Abstract
Increased or decreased hepatic lipase (HL) activity has been associated with coronary artery disease (CAD). This is consistent with the findings that gene variants that influence HL activity were associated with increased CAD risk in some population studies but not in others. In this review, we will explain the conditions that influence the effects of HL on CAD. Increased HL is associated with smaller and denser LDL (sdLDL) and HDL (HDL3) particles, while decreased HL is associated with larger and more buoyant LDL and HDL particles. The effect of HL activity on CAD risk is dependent on the underlying lipoprotein phenotype or disorder. Central obesity with hypertriglyceridemia (HTG) is associated with high HL activity that leads to the formation of sdLDL that is proatherogenic. In the absence of HTG, where large buoyant cholesteryl ester-enriched LDL is prominent, elevation of HL does not raise the risk for CAD. In HTG patients, drug therapy that decreases HL activity selectively decreases sdLDL particles, an antiatherogenic effect. Drug therapy that raises HDL2 cholesterol has not decreased the risk for CAD. In trials where inhibition of cholesterol ester transfer protein (CETP) or HL occurs, the increase in HDL2 most likely is due to inhibition of catabolism of HDL2 and impairment of reverse cholesterol transport (RCT). In patients with isolated hypercholesterolemia, but with normal triglyceride levels and big-buoyant LDL particles, an increase in HL activity is beneficial; possibly because it increases RCT. Drugs that lower HL activity might decrease the risk for CAD only in hypertriglyceridemic patients with sdLDL by selectively clearing sdLDL particles from plasma, which would override the potentially pro-atherogenic effect on RCT.
Keywords: Hepatic lipase, small-dense LDL, coronary artery disease, GWAS, triglyceride, reverse cholesterol transport
1. INTRODUCTION
Coronary artery disease (CAD) is a major cause of morbidity and mortality. Measures of LDL and HDL have been linked to CAD, particularly premature CAD, around the world [1]. HDL plays an important role in reverse cholesterol transport for protection against atherosclerosis. LDL and HDL levels as well as the size and density of these particles need to be considered. The variation in LDL and HDL size and density reported with premature CAD [2,3,4] seems to be related to proteins that remodel circulating lipoproteins, such as lipoprotein lipase (LPL), hepatic lipase (HL), cholesteryl ester transfer protein (CETP), phospholipid transfer protein (PLTP), apolipoprotein E (ApoE), endothelial lipase, and apolipoprotein CIII. HL has been linked to atherosclerosis in many studies. In some studies high HL activity was associated with increased atherosclerosis, in others, low HL was in those with atherosclerosis. In most population-based studies no effect of HL on CAD was noted at all. At best, the role of HL in atherosclerosis is controversial, possibly related to how the various study subjects were chosen. We will review these data and provide new data to suggest that the pro- or anti-atherogenicity of HL activity is dependent on the background lipoprotein phenotype.
2. HEPATIC LIPASE BIOLOGY
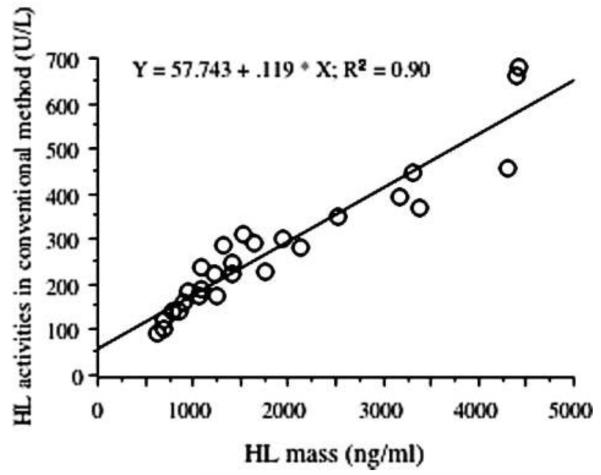
The gene for HL is on chromosome 15q21 and consists of 9 exons. Common polymorphisms in the HL promoter were shown to be associated with lower HL activity [5]. HL is a serine hydrolase with a catalytic triad with highest specificity for lipoprotein triglyceride and phospholipid. HL is synthesized in the liver and functions as a homodimer on the endothelial surface of the space of Disse [6]. At this site it is under regulation by angiopoeitin-like protein 3 [7]. Like LPL, HL is released into plasma with intravenous heparin. Post-heparin plasma is then incubated with a source of triglyceride in vitro and lipolysis is measured. HL activity can be measured in postheparin plasma when LPL is inactivated by an LPL-specific antibody. Postheparin plasma activity and mass are highly correlated [8] (Figure 1), except in HL deficiency [9].
Figure 1.
Hepatic lipase activity is highly correlated with hepatic lipase mass. The specific activity of hepatic lipase protein (neq FFA per min per ng protein) appears to be constant. From [7], with permission.
