Abstract
Objective
To examine the correlations between intra-hepatic and intra-thoracic (total, epicardial, and pericardial) fat deposition with cardiovascular disease (CVD) risk factors and subclinical atherosclerosis burden in healthy, recently postmenopausal women.
Methods
Women screened for the Kronos Early Estrogen Prevention Study (mean age 52.9 years) who underwent electron beam or multidetector computed tomography (CT) imaging for the quantification of intra-hepatic fat and thoracic adipose tissue, and coronary artery calcification (CAC) were included (n= 650).
Results
Higher levels of intra-hepatic and thoracic fat were each associated with CVD risk markers. After adjustment for BMI, the associations for intra-hepatic fat with hs-CRP and insulin persisted (r= 0.21 and 0.19, respectively; P<0.001), while those between thoracic fat indices and lipids persisted (r for total thoracic fat with HDL, LDL, and triglycerides= −0.16, 0.11, and 0.11, respectively, P<0.05). Total thoracic fat was associated with CAC after initial multivariable adjustment (odds ratio [OR] of 2nd, 3rd, and 4th vs. 1st quartile and [95% confidence intervals]: 0.8 [0.4–1.6], 1.5 [0.8–2.9], and 1.8 [1.0–3.4]; P for linear trend=0.017) and was only slightly attenuated after additional adjustment for BMI. Associations between total thoracic fat and CVD risk markers and CAC appeared due slightly more to associations with epicardial than pericardial fat.
Conclusion
While hepatic fat is related to hs-CRP and insulin, cardiac fat is associated with subclinical atherosclerosis as demonstrated by CAC. Cardiac fat may represent a useful marker for increased CVD risk beyond the standard adiposity measures of BMI and WC.
Keywords: coronary calcification, ectopic fat, cardiac fat, hepatic fat, risk factors, women
INTRODUCTION
Energy excess, rather than undernutrition, has long been the dominant nutritional problem in the US and is rapidly becoming so worldwide. Factors other than BMI may be key determinants of adiposity-associated CVD risk, as individuals of similar body size (body mass index [BMI]) have been shown to differ widely in their risk of cardiovascular disease (CVD).1–3 Ectopic fat, which includes triglyceride accumulation within lean tissues including the liver, muscle, pancreas, and kidneys, and excess adipose tissue in the epicardium and pericardium, may be one such factor. Excess hepatic triglyceride may be associated with up to a 4-fold increased risk of CVD.4–6 Excess fat in the epicardium and pericardium is also reported to be related to CVD risk.7–9 Previous investigations from the Framingham Heart Study have shown an association between cardiac fat and CVD and its risk factors.10–12 However, Framingham participants were a mean age of approximately 60 years at the time of the cardiac fat measurement, and additional studies have focused exclusively either on older patients or those at high risk for CVD, such as individuals with hypertension and glucose intolerance, leaving open the question of the relationship between cardiac fat and CVD risk in mid-life individuals with normal glucose and blood pressure.13–15
The purpose of the current study was to examine the associations of intra-hepatic fat and cardiac fat (total thoracic, epicardial, and pericardial) both with standard CVD risk markers and with subclinical atherosclerosis, independent of abdominal obesity, in healthy mid-life women. Due to the role of the liver in insulin resistance and inflammation, we hypothesized that intra-hepatic fat would be more strongly associated with inflammation, triglyceride levels and measures of glucose homeostasis; while cardiac fat, with its close proximity to the coronary circulation, would be more strongly associated with subclinical atherosclerosis, especially coronary artery calcification (CAC).
METHODS
Study Population
These results are derived from data from the screening visits of the Kronos Early Estrogen Prevention Study (KEEPS), a randomized, placebo-controlled, double-blinded, prospective trial of the effects of menopausal hormone therapy on subclinical atherosclerosis in recently menopausal women. KEEPS is a multi-center trial with 9 centers (University of Utah, University of California at San Francisco; Brigham and Women’s Hospital; Mayo Clinic, Rochester; Columbia University College of Physicians and Surgeons; University of Washington; Yale University; Montefiore Medical Center; Kronos Longevity Research Institute), at which subjects were recruited between July, 2005 and June, 2008, and are currently being followed. KEEPS inclusion criteria were age 42–58 years, having at least 6 months but no more than 36 months of amenorrhea and last spontaneous menses occurring after age 40, FSH values ≥35 ng/ml and/or estradiol levels <35 pg/ml, and good general health. Women reporting a history of clinical cardiovascular disease (CVD), current heavy smoking (greater than one half pack per day), a BMI ≥35 kg/m2, LDL cholesterol>190 mg/dL or triglycerides>400 mg/dL, diabetes, uncontrolled hypertension, or with moderate subclinical CVD, defined as a CAC score ≥50 Agatston units, were ineligible for randomization. A total of 1,046 women were screened between July 2005 to June 2008, with 775 of them passing earlier screening components and eventually receiving a CT scan for the purposes of confirming CAC Agatston score eligibility. Of those, 727 women were eventually enrolled into the study and subsequently randomized to two active treatment groups: oral conjugated equine estrogen (Premarin, 0.45mg/day), and transdermal 17β-estradiol (via skin patch, Climara, 50µg/day), and one daily placebo group (inactive pill/patch). Of the 775 women receiving a screening CT scan, 125 women had scans with insufficient visualization of the liver for calculation of liver attenuation. Therefore, these analyses include the 650 women with complete data for both hepatic and cardiac fat measures.
The institutional review boards of the participating institutions approved this study, and all women signed informed consent.
Cardiometabolic Risk Factor Measures
All blood specimens were collected following an overnight fast, then frozen at −70°C on site until they were either processed locally, or sent to the Kronos Science Laboratory (Phoenix, AZ, USA) for storage or assays. Complete blood count and chemistry panel were performed at the clinical laboratories at each recruiting center. C-reactive protein (CRP) and insulin were assayed by DPC Immulite 2000 (Diagnostic Products Corporation, Los Angeles, CA).
Anthropometric and Lifestyle Exposures
Baseline education (<college graduate, ≥college graduate), smoking status (current use yes/no), alcohol intake (current use yes/no), race-ethnicity, and levels of physical activity (Metabolic Equivalents, or METs, were calculated as total energy expenditure from recreational physical activity in kcal/week/kg, with the questionnaire intentionally worded without reference to a specific time frame to collect the “usual” patterns of physical activity) were obtained through self-reported questionnaire data. The height (cm) and weight (kg) of participants, wearing light clothing and without shoes, was measured with stadiometers and calibrated balance-beam scales, respectively. BMI was calculated as weight in kilograms divided by the square of height in meters. Waist circumference (cm) was measured with a non-stretchable tape after a normal expiration, at the smallest horizontal circumference between the ribs and iliac crest. Blood pressure was measured in the right arm in the seated position, after at least a 5 minute rest, and averaged across two readings.
