Abstract
N-methyl-D-aspartate receptors (NMDARs) are key components of neural signaling, playing roles in synaptic transmission and in the synaptic plasticity thought to underlie learning and memory. NMDAR activation can also have neurotoxic consequences contributing to several forms of neurodegeneration. Additionally, NMDARs can modulate neuronal function and regulate the ability of synapses to undergo synaptic plasticity. Evidence gathered over the past 20 years strongly supports the idea that untimely activation of NMDARs impairs the induction of long-term potentiation (LTP) by a form of metaplasticity. This metaplasticity can be triggered by multiple stimuli including physiological receptor activation, and metabolic and behavioral stressors. These latter findings raise the possibility that NMDARs contribute to cognitive dysfunction associated with neuropsychiatric disorders. This paper examines NMDAR metaplasticity and its potential role in cognition. Recent studies using NMDAR antagonists for therapeutic purposes also raise the possibility that metaplasticity may contribute to clinical effects of certain drugs.
Keywords: Synaptic plasticity, metaplasticity, delirium, dementia, hippocampus, ketamine, neurosteroids
1. Introduction
N-methyl-D-aspartate receptors (NMDARs) play key roles in brain function. On the one hand these receptors are critical for glutamate-mediated excitatory signaling, participating in synaptic transmission and triggering the synaptic plasticity that is thought to underlie learning and memory. If left unchecked, however, NMDARs can destroy neurons and initiate several forms of neuronal death (Watkins, 2000; Cull-Candy et al., 2001). In tribute to the dual actions of glutamate as excitatory transmitter and harbinger of neuronal death, Olney (1969) coined the term “excitotoxicity.” To keep these dual effects in check, NMDARs are highly regulated by a host of mechanisms, including the actions of ions such as magnesium, zinc, protons and calcium, and amino acids including glutamate, aspartate, glycine and D-serine among others (Aarts and Tymianski, 2004; Dingledine et al., 1999). More elaborate NMDAR regulation includes receptor phosphorylation, intramembranous receptor movement (Tovar and Westbrook, 1999) and intracellular receptor trafficking (Wenthold et al., 2003).
In addition to being highly regulated because of their toxic potential, NMDARs are highly regulated because of the unique roles that they play in brain function. Along with the AMPA class of glutamate receptors (AMPARs), NMDARs contribute to basal excitatory synaptic transmission, serving as cogs in fast information processing. The real power of NMDARs, however, lies in their contribution to synaptic plasticity. Here, NMDAR activation provides intracellular calcium signals that initiate several forms of synaptic plasticity including long-term potentiation (LTP) and long-term depression (LTD) (Malenka and Bear, 2004). LTP and LTD are leading mechanisms thought to underlie the synaptic changes associated with learning (Kemp and Manahan-Vaughan, 2007; Martin et al., 2000). In particular, LTP and LTD are “Hebbian” forms of plasticity, reflecting types of synaptic change originally postulated by Donald Hebb as being crucial for memory formation and in which coincident neuronal activity is a major determinant. Hebbian plasticity is the basis for the adage that “neurons that fire together wire together” and has been the subject of intense investigation since its initial conceptualization (Malenka and Bear, 2004; Martin et al., 2000).
Beyond excitotoxicity and synaptic plasticity, NMDARs are known to play even more complex roles in neural function. For example, in addition to driving the homosynaptic LTD (Dudek and Bear, 1992) that may contribute to certain types of learning (Kemp and Manahan-Vaughan, 2007), NMDAR activation can result in a form of synaptic resetting, referred to as LTP depotentiation (LTP-D) (Fujii et al., 1991). While LTD and LTP-D share some mechanisms, other evidence suggests that they are distinct processes (Zhu et al., 2005; McCormack et al., 2006). That is, changes in certain messengers such as protein kinase Mζ may contribute to depotentiation and not to LTD (Sacktor and Fenton, 2011) while the reverse may be true of phosphatidylinositol 3-kinaseγ (PI3Kγ) (Kim et al., 2011). Different mitogen activated protein kinases (MAPKs) also appear to be involved in LTD and LTP-D (Zhu et al., 2005), and there are forms of heterosynaptic stimulation that depotentiate Schaffer collateral synapses in the hippocampus without evoking LTD under baseline conditions (Izumi and Zorumski, 2008). To make matters more complex, there are also forms of NMDAR activation that do not produce either excitotoxicity or Hebbian plasticity, yet modulate neural function. Under some conditions, NMDAR activation regulates the ability of subsequent stimulation to induce either LTP or LTD. This latter form of modulation is referred to broadly as “metaplasticity,” a term originated by Abraham and Bear (1996) to describe the “plasticity of synaptic plasticity,” reflecting the concept that a neuron’s history influences its ability to undergo subsequent synaptic change.
While there are multiple forms of metaplasticity that include various glutamate receptors and other transmitter systems (Abraham, 2008; Abraham and Tate, 1997), we will focus on a specific type of NMDAR-dependent modulation. Under the conditions described, untimely NMDAR activation does not induce long-term changes in basal synaptic transmission or neuronal injury, but markedly impairs LTP induction. Importantly, under these conditions NMDAR antagonists have the ability to promote LTP, a form of plasticity dependent upon activation of these very receptors. The studies described have identified cellular and molecular events involved in metaplasticity and have explored conditions in which this mechanism may contribute to synaptic and cognitive dysfunction in neuropsychiatric disorders. Our focus will be on studies at Schaffer collateral synapses in the hippocampal CA1 region, an area that is critical for memory formation and that is involved in the pathophysiology of major psychiatric disorders (Tamminga et al., 2010; MacQueen and Frodl, 2011). Many mechanistic studies have been done in hippocampal slice preparations, but we will also highlight extensions of the work to living animals, stress, behavior and illnesses.
2. NMDARs and Synaptic Function
NMDARs are ionotropic receptors in which the binding of glutamate gates the opening of an intrinsic ion channel. Functional NMDARs contain four subunits of several types (NR1, NR2 and NR3, or GluN1, GluN2 and GluN3) (Cull-Candy et al., 2001; Dingledine et al., 1999; Paoletti, 2011). These subunits have similar overall structure including a large amino (N) terminal region that extends into the extracellular space followed by three membrane spanning regions with a re-entrant sequence between the first and second transmembrane regions (called a p-loop) that does not completely traverse the cell membrane but helps to form the ion channel (Mayer and Armstrong, 2004; Paoletti, 2011). NMDARs have an intracellular carboxy (C) terminus that varies among subtypes and is important for intracellular regulation and interactions with other proteins. There are eight splice variants of NR1 and this subunit contains an extracellular binding site for glycine, a necessary co-factor for receptor activation and ion channel gating. D-serine is also an endogenous ligand for the glycine regulatory site. There are four subtypes of NR2 subunits (NR2A, NR2B, NR2C and NR2D) and these contain glutamate binding domains in their N-termini. There are two NR3 subtypes that are expressed at highest levels during development and appear to negatively modulate channel function. There are also developmental changes in the expression of NR2A and NR2B subunits; NR2B predominates early in development and NR2A increases with maturation, although both are expressed into adulthood.
Most native NMDARs express NR1 with NR2 subunits with NR1/NR2A and NR1/NR2B being common receptors in the mammalian forebrain (Cull-Candy and Leszkiewicz, 2004; Tovar and Westbrook, 1999). Increasing evidence suggests that some, perhaps even the majority of synaptic receptors, are heterotrimers expressing NR1, NR2A and NR2B (Luo et al., 1997; Gray et al., 2011). Importantly, NMDARs are components of large protein complexes in which the receptor itself interacts with over 100 other proteins to accomplish intracellular and intercellular signaling (Nourry et al., 2003; Pocklington et al., 2006). While the functioning of this diverse protein network is only partially understood, it is clear that NMDARs trigger multiple intracellular responses that can affect short- and long-term information processing, including gene expression. This large protein network is a target for gene mutations and polymorphisms contributing to neuropsychiatric disorders including mental retardation, autism, schizophrenia, mood disorders and epilepsy among others (Bayes et al., 2011).
In addition to providing mechanisms for fast interneuronal (synaptic) communication, NMDARs are linchpins of synaptic plasticity. One of the important correlates of Hebbian plasticity is that long-term changes in function are triggered by coincident activity in presynaptic and postsynaptic neurons – it is this dynamic interaction and its timing that is critical for Hebbian change. This implies that neurons have mechanisms to detect activity occurring simultaneously in presynaptic and postsynaptic loci. NMDARs serve this function by requiring two things to allow effective channel opening. First, glutamate must bind the receptor (reflecting transmitter release from active presynaptic terminals). Second, the postsynaptic neuron must be simultaneously depolarized (activated). Neither action alone is sufficient to drive NMDAR channel gating. Glutamate release alone is insufficient to open NMDAR channels because the ion channels are blocked under physiological conditions by extracellular magnesium (Dingledine et al., 1999). Magnesium block is voltage dependent and relieved by depolarization (stimulation) of the neuronal membrane housing the receptors. At the resting membrane potential where neurons are largely inactive (about −70 mV), magnesium effectively blocks NMDAR channels. When neurons are depolarized, magnesium exits the channel and ions can flow through NMDARs to influence the receiving neuron. Thus, NMDARs monitor both presynaptic (glutamate release) and postsynaptic (depolarization) activity, and hence are called “coincidence detectors.” Only during simultaneous pre- and postsynaptic activity do NMDARs pass significant current. Under conditions that produce synaptic plasticity, several factors contribute to the required postsynaptic depolarization, but among these, the activation of AMPARs is particularly important.
NMDARs also have the important property that they are highly permeable to calcium ions, providing a significant intracellular calcium signal to the receiving neuron (Dingledine et al., 1999; Cull-Candy et al., 2004). This calcium influx activates the NMDAR protein network, including kinases, phosphatases and other messenger systems that drive the initial phases of synaptic change. Ultimately, the early events in synaptic plasticity activate gene expression and protein synthesis to support long-term synaptic modifications. Certain protein kinases (e.g., protein kinase Mζ) may also be persistently activated and contribute to longer-term modulation (Sacktor, 2011).
Many of the same events underlying synaptic plasticity also underlie the initial phases of excitotoxicity, with calcium influx again playing a key role. Thus, as noted, NMDARs are subject to a great deal of regulation. The role of magnesium ions was described above but other ions including extracellular zinc and hydrogen ions also inhibit NMDARs and play important roles in physiological and pathological processes. In addition, the amino acids glycine and D-serine are necessary co-factors for NMDAR activation and there is also modulation by other endogenous agents including polyamines (spermine and spermidine) and certain neurosteroids (pregnenolone sulfate), as well as posttranslational receptor modulation via phosphorylation (Dingledine et al., 1999; Cull-Candy et al., 2001; Cull-Candy and Leszkiewicz, 2004).
