Abstract
BACKGROUND
Thiazides and β-blockers cause adverse metabolic effects (AMEs), but whether these effects share predictors with blood pressure (BP) response is unknown. We aimed to determine whether AMEs are correlated with BP response in uncomplicated hypertensives.
METHODS
In a multicenter, open-label, parallel-group trial, we enrolled 569 persons, aged 17–65, with random assignment to 9 weeks of daily hydrochlorothiazide (HCTZ) or atenolol monotherapy, followed by 9 weeks of add-on therapy with the alternate agent. Measurements included home BP, averaged over 1 week, weight and fasting levels of serum glucose, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides, and uric acid (UA) before and after monotherapy and after add-on therapy.
RESULTS
Increases in UA correlated with reductions in systolic BP (SBP) (r = −0.18; P = 0.003) and diastolic BP (DBP) (r = −0.20; P = 0.001) following HCTZ monotherapy and add-on therapy (r = −0.27 and r = −0.21, respectively; both P < 0.001). After adjustment for age, race, gender, and baseline body mass index (BMI), only the correlation between UA and DBP response became nonsignificant. Reductions in HDL correlated with systolic response following atenolol monotherapy (r = 0.18; P = 0.002) and with systolic and diastolic response following add-on therapy (r = 0.30 and r = 0.24, respectively; both P < 0.0001). These correlations remained significant after covariate adjustment. BP responses were not correlated with changes in glucose, LDL, triglycerides, or weight following either therapy.
CONCLUSIONS
BP response correlated with changes in UA following HCTZ therapy and HDL following atenolol therapy. No other significant correlations were observed between BP response and AMEs, suggesting that these effects generally do not share predictors. Patients should be monitored for AMEs, regardless of BP response.
Keywords: thiazide diuretics, atenolol, β-blockers, blood pressure, hydrochlorothiazide, hypertension, metabolic effects
Hypertension is estimated to affect 1 billion of the world’s adult population and generally requires life-long treatment with one or more classes of antihypertensive therapy.1 Two antihypertensive classes, thiazide diuretics and β-blockers, are recommended as first-line therapies by current United States guidelines and are frequently used as initial therapy in newly diagnosed patients or as part of combination antihypertensive therapy.2 However, derangements in multiple metabolic parameters are common and well-known adverse effects of these antihypertensive classes. Both thiazide diuretics and β-blockers have been associated with increased glucose levels and an increased risk of diabetes.3–6 Additionally, adverse lipid effects, including increased low-density lipoprotein (LDL) and triglycerides, and decreased high-density lipoprotein (HDL) have been observed following thiazide diuretic and β-blockers therapy.7 Such effects may mitigate some of the cardiovascular benefits afforded by the blood pressure (BP)-lowering effects of these drugs. Finally, elevated serum uric acid (UA) during treatment with thiazide diuretics may increase the risk of developing gout.
Little is known of the mechanisms behind these common adverse effects, despite being extensively studied.8 Furthermore, relatively few variables are shown to consistently predict the occurrence or magnitude of these drug-induced adverse metabolic effects (AMEs).2,3 However, an important clinical question is whether those individuals with the greatest BP response to these agents are also the most likely to develop drug-induced AMEs during therapy. If these effects are correlated, AMEs may share predictors of BP response.
We aimed to determine whether BP response was associated with adverse effects on serum glucose, UA, LDL, HDL, triglycerides, and weight in a population of patients treated with hydrochlorothiazide (HCTZ) or atenolol.
METHODS
Design
The Pharmacogenomic Evaluation of Antihypertensive Response (PEAR) study (NIH U0I GM074492; ClinicalTrials.gov #NCT00246519) is a prospective, multicenter, randomized, open-label, parallel-group study with a primary focus of identifying genetic determinants of BP and adverse metabolic responses to a thiazide diuretic and β-blockers. Details of the PEAR trial design and purpose have been published previously.9 Participants included in this analysis were enrolled in PEAR from November 2005 through November 2009. PEAR is a multisite project that enrolled participants from the University of Florida (Gainesville, FL), Emory University (Atlanta, GA) and the Mayo Clinic (Rochester, MN). The study was conducted in accordance with the provisions of the Declaration of Helsinki. All participants provided voluntary, written informed consent and the institutional review boards of the participating study centers approved the study protocol.
