Abstract
Metaphors are fundamental to creative thought and expression. Newly coined metaphors regularly infiltrate our collective vocabulary and gradually become familiar, but how does this shift from novel to conventionalized meaning happen in the brain? We investigated the neural career of metaphors in a functional magnetic resonance imaging study using extensively normed new metaphors and simulated the ordinary, gradual experience of metaphor conventionalization by manipulating participants’ exposure to these metaphors. Results showed that the conventionalization of novel metaphors specifically tunes activity within bilateral inferior prefrontal cortex, left posterior middle temporal gyrus, and right postero-lateral occipital cortex. These results support theoretical accounts attributing a role for the right hemisphere in processing novel, low salience figurative meanings, but also show that conventionalization of metaphoric meaning is a bilaterally-mediated process. Metaphor conventionalization entails a decreased neural load within semantic networks rather than a hemispheric or regional shift across brain areas.
Keywords: career of metaphor, right hemisphere, figurative language, novelty, familiarity, fMRI
1. Introduction
Metaphoric language is ubiquitous in speech and writing, affording not just poetic flourish but a critical means to communicate about that which is abstract (Lakoff & Johnson, 1980). Moreover, metaphors are continually updated creatively as speakers contrive novel means to express ideas both eternal and timely. Consider the familiar expression, “She bit her tongue” and the novel one, “Her true opinion skulked behind her teeth”. Upon reflection, it is easy to determine that each of these statements means she refrained from saying aloud what she honestly thought. But what does this process of reflection actually entail? How is it that we come to know that skulking in this case does not literally refer to some kind of stealthy movement but rather to a state of concealment?
Behavioral studies of metaphor comprehension indicate the way we understand metaphors changes as they become familiar (Bowdle & Gentner, 2005). Initially, a novel metaphor is understood through a comparison between two semantically distant domains. For instance, in the expression “The purchase was a tiger pounce”, the pouncing behavior of tigers must be compared to the purchasing behavior of an implied buyer to identify their relevant common properties. This comparison purportedly evokes a common category subsuming both purchases and pounces – i.e. a category of swift, aggressive actions. The power of the metaphor comes from its ability to communicate creatively about a situation or concept. With repeated exposure, this metaphoric sense of “pounce” becomes conventional and then can be accessed directly without reliance on the more effortful comparison process. “Pounce” acquires dual reference (Glucksberg & Keysar, 1990) referring both to a narrow, concrete meaning (hunting behaviors of cats) as well as a more abstract sense (any swift, aggressive behavior). This gradual process by which words acquire additional and directly-accessible figurative meanings is known as the “career of metaphor” (Bowdle & Gentner, 2005).
This qualitative shift in cognitive processing from comparison to categorization is almost certainly accompanied by a parallel shift in neural processing. However, we know little about the neural underpinnings of metaphor and the way they evolve in the brain. The standard story about the neural substrates for metaphor has been that metaphor, like other forms of creative and non-literal language, relies upon processing unique to the right hemisphere. Attributing the right hemisphere a special sensitivity to metaphor reflects the hypothesis that novel linguistic associations require the flexible, open-ended semantic processing typical of the right hemisphere (Jung-Beeman, 2005). Despite the appeal of this hypothesis, the accumulated evidence for this traditional account is inconclusive (Cardillo et al., 2010; Schmidt et al., 2009). One alternative explanation for differences in hemispheric engagement is that familiarity rather than figurativeness determines lateralization, with the right hemisphere sensitive to novelty in general. An alternate hypothesis is that the right hemisphere is necessary to generate linguistic interpretations that are low in salience, where salience encompasses an expression’s familiarity as well as the conventionality, frequency, and predictability of its meaning (Giora, 2003). Common to both of these accounts is the prediction that the left hemisphere is sufficient for understanding familiar metaphors but novel metaphors require the right hemisphere.
However, even evidence for the preferential or additional engagement of the right hemisphere for novel metaphors is mixed. Some studies support this division of labor between the hemispheres, reporting right hemisphere or bilateral activation in response to novel metaphors (Arzouan et al., 2007; Bottini et al., 1994; Desai et al., 2011; Mashal et al., 2005, 2007; Sotillo et al., 2005), but others find only left-lateralized engagement (Kircher et al., 2007; Mashal et al., 2009; Rapp et al., 2004, 2007; Shibata et al., 2007). One possible reason for the conflicting evidence is that studies do not consider the potential import of metaphor variety. Metaphors are a motley family of expressions. Cognitive and neuropsychological research typically focuses on nominal metaphors, or figurative extensions of nouns. The basic syntactic form of these expressions take “An X is a Y”, where X is the literal target term being likened to the metaphorical sense of the base term Y. However, speakers frequently extend other grammatical classes metaphorically. Speakers also use adjectives (“the sexy design and the recalcitrant data”), prepositions (“She’s down for a drink; count me in too.”), and verbs (“I devoured the book”) metaphorically. Previous research has generally considered only one kind of metaphor in any given study, with the unexamined assumption that the effects associated with one type extend to all others. This assumption may not be appropriate, or perhaps in only certain circumstances (Cardillo et al., 2010; Chen et al., 2008; Schmidt et al., 2009). In noun-based, nominal metaphors semantic attributes of the base term are compared to those of the target term (Bowdle and Gentner, 2005), but in verb-based, predicate metaphors no such comparison between disparate entities occurs. The meaningfulness of predicate metaphors hinges instead on deriving a more abstract sense of a verb in which many of its concrete perceptual and motor features are shed. For instance, when we say a person has “run up a bill” we appeal to a sparse conceptualization of the verb, a pared down sense that implies a rapid change of state but no actual motion or involvement of the body (Chen et al., 2008; Torreano et al., 2005).
Given this proposed difference in how nominal and predicate metaphors are understood, it is not clear that we should expect identical neural processing associated with each. Indeed, a number of studies indicate that this process of verb abstraction draws upon brain areas not typically associated with metaphor comprehension. When action verbs are used figuratively, activity is instead observed in overlapping or adjacent brain areas as are involved in understanding the literal senses of action verbs (Chen et al., 2008; Saygin et al., 2009; Wallentin et al., 2005a b). Sentences with figuratively extended verbs activate either motion-sensitive area MT or adjacent cortex in postero-lateral temporal cortex and sentences with figuratively extended reaching and grasping verbs preferentially activate inferior parietal cortex, an area involved in recognizing reaching and grasping movements (Desai et al., 2011). We interpret this close parallel between the neural correlates for action perception, literal senses of dynamic action verbs, and figurative senses of dynamic action verbs to support an abstraction-based account of predicate metaphor processing. The more abstract the sense of a verb, the more its neural activity is shifted anteriorly relative to its perceptual point of entry (Chatterjee, 2010; Chen et al., 2008; Schmidt et al., 2009).
Other studies have specifically considered whether figurative senses of verbs activate premotor and motor cortex, another region associated with literal verb comprehension (Aziz-Zadeh & Damasio, 2008; Boulenger et al., 2009; Raposo et al., 2009). Evidence that these figurative senses activate motor areas is weak or absent. It is important to note that the stimuli in these studies entailed either idiomatic uses of verbs (”kick the bucket”) or very conventional metaphoric senses (“grasp the idea”). These expressions are so far along the novel-familiar continuum that deriving their meaning may be more akin to routine literal word recognition than an effortful abstraction away from sensory and motor features. Abstraction is likely to be most relevant when metaphoric expressions are unfamiliar (Desai et al., 2011).