3. NMDARs and Neuropsychiatry
The involvement of NMDARs in synaptic plasticity and excitotoxicity has implications for the pathophysiology of neurological and psychiatric disorders (Zorumski and Olney, 1993). Many of these disorders are associated with impaired learning and memory, and defects in synaptic plasticity are likely to play key roles in the cognitive dysfunction. It also appears, however, that aberrant synaptic plasticity contributes to other defects including the neural adaptations that drive the chronicity of substance abuse syndromes and the altered cognitive processing associated with other primary psychiatric disorders (Barkus et al., 2009; Ma et al., 2009; Mitchell and Baker, 2010, Marsden, 2011). Furthermore, excitotoxic processes are likely to contribute to acute and perhaps chronic neurodegenerative disorders including the acute neuronal loss associated with ischemia, hypoglycemia and repeated or prolonged seizures (Zorumski and Olney, 1993). The role of excitotoxins in chronic neurodegeneration is less certain, but illness such as Alzheimer’s disease, Huntington’s disease and Parkinson’s disease among others are likely to have components of glutamate-mediated neuronal damage or dysfunction (Chohan and Iqbal, 2006; Francis, 2009; Milnerwood and Raymond, 2010; Ondrejcak et al., 2010).
NMDARs are also sites of action of important neuroactive agents, including abused drugs such as ethanol and phencyclidine (PCP), and agents used in clinical medicine such as ketamine, nitrous oxide and memantine. Some of these chemicals have potent psychotomimetic properties (particularly PCP and ketamine), while others are used as anesthetics (ketamine, nitrous oxide) and neuroprotectants (memantine). Interestingly, ketamine, despite its psychotomimetic potential, is gaining recognition as a rapidly acting antidepressant for individuals with severe and refractory mood disorders (Zarate et al., 2006; Machado-Vieira et al., 2009).
NMDARs also participate in neurodevelopment, providing excitation that helps neurons survive and develop efficient connectivity (Mennerick and Zorumski, 2000). During certain periods of development, neurons are highly sensitive to agents that inhibit NMDARs, and NMDAR block results in substantial neuronal loss via programmed cell death (apoptosis). The period of greatest vulnerability to NMDAR antagonist-induced apoptosis is during the time when synapses are rapidly forming (Ikonomidou et al., 1999). This synaptogenesis period extends from the third trimester of pregnancy through the first several years of postnatal life in humans, and may be even more protracted in brain regions that are latest to mature such as prefrontal cortex. Developmental neuroapoptosis induced by NMDAR antagonists may be germane to several neurocognitive syndromes in childhood including fetal alcohol syndrome, the most common cause of non-genetic mental retardation (Ikonomidou et al., 2000; Izumi et al., 2005B). Furthermore, exposure to other NMDAR antagonists such as certain anesthetics and anticonvulsants may also have adverse impact on cognitive development and result in problems with learning and subsequent risk for adolescent and adult psychiatric disorders (Bittigau et al., 2002; Jevtovic-Todorovic et al., 2003).
In mature animals, NMDAR antagonists can also be toxic, inducing pathomorphological changes in regions of cortex and hippocampus (Olney et al., 1989). The posterior cingulate cortex, a component of the Default Mode Network (Raichle and Snyder, 2007), is particularly vulnerable to vacuolar changes in endoplasmic reticula and mitochondria resulting from NMDAR antagonist exposure. Coupled with the psychotomimetic properties of NMDAR antagonists, these observations have fostered the concept of NMDAR hypofunction as a pathogenetic mechanism in schizophrenia (Olney and Farber, 1995; Javitt, 2004).
4. NMDARs and Bidirectional Synaptic Plasticity
At first glance, it seems paradoxical that NMDARs drive both LTP and LTD. This has been shown, however, in multiple brain regions and has been studied extensively in the CA1 region of the hippocampus. In CA1, the timing and pattern of stimulation of afferent inputs (the Schaffer collateral pathway) determines the form of plasticity. Brief bursts of stimulation at high frequency (e.g. 100 Hz x 1 s) drive LTP, while more protracted lower frequency stimulation of the same pathway initiates LTD (e.g. 1–5 Hz for 10–15 min) (Malenka and Bear, 2004). Both forms of long-term plasticity require NMDAR activation during the period of stimulation; NMDAR antagonists are ineffective when administered following the high frequency (HFS) or low frequency stimulus (LFS). It appears that the degree and timing of calcium signals in postsynaptic neurons are major factors determining the type of synaptic plasticity. Brief, larger increases in calcium are important for LTP while more modest but prolonged increases promote LTD (Cormier et al., 2001; Franks and Sejnowski, 2002).
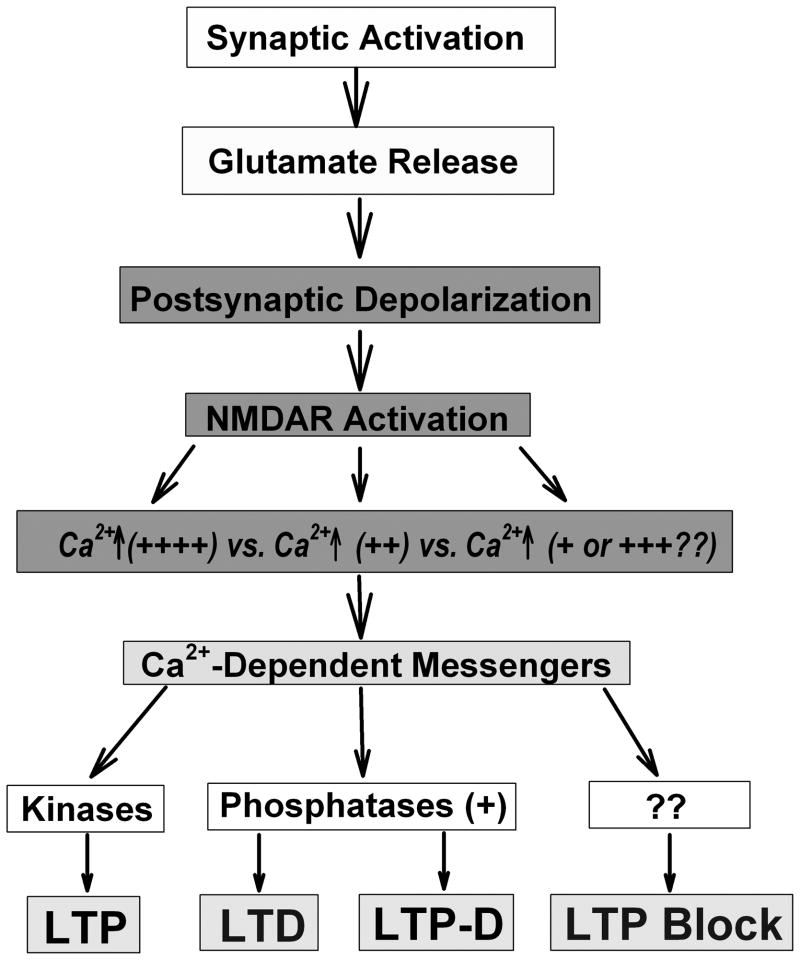
Intermediate frequencies of stimulation also have complex effects on synaptic function. For example, stimulation at ~10 Hz produces no net change in synaptic efficacy. Thus, the same number of stimuli (e.g. 900 pulses) administered at 1–5 Hz drives homosynaptic LTD while stimulation at frequencies above 30 Hz initiates LTP; 10 Hz stimulation results in no lasting change. This has led to the concept that there is a “frequency threshold” for synaptic plasticity (in this case 10 Hz) with stimuli above or below this threshold resulting in LTP or LTD, respectively (Dudek and Bear, 1992). This threshold concept is consistent with a model of experience-dependent synaptic change described by Bienenstock, Cooper and Munro (1982) that has had a significant impact on studies of synaptic plasticity. Importantly, the “threshold” can be shifted to the right or left by a variety of agents, including neuromodulators like norepinephrine (Katsuki et al., 1997; Izumi and Zorumski, 1999), phosphatase activity (Zeng et al., 2001) and sensory experience (Philpot et al., 2003; Sawtell et al., 2003). It remains unclear, however, what factors contribute to the lack of synaptic change at the threshold frequency. This is clearly above the threshold for LTD, but below the threshold for LTP. Ten Hz stimulation does result in NMDAR activation and activates a degree of calcium influx that Lisman (2001) has referred to as “no man’s land” (lying between the calcium levels required for the dominant forms of plasticity). Complicating things further, there are forms of NMDAR activation that are below the LTD threshold and have no lasting effect on synaptic efficacy, but markedly dampen the ability to induce LTP while, in many cases, enhancing LTD induction. It is these latter types of NMDAR activation that underlie the metaplastic states that will be the focus of the remainder of this paper. Figure 1 presents an overview of proximal events in the cascades leading to LTP, LTD, LTP-D and metaplasticity.
Figure 1.
The diagram depicts a simplified scheme of early events underlying homosynaptic LTP, LTD, depotentiation (LTP-D) and LTP block (metaplasticity), highlighting commonalities. Key events include NMDAR activation, varying degrees of calcium influx into postsynaptic neurons and activation of different calcium-dependent messengers. We have put LTD and LTP-D together because they share involvement of phosphatases, but are not necessarily the same process (see text). Longer-term changes include effects on AMPA receptor trafficking, gene expression and protein synthesis (not depicted).
5. NMDAR-mediated LTP Inhibition: A Specific Form of Metaplasticity
Much of the work we will discuss was done in hippocampal slices from juvenile (adolescent) rats where synaptic plasticity is highly robust and reliable. We will highlight these studies but also indicate where studies have been conducted in other species (particularly mice), in adult or aged animals, or in live animals. In the late 1980’s and early 1990’s, several groups found that untimely activation of NMDARs impaired LTP induction. By “untimely” we mean NMDAR activation occurring prior to (or sometimes immediately following) delivery of the stimulus required for LTP induction. In hippocampal slices, this NMDAR-mediated LTP inhibition can be induced in several ways. Initial studies showed that perfusion with solutions containing low concentrations of extracellular magnesium (which allow NMDARs to be activated tonically and during very low frequency stimulation) blocked LTP induction by a usually effective tetanus (Coan et al., 1989). This LTP inhibition resulted from NMDAR activation because it was overcome by NMDAR antagonists. Subsequently, it was found that perfusion of low concentrations of NMDA (e.g. 1 μM for 5 minutes in the presence of extracellular magnesium to mimic rises in ambient excitatory amino acids) markedly impaired LTP induction when administered either immediately before or immediately following HFS. In contrast, administration of 1 μM NMDA after LTP had been established had no effect (Izumi et al., 1992a). Similarly, weak tetanic stimulation of homosynaptic inputs (e.g. 50 Hz for 0.5 sec or less) also blocked LTP (Huang et al., 1992). In all of these cases, NMDAR activation had no lasting effect on basal synaptic transmission mediated by AMPA receptors, and the effects on LTP were prevented by co-administration of an NMDAR antagonist during the period of untimely NMDAR activation. This resulted in the counterintuitive finding that NMDAR antagonists, known to block LTP when administered at high concentrations during HFS, actually promote LTP under certain conditions when administered at low concentrations.
