Abstract
The developing nervous system is particularly vulnerable to chemical insults. Exposure to chemicals can results in neurobehavioural alterations, and these have been be used as sensitive readouts to assess neurotoxicity in animals and man. Deconstructing neurobehaviour into relevant cellular and molecular components may allow for detection of specific neurotoxic effects in cell-based systems, which in turn may allow an easier examination of neurotoxic pathways and modes of actions and eventually inform the regulatory assessment of chemicals with potential developmental neurotoxicity. Here, current developments towards these goals are reviewed. Imaging genetics (CB) provides new insights into the neurobiological correlates of cognitive function that are being used to delineate neurotoxic mechanisms. The gaps between in vivo neurobehaviour and real-time in vitro measurements of neuronal function are being bridged by ex vivo measurements of synaptic plasticity (RW). An example of solvent neurotoxicity demonstrates how an in vivo neurological defect can be linked via the N-methyl-D-aspartate (NMDA)-glutamate receptor as a common target to in vitro readouts (AB). Axonal and dendritic morphology in vitro proved to be good correlates of neuronal connectivity and neurobehaviour in animals exposed to polychlorinated biphenyls and organophosphorus pesticides (PJL). Similarly, chemically-induced changes in neuronal morphology affected the formation of neuronal networks on structured surfaces. Such network formation may become an important readout for developmental neurotoxicity in vitro (CvT), especially when combined with human neurons derived from embryonic stem cells (ML). We envision that future in vitro test systems for developmental neurotoxicity will combine the above approaches with exposure information, and we suggest a strategy for test system development and cell-based risk assessment.
Keywords: neurite growth, neurodifferentiation, neurotransmitter, electrophysiology neurogenetics, stem cells
1. Lessons from the past and new challenges for the future of neurotoxicity testing
Due to metabolic (e.g., high oxygen and energy demands) and cytoarchitectonic characteristics (e.g. considerable distances between somata and synaptic compartments), the nervous system is vulnerable to xenobiotics, such as environmental chemicals and pharmaceuticals. Accordingly, the CNS is often considered as the most frequent target organ of systemic toxicity (Klaassen, 2008). For more than 40 years (Reiter, 1978) behavioural readouts (e.g., motor activity, response acuity and speed) have been used in experimentally, environmentally or accidentally exposed laboratory animals and humans to identify the neurotoxic potency of particular compounds. Such neurobehavioural readouts are an integrative approach for investigating the integrity of the nervous system as a whole that does not require an understanding of the underlying neurobiological mechanisms. Triggered in part by the National Research Council (NRC, 2007) report of its vision of toxicity testing in the 21st century (Tox-21c), researchers from behavioural toxicology described some toxicity pathways associated with neurotoxic mechanisms of e.g. solvents, phthalates and thyroid signalling disrupters (Bushnell et al., 2010). Some of these toxicity pathways may involve neurobiological mechanisms implicated in cognitive function (e.g. Kandel, 2001), suggesting that assays that incorporate endpoints of relevance to these neurobiological mechanisms may provide mechanistically relevant surrogates of impaired performance of humans and experimental animals in neurobehavioural tests that assess different functional domains of the CNS (e.g. attention, memory, motor function). Thus, we propose that this emerging knowledge from molecular and cellular neuroscience and mechanistic neurotoxicology can be exploited to design in vitro tests that read out cellular and molecular endpoints that are predictive of behavioural signs of neurotoxic exposures in humans. For acute or more chronic neurotoxic effects the onset of these effects is temporally associated with the onset of the chemical exposure and usually follows a dose-response relationship. However, the discipline of developmental/ neurodevelopmental toxicology faces an additional problem. It is difficult to provide evidence for cause-effect relationships for processes with a long lag time, and to identify suitable test systems for delayed effects. Research in this particular area is also motivated by increasing incidence of neurodevelopmental disorders such as autism, ADHD and schizophrenia and the growing awareness that environmental factors influence susceptibility and/or severity of these diseases (Eubig et al. 2010; Herbert, 2010; Landrigen, 2010; Pessah and Lein, 2008, Winneke, 2011).
Developmental exposure to chemicals and drugs can alter behavioural function in young and adult animals (e.g. Werboff and Dembicki, 1962). Therefore, behavioural readouts have been used and validated since the 1960s as an apical measure of neurotoxicity. In the 1980s, the U.S. Environmental Protection Agency (U.S.E.P.A.) developed the first DNT guidelines and initiated the standardisation of this testing strategy by the Organisation of Economic Cooperation and Development (OECD). The pertinent OECD test guideline 426 was finally accepted in 2007 (Makris et al., 2009). A recent review (Makris et al., 2009) revealed that just over 100 compounds have been tested in studies using the OECD 426 draft guideline. Most of these compounds were pesticides (66%) and only 8 industrial chemicals were included. Another review identified about 174 compounds for which neurobehavioural risk assessment had been performed, in many cases also on the offspring of the exposed animals (F1 generation). Only 1% of these compounds were industrial chemicals (Middaugh et al., 2003). Thus, the available data regarding the developmental neurotoxicity of industrial chemicals is thus rather limited. For some compounds developmental neurotoxicity is the most sensitive of all toxicity endpoints evaluated in a broad safety evaluation battery. Thus, although developmental neurotoxicity appears to be an important domain of safety evaluation, test capacity is limited and test costs are extremely high. This puts pressure on the development of faster and cheaper in vitro systems that can predict developmental neurotoxicity, give information comparable to behavioural readouts, and facilitate screening or at least prioritization of relevant drugs and chemicals for further testing.
Endless numbers of in vitro tests would be required to model each biological process that may be affected by toxicants. However, many of the underlying mechanisms and signalling pathways are shared by the various biological processes. This thinking has led to the development of the Tox-21 strategy, which assumes that there is a limited number of toxicity pathways and mechanisms (NRC, 2007, Leist et al., 2008b). It suggests the setup of assays that examine quantitative cause-effect relationships with reference to relevant and convergent toxicity pathways. Then, prediction models (e.g., as probed in the ToxCast program) would integrate this information to arrive at a hazard assessment. Here, an initial basis is provided for ideas and approaches to utilize new knowledge and ideas for the design of such future test systems for DNT and probably neurotoxicity in the developed brain.
This review is arranged in three sections starting with the new and promising approach of genetic imaging that allows for a better understanding of neurobiological processes underlying specific behaviours in humans. The second section is composed of four chapters describing in vivo and in vitro approaches addressing specific toxicology pathways and functional endpoints (e.g., dendritic and axonal morphology, network formation) of existing cell systems. The last section will focus on the development of suitable in vitro test systems for developmental neurotoxicity based on pluripotent stem cells and neuronal precursors cells (NPCs).
2. Imaging genetics – SNPs and their relation to inter-individual differences in performing cognitive/ neurobehavioural tests
The field of ‘imaging genetics’ has attracted much interest in recent years. In behavioural sciences, researchers are faced with large inter-individual differences between people and there is growing interest in elucidating the neurobiological foundations that may contribute to such difference on a ‘behavioural phenotype’ level. In this regard a ‘candidate gene’ approach is widely used. This approach usually begins with selecting a biological aspect of a particular condition and then identifies variants in genes and meaningful DNA sequences within a candidate gene that are thought to impact the candidate biological process (Hariri & Weinberger, 2003). For the variant to be significant, it should have an impact at the molecular and cellular level in gene or protein function (i.e., be a functional variation) and the distribution of such effects at the level of brain systems involved in specific forms of information processing should be predictable (Hariri & Weinberger, 2003). Also, candidate genes with identified single nucleotide polymorphisms (SNPs) or other allele variants in coding or promoter regions with likely functional implications (e.g., non-conservative amino acid substitution or missense mutation in a promoter consensus sequence) involving circumscribed neuroanatomical systems would be attractive substrates (Hariri & Weinberger, 2003). When using this approach the main research question is how cognitive functions or performance in a given neuropsychological test vary for individuals that are characterized by different polymorphisms (Scerif & Karmiloff-Smith, 2005). In this way the ‘candidate gene approach’ serves as a bridge between different levels of investigation and hence integrates the behavioural level with the neurobiological level. Using such an approach one may assess the relevance of circumscribed neurobiological processes for a specific behaviour in vivo. This knowledge on key regulators of human behaviour may then be translated to refine in vitro models and to arrive at more sophisticated endpoints. The below example of BDNF polymorphism illustrate this approach:
Brain-derived neurotrophic factor (BDNF) is an important neuromodulator influencing cognitive processes. The functional BDNF Val66Met polymorphism has attracted much interest in recent years. This polymorphism alters the secretion of the mature peptide (Egan et al., 2003) and is relevant for cognitive functions (Bath et al., 2006). Compared to the met allele, the Val allele is associated with higher activity of the BDNF system (e.g., Rybakowski, 2008), leading to increased neural activity (Kafitz et al., 1999). Besides modulating processes in hippocampal brain structures that are important for learning and memory, BDNF plays also an important role in functional basal ganglia-prefrontal circuits (Han et al., 2010) that are relevant for other cognitive functions. Basal ganglia-prefrontal loops play an important role in processes related to the selection, control and inhibition of actions. Within these loops, BDNF modulates cortico-striatal synapses (Han et al., 2010; Foltynie et al., 2009). Moreover, BDNF has been shown to be an important regulator of gene expression in medium spiny neurons in the striatum (Saylor et al., 2006; Saylor & McGinty, 2008) and to modulate dopaminergic neural transmission (Do et al., 2007; Porritt et al., 2005; Narita et al., 2003; Guillin et al., 2001). BDNF can upregulate dopamine D1 receptors (Do et al., 2007), and dopamine D1-receptor signalling at a striatal level is partly mediated via TrkB-receptors (Iwakura et al., 2008), i.e., the receptor type that mediates neural excitation by binding BDNF (Kafitz et al., 1999). Obviously, BDNF plays a pivotal role in modulating striatal neurobiological processes and BDNF should impact action selection processes, as well.
Beste et al. (2010a) showed that processes related to the adaptation of actions after the commitment of response errors are affected by the BDNF Val66Met polymorphism. They showed that Met allele carriers revealed dysfunctional behavioural adaptation after the commitment of a response error, compared to a Val/Val genotype group. Examining neurophysiological processes underlying the behavioural effects, they showed that it is the degree of neural synchronization processes, which differs between genotypes and drives efficacy of post-error behavioural adaptation. After correct responses, neurophysiological processes were not modulated by the polymorphism, underlining that BDNF becomes especially necessary in situations requiring behavioural adaptation. BDNF may exert these error-specific effects either by augmenting phasic dopaminergic signalling or the efficacy of cortico-striatal synapses (Beste et al., 2010a). This result is well in line with numerous studies accounting for compromised cognitive processes in Met allele carriers. However, this raises a simple, but far reaching question: Why is an allele evolutionarily preserved that confers a disadvantage, or risk to its carriers? To be evolutionary sustained it is necessary that met alleles of the BDNF Val66Met polymorphism confer at least some advantage to its carriers (Tettamanti et al., 2009). In this regard, another cognitive function related to action selection processes (i.e., response inhibition functions) seems to reveal an opposite pattern of association. Beste et al., (2010b) showed that response inhibition processes are rendered more efficient in Met allele carriers. Also other studies accounted for benefits of the Met allele in some cognitive domains (Gasic et al., 2009). With respect to differences in post-error adaptation versus response inhibition processes, it is interesting that Parkinson’s disease patients reveal a similar pattern of results (Beste et al., 2010c; Beste et al., 2010d). The basal ganglia are fine-tuned by parallel inhibitory and excitatory loops, i.e., the direct and the indirect loops (DeLong and Wichmann, 2007). These loops reveal different characteristics (e.g. Gale et al., 2008): Decreases in nigro-striatal activity render the direct pathway less active while the indirect pathway becomes more active (Gale et al., 2008). It is possible that Met alleles may displace the balance between the direct and indirect pathway leaving a predominant indirect pathway (Beste et al., 2010b). This displacement may lead to the observed effects in post-error adaptation opposed to response inhibition processes. Such opposing directions of association have recently also been reported for gene coding for pro-inflammatory cytokines (Beste et al., 2010d; Beste et al., 2011b), as well as for the serotonin 1 A receptor (Beste et al., 2011a) and the NMDA receptor system (Beste et al., 2008).
These examples illustrate that the choice of cognitive-neurophysiological outcome measure or ‘read-out’ is critical, and needs careful translation into potential in vitro models. The effects of a specific neurobiological parameter on cognitive processes very much depend upon characteristics of the neuronal networks mediating a specific cognitive function. Depending on network characteristics, one neurobiological parameter (e.g. BDNF) can have opposing effects on cognitive functions. Generally, ‘imaging genetics’ may provide a useful tool to develop in vivo readouts that relate to cognitive-neurophysiological parameters in humans. However, even though this field offers a promising technique to gain insight into the neurobiological foundation of cognitive brain functions, when using these results for cellular of molecular endpoint selection for in vitro assays, the interpretation of the data is not always univocal. As Kovas and Plomin (2006) pointed out there are three possible mechanisms of effects of a single gene on the brain and associated cognitive processes. Due to the first mechanism a gene influences one area of the brain, which influences different, interrelated cognitive processes mediated by this brain area (Kovas & Plomin, 2006). Alternatively, a gene may influence several areas of the brain, with each area mediating a specific cognitive process, or a gene influences several brain areas that are all relevant for a specific cognitive function.
