Abstract
Alcohol use, and misuse, has been a part of human culture for thousands of years. In the modern medical era a great deal of attention has been justifiably focused on elucidating the mechanisms underlying the psychological and biological addiction to alcohol. However, a significant percentage, if not the majority, of alcohol-related morbidity and mortality occurs in individuals who do not meet the formal diagnostic criteria for alcohol use disorders. For example, many serious medical consequences of chronic alcohol ingestion can occur in individuals who do not have signs or symptoms of alcohol dependence. There is now clear evidence that even in otherwise healthy-appearing individuals who chronically consume excessive amounts of alcohol that alveolar macrophage immune capacity is impaired and, as a consequence, these individuals are at significantly increased risk of pneumonia. This brief review summarizes some of the key mechanisms underlying this phenomenon and proposes a hypothetical scheme by which alcohol interferes with zinc bioavailability within the alveolar space and thereby dampens macrophage function.
Keywords: pneumonia, ARDS, acute lung injury, glutathione, zinc
ALCOHOL ABUSE AND PNEUMONIA
Epidemiology
Alcohol consumption began many thousands of years ago and has been a popular aspect of human culture ever since. Legal alcohol consumption is prevalent, and complete abstinence by any given individual is the exception in most modern societies including the United States. In fact, according to the 2008 National Survey on Drug Use and Health, more than 50 percent of the adult population, or roughly 125 million people, in the United States consume alcohol. In addition to the many salutary ‘social benefits’ of alcohol ingestion that are touted by those who imbibe, there is in fact epidemiological evidence that moderate alcohol use can have positive health outcomes. For better or for worse, alcohol consumption has been a social norm in American society for centuries, even during our so-called ‘Prohibition Era’ in the early part of the 20th century. Given the pervasiveness of alcohol consumption in American culture, there is unfortunately a significant incidence of alcohol use disorders and ‘unsafe’ alcohol use has devastating consequences and places a major burden on society. It is estimated that there are 15–20 million alcoholics in the United States, and previous reports place the price tag on alcohol-related problems in our country at more than $185 billion per year. Data from the National Epidemiologic Survey on Alcohol and Related Conditions reported that the lifetime prevalence of alcohol abuse is an astonishing 18 percent, making alcohol not only the most widely used but also the most abused of all drugs.
A connection between excessive alcohol use and serious lung infections has been recognized for hundreds of years. Benjamin Rush, who was the first Surgeon General of the United States, made the observation more than two centuries ago that pneumonia and tuberculosis were more frequently encountered in those who had an affinity for alcohol. One hundred years later William Osler noted in his Principles and Practice of Medicine that alcohol was one of the greatest predisposing factors to the development of pneumonia. Despite the development of vaccines, antimicrobials and the medical technology to support critically ill individuals with respiratory failure over the past fifty years, pneumonia remains a major cause of morbidity and mortality in the United States as well as worldwide. According to recent data from the Centers for Disease Control, pneumonia is the eighth leading cause of death in the United States and continues to be the leading cause of death from infection 1. Accumulated data have confirmed that community-acquired pneumonia is more prevalent and poses a greater risk for poor outcomes in alcoholics 2. In 2000, Watari and colleagues reported on the mortality and prognostic factors in 231 patients admitted with community-acquired pneumonia and found that liver cirrhosis (as a marker of chronic alcohol abuse) was one of three factors that correlated with 30-day mortality, and that ongoing alcoholism correlated significantly with mortality at hospital discharge 3. In the same year, Marik reported on the outcomes, clinical and prognostic features, and the microbial pathogens derived from a database of patients presenting with septic shock secondary to community-acquired pneumonia 4. Of the 148 patients that met the study criteria, the survival at 28 days was 53%. As would be expected, Steptococcus pnuemoniae was the most frequently isolated pathogen but the mortality was particularly high (82%) in those patients infected with Pseudomonas aeruginosa or Acinetobacter species. Importantly, these more serious infections were significantly associated with a history of alcohol abuse. Subsequent studies have confirmed this association of alcoholism with more severe pulmonary infections.
