Abstract
Fatty acid synthase (FAS) catalyzes the de novo synthesis of fatty acids. In the liver, FAS has long been categorized as a housekeeping protein, producing fat for storage of energy when nutrients are present in excess. Most previous studies of FAS regulation have focused on the control of gene expression. However, recent findings suggest that hepatic FAS may also be involved in signaling processes that include activation of peroxisome proliferator-activated receptor α (PPARα). Moreover, reports of rapid alterations in FAS activity and as well as findings of post-translational modifications of the FAS protein support the notion that dynamic events in addition to transcription impact FAS regulation. These results indicate that FAS enzyme activity can impact liver physiology through signaling as well as energy storage and that its regulation may be complex.
Introduction
The liver is involved in the uptake, synthesis, storage, secretion, and catabolism of fatty acids and triglycerides. Fatty acid synthase (FAS), the enzyme catalyzing de novo synthesis of fatty acids, is traditionally thought of as a housekeeping protein, producing fatty acids that can be used for energy storage, membrane assembly and repair, and secretion in the form of lipoprotein triglycerides. However, the contribution by FAS to secreted triglycerides appears to be negligible compared to other sources of fat under common dietary conditions. An unexpected role for FAS as a signaling enzyme emerged with the finding that FAS can affect fatty acid oxidation through PPARα, the main mediator of the fasting response in the liver.
The possibility that FAS may be involved in promoting fat catabolism in addition to its known function of synthesizing fat raises new questions regarding the regulation of FAS. Are there multiple pools of FAS with distinct functions, allowing separate control of FAS-mediated signaling and FAS-mediated energy storage? How is FAS-PPARα signaling regulated in response to nutritional and hormonal stimuli, and how is it possible for FAS to be activated or inhibited rapidly? FAS has been thought to be regulated mostly at the transcriptional level, which might preclude an immediate response by FAS to changes in nutritional or hormonal stimuli since FAS mRNA is fairly stable. A role for FAS in signaling suggests the presence of rapid, post-translational mechanisms of FAS regulation. This review will address physiological functions of hepatic FAS, its regulation by nutrients and hormones, and mechanisms of regulation.
FAS may be a therapeutic target for treating fatty liver and dyslipidemia [1]. Both are common features of the metabolic syndrome [2, 3], which affects ~1 in 4 Americans [4]. Both are also independent risk factors for coronary artery disease [5–7], the most common cause of death worldwide. Identification of regulatory proteins and pathways distinguishing housekeeping FAS from signaling FAS could potentially lead to novel therapeutics that selectively target FAS function.
Hepatic triglyceride metabolism
Under nutrient-replete conditions, the primary fuel of the liver is glucose rather than fat. Fatty acids are not subjected to β-oxidation and instead are incorporated into triglycerides for storage in lipid droplets or secretion in very low-density lipoproteins (VLDL). Dietary fat in the form of chylomicron remnants is taken up by the liver; de novo synthesis of fatty acids by FAS may make a modest contribution to storing energy as fat when nutrients are present in excess.
During fasting, lipolysis in peripheral tissues (primarily adipose tissue) increases the levels of plasma free fatty acids (FFAs), which are taken up by the liver. Activation of the transcription factor peroxisome proliferator-activated receptor α (PPARα) mediates the adaptive response to fasting by promoting the transcription of genes involved in the uptake and catabolism of fatty acids [8–11]. Fatty acids derived from peripheral tissues or intrahepatic lipid droplets are catabolized through β-oxidation to produce ketone bodies, which are used as fuel when glucose is scarce.
In insulin resistance, insulin fails to suppress lipolysis in peripheral tissues even when nutrients are abundant, resulting in high circulating levels of FFAs that are taken up by the liver. Increased FFA uptake and perhaps increased de novo synthesis of fat in the liver overwhelms the capacity for fatty acid oxidation, leading to fat accumulation and eventually the development of hepatosteatosis or fatty liver.
There are thus three main sources of FFAs that contribute to liver triglyceride: plasma, de novo synthesis, and dietary fat delivered by chylomicron remnants. Triglycerides are secreted in VLDL, stored in lipid droplets, or catabolized through the action of lipases and β-oxidation. Fatty acid synthase appears to participate in liver triglyceride metabolism both by contributing de novo synthesized lipids for storage and secretion under nutrient-replete conditions and by promoting β-oxidation of fatty acids through activation of PPARα under nutrient-deficient conditions.
Fatty acid synthase
Fatty acid synthase (FAS, encoded by Fasn) catalyzes the biosynthesis of saturated fatty acids from simple precursors (de novo lipogenesis). The primary product of the FAS reaction is palmitate (C16:0), but stearate (C18:0) and shorter fatty acids may also be produced. FAS substrates are acetyl-CoA, malonyl-CoA, and NADPH. Acetyl-CoA functions as a primer for the reaction, while NADPH provides reducing equivalents. The fatty acid is elongated from the initial acetyl-CoA by repeated condensations with malonyl-CoA, which donates two carbons in each cycle of condensation. Palmitate synthesis thus requires seven cycles of malonyl-CoA addition to an acetyl-CoA primer to yield a saturated, 16-carbon fatty acid.
The FAS protein exists as a homodimer of 273 kDa subunits. Each monomer contains seven protein domains required for fatty acid synthesis: acyl carrier, acyl transferase, β-ketoacyl synthase, β-ketoacyl reductase, β-hydroxylacyl dehydratase, enoyl reductase, and thioesterase [12] (reviewed in [13–15]). However, FAS is only enzymatically active in the dimeric form [12]. The monomers were initially thought to be oriented head-to-tail to form the dimer [16, 17], but recent structural data demonstrate a head-to-head orientation of the monomers that are intertwined at their middle to form an X-shape [18–21]. Mammalian FAS is a type I FAS complex with the domains consolidated in a single peptide; prokaryotes and yeast have a type II FAS with separate proteins catalyzing the individual reactions. Type II FAS complexes capable of synthesizing short-chain (up to 14 carbons) fatty acids are also found in mammalian mitochondria [22].
