Summary
The Toll-like receptor adaptor protein MyD88 is essential for the regulation of intestinal homeostasis in mammals. In this study, we determined that Myd88-deficient mice are susceptible to colonic damage that is induced by dextran sulfate sodium (DSS) administration due to uncontrolled dissemination of intestinal commensal bacteria. The DSS-induced mortality of Myd88-deficient mice was completely prevented by antibiotic treatment to deplete commensal bacteria. By using cell type-specific Myd88-deficient mice, we established that B cell-intrinsic MyD88 signaling plays a central role in the resistance to DSS-induced colonic damage via the production of IgM and complement-mediated control of intestinal bacteria. Our results indicate that the lack of intact MyD88 signaling in B cells, coupled with impaired epithelial integrity, enables commensal bacteria to function as highly pathogenic organisms, causing rapid host death.
Introduction
The gastrointestinal tract is the primary site of interactions between the host tissue and the microbiota. Gut microbial communities have a broad impact on many aspects of host physiology, including the immune system. Strong evidence supports the hypothesis that eukaryotic hosts and their symbionts have co-evolved toward mutualistic interactions, in which each partner benefits from the association (Backhed et al., 2005; Dethlefsen et al., 2007). Homeostasis is achieved through multiple host defense mechanisms that constantly manage commensal bacteria via sophisticated mechanisms that rely on the activation of the innate and adaptive immune systems (Hill and Artis, 2010; Hooper and Macpherson, 2010; Saleh and Trinchieri, 2011). The factors required for intestinal homeostasis include antibacterial peptides expressed by epithelial cells (Ganz, 2003), mucus produced by goblet cells (Johansson et al., 2011), IgA secreted by B cells (Hapfelmeier et al., 2010; Shang et al., 2008; Fagarasan and Honjo, 2004) and pro- and anti-inflammatory cytokines expressed by NK and T cells (Cella et al., 2009; Littman and Rudensky, 2010; Satoh-Takayama et al., 2008; Zenewicz et al., 2008). Experiments using a DSS-induction model of colonic damage showed that the activation of the adaptor protein MyD88 by IL-18 and TLRs is critical for the regulation of intestinal homeostasis (Abraham and Medzhitov, 2011; Abreu, 2010; Asquith and Powrie, 2010; Rakoff-Nahoum et al., 2004; Saleh and Trinchieri, 2011). Under steady-state conditions, the lack of TLR and IL-18 signaling in Myd88-deficient mice is compensated for by other effector mechanisms, such as the production of IgA (Slack et al., 2009), but the induction of epithelial cell injury by treatment with dextran sodium sulfate (DSS) leads to severe intestinal pathology and rapid mortality in these mice (Rakoff-Nahoum et al., 2004). Because MyD88 functions in multiple cell types downstream of numerous TLRs and cytokines of the IL-1 family (Adachi et al., 1998; Kawai et al., 1999), the cellular mechanisms underlying MyD88-dependent host protection are unclear. Whereas MyD88 signaling in epithelial cells regulates cell proliferation and the induction of antimicrobial peptides (Brandl et al., 2010; Brown et al., 2007; Cash et al., 2006; Ismail et al., 2011), the activation of the MyD88 signaling pathway in macrophages and dendritic cells (DCs) regulates cell maturation and the secretion of proinflammatory cytokines (Hou et al., 2011a; Iwasaki and Medzhitov, 2010; Kelsall, 2008). Furthermore, MyD88 is indispensible for the proper activation of both T and B cells (Ehlers et al., 2006; Gelman et al., 2004; Hou et al., 2011b; Meyer-Bahlburg et al., 2007). Because of the intrinsic complexity of MyD88 signaling in various types of immune cells, we investigated the cell type-specific requirement for MyD88 in the regulation of intestinal homeostasis. We established that DSS-induced colon injury results in the lethal dissemination of commensal bacteria in complete and B cell-specific Myd88-deficient mice. At the same time, MyD88 signaling in other cell types was largely dispensable for controlling intestinal bacteria dissemination following DSS-induced intestinal damage. We also identified IgM and complement as the key host protection factors that are regulated by B cell-intrinsic MyD88 signaling and identified IgA as an additional factor that is involved in controlling intestinal bacteria following intestinal damage.
Results
Intestinal commensal bacteria are the cause of death in mice lacking MyD88 following DSS-induced colonic damage
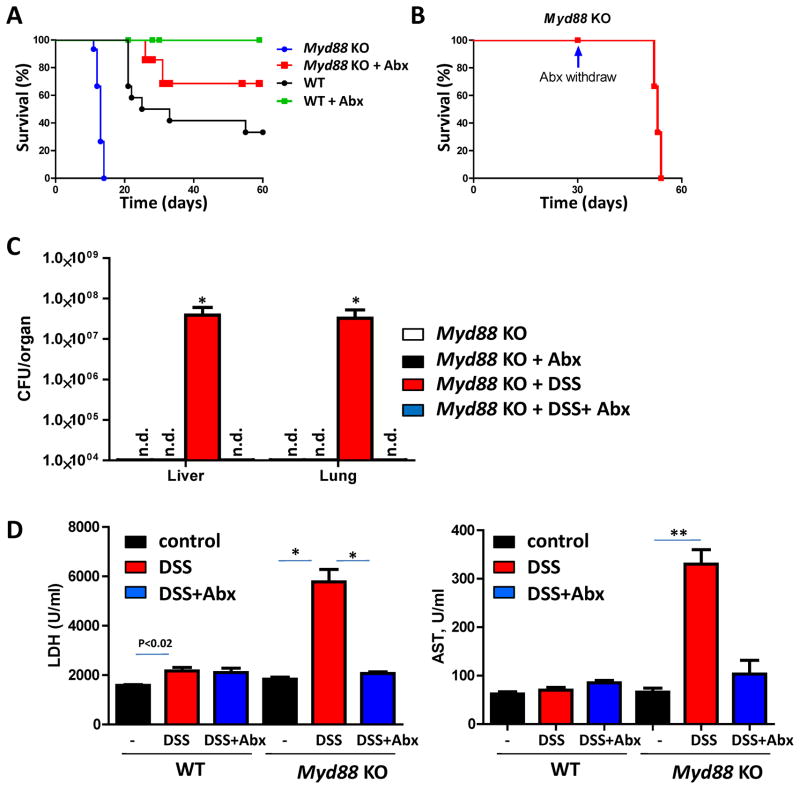
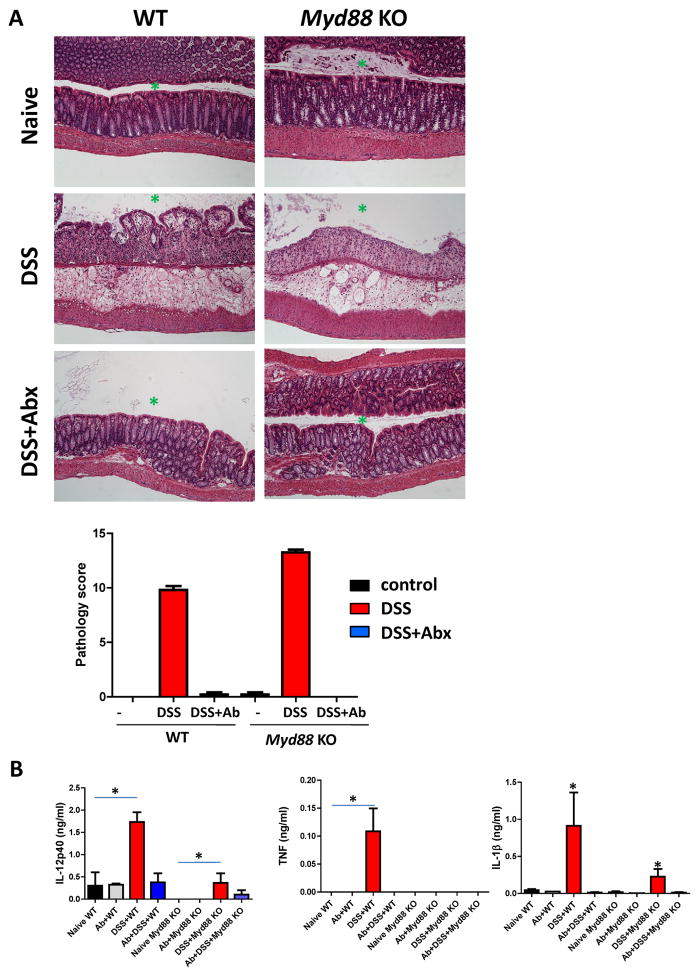
