Abstract
The growing family of interleukin (IL)-12-like cytokines produced by activated macrophages and dendritic cells became the important players in the control of infections, development of inflammation, autoimmunity and cancer. However, the role of one of them—heterodimer IL-23, which consists of IL12p40 and the unique p19 subunit in HIV-1 infection pathogenesis and progression to AIDS, represent special interest. We overviewed findings of IL-23 involvement in control of peripheral bacterial pathogens and opportunistic infection, central nervous system (CNS) viral infections and autoimmune disorders, and tumorogenesis, which potentially could be applicable to HIV-1 and AIDS.
Keywords: IL-23, Central nervous system, Bacterial infection, Interleukin-17, Autoimmune disorders, HIV-1
Introduction
Effective immune response towards a pathogen is invaluable in maintaining the integrity of the host. Initiation of an effective immune response requires close interactions between antigen-presenting cells of innate (macrophages and dendritic cells) and adaptive immunity (T and B cells). Cytokines produced by macrophages and dendritic cells (DC) are essential during host defense mechanisms in response to pathogens. In HIV-1 infection, the heterodimer of Interleukin (IL)-12 family—IL-23, represents special interest due to its possible involvement in the control of opportunistic infections, inflammation and tumorogenesis associated with AIDS.
Since its initial description (Oppmann et al. 2000), IL-23 has gained attention because of its unique heterodimer structure and similarity to key immune response cytokines IL-6/IL-12. IL-23 discovery had a significant impact on our understanding of myeloid cell derived cytokines and T-cell pathways that govern chronic inflammation (Langrish et al. 2004). IL-23 receptor (IL23R) represents one of the most significant human genetic polymorphisms in autoimmunity including ankylosing spondylitis, psoriasis, multiple sclerosis (MS), sarcoidosis and inflammatory bowel diseases (Duerr et al. 2006; Huber et al. 2008; Illes et al. 2008; Liu et al. 2008; Agarwal et al. 2009; Ban et al. 2009; Takaku et al. 2009; De Nitto et al. 2010). Unfortunately, in HIV-1 and other lentiviral infections, the role of IL-23 and its receptor-expressing cells remain indefinite. The first discussion of the possible involvement of IL-23 as a new player in cytokine control in HIV-1 pathogenesis was published by Alfano and co-authors (Alfano et al. 2008). It was observed that strong and sustained production of IL-23 by peripheral blood mononuclear cells in response to bacterial lipopolysaccharides (LPS) is associated with high viral load during primary infection (Louis et al. 2010). The reduced expression of IL-23 mRNA in immune-reconstituted by antiretroviral therapy patients (Lee et al. 2004b) with opportunistic infections brought our attention to the possible involvement of IL-23 in AIDS pathogenesis. Recently the discovery of the IL-23 receptor expression on cells that are involved in the development of the microbiota-induced tertiary lymphoid tissue (Aloisi and Pujol-Borrell 2006) during chronic inflammatory responses generates a suggestion that in the case of HIV-1-infection dysfunction or progressive loss of these cells may be the cause of the systemic opportunistic pathogens invasion.
In this article, we focus on the role of IL-23 in several inflammatory pathologies, antibacterial/antiviral protection from opportunistic infections associated with HIV-1, and with special emphasis on neurological disorders.
The infections associated with AIDS tend to fall into well-recognized patterns: the most common pathogens include Candida albicans, Pneumocystis carinii, Mycobacterium tuberculosis, Toxoplasma gondii, Cryptococcus neoformans, Mycobacterium avium intracellulare and cytomegalovirus (Lloyd 1996; Mamidi et al. 2002).
Malignant disease in patients with HIV infection also occurs as two prevalent tumors: Kaposi’s sarcoma of skin and mucosal surfaces; and non-Hodgkin’s lymphoma, which often arises within the central nervous system (Stanley et al. 1991; Cianfrocca and Roenn 1998; Hannon et al. 1998; Bohlius et al. 2009).
Autoimmune-like disorders often associated or exacerbated by HIV-1 infection are atopic dermatitis, psoriasis (Namazi 2004; Morar et al. 2010), inflammatory bowel syndrome (Kotler et al. 1993; Mohan et al. 2007; Cecchinato et al. 2008) and pneumonitis (Griffiths et al. 1995; Ingiliz et al. 2006; Segal et al. 2011).
Overall, autoimmunity in HIV-1-infected patients is associated with the detection of the antibodies to the variety of autoantigens: organ-specific autoantibodies such as antiplatelet antibodies, anti-cardiac myosin antibodies, anti-smooth muscle-specific antibodies, and anti-erythroid cell-specific antibodies; nonorgan-specific antibodies such as antinuclear antibodies, antihistone antibodies, anti-double-stranded DNA antibodies, and antiphospholipid antibodies; autoantibodies against cell surface molecules such as CD4, MHC class I and II. Some of these autoantibodies have been thought to contribute to systemic autoimmune disease [reviewed in (Onlamoon et al. 2005)].
Structure of IL-23 cytokine and regulation of secretion
IL-23 comprises of a 19-kD 4-fold helical core α subunit (IL-23p19), linked to an additional 40-kD distinct β subunit (IL-12p40) by disulfide bonding. IL-23 shares its p40 subunit with IL-12 but has a unique p19 subunit. IL-12 is composed of two covalently linked subunits, IL-12p35 and IL-12p40. The expression of the p35 and p40 genes is independently regulated. Production of p35 is rate limiting and the formation of biologically active IL-23 requires the synthesis of subunits, p40 and p19 within the same cell. Expression of the two subunits is tightly regulated and IL-23p19 is poorly secreted in the absence of IL-12p40. In vitro, p19 is secreted as a complex with the p40 subunit of IL-12. This association is necessary for its biological function, because purified p19 is biologically inert in vitro. IL-23 is produced by activated dendritic cells (DC) (Oppmann et al. 2000), macrophages (Pirhonen et al. 2002), monocytes (Lee et al. 2004b; Tchatalbachev et al. 2010). IL-23 rather than IL-12 is the primary type 1 cytokine produced by activated human pro-inflammatory macrophages (M1) (Verreck et al. 2004). Production of IL-23 is stimulated through activation of toll-like receptors (TLRs) by their ligands (including LPS, peptidoglycan, CpG DNA and poly I:C). Such interactions result in increased expression of p40 and p19, consequently enhancing the release of IL-23 (Langrish et al. 2004). However, generation pathway (blood-born or specific tissue residents), location in epithelial/endothelial barriers, program phenotype and ability to produce IL-23 in response to different pathogenic and non-pathogenic triggers for monocytes, macrophages and DC remains underinvestigated. Another aspect of IL-23 biology is the ability of non-hematopoietic epithelial/endothelial cells to directly produce or upregulate it production by resident macrophages/DC. Unfortunately, as of today, the only ability of human keratinocytes to express IL-23 mRNA and produce functional protein has been observed (Lee et al. 2004a; Piskin et al. 2006). The production of IL-23 p19 subunit by activated mouse astrocytes in response to LPS > LPS+IFNγ > IL-1β> IL-1β/IFNγ > IFNγ was also detected by PCR and confirmed by sequencing (Constantinescu et al. 2005; Xu and Drew 2007a).
Several factors are known to regulate the overall production of IL-23:
PRRs: The immune system is equipped with a variety of cell surface and cytoplasmic pattern recognition receptors (PRRs) that recognize conserved structures termed microbe-associated molecular patterns (MAMPs), which are expressed by a wide variety of organisms. A number of PRR families have been described, such as toll-like receptors (TLRs) and nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs) (Kawai and Akira 2009; Wang et al. 2011). Certain microbes possess a MAMP signature that specifically activates the IL-23 axis (Siegemund et al. 2007). This leads to preferential production of IL-23 at mucosal surfaces such as gut, as described in mucosal immunity section below. The preferential production of IL-23 in the gut may be a function of the pattern of PRR expression on intestinal immune cells as well as the nature of the PRR stimuli present in the intestinal lumen. Myeloid cells are differentially regulated in response to TLR signaling. IL-12 is strongly induced following TLR4, TLR8 stimulation. TLR2 agonists such as peptidoglycan (a cell wall component found in all bacteria, but particularly abundant in Gram-positive strains) are much more powerful inducers of IL-23 than TLR4 ligands such as LPS (a cell wall component of Gram-negative bacteria) (Re and Strominger 2001; Smits et al. 2004). There has been some evidence that activation of DC through TLR2 preferentially induces production of IL-23 over IL-12 (Langrish et al. 2004). TLR2 stimulation in combination with nucleotide oligomerization domain-containing protein 2 (NOD2) stimulation also induces expression of IL-23 (van Beelen et al. 2007). Stimulation of TLR3 and TLR7 also augments the expression of IL-23p19 by macrophages (Pirhonen et al. 2002; Al-Salleeh and Petro 2007). In addition, in mice with defective TLR4 signaling (TLR4lps-d), mice deficient in TLR9 (TLR9−/−) and double mutant mice (TLR4lps-d/TLR9−/−), the maximal classical activation of lung macrophages and expression of IL-23, required cooperative interactions between TLR4 and TLR9 (Bhan et al. 2010). Only synthetic TLR7/8 ligand, a mimic of viral ssRNA (such as HIV-1), induced IL-23 production by lamina propria (LP) intestinal CD1c+ DCs, and this proinflammatory cytokine response was synergistically enhanced following combined TLR7/8 and TLR4 stimulation (Dillon et al. 2010).
LPS: As the powerful inducer of IL-23 in human monocytes/macrophages/DC the regulation of LPS-induced expression of IL-23 is of special interest. LPS-induced expression of IL-23 could be down-regulated by interferon-stimulated response element (ISRE) in the Il23a promoter in macrophages (Sheikh et al. 2010, 2011). Global gene expression analysis of purified human monocytes showed that LPS-induced expression of IL-23 can be also regulated via triggering receptor expressed on myeloid cells-1 (TREM-1), which is an orphan immunoreceptor expressed on monocytes, macrophages, and neutrophils (Dower et al. 2008).
β-glucans in fungal cell walls and Dectin-1 receptor agonists such as curdlan triggers striking production of IL-23 by DC (LeibundGut-Landmann et al. 2007; Agrawal et al. 2010) and convert development of regulatory T cells in IL-17 producers (Osorio et al. 2008).
Granulocyte macrophage colony-stimulating factor (GM-MCSF): In a mouse model, the study of the relative ability of bone-marrow-derived dendritic cells (BMDCs) versus bone-marrow-derived macrophages (BMDMs) to produce IL-23 in response to S. pneumoniae infection were compared (Wang et al. 2011). BMDCs and BMDMs constitutively released IL-23 at very low levels. Following infection with S. pneumoniae or stimulation with single cognate TLR2 or −4 agonist (lipoteichoic acid or pneumolysin), the levels of IL-23 in the culture supernatants of BMDCs and BMDMs were significantly increased. Interestingly, treatment with cognate TLR9 or NOD2 agonist (CpG or MDP) did not result in a significant induction in IL-23 production in BMDCs and BMDMs. The levels of IL-23 were ~5-fold higher in culture supernatants of BMDCs compared with those of BMDMs in response to S. pneumoniae infection. This indicates that in vitro generated with GM-MCSF DCs are more potent producers of IL-23 in response to S. pneumoniae infection than macrophage expanded from bone marrow precursors with macrophage colony-stimulating factor (M-CSF) (Wang et al. 2011). GM-CSF- mediated polarization to M1 monocytes/macrophages was associated with increased expression of interferon-regulatory factor 5 (IRF5) (Krausgruber et al. 2011).
IFNγ→IL-23: IFNγ has a variety of effects on IL-23 production. Production of IL-12 from macrophages and DCs is dependent on IFNγ. Contrary, the expression of IL-23 in response to microbial stimulation seems to be less dependent on IFNγ. The addition of IFNγ has enhanced IL-23 expression slightly by type 1 macrophages (M1, GM-CSF-mediated), and has no effect on IL-23 production by monocyte-derived DCs in response to microbial stimulation (Ghilardi et al. 2004). Type 2 macrophages (M2, polarized in M-CSF) were unable to produce IL-23 in response to microbial stimulation (Ghilardi et al. 2004). However, pretreatment of macrophages with IFNγ enhanced p19 expression induced by viral stimulation (Pirhonen et al. 2002). These results were confirmed in relation to the intracellular pathogens (van de Wetering et al. 2009). GM-CSF and IFN-γ primed human monocytes enhanced IL-23 production (not only IL-12p40 but also IL-23p19 subunit) and M1 generated by culturing CD14+ monocytes for 6 days in the presence of GM-CSF were stronger producers of IL-23 compared with freshly isolated CD14+ monocytes. GM-CSF or IFN-γ (known to enhance the expression of the IL-23 subunit IL-12p40 could prime monocytes directly for enhanced IL-23 production in response to stimulation with heat killed Salmonella (van de Wetering et al. 2009). Thus, it appears that IFNγ controls IL-23 expression in some circumstances but plays a less stringent role than in IL-12 responses.
