Abstract
Previous studies suggest that individual preferences for medication- or psychotherapy-based treatments for depression may affect outcomes in clinical trials that compare these two forms of treatment. We assessed patients' beliefs about the causes of their depression, their preferred treatment, and strength of that preference in 80 patients participating in a 12-week clinical trial evaluating neuroimaging predictors of response to cognitive behavior therapy (CBT) or escitalopram. Forty-five patients expressed a preference for one of the 2 treatments, but being matched to preference did not influence remission or completion rates. Medication-preferring patients were more likely to terminate the trial early, regardless of treatment received. CBT-preferring patients rarely endorsed unknown causes for their depression, and medication-preferring patients were highly unlikely to identify pessimistic attitudes as a source of their depression. Among patients willing to be randomized to treatment, preference does not appear to strongly influence outcome. Specific preferences for CBT or medication may reflect differing conceptualizations about depressive illness, knowledge of which may enhance treatment retention and efficacy.
INTRODUCTION
Major depressive disorder (MDD) affects approximately 1 in 6 people during their lifetime and causes significant occupational and social impairments. (Kessler et al., 2003). Unfortunately, current treatments for MDD, produce remission in only about 30% of patients, though response short of remission occurs in about 50–75% (Kennedy et al., 2001; Nemeroff et al. 2008; Trivedi et al., 2006). Because there is so much variability in individual outcomes from MDD treatment, there are now increasing efforts to identify factors that may predict the optimal treatment modality for a given patient. Among the many factors thought to influence treatment response for MDD, there is increasing attention to patients' treatment preference and the strength of those preferences. Another potential predictor that has received less attention is patients' beliefs about the cause of their depression. Determining the extent to which patient treatment preferences and patients' beliefs affect MDD treatment outcome may have significant impact on both clinical practice and clinical trial design.
Preference is a complex construct that has been associated with gender, race and previous treatment experience (Churchill et al., 2000; Dwight-Johnson et al., 2000). Patient preferences have been associated with important features of treatment success such as treatment initiation (King et al., 2005; Raue et al., 2009), attrition rates (Kwan et al., 2010), adherence rate (Raue et al., 2009) and therapeutic alliance (Iacoviello et al., 2007; Kwan et al., 2010). Patients' preference for a treatment modality has been found to have a small effect in treatment outcomes in certain medical conditions and psychological disorders (King et al., 2005; Preference Collaborative Review Group, 2008; Swift & Callahan, 2009).
In studies of MDD, the relationship between treatment preference and treatment outcome has been inconsistent. Heterogeneity in trial design and in measures of preference and outcome in previous studies prevents definitive conclusions about the effect of patients' preferences treatment outcomes. Some studies suggest that patients matched to their treatment of preference had better treatment outcomes than patients who were not assigned to treatment that was congruent with their preference (Kocsis et al., 2009; Mergl et al., 2011). In contrast, other reports have not found evidence to support preference as a predictor in MDD treatment outcome (Bedi et al., 2000; Chilvers et al., 2001; Kwan et al. 2010; Leykin et al., 2007; Rokke et al., 1999; Raue et al. 2009; Ward et al., 2000). Others have suggested that strength of preference, rather than simple preference per se, may more strongly predict outcomes (Raue et al., 2009). Finally, it is possible that treatment preference itself, regardless of treatment received, may impact outcome by acting as a surrogate marker for other potential predictors of treatment response. For example, preference for medication may reflect personality factors, (e.g. interpersonal avoidance) or benefit experienced from participation in an earlier clinical trial that could be associated with probability of response.
Recently, the impact of patients' beliefs about the etiology of their depression has come under increased study. Patients endorse a variety of causes for depression such as biological causes, interpersonal causes, environmental stressors, and childhood events (Khalsa et al., 2011). Receipt of treatment congruent with the patient's beliefs about depression has been associated with features recognized to be important in treatment success, such as motivation to engage in treatment (Meyer & Garcia-Roberts, 2007), perceived helpfulness of treatment (Iselin & Addis, 2003), and satisfaction (Atkinson et al., 1991). Relatively little work has examined the effect of belief about the etiology of their depression as a predictor of treatment outcome. Depressed patients who endorsed the cause of their illness as being external, or outside their control, were less likely to improve following eight weeks of treatment with Saint John's Wort (Bann et al., 2004). In patients with dysthymia or minor depression, remission with paroxetine or placebo treatment was more likely among those who did not endorse a biological explanation of their depression (Sullivan et al. 2003).
It is commonly assumed that beliefs about the causes of depression and preference for specific treatment modality are related. However, this assumption has received little empirical testing. One recent randomized study found that patients preferring psychotherapy were more likely to attribute the cause of their depression to childhood reasons and complex causes than those who preferred medication (Khalsa et al., 2011). It is possible that beliefs may serve as better predictors of outcome than matching to treatment preference, particularly if patients select treatment preferences based on logistical (e.g. time, expense) or other competing factors (e.g. pressure from spouse). These possibilities suggest that separately exploring both beliefs and preferences is necessary to clarify their independent effects.
