Abstract
Myxomatous Mitral valve prolapse (MVP) is the most common cardiac valvular abnormality in industrialized countries and a leading cause of mitral valve surgery for isolated mitral regurgitation. The key role of valvular interstitial cells (VICs) during mitral valve development and homeostasis has been recently suggested, however little is known about the molecular pathways leading to MVP. We aim to characterize Bone Morphogenetic Protein 4 (BMP4) as a cellular regulator of mitral valvular interstitial cell activation towards a pathologic synthetic phenotype and to analyze the cellular phenotypic changes and extracellular matrix (ECM) reorganization associated with the development of myxomatous mitral valve prolapse. Microarray analysis showed significant up regulation of BMP4-mediated signaling molecules in myxomatous MVP when compared to controls. Histological analysis and cellular characterization suggest that during myxomatous MVP development, healthy quiescent mitral VICs undergo a phenotypic activation via up regulation of BMP4-mediated pathway. In vitro hBMP4 treatment of isolated human mitral VICs mimics the cellular activation and ECM remodeling as seen in MVP tissues. The present study characterizes the cell biology of mitral VICs in physiological and pathological conditions and provides insights into the molecular and cellular mechanisms mediated by BMP4 during MVP. The ability to test and control the plasticity of VICs using different molecules may help in developing new diagnostic and therapeutic strategies for myxomatous MVP.
Keywords: Myxomatous Mitral Valve Prolapse, Valvular Interstitial Cells, Bone Morphogenetic Protein 4, VIC synthetic phenotype, Extracellular matrix
Introduction
Myxomatous Mitral Valve Prolapse (MVP) is the most common indication for mitral valve surgery due to severe mitral regurgitation. Echocardiographically, MVP is defined as a single or bileaflet prolapse, at least 2 mm beyond the long-axis annular plane, with or without leaflet thickening (Freed et al., 1999). The prevalence of MVP is estimated at 2–3%, and is equally distributed between men and women. MVP is expected to occur in approximately 7.2 million individuals in the US, and over 144 million worldwide (Freed et al., 1999, 2001).
The process of Heart valve development is conserved along vertebrate species. The valve leaflets originate from mesenchymal outgrowths known as endocardial cushions, the precursors of valves and the cardiac septa. A subgroup of cells in the cushion-forming area, driven by signals from the underlying myocardium, undergoes endothelial to mesenchymal cell transition (EMT) and migrate into the cardiac jelly to form Valvular Interstitial Cells (VICs) (Schoen, 2008). VICs, from all four heart valves, share the same embryologic origin and maintain similar mesenchymal features once differentiated in adult cells. Although adult valves are sparsely populated with quiescent VICs, dynamic changes in ECM architecture and VIC phenotypes continue in response to altered mechanical and/or pathological conditions (Schoen, 2008; Coombs and Yutzey, 2009; Garg, 2006; Hinton and Yutzey, 2011). This concept has been investigated for aortic valve pathologies, however, little is known about the cellular plasticity of VICs during the pathogenesis of mitral valve diseases (Chen et al., 2009). Here, we investigated the role of VIC activation in human myxomatous MVP-derived tissues, and analyzed the direct effects of these phenotypic changes on ECM reorganization by testing MVP- and control-derived VICs plasticity.
VICs are the most abundant cell types in the mitral valves and are distributed throughout all its layers: a dense collagenous layer close to the outflow surface which provides the primary strength component (Fibrosa); a central core of loose connective tissue (Spongiosa); and a layer rich in elastin below the inflow surface (Atrialis) (Prunotto et al., 2010; Rabkin et al., 2001). The key microscopic changes in MVP appear to occur in the spongiosa and fibrosa, where the structural integrity of the entire valve resides. Alterations in matrix components (collagen, elastin, proteoglycans and matrix metalloproteinase and their tissue inhibitors) and ECM reorganization have been studied extensively, however very little is known about the cellular elements and molecular mechanisms leading to mitral VIC activation (Rabkin et al., 2004; Taylor et al., 2003).
Recent studies have suggested developmentally regulated expression of Bone Morphogenetic Proteins (BMPs) and Sox9 during the atrioventricular valve formation (Jiao et al., 2003). BMPs belong to TGF-β super family of cytokines, and have been implicated in numerous developmental processes including proper septation and valvulogenesis. BMP-2 and BMP-4 are known to be potent osteogenic morphogens and are shown to be present in ossified valves (Mohler et al., 2001). BMP4 is also a potent inducer of collagen, proteoglycan synthesis and matrix mineralization. It acts as a signal from the myocardium directly mediating atrioventricular septation, and defects in this process are shown to cause one of the most common human congenital heart abnormalities, atrioventricular canal defect (Jiao et al., 2003). A possible association of BMP signaling pathways is suggested in a MMP-2 transgenic mouse model of MVP (Mahimkar et al., 2009). The present study was carried out on the most common presentation of myxomatous mitral valve disease, posterior leaflet prolapse of the anatomic area described in the Carpentier’s classification as P2 or middle scallop of the posterior leaflet (Carpentier et al., 1980). Based on our tissue microarray analysis and MV-derived cell analysis we hypothesized that BMP4 mediates the activation of VICs from healthy quiescent cells to the pathologic synthetic phenotype during development of MVP. This study, for the first time, describes a molecular mechanism leading to the activation of quiescent VICs and its effect on ECM remodeling in human myxomatous Mitral Valve Prolapse.