Treatment of Myd88−/− mice with DSS reveals that commensal bacteria provide TLR- and IL-18-dependent signals, which are required for epithelial cell proliferation and the production of tissue protective factors. These data suggest that the activation of TLR by commensal bacteria is essential for intestinal homeostasis (Rakoff-Nahoum et al., 2004). Surprisingly, we observed that the depletion of commensal bacteria by antibiotic treatment protected Myd88-deficient mice from DSS-induced mortality not only after a short, 7-day treatment with DSS (Supplemental Figure 1) but also when the mice were treated with DSS for the entire duration of the experiments (Figure 1A). To further test whether commensal bacteria are responsible for the mortality observed in DSS-treated Myd88−/− mice, the antibiotics were withdrawn after 30 days of treatment with DSS. We observed that the reconstitution of the microbiota in DSS-treated Myd88-deficient mice resulted in the rapid death of these animals (Figure 1B), which strongly indicates that commensal bacteria cause the mortality of the Myd88−/− mice in response to DSS treatment. This hypothesis was confirmed by the recovery of a large number of bacteria from all of the examined tissues of DSS-treated Myd88−/− mice (Figure 1C and data not shown) and the dramatically elevated serum levels of tissue damage markers in these animals (Figure 1D). These results suggest that despite the many beneficial effects of commensal bacteria on host physiology, the lack of MyD88 enables microbiota to function as pathogenic bacteria that cause mortality in these mice during DSS-induced colonic damage. We also observed that antibiotic treatment not only prevented the mortality of Myd88−/− mice but also dramatically reduced the inflammation and epithelial cell erosion caused by DSS treatment (Figure 2A). Furthermore, we noted that the impairment of epithelial cell integrity by DSS treatment provoked potent, commensal bacteria-driven MyD88-dependent cytokine responses, similar to those typically observed in response to pathogens (Figure 2B). Taken together, our results suggest that the lack of a crucial component of the TLR, IL-1, and IL-18 signaling cascade changes the beneficial host-bacteria relationship into a highly pathogenic relationship, which causes the mortality of Myd88−/− mice following DSS treatment.
Figure 1. Commensal bacteria are responsible for the mortality of Myd88-deficient mice.
(A) WT and Myd88−/− mice received 2% DSS alone or 2% DSS+Abx (ampicillin + vancomycin + neomycin sulfate + metronidazole) in their drinking water for the duration of the experiment. Mice were monitored for 60 days after the initiation of DSS treatment. The results shown are representative of nine experiments, each of which included at least four mice per group. (B) Myd88−/− mice received 2% DSS+Abx for 30 days and were subsequently switched to drinking water containing DSS only. The results shown are representative of three experiments, each of which included at least four mice per group. (C) Myd88−/− mice (four mice per group) were left untreated (open bars), treated with Abx (black bars), treated with DSS (red bars) or treated with DSS+Abx (blue bars) in their drinking water, as described in Figure 1A. The bacterial loads in the liver and lungs were analyzed on day 10 post-treatment by plating tissue lysates on blood agar. Similar numbers of bacteria were detected under aerobic (shown here) and anaerobic conditions (not shown). The results shown are representative of four experiments, each of which included at least four mice per group. (D) Serum levels of lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) were analyzed in WT and Myd88−/− mice on day 10 post-treatment. The results shown are representative of three experiments. * P< 0.01, ** P< 0.001
Figure 2. The presence of commensal bacteria is essential for colon damage and the induction of cytokines following DSS treatment.
(A) WT and Myd88−/− mice were left untreated (top panel), treated with 2% DSS (middle panel) or treated with 2% DSS+Abx (ampicillin + vancomycin + neomycin sulfate + metronidazole, bottom panel) in their drinking water. The histological changes in the colon were analyzed on day 10 post-treatment based on the following additive scoring system: crypt integrity, 0 = normal, 1 = irregular crypts, 2 = mild crypt loss; 3 = severe crypt loss, 4 = complete crypt loss with intact epithelial cell layer, 5 = complete loss of crypts and surface epithelium (<10 crypt width), and 6 = complete loss of crypts and surface epithelium (>10 crypt width); infiltration of inflammatory cells into the mucosa, 0 = normal, 1 = mild; 2 = modest, and 3 = severe; infiltration into the submucosa, 0 = normal, 1 = mild; 2 = modest, and 3 = severe; and infiltration into the muscle, 0 = normal, 1 = mild; 2 = modest, and 3 = severe. The scores for each parameter were added, resulting in a total scoring range of 0 to 17. Antibiotic treatment alone had no detectable effects on the architecture of WT or Myd88−/− colons (not shown). (B) IL-12p40, TNF, and IL-1β secretion from the colons isolated from DSS- or DSS+Abx-treated WT and Myd88−/− mice on day 10 after the initiation of treatment. The results shown are representative of three experiments, each of which included at least four mice per group. A green asterisk indicates the position of the gut lumen in the histological images. * P< 0.01.