FAS is a soluble protein and thought to be localized in the cytoplasm, although the specifics of its subcellular localization are largely unexplored. Its tissue distribution is broad with highest levels in the liver, adipose tissue, and lungs [23, 24]. Whole-body knockout of FAS causes embryonic lethality in mice, suggesting that de novo lipogenesis is necessary early during development [25]. A likely possibility is that FAS is required to provide lipids for cell membranes of the growing embryo. Viable tissue-specific FAS knockout mice have been generated, including a liver-specific knockout (discussed below).
Function of FAS in hepatic lipid metabolism
Contribution of de novo synthesized lipids to stored and secreted hepatic triglycerides
Hepatic FAS synthesizes lipids that are stored as lipid droplets or secreted in VLDL in the fed state. In mice, the contribution of liver FAS to secreted VLDL is minor. Ob/ob mice have 10-fold increased hepatic de novo lipogenesis compared to lean mice, but no significant differences in serum triglycerides [26]. In mice with liver-specific knockout of FAS (FASKOL mice), serum triglycerides are normal on a chow diet [27].
The contribution of de novo lipogenesis to secreted triglycerides has been studied in humans in the setting of various diets. On diets low in fat and high in carbohydrate (10% of calories as fat and 75% as carbohydrate), de novo lipogenesis makes a significant contribution to circulating lipids as almost half of VLDL triglyceride is derived from de novo lipogenesis under these conditions [28]. However, a typical Western diet is high in fat as well as carbohydrates. In similar studies using diets higher in fat (30% fat, 55% carbohydrate or 40% fat, 45% carbohydrate), the contribution of de novo lipogenesis to VLDL triglycerides is undetectable or minor, at 0–10% [28, 29]. These diets are more representative of the high fat, high carbohydrate content of a typical Western diet, indicating that under common dietary conditions, de novo lipogenesis is not a significant contributor to VLDL triglycerides. Substituting starch for sugar in a high-carbohydrate diet also decreases the contribution of de novo lipogenesis to 0–1% or 5% [30, 31]. Obese individuals do not appear to have increased FAS-derived VLDL triglycerides compared to lean individuals [29]. Under the high-fat, high-carbohydrate dietary conditions common in the Western world today, hepatic FAS thus appears to be a minor contributor to VLDL triglycerides.
FAS may contribute to triglycerides stored in hepatic lipid droplets. In rats fed a chow diet, 11 ± 1% of hepatic triglycerides are derived from de novo lipogenesis [32]. On a high-fat diet, de novo lipogenesis is suppressed and only 1.0 ± 0.2% of hepatic triglycerides are derived from FAS [32]. FASKOL mice on a chow diet have normal, rather than decreased, liver triglyceride content [27]. It thus appears that the contribution of de novo lipogenesis to stored triglycerides is small in healthy liver.
In fatty liver, the contribution of FAS to intrahepatic triglycerides may be greater. Ob/ob mice have increased hepatic FAS activity and fatty liver [33], but a mechanistic link between the two has not been established. In humans with non-alcoholic fatty liver disease, one group has reported that 26 ± 7% of hepatic triglycerides are derived from de novo lipogenesis [34]. It is unknown how this compares to the triglyceride content of healthy human liver. However, even in the setting of hepatic over-accumulation of fat, the contribution of FAS appears to be less than that of fats derived from peripheral tissues or dietary fat.
Regulation of triglyceride metabolism through signaling lipids: ligand activation of PPARα
PPARα is a member of a family of ligand-activated nuclear receptors important for modulating metabolism and inflammation. During fasting, PPARα promotes lipid uptake and catabolism of fatty acids through β-oxidation to produce ketone bodies [9–11].
When liver-specific fatty acid synthase knockout (FASKOL) mice were generated, they were surprisingly not protected against hepatic lipid accumulation, but instead developed severe hepatic steatosis when on a zero-fat diet or with prolonged fasting [35]. The phenotype of fasted or zero-fat diet-fed FASKOL mice is similar to that of PPARα null mice: hypoglycemia, low serum ketone levels, marked hepatic steatosis, and deficient hepatic fatty acid oxidation [10, 35]. Much of this phenotype was corrected by administration of a known PPARα ligand. The deficient PPARα activation in the absence of both FAS and dietary fat led to the hypothesis that “new” fat, derived from de novo lipogenesis or dietary fat, can activate PPARα, whereas “old” fat, derived from peripheral tissues or stored in the liver, cannot. Hydrolysis of hepatic triglycerides has also been shown to mediate PPARα activation [36], suggesting that triglycerides of different origins (de novo synthesis vs. free fatty acids entering the liver following lipolysis in peripheral tissues) may occupy separate compartments in the hepatocyte. In addition to activating PPARα in liver, FAS has been shown to regulate PPARα in macrophages [37] and hypothalamus [38] as well.
Further study of the FASKOL mouse led to the identification of an endogenous ligand for hepatic PPARα: the phosphatidylcholine species 16:0/18:1-glycerophosphocholine [27]. The interaction of this species with PPARα is dependent on the activity of FAS, and inactivation of choline/ethanolamine phosphotransferase 1 (CEPT1), an enzyme catalyzing the final step in phosphatidylcholine biosynthesis, mimics the FASKOL phenotype [27]. FAS thus appears to contribute to PPARα activity by promoting the synthesis of one of its ligands.
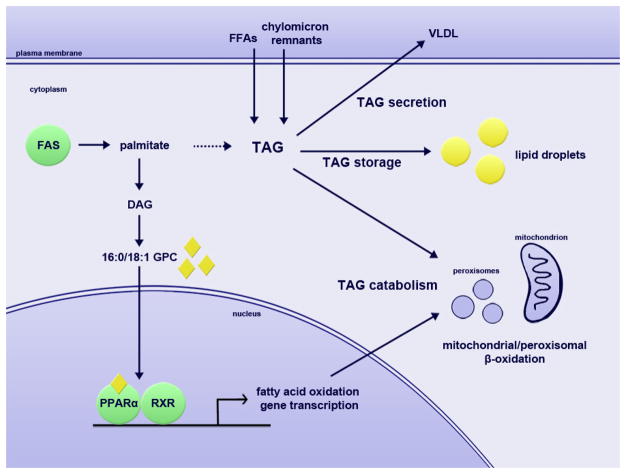
A summary of the impact of FAS on hepatic triglyceride metabolism is presented in Figure 1.