Ectopic Fat Measures
Cardiac and intra-hepatic fat measurements were all performed on the same non-contrast enhanced computer tomography (CT) axial scans, which were acquired either via beam CT using C150XP or C300 electron beam tomography scanners (GE/Imatron, Inc.), or via electron multidetector CT using the General Electric helical scanner or a Siemens multi-slice scanner (minimum requirement 4 detector heads). Comparability among centers was assured by regular calibration using a standard phantom. Results for all CT-derived fat measures were averaged across the two scans obtained for each woman. All imaging results were read centrally by experienced readers who were blinded to participant demographics.
Hepatic and splenic attenuation values were measured using regions of interest (ROI) greater than 100 mm2 in area. One ROI was placed in the right liver lobe, one ROI in the left liver lobe, and one ROI in the spleen. This was done on 3 consecutive slices for each region, and the average value was calculated. Whenever possible, ROIs with larger areas were used so that a greater area of the liver and spleen were included, but the regions of non-uniform parenchymal attenuation, such as hepatic vessels, were excluded. Although the spleen measure was collected as potential reference tissue for the liver density assessment, raw hepatic attenuation values were used in analyses since spleen attenuation values correlated with measures of adiposity. Since fat is less dense than surrounding tissues, higher levels of hepatic attenuation are indicative of less fat.
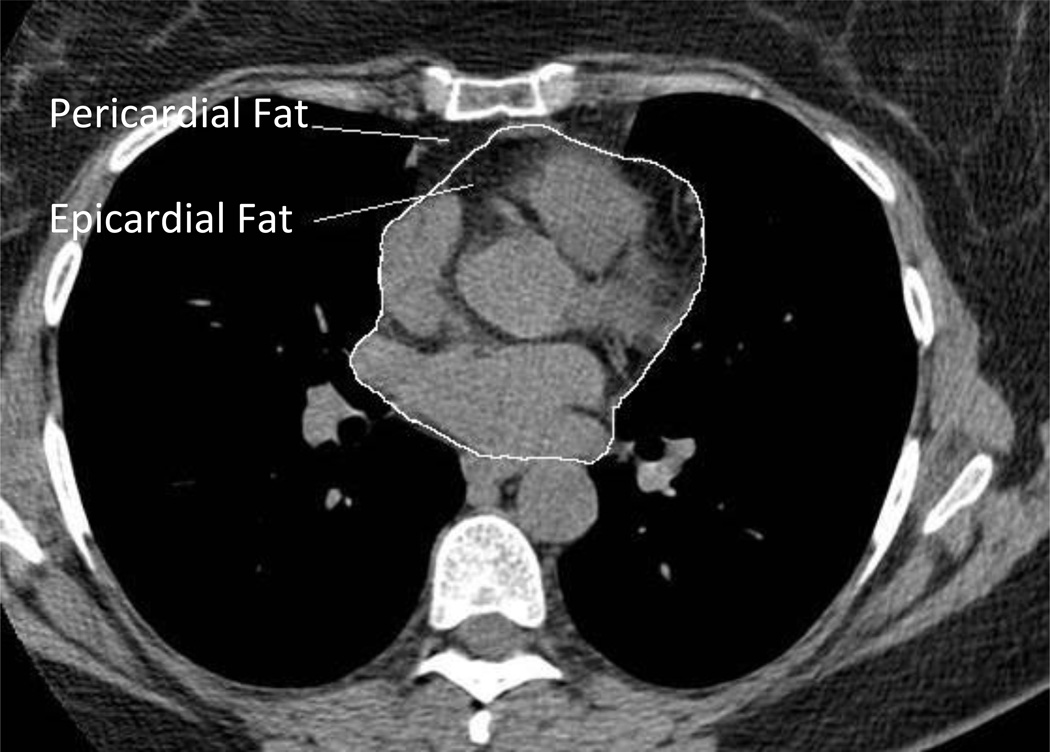
Using methods similar to a previously published study,16 epicardial adipose tissue (EAT) was defined as fat tissue inside the pericardial sac, pericardial adipose tissue (PAT) was defined as fat tissue outside the pericardial sac (Figure 1), and total thoracic adipose tissue (TAT) represented the combined total of EAT and PAT. Cardiac fat measurements were performed using CT images on axial data sets from 10mm above to 30mm below the superior extent of the left main coronary artery, the heart region that includes epicardial adipose tissue located around the proximal coronary arteries (left main coronary, left anterior descending coronary, left circumflex coronary, right coronary arteries). EAT was measured by manually tracing out the pericardium every 2 to 3 slices below the start point, and then using the software to automatically trace out the segments in between these selected slices. For thoracic fat measurements (EAT and PAT combined), the anterior border of the volume was defined by the chest wall and posterior border by the aorta and the bronchi. Adipose tissue present in the posterior mediastinum and para-aortic adipose tissue was not included. PAT was measured by subtracting EAT from total TAT fat volume. A threshold of −190 to −30 Hounsfield Units (HU) was used to discern fat from other tissues. Cardiac fat measures are volumes, with higher values indicating more fat.
Figure 1.
Epicardial and Pericardial Fat Depots
Reproducibility measurements of hepatic attenuation and cardiac fat volume measurements were performed on 20 randomly selected scans by two readers. For intra-reader reproducibility there were 18 scans available, and both Spearman and intra-class correlation coefficients between repeated readings of TAT, EAT, PAT, and hepatic attenuation were high (0.99 each for both TAT and EAT; 0.86 and 0.96, respectively, for PAT; and 0.99 each for hepatic attenuation). For inter-reader reproducibility, all 20 scans were available, and again both Spearman and intra-class correlation coefficients between repeated readings of TAT, EAT, PAT, and hepatic attenuation were high (TAT: 0.99 each; EAT: 0.98 each; PAT: 0.96 and 0.90, respectively; and hepatic attenuation: 0.95 and 0.98, respectively). We also compared measurement of hepatic attenuation from a single slice with average attenuation values derived from 3 consecutive slices. There was excellent correlation between the two (r values of 0.96, 0.98 for left and right hepatic lobes, respectively), and no significant differences were found between single slice vs average values. Fatty infiltration of liver is a diffuse process, so the use of average values from 3 slices provides a good measure of this parameter.