To better understand the impact of patient preference, strength of preference, and beliefs about causes of depression on treatment outcome, we assessed these factors prior to randomization in a 12-week trial comparing cognitive behavioral therapy versus escitalopram for the treatment of MDD. Our primary hypothesis was that patients matched to their preferred treatment would be more likely to remit than mismatched patients. We also hypothesized that endorsing beliefs congruent with the underlying explanatory model of their assigned treatment would remit at greater rates than those with beliefs incongruent with the theorized treatment mechanism. Finally, we predicted that mismatch between either preference or beliefs with assigned treatment would predict early termination from trial participation.
MATERIALS AND METHODS
Study Design
This analysis was derived from a randomized, double-blind clinical trial designed to identify neuroimaging and other biological predictors of remission to either escitalopram or cognitive behavioral therapy delivered over 12 weeks. The study was conducted through Emory University's Mood and Anxiety Disorders Program between January 2007 and May 2010. All patients were provided written informed consent prior to participating in the study. The study was conducted in accordance with the Declaration of Helsinki and its amendments, and approved by Emory's Institutional Review Board.
Patients
Patients were recruited through a combination of advertising and clinical referral. Eligible participants were adult outpatients between 18 and 60 years of age who met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for a primary diagnosis of MDD without psychotic features. The Structured Interview for DSM-IV (SCID) was used to determine the presence of MDD and the absence of any exclusionary diagnoses. In addition, participating patients were required to have a 17-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960) total score ≥18 at the screening visit and ≥15 at randomization.
Patients were excluded if they met lifetime criteria for bipolar disorder or a psychotic disorder, or currently met criteria for OCD. If present, comorbid current posttraumatic stress disorder (PTSD) could be of only mild severity and could not be the focus of treatment. Substance abuse in the past 3 months prior to screen, or substance dependence in the past year was also exclusionary. Patients who had failed to respond to previous treatment with escitalopram (at a minimum dose of 10 mg/day for 6 weeks) or manual-based CBT (at least 4 sessions) during the current episode were also excluded. Additional exclusionary criteria included: significant current suicidality or homicidality; a personality disorder, as assessed by the study psychiatrist's evaluation, that could interfere with study participation; electroconvulsive therapy within the past 6 months; contraindications to magnetic resonance imaging or positron emission tomography scans. Patients who were or intended to become pregnant or to breastfeed during the study, or who had a medical illness that might pose a risk to their safety or interfere with study assessments were excluded.
Interested patients were seen for a screening visit, which comprised a SCID interview and administration of the HDRS, Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979), and Hamilton Anxiety Rating Scale (HARS) (Hamilton, 1959) by trained raters. Patients completed the Quick Inventory of Depressive Symptoms (QIDS) (Rush et al., 2003), Beck Depression Inventory (BDI) (Beck et al., 1961), Childhood Trauma Questionnaire (CTQ) (Bernstein & Fink, 1998), and a form for demographic and past clinical information. Laboratory testing, electrocardiogram, and physical exam were performed.
As part of the informed consent document, patients read a paragraph describing escitalopram and CBT treatments for MDD. The study physician also explained to the patient that data indicates that, at a population level, people with MDD are equally likely (on average) to benefit from CBT or medication, and that the study's goal was to identify brain patterns that could be used to predict which treatment would be best for a specific individual with MDD. Patients were advised that it was alright for them to have a preference for one treatment or the other, but that in order to participate in the study, they would need to be willing to accept the type of treatment assigned to them by the randomization process.
Patients who continued to be eligible after the screening visit completed the neuroimaging components of the study: the positron emission tomography scan and structural and functional MRI scans. At the baseline visit, patients completed the HDRS, MADRS, HARS, QIDS and BDI.
Assessment of Beliefs and Preferences
At the baseline visit, prior to randomization, patients completed a seven-item questionnaire inquiring about their beliefs about the causes of their depression, whether they had a preferred treatment, and the strength of their preference, if any. Beliefs were rated on a six point scale, from strongly disagree to strongly agree. The 5 questions about were drawn from the Patient Attitudes and Beliefs Scale, which was developed for the National Institute of Mental Health Collaborative Study of Depression Treatments (Elkin et al., 1989). The questions used were: 1) “An imbalance in certain substances in my brain is the cause of my problem” (“BRAIN SUBSTANCES”); 2) “Pessimistic attitudes about many things is the cause of my problems” (“PESSIMISM”); 3) “Stressful or painful things that have happened are the cause of my problems” (“STRESSFUL EVENTS”); 4) “The cause of my problems comes from `out of the blue'” (“OUT OF BLUE”); 5) “An illness that affects me emotionally instead of physically is the cause of my problems” (“EMOTIONAL ILLNESS”).
Preference was assessed by a single question asking if their preferred treatment was: “No preference”, “Cognitive Behavioral Therapy,” or “Medication.” Those who indicated a preference then completed the last question, asking whether their strength of their preference was “Mild,” “Moderate,” or “Strong.”
Randomization and Treatments
Patients were randomized at a 1:1 ratio to receive 12 weeks of treatment with either escitalopram, flexibly dosed from 10–20 mg/d, or 16 sessions of CBT. Escitalopram was started at 10 mg/d, with the option to increasing the dose by week 3 to 20 mg/d. In rare cases of severe intolerance, the dose could be reduced to 5 mg/day for the first 2 weeks.