Methods
Sample population and Tissue collection
The present investigation conforms to the principles outlined in the Declaration of Helsinki. For the present study, from April 2009 to February 2011, we enrolled a total of 243 subjects, from the Hospital of the University of Pennsylvania and at the Penn Presbyterian Medical Center according to the approved IRB protocol# 809349. Out of 193, 72 patients underwent leaflet repair without resection. Of the remaining 121, we included 67 patients based on the exclusion criteria listed in Table 1. All the patients had a long history of mitral regurgitation and represented with leaflet thickening, annular dilatation and/or chordal rupture. The surgical technique involved partial resection of the prolapsed P2 segment with placement of a flexible annuloplasty band. There was no residual MR at the time of discharge and there were no surgical complications. Detailed surgical approach is described in the supplemental material. Controls (n=50), collected through the heart transplant and the Gift of Life program, or purchased from BioServe (Beltsville, MD) were asymptomatic with no significant history of medication (Table 2). All analyses were conducted primarily on control-harvested hearts showing normal features and no signs of any pathological conditions upon echocardiographic evaluation. Posterior P2 leaflet of MVP patients and controls were collected fresh in DMEM media (VIC isolation), formalin fixed (Immunohistochemisty) and frozen for RNA and protein analysis.
Table 1.
Patient enrollment
| MVP Patients | Transplant and donors | |
|---|---|---|
| Total subjects enrolled | 193 | 50 |
| Chordal repair without resection | 72 (37.3%) | N/A |
| MV tissue collected | ||
| - Osteogenesis imperfecta | - | - |
| - Marfan’s syndrome | 2 (1.0%) | - |
| - Ehlers Danlos | - | - |
| - Endocarditis | 2 (1.0%) | 1 (2.0%) |
| - Cancer | - | - |
| - Radiation therapy | - | - |
| - Rheumatic disease | 11 (5.7%) | 2 (4.0%) |
| - Ischemic MR | 2 (4.0%) | |
| - Mechanical bioprostheses | 1 (0.5%) | 2 (4.0%) |
| - Congenital valve disease | 2 (1.0%) | 1 (2.0%) |
| - Other connective disorders | - | - |
| - P1, P3 or anterior leaflet prolapse | 14 (7.3%) | - |
| - Mitral calcification | 8 (4.1%) | 1 (2.0%) |
| - Other | 14 (7.3%) | 14 (28.0%) |
| Inclusion criteria | 67 (34.7%) | 27 (54.0%) |
Table 2.
Patient demographics
| MVP Pts | Controls | p-value | |
|---|---|---|---|
| Age in years (Mean±SE) | 58.8±7.2 | 46±4.0 | <0.001 |
|
| |||
| Gender | |||
| Male | 54 (80.6%) | 17 (62.9%) | - |
| Female | 13(19.4%) | 10 (37.1%) | |
|
| |||
| Ejection fraction | 56.67 | 46.0 | NS |
|
| |||
| Mitral regurgitation | |||
| Moderate | - | 4 (14.8%) | - |
| Severe | 67(100%) | - | |
|
| |||
| Pulmonary hypertension | 3 (4.5%) | 1 (3.7%) | - |
|
| |||
| Hypertension | 27 (40.3%) | 4 (14.8%) | <0.01 |
|
| |||
| Hyperlipidemia | 22 (32.8%) | 5 (18.5%) | <0.01 |
|
| |||
| Diabetes mellitus | 6 (8.9%) | - | - |
|
| |||
| CAD | 7 (10.4%) | 4 (14.8%) | NS |
|
| |||
| Family history of MVP | 1 (1.5%) | - | - |
|
| |||
| Surgical procedure | |||
| Triangular resection | 50 (74.6%) | - | - |
| Quadrangular | 3 (4.5%) | ||
| Other | 14 (20.9%) | ||
Histological analysis
Histological analysis of the control and P2 prolapse tissues was performed by Hematoxylin and Eosin (H&E), Modified Movat Pentachrome staining (for proteoglycans, elastin and collagen) and Masson’s Trichrome staining (for collagen) according to the protocol of the Histology Laboratory of the University Of Pennsylvania School Of Medicine.
Microarray analysis
Microarray analysis was performed using the Human Whole Genome OneArray (Phalanx Biotech, Palo Alto, CA) as described in Supplement Material.
Validation of microarray data
Reverse Transcriptase PCR was performed to validate the expression of genes involved in the BMP4 pathway using oligonucleotides purchased from Integrated DNA Technologies (IA, USA) and Real time PCR LLC (Elkins Park, PA). RT-qPCR analysis was performed in triplicates using the 7500 Fast Real time PCR (Applied Biosystems, Carlsbad, CA). Relative expressions of genes were represented as RQ (Relative quantitation), which is calculated as 2−ΔΔct. The primers used for the analysis are listed in supplement table 1.
Isolation of Mitral VICs
Isolation of Mitral VICs was performed using a modified method described by Stephens et al (2007). See Supplemental Material and Methods. Characterization of the MVICs was performed as suggested by Rabkin et al (2001). All the experiments were performed on cultured VICs between their second and fourth passages.
BMP4-mediated VIC activation
In vitro experiments were performed using recombinant human BMP4 protein (Peprotech, Rocky Hill, NJ). Briefly, VICs were cultured in 60mm dishes until 50–60% confluence; media was changed to BMP4 enriched media (DMEM medium containing 1% FBS 10ng/ml FGF2, 10ng/ml PDGF and 100ng/ml hBMP4). hBMP4 (100ng/ml) treatment was done up to 21 days (Barry et al., 2010). Media was replaced every three days and fresh hBMP4 was added. After the 21 days of BMP4 treatment, RNA was extracted from the cells, converted to cDNA and amplified using the Fast SYBR protocol.
In vitro calcification assay
The in vitro calcification assay was performed as described in Poggio et al., (2010) using human-derived mitral VICs. Quantitation of calcium was carried out using Colorimetric assay (Biovision, Mountain View, CA).