Study population
Males or females with mild-to-moderate essential hypertension, of any race or ethnicity, between the ages of 17 and 65 were eligible for participation. Study participants were those with newly diagnosed hypertension, untreated hypertension or known hypertension previously treated with fewer than three antihypertensive drugs. Patients were excluded from participation if they had any secondary form of hypertension, a clinic systolic BP (SBP) >170 mm Hg during treatment with an antihypertensive, isolated systolic hypertension, other diseases requiring treatment with BP-lowering medications, a heart rate <55 beats/min, known cardiovascular disease, diabetes mellitus (type 1 or 2), renal insufficiency (defined as serum creatinine >1.5 mg/dl in males and 1.4 mg/dl in females), pregnancy or lactation, a history of Raynaud’s syndrome, chronic treatment with drugs known to elevate BP (nonsteroidal anti-inflammatory drugs, oral contraceptives), active alcoholism, or elevated liver enzymes.
Randomization and interventions
Participants with no exclusion criteria were further screened for inclusion based on untreated (for 3–6 weeks) home BP (average over 1 week seated diastolic BP (DBP) >85 mm Hg and ≤110 mm Hg and seated SBP <180 mm Hg) and clinic BP (seated DBP >90 mm Hg and ≤110 mm Hg and seated SBP <180 mm Hg). No lower cutoff was defined for home or office seated SBP. After this screening and before initiation of study medications, baseline studies included collection of home BP data, along with fasting blood, serum and urine samples. Following baseline studies, participants were randomly assigned to receive HCTZ 12.5 mg daily, titrated to 25 mg daily after 3 weeks if BP remained elevated (defined as SBP >120 or DBP >70 mm Hg) or atenolol 50 mg, titrated to 100 mg daily after 3 weeks if BP remained elevated. After 9 weeks, participants entered an add-on phase in which they received the alternate agent (i.e., those initially assigned HCTZ then received HCTZ-atenolol combination therapy and vice-versa) if their BP remained elevated. Following both the monotherapy (~9 weeks from initiation of the first study medication) and add-on phase (~18 weeks from initiation of first study medication), subjects were reassessed for home BP response and fasting blood sample collection was repeated.
Follow-up
Home BP was assessed daily using a Microlife model 3AC1-PC monitor (Microlife, Minneapolis, MN), measured in triplicate, then averaged, morning and evening over a 1-week period immediately preceding the 9-week visit. BP data were stored electronically and downloaded at prescribed clinic visits. At least five morning and five evening determinations were required during the 1-week period for inclusion in the analysis.
Fasting serum levels of glucose, lipids (LDL, HDL, and triglycerides) and UA were determined using an Hitachi 911 Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN). All laboratory parameters were determined at a central laboratory at the Mayo Clinic.
Statistical analysis
Descriptive statistics were used to represent demographic information. Mean ± s.d. of BP measurements were determined at the end of the monotherapy and add-on phases and the average change in BP was calculated within each treatment group. For all analyses, changes were coded as post-treatment minus baseline, such that a reduction in BP was coded as a negative value and an increase in laboratory parameters was coded as a positive value. We compared changes from baseline to on-treatment for BP, weight and laboratory parameters within each treatment arm using paired t-tests. For nonparametric data, we used the Wilcoxon signed-rank test.
We performed two identical primary analyses, one in each treatment group, focused on the correlation of treatment-related SBP and DBP reductions with adverse metabolic responses, using Pearson’s correlation coefficients. For all analyses, we estimated the partial correlations among these parameters with covariate adjustments for age, race, gender, and body mass index (BMI). As secondary analyses, we determined correlation coefficients stratified by race (blacks and whites only), gender and the presence/absence of abdominal obesity (defined as waist circumference >35 in females or > 40 in males). Fisher’s z transformation was used to test for differences in correlation coefficients between subgroups. Covariate adjustment for baseline serum potassium levels or the change in serum potassium levels was not performed in the final analyses because a previous analysis revealed no relationship between potassium and the aforementioned metabolic variables, including glucose.10
Finally, we performed replication analyses using data from the add-on phase to determine whether any significant correlations in the monotherapy analyses were also found when these drugs were used as add-on therapy. For example, for the replication analyses in HCTZ-treated patients, we determined correlation coefficients between the changes in SBP/DBP and metabolic parameters from the end of atenolol monotherapy to the end of the HCTZ add-on phase. Similar analyses were repeated for patients receiving atenolol as add-on therapy.
We defined statistical significance a priori as a P value <0.0042 to account for multiple comparisons. Based on the total sample size available for analysis and assuming a two-sided test using Fisher’s z transformation and an α-level of 0.05, we had ≥95% power to detect significant correlation coefficients of at least 0.10. All statistical analyses were performed with SAS 9.2 or 9.3 (SAS Institute, Cary, NC) or R 2.12.0 (R Development Core Team, http://www.r-project.org) statistical software.
RESULTS
Complete data were available for 286 subjects in the atenolol treatment group and 283 subjects in the HCTZ treatment group. Baseline demographics for both treatment groups are presented in Table 1. Overall, the mean age of study subjects was 49.1 years with a mean BMI of 30.7 kg/m2. Approximately 54% of study subjects were female and the majority of subjects self-identified as white (57.8%) or black (37.9%). Of those treated with atenolol, 251 (87.8%) were titrated to the maximum dose of 100 mg once daily, and 280 (98.9%) of those treated with HCTZ were titrated to the maximum dose of 25 mg once daily.