In conclusion, the various applications of imaging genetics might provide important insights into the neurobiological correlates of specific aspects of cognitive functioning (e.g. action selection). In vitro tests targeting any alternation of these correlates (e.g. deviant BNDF levels in glutamatergic cells) may be able to detect neurotoxic mechanisms that are also of relevance for neurobehavioural effect observed for a particular compound.
3. Bridging the gap between in vitro and in vivo neurotoxicity: do we need sophisticated models or functional endpoints?
Current (developmental) neurotoxicity testing largely relies on (behavioural) animal testing. This is due to the sensitivity of the model and test system, but also to the absence of validated and reliable in vitro alternatives that are complex enough to model the in vivo situation. Unfortunately, (behavioural) animal testing is still a 'black box' that provides limited mechanistic insights, whereas in vitro systems generally allow for a much larger 'experimental freedom' as well as mechanistic insight. In an attempt to bridge the gap between in vitro and in vivo neurotoxicity, the neurotoxic effects of the brominated flame retardant 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) have been investigated using a simple in vitro model (PC12 cells) as well as in ex vivo brain slices.
BDE-47 is globally dispersed throughout the environment due to its lipophilic, persistent and bioaccumulative properties and a large range of human tissue and serum levels have been reported (for a review see Dingemans et al., 2011). Early behavioural studies have demonstrated long-lasting adverse effects on learning and memory in mice following a single neonatal exposure to BDE-47 (Eriksson et al., 2001). To provide a functional basis for these behavioural effects, long-term potentiation (LTP) of field-excitatory postsynaptic potentials (fEPSPs) was measured ex vivo in hippocampal slices from BDE-47-exposed mice. LTP, which is induced by tetanic stimulation, strong depolarization and a large increase in intracellular Ca2+ level (for review see Lynch, 2004), has been used as an electrophysiological substrate for learning and memory for many years. In hippocampal slices from control mice, tetanic stimulation evoked a large and sustained (>30 min) increase of the f-EPSPs, indicative for LTP. However, in accordance with the observed behavioural effects, LTP was reduced by >50% in slices from BDE-47-exposed mice (Dingemans et al., 2007). Subsequent Western blot analysis of protein expression levels in the postsynaptic density (PSD) of the hippocampus revealed that the expression levels of the NMDA receptor subunit NR2B, the AMPA receptor subunit GluR1 and the autophosphorylated-active form of αCaMK-II were significantly reduced (Dingemans et al., 2007). As CamK-II autophosphorylation is essential for hippocampal NMDA-dependent LTP (Giese et al. 1998), this specific effect may underlie the reduction in synaptic plasticity resulting in behavioural impairments.
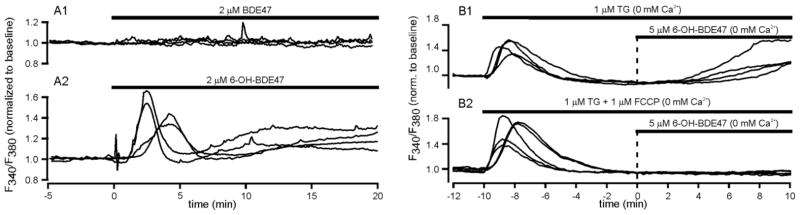
While it appears that ex vivo measurements of LTP and levels of postsynaptic proteins can serve as a first step, it will be difficult to bridge the gap with in vitro. In vitro measurements of the intracellular Ca2+ concentration ([Ca2+]i) can potentially aid in achieving this goal as [Ca2+]i plays an essential role in a large number of cellular processes, including neurotransmission (for reviews see: Garcia, et al. 2006, Westerink 2006) and gene expression (for review see: Carrasco and Hidalgo 2006). Therefore, acute effects of BDE-47 exposure on in vitro calcium homeostasis were studied in PC12 cells using single cell fluorescent microscopy. These in vitro data demonstrated only a modest increase in [Ca2+]i at 20 μM BDE-47 (Dingemans et al., 2007), whereas the doses of BDE-47 resulting in impaired learning and memory and LTP were estimated to result in peak brain concentrations of approximately 1 μM (Staskal et al. 2006). A major difference with the in vivo situation is the absence of metabolism in in vitro experiments. The effects of 6-OH-BDE-47, a hydroxylated metabolite of BDE-47, were therefore compared with the effects of BDE-47 on Ca2+ homeostasis. The data demonstrated that cells exposed to 2 μM BDE-47 did not show any increase in [Ca2+]i (Fig. 1A1), whereas cells exposed to 2 μM 6-OH-BDE-47 showed a clear increase in [Ca2+]i that consisted of an initial transient increase followed by an additional mores sustained increase (Fig. 1A2). These findings thus clearly demonstrate that hydroxylation, i.e., metabolism, increases the neurotoxic potential of BDE-47 (Dingemans et al., 2008).
Figure 1. Effects of BDE-47 and 6-OH-BDE-47 on calcium homeostasis.
Example recording of [Ca2+]i, expressed as normalized F340/F380, in PC12 cells after exposure to BDE-47 (2 μM, A1) or its metabolite 6-OH-BDE-47 (2 μM, A2) as indicated by the bar on top of the recordings. Exposure to 6-OH-BDE-47 induced a biphasic increase in [Ca2+]i, whereas BDE-47 was without effect. The biphasic increase in [Ca2+]i originated from ER and mitochondrial intracellular Ca2+ stores as the initial transient increase was absent following depletion of ER with TG and the late increase was absent when both ER and mitochondria were depleted using TG + FCCP (modified after Dingemans et al., 2008).
A major advantage of this in vitro approach is that it allows for investigation of the mechanisms underlying the biphasic increase in [Ca2+]i. Further experimentation under Ca2+-free conditions revealed that the increase in [Ca2+]i originates from intracellular stores. Additional Ca2+ imaging experiments demonstrated that 6-OH-BDE-47 was no longer able to evoke the initial transient increase in [Ca2+]i when the endoplasmic reticulum (ER) was depleted from Ca2+, though the late increase was still present (Fig. 1B1). When both ER and mitochondrial intracellular Ca2+ stores were depleted under Ca2+-free conditions, both the initial transient and the late increase in [Ca2+]i were completely absent (Fig. 1B2). These combined data thus indicate that the initial transient increase in [Ca2+]i is mainly due to intracellular Ca2+ release from the ER, whereas the late increase is mainly due to Ca2+ release from mitochondria (Dingemans et al., 2008).
As various hydroxylated metabolites of BDE-47 have been found to bioaccumulate in humans (for review see Dingemans et al., 2011), the acute effects of 6 OH-BDE 47, 6-OH-BDE-49, 5 OH-BDE-47, 3-OH-BDE-47, and 4-OH-BDE-49 on [Ca2+]i were investigated in PC12 cells. All investigated hydroxylated metabolites induced Ca2+ release from intracellular stores (mainly ER or both ER and mitochondria), although with different lowest observed effect concentrations (LOECs), ranging from 1–20 μM (Dingemans et al., 2010). Importantly, when the OH group was shielded on both sides by either the other phenyl ring and/or Br atoms (as in 6-OH-BDE-49 and 3-OH-BDE-47), the OH-PBDE increased [Ca2+]i less efficiently than when the OH group was shielded on only one side (as in 6 OH-BDE 47, 5 OH-BDE 47, and 4-OH-BDE-49). The toxicity of OH-PBDEs is thus attenuated by shielding of the OH group on both sides (Dingemans et al., 2010). These results thereby further demonstrate that oxidative metabolism should be included in human risk assessment of environmental pollutants.
When metabolites must also be taken into account for (in vitro) neurotoxicity, the number of compounds to be investigated increases dramatically. While single cell fluorescent microscopy is perfectly suited to measure real-time dynamic changes [Ca2+]i with high sensitivity, it is also labour intensive and time consuming. As a result, the demand for high-throughput screening (HTS) is increasing. Though HTS has proven powerful for static endpoints such as cell viability, the highly dynamic nature of Ca2+ regulation makes HTS for real-time kinetic measurements of [Ca2+]i challenging. Nonetheless, many laboratories now use plate reader-based HTS for measurements of [Ca2+]i, though these results have been questioned as data obtained with plate readers often differ between studies and in many cases do not match electrophysiological recordings and fluorescent microscopy data (Westerink and Hondebrink 2010). To evaluate the suitability of HTS for real-time kinetic measurements of [Ca2+]i, data obtained with single cell fluorescence microscopy and plate readers have been compared. These data demonstrate that the use of plate reader systems for high-throughput real-time measurements of [Ca2+]i is associated with many limitations and pitfalls, including limited sensitivity, limited possibility for exchange of saline solutions, lack of single cell resolution as well as erroneous sustained increases in fluorescence that are independent of changes in [Ca2+]i. Part of these limitations could be related to dye-leakage. However, when cells were pre-incubated with probenecid, which is widely used to prevent leakage, cells were no longer able to increase [Ca2+]i upon depolarization. Probenecid thus acts as an inhibitor of depolarization-evoked Ca2+-influx, limiting its use in HTS measurements of [Ca2+]i. Until solved, these limitations hamper HTS as well as mechanistic measurements of [Ca2+]i (Heusinkveld and Westerink, 2011).
In summary, ex vivo measurements of synaptic function are a good intermediate between in vivo and in vitro. An important advantage of in vitro experiments, even when using simple cell lines, includes the possibility to investigate the cellular and molecular mechanisms underlying the adverse neurotoxic effects. However, when performing in vitro experiments, oxidative metabolism should be considered. Consequently, the number of compounds to be investigated will increase, but great care should be taken when measuring functional neuronal readouts using HTS. Further developments in the field of HTS will definitely aid in bridging the gap between in vitro and in vivo experimentation, even in the absence of sophisticated in vitro models. Additionally, new in vitro models that better match the needs for human risk assessment, e.g., human-derived cells, developing cells (neurospheres) and (human) co-cultures with more complex intercellular interactions, may be a prerequisite for acceptation of in vitro data for the purpose of human risk assessment. As soon as these demands are met these new models for functional (high throughput) in vitro testing, combined with ex vivo validation, will prove a powerful tool for human neurotoxicological risk assessment.
4. Linkage of solvent neurotoxicity between in vitro and in vivo systems via the NMDA-glutamate receptor
The existing literature for solvent neurotoxicity is rich with regards to the observed neurological effects. Briefly, some of the neurological effects observed include cognitive disorders, auditory impairment, motor impairment, general CNS depressive effects, visual dysfunction, and decreased nerve conduction velocity. It was originally hypothesized that these neurological changes were due to perturbations of the lipid bilayer in the cellular membrane, as proposed by Meyer and Overton in their “lipid theory” published 1899, by a lipophilic molecule (including lipophilic solvents). More recent evidence has supported that solvents interact with lipophilic areas on protein receptors (for reviews see Balster, 1998; Bowen et al., 2006).
It was generally reported that solvents inhibit excitatory ion channel receptors such as the NMDA-glutamate receptor (Cruz et al., 1998, 2000), the nicotinic acetylcholine receptor (Bale et al., 2002, 2005a), and potentiate inhibitory ion channel receptors including the gamma aminobutyric acid-A (GABAA) (Beckstead et al., 2000), glycine (Beckstead et al., 2000, 2001), and serotonin (Lopreato et al., 2003). Although selected molecular targets for solvents were identified, it is unclear which targets contribute to the overall neurological change(s) observed after solvent exposure. Ultimately, it is important to ascertain if the molecular targets that are sensitive to solvents, in vitro, are relevant in vivo.
One approach used was to select an in vivo neurological endpoint that was sensitive to solvents and was also known to involve an in vitro molecular target/pathway for the solvent. Toluene was selected as the test solvent since the acute effects have been highly characterized (in comparison to other solvents) in humans and animals as well as having been extensively characterized in molecular in vitro studies and several targets have been identified. The NMDA-glutamate receptor was selected as the molecular target and steady state visual evoked potentials (VEPs) in Long-Evans rats were selected as the neurological test system since toluene is known to decrease VEP amplitude (Boyes et al., 2007a). The strategy that was used to evaluate the effects of toluene on the NMDA-glutamate receptor, in vivo, was to treat animals with an NMDA-glutamate receptor agonist (NMDA) or an antagonist (MK801), prior to toluene exposure and to measure steady state VEPs during a time course. Since toluene is known to decrease NMDA-glutamate receptor function in vitro, it was hypothesized that the agonist, NMDA, would block F2 amplitude decreases mediated by toluene. Similarly, a co-treatment of MK801 and toluene would exacerbate F2 amplitude decreases since both agents inhibit NMDA-glutamate receptor function. Prior to conducting the toluene and NMDA-glutamate receptor challenge studies, it was important to characterize optimal exposures and dosages for toluene, NMDA, and MK801 for the rats for steady state VEP measurements. For toluene exposures, rats were exposed in a head-only exposure chamber (see Boyes et al., 2005 for details) to toluene concentrations ranging from 1000–4000 ppm between 1–2 hours (Boyes et al., 2007a). Rats were presented with a sinusoidal ON-OFF pattern at a frequency of 4.55 Hz. The raw sinusoidal VEP was analyzed using a Fourier transform to record the amplitude at the frequency of pattern presentation (4.55 Hz; F1) and at double the frequency of pattern presentation (9 Hz; F2). Toluene was demonstrated to decrease F2 amplitude over the span of one hour without having an effect on F1 (Boyes et al., 2007a). A 1000 ppm concentration of toluene for one hour was selected as the optimal exposure because all of the exposures (1000–4000 ppm) resulted in a similar decrease in F2 amplitude over the time period. Additionally, animals were less lethargic at this concentration than at higher exposures.
