Abstract
Disease management programs that target patients with the highest risk of subsequent costs may help payers and providers control health care costs, but identifying these patients prospectively is challenging. We hypothesized that medical history and clinical data from a heart failure registry could be used to prospectively identify patients with heart failure most likely to incur high costs. We linked Medicare inpatient claims to clinical registry data for patients with heart failure and calculated total Medicare costs during the year after the index heart failure hospitalization. We defined patients as having high costs if they were in the upper 5% (> $76,500) of the distribution. We used logistic regression models to identify patient and clinical characteristics associated with high costs. Costs for the 40,317 patients in the study varied widely. Patients in the upper 5% of the cost distribution incurred mean costs of $117,193 ± $55,550 during 1 year of follow-up, compared with $17,086 ± $17,792 for the lower-cost group. Demographic and clinical characteristics associated with high costs included younger age and black race; history of anemia, chronic obstructive pulmonary disease, ischemic heart disease, diabetes mellitus, or peripheral vascular disease; serum creatinine level; and systolic blood pressure at admission. Mean 1-year Medicare costs for patients whom the model predicted would exceed the high-cost threshold were more than twice the costs for patients below the threshold. In conclusion, model based on variables from clinical registries can identify a group of patients with heart failure who, on average, will incur higher costs in the first year after hospitalization.
Keywords: Health Care Costs, Heart Failure, Medicare
Introduction
Disease management programs that target patients with the highest risk of subsequent costs may help payers and providers control health care costs.1-4 An inpatient episode of heart failure provides a unique opportunity to evaluate a patient's eligibility for postdischarge disease management, but the value of using inpatient medical history and clinical data to identify high-cost patients is unclear. Therefore, we used a nationwide sample of Medicare beneficiaries with heart failure from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) registry and the Get With the Guidelines-Heart Failure (GWTG-HF) registry to examine the value of clinical registry data to prospectively identify patients who are most likely to incur the highest costs.
Methods
We linked Medicare inpatient claims data from January 1, 2003, through December 31, 2006, with data from the OPTIMIZE-HF and GWTG-HF registries. In 2005, OPTIMIZE-HF transitioned to GWTG-HF under the sponsorship of the American Heart Association. The registries had the same design, inclusion criteria, and data collection methods.5,6 Outcome Sciences, Inc, serves as the data collection and coordination center for GWTG. The Duke Clinical Research Institute serves as the data analysis center and has an agreement to analyze aggregate de-identified data for research purposes. Patients were eligible for inclusion in the registries if they were admitted for an episode of worsening heart failure or had developed significant heart failure symptoms during a hospitalization for which heart failure was the primary discharge diagnosis. Hospital teams used heart failure case-ascertainment methods similar to those used by the Joint Commission7 and submitted data on medical history, signs and symptoms, medications, contraindications for or intolerance to medications, and diagnostic test results via a Web-based registry. The representativeness and validity of the OPTIMIZE-HF registry have been described previously.8
For each patient in this study, we obtained Medicare inpatient, outpatient, carrier, and denominator files from 2003 through 2007. These files include institutional claims for facility costs covered under Medicare Parts A and B, and noninstitutional claims for physician services covered under Medicare Part B. The denominator files include beneficiary demographic characteristics, dates of death, and program eligibility and enrollment information.
We used indirect identifiers to link data for patients 65 years or older from the OPTIMIZE-HF and GWTG-HF registries with inpatient Medicare claims files, a method described previously by Hammill et al.6 Using this method, we linked 62,311 (78%) of the 79,837 eligible OPTIMIZE-HF and GWTG-HF hospitalizations to Medicare inpatient claims. We included only persons living in the United States enrolled in fee-for-service Medicare who were discharged alive from a hospital participating fully in the OPTIMIZE-HF or GWTG-HF program. If a patient had multiple linked registry hospitalizations, we selected the first as the index hospitalization. The institutional review board of the Duke University Health System approved the study.
Patient-level information for risk adjustment was obtained from the registry data and included patient demographic characteristics, medical history, and results of admission laboratory tests and examinations. Race was recorded on the case report form as “Asian/Pacific Islander,” “black or African American,” “Caucasian,” “Native American,” “unknown,” or “other.” Consistent with previous analyses, we collapsed the categories into “black” and “other.”9,10 Variables in this analysis had low rates of missingness (ie, < 5% of records), with the exception of evaluation of left ventricular function (13.3%). Because the risk of biased parameter estimates is minimal with missing rates < 5%,11 we adopted an approach for missing variables used in the development of 30-day readmission and mortality risk models for patients with heart failure.12,13 For continuous variables and the variable for evaluation of left ventricular function, we created categorical variables that included a category for missing values. For the dichotomous smoking history variable, we imputed missing values to “no.”