Pathophysiology
Despite our longstanding awareness of the association between alcoholism and pneumonia, our understanding of the mechanisms responsible for this increased susceptibility to pulmonary infections is still incomplete. Fortunately, active investigation in both the clinical and laboratory-based settings is rapidly providing important clues. In general, pneumonia is a diverse syndrome and depends on several host and pathogen factors. The lungs are a unique organ in that they maintain constant contact with the outside environment and require a sophisticated defense system from the mouth to the gas-exchanging alveoli in order to preserve sterility of the lower airways. For the purposes of this review, we will begin by briefly discussing the upper airway defenses followed by a more thorough description of the lower airway responses, paying particular focus to innate immune function of the alveolar macrophage. Chronic exposure to alcohol undermines almost every aspect of these host defenses and therefore there are multiple mechanisms that, alone or in combination, likely explain the longstanding link between alcohol abuse and the development of pneumonia.
The mechanisms by which alcohol abuse increases the risk of pneumonia can be grouped into three general categories: 1) colonization of the oropharynx with pathogenic bacteria; 2) increased frequency of aspiration as a result of depressed level of consciousness and diminished gag and cough reflexes; and 3) impaired integrity of the host immune system. We will provide only a brief summary of the first two categories as the focus in this brief review is on how alcohol impairs alveolar macrophage function. More complete descriptions of the effects of alcohol on the upper airway defenses are provided in a previous review of this topic 5.
As the lower airways are typically a sterile environment, the majority of pneumonia cases are the direct result of aspiration of upper airway contents. Beginning with the mouth, one of the most important components of host defense is saliva, which contains numerous antimicrobial molecules including secretory immunoglobulin A (IgA). Chronic alcohol consumption causes sialosis, a disorder characterized by secretory and parenchymal alterations of the major salivary glands, thereby decreasing saliva output. Additionally, since saliva is important in its acid buffering functions, chronic alcohol abuse leads to accelerated gingivitis and favors the colonization of the mouth with anaerobic and gram-negative bacteria. Therefore, the lower airways are exposed to more virulent bacteria when alcoholics aspirate oropharyngeal contents. Moreover, alcohol compromises some of the anatomical defenses in the upper airway as well. Typically, aspiration into the trachea is difficult as it is protected by several structures including the epiglottis and vocal cords. These barriers prevent oropharyngeal contents from entering the airway by walling off these structures during swallowing and vigorously provoke the cough reflex when any such contents reach these areas. However, the activation of these reflexes is impaired during alcohol intoxication and this further exacerbates the risk of aspirating virulent anerobic and gram-negative bacteria into the lower airways with the subsequent development of pneumonia.
The majority of recent literature describing the effects of chronic alcohol consumption on pulmonary host defense has focused on two particular areas: alterations in host immunity, in particular the alveolar macrophage, which renders individuals susceptible to lung infections such as pneumonia; and alterations in barrier function, in particular the alveolar epithelium, which predisposes individuals to the development of lung injury and pulmonary edema. While both aspects are equally important, and act in tandem to contribute to the overall alcoholic lung phenotype, the effects of alcohol on the alveolar epithelial barrier are beyond the scope of this brief review and the rest of this section will focus on the effects of alcohol abuse on the alveolar macrophage.
The airways are continuously in contact with the external environment and, even under healthy conditions, various pathogens and particles get past the upper airway defenses and reach the alveolar space. Within this unique microenvironment, the resident alveolar macrophage is the first line of defense in terms of cellular immunity. As the primary phagocyte on the gas exchange surface of the lung, it has the capacity to efficiently ingest and clear any inhaled microbes and foreign particulate matter. However, alveolar macrophages in the alcoholic lung are severely impaired. Experimentally, alveolar macrophages isolated from rats fed an alcohol-containing diet for six weeks have significantly impaired bacterial phagocytic capacity 6,7. Further, experimental findings from multiple laboratories demonstrate that chronic alcohol ingestion interferes with the ability of alveolar macrophages to release cytokines, chemokines, and other factors that are essential for microbial killing and activation of the adaptive arm of the immune response 8. These experimental findings are consistent with defects in alveolar macrophage function in alcoholic human subjects 9. These and other studies implicate alveolar macrophage immune dysfunction as an important factor underlying the link between pulmonary infections and chronic alcohol consumption. Although the precise molecular mechanisms by which alcohol abuse interferes with alveolar macrophage function are not yet completely understood, recent studies have identified several unexpected perturbations within the alveolar space.