Statistical analysis
The data from all microarrays in each experimental set were passed to Omicsoft Array Studio software, control and missing features were removed, and the remaining signals were normalized and transformed to log2 values. Hypothesis testing and generation of fold change values: testing was performed by combining technical replicates and performing a standard student’s t-test to calculate raw p-values. Adjusted p values were calculated using the Benjamini and Hochberg method with a false discovery rate alpha-value of 0.05. Fold change for the gene expression was calculated based on the mean values of the technical replicates for each probe. Data filtering and selection of affected genes: a subset of genes was generated based on an internal intensity stringency filter. Furthermore a set of ‘affected genes’ was established using a raw p-value cutoff of 0.05 and a two-fold change cutoff. Gene Ontology (GO): Omicsoft Array Studio was used to evaluate the Gene Ontology enrichment. In addition p-values were calculated for each classification based on Fischer’s exact test using all the annotated probes on the array as a comparison set and the Benjamini and Hochberg method for p-value adjustment.
Results
Patient Selection
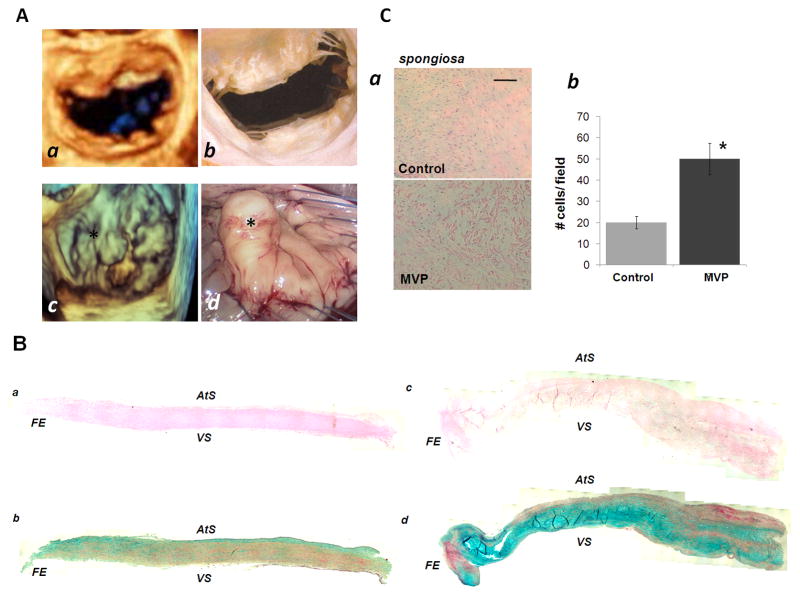
All myxomatous MVP patients shared similar intra-operative and echocardiographic appearance of the mitral valve. Macroscopically, our myxomatous mitral valves showed interchordal hooding due to some leaflet redundancy that includes both the rough (cooptation area) and clear (free atrial area) zones of the involved P2 segment. As a consequence of these histological changes, fibrosis of the surface of the leaflets, thinning and/or elongation of the chordae tendineae, and ventricular and atrial friction lesions were observed in the selected cases [Figure 1A and 1B]. To determine the histological structure of the resected mitral valve leaflets in pathological and physiological conditions, and the reorganization of the ECM, we performed H&E and modified Movat Pentachrome staining [Figure 1B]. High-definition images were taken of the entire leaflets and composite photomicrograph assembled. The structure of the mitral valve leaflet was well preserved in the control tissue, while the prolapsed segment showed an increase in the proteoglycan (blue) and collagen degradation (yellow) associated with increased cellularity, predominantly in the spongiosa [Figure 1B, 1C], suggests accumulation of VICs in myxomatous, but not in normal valves (Rabkin et al., 2001). These results generated the hypothesis that MVP is associated with the activation of VICs towards an active synthetic phenotype which, in turn, regulates ECM organization.
Figure 1. Macroscopic and Histological Analysis of myxomatous mitral valve and control.
[A] Comparison between normal mitral valve and patient with P2 prolapse and mitral regurgitation: (a) 3D Echocardiographic appearance of normal mitral valve with no evidence of prolapse, (b) Normal mitral valve in cadaver, (c) 3D echocardiographic appearance of P2 prolapse (marked by asterisk *) in patient with mitral regurgitation (d) intra-operative videoscopic view of prolapsed P2 scallop prior to resection. [B] Composite photomicrographs of sections of human mitral valve leaflets (transverse sections). Controls (a and b), and diseased valves (c and d) high-definition images have been assembled to rebuild the leaflet structures. H&E (a, c) and Movat Pentachrome staining (b, d) are reported. FE: free edge; VS: ventricularis, AtS: Atrialis [C] (a) Representative image of H&E section of the mitral valve spongiosa layers of control and MVP patient and relative number of VICs per field, (b) quantification of total cell number within selected fields of the spongiosa layers. Bar graph: 50 μm.
Gene Regulatory Pathways during Myxomatous Mitral Valve Prolapse
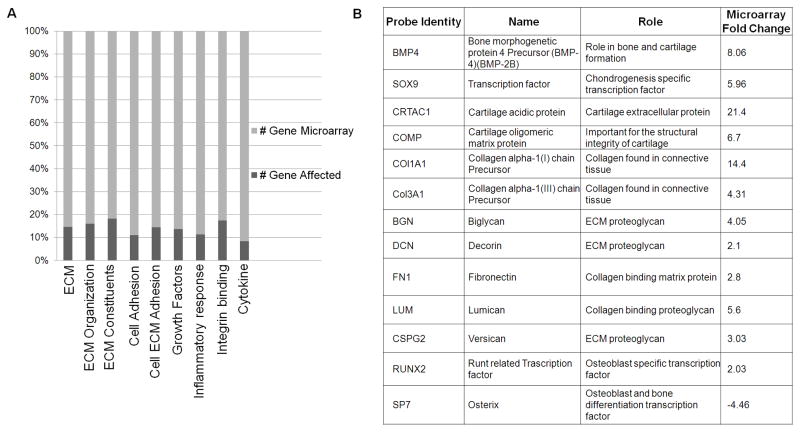
To identify the gene regulatory pathways involved in the activation of mitral VICs toward a pathologic phenotype, we performed a microarray study on 4 selected prolapsed P2 segments of patients with MVP, and 4 normal leaflets from healthy controls. Clinical characteristics of MVP patients used for microarray analysis are provided in supplemental table 2. Among the transcriptional activities of 19,553 human sequences determined by use of an oligonucleotide microarray, we found a total of 1,883 probe sets which fulfilled the criteria for differential expression. These transcripts represent genes showing at least a two-fold change in MVP tissue vs. controls. Out of 1,883 transcripts considered, 1,033 were upregulated (54.8%) and 850 down regulated (45.2%). The genes were characterized on the basis of their functional role [Figure 2A]. A detailed description of the gene profile summarized in Figure 2B could be found in the Supplemental Tables S2 to S6. To our knowledge, our study provides the first wide-scale evaluation of gene expression in human subjects with myxomatous MVP. Since both the cellular and the extracellular components of the mitral leaflets are affected in this pathological condition, we focused on the identification of possible regulatory elements that could be able to control both these aspects.