We followed up patients for 1 year after discharge from the index hospitalization. We calculated total costs to Medicare for subsequent hospitalizations by summing payment amounts and per diem adjustments for inpatient stays within 1 year of the index discharge date. Similarly, we calculated total outpatient facility costs by summing payment amounts from outpatient claims and total physician services costs by summing payment amounts from carrier claims. We adjusted all costs to 2007 US dollars based on the Consumer Price Index. We calculated total costs to Medicare as the sum of inpatient, outpatient, and carrier costs and created a binary indicator of “high costs”as those in the top 5% of total Medicare costs (> $76,500) during 1 year of follow-up. We defined patients in this top 5% group as high-cost and patients in the bottom 95% as lower-cost.
We were also interested in all-cause mortality and readmission. We obtained mortality information from the CMS denominator files. Using the inpatient claims files, we identified the first hospital readmission within 1 year after the index discharge date. Transfers to or from another hospital and admissions for rehabilitation (diagnosis related group 462 or an admission diagnosis code of V57.xx) did not count as readmissions.
For patient characteristics, we present categorical variables as frequencies and continuous variables as means with SDs and/or medians with interquartile ranges. We used Kaplan-Meier methods to calculate unadjusted 30-day, 90-day, 180-day, and 1-year mortality and log-rank tests to compare differences between the high-cost and lower-cost groups. To account for the competing risk of death, we used the cumulative incidence function to calculate unadjusted 30-day, 90-day, 180-day, and 1-year readmission at the patient level.14 We used Gray tests to assess differences in readmission rates between the high-cost and lower-cost groups.15
We used logistic regression models with hospital-level random effects to examine unadjusted and multivariable relationships between study variables and high costs. Covariates included age, female sex, and black race; medical history; clinical measures at admission, including systolic function, serum creatinine, systolic blood pressure, serum sodium, and hemoglobin; year of the index hospitalization; and a variable indicating whether length of stay for the index hospitalization was greater than 7 days.16 Hospital-level random effects account for variance in cost among hospitals so that the independent association between patient factors and cost can be estimated more accurately. In a sensitivity analysis, we excluded patients who were discharged from the index hospitalization to a skilled nursing facility (n = 7538) or hospice care (n = 637).
Using simple random selection, we constructed a derivation cohort composed of 75% of the study population and a validation cohort composed of the remaining 25% of the study population. We initially developed the logistic regression models in the derivation cohort, then used the results from these models to score records in the validation cohort. We evaluated the calibration and discrimination of all models in both cohorts and refit the models for the entire study population.17 On the basis of the refitted model, we generated predicted probabilities of incurring high costs in the year after the index hospitalization. We considered patients who were in the top 5%, top 10%, or top 25% of the predicted probabilities to be those whom the model predicted would incur high costs.18 At each threshold, we calculated the model's sensitivity (ie, the number of correctly predicted high-cost cases divided by the total number of actual high-cost cases), false-positive rate (ie, 1 minus the number of correctly predicted low-cost cases divided by the total number of actual low-cost cases), and positive predictive value (ie, the number of correctly predicted high-cost cases divided by the total number of cases predicted as high-cost), and the mean observed total costs. We used SAS version 9.2 (SAS Institute Inc, Cary, North Carolina) for all analyses.
Results
After linking hospitalizations from OPTIMIZE-HF and GWTG-HF to Medicare inpatient claims and applying the exclusion criteria, the study population included 40,317 index cases (Table 1). Ischemic heart disease, diabetes, and hypertension were the most common comorbid conditions. The 75% derivation cohort and the 25% validation cohort were comparable to the full cohort with respect to patient characteristics (data not shown).
Table 1. Baseline Characteristics of the Index Heart Failure Cohort.