Figure 1. The role of FAS in hepatic triglyceride metabolism.
Fatty acid synthase controls fatty acid catabolism through the synthesis of a ligand for PPARα, which activates fatty acid oxidation genes. FAS makes a minor contribution of lipids to stored and secreted triglycerides, with the major contributions coming from plasma free fatty acids and dietary fats from chylomicron remnants. 16:0/18:1 GPC, 16:0/18:1-glycerophosphocholine; DAG, diacylglycerol; FAS, fatty acid synthase; FFA, free fatty acid; PPARα, peroxisome proliferator-activated receptor alpha; RXR, retinoid X receptor; TAG, triacylglycerol (triglyceride); VLDL, very low-density lipoprotein.
Modulating hepatic FAS to treat disease
Ob/ob mice have increased hepatic FAS gene expression as well as increased hepatic FAS activity compared to lean mice [33]. Knockdown of the transcription factor carbohydrate response element binding protein (ChREBP), which promotes the expression of FAS as well as other genes, in ob/ob liver decreases hepatic lipid accumulation and decreases hepatic lipogenesis, suggesting a link between de novo lipogenesis by FAS and fatty liver [39]. However, in a gene expression profiling study of ob/ob animals separated into high glucose and lower glucose groups, mice with lower sugars (and thus likely to be more insulin sensitive) had higher hepatic expression levels of genes encoding lipogenic enzymes, including FAS, as compared to mice with higher sugars [40]. This finding suggests that while activation of lipogenic enzymes in the liver is associated with obesity, this effect is unlikely to be mechanistically linked to insulin resistance.
FAS inhibitors have been tested in mouse models of obesity and diabetes. Treatment of lean or obese mice with the FAS inhibitor C75 causes dramatic weight loss and improvement of hepatic steatosis in obese mice. However, the effect is primarily mediated by reduced food intake through inhibition of hypothalamic FAS (in addition to possible effects of this particular agent that are independent of FAS), obscuring the potential effects of inhibiting hepatic FAS [41].
The FAS inhibitor platensimycin is concentrated in the liver when administered orally and does not affect food intake [1]. Treatment of high-fructose diet-fed db/db mice with platensimycin reduces hepatic FAS activity, hepatic lipid accumulation, and hepatic fatty acid oxidation [1]. These data are consistent with roles for hepatic FAS both as a producer of fat that may accumulate in liver, and as a generator of lipid signals to nuclear receptors such as PPARα.
These data also highlight a caveat when considering FAS inhibitors as therapy for hepatic steatosis: inhibition of FAS can affect both lipid storage and lipid catabolism, and under conditions where baseline FAS activity is not particularly high, loss of FAS activity might aggravate rather than ameliorate hepatic steatosis, as seen in the liver-specific FAS knockout mice [35].
Regulation of FAS activity
Transcriptional regulation of FAS has been well characterized, but little is known about the post-translational regulation of FAS activity. Similarly, long-term effects of hormones and nutrients on FAS expression are clear but their immediate effects are poorly understood.
Hormonal and nutritional regulation of FAS
Hepatic FAS is known to be regulated by insulin, glucagon, cyclic AMP, fructose, glucose, and dietary fat.
Re-feeding mice or rats a high-carbohydrate diet following a prolonged fast causes a robust induction of FAS expression as compared to the fasted or the ad lib-fed state [42–45]. The effect of carbohydrate re-feeding is mediated by both insulin and glucose. Insulin regulates FAS through transcriptional and non-transcriptional mechanisms. Under nutrient-replete conditions, de novo lipogenesis may promote storage of excess energy in the form of hepatic triglycerides. Insulin promotes FAS expression through activation of the transcription factors sterol regulatory element binding protein 1c (SREBP-1c) [46] and upstream stimulatory factors 1 and 2 (USF1 and USF2) [47, 48]. Conversely, glucagon and cyclic AMP inhibit the increase in FAS activity induced by carbohydrate re-feeding in rats [42, 49, 50].
The effect of fasting compared to ad lib feeding on the activity of hepatic FAS is less clear. In mice, a 6 hour fast reduces FAS expression levels by 60% compared to ad lib feeding [44], and in rats, a 24 hour fast reduces FAS expression by over 90% compared to ad lib feeding [45]. However, a 14 hour fast in mice produces no change in FAS activity compared to ad lib-fed mice [51]. One potential explanation for the lack of change in FAS activity in some circumstances could be a relatively long half-life for the FAS protein. It is possible that changes in FAS gene expression might have little effect on FAS enzyme activity in response to certain physiologically relevant periods of fasting as compared to the ad lib fed condition.
While insulin promotes the expression of FAS, insulin also acutely inhibits the enzymatic activity of hepatic FAS, causing a decrease in FAS activity within minutes [51]. This inhibition is dependent on the presence of the carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), which is phosphorylated in response to insulin and subsequently associates with FAS [51]. This acute inhibition of FAS by insulin is blunted in hyperinsulinemic ob/ob mice [51]. While a clear physiological role for this acute inhibition of FAS activity has not been determined, it is possible that the acute effect on FAS by insulin primarily affects FAS lipid signals that impact PPARα. Acute inhibition of FAS in response to insulin could then serve to halt the fasting response by PPARα and decrease fatty acid oxidation when nutrients are abundant. Because this acute change alters the specific activity of FAS, the effect is likely post-translationally mediated. In contrast, the long-term effect of insulin on FAS is transcriptionally mediated and promotes FAS expression, enabling increased storage of energy as fat.