IL-23→IFNγ: The reverse situation was observed in regulation of IFNγ production by IL-23 in murine lymphocytes (Sieve et al. 2010). IL-23 antagonizes IL-12-induced secretion of IFNγ. When splenocytes or purified populations of T cells were cultured with IL-23, IFNγ secretion in response to IL-12 was dramatically reduced. According to this report, IL-23 receptor was not required and IL-23 inhibited signaling through the IL-12 receptor by reducing IL-12-induced signal transducer and activator of transcription 4 (STAT4) phosphorylation. These observations suggest that IL-23 may be an important factor in determining the responsiveness of lymphocytes to IL-12 and their subsequent secretion of IFNγ (Sieve et al. 2010).
CD40: IL-23 production can be enhanced via CD40-CD40L interaction, resulting in increased production of IL-23, and creating a potent positive feedback for augmenting IL-23 production by APC (Uhlig et al. 2006; Sender et al. 2010).
IL-10 is an important factor that downregulates the production of IL-23 by macrophages. IL-23 p19 expression was significantly enhanced in IL-10−/− mice compared with WT mice. Pretreatment with IL-10 inhibited IL-23 p19 expression in mouse peritoneal macrophages (Liu et al. 2009). Addition of neutralizing antibodies to IL-10R during IFNγ-stimulation of mouse monocytes/macrophages significantly upregulates expression of IL-23R (Parham et al. 2002).
IL-23 receptor (IL-23R)
IL-23 exerts its biological activities through the interaction with IL-23 receptors. The receptor complex is a heterodimer made up of IL-23R (unique for IL-23) and IL-12Rβ1 (shared with IL-12) subunits (Parham et al. 2002). The IL-23R, consisting of an extracellular N-terminal immunoglobulin-like domain and two cytokine receptor domains, binds to the IL-23p19, while the IL-12Rβ1 subunit contains three membrane-proximal fibronectin type III and two cytokine receptor domains that interact with IL-12/23p40 (Langrish et al. 2004). Human (h)IL-23R is a 629 amino acid type I transmembrane protein, with sequence homology with IL-12Rβ2 and gp130. The gene for hIL-23R is located on chromosome 1p31.2–32.1, which is very close to IL-12Rβ2, suggesting that a gene duplication occurred sometime during evolution (Lankford and Frucht 2003). The murine counterpart of hIL-23R is 644 amino acids in length and has 84% sequence homology with the protein-coding regions of the hIL-23R gene (Parham et al. 2002). The important difference was noted for the expression of human and mouse IL-23R. If murine IL-23R was detected on 1 week polarized Th1 and Th2 clones, hIL-23R was present only on Th1 clones. Mice with large B-cells Ig +, but not LPS-activated splenic B-cells had mIL-23R. In human, it was found on resting EBV-transformed B cells. The most dramatic differences were noted for DCs and monocyte/macrophages. Neither human LPS-activated nor monocyte/CD34+ cell-derived DCs stimulated with GM-CSF/TNF-α were strong IL-23R expressing cells. In contrast, murine BMDCs and LPS-stimulated BMDM showed significant levels of expression, which allows us suggest the possibility of autocrine regulation. It was also shown that among humans, natural killer cell populations with the highest levels of IL-23R expression were found on non-cytolitic clones (Parham et al. 2002).
The expression of IL-23R on non-lymphoid and lymphoid immune cells is tightly regulated by retinoid-related orphan nuclear receptors (ROR)α and (ROR) γt [reviewed in (Jetten 2009)]. RORγt is exclusively expressed in a few distinct cell types of the immune system. Overexpression of both greatly increased expression of IL-23R without cytokine stimulation. Deficiency of either reduced IL-23R expression (Yang et al. 2008). The balance of forkhead/winged-helix transcription factor (Foxp3) specific for natural and adaptive regulatory Tαβ cells (Treg) and RORγt expressing IL-17-producing Tαβ cells is an important factor of antibacterial and inflammatory responses (Lochner et al. 2008). Foxp3 was shown to bind to RORγt, suggesting that it directly regulates RORγt activity (Ichiyama et al. 2008). Moreover, the differentiation of human Th17 cells preferentially occurs from Foxp3 natural naive Treg in the presence of IL-2 and IL-1β and is increased by IL-23 and TGF-β (Valmori et al. 2010).
Expression of IL-23R was found on γδT cells. They show a rapid and robust response before the development of the adaptive immunity mediated by conventional T cells in tissues: in human intestine, in mice skin, in the uterine and vaginal epithelial, and they occur in tongue, lung, and mammary tissue (Moens et al. 2011).
In mice, Il23r is a candidate gene positively regulated by c-Maf [basic region/leucine zipper factors and binds to a consensus site [MARE (Maf recognition element)] during Th17 cell development. Authors suggested that c-Maf does not play an essential role in the early differentiation of Th17 cells but rather in the development and/or maintenance of memory Th17 cells (Sato et al. 2011).
Recently the expression of IL-23R was identified on a novel innate lymphoid cell population that accumulates in the inflamed tissues. These cells characterized by the expression of Thy1highSCA-1+RORγt, production of IL-17 and IFN-γ and is activated in response to microbes (Buonocore et al. 2010). Moreover, cells comparable in phenotype and location to the lymphoid tissue inducer (LTi) cells were identified in human lymphoid tissue, however they do have significant differences with mouse LTi (Spits and Di Santo 2011). LTi cells originate from hematopoietic precursor cells in the fetal liver characterized as CD45intCD4+CD3−CD127 (IL-7Rα)+Lin− cells in mice and in humans as lineage-negative CD45intCD4−CD3−CD127hiLin− cells. These cells did not show expression of IFNγ, but express high levels of IL-22 upon IL-23 stimulation (Kim et al. 2011; Sonnenberg et al. 2011).
The IL-23 receptor functional complex was also found on human plasma cells. In the presence of IL-2+IL-23 in culture medium, stimulation of IgM production was observed. Moreover, the bone marrow plasma cells also expressed IL-23R, but the functional relevance of these findings remains unknown (Cocco et al. 2010).
Likewise, polymorphisms in the gene encoding the IL-23 receptor are found to be important susceptibility factors for some disorders such as inflammatory bowel disease and psoriasis (Duerr et al. 2006; McGovern and Powrie 2007; Smith et al. 2008).
The signaling pathways
The signaling pathway of IL-23R is well investigated. It involves the Janus associated kinase 2 (Jak2), tyrosine kinase 2 (Tyk2) and several members of the signal transducer activator of transcription (STAT) family, including STAT1, STAT3, STAT4 and STAT5 (Parham et al. 2002). Stimulation of the receptor complex activates Jak2 and Tyk2, resulting in phosphorylation of the receptor complex, and formation of docking sites for STATs (1, 3, 4 and 5), same spectrum of Janus kinase (Jak)/signal transducers and activators of transcription (STAT) signaling molecules as IL-12 (Parham et al. 2002; Cua et al. 2003). The STATs are subsequently dimerized, phosphorylated, and translocated into the nucleus and activate target genes (Lankford and Frucht 2003). In lymphocytes, IL-23 induces a strong phosphorylation of STAT3 and a relative weak activation of STAT4, whereas the reverse is true for IL-12-induced phosphorylation of STAT4 and STAT3 (Cua et al. 2003; Smith et al. 2008). The phosphorylation of STAT3 is essential for development of IL-17-producing T helper (Th17) cells (Kikly et al. 2006) whereas, STAT4 is important for increasing IFNγ production and subsequent differentiation of Th1 cells (Ghilardi et al. 2004). Overall, this process orchestrates the cytokine cascade, activating the necessary immune cells involved in the eradication of any pathogenic/antigenic challenge.
IL-23 transgenic and knockout mice
The importance of IL-23 in the development and maintenance of host immunity and prevention of undesirable inflammatory responses were best exemplified in animal models of IL-23 manipulation. IL-23 transgenic (over expressing p19 subunit) mice displayed impaired growth accompanied by systemic inflammation, cytokine dysregulation, elevated neutrophil, lymphocyte and macrophage infiltration, and multi-organ dysfunction leading to premature death. Tissue-specific expression of p19 yielded two outcomes. Animals expressing p19 in bone marrow-derived cells had a similar phenotype of systemic inflammation as seen in animals with the ubiquitous p19 expression. In contrast, animals expressing p19 in liver were fertile, had a normal life span, and did not show signs of systemic or localized inflammation. These results indicate that hemo-poietic expression of p19 is necessary and sufficient to induce systemic inflammation, impaired growth and premature death (Wiekowski et al. 2001).
IL-23 knockout (KO) mice demonstrated impaired bacterial/parasitic clearance, reduced NK cell number, and reduced delayed type hypersensitivity response and a deficiency in T helper (Th) cell development (Ghilardi et al. 2004; Happel et al. 2005; Schulz et al. 2008a; Meeks et al. 2009; Riol-Blanco et al. 2010).
IL-23 and CD4+ cell subsets as a target for HIV-1
It is well established that HIV-1 destroys the immune system, which employs an array of specialized cells and effector mechanisms to control opportunistic infection. These include CD4+ T helper 1 (Th1) cells that produce IFN-γ, IL-2, and lymphotoxin and are involved in cellular immunity, CD4+ Th2 cells that produce IL-4, IL-5, IL-10, and IL-13 and provide help for B cells in their activation and differentiation leading to immunoglobulin production and humoral immune responses. Th1 cells provide help to cytotoxic CD8-positive T cells and macrophages to kill virus-infected cells and cells infected with intracellular pathogens. The production of immunoglobulin under the control of Th2 cells is one of the mechanisms that leads to elimination of extracellular bacteria, and, through the IgE mediated activation of mast cells, to protection against parasites. Another subset of CD4+ lymphocytes sensitive to HIV-1 is regulatory T cells (Tregs, CD3+CD4+CD25+FoxP3+ cells), which also gradually decline from peripheral blood of HIV-1-infected patients (Prendergast et al. 2010). At the same time Tregs accumulates in lymph nodes (Andersson et al. 2005; Nilsson et al. 2006; Kinter et al. 2007) and mucosal sites (Epple et al. 2006). Overall, the stage of disease is associated with different proportions of CD4+ cells of different phenotype and stage of development in peripheral blood, lymphoid and mucosal tissues (Dunham et al. 2008).
These concepts have been refined in recent years with the recognition of additional CD4+ T cell subsets producing IL-17 (Th17) cells in control of pathogenic and opportunistic infections (OI). Introduction of highly active anti-retroviral therapy (ART) in infected patients significantly slowed progression of disease to AIDS and the development of the most common and recurrent opportunistic infections—Pneumocystis carinii pneumonia (PCP), candidiasis, disseminated Mycobacterium avium complex (MAC) disease, toxoplasmosis, activation of cytomegalo-viral infection and other infections (Heaton et al. 2010; Li et al. 2010).
It was suggested that potential trigger for AIDS development in humans (HIV-1) and macaques (SIV) is the loss of CD4+ Th17, which is key for antibacterial defense in the gut. This process leads to microbial translocation, elevation of LPS and LPS control mechanisms in circulation, immune activation, possible conversion of Treg in Th17 (Osorio et al. 2008) and ongoing systemic loss of CD4+ cells (Schnittman and Fox 1997; Douek 2007; Ancuta et al. 2010; Klatt and Brenchley 2010; Prendergast et al. 2010).
Recent studies on SIV infected Rhesus macaques (Macaca mulatta) infected with 100 animal-infectious doses of uncloned pathogenic SIVmac251 intravenously showed that there was a ~10–25 fold increase in the expression of IL-23 (also IL-21, and TGFβ) genes in total jejunal cells in earlier stages of infection before the CD4 cell loss (7–17 days) (Kader et al. 2009). However, the same authors found a ~2–4 fold down regulation of IL-23 mRNA in peripheral blood mononuclear cells (as well as IL-21, TGFβ) compared to uninfected animals at 13 weeks post infection. The treatment with antiretroviral drugs did not restore the expression of IL-23 (Kader et al. 2009).
