM #162200) and subsequent studies of the functions of neurofibromin, the protein encoded by the NF1 gene. Identification of the genes that are affected in neurofibromatosis type 2 (NF2; OMIM # 101000), schwannomatosis (OMIM #162091) and Carney complex type 1 (CNC1; OMIM #160980) has been similarly important for deciphering the mechanisms involved in the pathogenesis of schwannomas. I will therefore begin each section presented below with a discussion of the cellular features of each tumor type, the relevant genetic diseases and the functions of the proteins encoded by the genes that are mutated in these diseases. I will then discuss how subsequent studies with both human tumors and GEM models of these genetic diseases has led to our current understanding of the mechanisms involved in the molecular pathogenesis of Schwann cell neoplasms.
NEUROFIBROMAS AND MPNSTs
Despite major differences in their clinical behavior, all neurofibroma subtypes are composed of an identical but complex mixture of cell types
All clinical disciplines recognize that there are several distinct neurofibroma subtypes. Unfortunately, these same clinical disciplines disagree as to precisely how to define these neurofibroma subtypes (see [24] and the review by Dr. Bernd Scheithauer in this issue for a detailed discussion of current neurofibroma classification schemes). In contrast, basic scientists studying neurofibromas simply categorize these neoplasms as either dermal neurofibromas (neurofibromas arising in skin) or plexiform neurofibromas (neurofibromas that occur in large, deeply situated nerves or nerve plexuses). Although this latter terminology glosses over some important clinical and anatomic considerations, it is practical as dermal and plexiform neurofibromas exhibit quite distinct patterns of clinical behavior. Dermal neurofibromas typically begin to appear in NF1 patients as they enter puberty; this observation, considered together with the fact that pregnant women with NF1 develop new dermal neurofibromas and demonstrate accelerated growth of existing tumors, has led to the suggestion that dermal neurofibromas are hormonally responsive. It is also notable that dermal neurofibromas have virtually no malignant potential. In contrast, plexiform neurofibromas are often congenital and show little clinical evidence of hormonal responsiveness. Plexiform neurofibromas are also prone to undergo malignant transformation and give rise to MPNSTs—an NF1 patient’s lifetime risk of developing an MPNST has been estimated at 8-13% [43] and 5.9-10.3% [125]. These distinct patterns of clinical behavior have led a number of investigators to ask whether dermal and plexiform neurofibromas arise via distinct molecular mechanisms and/or from different progenitors (see below).
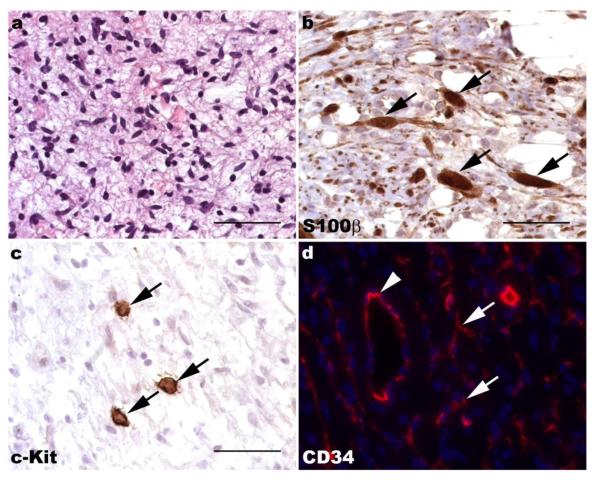
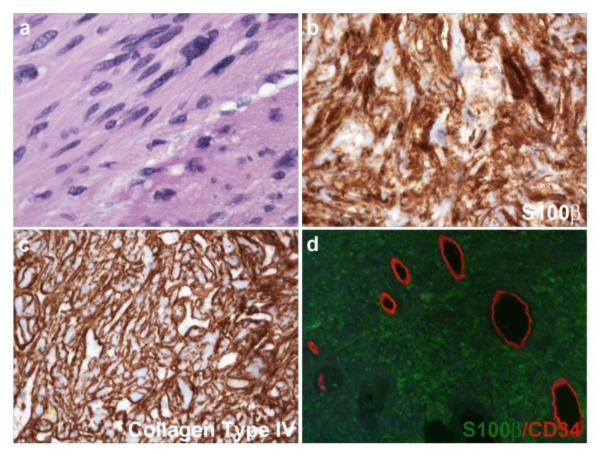
Despite their striking biological differences, dermal and plexiform neurofibromas have an identical, albeit complicated, cellular makeup. Microscopic examination of hematoxylin and eosin-stained sections of both dermal and plexiform neurofibromas show these lesions to be moderately hypercellular and often rather bland-appearing tumors in which spindled cells are set against a background rich in mucopolysaccharides and collagen (Fig. 1a). Immunohistochemistry for Schwann cell markers such as S100β (Fig. 1b) or the low affinity neurotrophin receptor (p75LNTR) shows that approximately 40-80% of the cells in a neurofibroma stain for these markers. The S100β-negative cells within these lesions represent a mixture of mast cells (Fig. 1c), fibroblasts, vascular elements and perineurial-like cells; these latter cells have ultrastructural findings characteristic of perineurial cells (e.g., numerous pinocytotic vesicles and a discontinuous basement membrane) but lack the epithelial membrane antigen immunoreactivity typically seen in the perineurium. Neurofibromas also contain a population of CD34-positive cells that have a morphology variously described as dendritic or fibroblastic (Fig. 1d). These cells have been suggested to be either a novel type of nerve sheath tumor cell that is distinct from fibroblasts and Schwann cells [193] or resident tissue macrophages [31]. However, their identity has not yet been clearly established.
Fig. 1.
Neurofibromas are composed of a complex mixture of cell types. (a) Hematoxylin and eosin stained section of a plexiform neurofibroma demonstrating the bland spindle cells set against a myxoid background that are typically seen in these lesions. (b) Immunohistochemistry for the Schwann cell marker S100β highlights several immunoreactive elements within this plexiform neurofibroma (arrows). Note that numerous S100β-negative cells are also present. (c) Immunoreactivity for c-Kit (CD117; arrows) is evident in numerous mast cells in neurofibromas. (d) CD34 immunoreactivity (red) is present within both vasculature (arrowhead) and a dendritic population (arrows) in neurofibromas. The density of CD34-immunoreactive dendritic cells is variable in most neurofibromas; this region is particularly abundant in these cells. a-c, 40x (scale bars, 50 μm); d, 60x.
Given this mixture of cell types, it is not surprising that the nature of neurofibromas was debated for decades. Some investigators felt that neurofibromas were endoneurial hamartomas or localized hyperplastic processes within nerves, while other considered them to be true neoplasms of either fibroblastic or Schwannian origin. This controversy was not resolved until the NF1 tumor suppressor gene was cloned.
Cloning of the NF1 gene establishes neurofibromas as neoplasms and Schwann cells as the neoplastic component within these tumors
In the general population, neurofibromas are commonly encountered as solitary lesions. However, patients with NF1 typically develop numerous neurofibromas at multiple sites throughout their body. NF1, which is inherited in an autosomal dominant fashion, is estimated to occur in 1 in 2500 to 1 in 3500 newborn infants. The gene affected in this condition has one of the highest rates of de novo mutation observed for any single gene disorder; curiously, these new mutations usually occur in the paternally derived chromosome [78]. Because of this high new mutation rate, about 50% of infants with NF1 are born into families with no previous history of the disease.
Although NF1 is completely penetrant, its manifestations are highly variable even within the same family. In addition to neurofibromas, NF1 patients often exhibit learning disabilities (i.e., intelligence quotients in the low average range) and can develop bony dysplasias. They are also prone to the development of multiple other tumor types including optic gliomas (pilocytic astrocytomas of the optic nerve), glioblastomas, pheochromocytomas, rhabdomyosarcomas, gastrointestinal stromal tumors and a rare leukemia known as juvenile chronic myelogenous leukemia. Finally, NF1 patients often manifest pigmentary lesions (melanocytic hamartomas) of the iris known as Lisch nodules, axillary freckling and café-au-lait macules; curiously, café-au-lait macules are commonly found overlying deeply situated neurofibromas.
Given the devastating clinical features of NF1, it is understandable that a great deal of effort was invested in identifying the gene responsible for this condition. These efforts culminated in 1990, when it was reported that a gene located on the long arm of chromosome 17 (17q11.2) was mutated in NF1 patients [186,189]. The NF1 gene is quite large, spanning nearly 283,000 base pairs and containing 60 exons, several of which are alternatively spliced in NF1 transcripts. Since the identification of the NF1 gene, comprehensive screening methods (a process that combines direct sequencing of all coding exons, copy number analysis and screening for deep intronic splice mutations) have been developed to identify mutations in patients potentially affected by this disease. With these methods, NF1 mutations are found in about 95% of non-founder classic NF1 patients (patients with neurofibromas in combination with other clinical features of NF1) [132]; the remaining 5% are suspected to have mutations in regions of the NF1 gene not covered by this screening procedure (e.g., promoter sequences regulating NF1 expression, the 3′ untranslated region of NF1 mRNA). Of note, there is a cadre of patients that present with the pigmentary lesions noted above in the absence of neurofibromas. Mutations of the NF1 gene are found in about 70% of these patients, with 19% having SPRED1 mutations (Legius syndrome) [131] and the remainder having mutations of other, as yet unidentified, genes. Thus, while neurofibroma pathogenesis is a very common feature of NF1, neurofibromas are not invariably present in patients with this disease.
Since the protein encoded by the NF1 gene, neurofibromin, contains multiple functional domains (see below) and some NF1 patients do not develop neurofibromas, the question arises as to whether mutations at specific locations in the NF1 gene predispose patients to the development of neurofibromas. Unfortunately, at present only sparse information is available regarding such genotype-phenotype correlations. As might be expected for a gene with such a high new mutation rate, the spectrum of mutations identified in the NF1 gene is highly variable. Total deletion of the NF1 gene is found in only about 5% of NF1 patients [199], with nonsense mutations, missense mutations, frameshift mutations [132] and mutations affecting mRNA splicing [198] being more typically encountered. Although these latter mutations do tend to cluster in certain locations within the NF1 gene (particularly exons 10a-c and 37), they can be found throughout this locus [132]. It has been observed that patients with whole gene deletions typically have large numbers of dermal neurofibromas together with dysmorphic features and substantial cognitive impairment [141]; these patients are also at increased risk for the development of plexiform neurofibromas and MPNSTs [121]. Beyond that, the only genotype-phenotype correlation relevant to neurofibroma pathogenesis identified to date is that patients with a 3 base pair in-frame deletion in exon 17 of the NF1 gene lack neurofibromas while demonstrating other features of NF1 [181]. Clearly, further investigation into the relationship between specific NF1 mutations and neurofibroma pathogenesis is needed.
The identification of the NF1 gene also made it possible to establish the nature of neurofibromas and the identity of the neoplastic cell type within these lesions. Both the autosomal dominant inheritance pattern of NF1 and the functional characteristics of the protein encoded by the NF1 locus (see below) suggested that NF1 was a tumor suppressor gene. If this postulate was correct, it was anticipated that NF1 patients would carry one mutated and one wild-type allele and that a “second hit” mutation of the remaining functional NF1 gene in a relevant cell type would result in tumorigenesis. In keeping with these predictions, NF1 loss of heterozygosity (LOH) was identified in cultured Schwann cells, but not fibroblasts, derived from neurofibromas [92] as well as in S100β immunoreactive cells within neurofibromas and MPNSTs [144]. These observations, together with subsequent studies of neurofibromin function and the generation of genetically engineered mouse models directly testing the consequences of Nf1 ablation (see below), clearly established that neurofibroma pathogenesis results from mutation of a tumor suppressor gene and that these lesions are thus true neoplasms. As NF1 LOH occurred specifically in Schwann cells, these studies also demonstrated that Schwann cells are the neoplastic component in neurofibromas.
The NF1 gene encodes neurofibromin, a protein that inhibits Ras signaling and has other actions
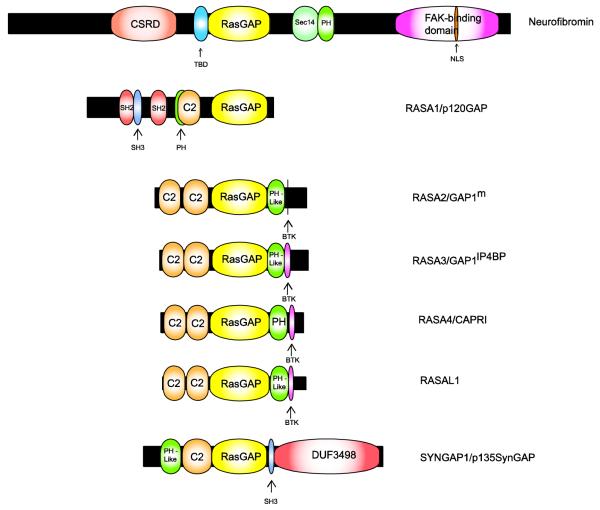
Neurofibromin, the 220 kDa protein encoded by the NF1 gene, spans 2,818 amino acids. The large size of neurofibromin made initial attempts to understand the function of this polypeptide difficult. However, shortly after the identification of the NF1 gene, it was noted that neurofibromin contains a domain (amino acids 1203-1549 in GenBank sequence NP_000258.1; Fig. 2) that is highly homologous to the Ras GTPase-activating protein (GAP) domain found in the yeast proteins IRA1 and IRA2 [205]. Ras proteins are highly conserved GTP-binding proteins (G-proteins) that, when activated, stimulate cytoplasmic signaling pathways that promote proliferation, survival, migration and a host of other essential cellular functions. Ras activation is triggered when Ras-bound GDP is exchanged for GTP, a process that is facilitated by a diverse class of molecules known as guanine nucleotide exchange factors (GEFs). Subsequent inactivation of activated Ras proteins is dependent upon the cleavage of a phosphate from Ras-bound GTP by a GTPase-activity intrinsic to Ras. However, this intrinsic GTPase activity is inefficient and, in isolation, cleaves GTP as a very low rate; GAPs such as IRA1 and IRA2 facilitate Ras inactivation by binding to Ras and enhancing its GTPase activity by several orders of magnitude. In keeping with their structural similarity, the neurofibromin GAP-related domain (GRD) proved capable of rescuing the heat shock-sensitive phenotype of IRA1- and IRA2-deficient yeast [204], suggesting that its function is both highly conserved and analogous to that of the yeast GAPs. Recombinant neurofibromin GRD also stimulated the GTPase activity of yeast RAS2 and human H-Ras [204], thus functionally verifying this neurofibromin domain’s ability to inactivate Ras.
Fig. 2.
Schematic illustrating the functional domains present in neurofibromin and comparing the structure of neurofibromin to that of other Ras GAPs. The lengths of each protein (indicated by the black bars) and domains within each Ras GAP (indicated by the colored expansions) are scaled to the actual number of amino acids in each. See the text for a detailed explanation of known functions of the domains contained within neurofibromin. Domain designations are as follows: CSRD, cysteine/serine-rich domain; TBD, tubulin-binding domain; RasGAP, Ras GTPase-activating protein; Sec14, Sec14-homology domain; PH, pleckstrin homology domain that can target protein to appropriate cellular location and bind inositol phosphates and other proteins; NLS, nuclear localization signal; SH2, domain that binds phosphotyrosine-containing ligands via two surface pockets (a phosphotyrosine pocket and a hydrophobic binding pocket); SH3, domain that binds proline-rich ligands with moderate affinity and selectivity (particularly PxxP motifs); C2, calcium-dependent membrane-targeting module that binds a variety of substrates including phospholipids, inositol polyphosphates and intracellular proteins; BTK, Bruton tyrosine kinase-like motif capable of binding zinc (always follows a PH domain); DUF3498, domain of unknown function.
It is now recognized that neurofibromin is a member of a family of mammalian Ras GAPs that includes RASA1 (p120GAP), RASA2 (Gap1m), RASA3 (GAP1IP4BP), RASA4 (CAPRI), RASAL1 and SYNGAP (p135SynGAP) (Fig. 2). Although the expression of some of these GAPs (e.g., SYNGAP) is restricted to specific cell types, others are expressed ubiquitously. Further, neurofibromin and RASA1 bind to the same target Ras proteins (see below for a discussion of these different Ras proteins) with similar affinities [2,3,157]. However, despite their overlapping expression patterns and the similarity of their interactions with Ras proteins, these other Ras GAPs cannot compensate for neurofibromin loss and thus prevent tumorigenesis (indeed, NF1 is the only Ras GAP gene that, when mutated, results in a tumor predisposition syndrome). This may reflect the fact that neurofibromin contains a number of structural domains that are not present in other Ras GAP family members (Fig. 2); some of these domains appear to modulate neurofibromin’s Ras GAP activity while others may serve different functions altogether. For instance, neurofibromin contains cysteine/serine-rich (CSRD) and tubulin-binding (TBD) domains that are located N-terminal to the GRD and act as regulators of neurofibromin-mediated inhibition of Ras signaling. Interestingly, these two domains can act in an opposing manner—tubulin binding to the TBD inhibits the GAP activity of neurofibromin [17], whereas phosphorylation of the CSRD by protein kinase Cα enhances this same activity [118]. Neurofibromin also contains a bipartite domain C-terminal to the GRD that contains both a segment homologous to the yeast Sec14p protein and a pleckstrin homology domain [34]. Although the function of this Sec14p homology domain is incompletely understood, it can specifically bind glycerophospholipids [194] and structural studies predict that mutations in this domain will interfere with as yet undefined protein-protein interactions [195]. A focal adhesion kinase (FAK)-interacting domain, which allows neurofibromin to modulate substrate adherence, is additionally present at the C-terminal end of neurofibromin [97]. Curiously, although nuclear actions have not yet been defined for neurofibromin, the FAK-binding domain contains a nuclear localization signal [184]. In comparison, the overwhelming majority of the non-GRD domains present in other Ras GAPs (Src homology 2 (SH2), Src homology 3 (SH3), calcium-binding C2 and Bruton tyrosine kinase-like domains) are not found in neurofibromin. Consequently, it is possible that other Ras GAPs do not compensate for neurofibromin loss because their other non-GRD domains impose regulatory and/or spatial constraints distinct from those acting on neurofibromin.
