Abstract
Our previous studies showed that the hypotensive effect of chronic ethanol in female rats is reduced by ovariectomy (OVX) rats and was restored after estrogen replacement (OVXE2). Further, in randomly cycling rats, chronic ethanol increased cardiac parasympathetic dominance and subsequently reduced myocardial contractility and blood pressure (BP). In this study, we tested the hypothesis that alterations in myocardial contractility and sympathovagal control account for the E2 exacerbation of the hemodynamic effects of ethanol. BP, myocardial contractility (+dP/dtmax), and spectral cardiovascular autonomic profiles were evaluated in radiotelemetered OVX, and OVXE2 rats receiving liquid diet with or without ethanol (5%, w/v) for 13 weeks. In OVX rats, ethanol caused modest hypotension along with significant increases in +dP/dtmax during weeks 2–5. The high-frequency (IBIHF, 0.75–3 Hz) and low-frequency (IBILF, 0.25–0.75 Hz) bands of interbeat intervals were briefly increased and decreased, respectively, by ethanol. Compared with its effects in OVX rats, chronic treatment of OVXE2 rats with ethanol elicited significantly greater and more sustained reductions in systolic (SBP) and diastolic (DBP) blood pressures and +dP/dtmax. Altered sympathovagal balance and parasympathetic overactivity were more evident in ethanol-treated OVXE2 rats as suggested by the sustained: (i) increases in high-frequency bands of interbeat intervals (IBIHF, 0.75–3 Hz), and (ii) decreases in low-frequency IBI bands (IBILF, 0.25–0.75 Hz), IBILF/HF ratio and +dP/dtmax. The plasma ethanol concentration was not affected by changes in the hormonal milieu. These findings suggest that estrogen exacerbates the ethanol-evoked reductions in myocardial contractility and BP and the associated parasympathetic overactivity in female rats.
Keywords: Ethanol, estrogen, blood pressure, myocardial contractility, hemodynamic variability, female rats
1. Introduction
Ethanol effect on BP follows a J-shaped relationship depending on the duration and amount of ethanol consumed (Klatsky et al., 1977). We have shown that acute ethanol administration reduces cardiac output and subsequently BP in female rats (El-Mas and Abdel-Rahman, 1999a, 1999b). These effects of ethanol are largely dependent on estrogen because they were reduced by OVX and restored after estrogen replacement (El-Mas and Abdel-Rahman, 1999a). Epidemiologically, ethanol lowers BP in young but not in older women (Klatsky, 1990), implying a role for ovarian hormones in the ethanol-evoked hypotension. Because ethanol increases plasma endotoxin (Rivera et al., 1998), which enhances the inducible nitric oxide synthase (iNOS) expression and causes myocardial depression and hypotension (Rudiger and Singer, 2007; Umar and van der Laarse, 2010), we tested in more recent studies the hypothesis that endotoxemia-induced upregulation of cardiac NOS mediates the acute hemodynamic actions of ethanol in female rats (El-Mas et al., 2008, 2009). Interestingly, the hypotensive effect of ethanol was associated with significant increases in plasma endotoxin and nitrite/nitrate levels as well as in myocardial iNOS and eNOS contents (El-Mas et al., 2008, 2009). Further, pharmacological inhibition of endogenous endotoxin, eNOS, or iNOS abrogated these effects, thus implicating endotoxin/myocardial NOS signaling in the acute hemodynamic effects of ethanol in female rats (El-Mas et al., 2008, 2009).
Similar to its acute effects, chronic ethanol administration also elicits estrogen-dependent hypotension in female rats (El-Mas and Abdel-Rahman, 2000, 2001). Endotoxemia and enhanced NOS signaling are also causally related to the chronic hypotensive effect of ethanol (El-Mas et al., 2011). In this latter study, spectral analyses presented evidence that ethanol caused remarkable shifts in the cardiac sympathovagal balance towards parasympathetic dominance, which resulted in weakening of the cardiac contractile force and lowered BP (El-Mas et al., 2011). These observations complement those of acute studies (El-Mas and Abdel-Rahman, 1999a; Choudhry et al., 2005), which highlighted a key role for reduced cardiac output in the ethanol-evoked hypotension (El-Mas and Abdel-Rahman, 1999a, 1999b).
Despite the evidence that implicated estrogen in the chronic hypotensive effect of ethanol (El-Mas and Abdel-Rahman, 2000, 2001), it is not known whether the detrimental effects of ethanol on myocardial contractility and sympathovagal balance (El-Mas et al., 2011) are also exacerbated by estrogen. Therefore, we conducted telemtry studies to investigate the chronic effects of ethanol on BP, heart rate, dP/dtmax of arterial pressure, and spectral indices of heart rate variability (HRV) in OVX rats with or without estrogen replacement. Cardiovascular variability is categorized into low (IBI-LF; 0.25–0.75 Hz) and high (IBI-HF; 0.75–3 Hz) frequency powers according to its oscillating frequency (Heart rate variability, 1996; Thomas, 2011). Whereas the LF band represents the sympathetic drive, the HF component corresponds to respiratory sinus arrhythmia and reflects the cardiac vagal control; the ratio of LF to HF (IBILF/HF) is a measure of the sympathovagal balance of the heart (Heart rate variability, 1996). Ethanol was given as 5% (w/v) in Lieber-DiCarli high-protein liquid diet for 13 weeks and control rats were pair-fed isocaloric diet, which allowed similar nutrient intake and fluid consumption to that of ethanol-fed rats.
2. Materials and methods
Female Sprague-Dawley rats (9–10 weeks; 190–225 g; Harlan, Indianapolis, IN) were used in the present study. Upon arrival, rats were housed individually in standard plastic cages and allowed free access to water and Purina chow and were maintained on a 12-12-hr light-dark cycle with light off at 4:00 p.m. Room temperature was maintained at 22±1°C. After 1 week acclimatization, rats were fed a standard Lieber-DiCarli high protein liquid diet (Dyets Inc., Bethlehem, PA) for another week before implantation of the telemetry device. Rats received the diet daily 30 min before the start of the dark cycle. All experiments were approved by the institutional animal care and use committee and carried out in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health.