Pharmacological characterization of rat pattern VEPs was undertaken (for details see Bale et al., 2005b) to determine optimal doses of NMDA and MK801 for the challenge studies. 2.5 mg/kg NMDA was selected since increases in F2 amplitude were observed and was opposite to toluene. 0.1 mg/kg MK801 was selected since no changes in body temperature were noted. In the first set of challenge studies (presented fully in Bale et al., 2007), 2.5 mg/kg NMDA or 0.1 mg/kg MK801 was administered to the rats 10 minutes prior to exposure to 1000 ppm toluene. VEPs were measured prior to any treatment (baseline measurement) and approximately every 10 minutes during the drug treatments for up to one hour. It was found that NMDA did not have any observable effect on the toluene-mediated F2 amplitude decreases. However, pretreatment with 0.1 mg/kg MK801 prior to 1000 ppm toluene exposure blocked toluene-mediated F2 amplitude decreases. In order to further elucidate the interaction between MK801 and toluene, the order of the challenge was switched. Animals were now exposed to 1000 ppm toluene for 20 minutes prior to MK801 administration. Toluene-mediated F2 amplitude decreases persisted and strongly suggest that the mechanism is not due to non-competitive inhibition. However, the study did demonstrate that there was an interaction between toluene and the NMDA-glutamate receptor, although apparently more complicated than originally thought.
One potential hypothetical mechanism is that toluene may preferentially act upstream of the NMDA-glutamate receptor by increasing the rate of glutamate release. Two separate studies have reported that acute toluene exposure results in increased glutamate release (Perrine et al., 2011; Win-Shwe et al., 2007). Westerink and Vijverberg (2002) demonstrated that toluene (30 μM-1000 μM) increased catecholamine release in PC12 cells and required the presence of calcium. This mechanism could be extended to increased glutamate release since increases in calcium influx by toluene could result in increased neurotransmitter release. It was observed that 10 mg/kg NMDA also decreased F2 amplitude similar to toluene exposure. Potentially, an increased glutamate release, due to an increase in basal calcium influx, presynaptically by toluene along with the exogenous addition of 2.5 mg/kg NMDA may mechanistically act similar to higher levels of NMDA and may have been the reason for the observed significant decrease in F2 amplitude. Similarly, when MK801 was pre-applied, the hypothesized glutamate release from toluene exposure would have occurred after MK801 occupied its binding site on the NMDA-glutamate receptor and would have prevented the released glutamate from activating the receptor. In the reverse treatment, it could be that the either toluene’s effect on F2 amplitude was too far along to be blocked my MK801 (i.e. MK801 should have been administered sooner) or a higher dose of MK801 was needed. It is highly unlikely that toluene is acting directly at the NMDA-glutamate receptor for this neurological effect (VEP) since toluene and MK801 both inhibit NMDA-glutamate receptor function. It should be noted that there are other molecular targets such as the neuronal nicotinic acetylcholine receptor that are involved producing the VEP (Bale et al., 2005b) and are more sensitive to inhibition to toluene (Bale et al., 2002; 2005a).
This evaluation demonstrated that there is an interaction between toluene and the NMDA-glutamate receptor, in vivo. However, a direct interaction with the NMDA-glutamate receptor and toluene may not be the primary mechanism for VEP changes. One lesson to be learned from this evaluation is that correlating one mechanistic component (in vitro) to a whole integrated system is challenging and requires extensive investigation.
5. Neuronal connectivity determines behavioural function
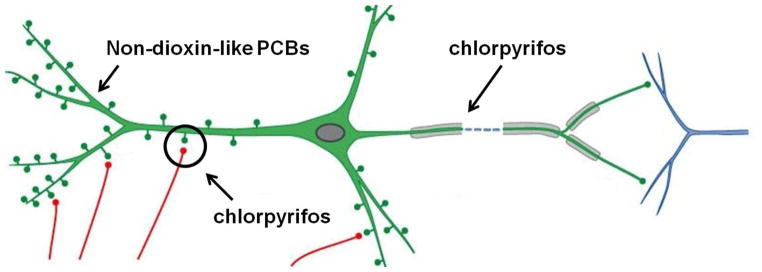
The functional properties of the vertebrate nervous system are determined by the connections formed between neurons during development and by the structural remodelling of these connections in response to experience. A critical determinant of neuronal connectivity is the cytoarchitecture of neurons, and in particular the shape of a neuron’s axonal plexus and dendritic arbor. Axonal and dendritic morphology determines the pattern of synaptic connections (Figure 2), which regulates the distribution of information within the nervous system.
Figure 2. Schematic of a vertebrate neuron illustrating the major structural components that determine neuronal connectivity.
Dendrites, which are the tapered, highly branched processes emanating from the cell body, are the major site of afferent input (the red processes representing axons of neurons upstream of the green neuron in the neural circuit) in the vertebrate nervous system. In excitatory neurons, synaptic connections typically are formed on dendritic spines (represented by the “knobs” protruding from the green dendritic shafts). Vertebrate neurons typically extend a single axon that is tapered at the axon hillock (the unmyelinated region of the axon immediately adjacent to the neuronal cell body) but then is of uniform diameter along the rest of the myelinated (represented by the gray ensheathing the green axon) axonal shaft. Axons are often much longer than dendrites and unbranched until they reach their targets (in this example, the blue dendritic processes of the downstream neuron in the neural circuit). Our data indicate that PCBs selectively interfere with dendritic growth and plasticity whereas low doses of CPF inhibit axons and potentially alter synapse formation.
The shape of these processes also affects signal processing within the neuron (Lasek, 1988). Experience (e.g., activity) influences axonal and dendritic morphology, and that structural remodelling of these components is necessary for adaptive brain functions such as learning and memory (Butz et al., 2009; Holtmaat and Svoboda, 2009). Experimental evidence indicates that even subtle perturbations of temporal or spatial aspects of axonal or dendritic growth can cause neurobehavioural deficits (Rice and Barone, 2000), and altered morphogenesis and/or structural plasticity of axons and dendrites is thought to contribute to clinical symptoms observed in both neurodevelopmental disorders (Zoghbi, 2003) and neurodegenerative diseases (Butz et al., 2009, Holtmaat and Svoboda, 2009). Collectively, these observations identify axonal and dendritic morphology as mechanistically relevant cellular correlates of neurobehaviour. This suggests the feasibility and scientific validity of using well-defined in vitro models of axonal and dendritic morphology (Lein et al., 2005) as mechanistically relevant platforms for not only screening chemicals to identify those with the potential to cause neurobehavioural deficits, but also for mechanistic studies of toxicant-induced neurobehavioural deficits. In the following sections, examples from work in our laboratory are presented in support of this suggestion.
Dendritic morphology as an in vitro correlate of PCB-induced neurobehavioural deficits
Population-based studies have consistently demonstrated that PCBs negatively impact neuropsychological function in exposed children (Carpenter, 2006, Korrick and Sagiv, 2008, Schantz et al., 2003). However, the cell and molecular mechanism(s) by which PCBs derail cognitive and psychomotor development remain speculative. We have been testing the hypothesis that PCBs elicit neurobehavioural deficits by interfering with dendritic growth and plasticity. As an initial test of this hypothesis, we examined dendritic morphology in the brains of weanling rats exposed to Aroclor 1254 (A1254) in the maternal diet throughout gestation and lactation (Yang et al., 2009). A subset of weanling rats from each treatment group were trained in the Morris water maze beginning on P24 to assess the effects of developmental PCB exposure on not only spatial learning and memory but also experience-dependent dendritic plasticity. Morphometric analyses of Purkinje cells indicated that in P31 littermates not trained in the Morris water maze, A1254 at 1 but not 6 mg/kg/d in the maternal diet significantly increased dendritic growth relative to vehicle controls (Yang et al., 2009). Morris water maze training increased dendritic complexity in control animals, and this effect was significantly attenuated in the 6 mg/kg treatment group and reversed in the 1 mg/kg treatment group. Inverted dose-related effects on dendritic growth in untrained animals and dendritic plasticity in trained animals were also observed in pyramidal neurons of neocortical layer IV in Golgi stained sections from the same animals (Yang et al., 2009). A similar inverted dose-related response similar was observed for subtle but statistically significant delays in learning and memory (Yang et al., 2009), suggesting a causal link between these responses.
Consistent with the observations of dendritic growth in non-maze-trained pups, exposure of primary cultures of rat neocortical neurons to nanomolar concentrations of PCB 95 significantly enhanced dendritic growth (Yang et al., 2009). Interestingly, micromolar concentrations of PCB 95 had no net effect on dendritic growth compared to controls, recapitulating the inverted dose-related effects of developmental A1254 exposure on dendritic growth in vivo. PCB 95 has been shown to increase intracellular Ca2+ concentrations in neurons via sensitization of the ryanodine receptor (RyR), a calcium induced calcium ion channel localized to the endoplasmic reticulum; whereas PCB 66 has negligible RyR activity (Pessah et al., 2006). Interestingly, PCB 66 had no effect on dendritic growth in these cultures, suggesting that PCBs interfere with dendritic growth and plasticity via effects on the RyR. In support of this hypothesis, the dendrite promoting activity of PCB 95 in cultured neocortical neurons was blocked by pharmacological antagonism of RyR activity (Yang et al., 2009). These data provide the first evidence linking a direct molecular effect of PCBs (RyR activation) to disruption of a specific neurodevelopmental event (dendritic morphogenesis).
Axonal morphology as an in vitro correlate of CPF-induced neurobehavioural deficits
Organophosphorus pesticides (OPs) are well documented albeit not well-understood developmental neurotoxicants. Data from animal models indicate that exposure to OPs during critical stages of brain development can cause persistent behavioural problems and cognitive deficits (Costa, 2006; Eaton et al., 2008), and an increasing number of human studies report an association between chronic exposure to low-level OPs and behavioural and cognitive problems in children (Bouchard et al., 2011; Engel et al., 2007, 2011; Eskenazi et al., 2007; Kofman et al., 2006; Lizardi et al., 2008; Rauh et al., 2011; Rohlman et al., 2005). It is widely posited that the developmental neurotoxicity of CPF reflects altered patterns of neuronal connectivity (Costa, 2006, Eaton et al., 2008). In support of this hypothesis, we observed that exposure to chlorpyrifos (CPF) or its oxon metabolite (CPFO) significantly decreased axonal growth in primary cultures of sympathetic neurons derived from neonatal rat superior cervical ganglia (SCG) (Howard et al., 2005) or sensory neurons derived from embryonic rat dorsal root ganglia (DRG) (Yang et al., 2008). These inhibitory effects are observed at concentrations that have no effect on cell viability, protein synthesis or the enzymatic activity of AChE. Comparative analyses of the effects of CPF and CPFO on axonal growth in DRG neurons cultured from AChE nullizygous (AChE−/−) versus wildtype (AChE+/+) mice indicated that while these OPs inhibited axonal growth in AChE+/+ DRG neurons, they had no effect on axonal growth in AChE−/− DRG neurons (Yang et al., 2008). However, transfection of AChE−/− DRG neurons with cDNA encoding full-length AChE restored the wildtype response to the axon inhibitory effects of OPs. These data indicate that inhibition of axonal growth by OPs requires AChE, but the mechanism involves inhibition of the morphogenic rather than enzymatic activity of AChE.
In subsequent studies, we used a zebrafish model to determine whether OPs alter spatiotemporal patterns of axonal growth in vivo (Yang et al., 2011) and whether such effects are coincident with neurobehavioural deficits. Static waterborne exposure to CPFO from 24 to 72 hours post fertilization (hpf) significantly decreased axonal growth in sensory neurons and primary motoneurons at 1.0 μM and in secondary motoneurons at > 0.1 μM. CPFO inhibited axonal growth in the absence of mortality, gross developmental defects or reduced numbers of secondary motoneurons, and the effects on axonal growth were reversible upon termination of CPFO exposure. These neuronal cell types detect touch stimuli and mediate an escape response in developing zebrafish (Clarke et al., 1984), and the touch-induced swimming response was significantly attenuated in zebrafish exposed to CPFO from 24 to 72 hpf at concentrations > 0.1 μM (Yang et al., 2011). Thus, both in vitro and in vivo data identify interference with axonal growth as a mechanism contributing to altered patterns of neuronal connectivity and support the functional relevance of axon inhibition to CPF-induced neurobehavioural deficits.
