Abstract
The sequential synaptic integration of adult-born neurons has been widely examined in rodents, but the mechanisms regulating the integration remain largely unknown. The primary cilium, a microtubule–based signaling center, plays essential roles in vertebrate development, including the development of the central nervous system. Here we examined the assembly and function of the primary cilium in the synaptic integration of adult-born hippocampal neurons. Strikingly, primary cilia are absent in young adult-born neurons but assemble precisely at the stage when newborn neurons approach their final destination, further extend dendrites and form synapses with entorhinal cortical projections. Conditional cilia deletion from adult-born neurons induced severe defects in dendritic refinement and synapse formation. Primary cilia deletion leads to enhanced Wnt/β-catenin signaling which may account for these developmental defects. Taken together, our study identifies the assembly of primary cilia as a critical regulatory event in the dendritic refinement and synaptic integration of adult-born neurons.
Introduction
Adult hippocampal neural progenitors continuously give rise to dentate granule cells (DGCs) throughout life1–2. The genesis of these DGCs is precisely controlled by many transcription factors3–4, epigenetic modulators3 and physiological stimuli4–6. After birth, newborn DGCs stereotypically integrate into existing neural circuits: they migrate into the granule cell layer2, extend dendrites, and form functional synapses7–10. This stepwise integration of newborn DGCs is regulated by mechanisms similar to those that regulate their initial genesis3–4. Despite the considerable progress in this area, many important questions remain, particularly regarding the mechanisms by which environmental or intrinsic signaling regulates sequential circuit integration.
One intriguing mechanism by which newborn DGCs could sequentially integrate into existing circuitry is via signaling cascades from the primary cilium, a highly specialized sensory organelle. Most vertebrate cells, including neural progenitors and neurons of the embryonic and adult brain, contain a primary cilium extending from a basal body. Specific architectural elements of primary cilia – including their polarized structure, high receptor and channel density, and close proximity to Golgi and vesicle transport systems11–13 – suggest that they participate in fundamental biological processes during development and adult homeostasis. The importance of primary cilia is emphasized by the increasing number of human genetic diseases associated with cilia dysfunction14. In animals, mutations of genes involved in the assembly or maintenance of the primary cilia cause early embryonic developmental defects in left-right asymmetry and in the formation of several organs11,15. Similar to the defects found in the developing nervous system11,13,16, two recent studies found that disrupting primary cilia assembly in adult neural progenitors impairs the formation, self-renewal and differentiation of these cells17–18. Primary cilia mediate critical signaling pathways such as sonic hedgehog, platelet-derived growth factor receptor-α, integrin and Wnt11,14,17–19. Disruption of these pathways may be the basis for the developmental abnormalities seen in both human diseases and animal models.
Here we asked whether primary cilia are present in adult-born DGCs and involved in regulating the sequential synaptic integration of adult-born neurons into functional circuits. We engineered inducible retroviral vectors to label and manipulate primary cilia in adult-born neurons during distinct phases of their development7–8. This approach allowed us to assess cell-autonomous functions of cilia without the confounding developmental defects or lethality common to germ-line knockout animals. We found that primary cilia begin to assemble when newborn DGCs complete their migration, refine dendritic arbors, and form glutamatergic synapses with entorhinal cortical (Ent) projections. Newborn DGCs lacking primary cilia display dendritic refinement defects and significantly diminished synaptic input from Ent projections. Moreover, we provide evidence that primary cilia deletion enhances Wnt/β-catenin signaling and that this enhancement is required and sufficient to impact the dendritic refinement. Thus, we conclude that timely assembled primary cilia serve as a key structure to sense and transduce environmental signals critical for dendritic refinement of newborn DGCs and their proper synapse formation with Ent projections.
Results
The timing of primary cilia assembly in newborn DGCs
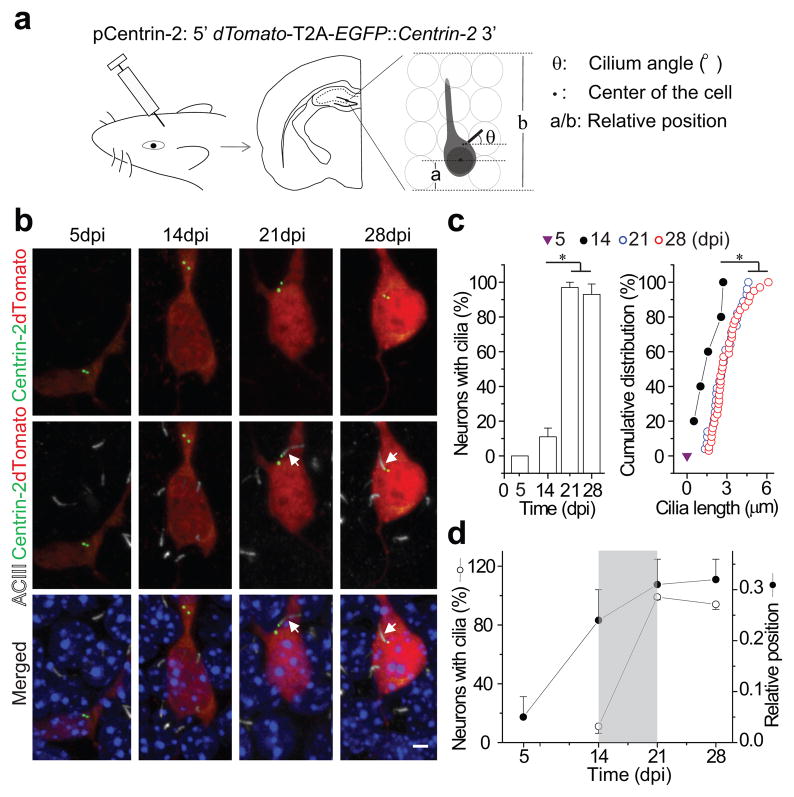
To examine the development of primary cilia in newborn DGCs and their potential functional roles, we used an oncoretrovirus-mediated approach to genetically label and manipulate these neurons in vivo7–8. We designed a retroviral vector (pCentrin-2, Fig. 1a) to co-express dTomato and Centrin-2 tagged with enhanced green fluorescent protein (EGFP) using a 2A peptide sequence20. High-titer retroviral stocks were injected into the hilar region of the hippocampus in adult mice. At 5, 14, 21 and 28 days post retroviral injection (dpi), we stained hippocampal sections with an antibody to adenylyl cyclase III (ACIII), a specific marker of primary cilia21, and used confocal microscopy to reconstruct 3-dimensional images of newborn DGCs. Infected DGCs contained diffuse cytoplasmic dTomato and EGFP+ centrioles (Fig. 1b). We then determined whether dTomato+ DGCs were ciliated or not based on co-localization of ACIII with their EGFP+ centrosomes (Mother centriole, Supplementary Video 1). We found that primary cilia were absent from dTomato+ DGCs at 5dpi but present at 14 dpi. At 14dpi, 11±4.7% contained primary cilia, with an average cilium length of 1.7±0.4 μm (n=5 of 48 newborn DGCs). At 21 and 28dpi, 99±1.9% and 94±3.4% of newborn DGCs contained primary cilia, averaging 2.8±0.5 μm and 3.0±0.4 μm in length, respectively (Fig. 1b, c).
Figure 1. Primary cilia assemble in developing adult-born neurons.
(a) A schematic diagram of the retroviral vector (pCentrin-2), retroviral injection, a sample brain section and the parameters measured from reconstructed images. (b) Confocal images of cilia formation in developing adult-born neurons showing EGFP (Centrin-2), dTomato, DAPI and immunostaining for ACIII. Arrows point to primary cilia associated with centrosomes. Scale bar: 3 μm. (c) Quantification of percentage of labeled adult-born neurons with primary cilia (left) and the distribution of ciliary length (right) at 5, 14, 21 and 28 dpi. (d) Percentage of retrovirally labeled neurons with primary cilia and their relative position within the dentate gyrus granule layer at 5, 14, 21 and 28 dpi. Values represent mean±SEM (n=32–48 neurons; *: p<0.01, ANOVA or Kolmogorov-Smirnov test). The analyses in c and d are from the same group of cells.