Some stimuli leading to LTP inhibition can depress NMDAR responses via receptor desensitization (Zorumski et al., 1989; Mennerick and Zorumski, 1996) or LTD of NMDA responses (Selig et al., 1995), providing a simple explanation for metaplasticity (Kato and Zorumski, 1993). However, this is not always the case and LTP inhibition can occur in the absence of changes in synaptic NMDAR responses (Izumi et al., 1992a; Kato et al., 1999). These early studies also found that the LTP inhibition was not associated with neuronal damage, but did require calcium during the period of NMDAR activation. LTP inhibition was also relatively slow to reverse, taking more than 30 min after 5 min exposure to low NMDA (Izumi et al., 1992a) and 60–90 min after weak tetanic stimulation (Huang et al., 1992). The LTP inhibition did not represent a complete loss of LTP induction but rather a shift in the relative ease with which LTP could be induced, and stronger tetanic stimulations or HFS in the presence of high extracellular calcium allowed LTP generation (Huang et al., 1992; Kato et al., 1999). Furthermore, modulators that enhanced calcium release from intracellular stores (e.g. norepinephrine) could promote LTP in the face of untimely NMDAR activation (Izumi et al., 1992b). It is important to note, however, that a formal test of whether the frequency thresholds for LTD and LTP change with NMDAR activation has not been done.
Early studies of this form of metaplasticity suggested a simple model in which an agonist (glutamate or other agonist) activated NMDARs and led to an intracellular calcium signal (Izumi et al., 1992a). This calcium signal then activated intracellular messengers that triggered short-and possibly longer-term changes in function (Figure 1). For synaptic activations driving metaplasticity, glutamate is the most likely agonist. Pharmacological studies showed that exogenous glutamate could reproduce the effect of NMDA as could exogenous aspartate (Izumi et al., 1992a). Activators of AMPARs or metabotropic (G-protein linked) glutamate receptors were ineffective. Other studies demonstrated links between LTP inhibition and conditions of neuronal stress. In particular, brief bouts of hypoxia in the presence of normal glucose (Izumi et al., 1998), low glucose alone (mimicking mild hypoglycemia) (Izumi and Zorumski, 1997) or exposure to ammonia (mimicking hepatotoxic states) (Izumi et al., 2005a) all resulted in impaired LTP induction in which basal neurotransmission was not persistently altered and in which NMDAR antagonists administered during the insult overcame the LTP inhibition. Later studies showed that treatments that relieved negative regulation of NMDARs by extracellular zinc (e.g. zinc chelators) also resulted in NMDAR-mediated LTP inhibition (Izumi et al., 2006).
6. Metaplasticity: NMDAR Subtypes and Messengers
NMDARs are complex signaling molecules with multiple subtypes and multiple interacting protein partners that differ by receptor subtype (Hardingham and Bading, 2003; 2010). In the early 2000’s, several groups found that subtypes of NMDARs may differentially contribute to LTP and LTD (Liu et al., 2004; Massey et al., 2004). Based on studies using selective subtype antagonists (particularly for NR1/NR2B receptors) and manipulations of gene expression, these studies suggested that LTP involved NMDARs expressing NR2A subunits while LTD involved NR2B-containing receptors. While not all studies agreed with these findings (Berberich et al., 2005; Hrabetova et al., 2000; Morishita et al., 2007), other studies supported the notion that NMDARs containing NR2B were important for LTD (Izumi et al., 2005c, 2006; Bartlett et al., 2007). NR1/NR2B receptors are important developmentally, and are expressed at highest synaptic levels early in development, waning in expression with maturation (Loftis and Janowsky, 2003; Molnar et al., 2002). As animals mature, NR2B-type receptors come to play important roles as extrasynaptic receptors, although NR2B subunits can also be expressed at synapses in mature animals; NR2A subunits can also be expressed extrasynaptically (Hardingham and Bading, 2003; 2010). Fewer studies have examined the role of NMDAR subtypes in LTP inhibition, but there is some evidence that the effects of pharmacological NMDAR activation in juvenile rodents are insensitive to block by antagonists with relative selectivity for NR1/NR2B receptors (Izumi et al., 2006). It is important to note that there are presently no completely selective NR1/NR2A antagonists (Traynelis et al., 2010), although there are several reasonably selective NR1/NR2B blockers and novel compounds with NR2A selectivity are being developed (Bettini et al., 2010); thus, conclusions about receptor subtypes remain tentative.
Given the role of calcium in NMDAR function and in NMDAR-mediated LTP inhibition, there has been interest in identifying calcium-dependent messengers that contribute to metaplasticity. Early studies suggested a role for nitric oxide synthase (NOS) and release of the volatile messenger nitric oxide (NO). A role for NO has been demonstrated in LTP inhibition induced by pharmacological NMDAR activation (Izumi et al., 1992c; Youssef et al., 2006), weak synaptic stimulation (Izumi et al., 1992c), brief hypoxia (Izumi et al., 1998), low glucose (Izumi et al., 1997), and extracellular zinc chelation (Izumi et al., 2006). While NO can inhibit NMDARs (Lei et al., 1992; Manzoni et al., 1992) and NO inhibitors can foster LTP by effects on NMDARs (Kato and Zorumski, 1993), this does not appear to be responsible for LTP inhibition (Izumi et al., 1992c; Kato et al., 1999) and focus has been on downstream targets of NO. These include guanylate cyclases, ADP ribosyltransferases and modulation of cellular energy metabolism via inhibition of glyceraldehyde phosphate dehydrogenase (GAPDH) and suppression of glycolysis, as well as effects on mitochondrial function (Guix et al., 2005; Calabrese et al., 2007).
Because LTP inhibition requires only low level NMDAR activation somewhat akin to LTD, there has also been interest in examining messengers involved in LTD. This is important because higher concentrations of NMDA (e.g. 20 μM for 3 min) can induce a form of “chemical” LTD (Lee et al., 1998), possibly providing a pharmacological way to disentangle LTD from metaplasticity. Emphasis has been placed on serine phosphatases, enzymes that play key roles in LTD (Malenka and Bear, 2004), and there is evidence suggesting roles for protein phosphatase 1 (PP1), PP2A and PP2B (calcineurin) in LTP inhibition (Kato et al., 1999; Izumi et al., 2006). Other studies indicate that tyrosine phosphatases such as STEP (striatal enriched phosphatase) may also participate in metaplasticity (Pelkey et al., 2002; Yang et al., 2006). Additionally, some (Izumi and Zorumski, 1993; Reyes-Harde, 1999) but not all (Cummings et al., 1994) studies indicate that NO plays a role in LTD, adding to possible interactions between metaplasticity and LTD. Release of adenosine may also contribute to both LTD and LTP inhibition (Fujii et al., 2000). Because of the overlap in mechanisms, there has also been interest in whether NMDAR-mediated metaplasticity promotes the induction of LTD. Indeed this appears to be the case and exposure to conditions that induce metaplasticity primes Schaffer collateral synapses for LTD (Mockett et al. 2002). In addition to homosynaptic forms of metaplasticity there are also forms of heterosynaptic modulation. For example, at perforant path synapses in the dentate gyrus, a form of heterosynaptic metaplasticity is mediated by NMDAR activation and interferes with LTP induction (Abraham et al., 2001; Gisabella et al., 2003). Intriguingly, induction of LTP also has metaplastic effects on LTD, resulting in a dampening of LTD induction via a mechanism involving glycogen synthase kinase 3β (GSK3β) (Peineau et al., 2007).
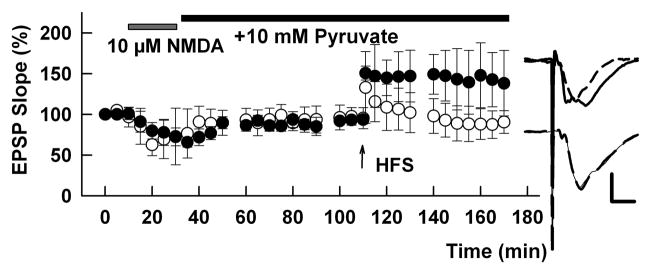
Protein kinases, such as PKC, also contribute to metaplasticity consistent with the ability of NMDAR activation to drive complex bidirectional effects on the phosphorylation status of synaptic proteins (Coba et al., 2009). MAPKs have also been linked to hippocampal synaptic plasticity and several lines of evidence suggest different roles for various MAPKs in LTP, LTD and depotentiation. Notably, extracellular signal-regulated kinases (ERK 1/2) appear to contribute to LTP (Sweatt, 2004), while other studies have linked p38 MAPK to LTD (Bolshakov et al., 2000; Anwyl, 2006) and c-Jun-N-terminal kinase (JNK) to LTP depotentiation (Zhu et al., 2005). While there is limited information about the role of MAPKs in metaplasticity, some evidence supports a role for p38 MAPK, but not ERK or JNK (Gisabella et al., 2003; Izumi et al., 2006). Studies using pharmacological activators and inhibitors of key enzymes suggest that metaplastic LTP inhibition involves a cascade that includes NMDAR activation (possibly NR1/NR2A), calcium influx, followed by calcineurin, NOS and p38 MAPK, likely in that order (Izumi et al., 2006). It is important to note that inhibitors of these steps in the cascade must be present during the period of untimely NMDAR activation; once NMDARs trigger the sequence, these inhibitors are ineffective. This raises important unanswered questions about factors that overcome LTP inhibition when administered in the post-NMDAR activation period, a period of great interest clinically. The one exception may be alternative energy substrates administered in the post-NMDAR period, including the monocarboxylate, pyruvate (Figure 2, see also Izumi and Zorumski, 2010). This latter observation may reflect inhibitory effects of NO on metabolism perhaps via GAPDH and glycolysis.
Figure 2.
Pyruvate overcomes LTP inhibition when applied following HFS. The graph shows the ability of 10 μM NMDA (hatched bar) to produce prolonged inhibition of LTP (white circles). In slices treated with 10 mM pyruvate (black bar) following NMDA and HFS, LTP could be readily induced (black circles). A 100 Hz x 1 sec HFS was delivered at the time denoted by the arrow. Note that the concentration of NMDA used in these experiments is higher than that needed to block LTP (1 μM) and produced depression during the NMDA exposure. Similar effects of pyruvate are observed following brief hypoxia (not shown). Traces to the right show representative excitatory postsynaptic potentials (EPSPs) obtained during baseline (solid lines) and 60 min following HFS (dashed lines) in control slices (bottom) and slices treated with pyruvate (top). Scale bar: 1mV, 5 ms.
7. Metaplasticity and Behavioral Stress: Implications for Psychiatry
The studies outlined above suggest that during conditions of mild to moderate metabolic stress, including brief hypoxia, low glucose and increased ammonia, untimely NMDAR activation could contribute to cognitive impairment, mental dysfunction and learning difficulties without resulting in neuronal death. This raises questions about whether other stressors, including behavioral stressors associated with psychiatric disorders, also negatively impact LTP and whether NMDARs are involved in these effects. Stress and reactions to stress are important contributors to the pathophysiology of major psychiatric disorders, including mood and anxiety disorders, psychotic illnesses and post-traumatic stress disorder, among others (McEwen, 2007). In their acute state, these illnesses are often associated with altered secretion of stress hormones, including glucocorticoids (Shin and Liberzon, 2010). These disorders also manifest an array of symptoms that include changes in emotion, motivation and cognition, including defects in learning and memory (Zorumski and Rubin, 2011). Importantly, cognitive dysfunction is a major contributor to work-related disability in psychiatric illnesses.
