Abstract
Background & aims
Only a fraction of IBS patients show increased perceptual sensitivity to rectal distension, suggesting possible differences in processing and/or modulation of visceral afferent signals within this group. The aim was to identify brain mechanisms which may underlie these perceptual differences.
Methods
44 women with IBS and 20 female healthy control subjects (HCs) were included. Symptom severity in IBS was determined by Severity Scoring System (IBS-SSS). Anxiety and depression symptoms were assessed using the Hospital anxiety & depression score (HAD). Blood oxygen level dependent (BOLD) signals were measured by functional Magnetic Resonance Imaging (fMRI) during expectation and delivery of high (45mmHg) and low (15mmHg) intensity rectal distensions. Perception thresholds to rectal distension were determined in the scanner. Brain imaging data from 18 normosensitive, 15 hypersensitive IBS patients and 18 HCs were compared. Results were reported significant if peak p-value ≤ 0.05 with family wise error correction in regions of interest.
Results
The two IBS subgroups were similar in age, symptom duration, psychological symptoms and IBS symptom severity. While brain responses to distension were similar in normosensitive patients and HCs, hypersensitive IBS demonstrated greater activation of insula and reduced deactivation in pregenual anterior cingulate cortex during noxious rectal distensions, compared to both HCs and normosensitive IBS. During expectation of rectal distension, normosensitive IBS had more activation in right hippocampus than HCs.
Conclusions
Despite similarities in symptoms, hyper- and normosensitive IBS patients differed substantially in cerebral response to standardized rectal distensions and their expectation, consistent with differences in ascending visceral afferent input.
Keywords: IBS, visceral sensitivity, functional MRI, brain
INTRODUCTION
Irritable bowel syndrome (IBS) is a common chronic abdominal pain disorder characterized by recurrent abdominal pain associated with altered bowel habits1, and a high prevalence of increased anxiety 2. The pathophysiology of IBS is incompletely understood; altered brain-gut interactions are thought to play an important role in the cardinal symptoms, particularly abdominal pain 3, 4. In the absence of generally agreed upon biomarkers, the diagnosis relies on symptom reports and exclusion of organic disease1.
Chronic visceral pain can be considered as a complex experience which signals an adverse condition in the gastrointestinal (GI) tract, requiring a behavioral response5. However, this experience is highly subjective and is modulated by several different emotional and cognitive factors, including anxiety, attention, and expectation 6. Chronic pain is therefore not linearly related to peripheral sensory input 7. For example, hypervigilance towards gastrointestinal sensations, anxiety and symptom related fears are common in functional GI disorder patients and play an important role in perceived symptom severity 8, 9. In IBS patients it is not known whether the reported abdominal pain reflects increased afferent input from the gut to the brain, or central alterations in the signals from the gut (“central pain amplification”), or both 10, 11. Multiple mechanisms for central pain modulation have been described in animal models, and evidence for altered engagement of some of these, including diffuse noxious inhibitory controls (DNIC), have been described in IBS patients 12 Recently Berman et al demonstrated that in IBS patients alterations during cued expectation of an aversive rectal stimulus correlated with subsequent brain responses to the delivered stimulus in bilateral pregenual anterior cingulate cortex (pACC), a subregion involved in descending inhibitory pain modulation 13.
As IBS patients and HCs generally differ in perception thresholds for reports of discomfort to controlled rectal balloon distension, this stimulus has been used for experimental pain provocation in numerous IBS studies 14,15,16,17,18. Enhanced perception of visceral stimuli is considered to be an important component in the pathophysiology of IBS 19, yet a large proportion of IBS patients have perception thresholds within the normal range 16,20, 21, even though all IBS patients present with a similar symptomatology 22, 23. Furthermore, there is a poor correlation between visceral hypersensitivity in IBS and symptom severity 24, 25,26,27
Results of brain imaging studies in IBS have varied or have been contradictory, perhaps due to different study designs, but perhaps also to heterogenous study populations, including patients with and without visceral hypersensitivity 28,29, 30. A recent meta analysis of published studies on brain responses to rectal distension supports the conclusion that brain responses to rectal distension differ between IBS patients and HCs 31,32. IBS patients showed greater activation during rectal distension in brain regions concerned with emotional arousal, cognitive modulation and interoceptive processing 32. However, similar to the poor correlations between perception thresholds to rectal stimuli and symptom severity, there were poor correlations between changes in brain responses to rectal stimulation during repeated stimulation and associated changes in symptom severity 9.
Given that visceral hypersensitivity is considered an important feature in the pathophysiology of IBS, it is of interest to examine whether there are differences in the cerebral response to standardized visceral pain stimuli between the patients with normal visceral sensitivity and those with increased visceral sensitivity. Here we refer to subjects with normal visceral sensitivity as “normo-sensitive” and subjects with increased sensitivity as “hyper-sensitive”. Such differences have not been investigated in any of the published studies. In the current study, using a validated paradigm of rectal distension and expectation of such distension, we aimed to answer the following questions: 1. Do brain responses to these stimuli differ between normosensitive and hypersensitive IBS patients, despite similar symptom burden? 2. Are brain responses of normosensitive IBS similar to those of HCs? We used a combination of aversive and non-aversive rectal distension, as well as an expectation of pain paradigm to differentiate top down and bottom up mechanisms which may play a role in the observed response patterns.
METHODS
Subjects
Forty-four right-handed women with IBS, fulfilling Rome III criteria, were included (mean age 35.5 years, range 20-60). Patients were referred by general practitioners to the Linköping University Hospital, Sweden. They were evaluated by a trained gastroenterologist; standard clinical investigations were performed to exclude organic GI disease. The control group consisted of 20 healthy right-handed women (mean age 32.2 years, range 21-54) recruited by advertisement. Exclusion criteria were organic gastrointestinal disease, metabolic, neurological or psychiatric disorders, nicotine intake, centrally acting medication, pacemaker, implanted metal in the brain and claustrophobia. Additional exclusion criterion for controls was a medical history of GI symptoms or complaints. All subjects completed the Hospital Anxiety and Depression (HAD) Scale 33 and IBS patients additionally completed the IBS Severity Scoring System (IBS SSS) 34 and Visceral Sensitivity Index (VSI) 35. Control subjects received a monetary compensation of 1000 SEK.
