Abstract
The ability of lymphocytes to migrate freely through connective tissues is vital to efficient immune function. How the extracellular matrix (ECM) may affect T cell adhesion and migration is not well understood. We have examined the adhesion and migration of activated human T lymphocytes on extracellular matrix made by fibroblast-like synoviocytes and lung fibroblasts. These cells were minimally interactive until treated with a viral mimetic, Poly I:C. This treatment promoted myofibroblast formation and engendered a higher-order structured ECM rich in versican and hyaluronan to which T cells avidly adhered in a hyaluronidase-sensitive manner. This Poly I:C-induced matrix impeded T-cell spreading and migration on and through synoviocyte monolayers, while hyaluronidase treatment or adding versican antibody during matrix formation reversed the effect on T-cell migration. Hyaluronidase also reversed the spread myofibroblast morphology. These data suggest that the viscous hyaluronan- and versican-rich matrix binds and constrains T lymphocytes. Using purified matrix components and solid state matrices of defined composition we uncovered a role for versican in modulating HA - T-cell interactions. Versican prevented T-cell binding to soluble hyaluronan, as well as the amoeboid shape change on hyaluronan coated dishes and T-cell penetration of collagen gels. Together, these data suggest that hyaluronan and versican play a role in T-cell trafficking and function in inflamed tissues.
Keywords: Hyaluronan, versican, lymphocyte, migration, myofibroblast, inflammation
1. Introduction
The ability of T-cells to adhere and migrate through connective tissue extracellular matrix (ECM) is vital to efficient immune responses (Korpos et al., 2009; Sorokin, 2010). T-cell migration is a multi-step process mediated by a complex assortment of integrins, matrix metalloproteases and other cell surface receptors, such as CD44, whose interactions initiate bidirectional signaling pathways (Denucci et al., 2009; Johnson and Ruffell, 2009). Among other effects, these signals result in T-cell activation and cytoskeleton rearrangements that are integral to T-cell adhesion and migration. When T-cells adhere to surfaces or tissues they usually adopt a “crawling” or “spreading” amoeboid morphology characterized by a broad lamellipodium at the leading edge and a handle-like protrusion or uropod at the rear. Cell scaffold–mediated migration strategies occur in lymph nodes, and it is likely that leukocytes move along stromal cells as part of their surveillance function in other tissues (Friedl and Weigelin, 2008). The transition from a rounded morphology to the amoeboid shape allows lymphocytes to squeeze through narrow spaces and move on collagen without reliance on matrix metalloproteases(Wolf et al., 2003). CD44 is thought to be important to crawling morphology and has been implicated in successful interstitial navigation of killer T cells and maintenance of stable migratory polarity(Mrass et al., 2008).
The ECM components that partner with cell-surface receptors at sites of inflammation are less well understood. This is because these molecules are dynamic, complex and difficult to study in isolation. Two relatively well characterized ECM components implicated in leukocyte adhesion are hyaluronan and versican. Hyaluronan is an extracellular matrix glycosaminoglycan that serves as a ligand for CD44 and is produced in connective tissues during inflammation in a number of contexts(Day and de la Motte, 2005). Versican is a chondroitin sulfate proteoglycan that aggregates with hyaluronan, and modulates cellular adhesion (Ernst et al., 1995; Yamagata and Kimata, 1994; Yamagata et al., 1989). Hyaluronan and versican are primary constituents of the cell coat (also known as the pericellular matrix or glycocalyx) of fibroblasts, myofibroblasts, smooth muscle cells and other cell types, and participate in the regulation of cell motility, proliferation, and myofibroblast differentiation (Evanko et al., 1999; Evanko et al., 2007; Meran et al., 2007; Toole, 2004). Versican is known to have a barrier/guidance function in neural crest migration and axonal growth (Dutt et al., 2006; Landolt et al., 1995). However, the influence of these ECM components on lymphocyte adhesion and migration is not well understood.
During chronic inflammation in both lung and synovial tissues, fibrosis and accumulation of myofibroblasts follow the accumulation of complex crosslinked hyaluronan matrices(Day and de la Motte, 2005; Kasperkovitz et al., 2005; Westergren-Thorsson et al., 2010), and this can be repeated in multiple inflammatory events. Thus, the fibroblast milieu may play a role in modulating inflammatory cell function, trafficking, and chronicity of inflammation. Inflammatory stimuli, such as viruses, viral mimics and inducers of endoplasmic reticulum (ER) stress, are known to induce production of adhesive higher order hyaluronan and versican-rich cable structures by smooth muscle cells and fibroblasts (de la Motte et al., 1999; Evanko et al., 2009b; Majors et al., 2003; Potter-Perigo et al., 2009; Selbi et al., 2006; Wang and Hascall, 2004), and there is evidence of similar matrices in vivo(de la Motte et al., 1999). We recently reported that the viral mimic poly inosine:cytidylic acid (Poly I:C), a ligand for TLR3, promotes the deposition of versican in the hyaluronan cables in lung fibroblasts (Potter-Perigo et al., 2009). Others have found that activation of TLR3 with Poly I:C can augment myofibroblast formation via production of TGF-β (Sugiura et al., 2009). Monocyte adhesion to the hyaluronan cables of fibroblasts and other cells is hyaluronan-dependent, as shown by abolishment using hyaluronidase, which destroys the pericellular cable and coat structures. This adhesion is also partly dependent on cell surface CD44 (de la Motte et al., 1999), as well as versican that is present in the matrix(Potter-Perigo et al., 2009). Although T-lymphocytes are known to express CD44 and bind hyaluronan(Bollyky et al., 2009a; Bollyky et al., 2007; Lesley et al., 1994; Ruffell and Johnson, 2008), the role of hyaluronan-based cable structures in T-cell adhesion and function is not well understood. Because hyaluronan is known to promote migration of several cell types, we hypothesized that hyaluronan cable structures formed by fibroblasts in vitro could provide a traction mechanism, thus promoting and supporting migration of lymphocytes.
In this study, we evaluate T-lymphocyte adhesion and migratory behavior on an inflammatory extracellular matrix that is rich in hyaluronan and versican made by fibroblasts in response to Poly I:C. We show that the retention of CD4+ T-cells by human lung fibroblasts and normal human synoviocytes is promoted by Poly I:C while migration is inhibited, and these effects are reversed by treatment with hyaluronidase and anti-versican antibody. To complement these studies with native, cell-derived matrices, we have also used defined synthetic matrices to evaluate the effect of hyaluronan and versican on T-cell migration. The hypothesis that hyaluronan-dependent, versican-rich cable structures would facilitate T-cell migration is not supported.
2. Materials and Methods
2.1 Reagents
Pharmaceutical grade hyaluronan with molecular weights of 1.53 MDa and 200 KDa was provided by Genzyme (Cambridge, MA, USA). Streptomyces hyaluronidase was obtained from Sigma-Aldrich (St Louis, MO, USA). Collagen (type I, rat tail) was from BD Biosciences (Bedford, MA). Poly I:C was from Invivogen (San Diego, CA). Biotinylated hyaluronan binding protein (b-HABP) was prepared from cartilage as described (Underhill et al. 1993). Biotinylated hyaluronan was prepared as described (Hoare et al., 1993). Monoclonal anti-versican antibodies, 2B1 and 12C5, came from Seikagaku Corp.; (East Falmouth, MA), and the Developmental Studies Hybridoma Bank (University of Iowa), respectively. Monoclonal antibody to human smooth muscle actin (clone 1A4) was from Dako North America, Inc.(Carpinteria, CA).
Versican was purified from bovine aorta by a combination of 4 M guanidinium HCL extraction, ion exchange and size exclusion chromatography, as described previously (Olin et al., 2001). The versican preparation was free of contaminants as assessed by SDS PAGE and coomassie blue and Alcian blue staining, and was endotoxin-free (<1.0 EU/µg) as assessed using a ToxinSensor™ LAL Endotoxin Assay Kit (GenScript Corp., Piscataway, NJ). The versican preparation bound specifically to biotinylated hyaluronan on ligand blots (Supplemental Fig. 1) and to versican specific antibodies on Western blots and comprised the V0 and V1 isoforms (data not shown).
A portion of the versican preparation was biotinylated, repurified on a hyaluronan affinity column, and used in an enzyme linked sorbent assay to assess the ability of 12C5 antibody to inhibit biotin-versican binding to hyaluronan coated plates and to assess direct binding of versican to T-cells. The biotinylation of versican and hyaluronan affinity column was done essentially as described for cartilage hyaluronan binding protein (Underhill et al., 1993), with slight modification. The trypsin step was omitted and versican was biotinylated in the presence of 100 µg/ml exogenous 200 KDa hyaluronan to preserve the binding site prior to putting it on the hyaluronan affinity column.
2.2 Human blood samples
Human peripheral blood mononuclear cell (PBMC) samples were obtained from healthy volunteers with informed consent, participating in a research protocol approved by the institutional review board of the Benaroya Research Institute at Virginia Mason (BRI, Seattle, WA, USA).
2.3 Isolation of leukocyte populations
Human PBMCs were prepared by centrifugation of peripheral blood over Ficoll–Hypaque gradients. CD4+ T cells were isolated using the Dynal CD4 Positive Isolation Kit (Invitrogen, Carlsbad, CA, USA) as per the manufacturer’s instructions. Purity of the resulting cell fractions was reliably 98% CD4+ by flow cytometry; anti-CD4 Ab (RPAT4), from BD-Biosciences (San Jose, CA) was used for this purpose. Cells were cultured in RPMI 1640 (Invitrogen) supplemented with 10% pooled human serum, 100 µg/ml penicillin, 100 U/ml streptomycin and 1 mM Na pyruvate (Invitrogen). CD4+ T-cells were activated with anti-CD3/28 coated beads (Invitrogen) in the setting of 100 IU of recombinant IL-2 (Chiron, Emeryville, CA, USA) for 72 hours prior to their use in binding studies and other assays.