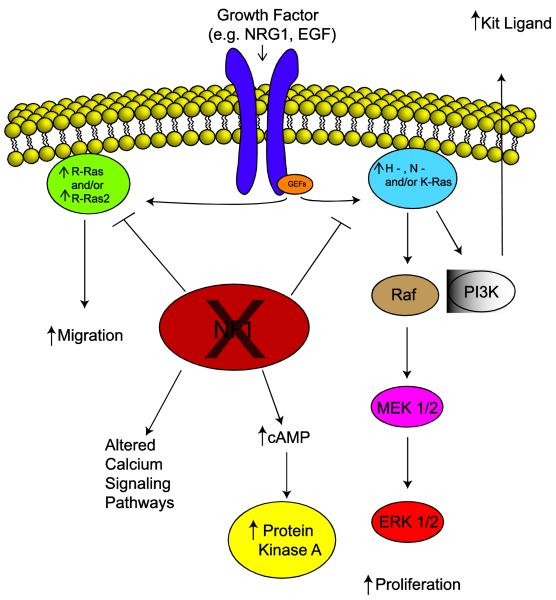
The functional validation of the neurofibromin GRD led to the expectation that neurofibromin loss would result in increased levels of activated Ras in NF1-associated peripheral nerve sheath tumors. It was subsequently verified that Ras activation is indeed increased in neurofibromas [166] and MPNSTs [14], which suggested that inhibiting Ras activity would be an effective means of treating these tumors. Consequently, a clinical trial with tipifarnib, an agent which inhibits Ras farnesylation (a modification essential for Ras maturation and appropriate localization), was initiated in children with plexiform neurofibromas. However, although tipifarnib performed well in the phase I trial [196], it proved ineffective in phase II. There are several potential explanations for this failure. However, one particularly attractive explanation is derived from the fact that neurofibromin regulates the activity of multiple Ras proteins in mammalian cells (Fig. 3), including members of both the classic Ras (H-Ras, N-Ras, K-Ras4A and K-Ras4B) and R-Ras (R-Ras, R-Ras2/TC21 and M-Ras/R-Ras3) subfamilies [139]; whereas farnesyl transferase inhibitors do effectively inhibit H-Ras action, other neurofibromin-regulated Ras proteins can bypass the effects of farnesyl transferase inhibitors by using alternative modifications (e.g., geranylgeranylation). At present, precisely what contribution each of member of the classic Ras and R-Ras subfamilies makes to peripheral nerve sheath tumorigenesis is unclear. Although the identity of the Ras proteins expressed in neurofibromas and MPNSTs has not yet been fully investigated, H-Ras, N-Ras, K-Ras, R-Ras and R-Ras2 are all present in murine wild-type and Nf1-/- Schwann cells [70]. Studies with H-Ras dominant negative mutants, which inhibit all members of the classic Ras subfamily, and R-Ras dominant negative mutants, which similarly inhibit R-Ras subfamily members, suggest that one or more classic Ras proteins mediate proliferation in these glia while an R-Ras family member (likely R-Ras2) promotes their migration [70]. Nonetheless, it remains to be established whether specific Ras proteins play a similar role in neoplastic Schwann cells and to what degree these proteins can compensate for each other. Given this uncertainty about the role individual Ras proteins play in neurofibromas and MPNSTs, it is understandable that attention has instead shifted to identifying key Ras-regulated signaling cascades that can be targeted therapeutically. In this regard, it is notable that recent evidence has implicated the PI3 kinase-Akt-TSC2-mTOR-S6 kinase cascade as a particularly important pathway in NF1-associated peripheral nerve sheath tumors [80,81]. Indeed, initial preclinical studies in which mice xenografted with MPNST cells were treated with the mTOR inhibitor rapamycin or rapamycin derivatives alone [15,82], or in combination with Akt inhibitors [212], have already shown promising effects.
Fig. 3.
A schematic demonstrating the cytoplasmic signaling pathways that are affected by neurofibromin loss and what impact these pathways have on key aspects of neurofibroma pathogenesis inducing tumor cell migration and proliferation and the secretion of factors such as Kit ligand.
Given its large size and the highly conserved nature of neurofibromin, the fact that mutations at multiple sites outside the GRD result in tumorigenesis and the finding that this protein contains multiple structural domains in addition to the GRD, it is reasonable to ask whether neurofibromin regulates the activity of cytoplasmic signaling events distinct from the canonical Ras-regulated cascades. At present, only limited information is available regarding potential neurofibromin effects on other signaling pathways (Fig. 3) and it is not clear what neurofibromin domains mediate these effects. There is evidence that that neurofibromin alters cAMP levels in astrocytes [37], non-neoplastic Schwann cells [85] and neoplastic Schwann cells [35], thereby regulating cAMP-dependent signaling pathways. Interestingly, neurofibromin loss increases cAMP levels in Schwann cells while decreasing them in astrocytes; these respective changes are both likely to be pro-proliferative. There is also evidence that neurofibromin loss influences calcium signaling. Keratinocytes isolated from NF1 patients have low resting intracellular calcium levels, reduced calcium stores and reduced capacitative calcium influx compared to controls [95]. Intercellular waves of calcium propagate abnormally in sheets of these cells, apparently as a result of abnormal localization of gap junction proteins. MPNST cells stimulated with platelet-derived growth factor-BB (PDGF-BB) also show an increase in intracellular calcium levels which is not evident in similarly treated wild-type human Schwann cells [36]. This increase is associated with enhanced phosphorylation of the calmodulin target CaMKII, which promotes mitogenesis. In keeping with these latter observations, we have recently found that tamoxifen potently inhibits MPNST proliferation and survival both in culture and in mice orthotopically xenografted with MPNST cells [22]. This effect is estrogen receptor-independent and appears instead to reflect tamoxifen action on calmodulin (another known tamoxifen target) as it is phenocopied by trifluoperazine, another well-established calmodulin inhibitor.
Genetically engineered mouse models confirm the key role Nf1 loss plays in neurofibroma pathogenesis and argue that non-neoplastic cells play an essential role in tumorigenesis
With the identification of the NF1 gene, it became feasible to directly test the role this tumor suppressor plays in neurofibroma pathogenesis by creating mice with null mutations of the murine Nf1 gene. Four years after the identification of the NF1 gene, it was reported that Nf1+/- mice were viable and developed some of the neoplasms (leukemia and pheochromocytomas) that are less commonly found in NF1 patients [77]. Unfortunately, however, these animals did not present with neurofibromas or MPNSTs. Further, it was not possible to determine whether complete Nf1 loss in Nf1-/- mice could induce peripheral nerve sheath tumor formation as these mice died in utero secondary to cardiac malformations [19] or exencephaly [98]. In an effort to bypass the consequences of neurofibromin loss in the developing heart and brain, chimeras were produced by fusing Nf1-/- and wild-type embryos. Encouragingly, some of these mice did develop tumors resembling neurofibromas in spinal nerve roots and other large nerves [29], suggesting that complete Nf1 loss in one or more cell types could trigger neurofibroma formation.
To definitively establish that it was complete Nf1 loss in the Schwann cell lineage that induced neurofibroma pathogenesis, a new mouse model was produced in which floxed Nf1 alleles were conditionally ablated in Schwann cells by Cre recombinase expressed under the control of the Krox20 promoter [211]. Surprisingly, although these animals developed microscopic hyperplastic lesions within nerves, they did not develop neurofibromas. However, noting that neurofibromas arising in human NF1 patients contain NF1 null Schwann cells embedded in a background of numerous NF1+/- non-neoplastic cell types, these investigators next constructed mice with complete Nf1 loss in Schwann cells and Nf1 haploinsufficiency in all other cell types (Krox20-Cre; Nf1flox/- mice). In contrast to their initial conditional knockout model, these Krox20-Cre; Nf1flox/- mice developed neurofibromas in multiple spinal nerve roots and cranial nerves. Based on these and other observations (see below), it was proposed that an NF1 haploinsufficient tumor microenvironment is required for neurofibroma pathogenesis. However, this hypothesis was subsequently challenged by studies of mouse models in which Nf1 was ablated in the Schwann cell lineage using Desert hedgehog (Dhh)-Cre [201] or proteolipid protein (Plp)-Cre [123] drivers. Both of these mouse models readily developed neurofibromas despite having two intact Nf1 alleles in other cell types present within their neurofibromas. In addition, it is clear that (barring the rare case of NF1 mosaicism) sporadic neurofibromas arising in the general population develop on a background of non-schwannian cells with intact NF1 function. At present, it remains unclear why Nf1 haploinsufficiency in non-schwannian cells is required for neurofibroma pathogenesis in some settings but not in others.
The suggestion that an NF1 haploinsufficient tumor microenvironment potentiates neurofibroma pathogenesis raises the question of which cell types within neurofibromas are essential for this process and what signaling molecules mediate these intercellular interactions. To date, the most convincing studies have focused on mast cells. Kit ligand (also known as stem cell factor), which is a growth factor that promotes the migration, maturation, survival and activation of mast cells, is secreted by Schwann cells in neurofibromas [68,154]. Nf1-/- Schwann cells secrete approximately 6 times more Kit ligand than either Nf1+/- or wild-type Schwann cells [207], indicating that a complete loss of neurofibromin expression enhances the release of this factor. The migration [76], proliferation and survival [75,76] of Nf1+/- mast cells challenged with Kit ligand is also enhanced compared to wild-type mast cells treated with this factor, demonstrating that Nf1 haploinsufficiency results in exaggerated mast cell responses to Kit ligand. Kit ligand also induces mast cell secretion of transforming growth factor-β (TGFβ), a process that is enhanced in Nf1+/- mast cells [206]. Nf1+/- fibroblasts treated with TGFβ show increased production of collagen relative to wild-type fibroblasts, suggesting that stimulation of intratumoral fibroblasts by this mast cell-derived factor may be responsible for the dense collagen deposits often observed in human neurofibromas.
Studies in knockout mice have confirmed the importance of mast cells in neurofibroma pathogenesis. As noted earlier, neurofibromas do not develop in Krox20-Cre;Nf1flox/flox mice. However, if Krox20-Cre;Nf1flox/flox mice are lethally irradiated and then grafted with Nf1+/- bone marrow, they develop large numbers of neurofibromas containing Nf1+/- mast cells [208]. Recruitment of mast cells and neurofibroma formation in these animals is clearly dependent on the action of Kit ligand as no tumor formation was observed in analogous experiments in which Krox20-Cre;Nf1flox/flox mice were grafted with bone marrow derived from mice with hypoactive c-Kit receptors (Nf1+/-;c-KitW41/W41 mice). Likewise, neurofibromas did not develop when lethally irradiated Krox20-Cre;Nf1flox/flox mice were grafted with wild-type bone marrow. It is also evident that mast cells potentiate continued growth of neurofibromas as treating Krox20-Cre;Nf1-/flox mice with established neurofibromas with the c-Kit inhibitor imatinib mesylate reduces tumor volumes and proliferation rates while increasing apoptosis. Having said that, it must be emphasized that it is still unclear precisely what functions mast cells perform upon reaching the developing neurofibroma that facilitate tumor initiation and subsequent growth.
Although the work noted above provides clear evidence that mast cells promote neurofibroma pathogenesis, the role other cell types play in this process remains undetermined. A host of reciprocal interactions between Schwann cells and other cell types (fibroblasts, vascular elements, perineurial-like cells, CD34+ cells) intrinsic to neurofibromas can easily be envisioned. Future investigations directed towards establishing whether these other cell types are required for tumorigenesis, what signals are involved in their recruitment and what function they perform after arriving in the developing tumor will be of great interest.
Genetically engineered mouse models suggest that dermal and plexiform neurofibromas arise from different progenitors
Given the presence of Schwann cells in neurofibromas and the fact that ablation of the Nf1 gene in the Schwann cell lineage results in neurofibroma pathogenesis, it is evident that Schwann cells are the neoplastic elements in neurofibromas. However, the work described above did not demonstrate which specific stage in Schwann cell differentiation serves as the source of these neoplastic elements. In addition, the major biologic differences between dermal and plexiform neurofibromas raise the question of whether these lesions are derived from distinct progenitors. As differences in the origin of these tumors can potentially influence their responsiveness to therapeutic agents, a great deal of effort has been invested in generating genetically engineered mouse models designed to identify the origin of the neoplastic cell type in these tumors.
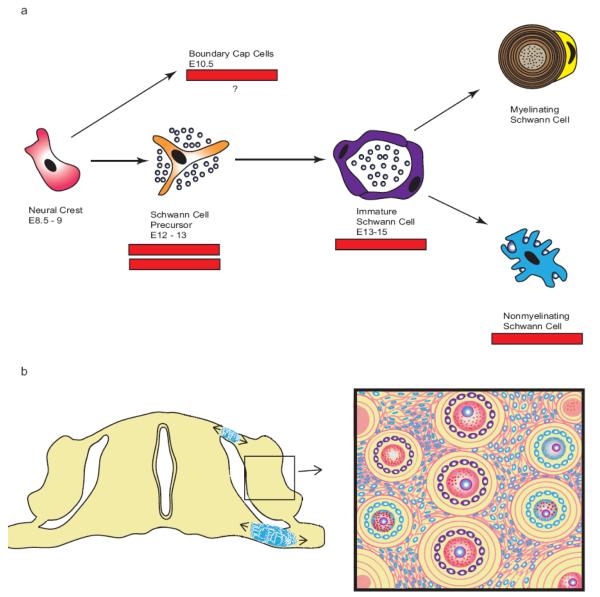
Conceptually, these efforts have focused on trying to relate the neurofibroma cell of origin to specific stages in Schwann cell development. The Schwann cell lineage, which has been most extensively studied in developing mouse sciatic nerve, arises from neural crest cells, a transient multipotent population of cells which appears in the mouse between embryonic days (E) 8.5 and 10.5 (Fig. 4a). Neural crest cells give rise to Schwann cell precursors (also known as neural crest stem cells) between E12-E13 which in turn become committed immature Schwann cells (E13-E15) that ultimately give rise to the two Schwann cell phenotypes (myelinating and non-myelinating) seen in adults. Ablation of Nf1 in neural crest cells within Wnt1-Cre;Nf1flox/-, Mpz-Cre;Nf1flox/- and Pax3-Cre;Nf1flox/- mice does not result in neurofibroma formation [84]. Granted, these mice die at birth, so it could be argued that they might have developed neurofibromas had they had survived for a longer period. However, Krox20-Cre;Nf1flox/- mice, which do not ablate Nf1 until later stages in the Schwann cell lineage, do develop neurofibromas which indicates that Nf1 loss in neural crest cells is not required for neurofibroma pathogenesis.
Fig. 4.
Multiple genetically engineered mouse models have been designed to determine whether specific stages in Schwann cell development are particularly susceptible to the pathogenesis of plexiform neurofibromas. (a) This scheme outlines the different stages of Schwann cell differentiation and the timing of their occurrence in the main trunk of the sciatic nerve; the embryonic days (E) indicated correspond to the periods at which these cell types first become evident during mouse embryogenesis. Bars beneath different stages indicate cell types in which Nf1 loss can result in neurofibroma pathogenesis, while the double bar indicates a stage of particular susceptibility to tumorigenesis. Boundary cap cells, a neural crest-derived population distinct from that giving rise to Schwann cells in the main trunk of the sciatic nerve, are also indicated in this scheme as they represent another population potentially capable of giving rise to plexiform neurofibromas. (b) The diagram to the left represents a cross-section of embryonic spinal cord together with its associated dorsal nerve roots, dorsal root ganglia (DRG) and ventral nerve roots. Boundary cap cells (indicated in blue) are initially found at the dorsal nerve roots entry zone and the ventral nerve root exit zone. They subsequently migrate into adjacent regions of the nerve roots and the dorsal root ganglia where they give rise to multiple cell types. The box to the right presents a magnified view of developing dorsal root ganglion that has been invaded by boundary cap cells that subsequently differentiated into Schwann cells, a subpopulation of nociceptive neurons and their associated satellite cells. Progeny derived from boundary cap cells are colored blue. Note that some DRG neurons and satellite cells are not blue. This indicates that these cellular elements are derived from precursors other than boundary cap cells.
In contrast, ablation of Nf1 in Schwann cell precursors does result in neurofibroma formation. P0 a-Cre;Nf1flox/- mice, which express Cre in Schwann cell precursors beginning at E12.5, develop neurofibromas by 15-20 months of age [84,210]. However, Schwann cell precursors could not be isolated from the sciatic nerves of P0a-Cre;Nf1flox/- mice and Schwann cell precursors isolated from E13 Nf1-/- mice were not tumorigenic when grafted into the sciatic nerves of Nf1+/- mice [84]. Based on these observations and the finding that proliferating cells within neurofibromas arising in P0a-Cre;Nf1flox/- mice lacked markers characteristic of Schwann cell precursors [e.g., brain lipid binding protein (BLBP), also known as fatty acid binding protein 7 (FABP7)], it was proposed that mature non-myelinating Schwann cells derived from Schwann cell precursors were the most likely progenitor for neurofibromas. In contrast, however, Dhh-Cre;Nf1flox/flox mice (which also express Cre in Schwann cell precursors beginning at E12.5) develop neurofibromas that contain numerous BLBP immunoreactive cells [201]. This was interpreted as indicating that a progenitor at the Schwann cell precursor-immature Schwann cell transition is a more likely source for the neoplastic cells within neurofibromas. In an attempt to resolve these conflicting views, PLP-CreERT2;Nf1flox/flox [123] and PLP-CreERT2;Nf1flox/- [103] mice, in which Cre-mediated gene ablation is induced by administration of tamoxifen, were generated and used to ablate Nf1 embryonically or in adulthood. Nf1 ablation at either of these times resulted in neurofibroma formation. However, tumorigenesis was more frequent when tamoxifen was administered embryonically. These observations suggest that, while Nf1 loss most effectively leads to tumorigenesis when occurring in Schwann cell precursors or immature Schwann cells, it can still lead to neurofibroma formation when occurring in mature Schwann cells. Alternatively, it is possible that an as yet undetected Schwann cell precursor population persists in adult nerve and is the source of these lesions. In addition, other potential sources of neoplastic Schwann cells within neurofibromas such as boundary cap cells (Fig. 4b) have not yet been ruled out. This latter possibility is consistent with the well-known clinical observation that neurofibromas commonly arise on dorsal spinal nerve roots in the vicinity of dorsal root ganglia.