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a 23-kDa peptide that is secreted by alveolar epithelial cells and is essential for terminal differentiation of circulating monocytes into mature, functional alveolar macrophages. GM-CSF was originally isolated from mouse lung extracts, but was named because of its potent effects on hematopoiesis. Interestingly, when the gene for GM-CSF was knocked out in mice, there was no appreciable effect on bone marrow maturation (contrary to what had been predicted) but instead the mice developed lung pathology that resembled pulmonary alveolar proteinosis (PAP), a rare human disease in which the alveolar macrophage fails to scavenge and clear surfactant protein and lipid within the airways. This serendipitous discovery led to the recognition that surfactant recycling by the alveolar macrophage required stimulation by a GM-CSF-mediated pathway, and that in fact most patients with the idiopathic form of PAP have acquired auto-antibodies to GM-CSF that prevent binding of the active peptide to its receptor on the macrophage membrane 10. Conversely, transgenic mice that over-express GM-CSF have alveolar epithelial cell hyperplasia, increased lung sizes, and improved removal of surfactant proteins from the alveolar space 11. It is now clear that although GM-CSF is indeed a potent stimulator of granulopoiesis and is used clinically to promote bone marrow recovery following chemotherapy, it is in fact absolutely required within the lung for the maturation and host immune function of the alveolar macrophage 12. Interestingly, there was some early evidence that the levels of GM-CSF within the alveolar space of patients with ARDS were associated with an improved survival. Therefore, several years ago we hypothesized that alcohol-mediated alveolar epithelial and macrophage dysfunction might involve impaired GM-CSF signaling. Consistent with the experimental findings in the alveolar epithelium discussed in the previous section, there is parallel experimental evidence that chronic alcohol ingestion interferes with GM-CSF-dependent ‘priming’ of the alveolar macrophage. Specifically, alcohol ingestion decreased expression of the GM-CSF receptor in the surface membrane of the alveolar macrophage and, in turn, dampened intracellular GM-CSF signaling as reflected by decreased expression and nuclear binding of PU.1, the master transcription factor for GM-CSF signaling 6. More importantly, treatment with exogenous GM-CSF restored GM-CSF receptor expression and PU.1 nuclear binding, and normalized bacterial phagocytic capacity in the alveolar macrophages of alcohol-fed animals 6. Although these experimental findings have not yet been confirmed in human studies, they provide the intriguing possibility that treatment with recombinant GM-CSF, as is done routinely to stimulate bone marrow recovery following chemotherapy for many malignancies, might improve alveolar macrophage (and epithelial) function in alcoholics with pneumonia and/or acute lung injury.
Alcohol-induced depletion of the critical anti-oxidant glutathione within the alveolar space also has profound implications for alveolar macrophage function. For example, chronic alcohol ingestion in experimental animals increases the susceptibility of the lung to group B streptococcus infection, and supplementation with dietary glutathione precursors improves clearance of bacteria and attenuates acute lung injury 13. In a similar study in neonatal guinea pigs, pups exposed to alcohol in utero had increased lung infection and sepsis, and the ability of their alveolar macrophages to phagocytose group B streptococcus was significantly impaired 14. However, when a glutathione precursor was supplemented in the maternal diet, lung and systemic infections were attenuated and macrophage phagocytosis was restored, suggesting that fetal alcohol exposure alters neonatal host lung defenses against bacterial infection and that treatment with exogenous glutathione could augment neonatal macrophage immune function. These findings have been corroborated in many other experimental models.
Zinc and Alcohol-Mediated Alveolar Macrophage Dysfunction
Another potential mediator of the macrophage dysfunction that is inherent to the alcoholic lung phenotype is zinc deficiency. Zinc is a key participant in normal host immune response, is critical for normal protein metabolism, is a co-factor required for the function of more than 300 metalloenzymes, and is necessary for membrane integrity 15. Additionally, adequate zinc levels are essential for healthy immune function, including both innate defenses (such as the alveolar macrophage) and adaptive defenses such as those provided by T and B lymphocytes. Alcohol abuse is often complicated by malnutrition, and zinc deficiency has been postulated to cause some of the skin changes and immunodeficiency associated with alcoholic liver disease. The primary source of zinc in the diet is meat, although vegetarians eating a balanced diet can have adequate intake and zinc transport in the intestine can be up-regulated significantly when dietary zinc is low. Therefore, the assumption has been that the zinc deficiency in alcoholics is the result of poor nutrition. However, there is recent experimental evidence in an animal model that chronic alcohol ingestion significantly decreases the expression of the primary zinc transporter in the intestinal epithelium, and that systemic zinc deficiency develops even when adequate zinc is present in the diet 7. Although not yet validated in humans, these findings nevertheless suggest that individuals who abuse alcohol could develop zinc deficiency even with a balanced diet, and that this zinc deficiency would be even more profound if their diet is poor. Consistent with this are other experimental findings in cell culture, animal models, and humans that alcohol can interfere with zinc transport and/or bioavailability, including maternal transfer to the fetus across the placental barrier 16.