The CBT provided followed a standardized protocol (Beck et al, 1979 The schedule for CBT delivery was 4 weeks of twice weekly sessions, followed by weekly sessions for the next 8 weeks, though twice weekly sessions could occur later during the trial if earlier sessions were missed. Concomitant hypnotic medications (zaleplon, eszopiclone, zolpidem, or diphenhydramine) up to 3 nights per week were permitted for patients who requested them, though not on the nights prior to study visits. Treatment with any other psychotropic medication during the trial was prohibited, including St. John's Wort. No supportive therapy was permitted during the trial, other than continued attendance at previously established substance abuse programs (e.g. Alcoholics Anonymous).
The study statistician (M.K.) created the randomization list, by randomly alternating blocks of 2 or 4, and the resulting sequential treatment assignments were sealed in opaque envelopes. At the baseline visit, after the patient had met eligibility to be randomized and completed their beliefs and preferences form, the study coordinator opened the next envelope in the sequence and informed the study physician of the treatment assignment, who then met with the patient to discuss their assigned treatment.
All patients, regardless of treatment, were assessed for symptom change weekly for the first 6 weeks and then every other week for the remaining six weeks. At assessment visits, experienced raters blind to treatment preference and assignment completed the HDRS, MADRS and HARS. The study physician, who was not blinded to treatment, met with the patient to assess safety and completed the Clinical Global Impression- Severity and –Change scales (Guy, 1979). Patients completed the QIDS and BDI at each assessment visit. Patients who did not meet study-defined remission criteria were offered the option to receive another 12 weeks of treatment, continuing on their initial treatment assignment (daily escitalopram or monthly CBT sessions) while starting on the alternative treatment for another 12 weeks. Remitting patients were seen monthly for 12 weeks, while continuing to receive escitalopram or monthly CBT sessions. This paper only reports on the first 12-week treatment period.
Efficacy Measures
The primary efficacy outcome was remission, defined as a HDRS ≤7 at the last visit (last observation carried forward, LOCF, method). Separate secondary analyses using the self-report questionnaires were also performed, using a last visit score of ≤5 to define remission for the QIDS analysis and ≤9 to define remission for the BDI analysis.
Statistical Analysis
All analyses were conducted on an as-treated basis using the LOCF sample, defined as all randomized patients who had ≥1 postbaseline, on-treatment evaluation on the primary efficacy endpoint.
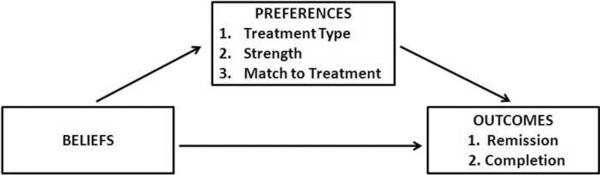
We tested associations between beliefs and preferences, beliefs and outcomes, and preferences and outcomes separately using both nominal (preferences) and logistic (outcomes) regression techniques (Figure 1). For the nominal regressions (also known as baseline category logistic), the user must specify a baseline group for comparison which is then compared to the other groups, thus we ran 2 separate models in order to obtain all possible odds ratios among the three preference groups (i.e., CBT/none, med/none, CBT/med). Beliefs were originally assigned scores of −3,−2,−1, 1, 2, and 3 to reflect responses from strongly disagree through strongly agree; however the distributions of the scores were heavily skewed, so beliefs were collapsed into 2 categories (agree or disagree) for simplification of multivariable analyses. Because this was an exploratory analysis, statistical tests were not adjusted for multiple comparisons.
Figure 1.
Model of potential impact of beliefs and preferences on treatment outcomes.
RESULTS
Study Sample and Treatment Outcomes
Eighty patients met all eligibility criteria and were randomized to treatment. Of these, 78 patients answered the questions about depression beliefs, and 79 indicated their treatment preference (No preference: 34; CBT: 23; Medication: 22). Of the 80 randomized patients, 3 had no post-treatment assessments and thus are excluded from the remission analyses. Demographic and clinical factors of the sample are presented in Table 1. Overall, 41 (51%) were treated with CBT and 39 (49%) with escitalopram. In total, 30/77 (39%) patients remitted with treatment by the HDRS. There was no difference in remission rates between the two treatments by the LOCF analysis (CBT: 37.5%, ESC: 40.5%, X2=0.075, p=0.785). Analysis of the completer sample (n=65 (81% of the randomized sample) also found no difference in remission rate.
Table 1.