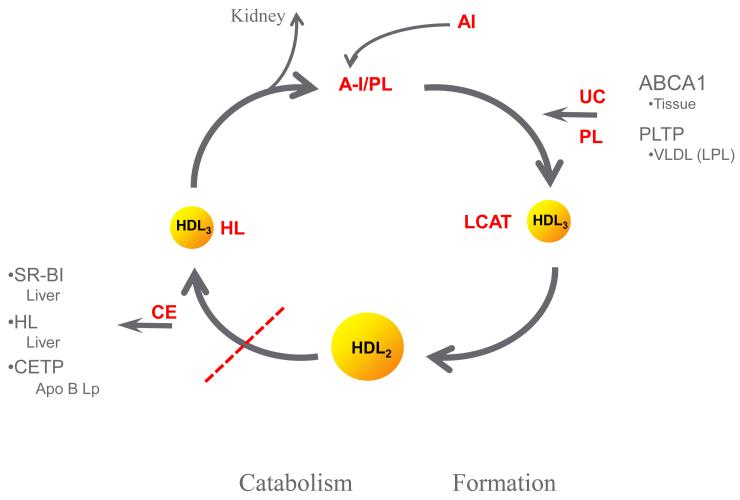
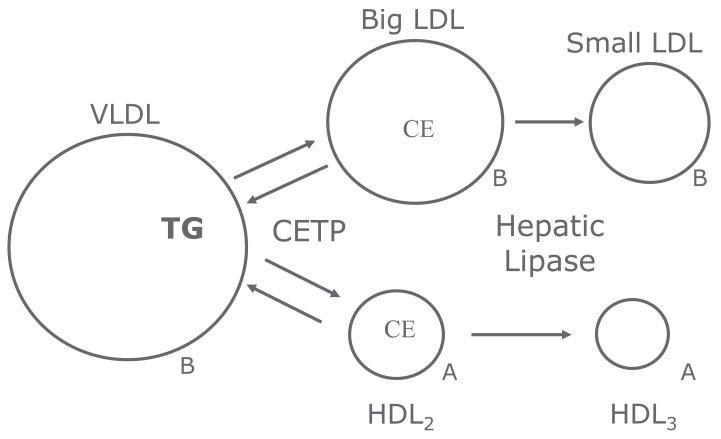
HL has effects on many sites in lipoprotein metabolism. HL acts as a factor in the complex for hepatic recognition of lipoprotein remnants of chylomicron and VLDL particles. This remnant recognition does not require catalytic activity in mice [10] or in humans [9]. In mice, this remnant recognition seems to be the predominant role for the enzyme, while in humans, remnant recognition is less important. In humans, HL activity plays an important role in the remodeling of LDL and HDL particles. HL activity is inversely related to the buoyancy and size of both LDL and HDL particles [11,12,13,14,15,16,17]. HDL2 cholesterol level is inversely related to HL activity in both healthy men and women (r=0.58) [18]. HL and LPL activities have opposite effects on HDL cholesterol, phospholipid and apoAI levels and are correlated to large and very large apoAI without apoAII HDL particles, as determined by immunoaffinity column chromatography [19] (Table 1). ApoAI with apoAII HDL particles do not seem to be affected by either LPL or HL, except for changes in phospholipid content. In the reverse cholesterol transport pathway, HL activity is involved in the catabolism of HDL2 particles generated by pre-beta HDL, ABC AI and LPL, combined with LCAT and PLTP [11,20,21] (Figure 2). Inhibition of HL activity or CETP activity would be expected to impair this reverse cholesterol transport by reducing the interaction of HL with hepatic SR B-1 receptors and the generation of smaller HDL and lipid-poor apoAI for recirculation of the pathway, and might be pro-atherogenic. HL facilitates the CETP-mediated uptake of cholesteryl ester via SR B-1 in humans [22]. This interaction of HL and SR B-1 promotes the transfer of cholesteryl ester from the HDL particle to the liver. In support of this is the family heterozygous for a mutation in SR B-1, in whom the affected relatives have elevated HDL cholesterol levels [23]. Endothelial lipase also contributes to this process [see review: [24]. Further, loss-of-function variants in EL are associated with increased HDL [25]. Thus, human mutations in CETP, HL, and EL are associated with increased HDL cholesterol. Similarly, LDL particles are large with very low HL activity as seen in HL deficiency [9] and in other situations as discussed below. The mechanism proposed for these effects is due an interaction between CETP and HL on LDL and HDL particle size and density (Figure 3). CETP activity mediates the transfer of triglyceride from triglyceride-rich particles in exchange for cholesteryl ester in LDL and HDL particles. HL activity proceeds to hydrolyze the triglyceride in the triglyceride-rich LDL and HDL leading to smaller and denser LDL and HDL. This process would be driven by particularly triglyceride-rich LDL particles and by central obesity and insulin resistance associated with high HL activity. While HL, along with CETP is important in HDL catabolism, LPL plays and important role in delivering unesterified cholesterol derived from the surface of triglyceride rich lipoproteins to smaller HDL particle via PLTP in the development of HDL2 in the anabolic side of reverse cholesterol transport [7,19]. Genetic variants in PLTP have decreased PLTP activity and mass and are associated with decrease in HDL particle number and mass, decreased level of large HDL and increased level of small HDL [26]. This is compatible with a decrease in the anabolic side of HDL-RCT [Figure 2]. The role of HL in the transfer of cholesteryl ester between HDL and LDL is less clear.