Fortunately, zinc deficiency is not very common in the general population in the United States. However, it contributes to a significant burden of disease in developing parts of the world such as sub-Saharan Africa and Southeast Asia 17. In these regions there is clear evidence, especially among children, that zinc deficiency is associated with pneumonia, and that dietary zinc supplementation in these children reduces both the incidence and severity of respiratory tract infections 18. These observations have led to a growing recognition that zinc is important for airway health 15, including experimental evidence that zinc deficiency interferes with the ability to mount an appropriate immune response to pneumococcal antigen and renders the host more susceptible to severe pneumococcal pneumonia 19. In this context, it is not surprising that we now have experimental evidence that zinc deficiency appears to be a major factor in the alveolar macrophage immune dysfunction that is characteristic of the alcoholic lung.
Our group recently determined that chronic alcohol ingestion in experimental animals alters the expression of zinc transporters and causes significant intracellular zinc deficiency in the alveolar macrophage 7. Remarkably, dietary zinc supplementation in these animals (in doses that are comparable to those used to treat zinc deficiency clinically) restored macrophage zinc levels and bacterial phagocytic function 7. In a subsequent study with even stronger potential clinical implications, dietary zinc supplementation restored the ability of these alcohol-fed animals to clear a bacterial challenge from their lungs as efficiently as animals on a control diet 20. In that study, dietary zinc supplementation also improved nuclear binding of PU.1, the master transcription factor for GM-CSF signaling, and of Nrf2, the master transcription factor required to activate the anti-oxidant response element (both discussed earlier) 20.
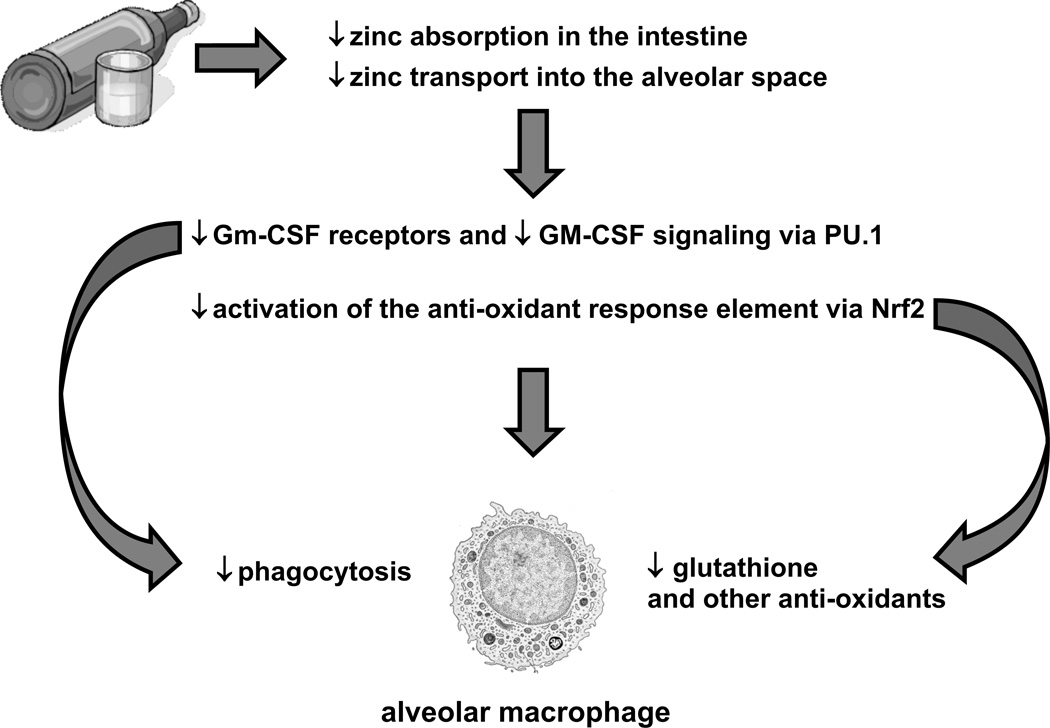
Taken together, the experimental findings discussed above reveal a complex interplay between alcohol-induced zinc deficiency and two fundamental signaling pathways that are required for robust host immune capacity within the alveolar macrophages. Specifically, it appears that alcohol interferes with dietary zinc absorption in the gut but also impairs zinc transport into the alveolar space and its uptake by the alveolar macrophage. As a consequence of this intracellular zinc deficiency, GM-CSF signaling is dampened as reflected by decreased surface expression of its receptor as well as decreased expression and binding of its master transcription factor, PU.1. As GM-CSF signaling is critical to ‘prime’ the mature