Demographic and clinical characteristics
| Characteristic | Total (N=79) | No Preference (N= 34) | Prefer CBT (N=23) | Prefer Med (N=22) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p | |
| Age (yrs) | 41.6 | 8.3 | 40.9 | 8.9 | 42.6 | 7.6 | 41.6 | 8.2 | 0.282 | 0.755 |
| Education (yrs) | 15.7 | 1.9 | 15.5 | 1.7 | 16.5 | 2.0 | 15.24 | 1.8 | 3.242 | 0.045* |
| Age at first episode (yrs) | 26.8 | 11.0 | 26.4 | 12.2 | 25.1 | 9.3 | 29.1 | 10.7 | 0.722 | 0.489 |
| Current episode duration (wks) | 185.1 | 339.9 | 200.3 | 423.1 | 155.1 | 200.7 | 194.3 | 331.8 | 0.127 | 0.881 |
| HDRS | 19.7 | 3.5 | 18.8 | 3.64 | 20.3 | 2.93 | 20.4 | 3.55 | 1.568 | 0.215 |
| QIDS-SR | 13.3 | 4.0 | 12.9 | 3.88 | 12.7 | 3.56 | 14.9 | 4.39 | 2.054 | 0.136 |
| BDI | 21.7 | 6.4 | 20.6 | 5.2 | 21.6 | 6.1 | 23.5 | 8.2 | 1.314 | 0.275 |
| HARS | 15.3 | 4.9 | 14.1 | 4.3 | 17.3 | 5.4 | 15.1 | 4.6 | 3.214 | 0.046* |
| CTQ Total | 48.0 | 14.3 | 49.0 | 15.3 | 48.7 | 14.0 | 45.6 | 13.5 | 0.417 | 0.661 |
| N | % | N | % | N | % | N | % | X2 | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | 0.408 | 0.815 | ||||||||
| Male | 34 | 43 | 16 | 47.1 | 9 | 26.5 | 9 | 26.5 | ||
| Female | 45 | 57 | 18 | 40.0 | 14 | 31.1 | 13 | 28.9 | ||
| Race | 5.584 | 0.232 | ||||||||
| Caucasian | 56 | 71 | 24 | 42.9 | 14 | 25.0 | 18 | 32.1 | ||
| Black | 17 | 22 | 8 | 47.1 | 5 | 29.4 | 4 | 23.5 | ||
| Other | 6 | 8 | 2 | 33.3 | 4 | 66.7 | 0 | 0 | ||
| Married/Cohabitating | 0.366 | 0.833 | ||||||||
| Yes | 35 | 44 | 16 | 45.7 | 9 | 25.7 | 10 | 28.6 | ||
| No | 44 | 56 | 18 | 40.9 | 14 | 31.8 | 12 | 27.3 | ||
| Employed full time | 7.723 | 0.021* | ||||||||
| Yes | 44 | 56 | 25 | 56.8 | 10 | 22.7 | 9 | 20.5 | ||
| No | 35 | 44 | 9 | 25.7 | 13 | 37.1 | 13 | 37.1 | ||
| Current Anxiety Disorder | 2.755 | 0.252 | ||||||||
| Yes | 32 | 41 | 11 | 34.4 | 9 | 28.1 | 12 | 37.5 | ||
| No | 47 | 59 | 23 | 48.9 | 14 | 29.8 | 10 | 21.3 | ||
| Previous Psychotherapy | 1.824 | 0.402 | ||||||||
| Yes | 43 | 54 | 18 | 41.9 | 15 | 34.9 | 10 | 23.3 | ||
| No | 36 | 46 | 16 | 44.4 | 8 | 22.2 | 12 | 33.3 | ||
| Previous Medication | 1.568 | 0.457 | ||||||||
| Yes | 65 | 82 | 29 | 44.6 | 17 | 26.2 | 19 | 29.2 | ||
| No | 14 | 18 | 5 | 35.7 | 6 | 42.9 | 3 | 21.4 | ||
| Previous Episodes | 2.423 | 0.659 | ||||||||
| 1 | 21 | 27 | 10 | 47.6 | 4 | 19.0 | 7 | 33.3 | ||
| 2 | 20 | 25 | 8 | 40.0 | 8 | 40.0 | 4 | 20.0 | ||
| ≥3 | 37 | 47 | 15 | 40.5 | 11 | 29.7 | 11 | 29.7 | ||
| Melancholic Subtype | 1.802 | 0.406 | ||||||||
| Yes | 39 | 49 | 17 | 43.6 | 9 | 23.1 | 13 | 33.3 | ||
| No | 40 | 59 | 17 | 42.5 | 14 | 35.0 | 9 | 22.5 |
p<0.5
BDI: Beck depression inventory; CTQ: Childhood trauma questionnaire; HARS: Hamilton anxiety rating scale HDRS: Hamilton depression rating scale; QIDS-SR: Quick inventory of depressive symptoms – self report;
Demographic and Clinical Factors Associated with Beliefs and Preferences
Full-time employed patients were less likely to express a treatment preference than unemployed or part-time working patients (p=.001), though there was no difference in type of treatment preferred in either group. Endorsing BRAIN SUBSTANCES as an etiologic factor was positively associated with higher education (p=.034), lower HDRS score at baseline (p =.017), being married (p=.02) but inversely associated with melancholic subtype (p=.032).
Higher CTQ scores were associated with endorsing STRESSFUL EVENTS (p=.008) as an etiologic depression factor. However, we did not find that clinically significant early life stress, as determined by CTQ scores, was associated with greater remission with CBT versus medication (p=.41),in contrast to an earlier study comparing nefazodone, cognitive behavioral analysis system of psychotherapy or their combination (Nemeroff et al., 2003).
Endorsed causes of current illness (beliefs)
The most commonly endorsed beliefs about depression etiology were STRESSFUL EVENTS (79.5%) and BRAIN SUBSTANCES (76.9%). PESSIMISM was endorsed by 67.9% of patients, and EMOTIONAL ILLNESS by 50.0%. Only 17.9% endorsed OUT OF BLUE. The initial assessment of beliefs included simple correlation coefficients to determine the extent of collinearity among the belief items. Correlations were mild to near zero (range −.04 to 0.34) indicating that the items could be used as multiple independent predictors without a negative effect on the estimates. The most significant relationships of note were positive associations between pessimistic attitudes and stressful events (r=0.34, p=0.002) and imbalance of brain substances and emotional illness (r=0.27, p=0.017).