2.4 Culture and Poly I:C treatment of fibroblasts
Human lung fibroblasts (HLFs) were derived from explants of the lung, following removal of both the pleura and parenchyma, and were a generous gift from Professor Ganesh Raghu, Divison of Pulmonary and Critical Care Medicine, University of Washington, Seattle. The cells were isolated as described previously in accordance with approval from the institution’s human subjects review committee (Raghu et al. 1988). HLFs were maintained in DMEM high-glucose medium supplemented with 10% FBS (HyClone; Logan, UT)), 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, 0.43 mg/ml GlutMAX-1, and penicillin-streptomycin (penicillin G sodium, 100 U/ml, and streptomycin sulfate, 0.10 mg/ml; Invitrogen Life Technologies, Carlsbad, CA) at 37C in 5% CO2. Cells were passaged with trypsin-EDTA (0.05% trypsin and 0.53 mM tetrasodium EDTA) and were used for experiments between passages 9 and 17 after initial isolation.
Normal human fibroblast-like synoviocytes (HFLS) were purchased from Cell Applications Inc. (San Diego, CA) and grown in synoviocyte growth medium (Cell Applications). They were arrested in DMEM with 0.1% FBS and stimulated in synoviocyte growth medium with and without poly I:C (20 µg/ml) for 20 h. HFLS were maintained in 5% CO2 at 37°C and passaged with trypsin-EDTA as for HLFs. Hyaluronan and versican in the cell layers were measured by ELSA using biotinylated HABP and Western blotting (2B1 antibody), respectively, as described previously(Potter-Perigo et al., 2009).
For imaging and immunohistochemistry, fibroblasts or synoviocytes were seeded on glass coverslips at 3.5 × 105/well in 6-well plates in 10% FBS DMEM or synoviocyte medium. After 24 hr, cells were growth arrested for 48 hr in medium containing 0.1% FBS, at which point the cells were nearly confluent. Following this period of serum deprivation, the cells tended to have very little hyaluronan on their surfaces and no cell coats by the particle exclusion assay (data not shown). Medium was then removed and cells were stimulated without or with poly I:C (20 µg/ml) in the presence of 10% FBS to stimulate the formation of hyaluronan-based matrix cable structures (de la Motte et al. 1999) for 20 hr. Alternatively, cells were sometimes seeded at 3.5 × 104/well in order to obtain sparse cultures to allow visualization of T-cells in warm up experiments. The fibroblast layers were washed with PBS and incubated with T-cells 0.5-1 × 106 at 4° C in synoviocyte medium or RPMI containing 10% FBS. Following binding, the non-adherent T-cells were rinsed by immersion of the inverted coverslip in 200 ml of PBS using forceps (6 dips of 1 sec duration).
Adhesion of calcein-labeled T-cells to fibroblasts and synoviocytes was assayed in 96 well plates as described previously for monocyte adhesion (Potter-Perigo et al., 2009). Some fibroblasts were treated with Streptomyces hyaluronidase (0.66 U/ml) in the growth medium for 30 min at 37°C prior to the adhesion assay.
2.5 Western Analysis and Ligand Blotting of Versican
For Western and ligand blotting, versican was digested by chondroitin ABC lyase, applied to a gradient of 4–12% SDS–PAGE, and electrophoretically transferred to 0.2 µm nitrocellulose membranes (GE Healthcare, Piscataway, NJ) using a BioRad Transblot SD Semi-Dry Transfer Cell (BioRad, Hercules, CA)(Olin et al., 1999). The transferred proteins were then detected with the primary antibody to versican, 2-B-1 (North Star Bioproducts), and enhanced chemiluminescence (Western-Light Chemiluminescent Detection System) with CSPD proprietary luminescent substrate (Applied Biosystems, Foster City, CA). Bands were scanned and quantitated using NIH Image J. For ligand blots to assess versican binding to biotinylated hyaluronan, 10 µg versican was electrophoresed under non-reducing and reducing conditions prior to transfer to nitrocellulose. Blot strips were incubated with biotinylated hyaluronan (prepared as described in (Hoare et al., 1993)). A control strip was preincubated with 500 µg/ml unlabeled hyaluronan.
2.6 Immunohistochemistry
Fibroblasts with bound T-cells on coverslips were fixed in acid-formalin-ethanol (3.7% formaldehyde-PBS, 70% ethanol, and 5% glacial acetic acid, all v/v) (Lin et al. 1997). Following rinsing in PBS, cells were stained for hyaluronan using b-HABP followed by either streptavidin-Alexafluor 488 or streptavidin-Texas Red in PBS containing 1% bovine serum albumin as previously described (Evanko and Wight 1999). Versican was localized using monoclonal antibody 2B1 (Seikagaku Corp.; East Falmouth, MA), followed by Alexafluor 488–conjugated secondary antibody (Invitrogen). As controls, fibroblast cultures were predigested with hyaluronan-specific Streptomyces hyaluronidase (before fixation), which abolished staining with the b-HABP (data not shown, see (Evanko et al., 2009a)), or incubated with normal mouse IgG as a control for versican staining (not shown). Smooth muscle actin antibody (Dako) was used at 1:50 (1.4 µg/ml) on cells fixed with 10% formalin. Cells were examined using a Leica DMIRB microscope under epifluroescence optics using a 63 × 0.70 numerical aperture objective, and images were acquired using a Spot cooled-CCD camera and imaging program.
2.7 Time lapse microscopy and determination of T-cell migration
CD4+ T-cells were applied to control and poly I:C-treated synoviocyte monolayers in synoviocyte medium containing 10% fetal bovine serum and 20 mM HEPES and allowed to settle for 15 minutes atop a microscope stage that was maintained at 37 °C. Sequential phase-contrast images were taken every 20 seconds for twenty minutes. Tracings of the migratory paths of 20 randomly selected lymphocytes from each condition were obtained with the aid of Image J (NIH). The net migration distance was used to calculate migration rate.
In some experiments, random migration through the matrix and monolayer in the Z axis was assessed. Calcein-labeled T cells (6×105 in 100 µl) were applied atop the synoviocytes and allowed to migrate for 20 min. at 37°C. Coverslips were fixed in 10% formalin for 30 min. Most of the T cells bound and trapped by the matrix were well above the cell layer in a different focal plane which facilitated counting the number of migrated T cells that had passed through the matrix and monolayer and were in focus at the level of the coverslip. The migrant cells were clearly discernable from the cells trapped well above. Some cultures were treated with Streptomyces hyaluronidase (1U/ml in the culture medium) prior to adding the T-cells to assess the contribution of the matrix to migration through the monolayer. Thirty fields per condition were measured using the 20 × objective. One-way ANOVA with Tukey’s post test was performed using GraphPad Prism version 5.03 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com. The data are representative of three independent experiments.
2.8 T-cell penetration of collagen gels
Collagen gels (1 mg/ml, 100 µl in DMEM) without or with added hyaluronan (100 µg/ml) or versican (5 or 20 µg/ml) or versican that was pretreated with Chondroitin ABC lyase, were cast atop flame-sterilized 22 mm coverslips. Control additives included chondroitin sulfate and chondroitinase enzyme alone. In some experiments, chemokines CCL19 (100 ng/ml) and CCL 21 (500 ng/ml) were included in the gels to enhance T-cell migration. Care was taken to spread the 100 µl collagen solutions over the entire coverslip. After polymerization of the collagen gels for 30 minutes at 37°, activated lymphocytes (2 × 105) were applied to the upper surface of the gel in 100 µl of RPMI medium containing 10% FBS and cells were allowed to penetrate the collagen for 3 h at 37°. Coverslips were fixed with formalin and the number of lymphocytes that penetrated to within 50 µm of the coverslip was counted using a 20 × objective with phase contrast optics and calibrated focusing. Alternatively, T-cell nuclei were stained with ToproRS (Invitrogen) and photographs were acquired at a depth of 100 µm into the gel using a Leica ICL SP5 confocal microscope, 10X objective, and the number of penetrated cells counted with the aid of Image J. A minimum of 30 fields per condition, randomly selected from the central 1 cm2 region of the coverslip were counted. One-way ANOVA with Tukey’s post test was performed as described above.
2.9 Two dimensional coatings and T-cell morphology
24 well culture dishes were coated overnight with 100 µg/ml of BSA-conjugated hyaluronan, rinsed with PBS, and then followed by nothing, versican or chondroitinase-treated versican (20 µg/ml) for 1 h. Lymphocytes were applied and allowed to settle for 20–30 minutes. Cells were fixed and the proportions of amoeboid versus round cells were quantitated from photographs.
2.10 Soluble HA and versican binding studies
FITC-labeled hyaluronan (Sigma) and unlabled versican were incubated together for 1 hour at 37° C before addition to T-cells. In the meantime, activated human T-cells (2×105) were washed and resuspended in 200µl/ml of RPMI 1640 not supplemented with serum. 100ml of 4-methylumbelliferone (4-MU) from Sigma-Aldrich (St. Louis, MO) was added to the cells at 50 µg/ml also for 1 hour. The FITC-labeled HA was then added (for a final concentration of 50 µg/ml) together with the indicated concentration of versican. The FITC-labeled HA and the T-cells were incubated for 1 hour at 37° C prior to analysis. Direct binding of biotinylated versican (5 µg/ml) to T cells pretreated with 4 MU and hyaluronidase was similarly assessed. Data were acquired on a FACSCaliber (Becton Dickinson). Analysis was performed using FlowJo (Treestar Inc.) software.
3. Results
3.1 Effect of Poly IC on ECM production
Poly IC had similar effects on ECM production by synoviocytes as was previously reported for lung fibroblasts (Potter-Perigo et al., 2009). In the poly I:C treated synoviocytes, hyaluronan was significantly increased in the cell layer by 8-fold (8.22 ± 2.34 fold, p < 0.01), and contained a higher proportion of high molecular weight hyaluronan as measured by gel filtration chromatography. As measured by Western blotting and densitometry, versican was increased 1.77-fold compared to controls, (1.77 ± 0.22, p < 0.01).