Table 1.
Relation of LPL and HL activity to HDL AI without AII particles in 28 men and women. LPL and HL activity were measured in postheparin plasma. Plasma HDL AI particles were separated from AI with AII particles by immunoaffinity column chromatography. HDL AI particles then were separated by size on gradient gel electrophoresis. Correlation coefficients are with the AI only (without AII) particle. LPL and HL activity did not correlate with the mass of the AI with AII particle. Modified from reference [16].
| Lipases and HDL Al Subspecies | ||
|---|---|---|
| LPL | HL | |
| HDL Al | r = +0.60 | −0.56 |
| Chol | r = +0.62 | −0.55 |
| PL | r = +0.58 | −0.55 |
| TG | ns | −0.53 |
| Small HDL | ns | ns |
| Medium | ns | ns |
| Large | r = +0.60 | −0.56 |
| Very Large | r = +0.51 | −0.48 |
Figure 2.
Pathway of formation and catabolism of HDL particles. ABC AI provides unesterified cholesterol and phospholipid to AI-phospholipid containing prebeta HDL. In humans PLTP may contribute up to 50% of the unesterified cholesterol and phospholipid for generation of HDL particles. These lipids are transferred from the redundant surface of triglyceride-rich lipoproteins following the action of LPL to decrease the core of these particles. LCAT esterifies cholesterol to cholesteryl ester to develop HDL3 and then HDL2 particles. Catabolism of HDL2 involves HL, CETP, SRB1 and an interaction with apoB containing particles. Modified from [11].
Figure 3.
CETP facilitates the exchange of triglyceride from triglyceride-rich particles with cholesteryl ester in LDL and HDL. The transfer of triglyceride to LDL and HDL makes them substrate for HL. Elevated plasma triglyceride levels and elevated HL activity enhance this pathway. Modified from [31].
In HTG, the exchange of triglyceride in chylomicrons and VLDL for cholesteryl ester in LDL and HDL [Figure 3] is exaggerated. The subsequent triglyceride-rich LDL and HDL are then substrate for elevated HL which leads to a decrease in size and buoyancy of both LDL and HDL particles, which results in formation of sdLDL particles, a decrease in HDL2 particles and increase in HDL3 particles. Some of these changes are potentially pro-atherogenic. The generation of small-dense LDL is probably pro-atherogenic while the decrease in HDL size and density probably reflects increased RCT, and reduced atherogenicity.
3. CLINICAL STUDIES
3.1 HEPATIC LIPASE IN OBESITY AND HYPERTRIGLCERIDEMIA
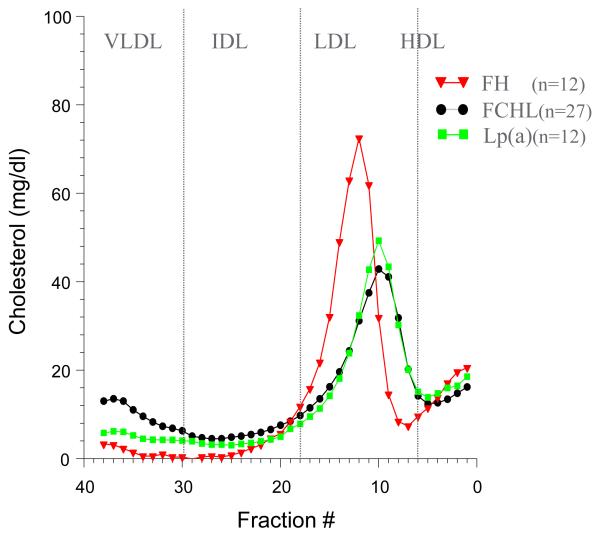
Hypertriglyceridemia is commonly associated with premature coronary artery disease [27] and ischemic stroke [28]. HTG individuals with premature CAD are often centrally obese and are said to have high TG-waist (triglyceride-times-waist product) [29]. Most primary HTG is due to a genetic disorder [30] and is often associated with central obesity. Familial combined hyperlipidemia (FCHL), familial hypo-alpha-lipoproteinemia (FHA), and the residual HTG seen in diabetic patients on glucose lowering agents (DM2) are genetic HTG disorders with central obesity and premature CAD [31]. The familial forms of HTG with central obesity usually have elevated HL activity [31]. Individuals with benign, monogenic, familial hypertriglyceridemia (FHTG) do not have premature CAD [30]. In FHTG HDL apoAI is normal, but HDL cholesterol is low, presumably due to replacement of cholesteryl ester with triglyceride [32]. Even though LDL are small and dense in FHTG, apoB and the number of these LDL particles is low. All of the HTG disorders are associated with small-dense LDL particles (Figure 4).