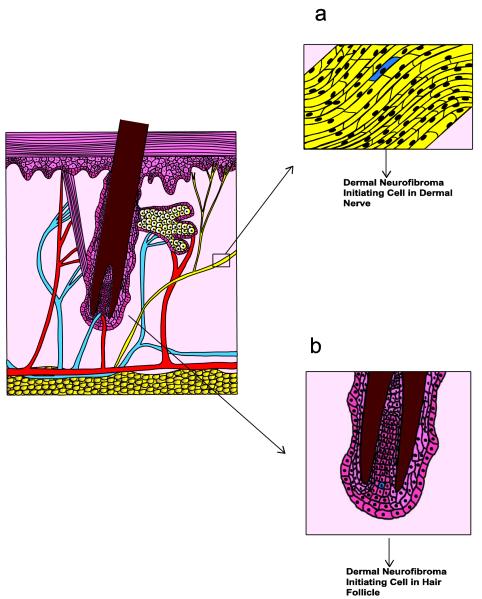
While it is clear that plexiform neurofibromas arise within peripheral nerves, the same cannot be said for dermal neurofibromas. Although it has long been assumed that dermal neurofibromas in NF1 patients arise from small cutaneous nerves (Fig. 5a), these tumors are often not clearly associated with a nerve; alternatively, if a nerve is present within a dermal neurofibroma, it is difficult to rule out the possibility that it is entrapped rather than being the source of the tumor. In addition, the Nf1 knockout models described above do not develop neurofibromas that are clearly equivalent to human dermal neurofibromas despite the use of a number of Cre driver lines that are presumably active in Schwann cells within small cutaneous nerves. These observations have led investigators to consider other possible sources for the neoplastic Schwann cells found within dermal neurofibromas such as skin-derived precursors (SKPs; Fig. 5b). SKPs are neural crest-derived multipotent progenitors that are capable of differentiating into either Schwann cells or melanocytes and are present within hair follicles in both mice and humans [48]. In keeping with the hypothesis that SKPs are the source of neoplastic Schwann cells within neurofibromas, painting tamoxifen onto the skin of CMV-CreERT2;Nf1flox/- mouse pups leads to the development of dermal neurofibromas at the site of administration [104]. Further, when SKPs are isolated from these mice, treated with tamoxifen and then grafted into the dermis of pregnant mice, neurofibromas develop at the graft site. These observations thus provide strong evidence that dermal and plexiform neurofibromas arise from fundamentally different progenitors. However, precisely what the functional differences between these progenitors are remains poorly understood. Indeed, attempts to define differences in the transcriptomes of dermal and plexiform neurofibromas have thus far been unsuccessful [133].
Fig. 5.
A comparison of two proposed sources of origin for dermal neurofibromas. The diagram to the left illustrates a cross-section of skin including the epidermis, dermis and subcutis. A hair shaft, together with its associated follicle and sebaceous gland, is evident in the center of this section. In this first scheme (a), dermal neurofibroma pathogenesis occurs when the remaining functional copy of NF1 is lost in a Schwann cell (indicated in blue) within a small cutaneous nonmyelinating nerve. Although this model has long been attractive, it does not explain why dermal neurofibromas typically do not develop in mice with conditional knockouts of Nf1; the promoters used to drive Cre recombinase expression in many of these animals would be expected to be active in small cutaneous nerves just as they are in larger nerves. An alternative source for dermal neurofibromas (b), which is now supported by findings made with recently derived genetically engineered mouse models, is that they are derived from skin-derived precursors (SKPs), a multipotent neural crest-derived precursor population that is located within hair follicles. In this scheme, neurofibroma pathogenesis is initiated when the remaining functional copy of NF1 is lost in a SKP (indicated as a blue cell within the hair follicle).
Neurofibroma-MPNST progression results from the accumulation of additional driver gene mutations
MPNSTs, like plexiform neurofibromas, demonstrate biallelic inactivation of their NF1 genes [106]. However, since MPNSTs arise via progression from plexiform neurofibromas, it is apparent that MPNSTs must accumulate mutations in additional driver genes (oncogenes and/or tumor suppressor genes) during this process. In keeping with this expectation, mutations of several tumor suppressor genes in the p19ARF-Mdm2-p53 and p16INK4A-cyclin D-Rb cell cycle regulatory cascades are commonly identified in MPNSTs. TP53 loss of function mutations are particularly common in MPNSTs [16,105,130], having been found in up to 75% of these sarcomas [69]. Interestingly, however, these mutations often affect only one copy of the TP53 gene, which has led some investigators to suggest that TP53 hemizygosity may suffice for MPNST pathogenesis [182]. Mutations of CDKN2A are also quite commonly encountered in MPNSTs, occurring in about 50% of these tumors [96,138]. CDKN2A mutations dysregulate both the p19ARF-Mdm2-p53 and the p16INK4A-cyclin D-Rb cascades as this tumor suppressor gene encodes both p19ARF and p16INK4A, each of which acts as an inhibitor of its respective cell cycle regulatory cascade. The p19ARF protein inhibits Mdm2, a ubiquitin ligase that ubiquitinates p53 and targets it for proteasomal degradation. The p16INK4A protein inhibits both cyclin-dependent kinase 4 (CDK4) and cyclin-dependent kinase 6 (CDK6) which phosphorylate the Retinoblastoma (Rb) protein, thereby promoting transition through the G1/S checkpoint of the cell cycle. Consistent with the importance of the p16INK4A-cyclin D-Rb cascade, loss of Rb is also seen in about 25% of MPNSTs [119,122].
Directly testing the significance of these additional tumor suppressor mutations in mouse Nf1 knockout models has proven to be challenging, as neurofibromas in these animals do not progress to become MPNSTs at a high frequency. Consequently, most investigators have instead taken the approach of generating mouse models that carry Nf1 mutations in combination with mutations of genes in the p19ARF-Mdm2-p53 and/or the p16INK4A-cyclin D-Rb cascades. To examine a potential interaction between the Nf1 and Trp53 genes (both of which are located on chromosome 11 in mice), tumorigenesis was examined in mice carrying these genes on the same copy of chromosome 11 (cis Nf1+/-;Trp53+/- mice) and in animals with the genes on different chromosomes (trans Nf1+/- ;Trp53+/- mice) [29,187]. As whole chromosomal loss is the most common means by which “second-hit” mutations occur in mice [111], it was anticipated that MPNSTs would occur at a higher frequency in cis Nf1+/-;Trp53+/- mice. In keeping with this expectation, 30% of cis Nf1+/-;Trp53+/- mice developed de novo MPNSTs at a young age (5 months), while trans Nf1+/-;Trp53+/- mice lived twice as long before succumbing to other types of soft tissue sarcomas. Similarly, 26% of mice with Nf1 haploinsufficiency and homozygous null mutations of CDKN2A (Nf1+/- ;p16Ink4a-/-/p19Arf-/- mice) develop de novo MPNSTs [84]. Of note, mice with mutations designed to affect only one of the two transcripts encoded by the CDKN2A locus (Nf1+/-;p19Arf-/- or Nf1+/-;p16Ink4a-/- mice) do not develop MPNSTs [84,86], indicating that simultaneous dysregulation of the p19ARF-Mdm2-p53 and p16INK4A-cyclin D-Rb pathways is required for MPNST pathogenesis.
Aberrant growth factor signaling also contributes to neurofibroma and MPNST pathogenesis
Neurofibromin loss can conceivably induce a basal increase in Ras activation in neurofibromas and MPNSTs. However, given the central regulatory role of Ras and the large number of upstream effectors (e.g., membrane tyrosine kinases) that serve to activate these G-proteins, it is highly likely that that activation of growth factor receptors further enhances Ras activation in neurofibromas and MPNSTs (the alternative possibility, namely that Ras action is further enhanced by mutations of the Ras protein itself, is unlikely as Ras mutations are uncommon in these neoplasms [192]). This suggestion is consistent with work from a number of laboratories that shows that maximal activation of Ras downstream effectors (e.g., Raf-1) requires both neoplastic activation of Ras and the action of membrane tyrosine kinase receptors (e.g., see [120]). A number of growth factor signaling systems have been identified that may play such a role in NF1-associated peripheral nerve sheath tumors (please see our previous review of this topic [26]). These include the EGF receptor, neuregulin-1 (NRG1) and its erbB receptors, hepatocyte growth factor (HGF) and its c-Met receptor and platelet-derived growth factor (PDGF) and its receptors. Consistent with the postulated interaction described above, pharmacologic inhibition of several of these growth factor receptors has been shown to impede the migration and/or proliferation of neoplastic Schwann cells. However, only the effects that the EGF receptor and NRG1 exert on peripheral nerve sheath tumorigenesis have been directly tested in genetically engineered mice.
The EGF receptor is expressed in human neurofibromas and MPNSTs (but not in wild-type neonatal Schwann cells) [38,55]. Further, this locus is amplified in some MPNSTs [143] and EGF promotes the growth and survival of serum-starved MPNST cells [38]. These observations provided the impetus for the generation of transgenic mice expressing the EGF receptor in Schwann cells (CNP-hEGFR mice) [109]. CNP-hEGFR mice demonstrated hyperproliferative changes within peripheral nerve associated with fibrosis, increased numbers of mast cells and Schwann cell-axon dissociation. However, the development of well-developed neurofibromas was very rare in CNP-hEGFR mice and crossing them to Nf1+/- mice did not further enhance their phenotype. On the other hand, crossing mice with an EGF receptor hypomorphic mutation (EGFRwa-2) to cis Nf1+/-;Trp53+/- mice impeded the development of MPNSTs, which clearly indicates that the EGF receptor acts to enhance MPNST pathogenesis. Considered collectively, these observations suggest that the EGFR mice promotes the pathogenesis of NF1-associated peripheral nerve sheath tumors, but is not itself sufficient for their pathogenesis.
NRG1 is a potent Schwann cell mitogen and its receptors (especially erbB2 and erbB3) are expressed throughout Schwann cell development [200] and adulthood [23]. In keeping with the hypothesis that NRG1/erbB signaling promotes PNS neoplasia, rodents exposed during specific windows in embryogenesis to the chemical carcinogen N-ethyl-N-nitrosourea (EtNU) develop MPNST-like neoplasms with activating erbB2 mutations [21] and the erbB2 locus is amplified in some MPNSTs [173]. More recently, it has been shown that NRG1 and its erbB receptors are coexpressed by neoplastic Schwann cells within human neurofibromas and MPNSTs [171]. These receptors are constitutively activated (phosphorylated) and treatment with erbB inhibitors (e.g., PD168393) abolishes erbB phosphorylation and inhibits the proliferation [171] and migration [42] of MPNST cells. Transgenic mice expressing a secreted NRG1 isoform (glial growth factor-β3; GGFβ3) in Schwann cells under the control of the myelin protein promoter (P0-GGFβ3 mice) [72] develop prominent Schwann cell hyperplasia [72] that is followed by the development of multiple neurofibromas throughout the PNS (unpublished observations). Some of these neurofibromas progress and give rise to highly aggressive MPNSTs [72].
The findings noted above provide strong evidence that aberrant EGF receptor and NRG1/erbB signaling contributes to the pathogenesis of neurofibromas and MPNSTs. However, it is unlikely that these are the only growth factor signaling cascades that are relevant to the development of these neoplasms, especially given the diverse mixture of cell types characteristic of neurofibromas. These growth factor receptors potentially represent key druggable targets in neurofibromas and MPNSTs. Consequently, identifying the growth factor signaling cascades that interact with Ras signaling to promote the neurofibroma and MPNST pathogenesis is likely to be highly important for the future development of effective new therapies for these neoplasms.
An initial scheme describing the process of neurofibroma-MPNST pathogenesis...with some significant caveats
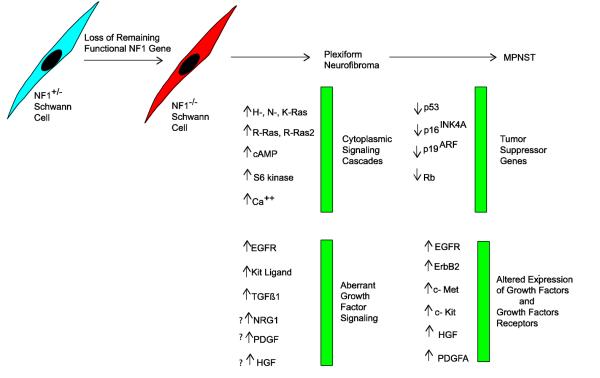
Considered jointly, these findings have led to a conceptual outline of the events responsible for the initial pathogenesis of plexiform neurofibromas and their subsequent progression to become MPNSTs (Fig. 6). In this scheme, loss of neurofibromin expression first occurs in a cell within the Schwann cell lineage. Neurofibromin loss, together with aberrant growth factor signaling, results in the hyperactivation of Ras proteins and signaling pathways regulated by Ras. This enhanced Ras signaling promotes the proliferative and invasive behavior of the neoplastic cells and their production of factors that recruit other NF1 haploinsufficient cell types into the nascent neurofibroma. The subsequent loss of additional tumor suppressor genes within the p19ARF-Mdm2-p53 and the p16INK4A-cyclin D Rb signaling cascades and amplification of key growth factor receptor genes then leads to the development of an MPNST derived from the neoplastic Schwann cells within the neurofibroma.
Fig. 6.
Schematic illustrating the changes in cytoplasmic signaling cascades that result from neurofibromin loss during the pathogenesis of neurofibromas and aberration in growth factor signaling that contribute to this same process. Also indicated are subsequently developing abnormalities in other tumor suppressor genes and additional alterations in growth factor signaling that contribute to the progression of a neurofibroma to become an MPNST.
This scheme is conceptually attractive and fits the available data. Nonetheless, this outline is almost certainly woefully incomplete and oversimplifies the process of neurofibroma pathogenesis and neurofibroma-MPNST progression. There are several lines of evidence that indicate this is the case. To begin with, it is highly likely that a loss of neurofibromin expression alone is not sufficient for tumorigenesis; a number of surveillance mechanisms exist to keep Ras activity in check and these must be overcome at least transiently for Ras hyperactivation to continue. This postulate is supported by experiments that have examined the consequences of ablating neurofibromin expression in fibroblasts. In these cells, neurofibromin loss triggers a transient activation of classic Ras proteins and their downstream effectors which is rapidly followed by inactivation of these proteins, growth arrest and the expression of senescence markers [32]. This negative feedback process, which is known as oncogene-induced senescence, relies upon the inhibition of GEFs and the enhanced activity of proteins opposing the action of Ras and other molecules in this signaling cascade [e.g., Sprouty proteins and dual specificity phosphatases (DUSPs)]. Senescent neurofibromin-negative Schwann cells are present in neurofibromas [32], which argues that these surveillance mechanisms are active at least initially in neoplastic Schwann cells. However, the growth of these lesions indicates that neurofibromin-null Schwann cells must have some means of overcoming oncogene-induced senescence. An alternative possibility is that neurofibroma pathogenesis is a more graduated process than is currently appreciated and that additional, as yet unidentified, mutations occur in neurofibromin-null Schwann cells that cooperate with NF1 loss. Consistent with this latter possibility, array comparative genomic hybridization (aCGH) studies of neurofibromas have identified regions of reproducible unbalanced chromosomal loss that are distinct from the NF1 gene [94].
There is also evidence to indicate that as yet unidentified modifier genes exist that influence the development of neurofibromas and MPNSTs. Epidemiologic studies of the occurrence of dermal and plexiform neurofibromas in patient cohorts that included pairs of monozygotic twins indicated that the likelihood of tumor occurrence correlated best between these twins relative to other members of their pedigrees [41]. In addition, neurofibroma occurrence correlated most closely between more closely related members of each pedigree. Mouse models also support the existence of such modifier genes. Strain background clearly influences the occurrence of MPNSTs in both cis Nf1+/- ;Trp53+/- [66] and P0-GGFβ3 (unpublished observations) mice. There is also evidence that two unlinked polymorphic loci referred to as nerve sheath tumor resistance 1 (Nstr1) and Nstr2 impact upon MPNST pathogenesis in cis Nf1+/-;Trp53+/- mice [147].
Genomic studies of MPNSTs and MPNST cell lines also suggest that a number of driver genes contributing to MPNST pathogenesis have not yet been identified. It has been widely noted that the karyotype of MPNSTs is highly complex and variable, with multiple regions of chromosomal gain (chromosomes 7, 8q, 15q) and loss (particularly involving chromosomes 1p, 9p, 11, 12p, 14q, 17q, 18, 22q, X and Y) being frequently observed in MPNSTs [25]. These regions of unbalanced chromosomal gains and losses do not correspond to known oncogenes and tumor suppressors that have been implicated in the pathogenesis of either neurofibromas or MPNSTs and thus may contain other genes contributing to the development of these tumors. Finally, it must be noted that some sporadic MPNSTs have intact, apparently functional copies of NF1 [134]. This argues that there may be pathways to MPNST pathogenesis that are independent of NF1 loss.
SCHWANNOMAS
In contrast to neurofibromas, schwannomas are composed solely of neoplastic Schwann cells and occur in association with multiple genetic diseases
Like neurofibromas, schwannomas (sometimes referred to as neurilemomas) are benign tumors of peripheral nerve. However, the similarities between these two tumor types largely end there. Whereas neurofibromas contain a complex mixture of cell types, schwannomas are composed solely of well-differentiated (albeit neoplastic) Schwann cells (Fig. 7). Schwannomas are typically encapsulated globular lesions that often ‘push’ the associated nerve to one side rather than infiltrating the nerve in the manner characteristic of neurofibromas. Further, malignant transformation of schwannomas is exceedingly rare. Although some uncommon schwannoma variants are periodically encountered [e.g., cellular schwannomas (a benign variant with cellularity higher than a conventional schwannoma), plexiform schwannomas (schwannomas with a growth pattern reminiscent of plexiform neurofibromas) and melanotic schwannomas (schwannomas containing extensive melanin and at times psammoma bodies), the primary significance of these variants is that they may be mistaken for a more aggressive tumor type (cellular schwannomas) or herald the presence of a genetic disease (plexiform schwannomas, melanotic schwannomas).