The primary purpose of this study was to look for the neural correlates of the so-called career of metaphor. A secondary goal was to consider the impact of syntactic form on the neural processing metaphors evoke, and whether different forms show different neural careers. In the past, various methodological shortcomings have made it difficult to determine how the brain responds to metaphors or becomes tuned to their interpretations. To overcome these shortcomings, we used an innovative design with three major strengths compared to other neuroimaging studies of metaphor comprehension. First, we created novel metaphors and normed them extensively (Cardillo et al., 2010). This set gave us unprecedented control over the stimuli in a way that is difficult to achieve with metaphors that are already in use. We also exercised unprecedented care in selecting our stimuli from this set. The contradictory nature of the literature on the neural basis of metaphor likely reflects, at least in part, a failure to adequately control for inadvertent differences in processing difficulty (Cardillo et al., 2010; Schmidt et al, 2009; Schmidt & Seger, 2009; Yang et al., 2009). Second, we simulated the gradual experience of metaphor conventionalization over time by experimentally manipulating participant familiarity with these new metaphors. This within-item manipulation prevents potential confounds associated with having different items in different conditions, the assumption that different individuals have identical degrees of experience with individual metaphors, and the artificiality of familiarity ratings on a Likert scale. It also increases our sensitivity to subtle neural shifts with familiarity because it relies upon a parametric rather than typical subtraction analysis. Third, in the same study we compared the neural processing associated with the two most widely-studied types of metaphors, nominal and predicate metaphors.
The existing literature motivates several hypotheses (Cardillo et al., 2010). Novel metaphors, like any other sentence, may require classic left hemisphere language areas for semantic and syntactic processing, as well as right hemisphere homologues in order to generate novel, low-salience semantic senses or cross-domain mappings. In contrast, familiar metaphors may be understood much like literal sentences and thus be mediated exclusively by classic perisylvian language areas of the left hemisphere. As described previously, this shift in hemispheric specialization for metaphors is consistent with the predictions of several independent accounts (Schmidt et al., 2009; Giora, 2003), and parallels the shift from comparison to categorization described by the Career of Metaphor model (Bowdle & Gentner, 2005).
However, it is unknown whether the brain processes all metaphors similarly, raising the possibility that the neural career of metaphor may vary with metaphor type. Our inclusion of equal numbers of nominal and predicate metaphors enabled us to consider two additional hypotheses (Cardillo et al., 2010). One possibility is that the syntactic form of a metaphor dictates the cognitive, and thus neural, processes required to understand it. Nominal metaphors may require the broader semantic associations typical of right hemisphere processing in order to meaningfully compare base and target terms. As familiarity facilitates a shift from this comparison process to categorization, the demand for right hemisphere semantic processing should diminish and activation should shift to typical left hemisphere language areas. Predicate metaphors, on the other hand, may involve a process of abstraction away from literal verb senses, drawing instead upon similar areas as those involved in literal interpretations of their base terms - i.e. left postero-lateral temporal cortex and premotor and motor cortex (Chatterjee, 2010; Watson & Chatterjee, 2011). As metaphoric meanings become more familiar, reliance on the concrete features of the base term may diminish, reducing activation in primary motor and secondary sensori-motor areas (Desai et al., 2011).
Alternatively, the semantic properties of the term being used metaphorically may drive the neural substrates for comprehension, regardless of the syntactic structure in which the base term appears. In all types of metaphors, the figurative sense of the base term entails a bleaching of some of the concrete sensory and motor features associated with it. The abstraction process proposed for predicate metaphors may apply to other grammatical forms too, with the neural basis of the abstraction depending on the specific sensori-motor properties of the literal sense of the base. By this logic, nominal metaphors based on the metaphoric extension of nominalized action verbs (e.g. “a slump”) should draw upon the same areas as predicate metaphors involving action verbs (e.g. “to slump”) since both types of metaphor entail abstract senses of action events. For both metaphors then, reliance on sensori-motor substrates in order to abstract novel meanings may become less relevant with conventionalization, with the activation associated with both shifting centripetally toward classic perisylvian language areas with increased familiarity (Schmidt et al., 2009; Chatterjee, 2010).
To summarize, this study tests several hypotheses regarding the neural processing associated with the conventionalization of metaphor meaning. One possibility is that novel metaphors recruit right hemisphere semantic processing. As metaphors become conventionalized a shift from right-sided to left-hemisphere mediated comprehension occurs. Another possibility is that nominal and predicate metaphors differ in their neural substrates, with nominal comprehension initially requiring comparison and predicate metaphor drawing instead upon abstraction. By this view, conventionalization of metaphor meaning is predicted to correlate with activation shifts both across hemispheres and within the left hemisphere. In the case of nominal metaphors, this shift would be observed as a decreased reliance on right hemisphere homologues with increased familiarity. In the case of predicate metaphors, the shift would be centripetally towards perisylvian cortex as abstraction away from sensory and motor representations becomes less relevant. We tested these possibilities by creating (Cardillo et al., 2010) and optimizing new nominal and predicate metaphors and marrying a novel, in situ conventionalization procedure outside the scanner with a parametric analysis of familiarity when reading metaphors inside the scanner.
2. Methods
2.1 Participants
Twenty paid volunteers participated in this experiment (mean age = 25.9, SD = 3.8). All participants were right-handed, native English speakers without history of neurological or psychiatric symptoms and provided written consent in accordance with the policies of the Institutional Review Board at the University of Pennsylvania. Eighteen different paid volunteers (mean age = 22.7, SD = 2.6) participated in a behavioral version of the task outside the scanner.
2.2 Stimuli
Stimuli consisted of 120 of the 280 metaphoric sentences found in the stimulus set we normed previously (Cardillo et al., 2010). Half of the stimuli were nominal metaphors and half were predicate metaphors. All nominal metaphors were of the form “The X was a Y” where Y was the word being used metaphorically. All predicate metaphors consisted of a noun phrase and an action verb followed by a prepositional phrase. In these items the verb was the word used metaphorically. Although the syntax differed between nominal and predicate metaphors, the word used metaphorically was similar semantically. In predicate metaphors the base was always the past tense form of an action verb and in nominal metaphors the base was always a nominalized action verb. This close matching between metaphor types ensures the strictest test that the syntactic form of a metaphor determines the kind of cognitive processes necessary to comprehend it. See Table 1 for examples of each metaphor type and Appendix A for the complete set.
Table 1.
Example stimuli
| Metaphor | Example |
|---|---|
| Nominal | The essay was a cruel snicker. |
| His handshake was a mumble. | |
| The man’s gaze was a shameless slurp. | |
| His work experience was a clumsy clamber. | |
| The reception was an icy swim. | |
| Predicate | The flowers purred in the sunlight |
| His curls roared amongst the bald men. | |
| The insults hopped on her tongue. | |
| The sad wife sidled up to the scotch. | |
| The urgent letter tugged at her sleeve. |
Appendix A.