Table 1.
Baseline demographics according to treatment group
| Characteristic | Hydrochlorothiazide (n = 283) |
Atenolol (n = 286) |
|---|---|---|
| Age, years, mean(range) | 49.4 (21–65) | 48.8 (18–65) |
| Females, N (%) | 142 (50.2) | 166 (58.0) |
| Race, N (%) | ||
| Caucasian | 164 (57.9) | 165 (57.7) |
| Black | 108 (38.2) | 108 (37.8) |
| Asian | 2 (0.7) | 4 (1.4) |
| Other | 9 (3.2) | 9 (3.2) |
| Systolic BP, mm Hg, mean ± s.d. | 147 ± 11 | 145 ± 9.6 |
| Diastolic BP, mm Hg, mean ± s.d. | 94 ± 5.9 | 93 ± 5.8 |
| Serum glucose, mg/dl, mean ± s.d. | 91.3 ± 10.3 | 90.3 ± 9.6 |
| Triglyceride, mg/dl, mean ± s.d. | 126.7 ± 93.1 | 120.6 ± 80.4 |
| HDL, mg/dl, mean ± s.d. | 49.0 ± 13.8 | 50.3 ± 14.6 |
| LDL, mg/dl, mean ± s.d. | 120.0 ± 30.7 | 121.6 ± 29.9 |
| Serum uric acid, mg/dl, mean ± s.d. | 5.7 ± 1.6 | 5.4 ± 1.4 |
| BMI, kg/m2, mean(range) ± s.d. | 30.7 (19.3–48.1) ± 5.1 | 30.7 (18.4–60.4) ± 6.0 |
| Waist circumference, cm, mean ± s.d. | 97.7 ± 13.4 | 97.3 ± 12.8 |
| Metabolic syndrome, N (%) | 123 (43.6) | 115 (40.6) |
BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P values are not presented since each treatment group was analyzed separately and no comparisons were performed between treatments with respect to blood pressure or metabolic effects.
Following 9 weeks of HCTZ monotherapy, SBP and DBP decreased by a mean of 9 and 5 mm Hg, respectively, whereas all other reported values, except HDL and weight, increased significantly (Table 2). After 9 weeks of atenolol monotherapy, SBP and DBP each decreased by a mean of 8 mm Hg. Likewise, HDL decreased significantly following 9 weeks of monotherapy, whereas serum glucose, UA, triglycerides, and weight increased significantly. LDL levels in atenolol-treated subjects were reduced slightly, but this change was not statistically significant.
Table 2.
Mean changes in study parameters after treatment with HCTZ and atenolol as monotherapy or add-on therapy
| Parameter | HCTZ | Atenolol | ||||||
|---|---|---|---|---|---|---|---|---|
| As monotherapy | As add-on therapy | As monotherapy | As add-on therapy | |||||
| Mean change ± s.d. |
P valuea | Mean change ± s.d. |
P valueb | Mean change ± s.d. |
P valuea | Mean change ± s.d. |
P valueb | |
| Systolic BP, mm Hg | −9.2 ± 9.2 | <0.0001 | −8.6 ± 10.1 | <0.0001 | −7.7 ± 10.6 | <0.0001 | −9.3 ± 8.0 | <0.0001 |
| Diastolic BP, mm Hg | −5.4 ± 6.0 | <0.0001 | −4.5 ± 6.5 | <0.0001 | −7.7 ± 7.0 | <0.0001 | −8.2 ± 6.1 | <0.0001 |
| Serum glucose, mg/dl | 3.5 ± 10.5 | <0.0001 | 2.4 ± 10.2 | <0.0001 | 2.6 ± 9.7 | <0.0001 | 1.4 ± 10.7 | 0.30 |
| Triglyceride, mg/dl | 10.7 ± 64.4 | 0.005 | 18.1 ± 76.9 | <0.0001 | 20.4 ± 66.3 | <0.0001 | 23.8 ± 87.6 | <0.0001 |
| HDL, mg/dl | −0.4 ± 5.9 | 0.22 | 2.2 ± 20.9 | 0.07 | −2.8 ± 6.7 | <0.0001 | −5.0 ± 18.9 | <0.0001 |
| LDL, mg/dl | 5.2 ± 20.7 | <0.0001 | −0.3 ± 7.1 | 0.46 | −2.1 ± 18.9 | 0.06 | −2.4 ± 6.4 | <0.0001 |
| Serum uric acid, mg/dl | 1.0 ± 0.9 | <0.0001 | 0.9 ± 0.9 | <0.0001 | 0.3 ± 0.2 | <0.0001 | 0.2 ± 0.9 | 0.007 |
| Weight, kg | −0.39 ± 7.5 | 0.46 | 0.09 ± 2.2 | 0.58 | 0.94 ± 2.5 | <0.0001 | 0.61 ± 1.8 | <0.0001 |
BP, blood pressure; HCTZ, hydrochlorothiazide; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P values represent the comparison between baseline (off all antihypertensive therapy) and the end of the monotherapy phase.