Carbohydrates directly promote the expression of hepatic FAS in the liver in addition to having an indirect effect by stimulating insulin secretion. Feeding mice a high-glucose or high-fructose diet for one week leads to 3-fold and 8-fold, respectively, increases in FAS protein [52]. The effect of glucose on FAS expression is mediated by ChREBP [53–56]. Hepatic metabolism of glucose by glucokinase (GK) is necessary for the glucose-mediated induction of FAS by ChREBP [57]. The insulin-induced activation of SREBP-1c and the glucose-induced activation of ChREBP act synergistically to promote FAS expression [57]. A connection between lipid/carbohydrate sensing and metabolism is suggested by the finding that stearoyl-CoA desaturase (SCD1), an enzyme catalyzing the synthesis of oleate, is involved in the carbohydrate-induced induction of FAS and other lipogenic enzymes [52].
Dietary fats inhibit FAS expression to decrease de novo lipogenesis when fats are already abundant. Polyunsaturated fatty acids (PUFAs) may decrease FAS expression through inhibition of SREBP-1c [58] and ChREBP [59] activity. Diets consisting of 10% oil inhibit hepatic FAS activity when fed to rats of over the course of 4 weeks, with the greatest reduction in rats fed fish oil [60]. Re-feeding rats a carbohydrate-free, high-fat diet following fasting suppresses FAS gene expression to levels as low as those seen in rats fasted for 24 hours [45].
Transcription and the FAS promoter
Much of the work on transcriptional regulation of FAS has been done in rats, but the FAS promoter is highly conserved between species suggesting that studies of the rat FAS promoter are likely to be relevant to mice and humans. Regulatory elements and transcription factor binding sites in the proximal mouse FAS promoter are shown in Figure 2.
Figure 2. The mouse proximal FAS promoter.
Regulatory elements and nuclear factor binding site nucleotides are highlighted in yellow. IRE, insulin response element; LXRE, liver X receptor element; Nf-Y, nuclear factor Y binding site; Sp1, specificity factor 1 binding site; SRE, sterol regulatory element.
As noted above, SREBP-1c is activated by insulin and under appropriate conditions promotes expression of lipogenic genes, including FAS. The FAS promoter contains a sterol regulatory element (SRE) at −150 as well as tandem SREs at positions −72 and −62 that are required for optimal SREBP-1c-mediated activation of FAS expression in rats [61–63].
An inverted CCAAT box at −94 is a binding site for nuclear factor Y (NF-Y) and is necessary for inhibition of FAS expression by cyclic AMP [64, 65]. A binding site for the transcription factor specificity factor 1 (Sp1) is located nearby at −91 [63]. NF-Y and Sp1 proteins interact [66] and mediate sterol-induced FAS expression synergistically with SREBP-1c [63, 67]. Another transcription factor, X-box binding protein 1 (XBP1), increases FAS promoter activity indirectly via SREBP-1c [68].
Also as noted above, ChREBP plays a central role in the glucose-induced transcriptional regulation of FAS as well as other lipogenic and glycolytic genes in the liver [53–56]. Glucose promotes the nuclear translocation and activation of ChREBP, while polyunsaturated fatty acids and cyclic AMP inhibit ChREBP activity [59, 69]. ChREBP binds to a carbohydrate response element (ChRE) located at −7214 in the distal FAS promoter in rats to activate FAS transcription [70]. ChREBP appears to be the main regulator of glucose-induced FAS expression, as glucose fails to induce an increase in FAS expression in ChREBP-null hepatocytes [53]. Mice fed a high-fructose diet have similar amounts of nuclear ChREBP protein and ChRE-bound ChREBP protein compared to mice fed a high-glucose diet, suggesting that dietary fructose and glucose have comparable effects on ChREBP [71].
In addition to the ChRE, a direct repeat-1 (DR-1) element located between −7110 and −7090 in the distal promoter of rat FAS is necessary for full glucose activation of FAS expression [72]. Hepatic nuclear factor-4α (HNF-4α) binds to the DR-1 element and interacts with ChREBP. Ablation of HNF-4α produces a corresponding decrease in glucose-induced FAS expression [72].
Liver X receptor (LXR), a transcription factor activated by oxysterols, upregulates FAS expression through direct and indirect mechanisms. Indirectly, LXR can promote FAS expression by binding to liver X receptor elements (LXREs) in the promoters of the SREBP [73] and ChREBP [74] genes to promote their transcription. SREBP and ChREBP in turn activate FAS transcription. The LXR-mediated activation of SREBP-1c is the primary mechanism of insulin-induced SREBP activation [73]. The physiological relevance of LXR-mediated transcriptional regulation of ChREBP is debated, as LXR is not necessary for the glucose-induced activation of ChREBP [56]. LXR can also bind directly to LXREs located at positions −686 to −672 of the mouse FAS promoter to activate FAS transcription [75].
An insulin response element (IRE) containing an E-box DNA binding motif is located at positions −71 to −50 of the FAS promoter, overlapping two tandem SREs. The IRE is necessary for insulin-induced FAS expression [76]. USF1 and USF2 bind to the IRE [48]. Mutation of the E-box prevents USF binding and abolishes insulin-induced FAS expression. However, the importance of USFs in insulin-stimulated FAS expression remains unclear, because mutation of the E-box also prevented SREBP-1c binding [47].
Post-translational regulation of FAS
Transcriptional regulation of FAS may require hours to affect protein levels since both FAS mRNA and protein are fairly stable, buffering sudden changes due to increased transcription and subsequent translation.
There are several reports of FAS protein being activated or inhibited in far shorter time frames, as well as reports of changes in FAS activity that do not correlate with changes in FAS protein levels. Insulin acutely decreases FAS enzyme activity. In hepatoma cells, FAS activity decreases linearly from 2 to 15 minutes after insulin treatment, followed by a increase in FAS activity for 75 minutes [51]. Peroxynitrate inhibits FAS activity in adipocytes within 10 minutes, without any effect on FAS protein levels [77]. Activation and inhibition of FAS without corresponding changes in FAS protein levels have been reported in a variety of cancer cell lines [78–80]. These data suggest the presence of post-translational regulation of FAS.