Two HCs and 11 patients had to be excluded from the data analysis: incomplete data collection (n=2), balloon leakage (n=1), excess motion (n=4), major scanner artefacts (n=2), intolerance to the procedure (n=4). Thus the data of 18 control subjects and 33 IBS patients were analysed.
Ethics
Written and oral informed consent was obtained from all subjects. The study protocol was approved by the Regional Research Committee for Ethical issues at Linköping, Sweden.
Questionnaires
Hospital Anxiety and Depression Scale (HAD)
HAD is a self-assessment scale that was developed for detecting states of depression and anxiety in medical outpatient settings 33. The scale consists of 7 items each for anxiety and depression. Responses are graded on a 4-point scale. The summed scores for anxiety and depression, respectively, range from 0 to 21, with a higher score indicating more pronounced symptoms.
IBS Severity Scoring System (IBS SSS)
The IBS SSS was developed for rating of IBS symptoms. 34 The scoring system incorporates 5 items: abdominal pain severity, pain frequency, bowel distension, bowel dysfunction and quality of life/global well-being. The items are scored on a visual analogue scale ranging from 0-100 mm. The maximum achievable score is 500. Mild, moderate and severe cases are indicated by scores of 75 to 175, 175 to 300 and > 300, respectively.
Visceral Sensitivity Index (VSI)
VSI consists of a 15-item scale to measure GI symptom-specific anxiety by assessing the cognitive, affective, and behavioural response to fear of GI sensations, symptoms, and the context in which these occurs. 35
Ratings of present intensity and unpleasantness of GI symptoms
A scale ranging from 0 to 10 was used to determine (A) intensity of present GI symptoms (low scores indicate faint intensity, and high scores indicate extremely intense symptoms) and (B) unpleasantness of subjects’ GI symptoms (low scores indicate neutral feelings, and high scores indicate intolerable unpleasantness).
Study protocol
The temporal overlap of menses period with the experimental day was avoided for all subjects. Subjects were required to cease medications and avoid alcohol at least 24 hours before the experiment. Subjects came to the experimental site after 4 hours of fasting. The barostat balloon was installed into the rectum with the subjects in left lateral position. The rectal balloon catheter consisted of a non-compliant polyethylene bag (maximal volume 520 mL), which was attached to a polyethylene tube. The subjects were then placed in the MRI-scanner and fitted with headphones (allowing two-way communication), high-resolution video MR-goggles for visual stimulus presentation in the MR scanner (Resonance Technology Inc., California, USA).
Determination of perception thresholds
After 5 minutes of rest in the scanner bed, a 10 minute resting scan was performed. Thereafter subjects underwent the thresholding procedure to test visceral sensitivity. Intermittent phasic isobaric rectal distensions of 30 seconds duration were administered by an electronic barostat (Dual Drive Barostat, Distender series II, G&J Electronics Inc.; Toronto, Canada). After each distension the balloon was emptied. Time interval between the onset of each distension was one minute. Visceral sensitivity was tested by using the ascending method of limits with pressure increments of 5mmHg. Thirty seconds after onset of each distension the subjects were shown a 4 point scale to grade their sensation and report it through the microphone: 0= no sensation; 1 =first sensation/some sensation; 2= urge to defecate; 3= maximum tolerable distension. After determination of sensitivity thresholds fMRI data were collected.
fMRI distension paradigm
Twenty high (45 mmHg) and 18 low (15 mmHg) rectal distensions with a duration of 15 seconds each were delivered in pseudorandom order. The distensions were preceded by a varied expectation period (5-8 s). In the beginning of each expectation period subjects were shown a red cue for the high distension and a blue cue for the low distension. Between the rectal distensions, 18 periods were pseudorandomly inserted during which subjects were shown grey cues with no subsequent distension (used as baseline). Subjects rated the present intensity and unpleasantness of GI symptoms before and after thresholding and at the end of the experiment. Directly after having completed the run subjects rated the last low and high stimulus, respectively (0= no sensation; 1 = some sensation; 2= urgency; 3= maximum tolerable pressure). In this paper, we focus on the differences between hyper- and normosensitive IBS patients according to their response to expectation and delivery of standardized low and high rectal distensions. The pressure threshold for classifying patients as normo- or hyper-sensitive was defined as the lower range of the maximum tolerable distension pressure of HCs. As in several previous fMRI studies of IBS patients, the experimental stimuli were equal for all subjects, regardless of the individual visceral sensitivity perception thresholds obtained using the ascending series of longer stimuli36,37,13,38. The intent of this design was to obtain a standardized peripheral visceral afferent input from the gut. The experimental protocol is illustrated in a Figure (supplemental material).
fMRI data acquisition
fMRI images were acquired by a Philips Achieva 1.5 T MR-scanner. For experimental design and presentation of information, Superlab Pro 4 software was used (Cedrus Corp., San Pedro, USA). Functional brain images were acquired by using a blood oxygen level dependent (BOLD) sensitive gradient echo sequence, employing the following acquisition parameters: TR = 3 s; TE = 40 ms; flip angle = 90°; voxel size: 3×3×3 mm3, 35 slices were acquired in interleaved mode with 0.5 mm slice gap.
Data analysis
Statistical parametric mapping 8 (SPM8) (Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) was used for the analysis of the BOLD fMRI data. Images were realigned to the first image of the time series, normalized to a standard brain atlas (MNI space) at 2 × 2 × 2 mm3 and smoothed using an 8 mm FWHM Gaussian kernel. Subjects were excluded from further analysis if the realignment parameters indicated excessive movement (> 3 mm) or the BOLD fMRI images contained major artefacts.
A first level general linear model was specified for each subject and modelled the conditions (1) high distension (45 mmHg), (2) low distension (15 mmHg), (3) expectation of high distension, (4) expectation of low distension and 5) baseline. In addition, motion parameters were included as regressors. Beta or contrast images comparing the active conditions to baseline were calculated, thresholded at p<.01 uncorrected, and entered into second level region of interest analyses.