Complete stimulus list
| Type | Itema | Base Term | Metaphor |
|---|---|---|---|
| Nominal | NMA06 | buzz | The ideas were a brain buzz. |
| Nominal | NMA07 | cackle | The legislation was a corporate cackle. |
| Nominal | NMA09 | chirp | His business card was an optimistic chirp. |
| Nominal | NMA13 | cough | The celebrity trial was a smothered cough. |
| Nominal | NMA14 | crackle | The seizure was a brain crackle. |
| Nominal | NMA16 | fart | His suggestion is an ill-timed fart. |
| Nominal | NMA17 | flush | His memoirs were a toilet flush. |
| Nominal | NMA18 | gasp | The logging was an environmentalist gasp. |
| Nominal | NMA19 | giggle | His ugly car is a giggle. |
| Nominal | NMA22 | grunt | The employee was a grunt. |
| Nominal | NMA24 | hiss | His posture was a cat’s hiss. |
| Nominal | NMA26 | huff | His framed degree was a proud huff. |
| Nominal | NMA33 | mumble | His handshake was a mumble. |
| Nominal | NMA35 | pop | The dad’s decision was a balloon pop. |
| Nominal | NMA36 | purr | Her smile was a cat’s purr. |
| Nominal | NMA40 | screech | The bank’s letter was a screech. |
| Nominal | NMA42 | shriek | The purchase was a gleeful shriek. |
| Nominal | NMA45 | slurp | The man’s gaze was a shameless slurp. |
| Nominal | NMA48 | sneeze | Her arrival was an unexpected sneeze. |
| Nominal | NMA49 | snicker | The essay was a cruel snicker. |
| Nominal | NMA50 | sniff | His interest was a mere sniff. |
| Nominal | NMA51 | snigger | The book was a sexist snigger. |
| Nominal | NMA55 | squawk | The banner was a patriotic squawk. |
| Nominal | NMA56 | squeal | The bill was a corrupt squeal. |
| Nominal | NMA57 | stammer | The demo tape was a shy stammer. |
| Nominal | NMA58 | stutter | Their courtship was a bashful stutter. |
| Nominal | NMA59 | Her shirt was a cheerful twitter. | |
| Nominal | NMA61 | wail | His hangover was his liver’s wail. |
| Nominal | NMA62 | weep | The film was a poignant weep. |
| Nominal | NMA63 | whimper | The flowers were a widow’s whimper. |
| Nominal | NMA65 | whinny | His message was a hopeful whinny. |
| Nominal | NMA67 | whisper | His glance was a furtive whisper. |
| Nominal | NMA68 | whistle | His smirk was a shameless whistle. |
| Nominal | NMA70 | yip | The opening was an eager yip. |
| Nominal | NMM01 | canter | His youth was a happy canter. |
| Nominal | NMM02 | cartwheel | The puzzle was a logic cartwheel. |
| Nominal | NMM04 | chop | The review was a karate chop. |
| Nominal | NMM05 | clamber | His work experience was a clumsy clamber. |
| Nominal | NMM11 | dig | The therapy was an archeological dig. |
| Nominal | NMM12 | dodge | His smile was a charming dodge. |
| Nominal | NMM14 | drive | The writer’s job is a lonely drive. |
| Nominal | NMM21 | glide | The art major was a glide. |
| Nominal | NMM23 | jog | The test review was a quick jog. |
| Nominal | NMM24 | jump | The home purchase was a bungee jump. |
| Nominal | NMM28 | limp | The winter was a heartbroken limp. |
| Nominal | NMM32 | polka | The friendship was a crazy polka. |
| Nominal | NMM33 | pounce | The purchase was a tiger pounce. |
| Nominal | NMM35 | pull | The road was an irresistible pull. |
| Nominal | NMM39 | roll | The new roommate was a dice roll. |
| Nominal | NMM42 | scamper | Her inquiries were a nervous scamper. |
| Nominal | NMM44 | skydive | The home purchase was a skydive. |
| Nominal | NMM47 | sleepwalk | The test was a sleepwalk. |
| Nominal | NMM48 | slither | The deal was a greedy slither. |
| Nominal | NMM49 | slouch | The poetry was a teenage slouch. |
| Nominal | NMM58 | swarm | The numbers were a brain swarm. |
| Nominal | NMM59 | sweep | The eviction was a mean sweep. |
| Nominal | NMM60 | swim | The reception was an icy swim. |
| Nominal | NMM66 | tug | The shop display was a gentle tug. |
| Nominal | NMM67 | wander | The anthology was a literary wander. |
| Nominal | NMM68 | wave | The letter was a goodbye wave. |
| Predicate | PMA01 | argue | The plaid pants argued with the paisley shirt. |
| Predicate | PMA04 | bleat | The wrinkled shirt bleated for an iron. |
| Predicate | PMA05 | blurt | His license blurted out his true age. |
| Predicate | PMA08 | chant | The waves chanted to the surfer. |
| Predicate | PMA09 | chat | Her short skirt chatted up all the men. |
| Predicate | PMA10 | cheer | The posters cheered for the candidate. |
| Predicate | PMA12 | chirp | His heart chirped at her name. |
| Predicate | PMA13 | chuckle | His eyes chuckled at the cute note. |
| Predicate | PMA14 | clamor | The banners clamored at the voters. |
| Predicate | PMA15 | clash | His smile clashed with his eyes. |
| Predicate | PMA18 | clomp | The student clomped through the task. |
| Predicate | PMA19 | coo | The cheap records cooed to the teenager. |
| Predicate | PMA22 | drone | The contract droned for many pages. |
| Predicate | PMA23 | drum | The liquor drummed through his body. |
| Predicate | PMA28 | growl | The cruise ship growled at the fishing boat. |
| Predicate | PMA29 | grumble | The sun grumbled at all the clouds. |
| Predicate | PMA30 | grunt | The truck grunted at the small parking space. |
| Predicate | PMA31 | hiss | The designer purse hissed at the fakes. |
| Predicate | PMA32 | holler | The bold packaging hollered at the shoppers. |
| Predicate | PMA33 | howl | His sore legs howled through the last mile. |
| Predicate | PMA36 | moan | His feet moaned for a massage. |
| Predicate | PMA40 | purr | The flowers purred in the sunlight. |
| Predicate | PMA41 | rant | The pamphlet ranted against the politician. |
| Predicate | PMA43 | roar | His curls roared amongst the bald men. |
| Predicate | PMA45 | screech | The date screeched to a stop. |
| Predicate | PMA47 | shout | Her tacky shirt shouted at the interviewer. |
| Predicate | PMA48 | shriek | Her pale skin shrieked in the sun. |
| Predicate | PMA49 | sigh | The editorial sighed over the riots. |
| Predicate | PMA50 | sing | The sunset sang to the lovers. |
| Predicate | PMA51 | sizzle | The dancers sizzled under the lights. |
| Predicate | PMA52 | snarl | The thorns snarled at the gardener. |
| Predicate | PMA53 | sniff | The hem of his trousers sniffed at the floor. |
| Predicate | PMA57 | sputter | The interview sputtered to a finish. |
| Predicate | PMA60 | stutter | His feet stuttered on the dance floor. |
| Predicate | PMA62 | wail | The street signs wailed at the lost driver. |
| Predicate | PMA64 | whimper | The plants whimpered in the shadows. |
| Predicate | PMA65 | whine | The garden whined for water. |
| Predicate | PMA70 | yowl | His tongue yowled at the spice. |
| Predicate | PMM01 | balloon | The kid’s courage ballooned during the fight. |
| Predicate | PMM05 | crawl | The banker crawled through the contract. |
| Predicate | PMM11 | flit | The model flitted between hair colors. |
| Predicate | PMM12 | flounder | The television show floundered in the spring. |
| Predicate | PMM14 | hobble | The gambler hobbled through the card game. |
| Predicate | PMM18 | lope | The rich loped through the recession. |
| Predicate | PMM20 | lurch | The football team lurched through the season. |
| Predicate | PMM21 | mosey | The friend moseyed through the photographs. |
| Predicate | PMM22 | plod | The surgeon plodded through the operation. |
| Predicate | PMM26 | puff | The coach puffed up the football team. |
| Predicate | PMM29 | reel | The colonel reeled in the officers. |
| Predicate | PMM30 | retreat | The painter retreated from his gloomy marriage. |
| Predicate | PMM33 | sail | The frank speaker sailed towards a finish. |
| Predicate | PMM34 | sashay | The divorcee sashayed through the paperwork. |
| Predicate | PMM36 | shuffle | The ex-boyfriend shuffled out of her life. |
| Predicate | PMM37 | sidle | The sad wife sidled up to the scotch. |
| Predicate | PMM40 | slide | The conversation slid into a wall. |
| Predicate | PMM43 | snake | The lies snaked through her story. |
| Predicate | PMM60 | totter | The cake shop tottered on bankruptcy. |
| Predicate | PMM61 | traipse | The artist traipsed through three marriages. |
| Predicate | PMM63 | tug | The urgent letter tugged at her sleeve. |
| Predicate | PMM67 | wander | The patient wandered through the magazine. |
Refers to item code (Cardillo et al., 2010)
Items were selected carefully in order to minimize lexical and sentential differences between metaphor types as much as possible. See Table 2 for complete details of the metaphor properties. Two-tailed independent t-tests with an alpha-criterion of .05 indicated nominal and predicate metaphors did not differ significantly in terms of familiarity, naturalness, imageability, figurativeness, interpretability, average frequency, valence, or the average time required to make a valence judgment. Critically, the familiarity ratings indicated both metaphor types were highly interpretable despite being fairly novel (averaging < 3.5 for familiarity on a 7-point scale). The only significant differences to emerge were that nominal metaphors were significantly shorter than predicates by all three length measures (characters: t118 = 6.97, p < .0005; words: t118 = 3.95, p < .0005; content words: t118 = 4.93, p < .0005) and their content words were, on average, slightly less concrete (t118 = 4.86, p < .0005). To ensure these differences did not obscure differences of interest between the neural signatures of nominal and predicate metaphors, length (in terms of number of content words) and average concreteness were included as covariates of non-interest in the image analysis.