Consistent with changes in cognition in psychiatric disorders, there is evidence that acute behavioral stress can impair LTP induction and hippocampal-dependent learning, and that this involves NMDAR activation (Kim and Diamond, 2002). While some early studies suggested that behavioral stress impaired LTP by inducing synaptic enhancement, this appears not to be the case, and exposure to acute behavioral stress of multiple types dampens LTP induction and enhances LTD (Foy et al., 1987; Xu et al., 1997; Ryan et al., 2010). In a key study, Kim and colleagues (1996) found that acute tail shock stress impaired LTP. This LTP inhibition was not altered by benzodiazepine (anti-anxiety) treatment but was prevented by co-administration of an NMDAR antagonist during the stress. Similar effects have been reported in a behavioral restraint-tail shock paradigm by Yang and colleagues (2008). This latter study pursued downstream mechanisms and found a role for phosphoinositide-3 kinase (PI3K), mammalian target of rapamycin (mTOR) and S6 kinase in the adverse effects of behavioral stress on synaptic function (Yang et al., 2008). Other work indicates that behavioral stressors promote the release of stress hormones such as corticosterone that modulate learning (Krugers, et al., 2011). Corticosterone has complex effects on synaptic plasticity depending on concentration (Joels, 2006). When present at higher concentrations during moderate to severe stress, corticosterone can dampen LTP (and promote LTD) via mechanisms that involve NMDAR activation (Diamond et al., 2005; Joels and Kruger, 2007). In particular, corticosterone appears to inhibit glutamate uptake resulting in untimely activation of extrasynaptic NMDARs expressing NR2B subunits (Sandi, 2011). This latter effect may also impair recollection of previously learned memories.
The studies outlined above indicate that during periods of metabolic (and behavioral) stress, including those that can sometimes lead to excitotoxic neuronal damage, glutamate accumulates in the extracellular space and activates NMDARs. This glutamate could come from ongoing spontaneous synaptic release, release from glia, or from changes in glutamate uptake (Espinosa and Kavalali, 2009; Nishizawa, 2001; Sandi, 2011). The role of extracellular glutamate in mediating the effects of these stressful conditions raises questions about whether low level NMDAR activation, such as that associated with chemical LTP inhibition, serves as a form of “stress” signal that triggers mechanisms to dampen neuronal activity and excitation. If this is the case, then a decrement in LTP induction might serve as an adaptive response to prevent excessive excitation of principal (excitatory) neurons during periods of metabolic (or behavioral) stress, but would have the deleterious effect of disrupting new memory formation, contributing to mental dysfunction.
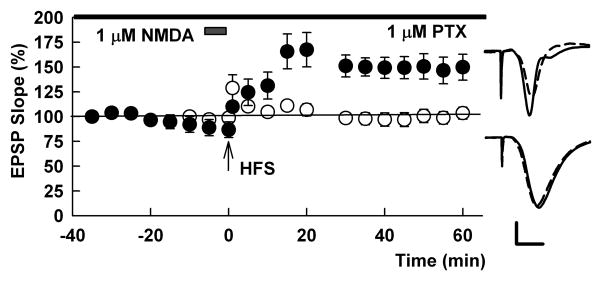
Related to the above discussion, we have been interested in determining whether low level NMDAR activation triggers mechanisms to dampen pyramidal neuron excitability. It is known that γ-aminobutyric acid type A receptor (GABAAR)-mediated inhibition dampens LTP induction, and that GABAAR inhibitors can facilitate LTP induction (Wigstrom and Gustafsson, 1983; Meredith et al., 2003). Consistent with this, we found that picrotoxin, a non-competitive GABAAR antagonist, overcomes the effects of low NMDA on LTP. While this effect of picrotoxin suggests a role for altered GABAergic function in NMDAR-mediated LTP inhibition, the known ability of picrotoxin to enhance neuronal network excitability complicates interpretation of these results (Figure 3).
Figure 3.
Picrotoxin overcomes LTP inhibition. The graph shows the ability of 1 μM NMDA administered for 5 minutes prior to HFS (gray bar) to inhibit LTP (white circles). In slices treated with 1 μM picrotoxin (top bar), the HFS induced robust LTP (black circles). HFS was delivered at the arrow. Traces show representative EPSPs from NMDA-treated slices (bottom) and NMDA + picrotoxin slices (top) obtained at baseline (dashed traces) and 60 min following HFS (solid traces). Although picrotoxin can alter neuronal excitability independent of effects on metaplasticity, the concentration used in these studies did not alter the magnitude of LTP in control slices (not shown). Scale bar: 1 mV, 5 ms.
Other recent studies are more directly consistent with a role for enhanced GABAergic activity in the effects of low NMDA on synaptic plasticity. NMDAR activation can enhance GABAergic function in hippocampal pyramidal neurons via presynaptic and postsynaptic actions, some of which involve NO (Xue et al., 2011). Furthermore, low level NMDAR activation is sufficient to promote the synthesis of GABAAR-enhancing neurosteroids such as allopregnanolone in hippocampal pyramidal neurons (Tokuda et al., 2011). These neurosteroids are potent and effective endogenous modulators of GABAARs (Covey et al., 2001). Under basal conditions, pyramidal neurons are the major cells in the hippocampus that express the machinery for cholesterol trafficking (the precursor for neurosteroids) (Valdez et al., 2010) and neurosteroid synthesis, including StAR (steroidogenic acute regulatory protein) (Kimoto et al., 2001; Lavaque et al., 2006), TSPO (translocator protein 18 kDa) (Tokuda et al., 2010), P450 cholesterol side chain cleavage enzyme (SCC) (Kimoto et al., 2001) and 5-alpha reductase (5AR) (Agis-Balboa et al., 2006). Neurosteroid synthesis is initiated by movement of cholesterol to the outer mitochondrial membrane via StAR, translocation to the inner mitochondrial membrane via TSPO (the “mitochondrial benzodiazepine receptor”), cleavage of cholesterol to pregnenolone by SCC, movement of pregnenolone out of mitochondria and eventual conversion to allopregnanolone via 5AR and 3α-hydroxysteroid dehydrogenase (Belelli and Lambert, 2005).
Under basal conditions, excitatory neurons are immunopositive for allopregnanolone and other 5α-reduced neurosteroids (Saalman et al., 2007; Tokuda et al., 2010, 2011). The levels of these steroids increase with NMDAR activation (Kimoto et al., 2001; Tokuda et al., 2011), and inhibitors of allopregnanolone synthesis overcome the effects of low concentrations of NMDA on LTP induction (Tokuda et al., 2011). It is presently unclear where NMDAR-driven neurosteroid production fits into the metaplasticity cascade relative to other messengers (e.g., calcium, calcineurin, NO and p38 MAPK), and it is possible that one or more of the already identified messengers involved in LTP inhibition trigger the synthesis of these steroids. It is also important to note that recent studies indicate that increases in neurosteroids are important for LTP inhibition by other agents (e.g. benzodiazepines and ethanol), and that increases in 5α-reduced neurosteroids appear to be necessary but not sufficient for LTP block (Izumi et al., 2007; Tokuda et al., 2010, 2011). This has prompted the concept that LTP inhibition by these agents requires “two hits” – increases in pyramidal neuron neurosteroids and a second process that may vary according to the causative condition. For example, in the case of benzodiazepines, LTP block required activation of BOTH central (GABAAR) and mitochondrial (TSPO) benzodiazepine receptors; activation of either receptor alone was insufficient to inhibit LTP (Tokuda et al., 2010).
The involvement of GABA-enhancing neurosteroids in the acute effects of untimely NMDAR activation on LTP is consistent with the role these steroids play in responses to stress. Prior studies have shown that behavioral stressors, including forced swim and foot shock, acutely increase allopregnanolone levels in brain and periphery (Purdy et al., 1991). However, chronic social isolation stress ultimately leads to a decrement in neurosteroid levels associated with diminished expression of 5AR in brain (Dong et al., 2001; Agis-Balboa et al., 2006). It remains to be determined how these latter changes affect hippocampal plasticity, although there is evidence for ongoing hippocampal dysfunction in chronic mild stress (Airan et al., 2007) and complementary changes in neurosteroid levels are found in human psychiatric disorders (Girdler and Klatzkin, 2007).
An intriguing twist on this story is that NMDAR-mediated metaplasticity may also contribute to the memory impairment associated with ethanol. Severe ethanol intoxication can induce an acute amnesic state, clinically called a memory “blackout” (White, 2003, Nelson et al., 2004). During a memory blackout individuals perform complex activities for which they have no subsequent recollection, reflecting a failure of acute memory formation. Ethanol is a known NMDAR antagonist and blocks LTP at high concentrations (White and Swartzwelder, 2004). This combination of effects has been thought to be the primary mechanism underlying clinical blackouts (McCool, 2011). Other studies, however, indicate that GABAergic inhibition also contributes to effects of ethanol on LTP (Schummers et al., 1997; Schummers and Browning, 2001). In our studies, the effects of ethanol on NMDAR-mediated synaptic transmission in the CA1 hippocampal region, at concentrations that block LTP, are only partial (about 50% inhibition by 60 mM ethanol), and largely dampen transmission by synaptic NR1/NR2B type NMDARs (Izumi et al., 2005c). In these studies, blocking NR1/NR2B receptors selectively inhibited induction of LTD, but not LTP. Ethanol, however, blocked both LTP and LTD. Effects on LTD correlated with inhibition of NR1/NR2B, but effects on LTP were more complex and persistent. Additionally, effects on LTP were overcome by picrotoxin, suggesting that LTP block involved altered GABAAR function (Izumi et al., 2005c). Ethanol enhances the production of 5α-reduced GABAergic neurosteroids such as allopregnanolone (Sanna et al., 2004), providing a potential tie to enhanced GABAergic function in the hippocampus. Consistent with this, effects on LTP were also overcome by agents that blocked either the production or actions of 5α-reduced neurosteroids (Izumi et al., 2007; Tokuda et al., 2011). How ethanol promotes the production of neurosteroids and LTP inhibition remained uncertain until recent studies found that both effects of ethanol were prevented by complete block of NMDARs with co-administration of a broad spectrum NMDAR antagonist during the period of ethanol exposure (Tokuda et al., 2011). Inhibitors of neurosteroid synthesis also prevented the effects of low NMDA on LTP. Thus, ethanol induced LTP inhibition appears to involve a contribution from NMDAR-dependent metaplasticity, in this case arising paradoxically via the activation of NMDARs that are not blocked acutely by ethanol. This mechanism appears to work in concert with other effects of ethanol, including partial NMDAR antagonism, to prevent LTP induction. How unblocked NMDARs are activated during ethanol exposure remains uncertain but could include enhanced release of glutamate or another excitatory amino acid from neurons or glia, and/or altered uptake of glutamate (Melendez et al., 2005; Salazar et al., 2008).