The general linear model and a region of interest approach were applied to test for group differences in brain activity during the distension and expectation conditions. Regions of interest (ROIs) included regions associated with emotional arousal and regions associated with processing of visceral sensations including pain, that is: amygdala, hippocampus, thalamus, periaqueductal gray (PAG), anterior midcingulate cortex (aMCC), subgenual anterior cingulate cortex (sgACC), pregenual anterior cingulate cortex (pACC), dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC) and 5 insula (INS) subregions: anterior ventral, anterior dorsal, posterior ventral, posterior dorsal and mid insula 32, 39. ROI center coordinates and volumes are shown in Table 1. ROIs with labels are shown in a figure in the supplementary. The regions of interest analyses were implemented using SPM8 small volume correction. Region of interest analyses were considered significant at p ≤ 0.05, family wise error (FWE) corrected.
Table 1.
ROI center coordinates (center of mass) and volumes
| ROI | x | y | z | Volume (mm) | |
|---|---|---|---|---|---|
| PAG | L | −1 | −31 | −8 | 160 |
| R | 3 | −31 | −9 | 152 | |
| Thalamus | L | −13 | −19 | 7 | 8456 |
| R | 13 | −19 | 7 | 8456 | |
| dlPFC | L | −33 | 32 | 34 | 47984 |
| R | 38 | 31 | 33 | 74272 | |
| vlPFC | L | −35 | 29 | −14 | 26968 |
| R | 40 | 31 | −13 | 27344 | |
| HIPP | L | −31 | −22 | −13 | 1880 |
| R | 28 | −22 | −13 | 2056 | |
| AMYG | L | −25 | −3 | −19 | 1992 |
| R | 22 | −3 | −19 | 2032 | |
| aMCC | L | −6 | 27 | 27 | 11208 |
| R | 7 | 29 | 27 | 11528 | |
| pACC | L | −7 | 43 | 3 | 14112 |
| R | 7 | 43 | 2 | 14344 | |
| sgACC | L | −4 | 26 | −11 | 3424 |
| R | 5 | 26 | −11 | 4272 | |
| Anterior INS, ventral | L | −33 | 17 | −9 | 2842 |
| R | 35 | 17 | −10 | 2433 | |
| Anterior INS, dorsal | L | −32 | 20 | 1 | 2884 |
| R | 34 | 19 | 2 | 2729 | |
| Mid INS | L | −37 | 2 | 1 | 3294 |
| R | 39 | 2 | 3 | 3234 | |
| Posterior INS, ventral | L | −37 | −12 | −4 | 4272 |
| R | 35 | 17 | −10 | 2433 | |
| Posterior INS, dorsal | L | −36 | −15 | 11 | 3403 |
| R | 36 | −16 | 11 | 2191 |
RESULTS
Clinical and psychophysical characterization of IBS subgroups
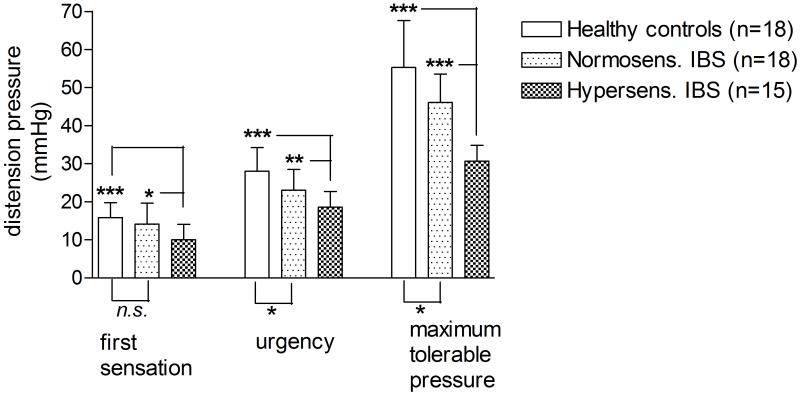
HCs (n=18) had a maximum tolerable rectal pressure of mean 55.28 mmHg (range 40-70 mmHg). Eighteen IBS patients had a maximum tolerable pressure of 40 mmHg or higher (mean 46.11 mmHg, range 40-65) and thus were considered as normosensitive. Fifteen IBS patients had a maximum tolerable rectal pressure less than 40 mmHg and were considered as hypersensitive (mean 30.67 mmHg (range 25-35)). There was no overlap between the hypersensitive IBS group and HC in terms of pressure thresholds. The hypersensitive group had significantly lower thresholds for first sensation and urgency than the normosensitive group and the HC group. The threshold for first sensation was mean 10.00 mmHg (5-15) for the hypersensitive group and 14.17 mmHg (5-25) and 15.83 mmHg (10-25) for the normosensitive IBS group and the HCs, respectively (Figure 1).
Figure 1.
The graph shows rectal distension sensitivity thresholds of hyper- and normosensitive IBS patients and HCs, obtained by the ascending method of limits before the imaging experiment, sorted by sensory level. Mean values and SD are shown. Differences are calculated with a non-paired t-test.
The clinical data for the two IBS groups are shown in Table 2. The two groups were similar in terms of disease duration, IBS symptom severity score, anxiety and depression symptoms or GI symptom related anxiety score (VSI). Eleven hypersensitive and ten normosensitive subjectshad severe symptoms according to SSS. IBS patients as a group had significantly higher anxiety and depression scores than HCs.
Table 2.