Endorsed causes of illness etiology as predictors of preferences
Table 2 summarizes the relationships between beliefs and preferences. Endorsement of brain substances and stressful events as the cause of the current illness were not predictive of preference for either CBT or medication. In contrast, those who endorsed an unknown cause (OUT OF BLUE) were less likely to prefer CBT. Those who endorsed pessimism were less likely to prefer medication, while those that endorsed emotional illness were more likely to prefer medication.
Table 2.
Endorsed causes of current illness as predictors of preferences
| Cause | Preference for CBT (n=22) vs No preference (n=34) | Preference for Medication (n=20) vs No preference (n=34) | Preference for CBT (n=22) vs Medication (n=20) | |||
|---|---|---|---|---|---|---|
| OR | p-value | OR | p-value | OR | p-value | |
| Brain substances | 1.12 | 0.87 | 1.10 | 0.90 | 1.011 | 0.990 |
| Pessimism | 1.04 | 0.96 | 0.16 | 0.01* | 6.487 | 0.013* |
| Stressful events | 1.05 | 0.95 | 0.98 | 0.98 | 1.080 | 0.934 |
| Out of blue | 0.10 | 0.046* | 0.47 | 0.39 | 0.220 | 0.247 |
| Emotional Illness | 0.67 | 0.50 | 4.14 | 0.045* | 0.163 | 0.017* |
OR: Odds ratio
p<.05
Effects of Preference on outcome
Remission
Of the 45 patients expressing a preference, 26 (57.8%) matched and 19 (42.2%) did not match to their preferred treatment type. Matched preference was not predictive of remission on any of the three designated outcomes (HDRS, QIDS, BDI), though the large proportion of patients that expressed no preference reduced the power to detect small effects. Even so, the OR of 1.122 for matched preference on HDRS remission would require a sample size of approximately 13,000 to reach clinical significance, so our data clearly does not support an association between matched preference and outcome. We also assessed the effects of treatment preference type (none, CBT, med) on remission. Although no significant associations were found for any of the remission criteria, it is interesting that the statement of a preference overall as well as matching treatment to preference increased the odds of remission on the HRSD, while it decreased the odds of remission using both BDI and QIDS criterion (Table 3). Strength of preference also was not significantly associated with outcomes.
Table 3.
Remission rates by treatment preference and matching to preference
| Treatment preference | Preference matching | |||||
|---|---|---|---|---|---|---|
| All patients (N=77) | No Preference (N=34) | Preference for CBT (N= 22) | Preference for Medication (N= 20) | Matched to Preference (N= 25) | Mismatched to Preference (N=17) | |
| Outcome | % | OR (p) | OR (p) | OR (p) | OR (p) | OR (p) |
| HDRS | 38.2% | 1.000 (---) | 1.742 (0.325) | 1.394 (0.570) | 1.122 (0.856) | 1.000 (---) |
| QIDS | 57.3% | 1.000 (---) | 0.825 (0.734) | 0.468 (0.188) | 0.963 (0.952) | 1.000 (---) |
| BDI | 70.7% | 1.000 (---) | 0.718 (0.605) | 0.404 (0.146) | 0.625 (0.484) | 1.000 (---) |
OR for treatment preference represents the odds of remission with “no preference” as the baseline rate (OR=1); OR for preference matching is the odds of remission with “mismatched to preference” as the baseline rate (OR=1).
Note: Number of patients with and without a preference sums to 76 because one patient did not complete the preference questions prior to randomization.
Completion
A larger percentage of matched patients (n=21/26, 80.8%) completed the trial than mismatched patients (n=13/19, 68.4%), although this result was not significant (p=.341). There was no difference in early termination rate between those with no preference [4/34 (11.8%)] versus those preferring CBT [3/23 (13%)]. However, patients who preferred medication [8/22 (36.4%)] were more likely to terminate prior to completion than those with no preference (Wald X2=4.41, df=1, p=.036).
Strength of Preference on outcome
Among patients expressing a treatment preference, the strength of preference was mild in 9 (21%), moderate in 21 (50%) and strong in 12 (29%). Strength of preference ratings were nearly identical between those preferring CBT and medication (p=.957). Strength of preference was not associated with outcome on any symptom measure. There was a linear association between strength of preference and probability of early termination (Mild: 11.1%; Moderate: 18.2%; Strong: 42.9%), though this was not statistically significant (p=.142).
Endorsed causes of illness etiology (beliefs) and outcome
None of the individual beliefs about etiology predicted remission on any of the outcome measures. Patients who endorsed PESSIMISM were more likely to complete the study (OR=3.71, p=.034); all other beliefs were not associated with completion.
DISCUSSION
Contrary to our hypotheses, we did not find that remission rates were associated with either: 1) being matched to treatment preference; or 2) beliefs about depression etiology consistent with the model underlying the assigned treatment. Moreover being mismatched to treatment did not predict early termination; neither did any specific belief about depression etiology.