2.1. Telemetry transmitter implantation
The description of the telemetry system (Data Sciences Int., St. Paul, MN) and the method used for the telemetry transmitter implantation are detailed in our previous studies (El-Mas and Abdel-Rahman, 2000, 2007).
2.2. Ovariectomy
Bilateral ovariectomy was performed at the same time of telemetry transmitter implantation as in our previous studies (El-Mas and Abdel-Rahman, 2000; El-Mas and Abdel-Rahman, 2001). The ovaries were isolated, tied off with sterile suture and removed.
2.3. Blood analyses
Plasma estrogen levels were measured at the end of the study by the radioimmunoassay (Diagnostic Systems Laboratories, Inc., Webster, TX) as described in our previous studies (El-Mas and Abdel-Rahman, 1999a, 1999b). The blood ethanol concentration was measured by the enzymatic method described by Bernt and Gutmann (1974) and used in our previous studies (El-Mas and Abdel-Rahman, 1993, 2000).
2.4. Protocols and experimental groups
Four groups of telemetered rats (2 OVX, and 2 OVXE2; n=6–7 each) matched for body weight and baseline BP were used in the present study to investigate the chronic hemodynamic effects of ethanol. All rats were fed control liquid diet 7 days before implantation of the telemetry transmitter. For estrogen replacement, subcutaneous silicone tubing (10 mm length, 1.57 mm inner diameter × 3.18 mm outer diameter, Silastic ®, Dow Corning) filled with approximately 25 mg of 17β-estradiol-3-benzoate [1,3,5(10)-estratriene-3,17β-diol-3-benzoate] were used as described elsewhere (McNeill et al., 2002). Silastic tubings were sealed with medical adhesive type A (Silastic ®, Dow Corning), gas sterilized, and implanted subcutaneously at the back of the neck in rats anesthetized with isoflurane. The two OVX groups of rats (OVX and OVX+ethanol) received empty silicone tubings. Silastic tubings were implanted 3 weeks after transmitter implantation and OVX operation. Rats in the 4 groups were pair-fed to allow similar nutrient and fluid consumption as in previous studies (El-Mas and Abdel-Rahman, 2000, 2001). Fresh diets were prepared every other day and stored in the refrigerator until dispensed. Rats were maintained on liquid diet for 13 weeks.
For the determination of plasma estrogen and ethanol levels, blood samples were withdrawn from the tail vein of all rats at the end of the study (week 13). The collected blood was centrifuged at 3220 g for 5 min and plasma was aspirated and stored at − 80 °C till analyzed as described above.
2.5. Telemetry data acquisition and analysis
Individual rat cages were placed on the top of the radio receivers and data were collected using a computerized data acquisition system (Dataquest A.R.T. 4.0, Data Sciences Int.). Hemodynamic measurements (BP and heart rate) started immediately after transmitter implantation to ensure proper operation of the system. Blood pressure waveforms were sampled at a rate of 1000 Hz for 20 s every 10 min. IBI was calculated from blood pressure waveforms. The maximum rate of rise of blood pressure waves (+dP/dtmax), index of myocardial contractility (Mehta et al., 1998; El-Mas and Abdel-Rahman, 2007), was computed by Data Sciences software. All parameters were averaged over a 7-day period for weekly values.
2.6. Spectral analysis of hemodynamic variability
Spectral hemodynamic fluctuations, quantitative indices of cardiovascular autonomic control (Stein et al., 1994; El-Mas and Abdel-Rahman, 2007), were used to reflect changes in sympathetic and vagal outflows. Hemodynamic variability was assessed by the frequency domain analysis of systolic blood pressure (SBP) and interbeat interval (IBI) data series as in previous studies including our own (Clifford and Tarassenko, 2004; El-Mas and Abdel-Rahman, 2007). Data Sciences software (Dataquest A.R.T. 4.0) uses the periodogram function of the rectangular window for direct transformation of data points into power spectral density graphs. Data were interpolated to obtain equally spaced samples with an effective sampling frequency of 10 Hz (0.1 s). A second-order quadratic was employed to fit a smooth curve to the existing data points and produce a smoother visual representation of data. Spectra were integrated into 2 specific frequency bands, LF (0.25–0.75 Hz) and HF (0.75–3 Hz) bands and expressed in normalized units (LFnu and HFnu). The ratio of LF to HF (IBILF/HF) is a measure of the sympathovagal balance of the heart. Parameters of hemodynamic variability were averaged over a 7-day period for weekly values as in our previous studies (El-Mas and Abdel-Rahman, 2007).
2.7. Drugs
Ketaject (ketamine), Xyla-ject (xylazine) (Phoenix Pharmaceuticals Inc., St Joseph, MI). Toradol (ketorolac tromethamine, Abbott Labs, Chicago, IL), Durapen (Penicillin G benzathine and penicillin G procaine, Vedco Inc., Overland Park, KS), 17β-estradiol-3-benzoate (Sigma Chemical Co., St. Louis, MO), and ethanol (Midwest Grain Products Co., Weston, MO) were purchased from commercial vendors.
2.8. Statistical analysis
Data are expressed as means±S.E.M. All parameters were averaged over a 7-day period for weekly values. The areas under the curves (AUC) of the changes in SBP and DBP caused by ethanol were calculated for individual experiments using trapezoidal integration (Graph pad prism, version 3.0). One-way analysis of variance (ANOVA), followed by a Newman-Keuls post-hoc test, was used for the analysis of the cumulative hypotensive effects (AUC) of ethanol in OVX and OVXE2 rats. The time-course effects of ethanol on hemodynamic and autonomic parameters wewe analyzed by repeated measures ANOVA. Probability levels less than 0.05 were considered significant.