Age, symptom duration, somatic and psychological evaluation in 33 female IBS patients with normal and increased visceral sensitivity compared to healthy women.
| Controls (n=18) |
Normo- sensitive IBS (n=18) |
p- valuea |
Hyper- sensitive IBS (n=15) |
p- valueb |
p- valuec |
|
|---|---|---|---|---|---|---|
| Age (years) (mean, range) |
32.5 (21-54) |
32.5 (20-60) |
0.987 | 40.3 (21-60) |
0.054 | 0.046 |
| HAD Anxiety (mean, range) |
3.0 (0-11) |
8.2 (0-17) |
0.002 | 7.4 (2-17) |
0.764 | 0.005 |
| HAD Depr. (mean, range) |
1.3 (0-3) |
3.7 (1-10) |
0.009 | 3.3 (0-8) |
0.850 | 0.011 |
| SSS intestinal (mean, range) |
319 (156-455) |
362.3 (271-484) |
0.099 | |||
| VSI (mean, range) |
44.3 (8-68) |
44.7 (16-63) |
0.950 |
Comparison between normosensitive IBS patients and healthy controls
Comparison between hypersensitive IBS patients and normosensitive IBS patients
Comparison between hypersensitive IBS patients and controls
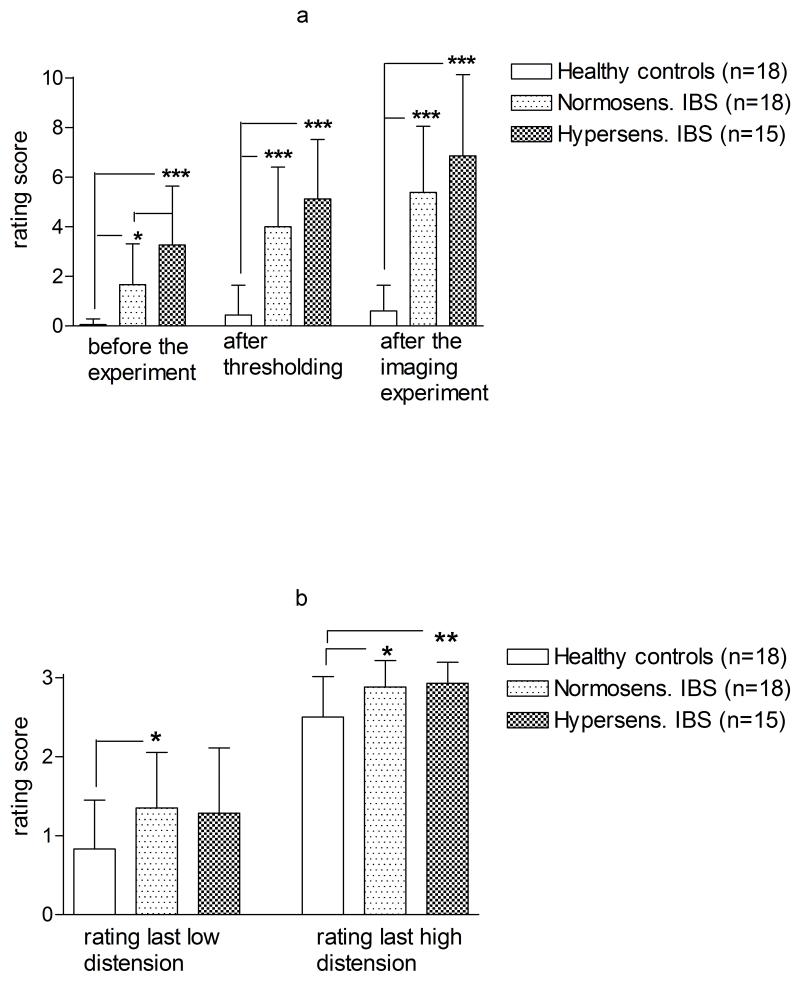
Hypersensitive IBS patients reported higher symptom intensity and unpleasantness ratings than the normosensitive (p=0.04) at the day of the distension paradigm, even though the difference reached statistical significance only prior to the thresholding procedure. Both IBS groups had significantly higher ratings of GI symptom intensity than HCs (Figure 2a). After the imaging experiment, both IBS subgroups rated the last low distension and the last high distension of the experiment significantly higher than the HCs. There was, however, no difference between the IBS subgroups (Figure 2b).
Figure 2.
a: Ratings of present intensity of gastrointestinal (GI) symptoms during the experiment. A scale ranging from 0 to 10 was used to determine (A) intensity of present GI symptoms with lowest score indicating faint intensity and highest score indicating extremely intense symptoms. Mean values and SD shown. Differences between groups are calculated with a non-paired t-test.
b: Ratings of the last low and high distension of the imaging experiment. Subjects rated on a 4 point scale as no sensation (=0); some sensation (=1); urgency (=2); maximum tolerable pressure (=3). Mean and SD are shown. Differences are calculated with a non-paired t-test.
Brain responses to distension and expectation of abdominal pain
As shown in Table 3a, IBS patients as a group had greater BOLD signals than HCs in the left VLPFC in response to the high distension and in the left middle INS in response to the low distension. During expectation of the high distension, IBS patients had more activation in the right anterior and middle INS and HIPP. There were no regions of significantly higher BOLD activation in HCs than in IBS patients.
Table 3.
Brain regions (ROIs) where the BOLD response was (a) greater in all IBS patients (n=33) than in healthy controls (n=18) (b) in hypersensitive IBS patients (n=15) than in normosensitive IBS patients (n=18)
| (a) IBS > controls | ||||||
|---|---|---|---|---|---|---|
| ROI | cluster size |
peak p(FWE) |
X- coord |
Y- coord |
Z- coord |
|
| 45 mmHg distension | ||||||
| VLPFC | L | 21 | 0.05 | −40 | 16 | −18 |
| 15 mmHg distension | ||||||
| insula mid | L | 59 | 0.04 | −40 | −8 | −8 |
|
Expectation of 45 mmHg
distension |
||||||
| insula anterior ventral | R | 10 | 0.04 | 32 | 22 | −14 |
| insula mid | R | 45 | 0.03 | 40 | −6 | −12 |
| hippocampus | R | 25 | 0.03 | 26 | −38 | 0 |
|
Expectation of 15 mmHg
distension |
||||||
| No significant findings | ||||||
| (b) Hypersensitive > normosensitive | ||||||
|---|---|---|---|---|---|---|
| ROI | cluster size |
peak p(FWE) |
X- coord |
Y- coord |
Z- coord |
|
| 45 mmHg distension | ||||||
| insula posterior | L | 69 | 0.02 | −34 | −26 | 6 |
| DLPFC | R | 345 | 0.04 | 22 | 48 | 8 |
| aMCC | R | 68 | 0.04 | 8 | 40 | 16 |
| pACC | L | 210 | 0.04 | −10 | 50 | 16 |
| pACC | R | 309 | 0.01 | 18 | 50 | 12 |
| 15 mmHg distension | ||||||
| No significant findings | ||||||
|
Expectation of 45 mmHg
distension |
||||||
| insula posterior | R | 71 | 0.01 | 36 | −14 | 20 |
| thalamus | R | 119 | 0.05 | 14 | −26 | 6 |
|
Expectation of 15 mmHg
distension |
||||||
| insula anterior ventral | R | 29 | <0.01 | 46 | 18 | −6 |
Hypersensitive IBS compared to normosensitive IBS
ROIs in which the hypersensitive IBS patients had larger BOLD signals than the normosensitive patients during expected and delivered distension are shown in Table 3b. They include several INS and cingulate subregions and lateral PFC regions.