Subclinical Atherosclerosis Measures
Intima-media thickness of the carotid arteries (CIMT) was measured via B-mode ultrasound. B-mode carotid artery images were acquired at each study center by certified ultrasound technicians trained at the core CIMT center (PI: Dr. Howard Hodis, USC) to perform a standard acquisition sequence. Image acquisition procedures were optimized for minimal measurement variability.17 The ultrasound power, echo detector gain, and dynamic range were recorded to establish identical conditions for serial examinations. All instruments were high-resolution imagers with a linear array 7.5 MHz probe. Electrocardiogram (ECG), external time code information, and ultrasound images were simultaneously recorded on digital videotape and processed images stored on CD’s. All images were evaluated by an experienced investigator at the core CIMT study center.
Coronary artery calcification (CAC) was measured on non-enhanced cardiac CT scans. Coronary calcium was defined as a plaque of at least 3 contiguous pixels (area 1.02 mm2) with a density of >130 HU. A total CAC score was determined by summing the individual Agatston scores from each of 4 anatomic sites (left main, left anterior descending, circumflex, and right coronary). A single experienced investigator, blinded to the subject identity, interpreted all the scans using commercially available software (TeraRecon, Foster City, CA).
Statistical Analyses
Mean (standard deviation) or prevalence of demographic, anthropometric, laboratory, and atherosclerosis characteristics were tabulated across hepatic attenuation, epicardial fat volume, and pericardial fat volume quartiles, separately. P-values for linear trend across quartiles was tabulated using general linear models with quartiles treated as a continuous variable for normally distributed continuous variables, using quantile regression with median values in each quartile treated as a continuous variable for non-normally distributed continuous variables, and using polytomous logistic regression for categorical variables. Partial Spearman correlation coefficients were tabulated in the case of cardiometabolic factors and CIMT, and multivariable logistic regression was utilized in the case of coronary artery calcification (>0 vs. 0) to evaluate associations between ectopic fat indices and outcomes after adjustment for important confounding factors. Initial adjustment was made for age, race (white vs. non-white), education level, smoking status, alcohol intake, physical activity level and study center. Further adjustment was then made for either BMI or waist circumference (WC). Statistical significance was considered at P <0.05, and all analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). Since lower hepatic attenuation values are indicative of more fat, hepatic attenuation quartiles are presented from high to low in Tables 1 and 3, in contrast to cardiac fat quartiles which are presented from low to high.
Table 1.
Demographic Characteristics and Cardiometabolic Risk Factor Levels across Quartiles of Hepatic and Intra-Thoracic Fat Indices
| ← Less Fat Hepatic Attenuation More Fat→ | |||||
|---|---|---|---|---|---|
| >67.0 | 64.0–67.0 | 59.0–63.0 | <58.0 | p-trend | |
| Age, years | 52.9 (0.2) | 52.8 (0.2) | 53.0 (0.2) | 53.0 (0.2) | 0.59 |
| n (%) White | 137 (77) | 120 (78) | 113 (72) | 113 (71) | 0.11 |
| Education | 0.15 | ||||
| n (%) ≤High School | 66 (39) | 50 (34) | 52 (34) | 48 (31) | |
| n (%) Some College | 48 (28) | 33 (22) | 39 (25) | 45 (29) | |
| n (%) ≥College | 56 (33) | 65 (44) | 64 (41) | 62 (40) | |
| n (%) Current Smoker | 9 (5) | 18 (12) | 10 (6) | 12 (8) | 0.73 |
| Physical Activity Levels, METS | 24.5 (1.6) | 22.9 (1.7) | 22.1 (1.4) | 18.4 (1.4) | 0.005 |
| Systolic Blood Pressure, mmHg | 117.0 (1.1) | 118.0 (1.4) | 119.4 (1.2) | 122.0 (1.1) | 0.003 |
| Diastolic Blood Pressure, mmHg | 74.2 (0.7) | 74.4 (0.8) | 75.7 (0.8) | 77.1 (0.7) | 0.003 |
| LDL Cholesterol, mg/dL | 127.0 (2.2) | 129.5 (2.3) | 128.1 (2.3) | 127.5 (2.3) | 0.950 |
| HDL Cholesterol, mg/dL | 65.9 (1.3) | 66.4 (1.3) | 66.5 (1.3) | 58.6 (1.4) | < 0.001 |
| Triglycerides, mg/dL* | 77.0 (58.0–106.0) | 74.5 (56.0–104.0) | 68.5 (51.5–100.5) | 92.5 (67.0–139.0) | < 0.001 |
| Glucose, mg/dL* | 88.0 (81.0–93.0) | 89.0 (82.0–95.0) | 88.0 (81.5–94.5) | 91.0 (86.0–97.0) | < 0.001 |
| Insulin, µIU/ml * | 4.8 (3.2–6.9) | 4.8 (3.2–7.5) | 5.9 (3.7–8.4) | 8.6 (4.8–11.8) | < 0.001 |
| BMI, Kg/m2 | 25.1 (0.3) | 25.3 (0.3) | 26.4 (0.4) | 29.0 (0.3) | < 0.001 |
| Waist Circumference, cm* | 78.7 (73.7–86.4) | 83.0 (72.5–89.8) | 83.2 (75.0–93.0) | 93.0 (84.5–101.0) | < 0.001 |
| C-reactive Protein, mg/dL* | 0.8 (0.5–1.6) | 1.3 (0.5–2.3) | 1.3 (0.6–3.3) | 2.4 (0.8–4.8) | < 0.001 |