3.1 T-cell adhesion to fibroblast and synoviocyte matrix
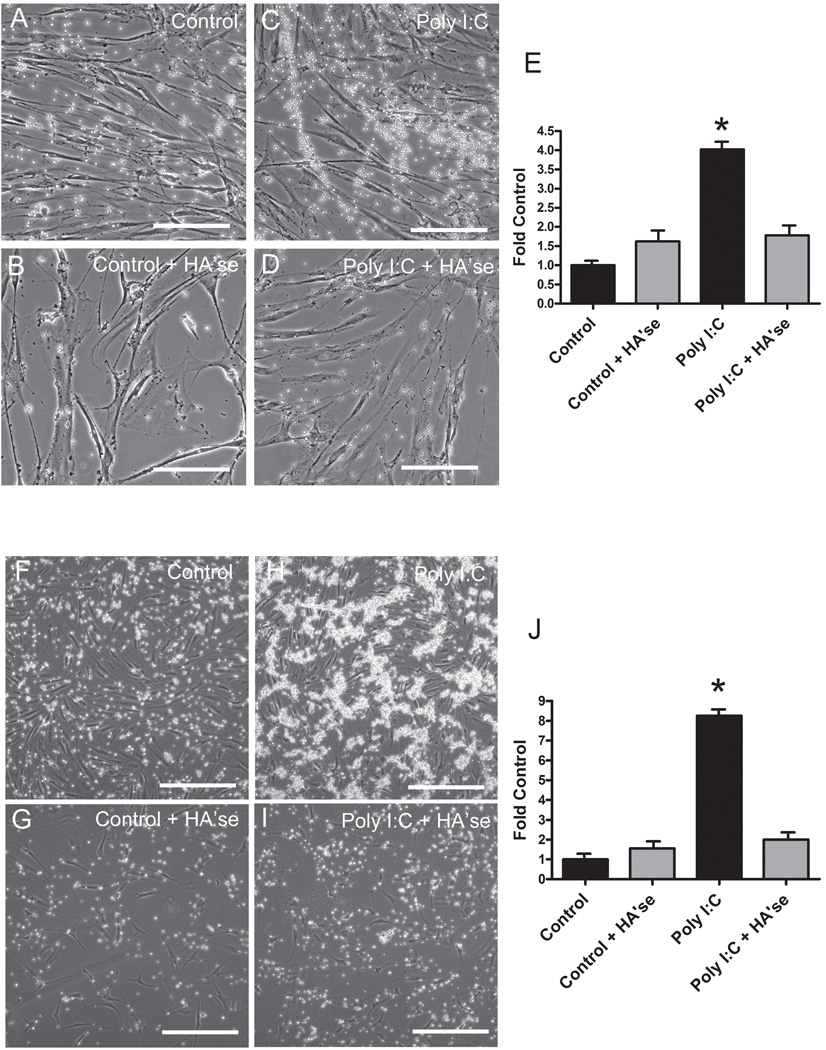
Compared to untreated control fibroblasts, several fold more activated human CD4+ T-lymphocytes bound to Poly I:C-treated human lung fibroblasts or fibroblast-like synoviocytes (Fig. 1). Treatment of the fibroblast monolayers with Streptomyces hyaluronidase abolished most of the lymphocyte adhesion to the Poly I:C-induced matrix, indicating that the adhesion was hyaluronan-dependent. Figure 1 also shows phase contrast images of the T-cells bound to the fibroblast cell layers and the effect of hyaluronidase treatment. In lung fibroblasts, the bound T-lymphocytes were typically arrayed along cable structures (Fig. 1C), as has been reported previously for binding of monocytes to smooth muscle cells or lung fibroblasts (de la Motte et al., 1999; Potter-Perigo et al., 2009). In contrast, the T-cells adhering to the synoviocyte matrix tended to form large clumps, while the extremely long cable structures were less apparent (Fig. 1H). T-cell subsets, such as CD4+CD25+ Treg, or TH1 and TH2 cells, were all capable of binding to the matrix of Poly I:C-treated fibroblasts in a hyaluronan-dependent manner (data not shown). Therefore, we limited the remainder of our studies to activated CD4+ T-cells.
Figure 1.
Phase contrast images and quantitation of activated human CD4+ T-lymphocytes binding to control and Poly I:C-treated human lung fibroblasts (A–E, upper panels) or synoviocytes (F–J, lower panels) without or following digestion of the matrix with hyaluronidase as indicated. Bars equal 100 µm in all images. Quantitiation of calcein labeled CD4+ T-lymphocyte binding to control and Poly I:C-treated human lung fibroblasts (E) or synoviocyte like fibroblasts (J) without (black bars) or following digestion of the matrix with hyaluronidase (gray bars). *p < 0.001 compared to control.
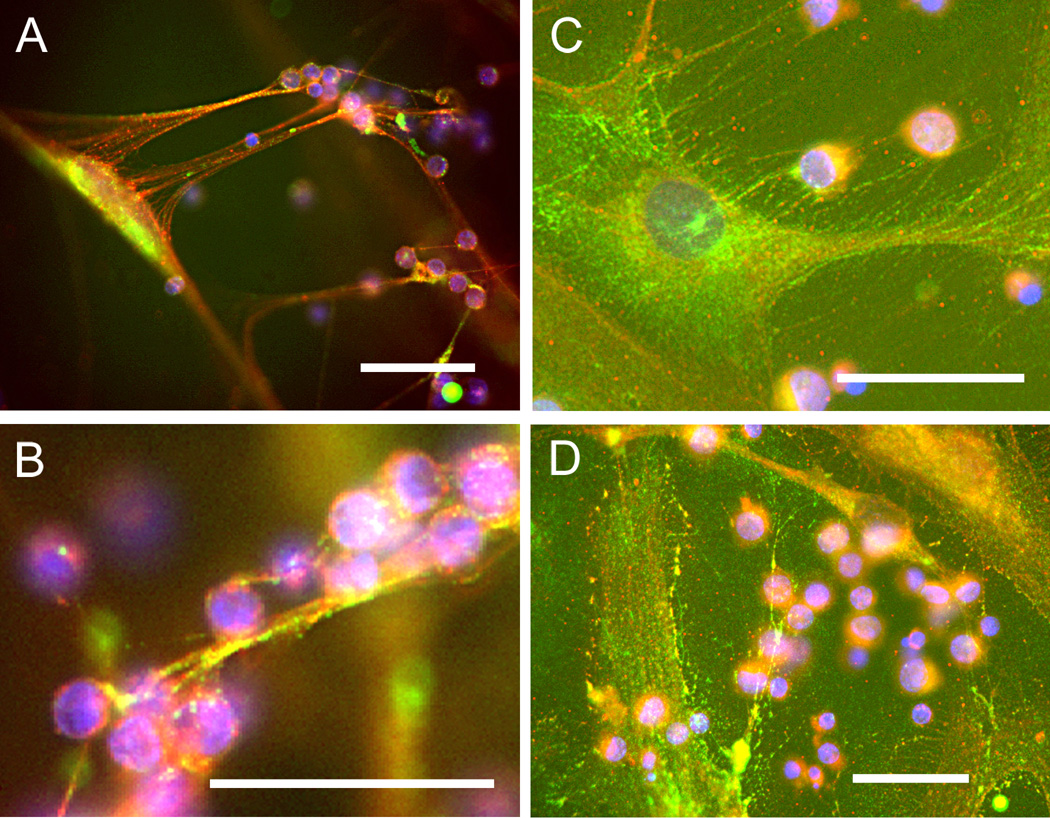
3.2 Versican and hyaluronan localization in adhesive matrix
Versican and hyaluronan were detected in the adhesive matrix of both fibroblast types, as shown by immunohistochemistry (Fig. 2). Following Poly I:C treatment of the fibroblasts and synoviocytes, most of the T-cells were bound in the matrix some distance (roughly 50–100 µm) above the fibroblast or synoviocyte cell layer (Fig. 2A), indicating that copious amounts of matrix were produced in response to the stimulus. Consistent with the phase contrast images, the hyaluronan- and versican-enriched matrix was present in the form of long cables in the lung fibroblasts, while in the synoviocytes, the hyaluronan and versican-rich matrix appeared as more of a dense mat or lawn and occasional broad cables, to which clumps of T-cells were bound. The synoviocyte matrix tended to have more intense versican staining than the lung fibroblasts as shown by the diffuse green signal over the entire cell layer in Figure 2C and D. Controls for staining included digestion of the cells with Streptomyces hyaluronidase, which abolished staining of the matrix with the hyaluronan binding probe, and normal mouse IgG, which was also negative (data not shown).
Figure 2.
T-cells bind to hyaluronan and versican-rich matrix. Following binding of human Tcells, lung fibroblasts (A, B) and synovial fibroblasts (C, D) were fixed with acid/alcohol/formalin and stained for hyaluronan (red) using bHABP and versican (green) using monoclonal antibody (2B1). Nuclei were counterstained with DAPI. Bars equal 50 µm in all images.