Hypersensitive IBS compared to HCs
Hypersensitive IBS patients had larger BOLD signals during the high rectal distension in left posterior INS (clustersize 64; [−36 −24 6], p=0.04); left pACC (clustersize 311; [−8 52 18], p=0.01) and left thalamus (clustersize 54; [−8 −8 14], p=0.04). Normosensitive IBS compared to HCs. Normosensitive IBS patients and HCs did not differ significantly in their BOLD response to either distension. During expectation of the high distension the normosensitive IBS group had more BOLD response than the HCs in the right HIPP ([28 −38 −2], p=0.01). HCs had more activation than the normosensitive IBS group during the low distension in the right anterior INS (p=0.02, [38 14 −4]).
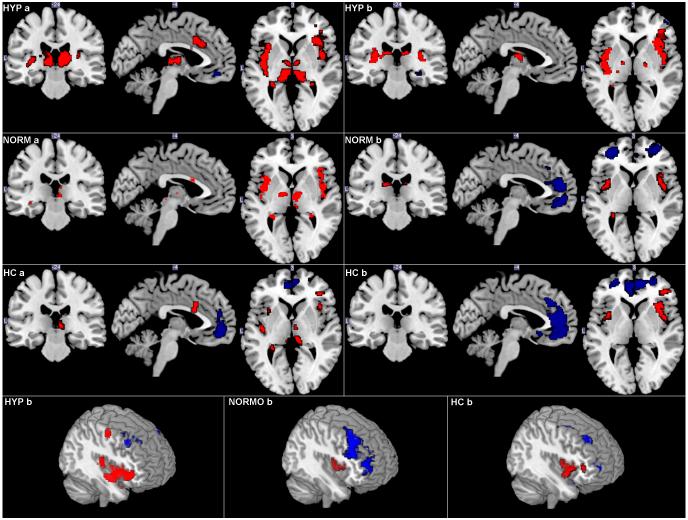
Cluster distributions of cerebral BOLD activation for IBS subgroups and HCs during expectation and delivery of the high distension are shown in Figure 3.
Figure 3.
BOLD response (<0.01) within the ROIs in HCs (n=18), normosensitive IBS (n=18) and hypersensitive IBS (n=15) during (a) high rectal distension (45 mmHg). (b) the expectation of high rectal distension (45 mmHg) Red = increased BOLD signal, blue = decreased BOLD signal. HYP = hypersensitive IBS, NORM= normosensitive IBS, HC = HCs
Tables with significant BOLD increases and decreases during the expectation and delivery of the low and high distension are added to the supplementary material.
DISCUSSION
The key findings of the study were: (1) Despite similarities in symptoms, hyper- and normosensitive IBS patients differed substantially in their BOLD response to high pressure rectal distensions, and to expectation of such distensions; (2) during rectal distension there were no significant differences in cerebral response between normosensitive IBS patients and HCs;(3) during expectation of the high pressure stimulus, normosensitive IBS patients had more activation in right hippocampus than HCs.
Group differences in brain responses during the high intensity distension were observed in primary interoceptive cortex (posterior INS (pINS)), suggesting that, this region is sensitized or it receives increased ascending input from the dorsal horn during rectal distensions. The fact that increased activity in mid-insular cortex and thalamus was also seen during the expectation of an aversive sensation, without any acute peripheral stimulus, suggests that differences in top down modulatory mechanisms, e.g. descending endogenous pain modulation systems, may also play an important role in the observed abnormalities.
The group comparison between HCs and all IBS patients confirms previous findings by showing greater INS responses (both anterior and middle subregions) during pain expectation in IBS patients 30,13 and greater activation of the ventrolateral PFC to aversive rectal distensions29,30,40,38,41. The fact that no group differences were observed during distension in other regions of the homeostatic afferent network (previously referred to as “pain matrix”; e.g. INS, MCC and thalamus) may be attributed to the fact that this difference was only contributed by the hypersensitive, but not by the normosenstive group, and therefore not detectable in the combined group (see table 3b and discussion below). The findings will be discussed in terms of the two primary hypotheses of the study.
Do brain responses to aversive rectal stimuli and their expectation differ between normosensitive and hypersensitive IBS patients?
Perceptual responses
The two IBS subgroups, defined by their subjective response to an acute rectal stimulus, did not differ in their subjective ratings of IBS symptom severity or symptoms of anxiety and depression. These results confirm previous reports demonstrating a poor correlation between perceptual responses to controlled rectal distension and subjective IBS symptoms 27 and emphasize that factors other than perception of an acute visceral stimulus contribute to patients’ reports of clinical symptoms. However, the mechanisms underlying this increased sensitivity in hypersensitive patients are incompletely understood. The hypersensitive group, as evaluated by the ascending method of limits (AML), had lower perception thresholds for first sensation and for first sensation of urgency compared to either normosensitive IBS patients or HCs. This leftward shift of the stimulus response curve in hypersensitive IBS patients has been described earlier 42 and could be caused by amplification of the experimental sensory inflow somewhere along the afferent pelvic visceral pathway: (1) at the gut receptor level (2) in the dorsal horn of the spinal cord (3) in the thalamus or (4) in the primary interoceptive cortex (pINS), or in the interoceptive association cortex, the middle and anterior INS. Alternatively, a leftward shift (with or without a reduction in threshold) could be caused by abnormal engagement of descending inhibitory mechanisms 43,29 or by greater activation of endogenous facilitatory systems in the hypersensitive IBS group. Both of the latter mechanisms could result in increased excitability of dorsal horn neurons, amplifying ascending afferent input to the brain 44. As previously pointed out, the AML paradigm maximizes selective attention to threat mechanisms, and it has been suggested that increased ratings may be more reflective of hypervigilance and selective attention to perceived discomfort, than sensory hypersensitivity 45.