P values represent the comparison between the end of monotherapy phase and the end of the add-on therapy phase.
Response correlations in the HCTZ treatment arms
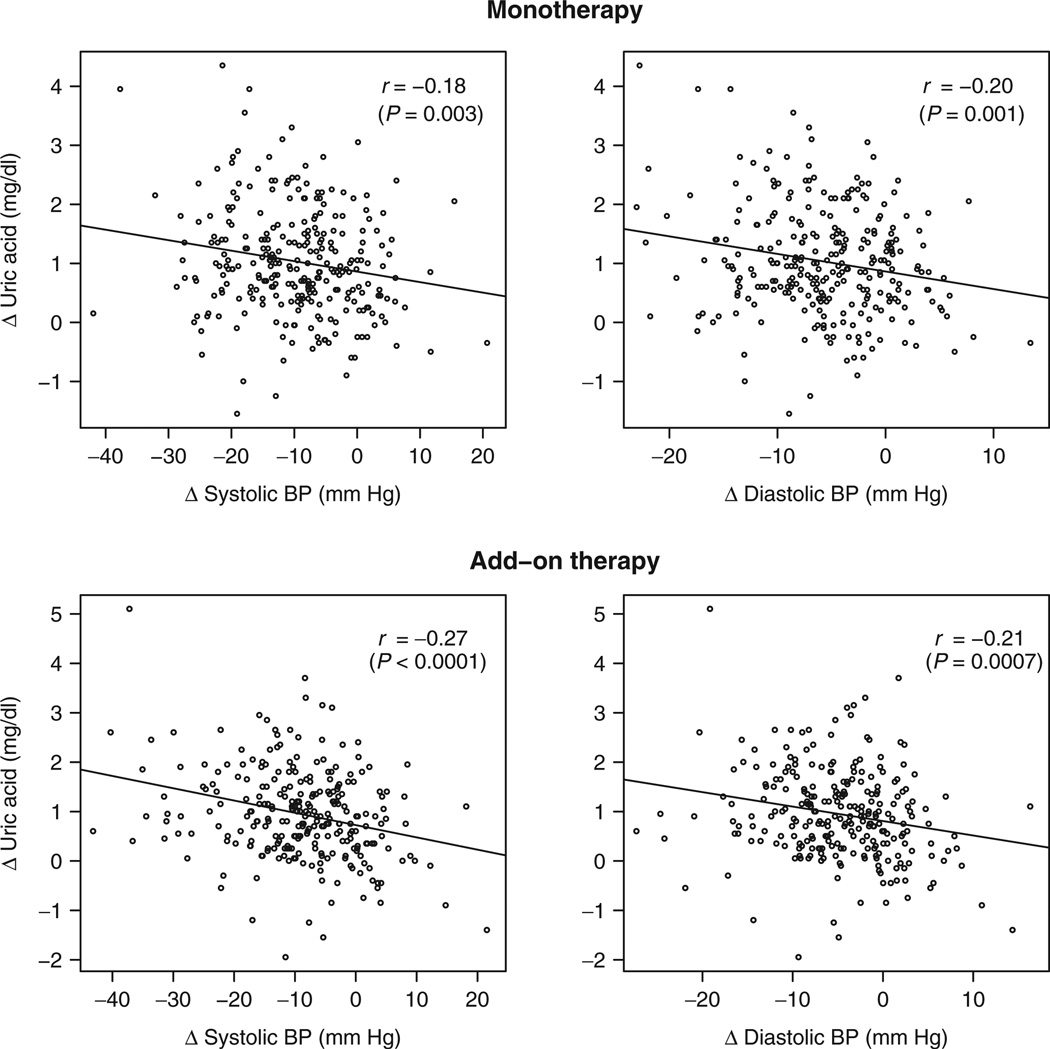
In the unadjusted analysis of subjects treated with HCTZ as monotherapy, greater elevations in serum UA levels were significantly correlated with greater reductions in SBP (r = −0.18; P = 0.003) and DBP (r = −0.20; P = 0.001) (Figure 1). These correlations remained significant after adjusting for age, race, gender, and baseline BMI (Table 3). No other adverse metabolic responses were correlated with either BP response following HCTZ monotherapy. In stratified analyses of the correlations between UA and SBP or DBP, correlation coefficients did not differ significantly between race, gender, or the presence/absence of abdominal obesity at baseline (data not shown).