3.3 T cell migration and interactions with fibroblasts
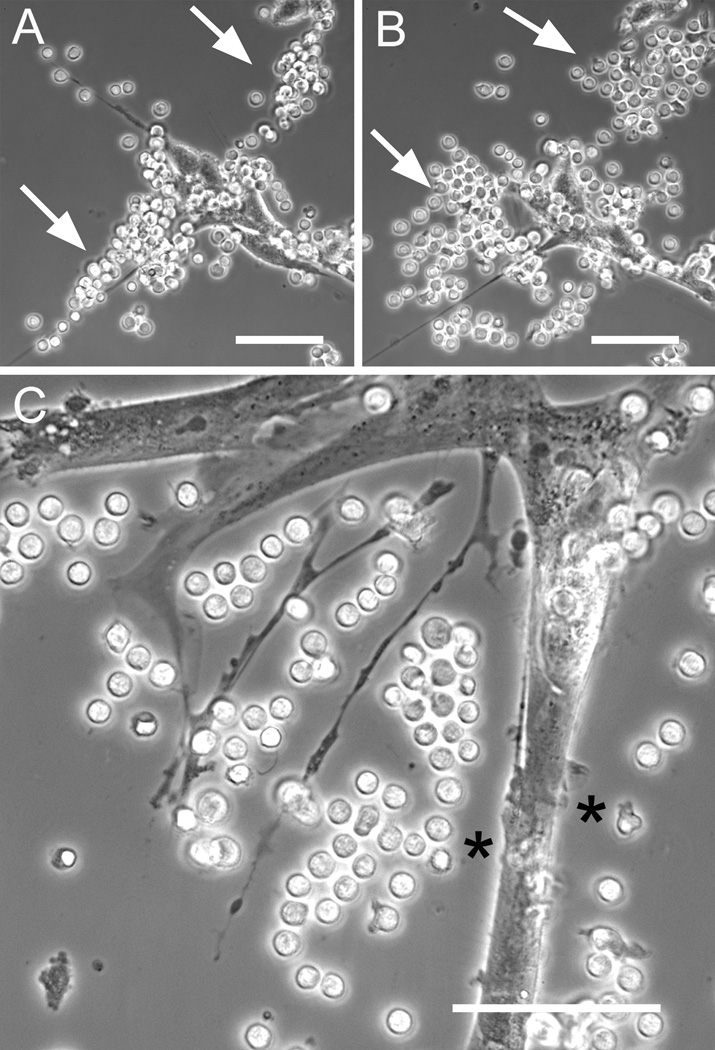
Beyond adhesion, the immediate functional consequence of T-cells encountering a matrix rich in hyaluronan and versican is not clear. We hypothesized that the cable structures made by Poly I:C treated lung fibroblasts could facilitate migratory behavior by the T-cells. However, following warm-up to physiological 37 °C, time-lapse microscopy showed no evidence for directional migration of T-cells along the hyaluronan cables. Instead, the T-cells aligned along the cables remained immobile and spherical, and eventually dispersed randomly and settled down onto the culture surface or fibroblast monolayer (Fig. 3A, B). This suggests that the cable structures made by lung fibroblasts in vitro do not support directional migration of T-cells under these conditions, and that the T-cells may, at least partially, degrade the matrix after binding. The cells tended to transition to the amoeboid shape only when they settled onto the culture surface (Fig. 3B, upper arrow). It was also evident that the pericellular matrix around the fibroblast acted as an exclusion barrier to the lymphocytes, preventing them from directly contacting the fibroblast surface in some places (Fig. 3C). This exclusion phenomenon using lymphocytes is similar to the exclusion of fixed erythrocytes in the widely used particle exclusion assay, used to identify pericellular hyaluronan coats (Evanko et al., 2007). T-cells were generally much more motile than the fibroblasts. Various kinds of direct physical interactions between the cell types were also noted. For example, T-cells crawling on the control fibroblast surfaces were sometimes deflected to change direction by microvilli on the fibroblasts. In other sequences, T-cells were physically pulled into tight clusters by the fibroblast, such as during occasional gross movements where the fibroblast retracted long cellular processes and matrix to which the T-cells had adhered (Supplemental video 1).
Figure 3.
T-cells bound to cable structures disperse randomly following warmup. T-cells were applied to sparse Poly I:C treated lung fibroblasts, rinsed, and then monitored with time lapse microscopy following warmup to physiological 37°C. A, immediately after washing the lymphocytes are bound along a putative hyaluronan cable (arrows) whose axis runs diagonally through the field. B, after 20 minutes at 37°C, the T cells have dispersed themselves mostly in directions perpendicular to the original axis of the cable. C. Another field showing how lymphocytes are excluded along portions of the fibroblast surface by the pericellular matrix (asterisks), much like the particle exclusion assay that employs fixed erythrocytes(Potter-Perigo et al., 2009). Bars equal 50 µm.
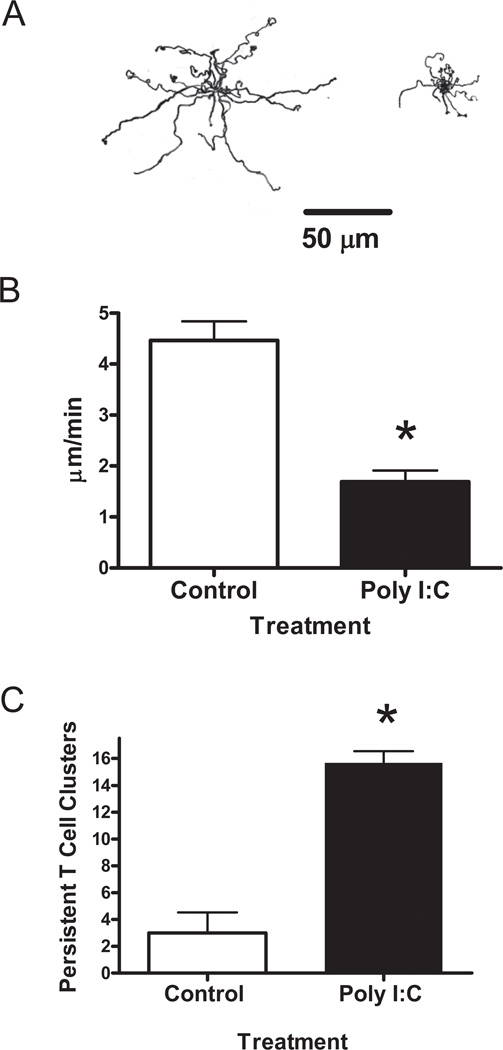
We performed additional time-lapse studies to assess T cell migration on monolayers of synoviocytes. (Supplemental videos 2 and 3 show T cells on Control and Poly I:C-treated synoviocytes, respectively). Given that hyaluronan tends to promote the migration of several cell types (Evanko et al., 2007), we also hypothesized that the interaction with the matrix would lead to faster migration of the T-cells. However, most of the T-cells remained clustered in the dense matrix of Poly I:C-treated synoviocytes and failed to disperse and migrate over the 20 minute period after encountering the matrix. Many of the T cells attempted to polarize by extending pseudopods in various directions, but appeared to be unable to gain traction or free themselves from the matrix. In contrast, the T-cells tended to crawl more directly on the surface of control synoviocytes, employing a cell scaffold–mediated migration strategy, and easily worked their way between and under the synoviocyte margins by amoeboid shape change. Tracings of the migratory paths of twenty representative T-cells on the ECM of control and Poly I:C treated synoviocytes are shown in Figure 4A. The rate of T-lymphocyte migration on the Poly I:C induced matrix (1.69 + 0.22 µm/min) was significantly decreased by about 55 % compared to the migration rate on control synoviocyte matrix (4.47 + 0.37µm/min) (Fig. 4B). Correspondingly, the number of persistent T-cell clusters was significantly increased on the Poly I:C-induced matrix (Fig. 4C).
Figure 4.
T-cell migration is impeded on matrix induced by Poly I:C. A. Tracings of the migratory paths of 20 randomly selected T-cells migrating on control (left) and Poly I:C-treated (right) synoviocytes for a twenty minute period. A bar indicating a distance of 50 µm is shown for reference. B. Migration rates were calculated using net distance of the migrating T-cells. C. Number of T-cell clusters that persisted for 10 minutes following warmup. *p < 0.001 compared to control.
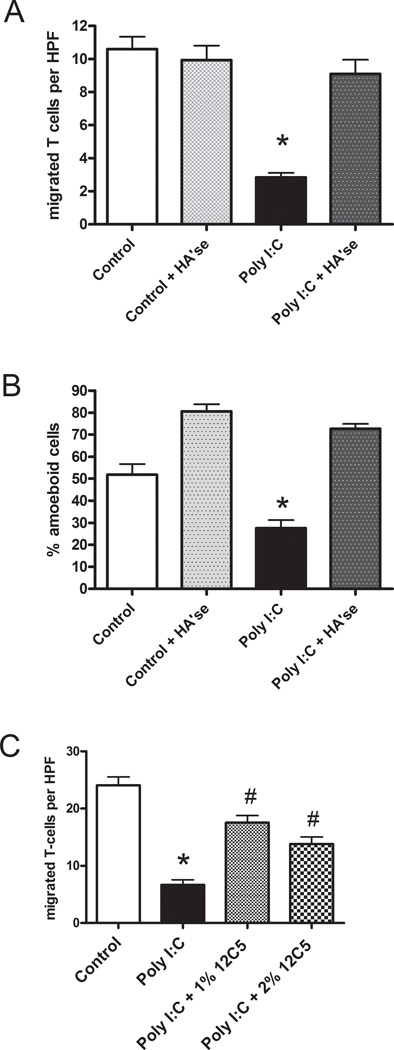
To assess migration along a vertical axis, the number of T-cells that passed through the synoviocyte monolayer to the level of the coverslip was measured after a twenty minute warmup period. Despite the increased binding potential of the matrix, the number of T-cells that migrated through the matrix and monolayer to the coverslip was significantly decreased in the Poly I:C-treated synoviocytes and this was reversed by hyaluronidase treatment (Fig. 5A). In addition, the proportion of the total bound T cells with the amoeboid morphology was also diminished in the Poly I:C treated cultures (Fig. 5B). Pre-treatment of the matrix with hyaluronidase before adding the T-cells promoted the amoeboid shape in the T-cells and abolished the inhibitory effect of the Poly I:C-induced matrix on T-cell penetration to the coverslip, indicating that hyaluronan integrity or its retention of versican may be crucial to T cell arrest and slower penetration of the fibroblast layer. Incubation of the synoviocytes with an antibody to the versican N-terminus (12C5) during the period of matrix formation also partially abolished the ability of the Poly I:C induced matrix to impede T-cell migration to the coverslip (Fig. 5C). This antibody had no effect on the amount of hyaluronan in the cell layer (data not shown). Although T-cell adhesion to the Poly I:C-induced matrix was increased, these results suggest that, rather than facilitating migration, the matrix may prevent polarization, promote rounding and constrain T-cell migration, and that versican may be one component that is partially responsible for this effect.