3. Results
3.1. Baseline data
The baseline values of SBP, DBP, heart rate, and +dP/dtmax obtained from conscious freely moving OVX, and OVXE2 rats before ethanol feeding were similar (Table 1). Low levels of plasma estrogen were detected in OVX rats that were significantly increased upon estrogen replacement (Table 1). The blood ethanol concentration and daily ethanol intake were similar in OVX and OVXE2 rats (Table 1).
Table 1.
Baseline values of systolic (SBP, mmHg) and diastolic (DBP, mmHg) blood pressure, heart rate (HR, beats/min) and myocardial contractility (+dP/dtmax, mmHg/sec) and the 13th-week values of plasma estrogen (E2, pg/ml) and ethanol (mg/dl) and daily ethanol intake (g/kg).
| Group | SBP | DBP | HR | dP/dtmax | E2 | Ethano | |
|---|---|---|---|---|---|---|---|
| week 0 | week 0 | week 0 | week 0 | Blood level |
Blood level |
Daily intake |
|
| OVX | 120±2 | 93±1 | 398±3 | 1595±89 | 4±1 | ------- | ------- |
| OVX/Etoh | 122±2 | 93±1 | 394±5 | 1448±40 | 4±1 | 103±3 | 7.3±0.1 |
| OVXE2 | 123±1 | 93±2 | 391±4 | 1487±67 | 52±4a | ------- | ------- |
| OVXE2/Etoh | 125±3 | 94±2 | 402±4 | 1507±77 | 41±4a | 120±7 | 7.4±0.2 |
Values are means±SEM of 6–7 observations.
P<0.05 vs. corresponding OVX values.
3.2. Cardiovascular effects of chronic ethanol feeding absence or presence of estrogen
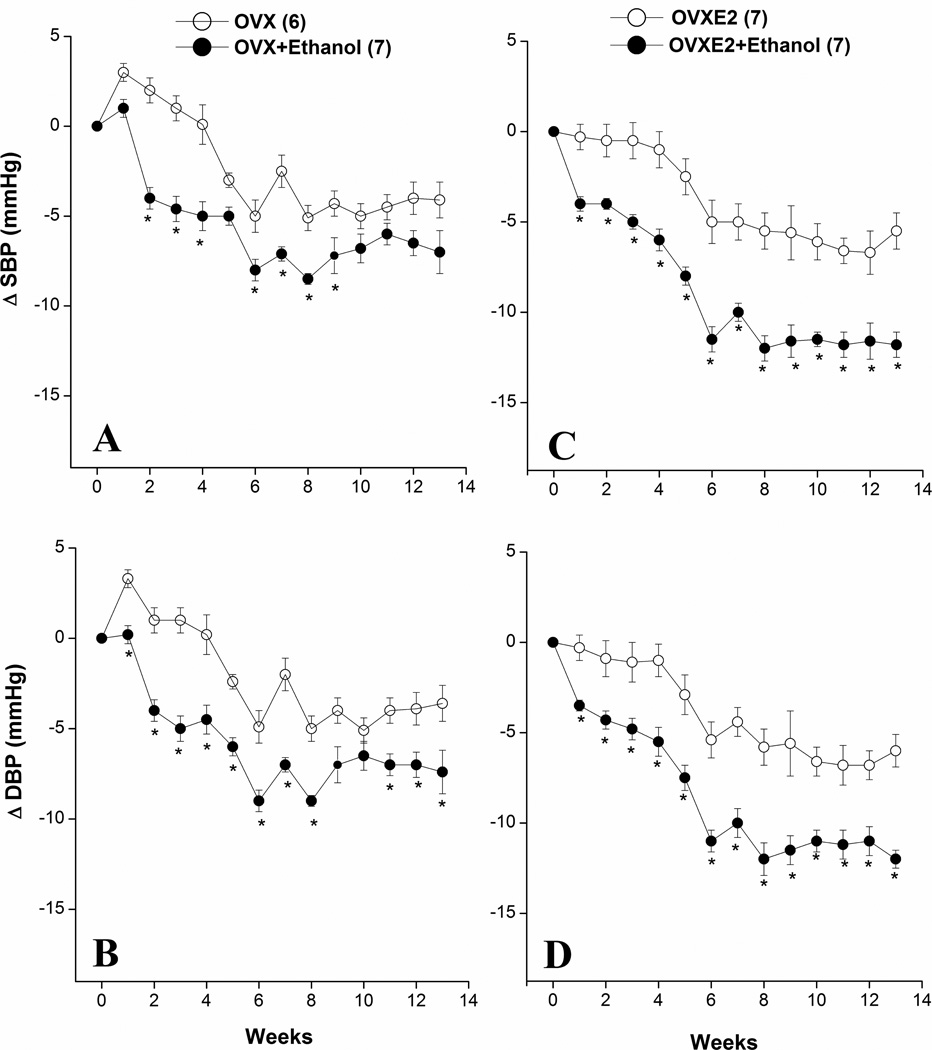
Changes evoked by chronic ethanol feeding in BP and myocardial contractility (+dP/dtmax) in OVX and OVXE2 rats are depicted in figures 1 and 2. Compared with pair-fed OVX rats, ethanol feeding to OVX rats produced modest hypotensive responses with near-maximal reductions in SBP and DBP observed at week 6 (Fig. 1A, 1B). Estrogen replacement in OVX rats significantly potentiated the hypotensive effect of ethanol (Figs. 1C, 1D). This finding was also demonstrated by the computation of the AUC, which measured the cumulative falls in SBP and DBP over the entire 13-week duration of the study. The AUC was significantly increased by ethanol feeding in both OVX and OVXE2 rats compared with respective values of ethanol-untreated rats (Table 2). However, the increase in the AUC caused by ethanol in OVXE2 rats was 50–60% greater than the corresponding value in OVX rats (Table 2). The myocardial contractility (+dP/dtmax) was not affected by OVX over the 13-week period of the study (Fig. 2A). Estrogen supplementation of OVX rats caused significant increases in +dP/dtmax that appeared during the first week of treatment (from 1487±67 to 1737±86 mmHg/sec, P<0.05) and continued thereafter (Fig. 2B). Ethanol feeding to OX rats caused brief (weeks 2–5) reductions in +dP/dtmax (Fig. 2A). In OVXE2 rats, ethanol elicited more sustained reductions in +dP/dtmax that continued for the entire experimentation period (Fig. 2B). Heart rate was not consistently affected by ethanol in all rat groups (data not shown).