During the high intensity rectal distension the hypersensitive group had more activation in the pINS and aMCC, key regions of a brain network consistently activated during interoceptive stimuli, including noxious stimuli (“homeostatic afferent network”) 46. Furthermore, they had a reduced deactivation in the pACC compared to HCs and normosensitive IBS, consistent with alterations in the engagement of descending pain modulation systems.
The pACC is considered to be the primary cortical region in the descending pain inhibition system 47, with a high concentration of opioid receptors 48. In most reported studies evaluating the endogenous descending pain inhibition system the peripheral stimulations were individually calibrated 49,50,47 and the present results might have been different if we had individually adjusted the high stimulus level to reflect distension tolerance.
However, according to the results of the pressure thresholds obtained with the AML, the range between the ratings of first sensation of urgency and maximum tolerable pressure was proportionally much larger for the normosensitive IBS patients and HCs than for the hypersensitive IBS group. This finding could indicate that the HCs as well as to some degree the normosensitive may mobilize mechanisms that helped them to tolerate the increasing visceral discomfort, allowing them to accept further pressure increments, whereas the hypersensitive IBS patients may lack this ability. This concept is consistent with our observation that hypersensitive IBS patients had more DLPFC activation than normosensitive patients. It has previously been shown that the expectation of pain relief is mediated by regions within both right and left DLPFC 47,51, and that the bilateral DLPFC was activated during the task to suppress the feeling of pain while enduring a tonic painful heat stimulation 49. Based on these findings, one may speculate that the hypersensitive IBS patients engage this prefrontal mechanism more than normosensitive patients in order to reduce the aversive experience, but that the mechanism doesn’t appear to be functioning appropriately.
In the present study a visual cue predicted the experimental stimuli. It has earlier been shown that expectation of a noxious stimulus can modulate the processing of a subsequent aversive stimulus in the brain 52,13. Engagement of aINS, ACC and thalamus have been observed during cued expectation of a somatic pain stimulus 53. IBS patients have been shown to engage aINS to a greater extent during pain expectation 13 and this has been interpreted as a possible prediction bias related to the expected pain intensity. Confirming this hypothesis, hypersensitive IBS patients, compared to the normosensitive IBS group, had more activation of the right aINS during the expectation of a non-aversive stimulus. During the expectation of the high pressure distension, greater activation of different INS subregions, e.g. the posterior/middle INS, as well as thalamus was observed. The greater activation of these homeostatic afferent regions is consistent with inappropriate engagement of descending pain facilitatory mechanisms triggered by the prediction of an aversive stimulus.
Are brain responses of normosensitive IBS similar to those of HCs?
No statistically significant differences between normosensitive IBS patients and HCs were observed in brain responses to rectal distension despite differences in HAD scores, suggesting that anxiety and depression symptoms were not a major modulator of brain responses to rectal distension. However, during the expectation of the high stimulus, normosensitive IBS patients had more activation in right hippocampus than HCs. Bingel et al recently showed that negative pain treatment expectancy was associated with increased activity in the HIPP, a brain region implicated in the anxiety-driven exacerbation of pain 54,55. In accordance with this, the present findings could be explained by enhanced negative expectations in the IBS patients compared to HCs, probably due to their generally increased anxiety levels compared to HCs but not due to a greater pain experience by the subsequent distension. The lack of influence of anxiety symptoms on visceral sensitivity is surprising, in view of several reports that psychological factors have an impact on pain perception, pain reporting and visceral sensitivity thresholds in IBS patients 56,8,45,57. Similarly, it has previously been reported that differences in brain responses to a visceral stimulus between normosensitive IBS patients and HCs were no longer seen when controlling for anxiety and depression scores as confounding variables 58.
Conclusions
Despite similarities in IBS symptoms, female IBS patients differentiated by normal or high perceptual sensitivity to acute rectal distension showed significantly different brain responses to both the actual stimulus and to its expectation. When viewed together, the findings are most consistent with the hypothesis that altered engagement of descending pain modulation systems increases the excitability of the dorsal horn, resulting in increased ascending input to brain regions processing interoceptive input. Brain responses were not significantly affected by general or symptom related anxiety, thus cognitive factors, such as prediction error, could be responsible for these differences. However, our findings cannot rule out alternative mechanisms, such as peripheral sensitization.
Supplementary Material
Figure with experimental protocol: Supplemental material: The time course of the imaging experiment is shown in the graph. Subjects rated their sensory thresholds during phase (C): 0= no sensation; 1 =first sensation/some sensation; 2= urge to defecate; 3= maximum tolerable distension.
Figure: Locations of Regions of Interest (ROIs): Supplemental material
Acknowledgments
Grant Support
County Council of Östergötland, Sweden, Lions forskningsfond för folksjukdomar National Institute of Health grants DK 64531, DK 48351, K23 DK73451.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have nothing to disclose
Author Contributions
MBO Larsson: Study design; acquisition of data; analysis and interpretation of data; drafting of manuscript
K Tillisch: Study design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content AD Craig: Analysis and interpretation of data; critical revision of the manuscript for important intellectual content
M Engström: Study concept and design; acquisition of data; analysis and interpretation of data; study supervision
J Labus: Study design; statistical analysis
B Naliboff: Study design
P Lundberg: Study concept; technical support, revision of the manuscript
M Ström: Study concept and design; Administrative support; obtained funding, revision of the manuscript
EA Mayer: Study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
SA Walter: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis; obtained funding; administrative, and material study supervision
Contributor Information
MBO Larsson, Department of Clinical and Experimental Medicine/Gastroenterology, Center for Medical Image Science and Visualization (CMIV), Linköping University; Department of Gastroenterology, UHL, County Council of Östergötland, Linköping, Sweden.