Figure 2. Microarray Analysis.
[A] Relative expression of mRNA transcripts over the total number of genes included in the microarray analysis. ECM organization, cell-cell and cell-matrix adhesion, growth factors and inflammatory response are reported. [B] BMP4 related VIC activation- and BMP4-mediated Osteogenesis induced gene transcripts up regulated or down regulated in the microarray analysis.
Activation of BMP4 pathway in human myxomatous mitral valve tissue
Our bio-informatics analysis highlighted the differential expression of several genes that directly or indirectly correlated with the BMP4 pathway [Figure 2A]. BMP4 has been reported to regulate the cellular activation of mesenchymal cells in embryologic development (Gupta et al., 2009). Since mitral VICs derive from the transdifferentiation of quiescent mesenchymal cells, we tested the hypothesis that BMP4 is involved in the activation of mitral VICs towards a pathologic synthetic phenotype. We therefore conducted a bioinformatics and molecular biology analysis of 20 selected patients with isolated posterior MVP and controls. Among the genes shown in Figure 2A, we validated the expression of BMP4 and its activators and effectors in surgically resected P2 segments of patients and controls by qRT-PCR [Figure 2B and Figure 3].
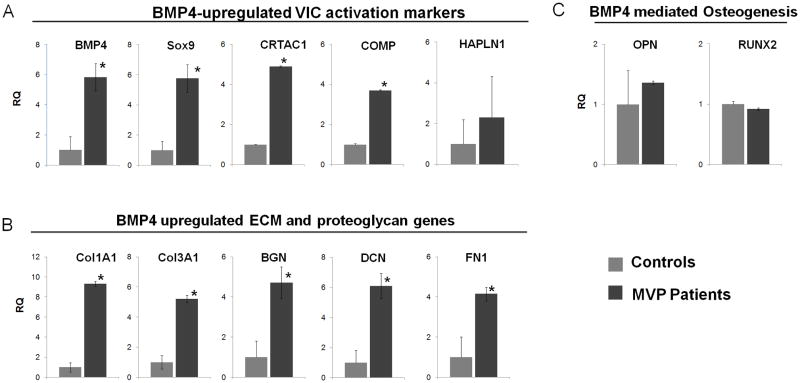
Figure 3. Graph bar representation of mRNA variations in myxomatous MVP vs. controls.
qPCR analysis were performed for 15 MVP and 15 control tissues. Relative transcript abundance of 18S-normalized PCR products is given, in which the control abundance of gene is assigned a value of 1. Data expressed as RQ ±dCT SE. [A] BMP4-upregulated VIC activation markers, [B] ECM remodeling markers, and [C] BMP4-mediated osteogenesis makers were analyzed as described (* denotes p<0.05).
BMP4 was up-regulated by 8.06 fold in our microarray analysis and more than 5.83 fold in our validation experiments when comparing MVP vs. control. Sox9 is a potent activator of collagen deposition and plays an important role in the BMP-mediated pathway. In our study, Sox9 is up-regulated 5.96 fold in the microarray analysis and 5.76 fold in the validation assays. Cartilage acidic protein 1 (CRTAC1), localized in the ECM, is stimulated directly by BMP4 and it was found up regulated by 21 fold in our bio-informatics analysis and 4.89 fold in our validation experiments. Cartilage Oligomeric Matrix Protein (COMP) plays a central role in the structural integrity of mesenchymal-derived tissue via its interaction with other extracellular matrix proteins such as collagens and fibronectin (FN) which were significantly up regulated in both microarray and q-PCR analysis. We found 6.7 fold up regulation of COMP in the microarray and 3.7 fold by qPCR. Decorin (DCN) and Biglycan (BGN) are small pericellular matrix proteoglycans that play a role in the assembling of collagen fibrils. These molecules were found up regulated more than 4 folds in MVP vs. control tissues. Taken together these results suggest the direct involvement of BMP4 pathway during the development of MVP and alterations associated with fibrillar collagens. Our results are in accordance with the activation of VICs during MVP development, with associated ECM reorganization, suggesting a direct role of BMP4 in these cellular and extracellular events.
Since BMP4 signaling pathways has been described as an activator of the aortic VICs chondro-osteogenic transdifferentiation during Calcific Aortic Valve Disease, we also analyzed the osteogenic-related genes activated by BMP4, to highlight differences and similarities between the VICs activation in aortic and mitral valve diseases. In microarray analysis and validation experiments we observed that Osteopontin, Osterix and RUNX2, MMP13 and alkaline phosphatase genes were either down regulated or unchanged in MVP vs. control. These results suggest that during MVP, BMP4 induced phenotypic activation of VICs is independent of BMP4-mediated osteogenic transformation seen in aortic VICs.