We reviewed IL-23-related effects below in regard of pathogenic and protective functions of IL-17-producing cells. IL-17 cytokine family has at least six (A-F) members and IL-17A/F were associated with IL-23-mediated induction (Kastelein et al. 2007). Virtually all human/mouse cells do have the receptor to IL-17A/F and engagement of these receptors on external (gut, skin, lung, mucosal and corneal epithelium), internal epithelial barrier (synovial epithelial cells, renal epithelium, choroid plexus) and endothelial cells (Pedroso et al. 2011) leads to the production of innate protective antibacterial/antiviral peptides, as well as wound healing (Li et al. 2011).
IL-23/IL-17 axis and autoimmunity
Upon activation, naive CD4+ T cells differentiate into different lineages of effector Th subsets. Each subset is characterized by its unique cytokine profile and biological functions. Th17, a newly described Th subset that produces IL-17, IL-17F and IL-22 in preference to other cytokines, has been shown to play an important role in clearing specific pathogens and in inducing autoimmune tissue inflammations. IL-23 support survival of highly pathogenic Th17 cells. Th17 cells produce IL-17 (IL-17A), IL-17F, IL-6, IL-22 and TNF-α but not IFNγ or IL-4 and, therefore, this subset is different from the classical Th1 or Th2 subset (Cua et al. 2003; McGovern and Powrie 2007). IL-17 enhances T cell priming and induces inflammation by stimulating fibroblasts, endothelial cells, macrophages and epithelial cells to produce multiple proinflammatory mediators, including IL-1, IL-6, TNF-α, NOS-2, metalloproteases and chemokines (Nakae et al. 2003; Kolls and Linden 2004). Th17 cell was a main pathogenic cell type in models of autoimmunity such as experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (CIA), animal models of multiple sclerosis (MS) and rheumatoid arthritis (RA), respectively, as well as in the pathogenesis of allergic responses such as contact hypersensitivity and delayed type hypersensitivity in IL-17-deficient mice (Nakae et al. 2002).
Role of IL-23 has been identified in patients with a variety of allergic and autoimmune diseases such as rheumatoid arthritis (Murphy et al. 2003), multiple sclerosis, inflammatory bowel disease (IBD) (Hue et al. 2006; Uhlig et al. 2006) and psoriasis suggesting the contribution of IL-17 to the induction and/or development of such diseases. Thus, the IL-23/Th17/IL-17 axis is likely to be associated with T cell-mediated autoimmune diseases.
IL-23/IL-17 axis and bacterial infections
Several researchers addressed the role of IL-23 in differentiation of naïve T cells to Th17 cells and their subsequent function. It was initially believed that IL-23 induces the differentiation of Th17 cells. Subsequent studies showed that was not true because naïve T cells do not express IL-23R. Later studies revealed that TGF-β, IL-6 and autocrine IL-21 direct the de novo generation of Th17 cells (Mangan et al. 2006; Veldhoen et al. 2006). TGFβ induces the generation of Foxp3 expressing regulatory Treg, but the presence of IL-6 deviates TGFβ driven Treg toward Th17 polarization. Combination of TGF-β and IL-6 also induces the expression of ROR-γt, a critical factor for Th17 lineage determination (Ivanov et al. 2006). More recently, other transcription factors including an additional member of ROR family, RORa, IRF4 (Dong 2008) and aryl hydrocarbon receptor (Quintana et al. 2008) have also been implicated in this pathway. Additionally, TGFβ and IL-6 up-regulate IL-23R expression on naive T cells (conferring responsiveness to IL-23), thus aiding IL-23 in terminal differentiation of Th17 cells. In the absence of IL-23, Th17 cells stalled at the early activation phase resulting in less proliferation and failed to maintain IL-17 production (McGeachy et al. 2009). IL-23 is also essential for Th17 proliferation and for full effector function (Parham et al. 2002; Duerr et al. 2006). IL-23 is required for survival of already differentiated Th17 cells, during restimulation of Th17 cells and essential for IL-17 production by Th17 cells. IL-23 stabilizes the phenotype of Th17 cells through transcription factor STAT3 pathway (Zhou et al. 2007; Yang et al. 2008). It is important to point out that virtually all cells in human/mice do have receptors for IL-17. It is not completely clear who is playing the leading role in control of bacterial infection of external epithelial barriers.
As mentioned before, IL-22 is also coordinately expressed in Th17 cells along with other cytokines and the expansion and maintenance of IL-22-secreting CD4+ T cells is dependent on IL-23 (Liang et al. 2006; Ness-Schwickerath et al. 2010). IL-23-dependent IL-22 secretion resulted in increased production of antimicrobial peptides by keratinocytes, offering an important mechanism by which IL-23 may perform a protective role during infection (Liang et al. 2006).
Recently it was shown that mouse CD4+ lymphoid tissue-inducer cells promote innate immunity in the gut to enteric rodent pathogen C. rodentium. Adult CD4+ LTi cells proliferate and upregulate IL-22 production within the first 6 days postinfection. CD4+ LTi cells were found to be the dominant source of IL-22, and infection-induced CD4+ LTi cell responses were predominantly IL-23 dependent (Sonnenberg et al. 2011). It was also shown for Rag2−/− immunodeficient mice that non-T, non-B LTi-like cells can rapidly produce IL-17 in vivo when challenged with the product of fungal pathogens. Moreover, consistent with their rapid responsiveness to IL-23, LTi-like cells constitutively expressed IL-23R mRNA at levels significantly greater than T cells, but expression levels of IL-12Rβ2 mRNA in CD4+ LTi-like cells were low compared with those in memory CD4+ T cells (Takatori et al. 2009).
IL-23-dependent IL-17 responses are important for protective immunity against extracellular bacterial infections for optimal induction of chemokines, recruitment of neutrophils, and bacterial killing. On exposure to bacterial pathogens, innate immune cells release cytokines such as TNFα to initiate the inflammatory response. Happel et al., showed that common p40 subunit of IL-12/23, IFNγ and IL-17 are critical for host defense against Klebsiella pneumonia (Happel et al. 2005). On intrapulmonary K. pneumoniae inoculation IL-12/23 p40–deficient mice are exquisitely sensitive and IL-23 p19−/−, IL-17R−/−, and IL-12 p35−/− mice also show increased susceptibility to infection. IL-12p35−/− mice show normal IL-17 and IL-17F but reduced IFNγ induction whereas IL-12/23p40−/− mice fail to generate pulmonary IFNγ, IL-17, or IL-17F responses to infection. In IL-23p19−/− mice pulmonary IL-17 and IL-17F production was severely impaired (despite normal IFNγ induction) and animals had substantial mortality from a sublethal dose of bacteria. In addition, administration of recombinant IL-17 restored bacterial control in IL-23p19-deficient mice, suggesting that IL-17 induced by IL-23 plays a significant role in the early host defense against K. pneumonia (Happel et al. 2005). The IL-23/IL-17 axis is also necessary for host protection against Citrobacter rodentium (Simmons et al. 2002). In another animal model of S. aureus cutaneous infection, it was found that mice deficient in γδ but not αβ T cells had substantially larger skin lesions with higher bacterial counts and impaired neutrophils recruitment compared with wild type (WT) mice. This neutrophil recruitment was dependent upon epidermal Vγ5+ γδ T cell production of IL-17, which requires IL-23. This was critical for host defense, since IL-17R deficient mice had a phenotype similar to that of γδ T cell–deficient mice. Importantly, γδ T cell–deficient mice inoculated with S. aureus and treated with a single dose of recombinant IL-17 had lesion sizes and bacterial counts resembling those of WT mice, demonstrating that IL-17 could restore the impaired immunity in these mice (Cho et al. 2010). In a well-established opiate abuse and S. pneumoniae lung infection mouse model it was demonstrated that Streptococcus pneumoniae induces IL-23 and IL-17 expression in the lungs as early as 2 h following infection. Morphine treatment causes a decrease in both IL-23 and IL-17 synthesis during the early stages of infection, leading to delayed neutrophil recruitment (Wang et al. 2011). This results in an increased bacterial burden within the lungs and the initiation of systemic disease. This study further established the critical role of IL-23→IL-17 cytokine pathway for protective immunity against extracellular bacterial infections (Ye et al. 2001; Happel et al. 2005).
In mouse models, Th17 cells provide protection against Klebsiella pneumoniae, Citrobacter rodentium, Staphylococcus aureus, Pseudomonas aeroginosa, and Candida albicans infections—all extracellular bacteria and fungi.
The importance of Th1 cell immunity in host resistance to the intracellular bacterium is well established (Cooper et al. 2002). However, the relative roles of interleukin IL-12-Th1 and IL-23/Th17 cell responses in immunity to intracellular pathogens have only recently been characterized (Lin et al. 2009). This was studied in an animal model of Francisella tularensis (F. tularensis). For F. tularensis infection, IL-17A, but not IL-17F or IL-22, induced IL-12 production in DCs, mediated Th1 responses and induced IL-12 and IFNγ production in macrophages mediated bacterial killing. Similar to other pulmonary infection models (Happel et al. 2005), early induction of IL-23p19 and the more sustained production of IL-12p35 in the F. tularensis live vaccine strain (LVS)-infected lungs suggests important IL-23 function during the early immune response. In vitro experimental exposure of human monocytes to L. monocytogenes, as well as S. aureus, S. pneumoniae upregulated the IL23A mRNA levels were 48 -, 30- and 6-fold, respectively. Despite donor specific gene variations and despite the different invasion strategies of the bacteria studied, the common program of gene expression induced by all three bacterial pathogens and a key cytokine—IL-23 was identified (Tchatalbachev et al. 2010).
Similarly, in a Bordetella pertussis model, it was shown that IL-17A treatment of macrophages enhanced bacterial clearance (Higgins et al. 2006) and was thought to be mediated by direct macrophage activation. This suggests that IL-17A can modulate the innate responses and contribute to immunity and clearance of some intracellular bacteria until the arrival of adaptive immune cells to the site of infection.
As mentioned earlier, it was earlier believed that for intracellular bacteria, such as Mycobacterium tuberculosis, IL-23 had no role in protection from infection (Khader et al. 2005; Wozniak et al. 2006). But recently, there is accumulating evidence for the role of IL-17 during mycobacterial infections. Pulmonary infection with Mycobacterium bovis bacille Calmette-Guerin (BCG) (Umemura et al. 2007) or M. tuberculosis (Khader et al. 2007) stimulated the early secretion of IL-17, within 1 and 14 days respectively, and this preceded the development of IFN-γ-secreting T cells. It is possible that the subsequent production of IFN-γ during mycobacterial infections down-regulates the IL-17-secreting T cell response (Khader et al. 2007), and therefore difficult to identify an independent protective effect of Th17 T cells early in the course of M. tuberculosis infection. During pulmonary BCG infection, IL-17 deficient mice showed reduced IFN-γ T cell and delayed type hypersensitivity responses to mycobacterial antigens and impaired granuloma formation in the lungs, suggesting IL-17 was required for the development of optimal Th1 T cell responses (Umemura et al. 2007). Infection of IL-17−/− mice with M. tuberculosis revealed that IL-17 was not essential to control the growth of M. tuberculosis during acute infection (Khader et al. 2007), and it was assumed that the emerging IFN-γ-secreting CD4+ and effector CD8+ T cells were sufficient to inhibit mycobacterial replication in absence of IL-17. More recently however, it has been suggested that IL-17 is necessary for the maintenance of Th1 T cell immunity during chronic M. tuberculosis infection, as infected IL-17−/− mice showing reduced survival late in the course of infection, as compared to wild type mice (Wozniak et al. 2010). Catabolism of tryptophan by IFNγ-inducible indoleamine-2,3-dioxygenase (Ido1) was important to control the balance between adaptive (Cooper and Khader 2008) and innate (Happel et al. 2005) control in murine model of M. tuberculosis infection (Desvignes and Ernst 2009). In IFNγ receptor knockout mice significant increase in IL-23p19 expression in chronic phase of disease with neutrophils recruitment to the lungs in large numbers due IL-17 overproduction was observed. Low concentrations of kynurenines were capable of controlling the development of Th17 cells in vitro, in an IL-23-dependent fashion (Desvignes and Ernst 2009).