| Characteristic | Patients (N = 40,317) |
|---|---|
| Age, mean ± SD (years) | 79.7 ± 7.9 |
| Female | 22,492 (55.8%) |
| Race | |
| Black | 4096 (10.2%) |
| Other | 36,221 (89.8%) |
| Medical history | |
| Anemia on the basis of standardized lab assessments | 7301 (18.1%) |
| Atrial arrhythmia | 14,650 (36.3%) |
| Chronic obstructive pulmonary disease | 11,259 (27.9%) |
| Chronic renal insufficiency | 7476 (18.5%) |
| Coronary artery disease/ischemic heart disease | 21,041 (52.2%) |
| Depression | 4131 (10.2%) |
| Diabetes mellitus | 15,844 (39.3%) |
| Current or previous treatment for hyperlipidemia | 13,943 (34.6%) |
| Current or previous treatment for hypertension | 28,745 (71.3%) |
| Peripheral vascular disease | 5684 (14.1%) |
| Prior cerebrovascular accident/transient ischemic attack | 6832 (16.9%) |
| Smoker within the past year | 3632 (9.0%) |
| Clinical measures at admission | |
| Systolic function | |
| Preserved systolic function | 20,128 (49.9%) |
| Left ventricular systolic dysfunction | 14,841 (36.8%) |
| Missing | 5348 (13.3%) |
| Serum creatinine, mean ± SD (mg/dL) | 1.7 ± 2.2 |
| Systolic blood pressure, mean ± SD (mm Hg) | 141.7 ± 30.6 |
| Serum sodium, mean ± SD (mEq/L) | 137.3 ± 8.2 |
| Hemoglobin, mean ± SD (g/dL) | 12.1 ± 5.8 |
| Length of stay for the index hospitalization (days) | |
| Mean ± SD | 5.5 ± 4.7 |
| Median (interquartile range) | 4.0 (3.0-7.0) |
| Index year | |
| 2003 | 9319 (23.1%) |
| 2004 | 14,551 (36.1%) |
| 2005 | 6445 (16.0%) |
| 2006 | 10,002 (24.8%) |
As shown in Table 2, unadjusted 1-year mortality was 34.4% and the 1-year readmission rate was 65.4%. Unadjusted 30-day, 90-day, and 180-day mortality were lower among patients who incurred the highest costs, and unadjusted 1-year mortality was slightly higher among patients with high costs. The rate of readmission at 30 days was twice as high in the high-cost group compared with the lower-cost group. Nearly all patients in the high-cost group were readmitted within 1 year, compared with 64% in the lower-cost group. Patients in the high-cost group incurred mean total 1-year costs of $117,193 and accounted for 27% of the total Medicare costs in the study population. Overall, the distribution of 1-year costs was right-skewed, and 30% of patients incurred less than $5000 in total costs.
Table 2. Unadjusted Outcomes at 1 Year After the Index Admission, Overall and by Cost Threshold.
| Outcome | All Patients (N = 40,317) |
Patients in the Upper 5% of Medicare Costs (n = 2029) |
Patients in the Lower 95% of Medicare Costs (n = 38,288) |
P Value |
|---|---|---|---|---|
| Mortality | ||||
| 30 days | 3106 (7.7%) | —* | 3101 (8.1%) | |
| 90 days | 6391 (15.9%) | 56 (2.8%) | 6335 (16.6%) | < .001 |
| 180 days | 9460 (23.6%) | 212 (10.5%) | 9248 (24.3%) | < .001 |
| 1 year | 13,698 (34.4%) | 728 (36.5%) | 12,970 (34.3%) | 0.15 |
| All-cause readmission, No. (cumulative incidence) | ||||
| 30 days | 8693 (21.6) | 866 (42.7) | 7827 (20.5) | < .001 |
| 90 days | 15,765 (39.2) | 1524 (75.1) | 14,241 (37.3) | < .001 |
| 180 days | 20,924 (52.2) | 1877 (92.6) | 19,047 (50.1) | < .001 |
| 1 year | 26,100 (65.4) | 2011 (99.3) | 24,089 (63.6) | < .001 |
| Costs to Medicare at 1 year | ||||
| Inpatient | < .001 | |||
| Mean ± SD | 14,619 ± 23,866 | 86,400 ± 48,707 | 10,815 ± 13,660 | |
| Median (interquartile range) | 6400 (0-19,130) | 74,749 (59,976-98,803) | 5780 (0-16,317) | |
| Outpatient | < .001 | |||
| Mean ± SD | 2572 ± 6336 | 11,124 ± 14,447 | 2119 ± 5209 | |
| Median (interquartile range) | 599 (78-1838) | 3438 (859-19,776) | 557 (67-1675) | |
| Carrier | < .001 | |||
| Mean ± SD | 4932 ± 8643 | 19,669 ± 30,347 | 4151 ± 4215 | |
| Median (interquartile range) | 3235 (1412-6316) | 16,134 (12,162-21,674) | 3006 (1327-5695) | |
| Total Medicare cost | < .001 | |||
| Mean ± SD | 22,124 ± 30,575 | 117,193 ± 55,550 | 17,086 ± 17,792 | |
| Median (interquartile range) | 11,849 (3190-29,324) | 100,779 (86,145-127,658) | 10,721 (2861-25,445) |
Cell sizes less than 11 are not shown.