Because both TLRs and IL-1 family members can activate MyD88 (Adachi et al., 1998; Kawai et al., 1999), we assessed the relative contribution of these activators to host protection from DSS-induced colonic damage. We evaluated mice lacking all of the major TLRs involved in bacterial recognition and established that the cooperation between TLR9 and caspase-1 plays a major role in protecting the host from commensal bacteria. Surprisingly, TLR2, TLR4, and TLR5 were dispensable for resistance to DSS-induced colonic damage (Supplemental Figure 2).
B cell-intrinsic MyD88 signaling plays a major role in host protection following DSS-induced colonic damage
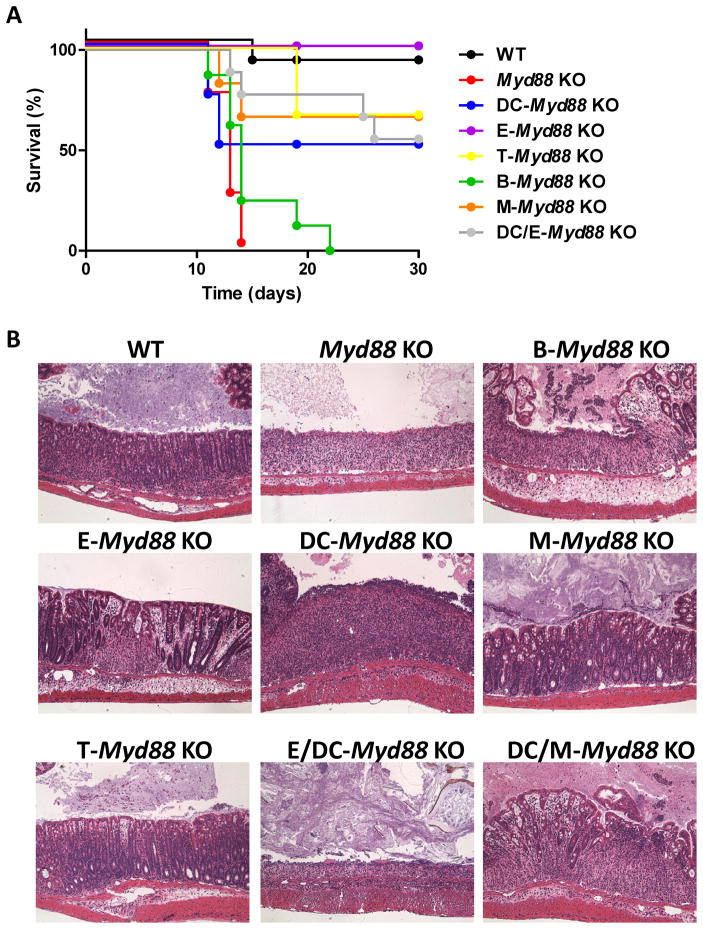
We addressed the question of how the cell-specific activation of MyD88 regulates host protection from DSS-induced intestinal damage by investigating a set of mice in which MyD88 is inactivated in a cell type-specific manner. It is well established that TLR and IL-18 signaling in epithelial cells play a major role in the innate control of microbiota via multiple mechanisms, including the induction of antimicrobial peptides and the regulation of cell proliferation (Abreu, 2010; Brown et al., 2007; Cash et al., 2006; Dupaul-Chicoine et al., 2010; Rakoff-Nahoum et al., 2004; Salcedo et al., 2010). Nevertheless, we observed that the deletion of the Myd88flox allele in epithelial cells, which was achieved by the expression of Cre recombinase under the Villin promoter, had no detectable effects on resistance to DSS-induced colitis (Figure 3A). Similarly, despite the requirement for TLR signaling in DCs for the regulation of innate and adaptive immunity (Iwasaki and Medzhitov, 2010; Kelsall, 2008; Niess et al., 2005), a DC-specific deletion of Myd88 only partially enhanced susceptibility to DSS treatment. Moreover, mice in which Myd88 was depleted in both DCs and epithelial cells were still relatively resistant to DSS treatment (Figure 3A). These results suggest that MyD88 signaling in cell types other than DCs and epithelial cells is required for host survival following DSS-induced colonic damage. In an additional attempt to identify cells involved in MyD88-dependent host protection against DSS-induced colonic injury, we generated mice lacking MyD88 in macrophage lineages (LysM-Cre × Myd88flox/flox, M-Myd88−/−) and in T (Lck-Cre × Myd88flox/flox, T-Myd88−/−) and B (CD19-Cre × Myd88flox/flox, B-Myd88−/−) cells. We observed that the selective inactivation of MyD88 in B cells rendered these mice highly susceptible to DSS-induced colitis (Figure 3A). In contrast, the cell-specific deletion of MyD88 in any other single cell type or a combined MyD88 deficiency in DCs and macrophages (DC/M-Myd88−/−) or in DCs and epithelial cells (DC/E-Myd88−/−) had limited effects on the survival of DSS-treated mice (Figure 3A). Histological analyses of the colon also revealed a major role for MyD88 signaling in B cells in the protection against DSS-induced colitis (Figure 3B). Whereas intact MyD88 in DCs was required to limit the severity of colitis, the lack of MyD88 signaling in macrophages and epithelial cells had no detectable effect on the resistance to DSS-induced tissue damage (Figure 3B). A crucial role for B cell-intrinsic MyD88 signaling in protecting the host from DSS-induced colonic damage was further supported by the observation that the adoptive transfer of WT B cells intoMyd88 −/− mice partially protected the recipients from DSS-induced mortality. In contrast, Myd88−/− B cells failed to protect the mice in the same experiment (Supplemental Figure 3A). Tlr9−/− B cells were also not capable of rescuing Myd88−/− mice from DSS-induced mortality, suggesting that MyD88 activation triggered by TLR9 is required for B cell-mediated host protection following DSS-induced colonic damage (Supplemental Figure 3A). An essential role for B cells in the resistance to DSS treatment was also observed in a mouse model in which the Jh gene has been deleted and mature B lymphocytes are therefore absent (Supplemental Figure 3B).
Figure 3. B cell-specific MyD88 signaling protects mice from DSS-induced colitis.
(A) WT mice (depicted in black), complete Myd88−/− mice (depicted in red) and mice with a cell type-specific deletion of MyD88 in epithelial cells (Villin-Cre ×Myd88 flox/flox, depicted in purple), dendritic cells (CD11c-Cre ×Myd88 flox/flox, depicted in blue), epithelial cells and DCs (Villin-Cre × CD11c-Cre ×Myd88 flox/flox, depicted in gray), monocytes and macrophages (LysM-Cre × Myd88flox/flox, depicted in orange), T cells (Lck-Cre ×Myd88 flox/flox, depicted in yellow) or B cells (CD19-Cre ×Myd88 flox/flox, depicted in green) were treated with 2% DSS in their drinking water and monitored for survival for 30 days. (B) The histological changes in the colon were analyzed on day 10 post-DSS treatment. The results are representative of six experiments, each of which included between four and six mice per group.