Cellular and Extracellular Changes within the Mitral Valve Leaflets during MVP
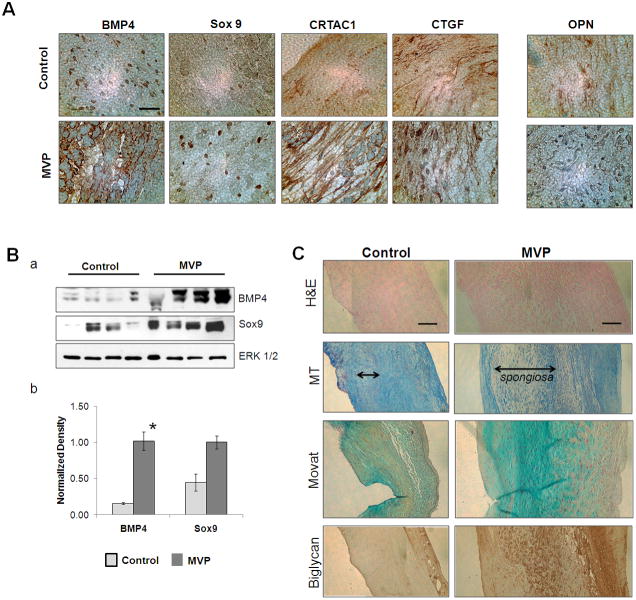
Similar to increased mRNA expression, increased protein levels of BMP4, Sox9, CRTAC1 and CTGF were detected in the MVP tissues when compared to control both by Immunohistochemistry and Western blot analysis [Figure 4A, B]. Expression of BMP4, CTGF, Biglycan and CRTAC1 were significantly increased in the atrial side and all over the spongiosa layer of the MVP sections while they were less abundant in the controls valves. Nuclear expression of Sox9 was seen mostly in the spongiosa of the MVP tissue section. Interestingly, the marker of BMP4-mediated osteoblastic transdifferentiation, Osteopontin, was not affected during the development of MVP [Figure 4A]. We also observed a differential organization of elastin, collagen, and proteoglycan, which in turn changes the biological and mechanical proprieties of the valve. Figure 4C shows an increased deposition of collagen and proteoglycan, and elastin disarray in a section of myxomatous mitral valve. The cross section of resected P2 segments shows significant rearrangement of the ECM with expression of BMP4-related ECM proteins such as fibronectin, the proteoglycans (mostly, Versican, Lumican, fibromodulin, and biglycan) along with alterations in the type of collagen expression (type I, III and IX) [Figure 4C]. As shown in these experiments, the key microscopic change in MVP appears to occur in the spongiosa.
Figure 4. BMP4-mediated pathways and ECM reorganization in Myxomatous MVP and controls.
[A] Immunohistochemistry of surgically resected myxomatous P2 prolapse and controls as indicated. 4 μm thick Cross sections; 63X Magnification, scale bar: 16μm. [B]. Shows western blot (a) and densitometry analysis (b) for expression of BMP4, Sox9 and ERK ½ (loading controls) in tissue lysates from controls and MVP leaflets (* denotes p<0.05). [C] H&E, Masson’s Trichrome (MT), Movat Pentachrome and Biglycan staining to visualize the extracellular structure, Valvular interstitial cell content, proteoglycans, collagen and elastin composition of the three valvular layers. Magnification 10X, Scale 100 μm.
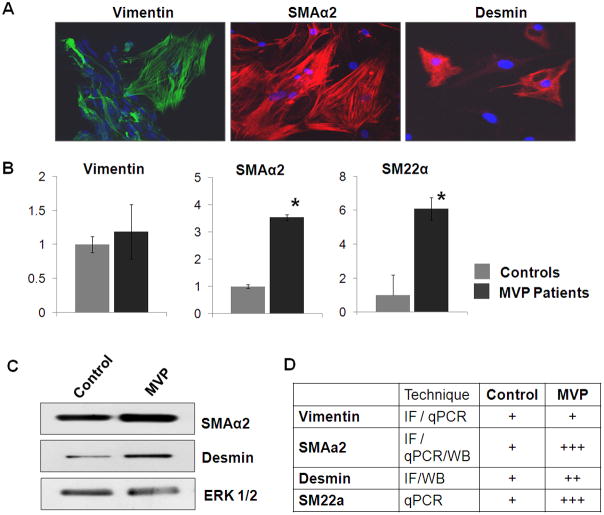
Mitral Valvular Interstitial Cell Characterization
Characterization of isolated VICs was performed as described earlier. SMAα2, Vimentin and desmin expression was detected by immunoflourescence staining and western blot analysis in all isolated cells [Figure 5A and C]. Elevated levels of SMAα2, Fibronectin and SM22α transcripts were detected in MV-derived cells confirm the activation of VICs for the cells isolated from MVP patients and a quiescent phenotype for control-derived cells (Figure 5B&C respectively). Figure 5D summarizes the expression of these genes/proteins in VICs. After the successful isolation and characterization of VICs, we used these cells to test the effects of recombinant human BMP4 protein in in vitro experiments.
Figure 5. Characterization of Mitral VICs.
[A] Immunofluorescence staining for Vimentin, SMAα2, and Desmin in the isolated VICs. Magnification: 40X, Scale: 50μm. [B] Relative abundance of transcripts for Vimentin, SMAα2 and SM22α in MVP-derived VICs, using qPCR. (* denotes p<0.05). [C] Western blots showing expression of SMAα2, desmin and ERK1/2 (loading control) [D] Schematic representation for expression of VIC markers.