| CIMT, mm | 0.74 (0.01) | 0.71 (0.01) | 0.73 (0.01) | 0.73 (0.01) | 0.65 |
| ← Less Fat Total TAT Volume (cm3) More Fat→ | |||||
| <32.24 | 32.29–44.53 | 44.71–58.21 | >58.6 | p-trend | |
| Age, years | 52.6 (0.2) | 52.9 (0.2) | 53.1 (0.2) | 53.1 (0.2) | 0.033 |
| n (%) White | 113 (70) | 117 (72) | 123 (75) | 130 (80) | 0.030 |
| Education | 0.030 | ||||
| n (%) ≤High School | 46 (29) | 57 (37) | 53 (34) | 60 (37) | |
| n (%) Some College | 31 (20) | 44 (29) | 44 (28) | 46 (29) | |
| n (%) ≥College | 79 (51) | 53 (34) | 60 (38) | 55 (34) | |
| n (%) Current Smoker | 9 (6) | 10 (6) | 15 (10) | 15 (9) | 0.12 |
| Physical Activity Levels, METS | 26.6 (1.7) | 20.9 (1.4) | 22.7 (1.6) | 18.0 (1.4) | < 0.001 |
| Systolic Blood Pressure, mmHg | 118.1 (1.4) | 117.1 (1.3) | 119.3 (1.1) | 121.6 (1.0) | 0.022 |
| Diastolic Blood Pressure, mmHg | 74.6 (0.8) | 73.8 (0.7) | 75.5 (0.8) | 77.4 (0.7) | 0.002 |
| LDL Cholesterol, mg/dL | 123.4 (2.3) | 126.5 (2.4) | 126.6 (2.2) | 135.5 (2.2) | < 0.001 |
| HDL Cholesterol, mg/dL | 70.9 (1.3) | 65.0 (1.3) | 64.6 (1.4) | 57.0 (1.2) | < 0.001 |
| Triglycerides, mg/dL* | 66.5 (50.0–84.5) | 74.0 (57.0–96.5) | 84.0 (60.0–113.0) | 101.5 (71.0–142.0) | < 0.001 |
| Glucose, mg/dL* | 85.0 (79.0–91.0) | 88.0 (81.0–94.5) | 90.0 (85.0–96.0) | 92.0 (87.0–98.0) | < 0.001 |
| Insulin, µIU/ml * | 4.4 (2.8–6.0) | 5.1 (3.7–7.5) | 5.8 (3.6–9.9) | 7.8 (5.0–11.0) | < 0.001 |
| BMI, Kg/m2 | 23.2 (0.3) | 25.1 (0.3) | 27.0 (0.3) | 30.4 (0.3) | < 0.001 |
| Waist Circumference, cm* | 75.5 (69.0–82.0) | 80.1 (73.7–88.9) | 84.3 (77.9–93.0) | 94.0 (88.9–101.0) | < 0.001 |
| C-reactive Protein, mg/dL* | 0.7 (0.4–1.5) | 1.1 (0.5–2.4) | 1.5 (0.7–3.5) | 2.3 (1.0–4.4) | < 0.001 |
| CIMT, mm | 0.73 (0.01) | 0.73 (0.01) | 0.72 (0.01) | 0.73 (0.01) | 0.89 |
Median (IQR). Others are mean (SE).
Table 3.
Multivariable-Adjusted Logistic Regression of CAC associated with Ectopic Fat Indices
| Model 1* | Model 2** | Model 3*** | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Hepatic Attenuation | ||||||
| >67.0 (ref) | 1.0 | 1.0 | 1.0 | |||
| 64.0–67.0 | 1.1 | 0.6–2.1 | 1.0 | 0.5–2.0 | 1.0 | 0.5–1.9 |
| 59.0–63.0 | 1.5 | 0.8–2.9 | 1.4 | 0.7–2.8 | 1.5 | 0.8–3.0 |
| <59.0 | 1.2 | 0.6–2.6 | 1.1 | 0.5–2.4 | 1.3 | 0.6–2.7 |
| P for linear trend | 0.51 | 0.71 | 0.46 | |||
| Total TAT Volume (cm3) | ||||||
| <32.29 (ref) | 1.0 | 1.0 | 1.0 | |||
| 32.29–44.70 | 0.8 | 0.4–1.6 | 0.8 | 0.4–1.6 | 0.7 | 0.3–1.4 |
| 44.71–58.69 | 1.5 | 0.8–2.9 | 1.4 | 0.7–2.9 | 1.5 | 0.7–2.9 |
| >58.69 | 1.8 | 1.0–3.4 | 1.8 | 0.8–3.8 | 1.7 | 0.9–3.3 |
| P for linear trend | 0.017 | 0.06 | 0.027 | |||
| EAT Volume (cm3) | ||||||
| <28.2 (ref) | 1.0 | 1.0 | 1.0 | |||
| 28.2–38.4 | 0.8 | 0.4–1.6 | 0.8 | 0.4–1.5 | 0.7 | 0.3–1.4 |
| 38.5–49.7 | 1.6 | 0.8–3.0 | 1.5 | 0.8–2.9 | 1.5 | 0.8–2.8 |
| >49.7 | 1.8 | 1.0–3.4 | 1.7 | 0.8–3.7 | 1.8 | 0.9–3.4 |
| P for linear trend | 0.017 | 0.06 | 0.020 | |||
| PAT Volume (cm3) | ||||||
| <2.76 (ref) | 1.0 | 1.0 | 1.0 | |||
| 2.76–5.58 | 1.4 | 0.6–3.2 | 1.3 | 0.5–2.9 | 1.4 | 0.6–3.5 |
| 5.59–10.41 | 1.3 | 0.6–3.0 | 1.2 | 0.5–2.8 | 1.4 | 0.6–3.4 |
| >10.41 | 1.9 | 0.9–4.4 | 1.6 | 0.7–4.1 | 2.1 | 0.9–5.0 |
| P for linear trend | 0.09 | 0.25 | 0.07 | |||
Model 1: Adjusted for age, race-ethnicity, education level, smoking status, alcohol intake, physical activity level and center
Model 2: Adjusted for Model 1, plus BMI
Model 3: Adjusted for Model 1, plus waist circumference
RESULTS
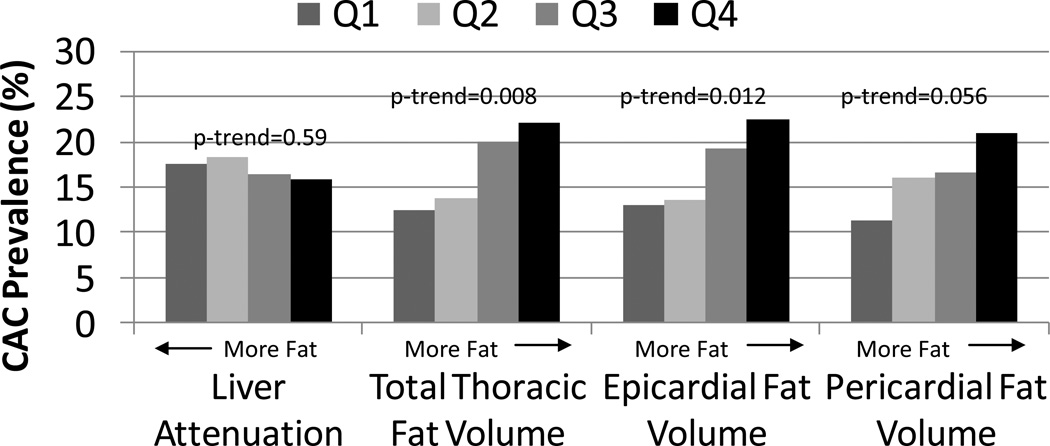
The mean age of these recently postmenopausal study participants was 52.9 (± 2.6) years and 3% (n=19) had evidence of non-alcoholic fatty liver disease determined as a hepatic attenuation value <40 HU. Demographic and laboratory characteristics of these participants stratified by quartiles of hepatic attenuation and total TAT volume are presented in Table 1. As the hepatic attenuation quartile decreased (indicative of more fat) and TAT quartiles increased, levels of cardiometabolic risk markers, with the exception of LDL-C, worsened. Additionally, those with higher levels of TAT were found to be slightly older, more often Caucasian, to report lesser education levels, and lower physical activity levels. The mean CIMT of the study sample was 0.73 (± 0.09) mm; 111 (17.1%) of women had a CAC score>0. CIMT values were not appreciably different across quartiles of either hepatic attenuation or TAT, or across PAT and EAT quartiles when they were evaluated. However, although the prevalence of CAC did not vary across hepatic attenuation quartiles, the prevalence of CAC was incrementally higher across cardiac fat quartiles, though this was only borderline statistically significant for PAT (Figure 2).