Fig. 7.
Unlike neurofibromas, schwannomas are composed almost exclusively of neoplastic Schwann cells and their supporting vasculature. (a) Hematoxylin and eosin stained section of an acoustic schwannoma resected from a 27 year old Caucasian man with NF2. (b, c) Acoustic schwannoma immunostained for the Schwann cell markers S100β (b) and collagen type IV (c). Note that immunoreactivity for the basement membrane protein collagen type IV invests each individual tumor cell. (d) Double label immunohistochemical preparation of an acoustic schwannoma stained for S100β (green) and CD34 (orange-red). In this tumor, unlike the neurofibroma illustrated in Fig. 1, CD34 immunoreactivity is only evident in intratumoral blood vessels.
Schwannomas are commonly encountered in the general population as solitary lesions. However, these peripheral nerve sheath tumors also occur multiply in patients with three distinct genetic diseases— neurofibromatosis type 2 (NF2), Carney complex type 1 and schwannomatosis. As the identification of the tumor suppressor genes that are affected in these disorders has provided key insights into the molecular mechanisms underlying the pathogenesis of schwannomas, I will discuss below each of these genetic diseases, our current understanding of the function of the protein encoded by the mutated genes and how their loss leads to neoplasia. Interestingly, the NF2, Carney complex type 1 and schwannomatosis tumor suppressor genes all potentially impact on a common cytoplasmic signaling cascade. This convergence points to key events that are likely essential for the development of schwannomas. I would also refer readers interested in more detailed descriptions of the clinical features of these diseases to the article by Rodriguez et al. in this issue.
Identification of the gene affected in NF2 points to a distinct set of signaling pathways capable of promoting Schwann cell neoplasia
NF2, the most common genetic disease associated with schwannoma pathogenesis, is estimated to affect 1 in 25,000 newborn infants. Schwannomas developing in NF2 patients typically arise in the vestibular branch of the eighth cranial nerve and may be unilateral or bilateral. Multiple schwannomas are also commonly found on cranial nerves or spinal nerve roots in these patients as well as cutaneously, where they are often plexiform. In addition to schwannomas, NF2 patients are prone to the development of meningiomas and, less commonly, ependymomas. Cataracts, retinal hamartomas and combined pigment epithelial and retinal hamartomas are also commonly found in these patients. Like NF1, NF2 is inherited in an autosomal dominant fashion, which is consistent with the NF2 gene encoding a tumor suppressor. The NF2 gene also has a similarly high rate of de novo mutation, resulting in about 50% of infants with NF2 being born into families with no previous history of the disease [47]; further, about a third of patients presenting with clinical features of NF2 demonstrate mosaicism for this mutation [47]. NF2 is completely penetrant in virtually all patients by the time they are 60 years old.
The NF2 tumor suppressor gene, which is located on chromosome 22 (22q12.2), was cloned in 1993 [152,180]. This locus spans 93,083 base pairs and includes 17 exons. The NF2 gene produces at least 10 protein isoforms via a combination of alternative splicing and the use of multiple transcription initiation sites [65,74,145]. However, isoforms I and II, which have distinct carboxy terminal sequences produced by alternative splicing of exons 16 and 17, respectively, predominate. The proteins encoded by the NF2 gene are highly unusual in that they do not contain catalytic domains such as are present in neurofibromin and many other tumor suppressors. These polypeptides are instead structurally similar to three molecules in the protein 4.1 superfamily that are known as ezrin, radixin and moesin (the ‘ERM’ proteins). These three ERM proteins link the actin cytoskeleton to membrane-spanning proteins, thereby organizing complex membranous domains and regulating cellular adhesion, migration, cellular morphology, exocytosis and endocytosis. Because of this structural similarity, the NF2 protein was dubbed ‘merlin’ (moesin-, ezrin-, radixin-like protein) [180]; as loss of the NF2 gene is associated with schwannoma pathogenesis, the NF2 protein is also sometimes referred to as schwannomin [152].
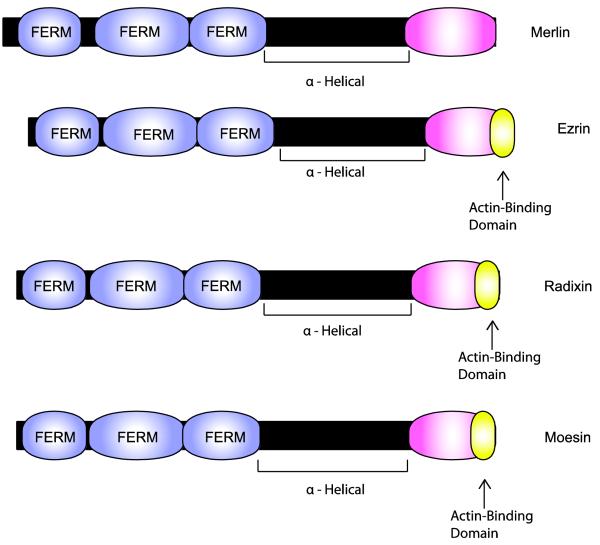
Merlin and the ERM proteins have some important functional differences, despite their structural similarity. Moesin, ezrin and radixin contain an amino-terminal FERM (protein 4.1-ezrin-radixin-moesin) domain that is linked, via an α-helical region, to a carboxy terminal ERM-binding domain (the C-ERMAD; Fig. 8) which includes an F-actin binding motif. The activity of the ERM proteins is regulated by intramolecular (‘head-to-tail’) interactions between the FERM domain and the carboxy terminal domain. In the ‘closed’ (head-to-tail interacting) configuration, the FERM domain binds the C-ERMAD and masks the actin-binding site, maintaining moesin, ezrin and radixin in an inactive state. Phosphatidylinositol (4,5)-bisphosphate (PIP2) binding to the FERM domain and subsequent phosphorylation of critical threonine residues within the carboxy terminal domain disrupts head-to-tail interactions within ERM proteins, resulting in their activation. Merlin shares the FERM/α-helical/C-ERMAD structure of ERM proteins, but its carboxy terminal domain lacks an actin-binding motif. Merlin isoform I (a 595 amino acid protein encoded by exons 1-15 and 17), like ERM proteins, is regulated by intramolecular interactions [165]. However, phosphorylation of Ser518 in the merlin C-ERMAD, which maintains merlin in the open configuration, results in the inactivation of merlin rather than its activation [5,151].
Fig. 8.
Schematic illustrating the functional domains present in merlin and comparing the structure of merlin to that of its closest relatives (ezrin, radixin and moesin). The lengths of each protein (indicated by the black bars) and domains within each of these proteins (indicated by the colored expansions) are scaled to the actual number of amino acids in each. Note that all four of these proteins contain a highly conserved amino terminal FERM domain composed of three related subdomains. In each of these proteins, the FERM domain is linked to a COOH domain by an α-helical region that allows these proteins to flex and facilitates intramolecular association between the FERM and COOH domains. As indicated in the text, this intramolecular association regulates the activity of these proteins. Curiously, however, merlin is apparently active in the “closed” configuration while this configuration is inactive for the other three proteins. A key signal regulating the configuration of these proteins is the phosphorylation of Ser518 in merlin and specific threonine residues in the COOH domain of ezrin, radixin and moesin. Ezrin, radixin and moesin all also contain a domain located at their extreme carboxy terminus that is capable of binding to filamentous (F) actin. This domain is not conserved in merlin.
In keeping with clinical observations suggesting that merlin is a tumor suppressor, germline DNA from NF2 patients contains a mutated NF2 gene and a functional wild-type allele while schwannomas developing in these patients carry two mutated NF2 genes [160]. The importance of NF2 mutations in schwannoma pathogenesis is further demonstrated by the observation that overwhelming majority of sporadic schwannomas have lost the expression of merlin, but not other ERM proteins [169]. Direct evidence that merlin functions as a tumor suppressor has been obtained by showing that overexpression of this protein inhibits both mitogenesis [112] and oncogene-induced transformation [178]. Further, re-expressing merlin in human schwannoma cells reduces both the proliferation and the survival of these cells [158]. Nonetheless, it is clear that merlin’s action as a tumor suppressor is rather unorthodox. While merlin does repress cyclin D1 expression [203], it is localized at sites where the cytoskeleton contacts the plasma membrane such as membrane ruffles and nascent cell-cell contact points [57,99,116]. Levels of dephosphorylated (active) merlin are increased at these sites in cells experiencing growth arrest secondary to stimuli such as contact inhibition, growth factor deprivation, exposure to the extracellular matrix component hyaluronic acid or a loss of adhesion to the extracellular matrix [60,99,135,163-165]. Consequently, it is thought that merlin inhibits proliferation by integrating extracellular signaling transmitted by membrane-spanning proteins with the action of molecules in multiple key cytoplasmic signaling pathways (see below).
As is evident from the discussion above, phosphorylation is a key means of regulating merlin’s activity. Merlin can be phosphorylated at multiple residues [163]. However, most studies to date have focused on the effects mediated by phosphorylation of Ser518 within merlin’s C-ERMAD domain. Events such as stimulation by growth factors, attachment of cells to substrate and cell density all regulate the phosphorylation of merlin at this serine [164]. Consistent with the postulated ability of merlin to integrate signals from disparate signaling pathways, several kinases are capable of phosphorylating merlin Ser518. Activation of the small G-protein Rac1 results in the phosphorylation of merlin at this critical residue [164] via the action of the Rac1 effector molecule p21-activated kinase (Pak; Fig. 9) [90,202]. Alternatively, protein kinase A, a cAMP-dependent signaling molecule, can phosphorylate merlin at this same site [5]. In Drosophila, the Sterile20-like kinase Slik also phosphorylates merlin at Ser518 [71]. However, it is currently unclear whether serine/threonine kinase 10 (STK10), the mammalian equivalent of Slik kinase, similarly phosphorylates merlin in mammalian cells. It is also likely that PIP2 binding to the merlin FERM domain acts cooperatively with Ser518 phosphorylation to regulate merlin activity as the PIP2 binding site identified in ezrin [10] is conserved in merlin.
Fig. 9.
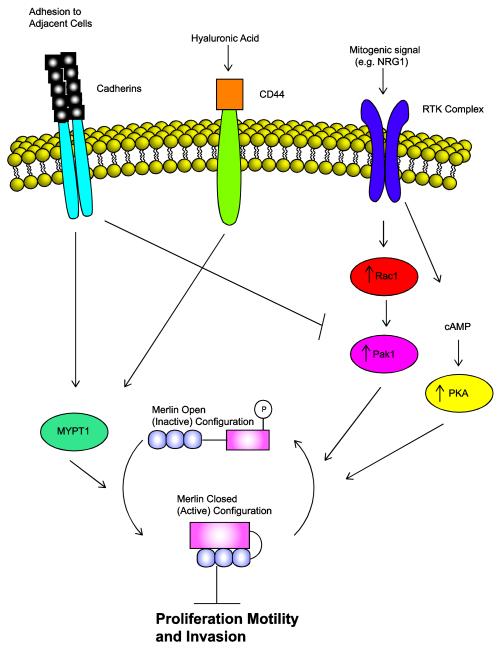
A schematic demonstrating the cycling of merlin between its active (dephosphorylated) and inactive (phosphorylated) configurations and how this activity affects major cytoplasmic signaling pathways important to Schwann cell biology. Note that different intramembranous receptors can inactivate [e.g. receptor tyrosine kinase (RTK) complexes] or activate (cadherins, CD44) merlin by activating kinases [protein kinase A (PKA), p21-activated kinase (Pak)] that phosphorylate merlin or phosphatases (MYPT1) that remove phosphate groups.
More recently, the functional significance of phosphorylation of Ser10, a residue within the N-terminus of merlin, has been examined. Ser10, which is conserved in humans, mice, rats and Xenopus, can be phosphorylated by either protein kinase A [101] or Akt [102]. Curiously, phosphorylation of merlin Ser10 does not affect Ser518 phosphorylation or promote heterodimerization with ezrin, an event previously demonstrated to occur following Ser518 phosphorylation [5]. Mutation of Ser10 to alanine (which is non-phosphorylatable) and subsequent expression of the mutated merlin protein in Nf2-/- fibroblasts instead is associated with the extension of long processes from the cells and inhibition of migration. In contrast, similarly introducing merlin with a Ser10 to glutamate (S10D) mutation (which mimics a phosphorylation event at this site) into fibroblasts causes the cells to elaborate numerous short filopodia-like protrusions and stabilizes actin in a filamentous form [101]. Considered collectively, these observations indicate that phosphorylation of Ser10 plays a key role in regulating cellular morphology.
Ser10 phosphorylation has also been reported to affect the half-life of merlin protein by enhancing the interaction of merlin with DCAF1 (DDB1- and Cul4-associated factor 1) [102], a protein that serves as a substrate receptor for the E3 ubiquitin ligase CRL4DCAF1. This interaction results in the ubiquitination of merlin and its subsequent degradation by the proteasome. In contrast, however, others have presented evidence that activated (dephosphorylated) merlin accumulates within the nucleus where it binds to the carboxy terminal region of DCAF1, resulting in suppression of CRL4DCAF1 activity [108]. Further, depletion of DCAF1 in merlin-null schwannoma cells inhibits the increased proliferation characteristic of these cells, indicating that enhanced CRL4DCAF1 activity occurring secondary to merlin loss promotes tumorigenesis. The importance of this interaction is underscored by the observation that specific merlin mutations identified in NF2 patients impair the interaction between merlin and DCAF1 [108]. Thus, it appears that merlin’s tumor suppressor function is strongly dependent upon its ability to translocate into the nucleus and block the action of CRL4DCAF1.
Given the presence of multiple functional domains and key phosphorylation sites in merlin, it is reasonable to ask both whether mutations at specific sites in the NF2 gene predispose patients to the pathogenesis of schwannomas and whether particular types of mutations result in increased numbers of schwannomas. Highly effective comprehensive screening approaches designed to detect frameshift mutations, nonsense mutations, missense mutations and small indels (direct sequencing of all coding sequences) as well as copy number alterations (multiple ligation-dependent probe amplification) within the NF2 gene have been developed and detect mutations in 93% of non-founder NF2 patients [45]. Genotype-phenotype correlations clearly indicate that patients with truncating nonsense mutations or frameshift mutations (which likely result in the production of an unstable protein product) diagnosed at a younger age and have a higher mean number of schwannomas [46,153]. In contrast, the disease phenotype is much milder in NF2 patients with large deletions, in-frame indels and missense mutations [153], while patients carrying mutations that affect RNA splice sites vary in the severity of their disease [12,93]. The missense mutations identified in the former patients tend to be clustered in the FERM domain, but are also often found in sequences encoding the α-helical and carboxy terminal domains of merlin [4,11]. Of note, most large clinical series agree that approximately 95% of NF2 patients ultimately develop bilateral acoustic schwannomas [44,167] and so the differences noted above primarily reflect differences in the numbers of schwannomas developing at sites other than the VIIIth cranial nerve (e.g., spinal nerve roots). This observation, as well as the fact that patients with schwannomatosis develop schwannomas on spinal nerve roots but not their acoustic nerves (see below), raises the question of whether there are other, as yet unidentified, site-specific pathogenic factors that affect schwannoma development.
Merlin links signaling from multiple cell surface receptors to essential cytoplasmic signaling pathways
Given their importance in Schwann cell biology, it is perhaps not surprising that the Schwann cell growth factor NRG1 and merlin play opposing roles in the control of Schwann cell mitogenesis (Fig. 9). Our laboratory [172] and others [64] have found that NF2-associated and sporadic schwannomas express multiple NRG1 isoforms together with activated forms of the NRG1 receptor subunits erbB2 and erbB3. In addition, erbB inhibitors effectively inhibit the proliferation of human schwannoma cells in vitro [1,6] and in xenografted immunodeficient mice [30]. In wild-type Schwann cells, the adaptor protein paxillin binds to merlin, directing merlin to the cell membrane where it forms a molecular complex that includes erbB2 and β1 integrin [49]. At the cell membrane, activated merlin represses the accumulation of both erbB2 and erbB3, resulting in an inhibition of Akt and MAPK signaling [100]. Thus, loss of merlin in schwannomas results in enhanced membranous localization of NRG1 receptors [100] which presumably results in elevated signaling by Akt and MAPK. Merlin also likely interacts indirectly with the NRG1 receptors by binding to cytoplasmic signaling molecules that interact with erbB2 and/or erbB3. For example, merlin binds to magicin [197], a molecule that forms a complex with the well known erbB effector molecule Grb2 [156]. Merlin also binds NHE-RF1 (also known as EBP50) [136,146], a molecule that in turn interacts with erbin, a PDZ domain protein that binds the cytoplasmic tail of erbB2 and stabilizes this membrane tyrosine kinase [176].
Although schwannomas typically do not express the EGF receptor [172], merlin inhibits the internalization of this kinase in other cell types, suggesting that merlin can also control growth factor signaling by regulating the intracellular trafficking of growth factor receptors. In keeping with this postulate, expression of the receptors for insulin-like growth factor 1 (IGF1) and platelet-derived growth factor (PDGF) is enhanced in peripheral nerves from Nf2 mutant mice and in human schwannomas [100]. Of note, the effect that merlin loss has on the accumulation of the IGF1 and PDGF receptors in Schwann cells likely is of significance for schwannoma pathogenesis. Both IGF1 and PDGF are well known Schwann cell mitogens. Further, merlin overexpression in schwannoma cells results in accelerated PDGF receptor degradation, an inhibition of mitogenesis and reduced activation of the Raf-ERK-MAPK and PI3 kinase-Akt signaling cascades [54]. Pharmacologic inhibitors of the PDGF receptor and c-Raf likewise effectively inhibit the proliferation of human schwannoma cells [7]. Thus, as in neurofibromas and MPNSTs, multiple growth factor signaling cascades may contribute to the pathogenesis of schwannomas and represent important therapeutic targets in these neoplasms.