Figure 5.
Penetration of synoviocyte monolayers by T-cells is inhibited by hyaluronan and versican-rich matrix following Poly I:C stimulation. A, the number of T-cells per high power field (HPF) that migrated to the underside of the synoviocytes without or following digestion of the formed matrix with hyaluronidase. B, the percentage of the total bound T-cells with an amoeboid morphology (same experiment as in A). C, In a separate experiment, an antibody to versican was added at two different concentrations to the fibroblasts during the period of matrix formation before addition of the T cells. *p < 0.001 compared to control. # p <0.001 compared to Poly I:C.
3.4 Poly I:C drives myofibroblast morphology
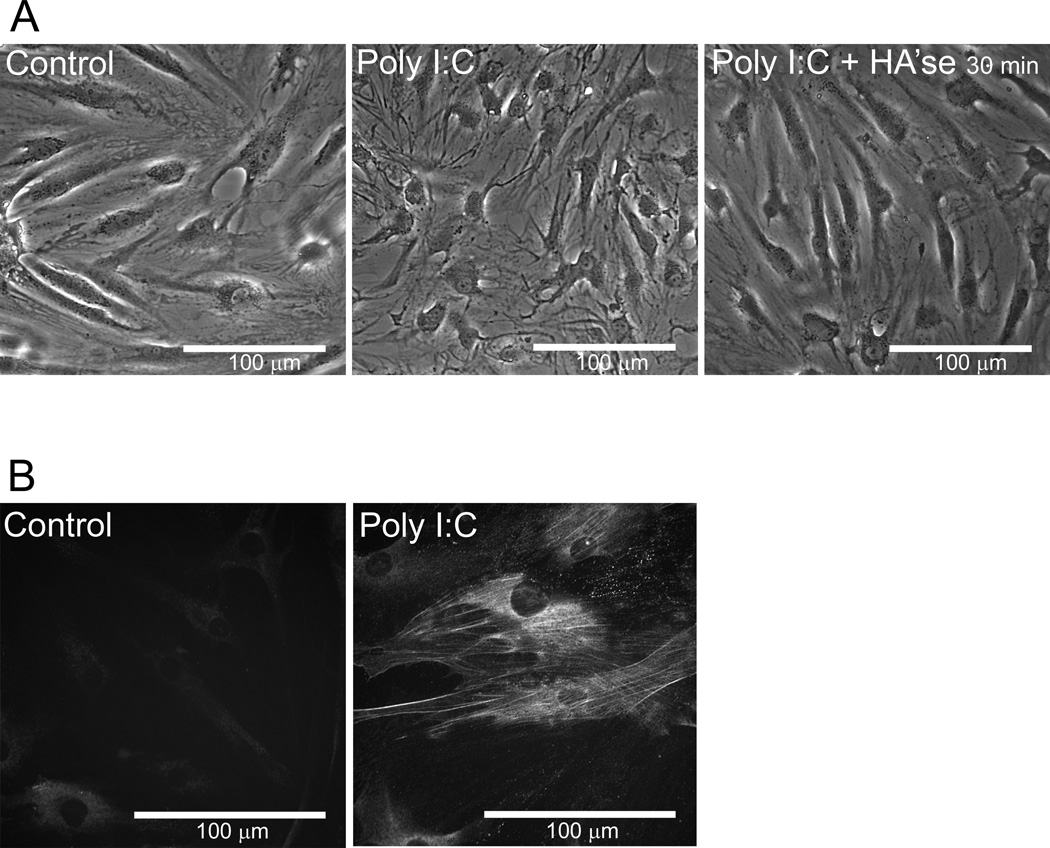
Treatment of fibroblast-like synoviocytes (Fig. 6) or lung fibroblasts (data not shown) with Poly I:C also caused a dramatic alteration in the morphology of these cells. The cells became much more spread and flattened, particularly around the nucleus (Fig. 6A). Digestion with Streptomyces hyaluronidase for 30 minutes caused a partial reversion of the synoviocytes back toward the control cell morphology. In addition, smooth muscle alpha actin was detected in Poly I:C treated synoviocytes but not in control cells (Fig. 6B). These data are consistent with previous studies showing increased stress fiber formation in lung fibroblasts (Evanko et al., 2009a), and augmented myofibroblast differentiation by activation of TLR3 with Poly I:C (Sugiura et al., 2009).
Figure 6.
Myofibroblast morphology and smooth muscle actin expression is induced by Poly I:C. A Phase contrast views of control (left panel) and poly I:C treated synoviocytes before and after digestion with Streptomyces hyaluronidase (middle and right panels) as indicated. B, Smooth muscle alpha actin staining in control and poly I:C treated cells. Bars in all images equal 100 µm.
3.5 Versican inhibits T-lymphocyte migration
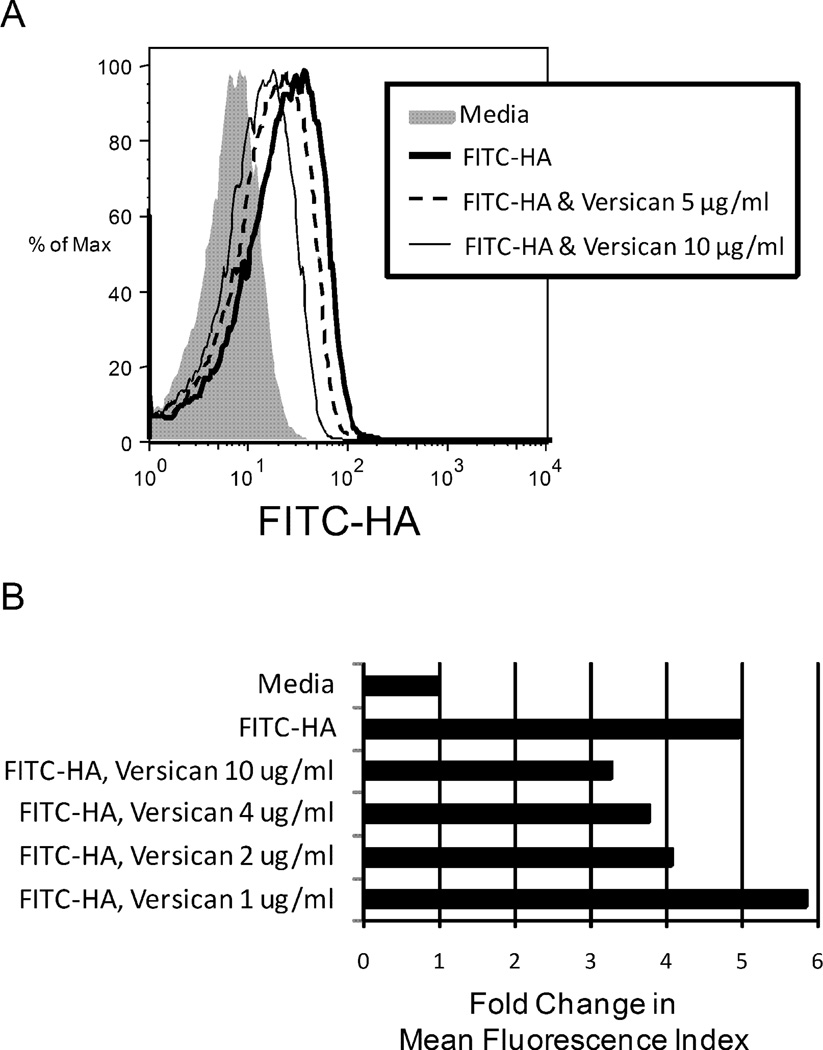
In order to simplify assessment of the role of versican and hyaluronan in T-cell function, we also studied the interaction of T-lymphocytes with purified matrix components. In solution binding experiments, versican partially inhibited the binding of fluorescein-labeled hyaluronan to activated CD4+ T-cells, as assessed by flow cytometry (Fig. 7A, B). The inhibition of hyaluronan binding to T-cells required preincubation of the versican with the hyaluronan, suggesting that it is the interaction of versican with hyaluronan that is important for the inhibition, rather than direct competition of versican with hyaluronan for CD44 or another receptor on the T-cells. We were unable to demonstrate binding of biotinylated versican directly to 4MU-treated T-cells (Supplemental Fig. 2) nor to untreated T-cells (data not shown). Consistent with earlier studies(Bollyky et al., 2007), the CD44 blocking antibody, BU75, only partially inhibited binding of FITC-hyaluronan to T-cells (Supplemental Fig. 2).
Figure 7.
Myofibroblast morphology and smooth muscle actin expression is induced by Poly I:C. A Phase contrast views of control (left panel) and poly I:C treated synoviocytes before and after digestion with Streptomyces hyaluronidase (middle and right panels) as indicated. B, Smooth muscle alpha actin staining in control and poly I:C treated cells. Bars in all images equal 100 µm.