Figure 2.
The Prevalence (%) of Coronary Artery Calcification (CAC) Score>0 by Quartiles of Ectopic Fat Indices.
Multivariable-Adjusted Associations with Density Attenuation in Liver Tissue
Lower hepatic attenuation remained associated with worse cardiometabolic risk factor levels after initial multivariable adjustment for age, race-ethnicity, education level, smoking status, alcohol intake, physical activity level, and study center (Table 2). These associations remained moderate to strong, with the exception of systolic blood pressure, triglyceride, and glucose levels, following additional adjustment for BMI or waist circumference.
Table 2.
Partial Spearman Correlation Coefficients for the Associations between Ectopic Fat Measures and Cardiometabolic Risk Factors.
| SBP | LDL | HDL | Triglycerides | Glucose | Insulin | Hs-CRP | |
|---|---|---|---|---|---|---|---|
| Partial 1 | |||||||
| Hepatic Attenuation | −0.17** | 0.01 | 0.20*** | −0.14** | −0.15** | −0.32*** | −0.33*** |
| Total TAT volume | 0.09 | 0.15** | −0.29*** | 0.24*** | 0.18** | 0.28*** | 0.26*** |
| EAT Volume | 0.08 | 0.18*** | −0.26*** | 0.22*** | 0.19*** | 0.27*** | 0.25*** |
| PAT Volume | 0.12* | −0.03 | −0.24*** | 0.16** | 0.08 | 0.22*** | 0.25*** |
| BMI | 0.23*** | 0.09 | −0.31*** | 0.29*** | 0.17** | 0.41*** | 0.50*** |
| Waist Circumference | 0.18** | 0.10 | −0.27*** | 0.21*** | 0.15** | 0.34*** | 0.41*** |
| Partial 2 | |||||||
| Hepatic Attenuation | −0.09 | 0.05 | 0.11* | −0.05 | −0.10 | −0.21*** | −0.19*** |
| Total TAT volume | −0.04 | 0.11* | −0.16** | 0.11* | 0.10 | 0.09 | <0.01 |
| EAT Volume | −0.04 | 0.15** | −0.14* | 0.10 | 0.13* | 0.09 | 0.02 |
| PAT Volume | 0.01 | −0.09 | −0.11* | 0.02 | −0.004 | 0.03 | 0.01 |
| Partial 3 | |||||||
| Hepatic Attenuation | −0.12* | 0.04 | 0.13* | −0.08 | −0.10 | −0.24*** | −0.23*** |
| Total TAT volume | 0.01 | 0.12* | 0.19*** | 0.16** | 0.11* | 0.15** | 0.08 |
| EAT Volume | −0.002 | 0.16** | −0.16** | 0.15** | 0.14** | 0.14* | 0.08 |
| PAT Volume | 0.06 | −0.08 | −0.15** | 0.08 | 0.02 | 0.10 | 0.10 |
Partial 1: Adjusted for age, race-ethnicity, education level, smoking status, alcohol intake, physical activity level and center
Partial 2: Adjusted for Model 1 plus BMI
Partial 3: Adjusted for model 1 plus waist circumference
p<0.001;
p<0.01;
p<0.05
The association of hepatic attenuation with CIMT was null, both before and after multivariable adjustment (r=0.02 to 0.04, depending on factors adjusted for, all P>0.05). Similarly, multivariable models revealed no statistically significant relationship between hepatic attenuation and CAC (Table 3).
Multivariable-Adjusted Associations with Cardiac Fat Measures
After initial multivariable adjustment, moderate to strong relationships existed between higher TAT or EAT and all cardiometabolic risk markers, with the exception of systolic blood pressure (Table 2). Findings were similar for PAT, though associations with LDL-C and glucose were weaker than for TAT or EAT. Additional adjustment for BMI diminished, but did not completely attenuate, associations between cardiac fat measures and cardiometabolic risk markers, while associations persisted to a greater extent after additional adjustment for waist circumference rather than BMI.
The associations of cardiac fat measures with CIMT were null, even after multivariable adjustment (r= −0.02, −0.03, and 0.02 for TAT, EAT, and PAT, respectively after initial multivariable adjustment; all P>0.05). Results were similar after additional adjustment for BMI or waist circumference. Logistic regression models revealed that the trend for a higher odds of CAC>0 with increasing cardiac fat quartiles persisted after adjustment for age, race-ethnicity, education level, smoking status, alcohol intake, physical activity level, and study center, though this trend was not statistically significant for PAT (Table 3). Findings were similar, though slightly diminished, after additional adjustment for BMI or waist circumference.
To gain insight into whether the associations between TAT and EAT with CAC might be explained by intermediary variables such as lipids, glucose, or insulin, we added adjustment for HDL-C, triglycerides, insulin, and glucose to the base models relating TAT and EAT quartiles to CAC. Resulting ORs and 95% CIs for CAC were 0.5 (0.2, 1.6), 1.3 (0.5, 3.3), and 1.8 (0.7, 4.5) for the 2nd, 3rd, and 4th quartiles of TAT vs. the 1st, respectively (p-trend=0.031), and 0.6 (0.2, 1.7), 1.1 (0.4, 2.8), and 1.8 (0.7, 4.2) for the 2nd, 3rd, and 4th quartiles of EAT vs. the 1st, respectively (p-trend=0.050).