In contrast, intramembranous receptors that transmit signals indicating contact with extracellular matrix or adjacent cells (contact inhibition) can cooperate with merlin to inhibit mitogenesis (Fig. 9). This first became evident when it was noted that merlin becomes activated (dephosphorylated) in confluent cells [135,163] and that this activation is both necessary and sufficient for contact inhibition [99,135,140]. CD44 and cadherins, which serve as extracellular matrix and adhesion receptors, appear to be particularly important for merlin activation in these situations. CD44 is a receptor for hyaluronic acid, an important component of the extracellular matrix in peripheral nerve [179]. Merlin binds directly to the intracellular (cytoplasmic) domain of CD44 [9,155], resulting in contact-mediated inhibition of proliferation [135]. In keratinocytes, merlin also associates with adherens junctions, where it interacts with complexes of E-cadherin and β-catenin [99]. Wild-type Schwann cells express both E-cadherin [33,129,209] and N-cadherin [107,190,191]; consistent with the findings delineated above, loss of merlin results in disruption of adherens junctions in primary cultures of schwannoma cells [53].
That is not to say that all intramembranous receptors involved in extracellular contacts cooperate with merlin to inhibit mitogenesis. As noted above, activated forms of the NRG1 receptors erbB2 and erbB3 associate with β1 integrin [49]. Simultaneous treatment of Schwann cells with NRG1β and the β1 integrin ligand laminin-1 synergistically promotes the inactivation (phosphorylation) of merlin [177]. Further, primary human schwannoma cells, which demonstrate pathologic adhesion to the extracellular matrix [52,183], express elevated levels of β1 and β4 integrins [183]. This overexpression of integrins, considered together with the fact that merlin loss results in other protumorigenic events such as integrin-dependent constitutive activation of mTORC1 and enhanced mRNA translation [110], suggests that integrins likely cooperate with associated growth factor receptors to promote schwannoma pathogenesis.
Considerable evidence indicates that the activation of merlin by growth factor receptors and integrins and its inactivation by CD44 and cadherins results in changes in the actin cytoskeleton that subsequently effect cellular motility, invasion and proliferation. In keeping with this, loss of merlin in schwannoma cells results in profound cytoskeletal changes manifest as altered membrane ruffling, changes in cell spreading and rearrangement of stress fibers [142]. These changes can be reversed by reexpressing merlin in schwannoma cells [13]. Nonetheless, although several of the molecules that mediate merlin’s effects on the cytoskeleton have been identified over the last decade, the interactions of these molecules with merlin are complicated and incompletely understood. For instance, it is apparent that negative feedback loops involving merlin and the Rho subfamily of small G-proteins (Rho, Rac, Cdc42) play a central role in shaping the actin cytoskeleton. As noted above, activation of the Rac-Pak signaling cascade results in merlin inactivation. Overexpression of merlin in turn inhibits Rac-dependent signaling cascades [164]; in keeping with this observation, Nf2-/- cells demonstrate changes analogous to cells with inappropriate activation of Rac proteins [164]. However, merlin also binds Rho guanine-dissociation inhibitor (Rho GDI) [116,175], an inhibitor of Rho and Rac that acts by impeding nucleotide release from these small G-proteins [39,40]; this results in activation of Rho family proteins. There is also evidence that merlin binds directly to and inhibits Pak [91]. Thus, the picture that is emerging is that merlin plays a complicated role in the regulation of Rho/Rac/Pak signaling, potentially affecting actin remodeling by acting at multiple sites in this key signaling cascade.
It has also been argued that merlin may more directly control the remodeling of the actin cytoskeleton. As noted above, merlin lacks the carboxy terminal actin-binding domain that is present in ezrin, radixin and moesin. However, merlin may instead bind actin directly via sequences in its amino terminus [20,79] or indirectly through interactions with other ERM proteins [59,128,137], paxillin [49] or the cytoskeletal protein βII-spectrin [159]. Merlin also binds to and inhibits the action of other proteins directly involved in remodeling of the actin cytoskeleton such as N-WASP [117]. N-WASP is an activator of Arp2/3, a complex which directs the nucleation of actin filaments and promotes the branching of actin filaments in membrane ruffles and adherens junctions [51]. Inhibition of N-WASP action by merlin therefore inhibits the cell’s ability to construct new actin assemblages.
In the last five years, considerable attention has been focused on the ability of merlin to regulate the Hippo signaling cascade, a pathway that plays an essential role in cellular proliferation and survival [67]. Evidence that merlin regulates the Hippo signaling cascade (which includes the Ste20 kinase Hippo, Salvador, Warts, Mats and Yorkie) has come predominantly from studies in Drosophila, where inactivating mutations of merlin result in enhanced mitogenesis and cell survival [63,124]. Mammalian homologs for each of these Drosophila proteins have been identified and several of them have been shown to play important roles in human cancers. However, molecules in the Hippo signaling cascade have not yet been shown to affect the pathogenesis of schwannomas.
Genetically engineered mouse models confirm a key role for Nf2 loss in schwannoma pathogenesis
Initial attempts to produce a mouse model of NF2 were directed towards producing genetically engineered mice with null mutations at the murine equivalent of sites that were frequently affected in human NF2 patients. Since a number of human mutations occurred within sequences encoding the merlin FERM domain (exons 2 and 3), a region that included half of exon 2 and all of exon 3 was deleted in the initial mouse model of NF2 [127]. When bred to homozygosity, these Nf2 null embryos failed to undergo gastrulation due to an absence of the extraembryonic ectoderm, a structure which normally produces signals required for the induction of mesoderm formation. Mice heterozygous for this Nf2 mutation frequently developed aggressive osteosarcomas as well as a number of other malignancies such as lymphomas, pulmonary adenocarcinomas, hepatocellular carcinomas and fibrosarcomas [126]. These malignancies demonstrated a strikingly high rate of metastasis, suggesting that Nf2 loss promotes metastasis. Unfortunately, however, Nf2+/- mice did not develop schwannomas, meningiomas, ependymomas or other manifestations characteristic of human NF2.
One possible explanation for the lack of schwannomas in Nf2+/- mice was that loss of the remaining functional Nf2 allele occurred at a very low rate in Schwann cells compared to other cell types. Consequently, mice expressing Cre recombinase under the control of the Schwann cell-specific myelin protein zero (P0) promoter were crossed to animals with floxed Nf2 genes, resulting in progeny with Nf2-null Schwann cells (P0-Cre;Nf2flox/flox mice) [56]. In contrast to Nf2+/- mice, these P0-Cre;Nf2flox/flox mice developed Schwann cell hyperplasia and schwannomas as well as cataracts, intracranial calcifications, osteomas and osteosarcomas. The schwannomas developing in P0- Cre;Nf2flox/flox mice have a cellular composition analogous to that of human schwannomas. However, the murine schwannomas do differ in some respects from their human counterparts. Of particular note, P0-Cre;Nf2flox/flox schwannomas, unlike human schwannomas, are not encapsulated and tend to infiltrate into adjacent soft tissue [168]. Nonetheless, the peripheral nerve sheath tumors arising in P0-Cre;Nf2flox/flox mice are clearly schwannomas and consequently this conditional knockout mouse model provides the best direct evidence available showing that NF2 loss promotes the pathogenesis of schwannomas.
Identification of the gene affected in Carney complex type 1 highlights an important role for protein kinase A in the pathogenesis of schwannomas
Patients with Carney complex (also known as Carney complex type 1, Carney myxoma-endocrine complex or Carney syndrome) are also prone to develop multiple schwannomas. Peculiarly, however, patients with this genetic disease develop an unusual schwannoma variant known as psammomatous melanotic schwannomas rather than the more conventional schwannomas seen in NF2 and schwannomatosis. Other clinical manifestations of Carney complex are the occurrence of myxomas in heart, skin or breast, lentiginous pigmentary lesions, blue nevi and endocrine overactivity. This condition is inherited in an autosomal dominant fashion, consistent with the affected gene encoding a tumor suppressor.
The occurrence of schwannomas in both NF2 patients and patients affected by Carney complex suggests that the genes affected in these conditions might act within interrelated signaling cascades that promote schwannoma pathogenesis. This suspicion was confirmed when the Carney complex gene located on the long arm of chromosome 17 (Carney complex type 1) was cloned and found to encode the type 1A regulatory subunit of protein kinase A (PRKAR1A) [87,89]. The PRKAR1A gene spans 20,798 base pairs and includes 11 exons encoding a 381 amino acid protein which contains two cAMP-binding domains and a protein dimerization domain. In its inactive state, protein kinase A is a tetramer that is composed of two regulatory subunits and two catalytic subunits that function as serine/threonine kinases. The binding of two cAMP molecules to each regulatory subunit elicits a conformational change in the subunit, resulting in the release of the active catalytic subunits. Initial analyses of PRKAR1A mutations indicated that the majority of mutations in Carney complex patients are functionally null [89]. Consistent with PRKAR1A functioning as a tumor suppressor, loss of heterozygosity for this locus is evident in tumors developing in Carney complex patients [87]; this loss of PRKAR1A expression results in increased total cAMP-stimulated kinase activity [18,149,150,170]. Subsequently, patients with expressed mutant proteins were identified. These mutations all resulted in enhanced protein kinase A activity which was attributed to decreased binding of mutant PRKAR1A protein to either the catalytic subunit or cAMP [58].
To directly test the role of PRKAR1A in the pathogenesis of schwannomas and other clinical features associated with Carney complex, Prkar1a+/- mice were generated [88]. Starting at 6 months of age, these mice developed nonpigmented schwannomas and fibro-osseous bone lesions. Further, mutation of the wild-type Prkar1a allele was evident in approximately a third of the cells within the murine schwannomas, consistent with the postulated role of Prkar1a as a tumor suppressor. Similarly, mice with complete loss of Prkar1a in facial neural crest cells (TEC3-Cre;Prkar1aflox/flox mice) developed schwannomas in the affected region [88]. A subsequent examination of candidate signaling molecules in these murine tumors showed that their expression of neurofibromin protein was decreased, presumably via a posttranslational mechanism [83]. Curiously, the loss of neurofibromin protein in TEC3-Cre;Prkar1aflox/flox schwannomas was associated with enhanced activation of Rac1, but not Ras proteins. Thus, PRKAR1A mutation both results in activation of the protein kinase A signaling pathways and effects other proteins previously implicated in the pathogenesis of peripheral nerve sheath tumors.
The pathogenesis of schwannomas in patients with schwannomatosis results from mutations in a novel type of tumor suppressor gene...possibly with assistance from a next-door neighbor
Patients with schwannomatosis (also known as neurilemmomatosis or neurofibromatosis type 3) present with multiple spinal nerve root schwannomas and cutaneous schwannomas that are often quite painful [8,28,113,115,174]. In contrast to patients with NF2, however, individuals affected by schwannomatosis do not develop vestibular schwannomas nor do they fulfill other diagnostic criteria for NF2 [50]. Schwannomatosis can occur sporadically or familially, although the familial form of this disease is strangely rare [113].
Given the overlap between the clinical features of NF2 and schwannomatosis, it is understandable that investigators long debated whether schwannomatosis was a distinct entity or simply an NF2 variant. However, linkage analyses ultimately excluded the NF2 locus as the site of the germline mutation in families with schwannomatosis and instead pointed to a different region on chromosome 22 that was located closer to the centromere [114]. Inactivating mutations of SMARCB1 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily B, member 1; also known as hSNF5 and INI1), a gene located at 22q11.2, were subsequently identified in families with schwannomatosis [73]. This gene was a known tumor suppressor, having been previously identified as the gene located within a region that is often deleted in malignant rhabdoid tumors and atypical teratoid/rhabdoid tumors (AT/RTs) [185]. Loss of function mutations of SMARCB1 had also been found to be the cause of rhabdoid predisposition syndrome-1 (OMIM #609322) [162].
The SMARCB1 tumor suppressor gene spans 47,554 base pairs and includes 9 exons which encode a 385 amino acid protein. This protein is a subunit of the SWI/SNF ATP-dependent chromatin remodeling complex which relaxes regions of repressed chromatin, thereby allowing the transcriptional machinery to access target genes. In malignant rhabdoid tumors, loss of SMARCB1 function results in polyploidy and chromosomal instability [188]; re-expression of SMARCB1 in these tumor cells couples cell cycle progression back to ploidy controls via a pathway that utilizes the cell cycle regulators p16Ink4a, cyclin D, CDK4, Rb and E2F. These observations therefore raised the possibility that SMARCB1 loss might similarly result in chromosomal instability in Schwann cells, leading to the development of schwannomas. However, analyses of somatic mutations in schwannomas from schwannomatosis patients suggested a more complicated possibility. Germline NF2 mutations are not typically found in schwannomatosis patients. Nonetheless, schwannomas arising in these patients frequently show biallelic inactivating mutations of NF2 [62,161]. Based on these observations, it was proposed that a ‘4-hit’ mechanism, in which both SMARCB1 and NF2 are inactivated, underlies the pathogenesis of schwannomas in patients with schwannomatosis.
Genetically engineered mouse models provide a potential means of determining which of these models better describes schwannoma development in the context of schwannomatosis. Unfortunately, although a conventional knockout model of Smarcb1 has been constructed [148], it has not provided a clear answer to this question. Smarcb1-/- mice die in utero at embryonic day 7, well before the appearance of Schwann cell progenitors. Smarcb1+/- mice appear normal until they begin to develop malignant rhabdoid tumors at 5 weeks of age, but have not been reported to develop schwannomas. Given the fact that apparently low levels of ‘second-hit’ mutations in Schwann cells did not lead to peripheral nerve sheath tumor formation in Nf1+/- and Nf2+/- mice, it is quite possible that the same issue limits the development of schwannomas in Smarcb1+/- mice. Consequently, it is likely that mice with conditional Smarcb1 knockouts in Schwann cells will be required to determine whether Smarcb1 loss alone is sufficient for schwannoma pathogenesis. Such mice have not yet been described in detail. However, a recent report from the 5th NCI Mouse Models of Human Cancers Consortium Nervous System Tumors Workshop noted that mice in which Smarcb1 was conditionally inactivated in Schwann cell precursors had been reported to develop peripheral nerve tumors and that mice in which both Nf2 and Smarcb1 are ablated in these glia are currently under development [61]. Hopefully, these new models will clarify the respective roles that Smarcb1 and Nf2 mutations play in the pathogenesis of schwannomas in schwannomatosis patients.
Conclusions and some unanswered questions regarding the molecular mechanisms involved in schwannoma pathogenesis
Based on the findings outlined above, a complex picture of schwannoma pathogenesis is emerging in which the loss of merlin plays a central role in the pathogenesis of both sporadic and NF2 associated schwannomas. The possibility that inactivation of both SMARCB1 and NF2 is necessary for the pathogenesis of schwannomas in the setting of schwannomatosis potentially links merlin loss to the development of these tumors as well. It also seems likely that PRKAR1A loss in the schwannomas developing in Carney complex patients results in inhibition of merlin signaling. The findings described above support the hypothesis that inappropriate activation of protein kinase A resulting from PRKAR1A loss promotes schwannoma pathogenesis. However, protein kinase A also phosphorylates and inactivates merlin, suggesting that merlin will be functionally repressed in Carney complex-associated schwannomas. Considered together, these findings argue that genetic or functional loss of merlin is a key step in the pathogenesis of most, if not all, schwannomas.
Nonetheless, it is quite clear that our understanding of the molecular mechanisms responsible for the pathogenesis of schwannomas is still very rudimentary. As was noted in the discussion above, major questions remain unanswered regarding the signaling cascades that are affected by merlin loss. The list of proteins that interact with merlin continues to grow at a rapid clip—moving forward, it will be necessary to determine which of these interactions are important for the development of schwannomas and what the consequences of these interactions are. In addition, only very limited genomic and epigenetic studies of schwannomas have been performed to date and it seems likely that there are other, as yet undiscovered genetic and epigenetic abnormalities occurring in schwannomas that interact with the mutations we already know about. Answering questions such as these will be critically important as basic scientists and clinicians work together to develop effective new therapies for schwannomas.