Phosphorylation has been proposed as a mechanism of FAS regulation in cancer cells, adipocytes, and liver. In livers from pigeons that were fasted and then re-fed, radiolabeled phosphate was incorporated into FAS only in the cytosolic fraction. The phosphorylation event was associated with low FAS activity, and dephosphorylation of FAS by incubation with phosphatases caused a 20-fold increase in FAS activity [81]. Another inhibitory phosphorylation was demonstrated in 3T3L1 adipocytes, where FAS threonine phosphorylation was associated with inhibition of FAS activity [77]. This phosphorylation event was shown to require AMP-activated kinase (AMPK), likely through indirect effects since in vitro kinase assays failed to demonstrate any incorporation of labeled phosphate into FAS in the presence of AMPK [77]. These findings suggest the presence of an unidentified intermediate kinase step.
In human and mouse breast cancer cell lines, the finding that large differences in FAS activity between cell lines did not correlate with FAS protein levels prompted an exploration of FAS phosphorylation as an alternative mechanism of FAS regulation [79]. Phosphoserine and phosphothreonine residues were detected in FAS in cell lines from both species, while FAS phosphotyrosine residues were detected in human cells only. Phosphorylation of FAS in these cell lines was associated with greater FAS activity [79]. Recently, tyrosine phosphorylation of FAS was noted in two different human breast cancer cell lines. Both FAS tyrosine phosphorylation and FAS activity were induced by overexpression of human epidermal growth factor receptor 2 (HER2) and decreased by HER2 inhibition, and FAS was phosphorylated when complexed with HER2 [80].
In addition to phosphorylation, FAS was one of a large number of hepatic metabolic enzymes recently found to be lysine acetylated [82]. Acetylation was linked with diverse effects on metabolic enzymes, including protein destabilization, activation, and inhibition, suggesting that acetylation may play a major role in metabolic regulation. Acetylation of FAS could represent a novel mechanism for controlling its activity.
Known examples of post-translational regulation of FAS are summarized in Table 1.
Table 1.
Post-translational modifications of FAS.
| Type of post-translational modification | Organism and tissue or cell type | Function |
|---|---|---|
| Phosphorylation [81] | Pigeon liver | FAS inhibition |
| Threonine phosphorylation [77] | 3T3-L1 adipocytes (mouse) | FAS inhibition |
| Threonine and serine phosphorylation [79] | NMuMG (mouse mammary epithelial cells), T1 (mouse mammary tumor cells), SKBr3 (human breast carcinoma cells) | Unknown, possibly FAS activation |
| Tyrosine phosphorylation [79, 80] | SKBr3 (human breast carcinoma cells) | FAS activation |
| Acetylation [82] | Human liver | Unknown |
Conclusions and future directions
Hepatic FAS is generally thought to be a housekeeping protein, synthesizing fatty acids for the partitioning and storage of excess energy. However, the contribution of FAS to stored and secreted triglycerides is minor under most physiological conditions. Studies of mice deficient in hepatic FAS have demonstrated that FAS also serves as a signaling protein, controlling the activation of PPARα under nutrient-deficient conditions to promote the adaptive response to fasting.
FAS is regulated in part through effects on gene expression. However, rapid changes in enzyme activity associated with alterations in nutritional status suggest that post-translational mechanisms underlie enzymatic responses to external stimuli. An approach to understanding these dynamic effects might include identifying post-translational modifications of FAS, characterizing FAS subcellular localization, searching for FAS interacting proteins, and pursuing other mechanisms that enable immediate control of FAS activity.
The existence of separate physiological functions for FAS implies that it might be possible to develop function-specific therapies. Exclusively modulating the cellular FAS pool that promotes fatty acid oxidation or exclusively modulating the pool that promotes synthesis of lipids for storage could provide new treatment options for fatty liver and other serious obesity-related conditions.
Highlights.
Fatty acid synthase (FAS) synthesizes lipids for energy storage and membrane integrity
FAS also has signaling functions that include activation of PPARalpha
Both transcriptional as well as post-translational mechanisms regulate FAS
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wu M, Singh SB, Wang J, Chung CC, Salituro G, Karanam BV, Lee SH, Powles M, Ellsworth KP, Lassman ME, Miller C, Myers RW, Tota MR, Zhang BB, Li C. Antidiabetic and antisteatotic effects of the selective fatty acid synthase (FAS) inhibitor platensimycin in mouse models of diabetes. Proc Natl Acad Sci U S A. 2011;108:5378–5383. doi: 10.1073/pnas.1002588108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 3.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 5.Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81:7B–12B. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 6.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 7.Gastaldelli A, Kozakova M, Hojlund K, Flyvbjerg A, Favuzzi A, Mitrakou A, Balkau B. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology. 2009;49:1537–1544. doi: 10.1002/hep.22845. [DOI] [PubMed] [Google Scholar]
- 8.Dreyer C, Keller H, Mahfoudi A, Laudet V, Krey G, Wahli W. Positive regulation of the peroxisomal beta-oxidation pathway by fatty acids through activation of peroxisome proliferator-activated receptors (PPAR) Biol Cell. 1993;77:67–76. doi: 10.1016/s0248-4900(05)80176-5. [DOI] [PubMed] [Google Scholar]