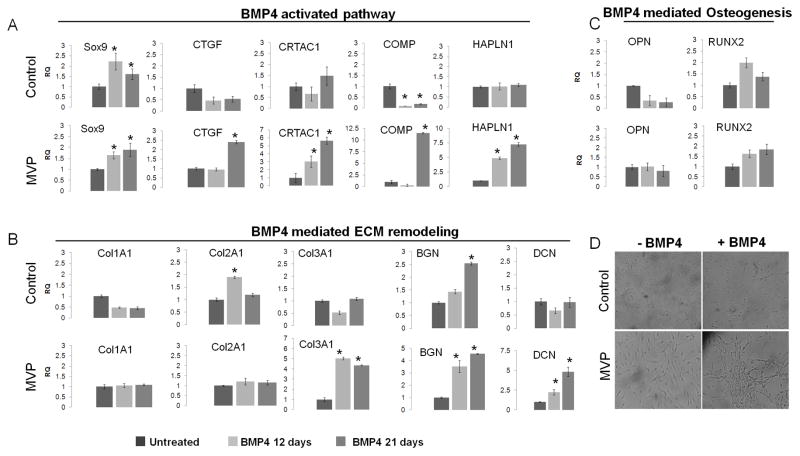
In vitro hBMP4 treatment mimics the phenotypic activation and ECM remodeling of human myxomatous MVP tissue
We investigated the ability of BMP4 to lead the activation of VIC and the expression of BMP-related markers using qPCR assays. BMP4 treatment increased expression of markers related to the activation of VICs, ECM genes, and morphological transformation of isolated mitral valve interstitial cells [Figure 6]. BMP4 treatment resulted in increased expression of Sox9, COMP, CRTAC1, CTGF and collagen type III, proteoglycan genes like BGN, DCN and elevated transcript levels of HAPLN1 as reported in MVP tissues. Conversely, the same treatments were mostly ineffective on isolated quiescent VICs isolated from controls [Figure 6A and B]: we observed no significant changes in Col1A1, Col3A1, and DCN; a small decrease in CTGF, and COMP in control-isolated cells. Among the analyzed markers Sox9, CRTAC1 and BGN were up regulated at 21 days, suggesting delayed effects of BMP4 in activating VICs from healthy controls. These results suggest there is an ongoing process of VIC activation at the time of mitral valve repair, whereas MV-derived cells from healthy control are mostly quiescent. In addition, these results indicate that BMP4 is able to mimic the process of cellular activation and ECM remodeling in vitro, opening new perspectives for the identification of specific therapeutic targets to halt the progression of the disease. Furthermore, and in agreement with our previous observations on tissue analysis, BMP4 treatment of mitral VICs did not induce changes in transcript levels of BMP4-related osteogenic markers, such as OPN or RUNX2 [Figure 6C], suggesting a different mechanism of action for BMP4 on these cells.
Figure 6. In vitro BMP4 treatment.
BMP4 regulated transcripts were detected after 12 or 21 days of BMP4 treatments of MV-derived cells from patients and controls. (A) BMP4 signaling molecules upregulated by BMP4 treatment in the MVP-derived VICs. (B) BMP4 mediated changes in ECM remodeling genes: Up regulation of Col3A1, Biglycan and DCN in MVP-isolated VICs was seen. An early up regulation of Col2A1 and Biglycan in control-isolated cells was observed. Relative transcript abundance of 18S-normalized PCR products is given, in which the control abundance of gene is assigned a value of 1. Data expressed as RQ±dCT SE. (C) BMP4-related osteogenesis markers No significant differences were noted on both cell populations. (D) Morphological analysis of MV-derived VIC from patients and controls. BMP4 treatment induces morphological changes on MVP-derived cells, but not in control-derived cells. Magnification: 10X, Scale bar 100μm (* denotes p<0.05).
Discussion
The mitral valve, often regarded as an inactive tissue with few components and limited cellular activity, is in fact extremely dynamic. MV leaflets are remarkably sophisticated structures containing a variety of cell types that produce and reside in a complex extracellular matrix. Specifically, the human valve consists mostly of valvular interstitial and endothelial cells. These cells interact within an extracellular matrix composed of proteoglycans, collagen, elastic fibers, glycosaminoglycans, and integrins (Rabkin et al., 2011; Rabkin et al., 2004; Gupta et al., 2009). Valvular cells continually engage in crosstalk with the external environment and respond to a multitude of mechanical and chemical stimuli. Valvular interstitial cells are responsible for the development of pathologic phenotypes and the overall structure of the valve (Rabkin et al., 2001). From the histopathalogical point of view, valvular diseases are mostly related to two, non-mutually exclusive patterns: myxomatous degeneration leading to insufficiency, or progressive fibrosis, resulting in stenosis/calcification (Caira et al., 2006; Lincoln et al., 2006). Extensive studies on calcific aortic valve stenosis have highlighted the central role of aortic VICs and the expression of several genes associated with osteogenesis including Osteopontin, Osteocalcin, Osterix, and alkaline phosphatase (Osman et al., 2006; Alexopoulos et al., 2010; Rajamannan et al., 2003). On the other end, the mechanisms leading to myxomatous degeneration remain vaguely understood, except for fibrillin-1 knockout mice model of Marfan syndrome which resembles only partially to human myxomatous MVP (Ng et al., 2003).
A possible implication of BMPs in the activation of VICs has been suggested in the setting of MVP (Mahimkar et al., 2009). However, the morphological changes and the molecular mechanisms leading to MVP directed by BMP4 are far from being elucidated. BMP4 is involved in the VIC transdifferentiation towards a pathologic phenotype in calcific aortic valve disease patients. In these patients, the VICs are activated towards the osteogenic phenotype in a process controlled by BMP-related proteins; Osteocalcin and alkaline phosphatase (Osman et al., 2006; Alexopoulos et al., 2010). Alexopoulos et al (2010) showed activation of Osteopontin, Osterix and RUNX-2 during the development of aortic valve stenosis.