Acknowledgements
This work was supported by the National Institute of Neurological Diseases and Stroke (R01 NS048353 to S.L.C.), the National Cancer Institute (R01 CA122804 to S.L.C.; R01 CA134773 to Kevin A. Roth and S.L.C.) and the Department of Defense (X81XWH-09-1-0086 to S.L.C.). We thank the Alabama Neuroscience Blueprint Core Center (P30 NS57098) for technical assistance with studies from our laboratory that are described in this review. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
References
- 1.Ahmad ZK, Brown CM, Cueva RA, Ryan AF, Doherty JK. ErbB expression, activation, and inhibition with lapatinib and tyrphostin (AG825) in human vestibular schwannomas. Otol Neurotol. 2011;32:841–847. doi: 10.1097/MAO.0b013e31821f7d88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmadian MR, Hoffmann U, Goody RS, Wittinghofer A. Individual rate constants for the interaction of Ras proteins with GTPase-activating proteins determined by fluorescence spectroscopy. Biochemistry. 1997;36:4535–4541. doi: 10.1021/bi962556y. [DOI] [PubMed] [Google Scholar]
- 3.Ahmadian MR, Kiel C, Stege P, Scheffzek K. Structural fingerprints of the Ras-GTPase activating proteins neurofibromin and p120GAP. J Mol Biol. 2003;329:699–710. doi: 10.1016/s0022-2836(03)00514-x. [DOI] [PubMed] [Google Scholar]
- 4.Ahronowitz I, Xin W, Kiely R, Sims K, MacCollin M, Nunes FP. Mutational spectrum of the NF2 gene: a meta-analysis of 12 years of research and diagnostic laboratory findings. Hum Mutat. 2007;28:1–12. doi: 10.1002/humu.20393. [DOI] [PubMed] [Google Scholar]
- 5.Alfthan K, Heiska L, Gronholm M, Renkema GH, Carpen O. Cyclic AMP-dependent protein kinase phosphorylates merlin at serine 518 independently of p21-activated kinase and promotes merlin-ezrin heterodimerization. J Biol Chem. 2004;279:18559–18566. doi: 10.1074/jbc.M313916200. [DOI] [PubMed] [Google Scholar]
- 6.Ammoun S, Cunliffe CH, Allen JC, et al. ErbB/HER receptor activation and preclinical efficacy of lapatinib in vestibular schwannoma. Neuro Oncol. 2010;12:834–843. doi: 10.1093/neuonc/noq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ammoun S, Flaiz C, Ristic N, Schuldt J, Hanemann CO. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 2008;68:5236–5245. doi: 10.1158/0008-5472.CAN-07-5849. [DOI] [PubMed] [Google Scholar]
- 8.Bacci C, Sestini R, Provenzano A, et al. Schwannomatosis associated with multiple meningiomas due to a familial SMARCB1 mutation. Neurogenetics. 2010;11:73–80. doi: 10.1007/s10048-009-0204-2. [DOI] [PubMed] [Google Scholar]
- 9.Bai Y, Liu YJ, Wang H, Xu Y, Stamenkovic I, Yu Q. Inhibition of the hyaluronan-CD44 interaction by merlin contributes to the tumor-suppressor activity of merlin. Oncogene. 2007;26:836–850. doi: 10.1038/sj.onc.1209849. [DOI] [PubMed] [Google Scholar]
- 10.Barret C, Roy C, Montcourrier P, Mangeat P, Niggli V. Mutagenesis of the phosphatidylinositol 4,5-bisphosphate (PIP(2)) binding site in the NH(2)-terminal domain of ezrin correlates with its altered cellular distribution. J Cell Biol. 2000;151:1067–1080. doi: 10.1083/jcb.151.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baser ME. The distribution of constitutional and somatic mutations in the neurofibromatosis 2 gene. Hum Mutat. 2006;27:297–306. doi: 10.1002/humu.20317. [DOI] [PubMed] [Google Scholar]
- 12.Baser ME, Kuramoto L, Woods R, et al. The location of constitutional neurofibromatosis 2 (NF2) splice site mutations is associated with the severity of NF2. J Med Genet. 2005;42:540–546. doi: 10.1136/jmg.2004.029504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bashour AM, Meng JJ, Ip W, MacCollin M, Ratner N. The neurofibromatosis type 2 gene product, merlin, reverses the F-actin cytoskeletal defects in primary human Schwannoma cells. Mol Cell Biol. 2002;22:1150–1157. doi: 10.1128/MCB.22.4.1150-1157.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356:713–715. doi: 10.1038/356713a0. [DOI] [PubMed] [Google Scholar]
- 15.Bhola P, Banerjee S, Mukherjee J, et al. Preclinical in vivo evaluation of rapamycin in human malignant peripheral nerve sheath explant xenograft. Int J Cancer. 2010;126:563–571. doi: 10.1002/ijc.24783. [DOI] [PubMed] [Google Scholar]
- 16.Birindelli S, Perrone F, Oggionni M, et al. Rb and TP53 pathway alterations in sporadic and NF1-related malignant peripheral nerve sheath tumors. Lab Invest. 2001;81:833–844. doi: 10.1038/labinvest.3780293. [DOI] [PubMed] [Google Scholar]
- 17.Bollag G, McCormick F, Clark R. Characterization of full-length neurofibromin: tubulin inhibits Ras GAP activity. EMBO J. 1993;12:1923–1927. doi: 10.1002/j.1460-2075.1993.tb05841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bossis I, Stratakis CA. Minireview: PRKAR1A: normal and abnormal functions. Endocrinology. 2004;145:5452–5458. doi: 10.1210/en.2004-0900. [DOI] [PubMed] [Google Scholar]
- 19.Brannan CI, Perkins AS, Vogel KS, et al. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev. 1994;8:1019–1029. doi: 10.1101/gad.8.9.1019. [DOI] [PubMed] [Google Scholar]
- 20.Brault E, Gautreau A, Lamarine M, Callebaut I, Thomas G, Goutebroze L. Normal membrane localization and actin association of the NF2 tumor suppressor protein are dependent on folding of its N-terminal domain. J Cell Sci. 2001;114:1901–1912. doi: 10.1242/jcs.114.10.1901. [DOI] [PubMed] [Google Scholar]
- 21.Buzard GS, Enomoto T, Anderson LM, Perantoni AO, Devor DE, Rice JM. Activation of neu by missense point mutation in the transmembrane domain in schwannomas induced in C3H/HeNCr mice by transplacental exposure to N-nitrosoethylurea. J Cancer Res Clin Oncol. 1999;125:653–659. doi: 10.1007/s004320050330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byer SJ, Eckert JM, Brossier NM, et al. Tamoxifen inhibits malignant peripheral nerve sheath tumor growth in an estrogen receptor-independent manner. Neuro Oncol. 2011;13:28–41. doi: 10.1093/neuonc/noq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carroll SL, Miller ML, Frohnert PW, Kim SS, Corbett JA. Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration. J Neurosci. 1997;17:1642–1659. doi: 10.1523/JNEUROSCI.17-05-01642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll SL, Ratner N. How does the Schwann cell lineage form tumors in NF1? Glia. 2008;56:1590–1605. doi: 10.1002/glia.20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carroll SL, Stonecypher MS. Tumor suppressor mutations and growth factor signaling in the pathogenesis of NF1-associated peripheral nerve sheath tumors. I. The role of tumor suppressor mutations. J Neuropathol Exp Neurol. 2004;63:1115–1123. doi: 10.1093/jnen/63.11.1115. [DOI] [PubMed] [Google Scholar]
- 26.Carroll SL, Stonecypher MS. Tumor suppressor mutations and growth factor signaling in the pathogenesis of NF1-associated peripheral nerve sheath tumors: II. The role of dysregulated growth factor signaling. J Neuropathol Exp Neurol. 2005;64:1–9. doi: 10.1093/jnen/64.1.1. [DOI] [PubMed] [Google Scholar]
- 27.CBTRUS CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2006. 2010 doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christiaans I, Kenter SB, Brink HC, et al. Germline SMARCB1 mutation and somatic NF2 mutations in familial multiple meningiomas. J Med Genet. 2011;48:93–97. doi: 10.1136/jmg.2010.082420. [DOI] [PubMed] [Google Scholar]
- 29.Cichowski K, Shih TS, Schmitt E, et al. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999;286:2172–2176. doi: 10.1126/science.286.5447.2172. [DOI] [PubMed] [Google Scholar]
- 30.Clark JJ, Provenzano M, Diggelmann HR, Xu N, Hansen SS, Hansen MR. The ErbB inhibitors trastuzumab and erlotinib inhibit growth of vestibular schwannoma xenografts in nude mice: a preliminary study. Otol Neurotol. 2008;29:846–853. doi: 10.1097/MAO.0b013e31817f7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen PR, Rapini RP, Farhood AI. Expression of the human hematopoietic progenitor cell antigen CD34 in vascular and spindle cell tumors. J Cutan Pathol. 1993;20:15–20. doi: 10.1111/j.1600-0560.1993.tb01243.x. [DOI] [PubMed] [Google Scholar]
- 32.Courtois-Cox S, Genther Williams SM, Reczek EE, et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10:459–472. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crawford AT, Desai D, Gokina P, Basak S, Kim HA. E-cadherin expression in postnatal Schwann cells is regulated by the cAMP-dependent protein kinase a pathway. Glia. 2008;56:1637–1647. doi: 10.1002/glia.20716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Angelo I, Welti S, Bonneau F, Scheffzek K. A novel bipartite phospholipid-binding module in the neurofibromatosis type 1 protein. EMBO Rep. 2006;7:174–179. doi: 10.1038/sj.embor.7400602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dang I, De Vries GH. Aberrant cAMP Metabolism in NF1 Malignant Peripheral Nerve Sheath Tumor Cells. Neurochem Res. 2011;36:1697–1705. doi: 10.1007/s11064-011-0433-2. [DOI] [PubMed] [Google Scholar]
- 36.Dang I, DeVries GH. Schwann cell lines derived from malignant peripheral nerve sheath tumors respond abnormally to platelet-derived growth factor-BB. J Neurosci Res. 2005;79:318–328. doi: 10.1002/jnr.20334. [DOI] [PubMed] [Google Scholar]
- 37.Dasgupta B, Dugan LL, Gutmann DH. The neurofibromatosis 1 gene product neurofibromin regulates pituitary adenylate cyclase-activating polypeptide-mediated signaling in astrocytes. J Neurosci. 2003;23:8949–8954. doi: 10.1523/JNEUROSCI.23-26-08949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeClue JE, Heffelfinger S, Benvenuto G, et al. Epidermal growth factor receptor expression in neurofibromatosis type 1-related tumors and NF1 animal models. J Clin Invest. 2000;105:1233–1241. doi: 10.1172/JCI7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Dovas A, Couchman JR. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem J. 2005;390:1–9. doi: 10.1042/BJ20050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Easton DF, Ponder MA, Huson SM, Ponder BA. An analysis of variation in expression of neurofibromatosis (NF) type 1 (NF1): evidence for modifying genes. Am J Hum Genet. 1993;53:305–313. [PMC free article] [PubMed] [Google Scholar]
- 42.Eckert JM, Byer SJ, Clodfelder-Miller BJ, Carroll SL. Neuregulin-1 beta and neuregulin-1 alpha differentially affect the migration and invasion of malignant peripheral nerve sheath tumor cells. Glia. 2009;57:1501–1520. doi: 10.1002/glia.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39:311–314. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans DG, Huson SM, Donnai D, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84:603–618. [PubMed] [Google Scholar]
- 45.Evans DG, Ramsden RT, Shenton A, et al. Mosaicism in neurofibromatosis type 2: an update of risk based on uni/bilaterality of vestibular schwannoma at presentation and sensitive mutation analysis including multiple ligation-dependent probe amplification. J Med Genet. 2007;44:424–428. doi: 10.1136/jmg.2006.047753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans DG, Trueman L, Wallace A, Collins S, Strachan T. Genotype/phenotype correlations in type 2 neurofibromatosis (NF2): evidence for more severe disease associated with truncating mutations. J Med Genet. 1998;35:450–455. doi: 10.1136/jmg.35.6.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans GR, Lloyd SK, Ramsden RT. Neurofibromatosis type 2. Adv Otorhinolaryngol. 2011;70:91–98. doi: 10.1159/000322482. [DOI] [PubMed] [Google Scholar]
- 48.Fernandes KJ, McKenzie IA, Mill P, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez-Valle C, Tang Y, Ricard J, et al. Paxillin binds schwannomin and regulates its density-dependent localization and effect on cell morphology. Nat Genet. 2002;31:354–362. doi: 10.1038/ng930. [DOI] [PubMed] [Google Scholar]
- 50.Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 2007;6:340–351. doi: 10.1016/S1474-4422(07)70075-3. [DOI] [PubMed] [Google Scholar]
- 51.Firat-Karalar EN, Welch MD. New mechanisms and functions of actin nucleation. Curr Opin Cell Biol. 2011;23:4–13. doi: 10.1016/j.ceb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flaiz C, Ammoun S, Biebl A, Hanemann CO. Altered adhesive structures and their relation to RhoGTPase activation in merlin-deficient Schwannoma. Brain Pathol. 2009;19:27–38. doi: 10.1111/j.1750-3639.2008.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flaiz C, Utermark T, Parkinson DB, Poetsch A, Hanemann CO. Impaired intercellular adhesion and immature adherens junctions in merlin-deficient human primary schwannoma cells. Glia. 2008;56:506–515. doi: 10.1002/glia.20629. [DOI] [PubMed] [Google Scholar]
- 54.Fraenzer JT, Pan H, Minimo L, Jr., Smith GM, Knauer D, Hung G. Overexpression of the NF2 gene inhibits schwannoma cell proliferation through promoting PDGFR degradation. Int J Oncol. 2003;23:1493–1500. [PubMed] [Google Scholar]
- 55.Frohnert PW, Stonecypher MS, Carroll SL. Constitutive activation of the neuregulin-1/ErbB receptor signaling pathway is essential for the proliferation of a neoplastic Schwann cell line. Glia. 2003;43:104–118. doi: 10.1002/glia.10232. [DOI] [PubMed] [Google Scholar]
- 56.Giovannini M, Robanus-Maandag E, van der Valk M, et al. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 2000;14:1617–1630. [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez-Agosti C, Xu L, Pinney D, et al. The merlin tumor suppressor localizes preferentially in membrane ruffles. Oncogene. 1996;13:1239–1247. [PubMed] [Google Scholar]
- 58.Greene EL, Horvath AD, Nesterova M, Giatzakis C, Bossis I, Stratakis CA. In vitro functional studies of naturally occurring pathogenic PRKAR1A mutations that are not subject to nonsense mRNA decay. Hum Mutat. 2008;29:633–639. doi: 10.1002/humu.20688. [DOI] [PubMed] [Google Scholar]
- 59.Gronholm M, Sainio M, Zhao F, Heiska L, Vaheri A, Carpen O. Homotypic and heterotypic interaction of the neurofibromatosis 2 tumor suppressor protein merlin and the ERM protein ezrin. J Cell Sci. 1999;112:895–904. doi: 10.1242/jcs.112.6.895. [DOI] [PubMed] [Google Scholar]
- 60.Gutmann DH, Sherman L, Seftor L, Haipek C, Hoang Lu K, Hendrix M. Increased expression of the NF2 tumor suppressor gene product, merlin, impairs cell motility, adhesionand spreading. Hum Mol Genet. 1999;8:267–275. doi: 10.1093/hmg/8.2.267. [DOI] [PubMed] [Google Scholar]
- 61.Gutmann DH, Stiles CD, Lowe SW, Bollag GE, Furnari FB, Charest AL. Report from the fifth National Cancer Institute Mouse Models of Human Cancers Consortium Nervous System Tumors Workshop. Neuro Oncol. 2011;13:692–699. doi: 10.1093/neuonc/nor080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hadfield KD, Newman WG, Bowers NL, et al. Molecular characterisation of SMARCB1 and NF2 in familial and sporadic schwannomatosis. J Med Genet. 2008;45:332–339. doi: 10.1136/jmg.2007.056499. [DOI] [PubMed] [Google Scholar]
- 63.Hamaratoglu F, Willecke M, Kango-Singh M, et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 64.Hansen MR, Roehm PC, Chatterjee P, Green SH. Constitutive neuregulin-1/ErbB signaling contributes to human vestibular schwannoma proliferation. Glia. 2006;53:593–600. doi: 10.1002/glia.20316. [DOI] [PubMed] [Google Scholar]
- 65.Hara T, Bianchi AB, Seizinger BR, Kley N. Molecular cloning and characterization of alternatively spliced transcripts of the mouse neurofibromatosis 2 gene. Cancer Res. 1994;54:330–335. [PubMed] [Google Scholar]
- 66.Hawes JJ, Tuskan RG, Reilly KM. Nf1 expression is dependent on strain background: implications for tumor suppressor haploinsufficiency studies. Neurogenetics. 2007;8:121–130. doi: 10.1007/s10048-006-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hergovich A, Hemmings BA. Mammalian NDR/LATS protein kinases in hippo tumor suppressor signaling. Biofactors. 2009;35:338–345. doi: 10.1002/biof.47. [DOI] [PubMed] [Google Scholar]
- 68.Hirota S, Nomura S, Asada H, Ito A, Morii E, Kitamura Y. Possible involvement of c-kit receptor and its ligand in increase of mast cells in neurofibroma tissues. Arch Pathol Lab Med. 1993;117:996–999. [PubMed] [Google Scholar]
- 69.Holtkamp N, Atallah I, Okuducu AF, et al. MMP-13 and p53 in the progression of malignant peripheral nerve sheath tumors. Neoplasia. 2007;9:671–677. doi: 10.1593/neo.07304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang Y, Rangwala F, Fulkerson PC, et al. Role of TC21/R-Ras2 in enhanced migration of neurofibromin-deficient Schwann cells. Oncogene. 2004;23:368–378. doi: 10.1038/sj.onc.1207075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hughes SC, Fehon RG. Phosphorylation and activity of the tumor suppressor Merlin and the ERM protein Moesin are coordinately regulated by the Slik kinase. J Cell Biol. 2006;175:305–313. doi: 10.1083/jcb.200608009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huijbregts RP, Roth KA, Schmidt RE, Carroll SL. Hypertrophic neuropathies and malignant peripheral nerve sheath tumors in transgenic mice overexpressing glial growth factor beta3 in myelinating Schwann cells. J Neurosci. 2003;23:7269–7280. doi: 10.1523/JNEUROSCI.23-19-07269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hulsebos TJ, Plomp AS, Wolterman RA, Robanus-Maandag EC, Baas F, Wesseling P. Germline mutation of INI1/SMARCB1 in familial schwannomatosis. Am J Hum Genet. 2007;80:805–810. doi: 10.1086/513207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huynh DP, Nechiporuk T, Pulst SM. Alternative transcripts in the mouse neurofibromatosis type 2 (NF2) gene are conserved and code for schwannomins with distinct C-terminal domains. Hum Mol Genet. 1994;3:1075–1079. doi: 10.1093/hmg/3.7.1075. [DOI] [PubMed] [Google Scholar]