Figure 1.
Relationship between change in serum uric acid level and change in systolic and diastolic blood pressure (BP) following hydrochlorothiazide (HCTZ) monotherapy (top panels) and HCTZ add-on therapy (bottom panels). r values and corresponding P values represent unadjusted Pearson correlation coefficients; adjusted correlation coefficients and P values are denoted in Table 3. Δ represents change in each parameter, calculated as on-treatment value minus baseline value.
Table 3.
Correlation analyses during 9 weeks of monotherapy and 9 weeks of add-on therapy with HCTZ and atenolol
| Treatment | Treatment phase | Parameter | Glucose | LDL | Triglycerides | HDL | Uric acid | Weight |
|---|---|---|---|---|---|---|---|---|
| HCTZ | Monotherapy | Systolic BP | −0.12 (0.046) | −0.04 (0.56) | −0.14 (0.02) | 0.12 (0.045) | −0.18a (0.003) | 0.01 (0.84) |
| Diastolic BP | −0.07 (0.27) | −0.02 (0.72) | −0.06 (0.30) | 0.07 (0.24) | −0.18a (0.003) | −0.001 (0.99) | ||
| Add-on | Systolic BP | −0.05 (0.39) | 0.04 (0.55) | −0.13 (0.044) | 0.18a (0.003) | −0.23a (0.0002) | 0.06 (0.43) | |
| Diastolic BP | −0.05 (0.43) | 0.02 (0.70) | −0.08 (0.19) | 0.20a (0.001) | −0.15 (0.02) | 0.06 (0.47) | ||
| Atenolol | Monotherapy | Systolic BP | 0.06 (0.29) | 0.13 (0.03) | −0.03 (0.62) | 0.22a (0.0002) | −0.01 (0.86) | 0.02 (0.80) |
| Diastolic BP | 0.07 (0.22) | 0.07 (0.27) | −0.01 (0.93) | 0.18a (0.002) | 0.03 (0.62) | −0.01 (0.88) | ||
| Add−on | Systolic BP | 0.03 (0.58) | 0.11 (0.09) | −0.11 (0.08) | 0.29a (<0.0001) | −0.13 (0.03) | −0.02 (0.75) | |
| Diastolic BP | 0.02 (0.70) | 0.14 (0.02) | −0.08 (0.20) | 0.22a (0.0003) | −0.05 (0.39) | −0.06 (0.41) |
Data are depicted as Pearson correlation coefficients, r, with P values in parentheses, for the analyses between change in BP parameters and adverse metabolic effects. All correlation coefficients are adjusted for race, baseline body mass index, gender, and age.
BP, blood pressure; HCTZ, hydrochlorothiazide; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Statistically significant (P < 0.0042 to adjust for multiple comparisons) correlation coefficients.
Similar results were obtained in the unadjusted analysis using data from participants receiving HCTZ as add-on therapy. However, elevations in serum UA levels were more strongly correlated with reductions in SBP (r = −0.27; P < 0.0001) and DBP (r = −0.21; P = 0.0007) with HCTZ add-on therapy compared with HCTZ monotherapy. After controlling for age, race, gender, and baseline BMI, only the relationship between UA and SBP remained significant (Table 3).