Table 2.
Lexical and sentential properties of metaphors
| Property | Nominal | Predicate | ||
|---|---|---|---|---|
| M | SD | M | SD | |
| Concretenessa | 439 | 63 | 488 | 47 |
| Frequencyb | 60 | 78 | 71 | 72 |
| Frequencyc | 62 | 94 | 59 | 64 |
| # Characters | 32.8 | 4.1 | 38.5 | 4.9 |
| # Words | 6.2 | 0.5 | 6.6 | 0.7 |
| # Content Words | 3.1 | 0.5 | 3.6 | 0.6 |
| Interpretability | .92 | 0.09 | .94 | 0.07 |
| Familiarity | 3.03 | 0.6 | 2.94 | 0.6 |
| Naturalness | 3.33 | 0.7 | 3.12 | 0.6 |
| Imageability | 3.48 | 0.6 | 3.28 | 0.7 |
| Figurativeness | 5.67 | 0.5 | 5.76 | 0.8 |
| Valence RT (ms) | 1518 | 235 | 1537 | 211 |
| Valence Ratio (Positive: Negative) | 0.24 | 0.28 | 0.18 | 0.26 |
Key:
Concreteness = values from MRC Psycholinguistic Database (Coltheart, 1981),
Frequency = values from a corpus of written American English (Kucera & Francis, 1967);
Frequency = SUBTLWF values from corpus of American English subtitles (Brysbaert & New, 2009).
Bold font indicates significant condition differences (p < .05); all other differences non-significant (p > .10).
2.3 Experimental design and procedure
Participants’ familiarity with the metaphors was carefully manipulated outside the scanner in a training task. The stimuli were initially novel for all participants. In order to simulate the process of familiarization that happens in everyday reading and speaking, we pre-exposed participants to a subset of the stimuli before scanning. In this way, at the time of scanning participants’ familiarity with each item could be assigned to one of three levels of familiarity: Novel, Moderate, or High. Novel items (N) were the third of the stimuli that a participant did not see at all before the scan. Moderate familiarity items (M) were the third that a participant was pre-exposed to twice and High familiarity items (H) were the third that a participant was pre-exposed to five times. To ensure that pre-exposure elicited thoughtful semantic processing, participants were required to make various semantic judgments of the stimuli in the same manner as when they were initially normed (Cardillo et al., 2010). For Moderate familiarity items, participants provided Familiarity and Naturalness ratings. For High familiarity items, participants provided Familiarity, Naturalness, Imageability, and Figurativeness ratings as well as an interpretation in their own words. For each participant, half of the Novel, Moderate, and High familiarity items were Nominal metaphors (NOM) and half were predicate metaphors (PRED). Assignment of items to familiarity levels was counterbalanced such that all items appeared in all conditions across participants.
After the pre-scan familiarization task, participants received 10 practice trials of the in-scan task. The in-scan task consisted of reading the full set of metaphoric stimuli and answering Yes/No comprehension questions following 15 % of items to ensure that participants’ were paying attention and reading for content. Metaphors and questions were presented centrally for five seconds in 18 point, white Arial font on a black background. Each participant read metaphors and questions in a unique, pseudo-random order interspersed with fixation trials of 3–12 seconds duration. Optimal orders for detecting differences in the BOLD response to each condition were determined using OptSeq (Dale, 1999). The presentation of stimuli was distributed across four runs of equal duration (261 seconds).
The pre-scan manipulation of initially novel metaphors ensured a precisely known parametric variation in familiarity. We remained concerned, however, that any neural correlation with this variation not reflect theoretically uninteresting increases in reading speed with increased familiarity. To control for this potential confound, we conducted a nearly identical behavioral version of the task outside the scanner with a different group of 18 volunteers. These participants completed the identical familiarization tasks and reading task as the scan participants with one additional requirement: they were instructed to press the space bar when they had finished reading each metaphor. This allowed us to calculate an average reading rate for each item at each of the three familiarity levels as well as an average reading rate for both metaphor types. Independent sample t-tests with an alpha-criterion of .05 indicated that reading rate did not significantly differ between Predicate and Nominal metaphors but that the High and Moderate familiarity metaphors were read significantly faster than Novel metaphors (Novel: mean = 1627, SD = 391; Moderate: mean = 1442, SD = 297; High: mean = 1393, SD = 339). To avoid a possible confounding influence of reading rate changes, these values were included as a covariate of non-interest in the data analyses.
2.4 fMRI image acquisition and analysis
Structural and functional data were collected on a 3T Siemens TRIO scanner using a standard eight-channel head coil. T2*-weighted echo-planar, blood oxygenation level dependent (BOLD) images were collected during the functional runs (TR = 3000ms, TE = 30ms, matrix size = 64 × 64, FOV = 220, voxel size = 3.4 × 3.4 ×3 mm, flip angle = 90) and a T1-weighted high-resolution anatomical image was collected using a 3D magnetization prepared rapid gradient echo (MPRAGE) sequence (TR = 1630ms, TE = 3.87ms, T1 = 1100ms, matrix size = 256 × 256, FOV = 240, voxel size = 1 × 1 × 1 mm, flip angle = 15). Eighty-seven volumes were collected during each of the functional runs, each consisting of 50 3-mm axial slices. The first four volumes of each run were discarded to allow for steady-state magnetization. All data was collected in a single session, following immediately after the familiarization procedure and practice trials.
A trigger signal from the scanner initiated each run and E-prime 1.0 software (Psychology Software Tools) controlled stimulus presentation and the recording of responses. Participants viewed stimuli projected on a rear projection screen via a mirror mounted on the head coil. Responses were transmitted via a fiber-optic response pad system (fORP, Current Design).
Image analysis was conducted using VoxBo (www.voxbo.org). Data pre-processing procedures included slice timing correction, standard realignment, smoothing, and thresholding to exclude extraparenchymal voxels. Anatomical images were then normalized to a standard Montreal Neurological Institute (MNI) template using the SPM2 normalization routine (http://www.fil.ion.ucl.ac.uk/spm/), and the functional data were registered to the normalized anatomical data. Functional data were realigned using rigid body alignment and sinc interpolation to register each volume with the participant’s first functional volume.
For each subject, a voxel-wise analysis of the data from the four concatenated runs was performed with a modified version of the general linear model (Worsley & Friston, 1995). Included in this model were covariates of interest modeling different task conditions (NOM, PRED, Familiarity (N, M, H), Questions) as well as three stimuli-related covariates of non-interest (Length, Concreteness, Reading Time), and an intercept, scan effects, and six motion covariates of non-interest (translation and rotation along x, y, and z axes). Task covariates were delta functions convolved with a standard hemodynamic response function, spatially smoothed using a full width at half maximum (FWHM) Gaussian kernel of 3 voxels, mean normalized, and high-pass filtered at .01 Hz.
Three different random effects analyses were performed on beta values obtained from the first-level analyses to test for hypothesized differences between conditions. First, we conducted a parametric analysis to determine regions for which activation was correlated with participant familiarity with metaphors. To test this hypothesis, we first created a functional mask of areas activated by all Metaphors more than baseline ((NOM + PRED) – Fixation) in order to restrict our hypothesis testing to meaningfully activated areas. This mask was intentionally thresholded leniently without correction for multiple comparisons (p < .05) since it was not intended for hypothesis testing so much as to minimize excessive statistical comparisons in the analyses of primary interest. We then tested within this mask for voxels whose signal was significantly positively or negatively correlated with our three-level Familiarity covariate, irrespective of metaphor type. The resulting statistical map was thresholded using a permutation-derived t-statistic corresponding to p < .05 (Nichols & Holmes, 2001).