Figure 4.
Density gradient ultracentrifugation pattern of cholesterol distribution for FCHL, hFH and Lp(a). Note differences in LDL peak density.
Central obesity is a risk factor for premature CAD [33]. One might think that elevated HL activity would be associated with premature CAD, mediated by central obesity and the effect of high HL activity to remodel lipoproteins. Indeed, many disorders associated with premature CAD have elevated HL activity [31]. First, elevated HL activity is associated with higher central body fat in obese men compared to lean men [29,34] and in obese women compared to lean women [35,36]. Second, men have a higher prevalence of central obesity than women and have higher HL activity [18] and CAD occurs 10 to 15 years earlier in men than in women. Third, HL activity increases with menopause, is associated with increased waist circumference and onset of premature CAD [37] (footnote 4). Finally, sedentary lifestyle is associated with elevated HL activity, central obesity and premature CAD [38,39,40,41,42,43,44,45].
[40] Weight loss following caloric restriction leads to a decrease in HL activity, an increase in HDL2 cholesterol and an increase in LDL particle size [34]. Weight loss also increases LPL activity in adipose tissue in men [46] and in women [47]. This increase in LPL may lead to increases in the delivery of triglyceride-rich lipoprotein surface unesterified cholesterol to HDL particles and increase reverse cholesterol transport, while the change in HL may lead to further increases in HDL2 due to inhibition of reverse cholesterol transport. Similarly, aerobic exercise selectively decreases central obesity, decreases premature CAD risk factors and is associated with a decrease in HL activity and may increase LPL activity [40]. Aerobic exercise is associated with decreased triglyceride and increased HDL2 cholesterol, in part due to changes in HL and LPL activity [32] and a change of sdLDL to bigger, more buoyant LDL particles [48].
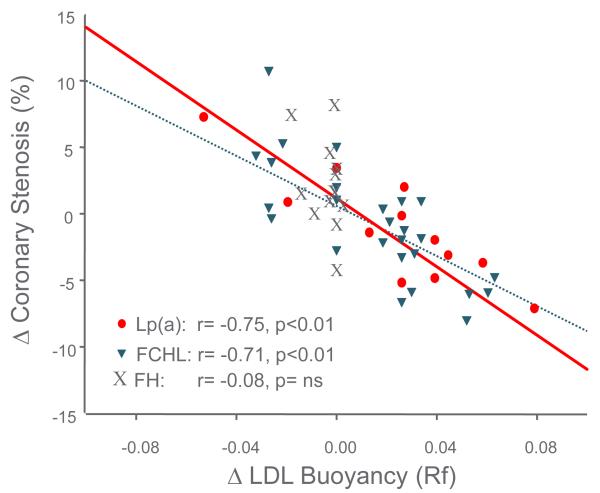
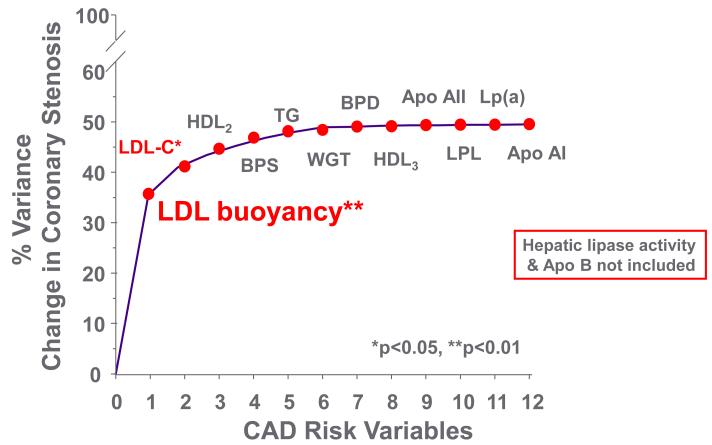
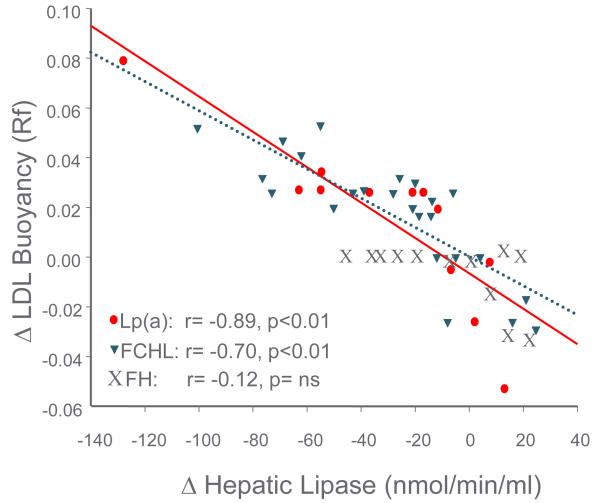
Decreases in HL activity played a role in the prevention of progression of premature CAD in the Familial Atherosclerosis Treatment Study (FATS) of B.G. Brown et al. in the 1980s. FATS was a study of combination drug lipid lowering therapy in middle-aged men with documented premature CAD, a positive family history of premature CAD and elevated apoB levels [49]. The subjects were evaluated with quantitative coronary angiography at baseline and after 2 ½ years of combination drug therapy. Bile acid binding resin in combination with niacin, or with lovastatin, resulted in less progression (and regression in some patients) of coronary stenosis compared to the group treated with resin and/or diet alone. The clinically positive change in stenosis was initially reported to be associated with a decrease in apoB (or in LDL cholesterol) level and an increase in HDL2 cholesterol level [49]. Lipoprotein heterogeneity was also studied with non-equilibrium density gradient untracentrifugation (DGUC) (Figure 4), and HL activity was measured on and off the drug combinations [14]. The density (DGUC) of the peak LDL cholesterol fraction (relative flotation or Rf) was used to evaluate the role of variation in LDL particle density with combination drug therapy and the effects on coronary progression or regression. The change in the peak density of