- 75.Ingram DA, Hiatt K, King AJ, et al. Hyperactivation of p21(ras) and the hematopoietic-specific Rho GTPase, Rac2, cooperate to alter the proliferation of neurofibromin-deficient mast cells in vivo and in vitro. J Exp Med. 2001;194:57–69. doi: 10.1084/jem.194.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ingram DA, Yang FC, Travers JB, et al. Genetic and biochemical evidence that haploinsufficiency of the Nf1 tumor suppressor gene modulates melanocyte and mast cell fates in vivo. J Exp Med. 2000;191:181–188. doi: 10.1084/jem.191.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nat Genet. 1994;7:353–361. doi: 10.1038/ng0794-353. [DOI] [PubMed] [Google Scholar]
- 78.Jadayel D, Fain P, Upadhyaya M, et al. Paternal origin of new mutations in von Recklinghausen neurofibromatosis. Nature. 1990;343:558–559. doi: 10.1038/343558a0. [DOI] [PubMed] [Google Scholar]
- 79.James MF, Manchanda N, Gonzalez-Agosti C, Hartwig JH, Ramesh V. The neurofibromatosis 2 protein product merlin selectively binds F-actin but not G-actin, and stabilizes the filaments through a lateral association. Biochem J. 2001;356:377–386. doi: 10.1042/0264-6021:3560377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johannessen CM, Johnson BW, Williams SM, et al. TORC1 is essential for NF1-associated malignancies. Curr Biol. 2008;18:56–62. doi: 10.1016/j.cub.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 81.Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005;102:8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johansson G, Mahller YY, Collins MH, et al. Effective in vivo targeting of the mammalian target of rapamycin pathway in malignant peripheral nerve sheath tumors. Mol Cancer Ther. 2008;7:1237–1245. doi: 10.1158/1535-7163.MCT-07-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones GN, Tep C, Towns WH, 2nd, et al. Tissue-specific ablation of Prkar1a causes schwannomas by suppressing neurofibromatosis protein production. Neoplasia. 2008;10:1213–1221. doi: 10.1593/neo.08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Joseph NM, Mosher JT, Buchstaller J, et al. The loss of Nf1 transiently promotes self-renewal but not tumorigenesis by neural crest stem cells. Cancer Cell. 2008;13:129–140. doi: 10.1016/j.ccr.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim HA, Ratner N, Roberts TM, Stiles CD. Schwann cell proliferative responses to cAMP and Nf1 are mediated by cyclin D1. J Neurosci. 2001;21:1110–1116. doi: 10.1523/JNEUROSCI.21-04-01110.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.King D, Yang G, Thompson MA, Hiebert SW. Loss of neurofibromatosis-1 and p19(ARF) cooperate to induce a multiple tumor phenotype. Oncogene. 2002;21:4978–4982. doi: 10.1038/sj.onc.1205632. [DOI] [PubMed] [Google Scholar]
- 87.Kirschner LS, Carney JA, Pack SD, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 88.Kirschner LS, Kusewitt DF, Matyakhina L, et al. A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res. 2005;65:4506–4514. doi: 10.1158/0008-5472.CAN-05-0580. [DOI] [PubMed] [Google Scholar]
- 89.Kirschner LS, Sandrini F, Monbo J, Lin JP, Carney JA, Stratakis CA. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the carney complex. Hum Mol Genet. 2000;9:3037–3046. doi: 10.1093/hmg/9.20.3037. [DOI] [PubMed] [Google Scholar]
- 90.Kissil JL, Johnson KC, Eckman MS, Jacks T. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem. 2002;277:10394–10399. doi: 10.1074/jbc.M200083200. [DOI] [PubMed] [Google Scholar]
- 91.Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell. 2003;12:841–849. doi: 10.1016/s1097-2765(03)00382-4. [DOI] [PubMed] [Google Scholar]
- 92.Kluwe L, Friedrich R, Mautner VF. Loss of NF1 allele in Schwann cells but not in fibroblasts derived from an NF1-associated neurofibroma. Genes Chromosomes Cancer. 1999;24:283–285. doi: 10.1002/(sici)1098-2264(199903)24:3<283::aid-gcc15>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 93.Kluwe L, MacCollin M, Tatagiba M, et al. Phenotypic variability associated with 14 splice-site mutations in the NF2 gene. Am J Med Genet. 1998;77:228–233. [PubMed] [Google Scholar]
- 94.Koga T, Iwasaki H, Ishiguro M, Matsuzaki A, Kikuchi M. Losses in chromosomes 17, 19, and 22q in neurofibromatosis type 1 and sporadic neurofibromas: a comparative genomic hybridization analysis. Cancer Genet Cytogenet. 2002;136:113–120. doi: 10.1016/s0165-4608(02)00527-7. [DOI] [PubMed] [Google Scholar]
- 95.Korkiamaki T, Yla-Outinen H, Koivunen J, Karvonen SL, Peltonen J. Altered calcium-mediated cell signaling in keratinocytes cultured from patients with neurofibromatosis type 1. Am J Pathol. 2002;160:1981–1990. doi: 10.1016/S0002-9440(10)61148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kourea HP, Orlow I, Scheithauer BW, Cordon-Cardo C, Woodruff JM. Deletions of the INK4A gene occur in malignant peripheral nerve sheath tumors but not in neurofibromas. Am J Pathol. 1999;155:1855–1860. doi: 10.1016/S0002-9440(10)65504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kweh F, Zheng M, Kurenova E, Wallace M, Golubovskaya V, Cance WG. Neurofibromin physically interacts with the N-terminal domain of focal adhesion kinase. Mol Carcinog. 2009;48:1005–1017. doi: 10.1002/mc.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lakkis MM, Golden JA, O’Shea KS, Epstein JA. Neurofibromin deficiency in mice causes exencephaly and is a modifier for Splotch neural tube defects. Dev Biol. 1999;212:80–92. doi: 10.1006/dbio.1999.9327. [DOI] [PubMed] [Google Scholar]
- 99.Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 2003;17:1090–1100. doi: 10.1101/gad.1054603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lallemand D, Manent J, Couvelard A, et al. Merlin regulates transmembrane receptor accumulation and signaling at the plasma membrane in primary mouse Schwann cells and in human schwannomas. Oncogene. 2009;28:854–865. doi: 10.1038/onc.2008.427. [DOI] [PubMed] [Google Scholar]
- 101.Laulajainen M, Muranen T, Carpen O, Gronholm M. Protein kinase A-mediated phosphorylation of the NF2 tumor suppressor protein merlin at serine 10 affects the actin cytoskeleton. Oncogene. 2008;27:3233–3243. doi: 10.1038/sj.onc.1210988. [DOI] [PubMed] [Google Scholar]
- 102.Laulajainen M, Muranen T, Nyman TA, Carpen O, Gronholm M. Multistep Phosphorylation by Oncogenic Kinases Enhances the Degradation of the NF2 Tumor Suppressor Merlin. Neoplasia. 2011;13:643–652. doi: 10.1593/neo.11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Le LQ, Liu C, Shipman T, Chen Z, Suter U, Parada LF. Susceptible Stages in Schwann Cells for NF1-Associated Plexiform Neurofibroma Development. Cancer Res. 2011;71:4686–4695. doi: 10.1158/0008-5472.CAN-10-4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Le LQ, Shipman T, Burns DK, Parada LF. Cell of origin and microenvironment contribution for NF1-associated dermal neurofibromas. Cell Stem Cell. 2009;4:453–463. doi: 10.1016/j.stem.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Legius E, Dierick H, Wu R, et al. TP53 mutations are frequent in malignant NF1 tumors. Genes Chromosomes Cancer. 1994;10:250–255. doi: 10.1002/gcc.2870100405. [DOI] [PubMed] [Google Scholar]
- 106.Legius E, Marchuk DA, Collins FS, Glover TW. Somatic deletion of the neurofibromatosis type 1 gene in a neurofibrosarcoma supports a tumour suppressor gene hypothesis. Nat Genet. 1993;3:122–126. doi: 10.1038/ng0293-122. [DOI] [PubMed] [Google Scholar]
- 107.Lewallen KA, Shen YA, De la Torre AR, Ng BK, Meijer D, Chan JR. Assessing the role of the cadherin/catenin complex at the Schwann cell-axon interface and in the initiation of myelination. J Neurosci. 2011;31:3032–3043. doi: 10.1523/JNEUROSCI.4345-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li W, You L, Cooper J, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140:477–490. doi: 10.1016/j.cell.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ling BC, Wu J, Miller SJ, et al. Role for the epidermal growth factor receptor in neurofibromatosis-related peripheral nerve tumorigenesis. Cancer Cell. 2005;7:65–75. doi: 10.1016/j.ccr.2004.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lopez-Lago MA, Okada T, Murillo MM, Socci N, Giancotti FG. Loss of the tumor suppressor gene NF2, encoding merlin, constitutively activates integrin-dependent mTORC1 signaling. Mol Cell Biol. 2009;29:4235–4249. doi: 10.1128/MCB.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Luongo C, Moser AR, Gledhill S, Dove WF. Loss of Apc+ in intestinal adenomas from Min mice. Cancer Res. 1994;54:5947–5952. [PubMed] [Google Scholar]
- 112.Lutchman M, Rouleau GA. The neurofibromatosis type 2 gene product, schwannomin, suppresses growth of NIH 3T3 cells. Cancer Res. 1995;55:2270–2274. [PubMed] [Google Scholar]
- 113.MacCollin M, Chiocca EA, Evans DG, et al. Diagnostic criteria for schwannomatosis. Neurology. 2005;64:1838–1845. doi: 10.1212/01.WNL.0000163982.78900.AD. [DOI] [PubMed] [Google Scholar]
- 114.MacCollin M, Willett C, Heinrich B, et al. Familial schwannomatosis: exclusion of the NF2 locus as the germline event. Neurology. 2003;60:1968–1974. doi: 10.1212/01.wnl.0000070184.08740.e0. [DOI] [PubMed] [Google Scholar]
- 115.MacCollin M, Woodfin W, Kronn D, Short MP. Schwannomatosis: a clinical and pathologic study. Neurology. 1996;46:1072–1079. doi: 10.1212/wnl.46.4.1072. [DOI] [PubMed] [Google Scholar]
- 116.Maeda M, Matsui T, Imamura M, Tsukita S. Expression level, subcellular distribution and rho-GDI binding affinity of merlin in comparison with Ezrin/Radixin/Moesin proteins. Oncogene. 1999;18:4788–4797. doi: 10.1038/sj.onc.1202871. [DOI] [PubMed] [Google Scholar]
- 117.Manchanda N, Lyubimova A, Ho HY, et al. The NF2 tumor suppressor Merlin and the ERM proteins interact with N-WASP and regulate its actin polymerization function. J Biol Chem. 2005;280:12517–12522. doi: 10.1074/jbc.C400583200. [DOI] [PubMed] [Google Scholar]
- 118.Mangoura D, Sun Y, Li C, et al. Phosphorylation of neurofibromin by PKC is a possible molecular switch in EGF receptor signaling in neural cells. Oncogene. 2006;25:735–745. doi: 10.1038/sj.onc.1209113. [DOI] [PubMed] [Google Scholar]
- 119.Mantripragada KK, Spurlock G, Kluwe L, et al. High-resolution DNA copy number profiling of malignant peripheral nerve sheath tumors using targeted microarray-based comparative genomic hybridization. Clin Cancer Res. 2008;14:1015–1024. doi: 10.1158/1078-0432.CCR-07-1305. [DOI] [PubMed] [Google Scholar]
- 120.Marais R, Light Y, Paterson HF, Mason CS, Marshall CJ. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem. 1997;272:4378–4383. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- 121.Mautner VF, Kluwe L, Friedrich RE, et al. Clinical characterisation of 29 neurofibromatosis type-1 patients with molecularly ascertained 1.4 Mb type-1 NF1 deletions. J Med Genet. 2010;47:623–630. doi: 10.1136/jmg.2009.075937. [DOI] [PubMed] [Google Scholar]
- 122.Mawrin C, Kirches E, Boltze C, Dietzmann K, Roessner A, Schneider-Stock R. Immunohistochemical and molecular analysis of p53, RB, and PTEN in malignant peripheral nerve sheath tumors. Virchows Arch. 2002;440:610–615. doi: 10.1007/s00428-001-0550-4. [DOI] [PubMed] [Google Scholar]
- 123.Mayes DA, Rizvi TA, Cancelas JA, et al. Perinatal or Adult Nf1 Inactivation Using Tamoxifen-Inducible PlpCre Each Cause Neurofibroma Formation. Cancer Res. 2011;71:4675–4685. doi: 10.1158/0008-5472.CAN-10-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McCartney BM, Kulikauskas RM, LaJeunesse DR, Fehon RG. The neurofibromatosis-2 homologue, Merlin, and the tumor suppressor expanded function together in Drosophila to regulate cell proliferation and differentiation. Development. 2000;127:1315–1324. doi: 10.1242/dev.127.6.1315. [DOI] [PubMed] [Google Scholar]
- 125.McCaughan JA, Holloway SM, Davidson R, Lam WW. Further evidence of the increased risk for malignant peripheral nerve sheath tumour from a Scottish cohort of patients with neurofibromatosis type 1. J Med Genet. 2007;44:463–466. doi: 10.1136/jmg.2006.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McClatchey AI, Saotome I, Mercer K, et al. Mice heterozygous for a mutation at the Nf2 tumor suppressor locus develop a range of highly metastatic tumors. Genes Dev. 1998;12:1121–1133. doi: 10.1101/gad.12.8.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McClatchey AI, Saotome I, Ramesh V, Gusella JF, Jacks T. The Nf2 tumor suppressor gene product is essential for extraembryonic development immediately prior to gastrulation. Genes Dev. 1997;11:1253–1265. doi: 10.1101/gad.11.10.1253. [DOI] [PubMed] [Google Scholar]
- 128.Meng JJ, Lowrie DJ, Sun H, et al. Interaction between two isoforms of the NF2 tumor suppressor protein, merlin, and between merlin and ezrin, suggests modulation of ERM proteins by merlin. J Neurosci Res. 2000;62:491–502. doi: 10.1002/1097-4547(20001115)62:4<491::AID-JNR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 129.Menichella DM, Arroyo EJ, Awatramani R, et al. Protein zero is necessary for E-cadherin-mediated adherens junction formation in Schwann cells. Mol Cell Neurosci. 2001;18:606–618. doi: 10.1006/mcne.2001.1041. [DOI] [PubMed] [Google Scholar]
- 130.Menon AG, Anderson KM, Riccardi VM, et al. Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in von Recklinghausen neurofibromatosis. Proc Natl Acad Sci U S A. 1990;87:5435–5439. doi: 10.1073/pnas.87.14.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Messiaen L, Yao S, Brems H, et al. Clinical and mutational spectrum of neurofibromatosis type 1-like syndrome. JAMA. 2009;302:2111–2118. doi: 10.1001/jama.2009.1663. [DOI] [PubMed] [Google Scholar]
- 132.Messiaen LM, Callens T, Mortier G, et al. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum Mutat. 2000;15:541–555. doi: 10.1002/1098-1004(200006)15:6<541::AID-HUMU6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 133.Miller SJ, Jessen WJ, Mehta T, et al. Integrative genomic analyses of neurofibromatosis tumours identify SOX9 as a biomarker and survival gene. EMBO Mol Med. 2009;1:236–248. doi: 10.1002/emmm.200900027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miller SJ, Rangwala F, Williams J, et al. Large-scale molecular comparison of human schwann cells to malignant peripheral nerve sheath tumor cell lines and tissues. Cancer Res. 2006;66:2584–2591. doi: 10.1158/0008-5472.CAN-05-3330. [DOI] [PubMed] [Google Scholar]
- 135.Morrison H, Sherman LS, Legg J, et al. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001;15:968–980. doi: 10.1101/gad.189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Murthy A, Gonzalez-Agosti C, Cordero E, et al. NHE-RF, a regulatory cofactor for Na(+)-H+ exchange, is a common interactor for merlin and ERM (MERM) proteins. J Biol Chem. 1998;273:1273–1276. doi: 10.1074/jbc.273.3.1273. [DOI] [PubMed] [Google Scholar]
- 137.Nguyen R, Reczek D, Bretscher A. Hierarchy of merlin and ezrin N- and C-terminal domain interactions in homo- and heterotypic associations and their relationship to binding of scaffolding proteins EBP50 and E3KARP. J Biol Chem. 2001;276:7621–7629. doi: 10.1074/jbc.M006708200. [DOI] [PubMed] [Google Scholar]
- 138.Nielsen GP, Stemmer-Rachamimov AO, Ino Y, Moller MB, Rosenberg AE, Louis DN. Malignant transformation of neurofibromas in neurofibromatosis 1 is associated with CDKN2A/p16 inactivation. Am J Pathol. 1999;155:1879–1884. doi: 10.1016/S0002-9440(10)65507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ohba Y, Mochizuki N, Yamashita S, et al. Regulatory proteins of R-Ras, TC21/R-Ras2, and M-Ras/R-Ras3. J Biol Chem. 2000;275:20020–20026. doi: 10.1074/jbc.M000981200. [DOI] [PubMed] [Google Scholar]
- 140.Okada T, Lopez-Lago M, Giancotti FG. Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J Cell Biol. 2005;171:361–371. doi: 10.1083/jcb.200503165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pasmant E, Sabbagh A, Spurlock G, et al. NF1 microdeletions in neurofibromatosis type 1: from genotype to phenotype. Hum Mutat. 2010;31:E1506–E1518. doi: 10.1002/humu.21271. [DOI] [PubMed] [Google Scholar]
- 142.Pelton PD, Sherman LS, Rizvi TA, et al. Ruffling membrane, stress fiber, cell spreading and proliferation abnormalities in human Schwannoma cells. Oncogene. 1998;17:2195–2209. doi: 10.1038/sj.onc.1202141. [DOI] [PubMed] [Google Scholar]