8. Does NMDAR-mediated LTP Inhibition Extend to Other Conditions?
The ability of untimely NMDAR activation to dampen LTP induction raises the possibility that this mechanism might contribute to disorders in which there is acute or on-going problems with memory formation (Abraham, 2008). One example would be the acute cognitive dysfunction associated with a variety of medical and neurological illnesses (Gofton, 2011). Multiple metabolic insults can produce acute memory impairment and altered cognition, including hypoxia/ischemia, hypoglycemia, and renal and hepatic insufficiency among others. The findings that NMDAR-mediated LTP inhibition is associated with brief hypoxia (Izumi et al., 1998), low glucose (Izumi and Zorumski, 1997) and elevated ammonia (Izumi et al., 2005a) are consistent with a role in cognitive dysfunction associated with these conditions, and raise the possibility that this may be a general mechanism contributing to similar clinical states. In the context of organ failure, metabolic stress, sometimes involving the accumulation of endogenous or exogenous toxins (such as ammonia in the case of liver failure) manifests, at least in part, via increases in extracellular glutamate and possibly the metaplastic changes outlined here (Beal et al., 1993; Lipton, 1999; Marcaida et al., 1992; Nishizawa, 2001). Memory impairment can also be induced by numerous neuroactive drugs and can arise by other mechanisms including direct effects on excitatory and inhibitory neurotransmission. Even here, however, it is important to consider overlap with metaplastic mechanisms, and the recent studies examining the effects of benzodiazepines and alcohol on LTP described above have found that the ability of these drugs to inhibit LTP and memory share at least one mechanism involving the generation of neurosteroids in hippocampal pyramidal neurons (Tokuda et al., 2010, 2011).
Whether metaplasticity contributes to chronic cognitive dysfunction is more speculative. Even here, however, there is some evidence that the long-term effects of metabolic illnesses such as diabetes also involve metaplastic changes in synaptic function (Artola, 2008). Furthermore, decrements in learning associated with aging may also have a metaplastic component, although there are other changes in synaptic function accompanying aging that also play a role (Artola, 2008). These changes, including altered neuronal excitability and intracellular calcium homeostasis, point to additional mechanisms that could contribute to or act in conjunction with metaplasticity (Burke and Barnes, 2010). Alzheimer’s disease (AD) is another interesting example. AD results, at least in part, from the accumulation of extracellular beta-amyloid (Aβ) peptides and the formation of amyloid plaques and neuritic tangles. In the fully developed disorder, AD results in massive degeneration within the brain. Much of the work on AD has rightfully focused on ways to prevent or halt this neurodegeneration. Other work, however, has considered that neuronal loss is a late manifestation of AD and that synaptic dysfunction, including defects in synaptic plasticity, may account for the earliest cognitive changes in the illness. This has prompted further examination of how Aβ peptides influence synaptic function. A recent study found that acute treatment of hippocampal slices with soluble Aβ oligomers impairs LTP induction and does so via a mechanism that involves untimely and/or excessive NMDAR activation (Li et al., 2011). In this case, Aβ oligomers resulted in activation of extrasynaptic NR2B containing NMDARs and this resulted in stimulation of p38 MAPK and downregulation of cyclic AMP response element binding protein (CREB). The effects of the Aβ oligomers were overcome by selective NR2B antagonists, similar to the ability of NMDAR antagonists to overcome other metaplastic effects, and were mimicked by an inhibitor of glutamate uptake suggesting a possible mechanism for the untimely NMDAR activation. Aβ peptides activate other mechanisms, including caspase-3, Akt1 and GSK-3β that also contribute to LTP inhibition (Jo et al., 2011), and some of these mechanisms are shared with the events underlying metaplasticity or LTD (Li et al., 2010b).
The role of metaplasticity in AD-associated synaptic dysfunction provides a possible explanation for the beneficial effects of memantine, an NMDAR antagonist used clinically in AD. Indeed, some evidence indicates that memantine does not block LTP acutely, but restores LTP induction and learning in conditions in which untimely NMDAR activation occurs (Frankiewicz and Parsons, 1999; Zajaczkowski et al., 1997). Similar considerations can be raised for recent findings showing that elevations in brain magnesium levels can have beneficial effects on LTP and learning, even in aged rodents (Slutsky et al., 2010). Chronic elevations in brain magnesium resulted in increased expression and activity of synaptic NR2B-expressing NMDARs, likely as a compensatory response to more complete block of baseline NMDARs.
The studies outlined above highlight several instructive points. First, even in chronic neurodegenerative conditions and aging, metaplastic changes may occur. Second, the mechanisms contributing to NMDAR-mediated metaplasticity may change with aging. The work we have outlined in most of this paper is based on experiments in juvenile (adolescent) rodents. Studies were done in these rodents because synaptic plasticity is highly robust and reliable at these ages, avoiding complications that can arise from aging alone. The studies in diabetes, aging and AD, however, were done in adult and aged animals, and raise the possibility that different subtypes of NMDARs may play a role in metaplasticity at different ages across development and aging. For example, studies in juvenile animals highlight the importance of NR1/NR2A receptors (Izumi et al., 2006) while findings in older animals with amyloid peptides (Li et al., 2011) and magnesium (Slutsky et al., 2010) involve NR1/NR2B receptors. In aged animals, changes in other currents that dampen excitability (e.g. enhanced potassium conductances underlying the action potential afterhyperpolarization) and altered calcium homeostasis also contribute along with changes in GABAergic inhibition (Bodhinathan et al., 2010). The underlying theme, however, is that untimely or excessive NMDAR activation can disrupt LTP induction in several pathological and physiological conditions.
9. Metaplasticity and the Treatment of Neuropsychiatric Disorders
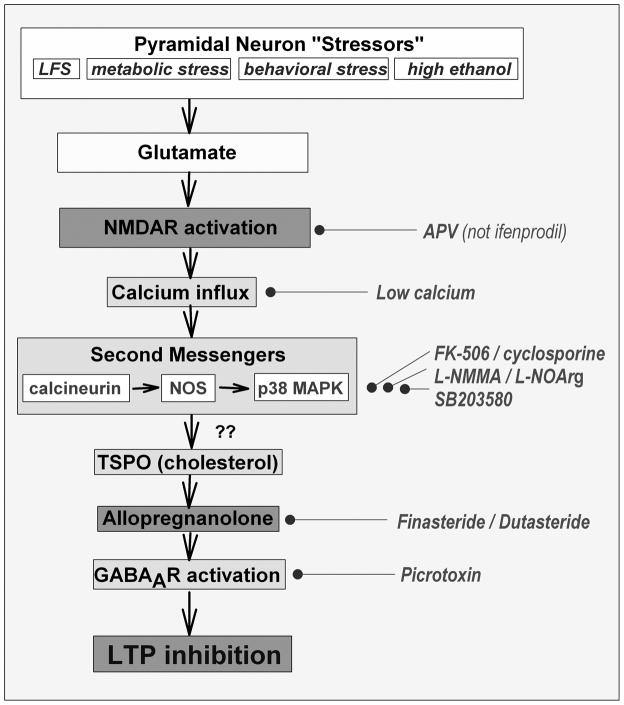
We conclude this review with several points and a few additional speculations about the potential role of metaplasticity in psychiatry. At the minimum, it is clear that metaplasticity involves a complex set of mechanisms. Simply treating hippocampal slices with a low concentration of NMDA for 5 minutes in the presence of physiological magnesium is sufficient to disrupt the machinery thought to underlie learning and memory. This low level receptor activation triggers multiple messengers, consistent with the dynamic nature and complexity of the NMDAR protein network (Coba et al., 2009). A summary of the current state of these mechanisms is shown in Figure 4, and the cascade is getting more complex with additional studies.
Figure 4.
Multiple messengers contribute to NMDAR-induced metaplasticity. Steps involving neurosteroids are recent additions. The question marks at these steps reflect uncertainty about whether activation of neurosteroid synthesis occurs subsequent to other steps in the pathway or reflects a parallel path to LTP inhibition. As described by Yang et al. (2008), other messengers such as PI3K, mTOR and S6 kinase are also likely to contribute to LTP inhibition. To the right of the main cascade, we list agents that inhibit the particular step and allow LTP induction. Key enzyme inhibitors include FK-506 and cyclosporine for calcineurin, L-N-monomethylarginine (L-NMMA) and L-nitroarginine (L-NOArg) for nitric oxide synthase (NOS) and SB203580 for p38 MAP kinase. Finasteride and dutasteride inhibit 5-alpha reductase a key enzyme in allopregnanolone synthesis and picrotoxin is a GABAAR antagonist. As noted in Figure 3, interpretation of the effects of picrotoxin is complex because of effects on network excitability.
To summarize the relevance of metaplasticity to psychiatry, we emphasize several points. NMDAR-induced metaplasticity represents a higher order form of synaptic dysfunction. It is triggered by numerous insults (“stressors”), including acute behavioral stress. This process has a negative impact on learning and network function but does not completely eliminate the ability to induce LTP – rather, it makes it more difficult to induce LTP and likely makes it easier to induce LTD, potentially shifting the balance of synaptic activity and the ratio of excitatory to inhibitory connectivity. Beyond acute stress-induced memory impairment, we suggest that metaplasticity could contribute to other aspects of synaptic dysfunction in psychiatry. In particular, metaplasticity is a form of “NMDAR hypofunction,” one of the mechanisms that may contribute to the pathophysiology of psychotic disorders such as schizophrenia (Olney and Farber, 1995; Javitt, 2004). By dampening LTP generation, metaplastic mechanisms could contribute to the cognitive impairment, including learning difficulties and hippocampal dysfunction, associated with major mental disorders and perhaps to psychosis, given the propensity of NMDAR antagonists such as PCP to induce delusions, hallucinations and thought disorder.
We also wonder about the possible role of metaplasticity in the effects of clinically-used NMDAR antagonists. Here, studies of ethanol-induced LTP inhibition could be instructive. Partial NMDAR antagonism, particularly affecting a subtype of NMDARs, can result in metaplastic effects via activation of unblocked NMDARs (Tokuda et al., 2011). Earlier, we noted the ability of memantine to overcome metaplastic effects of NMDA when administered acutely (Frankiewicz and Parsons, 1999). Low dose memantine, however, has been associated with memory impairment in rodents and the drug is only marginally effective in AD (Creeley et al., 2006). This raises the possibility that longer term use of memantine may actually work against its acute beneficial actions perhaps via longer-lived metaplastic changes.