- 9.Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. J Biol Chem. 1998;273:16710–16714. doi: 10.1074/jbc.273.27.16710. [DOI] [PubMed] [Google Scholar]
- 10.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) J Biol Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- 12.Stoops JK, Ross P, Arslanian MJ, Aune KC, Wakil SJ, Oliver RM. Physicochemical studies of the rat liver and adipose fatty acid synthetases. J Biol Chem. 1979;254:7418–7426. [PubMed] [Google Scholar]
- 13.Wakil SJ. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry. 1989;28:4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- 14.Chirala SS, Wakil SJ. Structure and function of animal fatty acid synthase. Lipids. 2004;39:1045–1053. doi: 10.1007/s11745-004-1329-9. [DOI] [PubMed] [Google Scholar]
- 15.Smith S. The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. FASEB J. 1994;8:1248–1259. [PubMed] [Google Scholar]
- 16.Stoops JK, Wakil SJ. Animal fatty acid synthetase. A novel arrangement of the beta-ketoacyl synthetase sites comprising domains of the two subunits. J Biol Chem. 1981;256:5128–5133. [PubMed] [Google Scholar]
- 17.Stoops JK, Wakil SJ, Uberbacher EC, Bunick GJ. Small-angle neutron-scattering and electron microscope studies of the chicken liver fatty acid synthase. J Biol Chem. 1987;262:10246–10251. [PubMed] [Google Scholar]
- 18.Maier T, Jenni S, Ban N. Architecture of mammalian fatty acid synthase at 4.5 A resolution. Science. 2006;311:1258–1262. doi: 10.1126/science.1123248. [DOI] [PubMed] [Google Scholar]
- 19.Maier T, Leibundgut M, Ban N. The crystal structure of a mammalian fatty acid synthase. Science. 2008;321:1315–1322. doi: 10.1126/science.1161269. [DOI] [PubMed] [Google Scholar]
- 20.Asturias FJ, Chadick JZ, Cheung IK, Stark H, Witkowski A, Joshi AK, Smith S. Structure and molecular organization of mammalian fatty acid synthase. Nat Struct Mol Biol. 2005;12:225–232. doi: 10.1038/nsmb899. [DOI] [PubMed] [Google Scholar]
- 21.Witkowski A, Ghosal A, Joshi AK, Witkowska HE, Asturias FJ, Smith S. Head-to-head coiled arrangement of the subunits of the animal fatty acid synthase. Chem Biol. 2004;11:1667–1676. doi: 10.1016/j.chembiol.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Witkowski A, Joshi AK, Smith S. Coupling of the de novo fatty acid biosynthesis and lipoylation pathways in mammalian mitochondria. J Biol Chem. 2007;282:14178–14185. doi: 10.1074/jbc.M701486200. [DOI] [PubMed] [Google Scholar]
- 23.Semenkovich CF, Coleman T, Fiedorek FT., Jr Human fatty acid synthase mRNA: tissue distribution, genetic mapping, and kinetics of decay after glucose deprivation. J Lipid Res. 1995;36:1507–1521. [PubMed] [Google Scholar]
- 24.Jayakumar A, Tai MH, Huang WY, al-Feel W, Hsu M, Abu-Elheiga L, Chirala SS, Wakil SJ. Human fatty acid synthase: properties and molecular cloning. Proc Natl Acad Sci U S A. 1995;92:8695–8699. doi: 10.1073/pnas.92.19.8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chirala SS, Chang H, Matzuk M, Abu-Elheiga L, Mao J, Mahon K, Finegold M, Wakil SJ. Fatty acid synthesis is essential in embryonic development: fatty acid synthase null mutants and most of the heterozygotes die in utero. Proc Natl Acad Sci U S A. 2003;100:6358–6363. doi: 10.1073/pnas.0931394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiegman CH, Bandsma RH, Ouwens M, van der Sluijs FH, Havinga R, Boer T, Reijngoud DJ, Romijn JA, Kuipers F. Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to the suppressive effects of insulin. Diabetes. 2003;52:1081–1089. doi: 10.2337/diabetes.52.5.1081. [DOI] [PubMed] [Google Scholar]
- 27.Chakravarthy MV, Lodhi IJ, Yin L, Malapaka RR, Xu HE, Turk J, Semenkovich CF. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell. 2009;138:476–488. doi: 10.1016/j.cell.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudgins LC, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest. 1996;97:2081–2091. doi: 10.1172/JCI118645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudgins LC, Hellerstein MK, Seidman CE, Neese RA, Tremaroli JD, Hirsch J. Relationship between carbohydrate-induced hypertriglyceridemia and fatty acid synthesis in lean and obese subjects. J Lipid Res. 2000;41:595–604. [PubMed] [Google Scholar]
- 30.Hudgins LC, Seidman CE, Diakun J, Hirsch J. Human fatty acid synthesis is reduced after the substitution of dietary starch for sugar. Am J Clin Nutr. 1998;67:631–639. doi: 10.1093/ajcn/67.4.631. [DOI] [PubMed] [Google Scholar]
- 31.Parks EJ, Krauss RM, Christiansen MP, Neese RA, Hellerstein MK. Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production, and clearance. J Clin Invest. 1999;104:1087–1096. doi: 10.1172/JCI6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delgado TC, Pinheiro D, Caldeira M, Castro MM, Geraldes CF, Lopez-Larrubia P, Cerdan S, Jones JG. Sources of hepatic triglyceride accumulation during high-fat feeding in the healthy rat. NMR Biomed. 2009;22:310–317. doi: 10.1002/nbm.1327. [DOI] [PubMed] [Google Scholar]
- 33.Iizuka K, Miller B, Uyeda K. Deficiency of carbohydrate-activated transcription factor ChREBP prevents obesity and improves plasma glucose control in leptin-deficient (ob/ob) mice. Am J Physiol Endocrinol Metab. 2006;291:E358–364. doi: 10.1152/ajpendo.00027.2006. [DOI] [PubMed] [Google Scholar]