- 143.Perry A, Kunz SN, Fuller CE, et al. Differential NF1, p16, and EGFR patterns by interphase cytogenetics (FISH) in malignant peripheral nerve sheath tumor (MPNST) and morphologically similar spindle cell neoplasms. J Neuropathol Exp Neurol. 2002;61:702–709. doi: 10.1093/jnen/61.8.702. [DOI] [PubMed] [Google Scholar]
- 144.Perry A, Roth KA, Banerjee R, Fuller CE, Gutmann DH. NF1 deletions in S-100 protein-positive and negative cells of sporadic and neurofibromatosis 1 (NF1)-associated plexiform neurofibromas and malignant peripheral nerve sheath tumors. Am J Pathol. 2001;159:57–61. doi: 10.1016/S0002-9440(10)61673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pykett MJ, Murphy M, Harnish PR, George DL. The neurofibromatosis 2 (NF2) tumor suppressor gene encodes multiple alternatively spliced transcripts. Hum Mol Genet. 1994;3:559–564. doi: 10.1093/hmg/3.4.559. [DOI] [PubMed] [Google Scholar]
- 146.Rangwala R, Banine F, Borg JP, Sherman LS. Erbin regulates mitogen-activated protein (MAP) kinase activation and MAP kinase-dependent interactions between Merlin and adherens junction protein complexes in Schwann cells. J Biol Chem. 2005;280:11790–11797. doi: 10.1074/jbc.M414154200. [DOI] [PubMed] [Google Scholar]
- 147.Reilly KM, Broman KW, Bronson RT, et al. An imprinted locus epistatically influences Nstr1 and Nstr2 to control resistance to nerve sheath tumors in a neurofibromatosis type 1 mouse model. Cancer Res. 2006;66:62–68. doi: 10.1158/0008-5472.CAN-05-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Roberts CW, Galusha SA, McMenamin ME, Fletcher CD, Orkin SH. Haploinsufficiency of Snf5 (integrase interactor 1) predisposes to malignant rhabdoid tumors in mice. Proc Natl Acad Sci U S A. 2000;97:13796–13800. doi: 10.1073/pnas.250492697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Robinson-White A, Hundley TR, Shiferaw M, Bertherat J, Sandrini F, Stratakis CA. Protein kinase-A activity in PRKAR1A-mutant cells, and regulation of mitogen-activated protein kinases ERK1/2. Hum Mol Genet. 2003;12:1475–1484. doi: 10.1093/hmg/ddg160. [DOI] [PubMed] [Google Scholar]
- 150.Robinson-White A, Meoli E, Stergiopoulos S, et al. PRKAR1A Mutations and protein kinase A interactions with other signaling pathways in the adrenal cortex. J Clin Endocrinol Metab. 2006;91:2380–2388. doi: 10.1210/jc.2006-0188. [DOI] [PubMed] [Google Scholar]
- 151.Rong R, Surace EI, Haipek CA, Gutmann DH, Ye K. Serine 518 phosphorylation modulates merlin intramolecular association and binding to critical effectors important for NF2 growth suppression. Oncogene. 2004;23:8447–8454. doi: 10.1038/sj.onc.1207794. [DOI] [PubMed] [Google Scholar]
- 152.Rouleau GA, Merel P, Lutchman M, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- 153.Ruttledge MH, Andermann AA, Phelan CM, et al. Type of mutation in the neurofibromatosis type 2 gene (NF2) frequently determines severity of disease. Am J Hum Genet. 1996;59:331–342. [PMC free article] [PubMed] [Google Scholar]
- 154.Ryan JJ, Klein KA, Neuberger TJ, et al. Role for the stem cell factor/KIT complex in Schwann cell neoplasia and mast cell proliferation associated with neurofibromatosis. J Neurosci Res. 1994;37:415–432. doi: 10.1002/jnr.490370314. [DOI] [PubMed] [Google Scholar]
- 155.Sainio M, Zhao F, Heiska L, et al. Neurofibromatosis 2 tumor suppressor protein colocalizes with ezrin and CD44 and associates with actin-containing cytoskeleton. J Cell Sci. 1997;110(Pt 18):2249–2260. doi: 10.1242/jcs.110.18.2249. [DOI] [PubMed] [Google Scholar]
- 156.Schade B, Lam SH, Cernea D, et al. Distinct ErbB-2 coupled signaling pathways promote mammary tumors with unique pathologic and transcriptional profiles. Cancer Res. 2007;67:7579–7588. doi: 10.1158/0008-5472.CAN-06-4724. [DOI] [PubMed] [Google Scholar]
- 157.Scheffzek K, Ahmadian MR, Kabsch W, et al. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997;277:333–338. doi: 10.1126/science.277.5324.333. [DOI] [PubMed] [Google Scholar]
- 158.Schulze KM, Hanemann CO, Muller HW, Hanenberg H. Transduction of wild-type merlin into human schwannoma cells decreases schwannoma cell growth and induces apoptosis. Hum Mol Genet. 2002;11:69–76. doi: 10.1093/hmg/11.1.69. [DOI] [PubMed] [Google Scholar]
- 159.Scoles DR, Huynh DP, Morcos PA, et al. Neurofibromatosis 2 tumour suppressor schwannomin interacts with betaII-spectrin. Nat Genet. 1998;18:354–359. doi: 10.1038/ng0498-354. [DOI] [PubMed] [Google Scholar]
- 160.Seizinger BR, Rouleau G, Ozelius LJ, et al. Common pathogenetic mechanism for three tumor types in bilateral acoustic neurofibromatosis. Science. 1987;236:317–319. doi: 10.1126/science.3105060. [DOI] [PubMed] [Google Scholar]
- 161.Sestini R, Bacci C, Provenzano A, Genuardi M, Papi L. Evidence of a four-hit mechanism involving SMARCB1 and NF2 in schwannomatosis-associated schwannomas. Hum Mutat. 2008;29:227–231. doi: 10.1002/humu.20679. [DOI] [PubMed] [Google Scholar]
- 162.Sevenet N, Sheridan E, Amram D, Schneider P, Handgretinger R, Delattre O. Constitutional mutations of the hSNF5/INI1 gene predispose to a variety of cancers. Am J Hum Genet. 1999;65:1342–1348. doi: 10.1086/302639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shaw RJ, McClatchey AI, Jacks T. Regulation of the neurofibromatosis type 2 tumor suppressor protein, merlin, by adhesion and growth arrest stimuli. J Biol Chem. 1998;273:7757–7764. doi: 10.1074/jbc.273.13.7757. [DOI] [PubMed] [Google Scholar]
- 164.Shaw RJ, Paez JG, Curto M, et al. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell. 2001;1:63–72. doi: 10.1016/s1534-5807(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 165.Sherman L, Xu HM, Geist RT, et al. Interdomain binding mediates tumor growth suppression by the NF2 gene product. Oncogene. 1997;15:2505–2509. doi: 10.1038/sj.onc.1201418. [DOI] [PubMed] [Google Scholar]
- 166.Sherman LS, Atit R, Rosenbaum T, Cox AD, Ratner N. Single cell Ras-GTP analysis reveals altered Ras activity in a subpopulation of neurofibroma Schwann cells but not fibroblasts. J Biol Chem. 2000;275:30740–30745. doi: 10.1074/jbc.M001702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sobel RA. Vestibular (acoustic) schwannomas: histologic features in neurofibromatosis 2 and in unilateral cases. J Neuropathol Exp Neurol. 1993;52:106–113. doi: 10.1097/00005072-199303000-00002. [DOI] [PubMed] [Google Scholar]
- 168.Rachamimov AO, Louis DN, Nielsen GP, et al. Comparative pathology of nerve sheath tumors in mouse models and humans. Cancer Res. 2004;64:3718–3724. doi: 10.1158/0008-5472.CAN-03-4079. [DOI] [PubMed] [Google Scholar]
- 169.Stemmer-Rachamimov AO, Xu L, Gonzalez-Agosti C, et al. Universal absence of merlin, but not other ERM family members, in schwannomas. Am J Pathol. 1997;151:1649–1654. [PMC free article] [PubMed] [Google Scholar]
- 170.Stergiopoulos SG, Stratakis CA. Human tumors associated with Carney complex and germline PRKAR1A mutations: a protein kinase A disease! FEBS Lett. 2003;546:59–64. doi: 10.1016/s0014-5793(03)00452-6. [DOI] [PubMed] [Google Scholar]
- 171.Stonecypher MS, Byer SJ, Grizzle WE, Carroll SL. Activation of the neuregulin-1/ErbB signaling pathway promotes the proliferation of neoplastic Schwann cells in human malignant peripheral nerve sheath tumors. Oncogene. 2005;24:5589–5605. doi: 10.1038/sj.onc.1208730. [DOI] [PubMed] [Google Scholar]
- 172.Stonecypher MS, Chaudhury AR, Byer SJ, Carroll SL. Neuregulin growth factors and their ErbB receptors form a potential signaling network for schwannoma tumorigenesis. J Neuropathol Exp Neurol. 2006;65:162–175. doi: 10.1097/01.jnen.0000199575.93794.2f. [DOI] [PubMed] [Google Scholar]
- 173.Storlazzi CT, Brekke HR, Mandahl N, et al. Identification of a novel amplicon at distal 17q containing the BIRC5/SURVIVIN gene in malignant peripheral nerve sheath tumours. J Pathol. 2006;209:492–500. doi: 10.1002/path.1998. [DOI] [PubMed] [Google Scholar]
- 174.Swensen JJ, Keyser J, Coffin CM, Biegel JA, Viskochil DH, Williams MS. Familial occurrence of schwannomas and malignant rhabdoid tumour associated with a duplication in SMARCB1. J Med Genet. 2009;46:68–72. doi: 10.1136/jmg.2008.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Takahashi K, Sasaki T, Mammoto A, et al. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem. 1997;272:23371–23375. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- 176.Tao Y, Dai P, Liu Y, et al. Erbin regulates NRG1 signaling and myelination. Proc Natl Acad Sci U S A. 2009;106:9477–9482. doi: 10.1073/pnas.0901844106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Thaxton C, Lopera J, Bott M, Fernandez-Valle C. Neuregulin and laminin stimulate phosphorylation of the NF2 tumor suppressor in Schwann cells by distinct protein kinase A and p21-activated kinase-dependent pathways. Oncogene. 2008;27:2705–2715. doi: 10.1038/sj.onc.1210923. [DOI] [PubMed] [Google Scholar]
- 178.Tikoo A, Varga M, Ramesh V, Gusella J, Maruta H. An anti-Ras function of neurofibromatosis type 2 gene product (NF2/Merlin) J Biol Chem. 1994;269:23387–23390. [PubMed] [Google Scholar]
- 179.Tona A, Perides G, Rahemtulla F, Dahl D. Extracellular matrix in regenerating rat sciatic nerve: a comparative study on the localization of laminin, hyaluronic acid, and chondroitin sulfate proteoglycans, including versican. J Histochem Cytochem. 1993;41:593–599. doi: 10.1177/41.4.8450198. [DOI] [PubMed] [Google Scholar]
- 180.Trofatter JA, MacCollin MM, Rutter JL, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;75:826. doi: 10.1016/0092-8674(93)90501-g. [DOI] [PubMed] [Google Scholar]
- 181.Upadhyaya M, Huson SM, Davies M, et al. An absence of cutaneous neurofibromas associated with a 3-bp inframe deletion in exon 17 of the NF1 gene (c.2970-2972 delAAT): evidence of a clinically significant NF1 genotype-phenotype correlation. Am J Hum Genet. 2007;80:140–151. doi: 10.1086/510781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Upadhyaya M, Kluwe L, Spurlock G, et al. Germline and somatic NF1 gene mutation spectrum in NF1-associated malignant peripheral nerve sheath tumors (MPNSTs) Hum Mutat. 2008;29:74–82. doi: 10.1002/humu.20601. [DOI] [PubMed] [Google Scholar]
- 183.Utermark T, Kaempchen K, Hanemann CO. Pathological adhesion of primary human schwannoma cells is dependent on altered expression of integrins. Brain Pathol. 2003;13:352–363. doi: 10.1111/j.1750-3639.2003.tb00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vandenbroucke I, Van Oostveldt P, Coene E, De Paepe A, Messiaen L. Neurofibromin is actively transported to the nucleus. FEBS Lett. 2004;560:98–102. doi: 10.1016/S0014-5793(04)00078-X. [DOI] [PubMed] [Google Scholar]
- 185.Versteege I, Sevenet N, Lange J, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394:203–206. doi: 10.1038/28212. [DOI] [PubMed] [Google Scholar]
- 186.Viskochil D, Buchberg AM, Xu G, et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62:187–192. doi: 10.1016/0092-8674(90)90252-a. [DOI] [PubMed] [Google Scholar]
- 187.Vogel KS, Klesse LJ, Velasco-Miguel S, Meyers K, Rushing EJ, Parada LF. Mouse tumor model for neurofibromatosis type 1. Science. 1999;286:2176–2179. doi: 10.1126/science.286.5447.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vries RG, Bezrookove V, Zuijderduijn LM, et al. Cancer-associated mutations in chromatin remodeler hSNF5 promote chromosomal instability by compromising the mitotic checkpoint. Genes Dev. 2005;19:665–670. doi: 10.1101/gad.335805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wallace MR, Marchuk DA, Andersen LB, et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249:181–186. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- 190.Wanner IB, Guerra NK, Mahoney J, et al. Role of N-cadherin in Schwann cell precursors of growing nerves. Glia. 2006;54:439–459. doi: 10.1002/glia.20390. [DOI] [PubMed] [Google Scholar]
- 191.Wanner IB, Wood PM. N-cadherin mediates axon-aligned process growth and cell-cell interaction in rat Schwann cells. J Neurosci. 2002;22:4066–4079. doi: 10.1523/JNEUROSCI.22-10-04066.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Watanabe T, Sakamoto A, Tamiya S, Oda Y, Masuda K, Tsuneyoshi M. H-ras-1 point mutation in malignant peripheral nerve sheath tumors: polymerase chain reaction restriction fragment length polymorphism analysis and direct sequencing from paraffin-embedded tissues. Int J Mol Med. 2000;5:605–608. doi: 10.3892/ijmm.5.6.605. [DOI] [PubMed] [Google Scholar]
- 193.Weiss SW, Nickoloff BJ. CD-34 is expressed by a distinctive cell population in peripheral nerve, nerve sheath tumors, and related lesions. Am J Surg Pathol. 1993;17:1039–1045. doi: 10.1097/00000478-199310000-00009. [DOI] [PubMed] [Google Scholar]
- 194.Welti S, Fraterman S, D’Angelo I, Wilm M, Scheffzek K. The sec14 homology module of neurofibromin binds cellular glycerophospholipids: mass spectrometry and structure of a lipid complex. J Mol Biol. 2007;366:551–562. doi: 10.1016/j.jmb.2006.11.055. [DOI] [PubMed] [Google Scholar]
- 195.Welti S, Kuhn S, D’Angelo I, Brugger B, Kaufmann D, Scheffzek K. Structural and biochemical consequences of NF1 associated nontruncating mutations in the Sec14-PH module of neurofibromin. Hum Mutat. 2011;32:191–197. doi: 10.1002/humu.21405. [DOI] [PubMed] [Google Scholar]
- 196.Widemann BC, Salzer WL, Arceci RJ, et al. Phase I trial and pharmacokinetic study of the farnesyltransferase inhibitor tipifarnib in children with refractory solid tumors or neurofibromatosis type I and plexiform neurofibromas. J Clin Oncol. 2006;24:507–516. doi: 10.1200/JCO.2005.03.8638. [DOI] [PubMed] [Google Scholar]
- 197.Wiederhold T, Lee MF, James M, et al. Magicin, a novel cytoskeletal protein associates with the NF2 tumor suppressor merlin and Grb2. Oncogene. 2004;23:8815–8825. doi: 10.1038/sj.onc.1208110. [DOI] [PubMed] [Google Scholar]
- 198.Wimmer K, Roca X, Beiglbock H, et al. Extensive in silico analysis of NF1 splicing defects uncovers determinants for splicing outcome upon 5′ splice-site disruption. Hum Mutat. 2007;28:599–612. doi: 10.1002/humu.20493. [DOI] [PubMed] [Google Scholar]
- 199.Wimmer K, Yao S, Claes K, et al. Spectrum of single- and multiexon NF1 copy number changes in a cohort of 1,100 unselected NF1 patients. Genes Chromosomes Cancer. 2006;45:265–276. doi: 10.1002/gcc.20289. [DOI] [PubMed] [Google Scholar]
- 200.Woodhoo A, Sommer L. Development of the Schwann cell lineage: from the neural crest to the myelinated nerve. Glia. 2008;56:1481–1490. doi: 10.1002/glia.20723. [DOI] [PubMed] [Google Scholar]
- 201.Wu J, Williams JP, Rizvi TA, et al. Plexiform and dermal neurofibromas and pigmentation are caused by Nf1 loss in desert hedgehog-expressing cells. Cancer Cell. 2008;13:105–116. doi: 10.1016/j.ccr.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Xiao GH, Beeser A, Chernoff J, Testa JR. p21-activated kinase links Rac/Cdc42 signaling to merlin. J Biol Chem. 2002;277:883–886. doi: 10.1074/jbc.C100553200. [DOI] [PubMed] [Google Scholar]
- 203.Xiao GH, Gallagher R, Shetler J, et al. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol Cell Biol. 2005;25:2384–2394. doi: 10.1128/MCB.25.6.2384-2394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xu GF, Lin B, Tanaka K, et al. The catalytic domain of the neurofibromatosis type 1 gene product stimulates ras GTPase and complements ira mutants of S. cerevisiae. Cell. 1990;63:835–841. doi: 10.1016/0092-8674(90)90149-9. [DOI] [PubMed] [Google Scholar]
- 205.Xu GF, O’Connell P, Viskochil D, et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62:599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- 206.Yang FC, Chen S, Clegg T, et al. Nf1+/- mast cells induce neurofibroma like phenotypes through secreted TGF-beta signaling. Hum Mol Genet. 2006;15:2421–2437. doi: 10.1093/hmg/ddl165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yang FC, Ingram DA, Chen S, et al. Neurofibromin-deficient Schwann cells secrete a potent migratory stimulus for Nf1+/- mast cells. J Clin Invest. 2003;112:1851–1861. doi: 10.1172/JCI19195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yang FC, Ingram DA, Chen S, et al. Nf1-dependent tumors require a microenvironment containing Nf1+/-- and c-kit-dependent bone marrow. Cell. 2008;135:437–448. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Young P, Boussadia O, Berger P, et al. E-cadherin is required for the correct formation of autotypic adherens junctions of the outer mesaxon but not for the integrity of myelinated fibers of peripheral nerves. Mol Cell Neurosci. 2002;21:341–351. doi: 10.1006/mcne.2002.1177. [DOI] [PubMed] [Google Scholar]
- 210.Zheng H, Chang L, Patel N, et al. Induction of abnormal proliferation by nonmyelinating schwann cells triggers neurofibroma formation. Cancer Cell. 2008;13:117–128. doi: 10.1016/j.ccr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 211.Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zou CY, Smith KD, Zhu QS, et al. Dual targeting of AKT and mammalian target of rapamycin: a potential therapeutic approach for malignant peripheral nerve sheath tumor. Mol Cancer Ther. 2009;8:1157–1168. doi: 10.1158/1535-7163.MCT-08-1008. [DOI] [PubMed] [Google Scholar]