Figure 1.
Changes in systolic (SBP) and diastolic (DBP) blood pressures caused by chronic ethanol feeding (5% w/v) or isocaloric control diet in ovariectomized rats in the absence (OVX, panels A–B) or presence (OVXE2, panels C–D) of estrogen replacement. Values are means±S.E.M. of 6–7 observations. *P<0.05 vs. respective control (ethanol-untreated) values.
Figure 2.
Changes in myocardial contractility (+dP/dtmax) caused by chronic ethanol feeding (5% w/v) or isocaloric control diet in ovariectomized rats in the absence (OVX, panel A) or presence (OVXE2, panel B) of estrogen replacement. Values are means±S.E.M. of 6–7 observations. *P<0.05 vs. respective control (ethanol-untreated) values.
Table 2.
Areas under the curves (mmHg.week) of the cumulative falls in systolic (SBP) and diastolic (DBP) blood pressures over the entire 13-week duration of the study.
| Group | SBP | DBP | ||
|---|---|---|---|---|
| AUC | AUC difference | AUC | AUC difference | |
| OVX | 32±4 | 42 | 35±3 | 44 |
| OVX/Etoh | 74±6a | 79±5a | ||
| OVXE2 | 50±4 | 69 | 53±5 | 63 |
| OVXE2/Etoh | 119±6ab | 115±6ab | ||
Values are means±SEM of 6–7 observations.
P<0.05 vs. respective OVX or OVXE2 values,
P<0.05 vs. OVX/Etoh values.
3.3. Autonomic effects of chronic ethanol feeding in absence or presence of estrogen
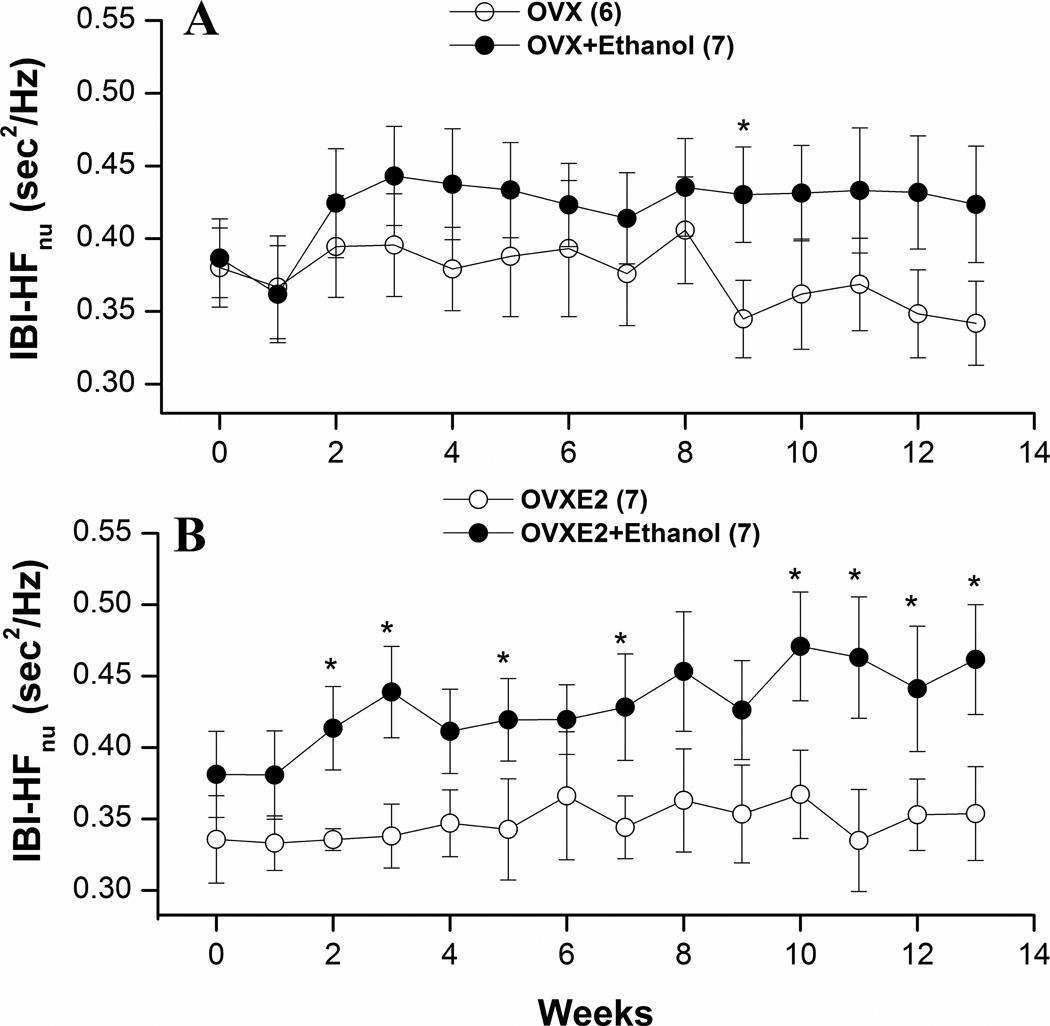
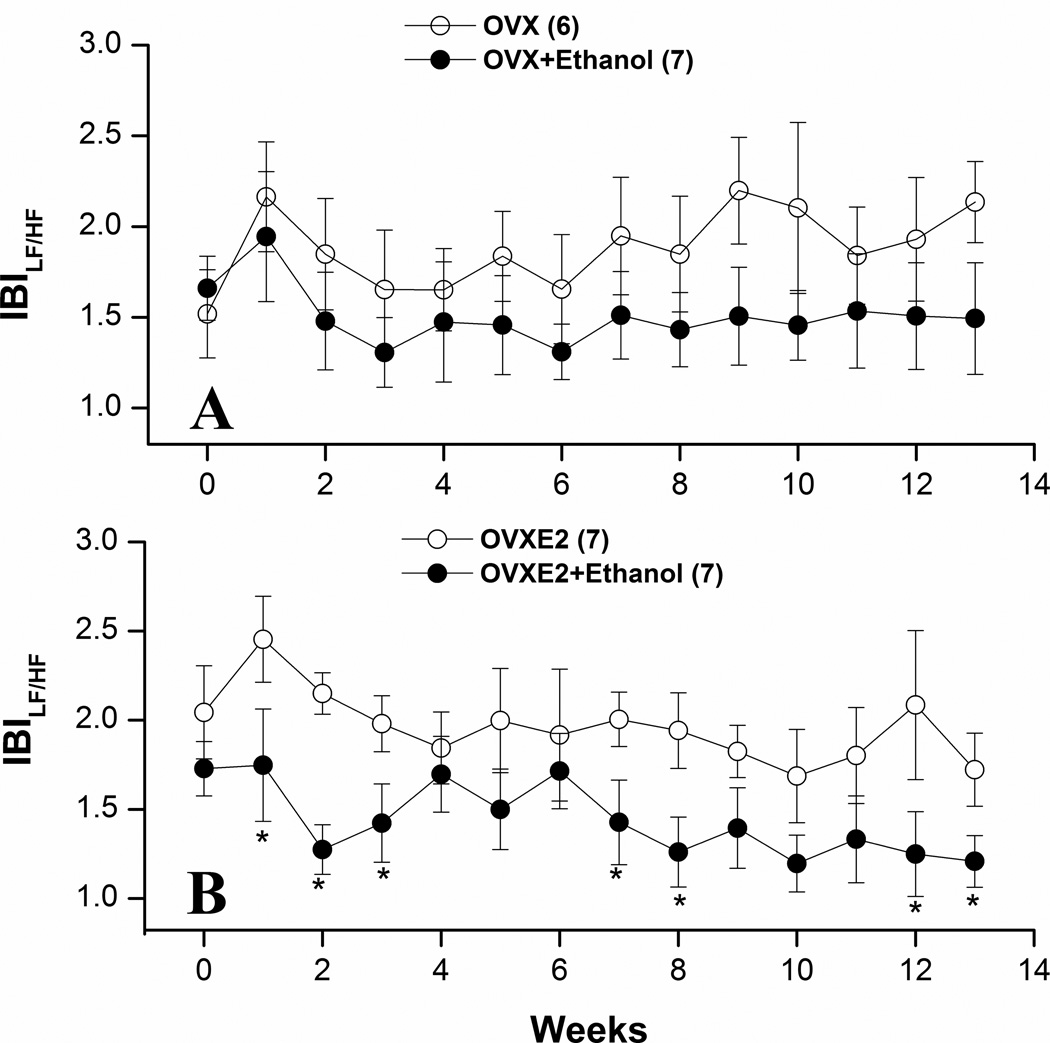
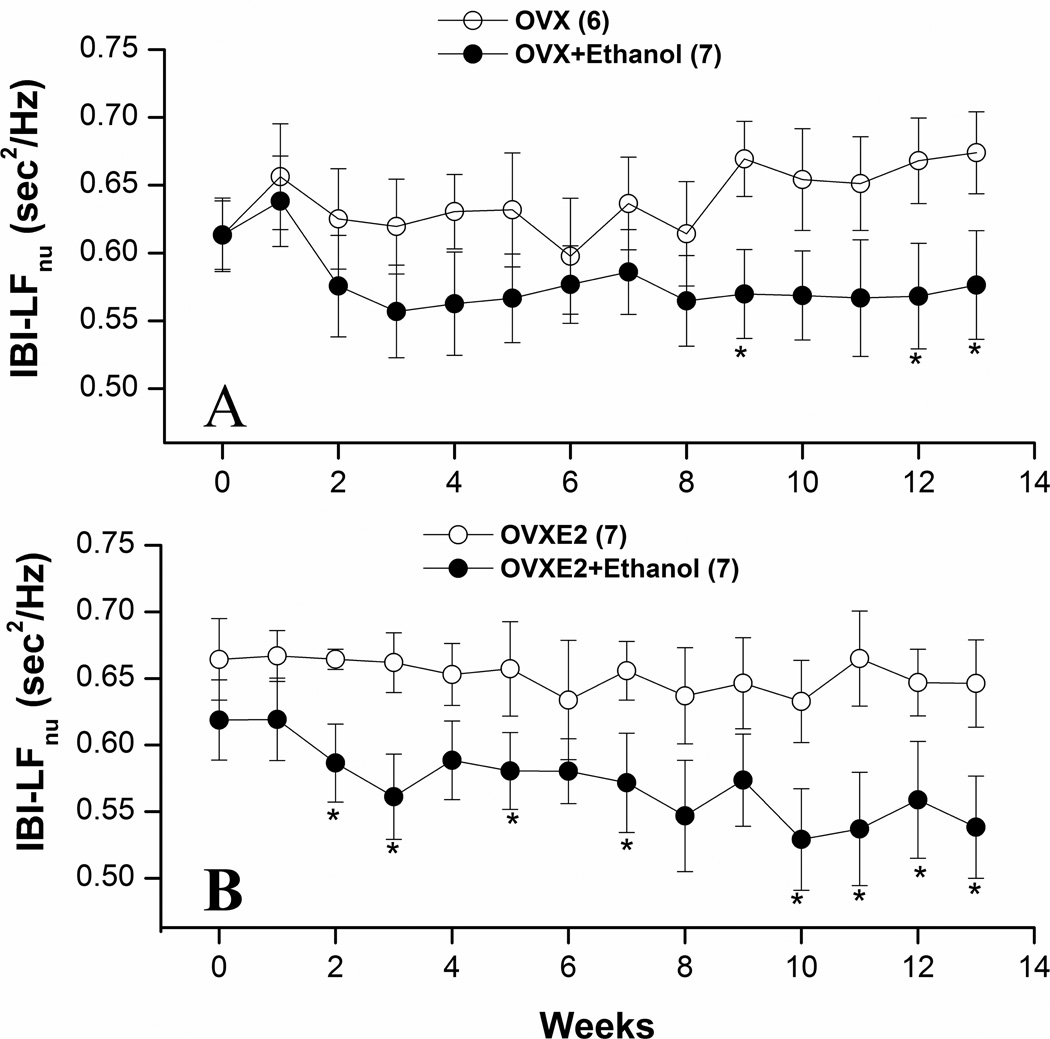
Figures 3–5 illustrate the effects of ethanol feeding on spectral measures of cardiovascular autonomic control in OVX and OVXE2. Except for a significant increase at week 9, the spectral density of IBI in the HF range (0.75–3 Hz) was not influenced by ethanol feeding in OVX rats (Fig. 3A). The LF (0.25–0.75 Hz) oscillations of IBI were significantly reduced by ethanol feeding of OVX rats at weeks 9, 12, and 13 (Fig. 4A). In OVXE2 rats, ethanol feeding caused more sustained increases in the HF (Fig. 3B) and decreases in the LF (Fig. 4) oscillations. The IBILF/HF ratio, a measure of cardiac sympathovagal balance, was significantly reduced by ethanol in OVXE2 rats (Fig. 5B) in contrast to no effect in OVX rats (Fig. 5A). On the other hand, SBP spectra in the LF range were not affected by ethanol treatment (data not shown).