B cell-intrinsic MyD88 signaling restricts the dissemination of commensal bacteria during DSS-induced colon damage
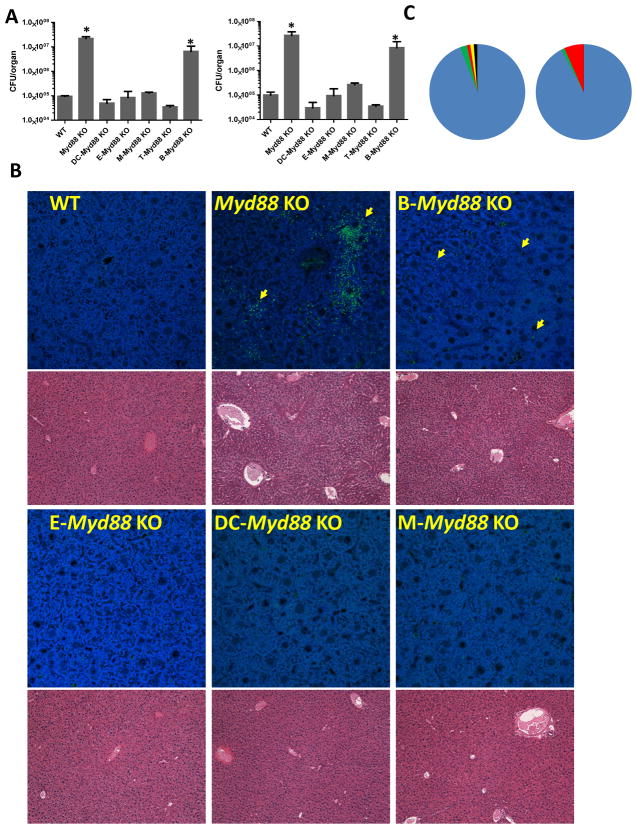
Similar to the result observed in complete Myd88-deficient mice, a large number of live bacteria were recovered under aerobic and anaerobic conditions from the liver, lung and other examined tissues of B cell-specific Myd88-deficient mice (Figure 4A and data not shown). On average, approximately 107 colonies were recovered from each liver or lung from mice lacking MyD88 in all cell types. High, but still reduced, bacterial loads were detected in the tissues of B cell-specific Myd88−/− mice. All of the other examined cell type-specific Myd88-knockout mice were statistically indistinguishable from the WT controls with respect to the bacterial loads detected in the tissues examined (Figure 4A and data not shown). These results strongly suggest that MyD88 signaling in B cells plays an integral role in preventing the dissemination of commensal bacteria following colonic damage. Because many commensal gut bacteria cannot be grown under standard culturing techniques (Garrett et al., 2010; Ley et al., 2008), we also applied a non-culture-based method for the visualization and identification of commensal bacteria. We visualized commensal bacteria by in situ hybridization to their 16S RNA sequences, confirming the presence of bacteria in the livers of DSS-treated Myd88−/− and B-Myd88−/− mice, but not in the other examined cell-specific Myd88-deficient animals (Figure 4B). Additionally, sequencing analysis identified Firmicutes as a dominant phylogenetic group in the livers of the DSS-treated Myd88−/− and B cell-Myd88−/− mice (Figure 4C). The reduced numbers of bacteria observed in B cell-specific Myd88-deficient mice compared with complete Myd88−/− mice suggest that B cell-intrinsic MyD88 plays a major role in controlling host resistance to DSS treatment, whereas MyD88 signaling in cells other than B cells is also involved in controlling intestinal bacteria and in preventing their dissemination into peripheral tissues. This model is also supported by the higher degree of liver damage observed in complete Myd88−/− mice compared to B cell-specific Myd88-deficient mice (Figure 4B). Nevertheless, it is important to note that commensal bacteria were not detected in the livers of mice lacking MyD88 in epithelial cells, DCs, macrophages or T cells, all of which have an intact MyD88 signaling pathway in B cells (Figure 4 and data not shown). Taken together, these results indicate that MyD88 signaling in B cells but not in epithelial cell or DC restricts the dissemination of commensal bacteria during DSS-induced colon damage.
Figure 4. B cell-intrinsic MyD88 signaling restricts the dissemination of commensal bacteria following DSS-induced colonic damage.
(A) Mice with a cell -specific deletion of MyD88 in epithelial cells, DCs, monocytes/macrophages, T cells and B cells and control animals were treated with 2% DSS in their drinking water. Aerobic (left panel) and anaerobic (right panel) bacteria were recovered from the livers of the mice on day 10 post-DSS treatment. The colonies were analyzed by plating the bacteria on blood agar. * P< 0.01(B) The dissemination of bacteria in the livers of WT mice and of mice lacking MyD88 in all tissues was analyzed compared to the cell type-specific Myd88-deficient mice on day 10 post-DSS treatment by in situ hybridization. Yellow arrows point to the detected bacteria. Histopathological analysis of livers from the same mice after H&E staining is shown below the in situ hybridization images. The results shown are representative of at least four experiments including three or four mice per group, and the images shown are representative of multiple sections examined. (C) Bacterial DNA was isolated from the livers of Myd88−/− (left) and B cell-Myd88−/− mice (right), and the relative frequencies of the major phylogenetic groups were analyzed by 16SRNA sequencing (blue: Firmicutes, green: Bacteroidetes, red: Proteobacteria, yellow: Deferribacteres, and black: Actinobacteria).
A role for IgM and IgA in the protection against DSS-induced colonic damage
One well-characterized function of TLR signaling is the regulation of antibody production in B cells (Ehlers et al., 2006; Hou et al., 2011b; Shang et al., 2008; Shlomchik, 2009). In the context of intestinal homeostasis, IgA is known to play a central role in restraining commensal bacteria (Baker et al., 2010; Fagarasan et al., 2010; Macpherson et al., 2008). Contrary to our expectations, we observed that a B cell-specific deletion of Myd88 had little effect on commensal bacteria-specific IgA production, as measured using a flow cytometric approach (Supplemental Figure 4). Furthermore, the analysis of total IgA levels by ELISA and immunohistochemical methods failed to reveal a B cell-intrinsic role for MyD88 in the regulation of IgA production. This contrasts with the substantially reduced titers of commensal bacteria-specific IgA that was observed in complete Myd88−/− mice (Supplemental Figure 4). Moreover, the flow cytometry-based quantification of IgA-positive cells in the lamina propria and Peyer’s patches of naïve and DSS-treated Myd88−/− mice strongly suggested that although MyD88 signaling regulates IgA production by B cells, intact MyD88 in cell types other than B cells is required for this process (Supplemental Figure 5).