- 34.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Sapiro JM, Mashek MT, Greenberg AS, Mashek DG. Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity. J Lipid Res. 2009;50:1621–1629. doi: 10.1194/jlr.M800614-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider JG, Yang Z, Chakravarthy MV, Lodhi IJ, Wei X, Turk J, Semenkovich CF. Macrophage fatty-acid synthase deficiency decreases diet-induced atherosclerosis. J Biol Chem. 2010;285:23398–23409. doi: 10.1074/jbc.M110.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakravarthy MV, Zhu Y, Lopez M, Yin L, Wozniak DF, Coleman T, Hu Z, Wolfgang M, Vidal-Puig A, Lane MD, Semenkovich CF. Brain fatty acid synthase activates PPARalpha to maintain energy homeostasis. J Clin Invest. 2007;117:2539–2552. doi: 10.1172/JCI31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dentin R, Benhamed F, Hainault I, Fauveau V, Foufelle F, Dyck JR, Girard J, Postic C. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 2006;55:2159–2170. doi: 10.2337/db06-0200. [DOI] [PubMed] [Google Scholar]
- 40.Lan H, Rabaglia ME, Stoehr JP, Nadler ST, Schueler KL, Zou F, Yandell BS, Attie AD. Gene expression profiles of nondiabetic and diabetic obese mice suggest a role of hepatic lipogenic capacity in diabetes susceptibility. Diabetes. 2003;52:688–700. doi: 10.2337/diabetes.52.3.688. [DOI] [PubMed] [Google Scholar]
- 41.Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, Lane MD, Kuhajda FP. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 42.Nepokroeff CM, Lakshmanan MR, Ness GC, Muesing RA, Kleinsek DA, Porter JW. Coordinate control of rat liver lipogenic enzymes by insulin. Arch Biochem Biophys. 1974;162:340–344. doi: 10.1016/0003-9861(74)90191-x. [DOI] [PubMed] [Google Scholar]
- 43.Clarke SD, Armstrong MK, Jump DB. Nutritional control of rat liver fatty acid synthase and S14 mRNA abundance. J Nutr. 1990;120:218–224. doi: 10.1093/jn/120.2.218. [DOI] [PubMed] [Google Scholar]
- 44.Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci U S A. 1998;95:5987–5992. doi: 10.1073/pnas.95.11.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez J, Palou A, Pico C. Response to carbohydrate and fat refeeding in the expression of genes involved in nutrient partitioning and metabolism: striking effects on fibroblast growth factor-21 induction. Endocrinology. 2009;150:5341–5350. doi: 10.1210/en.2009-0466. [DOI] [PubMed] [Google Scholar]
- 46.Foretz M, Guichard C, Ferre P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci U S A. 1999;96:12737–12742. doi: 10.1073/pnas.96.22.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang D, Sul HS. Upstream stimulatory factor binding to the E-box at -65 is required for insulin regulation of the fatty acid synthase promoter. J Biol Chem. 1997;272:26367–26374. doi: 10.1074/jbc.272.42.26367. [DOI] [PubMed] [Google Scholar]
- 48.Wang D, Sul HS. Upstream stimulatory factors bind to insulin response sequence of the fatty acid synthase promoter. USF1 is regulated. J Biol Chem. 1995;270:28716–28722. doi: 10.1074/jbc.270.48.28716. [DOI] [PubMed] [Google Scholar]
- 49.Lakshmanan MR, Nepokroeff CM, Porter JW. Control of the synthesis of fatty-acid synthetase in rat liver by insulin, glucagon, and adenosine 3′:5′ cyclic monophosphate. Proc Natl Acad Sci U S A. 1972;69:3516–3519. doi: 10.1073/pnas.69.12.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paulauskis JD, Sul HS. Hormonal regulation of mouse fatty acid synthase gene transcription in liver. J Biol Chem. 1989;264:574–577. [PubMed] [Google Scholar]
- 51.Najjar SM, Yang Y, Fernstrom MA, Lee SJ, Deangelis AM, Rjaily GA, Al-Share QY, Dai T, Miller TA, Ratnam S, Ruch RJ, Smith S, Lin SH, Beauchemin N, Oyarce AM. Insulin acutely decreases hepatic fatty acid synthase activity. Cell Metab. 2005;2:43–53. doi: 10.1016/j.cmet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim HJ, Ntambi JM. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem. 2004;279:25164–25171. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- 53.Ishii S, Iizuka K, Miller BC, Uyeda K. Carbohydrate response element binding protein directly promotes lipogenic enzyme gene transcription. Proc Natl Acad Sci U S A. 2004;101:15597–15602. doi: 10.1073/pnas.0405238101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamashita H, Takenoshita M, Sakurai M, Bruick RK, Henzel WJ, Shillinglaw W, Arnot D, Uyeda K. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci U S A. 2001;98:9116–9121. doi: 10.1073/pnas.161284298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma L, Robinson LN, Towle HC. ChREBP-Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem. 2006;281:28721–28730. doi: 10.1074/jbc.M601576200. [DOI] [PubMed] [Google Scholar]
- 56.Denechaud PD, Bossard P, Lobaccaro JM, Millatt L, Staels B, Girard J, Postic C. ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver. J Clin Invest. 2008;118:956–964. doi: 10.1172/JCI34314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dentin R, Pegorier JP, Benhamed F, Foufelle F, Ferre P, Fauveau V, Magnuson MA, Girard J, Postic C. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J Biol Chem. 2004;279:20314–20326. doi: 10.1074/jbc.M312475200. [DOI] [PubMed] [Google Scholar]
- 58.Moon YS, Latasa MJ, Griffin MJ, Sul HS. Suppression of fatty acid synthase promoter by polyunsaturated fatty acids. J Lipid Res. 2002;43:691–698. [PubMed] [Google Scholar]
- 59.Dentin R, Benhamed F, Pegorier JP, Foufelle F, Viollet B, Vaulont S, Girard J, Postic C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J Clin Invest. 2005;115:2843–2854. doi: 10.1172/JCI25256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim HK, Choi S, Choi H. Suppression of hepatic fatty acid synthase by feeding alpha-linolenic acid rich perilla oil lowers plasma triacylglycerol level in rats. J Nutr Biochem. 2004;15:485–492. doi: 10.1016/j.jnutbio.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 61.Magana MM, Osborne TF. Two tandem binding sites for sterol regulatory element binding proteins are required for sterol regulation of fatty-acid synthase promoter. J Biol Chem. 1996;271:32689–32694. doi: 10.1074/jbc.271.51.32689. [DOI] [PubMed] [Google Scholar]