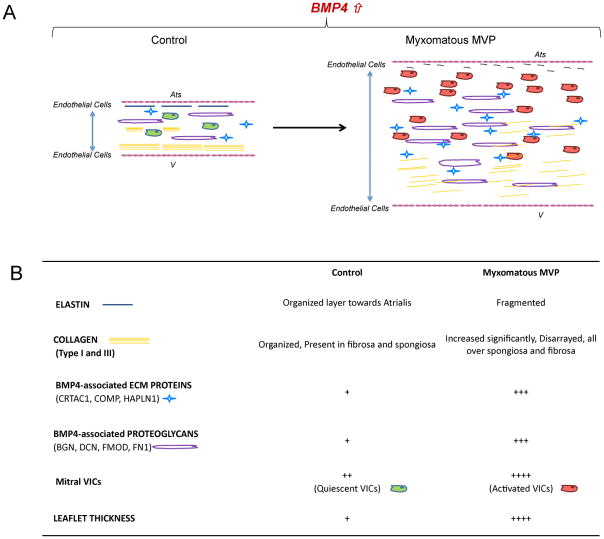
Studies on mesenchymal cell transdifferentiation have suggested a dual, reversible, mechanism of phenotypic changes towards prechondrocyte-chondrocyte phenotype or towards osteogenesis (Barry et al., 2001). Chondrocyte-like differentiation in presence of BMP4 leads to expression of Sox9, Collagen type I and III, fibronectin, COMP and other matrix proteins (Steinert et al., 2009). On the other hand, during osteoblastic transdifferentiation of mesenchymal cells, RUNX2 and Wnt signaling enhance the mineralization of the cartilage matrix and maintain the osteogenic phenotype (Komori et al., 2008). In our study we observed increased matrix production and proteoglycan deposition in the MVP tissues and in in vitro analysis, which correlates with the changes in ECM organization with VIC transdifferentiation towards an activated or synthetic phenotype. Similar increase in collagen and fibronectin expression upon BMP4 treatment has been reported in the airway epithelial cells (Molloy et al., 2008). However, this effect in our VICs was independent from the BMP4 induced osteogenic-like transdifferentiation as seen in aortic valve interstitial cells. These changes highlight that there is a transition of VICs from quiescent to pathologic phenotype which in turn changes the biological and mechanical proprieties of the valve [Figure 7].
Figure 7. Human Myxomatous Mitral Valve Prolapse Tissue: Role of Bone Morphogenetic Protein 4 in valvular interstitial cell activation and Extracellular Matrix Homeostasis.
(A) Schematic representation of the role of BMP4 in cellular activation and ECM remodeling in Myxomatous MVP leaflet and control. Ats: Atrialis; V: ventricularis (B) Table of cellular and extracellular changes associated with the increased levels of BMP4 in Myxomatous MVP and Controls.
These reports generate the following questions: are valvular pathologies the result of an active, reversible, cellular transdifferentiation process? Can the plasticity of VICs be controlled towards a specific phenotype? Here we have reported BMP4 as a mediator of the mitral valvular interstitial cells activation and, in turn, ECM reorganization. Interestingly, Mitral VICs isolated from healthy controls and myxomatous MVP patients, undergo biomineralization when grown in inorganic phosphate medium suggesting that different physiological responses can be triggered under different specific stimuli [Supplemental Figure 1].
Our study population i.e. patients with the most common type of MVP (P2 prolapse) undergoing mitral valve repair have their valve repaired using a limited resection of this scallop with or without sliding plasty or by re-suspending this segment with artificial chordae to a height equal to that of the neighboring scallops of the posterior leaflet. The annulus, which, in most cases, is distorted and/or dilated, is then stabilized with an annuloplasty ring or band (Verma and Mesana, 2009). The longevity of these repair techniques, in experienced hands, is remarkable. The mechanical stress forces present prior to the operation disappear postoperatively, and the valve dynamics are reset. The removal of these mechanical forces upon the valve leaflets by either excising or fixating of the elongated prolapsed segments in conjunction with a remodeling annuloplasty, allows for a significantly improved leaflet cooptation and valve performance. We thereby speculate that the removal of the previously present stress forces onto the valve leaflets is a fundamental factor in the resetting of normal valvular cellular physiology and directly responsible for the long-term durability of this type of operation. Furthermore, comparing the cell biology based studies with new echocardiographic finite element analysis of the stress forces present on the leaflets of prolapsing mitral valves, will help us better understand when quiescent VICs get activated and start remodeling ECM and lead to has started in patients with this condition.
A limitation of this study is the absence of mechanical stimuli to mimic the mitral hemodynamic conditions in valvular interstitial cells culture. Although our cell culture results are consistent with the analysis of the explanted tissue, to further investigate the role of VICs and their direct role on ECM reorganization, an in vivo or ex-vivo system able to test changes in the valvular geometry should be used. Unveiling VIC plasticity and being able to better understand their ability to differentiate and/or being reprogrammed could help in designing tissue-engineering valves that carry the patient’s own cells, and also develop new diagnostic and therapeutic strategies for patients with MVP.
Supplementary Material
Acknowledgments
Funding
This project is partially supported by award number RC1HL100035 from the National Heart, Lung, And Blood Institute, NIH (GF) and by the “Harrison Memorial Fund” of the University of Pennsylvania School of Medicine.
We thank Dr. Kenneth B. Margulies, from the Heart Failure Research Department at the University of Pennsylvania for his precious collaboration in the collection of heart valve specimens.