To confirm that our ACIII staining was accurately and reliably birth-dating the formation of primary cilia, we constructed a retroviral vector to co-express intraflagellar transport protein 88 (Ift88), a component of the transport machinery required for primary cilia assembly22. As with Centrin-2 experiments, Ift88 was fused with EGFP and paired with dTomato using a 2A peptide sequence. We analyzed the percentage of dTomato+ DGCs with EGFP+ primary cilia at 5, 14 and 21 dpi, and again found that most cilia are generated between 14 and 21 days post-birth, consistent with ACIII staining results (Fig. 1c and Supplementary Fig. 1a, b). To further exclude any potential confounding effects of ectopic Centrin-2 expression during cilia assembly, we then constructed an inducible retrovirus for Centrin-2 and induced expression at 12dpi time point. Two days later, 13±3.1% of newborn DGCs harbored ACIII+ primary cilia, similar to the percentage of labeled cells having cilia after 14 days of continual EGFP-Centrin-2 expression (Supplementary Fig. 1c), suggesting that ectopic Centrin-2 expression is unlikely to alter cilia assembly. Together, these data show that most primary cilia assemble between 2 and 3 weeks after the birth of newborn DGCs.
Newborn DGCs migrate radially into the granular cell layer during their initial development4,8,10. In migrating cultured fibroblasts, primary cilia are found in the leading edge where they point in the direction of migration23. To determine whether primary cilia play a similar role in the migration of newborn DGCs, we analyzed their cellular position and orientation (Supplementary Fig. 2a, b, c). While the majority of primary cilia protrude from the leading edge (between the nucleus and root of apical dendrite24), they orient randomly. Moreover, when we analyzed the migration distance of newborn DGCs at different stages, we found that the majority of newborn DGCs had completed most of their migration into the DGC layer by 14dpi, prior to the elaboration of a primary cilium (Fig. 1a, d)25. This analysis demonstrates that primary cilia assembly is initiated as newborn DGCs near their final destination. Thus, primary cilia, or primary cilia assembly, may not be required for migration or at least the initial phase of migration.
Synapse formation during primary cilia assembly
From 14dpi, newborn DGCs further extend their dendrites and form functional synapses with existing neural circuits7–10. The coincident timing of cilia assembly and synapse formation suggested that cilia could play a role in synaptic integration.
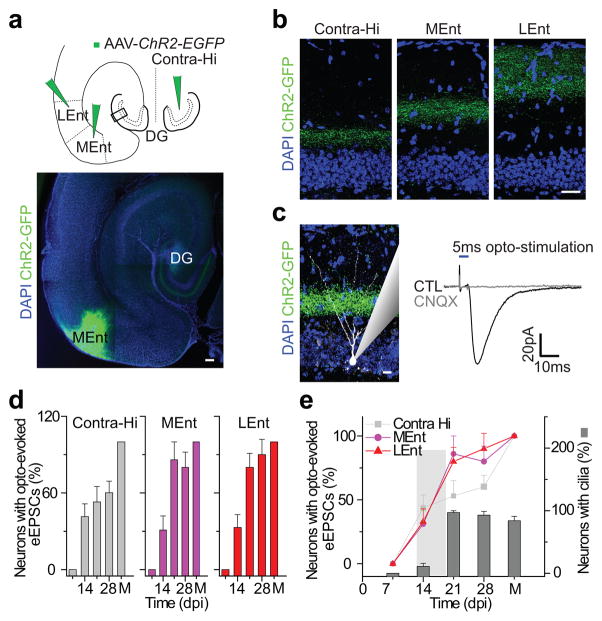
A mature DGC receives glutamatergic synaptic inputs in a laminar pattern: the inner molecular layer is innervated by contra- and ipsi-lateral hilar mossy cell projections, while the middle and outer layers receive medial and lateral Ent projections, respectively26. These glutamatergic inputs become detectable at ~14 dpi in newborn DGCs8,10. To examine the development of laminar glutamatergic innervations onto newborn DGCs and determine the specific roles of the primary cilium in this process, we dissected laminar glutamatergic inputs using optogenetic stimulation (Fig. 2a)27. We infused the contralateral hippocampal hilus, medial entorhinal or lateral entorhinal cortex with an adeno-associated virus expressing Channelrhodopsin 2 tagged with EGFP (AAV-ChR2; Fig. 2a; Methods). We showed sample axonal projections to the dentate gyrus from these areas (Fig. 2b). Fourteen days after AAV injections, laminar axonal projections were clearly visible and 473 nm blue-light stimulation could reliably induce evoked excitatory postsynaptic currents (eEPSCs) in randomly selected DGCs (Fig. 2c). Newborn DGCs were labeled with retrovirally expressed dTomato and analyzed at 7, 14, 21, and 28 dpi; for each of these time points, animals were injected with AAV-ChR2 14 days prior. Whole cell recordings were collected from dTomato+ DGCs in acute brain slices (Methods) 8,25. Simultaneously, an additional recording was made from a dTomato negative DGC in the outer edge of the granule cell layer, where DGCs are considered fully mature28–29. eEPSCs were elicited by short pulses of 473 nm light illumination in the presence of 5 μm bicuculline to block GABAergic activity. The recordings from the mature DGCs confirmed that functional laminar excitation could be elicited by short pulses of blue light. Only when the mature DGC showed reliable eEPSCs was the newborn neuron included for subsequent analysis. We found that 43±10% of the recorded dTomato+ DGCs at 14dpi received functional contralateral mossy cell projections (Fig. 2d). This proportion remained fairly constant until 28 dpi. In contrast, while 31±11% of newborn DGCs showed responses to light stimulation of inputs from the medial entorhinal cortex at 14 dpi, this proportion increased significantly to 86±14% at 21 dpi. A similar increase in the extent of innervations from the lateral entorhinal cortex also occurred between 14 and 21dpi (33±10% at 14dpi and 80±11% at 21 dpi, Fig. 2d). These results show that robust glutamatergic synaptic development between newborn DGCs and Ent projections occurs 14–21 days after the neurons are born, a time coincident with cilia assembly (Fig. 2e).
Figure 2. Entorhinal cortical innervations of adult-born neurons accompany primary cilia assembly.
(a) A schematic diagram showing the AAV-ChR2-EGFP injection sites in the contralateral hilus (Contra-Hi), lateral (LEnt) and medial (MEnt) entorhinal cortex. The sample image (EGFP) shows the MEnt injection site and axonal projections in the molecular layer of the dentate gyrus. Scale bar: 150 μm. (b) Laminar glutamatergic innervation pattern in the molecular layer of the dentate gyrus. The images, taken from the location marked by a rectangle in a, show axon terminals (EGFP) in the molecular layer originating from the contralateral hilus, medial and lateral entorhinal cortex, respectively. Scale bar: 50 μm. (c) Opto-evoked glutamatergic synaptic transmission in a randomly picked DGC using a 5ms pulse of 473 nm light stimulation. Shown to the left are MEnt axonal terminals (EGFP) and a recorded DGC (white). A recording trace is shown to the right, which could be completely blocked by 50 μm CNQX. Scale bar: 20 μm. (d) Functional glutamatergic synapse formation in the developing adult-born neurons. Shown are the percentages of recorded mature and adult-born DGCs at 7, 14, 21 or 28 dpi with detectable evoked eEPSCs after opto-stimulation of a single layer. (e) Correlations of functional entorhinal cortical innervations with primary cilia assembly. Shown is a summary of the contralateral, lateral and medial entorhinal cortical innervations an d cilia assembly of adult-bornDGCs at 7, 14, 21 and 28 dpi. Values represent mean±SEM (n=10–12 neurons). M, mature DGCs.