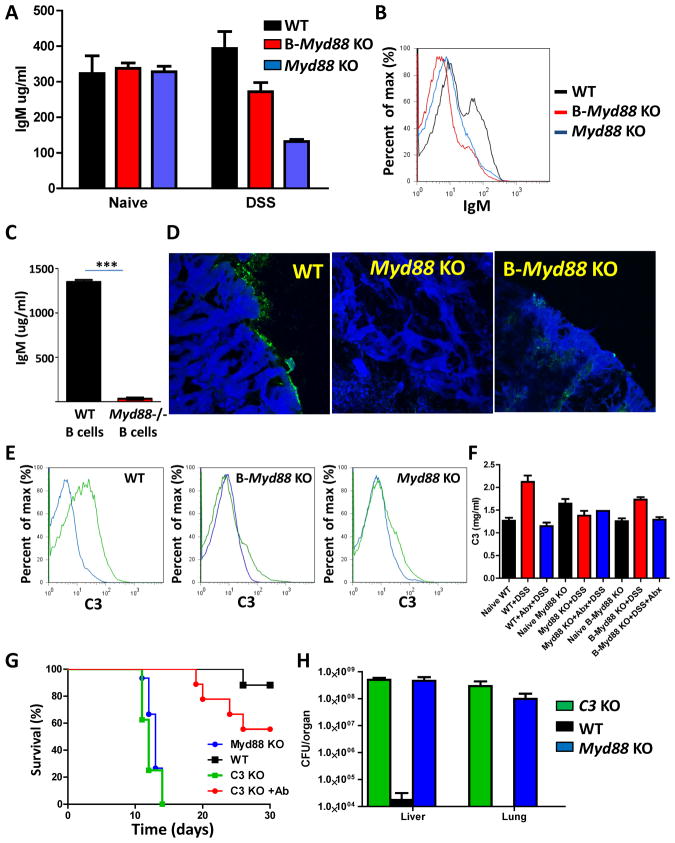
In search of a B cell-intrinsic role for MyD88 in the regulation of protection from intestinal bacteria, we analyzed IgM production in the colons of naïve and DSS-treated mice. We focused on this immunoglobulin isotype because, in addition to IgA, large quantities of IgM are present in mucosal tissues (Cerutti et al., 2011; Johansen et al., 2000). No differences in the levels of IgM were observed between WT and any of the examined MyD88-deficient mice under steady-state conditions (Figure 5A). In contrast, the lack of MyD88 resulted in decreased levels of IgM in the lumen of DSS-treated mice, and the B cell-restricted deletion of Myd88 partially recapitulated the IgM deficiency observed in the complete Myd88−/− mice (Figure 5A). Flow cytometry revealed that while a large fraction of intestinal bacteria were coated with IgM in WT mice, the loss of MyD88 specifically in B cells dramatically reduced IgM-coated commensal bacteria (Figure 5B). This result suggests that B cell-intrinsic MyD88 signaling is essential for the IgM-mediated control of commensal bacteria (Figure 5B).
Figure 5. B cell-intrinsic MyD88 signaling regulates complement-mediated host protection from commensal bacteria during colonic damage.
The IgM levels in the stools of naïve and DSS-treated WT, Myd88−/− and B cell-Myd88−/− mice were measured by (A) ELISA or (B) flow cytometry. The experiment shown is representative of three experiments, each of which included at least four mice per group. The black bars and histograms depict WT mice, and the red and blue bars and histograms depict B cell-specific and complete Myd88−/− mice, respectively. (C) IgM secretion by purified WT and Myd88−/− B cells stimulated with commensal bacterial extract (10 μg/ml) for 72 hours. IgM levels in the cell culture supernatant were measured by ELISA. The results shown are representative of three experiments. *** P< 0.0001. (D) Complement deposition in the colons of WT, Myd88−/− and B cell-Myd88−/− mice treated with DSS was visualized by anti-C3 staining. (E) The percentages of commensal bacteria covered with C3 (green curves) were analyzed by flow cytometry. Black curves represent isotype controls. The experiment shown is representative of three experiments, each of which included at least four mice per group. (F) WT, Myd88−/−, and B-Myd88−/− mice were left untreated (naïve), treated with 2% DSS, or treated with 2% DSS+Abx in their drinking water. Serum levels of C3 were analyzed on day 10 post-treatment. The results shown are representative of three experiments. (G) The survival and (H) the dissemination of commensal bacteria in the livers and lungs of WT, Myd88−/−, and C3−/− mice were analyzed on day 10 post-DSS or DSS+Abx treatment. The experiment shown is representative of four experiments, each of which included at least four mice per group.
Although we observed no difference in the frequencies of IgM-positive B cells in the lamina propria and Payer’s patches of naive and DSS-treated B-Myd88−/− mice (Supplemental Figures 5C and 5D), intact MyD88 was essential for IgM secretion in response to commensal bacteria (Figure 5C and Supplemental Figure 5E). A comparative analysis of IgA secretion under the same conditions revealed a partial requirement for MyD88 in the regulation of IgA secretion (Supplemental Figure 5F). Finally, we directly examined the susceptibility of IgM- and IgA-deficient mice to DSS treatment. We observed rapid mortality of IgM and IgA deficient mice in response to DSS treatment (Supplemental Figure 6), which closely resembled the susceptibility of B-Myd88−/− mice.
Taken together, our results suggest that B cell-intrinsic MyD88 signaling regulates IgM-mediated host protection. In addition, B cells provide IgA-mediated resistance to DSS treatment that depends in part on intact MyD88 signaling.
A role for complement in the protection against DSS-induced colonic damage
Antibodies mediate protection via multiple mechanisms, such as activating the classical complement system, facilitating the uptake of opsonized bacteria for rapid killing by macrophages and enhancing the production of proinflammatory cytokines (Cerutti et al., 2011). IgM is considered to be a poor opsonin, but it is a potent activator of the complement system (Carroll, 1998). We observed that in WT mice, epithelial cells and commensal bacteria were segregated by the deposition of complement factor C3, which was not observed in B cell-specific or complete Myd88-knockout mice (Figure 5D). Furthermore, a significant fraction of luminal bacteria were coated with factor C3 in WT mice but not in Myd88−/− or B-Myd88−/− mice (Figure 5E). Although DSS treatment resulted in the commensal bacteria- and MyD88-dependent upregulation of C3 production, a lack of MyD88 in only B cells had no effect on the induction of C3 in response to DSS treatment (Figure 5F). Thus, it is likely that B cell-intrinsic MyD88 is essential for C3 activation rather than C3 production. These data, combined with the observation that commensal bacteria-specific IgM is reduced in B-Myd88−/− mice, suggest that B cell-intrinsic MyD88 is essential for IgM-mediated deposition of complement factor C3 on commensal bacteria.