Next, we considered our secondary question regarding metaphor type, by conducting a subtraction analysis to determine whether nominal and predicate metaphors activate non-overlapping neural systems. For this analysis we again restricted our hypothesis testing to the regions defined by the functional mask of all Metaphors, testing for differences in activation for nominal metaphors compared to predicate metaphors and vice versa, collapsing across familiarity level. For these subtractions (NOM − PRED and PRED − NOM), we again used permutation thresholding at p < .05 to correct for multiple comparisons. The inclusion of concreteness and length as covariates of non-interest ensured that any variance predicted by the slightly longer, more concrete nature of the predicate metaphors did not contribute to its parameter estimate and inadvertently bias us towards finding a statistical difference between metaphor types.
To increase our sensitivity to possible nominal/predicate differences, we also performed a random effects analysis for six anatomically defined regions of interest (ROIs) in each hemisphere. These regions were chosen based on their frequent activation during a diverse set of sentence comprehension tasks: pars opercularis (Pop), pars triangularis (Ptr), and pars orbitalis (Por) of the inferior frontal gyrus and the superior (STG) middle (MTG), and inferior (ITG) gyri of the temporal lobe. Masks were drawn on the MNI Colin brain template by graduate and medical student trainees and then reviewed by a senior neurologist. Time series for each participant were averaged across all voxels within each anatomical ROI and then a normalized beta value for each covariate of interest was derived from each participant’s GLM. One-sample t-tests were then used to determine if a given contrast was consistently greater than zero within the ROI across participants.
3. Results
3.1 Behavioral
High accuracy on the comprehension questions indicated participants attended to metaphors and understood them well (mean accuracy = 96.9%, SD = 3.7).
3.2 Activations correlated with metaphor familiarity
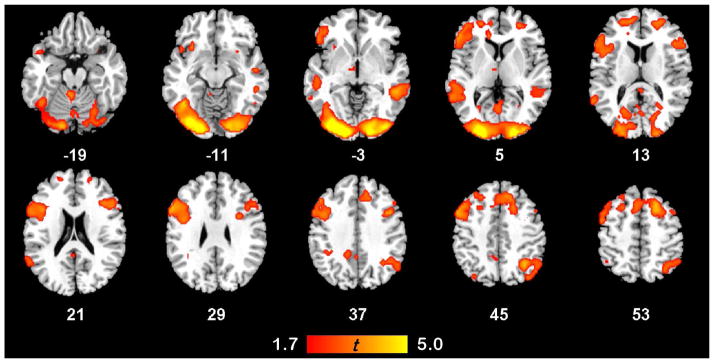
The functional mask determined by the subtraction of baseline activity from the activation for reading nominal and predicate metaphors revealed a typical, left-lateralized network of language-sensitive brain areas (see Fig. 1). Reading metaphors strongly activated pars triangularis and pars opercularis of the left inferior frontal gyrus, as well as pars orbitalis. This activation extended posteriorly and ventrally into the insula and anterior temporal lobe, and also included the posterior extent of the middle temporal gyri, as well as the inferior temporal and fusiform gyri. Activation was also seen in supplementary motor cortex, the precentral gyrus, and thalamus of the left hemisphere. Robust bilateral activation of visual cortex in the occipital lobe was observed, as well as more modest bilateral activity in the cerebellum, posterior cingulate, temporal poles, and superior and middle frontal gyri. Activation of right hemisphere homologues of language areas was also observed, although the extent and intensity of this activation within prefrontal cortex and middle temporal gyrus was weaker than in the left. Strongly right-lateralized activity was restricted to the inferior parietal cortex.
Figure 1.
Functional Mask of All Metaphors. Areas activated by the contrast of All Metaphors (Nominals + Predicates) versus the baseline condition (fixation). Intensity scale reflects t-statistic range. Axial slice numbers correspond to MNI Colin template. Images are thresholded at p < .05, uncorrected.
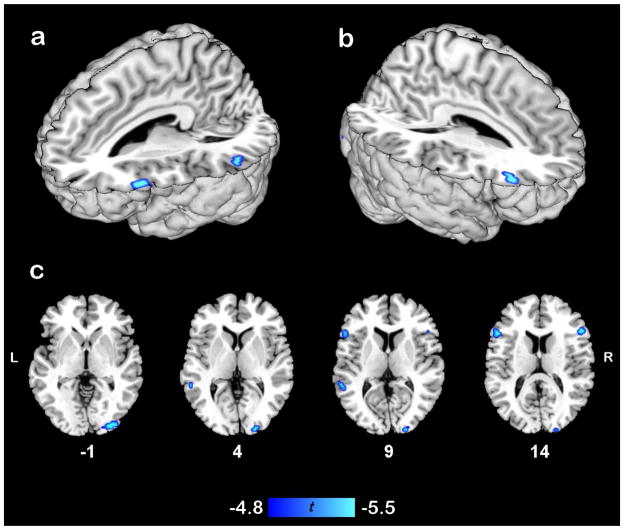
The parametric analysis of familiarity calculated within this functional mask revealed four areas that were sensitive to familiarity: bilateral pars triangularis of the inferior frontal gyrus, left posterior middle temporal gyrus, and right postero-lateral occipital gyri (see Table 1 and Fig. 2). BOLD signal in all four regions was significantly negatively correlated with familiarity level, indicating activity in these regions decreased as familiarity increased. In contrast, this analysis revealed no brain regions for which activation increased as metaphor familiarity increased.
Figure 2.
Parametric Familiarity Effect. Areas within functional mask in which BOLD signal significantly negatively correlated with participant familiarity with metaphors: a) 3D rendering of significant effects in the left hemisphere, b) 3D rendering of significant effects in the right hemisphere, and c) axial views of clusters in inferior frontal gyrus, posterior middle temporal gyrus, and postero-lateral occipital cortex. Sagittal slice numbers correspond to MNI Colin template. Images are thresholded at a permutation-derived t-statistic corresponding to p < .05.
It is important to note that the inclusion of reaction time as a covariate of non-interest in our model allowed us to account for (and disregard) any activations uniquely attributable to a simple speeding of reading rate with increased familiarity. However, given the modest correlation between Familiarity and RT (r=.37), we also considered whether activation in some voxels might correlate with both Familiarity and RT, a possibility that our original confound removal procedure would still allow. In most experimental designs, reaction time differences between conditions are an inadvertent confound. More difficult, time-consuming conditions are likely to more strongly engage the same areas necessary for the easier trials and additionally recruit bilateral working memory and attentional control areas that are not specific to the task at hand (Binder et al., 2009). We have noted elsewhere (Cardillo et al, 2010) that such difficulty differences may have fueled reported differences, for instance, between metaphoric and literal stimuli and contributed to ambiguity about the neural substrates for metaphor. However, in studies of priming or designs such as this one, a speeding of reading rate is an index of the cognitive process of theoretical interest (i.e. increased neural efficiency when accessing a figurative meaning), rather than an inadvertent condition difference. In such circumstances, fully disentangling response time from cognitive process may be theoretically inappropriate and cause one to overlook relevant brain areas. For this reason, we allowed our initial analysis to implicate areas whose activation may have been predicted by both familiarity and RT. To clarify this observed pattern, we also ran a further analysis in which we looked for areas uniquely accounted for by our manipulation of familiarity, independent of reading rate changes. For this analysis, we re-ran the parametric test of Familiarity with Familiarity orthogonalized with respect to RT, once again testing within our functionally derived Metaphor mask and using permutation thresholding at p < .05. Results indicated that only left inferior prefrontal cortex still showed a significant negative correlation with Familiarity above and beyond any effect associated with speeded reading times.