Our finding that matching preference to treatment did not impact remission is consistent with most, but not all previous studies. Our trial design is most similar to that of Leykin et al. (2007), who compared CBT versus medication in 2 academic medical centers, and also found no difference in outcome by matching to treatment preference. Notably, the two studies that did find that receiving one's preferred treatment affected outcome had unique aspects to their design, and had somewhat contradictory findings. Kocsis et al. enrolled only patients with chronic MDD, and offered the option of combination medication and psychotherapy; few participants specifically desired one of the monotherapies alone. They found significantly lower remission rates in patients preferring psychotherapy who were assigned to medication (Kocsis et al., 2009). In contrast, Mergl and colleagues compared an SSRI versus group therapy for patients with minor depression, and found that no medication-preferring patients remitted if assigned to psychotherapy (Mergl et al., 2011). For patients preferring medication treatment, group psychotherapy may be particularly undesired and difficult to engage in. Moreover, our data suggest increased attention should be paid to differences between clinician- and self-rated outcomes of treatment (Dunlop et al., 2011).
A limitation in all randomized studies comparing highly differing treatment modalities is that patients with the strongest preferences about treatment are typically not included. In our study, all patients were advised prior to signing consent that even though they may have a preference for treatment type, they needed to be willing to accept their randomization assignment to participate. This instruction helped prevent enrolling patients who might withdraw from the study upon learning of their treatment assignment, but limits the generalizability of the findings to the broader clinical population. There is little data to inform an estimate of what proportion of patients with MDD would absolutely refuse treatment with CBT or with medication.
We did not find that failure to receive preferred treatment was associated with early termination. This finding is consistent with the findings from larger studies (Kocsis et al., 2009; Leykin et al., 2007), but differs from others (Kwan, et al., 2010; Raue et al., 2009). Inconsistent findings between trials on drop-out rates may arise from variability in patient education prior to randomization. In our trial, medication-preferring patients were more likely to drop-out (regardless of treatment assignment) than patients with no preference; this may have reflected unrealistic expectations about medication effects. Alternatively, preferring medication may reflect a desire for a “quick fix” on behalf of some patients, which may be associated with discouragement and early termination.
The relatively limited number of associations we found between beliefs and treatment preferences was unexpected. It may be that preference was determined largely by factors others than beliefs, including treatment convenience, fear of emotions arising through therapy, or previous treatment experiences. Moreover, patients may have had unmeasured beliefs that conflicted with the beliefs we examined. For example, patients may have believed altered neurochemistry was a cause for their depression, but also had beliefs about dangerous side effects or “addicting” effects of medication, leading them to express a preference for psychotherapy.
The great majority of patients endorsed the beliefs that stress and disturbances in substances related to brain function contribute to their depression. That patients endorsing neurochemical causes were equally likely to prefer medication, CBT or no treatment indicates that acceptance of such a medical model does not equate to desire for medication treatment. It is possible that many patients in our sample of highly educated depressed patients believed that CBT can change biological facets of their brain function, given that they consented to participate in a study in which one of the aims was to identify changes in brain activity with the two treatments.
The high education level (mean 15.7 years) of our sample may be a factor that limits the generalizability of our findings to the broader population of treatment-seeking MDD patients. Acceptance of CBT as a treatment for MDD appears to be related to level of education. In a large trial for moderately ill MDD patients comparing cognitive therapy versus medication, the mean education level was 15 years (DeRubeis et al., 2005). Similarly in STAR*D, the proportion of Step 2 participants who were willing to accept cognitive therapy as a Step 2 treatment increased with increasing level of education (Wisniewski et al., 2007). Although we did not find an interaction between education level and beliefs or preferences, a sample with broader educational range may have identified such relationships.
Roughly two-thirds of the patients endorsed pessimistic attitudes as a cause of their depression, and these patients were significantly less likely to prefer medication treatment. Combined with our finding that CBT-preferring patients rarely endorsed unknown causes for their depression, this association suggests that patients who more fully conceptualize their depression through cause-effect relationships are unlikely to desire medication-based treatments. Furthermore, pessimism was associated with a higher completion rate, suggesting that acknowledging pessimism may reflect some level “ownership” of one's depression and thereby increase motivation to complete treatment.
Patients with higher levels of childhood trauma were more likely to endorse stressful or painful events as a cause of their depression, which may reflect greater susceptibility to stress in these patients (Heim et al., 2000). Somewhat counter-intuitively, patients with melancholia and greater baseline depression severity were less likely to endorse disturbed neurochemistry as a cause of their illness, which is not consistent with proposed explanations for outcomes in patients participating in trials with psychotherapy arms (Dunlop, 2010).
Strengths of this study are the pre-randomization assessment of beliefs, preferences and strength of preferences. To our knowledge, this is the only study that has evaluated both beliefs about depression and treatment preferences as predictors of outcome.
The limitations to our study include a moderate sample size, though the sample was large enough to detect moderate to large effects. We do not believe our results reflect a type II error due to low power; the effect sizes of matching to preference were very small. It is possible that our model could have identified the expected differences in a more unselected sample of patients than our study patients, who were highly educated and willing to be randomized. It is also possible that our model, though intuitive, was inadequately specified to identify the active forces that drive preferences and outcomes. Most significantly, we did not ask about strength of dislike of treatments, nor about practical factors impacting expressed preference. By only asking about preference, we assessed positive, but not negative, valences regarding treatments, and thus did not capture a full picture of preferences. We did not inquire about past positive or negative experiences with medications or other forms of psychotherapy which could have had some conditioning effect to set up a positive or negative expectation of outcome, and thus treatment preference. Additionally, our assessment of beliefs was limited, and other scales exist (e.g., Addis et al., 1995; Budd et al., 2008 ) that could more fully capture belief structures that may more adequately predict preference, and perhaps outcomes. Another limitation is that we did not reassess beliefs after treatment, to explore whether alterations in beliefs resulting from treatment was associated with remission.