Figure 3.
Effects of chronic ethanol feeding (5% w/v) or isocaloric control diet on the high-frequency IBI spectral density (IBI-HFnu, 0.75–3 Hz) in ovariectomized rats in the absence (OVX, panel A) or presence (OVXE2, panel B) of estrogen replacement. Values are means±S.E.M. of 6–7 observations. *P<0.05 vs. respective control (ethanol-untreated) values.
Figure 5.
Effects of chronic ethanol feeding (5% w/v) or isocaloric control diet on the LF/HF ratio of IBI in ovariectomized rats in the absence (OVX, panel A) or presence (OVXE2, panel B) of estrogen replacement. Values are means±S.E.M. of 6–7 observations. *P<0.05 vs. respective control (ethanol-untreated) values.
Figure 4.
Effects of chronic ethanol feeding (5% w/v) or isocaloric control diet on the low-frequency IBI spectral density (IBI-LFnu, 0.25–0.75 Hz) in ovariectomized rats in the absence (OVX, panel A) or presence (OVXE2, panel B) of estrogen replacement. Values are means±S.E.M. of 6–7 observations. *P<0.05 vs. respective control (ethanol-untreated) values.
4. Discussion
Our recent findings inferred an important role for alterations in autonomic control of the heart and the associated reduction in cardiac contractility in the ethanol-evoked hypotension in randomly cycling female rats (El-Mas et al., 2011). In this report, we tested the hypothesis that estrogen exacerbations of the alterations in myocardial contractility and cardiac autonomic activity constitute an important hemodynamic mechanism for estrogen-dependent hypotensive effect of ethanol in female rats. The results showed that in the absence of ovarian hormones, ethanol feeding caused modest reductions in BP, +dP/dtmax, IBILF and LF/HFIBI ratio and increases in IBIHF in telemetered OVX rats. These hemodynamic responses were exacerbated following estrogen replacement in ethanol-fed OVXE2 rats. These findings suggest a modulatory role for estrogen in the enhancement and suppression of cardiac parasympathetic and sympathetic control, respectively, which ultimately contribute to the reductions in myocardial contractility and BP caused by ethanol in female rats.