6. The NFA a new in vitro tool for the assessment of neurotoxicity
As outlined in the previous section the integrity of the nervous system, and thus unimpaired behaviour, is achieved by interconnected neurons and changes in the morphology of these communicating axons and dendrites is crucial. Thus, examining the formation and degeneration of neuronal networks in vitro could allow for screening for developmental and acute/chronic neurotoxicity. During the last decades of the bygone millennium several epidemiological studies revealed a dramatic impairment of nervous system functioning in children after pre- and postnatal exposures to environmental chemicals like lead, methylmercury or PCBs (Bellinger and Stiles, 1993, Grandjean et al., 1995, Walkowiak et al., 2001). Due to these convincing evidence and the reasonable suspicion for similar effects of compounds like the anticonvulsants phenytoin (Adams et al., 1990) or valporic acid (Ogawa et al., 2007), manganese (Brenneman et al., 1999) or toluene (Lin et al., 2008) neurodevelopment disorders have been considered as one of the major health risks of the 21st century (Grandjean and Landrigan, 2006). To cover the important endpoint of developmental neurotoxicity (DNT) in toxicological risk assessment a test battery (e.g. assessment of sensory and motor function, learning and memory in the offspring of exposed animals) has been developed and meanwhile guidelines for animal testing (OECD TG 426) are available. Due to this obvious sensitivity and the known vulnerability of the developing brain towards neurotoxins (Grandjean and Landrigan, 2006), DNT has been mentioned when in vitro tests methods are discussed that provide an understanding of the molecular/cellular mechanisms involved in neurotoxicity (Bal-Price et al., 2010a). One of the most promising readouts that have been proposed to measure neurotoxicity/developmental neurotoxicity is the inhibition of neurite outgrowth (Nordin-Andersson et al., 1998, Radio et al., 2008, Radio and Mundy, 2008), a hallmark of neuronal differentiation. Recently, it could has been shown that neurite outgrowth was significantly inhibited by methylmercury at 0.001 μM, a concentration far below the cytotoxic concentration (1 μM) of this neurotoxin in PC12 cells that have been used in this assay (Radio et al., 2010). Such higher susceptibility for inhibited neurite growth compared to cytotoxicity have also been systematically documented for a larger panel of compounds in human neurons (Stiegler et al., 2011) In order to quantify neurite outgrowth, several measures (read-outs), such as number of neurites per cell, cells with neurites, total or average neurite length, etc., are used. These methods are labor-intensive since most of the parameters require the manual quantification of outgrowth length, e.g. in comparison to the diameter of the cell body, in order to identify small excrescences of cells as neurites. To overcome these obstacles our group recently proposed a new method, the network formation assay (NFA), which combines microtechnology (i.e., microcontact printing) and cell biology (Frimat et al., 2010). By chemical treatment of the surface of the cell-substrate a spatially standardized pattern for cell adhesion was created. On this hexagonal array of adhesion nodes (diameter 70 μm) with a distance of 100 μm to all neighboring nodes the human SH-SY5Y neuroblastoma cell-line (DSMZ, Germany) occupied approximately 80% of the adhesion nodes (on average 7 cells per node) within 24 hours after seeding. Over a period of 72 hours a network of neurites between neighboring cells was formed, the number of these connections was counted manually, and the number of connections per node (cpn) was used to quantity the “network formation” of the assay. Using the known human neurotoxin acrylamide (Calleman et al., 1994) that also inhibits neurite outgrowth in vitro (Nordin-Andersson et al., 2003) we demonstrated the feasibility of this new assay to pick up the neurotoxic effect of acrylamide. By calculating the relative potency, a statistical measure for the comparison of different dose-response curves (e.g. Villeneuve et al., 2000), we showed that the NFA detects the organ-specific toxicity (i.e. neurotoxicity) of acrylamide at concentrations 7.55-times lower than the unspecific, cytotoxic effect, as measured by the CellTiter-Blue® cell viability assay (Promega) in the SH-SY5Y cells. This result illustrates that the NFA might be a useful approach that could be integrated into broader battery of screening tests (Smith, 2009) assessing developmental neurotoxicity of drugs and chemicals.
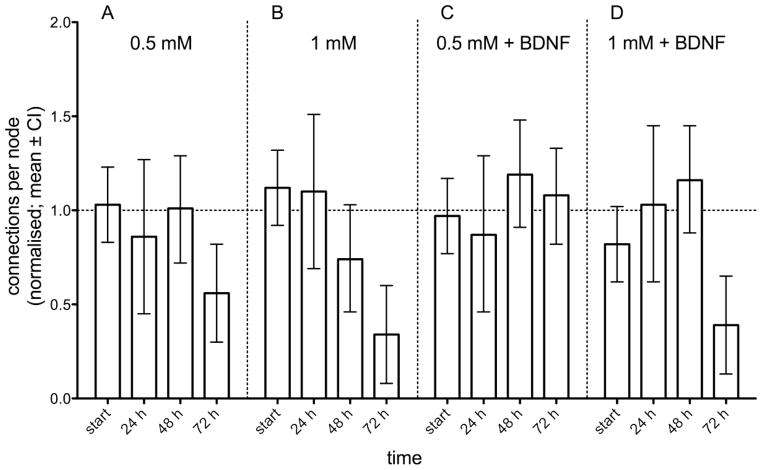
However, the NFA has some neurobiological advantages that haven’t been exploited yet. The endpoint of this new assay is network formation among neurons that have been placed on spatially predefined adhesion nodes. The development of networks in the brain is essential during brain development but existing networks are also modified activity-dependently (Nelson et al., 1990) and neuronal plasticity is one of the basic characteristics of the brain (Kempermann et al., 2000). By means of synaptic plasticity the organism can adapt to new situations and challenges in its environment, and thus, network dynamics serve as neurobiological principle of learning and memory (Kandel, 2001). The assessment of so-called functional domains, like cognitive functions related to learning and memory, has been proposed to increase the comparability of neurobehavioural toxicology in humans and animals (Boyes et al., 2007b) and to exploit the complementary advantages of the respective approaches for toxicological risk assessment. Since several molecular key players in neuronal plasticity have already been identified, in vitro assays in neurotoxicology might also benefit from research in molecular neuroscience (see section ‘imaging genetics’). The neurotrophin BDNF is known to modulate differentiation and survival of neurons of the CNS. Furthermore, it is one of the molecular key players underlying learning and memory in the adult brain (Cunha et al., 2010). From experiments investigating hippocampal long-term potentiation (LTP), typically induced by high-frequency stimulation (HFS) of excitatory input in glutamatergic neurons (see also section ‘bridging the gap …’), it is known that BDNF is involved in LTP and subsequent Arc-dependent (Arc: activity-regulated cytoskeleton-associated protein) consolidation processes (Bramham and Messaoudi, 2005). Partly based on such neurobiological processes neuronal networks might be optimized via BDNF signaling and thus, we stimulated the NFA by pre-incubation with BDNF. In a range finding experiment we showed that incubation of SH-SY5Y cells (pre-differentiated with 10 μM retinoic acid (RA) for 4 days) with 100 ng/mL BDNF for 72 hours increased the connection per nodes of the NFA from approximately 0.5 to 1.5 cpn, while treatment with RA or lower BDNF concentrations had no sustained effect on the network complexity. In a recent experiment we tried to use the NFA as an in vitro assay to investigate (a) the degenerative effect of acrylamide on existing networks and (b) the effect of co-incubation with BDNF on network degeneration (Hardelauf et al., 2011). Compared to the control condition both acrylamide concentrations (0.5 and 1 mM) significantly degenerated the existing network after 72 hour and for the 1 mM condition the degeneration was almost significant after 48 hours (see confidence interval in figure 3). The additional co-treatment with BDNF counteracts the degenerative effect of both acrylamide concentrations for up to 48 hours and the 1 mM concentrations significantly degenerated the network after 72 hours.
Figure 3. Degeneration of existing networks among SH-SY5Y cells during three days of acrylamide incubation.
The existing network of neurites connecting clusters of human neuroblastoma cells attached to the spatially standardized adhesions nodes of the NFA-chips degenerated dose-dependently when exposed to 0.5 (panel A) and 1 mM (panel B) of acrylamide. Co-treatment of the assay with BDNF (100 ng/ml) (i) increased the network formation for 48 hours (panel C and D) and (ii) counteracts the degenerative effect of 0.5 mM acrylamide (panel C) but not the strong effect of 1 mM acrylamide after 72 hours (see panel B and D).
The underlying biological mechanisms of this antagonizing effect are not totally understood yet, but it seems reasonable that down-stream signalling of BNDF via TrkB receptors promoting network formation, is not or less affected by acrylamide. Mechanistically, disruption of neurotransmission at presynaptic sites (LoPachin et al., 2004) and elevated [Ca2+]i (Nordin-Andersson et al., 2003) are more likely to underlie the neurotoxic effect of acrylamide. However, these results showed that the NFA is also suitable to investigate the degenerative effect of toxicants on more mature networks and thus, expanding its usability from DNT to neurotoxicity in general. Moreover, the system appears to be suitable for pharmacological interventions that are necessary to confirm ‘toxicity pathways’. The growing knowledge from molecular neuroscience about neurobiological factors affecting and/or supporting cognitive functions like learning and memory has already been used for the design of in vitro assays (Chen et al., 2011). This paper showed that BDNF-induced arc expression in SH-SY5Y cells was reduced after aluminium exposure a neurotoxic metal affecting neurobehavioural test results in humans (Meyer-Baron et al., 2007). However, the concentration of arc was measured in cell lysate and protein localization within the network/neurons was not possible. The spatially standardized cell patterning of the NFA will facilitated such protein localization studies and further experiments with the NFA will address this issue. We are currently preparing a new NFA chip generation that can be used with different cell lines (e.g. LUHMES cells (Stiegler et al., 2011)), NPCs from murine ESCs, and primary mouse neurons. Thereby, the relevance of possible toxicity pathways can be compared across species, a highly relevant source of uncertainty in human risk assessment.
7. Use of in vitro models for developmental neurotoxicity assessment
7.1.What is required for cellular in vitro models?
The nervous system consists of many different cell populations that may be affected by DNT. Moreover, toxic effects can have behavioural and functional consequences (e.g. on regulation of mood, intelligence, attention, sensory function, motor activity) without obvious morphological correlates. This needs to be taken into account when test systems are being developed. For instance, the difference in the ratio between different neuronal populations needs to be detectable in the absence of an overall loss of cells.
As different brain regions develop during different time windows, they display different sensitivities to neurotoxicants at different times. For instance, the DNT compound methylazoxymethanol (MAM) has different effects on the brain when given on different days of embryonic development (Penschuck et al., 2006 and references therein). Thus DNT test systems must also provide the option to apply potential toxicants in different phases of development.
Neurodevelopment is a highly complex biological process that involves proliferation, migration, cell death, differentiation, synaptogenesis, neurite and network formation, as well as gliogenesis and myelination. All these processes need not only to be functional, but also require correct timing and complicated balances within a microenvironment often referred to as a “niche”. Therefore, one single type of endpoint and one single cell type are unlikely to be sufficient for a comprehensive testing of DNT.
Potential experimental endpoints of a test battery comprise electrophysiology, neurotransmitter release, immunostaining and other methods of protein quantification including proteomics techniques, methods of RNA quantification (including transcriptomics), cellular metabolism assays (including metabolomics) and evaluations of cellular morphology and movement. More specialised approaches would address specific pathologies, such as ongoing inflammation and glial activation (Falsig et al., 2004; Lund et al., 2006) or specific forms of cell death (Blomgren et al., 2007) and the elimination of dying cells (Hirt and Leist, 2003) Practical limitations are often due to the heterogeneity of the cultures, which precludes certain methods of quantification. This heterogeneity may be desired, e.g. for generation of “organ simulating tissues”. In most cases it is accidental or stochastic, as currently-used protocols lead to the generation of different cell populations that are not homogeneously distributed but may rather grow in patches or islands within a dish. Moreover, some cells grow preferentially on top of or under other cells. In this situation it is particularly important to select endpoints that guarantee robustness (reproducible results, also when experimental conditions vary slightly).
For test system development some issues require particular attention:
Developmental neurotoxicity may arise directly from “toxicity for cells present during development”. This may be tested in “static” test systems that do not change composition over time (e.g. self-renewing neural stem cell cultures). However, DNT may also arise from the “inhibition of a developmental function” in the absence of cytotoxicity to a cell. This requires dynamic test systems showing such changes over time. The endpoints for these two approaches are very different.
In vitro models are often designed to reflect a specific mode of action (MoA) in vivo, (e.g. inhibition of neurite growth or of migration). They have therefore a limited applicability domain. For instance, a model optimised to detect compounds disturbing differentiation may not identify compounds inhibiting myelination. Although this appears trivial, it has a major implication for test design and interpretation, as well as dynamic range with respect to modes of action of chemicals. However, also apparently different biological processes such as neurite growth and migration share some basic mechanisms such as rho kinase activation. Thus, for some toxicants effects on one of these processes (migration) may be predictive of effects on the other process (neurite outgrowth). The design of tests based on such convergent mechanisms of action would allow for smaller panels of assays to screen for a broad range of chemicals.
Models can only answer certain predefined questions. Unfortunately, they will always yield data, even if the wrong question is asked. This puts high responsibility on the researcher as even technically sound and statistically controlled data can be toxicologically/biologically meaningless when the question was not appropriate.