Response correlations in the atenolol treatment arms
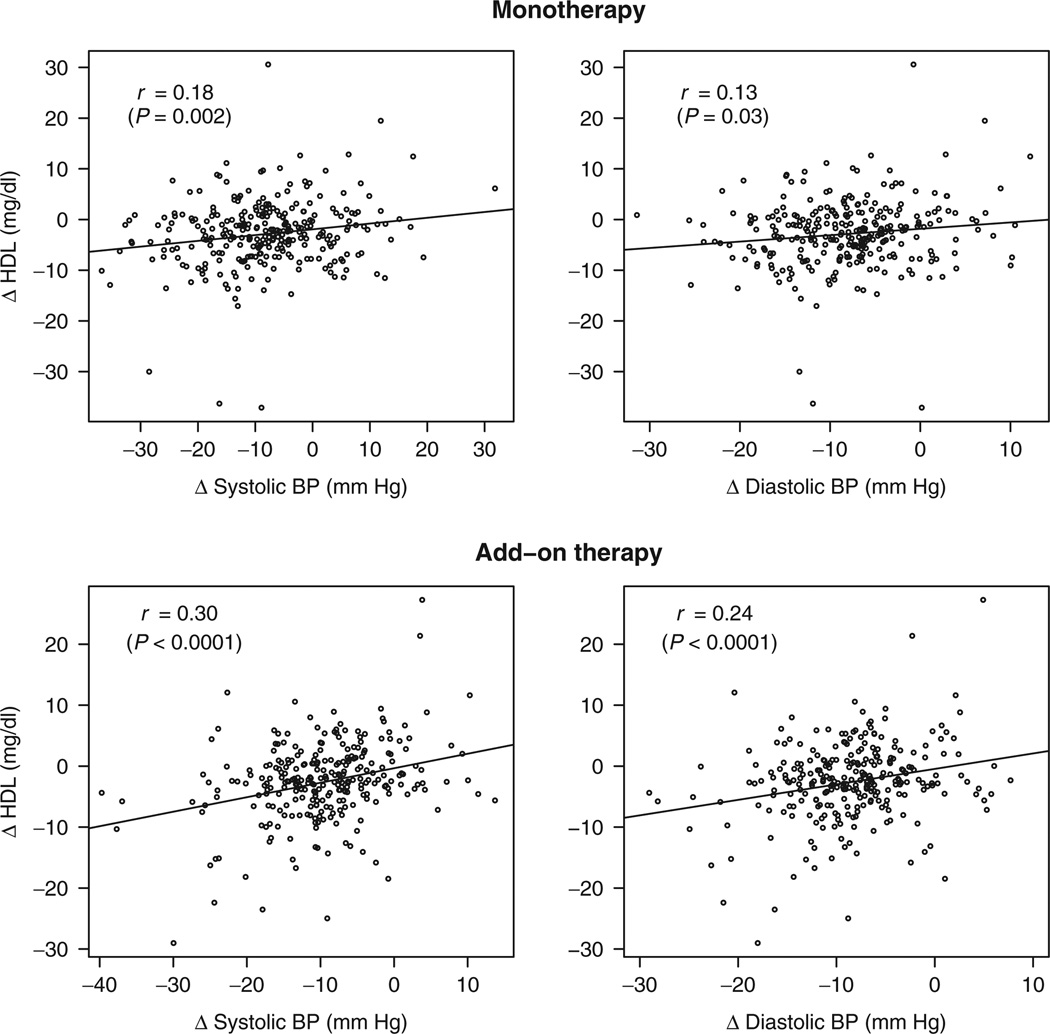
In the unadjusted analysis of subjects receiving atenolol monotherapy, a greater reduction in HDL was significantly correlated with a greater SBP response (r = 0.18; P = 0.002), but not DBP response (r = 0.13; P = 0.03) (Figure 2). After controlling for age, race, gender, and BMI, a greater SBP response (r = 0.22; P = 0.0002) and a greater DBP response (r = 0.18; P = 0.002) were significantly correlated with greater reductions in HDL (Table 3). In stratified analyses, these correlations did not differ significantly between races, genders, or the presence/absence of abdominal obesity at baseline (data not shown). Stronger correlations (r = 0.29; P < 0.0001 for SBP response, and r = 0.22; P = 0.0003 for DBP response) were observed between each of these parameters and HDL during treatment with atenolol as add-on therapy (Table 3). We also observed significant correlations between reductions in HDL and SBP response (r = 0.18; P = 0.003) and DBP response (r = 0.20; P = 0.001) during atenolol + HCTZ add-on therapy. A sensitivity analysis excluding subjects who started a statin during the trial (n = 3 per treatment group) did not appreciably alter any of the aforementioned results (data not shown). Otherwise, no significant correlations were found between BP response and changes in reported parameters following atenolol treatment as monotherapy or add-on therapy.
Figure 2.
Relationship between change in serum high-density lipoprotein (HDL) and systolic and diastolic blood pressure (BP) following atenolol monotherapy (top panels) and atenolol add-on therapy (bottom panels). r values represent unadjusted Pearson correlation coefficients; adjusted correlation coefficients and P values are denoted in Table 3. Δ represents change in each parameter, calculated as on-treatment value minus baseline value.
DISCUSSION
Following 9 weeks of monotherapy with HCTZ, SBP and DBP were reduced significantly, whereas serum glucose, UA, LDL, and triglycerides levels increased. In the HCTZ monotherapy and add-on groups, reductions in SBP and DBP were correlated with changes in serum UA. These significant correlation coefficients did not differ significantly among subgroups in analyses stratified by race, gender, and the presence/absence of abdominal obesity. A significant correlation between changes in serum UA and BP response in HCTZ-treated patients may be attributable to thiazide-induced volume depletion which contributes significantly to initial BP response and increases serum UA by increasing net reabsorption of urate at the renal tubule, either through enhanced reabsorption or reduced secretion.11 This effect on urate is mitigated in diuretic-treated patients receiving volume replacement.12 Consequently, the shared predictor between BP response and changes in serum UA may be the degree of volume depletion incurred following HCTZ treatment. In support of this hypothesis, a follow-up analysis found that treatment-induced changes in plasma renin activity were correlated with changes in serum UA during HCTZ monotherapy (r = 0.26; P < 0.0001), HCTZ add-on therapy (r = 0.31; P < 0.0001), and during atenolol add-on therapy (e.g., HCTZ + atenolol therapy; r = 0.22; P = 0.0002), but not during atenolol monotherapy (r = 0.09; P = 0.12). These data support the hypothesis that volume depletion may be a shared predictor during HCTZ therapy because as plasma renin activity increases (and, ostensibly, volume decreases), serum UA also increases.