K Tillisch, Oppenheimer Family Center for Neurobiology of Stress, Division of Digestive, Diseases, Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, USA.
A.D. (Bud) Craig, Barrow Neurological Institute, Phoenix, AZ, USA; Visiting Professor at the Department of Clinical and Experimental Medicine, Linköping University, Sweden.
M Engström, Department of Medical and Health Sciences (IMH)/Radiology, Linköping University, Sweden; Center for Medical Image Science and Visualization (CMIV), Linköping University, Sweden.
J Labus, Oppenheimer Family Center for Neurobiology of Stress, Department of Psychiatry, David Geffen School of Medicine at UCLA, Los Angeles, USA.
B Naliboff, Oppenheimer Family Center for Neurobiology of Stress, Department of Psychiatry, David Geffen School of Medicine at UCLA, Los Angeles, USA.
P Lundberg, Center for Medical Image Science and Visualization, CMIV; Radiation Physics, Department of Medical and Health Sciences, Faculty of Health Sciences, Linköping University; Department of Radiation Physics UHL, County Council of Östergötland, Linköping, Sweden; Center for Medical Image Science and Visualization, CMIV; Radiology, Department of Medical and Health Sciences, Faculty of Health Sciences, Linköping University; Department of Radiology UHL, County Council of Östergötland, Linköping, Sweden.
M Ström, Department of Clinical and Experimental Medicine/Gastroenterology, Linköping University; Department of Gastroenterology, UHL, County Council of Östergötland, Linköping, Sweden.
EA Mayer, Oppenheimer Family Center for Neurobiology of Stress, Division of Digestive Diseases, Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, USA.
SA Walter, Department of Clinical and Experimental Medicine/Gastroenterology, Center for Medical Image Science and Visualization (CMIV), Linköping University; Department of Gastroenterology, UHL, County Council of Östergötland, Linköping, Sweden.
References
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Svedlund J, Sjodin I, Dotevall G, Gillberg R. Upper gastrointestinal and mental symptoms in the irritable bowel syndrome. Scand J Gastroenterol. 1985;20:595–601. doi: 10.3109/00365528509089702. [DOI] [PubMed] [Google Scholar]
- 3.Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519–24. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- 4.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237–41. [PubMed] [Google Scholar]
- 5.Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003;26:303–7. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 6.Valet M, Sprenger T, Tolle TR. [Studies on cerebral processing of pain using functional imaging: Somatosensory, emotional, cognitive, autonomic and motor aspects] Schmerz. 24:114–21. doi: 10.1007/s00482-010-0896-0. [DOI] [PubMed] [Google Scholar]
- 7.Tracey I. Imaging pain. Br J Anaesth. 2008;101:32–9. doi: 10.1093/bja/aen102. [DOI] [PubMed] [Google Scholar]
- 8.Whitehead WE, Palsson OS. Is rectal pain sensitivity a biological marker for irritable bowel syndrome: psychological influences on pain perception. Gastroenterology. 1998;115:1263–71. doi: 10.1016/s0016-5085(98)70099-x. [DOI] [PubMed] [Google Scholar]
- 9.Naliboff BD, Berman S, Suyenobu B, Labus JS, Chang L, Stains J, Mandelkern MA, Mayer EA. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131:352–65. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–42. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q, Fillingim RB, Riley JL, 3rd, Malarkey WB, Verne GN. Central and peripheral hypersensitivity in the irritable bowel syndrome. Pain. 148:454–61. doi: 10.1016/j.pain.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heymen S, Maixner W, Whitehead WE, Klatzkin RR, Mechlin B, Light KC. Central processing of noxious somatic stimuli in patients with irritable bowel syndrome compared with healthy controls. The Clinical journal of pain. 2010;26:104–9. doi: 10.1097/AJP.0b013e3181bff800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller JA, Ruby K, Jarcho J, Mayer EA. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28:349–59. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 15.Harraf F, Schmulson M, Saba L, Niazi N, Fass R, Munakata J, Diehl D, Mertz H, Naliboff B, Mayer EA. Subtypes of constipation predominant irritable bowel syndrome based on rectal perception. Gut. 1998;43:388–394. doi: 10.1136/gut.43.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganière M, Verrier P, Poitras P. Rectal distention testing in patients with irritable bowel syndrome: Sensitivity, specitivity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771–1777. doi: 10.1053/gast.2002.33601. [DOI] [PubMed] [Google Scholar]
- 17.Dong WZ, Zou DW, Li ZS, Zou XP, Zhu AY, Xu GM, Yin N, Gong YF, Sun ZX, Man XH. Study of visceral hypersensitivity in irritable bowel syndrome. Chin J Dig Dis. 2004;5:103–9. doi: 10.1111/j.1443-9573.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- 18.Kwan CL, Diamant NE, Mikula K, Davis KD. Characteristics of rectal perception are altered in irritable bowel syndrome. Pain. 2005;113:160–171. doi: 10.1016/j.pain.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, Spiller RC. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19:62–88. doi: 10.1111/j.1365-2982.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee KJ, Kim JH, Cho SW. Relationship of underlying abnormalities in rectal sensitivity and compliance to distension with symptoms in irritable bowel syndrome. Digestion. 2006;73:133–41. doi: 10.1159/000094099. [DOI] [PubMed] [Google Scholar]
- 21.Wilder-Smith CH, Robert-Yap J. Abnormal endogenous pain modulation and somatic and visceral hypersensitivity in female patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:3699–704. doi: 10.3748/wjg.v13.i27.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuiken SD, Lindeboom R, Tytgat GN, Boeckxstaens GE. Relationship between symptoms and hypersensitivity to rectal distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22:157–64. doi: 10.1111/j.1365-2036.2005.02524.x. [DOI] [PubMed] [Google Scholar]