Similar to our previous studies (El-Mas and Abdel-Rahman, 2001), the present study showed that the hypotensive effect of ethanol is exacerbated in the presence of estrogen. Because, as stated above, changes in myocardial contractility and autonomic control mediated the hypotensive action of ethanol in randomly cycling rats (El-Mas et al., 2011), we tested the hypothesis that estrogen exacerbation of the deleterious effects of chronic ethanol on cardiac dynamics underlies the exaggerated ethanol-evoked hypotensive response in the presence of estrogen. In support of this hypothesis, are the findings that the depressant effect of ethanol on myocardial contractility (+dP/dtmax) was exacerbated following estrogen replacement in OVXE2 rats. Similarly, the increased parasympathetic dominance, reflected by the increases in IBIHF and decreases in IBILF and LF/HFIBI ratio, seen in ethanol-fed, randomly cycling rats, in our recent study (El-Mas et al., 2011) was replicated in ethanol-fed OVXE2 in the present study. Interestingly, comparisons of the data reveal virtually identical time-course profiles for the hypotensive, autonomic, and myocardial depressant effects of ethanol in randomly cycling rats (El-Mas et al., 2011) and OVXE2 rats (present study). These findings contrast with the smaller and shorter-lived changes in myocardial contractility and spectral profiles in response to ethanol in OVX rats. These findings highlight a temporal and possibly causal relationship between autonomic imbalances caused by ethanol on the one hand, and the reductions in myocardial contractility and BP on the other. However, this conclusion suffered two limitations. First, despite the apparently exaggerated autonomic effects of ethanol in OVXE2, the changes caused by ethanol were not maintained throughout the 13 weeks of the study and at some time points the data obtained from ethanol-treated and untreated OVX rats were not statistically different (see Figs. 3B, 4B, and 5B). The second limitation pertained to the observation that estrogen supplementation of OVX rats caused immediate and maintained increases in +dP/dtmax. As such, the elevated +dP/dtmax in OVXE2 rats may account, at least partly, for the exaggerated myocardial depressant effect of ethanol in OVXE2 rats.