The unique requirement for the IL-23-Th17 cell pathway in induction of Th1 cell responses during F. tularensis LVS (Butchar et al. 2007; Lin et al. 2009; Markel et al. 2010), M. tuberculosis (Khader et al. 2005; Desvignes and Ernst 2009), but not other intracellular infections is intriguing. This suggests that different intracellular bacteria may stimulate differential TLRs on APCs and produce distinct polarizing cytokines that impact host immune response to infection. It is likely that some intracellular bacterial infections can effectively induce IL-12-IFNγ responses in the host, whereas other pathogens require the IL-23-IL-17A pathway for effective induction of host IL-12-IFNγ responses for pathogen control. Induction of IL-12 by F. tularensis LVS infection is dependent on TLR-2 signaling (Hong et al. 2007), whereas induction of IL-12p40 by a heat-shock protein of Francisella is dependent on TLR-4 (Ashtekar et al. 2008). BCG lipomannans induce IL-12p40 through TLR-2 (Quesniaux et al. 2004). In contrast, long-term up-regulation of the IL-23p19 message by BCG in human monocytes was observed (Begum et al. 2004).
Toxoplasma gondii is an opportunistic pathogen in AIDS patients. Neutralization of IL-12 enhanced mortality in mice infected with T. gondi (Hunter et al. 1994). IL-12p40 and p35 KO mice were infected with T. gondii and their response to infection was compared with wild type (WT) animals. In this study, none of WT mice succumbed after infection. In contrast, in p40 −/− mice, 100% of the mice had succumbed by 20 days post infection. The p35−/− mice exhibited intermediate sensitivity to T. gondii compared to WT and p40−/− mice (Lieberman et al. 2004). The p35−/− p40−/− mice exhibited higher parasite burden than WT mice, however, IL-23p19−/− mice were as resistant to T. gondii as WT (Vossenkamper et al. 2004). Thus, IL-12 plays a primary role in resistance to T. gondii, but IL-23 can also contribute to resistance in the absence of IL-12. A similar role was reported in the pathogenesis of Leishmania, another major intracellular protozoan parasite (Murray 1997; Engwerda et al. 1998). A recent study showed that IL-23 regulates the T. gondii mediated small bowel inflammation via IL-22 but is independent of IL-17 (Munoz et al. 2009).
Candida albicans—the ubiquitious yeast (fungus) living in warm, moist areas of the body becomes a generalized infection for HIV-1 immunosuppressed patients. For a long time the Th1 and IFNγ were believed to be critical in host defense against C. albicans. In a murine model of cutaneous candidiasis the dependence upon IL-23, for an effective immune response against C. albicans within skin as well as epidermal hyperplasia was shown (Kagami et al. 2010). However, the evaluation of the contribution of the IL-23/IL-17 pathway to C. albicans infection in mice with gastrointestinal infection led to the different conclusion (Zelante et al. 2007). Comparatively assessed p19−/−, p35−/−, p40−/− and C57BL/6 mice for survival, fungal growth, tissue pathology and parameters of inflammatory and adaptive Th1/Th17 immunity showed that IL-23/IL-17 pathway impaired anti-fungal immune resistance (Zelante et al. 2007). IL-23 acted as a molecular connection between uncontrolled fungal growth and inflammation, being produced by dendritic cells in response to a high fungal burden and counter-regulating IL-12p70 production. Both IL-23 and IL-17 subverted the inflammatory program of neutrophils, which resulted in severe tissue inflammatory pathology associated with infection. The role of IL-23 in candidiasis was recently reviewed by (Wei et al. 2011).
In relation to protection against bacteria we have to point out that IL-23 also regulates granulopoiesis in a neutrostat regulatory feedback loop through IL-17A-producing neutrophil regulatory (Tn) cells, most of which express γδ TCR. Both normal and neutrophilic (Itgb2−/−) mice showed reduced circulating neutrophil counts when deficient in IL-12 p40. Injection of rmIL-23, but not rmIL-12 into p40-deficient mice restored circulating neutrophil numbers (Smith et al. 2007).
IL-23 and mucosal immunity
For an effective mucosal homeostasis, immune responses are tightly regulated to ensure protective immunity to the host, and it is of utmost importance that the adaptive and innate immune systems are able to recognize pathogenic organisms while ignoring the commensal flora. IL-23 has recently been shown to be a key player in influencing the balance between tolerance and immunity at mucosal surfaces such as in the gut, the lung, and the skin. IL-23 orchestrates T- cell-dependent and T-cell-independent pathways through effects on Th1 and T17- associated cytokines (Ahern et al. 2008). Of various immune regulators, Treg cells, by secreting TGF-β and IL-10, prevent unwanted immune responses and maintain a ‘high threshold’ for immune activation. Treg cells express high levels of the transcription factor foxp3 and play a non-redundant role in immune homeostasis. IL-23, by acting as a switch factor, allows induction of cellular immunity during an infection by overcoming the suppressive effects of Treg cells in the intestine. This is evident by a dramatic increase in the Tregs in the intestine when IL-23 is absent (Izcue et al. 2008), and the inability to elicit an immune response against gut pathogens in IL-23p19−/− mice (Mangan et al. 2006). From these observations, it has become clear that aberrant expression of IL-23 in the gut certainly can be a major driver of chronic inflammation leading to autoimmune colitis. There is ever-growing evidence that IL-23 seems to play a more dominant role in mucosal tissues. Many gut pathogens evoke a strong IL-23 response, which is essential for host defense. IL-23 activates the adoptive and innate immune systems to produce IL-17A, IL-17F, IL-22, and TNF, all of which help to stimulate epithelial cells to produce antimicrobial factors. These properties are important in host defense against a number of infections as mentioned earlier, such as Klebsiella pneumonia, Candida albicans and Toxoplasma gondii. IL-23 thus provides a robust innate immune response for first-line defense against environmental assaults, such as in the gut, the lung, and the skin. It is when this response goes unchecked that its pro-inflammatory actions overcome the beneficial role of IL-23 (Tato and Cua 2008). The source of intestinal IL-23 could be the unique subset of CD14+ population of macrophages that not only produce IL-23 in response to commensal flora, but increased production of IFNγ by lamina propria infiltrating lymphocytes, which in turn trigger further abnormal macrophage differentiation with an IL-23-hyperproducing phenotype (Kamada et al. 2008). An important subset of CD4+ Th17 and their gut-homing precursors CD4+CD161+ appears to be preferentially infected and depleted during acute HIV/SIV infection (Macal et al. 2008; Ancuta et al. 2010; Prendergast et al. 2010). Th17 cells secrete both IL-17 and IL-22, which in turn induce the production of other cytokines, β-defensins, and other antimicrobial peptides important for host defense (Schulz et al. 2008b). These cells are considered particularly important for mucosal defense against opportunistic pathogens, including fungi such as Candida albicans, and for maintaining epithelial integrity. Relative to controls, SIV-infected macaques coinfected with Salmonella typhimurium showed increased dissemination of Salmonella, which was associated with depletion of mucosal Th17 cells (Raffatellu et al. 2008). This finding suggested that IL-17 deficiency contributes to defective intestinal barrier function and host protection. However, the association of IL-23-producing activated dendritic cells/macrophages and development and survival of IL-17-producing cells in gut-associated immune compartment was not rigorously investigated.
In summary, IL-23 mediates both protective and pathological functions. Under homeostatic conditions, low levels of IL-23 may contribute to mucosal barrier function and microbicidal activity through IL-17 and IL-22. In the presence of a strong inflammatory stimulus, there is increased production of IL-23 and other inflammatory mediators that inhibit Treg responses leading chronic inflammatory pathways at mucosal barrier as seen in inflammatory bowel disease (Ahern et al. 2008).
IL 23 and neurological disorders
Experimental autoimmune encephalitis and multiple sclerosis (EAE and MS)
Historically, the most well-established murine model of brain inflammation is EAE. It was previously believed that IL-12 induced Th1 cells triggered autoimmune diseases such as EAE (Gately et al. 1998; El-Behi et al. 2011). However subsequent studies have demonstrated that the similar structure, IL-23, not IL-12, is essential for the development of these autoimmune diseases (Cua et al. 2003; Ciric et al. 2009;). Mice deficient in IFN-γ, IFN-γR, IL-12βR2 and STAT1, which are critical molecules in IL-12/IFN-γ mediated responses, were not resistant but, were more susceptible to EAE (Engwerda et al. 1998; Chen et al. 2006). Subsequently targeting IL-12 subunits further questioned IL-12 association with chronic inflammatory conditions. Mice deficient in either the IL-23p19 subunit or the IL-12/23p40 subunit are resistant to EAE, whereas IL-12p35-deficient mice remain susceptible to these diseases (Cua et al. 2003; Murphy et al. 2003).
Targeting of p19 and p35 subunits revealed that IL-23, rather than IL-12, is crucial during the pathogenesis of these autoimmune diseases. Genetic deficiency or in vivo neutralization of IL-23p19 chain confers resistance against EAE (Cua et al. 2003; Chen et al. 2006; Thakker et al. 2007). IL-23 p19 deficient mice were still able to mount Th1 response but were resistant to EAE (Cua et al. 2003). Anti-IL-23 therapy reduced the serum levels of IL-17 as well as CNS expression of IFN-γ, IL-17, IL-6 and TNF α and ameliorated EAE (Chen et al. 2006). In addition, IL-23-stimulated peripheral blood CD4+ lymphocytes are potentially efficient at penetrating brain derived microvascular endothelial monolayers in vitro (Kebir et al. 2007). This could be secondary to IL-23 driven upregulation of adhesion molecules on CD4+ T cells that enhance their interactions with endothelium. IL-23 could also stimulate CD4+ T cells to secrete soluble factors and/or upregulates cell surface adhesion molecule expression on endothelial cells, or disrupt junctions between endothelial cells, in their vicinity. IL-23 could influence T cell trafficking by modulation of chemokines receptor profiles.
Effects of IL-23 cannot be explained by actions of IL-17 alone because anti-IL-17 treatment did not prevent the onset and relapse of EAE with the same efficiency as anti-IL-23p19 or anti IL-12/IL-23p40 treatment. IL-23 may directly activate a subset of mouse macrophages and dendritic cells expressing IL-23R, resulting in the production TNF-α and IL-1 (Cua et al. 2003). IL-6 and IL-1 likely play role in the development of EAE since IL-6−/− and IL-1−/− mice are significantly resistant and IL-1 receptor antagonist deficient mice are more susceptible to EAE (Chen et al. 2006). In the EAE model, it was shown that DCs also expressed IL-17R and secreted IL-23 in responses to IL-17, which was enhanced by LPS and blocked by addition of IL-17RFc. Furthermore, DCs expressed IL-1β, IL-6, TGF-β, CCL2, and CXCL10 in response to IL-17 alone or in synergy with a TLR agonist. These findings demonstrate that γδ T cell-derived IL-17 promotes further IL-17 production by Th17 cells, both directly by interacting with IL-17R on CD4+ T cell and indirectly by enhancing the production of IL-23 and other cytokines and chemokines from DCs that promote the development, expansion, and recruitment of Th17 cells in the brain (Sutton et al. 2009).
LPS-stimulated human microglial cells efficiently express IL-23p19 as well as perivascular dendritic cells in areas affected by MS. Effective treatment of MS patients with type 1 interferon-beta reduced the expression of IL-23p19 (Li et al. 2003; Sonobe et al. 2005; Xu and Drew 2007).
Though there was considerable progress made in delineating the pathophysiology of EAE, the animal model of MS, and having preclinical studies using animal models yielded promising results, translation into the clinic was disappointing (Codarri et al. 2010). So, further understanding of pathophysiology is required at this stage to be able to translate the knowledge into clinical practice successfully. MS is one of several autoimmune diseases, where different types of blocking therapeutic antibodies for IL-23 and IL-23p19 were used (Kasper et al. 2006; Wittig 2007; Segal et al. 2008; Kurzeja et al. 2011).
CNS viral infections
IL-23 is also involved in the control of viral infections in the brain. First it was shown for experimental ocular herpes simplex virus type 1 (HSV-1) infection in mice (Kim et al. 2008), where upregulation of IL-23p19 was detected as soon as 3 days post infection in trigeminal ganglia (Broberg et al. 2002, 2004). In West Nile virus (WNV), a mosquito-transmitted single-stranded RNA (ssRNA) flavivirus, causes human CNS disease of variable severity, TLR7 and IL-23-dependent responses represented a vital host defense mechanism (Town et al. 2009). In autoimmune demyelinating disease following Theiler’s virus (TMEV) infection Thomas M. Petro and co-authors have shown that TLR3 and TLR7 contribute to IL-23 p19 expression during challenge of macrophages with TMEV (Al-Salleeh and Petro 2007, 2008).