Primary ciliogenesis is critical for synapse formation
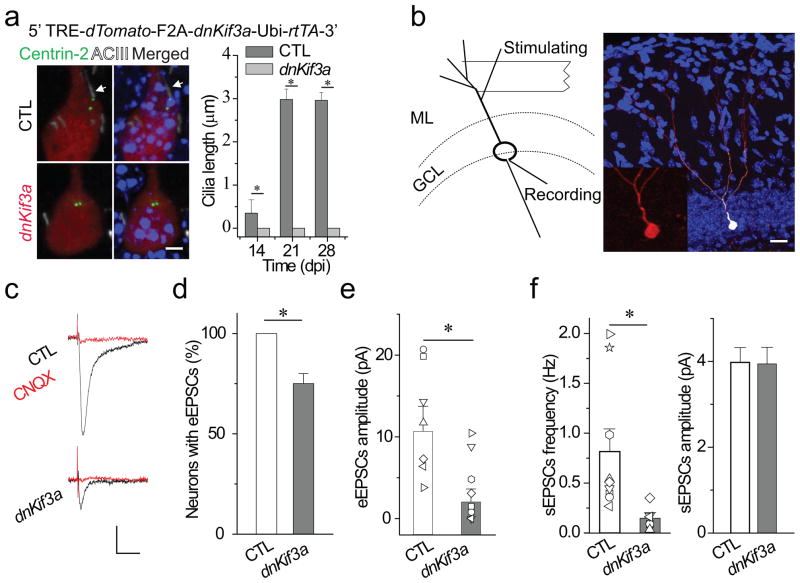
We next asked whether primary cilia assembly in developing newborn DGCs plays a functional role in synapse formation with the Ent projections by deleting primary cilia of newborn DGCs during the period of synapse formation. Since ectopic expression of dominant-negative Kinesin II motor protein (Kif3a) or knockout of Kif3a prevents primary cilia assembly from various cell types, including adult neural progenitors17,19,30, we constructed an inducible retroviral vector to express dominant-negative Kif3a (dnKif3a) in newborn DGCs. We co-injected an EGFP-tagged Centrin-2 retrovirus with either the inducible dnKif3a retrovirus or an inducible retrovirus expressing dTomato as a control group (CTL) into the hilus of the adult dentate gyrus. We induced dnKif3a expression at 7–11 dpi with doxycycline (dox), during which time retrovirus-labeled DGCs have already formed stereotypical dendritic arbors and axonal projections4,8,10. In this way, we avoided any possible confounding effects due to altered proliferation or differentiation caused by dnKif3a-induced primary cilia deletion 17–18,31–32. As expected, we found that dox-induced ectopic expression of dnKif3a efficiently eliminated primary cilia in newborn DGCs at 14, 21 and 28 dpi (Fig. 3a).
Figure 3. Primary cilia deletion caused by expressing dnKif3a severely disrupts functional glutamatergic synapse formation in adult-born neurons.
(a) Primary cilia deletion in adult-born DGCs by dnKif3a ectopic expression. At top, a diagram of the inducible retroviral vector (dnKif3a), below, typical adult-born DGCs labeled with markers of cilia (White, ACIII staining), centrioles (Green, Centrin-2-EGFP) and adult-born DGCs (Red, dTomato). Arrows point to centrosome. Scale bar: 5 μm.At right, a comparison of cilia length in control and dnKif3a+ adult-born DGCs. (b) A schematic diagram of the electrophysiological testing and an image of a recorded newborn DGC filled with biocytin through the recording pipette. The stimulating electrode was placed in the outer molecular layer to excite principally the entorhinal cortical projections. The inset is an enlarged image of the recorded adult-born DGC with dTomato signal. GCL, granule cell layer. ML, molecular layer. Scale bar: 25 μm. (c–f) Glutamatergic synaptic transmission recorded from control and dnKif3a+ adult-born DGCs at 21 dpi. c) Sample traces of glutamatergic synaptic transmission in the presence of 5 μ bicuculline. Cells were held at Vm=-65 mV. Scale bars: 10 pA and 15 ms. d) The percent of recorded newborn DGCs with detectable synaptic transmission. e) eEPSCs amplitude. Shown are individual and averaged eEPSCs amplitudes from control and dnKif3a+ adult-born DGCs. f) Spontaneous glutamateric synaptic transmission, frequency and amplitude. In a, d–f, all values represent mean±SEM (n=7–12 neurons; *: p<0.01, ANOVA).
We then measured functional synapse formation onto newborn DGCs by assessing the profile of evoked synaptic transmission. We performed whole cell recording in control and dnKif3a+ newborn DGCs at 21dpi while electrically stimulating the outer two-third of the molecular layer of the dentate gyrus to activate Ent projections (Fig. 3b)26,28. Following electrophysiological recordings, biocytin-labeling confirmed that recorded cells were indeed retrovirus-labeled newborn DGCs (Fig. 3b). We analyzed eEPSCs in the presence of 5 μM bicuculline as previously described8,10. Successful synaptic transmission was recorded from 100% of control newborn DGCs but from only 75±5.6% of dnKif3a+ DGCs. The mean peak amplitude of eEPSCs in responsive dnKif3a+ DGCs was smaller than controls (Fig. 3c, d, e). The frequency of spontaneous glutamatergic synaptic currents (sEPSCs) of control newborn DGCs was 0.82±0.22 Hz as compared to 0.15±0.05 Hz in dnKif3a+ DGCs. There was no significant difference in sEPSCs mean amplitude (Fig. 3f).
In addition to its ciliary role, Kif3a forms a motor complex involved in plus-end directed trafficking in cultured cells32–33. Knock-down of Kif3a in epithelial cells causes endosome/lysosome clusters33. It is unclear whether Kif3a knock-down might similarly affect minus-end directed endosome/lysosome trafficking in neuronal dendrites. To assess this, we briefly stained dnKif3a+ DGCs at 21 dpi with LAMP1 antibody, a marker for the late endosome/lysosome33. We did not observe endosome/lysosome clusters in either the soma or proximal apical dendrite of dnKif3a+ DGCs (Supplementary Fig. 3). This suggests that Kif3a is likely not involved in minus-end directed endosome trafficking, consistent with a previous report33. To further rule out possible non-ciliary effects of dnKif3a expression on synapse formation, we expressed dnKif3a in mature DGCs in vivo using AAV-dnKif3a and recorded dendritic synaptic activity by stimulating Ent projections. There was no difference in the dendritic synaptic responses from control and mature dnKif3a+ DGCs (Supplementary Fig. 4). Overall, these results suggest that the expression of dnKif3a causes defects in dendritic synapse formation in newborn DGCs and these defects arise due to the failure of cilia assembly. Thus, primary cilia assembly is required for the proper establishment of functional synapses between Ent projections and newborn DGCs.
Role of primary cilia in dendritic refinement
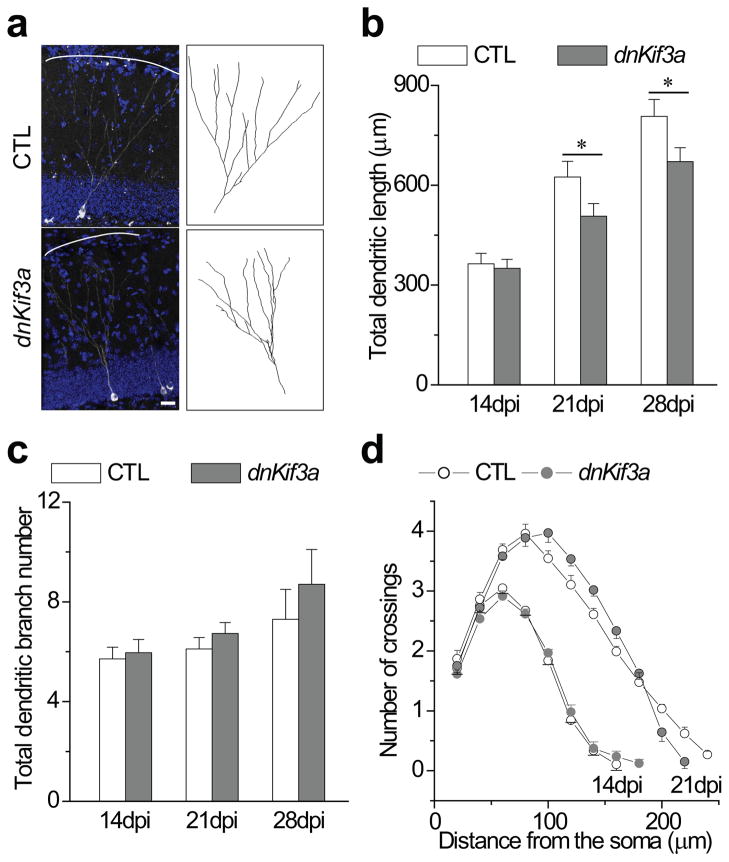
Newborn DGCs further elaborate their dendrites (dendritic refinement) between 14 and 21 dpi8,25, during which they assemble primary cilia and form glutamatergic synapses with Ent projections. Accordingly, we next asked whether the assembly of primary cilia in newborn DGCs was required for dendritic refinement by measuring dendritic length and branching in dnKif3a+ and control newborn DGCs. Using confocal microscopy methods8,25, we reconstructed the dendritic arborization of dnKif3a+ DGCs at 28 dpi (Fig. 4a and Methods). We then measured the total dendritic length of control and dnKif3a+ newborn DGCs. At 28 dpi, dnKif3a+ DGCs had significantly shorter total dendritic arbor length (671±42μm) compared to control newborn DGCs (807±51μm; Fig. 4b). In contrast, analysis of dendritic branching revealed that dnKif3a+ DGCs have 8.6±1.4 branches in average, similar to control newborn DGCs (7.4±1.5, Fig. 4b). We further analyzed dendritic length and branching of dnKif3a+ and control newborn DGCs at 14 and 21 dpi (Fig. 4c, d). At 14 dpi there was no difference in either total dendritic length or branch number between control and dnKif3a+ cells, but by 21dpi, defects similar to those observed at 28 dpi. The lack of an effect of dnKif3a expression at time prior to the formation of cilia but a robust effect after the period of cilia formation suggests that the failure to elaborate dendrites is due to the lack of a primary cilium and not due to a non-ciliary function of Kif3a.