The quality of the model and of the input determines the quality of the output (Crofton et al., 2011; Leist et al., 2010). As in the previous point, data are also obtained from poorly controlled systems, but in this case their toxicological significance cannot be judged although they may look statistically significant. This point and point #3, distinguish in vitro models fundamentally from animal models. Uncontrolled and inappropriately applied animal tests will, in many cases, still yield data on the effects of a compound on the animal – i.e. the model itself remains. Under in vitro conditions, changes of parameters, such as the initial cell number, result in a different model that requires completely new characterization. Thus, data obtained under such situations cannot be compared to other/older data, as they were derived from a different model
A multi-stage developmental process will therefore require several in vitro models for DNT testing. It may appear attractive on first sight, to establish a complex model incorporating many successive steps and to expose this model to the test chemical over the entire time period. However, changes in one step by a test compound will in many cases change the initial conditions for the next step, and therefore change the model. One approach to overcome this limitation, is the testing of compounds in different shorter and defined experimental windows of the same more complex model (e.g. day 0–7, 7–14 and 14–21 of a 21-day model)
At the present stage, many different options are available. To find the best approaches, more exploratory testing and standardisation of protocols, are necessary. Before a final decision on the best system for regulatory purposes can be reached, a large variety of different approaches should be promoted and explored for a sufficiently long time before a rational selection process can be initiated with the goal of identifying a smaller set of assays that may be used eventually for risk assessment.
7.2. Assays for disturbed development of pluripotent stem cells to neural precursors
This type of test addresses the very first step of differentiation during the generation of nervous tissue precursor cells. It corresponds approximately to the period of germ layer formation and initial development in the early embryo. This phase is experimentally difficult to address in vivo, and therefore only few data are available from animals (Stodgell et al., 2006). The timing would correspond to about week 3 and 4 (past fertilization) in human development. This corresponds to the window of sensitivity for thalidomide (Kim and Scialli, 2011), but other toxicological information on this time period is still sparse. In vitro models use pluripotent stem cells like embryonic stem cells (ESC) or induced pluripotent stem cells (iPSC). These cells are then triggered to differentiate towards neuroectoderm and the neural lineage. A number of questions arise when one considers using such cells as potential test systems for DNT (Leist et al., 2008a). These involve species of the stem cells, the source of the cells, their genotype, their exact developmental status at the beginning and end of the experiment, the desired throughput and, last but not least, the endpoints chosen as readout of the model system.
Several labs have used murine ESCs as an experimental DNT system. The generation of neural precursors occurs within 5–7 days with high efficiency and it can be monitored by FACS, immunostaining, or by using mRNA markers. This phase may be particularly sensitive to toxicants that inhibit proliferation, but for instance also methylmercury has affected this differentiation step (Zimmer et al., 2011b; Stummann et al., 2007; Klemm and Schrattenholz, 2004; Coecke et al., 2007; Groebe et al., 2010). A special case of this approach is the investigation of toxicity directly to the ESC (Uibel et al., 2010). More recently, human ESCs have also been used (Stummann et al., 2009; Schrattenholz and Klemm, 2007). The initial phase of differentiation is characterized by particularly strong epigenetic changes (Zimmer et al., 2011a), and it may be susceptible to long-term toxic effects that are manifest only later in life due to epigenetic memory. The unravelling of the underlying mechanisms is a focus of current research, and requires controlled models of synchronous differentiation, that allow the application of quantitative biochemical endpoints.
7.3. Functional integrity of neural precursors
Neural precursors may be isolated from rodents as neurospheres. They differ according to the brain region from which they are derived from and whether they are derived from fetal, neonatal or adult brain. This indicates large functional heterogeneity of neural stem cells (Robel et al., 2011; Kriegstein and Alvarez-Buylla, 2009; Ma et al., 2010; Suh et al., 2009), and the terms “neural stem cell” or “neural precursor (NPC)” need to be used with care. Rodent NPC have been used for toxicity testing, and comparisons between human and murine NPC have revealed interesting differences (Gassmann et al., 2010). Human NPC can be obtained from foetuses at different stages, and they also show large heterogeneity depending on the age/source of the donor. Such cells from commercial sources have been used successfully for DNT testing (Schreiber et al., 2010). One of the major functions of NPC is the migration to the appropriate position in the brain, and it is possible to screen for disturbances by toxicants of this function can be screened for (Breier, 2010). Another emerging research model comprises neural crest stem cells, which are the NPC for the peripheral nervous system. The migration distance requirements for these cells are particularly high, and the cells can differentiate both towards the neural lineage, but also to other lineages. Thus, testing of function and differentiation of this specific subpopulation of NPC is an important emerging theme in DNT testing.
In addition to primary cells and ESC-derived cells, several NPC cell lines and immortalized cells have been used successfully for DNT testing, but a rigorous comparison of cell lines and primary cells has not been performed (Buzanska et al., 2009; Tamm et al., 2008; Breier et al., 2008)
7.4. Development of neural precursors to neurons
Neuronal development is associated with dramatic functional and morphological changes that may be used as assay readouts. For instance the growth of neurites has often been evaluated as target for toxic effects of chemicals (Radio et al., 2008; Yang et al, 2008; Stiegler et al, 2011; Frimat et al, 2010). A major issue of neurite toxicity assays is the distinction between specific chemical effects on the neurite and more general toxicity affecting the overall viability of the cell (Volbracht et al., 1999; Berliocchi et al., 2005; Lotharius et al., 2005). Moreover, relatively little is known on the difference of mechanisms responsible for the direct inhibition of neurite growth (or for excessive growth acceleration) in comparison to those responsible for the toxicity of compounds for developed neurites. An emerging field is also the differential toxicity of compounds for dendrites vs axons (Kim et al., 2009; Lein et al., 2007). Different human based model systems have been described (Harrill et al. 2011b; Stiegler et al., 2011) and may be used for such purposes.
A second feature of developing neurons is the dramatic change of the transcriptional profile. This has been used as endpoint for examining toxicant action (Bal-Price et al., 2010b; Zimmer et al., 2011a; 2011b; Buzanska et al., 2009; Stummann et al., 2007, 2009; Kuegler et al., 2010). The problem of how to translate this endpoint into behaviourally-relevant in vivo effects is still unsolved, but functional neurochemical follow-up assays may build a bridge here (Zimmer et al., 2011b). Issues that need to be addressed are criteria for the validation of such assays and endpoints, and the definition of specificity (Leist et al., 2010). The sensitivity of the method is potentially very high, but it requires good control of data normalization, especially when mRNA pools from different cell populations (differentiated vs non-differentiated) are compared.
7.5. Assays for the maturation and functional integrity of neurons
Disturbances of the functional maturation of neurons might link directly to behavioural deficits. Different processes have been examined as an endpoint for DNT. These include myelination, synapse formation and electrical activity (Hogberg et al., 2011; Johnstone et al., 2010; Harrill et al., 2011a). Some of these assays require specialised technical platforms, such as high-content imagers and microelectrode arrays. This field is developing dynamically, and is moving towards higher throughput and to the use of human cells for such evaluations. At present most studies are still at the proof of concept stage, although some new information on potential inhibitors of toxic processes has been found (Zimmer et al., 2011b; Zurich and Monnet-Tschudi, 2009)
8. Notes on the use of cell-based in vitro models for risk evaluation
As discussed in this review, it is obvious that nervous system functioning heavily relies on fine-tuned networks of specialized cell populations that need to interact with high precision. Even mild deviations from normal conditions might be associated with measurable differences in behaviour (e.g. neurobehavioural task performance). Some of these deviations arise from exposures to xenobiotics in adulthood, but exposure during brain development might affect nervous system functioning even more severely since these networks are not fully developed. However, there is also the counter argument that the developing brain is more plastic than the adult brain and the capability to recover from toxic insult might be higher. These opposed arguments indicate that more research on this issue is needed and recent developments in neurosciences (e.g. neuroimaging and electrophysiology, genomics and proteomics, etc.) enable neurotoxicologists to exploit emerging evidence identifying cellular and molecular correlates of behaviour in humans and animals.
There is compelling evidence from both experimental animal and clinical studies that axonal and dendritic morphology critically influence neurobehavioural endpoints. For instance, the studies of PCB and CPF developmental neurotoxicity link changes in axonal and dendritic morphology in vivo to neurobehavioural deficits and demonstrate comparable in vivo and in vitro effects of these neurotoxicants on structural components of neuronal connectivity. Of importance is that these structurally and mechanistically diverse chemicals exert qualitatively different effects on neuronal morphology with PCBs enhancing basal dendritic growth and attenuating activity-dependent dendritic plasticity and CPF inhibiting axonal growth and possibly also synapse formation and/or stability (see Figure 2). Moreover, these effects on neuronal cytoarchitecture have been causally linked to specific molecular mechanisms. These observations indicate that the effects of the toxicants on axonal and dendritic morphology are specific and not an indirect effect of generalized systemic or cellular toxicity, a conclusion supported by data indicating that PCBs and CPF affect dendritic and axonal growth, respectively, at levels that do not cause signs of systemic toxicity in vivo or decreased neuronal cell viability in vitro. Collectively, these observations support the feasibility of using axonal and dendritic morphology in primary neuronal cell cultures as in vitro correlates of toxicant-induced neurobehavioural deficits.
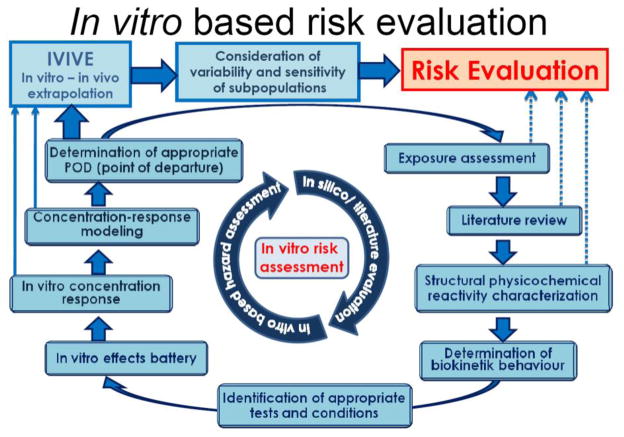
It is yet unclear, how such in vitro data will eventually be used for regulatory purposes. However, generic strategies can be designed that would provide a scientific framework for employing in vitro information in the risk evaluation process. This may lead to more mechanism-based data and to a reduction of animal use (Leist et al., 2008a; Hartung and Leist 2008). A brief outline of the process is presented in Figure 4.
Figure 4. In vitro risk assessment.
Future regulatory decisions based on in vitro methods will require the integration of literature knowledge, e.g. on QSAR or genetic variability, and of in vitro hazard assessment to arrive at a point-of-departure (POD) for risk evaluation. In contrast to current methods, this POD will only be useful after development and application of reliable in vitro-in vivo extrapolation procedures that take pharmacokinetic parameters into account. The figure depicts a potential strategy. QSAR: quantitative structure activity relationship.
It would most effectively start with in silico-based methods and information mining, for instance on QSAR and exposure data. An important step before the choice of appropriate in vitro systems would be the evaluation of the biokinetic behaviour of the test compound under typical culture conditions (volatility, protein binding, plastic adsorption, stability, potential formation of metabolites). An in vitro test battery would then be used to assess the in vitro hazard on the basis of concentration-response modelling. The data would be used to obtain a point-of-departure (POD) for in vitro→in vivo extrapolation. This would involve physiology-based pharmacokinetic modelling. In a last step, variability and sensitivity of subpopulations would be considered. Here, new genetic information on allelic variation and polymorphisms, and their biological, roles would be used to finally arrive at a meaningful risk evaluation.
In conclusion, the CAAT symposium in Xi’an and this review aimed at a dialogue between neuroscience and neurotoxicolgy to design in vitro methods and readouts that can be used (a) for basic research on toxicology pathways in the nervous system as well as (b) for high-throughput screening (HTS) in drug-development and risk assessment.
Acknowledgments
We are indebted to many colleagues for valuable contributions and insightful discussions. The work was supported by grants from the Doerenkamp-Zbinden foundation (M.L.) and the European Community’s Seventh Framework Programme (FP7/2007-2013; ESNATS project (M.L. and C.v.T.)), the National Institutes of Health (N.I.H.) (R01 ES016308 to (W.K.A. and P.J.L.); R01 ES014901 (P.J.L. and I.N.P.); R03 HD40936 (P.J.L.): R21 ES11771 (P.J.L.)), the UC Davis M.I.N.D. Institute (P.J.L.) and the Johns Hopkins University Center for Alternatives to Animal Testing (P.J.L.).