3.3 Activations specific to nominal or predicate metaphors
Both comparisons of nominal and predicate metaphors (NOM − PRED and PRED − NOM) within the functionally derived Metaphor mask failed to reveal any significant differences. Further, no significant differences between nominal and predicate metaphors emerged in any of the anatomical ROI analyses in either hemisphere even at levels uncorrected for multiple comparisons.
4. Discussion
Using a novel familiarization procedure, this study reframes a prominent cognitive model of metaphor comprehension, the Career of Metaphor (Bowdle & Gentner, 2005), in neural terms. Our primary objective was to understand how neural responses to novel metaphors are tuned by experience. We found neural activity decreased within bilateral inferior frontal gyri, left posterior middle temporal cortex, and right postero-lateral occipital cortex as metaphors became more familiar, and found no areas with increased neural signal associated with increased familiarity. Our secondary goal was to consider whether metaphoric diversity based on syntax or on semantics is a meaningful distinction at a neural level. We found no evidence for a neural differentiation between nominal and predicate metaphors based on inherent syntactic differences. In the following sections we discuss each of these findings in turn.
The most significant finding of our study was that the conventionalization of a novel metaphoric meaning occurs within a bilaterally distributed network. Foremost, the inferior frontal gyrus of the left hemisphere (LIFG) and its right hemisphere homologue were tuned to the evolving career of metaphors. LIFG activation is observed in a surprisingly diverse range of stimuli types and tasks. When tasks require comprehension of linguistic materials, this region is engaged by conditions that place high demands on the need to choose between competing semantic representations. For instance, verb generation tasks more strongly engage LIFG when there are at least two salient possible responses (Thompson-Schill et al., 1997). Accessing non-dominant meanings of lexically ambiguous words also increases activity in inferior frontal cortex (Rodd et al., 2005) and left hemisphere injured patients with damage to this area are impaired in this ability (Vuong & Martin, 2011). LIFG is also recruited during sentence comprehension tasks when two or more semantic representations compete, such as when sentence endings are semantically unpredictable, implausible, or anomalous (Cardillo et al., 2004; Kiehl et al., 2002; Zempleni et al., 2007). An extensive review of the evidence indicates that two of this region’s primary functions during sentence comprehension are to detect semantic conflict and initiate re-analysis after misinterpretation (Novick et al., 2005). Although our dataset was not designed to test this interpretation the LIFG may be more strongly recruited during novel metaphor comprehension because these expressions initially evoke competing, nonsensical literal interpretations of the base term that require suppression. As the metaphorical sense of the base term becomes familiar it is more readily activated, thereby diminishing the cognitive control needed to suppress the inappropriate interpretation and decreasing reliance on LIFG.
Alternatively or in addition, LIFG may be recruited to adjudicate between competing metaphorical senses of the base term. For some items, base terms may lend themselves to more than one novel figurative sense, creating interpretive ambiguity at the sentence level. For instance, “The day’s events were a whir” can reasonably be interpreted to mean the day’s events passed quickly, that they are indistinct in hindsight, or both. In our norming task (Cardillo et al, 2010), we calculated intelligibility based on whether items consistently evoked plausible metaphorical interpretations, not whether they evoked only a single interpretation, because we felt this to be more reflective of natural language. It may be that as participants become more familiar with novel metaphors, they commit to a single interpretation, and LIFG tracks this reduction in uncertainty.
We propose that the observed decreases in right prefrontal cortex with increased familiarity serve a similar function. Although the right inferior frontal gyrus is commonly associated with the need to inhibit prepotent responses (e.g. Aron et al, 2003; for review, see Aron, Robbins, & Poldrack, 2004), such a mechanism would not be required by our passive reading task. More relevant, neuroimaging studies showing that activity in right inferior prefrontal cortex is modulated by the complexity of a language task, suggesting it plays a supportive role when cognitive demands on the left-lateralized language system are high (Jung-Beeman, 2005). For instance, comprehension tests demonstrate that right prefrontal activity increases as the syntactic complexity of sentences increases (Just et al, 1996; Constable et al, 2004), as the abstractness of verbs increases (Rodriguez-Ferreiro et al, 2010), and as the difficulty of a semantic judgment increases (Yang et al, 2009). Studies of analogical reasoning also indicate increased loading on RIFG during analogical reasoning (Cho et al, 2009; Watson and Chatterjee, in press), a suggestive parallel in light of arguments that metaphors are a species of analogy and understood using comparable mechanisms (Gentner, et al, 2001). A recent meta-analysis concluded that right inferior prefrontal cortex supports left inferior prefrontal executive function across a variety of language tasks, its recruitment increasing as task demands increase (Vigneau et al, 2011). With respect to our own study, we suggest that as semantic selection demands and ambiguity decrease with increased metaphor familiarity, so too does the supportive role of LIFG’s right hemisphere homologue.
We further speculate that the function of the left posterior middle temporal sensitivity to metaphor familiarity is of a different nature. While prefrontal cortex operates in a domain-general fashion to resolve competition between representations, in our study left pMTG is likely to be the substrate for these competing representations. Postero-lateral temporal cortex is commonly activated by studies involving either action pictures and videos or the words used to describe them (Kable et al., 2002, 2005) as well as the figurative extensions of these verbs (Chen et al. 2008; Saygin et al., 2009; Wallentin et al., 2005a b). Given its proximity to motion-sensitive area V5, this pattern reflects a close parallel between the neural substrates for motion perception and the words describing motion events. However, some studies report that this area is activated preferentially for most verbs, not just motion verbs (Bedny et al., 2008), suggesting that postero-lateral temporal cortex may be sensitive to event semantics, independent of grammatical class or motion features. This interpretation accords well with our stimuli, which included some verbs of sound as well as verbs of motion in our predicate metaphors, and some nominalized verbs of sound as well as nominalized verbs of motion in our nominal metaphors. We propose that left pMTG mediates the abstraction process necessary to derive a metaphorical sense of action events. As the metaphorical sense becomes more familiar, the abstraction process becomes more neurally efficient, thereby lessening its demand on the region.
We also observed familiarity-correlated decreases in right postero-lateral occipital cortex, an unexpected result. This region, a visual association area, is generally associated with the processing of visual forms and features. In construing the metaphorical sense of a word, concrete sensory details relating to the visual features of the word’s referent are minimally relevant. With increased familiarity with this abstract word sense, postero-lateral occipital cortex processing may be less necessary for comprehension. In this case, the function of postero-lateral occipital cortex during metaphor comprehension is similar to that of left pMTG: both exhibit decreases in activity as metaphors become more abstract and less linked to their real-world referents.
We acknowledge that the above interpretations of our results are speculative at this point and require additional studies to test them. However, our results are not likely to simply index difficulty, a heterogeneous construct commonly operationalized in terms of reaction time. By co-varying out any unique effects of reaction time, we ensure a strict report of neural areas responsive to changes in familiarity. Moreover, our follow-up orthogonalization analysis indicates that even with modest reaction time differences between conditions, when items are well-matched on other lexical and sentential characteristics affecting comprehension, reaction time does not predict the observed neural differences in LIFG. This pattern suggests two possibilities: either LIFG mediates a cognitive process during metaphor comprehension that is qualitatively different from the other familiarity sensitive areas, or activity in LIFG exhibits greater decreases as a result of increased familiarity than is predicted by reaction time alone. Determination of the precise cognitive mechanisms driving these novelty –processing speed relationships remains a question for future research.
With respect to the neural metaphor literature, our study supports and refines the right hemisphere novelty and salience hypotheses. Both accounts, as well as our previous speculations (Schmidt et al., 2009), predicted a shift in lateralization from the right hemisphere to the left hemisphere as metaphors become familiar. Our conventionalization procedure indicated instead that the career of metaphor is mediated by decreased processing within both hemispheres, rather than a decrease in the right and increase in the left with increased familiarity. This bilateral frontal pattern accords with other metaphor studies directly contrasting semantic processing of novel and conventional metaphors (Arzouan et al, 2007; Mashal et al, 2007; Yang et al, 2009; but see Schmidt & Seger, 2009), refining the right hemisphere novelty and salience hypotheses to incorporate neural decreases, but not increases, within the distributed network of regions subserving novel metaphor comprehension.