Figure 4. Primary cilia deletion in adult-born neurons results in defective dendritic refinement.
(a) Defective dendritic refinement of dnKif3a+ DGCs. Shown on the left are the images of control and dnKif3a+ DGCs at 28 dpi. Solid lines mark the edges of the outer molecular layer. Shown to the right are drawings from the images. Scale bar: 20 μm. (b) A summary of total dendritic length for control and dnKif3a+ adult-born DGCs at 14, 21, and 28 dpi. (c) Dendritic branch number for control and dnKif3a+ adult-born DGCs at 14, 21, and 28 dpi. (d) Sholl analysis of the dendritic tree of control and dnKif3a adult-born neurons at 14 and 21dpi. (For b, c and d, n=25–38 neurons; for b and c, *: p<0.01, ANOVA; for d, statistical significance was determined by Student’s t-test). All values represent mean±SEM.
To further rule out possible nonciliary function of dnKif3a, we expressed dnKif3a from 5 dpi and analyzed the dendrites of newborn DGCs at 7 dpi, during which time there were no primary cilia present (Fig. 1c). There were no significant defects in dendritic length or branching (Supplementary Fig. 5). These results indicate that defects in dendritic refinement in dnKif3a+ DGCs occurred indeed after the initiation of cilia assembly. To further confirm that the dendritic defects in dnKif3a+ DGCs are due to a failure of cilia assembly, we disrupted primary cilia assembly by an alternate approach, shRNA-mediated knockdown of Ift88 (Supplementary Fig. 6a, b). We analyzed the dendrites of shIft88#4+ DGCs at 28dpi. Similar to the findings in dnKif3a+ DGCs (Fig. 4b, c), we found that shIft88#4+ DGCs had significantly shorter dendrites but similar dendritic branch number compared to control shRNA+ DGCs (Supplementary Fig. 6c, d). Ift88 has been implicated in spindle formation and centrosome function34–35. To exclude the possibility that these non-ciliary functions might alter dendritic refinement, we examined centrosome localization in shRNA88#4+ DGCs at 7, 14 and 21 dpi. Knock-down cells had normal centrosome localization (Supplementary Fig. 7a, b), suggesting no major effects of knock-down on either spindle or centrosome formation. Together, these results show that either the expression of dnKif3a or knock-down of Ift88 disrupt cilia assembly and lead to defects in dendritic refinement. Furthermore, it is likely that in the absence of cilia, the resulting shorter dendrites reduces the number of active glutamatergic synapses from Ent projections; however, future studies will be required to confirm this hypothesis.
Enhanced Wnt/β-catenin signaling following cilia loss
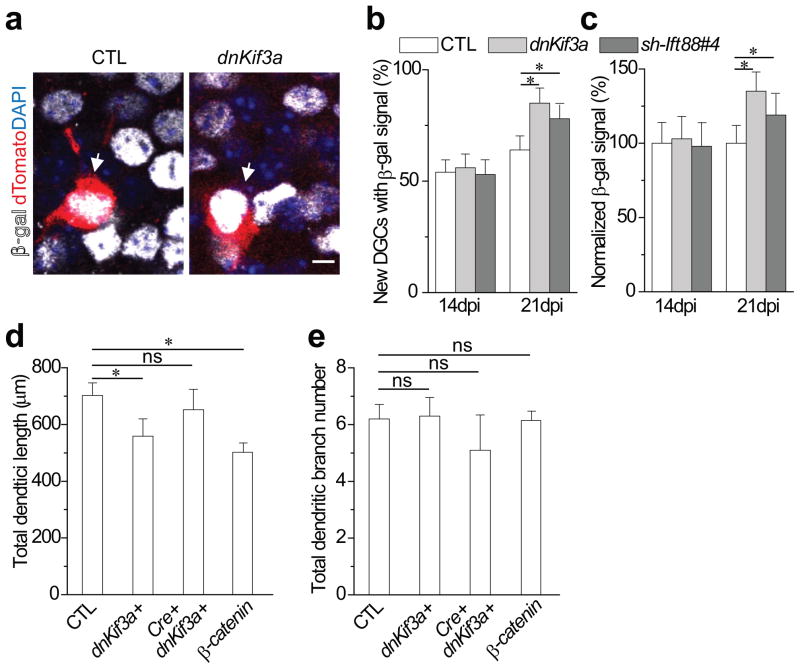
One function of primary cilia is the regulation of Wnt/β-catenin signaling. In adult hippocampal neural progenitors, the Wnt/β-catenin signaling pathway is active and regulates neuronal proliferation and differentiation36. Whether Wnt/β-catenin signaling is active in newborn DGCs, and whether it plays a role in dendritic refinement during cilia assembly, remains unknown. Thus, we first assessed Wnt/β-catenin signaling activity in newborn DGCs using β-catenin reporter (BAT-gal) mice36–37. At 14 dpi, 54±5.6% of newborn DGCs showed detectable β-gal signals, increasing to 64±6.3% by 21 dpi (Fig. 5a, b). We next asked whether primary cilia deletion alters Wnt/β-catenin signaling activity in newborn DGCs as previously suggested in other cell types11,38. At 21 dpi, β-gal signal was detectable in 85±6.8% of dnKif3a+ compared with 64±6.3% of control newborn DGCs. The mean β-gal intensity in dnKif3a+ DGCs was nearly 30% stronger than that of control DGCs (Fig. 5c), suggesting that not only is signaling active in more cells but that signal strength is enhanced. At 14 dpi, when most DGCs lack cilia, there was no difference in either the number of β-gal-positive cells (56±6.2% of dnKif3a versus 54±5.6% of control) or the intensity of the β-gal signal (Fig. 5b, c). This result shows that the expression of dnKif3a in newborn DGCs modulates Wnt/β-catenin signaling only after primary cilia assembly. Enhanced β-catenin signaling activity was likewise observed in shIft88#4+ DGCs displaying disrupted cilia assembly and shorter cilia (Fig. 5b and Supplementary Fig. 6). We also found a significant increase of β-catenin signaling in shIft88#4+ DGCs at 21 dpi but not 14 dpi. Together these results indicate that disrupted cilia assembly leads to enhanced β-catenin signaling in newborn DGCs.
Figure 5. Altered Wnt/β-catenin signaling activity upon primary cilia deletion regulates the dendritic refinement of adult-born neurons.
(a) Wnt/β-catenin signaling in developing adult-born DGCs. Shown are sample single plane images of β-gal signal in control and dnKif3a+ DGCS at 21 dpi using BAT-gal reporter mice. Arrows point to newborn DGCs. Scale bar: 10μm. (b, c) Enhanced β-gal signal upon primary cilia deletion at 21 dpi. Shown in (b) is a summary of the percent of the control or dnKif3a+ DGCs having β-gal signal. Shown in (c) is a summary of normalized intensity of the β-gal signal for control or dnKif3a+ adult-born DGCs at 21 dpi. All values represent mean±SEM (n=22–29; *: p<0.01, ANOVA). (d)Cre-mediated excision of β-catenin restores normal dendritic length in dnKif3a+ adult-born neurons at 21 dpi. β-cateninfl/fl mice were injected with dnKif3a and inducible Cre retroviruses as described in the Methods and dendritic lengths measured at 21 dpi. S33Y β-catenin was induced at 14 dpi and dendritic lengths determined at 21 dpi. (e) Dendritic branch number for the same cohort of neurons shown in d. All values represent mean±SEM (n=35–40; *: p<0.01, ANOVA; ns represents no significance).