LDL from a dense particle to a more buoyant particle predicted decreased coronary stenosis progression (Figure 5). With multiple-regression analysis the change in LDL to a more buoyant LDL particle displaced LDL and HDL2 as the major predictors of change in coronary stenosis progression (Figure 6). Further, a decrease in HL activity was correlated with the increase in LDL buoyancy (Figure 7), and could replace the change in density in the statistical analysis of coronary stenosis. The decrease in HL activity was also associated with an increase in HDL2 cholesterol levels which was not significant after adjusting for change in sdLDL and LDL cholesterol levels. [14]. In a second study of BG Brown et al. [50], the HDL Atherosclerosis Treatment Study (HATS) in men and women with premature CAD and low HDL cholesterol and elevated triglyceride, a similar change in LDL particles was noted to be associated with change in coronary artery stenosis and CAD death. The increase in HDL cholesterol again did not predict the change in coronary stenosis or in cardiovascular events (Zambon A., unpublished data). The results of the FATS and HATS studies above support the hypothesis derived from studies of the familial forms of HTG, gender, menopause and central obesity that high HL activity is associated with more premature CAD; and that reduction of HL activity improves outcomes, despite potentially detrimental effects on RCT
Figure 5.
The change in coronary stenosis during combination drug and placebo therapy in FATS is related to the change in LDL peak buoyancy: an increase in LDL buoyancy is associated with less coronary stenosis. Patients with FCHL and elevated Lp(a) treated with combination therapy had and increase in buoyancy, while those with hFH did not. Includes subjects treated with placebo. Modified from [14].
Figure 6.
Stepwise multiple regression analysis indicates change in LDL buoyancy (or HL activity) was the best predictor of decreased coronary stenosis in FATS. Change in LDL cholesterol was next. The changes in HDL were not related to changes in stenosis. HL activity and LDL buoyancy were highly collinear as were LDL cholesterol and apoB. Modified from [41].
Figure 7.
Change in LDL peak buoyancy with drug and placebo in FATS was inversely related to change in HL activity. Patients with FCHL and elevated Lp(a) changed LDL peak buoyancy. Patients with hFH did not change LDL buoyancy even though HL lipase activity changed. Modified from [14]. Includes subjects treated with placebo. Modified from [14].
3.2. HEPATIC LIPASE IN HYPERCHOLESTEROLEMIA
However, in contrast, several studies in patients with clinical coronary artery disease found that low HL activity was associated with increased CAD [51,52]. This is the exact opposite from what was predicted from the previous FATS and HATS studies noted above. Jansen et al. reported that the hepatic lipase promoter polymorphism, which leads to decreased HL activity, was significantly more common in patients with mild hypercholesterolemia and clinical coronary disease than in control subjects [53]. Dugi et al. found coronary calcification to be greater in patients who are heterozygous for familial hypercholesterolemia who had low HL activity than those with higher HL activity [51]. Dugi et al. also found a weak inverse correlation between HL activity and extent of coronary atherosclerosis in 190 men with abnormal coronary angiograms compared to 40 with normal angiograms.