Abbreviation
- BMP4
Bone morphogenetic protein 4
- VIC
Valvular Interstitial cells
- MVP
Mitral Valve Prolapse
- MR
Mitral Regurgitation
Footnotes
Conflicts of Interest
None
References
- Alexopoulos A, Bravou V, Peroukides S, Kaklamanis L, Varakis J, Alexopoulos D, Papadaki H. Bone regulatory factors NFATc1 and Osterix in human calcific aortic valves. Int J Cardiol. 2010;139:142–149. doi: 10.1016/j.ijcard.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- Caira F, Stock S, Gleason T, McGee E, Huang J, Bonow R, Spelsberg T, McCarthy P, Rahimtoola S, Rajamannan N. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediate bone formation. J Am Coll Cardiol. 2006;47:1707–1712. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier A, Chauvaud S, Fabiani JN. Reconstructive surgery of mitral valve incompetence: ten-year appraisal. J Thorac Cardiovasc Surg. 1980;79:338–348. [PubMed] [Google Scholar]
- Chen JH, Yip CY, Sone ED, Simmons CA. Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am J Pathol. 2009;174:1109–1119. doi: 10.2353/ajpath.2009.080750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, Dietz HC. TGF-β dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs M, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105:408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed L, Benjamin E, Levy D, Larson M, Evans J, Fuller D, Lehman B, Levine R. Mitral valve prolapse in the general population: the benign nature of echocardiographic features in the Framingham Heart Study. J Am Coll Cardiol. 2002;40:1298–1304. doi: 10.1016/s0735-1097(02)02161-7. [DOI] [PubMed] [Google Scholar]
- Freed L, Levy D, Levine R, Larson M, Evans J, Fuller D, Lehman B, Benjamin E. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1–7. doi: 10.1056/NEJM199907013410101. [DOI] [PubMed] [Google Scholar]
- Garg V. Molecular genetics of aortic valve disease. Curr Opin Cardiol. 2006;21:180–184. doi: 10.1097/01.hco.0000221578.18254.70. [DOI] [PubMed] [Google Scholar]
- Gupta V, Barzilla JE, Mendez JS, Stephens EH, Lee EL, Collard CD, Laucirica R, Weigel PH, Grande-Allen KJ. Abundance and location of proteoglycans and hyaluronan within normal and myxomatous mitral valves. Cardiovasc Pathol. 2009;18:191–197. doi: 10.1016/j.carpath.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton RB, Yutzey KE. Heart valve structure and function in Development and Disease. Annu Rev Physiology. 2011;73:29–46. doi: 10.1146/annurev-physiol-012110-142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T. Regulation of bone development and maintenance by Runx2. Front Biosci. 2008;1:898–903. doi: 10.2741/2730. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Lange AW, Yutzey KE. Hearts and bones: shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev Biol. 2006;294:292–302. doi: 10.1016/j.ydbio.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Mahimkar R, Nguyen A, Mann M, Yeh CC, Zhu BQ, Karliner JS, Lovett DH. Cardiac transgenic matrix metalloproteinase-2 expression induces myxomatous valve degeneration: a potential model of mitral valve prolapse disease. Cardiovasc Pathol. 2009;18:253–261. doi: 10.1016/j.carpath.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler ER, III, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- Molloy EL, Adams A, Moore JB, Masterson JC, Madrigal-Estebas L, Mahon BP, O’Dea S. BMP4 induces an epithelial-mesenchymal transition-like response in adult airway epithelial cells. Growth Factors. 2008;26:12–22. doi: 10.1080/08977190801987166. [DOI] [PubMed] [Google Scholar]
- Nasuti JF, Zhang PJ, Feldman MD, Pasha T, Khurana JS, Gorman JH, 3rd, Gorman RC, Narula J, Narula N. Fibrillin and other matrix proteins in mitral valve prolapse syndrome. Ann Thorac Surg. 2004;77:532–536. doi: 10.1016/S0003-4975(03)01584-4. [DOI] [PubMed] [Google Scholar]
- Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation. 2006;114:I547–I552. doi: 10.1161/CIRCULATIONAHA.105.001115. [DOI] [PubMed] [Google Scholar]
- Poggio P, Grau JB, Field BC, Sainger R, Seefried WF, Rizzolio F, Ferrari G. Osteopontin controls endothelial cell migration in vitro and in excised human valvular tissue from patients with Calcific Aortic Stenosis and controls. J Cell Physiol. 2011;226:2139–2149. doi: 10.1002/jcp.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunotto M, Caimmi PP, Bongiovanni M. Cellular pathology of mitral valve prolapse. Cardiovasc Pathol. 2010;19:e113–117. doi: 10.1016/j.carpath.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Rabkin E, Aikawa M, Stone J, Fukumoto Y, Libby P, Schoen F. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- Rabkin-Aikawa E, Farber M, Aikawa M, Schoen FJ. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis. 2004;13:841–847. [PubMed] [Google Scholar]
- Rajamannan N, Subramaniam M, Rickard D, Stock S, Donovan J, Springett M, Orszulak T, Fullerton D, Tajik A, Bonow R, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen F. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 2008;118:1864–1880. doi: 10.1161/CIRCULATIONAHA.108.805911. [DOI] [PubMed] [Google Scholar]
- Steinert AF, Proffen B, Kunz M, Hendrich C, Ghivizzani SC, Nöth U, Rethwilm A, Eulert J, Evans CH. Hypertrophy is induced during the in vitro chondrogenic differentiation of human mesenchymal stem cells by bone morphogenetic protein-2 and bone morphogenetic protein-4 gene transfer. Arthritis Res Ther. 2009;11:R148. doi: 10.1186/ar2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens E, Carroll J, Grande-Allen K. The use of collagenase III for the isolation of porcine aortic valvular interstitial cells: rationale and optimization. J Heart Valve Dis. 2003;16:175–183. [PubMed] [Google Scholar]
- Taylor PM, Batten P, Brand NJ, Thomas PS, Yacoub MH. The cardiac valve interstitial cell. Int J Biochem Cell Biol. 2003;35:113–118. doi: 10.1016/s1357-2725(02)00100-0. [DOI] [PubMed] [Google Scholar]
- Verma S, Mesana TG. Mitral-valve repair for mitral-valve prolapse. N Engl J Med. 2009;361:2261–2269. doi: 10.1056/NEJMct0806111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.