Multivariable-Adjusted Associations with BMI and WC
Multivariable adjusted correlations between BMI or WC with cardiometabolic risk markers were similar to those between ectopic fat indices and cardiometabolic risk markers, though slightly stronger. In addition, as with ectopic fat variables, null associations were found between both BMI and WC with CIMT (r=0.05 and 0.06, respectively, after initial multivariable adjustment; both P >0.05). There was also no linear increase in odds of CAC >0 with increasing quartiles of BMI or WC (P for linear tread= 0.21 and 0.12, respectively).
DISCUSSION
Among these healthy, recently postmenopausal women, even after accounting for overall and central adiposity, intra-hepatic fat was associated with greater insulin and hs-CRP levels, while TAT (EAT more so than PAT) was associated with adverse lipid levels and markers of glucose homeostasis, as well as with the presence of CAC. The finding that intra-hepatic fat was more strongly related to both CRP and insulin than cardiac fat, whereas cardiac fat was more strongly associated with CAC supports our hypothesis that intra-hepatic fat predisposes to inflammation and insulin resistance, while cardiac fat predicts atherosclerosis.
CRP is synthesized by the liver, while insulin resistance is now known to be a primary trigger of hepatic fat accumulation. As such, prior studies have shown that subjects with nonalcoholic fatty liver disease (NAFLD) have higher levels of both CRP18 and insulin resistance19 compared to those without NAFLD. Conversely, Kim et al. demonstrated similar CIMT values among metabolic syndrome-negative women with vs. without NAFLD.20 The present study extends these findings by also showing a relationship between intra-hepatic fat attenuation and both CRP and insulin resistance, while also demonstrating null findings between intra-hepatic fat and CIMT, in a population of women with low prevalence of both cardiometabolic risk markers and NAFLD (3%). These associations persisted even after accounting for possible confounders, such as race-ethnicity, physical activity level, and BMI.
Few studies have compared the associations of total TAT, EAT, and PAT. In these healthy, recently postmenopausal women, we found that total TAT, an additive variable of EAT and PAT measures, was associated both with CVD risk markers and CAC, and that this appeared to be driven slightly more so by associations with EAT than with PAT. When EAT and PAT were examined separately, associations with both CVD risk markers and CAC were more consistent for EAT. However, it should be noted that in the case of CAC, the magnitude of odds ratios were similar between EAT and PAT, but only achieved statistical significance for EAT. Among 111 consecutive asymptomatic patients undergoing CAC scanning (mean age 60 ± 10 years), EAT was more strongly associated with the presence and severity of CAC than either total TAT or PAT, even after adjustment for multiple CVD risk markers and BMI.21 Limited prior research from the Framingham Offspring and EISNER (Early Identification of Subclinical Atherosclerosis using Noninvasive Imaging Research) studies have compared a measure of total TAT (a combined measure that includes, in part, both PAT and EAT as we have defined them) to what corresponds to our measure of EAT (fat within the pericardial sac). Among older Framingham Offspring participants (mean age approximately 60 ± 9 years), total TAT was more strongly associated with CVD risk markers than was EAT, but EAT was more strongly associated with CAC and CVD events than was total thoracic fat, and many of these relationships were stronger in women, among whom there was more EAT than PAT.10, 11 Specifically, more EAT was associated with a higher odds of CAC after adjustment for traditional risk factors, abdominal visceral fat, BMI, and waist circumference, while the association with total TAT was no longer statistically significant after these adjustments.10 Similarly, although neither EAT nor total TAT was associated with incident CVD events independent of traditional CVD risk markers, after initial adjustment for age, BMI, and waist circumference, more EAT was statistically significantly associated with a higher risk for CVD events, while total thoracic fat was not.11 Similarly, among EISNER participants (age 45–54 years for men and 55–64 years for women with at least 1 CVD risk factor), EAT, but not total TAT, significantly improved the sensitivity and overall accuracy for the prediction of major adverse cardiac events when added to a predictive model that included CAC.22 Consistent with our findings, these publications suggest the possibility of a stronger local cardiac effect for EAT, specifically. However, two very recent, though small (n=113 and 49, respectively) studies suggest stronger associations between cardiometabolic risk factors and PAT than EAT.23, 24 These discordant results may result from the very high proportion of men vs. women in these most recent studies. Although sex-specific data are limited, men appear to have more PAT than EAT, while the reverse appears true for women, and as noted above in the Framingham Study, associations with EAT, at least, appear to differ between men and women.
EAT is metabolically active and releases a number of pro-atherogenic adipocytokines, including IL-6, TNF-α, and MCP-1,25 and there is evidence for diffusion of adipocytokines from epicardial adipocytes into the coronary artery.26 In addition, EAT has greater fatty acid synthesis and breakdown, and greater insulin-induced lipogenesis than do other fat depots, including PAT.8 These properties of EAT may contribute to vascular inflammation and coronary atherosclerosis.
The measures of subclinical atherosclerosis used in this study, CIMT and CAC, revealed differences in their associations with the ectopic fat indices. Null findings were present between all ectopic fat measures and CIMT, whereas significant associations between cardiac fat and CAC were found. These results are similar to those demonstrated in the Multiethnic Study of Atherosclerosis (MESA), which found that higher cardiac fat was significantly associated with CHD and CAC after adjustment for traditional CVD risk markers and BMI, but was not associated with CIMT independent of CVD risk markers.16, 27, 28 This pattern might be expected in light of the local paracrine effects noted above for EAT and the anatomical locations of CAC (coronary arteries) versus CIMT (carotid arteries) measurements. Similarly, the local nature of the diffusion of adipocytokines from epicardial adipocytes into the coronary artery would also make plausible the stronger associations we demonstrated between EAT and coronary atherosclerosis than for abdominal (waist circumference) or overall (BMI) adiposity. A direct local effect is further supported by our finding that EAT was associated with CAC independent of lipid, glucose, and insulin values.