Similar considerations can be raised about the use of ketamine to treat refractory depression. Here, low doses of ketamine infused over an hour or so result in an acute antidepressant effect that can persist for days (Zarate et al., 2006). Why an NMDAR antagonist is effective in depression is uncertain, but is consistent with the idea that behavioral stress and depression may reflect hyperglutamatergic states (Marsden, 2011). Mechanistic studies highlight acute effects of ketamine on cortical and hippocampal synapses that may contribute to antidepressant actions (Li et al., 2010a; Autry et al., 2011). In a scheme described by Li and colleagues (2010a), low dose ketamine (or an NR2B-selective NMDAR antagonist) results in increased glutamate release, activation of AMPA receptors, release of brain-derived neurotrophic factor (BDNF), activation of mTOR and p70S6 kinase, protein synthesis and synaptogenesis. The net physiological effect is an increase in AMPA receptor-mediated synaptic currents. While this scheme differs from the one we outlined for metaplasticity (Figure 3), we note that these effects of ketamine were observed only at low doses. When higher doses were administered, the effects of ketamine on synaptic function were abolished (Li et al., 2010a). Ketamine is a non-selective NMDAR antagonist and higher (anesthetic) doses block a greater proportion of NMDARs, in addition to other effects. This raises the possibility that activation of unblocked NMDARs may contribute to the beneficial effects and may also have metaplastic actions. We believe this speculation is not unreasonable. For example, ketamine (and other NMDAR antagonists) are known to adversely affect memory, and there is evidence that a single in vivo administration of ketamine, MK-801or PCP can cause defects in LTP and spatial memory (as well as psychotomimetic behaviors) that outlive the lives of the drugs, sometimes persisting for a week (Manahan-Vaughan et al., 2008). Studies of NMDAR-induced metaplasticity in vitro indicate that effects of untimely NMDAR activation typically reverse over several hours (Huang et al., 1992; Izumi et al., 1992a,c). Thus, persistent effects on memory could reflect a metaplastic component, although other mechanisms also likely contribute. The acute memory defects caused by ketamine and MK-801 are also overcome by inhibitors of NOS, suggesting another tie to metaplasticity (Boultadakis and Pitsikas, 2010). Prior work by Yang and colleagues (2008) further indicates that mTOR and S6 kinase contribute to NMDAR-mediated effects of behavioral stress on LTP, in addition to their roles in ketamine’s antidepressant effects on cortical synapses (Li et al., 2010a). Ketamine, however, has other effects, particularly effects on spontaneous excitatory transmission, BDNF and protein synthesis that may contribute to therapeutic actions in depression (Autry et al., 2011). Furthermore, NMDAR-induced metaplasticity can have neuroprotective effects and may contribute to beneficial effects of preconditioning against excitotoxins (Soriano et al., 2006; Youssef et al., 2006). The antidepressant effects of ketamine also occur rapidly following infusion; thus, ketamine’s ability to block NMDARs may help to improve a hyperglutamatergic state in the short run, perhaps via acute anti-metaplastic actions.
10. Summary
We have described an expanding body of work spanning more than 20 years focused on a form of NMDAR-induced metaplasticity. These studies have detailed a unique form of modulation that may contribute to both physiological modulation of synaptic function and to multiple pathological conditions and their treatments. These studies raise the possibility that strategies that modulate this form of metaplasticity could have therapeutic potential in a variety of neuropsychiatric disorders.
HIGHLIGHTS.
NMDA receptors play key roles in synaptic function and plasticity
NMDA receptors also modulate neuronal function and inhibit LTP via metaplasticity
Metaplasticity contributes to dysfunction in multiple neuropsychiatric disorders
Acknowledgments
Work in the authors’ laboratory is supported by grants MH07791, GM47969, AA017413 and NS057105 from the National Institutes of Health, and the Bantly Foundation. CFZ serves as a consultant for Sage Therapeutics.
Abbreviations
- AD
Alzheimer’s disease
- 5AR
5-alpha reductase
- AMPARs
AMPA class of glutamate receptors
- Aβ
beta-amyloid
- BDNF
brain-derived neurotrophic factor
- JNK
c-Jun-N-terminal kinase
- CREB
cyclic AMP response element binding protein
- LTP-D
depotentiation
- EPSPs
excitatory postsynaptic potentials
- ERK
extracellular signal related kinase
- GABAARs
γ-aminobutyric acid type A receptors
- GAPDH
glyceraldehyde phosphate dehydrogenase
- GSK3β
glycogen synthase kinase 3β
- HFS
high frequency stimulus
- LTD
long-term depression
- LTP
long-term potentiation
- LFS
low frequency stimulus
- mTOR
mammalian target of rapamycin
- MAPKs
mitogen-activated protein kinases
- NMDARs
N-methyl-D-aspartate receptors
- NO
nitric oxide
- NOS
nitric oxide synthase
- SCC
P450 cholesterol side chain cleavage enzyme
- PCP
phencyclidine
- PI3K
phosphoinositide-3 kinase
- PKC
protein kinase C
- StAR
steroidogenic acute regulatory protein
- STEP
striatal enriched phosphatase
- TSPO
translocator protein 18 kDa
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts MM, Tymianski M. Molecular mechanisms underlying specificity of excitotoxic signaling in neurons. Curr Mol Med. 2004;4:137–147. doi: 10.2174/1566524043479202. [DOI] [PubMed] [Google Scholar]
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nature Rev Neurosci. 2008;9:387–399. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Mason-Parker SE, Bear MF, Webb S, Tate WP. Heterosynaptic metaplasticity in the hippocampus in vivo: a BCM-like modifiable threshold for LTP. Proc Natl Acad Sci (USA) 2001;98:10924–10929. doi: 10.1073/pnas.181342098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WC, Tate WP. Metaplasticity: a new vista across the field of synaptic plasticity. Prog Neurobiol. 1997;52:303–323. doi: 10.1016/s0301-0082(97)00018-x. [DOI] [PubMed] [Google Scholar]
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci (USA) 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Induction and expression mechanisms of postsynaptic NMDA receptor- independent homosynaptic long-term depression. Prog Neurobiol. 2006;78:17–37. doi: 10.1016/j.pneurobio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Artola A. Diabetes-, stress- and aging-related changes in synaptic plasticity in hippocampus and neocortex – the same metaplastic process? Eur J Pharmacol. 2008;585:153–162. doi: 10.1016/j.ejphar.2007.11.084. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioral antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur J Pharmacol. 2009;626:49–56. doi: 10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacol. 2007;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Bayes A, van de Lagemaat LN, Collins MO, Croning MD, Whittle IR, Choudhary JS, Grant SG. Characterization of the proteome, diseases and evolution of the human postsynaptic density. Nature Neurosci. 2011;14:19–21. doi: 10.1038/nn.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF, Hyman BT, Koroshetz W. Do defects in mitochondrial energy metabolism underlie the pathology of neurodegenerative diseases? Trends Neurosci. 1993;16:125–131. doi: 10.1016/0166-2236(93)90117-5. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nature Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen M, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettini E, Sava A, Griffante C, Carignani C, Buson A, Capelli AM, Negri M, Andreetta F, Senar-Sancho SA, Guiral L, Cardullo F. Identification and characterization of novel NMDA receptor antagonists selective for NR2A- over NR2B-containing receptors. J Pharmacol Exp Ther. 2010;335:636–644. doi: 10.1124/jpet.110.172544. [DOI] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittigau P, Sifringer M, Genz K, Reith K, Pospischil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci (USA) 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhinathan K, Kumar A, Foster TC. Redox sensitive calcium stores underlie enhanced after hyperpolarization of aged neurons: role for ryanodine receptor mediated calcium signaling. J Neurophysiol. 2010;104:2586–2593. doi: 10.1152/jn.00577.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakov VY, Carboni L, Cobb MH, Siegelbaum SA, Belardetti F. Dual MAP kinase pathways mediate opposing forms of long-term plasticity at CA3-CA1 synapses. Nature Neurosci. 2000;3:1107–1112. doi: 10.1038/80624. [DOI] [PubMed] [Google Scholar]
- Boultadakis A, Pitsikas N. Effects of the nitric oxide synthase inhibitor L-NAME on recognition and spatial memory deficits produced by different NMDA receptor antagonists in the rat. Neuropsychopharmacol. 2010;35:2357–2366. doi: 10.1038/npp.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci. 2010;33:153–161. doi: 10.1016/j.tins.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AMG. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nature Rev Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- Chohan MO, Iqbal K. From tau to toxicity: emerging roles of NMDA receptor in Alzheimer’s disease. J Alzheimers Dis. 2006;10:81–87. doi: 10.3233/jad-2006-10112. [DOI] [PubMed] [Google Scholar]
- Coan EJ, Irving AJ, Collingridge GL. Low frequency activation of the NMDA receptor system can prevent the induction of LTP. Neurosci Lett. 1989;105:205–210. doi: 10.1016/0304-3940(89)90038-4. [DOI] [PubMed] [Google Scholar]
- Coba MP, Pocklington AJ, Collins MO, Kopanitsa MV, Uren RT, Swamy S, Croning MDR, Choudhary JS, Grant SGN. Neurotransmitters drive combinatorial multistate postsynaptic density networks. Sci Signal. 2009;2:ra19. doi: 10.1126/scisignal.2000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier RJ, Greenwood AC, Connor JA. Bidirectional synaptic plasticity correlated with the magnitude of dendritic calcium transients above a threshold. J Neurophysiol. 2001;85:399–406. doi: 10.1152/jn.2001.85.1.399. [DOI] [PubMed] [Google Scholar]
- Covey DF, Evers AS, Mennerick S, Zorumski CF, Purdy RH. Recent developments in structure-activity relationships for steroid modulators of GABAA receptors. Brain Res Rev. 2001;37:91–97. doi: 10.1016/s0165-0173(01)00126-6. [DOI] [PubMed] [Google Scholar]
- Creeley C, Wozniak DF, Labruyere J, Taylor GT, Olney JW. Low doses of memantine disrupt memory in adult rats. J Neurosci. 2006;26:3923–3932. doi: 10.1523/JNEUROSCI.4883-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Op Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Science STKE. 2004;255:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Nicola SM, Malenka RC. Induction in the rat hippocampus of long-term potentiation (LTP) and long-term depression (LTD) in the presence of a nitric oxide synthase inhibitor. Neurosci Lett. 1994;176:110–114. doi: 10.1016/0304-3940(94)90883-4. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Campbell AM, Woodson JC. Competitive interactions between endogenous LTD and LTP in the hippocampus underlie the storage of emotional memories and stress-induced amnesia. Hippocampus. 2005;15:1006–1025. doi: 10.1002/hipo.20107. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, Guidotti A. Brain 5-alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci (USA) 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci (USA) 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa F, Kavalali ET. NMDA receptor activation by spontaneous glutamatergic neurotransmission. J Neurophysiol. 2009;101:2290–2296. doi: 10.1152/jn.90754.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Stanton ME, Levine S, Thompson RF. Behavioral stress impairs long-term potentiation in rodent hippocampus. Behav Neural Biol. 1987;48:138–149. doi: 10.1016/s0163-1047(87)90664-9. [DOI] [PubMed] [Google Scholar]