- 23.Sabate JM, Veyrac M, Mion F, Siproudhis L, Ducrotte P, Zerbib F, Grimaud JC, Dapoigny M, Dyard F, Coffin B. Relationship between rectal sensitivity, symptoms intensity and quality of life in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:484–90. doi: 10.1111/j.1365-2036.2008.03759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zar S, Benson MJ, Kumar D. Rectal afferent hypersensitivity and compliance in irritable bowel syndrome: differences between diarrhoea-predominant and constipation-predominant subgroups. Eur J Gastroenterol Hepatol. 2006;18:151–8. doi: 10.1097/00042737-200602000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Posserud I, Syrous A, Lindstrom L, Tack J, Abrahamsson H, Simren M. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology. 2007;133:1113–23. doi: 10.1053/j.gastro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 26.van der Veek PP, Van Rood YR, Masclee AA. Symptom severity but not psychopathology predicts visceral hypersensitivity in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:321–8. doi: 10.1016/j.cgh.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Mayer EA, Bradesi S, Chang L, Spiegel BM, Bueller JA, Naliboff BD. Functional GI disorders: from animal models to drug development. Gut. 2008;57:384–404. doi: 10.1136/gut.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rapps N, van Oudenhove L, Enck P, Aziz Q. Brain imaging of visceral functions in healthy volunteers and IBS patients. J Psychosom Res. 2008;64:599–604. doi: 10.1016/j.jpsychores.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Derbyshire SW. A systematic review of neuroimaging data during visceral stimulation. Am J Gastroenterol. 2003;98:12–20. doi: 10.1111/j.1572-0241.2003.07168.x. [DOI] [PubMed] [Google Scholar]
- 32.Tillisch K, Mayer EA, Labus JS. Quantitative Meta-Analysis Identifies Brain Regions Activated During Rectal Distension in Irritable Bowel Syndrome. Gastroenterology. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 34.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 35.Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, Naliboff BD. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 36.Berman S, Munakata J, Naliboff BD, Chang L, Mandelkern M, Silverman D, Kovalik E, Mayer EA. Gender differences in regional brain response to visceral pressure in IBS patients. Eur J Pain. 2000;4:157–72. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- 37.Naliboff BD, Derbyshire SWG, Munakata J, Chang L, Mayer EA. Cerebral Activation in Patients with Irritable Bowel Syndrome and Control Subjects During Rectosigmoid Stimulation. Psychosomatic Medicine. 2001;63:365–375. doi: 10.1097/00006842-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, Price DD. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- 39.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silverman DH, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112:64–72. doi: 10.1016/s0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- 41.Yuan YZ, Tao RJ, Xu B, Sun J, Chen KM, Miao F, Zhang ZW, Xu JY. Functional brain imaging in irritable bowel syndrome with rectal balloon-distention by using fMRI. World J Gastroenterol. 2003;9:1356–60. doi: 10.3748/wjg.v9.i6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naliboff BD, Munakata J, Fullerton S, Gracely RH, Kodner A, Harraf F, Mayer EA. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ringel Y, Drossman DA, Leserman JL, Suyenobu BY, Wilber K, Lin W, Whitehead WE, Naliboff BD, Berman S, Mayer EA. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an FMRI study. Gastroenterology. 2008;134:396–404. doi: 10.1053/j.gastro.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 44.Lagraize SC, Guo W, Yang K, Wei F, Ren K, Dubner R. Spinal cord mechanisms mediating behavioral hyperalgesia induced by neurokinin-1 tachykinin receptor activation in the rostral ventromedial medulla. Neuroscience. 171:1341–56. doi: 10.1016/j.neuroscience.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dorn SD, Palsson OS, Thiwan SI, Kanazawa M, Clark WC, van Tilburg MA, Drossman DA, Scarlett Y, Levy RL, Ringel Y, Crowell MD, Olden KW, Whitehead WE. Increased colonic pain sensitivity in irritable bowel syndrome is the result of an increased tendency to report pain rather than increased neurosensory sensitivity. Gut. 2007;56:1202–9. doi: 10.1136/gut.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 47.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 48.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295:1737–40. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 49.Freund W, Klug R, Weber F, Stuber G, Schmitz B, Wunderlich AP. Perception and suppression of thermally induced pain: a fMRI study. Somatosens Mot Res. 2009;26:1–10. doi: 10.1080/08990220902738243. [DOI] [PubMed] [Google Scholar]
- 50.Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Giesecke T, Mainguy Y, Gracely R, Ingvar M. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain. 2009;144:95–100. doi: 10.1016/j.pain.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 51.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–91. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 52.Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. Neuroimage. 2006;32:1804–14. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 53.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 30:12964–77. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:9896–903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Science translational medicine. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 56.Jerndal P, Ringstrom G, Agerforz P, Karpefors M, Akkermans LM, Bayati A, Simren M. Gastrointestinal-specific anxiety: an important factor for severity of GI symptoms and quality of life in IBS. Neurogastroenterol Motil. 2010;22:646–e179. doi: 10.1111/j.1365-2982.2010.01493.x. [DOI] [PubMed] [Google Scholar]
- 57.Guthrie E, Barlow J, Fernandes L, Ratcliffe J, Read N, Thompson DG, Tomenson B, Creed F. Changes in tolerance to rectal distension correlate with changes in psychological state in patients with severe irritable bowel syndrome. Psychosom Med. 2004;66:578–82. doi: 10.1097/01.psy.0000128899.22514.c0. [DOI] [PubMed] [Google Scholar]
- 58.Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. 2009;59:489–95. doi: 10.1136/gut.2008.175000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure with experimental protocol: Supplemental material: The time course of the imaging experiment is shown in the graph. Subjects rated their sensory thresholds during phase (C): 0= no sensation; 1 =first sensation/some sensation; 2= urge to defecate; 3= maximum tolerable distension.
Figure: Locations of Regions of Interest (ROIs): Supplemental material