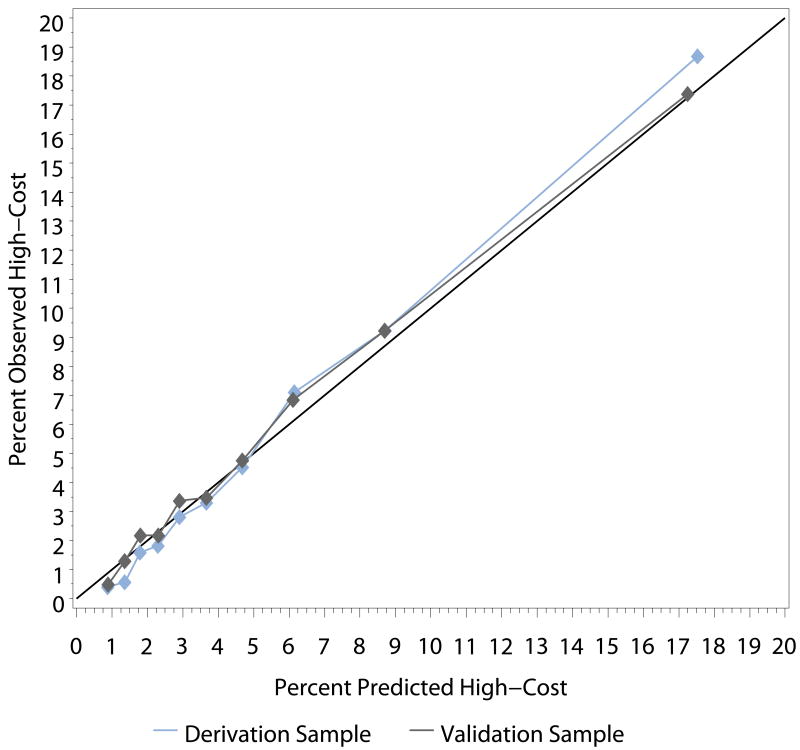
The regression models were well-calibrated for predicting which patients would have high costs. Predicted probability distributions for the derivation and validation cohorts were highly consistent, and the percentages of predicted and observed high-cost patients were similar within deciles (Figure). Table 3 shows the results of the refitted unadjusted and multivariable models examining relationships between patient characteristics and high-cost status. After adjustment, black race and a history of anemia, chronic obstructive pulmonary disease, ischemic heart disease, diabetes mellitus, and peripheral vascular disease were positively associated with greater odds of high costs in the year after the index hospitalization. Older age, because of its association with a higher risk of mortality, was associated with lower costs during the 1-year follow-up. The odds of incurring high costs were more than twice as high in patients with serum creatinine ≥ 2 mg/dL compared with patients with serum creatinine < 1.5 mg/dL. Systolic blood pressure over 120 mm Hg was also associated with higher costs.
Figure. Percent Predicted High-Cost vs Observed High-Cost Within Deciles.
Table 3. Predictors of High Inpatient Costs (Upper 5%) in the Year After the Index Admission.
| Variable | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|
| Age (years) | ||||
| 65-69 | 1.00 [Reference] | 1.00 [Reference] | ||
| 70-74 | 0.77 (0.68-0.88) | < 0.001 | 0.79 (0.69-0.91) | < 0.001 |
| 75-79 | 0.59 (0.51-0.67) | < 0.001 | 0.62 (0.54-0.71) | < 0.001 |
| ≥80 | 0.25 (0.22-0.29) | < 0.001 | 0.28 (0.25-0.33) | < 0.001 |
| Women | 0.80 (0.73-0.88) | < 0.001 | 0.96 (0.87-1.06) | 0.45 |
| Race | ||||
| Black | 2.15 (1.88-2.45) | < 0.001 | 1.70 (1.48-1.96) | < 0.001 |
| Other | 1.00 [Reference] | 1.00 [Reference] | ||
| Medical history | ||||
| Anemia | 1.45 (1.29-1.62) | < 0.001 | 1.18 (1.05-1.34) | 0.006 |
| Atrial arrhythmia | 0.75 (0.68-0.83) | < 0.001 | 0.91 (0.82-1.01) | 0.07 |
| Chronic obstructive pulmonary disease | 1.22 (1.11-1.35) | < 0.001 | 1.17 (1.05-1.30) | 0.003 |
| Chronic renal insufficiency | 2.12 (1.92-2.35) | < 0.001 | 1.10 (0.96-1.25) | 0.16 |
| Coronary artery disease/ischemic heart disease | 1.27 (1.16-1.39) | < 0.001 | 1.14 (1.04-1.26) | 0.007 |
| Depression | 1.05 (0.90-1.23) | 0.50 | 1.05 (0.90-1.24) | 0.52 |