- Francis PT. Altered glutamate neurotransmission and behavior in dementia: evidence from studies of memantine. Curr Mol Pharmacol. 2009;2:77–82. doi: 10.2174/1874467210902010077. [DOI] [PubMed] [Google Scholar]
- Frankiewicz T, Parsons CG. Memantine restores long-term potentiation impaired by tonic N-methyl-D-aspartate (NMDA) receptor activation following reduction of Mg2+ in hippocampal slices. Neuropharmacol. 1999;38:1253–1259. doi: 10.1016/s0028-3908(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Franks KM, Sejnowski TJ. Complexity of calcium signaling in synaptic spines. Bioessays. 2002;24:1130–1144. doi: 10.1002/bies.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Kuroda Y, Ito K-I, Yoshioka M, Kaneko K, Yamazaki Y, Sasaki H, Kato H. Endogenous adenosine regulates the effects of low-frequency stimulation on the induction of long-term potentiation in CA1 neurons of guinea pig hippocampal slices. Neurosci Lett. 2000;279:121–1224. doi: 10.1016/s0304-3940(99)00980-5. [DOI] [PubMed] [Google Scholar]
- Fujii S, Saito K, Miyakawa H, Ito K, Kato H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Res. 1991;555:112–122. doi: 10.1016/0006-8993(91)90867-u. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol Therap. 2007;116:125–139. doi: 10.1016/j.pharmthera.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisabella B, Rowan MJ, Anwyl R. Mechanisms underlying the inhibition of long-term potentiation by preconditioning stimulation in the hippocampus in vitro. Neuroscience. 2003;121:297–305. doi: 10.1016/s0306-4522(03)00440-8. [DOI] [PubMed] [Google Scholar]
- Gofton TE. Delirium: a review. Can J Neurol Sci. 2011;38:673–680. doi: 10.1017/s0317167100012269. [DOI] [PubMed] [Google Scholar]
- Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoll RA. Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron. 2011;71:1085–1101. doi: 10.1016/j.neuron.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guix FX, Uribesalgo I, Coma M, Munoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005;76:126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. The yin and yang of NMDA receptor signaling. Trends Neurosci. 2003;26:81–89. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signaling: implications for neurodegenerative disorders. Nature Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabetova S, Serrano P, Blace N, Tse HW, Skifter DA, Jane DE, Monaghan DT, Saktor TC. Distinct NMDA receptor subpopulations contribute to long-term potentiation and long-term depression induction. J Neurosci. 2000;20:RC81. doi: 10.1523/JNEUROSCI.20-12-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Colino A, Selig DK, Malenka RC. The influence of prior synaptic activity on the induction of long-term potentiation. Science. 1992;255:730–733. doi: 10.1126/science.1346729. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniakm DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and the fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Auberson YP, Zorumski CF. Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors. J Neurosci. 2006;26:7181–7188. doi: 10.1523/JNEUROSCI.1258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Clifford DB, Zorumski CF. Low concentrations of N-methyl-D-aspartate inhibit the induction of long-term potentiation in rat hippocampal slices. Neurosci, Lett. 1992a;137:245–248. doi: 10.1016/0304-3940(92)90414-3. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Clifford DB, Zorumski CF. Norepinephrine reverses N-methyl-D-aspartate-mediated inhibition of long-term potentiation in rat hippocampal slices. Neurosci Lett. 1992b;142:163–166. doi: 10.1016/0304-3940(92)90364-d. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Clifford DB, Zorumski CF. Inhibition of long-term potentiation by NMDA-mediated nitric oxide release. Science. 1992c;257:1273–1276. doi: 10.1126/science.1519065. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Izumi M, Matsukawa M, Funatsu M, Zorumski CF. Ammonia-mediated LTP inhibition: effects of NMDA receptor antagonists and L-carnitine. Neurobiol Disease. 2005a;20:615–624. doi: 10.1016/j.nbd.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Katsuki H, Benz AM, Zorumski CF. Oxygen deprivation produces delayed inhibition of LTP by activation of NMDA receptors and nitric oxide synthase. J Cerebral Bl Fl Metab. 1998;18:97–108. doi: 10.1097/00004647-199801000-00010. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Kitabayashi R, Funatsu M, Izumi M, Yuede C, Hartman RE, Wozniak DF, Zorumski CF. A single day of ethanol exposure during development has persistent effects on bi-directional plasticity, NMDA receptor function and ethanol sensitivity. Neuroscience. 2005b;136:269–279. doi: 10.1016/j.neuroscience.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Murayama K, Tokuda K, Krishnan K, Covey DF, Zorumski CF. GABAergic neurosteroids mediate the effects of ethanol on long-term potentiation in rat hippocampal slices. Eur J Neurosci. 2007;26:1881–1888. doi: 10.1111/j.1460-9568.2007.05809.x. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Nagashima K, Murayama K, Zorumski CF. Acute effects of ethanol on hippocampal LTP and LTD are mediated by different mechanisms. Neuroscience. 2005c;136:509–517. doi: 10.1016/j.neuroscience.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. Nitric oxide and long-term synaptic depression in the rat hippocampus. NeuroReport. 1993;4:1131–1134. [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. Involvement of nitric oxide in low glucose-mediated inhibition of hippocampal long-term potentiation. Synapse. 1997;25:258–262. doi: 10.1002/(SICI)1098-2396(199703)25:3<258::AID-SYN4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. Norepinephrine promotes long-term potentiation in the adult rat hippocampus in vitro. Synapse. 1999;31:196–202. doi: 10.1002/(SICI)1098-2396(19990301)31:3<196::AID-SYN4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. Direct cortical inputs erase long-term potentiation at Schaffer collateral synapses. J Neurosci. 2008;28:9557–9563. doi: 10.1523/JNEUROSCI.3346-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. Neuroprotective effects of pyruvate following NMDA-mediated excitotoxic insults in hippocampal slices. Neurosci Lett. 2010;478:131–135. doi: 10.1016/j.neulet.2010.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry. 2004;9:984–997. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL, Lo S-C, Bru-Mercier G, Dickinson B, Scullion S, Sheng M, Collingridge G, Cho K. Aβ1–42 inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3β. Nature Neurosci. 2011;14:545–547. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- Joels M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci. 2006;27:244–250. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Joels M, Kruger HJ. LTP after stress: up or down? Neural Plast. 2007:93202. doi: 10.1155/2007/93202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Li ST, Zorumski CF. Modulation of LTP induction in the hippocampus by NMDA-mediated presynaptic inhibition. Neuroscience. 1999;92:1261–1272. doi: 10.1016/s0306-4522(99)00080-9. [DOI] [PubMed] [Google Scholar]
- Kato K, Zorumski CF. Nitric oxide inhibitors facilitate the induction of hippocampal long-term potentiation by modulating NMDA responses. J Neurophysiol. 1993;70:1260–1263. doi: 10.1152/jn.1993.70.3.1260. [DOI] [PubMed] [Google Scholar]
- Katsuki H, Izumi Y, Zorumski CF. Noradrenergic regulation of synaptic plasticity in the hippocampal CA1 region. J Neurophysiol. 1997;77:3013–3020. doi: 10.1152/jn.1997.77.6.3013. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proc Natl Acad Sci (USA) 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-I, Lee H-R, Sim S, Baek J, Yu N-K, Choi J-H, Ko H-G, Lee Y-S, Park S-W, Kwak C, Ahn S-J, Choi SY, Kim H, Kim K-H, Backx PH, Bradley CA, Kim E, Jang D-J, Lee K, Kim SJ, Zhuo M, Collingridge GL, Kaang B-K. PI3Kγ is required for NMDA receptor-dependent long-term depression and behavioral flexibility. Nature Neurosci. 2011;14:1447–1454. doi: 10.1038/nn.2937. [DOI] [PubMed] [Google Scholar]
- Kimoto T, Tsurugizwa T, Ohta Y, Makino J, Tamura HO, Hojo Y, Takata N, Kawato S. Neurosteroid synthesis by cytochrome p450-containing systems localized in rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology. 2001;142:3578–3589. doi: 10.1210/endo.142.8.8327. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Zhou M, Joels M, Kindt M. Regulation of excitatory synapses and fearful memories by stress hormones. Front Behav Neurosci. 2011;5:1–11. doi: 10.3389/fnbeh.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavaque E, Sierra A, Azcoitia I, Garcia-Segura LM. Steroidogenic acute regulatory protein in the brain. Neuroscience. 2006;138:741–747. doi: 10.1016/j.neuroscience.2005.05.060. [DOI] [PubMed] [Google Scholar]
- Lee HK, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors I hippocampus. Neuron. 1998;21:1151–1162. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- Lei SZ, Pan ZH, Aggarwal SK, Chen H-SC, Hartman J, Sucher NJ, Lipton SA. Effect of nitric oxide production on the redox modulatory site of the NMDA receptor-channel complex. Neuron. 1992;8:1087–1099. doi: 10.1016/0896-6273(92)90130-6. [DOI] [PubMed] [Google Scholar]
- Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010a;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jo J, Jia JM, Lo SC, Whitcomb DJ, Jiao S, Cho K, Sheng M. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010b;141:859–871. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Augerson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Three Ca2+ levels affect plasticity differently: the LTP zone, the LTD zone and no man’s land. J Physiol. 2001;532:1469–1473. doi: 10.1111/j.1469-7793.2001.0285f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation and clinical implications. Pharmacol Therap. 2003;97:55–85. doi: 10.1016/s0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- Luo J, Wang Y, Yasuda RP, Dunah AW, Wolfe BB. The majority of N-methyl-D-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B) Mol Pharmacol. 1997;51:79–86. doi: 10.1124/mol.51.1.79. [DOI] [PubMed] [Google Scholar]
- Ma YY, Cepeda C, Cui CL. The role of striatal NMDA receptors in drug addiction. Int Rev Neurobiol. 2009;89:131–146. doi: 10.1016/S0074-7742(09)89006-5. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA. Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther. 2009;123:143–150. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 2011;16:252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarassment of riches. Neuron. 2004;44:5–12. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, von Haebler D, Winter C, Juckel G, Heinemann U. A single application of MK-801 causes symptoms of psychosis, deficits in spatial memory, and impairment of synaptic plasticity in rats. Hippocampus. 2008;18:125–134. doi: 10.1002/hipo.20367. [DOI] [PubMed] [Google Scholar]
- Manzoni O, Prezeau L, Marin P, Deshager S, Bockaert J, Fagni L. Nitric oxide-induced blockade of NMDA receptors. Neuron. 1992;8:653–662. doi: 10.1016/0896-6273(92)90087-t. [DOI] [PubMed] [Google Scholar]