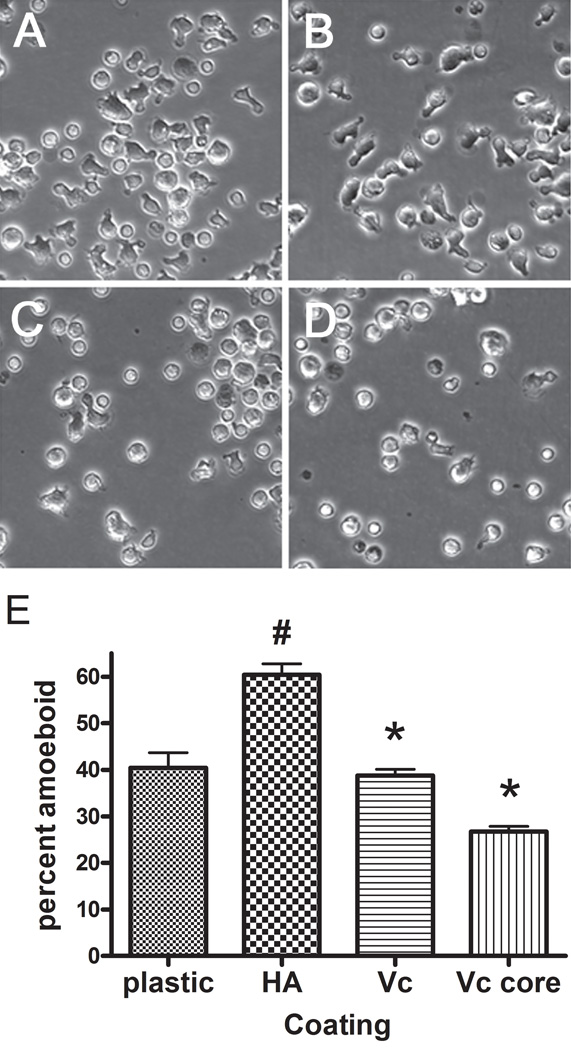
When T-cells were plated in dishes coated with hyaluronan alone, there was a significant increase in the proportion of cells with an amoeboid shape compared to tissue culture plastic (Fig. 8). Preincubation of the hyaluronan coated dishes with versican or versican core protein caused a significant reduction in the proportion of T-cells with the amoeboid shape compared to hyaluronan alone. These data indicate that versican may interrupt adhesion of T-cells to plate-bound hyaluronan, promote rounding, and potentially influence the migratory ability of the T cells.
Figure 8.
Versican blocks amoeboid shape change induced by hyaluronan. A–D, phase contrast images of T-cells plated on A, tissue culture plastic; B, hyaluronan coated surface, C, hyaluronan coated dish followed by versican or D, versican core protein. E, quantitation of the number of amoeboid cells. # p < 0.001 compared to plastic. * p < 0.001 compared to hyaluronan alone.
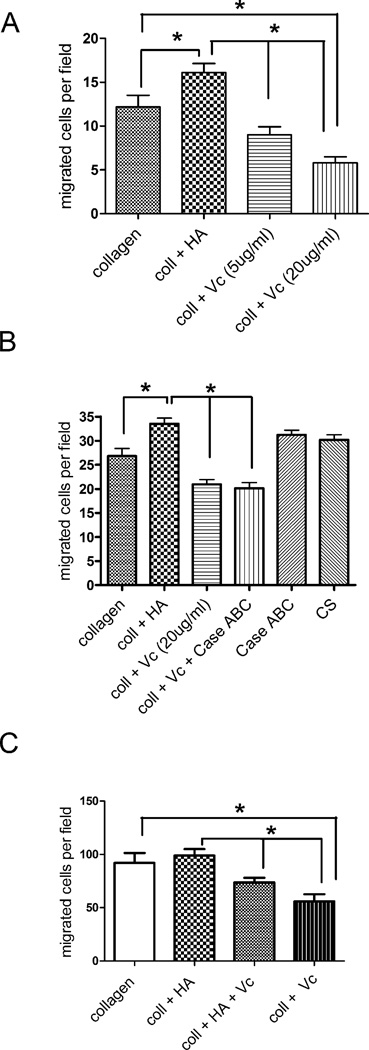
To assess the contribution of the matrix components on T cell migration in a three dimensional system, collagen gels were cast on coverslips without or with the inclusion of purified versican alone or in combination with hyaluronan, as well as the versican core protein and T-cell penetration into the gels over a three hour period was measured (Fig. 9). Hyaluronan alone significantly promoted random T-cell penetration into the collagen gels in 4 out of 7 experiments (two examples are given in Fig. 9 A, B). Versican and versican core protein significantly inhibited T-cell penetration into the collagen gels (Fig. 9B). Neither chondroitin sulfate nor the chondroitin ABC lyase enzyme used to generate the versican core protein had an effect on T-cell penetration into the collagen (Fig. 9B). Similar results were seen when versican or hyaluronan was added to the gels after the collagen had polymerized, suggesting that the diminished penetration by randomly migrating T-cells was not due to an effect of the additives on collagen polymerization or pore size (data not shown). When chemokines CCL19 and CCL 21 were included in the gel to increase T cell migration, the promotional effect of hyaluronan alone was lost, while versican alone, or added with hyaluronan, significantly reduced T cell penetration into the collagen (Fig. 9C).
Figure 9.
Versican inhibits T-cell penetration of collagen gels. A, T-cells were applied to the top of gels containing collagen alone or with added hyaluronan (50 µg/ml) or versican at 5 and 20 µg/ml hyaluronan (50 µg/ml), and the number of T-cells penetrating to a depth of 100 µm after 3 h were counted. B, Another experiment using hyaluronan, versican, versican core protein (Vc + Case ABC), chondroitin sulfate (30 µg/ml), or chondroitinase ABC (10 mU). C, A third experiment in which the chemokines CCL19 and CCL21 were added to the gel to promote T-cell migration. * p < 0.05.
4. Discussion
The present study examines the effects of T-lymphocytes encountering matrix that is rich in hyaluronan and the hyaluronan-binding proteoglycan, versican and derived from treatment of lung fibroblasts and synoviocytes with a viral mimetic that is known to promote myofibroblast formation and hyaluronan cable formation. Others have previously shown that monocytes adhere to hyaluronan and versican-rich cable structures induced by Poly I:C, and this adhesion is partly dependent on CD44 (de la Motte et al., 1999; Evanko et al., 2009b; Majors et al., 2003; Potter-Perigo et al., 2009; Selbi et al., 2006; Wang and Hascall, 2004). Our results extend these observations to activated CD4+ T-lymphocytes.
Unexpectedly, increased binding of T-lymphocytes to the hyaluronan and versican-rich cables made by lung fibroblasts in response to Poly I:C did not translate into directional migration along the cables. Instead, the matrix promoted clustering, rounding and arrest of the lymphocytes and thus may interfere with polarization. The matrix also constrained T-cell migration on the surface and to the underside of the synoviocyte monolayer. T-cell penetration of the monolayer and the number of T-cells with the amoeboid shape was increased after hyaluronidase digestion of the matrix, indicating that the structural integrity of hyaluronan-based matrix was vital, and that it was not due to an effect of any residual Poly I:C in the cultures. In addition to the reversion to normal fibroblast morphology (i.e., less spread) by hyaluronidase treatment reported here, hyaluronidase digestion has been shown to induce the retraction of fine microvillous protrusions that are involved in pericellular matrix formation in fibroblasts and other cells (Kultti et al., 2006) and this may also facilitate T-cells passing through the monolayer. These data also suggest that factors which impact local production of hyaluronidases may be important in the transition from T-cell adhesion to polarized migration.
Inflammation can result in a mucinous ECM/extracellular fluid environment where high viscosity, negative charge density and other attributes of the proteoglycans may contribute to alterations in leukocyte retention and rate of migration, and this ECM tends to precede fibrosis. Hyaluronan and versican have both been shown to modulate differentiation of myofibroblasts (Hattori et al., Epub 2011; Meran et al., 2007; Webber et al., 2009). During chronic inflammation in both lung and synovial tissues, fibrosis and accumulation of myofibroblasts tend to follow the accumulation of complex hyaluronan matrices (Day and de la Motte, 2005; Kasperkovitz et al., 2005; Westergren-Thorsson et al., 2010), and this can be repeated in multiple inflammatory events. Our data suggest that the milieu of the fibroblast or synoviocyte may modulate inflammatory cell migration and surveillance.
Previous studies have shown a selective association of myofibroblasts with high inflammation synovial tissues (Kasperkovitz et al., 2005), and fibrosis in lung tissue is common following chronic inflammation (Westergren-Thorsson et al., 2010). Our results are consistent with previous studies showing that Poly I:C augmented myofibroblast formation (Sugiura et al., 2009) as well as stress fibers and microvillous protrusions in HLF (Evanko et al., 2009a). Together these results imply that this ECM of myofibroblasts induced by a viral mimetic may inhibit the migration of lymphocytes, partly through a trapping effect. In a study of lymphocyte migration in infected brain, parasite specific CD8+ T cells migrated on a system of inflammation-associated reticular fibers of unknown composition visualized by second harmonics (Wilson et al., 2009). The lymphocytes exhibited various other behaviors such as clustering, or rapid migration followed by constraint and rounding, but it was not clear if hyaluronan and versican, known matrix components in brain (Dours-Zimmermann et al., 2009), may have influenced migratory behavior in these studies.
Lymphocyte penetration of the synoviocyte monolayer was increased in Poly I:C treated cultures when an antibody to an epitope in the N-terminal globular domain of versican (12C5) was added during the period of matrix formation, suggesting that versican’s interaction with hyaluronan may be important for the ability of the matrix to impede T-cell migration. This antibody was previously shown to inhibit monocyte binding to the matrix of lung fibroblasts(Potter-Perigo et al., 2009). However, the 12C5 antibody was unable to block the binding of biotinylated versican to hyaluronan coated dishes (data not shown), suggesting that it may work by other mechanisms, such as interfering with aggregate stability or versican turnover. More experiments will be required to understand how this antibody is working.
In addition, the fibroblast cell coat acted as a barrier to direct contact between T-cells and the fibroblasts, potentially impairing cell scaffold–mediated migration strategies. This, together with time-lapse video data, suggests that T-cell migration may be more efficient when direct contact is made between lymphocytes and stromal cells. The more flattened and adherent nature of myofibroblasts may also help to limit the potential of the T-cells to slip between cell margins. Overwhelming amounts of the matrix may prevent proper T cell polarization. Further study will be required to better understand the roles of hyaluronan, versican, and the participation of myofibroblasts in the inflammatory process.