Atenolol was associated with significant reductions in SBP, DBP, and HDL and a significant increase in serum glucose, UA, and triglyceride levels. Following atenolol therapy, whether as monotherapy or in combination with HCTZ, reductions in HDL were correlated with reductions in SBP and DBP, with the strongest correlations observed following add-on atenolol therapy to HCTZ. A reduction in HDL levels following β-blockers therapy has been consistently demonstrated, particularly with atenolol.13–16 However, the mechanism behind this AME has not been fully elucidated. Given that the correlations were replicated in both the atenolol monotherapy and add-on therapy groups as well as the HCTZ add-on group (e.g., HCTZ added on to atenolol therapy), this consistent finding suggests that BP response and adverse effects on HDL in atenolol-treated patients may share common predictors. As with the significant correlations observed between UA and BP response during HCTZ treatment, the correlation coefficients in these analyses were relatively low, suggesting that only a small portion of the variation in changes in HDL levels is related to BP response following atenolol treatment.
We found no evidence of a relationship between BP response and changes in glucose, LDL, triglycerides, or weight during treatment with either drug. These findings suggest that treatment-related BP response does not share similar predictors with these AMEs during HCTZ or atenolol therapy. These findings are important since we found no evidence that persons most likely to have a greater BP response to thiazide or β-blocker therapy will likewise experience the greatest adverse effects on these metabolic parameters. From a clinical standpoint, measurement of BP response during therapy is unlikely to provide additional insight into potential effects on glucose, LDL, triglycerides, and weight. Consequently, monitoring for these AMEs during therapy is essential, regardless of BP response to these drugs. The present findings also highlight the need for future research to identify determinants of these antihypertensive-associated AMEs that, according to our results, should differ substantially from determinants of BP response to these agents. These determinants, whether clinical or genetic, will allow clinicians to identify persons most likely to benefit from the BP-lowering effects of these drugs while minimizing most AMEs.
Three limitations of this analysis are noteworthy. First, the maximum allowed dose of HCTZ was 25 mg/day. Higher doses may cause greater AMEs and greater BP response. However, this dose reflects current prescribing patterns and current guideline recommendations. Whether higher doses alter the relationship (or lack of relationship) between treatment-induced BP response and AMEs is unknown. Second, we did not objectively measure volume depletion during diuretic therapy and thus were unable to fully answer whether volume depletion may be link between treatment-induced changes in UA and BP response. Finally, this analysis included a low number of participants that were not white or black which precluded conducting correlation analyses in other racial subgroups.
In conclusion, we found that BP response and increases in serum UA were correlated during HCTZ therapy, while BP response and reductions in HDL were correlated during atenolol therapy. While the effects of these drugs on UA and HDL has long been recognized, our study is the first to our knowledge to describe a relationship between BP response and these metabolic parameters. Additionally, we found no significant evidence that other AMEs were correlated with BP response during therapy with either HCTZ or atenolol. Excepting UA and HDL, BP response and AMEs are unlikely to share significant predictors and thus clinicians should remain vigilant in assessing potential antihypertensive-induced AMEs regardless of a patient’s BP response to these medications. Future research, including the forthcoming results from the PEAR study, should help to identify genetic predictors of antihypertensive-induced AMEs. Combined with clinical and laboratory predictors, these findings will be useful in maximizing the benefit-to-risk ratio for patients with indications for thiazide or β-blocker therapy.