| Diabetes mellitus | 1.68 (1.53-1.84) | < 0.001 | 1.16 (1.05-1.28) | 0.003 |
| Hyperlipidemia | 1.23 (1.12-1.35) | < 0.001 | 1.04 (0.94-1.15) | 0.47 |
| Hypertension | 1.17 (1.05-1.30) | 0.004 | 1.01 (0.91-1.14) | 0.80 |
| Peripheral vascular disease | 1.41 (1.24-1.59) | < 0.001 | 1.14 (1.00-1.29) | 0.05 |
| Prior cerebrovascular accident/transient ischemic attack | 0.97 (0.85-1.09) | 0.57 | 0.94 (0.83-1.06) | 0.32 |
| Smoker within the past year | 1.21 (1.04-1.41) | 0.01 | 0.90 (0.77-1.06) | 0.22 |
| Findings on admission | ||||
| Systolic function | ||||
| Preserved systolic function | 1.00 [Reference] | 1.00 [Reference] | ||
| Left ventricular systolic dysfunction | 1.07 (0.97-1.18) | 0.18 | 0.99 (0.89-1.10) | 0.82 |
| Missing | 1.13 (0.98-1.30) | 0.09 | 1.12 (0.97-1.29) | 0.13 |
| Serum creatinine, mg/dL | ||||
| < 1.5 | 1.00 [Reference] | 1.00 [Reference] | ||
| 1.5-<2.0 | 1.32 (1.16-1.50) | < 0.001 | 1.26 (1.10-1.44) | < 0.001 |
| ≥2.0 | 2.93 (2.64-3.25) | < 0.001 | 2.34 (2.05-2.67) | < 0.001 |
| Missing | 1.15 (0.57-2.35) | 0.70 | 1.20 (0.57-2.55) | 0.63 |
| Systolic blood pressure, mm Hg | ||||
| < 120 | 1.00 [Reference] | 1.00 [Reference] | ||
| ≥ 120 and < 140 | 1.10 (0.96-1.25) | 0.17 | 1.18 (1.03-1.35) | 0.02 |
| ≥ 140 and < 160 | 1.04 (0.90-1.19) | 0.61 | 1.10 (0.96-1.27) | 0.19 |
| ≥ 160 | 1.17 (1.03-1.34) | 0.02 | 1.18 (1.02-1.35) | 0.02 |
| Missing | 0.99 (0.48-2.07) | 0.99 | 1.30 (0.63-2.68) | 0.48 |
| Serum sodium, mEq/L | ||||
| < 135 | 1.00 [Reference] | 1.00 [Reference] | ||
| ≥ 135 and < 145 | 1.15 (1.02-1.30) | 0.02 | 1.13 (1.00-1.29) | 0.05 |
| ≥ 145 | 1.05 (0.82-1.34) | 0.71 | 1.00 (0.78-1.30) | 0.97 |
| Missing | 0.78 (0.46-1.32) | 0.35 | 0.90 (0.50-1.63) | 0.73 |
| Hemoglobin, g/dL | ||||
| < 9 | 1.00 [Reference] | 1.00 [Reference] | ||
| ≥ 9 and < 12 | 0.98 (0.81-1.17) | 0.79 | 1.14 (0.94-1.38) | 0.18 |
| ≥ 12 | 0.67 (0.56-0.81) | < 0.001 | 0.92 (0.75-1.13) | 0.43 |
| Missing | 0.64 (0.45-0.89) | 0.009 | 0.81 (0.56-1.17) | 0.26 |
| Length of index stay >7 days | 1.24 (1.12-1.38) | < 0.001 | 1.10 (0.99-1.23) | 0.08 |
| Index year | ||||
| 2003 | 1.00 [Reference] | 1.00 [Reference] | ||
| 2004 | 1.13 (1.00-1.29) | 0.06 | 1.17 (1.03-1.34) | 0.02 |
| 2005 | 1.09 (0.90-1.32) | 0.36 | 1.15 (0.95-1.39) | 0.16 |
| 2006 | 1.04 (0.86-1.24) | 0.70 | 1.17 (0.97-1.40) | 0.11 |
Abbreviations: OR, odds ratio; CI, confidence interval.
The multivariable model includes all of the variables listed.