Our study also considered whether different types of metaphors rely upon different neural substrates. Cognitive models for metaphor comprehension are almost exclusively concerned with nominal metaphors. Glucksberg (2003) has argued that the same categorization mechanism for comprehending nominal metaphors applies to predicate metaphors, but behavioral studies on predicate metaphors are scarce (Torreano & Glucksberg, 2005). We are unaware of any studies contrasting them directly.
Our finding that the neural processes associated with nominal and predicate metaphors do not differ suggests that semantic rather than syntactic features are critical to metaphor processing. When the semantic features of the figuratively used word are matched across metaphor types, and other lexical and sentential differences are controlled, nominal metaphors and predicate metaphors are processed similarly. More specifically, the three features distinguishing different types of metaphor - syntactic construction, grammatical class of the base term, and semantic properties of the base term – do not appear of equal importance. Typically, these features are confounded in studies. Nominal metaphors entail “X is a Y” assertions with concrete object nouns typically used as the base term. Predicate metaphors entail a different syntax and have previously always involved dynamic verbs of motion. In our study, however, we intentionally equated semantic features of the base term by using nominalized verbs as base terms in the nominal condition. We attribute the similarity of nominal and predicate activation patterns to this critical semantic similarity between conditions. Semantics trumps syntactic structure and grammatical class as determinants of neural processing of metaphors. Future studies contrasting predicate metaphors with nominal metaphors using concrete, object nouns as the base term might be more likely to reveal differences, with nominal metaphors more likely in this case to draw upon typical object/noun processing areas.
5. Conclusions
The Career of Metaphor model (Bowdle & Gentner, 2005) integrates in a single cognitive model a variety of patterns observed in behavioral studies of metaphor comprehension. The model’s chief strength is that it accounts well for behavioral evidence indicating two different cognitive strategies for metaphor comprehension, comparison and categorization. However, how these cognitive mechanisms translate to neural processes has not been explicit. Our hypothesis is that the familiarity-dependent changes we observed in prefrontal and left posterior temporal areas relate to comparison and categorization mechanisms, respectively. On the one hand, the cross-domain mapping entailed by a comparison process might reasonably be mediated by prefrontal cortex given this region’s involvement in cognitive control, or the ability to select contextually-appropriate representations in the presence of competing, pre-potent options (Novick et al., 2005). Novel metaphors place high demands on such control because they require suppression of the literal sense of the sentence as well as the suppression of the irrelevant features of the words being used abstractly (in predicates) or likened to a different domain (in nominals). In contrast, a familiar metaphor might be understood much like a literal statement and retrieving metaphorical senses of words might be much like the fairly mundane, less effortful retrieval of the contextually-appropriate senses of literal words, thereby reducing demand on prefrontal cortex.
On the other hand, our abstraction interpretation of the role of posterior middle temporal gyrus engagement is similar in spirit to the categorization process that the Career of Metaphor model (Bowdle & Gentner, 2005) posits predominates once metaphors become familiar. As we observed decreases in this area with increased familiarity, our data reveal that switching from comparison to categorization entails a honing of the neural networks within a region as opposed to across regions. One possibility is that, at a neural level, categorization is a learning process that operates much like the sharpening accounts of repetition suppression effects (Grill-Spector et al., 2006). More selective neural engagement within the cortices most relevant to the literal sense of metaphorically extended words may reflect more efficient activation of only the most relevant features of the base word. Consistent with this view is a recent report of decreased activation in primary motor and biological motion perception areas for more familiar predicate metaphors (Desai et al., 2011).
That metaphor is a pervasive and creative form of linguistic expression is self-evident, and that metaphors change over time is uncontroversial. Yet, we know little of the neural instantiation and evolution of metaphors. Our data are a first step towards a common accounting of the cognitive and neural careers of metaphors, emphasizing the critical role of both hemispheres in deriving and refining creative meanings.
Table 3.
Areas significantly modulated by parametric variation of familiarity
| Area | Cluster size (# voxels)a | Peak Voxel Coordinatesb | Max t-statistic | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Left Inferior Frontal Gyrus | 23 | −54 | 25 | 10 | −5.84 |
| *After orthogonalization with respect to RT | 10 | −54 | 25 | 10 | −2.18 |
| Right Inferior Frontal Gyrus | 16 | 56 | 27 | 16 | −6.19 |
| Left Posterior Middle Temporal Gyrus | 11 | −57 | −41 | 10 | −5.50 |
| Right Postero-lateral Occipital cortex | 39 | 33 | −92 | −5 | −6.85 |
Voxel dimensions = 3.4 × 3.4 × 3.0;
Coordinates correspond to MNI stereotaxic space.
Acknowledgments
We thank Bianca Bromberger and Matt Lehet for their assistance with behavioral data collection. We are also indebted to Dan Kimberg, Geoff Aguirre, and two anonymous reviewers for guidance on experimental design and/or image analysis.
ROLE OF FUNDING SOURCE
This study was funded by National Institute of Health grants ROI-HD-050199 and RO1-DC008779 awarded to Anjan Chatterjee. NIH had no role in study design, data collection and analysis, interpretation of the results, writing of the manuscript, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron A, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Arzouan Y, Goldstein A, Faust M. Dynamics of hemispheric activity during metaphor comprehension: Electrophysiological measures. NeuroImage. 2007;36:222–231. doi: 10.1016/j.neuroimage.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Damasio A. Embodied semantics for actions: Findings from functional brain imaging. J Phys Paris. 2008;102:35–39. doi: 10.1016/j.jphysparis.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Bedny M, Caramazza A, Grossman E, Pascual-Leone A, Saxe R. Concepts are more than percepts: The case of action verbs. J Neurosci. 2008;28:11347–11353. doi: 10.1523/JNEUROSCI.3039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A Critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini G, Corcoran R, Sterzi R, Paulesu E, Schenone P, Scarpa P, et al. The role of the right hemisphere in the interpretation of figurative aspects of language: A positron emission tomography activation study. Brain. 1994;117:1241–1253. doi: 10.1093/brain/117.6.1241. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Hauk O, Pulvermuller F. Grasping ideas with the motor system: Semantic somatotopy in idiom comprehension. Cereb Cortex. 2009;19:1905–1914. doi: 10.1093/cercor/bhn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowdle BF, Gentner D. The Career of Metaphor. Psychol Rev. 2005;112:193–216. doi: 10.1037/0033-295X.112.1.193. [DOI] [PubMed] [Google Scholar]
- Brysbaert M, New B. Moving beyond Kucera and Francis: A Critical Evaluation of current word frequency norms and the introduction of a new and improved word frequency measure for American English. Beh Res Meth. 2009;41:977–990. doi: 10.3758/BRM.41.4.977. [DOI] [PubMed] [Google Scholar]