To investigate the relevance of this observation to host protection following DSS treatment, we investigated the survival of C3−/− mice during colonic damage. We found that C3-deficient mice succumbed to DSS treatment very rapidly, and these mice had essentially the same mortality kinetics as did Myd88−/− mice (Figure 5G). Furthermore, we observed that the lack of C3 resulted in the dissemination of commensal bacteria to the peripheral tissue at levels comparable to those observed in mice lacking the TLR adaptor protein (Figure 5H). Importantly, the antibiotic-mediated depletion of intestinal bacteria protected C3−/− mice from DSS-induced mortality (Figure 5G). These results revealed an essential role for the complement system in controlling intestinal homeostasis during colonic damage via the restriction of the dissemination of commensal bacteria.
Discussion
Intestinal commensal bacteria communicate continuously with host epithelial and innate and adaptive immune cells under both steady-state and stressful conditions (Hooper and Macpherson, 2010; Lee and Mazmanian, 2010; Ley et al., 2008). These interactions have abundant beneficial effects on host metabolism and immune function. However, these interactions require host factors for the maintenance of immune homeostasis. The oral administration of DSS, which is directly toxic to enterocytes, has been a crucial tool for the identification of the cell signaling pathways involved in the regulation of host-commensal bacteria interactions (Saleh and Trinchieri, 2011). Among these factors, the TLR and IL-1 family receptor signaling adaptor protein MyD88 plays a central role in resistance to experimental DSS-induced colitis. Myd88−/− mice, which cannot signal through IL-1 family receptors or most TLRs, are acutely susceptible to DSS-induced colonic injury (Rakoff-Nahoum et al., 2004). It appears that both TLR-dependent and IL-18-dependent activation of MyD88 are essential for host resistance to DSS-induced colonic damage (Dupaul-Chicoine et al., 2010; Rakoff-Nahoum et al., 2004; Salcedo et al., 2010; Saleh and Trinchieri, 2011; Zaki et al., 2010).
Our results in mice simultaneously lacking multiple TLRs that are involved in bacterial recognition suggested that TLR9- and caspase-1-dependent activation of MyD88 plays a major role in host resistance to DSS-induced colonic damage. We also determined that MyD88 signaling in B cells is required to control commensal bacteria following intestinal damage. Our results highlighted the physiological importance of TLR signaling in B cells for IgM- and complement-mediated host protection following DSS-induced colonic damage.
It is well known that the lack of MyD88 in all cell types results in rapid mortality following DSS treatment in mice (Abraham and Medzhitov, 2011; Saleh and Elson, 2011). Based on the results of previous studies, this outcome can be attributed to the deregulated epithelial cell homeostasis that is a result of the impaired recognition of commensal bacteria by the cells. The intestinal epithelium provides not only a physical barrier against the excessive entry of luminal microbiota but also executes the MyD88-dependent microbe sensing programs that are required for the restriction of intestinal bacteria via the production of antimicrobial peptides and epithelial cell proliferation (Hooper and Macpherson, 2010). Surprisingly, the lack of MyD88 in epithelial cells had no effect on the survival of mice following DSS treatment. Furthermore, colonic damage was comparable between epithelial cell-specific Myd88-deficient mice (Villin-Cre × Myd88flox/flox) and their WT counterparts (Myd88flox/flox mice without Cre expression or Villin-Cre × Myd88flox/wt mice).
We observed that the uncontrolled dissemination of commensal bacteria was responsible for the mortality of Myd88−/− mice during prolonged DSS-induced colonic damage. The depletion of commensal bacteria by antibiotic treatment during DSS-induced colonic damage completely rescued Myd88−/− mice from the mortality that typically occurs in DSS-treated mice. Furthermore, reconstitution of the intestinal microbiota in DSS-treated Myd88−/− mice reverted their resistance and resulted in the mortality that is associated with the dissemination of commensal bacteria throughout the peripheral tissues. These results formally demonstrated that MyD88 is indispensible in restricting intestinal bacteria from invasion and dissemination when the integrity of the epithelial cell layer is compromised. In our attempts to identify the cell-specific requirements for MyD88 signaling, we observed that whereas TLR signaling in DCs and macrophages contributed to host protection, B cell-intrinsic MyD88 played the most critical role in orchestrating host protection following DSS-induced colonic injury. Although MyD88 activation has multiple cell-specific downstream effects, our results suggested that B cell-intrinsic MyD88 signaling is required to coordinate the IgM- and complement-mediated control of intestinal bacteria following DSS-induced colonic damage.
Because the fraction of intestinal bacteria that was coated with IgM and complement C3 fragments was substantially decreased by the deletion of MyD88 in B cells, it is likely that the key protective function of MyD88 in B cells is to promote the production of IgM antibodies that subsequently bind to commensal organisms. This function is essential for preventing bacteria from gaining access to the subepithelial tissue following DSS-induced breaches of the epithelial barrier.
Our work also revealed an important role for IgA in conferring protection against DSS-induced colitis. However, IgA responses were only partially regulated by MyD88, and it is likely that TLR, IL-1R, and IL-18R signaling in cell types other than B cells is involved in the regulation of commensal bacteria-specific IgA responses. In addition, because mutations in the antigen-binding variable region of IgA are crucial for the control of commensal bacteria, further studies will be needed to investigate whether B cell intrinsic or extrinsic MyD88 signaling is involved in somatic hypermutation (Wei et al., 2011). Our experiments cannot rule out the possibility that the lack of B cell-intrinsic MyD88 reduces protection against colitis by impairing the induction of somatic hypermutations.
While the mechanism we identified does not exclude additional functions for TLR signaling in B cells and other innate and adaptive immune cells, our work does emphasize the importance of B cell-intrinsic MyD88 in the regulation of intestinal homeostasis via the restriction of commensal bacteria from dissemination into the peripheral tissues. Our results suggest that impaired MyD88-dependent recognition of commensal bacteria causes normal microbiota to function as pathogenic microorganisms and induces mortality in the mammalian host when the barrier function of the epithelial layer is compromised.
Experimental Procedures
Animals
C57BL/6 mice were obtained from the University of Texas Southwestern (UTSW) Medical Center Mouse Breeding Core Facility. Tlr2−/−, Tlr4−/−, Tlr9−/−, Myd88−/− and Caspase-1−/− mice were generously provided by Drs. S. Akira and R. Flavell. Tlr5−/− and Ighm−/− (Ighmtm1Cgn) mice were obtained from Jackson Laboratory. Igha−/− and Jh−/− mice were a gift from Dr. L. Hooper. Myd88floxmice (B6.129P2-Myd88 tm1Defr) and Myd88flox/flox × CD11c-Cre (DC-Myd88−/−) mice have been described previously (Hou et al., 2008; Hou et al., 2011a). In this study, the Myd88flox mice were additionally crossed to Villin-Cre (Madison et al., 2002), CD19-Cre (Rickert et al., 1997), LysM-Cre (Clausen et al., 1999), CD11c-Cre × Villin-Cre, LysM-Cre × CD11c-Cre and Lck-Cre deleter mice, which were obtained from Jackson Laboratory. Control and experimental mice were age-matched within individual experiments.