We next asked whether the increased β-cateinin signaling resulting from cilia loss is necessary for the impaired dendritic refinement. To test this, we expressed dnKif3a in β-catenin-deleted newborn DGCs and assessed their dendritic refinement. To eliminate Wnt/β-catenin signaling, we co-injected inducible EGFP-tagged Cre recombinase with control or dnKif3a retroviruses into the hippocampus of conditional β-catenin−/−(β-cateninfl/fl) mice39. Gene expression was induced beginning at 7 dpi and dendritic length and branching were measured at 21 dpi. The induction of dnKif3a in newborn DGCs with otherwise normal Wnt/β-catenin signaling caused a significant decrease in mean total dendritic length (from 702±45μm in control newborn to 559±61μm in dnKif3a+DGCs) with no change in mean dendritic branching number (Fig. 5d, e, comparable to Fig. 4). In contrast, the specific induction of dnKif3a in newborn DGCs also deficient for Wnt/β-catenin signaling resulted in a significant increase in mean total dendritic length (652±72μm compared to 559±61μm in cilia-deficient control animals). The mean number of dendritic branches in β-cateninfl/fl and Cre+dnKif3a+ DGCs was not significantly different from that of β-cateninfl/fl and dnKif3a+ DGCs (Fig. 5e). This data shows that knockout of β-catenin in newborn DGCs reversed the defective dendritic refinement caused by dnKif3a expression, suggesting that the increase in β-catenin signaling may contribute to defective dendritic refinement.
To determine whether enhanced β-catenin activity is sufficient to perturb dendritic refinement, we expressed constitutively active S33Y β-catenin40 in newborn DGCs using an inducible retrovirus. To avoid any potentially confounding effects on the initial development of the newborn DGCs41, we induced S33Y β-catenin at 14dpi and analyzed dendrites at 21 dpi. S33Y β-catenin+ DGCs had significantly shorter dendrites (Fig. 5d, e), similar in length to dnKif3a+ DGCs (Fig. 4). There was no difference in dendritic branch number. This result demonstrates that an increase in β-catenin signaling can suppress dendritic refinement of newborn DGCs between 14 and 21 dpi.
In summary, these data show that the assembly of primary cilia, which occurs in newborn DGCs between 14 and 21 dpi, is required for proper dendritic refinement. Perturbing cilia assembly impairs dendritic refinement at least partially by enhancing Wnt/β-catenin signaling.
Discussion
Here we have shown that primary cilia assemble in newborn DGCs during the time when these neurons are being actively innervated by the Ent projections, raising the possibility that cilia assembly could be essential to synaptic integration. Due to the embryonic lethality of cilia loss and technical limitations for labeling and controlling primary cilia, cilia function during neuronal development and the underlying mechanisms remain unknown. Recently, several transgenic mice with conditional deletion of primary cilia have revealed the essential roles of primary cilia in neuronal proliferation and differentiation17–18,31. Because of the limited availability of neuron-specific Cre transgenic lines and potential developmental compensation, however, these inducible lines have not been used to examine the role of primary cilia in neuronal development. Using an ‘in vivo single cell genetic approach’7–8, we analyzed cilia formation in newborn DGCs and the developmental impact of cilia deletion. We found that primary cilia are absent from most young newborn neurons but form between 14 and 21 dpi, during which these neurons form synapses with Ent projections (Supplementary Fig. 9). Deletion of primary cilia during this period disrupted not only glutamatergic synapse formation but also dendritic refinement. Furthermore, we showed that these defects in dendritic refinement are likely due to elevated Wnt/β-catenin signaling activity. These results demonstrate the requirement of primary cilia assembly in newborn DGCs for their successful synaptic integration.
Essential roles of primary cilia in neuronal development
In rodent embryos, disruption of primary cilia by altered expression of cilia-associated genes such as Ift88, Kif3a, Smoothened or Stumpy causes aberrant formation of numerous brain structures, presumably due to impaired expansion of different neural progenitor pools17–18,31,42. These findings demonstrate that primary cilia regulate the early stages of brain morphogenesis, likely by regulating progenitor proliferation. In their knockout mice, there are a reduced pool of adult hippocampal neural stem cells17–18, suggesting a role for primary cilia in maintaining neural progenitor cells.
Here we deleted primary cilia by expressing dnKif3a or shRNA against Ift88 in newborn DGCs. Both dnKif3a expression and Ift88 knock-down impaired dendritic refinement, while there were no apparent defects in non-ciliary functions such as neuronal polarization and endosomal trafficking. With these genetic manipulations, the development of DGCs was normal until 14 dpi, the period when cilia assembly commences. We consider it likely that the impaired dendritic refinement and synapse formation are the direct consequences of a failure to properly assemble and maintain a primary cilium.
The assembly of primary cilia in newborn DGCs occurs precisely between 14 and 21 dpi during which time newborn DCGs begin to form dendritic synapses with Ent projections. Primary cilia deletion severely disrupted this synaptic integration. Cells with disrupted cilia also displayed defective dendritic refinement, showing shorter dendrites. Although we found that the amplitude of sEPSCs was not affected by cilia loss, the frequency was reduced significantly. This suggests that individual synapses of cilia-deleted newborn neurons can form normally but are fewer in number. The decreased frequency is presumably due to a reduction in synapse number as would be expected from shorter dendrites, although the possibility of other dendritic defects remains to be examined. In the sholl analysis of dendrites in 14, 21 and 28 dpi (Fig. 4d), we did not find a significant change of dendritic branching. Branch formation and elongation may therefore be controlled differentially at least at this developmental stage. We also briefly analyzed dendritic length of control and dnKif3a+ newborn DGCs at 28 dpi in the inner molecular layer and granular cell layer, showing no significant change (data not shown). Since glutamatergic synapses in the inner molecular layer develop prior to cilia formation and the extent of glutamatergic synaptic innervations remains relatively stable till 28 dpi (Fig. 2d, e), primary cilia may not be critical for synapses formed by contralateral projections to the dentate gyrus. Rather, cilia formation appears to be essential for newborn DGCs to form synapses with Ent projections.
Signaling pathways through primary cilia
It has become clear that various signaling pathways in vertebrate embryonic development require the presence of primary cilia13. In our studies, primary cilia deletion significantly enhanced Wnt/β-catenin signaling, consistent with findings in other systems11,19,38. Our results raise two important questions: 1) Does disrupting cilia assembly lead to enhanced Wnt/β-catenin signaling (and how?); and 2) Is increased Wnt/β-catenin signaling responsible for the observed reduced dendritic length? The data (Fig. 5) shows a significant increase of Wnt/β-catenin signaling in dnKif3a or Ift88 shRNA expressing newborn DGCs at 21 dpi. Since there is no significant increase before cilia formation, Kif3a or Ift88 likely modulates this pathway through its ciliary function rather than both ciliary and non-ciliary functions, as previously reported19. Furthermore, the lack of Smoothened expression in the granule cell and molecular layers in the dentate gyrus makes it unlikely that hedgehog signaling is involved in dendritic refinement during this period43–44. With regard to the second question, the data (Fig. 5) show that genetic removal of β-catenin in newborn DGCs rescues the dendritic defects caused by dnKif3a expression and that the expression of a constitutively active form of β-catenin is sufficient to cause shorter dendritic length in newborn DGCs. In summary, we surmise that the assembly of primary cilia between 14 and 21 dpi in newborn DGCs is essential for dendritic refinement at least in part through regulating Wnt/β-catenin signaling. Indeed dendritic development is regulated in other types of neurons by several different Wnt/β-catenin signaling pathways45–47. Thus in addition to its function in other brain circuits, Wnt/β-catenin signaling may play complex roles in the formation and maintenance of entorhinal cortex-dentate gyrus circuitry.
Primary cilia may sense local environmental signals to regulate neuronal development. For example, sonic hedgehog signaling requires the presence of primary cilia and is essential for the establishment, proliferation and differentiation of adult neural progenitor cells16. Our data suggests that the Wnt/β-catenin signaling pathway is altered by the assembly of primary cilia and in turn impacts the stepwise synaptic integration of newborn DGCs (Supplementary Fig. 9). Thus, different signaling pathways are mediated by primary cilia at distinct developmental stages. Furthermore, we found that there is a sustained period of neuronal migration during which newborn neurons lack primary cilia and show no response to experimental manipulations of cilia-associated proteins. Such stage-specific differences in the pathways requiring functional primary cilia may explain why other studies found that primary cilia play no predominant roles in transducing canonical Wnt signaling in developing organisms48–49. Thus, further study of the precise time course of cilia development and associated signaling pathways is required.
Defects in primary cilia are associated with several human diseases, including retinal blindness, brain dysgenesis and neurocognitive impairments14. In ciliopathies such as Joubert syndrome and related disorders, there are numerous structural brain lesions. Patients with Meckel-Gruber or Bardet-Biedl syndrome also display structural defects in the central nervous system and defective neocortical development is associated with cilia disorders12,14,50. Thus, cilia function is likely required for many aspects of brain development, although the detailed signaling pathways and mechanisms remain to be determined. Here we identified a new role for the primary cilium in the sequential synaptic integration of adult-born neurons in vivo. This study also sheds light on the mechanisms of neural development in the adult organism, and possibly on the etiology of cilia associated-brain disorders.