Although intra-hepatic fat was more strongly associated with both hsCRP and insulin compared to EAT, in contrast to our hypothesis, our results show that EAT had the strongest association with glucose and triglyceride levels. The current findings also demonstrated that EAT was more strongly related to LDL-C as compared with PAT or intra-hepatic fat. These results are surprising, since intra-hepatic fat is believed to be in direct pathophysiologic relation to dysregulation of glucose and lipid metabolism. The finding that cardiac fat is associated with lipids and glucose, however, is not unprecedented. In a sample of 127 consecutive patients, Wang, et al demonstrated a correlation between EAT and both glucose and triglyceride levels in analyses adjusted for age and gender.15 Similar associations were reported after age adjustment in healthy post-menopausal women in the PROSPECT study.29 Among 71 men and women who underwent cardiac CT and coronary angiography for effort angina evaluation, EAT was found to be related to LDL-C in unadjusted analysis.14 In the present study, EAT remained associated with CAC after adjustment for lipids and glucose.
Although the current study utilized CT for quantification of cardiac fat, it is relevant to the potential clinical implications of cardiac fat that relationships have also been observed between echocardiographic cardiac fat and both cardiometabolic risk factors and coronary artery disease. A recent review paper by Iacobellis and Willens suggests specific cut-off values ranging from ≥3 mm to ≥11 mm, depending on the outcome, for the thickness of the echo-free space between the outer wall of the myocardium and the visceral layer of the pericardium observed using two-dimensional echocardiography for potential clinical utilization in the identification of patients at high risk of metabolic syndrome, insulin resistance, coronary artery disease, low coronary flow reserve, or subclinical atherosclerosis.30
Some limitations of this study must be taken into consideration. The design of this study was cross-sectional, so the results do not imply causality. Our study sample also included only healthy, recently menopausal women. Therefore, our results may not be applicable to an older population with greater prevalence of atherosclerosis. Additionally, because glucose was measured during screening, while insulin was obtained from blood collected at the baseline randomization examination, we were unable to estimate insulin resistance using the homeostasis model assessment (HOMA-IR). Instead, we have used glucose and insulin levels separately as indicators of glucose homeostasis.
One of the strengths of this study is the relatively large sample size from the KEEPS trial. In addition, this is the largest study to have simultaneously reported associations with total TAT, EAT, and PAT, and the first to compare the strength of each of these associations to those for intra-hepatic fat in the same sample of individuals. Further, because healthy women of various body sizes and education levels were enrolled in this study, the results may apply broadly to recently postmenopausal women. Lastly, our tests of reliability confirmed that each of the non-invasive CT-derived measures of ectopic fat are highly reproducible, suggesting the possibility of clinical use.
In conclusion, these analyses in healthy, midlife women suggest that while intra-hepatic fat is more closely related to measures of inflammation and insulin, cardiac fat is substantially more strongly associated with CAC. BMI and WC, measures of general adiposity, were more closely related to cardiometabolic risk factors than any ectopic fat measure, but their association with both CIMT and CAC was null. Taken together, this study suggests that cardiac fat may be a useful marker for increased CVD risk beyond the standard adiposity measures BMI and waist circumference.
ACKNOWLEDGMENTS
Funding/Support: KEEPS is funded by grants from the Aurora Foundation to the Kronos Longevity Research Institute, NIH HL90639 to VMM, 1 UL1 RR024150, and the Mayo Foundation. Study medications were supplied in part by Bayer Health Care and by Abbott Pharmaceuticals. Ectopic fat measures reported herein were funded by NIH grant number HL094581 to Dr. Wildman.
Role of the Sponsors: The Aurora Foundation did not have input into the design or conduct of the study or the review or approval.
Additional Contributions: We gratefully acknowledge the dedicated efforts of all the investigators and staff at the KEEPS clinical centers, the KEEPS Data Coordinating Center at KLRI, and the NIH Institutes supporting ancillary studies. Above all, we recognize and thank the KEEPS participants for their dedication and commitment to the KEEPS research program.
KEEPs: Investigators and Staff
Albert Einstein College of Medicine: Genevieve Neal-Perry, Ruth Freeman, Hussein Amin
Brigham and Women’s Hospital/Harvard Medical School: JoAnn Manson, Maria Bueche, Marie Gerhard- Herman, Kate Kalan, Jan Lieson, Kathryn M. Rexrode, Frank Rybicki, Barbara Richmond
Columbia College of Physicians and Surgeons: Rogerio Lobo, Luz Sanabria, Maria Soto, Michelle P. Warren, Ralf C. Zimmerman
Kronos Longevity Research Institute: S. Mitchell Harman, Mary Dunn, Panayiotis D. Tsitouras, Viola Zepeda
Mayo Clinic: Virginia M. Miller, Philip A. Araoz, Rebecca Beck, Dalene Bott-Kitslaar, Sharon L. Mulvagh, Lynne T. Shuster, Teresa G. Zais
University of California, Los Angeles, CAC Reading Center: Matthew Budoff, Chris Dailing, Yanlin Gao, Angel Solano
University of California, San Francisco: Marcelle Cedars, Nancy Jancar, Grechen Good; Statistical Reading Center: Lisa Palermo
University of Southern California, Atherosclerosis Research Unit: Howard Hodis, Yanjie Li
University of Utah School of Medicine: Eliot Brinton, M. Nazeem Nanjee, Paul N. Hopkins, Kirtly Jones, Timothy Beals, Stacey Larrinaga-Shum
VA Puget Sound Health Care System and University of Washington School of Medicine: George Merriam, Pamela Asberry, SueAnn Brickle, Colleen Carney, Molly
Carr, Monica Kletke, Lynna C. Smith Yale University, School of Medicine: Hugh Taylor, Kathryn Czarkowski, Lubna Pal, Linda MacDonald, Mary Jane Minkin, Diane Wall.