Table 4 summarizes the performance of the models for predicting which patients would incur high costs after the index hospitalization. Based on the top 5% of predicted probabilities, 22.6% of the patients who actually incurred high costs were predicted to incur high costs. The sensitivity of the model improved as the prediction threshold became less stringent. Based on the top 25% of predicted probabilities, 63% of the patients who actually incurred high costs were predicted to incur high costs. Among patients who did not incur high costs, the model falsely predicted that 4% would incur high costs, based on the top 5% of predicted probabilities. The false-positive rate increased as the prediction threshold became less stringent. Among patients predicted to incur high costs based on the top 5% of predicted probabilities, approximately 23% actually incurred high costs. This positive predictive value dropped for higher prediction thresholds. The mean observed cost among the 2015 patients who were predicted to incur high costs using the 5% threshold was 2.4 times higher than the mean cost among the 38,302 patients who were predicted to incur lower costs. The ratio of the mean cost among patients predicted to have high costs to the mean cost among patients predicted to have low costs was slightly lower using the 10% threshold (ratio, 2.3) and the 25% threshold (ratio, 2.0). The area under the curve was 0.778. Results were similar in the sensitivity model in which we excluded patients discharged from the index hospitalization to a skilled nursing facility or hospice care.
Table 4. Performance of the Model Predicting Patients With High Costs in the Year After the Index Admission.
| Performance Measure | High-Cost Prediction Threshold | ||
|---|---|---|---|
| Upper 5% | Upper 10% | Upper 25% | |
| No. of patients | 2015 | 4031 | 10,079 |
| Sensitivity, % | 22.6 | 37.4 | 63.1 |
| False-positive rate, % | 4.1 | 8.5 | 23.0 |
| Positive predictive value, % | 22.8 | 18.8 | 12.7 |
| Mean actual cost ± SD - predicted high, $ | 49,986 ± 52,879 | 44,733 ± 49,214 | 35,225 ± 42,066 |
| Mean actual cost ± SD - predicted low, $ | 20,658 ± 28,178 | 19,612 ± 26,582 | 17,757 ± 24,091 |
| Area under the curve | 0.778 | ||
As shown in the Supplemental Table, mean observed costs increased by quintile of predicted probability of high 1-year costs. Although costs increased gradually in the lower quintiles, there was a much steeper increase from quintile 4 to quintile 5. Comparing the highest and lowest quintiles, mean inpatient costs were 2.8 times higher, outpatient costs were 4.6 times higher, and carrier costs were 2.5 times higher.
Discussion
We used clinical registry data to derive and validate a method for prospectively identifying patients with heart failure who are most likely to incur high costs in the year after a heart failure hospitalization. Although prospective identification of these patients is challenging, it is possible to create a model with adequate discrimination and calibration using data readily available from an inpatient episode of heart failure.
The approach taken to allow prospective identification of high cost heart failure patients was to develop a model to predict costs in the top 5% of the distribution (which accounts for 27% of total costs) and evaluate model performance at various thresholds of the distribution of predicted high-cost probabilities. The model had greatest sensitivity when the threshold included the top 25% of the distribution. However, the trade-off of casting a wider net was a higher false-positive rate. Although we had only modest success in accurately predicting which patients would incur high costs, the results allowed us to compare actual mean costs for the groups of patients identified by the model as having high costs or low costs. Patients who were predicted to have high costs incurred, on average, more than twice the costs of patients not predicted to have high costs. This finding was consistent at every prediction threshold. Moreover, mean observed costs by quintile of predicted probability of high 1-year costs suggested a higher proportion of quintile 5 patients in the tail of the cost distribution, which the prediction model was designed to target.
Our findings highlight the challenges associated with identifying patients with heart failure who will incur the highest costs. First, the risk of death is high among older Medicare beneficiaries with heart failure, and early mortality translates into lower costs over time. Therefore, variables associated with early postdischarge mortality tend to be associated with lower costs. In this study, advanced age was associated with lower costs, likely reflecting early mortality among these patients. Compared with patients who had lower costs, patients with high costs had lower mortality during 6 months of follow-up. At 1 year, mortality among patients with high costs slightly exceeded mortality among patients with low costs. Unlike a model predicting mortality, a model predicting costs must, in general, simultaneously predict which patients are healthy enough to survive during the year after discharge but sick enough to require readmission during that year. In addition, patients with heart failure incur higher-than-average medical costs, so the discriminatory ability of the model may reflect the difficulty of distinguishing very high costs in a high-cost population.
Our study offers insights into the risk factors associated with high inpatient costs. The presence of multiple comorbid conditions has been consistently linked with higher health care expenditures.19-22 In particular, Wolff et al.22 found that the risk of hospitalization increases dramatically with each additional chronic condition. Management of heart failure and concurrent chronic conditions is complex. The high burden of comorbidity limits the ability to use comorbid conditions to discern which patients are likely to incur the highest costs.