Methods
Labeling and manipulation of newborn DGCs
Engineered self-inactivating murine oncoretroviruses were used to deliver genes of interest and to express shRNAs against targeted gene transcripts specifically in proliferating cells and their progeny7–8. The retroviral packaging vectors and cell line were kindly provided by Fred Gage’s laboratory (The Salk Institute). The retroviral vectors were originally obtained from Dr. Hongjun Song’s laboratory (The Johns Hopkins University). dTomato, Centrin-2, dnKif3a, Cre and short hairpin sequences were inserted into different retroviral vectors as described in the text. Four shRNAs against different regions of Ift88 and a control shRNA not against any known genes were produced (Supplementary Fig. 6). To validate the specificity and efficiency of shRNAs, retroviral shRNA vectors and expression constructs for EGFP-tagged full-length mouse Ift88 were co-transfected into 293 cells. Cell lysates were then prepared for Western blot analysis using anti-GFP antibodies.
High titers of engineered retroviruses were stereotaxically injected into ~5 weeks old adult female C57BL/6 mice (Charles River), β-gal reporter mice37 and β-cateninfl/fl mice (The Jackson laboratory) housed under standard conditions8. Doxycycline, where used, was administered daily through drinking water with 5% sucrose. All animal procedures were in accordance with institutional guidelines.
Genetical delivery of Channelrhodopsin-2 and dnKif3a
Adeno-associated viral vector expressing Channelrhdopsin-2 (AAV-ChR2) was kindly provided by Karl Deisseroth’s laboratory. High-titer AAV-ChR2 or dnKif3a was produced and purified by the University of North Carolina Vector Core Facility. The AAV injection procedure was the same as used for retroviral injections. For the AAV-ChR2, the contralateral hilus received two injections (0.1μl each, 1×1012 viral particles/ml) along the anterior-posterior axis to infect nearly the entire hilus of the hippocampus. Lateral or medial entorhinal cortex received only one injection per hemisphere. As described in the text, 14 days after AAV-ChR2 injection, mice were used for electrophysiological tests. The AAV-dnKif3a was injected into the dentate gyrus to infect DGCs with the same procedure as that for AAV-ChR2 injection.
Immunostaining, confocal imaging and image analysis
Coronal brain sections (40 μm thick) were prepared from different retrovirus-injected mice and processed for immunostaining, as previously described8, using ACIII primary antibody (goat, 1:250, Santa Cruz). Images were acquired on an Olympus FV1000 confocal system using a multi-track configuration. For analysis of primary cilia and cell morphology, Z-series stacks of 640×640 pixel images were taken with a 60X/1.4 NA oil objective using a Z-resolution of 0.5 μm. For dendritic analysis, Z-series stacks of 640×640 pixel images were taken using a 40X/1.0 NA oil objective using a Z-resolution of 1 μm. The resulting three-dimensional images were analyzed using the Imaris V6.4.
For analysis of neuronal positioning, single section confocal images of dTomato+ neurons counterstained with 4′,6-diaminodino-2-phenylindole (DAPI, 1:5000) in the mounting media were used to localize the cell, as previously reported25. We defined the inner or outer granule cell layer by drawing a tangential line across three DAPI-stained cells approximately 50 μm apart in the inner or outermost cell layer. We then measured the position of these neurons as defined above (Fig. 1a). For analysis of primary cilia development and length, we used reconstructed 3D primary cilia images of newborn DGCs. All the co-localization of primary cilia and centrosome were confirmed by rotating these images. Using the above measurement definition (supplementary Fig. 2a), we measured cilia position and angle with single Z-plane 2D images containing two centrioles. A minimum of 25 neurons from randomly chosen sections of at least 3 animals were analyzed for each experimental condition. For analysis of dendritic refinement, 3D reconstructions of entire dendritic arbors were made from Z-series stacks of confocal images using Imaris. Those dTomato+ dentate granule cells with largely intact dendritic trees were analyzed for dendritic length and branch number (Fig. 4b, c), the same as previously reported methods8,25. Data shown are from at least 17 individual dTomato+ neurons from 3 or more animals for each condition.
The β-gal antigen was stained with an antibody from MP Biomedicals, LLC (Rabbit, 1:500). LAMP-1 was stained with an antibody from Saint Cruz (Rabbit, 1:250). To minimize variation between samples, CTL and experimental brain sections were immunostained side by side and imaged on the same day using identical imaging parameters. Ten scans across the cell were collected, and the image with the largest nuclear area was analyzed. When any procedure failed, the selected cell was discarded. The intensity of cellular β-gal or LAMP-1 was measured with Image J software (NIH).
Significance in all statistical tests was determined using either ANOVA or the Kolmogorov-Smirnov test.
Electrophysiology
Mice were housed under standard conditions and processed at 7, 14, 21 and 28 dpi for electrophysiological recordings at 32–34°C, as previously described8. To examine contralateral mossy cell and lateral or medial entorhinal cortical glutamatergic inputs, cells were held at −65 mV and exposed to short pulses of blue light from a 473 nm laser launched into a Zeiss upright microscope through the epifluoresence light path. The 50mW laser was controlled by a standard TTL board; ending power on brain slices was ~5 mW. Electrical stimulation experiments of the entorhinal perforant path used standard bipolar electrodes to determine evoked glutamatergic synaptic transmission in cells held at −65 mV (Fig. 3b). The stimulus intensity was maintained for all tests. Any failure to evoke a response in recorded neurons was further confirmed by increasing the stimulus intensity8(Supplementary Fig. 8). Spontaneous synaptic activity was examined in the presence of 1 μM TTX during five-minute continuous sweeps recorded under voltage-clamp at −65 mV in the presence of 5 μM bicuculline. All chemicals used were purchased from Sigma.
Supplementary Material
Supplementary figure 1. Primary cilia assembly in developing adult-born neurons. (a) A schematic diagram of the inducible retroviral vector for co-expression of dTomato nd Ift88 fused with EGFP. Shown are representative confocal images (a-1, a-2 and a-3) of adult-born neurons at 21 dpi expressing the dTomato and Ift88-EGFP, counterstained for DAPI and immunostained for ACIII. Arrows indicate primary cilia. Scale bar: 2.5 μm. (b) Quantification of primary cilia development in adult-born neurons at 5, 14 and 21 dpi. (c) Ectopic expression of Centrin-2 has no significant effect on cilium assembly at 14 dpi. Shown is the percent of adult-born DGCs with cilia after continuous expression of Centrin-2 for 14 days versus induced expression from12 dpi to 14 dpi by the administration of doxycycline. The group of neurons continuously expressing Centrin-2 is also presented in Fig. 1c. Values represent mean±SEM (n=25–48 neurons; *: p<0.01, ANOVA).
Supplementary figure 2. Primary cilia consistently originate from the leading edge of migrating DGCs but do not protrude in a fixed direction. (a) A sample image and schematic diagram of a developing newborn DGC, indicating the subgranular zone (SGZ), the granule cell layer (GCL), the stereotypic direction of migration (arrow), the leading edge of the nucleus (middle dashed line), centrosome (dots), primary cilium (solid line), and cilium angle (θ). (b) Percent of newborn DGCs at 14 or 21 dpi that assemble primary cilia in the leading side of the nucleus. Values represent mean±SEM (n=32–48 neurons; *: p<0.01, ANOVA). (c) Radial plot of cilia angle and length at 14, 21 and 28dpi. Radial unit: 2 μm.
Supplementary figure 3. Absence of late endosome/lysosome clusters in adult-born DGCs after ectopic expression of dnKif3a. (a–b) Comparison of the intensity of LAMP-1 endosome immunostaining in wild-type versus experimental adult-born DGCs at 21 dpi. Randomly selected cells from each group were analyzed along their major (center of nucleus to proximal apical dendrite) and minor axes, as indicated in the schematic overlay of the first panel. (a) At top, representative confocal images of three wild-type adult-born neurons immunostained for LAMP-1 (green panels), as visualized with retroviral dTomato and DAPI (merged red-blue panels). At bottom, linear plots of LAMP-1 intensity along the axes. (b) LAMP-1 staining and measurement in three dnKif3a expressing DGCs. Scale bar: 5 μm.