- 62.Latasa MJ, Moon YS, Kim KH, Sul HS. Nutritional regulation of the fatty acid synthase promoter in vivo: sterol regulatory element binding protein functions through an upstream region containing a sterol regulatory element. Proc Natl Acad Sci U S A. 2000;97:10619–10624. doi: 10.1073/pnas.180306597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bennett MK, Lopez JM, Sanchez HB, Osborne TF. Sterol regulation of fatty acid synthase promoter. Coordinate feedback regulation of two major lipid pathways. J Biol Chem. 1995;270:25578–25583. doi: 10.1074/jbc.270.43.25578. [DOI] [PubMed] [Google Scholar]
- 64.Rangan VS, Oskouian B, Smith S. Identification of an inverted CCAAT box motif in the fatty-acid synthase gene as an essential element for modification of transcriptional regulation by cAMP. J Biol Chem. 1996;271:2307–2312. doi: 10.1074/jbc.271.4.2307. [DOI] [PubMed] [Google Scholar]
- 65.Roder K, Wolf SS, Beck KF, Sickinger S, Schweizer M. NF-Y binds to the inverted CCAAT box, an essential element for cAMP-dependent regulation of the rat fatty acid synthase (FAS) gene. Gene. 1997;184:21–26. doi: 10.1016/s0378-1119(96)00568-9. [DOI] [PubMed] [Google Scholar]
- 66.Roder K, Wolf SS, Larkin KJ, Schweizer M. Interaction between the two ubiquitously expressed transcription factors NF-Y and Sp1. Gene. 1999;234:61–69. doi: 10.1016/s0378-1119(99)00180-8. [DOI] [PubMed] [Google Scholar]
- 67.Xiong S, Chirala SS, Wakil SJ. Sterol regulation of human fatty acid synthase promoter I requires nuclear factor-Y- and Sp-1-binding sites. Proc Natl Acad Sci U S A. 2000;97:3948–3953. doi: 10.1073/pnas.040574197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ning J, Hong T, Ward A, Pi J, Liu Z, Liu HY, Cao W. Constitutive Role for IRE1{alpha}-XBP1 Signaling Pathway in the Insulin-Mediated Hepatic Lipogenic Program. Endocrinology. 2011 doi: 10.1210/en.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawaguchi T, Takenoshita M, Kabashima T, Uyeda K. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc Natl Acad Sci U S A. 2001;98:13710–13715. doi: 10.1073/pnas.231370798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rufo C, Teran-Garcia M, Nakamura MT, Koo SH, Towle HC, Clarke SD. Involvement of a unique carbohydrate-responsive factor in the glucose regulation of rat liver fatty-acid synthase gene transcription. J Biol Chem. 2001;276:21969–21975. doi: 10.1074/jbc.M100461200. [DOI] [PubMed] [Google Scholar]
- 71.Koo HY, Miyashita M, Cho BH, Nakamura MT. Replacing dietary glucose with fructose increases ChREBP activity and SREBP-1 protein in rat liver nucleus. Biochem Biophys Res Commun. 2009;390:285–289. doi: 10.1016/j.bbrc.2009.09.109. [DOI] [PubMed] [Google Scholar]
- 72.Adamson AW, Suchankova G, Rufo C, Nakamura MT, Teran-Garcia M, Clarke SD, Gettys TW. Hepatocyte nuclear factor-4alpha contributes to carbohydrate-induced transcriptional activation of hepatic fatty acid synthase. Biochem J. 2006;399:285–295. doi: 10.1042/BJ20060659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci U S A. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cha JY, Repa JJ. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem. 2007;282:743–751. doi: 10.1074/jbc.M605023200. [DOI] [PubMed] [Google Scholar]
- 75.Joseph SB, Laffitte BA, Patel PH, Watson MA, Matsukuma KE, Walczak R, Collins JL, Osborne TF, Tontonoz P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J Biol Chem. 2002;277:11019–11025. doi: 10.1074/jbc.M111041200. [DOI] [PubMed] [Google Scholar]
- 76.Moustaid N, Beyer RS, Sul HS. Identification of an insulin response element in the fatty acid synthase promoter. J Biol Chem. 1994;269:5629–5634. [PubMed] [Google Scholar]
- 77.An Z, Wang H, Song P, Zhang M, Geng X, Zou MH. Nicotine-induced activation of AMP-activated protein kinase inhibits fatty acid synthase in 3T3L1 adipocytes: a role for oxidant stress. J Biol Chem. 2007;282:26793–26801. doi: 10.1074/jbc.M703701200. [DOI] [PubMed] [Google Scholar]
- 78.Sabbisetti V, Di Napoli A, Seeley A, Amato AM, O’Regan E, Ghebremichael M, Loda M, Signoretti S. p63 promotes cell survival through fatty acid synthase. PLoS One. 2009;4:e5877. doi: 10.1371/journal.pone.0005877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hennigar RA, Pochet M, Hunt DA, Lukacher AE, Venema VJ, Seal E, Marrero MB. Characterization of fatty acid synthase in cell lines derived from experimental mammary tumors. Biochim Biophys Acta. 1998;1392:85–100. doi: 10.1016/s0005-2760(98)00023-x. [DOI] [PubMed] [Google Scholar]
- 80.Jin Q, Yuan LX, Boulbes D, Baek JM, Wang YN, Gomez-Cabello D, Hawke DH, Yeung SC, Lee MH, Hortobagyi GN, Hung MC, Esteva FJ. Fatty acid synthase phosphorylation: a novel therapeutic target in HER2-overexpressing breast cancer cells. Breast Cancer Res. 2010;12:R96. doi: 10.1186/bcr2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qureshi AA, Jenik RA, Kim M, Lornitzo FA, Porter JW. Separation of two active forms (holo-a and holo-b) of pigeon liver fatty acid synthetase and their interconversion by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 1975;66:344–351. doi: 10.1016/s0006-291x(75)80334-2. [DOI] [PubMed] [Google Scholar]
- 82.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]