We conclude that beliefs about depression etiology and treatment preferences are poor predictors of treatment response among patients willing to be randomized to medication or psychotherapy. For patients willing to start treatment, conceptualizations of illness and treatment appear to be cognitive epiphenomena unrelated to factors mediating treatment response. However, these cognitions may act to diminish willingness to start treatment, and thus could represent poor prognostic factors for the broader population of depressed patients (Van Voorhees et al., 2005).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Addis ME, Truax P, Jacobson NS. Why do people think they are depressed?: The Reasons for Depression Questionnaire. Psychotherapy. 1995;32:476–483. [Google Scholar]
- Atkinson DR, Worthington RL, Dana DM, Good GE. Etiology beliefs, preferences for counseling orientations, and counseling effectiveness. Journal of Counseling Psychology. 1991;38:258–264. [Google Scholar]
- Bann CM, Parker CB, Bradwejn J, Davidson JRT, Vitiello B, Gadde KM. Assessing patient beliefs in a clinical trial of Hypericumperforatum in major depression. Depression and Anxiety. 2004;20:114–122. doi: 10.1002/da.20036. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF. Cognitive Therapy of Depression. Guilford Press; New York: 1979. [Google Scholar]
- Bedi N, Chilvers C, Churchill R, Dewey M, Duggan C, Fielding K, Gretton V, Miller P, Harrison G, Lee A, Williams I. Assessing effectiveness of treatment of depression in primary care. Partially randomised preference trial. British Journal of Psychiatry. 2000;177:312–318. doi: 10.1192/bjp.177.4.312. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Psychological Corporation; San Antonio, TX: 1998. Childhood trauma questionnaire manual. [Google Scholar]
- Budd R, James D, Hughes I. Patients' explanations for depression: a factor analytic study. Clinical Psychology and Psychotherapy. 2008;15:28–37. doi: 10.1002/cpp.558. [DOI] [PubMed] [Google Scholar]
- Chilvers C, Dewey M, Fielding K, Gretton V, Miller P, Palmer B, Weller D, Churchill R, Williams I, Bedi N, Duggan C, Lee A, Harrison G. Antidepressant drugs and generic counselling for treatment of major depression in primary care: randomised trial with patient preference arms. British Medical Journal. 2001;322:772–775. doi: 10.1136/bmj.322.7289.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill R, Khaira M, Gretton V, Chilvers C, Dewey M, Duggan C, Lee A. Treating depression in general practice: factors affecting patients' treatment preferences. British Journal of General Practice. 2000;50:905–906. [PMC free article] [PubMed] [Google Scholar]
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, O'Reardon JP, Lovett ML, Gladis MM, Brown LL, Gallop R. Cognitive therapy vs medications in the treatment of moderate to severe depression. Archives of General Psychiatry. 2005;62:409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- Dunlop BW. An additional consideration for comparisons of antidepressants versus placebo. Journal of Clinical Psychopharmacology. 2010;30:641–642. doi: 10.1097/JCP.0b013e3181f13402. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Li T, Kornstein SG, Friedman ES, Rothschild AJ, Pedersen R, Ninan PT, Keller M, Trivedi MH. Concordance between clinician and patient ratings as predictors of response, remission and recurrence in major depressive disorder. Journal of Psychiatric Research. 2011;45:96–103. doi: 10.1016/j.jpsychires.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwight-Johnson M, Sherbourne CD, Liao D, Wells KB. Treatment preferences among depressed primary Care Patients. Journal of General Internal Medicine. 2000;15:527–534. doi: 10.1046/j.1525-1497.2000.08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Archives of General Psychiatry. 1989;46:871–982. doi: 10.1001/archpsyc.1989.01810110013002. [DOI] [PubMed] [Google Scholar]
- Guy W. Clinical Global Impressions. In: Guy W, editor. ECDEU assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare; Rockville, MD: 1976b. pp. 217–222. Revised, ADM 76-338. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegerl U, Hautzinger M, Mergl R, Kohnen R, Schütze M, Scheunemann W, Allgaier A, Coyne J, Henkel V. Effects of pharmacotherapy and psychotherapy in depressed primary-care patients: a randomized, controlled trial including a patients' choice arm. International Journal of Neuropsychopharmacology. 2010;13:31–44. doi: 10.1017/S1461145709000224. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Journal of the American Medical Association. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Iacoviello BM, McCarthy KS, Barrett MS, Rynn M, Gallop R, Barber JP. Treatment preferences affect the therapeutic alliance: Implications for randomized controlled trials. Journal of Consulting and Clinical Psychology. 2007;75:194–198. doi: 10.1037/0022-006X.75.1.194. [DOI] [PubMed] [Google Scholar]
- Iselin M, Addis ME. Effects of etiology on perceived helpfulness of treatments for depression. Cognitive Therapy & Research. 2003;27:205. [Google Scholar]