Supplementary figure 4. Mature DGCs expressing dnKif3a have normal dendritic synaptic activity. (a) Mature DGCs infected by AAV-dnKif3a injected into the dentate gyrus. Shown are confocal images of two recorded DGCs filled with biocytin through the recording pipette. The left-most cell is negative for dnKif3a (left inset), and the right-most is positive for dnKif3a (right panels). The recording paradigm was the same as that in Fig. 3b. Scale bar: 20 μm. (b) Synaptic responses as measured by evoked excitatory post-synaptic currents (eEPSCs) in mature dnKif3a-and dnKif3a+ DGCs. All values represent mean±SEM (n=5–12 neurons; p<0.01, ANOVA).
Supplementary figure 5. Dendritic growth of control and dnKif3a+ adult-born DGCs at 7dpi. 7dpi control neurons versus dnKif3a-induced (5 dpi to 7 dpi) neurons were analyzed for (a) total dendritic length and (b) total dendritic branching number (n=25–26 cells; *: p<0.01, ANOVA). All values represent mean±SEM.
Supplementary figure 6. Primary cilia deletion via shRNA knockdown in adult-born neurons results in defective dendritic refinement in the entorhinal cortex projection field of the dentate gyrus. (a) Validation of the efficacy of various shRNAs against mouse Ift88 in vitro. Retroviral constructs expressing control (randomly-generated sequence not complementary to any known gene) or different shRNAs against mouse Ift88 were co-transfected together with an expression construct for mIft88-EGFP into HEK293 cells. The top panel shows a representative immunoblot using anti-GFP and anti-GAPDH antibodies. Shown below is the densitometric quantification of the relative amounts of mIft88-EGFP. All values represent mean±SEM (n=3; *: p<0.01, ANOVA). (b) Disruption of primary cilia assembly upon Ift88 knock-down. At left, sample images of control and shIft88#4+ DGCs at 28 dpi, immunostained for ACIII and counterstained with DAPI. At right, quantification of cilia length. Scale bar: 5 μm. (c) Dendritic lengths of 28 dpi control and shIft88#4+ DGCs. (d) Dendritic branch number of 28 dpi control and shIft88#4+. (For b, c and d, n=30–35 cells, *: p<0.01, ANOVA). All values represent mean±SEM.
Supplementary figure 7. Developing adult-born DGCs with deleted primary cilia display normal centrosome migration. (a) Representative images of Centrin-2-labeled centrosomes in adult-born DGCs. Shown are single plane images of the centrioles (arrows) at 7, 14 and 28 dpi, oriented with the SGZ at the bottom. Scale bar: 10 μm. (b) Centrosome position within developing adult-born DGCs. At left, the distance between the centrosome and the center of nucleus in control adult-born DGCs at 5, 14, 21 and 28 dpi, showing the transient migration of centrosomes away from the nucleus during development. At right, a summary of the distance between centrosome and nucleus in control and shIft88#4 expressing adult-born DGCs at identical time points. Values in b represent mean ± SEM (n=32–34 cells; control values in both figures of b were from the same group of cells; *: p<0.05, ANOVA).
Supplementary figure 8. eEPSCs amplitude in control and dnKif3a+ adult-born neurons. Increasing the stimulus intensity did not evoke eEPSCs in cilia-deleted neurons without synaptic response.
Supplementary figure 9. A model of the effects of primary cilia assembly on dendritic refinement and synapse formation with entorhinal cortical projections in adult-born DGCs. At 14 dpi, cilia have not yet assembled, Wnt/β-catenin signaling is active and dendrites are immature. At 21 dpi, newly formed cilia suppress Wnt/β-catenin signaling allowing for further growth of dendrites. GCL, granule cell layer. MC, mossy cell projecting layer.
Supplementary Video 1. Three dimensional reconstruction of Z-series confocal images of primary cilia. Shown is a primary cilium image of an adult-born neuron at 21 dpi expressing the dTomato and EGFP-Centrin-2, counterstained for DAPI and immunostained for ACIII (see Methods).
Acknowledgments
We would like to thank Simon Halegoua, Maurice Kernan, Gary Matthews, Lorna Role and Hongjun Song for critical comments, and Feng-Qian Li, Justin Rodriguez and Qiaojie Xiong for technical support. This work was supported by grants from NIH (NS065915), AHA (0930067N), and SUNY REACH to S.G., and NIH (HL107493) to K.I.T.
Footnotes
Author contributions
N.K. engineered retroviral constructs. N.K. and J.W. did immunohistochemistry and confocal imaging analysis. Y.G. did all physiology analysis and took some images. S.J. helped analyzing some images and editing the manuscript. K.T. helped characterizing retroviral vectors. S.G. supervised the project. S.G. and J.L. wrote the manuscript. All authors read and discussed the manuscript.
References
- 1.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 2.Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- 3.Ma DK, et al. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat Neurosci. 2010;13:1338–1344. doi: 10.1038/nn.2672. nn.2672 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. S0092-8674(08)00134-7 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. S0166-2236(06)00263-3 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Kelsch W, Sim S, Lois C. Watching synaptogenesis in the adult brain. Annu Rev Neurosci. 2010;33:131–149. doi: 10.1146/annurev-neuro-060909-153252. [DOI] [PubMed] [Google Scholar]
- 7.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. 4151030a [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. nature04404 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overstreet Wadiche L, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol. 2005;94:4528–4532. doi: 10.1152/jn.00633.2005. 00633.2005 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Esposito MS, et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. 25/44/10074 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. S0092-8674(09)00322-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JE, Gleeson JG. Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Curr Opin Neurol. 2011;24:98–105. doi: 10.1097/WCO.0b013e3283444d05. 00019052-201104000-00003 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. nrg2774 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lancaster MA, Gleeson JG. The primary cilium as a cellular signaling center: lessons from disease. Curr Opin Genet Dev. 2009;19:220–229. doi: 10.1016/j.gde.2009.04.008. S0959-437X(09)00086-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bisgrove BW, Yost HJ. The roles of cilia in developmental disorders and disease. Development. 2006;133:4131–4143. doi: 10.1242/dev.02595. dev.02595 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Han YG, Alvarez-Buylla A. Role of primary cilia in brain development and cancer. Curr Opin Neurobiol. 2010;20:58–67. doi: 10.1016/j.conb.2009.12.002. S0959-4388(09)00178-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han YG, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. nn2059 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Breunig JJ, et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2008;105:13127–13132. doi: 10.1073/pnas.0804558105. 0804558105 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corbit KC, et al. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. ncb1670 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Szymczak AL, et al. Correction of multi-gene deficiency in vivo using a single’self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. nbt957 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Bishop GA, Berbari NF, Lewis J, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 2007;505:562–571. doi: 10.1002/cne.21510. [DOI] [PubMed] [Google Scholar]
- 22.Pazour GJ, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider L, et al. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem. 2010;25:279–292. doi: 10.1159/000276562. 000276562 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai LH, Gleeson JG. Nucleokinesis in neuronal migration. Neuron. 2005;46:383–388. doi: 10.1016/j.neuron.2005.04.013. S0896-6273(05)00349-1 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Duan X, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. S0092-8674(07)00897-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) Prog Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. S0079-6123(07)63001-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang F, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. nature05744 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. S0896-6273(07)00334-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 30.Rohatgi R, Milenkovic L, Corcoran RB, Scott MP. Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proc Natl Acad Sci U S A. 2009;106:3196–3201. doi: 10.1073/pnas.0813373106. 0813373106 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willaredt MA, et al. A crucial role for primary cilia in cortical morphogenesis. J Neurosci. 2008;28:12887–12900. doi: 10.1523/JNEUROSCI.2084-08.2008. 28/48/12887 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura T, et al. Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat Cell Biol. 2004;6:328–334. doi: 10.1038/ncb1118. ncb1118 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Brown CL, et al. Kinesin-2 is a motor for late endosomes and lysosomes. Traffic. 2005;6:1114–1124. doi: 10.1111/j.1600-0854.2005.00347.x. TRA347 [pii] [DOI] [PubMed] [Google Scholar]