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interest. The content is solely the authors’ responsibility and does not necessarily represent official views of the N.I.H. or the United States Environmental Protection Agency (U.S.E.P.A.).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- Adams J, Vorhees CV, Middaugh LD. Developmental neurotoxicity of anticonvulsants: human and animal evidence on phenytoin. Neurotoxicol Teratol. 1990;12:203–14. doi: 10.1016/0892-0362(90)90092-q. [DOI] [PubMed] [Google Scholar]
- Bal-Price AK, Hogberg HT, Buzanska L, Coecke S. Relevance of in vitro neurotoxicity testing for regulatory requirements: challenges to be considered. Neurotoxicology and Teratology. 2010a;32:36–41. doi: 10.1016/j.ntt.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Bal-Price AK, Hogberg HT, Buzanska L, Lenas P, van Vliet E, Hartung T. In vitro developmental neurotoxicity (DNT) testing: relevant models and endpoints. Neurotoxicology. 2010b;31:545–54. doi: 10.1016/j.neuro.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Bale AS, Smothers CT, Woodward JJ. Inhibition of neuronal nicotinic acetylcholine receptors by the abused solvent, toluene. Br J Pharmacol. 2002;137:375–83. doi: 10.1038/sj.bjp.0704874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale AS, Meacham CA, Benignus VA, Bushnell PJ, Shafer TJ. Volatile organic compounds inhibit human and rat neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Toxicol Appl Pharmacol. 2005a;205:77–88. doi: 10.1016/j.taap.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Bale AS, Adams TL, Bushnell PJ, Shafer TJ, Boyes WK. Role of NMDA, nicotinic, and GABA receptors in the steady-state visual-evoked potential in rats. Pharmacol Biochem Behav. 2005b;82:635–45. doi: 10.1016/j.pbb.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Bale AS, Jackson MD, Krantz QT, Benignus VA, Bushnell PJ, Shafer TJ, Boyes WK. Evaluating the NMDA-glutamate receptor as a site of action for toluene, in vivo. Toxicol Sci. 2007;98:159–66. doi: 10.1093/toxsci/kfm080. [DOI] [PubMed] [Google Scholar]
- Balster RL. Neural basis of inhalant abuse. Drug Alcohol Depend. 1998;51:207–14. doi: 10.1016/s0376-8716(98)00078-7. [DOI] [PubMed] [Google Scholar]
- Bath KG, Lee FS. Variant BDNF (Val66Met) impact on brain structure and function. Cogn Affect Behav Neurosci. 2006;6:79–85. doi: 10.3758/cabn.6.1.79. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Weiner JL, Eger EI, 2nd, Gong DH, Mihic SJ. Glycine and gamma-aminobutyric acid(A) receptor function is enhanced by inhaled drugs of abuse. Mol Pharmacol. 2000;57:1199–1205. [PubMed] [Google Scholar]
- Beckstead MJ, Phelan R, Mihic SJ. Antagonism of inhalant and volatile anesthetic enhancement of glycine receptor function. J Biol Chem. 2001;276:24959–64. doi: 10.1074/jbc.M011627200. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Stiles KM. Epidemiologic approaches to assessing the developmental toxicity of lead. Neurotoxicology. 1993;14:151–60. [PubMed] [Google Scholar]
- Berliocchi L, Fava E, Leist M, Horvat V, Dinsdale D, Read D, Nicotera P. Botulinum neurotoxin C initiates two different programs for neurite degeneration and neuronal apoptosis. J Cell Biol. 2005;168:607–18. doi: 10.1083/jcb.200406126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C, Kolev V, Yordanova J, Domschke K, Falkenstein M, Baune BT, Konrad C. The role of the BDNF Val66Met polymorphism for the synchronization of error-specific neural networks. J Neurosci. 2010a;30:10727–33. doi: 10.1523/JNEUROSCI.2493-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C, Baune BT, Domschke K, Falkenstein M, Konrad C. Paradoxical association of the brain-derived-neurotrophic factor val66met genotype with response inhibition. Neuroscience. 2010b;166:178–84. doi: 10.1016/j.neuroscience.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Beste C, Willemssen R, Saft C, Falkenstein M. Response inhibition subprocesses and dopaminergic pathways: basal ganglia disease effects. Neuropsychologia. 2010c;48:366–73. doi: 10.1016/j.neuropsychologia.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Beste C, Baune Bt, Falkenstein M, Konrad C. Variations in the TNF-α gene (TNF-α-308G =>A) affect attention and action selection mechanisms in a dissociated fashion. J Neurophysiol. 2010d;104:2523–31. doi: 10.1152/jn.00561.2010. [DOI] [PubMed] [Google Scholar]
- Beste C, Domschke K, Radenz B, Falkenstein M, Konrad C. The functional 5-HT1A receptor polymorphism affects response inhibition processes in a context-dependent manner. Neuropsychologia. 2011a;49:2664–72. doi: 10.1016/j.neuropsychologia.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Beste C, Güntürkün O, Baune BT, Domschke K, Falkenstein M, Konrad C. Double dissociated effects of the functional TNF-α-308G/A polymorphism on proceses of cognitive control. Neuropsychologia. 2011b;49:196–202. doi: 10.1016/j.neuropsychologia.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Beste C, Saft C, Güntürkün O, Falkenstein M. Increased cognitive functioning in symptomatic Huntington's disease as revealed by behavioural and event-related potential indices of auditory sensory memory and attention. J Neurosci. 2008;58:11695–702. doi: 10.1523/JNEUROSCI.2659-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C, Willemssen R, Saft C, Falkenstein M. Error processing in normal aging and in basal ganglia disorders. Neuroscience. 2009;159:143–49. doi: 10.1016/j.neuroscience.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Blomgren K, Leist M, Groc L. Pathological apoptosis in the developing brain. Apoptosis. 2007;12:993–1010. doi: 10.1007/s10495-007-0754-4. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. Prenatal Exposure to Organophosphate Pesticides and IQ in 7-Year Old Children. Environ Health Perspect. 2011 doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, Batis JC, Paez-Martinez N, Cruz SL. The last decade of solvent research in animal models of abuse: mechanistic and behavioural studies. Neurotoxicol Teratol. 2006;28:636–47. doi: 10.1016/j.ntt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Boyes WK, Bercegeay M, Krantz T, Evans M, Benignus V, Simmons JE. Momentary brain concentration of trichloroethylene predicts the effects on rat visual function. Toxicol Sci. 2005;87:187–96. doi: 10.1093/toxsci/kfi242. [DOI] [PubMed] [Google Scholar]
- Boyes WK, Bercegeay M, Krantz QT, Kenyon EM, Bale AS, Shafer TJ, Bushnell PJ, Benignus VA. Acute toluene exposure and rat visual function in proportion to momentary brain concentration. Toxicol Sci. 2007a;99:572–81. doi: 10.1093/toxsci/kfm172. [DOI] [PubMed] [Google Scholar]
- Boyes WK, Moser VC, Geller AM, Benignus VA, Bushnell PJ, Kamel F. Integrating epidemiology and toxicology in neurotoxicity risk assessment. Hum Exp Toxicol. 2007b;26:283–93. doi: 10.1177/0960327106070481. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Progress in neurobiology. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Breier JM, Gassmann K, Kayser R, Stegeman H, De Groot D, Fritsche E, Shafer TJ. Neural progenitor cells as models for high-throughput screens of developmental neurotoxicity: state of the science. Neurotoxicol Teratol. 2010;32:4–15. doi: 10.1016/j.ntt.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Breier JM, Radio NM, Mundy WR, Shafer TJ. Development of a high-throughput screening assay for chemical effects on proliferation and viability of immortalized human neural progenitor cells. Toxicol Sci. 2008;105:119–33. doi: 10.1093/toxsci/kfn115. [DOI] [PubMed] [Google Scholar]
- Brenneman KA, Cattley RC, Ali SF, Dorman DC. Manganese-induced developmental neurotoxicity in the CD rat: is oxidative damage a mechanism of action? Neurotoxicology. 1999;20:477–87. [PubMed] [Google Scholar]
- Bushnell PJ, Boyes WK, Shafer TJ, Bale AS, Benignus VA. Approaches to extrapolating animal toxicity data on organic solvents to public health. Neurotoxicology. 2007;28:221–6. doi: 10.1016/j.neuro.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Kavlock RJ, Crofton KM, Weiss B, Rice DC. Behavioural toxicology in the 21st century: challenges and opportunities for behavioural scientists. Summary of a symposium presented at the annual meeting of the neurobehavioural teratology society, June, 2009. Neurotoxicol Teratol. 2010;32:313–28. doi: 10.1016/j.ntt.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Butz M, Worgotter F, van Ooyen A. Activity-dependent structural plasticity. Brain Res Rev. 2009;60:287–305. doi: 10.1016/j.brainresrev.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Buzanska L, Sypecka J, Nerini-Molteni S, Compagnoni A, Hogberg HT, del Torchio R, Domanska-Janik K, Zimmer J, Coecke S. A human stem cell-based model for identifying adverse effects of organic and inorganic chemicals on the developing nervous system. Stem Cells. 2009;27:2591–601. doi: 10.1002/stem.179. [DOI] [PubMed] [Google Scholar]
- Calleman CJ, Wu Y, He F, Tian G, Bergmark E, Zhang S, et al. Relationships between biomarkers of exposure and neurological effects in a group of workers exposed to acrylamide. Toxicol Appl Pharmacol. 1994;126:361–71. doi: 10.1006/taap.1994.1127. [DOI] [PubMed] [Google Scholar]
- Carpenter DO. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev Environ Health. 2006;21:1–23. doi: 10.1515/reveh.2006.21.1.1. [DOI] [PubMed] [Google Scholar]
- Carrasco MA, Hidalgo C. Calcium microdomains and gene expression in neurons and skeletal muscle cells. Cell Calcium. 2006;40:575–83. doi: 10.1016/j.ceca.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Chen T-J, Cheng H-M, Wang D-C, Hung H-S. Nonlethal aluminum maltolate can reduce brain-derived neurotrophic factor-induced Arc expression through interrupting the ERK signaling in SH-SY5Y neuroblastoma cells. Toxicology Letters. 2011;200:67–76. doi: 10.1016/j.toxlet.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Clarke JD, Hayes BP, Hunt SP, Roberts A. Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J Physiol. 1984;348:511–25. doi: 10.1113/jphysiol.1984.sp015122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coecke S, Goldberg AM, Allen S, Buzanska L, Calamandrei G, Crofton K, Hareng L, Hartung T, Knaut H, Honegger P, Jacobs M, Lein P, Li A, Mundy W, Owen D, Schneider S, Silbergeld E, Reum T, Trnovec T, Monnet-Tschudi F, Bal-Price A. Workgroup report: incorporating in vitro alternative methods for developmental neurotoxicity into international hazard and risk assessment strategies. Environ Health Perspect. 2007;115:924–31. doi: 10.1289/ehp.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG. Current issues in organophosphate toxicology. Clinica chimica acta; international journal of clinical chemistry. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Mundy WR, Lein PJ, Bal-Price A, Coecke S, Seiler AE, Knaut H, Buzanska L, Goldberg A. Developmental neurotoxicity testing: recommendations for developing alternative methods for the screening and prioritization of chemicals. ALTEX. 2011;28:9–15. [PubMed] [Google Scholar]
- Cruz SL, Mirshahi T, Thomas B, Balster RL, Woodward JJ. Effects of the abused solvent toluene on recombinant N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1998;286:334–40. [PubMed] [Google Scholar]
- Cruz SL, Balster RL, Woodward JJ. Effects of volatile solvents on recombinant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Br J Pharmacol. 2000;131:1303–8. doi: 10.1038/sj.bjp.0703666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci. 2010;3:1–14. doi: 10.3389/neuro.02.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–4. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Dingemans MML, de Groot A, van Kleef RGDM, Bergman Å, van den Berg M, Vijverberg HPM, Westerink RHS. Hydroxylation increases the neurotoxic potential of BDE-47 to affect exocytosis and calcium homeostasis in PC12 cells. Environ Health Perspect. 2008;116:637–43. doi: 10.1289/ehp.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MML, Heusinkveld HJ, Bergman A, van den Berg M, Westerink RHS. Bromination pattern of hydroxylated metabolites of BDE-47 affects their potency to release calcium from intracellular stores in PC12 cells. Environ Health Perspect. 2010;118:519–25. doi: 10.1289/ehp.0901339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MML, Ramakers GMJ, Gardoni F, van Kleef RGDM, Bergman Å, Di Luca M, van den Berg M, Westerink RHS, Vijverberg HPM. Neonatal exposure to brominated flame retardant BDE-47 reduces long-term potentiation and postsynaptic protein levels in mouse hippocampus. Environ Health Perspect. 2007;115:865–70. doi: 10.1289/ehp.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MML, van den Berg M, Westerink RHS. Neurotoxicity of brominated flame retardants: (in-)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ Health Perspect. 2011;119:900–7. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do T, Kerr B, Kuzhikandathil EV. Brain-derived neurotrophic factor regulates the expression of D1 dopamine receptors. J Neurochem. 2007;100:416–28. doi: 10.1111/j.1471-4159.2006.04249.x. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, Coyle J, McKhann G, Mobley WC, Nadel L, Neubert D, Schulte-Hermann R, Spencer PS. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol. 2008;38(Suppl 2):1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolchana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, Wetmur JG, Wolff MS. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioural Assessment Scale in a multiethnic pregnancy cohort. Am J Epidemiol. 2007;165:1397–404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, Wolff MS. Prenatal Exposure to Organophosphates, Paraoxonase 1, and Cognitive Development in Childhood. Environ Health Perspect. 2011 doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P, Jakobsson E, Fredriksson A. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment? Environ Health Perspect. 2001;109:903–908. doi: 10.1289/ehp.01109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–8. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubig PA, Aguiar A, Schantz SL. Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ Health Perspect. 2010;118:1654–67. doi: 10.1289/ehp.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsig J, Latta M, Leist M. Defined inflammatory states in astrocyte cultures: correlation with susceptibility towards CD95-driven apoptosis. J Neurochem. 2004;88:181–93. doi: 10.1111/j.1471-4159.2004.02144.x. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Cheeran B, Williams-Gray CH, Edwards MJ, Schneider SA, Weinberger D, Rothwell JC, Barker RA, Bhatia KP. BDNF val66met influences time to onset of levodopa induced dyskinesia in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2009;80:141–4. doi: 10.1136/jnnp.2008.154294. [DOI] [PubMed] [Google Scholar]