An extreme example of thr proatherogenic role of HL in the absence of elevated VLDL triglyceride levels occurred in a patient with premature atherosclerosis and deficiency of HL activity, but normal HL protein [9]. VLDL levels were presumably normal due to the noncatalytic role of HL protein [10], but he had TG-rich LDL and HDL due to the loss of HL activity [54].These large buoyant HDL particles are hypothesized to be due to the absence of HL in RCT. Patients missing both HL activity and mass have accumulation of remnants lipoproteins in addition to TG-rich LDL and HDL [9]. Both of these abnormalities probably account for the premature CAD in HL deficiency.
3.3 HEPATIC LIPASE PROMOTER VARIATION IN POPULATION BASED STUDIES
Large prospective population-based studies have generally been unable to come to conclusions as to the association between the rare HL promoter variant with lower HL activity and CAD. Some have observed associations and others have not [55]. In over 9000 individuals in the Copenhagen City Heart Study followed for 28 years, the cumulative incidence of ischemic cardiovascular disease was no different for CC, CT or TT genotypes [56]. In two population-based studies (n= 5933) in Australia and one CAD case control study (n=556) no evidence for HL genotype and coronary heart disease was found [57]. In one prospective, population-based study (n=966), the HL promoter variant was found to predict CHD [38]. Why this last study differed from the other studies is not clear. Most of the effect in this study was in Hispanic individuals who have higher frequency of the rare allele.
It is possible that the type of patients studied may make a difference in the association of the HL promoter variant with atherogenicity. In a small study of CAD in 39 men, it was suggested that high HL activity was anti-atherogenic in hypercholesterolemia, but pro-atherogenic in HTG [15]. Jansen et al. expanded this observation to show that dense LDL particles were associated with high HL activity and big LDL particles with low HL activity [52,53]. Based on the combined findings [15,51,52,53]. Jansen et al. hypothesized that increased HL activity was pro-atherogenic in hypertriglyceridemic CAD patients and anti-atherogenic in normo-triglyceridemic patients [13,58]. Thus, the atherogenicity of the HL promoter variant was dependent on the type of underlying lipid abnormality [58]. This might be due to reduced conversion of HDL2 and, therefore, reduced RCT in the absence of small-dense LDL.
3.4 THE FAMILIAL ATHEROSCLEROSIS TREATMENT STUDY
To evaluate the relation of increases and decreases in HL with CAD in different lipoprotein backgrounds the FATS study of B.G. Brown et al. was reevaluated to determine the interaction of HL activity with the specific underlying familial dyslipidemia. The families of the probands who had been selected for elevated apoB level and premature CAD were divided into three distinct groups by three independent investigators [32]. The three distinct genetic disorders were: heterozygous familial hypercholesterolemia (hFH), familial combined hyperlipidemia (FCHL) and families with elevated Lp(a). All three disorders were associated with elevated apoB levels. If the proband or his family members had tendonous xanthomas, the family was diagnosed as hFH. If the proband had an Lp(a) level above the 95th percentile, this determined the diagnosis. In the remaining families with elevated apoB levels variation in LDL cholesterol and triglyceride were seen, characteristic of FCHL. In subjects with dense LDL particles [FCHL & Lp(a)] high HL activity was associated with coronary stenosis, and the decrease in HL activity caused an increase in the buoyancy of the dense LDL particles to those of bigger size, and predicted the regression or lack of progression of coronary stenosis [14] (Figures 5 and 7). The changes in the two groups were virtually identical even though the reason for the dense LDL particles was different. However, Lp(a) particles also are generally smaller than LDL [59]. The probands from the hFH families had a decrease in HL activity, but their large LDL particles at baseline did not undergo the further enlargement as seen in FCHL and Lp(a). Although regression of premature CAD was seen in FCHL and high Lp(a) patients, only a slowing of progression of coronary stenosis occurred in the hFH patients [60].
These studies support the suggestion of Jansen and his collaborators [58] that the underlying lipid disorder determines the atherogeneity of HL. It further suggests that the change in LDL size and density to bigger, more buoyant LDL particles is anti-atherogenic. They also suggest that the increase in HDL size and density via decreased HL activity may not be anti-atherogenic. The increase in HDL size and density may reflect an accumulation of HDL2 because of a decrease in the catabolism of the HDL particles due to partial inhibition of reverse cholesterol transport. It has been suggested the increased HDL cholesterol level seen with the rarer HL promoter allele does not provide protection against LDL particle mediated risk for premature CAD [56,61] even though the increase in HDL cholesterol was in HDL2.