- 34.Delaval B, Bright A, Lawson ND, Doxsey S. The cilia protein IFT88 is required for spindle orientation in mitosis. Nat Cell Biol. 2011;13:461–468. doi: 10.1038/ncb2202. ncb2202 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robert A, et al. The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J Cell Sci. 2007;120:628–637. doi: 10.1242/jcs.03366. jcs.03366 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Lie DC, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. nature04108 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Maretto S, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. 0434590100 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDermott KM, Liu BY, Tlsty TD, Pazour GJ. Primary Cilia Regulate Branching Morphogenesis during Mammary Gland Development. Curr Biol. 2010 doi: 10.1016/j.cub.2010.02.048. S0960-9822(10)00236-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brault V, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 40.Li FQ, Mofunanya A, Fischer V, Hall J, Takemaru K. Nuclear-cytoplasmic shuttling of Chibby controls beta-catenin signaling. Mol Biol Cell. 2010;21:311–322. doi: 10.1091/mbc.E09-05-0437. E09-05-0437 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Budnik V, Salinas PC. Wnt signaling during synaptic development and plasticity. Curr Opin Neurobiol. 2011;21:151–159. doi: 10.1016/j.conb.2010.12.002. S0959-4388(10)00204-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chizhikov VV, et al. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. 27/36/9780 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masdeu C, Bernard V, Faure H, Traiffort E, Ruat M. Distribution of Smoothened at hippocampal mossy fiber synapses. Neuroreport. 2007;18:395–399. doi: 10.1097/WNR.0b013e32801421ce. 00001756-200703050-00020 [pii] [DOI] [PubMed] [Google Scholar]
- 44.Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–362. doi: 10.1016/j.neuron.2009.03.022. S0896-6273(09)00247-5 [pii] [DOI] [PubMed] [Google Scholar]
- 45.Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. nn1132 [pii] [DOI] [PubMed] [Google Scholar]
- 46.Gao X, Arlotta P, Macklis JD, Chen J. Conditional knock-out of beta-catenin in postnatal-born dentate gyrus granule neurons results in dendritic malformation. J Neurosci. 2007;27:14317–14325. doi: 10.1523/JNEUROSCI.3206-07.2007. 27/52/14317 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. nn1374 [pii] [DOI] [PubMed] [Google Scholar]
- 48.Ocbina PJ, Tuson M, Anderson KV. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS One. 2009;4:e6839. doi: 10.1371/journal.pone.0006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development. 2009;136:3089–3098. doi: 10.1242/dev.041343. 136/18/3089 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spassky N, et al. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008;317:246–259. doi: 10.1016/j.ydbio.2008.02.026. S0012-1606(08)00139-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. Primary cilia assembly in developing adult-born neurons. (a) A schematic diagram of the inducible retroviral vector for co-expression of dTomato nd Ift88 fused with EGFP. Shown are representative confocal images (a-1, a-2 and a-3) of adult-born neurons at 21 dpi expressing the dTomato and Ift88-EGFP, counterstained for DAPI and immunostained for ACIII. Arrows indicate primary cilia. Scale bar: 2.5 μm. (b) Quantification of primary cilia development in adult-born neurons at 5, 14 and 21 dpi. (c) Ectopic expression of Centrin-2 has no significant effect on cilium assembly at 14 dpi. Shown is the percent of adult-born DGCs with cilia after continuous expression of Centrin-2 for 14 days versus induced expression from12 dpi to 14 dpi by the administration of doxycycline. The group of neurons continuously expressing Centrin-2 is also presented in Fig. 1c. Values represent mean±SEM (n=25–48 neurons; *: p<0.01, ANOVA).
Supplementary figure 2. Primary cilia consistently originate from the leading edge of migrating DGCs but do not protrude in a fixed direction. (a) A sample image and schematic diagram of a developing newborn DGC, indicating the subgranular zone (SGZ), the granule cell layer (GCL), the stereotypic direction of migration (arrow), the leading edge of the nucleus (middle dashed line), centrosome (dots), primary cilium (solid line), and cilium angle (θ). (b) Percent of newborn DGCs at 14 or 21 dpi that assemble primary cilia in the leading side of the nucleus. Values represent mean±SEM (n=32–48 neurons; *: p<0.01, ANOVA). (c) Radial plot of cilia angle and length at 14, 21 and 28dpi. Radial unit: 2 μm.
Supplementary figure 3. Absence of late endosome/lysosome clusters in adult-born DGCs after ectopic expression of dnKif3a. (a–b) Comparison of the intensity of LAMP-1 endosome immunostaining in wild-type versus experimental adult-born DGCs at 21 dpi. Randomly selected cells from each group were analyzed along their major (center of nucleus to proximal apical dendrite) and minor axes, as indicated in the schematic overlay of the first panel. (a) At top, representative confocal images of three wild-type adult-born neurons immunostained for LAMP-1 (green panels), as visualized with retroviral dTomato and DAPI (merged red-blue panels). At bottom, linear plots of LAMP-1 intensity along the axes. (b) LAMP-1 staining and measurement in three dnKif3a expressing DGCs. Scale bar: 5 μm.
Supplementary figure 4. Mature DGCs expressing dnKif3a have normal dendritic synaptic activity. (a) Mature DGCs infected by AAV-dnKif3a injected into the dentate gyrus. Shown are confocal images of two recorded DGCs filled with biocytin through the recording pipette. The left-most cell is negative for dnKif3a (left inset), and the right-most is positive for dnKif3a (right panels). The recording paradigm was the same as that in Fig. 3b. Scale bar: 20 μm. (b) Synaptic responses as measured by evoked excitatory post-synaptic currents (eEPSCs) in mature dnKif3a-and dnKif3a+ DGCs. All values represent mean±SEM (n=5–12 neurons; p<0.01, ANOVA).
Supplementary figure 5. Dendritic growth of control and dnKif3a+ adult-born DGCs at 7dpi. 7dpi control neurons versus dnKif3a-induced (5 dpi to 7 dpi) neurons were analyzed for (a) total dendritic length and (b) total dendritic branching number (n=25–26 cells; *: p<0.01, ANOVA). All values represent mean±SEM.
Supplementary figure 6. Primary cilia deletion via shRNA knockdown in adult-born neurons results in defective dendritic refinement in the entorhinal cortex projection field of the dentate gyrus. (a) Validation of the efficacy of various shRNAs against mouse Ift88 in vitro. Retroviral constructs expressing control (randomly-generated sequence not complementary to any known gene) or different shRNAs against mouse Ift88 were co-transfected together with an expression construct for mIft88-EGFP into HEK293 cells. The top panel shows a representative immunoblot using anti-GFP and anti-GAPDH antibodies. Shown below is the densitometric quantification of the relative amounts of mIft88-EGFP. All values represent mean±SEM (n=3; *: p<0.01, ANOVA). (b) Disruption of primary cilia assembly upon Ift88 knock-down. At left, sample images of control and shIft88#4+ DGCs at 28 dpi, immunostained for ACIII and counterstained with DAPI. At right, quantification of cilia length. Scale bar: 5 μm. (c) Dendritic lengths of 28 dpi control and shIft88#4+ DGCs. (d) Dendritic branch number of 28 dpi control and shIft88#4+. (For b, c and d, n=30–35 cells, *: p<0.01, ANOVA). All values represent mean±SEM.
Supplementary figure 7. Developing adult-born DGCs with deleted primary cilia display normal centrosome migration. (a) Representative images of Centrin-2-labeled centrosomes in adult-born DGCs. Shown are single plane images of the centrioles (arrows) at 7, 14 and 28 dpi, oriented with the SGZ at the bottom. Scale bar: 10 μm. (b) Centrosome position within developing adult-born DGCs. At left, the distance between the centrosome and the center of nucleus in control adult-born DGCs at 5, 14, 21 and 28 dpi, showing the transient migration of centrosomes away from the nucleus during development. At right, a summary of the distance between centrosome and nucleus in control and shIft88#4 expressing adult-born DGCs at identical time points. Values in b represent mean ± SEM (n=32–34 cells; control values in both figures of b were from the same group of cells; *: p<0.05, ANOVA).
Supplementary figure 8. eEPSCs amplitude in control and dnKif3a+ adult-born neurons. Increasing the stimulus intensity did not evoke eEPSCs in cilia-deleted neurons without synaptic response.
Supplementary figure 9. A model of the effects of primary cilia assembly on dendritic refinement and synapse formation with entorhinal cortical projections in adult-born DGCs. At 14 dpi, cilia have not yet assembled, Wnt/β-catenin signaling is active and dendrites are immature. At 21 dpi, newly formed cilia suppress Wnt/β-catenin signaling allowing for further growth of dendrites. GCL, granule cell layer. MC, mossy cell projecting layer.
Supplementary Video 1. Three dimensional reconstruction of Z-series confocal images of primary cilia. Shown is a primary cilium image of an adult-born neuron at 21 dpi expressing the dTomato and EGFP-Centrin-2, counterstained for DAPI and immunostained for ACIII (see Methods).