immune function of the alveolar macrophage, the result is decreased phagocytic function. In parallel, alcohol-induced zinc deficiency dampens activation of the anti-oxidant response element by decreasing the expression and nuclear binding of its master transcription factor, Nrf2. As a result, the production of a broad array of critical anti-oxidants, including glutathione, is severely limited and leaves the macrophage susceptible to the oxidative stress that accompanies acute insults such as pneumonia. Although these recent and exciting experimental findings need to be confirmed in clinical studies (that are in progress), they elucidate discrete molecular targets by which alcohol abuse disrupts signaling pathways in the alveolar macrophage and thereby renders the lung susceptible to infection. The figure below depicts this hypothetical scheme. In addition to identifying potential mechanisms by which alcohol impairs lung health, it suggests specific therapeutic interventions, most notably zinc and glutathione supplementation, which we hypothesize could decrease the enormous morbidity and mortality associated with the ‘alcoholic lung’ in our society.
It remains to be seen if these experimental studies that are elucidating the underlying mechanisms by which chronic alcohol ingestions renders the lung susceptible to various injuries can be translated into novel therapies that can improve or even restore lung health in these vulnerable individuals. Although many challenges remain in diminishing the devastating effects of alcohol use disorders in our society, it would appear that we are at least getting closer to developing comprehensive treatment regimens that can address the medical consequences of this age-old problem.
Figure 1. Hypothetical scheme for alcohol-mediated alveolar macrophage dysfunction.
There is evolving experimental evidence that chronic alcohol abuse inhibits transport-mediated absorption of dietary zinc in the small intestine and, in parallel, decreases zinc transport into the alveolar space and uptake by the alveolar macrophage. As a consequence, the surface expression of GM-CSF receptors and the intracellular signaling of GM-CSF, via its master transcription factor, PU.1, are both decreased and thereby impair alveolar macrophage phagocytic capacity. In parallel, the alcohol-mediated zinc deficiency inhibits activation of the anti-oxidant response element by decreasing signaling through its master transcription factor, Nrf2. As a result, the alveolar macrophage becomes profoundly deficient in glutathione and other anti-oxidants, and is therefore vulnerable to further cellular damage and dysfunction during the acute oxidative stress imposed by pneumonia or sepsis.