The mechanism by which estrogen modulates the cardiovascular and autonomic effects of ethanol is not clear. Pharmacokinetic factors are unlikely to contribute to the estrogen exacerbation of the hemodynamic effects of ethanol because neither the plasma alcohol level was affected by the hormonal (estrogen) status nor the plasma estrogen level was altered by ethanol feeding. Alternatively, the dependence of ethanol-evoked hypotension on estrogen may be explained in view of the similarity of the vascular effects of the two drugs. For example, both drugs inhibit calcium influx (Vasdev et al., 2006; Babaei and Azarmi, 2008) and facilitate NOS activity (Rekik et al., 2002; LeBlanc et al., 2009). Upregulation of cardiac NOS mediates the reductions in cardiac output (El-Mas et al., 2008, 2009) and cardiac contractile force (El-Mas et al., 2011) caused by ethanol in female rats. Further, decreased intracellular calcium in cardiomyocytes correlates with heart failure (Sossalla et al. 2008). It is conceivable, therefore, to assume that ethanol may interact synergistically with estrogen to produce cardiovascular changes that trigger myocardial depression and hypotension. However, more dose-response studies are needed to investigate this possibility.
Despite the importance of spectral analysis of heart rate variability in defining the autonomic control of the circulation, the possibility should be considered that heart rate variability might also be influenced by other mechanisms unrelated to autonomic control of cardiac function. For instance, the vagal cardiomotor activity is not the only determinant of the HF oscillations of HRV. In fact, residual HF oscillations remain after autonomic blockade or cardiac transplantation, which have been attributed to mechanical modulation of sinus node by respiration (Bernardi et al., 1989). Also, in addition to the sympathetic drive, other factors including the parasympathetic control as well as other factors such as gender, and age can influence the LF component of HRV (Parati et al., 2006). The representation of LF and HF powers in normalized units and the computation of the LF/HF power ratio have been suggested to increase the reliability of spectral parameters in reflecting cardiac autonomic modulation (Heart rate variability, 1996; Parati et al., 2006). Therefore, we employed both maneuvers in the current study.
In conclusion, the present study showed that chronically administered ethanol in female rats increased vagal cardiomotor activity, which apparently resulted in weakening myocardial contractility and lowering BP. Compared to OVX rats, these effects of ethanol were exacerbated in estrogen-replaced OVX rats. Therefore, it is conceivable to suggest a key role for estrogen in the autonomic effects of ethanol, which influence the cardiac mechanics, in female rats. Importantly, the dose of ethanol used in the present study (5% w/v) produced blood ethanol concentrations comparable to those attained in humans following consumption of moderate to intoxicating amounts of ethanol (Eddleston et al., 2009; Schaller et al., 2010). Further, our findings yield insight into the greater hypotensive response caused by ethanol in younger, compared with older, women (Klatsky, 1990). Overall, these findings highlight the clinical relevance of the present study and the need to conduct future studies on the molecular mechanisms implicated in estrogen exacerbation of the deleterious myocardial effects of ethanol in females.
Acknowledgments
Supported by Grant R01 AA014441 from the National Institute on Alcohol Abuse and Alcoholism. The authors thank Ms. Kui Sun for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babaei H, Azarmi Y. 17beta-estradiol inhibits calcium-dependent and -independent contractions in isolated human saphenous vein. Steroids. 2008;73:844–850. doi: 10.1016/j.steroids.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Keller F, Sanders M, Reddy PS, Griffith B, Meno F, Pinsky MR. Respiratory sinus arrhythmia in the denervated human heart. J. Appl. Physiol. 1989;67:1447–1455. doi: 10.1152/jappl.1989.67.4.1447. [DOI] [PubMed] [Google Scholar]
- Bernt E, Gutmann I. Ethanol: Determination with alcohol dehydrogenase and NAD. In: Bergmeyer HU, editor. "Methods of Enzymatic Analysis" 1974. 2nd edition. Volume 3. New York, San Francisco, London: Academic Press; 1974. pp. 1499–1502. [Google Scholar]
- Choudhry MA, Ba ZF, Rana SN, Bland KI, Chaudry IH. Alcohol ingestion before burn injury decreases splanchnic blood flow and oxygen delivery. Am J. Physiol. Heart Circ. Physiol. 2005;288:H716–H721. doi: 10.1152/ajpheart.00797.2004. [DOI] [PubMed] [Google Scholar]
- Clifford GD, Tarassenko L. Segmenting cardiac-related data using sleep stages increases separation between normal subjects and apnoeic patients. Physiol. Meas. 2004;25:N27–N35. doi: 10.1088/0967-3334/25/6/n03. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Gunnell D, von Meyer L, Eyer P. Relationship between blood alcohol concentration on admission and outcome in dimethoate organophosphorus self-poisoning. BrJ Clin. Pharmacol. 2009;68:916–919. doi: 10.1111/j.1365-2125.2009.03533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. An association between the estrogen-dependent hypotensive effect of ethanol and an elevated brainstem c-jun mRNA in female rats. Brain Res. 2001;912:79–88. doi: 10.1016/s0006-8993(01)02727-5. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. Upregulation of cardiac NOS due to endotoxemia and vagal overactivity contributes to the hypotensive effect of chronic ethanol in female rats. Eur. J. Pharmacol. 2011;650:317–323. doi: 10.1016/j.ejphar.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Direct evidence for selective involvement of aortic baroreceptors in ethanol-induced impairment of baroreflex control of heart rate. J. Pharmacol. Exp. Ther. 1993;264:1198–1205. [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Estrogen-dependent hypotensive effects of ethanol in conscious female rats. Alcohol. Clin. Exp. Res. 1999a;23:624–632. [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Intermittent clonidine regimen abolishes tolerance to its antihypertensive effect: a spectral study. J. Cardiovasc. Pharmacol. 2007;49:174–181. doi: 10.1097/FJC.0b013e3180318afb. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Ovariectomy alters the chronic hemodynamic and sympathetic effects of ethanol in radiotelemetered female rats. Clin. Exp. Hypertens. 2000;22:109–126. doi: 10.1081/ceh-100100066. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Sexually dimorphic hemodynamic effects of intragastric ethanol in conscious rats. Clin. Exp. Hypertens. 1999b;21:1429–1445. doi: 10.3109/10641969909070858. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. Endotoxemia-mediated induction of cardiac inducible nitric-oxide synthase expression accounts for the hypotensive effect of ethanol in female rats. J. Pharmacol. Exp. Ther. 2008;324:368–375. doi: 10.1124/jpet.107.127498. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. Facilitation of myocardial PI3K/Akt/nNOS signaling contributes to ethanol-evoked hypotension in female rats. Alcohol. Clin. Exp. Res. 2009;33:1158–1168. doi: 10.1111/j.1530-0277.2009.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Klatsky AL, Friedman GD, Siegelaub AB, Gerard MJ. Alcohol consumption and blood pressure. N. Engl. J. Med. 1977;296:1194–1200. doi: 10.1056/NEJM197705262962103. [DOI] [PubMed] [Google Scholar]
- Klatsky AL. Blood pressure and alcohol intake. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis, and Management. Raven Press Ltd; 1990. pp. 277–294. [Google Scholar]
- LeBlanc AJ, Reyes R, Kang LS, Dailey RA, Stallone JN, Moningka NC, Muller-Delp JM. Estrogen replacement restores flow-induced vasodilation in coronary arterioles of aged and ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R1713–R1723. doi: 10.1152/ajpregu.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill AM, Zhang C, Stanczyk FZ, Duckles SP, Krause DN. Estrogen increases endothelial nitric oxide synthase via estrogen receptors in rat cerebral blood vessels: effect preserved after concurrent treatment with medroxyprogesterone acetate or progesterone. Stroke. 2002;33:1685–1691. doi: 10.1161/01.str.0000016325.54374.93. [DOI] [PubMed] [Google Scholar]
- Mehta MC, Jain AC, Billie M. Combined effects of alcohol and nicotine on cardiovascular performance in a canine model. J. Cardiovasc. Pharmacol. 1998;31:930–936. doi: 10.1097/00005344-199806000-00018. [DOI] [PubMed] [Google Scholar]
- Parati G, Mancia G, Di Rienzo M, Castiglioni P, Taylor JA, Studinger P. Point: Counterpoint: Cardiovascular variability is/is not an index of autonomic control of circulation. J. Appl. Physiol. 2006;101:676–682. doi: 10.1152/japplphysiol.00446.2006. [DOI] [PubMed] [Google Scholar]
- Rekik M, El-Mas MM, Mustafa SJ, Abdel-Rahman AA. Role of endothelial adenosine receptor-mediated vasorelaxation in ethanol-induced hypotension in hypertensive rats. Eur. J. Pharmacol. 2002;452:205–214. doi: 10.1016/s0014-2999(02)02304-x. [DOI] [PubMed] [Google Scholar]
- Rivera CA, Bradford BU, Seabra V, Thurman RG. Role of endotoxin in the hypermetabolic state after acute ethanol exposure. Am. J. Physiol. 2007;275:G1252–G1258. doi: 10.1152/ajpgi.1998.275.6.G1252. [DOI] [PubMed] [Google Scholar]
- Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit. Care Med. 2007;35:1599–1608. doi: 10.1097/01.CCM.0000266683.64081.02. [DOI] [PubMed] [Google Scholar]
- Schaller G, Kretschmer S, Gouya G, Haider DG, Mittermayer F, Riedl M, Wagner O, Pacini G, Wolzt M, Ludvik B. Alcohol acutely increases vascular reactivity together with insulin sensitivity in type 2 diabetic men. Exp. Clin. Endocrinol. Diabetes. 2010;118:57–60. doi: 10.1055/s-0029-1233453. [DOI] [PubMed] [Google Scholar]
- Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schöndube FA, Tirilomis T, Tenderich G, Hasenfuss G, Belardinelli L, Maier LS. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts--role of late sodium current and intracellular ion accumulation. J. Mol. Cell Cardiol. 2008;45:32–43. doi: 10.1016/j.yjmcc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Stein PK, Bosner MS, Kleiger RE, Conger BM. Heart rate variability: a measure of cardiac autonomic tone. Am. Heart J. 1994;127:1376–1381. doi: 10.1016/0002-8703(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Thomas GD. Neural control of the circulation. Adv. Physiol. Educ. 2011;35:28–32. doi: 10.1152/advan.00114.2010. [DOI] [PubMed] [Google Scholar]
- Umar S, van der Laarse A. Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol. Cell Biochem. 2010;333:191–201. doi: 10.1007/s11010-009-0219-x. [DOI] [PubMed] [Google Scholar]
- Vasdev S, Gill V, Singal PK. Beneficial effect of low ethanol intake on the cardiovascular system: possible biochemical mechanisms. Vasc. Health Risk Manag. 2006;2:263–276. doi: 10.2147/vhrm.2006.2.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]