All mice were maintained at the American Association of Laboratory Animal Care-accredited animal facility at the UTSW Medical Center. All of the animals that were used were age- and sex-matched and were maintained in the same animal room. All experiments were performed using protocols approved by the Institutional Animal Care and Use Committees of the UT Southwestern Medical Center.
DSS and antibiotic treatment
Mice were treated with 2%(w/v) DSS (M.W. = 36,000–50,000 Da; MP Biomedicals) in their drinking water for the duration of the experiments. In the experiments shown in Supplemental Figure 1, the mice were treated with DSS for 7 days and subsequently switched to regular drinking water. To deplete the gut commensal microflora, WT, Myd88−/−or cell type-specific Myd88-deficientmice were treated with ampicillin (1 g/L), vancomycin (500 mg/L), neomycin sulfate (1 g/L), and metronidazole (1 g/L) along with 2% DSS in their drinking water. The efficiency of commensal bacteria depletion was verified by bacteriological analysis of the feces, which was performed by cultivating aerobic and anaerobic bacteria on blood agar. Control animals were treated with regular water or antibiotics alone without DSS. No mortality of Myd88−/−, WT or any cell type-specific Myd88-deficient mice was observed under the control conditions. As indicated in Supplemental Figure 3, in some experiments, DSS-treated Myd88−/− mice also received 2×106 purified WT B cells that had been isolated from the spleens of naïve WT, Myd88−/−, or Tlr9−/− mice using the Miltenyi B Cell Isolation Kit.
Histopathology and in situ hybridization
Mice were left untreated or were treated with 2% (w/v) DSS in their drinking water for ten days and subsequently necropsied. Portions of the colon and liver were fixed in Bouin’s fixative, embedded in paraffin, cut into 5-μm sections and stained with hematoxylin and eosin (H&E). For in situ hybridization, the livers were fixed in Carnoy’s fixative solution instead of Bouin’s fixative. After deparaffinization and rehydration in hybridization buffer (0.9 M NaCl, 0.1% SDS and 20 mM Tris-HCl, pH 7.4), the colon and liver sections were incubated overnight at 50°C in the dark with an Alexa-488-conjugated EUB338 general bacterial probe for a bacterial 16S rRNA gene (5′-GCT GCC TCC CGT AGG AGT-3′), which was diluted to a final concentration of 1 ng/μl in hybridization solution. The sections were then washed three times with hybridization solution for 15 minutes, counterstained with SYTO62, and mounted using ProLong Gold Antifade reagent (Invitrogen). The sections were imaged using a Leica SPE system fitted with a Leica 63×objective NA 1.4. The datasets were processed using Leica Advanced Fluorescence software (Leica).
Histological changes were analyzed in a double-blind fashion. The severity of intestinal pathology was analyzed based on the following additive scoring system: crypt integrity, 0 = normal, 1 = irregular crypts, 2 = mild crypt loss; 3 = severe crypt loss, 4 = complete crypt loss with an intact epithelial cell layer, 5 = complete loss of crypts and surface epithelium (<10 crypt width), and 6 = complete loss of crypts and surface epithelium (>10 crypts); infiltration of inflammatory cells into the mucosa, 0 = normal, 1 = mild; 2 = modest, and 3 = severe; infiltration of the submucosa, 0 = normal, 1 = mild; 2 = modest, and 3 = severe; and infiltration of the muscle, 0 = normal, 1 = mild; 2 = modest, and 3 = severe. Theses cores were added, resulting in a total scoring range of 0 to 17.
Bacterial cultures
To detect anaerobic and aerobic bacterial colonies in the liver and lung tissues during DSS treatment, these organs were removed on day 10 post-DSS treatment, placed into 15-ml conical tubes with PBS and homogenized. Aerobic bacteria were grown on LB agar or blood agar. Anaerobic bacteria were grown on blood agar using an anaerobic vented jar (BD Biosciences). Culturable bacteria counts were obtained by plating serial dilutions of bacteria on the corresponding media for 48 hr (aerobes)or 72 hr (anaerobes) (Benson et al., 2009). In some cases, bacterial genomic DNA was extracted from the livers of DSS-treated Myd88−/− and B cell -Myd88−/− mice on day 10 post -treatment using the Qiagen DNeasy kit according to the manufacturer’s instructions. Broad-range 16S rDNA PCR (forward primer: 5′-AGAGTTTGATYMTGGCTCAG-3′ nd reverse primer: 5′-ACGGYTACCTTGTTACGACTT-3′) was used to amplify and clone the bacterial DNA present in individual samples without any prior cultivation. To minimize contamination, all extractions and PCR amplifications were prepared using sterile solutions and filter tips. Cloned inserts were identified through sequencing of the 16S rDNA gene fragments using the pCR2.1 vector-specific M13 reverse and T7 promoter primers. The DNA sequences were interpreted by comparing the retrieved sequences with those stored in the GenBank Data System using the basic local alignment software tool (BLAST; National Center for Biotechnology Information) and the Seqmatch tool (Ribosomal Database Project).
Flow cytometry and ELISA
Fecal pellets were disrupted in PBS by vortexing for 5 minutes, and insoluble material was removed by filtering through a 100-μm filter (Millipore). The bacteria were washed twice in PBS/5 mM EDTA/2% FBS before resuspension in Alexa 488-conjugated anti-IgA or anti-IgM monoclonal antibodies (BD Pharmingen). Commensal bacteria-specific IgA and IgM were assessed by staining luminal bacteria for 30 minutes on ice. The data were acquired using a FACSCalibur cytometer (BD Pharmingen) and were analyzed using FlowJo software (TreeStar, USA). The total IgA and IgM levels in the fecal extracts were assessed by ELISA (Bethyl Laboratories) according to the manufacturer’s instructions.
To assay cytokine responses in mice treated with DSS, colons were harvested from the mice on day 7 or 10 post-treatment, extensively washed with PBS, cut longitudinally and incubated overnight in cell culture media (RPMI+10% FBS). The IL-12p40, TNF, IL-1β, and IL-6 concentrations in the supernatants were determined using ELISA kits from eBioscience according to the manufacturer’s instructions.
Statistical analysis
All data were analyzed with Prism (Version 5; GraphPad). The data were considered statistically significant when P values were less than 0.05 using a two-tailed t-test.
Supplementary Material
Highlights.