- Frimat JP, Sisnaiske J, Subbiah S, Menne H, Godoy P, Lampen P, Leist M, Franzke J, Hengstler JG, van Thriel C, West J. The network formation assay: a spatially standardized neurite outgrowth analytical display for neurotoxicity screening. Lab Chip. 2010;10:701–9. doi: 10.1039/b922193j. [DOI] [PubMed] [Google Scholar]
- Gale JT, Amirnovin R, Williams ZM, Flaherty AW, Eskandar EN. From symphony to cacophony: pathophysiology of the human basal ganglia in Parkinson disease. Neurosci Biobehav Rev. 2008;32:378–87. doi: 10.1016/j.neubiorev.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Garcia AG, Garcia-De-Diego AM, Gandia L, Borges R, Garcia-Sancho J. Calcium signaling and exocytosis in adrenal chromaffin cells. Physiol Rev. 2006;86:1093–1131. doi: 10.1152/physrev.00039.2005. [DOI] [PubMed] [Google Scholar]
- Gasic GP, Smoller JW, Perlis RH, Sun M, Lee S, Kim BW, Lee MJ, et al. BDNF, relative preference, and reward circuitry responses to emotional communication. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:762–81. doi: 10.1002/ajmg.b.30944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann K, Abel J, Bothe H, Haarmann-Stemmann T, Merk HF, Quasthoff KN, Rockel TD, Schreiber T, Fritsche E. Species-specific differential AhR expression protects human neural progenitor cells against developmental neurotoxicity of PAHs. Environ Health Perspect. 2010;118:1571–7. doi: 10.1289/ehp.0901545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;6:870–3. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–78. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF. Milestone development in infants exposed to methylmercury from human milk. Neurotoxicology. 1995;16:27–33. [PubMed] [Google Scholar]
- Groebe K, Hayess K, Klemm-Manns M, Schwall G, Wozny W, Steemans M, Peters AK, Sastri C, Jaeckel P, Stegmann W, Zengerling H, Schöpf R, Poznanovic S, Stummann TC, Seiler A, Spielmann H, Schrattenholz A. Unexpected common mechanistic pathways for embryotoxicity of warfarin and lovastatin. Reprod Toxicol. 2010;30:121–30. doi: 10.1016/j.reprotox.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Guillin O, Diaz J, Caroll P, Griffon N, Schwartz JC, Sokoloff P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411:86–9. doi: 10.1038/35075076. [DOI] [PubMed] [Google Scholar]
- Han I, You Y, Kordower JH, Brady ST, Morfini GA. Differential vulnerability of neurons in Huntington's disease: the role of cell type-specific features. J Neurochem. 2010;113:1073–91. doi: 10.1111/j.1471-4159.2010.06672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardelauf H, Sisnaiske J, Taghipour-Anvari AA, Jacob P, Drabiniok E, Marggraf U, Frimat JP, Hengstler JG, Neyer A, van Thriel C, West J. High fidelity neuronal networks formed by plasma masking with a bilayer membrane: analysis of neurodegenerative and neuroprotective processes. Lab on a chip. 2011 doi: 10.1039/C1LC20257J. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Weinberger D. Imaging genomics. Br Med Bull. 2003;65:259–70. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- Harrill JA, Robinette BL, Mundy WR. Use of high content image analysis to detect chemical-induced changes in synaptogenesis in vitro. Toxicol In Vitro. 2011a;25:368–87. doi: 10.1016/j.tiv.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Harrill JA, Freudenrich TM, Robinette BL, Mundy WR. Comparative sensitivity of human and rat neural cultures to chemical-induced inhibition of neurite outgrowth. Toxicol Appl Pharmacol. 2011b doi: 10.1016/j.taap.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hartung T, Leist M. Food for thought ..on the evolution of toxicology and the phasing out of animal testing. ALTEX. 2008;25:91–102. doi: 10.14573/altex.2008.2.91. [DOI] [PubMed] [Google Scholar]
- Herbert MR. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol. 2010;23:103–10. doi: 10.1097/WCO.0b013e328336a01f. [DOI] [PubMed] [Google Scholar]
- Heusinkveld HJ, Westerink RHS. Caveats and limitations of plate reader-based high throughput kinetic measurements of intracellular calcium levels. Toxicol Appl Pharmacol. 2011 doi: 10.1016/j.taap.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Hirt UA, Leist M. Rapid, noninflammatory and PS-dependent phagocytic clearance of necrotic cells. Cell Death Differ. 2003;10:1156–64. doi: 10.1038/sj.cdd.4401286. [DOI] [PubMed] [Google Scholar]
- Hogberg HT, Sobanski T, Novellino A, Whelan M, Weiss DG, Bal-Price AK. Application of micro-electrode arrays (MEAs) as an emerging technology for developmental neurotoxicity: evaluation of domoic acid-induced effects in primary cultures of rat cortical neurons. Neurotoxicology. 2011;32:158–68. doi: 10.1016/j.neuro.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–58. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Howard AS, Bucelli R, Jett DA, Bruun D, Yang D, Lein PJ. Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicol Appl Pharmacol. 2005;207:112–24. doi: 10.1016/j.taap.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Iwakura Y, Nawa H, Sora I, Chao MV. Dopamine D1 receptor-induced signaling through TrkB receptors in striatal neurons. J Biol Chem. 2008;283:15799–806. doi: 10.1074/jbc.M801553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone AF, Gross GW, Weiss DG, Schroeder OH, Gramowski A, Shafer TJ. Microelectrode arrays: a physiologically based neurotoxicity testing platform for the 21st century. Neurotoxicology. 2010;31:331–50. doi: 10.1016/j.neuro.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999;401:918–21. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–8. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kempermann G, van Praag H, Gage FH. Activity-dependent regulation of neuronal plasticity and self repair. Progress in brain research. 2000;127:35–48. doi: 10.1016/s0079-6123(00)27004-0. [DOI] [PubMed] [Google Scholar]
- Kim JH, Scialli AR. Thalidomide: the tragedy of birth defects and the effective treatment of disease. Toxicol Sci. 2011;122:1–6. doi: 10.1093/toxsci/kfr088. [DOI] [PubMed] [Google Scholar]
- Kim WY, Gonsiorek EA, Barnhart C, Davare MA, Engebose AJ, Lauridsen H, Bruun D, Lesiak A, Wayman G, Bucelli R, Higgins D, Lein PJ. Statins decrease dendritic arborization in rat sympathetic neurons by blocking RhoA activation. J Neurochem. 2009;108:1057–71. doi: 10.1111/j.1471-4159.2008.05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD. Casarett and Doull's Toxicology - The Basic Science of Poisons. 7. New York: McGraw-Hill; 2008. [Google Scholar]
- Klemm M, Schrattenholz A. Neurotoxicity of active compounds--establishment of hESC-lines and proteomics technologies for human embryo- and neurotoxicity screening and biomarker identification. ALTEX. 2004;21 (Suppl 3):41–8. [PubMed] [Google Scholar]
- Kofman O, Berger A, Massarwa A, Friedman A, Jaffar AA. Motor inhibition and learning impairments in school-aged children following exposure to organophosphate pesticides in infancy. Pediatric research. 2006;60:88–92. doi: 10.1203/01.pdr.0000219467.47013.35. [DOI] [PubMed] [Google Scholar]
- Korrick SA, Sagiv SK. Polychlorinated biphenyls, organochlorine pesticides and neurodevelopment. Current opinion in pediatrics. 2008;20:198–204. doi: 10.1097/MOP.0b013e3282f6a4e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovas Y, Plomin R. Generalist genes: implications for the cognitive sciences. Trends Cogn Sci. 2006;10:198–203. doi: 10.1016/j.tics.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–84. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuegler PB, Zimmer B, Waldmann T, Baudis B, Ilmjärv S, Hescheler J, Gaughwin P, Brundin P, Mundy W, Bal-Price AK, Schrattenholz A, Krause KH, van Thriel C, Rao MS, Kadereit S, Leist M. Markers of murine embryonic and neural stem cells, neurons and astrocytes: reference points for developmental neurotoxicity testing. ALTEX. 2010;27:17–42. doi: 10.14573/altex.2010.1.16. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ. What causes autism? Exploring the environmental contribution Curr Opin Pediatr. 2010;22:219–25. doi: 10.1097/MOP.0b013e328336eb9a. [DOI] [PubMed] [Google Scholar]
- Lasek R. Studying the intrinsic determinants of neuronal form and function. In: Lasek R, Black MB, editors. Intrinsic Determinants of Neuronal Form and Function. New York: A.R. Liss; 1988. pp. 3–58. [Google Scholar]
- Lein P, Goldberg A, Locke P, Silbergeld E. In vitro and other alternative approaches to developmental neurotoxicity testing (DNT) Environ Toxicol Pharmacol Appl Physiol. 2005;19:735–44. doi: 10.1016/j.etap.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lein PJ, Guo X, Shi GX, Moholt-Siebert M, Bruun D, Andres DA. The novel GTPase Rit differentially regulates axonal and dendritic growth. J Neurosci. 2007;27:4725–36. doi: 10.1523/JNEUROSCI.5633-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist M, Bremer S, Brundin P, Hescheler J, Kirkeby A, Krause KH, Poerzgen P, Puceat M, Schmidt M, Schrattenholz A, Zak NB, Hentze H. The biological and ethical basis of the use of human embryonic stem cells for in vitro test systems or cell therapy. ALTEX. 2008a;25:163–90. [PubMed] [Google Scholar]
- Leist M, Hartung T, Nicotera P. The dawning of a new age of toxicology. ALTEX. 2008b;25:103–14. [PubMed] [Google Scholar]
- Leist M, Efremova L, Karreman C. Food for thought ..considerations and guidelines for basic test method descriptions in toxicology. ALTEX. 2010;27:309–17. doi: 10.14573/altex.2010.4.309. [DOI] [PubMed] [Google Scholar]
- Lin HM, Liu CY, Jow GM, Tang CY. Toluene disrupts synaptogenesis in cultured hippocampal neurons. Toxicol Lett. 2009;184:90–6. doi: 10.1016/j.toxlet.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Lizardi PS, O'Rourke MK, Morris RJ. The effects of organophosphate pesticide exposure on Hispanic children's cognitive and behavioural functioning. J Pediatr Psychol. 2008;33:91–101. doi: 10.1093/jpepsy/jsm047. [DOI] [PubMed] [Google Scholar]
- LoPachin RM, Schwarcz AI, Gaughan CL, Mansukhani S, Das S. In vivo and in vitro effects of acrylamide on synaptosomal neurotransmitter uptake and release. Neurotoxicology. 2004;25:349–63. doi: 10.1016/S0161-813X(03)00149-9. [DOI] [PubMed] [Google Scholar]
- Lopreato GF, Phelan R, Borghese CM, Beckstead MJ, Mihic SJ. Inhaled drugs of abuse enhance serotonin-3 receptor function. Drug Alcohol Depend. 2003;70:11–5. doi: 10.1016/s0376-8716(02)00330-7. [DOI] [PubMed] [Google Scholar]
- Lotharius J, Falsig J, van Beek J, Payne S, Dringen R, Brundin P, Leist M. Progressive degeneration of human mesencephalic neuron-derived cells triggered by dopamine-dependent oxidative stress is dependent on the mixed-lineage kinase pathway. J Neurosci. 2005;25:6329–42. doi: 10.1523/JNEUROSCI.1746-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S, Christensen KV, Hedtjärn M, Mortensen AL, Hagberg H, Falsig J, Hasseldam H, Schrattenholz A, Pörzgen P, Leist M. The dynamics of the LPS triggered inflammatory response of murine microglia under different culture and in vivo conditions. J Neuroimmunol. 2006;180:71–87. doi: 10.1016/j.jneuroim.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Ma DK, Marchetto MC, Guo JU, Ming GL, Gage FH, Song H. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat Neurosci. 2010;13:1338–44. doi: 10.1038/nn.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris SL, Raffaele K, Allen S, Bowers WJ, Hass U, Alleva E, Calamandrei G, Sheets L, Amcoff P, Delrue N, Crofton KM. A retrospective performance assessment of the developmental neurotoxicity study in support of OECD test guideline 426. Environ Health Perspect. 2009;117:17–25. doi: 10.1289/ehp.11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Baron M, Schaper M, Knapp G, van Thriel C. Occupational aluminum exposure: Evidence in support of its neurobehavioural impact. Neurotoxicology. 2007;28:1068–78. doi: 10.1016/j.neuro.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Dow-Edwards D, Li AA, Sandler JD, Seed J, Sheets LP, Shuey DL, Slikker W, Jr, Weisenburger WP, Wise LD, Selwyn MR. Neurobehavioral assessment: a survey of use and value in safety assessment studies. Toxicol Sci. 2003;76:250–61. doi: 10.1093/toxsci/kfg211. [DOI] [PubMed] [Google Scholar]