Acknowledgments
This work was supported by a VA Career Development Award for Dr. Mehta, and a VA Merit Review as well as NIH awards (AA P50 013757 and AA 017627) for Dr. Guidot
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Xu J, Kochanek KD, Murphy SL, et al. Deaths: Final Data for 2007. Natl Vital Stat Rep. 2010;58(19):1–135. [PubMed] [Google Scholar]
- 2.de Roux A, Cavalcanti M, Marcos MA, et al. Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest. 2006;129(5):1219–1225. doi: 10.1378/chest.129.5.1219. [DOI] [PubMed] [Google Scholar]
- 3.Watari M, Ohe M, Kunimoto E, et al. [Mortality and prognostic factors in patients with community-acquired pneumonia: an analysis of 231 cases] Nihon Kokyuki Gakkai Zasshi. 2000;38(7):509–517. [PubMed] [Google Scholar]
- 4.Marik PE. The clinical features of severe community-acquired pneumonia presenting as septic shock. Norasept II Study Investigators. J Crit Care. 2000;15(3):85–90. doi: 10.1053/jcrc.2000.16460. [DOI] [PubMed] [Google Scholar]
- 5.Joshi PC, Guidot DM. The alcoholic lung: epidemiology, pathophysiology, and potential therapies. Am J Physiol Lung Cell Mol Physiol. 2007;292(4):L813–L823. doi: 10.1152/ajplung.00348.2006. [DOI] [PubMed] [Google Scholar]
- 6.Joshi PC, Applewhite L, Ritzenthaler JD, et al. Chronic ethanol ingestion in rats decreases granulocyte-macrophage colony-stimulating factor receptor expression and downstream signaling in the alveolar macrophage. J Immunol. 2005;175(10):6837–6845. doi: 10.4049/jimmunol.175.10.6837. [DOI] [PubMed] [Google Scholar]
- 7.Joshi PC, Mehta A, Jabber WS, et al. Zinc deficiency mediates alcohol-induced alveolar epithelial and macrophage dysfunction in rats. Am J Respir Cell Mol Biol. 2009;41(2):207–216. doi: 10.1165/rcmb.2008-0209OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Souza NB, Nelson S, Summer WR, et al. Alcohol modulates alveolar macrophage tumor necrosis factor-alpha, superoxide anion, and nitric oxide secretion in the rat. Alcohol Clin Exp Res. 1996;20(1):156–163. doi: 10.1111/j.1530-0277.1996.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 9.Wallaert B, Aerts C, Colombel JF, et al. Human alveolar macrophage antibacterial activity in the alcoholic lung. Am Rev Respir Dis. 1991;144(2):278–283. doi: 10.1164/ajrccm/144.2.278. [DOI] [PubMed] [Google Scholar]
- 10.Huffman JA, Hull WM, Dranoff G, et al. Pulmonary epithelial cell expression of GM-CSF corrects the alveolar proteinosis in GM-CSF-deficient mice. J Clin Invest. 1996;97(3):649–655. doi: 10.1172/JCI118461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikegami M, Jobe AH, Huffman Reed JA, et al. Surfactant metabolic consequences of overexpression of GM-CSF in the epithelium of GM-CSF-deficient mice. Am J Physiol. 1997;273(4 Pt 1):L709–L714. doi: 10.1152/ajplung.1997.273.4.L709. [DOI] [PubMed] [Google Scholar]
- 12.Trapnell BC, Whitsett JA. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 13.Tang SM, Gabelaia L, Gauthier TW, et al. N-acetylcysteine improves group B streptococcus clearance in a rat model of chronic ethanol ingestion. Alcohol Clin Exp Res. 2009;33(7):1197–1201. doi: 10.1111/j.1530-0277.2009.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauthier TW, Young PA, Gabelaia L, et al. In utero ethanol exposure impairs defenses against experimental group B streptococcus in the term Guinea pig lung. Alcohol Clin Exp Res. 2009;33(2):300–306. doi: 10.1111/j.1530-0277.2008.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zalewski PD. Zinc metabolism in the airway: basic mechanisms and drug targets. Curr Opin Pharmacol. 2006;6(3):237–243. doi: 10.1016/j.coph.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Carey LC, Coyle P, Philcox JC, et al. Zinc supplementation at the time of ethanol exposure ameliorates teratogenicity in mice. Alcohol Clin Exp Res. 2003;27(1):107–110. doi: 10.1097/01.ALC.0000046337.19144.7D. [DOI] [PubMed] [Google Scholar]
- 17.Walsh CT, Sandstead HH, Prasad AS, et al. Zinc: health effects and research priorities for the 1990s. Environ Health Perspect. 1994;102 Suppl 2:5–46. doi: 10.1289/ehp.941025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhandari N, Bahl R, Taneja S, et al. Effect of routine zinc supplementation on pneumonia in children aged 6 months to 3 years: randomised controlled trial in an urban slum. BMJ. 2002;324(7350):1358. doi: 10.1136/bmj.324.7350.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strand TA, Hollingshead SK, Julshamn K, et al. Effects of zinc deficiency and pneumococcal surface protein a immunization on zinc status and the risk of severe infection in mice. Infect Immun. 2003;71(4):2009–2013. doi: 10.1128/IAI.71.4.2009-2013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta AJ, Joshi PC, Fan X, et al. Zinc Supplementation Restores PU.1 and Nrf2 Nuclear Binding in Alveolar Macrophages and Improves Redox Balance and Bacterial Clearance in the Lungs of Alcohol-Fed Rats. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]