- Cardillo ER, Aydelott J, Matthews PM, Devlin JT. Left inferior prefrontal cortex activity reflects inhibitory rather than facilitatory priming. J Cogn Neurosci. 2004;16:1552–1561. doi: 10.1162/0898929042568523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo ER, Schmidt GL, Kranjec A, Chatterjee A. Stimulus design is an obstacle course: 560 matched literal and metaphorical sentences for testing neural hypotheses about metaphor. Beh Res Meth. 2010;42:651–664. doi: 10.3758/BRM.42.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. Disembodying Cognition. Lang Cogn. 2010;2:79–116. doi: 10.1515/LANGCOG.2010.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Widick P, Chatterjee A. Functional-anatomical organization of predicate metaphor processing. Brain Lang. 2008;107:194–202. doi: 10.1016/j.bandl.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Moody TD, Fernandino L, Mumford JA, Poldrack RA, Cannon TD, et al. Common and dissociable prefrontal loci associated with component mechanisms of analogical reasoning. Cereb Cortex. 2010;20:524–533. doi: 10.1093/cercor/bhp121. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC Psycholinguistic Database. Qtr J Exp Psychol, Sec A: Hum Exp Psychol. 1981;33:497–505. [Google Scholar]
- Dale A. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RH, Binder JR, Conant LL, Mano QR, Seidenberg MS. The neural career of sensory-motor metaphors. J Cogn Neurosci. 2011;23:2376–2386. doi: 10.1162/jocn.2010.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner D, Bowdle BF, Wolff P, Boronat C. Metaphor is like analogy. In: Gentner D, Holyoak KJ, Kokinov BN, editors. The analogical mind: Perspectives from cognitive science. Cambridge MA: MIT Press; 2001. pp. 199–253. [Google Scholar]
- Giora R. On Our Mind: Salience, Context, and Figurative Language. Oxford: Oxford University Press; 2003. [Google Scholar]
- Glucksberg S, Keysar B. Understanding metaphorical comparisons: Beyond similarity. Psychol Rev. 1990;97:3–18. [Google Scholar]
- Glucksberg S. The Psycholinguistics of metaphor. Trends Cogn Sci. 2003;7:92–96. doi: 10.1016/s1364-6613(02)00040-2. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Hensen R, Martin A. Repetition and the brain: Neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending nautral language. Trends Cogn Sci. 2005;9:512–518. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kable JW, Lease-Spellmeyer J, Chatterjee A. Neural substrates of action event knowledge. J Cogn Neurosci. 2002;14:795–805. doi: 10.1162/08989290260138681. [DOI] [PubMed] [Google Scholar]
- Kable JW, Kan IP, Wilson A, Thompson-Schill SL, Chatterjee A. Conceptual representations of action in the lateral yemporal cortex. J Cogn Neurosci. 2005;17:1855–1870. doi: 10.1162/089892905775008625. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Liddle PF. Reading anomalous sentences: An event-related fMRI study of semantic processing. NeuroImage. 2002;17:842–850. [PubMed] [Google Scholar]
- Kircher TT, Leube DT, Erb M, Grodd W, Rapp AM. Neural correlates of metaphor processing in schizophrenia. NeuroImage. 2007;34:281–289. doi: 10.1016/j.neuroimage.2006.08.044. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational analysis of present-day American-English. Providence, RI: Brown University Press; 1967. [Google Scholar]
- Lakoff G, Johnson M. Metaphors We Live By. Chicago: University of Chicago Press; 1980. [Google Scholar]
- Mashal N, Faust M, Hendler T. The role of the right hemisphere in processing nonsalient metaphorical meanings: Application of principal components analysis to fMRI data. Neuropsychologia. 2005;43:2084–2100. doi: 10.1016/j.neuropsychologia.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T, Jung-Beeman M. An fMRI investigation of the neural correlates underlying the processing of novel metaphoric expressions. Brain Lang. 2007;100:115–126. doi: 10.1016/j.bandl.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T, Jung-Beeman M. An fMRI-study of processing novel metaphoric sentences. Laterality. 2009;14:30. doi: 10.1080/13576500802049433. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Br Mapp. 2001;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, Thompson-Schill SL. Cognitive control and parsing: Reexamining the role of Broca’s area in sentence comprehension. Cogn Affect Behav Neurosci. 2005;5:263–281. doi: 10.3758/cabn.5.3.263. [DOI] [PubMed] [Google Scholar]
- Pobric G, Mashal N, Faust M, Lavidor M. The Role of the right cerebral hemisphere in processing novel metaphoric expressions: A transcranial magnetic stimulation study. J Cogn Neurosci. 2008;20:170–181. doi: 10.1162/jocn.2008.20005. [DOI] [PubMed] [Google Scholar]
- Raposo A, Moss HE, Stamatakis EA, Tyler LK. Modulation of motor and premotor cortices by actions, action words and action sentences. Neuropsychologia. 2009;47:388–396. doi: 10.1016/j.neuropsychologia.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Rapp AM, Leube DT, Erb M, Grodd W, Kircher TTJ. Neural correlates of metaphor processing. Cogn Brain Res. 2004;20:395–402. doi: 10.1016/j.cogbrainres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Rapp AM, Leube DT, Erb M, Grodd W, Kircher TT. Laterality in metaphor processing: Lack of evidence from functional magnetic resonance imaging for the right hemisphere theory. Brain Lang. 2007;100:142–149. doi: 10.1016/j.bandl.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Davis MH, Johnsrude IS. The Neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cereb Cortex. 2005;15:1261–1269. doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- Saygin AP, McCullough S, Alac M, Emmorey K. Modulation of BOLD Response in Motion-sensitive Lateral Temporal Cortex by Real and Fictive Motion Sentences. J Cogn Neurosci. 2009;22:2480–2490. doi: 10.1162/jocn.2009.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt GL, Cardillo ER, Kranjec A, Chatterjee A. Beyond laterality: A critical assessment of research on the neural basis of metaphor. Int J Neuropsych. 2009:1–5. doi: 10.1017/S1355617709990543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt GL, Seger CA. Neural correlates of metaphor processing: The roles of figurativeness, familiarity and difficulty. Brain Cogn. 2009;71:375–386. doi: 10.1016/j.bandc.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Abe J, Terao A, Miyamoto T. Neural mechanisms involved in the comprehension of metaphoric and literal sentences: An fMRI study. Brain Res. 2007;1166:92–102. doi: 10.1016/j.brainres.2007.06.040. [DOI] [PubMed] [Google Scholar]
- Sotillo M, Carretié L, Hinojosa JA, Tapia M, Mercado F, López-Martín S, et al. Neural activity associated with metaphor comprehension: spatial analysis. Neurosci Lett. 2005;373:5–9. doi: 10.1016/j.neulet.2004.09.071. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left prefrontal cortex in retrieval of semantic knowledge: A re-evaluation. Proc Natl Acad Sci. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torreano LA, Cacciari C, Glucksberg S. When dogs can fly: Level of abstraction as a cue to metaphorical use of verbs. Met Sym. 2005;20:259–274. [Google Scholar]
- Vigneau M, Beaucousin V, Herve P, Jobard G, Petit L, Crivello F, Mellet E, Zago L, Mazoyer B, Tzourio-Mazoyer N. What is right-hemisphere contribution to phonological, lexico-semantic, and sentence processing? Insights from a meta-analysis. NeuroImage. 2011;54:577–593. doi: 10.1016/j.neuroimage.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Vuong LC, Martin RC. LIFG-based attentional control and the resolution of lexical ambiguities in sentence context. Brain Lang. 2011;116:22–32. doi: 10.1016/j.bandl.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallentin M, Lund TE, Østergaard S, Østergaard L, Roepstorff A. Motion verb sentences activate left posterior middle temporal cortex despite static context. Neuroreport. 2005a;16:649–652. doi: 10.1097/00001756-200504250-00027. [DOI] [PubMed] [Google Scholar]
- Wallentin M, Østergaard S, Lund TE, Østergaard L, Roepstorff A. Concrete spatial language: See what I mean? Brain Lang. 2005b;92:221–233. doi: 10.1016/j.bandl.2004.06.106. [DOI] [PubMed] [Google Scholar]
- Watson CE, Chatterjee A. The functional neuroanatomy of actions. Neurol. 2011;77:1428–1434. doi: 10.1212/WNL.0b013e3182166e2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CE, Chatterjee A. A bilateral frontoparietal network underlies visuospatial analogical reasoning. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.09.030. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI Time-Series Revisited-Again. NeuroImage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yang FP, Edens J, Simpson C, Krawczyk DC. Differences in task demands influence the hemispheric lateralization and neural correlates of metaphor. Brain Lang. 2009;111:114–124. doi: 10.1016/j.bandl.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Zempleni MZ, Renken R, Hoeks JCJ, Hoogduin JM, Stowe LA. Semantic ambiguity processing sentence context: Evidence from event-related fMRI. NeuroImage. 2007;34:1270–1279. doi: 10.1016/j.neuroimage.2006.09.048. [DOI] [PubMed] [Google Scholar]