Acknowledgments
We acknowledge and thank the valuable contributions of the study participants, support staff, and study physicians: Drs George Baramidze, Carmen Bray, R. Whit Curry, Karen Hall, Frederic Rabari-Oskoui, Dan Rubin, and Seigfried Schmidt. This work is supported by a grant from the National Institutes of Health (Bethesda, MD), grant # U01 GM074492, funded as part of the Pharmacogenetics Research Network. Additional support for this work includes: K23 grants HL091120 (A.L.B.) and HL086558 (R.M.C.-D); CTSA grants UL1-RR029890 (University of Florida), UL1-RR025008 (Emory University), and UL1-RR024150 (Mayo Clinic); and funds from the Mayo Foundation. The authors are solely responsible for the design and conduct of the study, all study analyses, the drafting and editing of the manuscript, its final contents, and the decision to submit for publication.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Barzilay JI, Davis BR, Cutler JA, Pressel SL, Whelton PK, Basile J, Margolis KL, Ong ST, Sadler LS, Summerson J ALLHAT Collaborative Research Group. Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Arch Intern Med. 2006;166:2191–2201. doi: 10.1001/archinte.166.20.2191. [DOI] [PubMed] [Google Scholar]
- 4.Sowers JR, Bakris GL. Antihypertensive therapy and the risk of type 2 diabetes mellitus. N Engl J Med. 2000;342:969–970. doi: 10.1056/NEJM200003303421310. [DOI] [PubMed] [Google Scholar]
- 5.Cooper-DeHoff RM, Pacanowski MA, Pepine CJ. Cardiovascular therapies and associated glucose homeostasis: implications across the dysglycemia continuum. J Am Coll Cardiol. 2009;53:S28–S34. doi: 10.1016/j.jacc.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zillich AJ, Garg J, Basu S, Bakris GL, Carter BL. Thiazide diuretics, potassium, and the development of diabetes: a quantitative review. Hypertension. 2006;48:219–224. doi: 10.1161/01.HYP.0000231552.10054.aa. [DOI] [PubMed] [Google Scholar]
- 7.Lithell HO. Effect of antihypertensive drugs on insulin, glucose, and lipid metabolism. Diabetes Care. 1991;14:203–209. doi: 10.2337/diacare.14.3.203. [DOI] [PubMed] [Google Scholar]
- 8.Carter BL, Einhorn PT, Brands M, He J, Cutler JA, Whelton PK, Bakris GL, Brancati FL, Cushman WC, Oparil S, Wright JT, Jr Working Group from the National Heart, Lung, and Blood Institute. Thiazide-induced dysglycemia: call for research from a working group from the national heart, lung, and blood institute. Hypertension. 2008;52:30–36. doi: 10.1161/HYPERTENSIONAHA.108.114389. [DOI] [PubMed] [Google Scholar]
- 9.Johnson JA, Boerwinkle E, Zineh I, Chapman AB, Bailey K, Cooper-DeHoff RM, Gums J, Curry RW, Gong Y, Beitelshees AL, Schwartz G, Turner ST. Pharmacogenomics of antihypertensive drugs: rationale and design of the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) study. Am Heart J. 2009;157:442–449. doi: 10.1016/j.ahj.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith SM, Anderson SD, Wen S, Gong Y, Turner ST, Cooper-Dehoff RM, Schwartz GL, Bailey K, Chapman A, Hall KL, Feng H, Boerwinkle E, Johnson JA, Gums JG. Lack of correlation between thiazide-induced hyperglycemia and hypokalemia: subgroup analysis of results from the pharmacogenomic evaluation of antihypertensive responses (PEAR) study. Pharmacotherapy. 2009;29:1157–1165. doi: 10.1592/phco.29.10.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn AM. Effect of diuretics on the renal handling of urate. Semin Nephrol. 1988;8:305–314. [PubMed] [Google Scholar]
- 12.Steele TH, Oppenheimer S. Factors affecting urate excretion following diuretic administration in man. Am J Med. 1969;47:564–574. doi: 10.1016/0002-9343(69)90187-9. [DOI] [PubMed] [Google Scholar]
- 13.Fogari R, Zoppi A, Corradi L, Preti P, Mugellini A, Lusardi P. â-blocker effects on plasma lipids during prolonged treatment of hypertensive patients with hypercholesterolemia. J Cardiovasc Pharmacol. 1999;33:534–539. doi: 10.1097/00005344-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Bell DS, Bakris GL, McGill JB. Comparison of carvedilol and metoprolol on serum lipid concentration in diabetic hypertensive patients. Diabetes Obes Metab. 2009;11:234–238. doi: 10.1111/j.1463-1326.2008.00927.x. [DOI] [PubMed] [Google Scholar]
- 15.Giugliano D, Acampora R, Marfella R, De Rosa N, Ziccardi P, Ragone R, De Angelis L, D’Onofrio F. Metabolic and cardiovascular effects of carvedilol and atenolol in non-insulin-dependent diabetes mellitus and hypertension. A randomized, controlled trial. Ann Intern Med. 1997;126:955–959. doi: 10.7326/0003-4819-126-12-199706150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Maitland-van der Zee AH, Klungel OH, Kloosterman JM, Seidell JC, Leufkens HG, de Boer A. The association between antihypertensive drug therapies and plasma lipid levels in the general population. J Hum Hypertens. 2001;15:701–705. doi: 10.1038/sj.jhh.1001197. [DOI] [PubMed] [Google Scholar]