Using purified matrix components, we found that versican (V0, V1) partially prevented the binding of soluble hyaluronan to T-lymphocytes. Versican added to plate bound hyaluronan also inhibited the amoeboid shape change induced by the hyaluronan coating alone. In a three dimensional system, we found that addition of versican or versican core protein alone, or in combination with hyaluronan, blocked T-cell migration into collagen gels. Earlier studies have found that versican can have both adhesive and antiadhesive functions (Ernst et al., 1995; Yamagata et al., 1993; Yamagata et al., 1989) and this depends in part on the geometry of the assay system. These differences may also reflect the presence of higher order hyaluronan structures, such as cables. Others have shown that versican V0, V1 and the core protein inhibited or guided migration of neural crest cells on fibronectin by interfering with substrate adhesion (Dutt et al., 2006). Addition of aggregating proteoglycans or their G1 domains to hyaluronan can affect length and conformation of hyaluronan (Morgelin et al., 1995) and material properties, such as viscosity(Mow et al., 1984). Excess amounts of hyaluronan binding domains of the proteoglycan can destabilize hyaluronan networks (Brewton and Mayne, 1992; Morgelin et al., 1995). Thus the effects of versican on the interactions of hyaluronan with the T cell surface may be quite complex. Previous studies have shown that versican V3 expression and CD44 silencing in melanoma cells blocked CD44-dependent hyaluronan internalization, leading to an accumulation of hyaluronan in the pericellular matrix and to changes in cell migration on hyaluronan (Hernandez et al., 2010). Our results suggest there may be a role for versican V0, V1 in modulating effects of hyaluronan on T cell adhesion and migration.
The physiologic relevance of these findings extends beyond T-cell trafficking. Hyaluronan can play concomitant pro-tolerogenic or pro-inflammatory roles depending on its size and amount (Day and de la Motte, 2005; Noble, 2002). Several studies have looked at the influence of hyaluronan on leukocyte function. For example, we have previously reported that high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+regulatory T cells in part through production of the immunosuppressive cytokine IL-10 (Bollyky et al., 2009a; Bollyky et al., 2009b; Bollyky et al., 2007). In subsequent work we have found that HMW-HA also promotes IL-10 production by conventional T-cells (manuscript submitted). The data presented here indicate that the presence of versican may be one way in which the capacity of hyaluronan to affect T cell biology is regulated.
Collectively, these results suggest that versican and hyaluronan in complex extracellular matrices synthesized by myofibroblasts may influence the rate of lymphocyte trafficking to and from inflamed tissues.
Highlights.
Poly I:C-induced hyaluronan and versican rich matrix promoted T-cell adhesion but impeded T-cell spreading and migration on and through synoviocyte monolayers.
Hyaluronidase treatment or adding versican antibody during matrix formation reversed the effect on T-cell migration (and reversed myofibroblast morphology).
The viscous hyaluronan- and versican-rich matrix binds and constrains T lymphocytes.
Versican prevented T-cell binding to soluble hyaluronan, as well as the amoeboid shape change on hyaluronan coated dishes and T-cell penetration of collagen gels.
Hyaluronan and versican play a role in T-cell trafficking and function in inflamed tissues.
Supplementary Material
Supplemental Figure 1. Versican preparation binds to biotinylated hyaluronan. Versican (10 µg/lane) was electrophoresed on 4–12 % gradient SDS PAGE under non-reducing (lanes 1 and 2) and reducing (lane 3) conditions, transferred to nitrocellulose and incubated with biotinylated hyaluronan. In lane 2, the strip was preincubated with 500 µg/ml unlabeled hyaluronan to demonstrate specificity. Detection was done with streptavidin-peroxidase and enhanced chemiluminescence as described in the methods section. The position of V0 and V1 bands are indicated.
Supplemental Figure 2. A, An anti-CD44 antibody blocks FITC-HA binding to T-cells. Human CD4+ T-cells were activated for 48 hours and then treated with hyaluronidase for 30’. After washing, either PBS, an irrelevant rat anti-mouse IgG control antibody, or BU75 (an anti-CD44 Ab we and others have previously demonstrated blocks the binding of HA to CD44 (Sugiyama et al., 1999; Bollyky, et al., 2007) was added for 30’. Following another wash, cells were resuspended in serum free media to which FITC-HA (Sigma) or an equivalent volume of PBS was added and incubated at 37 degrees for 60 minutes. Cells were then directly analyzed on a flow cytometer. B, Biotinylated Versican (bVersican) binding to activated human CD4+ T-cells. Human CD4+ T-cells were activated for 48 hours. Cells were treated either with 4-MU and HA’ase or with PBS and then washed prior to addition of bVersican. Incubation was at 37 degrees for 30 minutes. Cells were again washed and streptavidin (SA) was added for an additional 15 minute incubation. Cells were again washed and run on a flow cytometer.
Acknowledgements
We would like to thank Dr. Inkyung Kang for helpful discussions, Rebecca Wu for technical assistance, and Dr. Virginia M. Green for manuscript preparation. This work was supported by National Institutes of Health grants DK046635 (to GTN); DK080178 and DK089128 (to PLB); and HL018645 and a BIRT supplement to AR037296 (to TNW). This work was also supported by grants from the Juvenile Diabetes Research Foundation (nPOD 25-2010-648 (to TNW)), and The Center for Translational Research at BRI (to GTN).
The abbreviations used are
- ECM
extracellular matrix
- poly I:C
polyinosinic-polycytidylic acid
- HMWHA
high molecular weight hyaluronan
- b-HABP
biotinylated hyaluronan binding protein
- nTreg
natural T-reg
- PBMC
peripheral blood mononuclear cell
- HLFs
human lung fibroblasts
- HFLS
human fibroblast-like synoviocytes
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bollyky PL, Falk BA, Long SA, Preisinger A, Braun KR, Wu RP, Evanko SP, Buckner JH, Wight TN, Nepom GT. CD44 costimulation promotes FoxP3+ regulatory T cell persistence and function via production of IL-2, IL-10, and TGF-beta. J Immunol. 2009a;183:2232–2241. doi: 10.4049/jimmunol.0900191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollyky PL, Falk BA, Wu RP, Buckner JH, Wight TN, Nepom GT. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells. J Leukoc Biol. 2009b;86:567–572. doi: 10.1189/jlb.0109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollyky PL, Lord JD, Masewicz SA, Evanko SP, Buckner JH, Wight TN, Nepom GT. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:744–747. doi: 10.4049/jimmunol.179.2.744. [DOI] [PubMed] [Google Scholar]
- Brewton RG, Mayne R. Mammalian vitreous humor contains networks of hyaluronan molecules: electron microscopic analysis using the hyaluronan-binding region (G1) of aggrecan and link protein. Exp Cell Res. 1992;198:237–249. doi: 10.1016/0014-4827(92)90376-j. [DOI] [PubMed] [Google Scholar]
- Day AJ, de la Motte CA. Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 2005;26:637–643. doi: 10.1016/j.it.2005.09.009. [DOI] [PubMed] [Google Scholar]
- de la Motte CA, Hascall VC, Calabro A, Yen-Lieberman B, Strong SA. Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly(I.C) J Biol Chem. 1999;274:30747–30755. doi: 10.1074/jbc.274.43.30747. [DOI] [PubMed] [Google Scholar]
- Denucci CC, Mitchell JS, Shimizu Y. Integrin function in T-cell homing to lymphoid and nonlymphoid sites: getting there and staying there. Crit Rev Immunol. 2009;29:87–109. doi: 10.1615/critrevimmunol.v29.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dours-Zimmermann MT, Maurer K, Rauch U, Stoffel W, Fassler R, Zimmermann DR. Versican V2 assembles the extracellular matrix surrounding the nodes of ranvier in the CNS. J Neurosci. 2009;29:7731–7742. doi: 10.1523/JNEUROSCI.4158-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt S, Kleber M, Matasci M, Sommer L, Zimmermann DR. Versican V0 and V1 guide migratory neural crest cells. J Biol Chem. 2006;281:12123–12131. doi: 10.1074/jbc.M510834200. [DOI] [PubMed] [Google Scholar]
- Ernst H, Zanin MKB, Everman D, Hoffman S. Receptor-mediated adhesive and anti-adhesive functions of chondroitin sulfate proteoglycan preparations from embryonic chicken brain. J Cell Sci. 1995;108:3807–3816. doi: 10.1242/jcs.108.12.3807. [DOI] [PubMed] [Google Scholar]
- Evanko SP, Angello JC, Wight TN. Formation of hyaluronan and versican rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler.Thromb Vasc Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- Evanko SP, Potter-Perigo S, Johnson PY, Wight TN. Organization of hyaluronan and versican in the extracellular matrix of human fibroblasts treated with the viral mimetic poly I:C. J Histochem Cytochem. 2009a;57:1041–1060. doi: 10.1369/jhc.2009.953802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanko SP, Potter-Perigo S, Johnson PY, Wight TN. Organization of Hyaluronan and Versican in the Extracellular Matrix of Human Fibroblasts Treated With the Viral Mimetic, poly I:C. J Histochem Cytochem. 2009b doi: 10.1369/jhc.2009.953802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanko SP, Tammi MI, Tammi RH, Wight TN. Hyaluronan-dependent pericellular matrix. Adv Drug Deliv Rev. 2007;59:1351–1365. doi: 10.1016/j.addr.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- Hattori N, Carrino DA, Lauer ME, Vasanji A, Wylie JD, Nelson CM, Apte SS. Pericellular versican regulates the fibroblast-myofibroblast transition. A role for ADAMTS5-mediated proteolysis. J Biol Chem. 2011 doi: 10.1074/jbc.M111.254938. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez D, Miquel-Serra L, Docampo MJ, Marco-Ramell A, Cabrera J, Fabra A, Bassols A. V3 versican isoform alters the behavior of human melanoma cells by interfering with CD44/ErbB-dependent signaling. J Biol Chem. 2010;286:1475–1485. doi: 10.1074/jbc.M110.127522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare K, Savani RC, Wang C, Yang B, Turley EA. Identification of hyaluronan binding proteins using a biotinylated hyaluronan probe. Connect Tissue Res. 1993;30:117–126. doi: 10.3109/03008209309041327. [DOI] [PubMed] [Google Scholar]