CNS HIV-1 infection
HIV-1 infects brain mononuclear phagocytic cells (MP, macrophages/micrglia) and activated MP are tightly associated with the development of neurologic complications in infected patients (Price et al. 1988; Budka 1990; Gendelman and Tardieu 1994; Newton 1995; Antinori et al. 2007; Yadav and Collman 2009). However, involvement of IL-23 in pathogenesis of such conditions is not clear. The peripheral immune cells, which pass through the BBB and scatter through the brain parenchyma (white matter tracts) or become entrapped in perivascular spaces, can activate microglial cells. Activated microglia can persistently contribute to the loss of neuronal function, with or without productive HIV-1-infection (Spencer and Price 1992; Kielian 2004). Microglial cells are the major sensor of the neuronal microenvironment and respond to neuronal dysfunction by multiple mechanisms (Kramer-Hammerle et al. 2005; Yadav and Collman 2009). The ability of activated lymphocytes, primarily functionally mature CD4 T helper 1 (Th1) T cells and CD8+ cytotoxic lymphocytes, NK (Shieh et al. 2001; Clements et al. 2002; Bissel et al. 2008; Sadagopal et al. 2008), and to a lesser extent in HIV-1/SIV brain infection, NK T cells and γ δ T cells, to prime microglial cells via IFNγ is part of the cascade of cytokines and events associated with the adaptive immune response. Activated microglia by IL-23 secretion can stimulate T cells differentiation into Th17 (IL17-, IL-21- and IFNγ producing cells), which contribute to inflammation in the brain (Gottfried-Blackmore et al. 2009; Goverman 2009; Louis et al. 2010); and play a significant role in protecting the host from opportunistic bacterial infections (Jin et al. 2004; Khader et al. 2005; Desvignes and Ernst 2009; Dietlin et al. 2009; Martin et al. 2009; Meeks et al. 2009; Riol-Blanco et al. 2010). The decline of protective HIV-1-specific CD4+ cells due to HIV-1 infection may be associated with increased migration and local CNS expansion of antiviral/antibacterial T cells, which produce IL-17 and IFNγ in response to damage signals that destroy infected cells by MHC-independent mechanisms (Fenoglio et al. 2009). The presence and dynamics of these cell populations (NK T cells and T cells) in the brain during HIV-1/SIV infection has not been vigorously investigated. Moreover, in the brain, the balance between effector and regulatory CD4+ cells, which are susceptible to HIV-1 infection and could contribute to the brain inflammatory responses is unknown.
IL-23 and tumors
Many cancers arise from the sites of infection, chronic irritation and inflammation, caused by infectious agents such as Helicobacter pylori and hepatitis viruses or non-infectious agents such as asbestos etc. Inflammatory cells in tumor microenvironment play a significant role in the neoplastic process by altering proliferation and survival of tumor cells (Coussens and Werb 2002). Innate immune cells in chronically inflamed tissues secrete several cytokines and metalloproteinases, along with factors affecting angiogenesis, such as vascular endothelial growth factor, thereby altering tumor growth. Locally expressed cytokines and various growth factors may not only aid in tissue remodeling and angiogenesis, but also protect the tumor from immune mediated elimination (Ganss et al. 2004). In contrast, tumor infiltrating T cells and intraepithelial lymphocytes provide immune surveillance by eliminating early malignant lesions (Dunn et al. 2004). HIV-1-induced immunodeficiency significantly increases development of malignancies, such as Kaposhi sarcoma, non-Hodgkin lymphoma and other different types of carcinomas (De Vuyst et al. 2008; Silverberg et al. 2009).
Infectious inflammation is associated with the secretion of several cytokines by innate immune cells in response to pathogen-associated molecular stimuli. Recent studies showed that IL-23, and not IL-12, is over-expressed by macrophages and DCs in human and mouse tumors and antagonistically regulates local inflammatory responses in the tumor microenvironment and infiltration of intraepithelial lymphocytes. IL-23 upregulates proinflammatory cytokines IL-17 and IL-22, matrix metalloproteinase MMP9 and increases angiogenesis. In addition, IL-23 reduces tumor infiltration of CD8+ T cells. In contrast, IL-12 promotes infiltration of cytotoxic T cells. Genetic deletion or antibody-mediated elimination of IL-23 leads to increased infiltration of cytotoxic T cells into the transformed tissues, thus protecting against chemically induced carcinogenesis (Langowski et al. 2006). In addition, mice depleted for IL-23 or IL-23R showed restricted growth in transplanted tumors (Langowski et al. 2006). It was also shown that Th17 cells are gradually increased in the tumor microenvironment during tumor development (Kryczek et al. 2007) and that IL-17 upregulates the production of a variety of proinflammatory cytokines (Kolls and Linden 2004) and proangiogenic factors (Takahashi et al. 2005) to promote tumor development (Numasaki et al. 2003). Therefore, the activation of the IL-23/IL-17 pathway promotes the incidence and growth of tumors by inducing local inflammatory responses (Shime et al. 2008). The relationship between microbial stimulation, inflammation and cancer was in deep review by (von Hertzen et al. 2011). In addition, in murine models it was shown that IL-23p19 suppressed controlled by NK cell-mediated immunity. In particular, NK cell perforin and IFN-γ effector functions appear to be suppressed by host IL-23p19, and suppression was independent of host IL-17A (Teng et al. 2010).
Conclusions
The actual involvement of IL-23 in controlling OI and cancer in AIDS remains understudied. Taking the fact that the most powerful inducers of this cytokine are bacterial/fungal products, the microbial/fungal local persistence is impossible due to strong innate immunity mediated by microbicidal peptides at all external body barriers. The deficiency of the barriers and translocation of bacterial/fungal products in systemic lymphatic tissues should continuously stimulate macrophages/DC to produce IL-23/IL-12 and support the innate barrier protection with CD4+ IL-17-producing cells as well as all arms of adaptive immunity until complete resolution. In HIV-1 infection, overproduction of IL-23 should counterbalance the anti-bacterial/fungal protection by stimulation of local accumulation of innate immune cells. Unfortunately, in the absence of Th1 and Th2 types effectors these cells do not have the power to compensate adaptive immunity, but will attract more granulacytes/macrophages with high tissue destructive potential. Schematic representation of this hypothesis is shown on Fig. 1. Another arm of immune suppression and actual reduction of IL-23 production by activated macrophages in chronic HIV-1 infection could be disproportional accumulation of IL-10-producing Treg and/or changes in macrophage polarization from M1 IL-23-producing to M2 IL-10-producing cells. Antiretroviral treatment induced restoration of CD4+ cell (Th1) system may not come with the restoration of IL-23 production as was shown by a few humans and SIV-infected monkey observations.
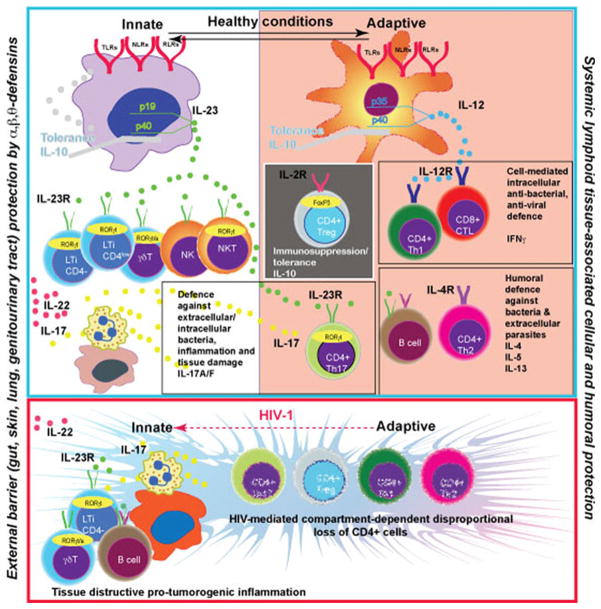
Fig. 1.
IL-23 is a key cytokine produced by macrophages at the external body barriers – gut, skin, lung, genitourinary tract. IL-23 stimulates the protective function in a variety of innate immune cells. It includes IL-17, IL-22 secretion, granulocytes attraction and the release of bactericidal peptides. The barrier prevents generalization of bacterial/fungal infections and maintains the balance with systemic lymphoid tissue associated IL-12→IFNγ and IL-4 axis of the Th1/Th2 mediated adaptive immune system. The protection generated by T cells of adaptive immunity (antibody production and cytotoxic T cells) at external barriers now includes CD4+ IL-17 producing cells. These cells are in a balance with immunosuppressive Treg. Microbial environment at the external barriers and cytokine milieu induced by microbiota dictates the organ-specific balance between Th17 and Treg cells. The transcriptional factors FoxP3 and RORγt are involved in phenotypic control of these cells. There is ongoing loss of CD4+ T cells and an associated adaptive immunity contribution in antibacterial/antifungal control during HIV-1 infection. External barrier macrophages upregulate expression of IL-23 in response to pathogens and attract more innate immune cells, B cells and neutrophils to generate a new type of barrier (tertiary lymphoid structures in bowel, lung, skin). Expanded proliferation of epithelial cells and tissue destruction (inflammation) is a pro-tumorogenic environment. Anti-retroviral treatment will induce partial restoration of Th1/Th2 immune responses and such inflammation could be increased. However, it will not compensate the innate immune cell protective function. The secretion of IL-23 will be reduced as a result of a combination of several negative regulatory factors, such as IL-10, IFNγ and IL-4, and facilitate the development of opportunistic infections
Based on reviewed findings we suggest that overproduction of IL-23 by activated macrophages, DC and brain resident microglial cells could be a pathogenetic factor and a therapeutic target for prevention of HIV-1-induced immune activation, inflammation, neurodegeneration and tumorogenesis. However, down regulation of IL-23 expression in advanced HIV/SIV infection raised the question of the mechanisms involved in regulation of its production by cells of myeloid lineage (macrophages and dendritic cells) and capability to support the protective antibacterial/antifungal function of Th17 cells as well as all other IL-23R expressing populations. All these aspects require attention and thoughtful investigation.
Acknowledgments
This work was supported by National Institutes of Health Grants 5 P20 RR021937-02, 5 P01 NS043985-07. We acknowledge Deepa Roy, Jaclyn Knibbe, Edward Makarov, Santhi Gorantla for assistance.
Footnotes
Conflict of interest disclosure There are no conflicts of interest for any of the authors.
Contributor Information
Govardhana Rao Yannam, Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, Omaha, NE, USA.
Tanuja Gutti, Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, Omaha, NE, USA.