- Khalsa S, McCarthy KS, Sharpless BA, Barrett MS, Barber JP. Beliefs about the causes of depression and treatment preferences. Journal of Clinical Psychology. 2011;67:539–549. doi: 10.1002/jclp.20785. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Eisfeld BS, Meyer JH, Bagby M. Antidepressants in clinical practice: limitations of assessment methods and drug response. Human Psychopharmacology: Clinical & Experimental. 2001;16:105–114. doi: 10.1002/hup.189. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, Byford S. Randomised controlled trial of non-directive counselling, cognitive-behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care. Health Technology Assessment. 2000;4:1–83. [PubMed] [Google Scholar]
- King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, Sibbald B, Lai R. Impact of Participant and Physician Intervention Preferences on Randomized Trials: A Systematic Review. Journal of the American Medical Association. 2005;293:1089–1099. doi: 10.1001/jama.293.9.1089. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Leon AC, Markowitz JC, Manber R, Arnow B, Klein DN, Thase ME. Patient preference as a moderator of outcome for chronic forms of major depressive disorder treated with nefazodone, cognitive behavioral analysis system of psychotherapy, or their combination. Journal of Clinical Psychiatry. 2009;70:354–361. doi: 10.4088/jcp.08m04371. [DOI] [PubMed] [Google Scholar]
- Kwan BM, Dimidjian S, Rizvi SL. Treatment preference, engagement, and clinical improvement in pharmacotherapy versus psychotherapy for depression. Behavioral Research and Therapy. 2010;48:799–804. doi: 10.1016/j.brat.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leykin Y, DeRubeis RJ, Gallop R, Amsterdam JD, Shelton RC, Hollon SD. The Relation of Patients' Treatment Preferences to Outcome in a Randomized Clinical Trial. Behavior Therapy. 2007;38:209–217. doi: 10.1016/j.beth.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Mergl R, Henkel V, Allgaier A, Kramer D, Hautzinger M, Kohnen R, Coyne J, Hegerl U. Are treatment preferences relevant in response to serotonergic antidepressants and cognitive-behavioral therapy in depressed primary care patients? Results from a randomized controlled trial including a patients' choice arm. Psychotherapy and Psychosomatics. 2011;80:39–47. doi: 10.1159/000318772. [DOI] [PubMed] [Google Scholar]
- Meyer B, Garcia-Roberts L. Congruence between reasons for depression and motivations for specific interventions. Psychology & Psychotherapy: Theory, Research & Practice. 2007;80:525–542. doi: 10.1348/147608306X169982. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP, Weiss PM, Dunner DL, Rothbaum BO, Kornstein S, Keitner G, Keller MB. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2003;100:14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Entsuah R, Benattia I, Demitrack M, Sloan DM, Thase ME. Comprehensive analysis of remission (COMPARE) with venlafaxine versus SSRIs. Biological Psychiatry. 2008;63:424–434. doi: 10.1016/j.biopsych.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Preference Collaborative Review Group Patients' preferences within randomised trials: systematic review and patient level meta-analysis. British Medical Journal. 2008;337:a1864. doi: 10.1136/bmj.a1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raue PJ, Schulberg HC, Heo M, Klimstra S, Bruce ML. Patients' depression treatment preferences and initiation, adherence, and outcome: a randomized primary care study. Psychiatry Services. 2009;60:337–343. doi: 10.1176/appi.ps.60.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokke PD, Tomhave JA, Jocic Z. The role of client choice and target selection in self-management therapy for depression in older adults. Psychology and Aging. 1999;14:155–169. doi: 10.1037//0882-7974.14.1.155. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-item Quick Inventory of Depressive Symptomatology (QIDS) Clinician Rating (QIDS-C) and Self-Report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Katon WJ, Russo JE, Frank E, Barrett JE, Oxman TE, Williams JJr. Patient beliefs predict response to paroxetine among primary care patients with dysthymia and minor depression. Journal of the American Board of Family Practice. 2003;16:22–31. doi: 10.3122/jabfm.16.1.22. [DOI] [PubMed] [Google Scholar]
- Swift JK, Callahan JL. The impact of client treatment preferences on outcome: A metaanalysis. Journal of Clinical Psychology. 2009;65:368–381. doi: 10.1002/jclp.20553. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. American Journal of Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Van Vorhees BW, Fogel J, Houston TK, Cooper LA, Wang N-Y, Ford DE. Beliefs and attitudes associated with intention not to accept the diagnosis of depression among young adults. Annals of Family Medicine. 2005;3:38–45. doi: 10.1370/afm.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E, King M, Lloyd M, et al. Randomised controlled trial of non-directive counselling, cognitive behaviour therapy and usual general practitioner care for patients with depression, I: clinical effectiveness. British Medical Journal. 2000;321:1383–1388. doi: 10.1136/bmj.321.7273.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski SR, Fava M, Trivedi MH, Thase ME, Warden D, Niederehe G, Friedman ES, Biggs MM, Sackeim HA, Shores-Wilson K, McGrath PJ, Lavori PW, Miyahara S, Rush AJ. Acceptability of second-step treatments to depressed outpatients: A STAR*D report. American Journal of Psychiatry. 2007;164:753–760. doi: 10.1176/ajp.2007.164.5.753. [DOI] [PubMed] [Google Scholar]