Provisions in the Patient Protection and Affordable Care Act of 2010 aim to reduce health care costs and improve patient care by incentivizing hospitals to coordinate care for chronic illnesses like heart failure through models such as accountable care organizations (ACOs), as well as by penalizing hospitals with high risk-adjusted 30-day readmission rates.23,24 The Medicare Payment Advisory Commission recently proposed to strengthen these incentives by giving ACOs opportunities to share not only in savings below a certain spending threshold but also in costs when spending exceeds that threshold.25 Therefore, identifying beneficiaries at risk for readmission and substantially higher costs will be critical for providers participating in ACOs. Moreover, timely risk assessment will be critical to achieving the patient-focused goals outlined in the current proposal for ACOs from the Department of Health and Human Services— shared decision making, informed choices, and higher-quality care.26 However, identifying beneficiaries at risk for substantially higher costs may shift the therapeutic focus to those with the highest costs and away from patients at highest risk. In the context of the broader imperative to improve health care, such a shift would be undesirable.
Other findings are noteworthy. Black patients had higher odds of incurring high costs, consistent with a previous analysis in which the hazard of all-cause readmission was higher among black patients in the year after an index heart failure hospitalization, whereas the hazard of all-cause mortality was lower.10 Elevated serum creatinine at admission was also associated with higher costs in the subsequent year. A recent study showed that elevated creatinine is a risk factor for long-term outcomes among patients with heart failure.27
Our study has some limitations. First, we defined patients at high risk on the basis of total Medicare costs. Other domains, such as functional status and health-related quality of life, were not available but should be considered in future studies. Second, hospitalization for heart failure was an inclusion criterion for the study, and patients with heart failure who have at least 1 hospitalization are at higher risk for future hospitalizations and higher costs.28 Consequently, the study may have included a disproportionate number of patients at higher risk for whom it is even more difficult to predict future resource use. Third, the analysis was restricted to fee-for-service Medicare beneficiaries, so the generalizability to Medicare beneficiaries in managed care plans is unclear. Similarly, hospitals that voluntarily participate in quality-improvement registries differ from nonparticipating hospitals.8 Fourth, we could not assess optimal medical therapy, which may be associated with lower long-term costs. Although assessment of optimal medical therapy might improve the performance of the high-cost prediction model, the practicality of assessing future patient adherence to therapy at the time of initial hospitalization is unclear. Fifth, clinical measures that were not consistently collected in the heart failure registries (eg, serum urea nitrogen, serum potassium) could improve the performance of the high-cost prediction model. Finally, the study included only beneficiaries 65 years or older and may not be generalizable to younger patients.
Supplementary Material
Acknowledgments
Funding/Support: Get With the Guidelines–Heart Failure (GWTG-HF) is a program of the American Heart Association and is supported by Medtronic, Ortho-McNeil, and the American Heart Association Pharmaceutical Roundtable. GWTG-HF has been funded in the past through support from GlaxoSmithKline. The Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) was funded by GlaxoSmithKline. This study was supported by American Heart Association Pharmaceutical Roundtable Outcomes Center grant 087512N, grant U18HS10548 from the Agency for Healthcare Research and Quality, grant R01AG026038 from the National Institute on Aging, and grant U01HL066461 from the National Heart, Lung, and Blood Institute. Dr. Hernandez is a recipient of an American Heart Association Pharmaceutical Roundtable grant (0675060N). Dr. Fonarow is supported by the Ahmanson Foundation and the Corday Family Foundation.
Footnotes
Information about funding for this work is provided in the Acknowledgments.
Additional Contributions: Damon M. Seils, MA, Duke University, provided editorial assistance and prepared the manuscript. Mr Seils did not receive compensation for his assistance apart from his employment at the institution where the study was conducted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 2.Riegel B, Carlson B, Glaser D, Hoagland P. Which patients with heart failure respond best to multidisciplinary disease management? J Card Fail. 2000;6:290–299. doi: 10.1054/jcaf.2000.19226. [DOI] [PubMed] [Google Scholar]
- 3.Smith B, Hughes-Cromwick PF, Forkner E, Galbreath AD. Cost-effectiveness of telephonic disease management in heart failure. Am J Manag Care. 2008;14:106–115. [PubMed] [Google Scholar]
- 4.Eapen ZJ, Reed SD, Curtis LH, Hernandez AF, Peterson ED. Do heart failure disease management programs make financial sense under a bundled payment system? Am Heart J. 2011;161:916–922. doi: 10.1016/j.ahj.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Fonarow GC, Abraham WT, Albert NM, Gattis WA, Gheorghiade M, Greenberg B, O'Connor CM, Yancy CW, Young J. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148:43–51. doi: 10.1016/j.ahj.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:996–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joint Commission. Specifications Manual for National Hospital Quality Measures. [Accessed December 21, 2009];2006 http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Historical+NHQM+manuals.htm.