- Johnson P, Ruffell B. CD44 and its role in inflammation and inflammatory diseases. Inflamm Allergy Drug Targets. 2009;8:208–220. doi: 10.2174/187152809788680994. [DOI] [PubMed] [Google Scholar]
- Kasperkovitz PV, Timmer TC, Smeets TJ, Verbeet NL, Tak PP, van Baarsen LG, Baltus B, Huizinga TW, Pieterman E, Fero M, Firestein GS, van der Pouw Kraan TC, Verweij CL. Fibroblast-like synoviocytes derived from patients with rheumatoid arthritis show the imprint of synovial tissue heterogeneity: evidence of a link between an increased myofibroblast-like phenotype and high-inflammation synovitis. Arthritis Rheum. 2005;52:430–441. doi: 10.1002/art.20811. [DOI] [PubMed] [Google Scholar]
- Korpos E, Wu C, Song J, Hallmann R, Sorokin L. Role of the extracellular matrix in lymphocyte migration. Cell Tissue Res. 2009;339:47–57. doi: 10.1007/s00441-009-0853-3. [DOI] [PubMed] [Google Scholar]
- Kultti A, Rilla K, Tiihonen R, Spicer AP, Tammi RH, Tammi MI. Hyaluronan synthesis induces microvillus-like cell surface protrusions. J Biol Chem. 2006;281:15821–15828. doi: 10.1074/jbc.M512840200. [DOI] [PubMed] [Google Scholar]
- Landolt RM, Vaughan L, Winterhalter KH, Zimmermann DR. Versican is selectively expressed in embryonic tissues that act as barriers to neural crest cell migration and axon outgrowth. Development. 1995;121:2303–2312. doi: 10.1242/dev.121.8.2303. [DOI] [PubMed] [Google Scholar]
- Lesley J, Howes N, Perschl A, Hyman R. Hyaluronan binding function of CD44 is transiently activated on T cells during an in vivo immune response. J Exp Med. 1994;180:383–387. doi: 10.1084/jem.180.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors AK, Austin RC, de la Motte CA, Pyeritz RE, Hascall VC, Kessler SP, Sen G, Strong SA. Endoplasmic reticulum stress induces hyaluronan deposition and leukocyte adhesion. J Biol Chem. 2003;278:47223–47231. doi: 10.1074/jbc.M304871200. [DOI] [PubMed] [Google Scholar]
- Meran S, Thomas D, Stephens P, Martin J, Bowen T, Phillips A, Steadman R. Involvement of hyaluronan in regulation of fibroblast phenotype. J Biol Chem. 2007;282:25687–25697. doi: 10.1074/jbc.M700773200. [DOI] [PubMed] [Google Scholar]
- Morgelin M, Paulsson M, Heinegard D, Aebi U, Engel J. Evidence of a defined spatial arrangement of hyaluronate in the central filament of cartilage proteoglycan aggregates. Biochem J. 1995;307:595–601. doi: 10.1042/bj3070595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mow VC, Mak AF, Lai WM, Rosenberg LC, Tang LH. Viscoelastic properties of proteoglycan subunits and aggregates in varying solution concentrations. J Biomech. 1984;17:325–338. doi: 10.1016/0021-9290(84)90027-7. [DOI] [PubMed] [Google Scholar]
- Mrass P, Kinjyo I, Ng LG, Reiner SL, Pure E, Weninger W. CD44 mediates successful interstitial navigation by killer T cells and enables efficient antitumor immunity. Immunity. 2008;29:971–985. doi: 10.1016/j.immuni.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21:25–29. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- Olin KL, Potter-Perigo S, Barrett PH, Wight TN, Chait A. Lipoprotein lipase enhances the binding of native and oxidized low density lipoproteins to versican and biglycan synthesized by cultured arterial smooth muscle cells. J Biol Chem. 1999;274:34629–34636. doi: 10.1074/jbc.274.49.34629. [DOI] [PubMed] [Google Scholar]
- Olin KL, Potter-Perigo S, Barrett PH, Wight TN, Chait A. Biglycan, a vascular proteoglycan, binds differently to HDL2 and HDL3: role of apoE. Arterioscler Thromb Vasc Biol. 2001;21:129–135. doi: 10.1161/01.atv.21.1.129. [DOI] [PubMed] [Google Scholar]
- Potter-Perigo S, Johnson PY, Evanko SP, Chan CK, Braun KR, Wilkinson TS, Altman LC, Wight TN. Poly I:C Stimulates Versican Accumulation in the Extracellular Matrix Promoting Monocyte Adhesion. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2009-0081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Johnson P. Hyaluronan induces cell death in activated T cells through CD44. J Immunol. 2008;181:7044–7054. doi: 10.4049/jimmunol.181.10.7044. [DOI] [PubMed] [Google Scholar]
- Selbi W, de la Motte CA, Hascall VC, Day AJ, Bowen T, Phillips AO. Characterization of hyaluronan cable structure and function in renal proximal tubular epithelial cells. Kidney Int. 2006;70:1287–1295. doi: 10.1038/sj.ki.5001760. [DOI] [PubMed] [Google Scholar]
- Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10:712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- Sugiura H, Ichikawa T, Koarai A, Yanagisawa S, Minakata Y, Matsunaga K, Hirano T, Akamatsu K, Ichinose M. Activation of Toll-like receptor 3 augments myofibroblast differentiation. Am J Respir Cell Mol Biol. 2009;40:654–662. doi: 10.1165/rcmb.2008-0371OC. [DOI] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- Underhill CB, Nguyen H, Shizari M, Culty M. CD44 positive macrophages take up hyaluronan during lung development. Dev Biol. 1993;155:324–336. doi: 10.1006/dbio.1993.1032. [DOI] [PubMed] [Google Scholar]
- Wang A, Hascall VC. Hyaluronan structures synthesized by rat mesangial cells in response to hyperglycemia induce monocyte adhesion. J Biol Chem. 2004;279:10279–10285. doi: 10.1074/jbc.M312045200. [DOI] [PubMed] [Google Scholar]
- Webber J, Jenkins RH, Meran S, Phillips A, Steadman R. Modulation of TGFbeta1-dependent myofibroblast differentiation by hyaluronan. Am J Pathol. 2009;175:148–160. doi: 10.2353/ajpath.2009.080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergren-Thorsson G, Larsen K, Nihlberg K, Andersson-Sjoland A, Hallgren O, Marko-Varga G, Bjermer L. Pathological airway remodelling in inflammation. Clin Respir J. 2010;4 Suppl 1:1–8. doi: 10.1111/j.1752-699X.2010.00190.x. [DOI] [PubMed] [Google Scholar]
- Wilson EH, Harris TH, Mrass P, John B, Tait ED, Wu GF, Pepper M, Wherry EJ, Dzierzinski F, Roos D, Haydon PG, Laufer TM, Weninger W, Hunter CA. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity. 2009;30:300–311. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Muller R, Borgmann S, Brocker EB, Friedl P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood. 2003;102:3262–3269. doi: 10.1182/blood-2002-12-3791. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Kimata K. Repression of a malignant cell-substratum adhesion phenotype by inhibiting the production of the anti-adhesive proteoglycan, PG-M/versican. J Cell Sci. 1994;107:2581–2590. doi: 10.1242/jcs.107.9.2581. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Saga S, Kato M, Bernfield M, Kimata K. Selective distributions of proteoglycans and their ligands in pericellular matrix of cultured fibroblasts. Implications for their roles in cell-substratum adhesion. J Cell Sci. 1993;106:55–65. doi: 10.1242/jcs.106.1.55. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Suzuki S, Akiyama S, Yamada K, Kimata K. Regulation of cell-substrate adhesion by proteoglycans immobilized on extracellular substrates. J Biol Chem. 1989;264:8012–8018. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Versican preparation binds to biotinylated hyaluronan. Versican (10 µg/lane) was electrophoresed on 4–12 % gradient SDS PAGE under non-reducing (lanes 1 and 2) and reducing (lane 3) conditions, transferred to nitrocellulose and incubated with biotinylated hyaluronan. In lane 2, the strip was preincubated with 500 µg/ml unlabeled hyaluronan to demonstrate specificity. Detection was done with streptavidin-peroxidase and enhanced chemiluminescence as described in the methods section. The position of V0 and V1 bands are indicated.
Supplemental Figure 2. A, An anti-CD44 antibody blocks FITC-HA binding to T-cells. Human CD4+ T-cells were activated for 48 hours and then treated with hyaluronidase for 30’. After washing, either PBS, an irrelevant rat anti-mouse IgG control antibody, or BU75 (an anti-CD44 Ab we and others have previously demonstrated blocks the binding of HA to CD44 (Sugiyama et al., 1999; Bollyky, et al., 2007) was added for 30’. Following another wash, cells were resuspended in serum free media to which FITC-HA (Sigma) or an equivalent volume of PBS was added and incubated at 37 degrees for 60 minutes. Cells were then directly analyzed on a flow cytometer. B, Biotinylated Versican (bVersican) binding to activated human CD4+ T-cells. Human CD4+ T-cells were activated for 48 hours. Cells were treated either with 4-MU and HA’ase or with PBS and then washed prior to addition of bVersican. Incubation was at 37 degrees for 30 minutes. Cells were again washed and streptavidin (SA) was added for an additional 15 minute incubation. Cells were again washed and run on a flow cytometer.