4. DRUG EFFECTS ON HL AND HDL AND LDL PARTICLES
While the value of HDL cholesterol as a predictor of CAD risk is accepted, and occurs across all LDL cholesterol levels [62], with meta-analysis the increases seen in HDL cholesterol level with drug therapy have not been associated with decreased CAD in spite of the concomitant increases in HDL cholesterol [63]. The CETP inhibitor, torcetrapid, increased HDL cholesterol without a decrease in atherosclerosis [64]. It has been suggested this lack of benefit was due to an increase in blood pressure with this specific drug. An alternative reason for the failure of CETP inhibition to reduce CAD is inhibition of RCT that would not be expected to be beneficial. The recent discontinuation by the NIH (NIH release May 26, 2011) of the AIMHIGH study of addition of niacin to statin was due to no potential benefit, in spite of the increase in HDL cholesterol. This result supports the idea that patients with isolated low HDL cholesterol, niacin-induced increase in HDL had no benefit. In AIMHIGH, patients with pre-existing atherosclerosis on statin therapy, were randomized to additional niacin therapy. At baseline entry into AIMHIGH, the mean LDL cholesterol was 71mg/dl, triglyceride was 161 mg/dl and the mean age was 64 years [65]. In absence of other lipoprotein abnormalities, raising HDL cholesterol with niacin failed to prevent CAD. Niacin acts at many sites in lipoprotein metabolism. Niacin may decrease hepatic TG-rich lipoprotein secretion by decreasing post-prandial free fatty acid levels and by decreasing hepatic diacylglycerol transferase (DGAT) levels [66,67,68]. Niacin, in combination with colestipol [14] or alone [69] decreased sdLDL levels and increased HDL2 levels. The changes in sdLDL and HDL2 are mediated by changes in HL activity. Finally, niacin increased HDL cholesterol by increasing apoAI secretion [67,68] and by impairing HDL catabolism [70], presumably, by decreasing HL activity.
GENOME WIDE ASSOCIATION STUDIES
5.1 Association of common gene variants with CAD
CAD is a complex multifactorial disease affected by dyslipidemia, hypertension, diabetes, obesity and smoking. Genetic factors play an important role since it is estimated, based on twin studies, that inheritance of CAD is 30-60% [71,72]. Massive genome-wide-association studies (GWAS) and meta-analysis have been performed to identify common gene variants that influence susceptibility to CAD. So far, about 28 loci have been have been identified among Caucasians and African Americans [73,74,75,76,77,78,79,80]. Some of these loci are also associated with risks for other diseases and traits. Among these loci associated with CAD that are known to play a role in lipoprotein metabolism are: LDLR, SORT1 (sortilin1, regulator of hepatic lipoprotein export), LIPA (Lysosomal acid lipase/cholesteryl ester hydrolase), PCSK9 (protein convertase subtilisin/kexin type 9), LPA (lipoprotein (a)), Apo E and the APOA1-C3-A4-A5 locus. Several other loci associated with CAD risk are located in genes that do not influence traditional risk factors [78], suggesting the existence of other novel pathways of CAD. Currently, about 13% of the total heritability of CAD has been identified. GWAS results demonstrated risk ratios of only 1.1-1.8 per risk allele and account for about 4% of interindividual variation in CAD risk. Therefore, many CAD genes are currently undiscovered. Future studies using genome-wide and exome resequencing will discover novel lower-frequency variants associated with CAD.
5.2 Association of common gene variants with circulating lipoproteins
GWAS identified variants of the following genes that are related to circulating lipoproteins are associated with HDL-C levels: CETP, LPL, LIPC, LIPG (endothelial lipase), LCAT, FADS 1-3 (fatty acid desaturases 1-3), PLTP (phospholipid transfer protein), and ABCA1 (ATP binding cassette transporter A1). Variants at the LDLR, ApoE, LPA, SORT1, APOB are mainly associated with LDL-C levels. Variants in LIPC, LPL, APOA1-C3-A4-A5 loci are mainly associated with triglyceride levels [81]. These results indicate that the risk for CAD is partially associated with common variants in genes such as LDLR, SORT1 and APOA1-C3-A4-A5, via the control of HDL and LDL particles that varies with the background lipid phenotype. In order to increase the level of association with CAD, combinations of certain lipid (such as triglycerides) lipoprotein (density and size) and central obesity phenotypes, with single and combinatorial gene variants need to be performed. For example, a synergistic role of CETP-LIPC gene variants in CAD risk was observed (van Acker et al, 2008). The CETP-B2B2-LIPC-TT double homozygote individuals, who had lower plasma CETP and HL, had a higher risk for CAD, even though their HDL levels were increased. This is consistent with the potential effect of these variants on lowering the rate of reverse cholesterol transport.