- 8.Curtis LH, Greiner MA, Hammill BG, DiMartino LD, Shea AM, Hernandez AF, Fonarow GC. Representativeness of a national heart failure quality-of-care registry: comparison of OPTIMIZE-HF and non–OPTIMIZE-HF Medicare patients. Circ Cardiovasc Qual Outcomes. 2009;2:377–384. doi: 10.1161/CIRCOUTCOMES.108.822692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whellan DJ, Greiner MA, Schulman KA, Curtis LH. Costs of inpatient care among Medicare beneficiaries with heart failure, 2001 to 2004. Circ Cardiovasc Qual Outcomes. 2010;3:33–40. doi: 10.1161/CIRCOUTCOMES.109.854760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis LH, Greiner MA, Hammill BG, Kramer JM, Whellan DJ, Schulman KA, Hernandez AF. Early and long-term outcomes of heart failure in elderly persons, 2001-2005. Arch Intern Med. 2008;168:2481–2488. doi: 10.1001/archinte.168.22.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrell FE., Jr . Regression Modeling Strategies. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- 12.Keenan PS, Normand SL, Lin Z, Drye EE, Bhat KR, Ross JS, Schuur JD, Stauffer BD, Bernheim SM, Epstein AJ, Wang Y, Herrin J, Chen J, Federer JJ, Mattera JA, Wang Y, Krumholz HM. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1:29–37. doi: 10.1161/CIRCOUTCOMES.108.802686. [DOI] [PubMed] [Google Scholar]
- 13.Krumholz HM, Wang Y, Mattera JA, Wang Y, Han LF, Ingber MJ, Roman S, Normand SL. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–1701. doi: 10.1161/CIRCULATIONAHA.105.611194. [DOI] [PubMed] [Google Scholar]
- 14.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 15.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 16.Krumholz HM, Parent EM, Tu N, Vaccarino V, Wang Y, Radford MJ, Hennen J. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99–104. [PubMed] [Google Scholar]
- 17.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd. New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- 18.Meenan RT, O'Keeffe-Rosetti C, Hornbrook MC, Bachman DJ, Goodman MJ, Fishman PA, Hurtado AV. The sensitivity and specificity of forecasting high-cost users of medical care. Med Care. 1999;37:815–823. doi: 10.1097/00005650-199908000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Liao L, Anstrom KJ, Gottdiener JS, Pappas PA, Whellan DJ, Kitzman DW, Aurigemma GP, Mark DB, Schulman KA, Jollis JG. Long-term costs and resource use in elderly participants with congestive heart failure in the Cardiovascular Health Study. Am Heart J. 2007;153:245–252. doi: 10.1016/j.ahj.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Wexler DJ, Chen J, Smith GL, Radford MJ, Yaari S, Bradford WD, Krumholz HM. Predictors of costs of caring for elderly patients discharged with heart failure. Am Heart J. 2001;142:350–357. doi: 10.1067/mhj.2001.116476. [DOI] [PubMed] [Google Scholar]
- 21.Charlson M, Charlson RE, Briggs W, Hollenberg J. Can disease management target patients most likely to generate high costs? The impact of comorbidity. J Gen Intern Med. 2007;22:464–469. doi: 10.1007/s11606-007-0130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162:2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 23.Orszag PR, Emanuel EJ. Health care reform and cost control. N Engl J Med. 2010;363:601–603. doi: 10.1056/NEJMp1006571. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser Family Foundation. Summary of Key Changes to Medicare in 2010 Health Reform Law. [Accessed December 1, 2010]; http://www.kff.org/healthreform/7948.cfm.
- 25.Hackbarth GM. Letter from Glenn M. Hackbarth, chairman of the Medicare Payment Advisory Commission (MedPAC), to Donald M Berwick, administrator of the Centers for Medicare & Medicaid Services. [Accessed December 1, 2010];2010 November 22; http://www.medpac.gov/documents/11222010_ACO_COMMENT_MedPAC.pdf.
- 26.Berwick D. Launching accountable care organizations—the proposed rule for Medicare shared savings program. N Engl J Med. 2011 Mar 31; doi: 10.1056/NEJMp1103602. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Kociol RD, Greiner MA, Hammill BG, Phatak H, Fonarow GC, Curtis LH, Hernandez AF. Long-term outcomes of Medicare beneficiaries with worsening renal function during hospitalization for heart failure. Am J Cardiol. 2010;105:1786–1793. doi: 10.1016/j.amjcard.2010.01.361. [DOI] [PubMed] [Google Scholar]
- 28.Chan DC, Heidenreich PA, Weinstein MC, Fonarow GC. Heart failure disease management programs: a cost-effectiveness analysis. Am Heart J. 2008;155:332–338. doi: 10.1016/j.ahj.2007.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.