Colonic injury results in the lethal dissemination of commensal bacteria in Myd88−/− mice
B cell-intrinsic MyD88 protects the host from intestinal commensal bacteria
B cell-MyD88 signaling regulates IgM- and complement-dependent host protection
Acknowledgments
This research was supported by NIH R01 AI085263 to F.Y. and P01AI078869 to A.L.D. We thank D. Rakheja and J. Shelton for their help with the histology and pathology scoring.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham C, Medzhitov R. Interactions Between the Host Innate Immune System and Microbes in Inflammatory Bowel Disease. Gastroenterology. 2011;140:1729–1737. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–143. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Asquith M, Powrie F. An innately dangerous balancing act: intestinal homeostasis, inflammation, and colitis-associated cancer. Journal of Experimental Medicine. 2010;207:1573–1577. doi: 10.1084/jem.20101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Baker K, Lencer WI, Blumberg RS. Beyond IgA: the mucosal immunoglobulin alphabet. Mucosal Immunology. 2010;3:324–325. [Google Scholar]
- Benson A, Pifer R, Behrendt CL, Hooper LV, Yarovinsky F. Gut Commensal Bacteria Direct a Protective Immune Response against Toxoplasma gondii. Cell Host Microbe. 2009;6:187–196. doi: 10.1016/j.chom.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl K, Sun L, Neppl C, Siggs OM, Le Gall SM, Tomisato W, Li XH, Du X, Maennel DN, Blobel CP, et al. MyD88 signaling in nonhematopoietic cells protects mice against induced colitis by regulating specific EGF receptor ligands. Proc Natl Acad Sci U S A. 2010;107:19967–19972. doi: 10.1073/pnas.1014669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, Stappenbeck TS. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. Journal of Clinical Investigation. 2007;117:258–269. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MC. The role of complement and complement receptors in induction and regulation of immunity. Annual Review of Immunology. 1998;16:545–568. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JKM, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A, Chen K, Chorny A. Immunoglobulin Responses at the Mucosal Interface. Annual Review of Immunology. 2011;29:273–293. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Research. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KSB, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N, et al. Control of Intestinal Homeostasis, Colitis, and Colitis-Associated Colorectal Cancer by the Inflammatory Caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. Journal of Experimental Medicine. 2006;203:553–561. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagarasan S, Honjo T. Regulation of IgA synthesis at mucosal surfaces. Current Opinion in Immunology. 2004;16:277–283. doi: 10.1016/j.coi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive Immune Regulation in the Gut: T Cell-Dependent and T Cell-Independent IgA Synthesis. Annual Review of Immunology. 2010;28:243–273. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- Ganz T. Defensins: Antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Garrett WS, Gordon JI, Glimcher LH. Homeostasis and Inflammation in the Intestine. Cell. 2010;140:859–870. doi: 10.1016/j.cell.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman AE, Zhang JD, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4(+) T cell survival. Journal of Immunology. 2004;172:6065–6073. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapfelmeier S, Lawson MAE, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, et al. Reversible Microbial Colonization of Germ-Free Mice Reveals the Dynamics of IgA Immune Responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DA, Artis D. Intestinal Bacteria and the Regulation of Immune Cell Homeostasis. Annual Review of Immunology. 2010;28:623–667. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29:272–282. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou BD, Benson A, Kuzmich L, DeFranco AL, Yarovinsky F. Critical coordination of innate immune defense against Toxoplasma gondii by dendritic cells responding via their Toll-like receptors. Proc Natl Acad Sci U S A. 2011a;108:278–283. doi: 10.1073/pnas.1011549108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou BD, Saudan P, Ott G, Wheeler ML, Ji M, Kuzmich L, Lee LM, Coffman RL, Bachmann MF, DeFranco AL. Selective Utilization of Toll-like Receptor and MyD88 Signaling in B Cells for Enhancement of the Antiviral Germinal Center Response. Immunity. 2011b;34:375–384. doi: 10.1016/j.immuni.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AS, Severson KM, Vaishnava S, Behrendt CL, Yu XF, Benjamin JL, Ruhn KA, Hou BD, DeFranco AL, Yarovinsky F, et al. gamma delta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci U S A. 2011;108:8743–8748. doi: 10.1073/pnas.1019574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of Adaptive Immunity by the Innate Immune System. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen FE, Braathen R, Brandtzaeg P. Role of J chain in secretory immunoglobulin formation. Scandinavian Journal of Immunology. 2000;52:240–248. doi: 10.1046/j.1365-3083.2000.00790.x. [DOI] [PubMed] [Google Scholar]
- Johansson MEV, Larsson JMH, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U S A. 2011;108:4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Kelsall B. Recent progress in understanding the phenotype and function of intestinal dendritic cells and macrophages. Mucosal Immunology. 2008;1:460–469. doi: 10.1038/mi.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Mazmanian SK. Has the Microbiota Played a Critical Role in the Evolution of the Adaptive Immune System? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nature Reviews Microbiology. 2008;6:776–788. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman DR, Rudensky AY. Th17 and Regulatory T Cells in Mediating and Restraining Inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunology. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- Madison BB, Dunbar L, Qiao XTT, Braunstein K, Braunstein E, Gumucio DL. cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. Journal of Biological Chemistry. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg A, Khim S, Rawlings DJ. B cell-intrinsic TLR signals amplify but are not required for humoral immunity. Journal of Experimental Medicine. 2007;204:3095–3101. doi: 10.1084/jem.20071250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niess JH, Brand S, Gu XB, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. CX(3)CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Research. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, Wang EN, Ma W, Haines D, O’HUigin C, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. Journal of Experimental Medicine. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M, Elson CO. Experimental Inflammatory Bowel Disease: Insights into the Host-Microbiota Dialog. Immunity. 2011;34:293–302. doi: 10.1016/j.immuni.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol. 2011;11:9–20. doi: 10.1038/nri2891. [DOI] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CAJ, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial Flora Drives Interleukin 22 Production in Intestinal NKp46(+) Cells that Provide Innate Mucosal Immune Defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Shang LM, Fukata M, Thirunarayanan N, Martin AP, Arnaboldi P, Maussang D, Berin C, Unkeless JC, Mayer L, Abreu MT, et al. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135:529–538. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik MJ. Activating systemic autoimmunity: B’s, T’s, and tolls. Current Opinion in Immunology. 2009;21:626–633. doi: 10.1016/j.coi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MAE, Geuking MB, Beutler B, Tedder TF, Hardt WD, et al. Innate and Adaptive Immunity Cooperate Flexibly to Maintain Host-Microbiota Mutualism. Science. 2009;325:617–620. doi: 10.1126/science.1172747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Shinkura R, Doi Y, Maruya M, Fagarasan S, Honjo T. Mice carrying a knock-in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense. Nat Immunol. 2011;12:264–U102. doi: 10.1038/ni.1991. [DOI] [PubMed] [Google Scholar]
- Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 Inflammasome Protects against Loss of Epithelial Integrity and Mortality during Experimental Colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and Adaptive Interleukin-22 Protects Mice from Inflammatory Bowel Disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.