- Narita M, Aoki K, Takagi M, Yajima Y, Suzuki T. Implication of brain-derived neurotrophic factor in the release of dopamine and dopamine-related behaviours induced by methamphetamine. Neuroscience. 2003;119:767–75. doi: 10.1016/s0306-4522(03)00099-x. [DOI] [PubMed] [Google Scholar]
- NRC. Toxicity Testing in the 21st Century: a vision and a strategy. Washington, D.C: The National Academies Press; 2007. [Google Scholar]
- Nelson PG, Fields RD, Yu C, Neale EA. Mechanisms involved in activity-dependent synapse formation in mammalian central nervous system cell cultures. Journal of neurobiology. 1990;21:138–56. doi: 10.1002/neu.480210110. [DOI] [PubMed] [Google Scholar]
- Nordin-Andersson M, Forsby A, Heldring N, Dejongh J, Kjellstrand P, Walum E. Neurite degeneration in differentiated human neuroblastoma cells. Toxicol In Vitro. 1998;12:557–60. doi: 10.1016/s0887-2333(98)00035-6. [DOI] [PubMed] [Google Scholar]
- Nordin-Andersson M, Walum E, Kjellstrand P, Forsby A. Acrylamide-induced effects on general and neurospecific cellular functions during exposure and recovery. Cell Biol Toxicol. 2003;19:43–51. doi: 10.1023/a:1022017731328. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Kuwagata M, Hori Y, Shioda S. Valproate-induced developmental neurotoxicity is affected by maternal conditions including shipping stress and environmental change during early pregnancy. Toxicol Lett. 2007;174:18–24. doi: 10.1016/j.toxlet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Penschuck S, Flagstad P, Didriksen M, Leist M, Michael-Titus AT. Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. Eur J Neurosci. 2006;23:279–84. doi: 10.1111/j.1460-9568.2005.04536.x. [DOI] [PubMed] [Google Scholar]
- Perrine SA, O'Leary-Moore SK, Galloway MP, Hannigan JH, Bowen SE. Binge toluene exposure alters glutamate, glutamine and GABA in the adolescent rat brain as measured by proton magnetic resonance spectroscopy. Drug Alcohol Depend. 2011;115:101–6. doi: 10.1016/j.drugalcdep.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Hansen LG, Albertson TE, Garner CE, Ta TA, Do Z, Kim KH, Wong PW. Structure-activity relationship for noncoplanar polychlorinated biphenyl congeners toward the ryanodine receptor-Ca2+ channel complex type 1 (RyR1) Chem Res Toxicol. 2006;19:92–101. doi: 10.1021/tx050196m. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Lein PJ. Evidence for Environmental Susceptibility in Autism: What We Need to Know about Gene x Environment Interactions. In: Zimmerman AW, editor. Autism: Current Theories and Evidence. Totowa, NJ: Humana Press; 2008. pp. 409–28. [Google Scholar]
- Porritt MJ, Batchelor PE, Howells DW. Inhibiting BDNF expression by antisense oligonucleotide infusion causes loss of nigral dopaminergic neurons. Exp Neurol. 2005;192:226–34. doi: 10.1016/j.expneurol.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Radio NM, Breier JM, Shafer TJ, Mundy WR. Assessment of chemical effects on neurite outgrowth in PC12 cells using high content screening. Toxicol Sci. 2008;105:106–18. doi: 10.1093/toxsci/kfn114. [DOI] [PubMed] [Google Scholar]
- Radio NM, Freudenrich TM, Robinette BL, Crofton KM, Mundy WR. Comparison of PC12 and cerebellar granule cell cultures for evaluating neurite outgrowth using high content analysis. Neurotoxicol Teratol. 2010;32:25–35. doi: 10.1016/j.ntt.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Radio NM, Mundy WR. Developmental neurotoxicity testing in vitro: models for assessing chemical effects on neurite outgrowth. Neurotoxicology. 2008;29:361–76. doi: 10.1016/j.neuro.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R. 7-Year Neurodevelopmental Scores and Prenatal Exposure to Chlorpyrifos, a Common Agricultural Pesticide. Environ Health Perspect. 2011 doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter L. An introduction to neurobehavioural toxicology. Environ Health Perspect. 1978;26:5–7. doi: 10.1289/ehp.78265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108 (Suppl 3):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robel S, Berninger B, Götz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011;12:88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- Rohlman DS, Arcury TA, Quandt SA, Lasarev M, Rothlein J, Travers R, et al. Neurobehavioural performance in preschool children from agricultural and non-agricultural communities in Oregon and North Carolina. Neurotoxicology. 2005;26:589–98. doi: 10.1016/j.neuro.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK. BDNF gene: functional Val66Met polymorphism in mood disorders and schizophrenia. Pharmacogenomics. 2008;9:1589–93. doi: 10.2217/14622416.9.11.1589. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Kubota M, Liu XJ, Murata K, Nakai K, Satoh H. Maternal and fetal mercury and n-3 polyunsaturated fatty acids as a risk and benefit of fish consumption to fetus. Environ Sci Technol. 2004;38:3860–3. doi: 10.1021/es034983m. [DOI] [PubMed] [Google Scholar]
- Saylor AJ, McGinty JF. Amphetamine-induced locomotion and gene expression are altered in BDNF heterozygous mice. Genes Brain Behav. 2008;7:906–14. doi: 10.1111/j.1601-183X.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor AJ, Meredith GE, Vercillo MS, Zahm DS, McGinty JF. BDNF heterozygous mice demonstrate age-related changes in striatal and nigral gene expression. Exp Neurol. 2006;199:362–72. doi: 10.1016/j.expneurol.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Scerif G, Karmiloff-Smith A. The dawn of cognitive genetics? Crucial developmental caveats. Trends Cogn Sci. 2005;9:126–35. doi: 10.1016/j.tics.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ, Rice DC. Effects of PCB exposure on neuropsychological function in children. Environmental health perspectives. 2003;111:357–576. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrattenholz A, Klemm M. Neuronal cell culture from human embryonic stem cells as in vitro model for neuroprotection. ALTEX. 2007;24:9–15. doi: 10.14573/altex.2007.1.9. [DOI] [PubMed] [Google Scholar]
- Schreiber T, Gassmann K, Götz C, Hübenthal U, Moors M, Krause G, Merk HF, Nguyen NH, Scanlan TS, Abel J, Rose CR, Fritsche E. Polybrominated diphenyl ethers induce developmental neurotoxicity in a human in vitro model: evidence for endocrine disruption. Environ Health Perspect. 2010;118:572–8. doi: 10.1289/ehp.0901435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA. Twenty-first century challenges for in vitro neurotoxicity. Altern Lab Anim. 2009;37:367–75. doi: 10.1177/026119290903700407. [DOI] [PubMed] [Google Scholar]
- Staskal DF, Diliberto JJ, Birnbaum LS. Disposition of BDE 47 in developing mice. Toxicol Sci. 2006;90:309–16. doi: 10.1093/toxsci/kfj098. [DOI] [PubMed] [Google Scholar]
- Stiegler NV, Krug AK, Matt F, Leist M. Assessment of chemical-induced impairment of human neurite outgrowth by multiparametric live cell imaging in high-density cultures. Toxicol Sci. 2011;121:73–87. doi: 10.1093/toxsci/kfr034. [DOI] [PubMed] [Google Scholar]
- Stodgell CJ, Ingram JL, O'Bara M, Tisdale BK, Nau H, Rodier PM. Induction of the homeotic gene Hoxa1 through valproic acid's teratogenic mechanism of action. Neurotoxicol Teratol. 2006;28:617–24. doi: 10.1016/j.ntt.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Stummann TC, Hareng L, Bremer S. Embryotoxicity hazard assessment of methylmercury and chromium using embryonic stem cells. Toxicology. 2007;242:130–43. doi: 10.1016/j.tox.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Stummann TC, Hareng L, Bremer S. Hazard assessment of methylmercury toxicity to neuronal induction in embryogenesis using human embryonic stem cells. Toxicology. 2009;257:117–26. doi: 10.1016/j.tox.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009;25:253–75. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- Tamm C, Duckworth JK, Hermanson O, Ceccatelli S. Methylmercury inhibits differentiation of rat neural stem cells via Notch signalling. Neuroreport. 2008;19:339–43. doi: 10.1097/WNR.0b013e3282f50ca4. [DOI] [PubMed] [Google Scholar]
- Tettamanti G, Cattaneo AG, Gornati R, de Equileor M, Bernardini G, Binelli G. Phylogenesis of brain-derived neurotrophic factor (BDNF) in vertebrates. Gene. 2010;450:85–93. doi: 10.1016/j.gene.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Uibel F, Mühleisen A, Köhle C, Weimer M, Stummann TC, Bremer S, Schwarz M. ReProGlo: a new stem cell-based reporter assay aimed to predict embryotoxic potential of drugs and chemicals. Reprod Toxicol. 2010;30:103–12. doi: 10.1016/j.reprotox.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Blankenship AL, Giesy JP. Derivation and application of relative potency estimates based on in vitro bioassay results. Environmental Toxicology and Chemistry. 2000;19:2835–43. [Google Scholar]
- Volbracht C, Leist M, Nicotera P. ATP controls neuronal apoptosis triggered by microtubule breakdown or potassium deprivation. Mol Med. 1999;5:477–89. [PMC free article] [PubMed] [Google Scholar]
- Walkowiak J, Wiener JA, Fastabend A, Heinzow B, Kramer U, Schmidt E, Steingrüber HJ, Wundram S, Winneke G. Environmental exposure to polychlorinated biphenyls and quality of the home environment: effects on psychodevelopment in early childhood. Lancet. 2001;358:1602–7. doi: 10.1016/S0140-6736(01)06654-5. [DOI] [PubMed] [Google Scholar]
- Werboff J, Dembicki EL. Toxic effects of tranquilizers administered to gravid rats. J Neuropsychiatry. 1962;4:87–91. [PubMed] [Google Scholar]
- Westerink RH, Vijverberg HP. Toluene-induced, Ca(2+)-dependent vesicular catecholamine release in rat PC12 cells. Neurosci Lett. 2002;326:81–4. doi: 10.1016/s0304-3940(02)00315-4. [DOI] [PubMed] [Google Scholar]
- Westerink RHS, Hondebrink L. Are high-throughput measurements of intracellular calcium using plate-readers sufficiently accurate and reliable? Toxicol Appl Pharmacol. 2010;249:247–8. doi: 10.1016/j.taap.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Westerink RHS. Targeting Exocytosis: Ins and outs of the modulation of quantal dopamine release. CNS and Neurological Disorders - Drug Targets. 2006;5:57–77. doi: 10.2174/187152706784111597. [DOI] [PubMed] [Google Scholar]
- Winneke G. Developmental aspects of environmental neurotoxicology: Lessons from lead and polychlorinated biphenyls. J Neurol Sci. 2011 doi: 10.1016/j.jns.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Win-Shwe TT, Mitsushima D, Nakajima D, Ahmed S, Yamamoto S, Tsukahara S, Kakeyama M, Goto S, Fujimaki H. Toluene induces rapid and reversible rise of hippocampal glutamate and taurine neurotransmitter levels in mice. Toxicol Lett. 2007;168:75–82. doi: 10.1016/j.toxlet.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Yang D, Howard A, Bruun D, Ajua-Alemanj M, Pickart C, Lein PJ. Chlorpyrifos and chlorpyrifos-oxon inhibit axonal growth by interfering with the morphogenic activity of acetylcholinesterase. Toxicol Appl Pharmacol. 2008;228:32–41. doi: 10.1016/j.taap.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Kim KH, Phimister A, Bachstetter AD, Ward TR, Stackman RW, Mervis RF, Wisniewski AB, Klein SL, Kodavanti PR, Anderson KA, Wayman G, Pessah IN, Lein PJ. Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ Health Perspect. 2009;117:426–35. doi: 10.1289/ehp.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Lauridsen H, Buels K, Chi LH, La Du J, Bruun DA, Olson JR, Tanguay RL, Lein PJ. Chlorpyrifos-oxon disrupts zebrafish axonal growth and motor behaviour. Toxicol Sci. 2011;121:146–59. doi: 10.1093/toxsci/kfr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer B, Kuegler PB, Baudis B, Genewsky A, Tanavde V, Koh W, Tan B, Waldmann T, Kadereit S, Leist M. Coordinated waves of gene expression during neuronal differentiation of embryonic stem cells as basis for novel approaches to developmental neurotoxicity testing. Cell Death Differ. 2011a;18:383–95. doi: 10.1038/cdd.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer B, Schildknecht S, Kuegler PB, Tanavde V, Kadereit S, Leist M. Sensitivity of dopaminergic neuron differentiation from stem cells to chronic low-dose methylmercury exposure. Toxicol Sci. 2011b;121:357–67. doi: 10.1093/toxsci/kfr054. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY. Postnatal neurodevelopmental disorders: meeting at the synapse? Science. 2003;302:826–30. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]
- Zurich MG, Monnet-Tschudi F. Contribution of in vitro neurotoxicology studies to the elucidation of neurodegenerative processes. Brain Res Bull. 2009;80:211–6. doi: 10.1016/j.brainresbull.2009.06.008. [DOI] [PubMed] [Google Scholar]