Larisa Y. Poluektova, Email: lpoluekt@unmc.edu, Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, Omaha, NE, USA. 985880 Nebraska Medical Center, Omaha, NE, USA
References
- Agarwal SK, Gourh P, Shete S, Paz G, Divecha D, Reveille JD, Assassi S, Tan FK, Mayes MD, Arnett FC. Association of interleukin 23 receptor polymorphisms with anti-topoisomerase-I positivity and pulmonary hypertension in systemic sclerosis. J Rheumatol. 2009;36:2715–2723. doi: 10.3899/jrheum.090421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S, Gupta S, Agrawal A. Human dendritic cells activated via dectin-1 are efficient at priming Th17, cytotoxic CD8 T and B cell responses. PLoS ONE. 2010;5:e13418. doi: 10.1371/journal.pone.0013418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern PP, Izcue A, Maloy KJ, Powrie F. The interleukin-23 axis in intestinal inflammation. Immunol Rev. 2008;226:147–159. doi: 10.1111/j.1600-065X.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- Alfano M, Crotti A, Vicenzi E, Poli G. New players in cytokine control of HIV infection. Curr HIV/AIDS Rep. 2008;5:27–32. doi: 10.1007/s11904-008-0005-5. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- Al-Salleeh F, Petro TM. TLR3 and TLR7 are involved in expression of IL-23 subunits while TLR3 but not TLR7 is involved in expression of IFN-beta by Theiler’s virus-infected RAW264.7 cells. Microb nfect. 2007;9:1384–1392. doi: 10.1016/j.micinf.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Al-Salleeh F, Petro TM. Promoter analysis reveals critical roles for SMAD-3 and ATF-2 in expression of IL-23 p19 in macrophages. J Immunol. 2008;181:4523–4533. doi: 10.4049/jimmunol.181.7.4523. [DOI] [PubMed] [Google Scholar]
- Ancuta P, Monteiro P, Sekaly RP. Th17 lineage commitment and HIV-1 pathogenesis. Curr Opin HIV AIDS. 2010;5:158–165. doi: 10.1097/COH.0b013e3283364733. [DOI] [PubMed] [Google Scholar]
- Andersson J, Boasso A, Nilsson J, Zhang R, Shire NJ, Lindback S, Shearer GM, Chougnet CA. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol. 2005;174:3143–3147. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- Antinori A, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtekar AR, Zhang P, Katz J, Deivanayagam CC, Rallabhandi P, Vogel SN, Michalek SM. TLR4-mediated activation of dendritic cells by the heat shock protein DnaK from Francisella tularensis. J Leukoc Biol. 2008;84:1434–1446. doi: 10.1189/jlb.0308215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban Y, Tozaki T, Taniyama M, Nakano Y, Yoneyama K, Hirano T. Association studies of the IL-23R gene in autoimmune thyroid disease in the Japanese population. Autoimmunity. 2009;42:126–130. doi: 10.1080/08916930802422265. [DOI] [PubMed] [Google Scholar]
- Begum NA, Ishii K, Kurita-Taniguchi M, Tanabe M, Kobayashi M, Moriwaki Y, Matsumoto M, Fukumori Y, Azuma I, Toyoshima K, Seya T. Mycobacterium bovis BCG cell wall-specific differentially expressed genes identified by differential display and cDNA subtraction in human macrophages. Infect Immun. 2004;72:937–948. doi: 10.1128/IAI.72.2.937-948.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan U, Ballinger MN, Zeng X, Newstead MJ, Cornicelli MD, Standiford TJ. Cooperative interactions between TLR4 and TLR9 regulate interleukin 23 and 17 production in a murine model of gram negative bacterial pneumonia. PLoS ONE. 2010;5:e9896. doi: 10.1371/journal.pone.0009896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissel SJ, Wang G, Bonneh-Barkay D, Starkey A, Trichel AM, Murphey-Corb M, Wiley CA. Systemic and brain macrophage infections in relation to the development of simian immunodeficiency virus encephalitis. J Virol. 2008;82:5031–5042. doi: 10.1128/JVI.02069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlius J, Schmidlin K, Costagliola D, Fatkenheuer G, May M, Caro-Murillo AM, Mocroft A, Bonnet F, Clifford G, Karafoulidou A, Miro JM, Lundgren J, Chene G, Egger M. Incidence and risk factors of HIV-related non-Hodgkin’s lymphoma in the era of combination antiretroviral therapy: a European multicohort study. Antivir Ther. 2009;14:1065–1074. doi: 10.3851/IMP1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberg EK, Setälä N, Erälinna JP, Salmi AA, Röyttä M, Hukkanen V. Herpes simplex virus type 1 infection induces upregulation of interleukin-23 (p19) mRNA expression in trigeminal ganglia of BALB/c mice. J Interferon Cytokine Res. 2002;22:641–651. doi: 10.1089/10799900260100123. [DOI] [PubMed] [Google Scholar]
- Broberg EK, Peltoniemi J, Nygardas M, Vahlberg T, Roytta M, Hukkanen V. Spread and replication of and immune response to gamma134.5-negative herpes simplex virus type 1 vectors in BALB/c mice. J Virol. 2004;78:13139–13152. doi: 10.1128/JVI.78.23.13139-13152.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budka H. Human immunodeficiency virus (HIV) envelope and core proteins in CNS tissues of patients with the acquired immune deficiency syndrome (AIDS) Acta Neuropathol. 1990;79:611–619. doi: 10.1007/BF00294238. [DOI] [PubMed] [Google Scholar]
- Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchar JP, Rajaram MV, Ganesan LP, Parsa KV, Clay CD, Schlesinger LS, Tridandapani S. Francisella tularensis induces IL-23 production in human monocytes. J Immunol. 2007;178:4445–4454. doi: 10.4049/jimmunol.178.7.4445. [DOI] [PubMed] [Google Scholar]
- Cecchinato V, Tryniszewska E, Ma ZM, Vaccari M, Boasso A, Tsai WP, Petrovas C, Fuchs D, Heraud JM, Venzon D, Shearer GM, Koup RA, Lowy I, Miller CJ, Franchini G. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol. 2008;180:5439–5447. doi: 10.4049/jimmunol.180.8.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates auto-immune encephalomyelitis. J Clin Investig. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Investig. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianfrocca M, Roenn JH. Epidemic Kaposi’s sarcoma. Oncology (Williston Park) 1998;12:1375–1381. 1385. discussion 1385–1390. [PubMed] [Google Scholar]
- Ciric B, El-behi M, Cabrera R, Zhang GX, Rostami A. IL-23 drives pathogenic IL-17-producing CD8+ T cells. J Immunol. 2009;182:5296–5305. doi: 10.4049/jimmunol.0900036. [DOI] [PubMed] [Google Scholar]
- Clements JE, Babas T, Mankowski JL, Suryanarayana K, Piatak M, Jr, Tarwater PM, Lifson JD, Zink MC. The central nervous system as a reservoir for simian immunodeficiency virus (SIV): steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J Infect Dis. 2002;186:905–913. doi: 10.1086/343768. [DOI] [PubMed] [Google Scholar]
- Cocco C, Canale S, Frasson C, Di Carlo E, Ognio E, Ribatti D, Prigione I, Basso G, Airoldi I. Interleukin-23 acts as antitumor agent on childhood B-acute lymphoblastic leukemia cells. Blood. 2010;116:3887–3898. doi: 10.1182/blood-2009-10-248245. [DOI] [PubMed] [Google Scholar]
- Codarri L, Fontana A, Becher B. Cytokine networks in multiple sclerosis: lost in translation. Curr Opin Neurol. 2010;23:205–211. doi: 10.1097/WCO.0b013e3283391feb. [DOI] [PubMed] [Google Scholar]
- Constantinescu CS, Tani M, Ransohoff RM, Wysocka M, Hilliard B, Fujioka T, Murphy S, Tighe PJ, Sarma JD, Trinchieri G, Rostami A. Astrocytes as antigen-presenting cells: expression of IL-12/IL-23. J Neurochem. 2005;95:331–340. doi: 10.1111/j.1471-4159.2005.03368.x. [DOI] [PubMed] [Google Scholar]
- Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol. 2002;168:1322–1327. doi: 10.4049/jimmunol.168.3.1322. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- De Nitto D, Sarra M, Cupi ML, Pallone F, Monteleone G. Targeting IL-23 and Th17-cytokines in inflammatory bowel diseases. Curr Pharm Des. 2010;16:3656–3660. doi: 10.2174/138161210794079164. [DOI] [PubMed] [Google Scholar]
- De Vuyst H, Lillo F, Broutet N, Smith JS. HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. Eur J Canc Prev. 2008;17:545–554. doi: 10.1097/CEJ.0b013e3282f75ea1. [DOI] [PubMed] [Google Scholar]
- Desvignes L, Ernst JD. Interferon-gamma-responsive non-hematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity. 2009;31:974–985. doi: 10.1016/j.immuni.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietlin TA, Cua DJ, Burke KA, Lund BT, van der Veen RC. Role of IL-23 in mobilization of immunoregulatory nitric oxide-or superoxide-producing Gr-1+ cells from bone marrow. Free Radic Biol Med. 2009;47:357–363. doi: 10.1016/j.freeradbiomed.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Dillon SM, Rogers LM, Howe R, Hostetler LA, Buhrman J, McCarter MD, Wilson CC. Human intestinal lamina propria CD1c+ dendritic cells display an activated phenotype at steady state and produce IL-23 in response to TLR7/8 stimulation. J Immunol. 2010;184:6612–6621. doi: 10.4049/jimmunol.1000041. [DOI] [PubMed] [Google Scholar]
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- Douek D. HIV disease progression: immune activation, microbes, and a leaky gut. Top HIV Med. 2007;15:114–117. [PubMed] [Google Scholar]
- Dower K, Ellis DK, Saraf K, Jelinsky SA, Lin LL. Innate immune responses to TREM-1 activation: overlap, divergence, and positive and negative cross-talk with bacterial lipopolysaccharide. J Immunol. 2008;180:3520–3534. doi: 10.4049/jimmunol.180.5.3520. [DOI] [PubMed] [Google Scholar]
- Duerr RH, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham RM, Cervasi B, Brenchley JM, Albrecht H, Weintrob A, Sumpter B, Engram J, Gordon S, Klatt NR, Frank I, Sodora DL, Douek DC, Paiardini M, Silvestri G. CD127 and CD25 expression defines CD4+ T cell subsets that are differentially depleted during HIV infection. J Immunol. 2008;180:5582–5592. doi: 10.4049/jimmunol.180.8.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engwerda CR, Murphy ML, Cotterell SE, Smelt SC, Kaye PM. Neutralization of IL-12 demonstrates the existence of discrete organ-specific phases in the control of Leishmania donovani. Eur J Immunol. 1998;28:669–680. doi: 10.1002/(SICI)1521-4141(199802)28:02<669::AID-IMMU669>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Epple HJ, Loddenkemper C, Kunkel D, Troger H, Maul J, Moos V, Berg E, Ullrich R, Schulzke JD, Stein H, Duchmann R, Zeitz M, Schneider T. Mucosal but not peripheral FOXP3+ regulatory T cells are highly increased in untreated HIV infection and normalize after suppressive HAART. Blood. 2006;108:3072–3078. doi: 10.1182/blood-2006-04-016923. [DOI] [PubMed] [Google Scholar]
- Fenoglio D, Poggi A, Catellani S, Battaglia F, Ferrera A, Setti M, Murdaca G, Zocchi MR. Vdelta1 T lymphocytes producing IFN-gamma and IL-17 are expanded in HIV-1-infected patients and respond to Candida albicans. Blood. 2009;113:6611–6618. doi: 10.1182/blood-2009-01-198028. [DOI] [PubMed] [Google Scholar]
- Ganss R, Arnold B, Hammerling GJ. Mini-review: overcoming tumor-intrinsic resistance to immune effector function. Eur J Immunol. 2004;34:2635–2641. doi: 10.1002/eji.200425474. [DOI] [PubMed] [Google Scholar]
- Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- Gendelman H, Tardieu M. Macrophages/microglia and the pathophysiology of CNS injuries in AIDS. J Leukoc Biol. 1994;56:387–388. doi: 10.1002/jlb.56.3.387. [DOI] [PubMed] [Google Scholar]
- Ghilardi N, Kljavin N, Chen Q, Lucas S, Gurney AL, De Sauvage FJ. Compromised humoral and delayed-type hypersensitivity responses in IL-23-deficient mice. J Immunol. 2004;172:2827–2833. doi: 10.4049/jimmunol.172.5.2827. [DOI] [PubMed] [Google Scholar]
- Gottfried-Blackmore A, Kaunzner UW, Idoyaga J, Felger JC, McEwen BS, Bulloch K. Acute in vivo exposure to interferon-{gamma} enables resident brain dendritic cells to become effective antigen presenting cells. Proc Natl Acad Sci USA. 2009;106(49):20918–23. doi: 10.1073/pnas.0911509106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths MH, Miller RF, Semple SJ. Interstitial pneumonitis in patients infected with the human immunodeficiency virus. Thorax. 1995;50:1141–1146. doi: 10.1136/thx.50.11.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon FB, Easterbrook PJ, Padley S, Boag F, Goodall R, Phillips RH. Bronchopulmonary Kaposi’s sarcoma in 106 HIV-1 infected patients. Int J STD AIDS. 1998;9:518–525. doi: 10.1258/0956462981922755. [DOI] [PubMed] [Google Scholar]
- Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J NeuroVirol. 2010;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins SC, Jarnicki AG, Lavelle EC, Mills KH. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol. 2006;177:7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- Hong KJ, Wickstrum JR, Yeh HW, Parmely MJ. Toll-like receptor 2 controls the gamma interferon response to Francisella tularensis by mouse liver lymphocytes. Infect Immun. 2007;75:5338–5345. doi: 10.1128/IAI.00561-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AK, Jacobson EM, Jazdzewski K, Concepcion ES, Tomer Y. Interleukin (IL)-23 receptor is a major susceptibility gene for Graves’ ophthalmopathy: the IL-23/T-helper 17 axis extends to thyroid autoimmunity. J Clin Endocrinol Metab. 2008;93:1077–1081. doi: 10.1210/jc.2007-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CA, Subauste CS, Van Cleave VH, Remington JS. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun. 1994;62:2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, Takaesu G, Hori S, Yoshimura A, Kobayashi T. Foxp3 inhibits RORγt-mediated IL-17A mRNA transcription through direct interaction with RORγt. J Biol Chem. 2008;283:17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- Illes Z, Safrany E, Peterfalvi A, Magyari L, Farago B, Pozsonyi E, Rozsa C, Komoly S, Melegh B. 3′UTR C2370A allele of the IL-23 receptor gene is associated with relapsing-remitting multiple sclerosis. Neurosci Lett. 2008;431:36–38. doi: 10.1016/j.neulet.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Ingiliz P, Appenrodt B, Gruenhage F, Vogel M, Tschampa H, Tasci S, Rockstroh JK. Lymphoid pneumonitis as an immune reconstitution inflammatory syndrome in a patient with CD4 cell recovery after HAART initiation. HIV Med. 2006;7:411–414. doi: 10.1111/j.1468-1293.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Izcue A, Hue S, Buonocore S, Arancibia-Carcamo CV, Ahern PP, Iwakura Y, Maloy KJ, Powrie F. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Lundkvist G, Dons L, Kristensson K, Rottenberg ME. Interferon-gamma mediates neuronal killing of intracellular bacteria. Scand J Immunol. 2004;60:437–448. doi: 10.1111/j.0300-9475.2004.01500.x. [DOI] [PubMed] [Google Scholar]
- Kader M, Bixler S, Piatak M, Lifson J, Mattapallil JJ. Anti-retroviral therapy fails to restore the severe Th-17: Tc-17 imbalance observed in peripheral blood during simian immunodeficiency virus infection. J Med Primatol. 2009;38(Suppl 1):32–38. doi: 10.1111/j.1600-0684.2009.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol. 2010;185:5453–5462. doi: 10.4049/jimmunol.1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, Sato T, Sakuraba A, Kitazume MT, Sugita A, Koganei K, Akagawa KS, Hibi T. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Investig. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LH, Everitt D, Leist TP, Ryan KA, Mascelli MA, Johnson K, Raychaudhuri A, Vollmer T. A phase I trial of an interleukin-12/23 monoclonal antibody in relapsing multiple sclerosis. Curr Med Res Opin. 2006;22:1671–1678. doi: 10.1185/030079906X120931. [DOI] [PubMed] [Google Scholar]
- Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, Ghilardi N, deSauvage F, Cooper AM. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- Kielian T. Microglia and chemokines in infectious diseases of the nervous system: views and reviews. Front Biosci. 2004;9:732–750. doi: 10.2741/1266. [DOI] [PubMed] [Google Scholar]
- Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18:670–675. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Kim B, Sarangi PP, Azkur AK, Kaistha SD, Rouse BT. Enhanced viral immunoinflammatory lesions in mice lacking IL-23 responses. Microb Infect. 2008;10:302–312. doi: 10.1016/j.micinf.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Han S, Withers DR, Gaspal F, Bae J, Baik S, Shin HC, Kim KS, Bekiaris V, Anderson G, Lane P, Kim MY. CD117 CD3 CD56 OX40Lhigh cells express IL-22 and display an LTi phenotype in human secondary lymphoid tissues. Eur J Immunol. 2011;41:1563–1572. doi: 10.1002/eji.201040915. [DOI] [PubMed] [Google Scholar]
- Kinter A, McNally J, Riggin L, Jackson R, Roby G, Fauci AS. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proc Natl Acad Sci USA. 2007;104:3390–3395. doi: 10.1073/pnas.0611423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt NR, Brenchley JM. Th17 cell dynamics in HIV infection. Curr Opin HIV AIDS. 2010;5:135–140. doi: 10.1097/COH.0b013e3283364846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Kotler DP, Reka S, Clayton F. Intestinal mucosal inflammation associated with human immunodeficiency virus infection. Dig Dis Sci. 1993;38:1119–1127. doi: 10.1007/BF01295730. [DOI] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- Kurzeja M, Rudnicka L, Olszewska M. New interleukin-23 pathway inhibitors in dermatology: ustekinumab, briakinumab, and secukinumab. Am J Clin Dermatol. 2011;12:113–125. doi: 10.2165/11538950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- Langrish CL, McKenzie BS, Wilson NJ, De Waal MR, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- Lankford CS, Frucht DM. A unique role for IL-23 in promoting cellular immunity. J Leukoc Biol. 2003;73:49–56. doi: 10.1189/jlb.0602326. [DOI] [PubMed] [Google Scholar]
- Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, Dhodapkar M, Krueger JG. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004a;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, French MA, Price P. IL-23 and IFN-gamma deficiency in immunodeficient HIV patients who achieved a long-term increase in CD4 T-cell counts on highly active antiretroviral therapy. AIDS. 2004b;18:1337–1340. doi: 10.1097/00002030-200406180-00014. [DOI] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- Li J, Gran B, Zhang GX, Ventura ES, Siglienti I, Rostami A, Kamoun M. Differential expression and regulation of IL-23 and IL-12 subunits and receptors in adult mouse microglia. J Neurol Sci. 2003;215:95–103. doi: 10.1016/s0022-510x(03)00203-x. [DOI] [PubMed] [Google Scholar]
- Li JC, Yim HC, Lau AS. Role of HIV-1 Tat in AIDS pathogenesis: its effects on cytokine dysregulation and contributions to the pathogenesis of opportunistic infection. AIDS. 2010;24:1609–1623. doi: 10.1097/QAD.0b013e32833ac6a0. [DOI] [PubMed] [Google Scholar]
- Li Q, Ke F, Zhang W, Shen X, Xu Q, Wang H, Yu XZ, Leng Q. Plasmin plays an essential role in amplification of psoriasiform skin inflammation in mice. PLoS ONE. 2011;6:e16483. doi: 10.1371/journal.pone.0016483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman LA, Cardillo F, Owyang AM, Rennick DM, Cua DJ, Kastelein RA, Hunter CA. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J Immunol. 2004;173:1887–1893. doi: 10.4049/jimmunol.173.3.1887. [DOI] [PubMed] [Google Scholar]
- Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, Onishi R, Nyugen N, Walter MJ, Pociask D, Randall TD, Gaffen SL, Iwakura Y, Kolls JK, Khader SA. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lin F, Strainic MG, An F, Miller RH, Altuntas CZ, Heeger PS, Tuohy VK, Medof ME. IFN-gamma and IL-17 production in experimental autoimmune encephalomyelitis depends on local APC-T cell complement production. J Immunol. 2008;180:5882–5889. doi: 10.4049/jimmunol.180.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Ouyang X, Yang J, Liu J, Li Q, Gu Y, Fukata M, Lin T, He JC, Abreu M, Unkeless JC, Mayer L, Xiong H. AP-1 activated by toll-like receptors regulates expression of IL-23 p19. J Biol Chem. 2009;284:24006–24016. doi: 10.1074/jbc.M109.025528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A. HIV infection and AIDS. P N G Med J. 1996;39:174–180. [PubMed] [Google Scholar]
- Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis S, Dutertre C-A, Vimeux L, Fery L, Henno L, Diocou S, Kahi S, Deveau C, Meyer L, Goujard C, Hosmalin A. IL-23 and IL-12p70 production by monocytes and dendritic cells in primary HIV-1 infection. J Leukoc Biol. 2010:jlb.1009684. doi: 10.1189/jlb.1009684. [DOI] [PubMed] [Google Scholar]
- Macal M, Sankaran S, Chun TW, Reay E, Flamm J, Prindiville TJ, Dandekar S. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 2008;1:475–488. doi: 10.1038/mi.2008.35. [DOI] [PubMed] [Google Scholar]
- Mamidi A, DeSimone JA, Pomerantz RJ. Central nervous system infections in individuals with HIV-1 infection. J Neuro-Virol. 2002;8:158–167. doi: 10.1080/13550280290049723. [DOI] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T (H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Markel G, Bar-Haim E, Zahavy E, Cohen H, Cohen O, Shafferman A, Velan B. The involvement of IL-17A in the murine response to sublethal inhalational infection with Francisella tularensis. PLoS ONE. 2010;5:e11176. doi: 10.1371/journal.pone.0011176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern D, Powrie F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut. 2007;56:1333–1336. doi: 10.1136/gut.2006.115402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks KD, Sieve AN, Kolls JK, Ghilardi N, Berg RE. IL-23 is required for protection against systemic infection with Listeria monocytogenes. J Immunol. 2009;183:8026–8034. doi: 10.4049/jimmunol.0901588. [DOI] [PubMed] [Google Scholar]
- Moens E, Brouwer M, Dimova T, Goldman M, Willems F, Vermijlen D. IL-23R and TCR signaling drives the generation of neonatal Vgamma9Vdelta2 T cells expressing high levels of cytotoxic mediators and producing IFN-gamma and IL-17. J Leukoc Biol. 2011;89:743–752. doi: 10.1189/jlb.0910501. [DOI] [PubMed] [Google Scholar]
- Mohan H, Bal A, Garg S, Dalal U. Cytomegalovirus-associated pseudotumor simulating gastric malignancy in acquired immunodeficiency syndrome: a case report with review of literature. Jpn J Infect Dis. 2007;60:134–136. [PubMed] [Google Scholar]
- Morar N, Willis-Owen SA, Maurer T, Bunker CB. HIV-associated psoriasis: pathogenesis, clinical features, and management. Lancet Infect Dis. 2010;10:470–478. doi: 10.1016/S1473-3099(10)70101-8. [DOI] [PubMed] [Google Scholar]
- Munoz M, et al. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009;206:3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray HW. Endogenous interleukin-12 regulates acquired resistance in experimental visceral leishmaniasis. J Infect Dis. 1997;175:1477–1479. doi: 10.1086/516482. [DOI] [PubMed] [Google Scholar]
- Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namazi MR. Paradoxical exacerbation of psoriasis in AIDS: proposed explanations including the potential roles of substance P and gram-negative bacteria. Autoimmunity. 2004;37:67–71. doi: 10.1080/08916930310001637986. [DOI] [PubMed] [Google Scholar]
- Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vγ2Vδ T Cells. J Immunol. 2010;184:7268–7280. doi: 10.4049/jimmunol.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton HB. Common neurologic complications of HIV-1 infection and AIDS. Am Fam Physician. 1995;51:387–398. [PubMed] [Google Scholar]
- Nilsson J, Boasso A, Velilla PA, Zhang R, Vaccari M, Franchini G, Shearer GM, Andersson J, Chougnet C. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- Onlamoon N, Pattanapanyasat K, Ansari AA. Human and nonhuman primate lentiviral infection and autoimmunity. Ann N Y Acad Sci. 2005;1050:397–409. doi: 10.1196/annals.1313.091. [DOI] [PubMed] [Google Scholar]
- Oppmann B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Osorio F, LeibundGut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G, Reis e Sousa C. DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol. 2008;38:3274–3281. doi: 10.1002/eji.200838950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham C, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- Pedroso DC, Tellechea A, Moura L, Fidalgo-Carvalho I, Duarte J, Carvalho E, Ferreira L. Improved survival, vascular differentiation and wound healing potential of stem cells co-cultured with endothelial cells. PLoS ONE. 2011;6:e16114. doi: 10.1371/journal.pone.0016114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirhonen J, Matikainen S, Julkunen I. Regulation of virus-induced IL-12 and IL-23 expression in human macrophages. J Immunol. 2002;169:5673–5678. doi: 10.4049/jimmunol.169.10.5673. [DOI] [PubMed] [Google Scholar]
- Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol. 2006;176:1908–1915. doi: 10.4049/jimmunol.176.3.1908. [DOI] [PubMed] [Google Scholar]
- Prendergast A, Prado JG, Kang YH, Chen F, Riddell LA, Luzzi G, Goulder P, Klenerman P. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. AIDS. 2010;24:491–502. doi: 10.1097/QAD.0b013e3283344895. [DOI] [PubMed] [Google Scholar]
- Price R, Brew B, Sidtis J, Rosenblum M, Scheck A, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Quesniaux VJ, Nicolle DM, Torres D, Kremer L, Guerardel Y, Nigou J, Puzo G, Erard F, Ryffel B. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J Immunol. 2004;172:4425–4434. doi: 10.4049/jimmunol.172.7.4425. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;276:37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- Riol-Blanco L, Lazarevic V, Awasthi A, Mitsdoerffer M, Wilson BS, Croxford A, Waisman A, Kuchroo VK, Glimcher LH, Oukka M. IL-23 receptor regulates unconventional IL-17-producing T cells that control bacterial infections. J Immunol. 2010;184:1710–1720. doi: 10.4049/jimmunol.0902796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagopal S, Lorey SL, Barnett L, Basham R, Lebo L, Erdem H, Haman K, Avison M, Waddell K, Haas DW, Kalams SA. Enhancement of human immunodeficiency virus (HIV)-specific CD8+ T cells in cerebrospinal fluid compared to those in blood among antiretroviral therapy-naive HIV-positive subjects. J Virol. 2008;82:10418–10428. doi: 10.1128/JVI.01190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Miyoshi F, Yokota K, Araki Y, Asanuma Y, Akiyama Y, Yoh K, Takahashi S, Aburatani H, Mimura T. Marked induction of c-Maf potein during Th17 cell differentiation and its implication in memory Th cell development. J Biol Chem. 2011;286:14963–14971. doi: 10.1074/jbc.M111.218867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittman SM, Fox L. Preliminary evidence for partial restoration of immune function in HIV type 1 infection with potent antiretroviral therapies: clues from the Fourth Conference on Retroviruses and Opportunistic Diseases. AIDS Res Hum Retrovir. 1997;13:815–818. doi: 10.1089/aid.1997.13.815. [DOI] [PubMed] [Google Scholar]
- Schulz SM, Kohler G, Schutze N, Knauer J, Straubinger RK, Chackerian AA, Witte E, Wolk K, Sabat R, Iwakura Y, Holscher C, Muller U, Kastelein RA, Alber G. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J Immunol. 2008a;181:7891–7901. doi: 10.4049/jimmunol.181.11.7891. [DOI] [PubMed] [Google Scholar]
- Schulz SM, Köhler G, Schütze N, Knauer J, Straubinger RK, Chackerian AA, Witte E, Wolk K, Sabat R, Iwakura Y, Holscher C, Müller U, Kastelein RA, Alber G. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J Immunol. 2008b;181:7891–7901. doi: 10.4049/jimmunol.181.11.7891. [DOI] [PubMed] [Google Scholar]
- Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7:796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- Segal LN, Methe BA, Nolan A, Hoshino Y, Rom WN, Dawson R, Bateman E, Weiden MD. HIV-1 and bacterial pneumonia in the era of antiretroviral therapy. Proc Am Thorac Soc. 2011;8:282–287. doi: 10.1513/pats.201006-044WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender LY, Gibbert K, Suezer Y, Radeke HH, Kalinke U, Waibler Z. CD40 ligand-triggered human dendritic cells mount interleukin-23 responses that are further enhanced by danger signals. Mol Immunol. 2010;47:1255–1261. doi: 10.1016/j.molimm.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Sheikh SZ, Matsuoka K, Kobayashi T, Li F, Rubinas T, Plevy SE. Cutting edge: IFN-gamma is a negative regulator of IL-23 in murine macrophages and experimental colitis. J Immunol. 2010;184:4069–4073. doi: 10.4049/jimmunol.0903600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh SZ, Kobayashi T, Matsuoka K, Onyiah JC, Plevy SE. Characterization of an interferon-stimulated response element (ISRE) in the Il23a promoter. J Biol Chem. 2011;286:1174–1180. doi: 10.1074/jbc.M110.147884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh TM, Carter DL, Blosser RL, Mankowski JL, Zink MC, Clements JE. Functional analyses of natural killer cells in macaques infected with neurovirulent simian immunodeficiency virus. J NeuroVirol. 2001;7:11–24. doi: 10.1080/135502801300069593. [DOI] [PubMed] [Google Scholar]
- Shime H, Yabu M, Akazawa T, Kodama K, Matsumoto M, Seya T, Inoue N. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J Immunol. 2008;180:7175–7183. doi: 10.4049/jimmunol.180.11.7175. [DOI] [PubMed] [Google Scholar]
- Siegemund S, Schutze N, Freudenberg MA, Lutz MB, Straubinger RK, Alber G. Production of IL-12, IL-23 and IL-27p28 by bone marrow-derived conventional dendritic cells rather than macrophages after LPS/TLR4-dependent induction by Salmonella Enteritidis. Immunobiology. 2007;212:739–750. doi: 10.1016/j.imbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Sieve AN, Meeks KD, Lee S, Berg RE. A novel immunoregulatory function for IL-23: Inhibition of IL-12-dependent IFN-gamma production. Eur J Immunol. 2010;40:2236–2247. doi: 10.1002/eji.200939759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg MJ, Chao C, Leyden WA, Xu L, Tang B, Horberg MA, Klein D, Quesenberry CP, Jr, Towner WJ, Abrams DI. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23:2337–2345. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons CP, Goncalves NS, Ghaem-Maghami M, Bajaj-Elliott M, Clare S, Neves B, Frankel G, Dougan G, MacDonald TT. Impaired resistance and enhanced pathology during infection with a noninvasive, attaching-effacing enteric bacterial pathogen, Citrobacter rodentium, in mice lacking IL-12 or IFN-gamma. J Immunol. 2002;168:1804–1812. doi: 10.4049/jimmunol.168.4.1804. [DOI] [PubMed] [Google Scholar]
- Smith E, Zarbock A, Stark MA, Burcin TL, Bruce AC, Foley P, Ley K. IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice. J Immunol. 2007;179:8274–8279. doi: 10.4049/jimmunol.179.12.8274. [DOI] [PubMed] [Google Scholar]
- Smith RL, Warren RB, Eyre S, Ho P, Ke X, Young HS, Griffiths CE, Worthington J. Polymorphisms in the IL-12beta and IL-23R genes are associated with psoriasis of early onset in a UK cohort. J Investig Dermatol. 2008;128:1325–1327. doi: 10.1038/sj.jid.5701140. [DOI] [PubMed] [Google Scholar]
- Smits HH, van Beelen AJ, Hessle C, Westland R, de Jong E, Soeteman E, Wold A, Wierenga EA, Kapsenberg ML. Commensal Gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. Eur J Immunol. 2004;34:1371–1380. doi: 10.1002/eji.200324815. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- Sonobe Y, Yawata I, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Production of IL-27 and other IL-12 family cytokines by microglia and their subpopulations. Brain Res. 2005;1040:202–207. doi: 10.1016/j.brainres.2005.01.100. [DOI] [PubMed] [Google Scholar]
- Spencer DC, Price RW. Human immunodeficiency virus and the central nervous system. Annu Rev Microbiol. 1992;46:655–693. doi: 10.1146/annurev.mi.46.100192.003255. [DOI] [PubMed] [Google Scholar]
- Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- Stanley H, Fluetsch-Bloom M, Bunce-Clyma M. HIV-related non-Hodgkin’s lymphoma. Oncol Nurs Forum. 1991;18:875–880. [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Numasaki M, Lotze MT, Sasaki H. Interleukin-17 enhances bFGF-, HGF- and VEGF-induced growth of vascular endothelial cells. Immunol Lett. 2005;98:189–193. doi: 10.1016/j.imlet.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Takaku T, Calado RT, Kajigaya S, Young NS. Interleukin-23 receptor (IL-23R) gene polymorphisms in acquired aplastic anemia. Ann Hematol. 2009;88:653–657. doi: 10.1007/s00277-008-0666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O’Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tato CM, Cua DJ. Reconciling id, ego, and superego within interleukin-23. Immunol Rev. 2008;226:103–111. doi: 10.1111/j.1600-065X.2008.00715.x. [DOI] [PubMed] [Google Scholar]
- Tchatalbachev S, Ghai R, Hossain H, Chakraborty T. Gram-positive pathogenic bacteria induce a common early response in human monocytes. BMC Microbiol. 2010;10:275. doi: 10.1186/1471-2180-10-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng MW, Andrews DM, McLaughlin N, von Scheidt B, Ngiow SF, Moller A, Hill GR, Iwakura Y, Oft M, Smyth MJ. IL-23 suppresses innate immune response independently of IL-17A during carcinogenesis and metastasis. Proc Natl Acad Sci USA. 2010;107:8328–8333. doi: 10.1073/pnas.1003251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker P, Leach MW, Kuang W, Benoit SE, Leonard JP, Marusic S. IL-23 is critical in the induction but not in the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:2589–2598. doi: 10.4049/jimmunol.178.4.2589. [DOI] [PubMed] [Google Scholar]
- Town T, Bai F, Wang T, Kaplan AT, Qian F, Montgomery RR, Anderson JF, Flavell RA, Fikrig E. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity. 2009;30:242–253. doi: 10.1016/j.immuni.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- Valmori D, Raffin C, Raimbaud I, Ayyoub M. Human RORgammat+ TH17 cells preferentially differentiate from naive FOXP3+Treg in the presence of lineage-specific polarizing factors. Proc Natl Acad Sci USA. 2010;107:19402–19407. doi: 10.1073/pnas.1008247107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SAJ, Kapsenberg ML, de Jong EC. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- van de Wetering D, de Paus RA, van Dissel JT, van de Vosse E. Salmonella induced IL-23 and IL-1b allow for IL-12 production by monocytes and Mϕ1 through induction of IFN-γ in CD56+ NK/NK-like T cells. PLoS ONE. 2009;4:e8396. doi: 10.1371/journal.pone.0008396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA. 2004;101:4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hertzen L, Joensuu H, Haahtela T. Microbial deprivation, inflammation and cancer. Canc Metastasis Rev. 2011;30:211–223. doi: 10.1007/s10555-011-9284-1. [DOI] [PubMed] [Google Scholar]
- Vossenkamper A, Struck D, Alvarado-Esquivel C, Went T, Takeda K, Akira S, Pfeffer K, Alber G, Lochner M, Forster I, Liesenfeld O. Both IL-12 and IL-18 contribute to small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii, but IL-12 is dominant over IL-18 in parasite control. Eur J Immunol. 2004;34:3197–3207. doi: 10.1002/eji.200424993. [DOI] [PubMed] [Google Scholar]
- Wang J, Ma J, Charboneau R, Barke R, Roy S. Morphine inhibits murine dendritic cell interleukin 23 production by modulating TLR2 and Nod2 signaling. J Biol Chem. 2011;286(12):10225–32. doi: 10.1074/jbc.M110.188680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei XQ, Rogers H, Lewis MA, Williams DW. The role of the IL-12 cytokine family in directing T-cell responses in oral candidosis. Clin Dev Immunol. 2011;2011:697340. doi: 10.1155/2011/697340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiekowski MT, Leach MW, Evans EW, Sullivan L, Chen SC, Vassileva G, Bazan JF, Gorman DM, Kastelein RA, Narula S, Lira SA. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J Immunol. 2001;166:7563–7570. doi: 10.4049/jimmunol.166.12.7563. [DOI] [PubMed] [Google Scholar]
- Wittig BM. Drug evaluation: CNTO-1275, a mAb against IL-12/IL-23p40 for the potential treatment of inflammatory diseases. Curr Opin Investig Drugs. 2007;8:947–954. [PubMed] [Google Scholar]
- Wozniak TM, Ryan AA, Britton WJ. Interleukin-23 restores immunity to Mycobacterium tuberculosis infection in IL-12p40-deficient mice and is not required for the development of IL-17-secreting T cell responses. J Immunol. 2006;177:8684–8692. doi: 10.4049/jimmunol.177.12.8684. [DOI] [PubMed] [Google Scholar]
- Wozniak TM, Saunders BM, Ryan AA, Britton WJ. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infect Immun. 2010;78:4187–4194. doi: 10.1128/IAI.01392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists suppress the production of IL-12 family cytokines by activated glia. J Immunol. 2007;178:1904–1913. doi: 10.4049/jimmunol.178.3.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A, Collman RG. CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J Neuro-immune Pharmacol. 2009;4:430–447. doi: 10.1007/s11481-009-9174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, Bistoni F, Puccetti P, Kastelein RA, Kopf M, Romani L. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]