- Marcaida G, Felipo V, Hermenegildo C, Minana MD, Grisolia S. Acute ammonia toxicity is mediated by the NMDA type of glutamate receptors. FEBS. 1992;296:67–68. doi: 10.1016/0014-5793(92)80404-5. [DOI] [PubMed] [Google Scholar]
- Marsden WN. Stressor-induced NMDAR dysfunction as a unifying hypothesis for the aetiology, pathogenesis and comorbidity of clinical depression. Medical Hypotheses. 2011 doi: 10.1016/j.mehy.2011.06.021. (epub) [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RGM. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Armstrong N. Structure and function of glutamate receptor ion channels. Annu Rev Physiol. 2004;66:161–181. doi: 10.1146/annurev.physiol.66.050802.084104. [DOI] [PubMed] [Google Scholar]
- McCool BA. Ethanol modulation of synaptic plasticity. Neuropharmacol. 2011;61:1097–1108. doi: 10.1016/j.neuropharm.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack SG, Stornetta RL, Zhu JJ. Synaptic AMPA receptor exchange maintains bidirectional plasticity. Neuron. 2006;50:75–88. doi: 10.1016/j.neuron.2006.02.027. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Meredith RM, Floyer-Lea A, Paulsen O. Maturation of long-term potentiation induction rules in rodent hippocampus: role of GABAergic inhibition. J Neurosci. 2003;23:11142–11146. doi: 10.1523/JNEUROSCI.23-35-11142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Postsynaptic modulation of NMDA synaptic currents in rat hippocampal microcultures by paired pulse stimulation. J Physiology (London) 1996;490:405–417. doi: 10.1113/jphysiol.1996.sp021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Survival and neural activity in the developing nervous system. Mol Neurobiol. 2000;22:41–54. doi: 10.1385/MN:22:1-3:041. [DOI] [PubMed] [Google Scholar]
- Milnerwood AJ, Raymond LA. Early synaptic pathophysiology in neurodegeneration: insights from Huntington’s disease. Trends Neurosci. 2010;33:513–523. doi: 10.1016/j.tins.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Mitchell ND, Baker GB. An update on the role of glutamate in the pathophysiology of depression. Acta Psychiatr Scand. 2010;122:192–210. doi: 10.1111/j.1600-0447.2009.01529.x. [DOI] [PubMed] [Google Scholar]
- Mockett B, Coussens C, Abraham WC. NMDA receptor-mediated metaplasticity during the induction of long-term depression by low-frequency stimulation. Eur J Neurosci. 2002;15:1819–1826. doi: 10.1046/j.1460-9568.2002.02008.x. [DOI] [PubMed] [Google Scholar]
- Molnar E, Pickard L, Duckworth JK. Developmental changes in ionotropic glutamate receptors: lessons from hippocampal synapses. Neuroscientist. 2002;8:143–153. doi: 10.1177/107385840200800210. [DOI] [PubMed] [Google Scholar]
- Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacol. 2007;52:71–76. doi: 10.1016/j.neuropharm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Heath AC, Bucholz KK, Madden PA, Fu Q, Knopik V, Lynskey MT, Whitfield JB, Statham DJ, Martin NG. Genetic epidemiology of alcohol-induced blackouts. Arch Gen Psychiatry. 2004;61:257–263. doi: 10.1001/archpsyc.61.3.257. [DOI] [PubMed] [Google Scholar]
- Nishizawa Y. Glutamate release and neuronal damage in ischemia. Life Sci. 2001;69:369–381. doi: 10.1016/s0024-3205(01)01142-0. [DOI] [PubMed] [Google Scholar]
- Nourry C, Grant SG, Borg JP. PDZ domain proteins: plug and play. Sci STKE. 2003;179:re7. doi: 10.1126/stke.2003.179.re7. [DOI] [PubMed] [Google Scholar]
- Olney JW. Brain lesions, obesity and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244:1360–1362. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- Ondrejcak T, Klyubin I, Hu NW, Barry AE, Cullen WK, Rowan MJ. Alzheimer’s disease amyloid beta-protein and synaptic function. Neuromolecular Med. 2010;12:13–26. doi: 10.1007/s12017-009-8091-0. [DOI] [PubMed] [Google Scholar]
- Paoletti P. Molecular basis of NMDA receptor functional diversity. Eur J Neurosci. 2011;33:1351–1365. doi: 10.1111/j.1460-9568.2011.07628.x. [DOI] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JTR, Bortolotto ZA, Wang YT, Collingridge GL. LTP inhibits LTD in the hippocampus via regulation of GSK3β. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Askalan R, Paul S, Kalia LV, Nguyen TH, Pitcher GM, Salter MW, Lombroso PJ. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34:127–138. doi: 10.1016/s0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci. 2003;23:5583–5588. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocklington AJ, Armstrong JD, Grant SG. Organization of brain complexity – synapse proteome form and function. Brief Funct Genomic Proteomic. 2006;5:66–73. doi: 10.1093/bfgp/ell013. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Paul SM. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci (USA) 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Reyes-Harde M, Potter BV, Galione A, Stanton PK. Induction of hippocampal LTD requires nitric oxide-stimulated PKG activity and Ca2+ release from cyclic ADP-ribose-sensitive stores. J Neurophysiol. 1999;82:1569–1576. doi: 10.1152/jn.1999.82.3.1569. [DOI] [PubMed] [Google Scholar]
- Ryan BK, Vollmayr B, Klyubin I, Gass P, Rowan MJ. Persistent Inhibition of hippocampal long-term potentiation in vivo by learned helplessness stress. Hippocampus. 2010;20:758–767. doi: 10.1002/hipo.20677. [DOI] [PubMed] [Google Scholar]
- Saalman YB, Kirkcaldie MT, Waldron S, Calford MB. Cellular distribution of the GABAA receptor-modulating 3α-hydrox, 5α-reduced pregnane steroids in the adult rat brain. J Neuroendocrinol. 2007;19:272–284. doi: 10.1111/j.1365-2826.2006.01527.x. [DOI] [PubMed] [Google Scholar]
- Sacktor TC. How does PKMζ maintain long-term memory? Nat Rev Neurosci. 2011;12:9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- Sacktor TC, Fenton AA. Appropriate application of ZIP for PKMζ inhibition, LTP reversal and memory erasure. Hippocampus. 2011 doi: 10.1002/hipo.20978. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Salazar M, Pariente JA, Salido GM, Gonzalez A. Ethanol induces glutamate secretion by Ca2+ mobilization and ROS generation in rat hippocampal astrocytes. Neurochem Int. 2008;52:1061–1067. doi: 10.1016/j.neuint.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Sandi C. Glucocorticoids act on glutamatergic pathways to affect memory processes. Trends Neurosci. 2011;34:165–176. doi: 10.1016/j.tins.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABA-A receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- Schummers J, Bentz S, Browning MD. Ethanol’s inhibition of LTP may not be mediated solely via direct effects on the NMDA receptor. Alcohol Clin Exp Res. 1997;21:404–408. doi: 10.1111/j.1530-0277.1997.tb03783.x. [DOI] [PubMed] [Google Scholar]
- Schummers J, Browning MD. Evidence for a role for GABAA and NMDA receptors in ethanol inhibition of long-term potentiation. Mol Brain Res. 2001;94:9–14. doi: 10.1016/s0169-328x(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Selig DK, Hjelmstad GO, Herron C, Nicoll RA, Malenka RC. Independent mechanisms for long-term depression of AMPA and NMDA responses. Neuron. 1995;15:417–426. doi: 10.1016/0896-6273(95)90045-4. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress and anxiety disorders. Neuropsychopharmacol Rev. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky I, Abumaria N, Wu LJ, Huang C, Zhang L, Li B, Zhao X, Govindarajan A, Zhao MG, Zhuo M, Tonegawa S, Liu G. Enhancement of learning and memory by elevating brain magnesium. Neuron. 2010;65:165–177. doi: 10.1016/j.neuron.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Soriano FX, Papadia S, Hofmann F, Hardingham NR, Bading H, Hardingham GE. Preconditioning doses of NMDA promote neuroprotection by enhancing neuronal excitability. J Neurosci. 2006;26:4509–4518. doi: 10.1523/JNEUROSCI.0455-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Op Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Tokuda K, Izumi Y, Zorumski CF. Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. J Neurosci. 2011;31:9905–9909. doi: 10.1523/JNEUROSCI.1660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K, O’Dell KA, Izumi Y, Zorumski CF. Midazolam inhibits hippocampal long-term potentiation and learning through dual central and peripheral benzodiazepine receptor activation and neurosteroidogenesis. J Neurosci. 2010;30:16788–16795. doi: 10.1523/JNEUROSCI.4101-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez CM, Smith MA, Perry G, Phelix CF, Santamaria F. Cholesterol homeostasis markers are localized to mouse hippocampal pyramidal and granule layers. Hippocampus. 2010;20:902–905. doi: 10.1002/hipo.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins JC. L-glutamate as a central transmitter: looking back. Biochem Soc Trans. 2000;28:297–309. [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol. 2003;43:335–358. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- White AM. What happened? Alcohol, memory blackouts and the brain. Alcohol Res Health. 2003;27:186–196. [PMC free article] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Ann NY Acad Sci. 2004;1021:206–220. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. Facilitated induction of hippocampal long-lasting potentiation during blockade of inhibition. Nature. 1983;301:603–604. doi: 10.1038/301603a0. [DOI] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. Behavioral stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- Xue J-G, Masuoka T, Gong X-D, Chen K-S, Yanagawa Y, Law SKA, Konishi S. NMDA receptor activation enhances inhibitory GABAergic transmission onto hippocampal pyramidal neurons via presynaptic and postsynaptic mechanisms. J Neurophysiol. 2011;105:2897–2906. doi: 10.1152/jn.00287.2010. [DOI] [PubMed] [Google Scholar]
- Yang CH, Huang CC, Hsu KS. Novelty exploration elicits a reversal of acute stress-induced modulation of hippocampal synaptic plasticity in the rat. J Physiol. 2006;577:601–615. doi: 10.1113/jphysiol.2006.120386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PC, Yang CH, Huang CC, Hsu KS. Phosphatidylinositol 3-kinase activation is required for stress protocol-induced modification of hippocampal synaptic plasticity. J Biol Chem. 2008;283:2631–2643. doi: 10.1074/jbc.M706954200. [DOI] [PubMed] [Google Scholar]
- Youssef FF, Addae JI, Stone TW. NMDA-induced preconditioning attenuates synaptic plasticity in the rat hippocampus. Brain Res. 2006;1073–1074:183–189. doi: 10.1016/j.brainres.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Zajaczkowski W, Frankiewicz T, Parsons CG, Danysz W. Uncompetitive NMDA receptor antagonists attenuate NMDA-induced impairment of passive avoidance learning and LTP. Neuropharmacol. 1997;36:961–971. doi: 10.1016/s0028-3908(97)00070-1. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot BD, Miyakawa T, Bear MF, Tonegawa S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Pak D, Qin Y, McCormack SG, Kim MJ, Baumgart JP, Velamoor V, Auberson YP, Osten P, van Aelst L, Sheng M, Zhu JJ. Rap-2-JNK removes synaptic AMPA receptors during depotentiation. Neuron. 2005;46:905–916. doi: 10.1016/j.neuron.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Olney JW. Excitotoxic neuronal damage and neuropsychiatric disorders. Pharmacol Ther. 1993;59:145–162. doi: 10.1016/0163-7258(93)90043-d. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Rubin EH. Psychiatry and Clinical Neuroscience: A Primer. Oxford University Press; New York: 2011. [Google Scholar]
- Zorumski CF, Yang J, Fischbach GD. Calcium dependent, slow desensitization distinguishes different types of glutamate receptors. Cell Molec Neurobiol. 1989;9:95–104. doi: 10.1007/BF00711446. [DOI] [PMC free article] [PubMed] [Google Scholar]