Others: Frederick Naftolin, Nanette Santoro
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kip KE, Marroquin OC, Kelley DE, Johnson BD, Kelsey SF, Shaw LJ, Rogers WJ, Reis SE. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: A report from the women's ischemia syndrome evaluation (wise) study. Circulation. 2004;109:706–713. doi: 10.1161/01.CIR.0000115514.44135.A8. [DOI] [PubMed] [Google Scholar]
- 2.Song Y, Manson JE, Meigs JB, Ridker PM, Buring JE, Liu S. Comparison of usefulness of body mass index versus metabolic risk factors in predicting 10-year risk of cardiovascular events in women. Am J Cardiol. 2007;100:1654–1658. doi: 10.1016/j.amjcard.2007.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wildman RP, McGinn AP, Lin J, Wang D, Muntner P, Cohen HW, Reynolds K, Fonseca V, Sowers MR. Cardiovascular disease risk of abdominal obesity vs. Metabolic abnormalities. Obesity. 2011;19:853–860. doi: 10.1038/oby.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 5.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, Okuda J, Ida K, Yoshikawa T. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–1584. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer CD, Heine RJ, Diamant M. Alanine aminotransferase predicts coronary heart disease events: A 10-year follow-up of the hoorn study. Atherosclerosis. 2007;191:391–396. doi: 10.1016/j.atherosclerosis.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Taguchi R, Takasu J, Itani Y, Yamamoto R, Yokoyama K, Watanabe S, Masuda Y. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis. 2001;157:203–209. doi: 10.1016/s0021-9150(00)00709-7. [DOI] [PubMed] [Google Scholar]
- 8.Rabkin SW. Epicardial fat: Properties, function and relationship to obesity. Obes Rev. 2007;8:253–261. doi: 10.1111/j.1467-789X.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- 9.Cikim AS, Topal E, Harputluoglu M, Keskin L, Zengin Z, Cikim K, Ozdemir R, Aladag M, Yologlu S. Epicardial adipose tissue, hepatic steatosis and obesity. J Endocrinol Invest. 2007;30:459–464. doi: 10.1007/BF03346328. [DOI] [PubMed] [Google Scholar]
- 10.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O'Donnell CJ, Fox CS. Pericardial, fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: The Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 11.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, O'Donnell CJ, Fox CS, Hoffmann U. Association of pericardial, fat, intrathoracic, fat, and visceral abdominal fat with cardiovascular disease burden: The Framingham Heart Study. Eur Heart J. 2009;30:850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thanassoulis G, Massaro JM, Hoffmann U, Mahabadi AA, Vasan RS, O'Donnell CJ, Fox CS. Prevalence, distribution, and risk factor correlates of high pericardial and intrathoracic fat depots in the framingham heart study. Circ Cardiovasc Imaging. 2010;3:559–566. doi: 10.1161/CIRCIMAGING.110.956706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn SG, Lim HS, Joe DY, Kang SJ, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ, Shin JH. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart. 2008;94:e7. doi: 10.1136/hrt.2007.118471. [DOI] [PubMed] [Google Scholar]
- 14.Ueno K, Anzai T, Jinzaki M, Yamada M, Jo Y, Maekawa Y, Kawamura A, Yoshikawa T, Tanami Y, Sato K, Kuribayashi S, Ogawa S. Increased epicardial fat volume quantified by 64-multidetector computed tomography is associated with coronary atherosclerosis and totally occlusive lesions. Circ J. 2009;73:1927–1933. doi: 10.1253/circj.cj-09-0266. [DOI] [PubMed] [Google Scholar]
- 15.Wang CP, Hsu HL, Hung WC, Yu TH, Chen YH, Chiu CA, Lu LF, Chung FM, Shin SJ, Lee YJ. Increased epicardial adipose tissue (eat) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin Endocrinol. 2009;70:876–882. doi: 10.1111/j.1365-2265.2008.03411.x. [DOI] [PubMed] [Google Scholar]
- 16.Ding J, Kritchevsky SB, Harris TB, Burke GL, Detrano RC, Szklo M, Jeffrey Carr J. The association of pericardial fat with calcified coronary plaque. Obesity. 2008;16:1914–1919. doi: 10.1038/oby.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154:185–193. doi: 10.1016/s0021-9150(00)00461-5. [DOI] [PubMed] [Google Scholar]
- 18.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: Sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 19.Bugianesi E, Pagotto U, Manini R, Vanni E, Gastaldelli A, de Iasio R, Gentilcore E, Natale S, Cassader M, Rizzetto M, Pasquali R, Marchesini G. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab. 2005;90:3498–3504. doi: 10.1210/jc.2004-2240. [DOI] [PubMed] [Google Scholar]
- 20.Kim HC, Kim DJ, Huh KB. Association between nonalcoholic fatty liver disease and carotid intima-media thickness according to the presence of metabolic syndrome. Atherosclerosis. 2009;204:521–525. doi: 10.1016/j.atherosclerosis.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Ahmadi N, Nabavi V, Yang E, Hajsadeghi F, Lakis M, Flores F, Zeb I, Bevinal M, Ebrahimi R, Budoff M. Increased epicardial, pericardial, and subcutaneous adipose tissue is associated with the presence and severity of coronary artery calcium. Academic Radiol. 2010;17:1518–1524. doi: 10.1016/j.acra.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Cheng VY, Dey D, Tamarappoo B, Nakazato R, Gransar H, Miranda-Peats R, Ramesh A, Wong ND, Shaw LJ, Slomka PJ, Berman DS. Pericardial fat burden on ecg-gated noncontrast ct in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging. 2010;3:352–360. doi: 10.1016/j.jcmg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sironi AM, Petz R, De Marchi D, Buzzigoli E, Ciociaro D, Positano V, Lombardi M, Ferrannini E, Gastaldelli A. Impact of increased visceral and cardiac fat on cardiometabolic risk and disease. Diab Med. 2011 doi: 10.1111/j.1464-5491.2011.03503.x. [DOI] [PubMed] [Google Scholar]
- 24.Sicari R, Sironi AM, Petz R, Frassi F, Chubuchny V, De Marchi D, Positano V, Lombardi M, Picano E, Gastaldelli A. Pericardial rather than epicardial fat is a cardiometabolic risk marker: An mri vs echo study. JASE. 2011;24:1156–1162. doi: 10.1016/j.echo.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 25.McLean DSS AE. Epicardial adipose tissue as a cardiovascular risk marker. Clin Lipidol. 2009;4:55–62. [Google Scholar]
- 26.Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 27.Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, Ouyang P, Espeland MA, Lohman KK, Criqui MH, Allison M, Bluemke DA, Carr JJ. The association of pericardial fat with incident coronary heart disease: The Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soliman EZ, Ding J, Hsu FC, Carr JJ, Polak JF, Goff DC., Jr Association between carotid intima-media thickness and pericardial fat in the multi-ethnic study of atherosclerosis (mesa) J Stroke Cerebrovasc Dis. 2010;19:58–65. doi: 10.1016/j.jstrokecerebrovasdis.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Vos AM, Prokop M, Roos CJ, Meijs MF, van der Schouw YT, Rutten A, Gorter PM, Cramer MJ, Doevendans PA, Rensing BJ, Bartelink ML, Velthuis BK, Mosterd A, Bots ML. Peri-coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post-menopausal women. Eur Heart J. 2008;29:777–783. doi: 10.1093/eurheartj/ehm564. [DOI] [PubMed] [Google Scholar]
- 30.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: A review of research and clinical applications. JASE. 2009;22:1311–1319. doi: 10.1016/j.echo.2009.10.013. [DOI] [PubMed] [Google Scholar]