In addition, less common single-base variants that cannot be captured by GWAS are in the process of being determined by exome sequencing and genome-wide copy number variations (CNV) analysis. Recent genome-wide mapping studies have demonstrated the existence of common CNV in the general population. Association of CNVs with lipoprotein disorders and CAD risk is in progression. Variation in apolipoprotein (a) kringle IV repeat number has already been shown to be associated with risk for coronary heart disease. In addition, CNV analysis has revealed a number of additional causative mutations in the LDLR gene in patients with familial hypercholesterolemia (Reviewed by [82]). Relatively rare CNVs identified in the LPL, LIPC and LIPG genes were found to be associated with loss of enzymatic activity [83]. No association between HL and CAD has been found by GWAS. Association between LIPC promoter polymorphisms, which influence HL activity levels, and CAD has been investigated. No conclusive results have been observed [55]. This may be due to the variable risk for CAD due to either increasing or decreasing HL activity. As discussed above, the variability in the impact of HL on CAD is significantly dependent on the background lipoprotein phenotype.
5.3 Association of common gene variants with HDL and LDL-particle size
The size of lipoprotein particles is not homogeneous and plays a role in influencing the risk for CAD. In a large-scale candidate gene association study, it was observed that association of candidate gene loci with HDL particle size gave additional associations with LIPC, CETP and PLTP (7), suggesting that HDL-C and particle size phenotypes are influenced by independent pathways of cholesterol and triglyceride/phospholipid content. The use of lipoprotein particle characteristic phenotypes in GWAS appears to provide higher sensitivity for detecting associations.
Unfortunately, no GWAS has been used to identify gene variants associated with LDL particle size, nor has a GWAS been performed in hypertriglyceridemic subjects with CAD. However, we previously reported that polymorphisms at the LIPC promoter are associated with LDL particle size [84]. The observation that variation in HL activity may increase or decrease risk for CAD, depending on lipid phenotypic background, explains the lack of association of common LIPC promoter variants with CAD observed in most GWAS. As mentioned above, LIPC variants associated with higher levels of HL would increase the risk of CAD in subjects with high triglyceride levels leading to increased prevalence of small-dense LDL particles [85].
6. SUMMARY AND IMPLICATIONS
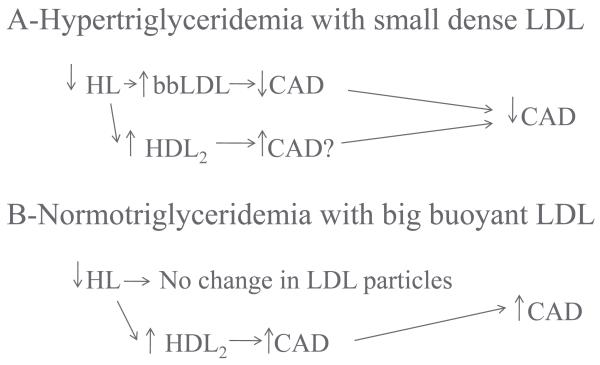
Hepatic lipase plays an important role in determining the size and density of LDL and HDL particles. It has previously been suggested that the increase in both LDL and HDL size and buoyancy, caused by lipid-lowering drug combinations which include niacin, is beneficial [14], due to a decrease in HL activity. It is now suggested that the increase in LDL size and density is atheroprotective due to decrease in the atherogenic sdLDL particles (Figure 8). On the other hand the increase in HDL2 cholesterol and decrease in HDL3 cholesterol is possibly proatherogenic, due to inhibition of reverse cholesterol transport. The result is an increase in HDL2 particles, with a long residence time, which may be non-functional. In non-dyslipidemic individuals and those with heterozygous familial hypercholesterolemia, the LDL particles are large and no further increase in size can occur with lipid lowering therapy. In this situation the positive effects to increase hepatic lipase activity and increase reverse cholesterol transport would offset the lack of benefit in the LDL particle range. This has major implications for the development of pharmaceutical agents designed to modify hepatic lipase activity or function. A lower HL activity would be desired in hypertriglyceridemic subjects with sdLDL particles. In contrast, an increase in hepatic lipase activity in normo-triglyceridemic individuals may increase reverse cholesterol transport, with paradoxically lower HDL cholesterol levels and a decrease in risk for premature CAD.
Figure 8.
Effect of drug therapy on hepatic lipase activity is constant across common familial from of dyslipidemia. However, the responses to changes in HL activity are dependent on the background lipid phenotype. In hypertriglyceridemic patients who have sdLDL and decreased HDL2 particles, a decrease in HL activity is associated with an increase in the size and buoyancy of LDL, with selective clearance of sdLDL. This would be expected to be antiatherogenic. The concomitant increase in HDL2 might be proatherogenic. In patients with normal triglyceride levels and bbLDL, a decrease in HL activity would have no effect on LDL particles, while the increase in HLD2, probably due to decreased reverse cholesterol transport, might be proatherogenic, leading overall to a proatherogenic state.
Residual risk for CAD persists after treatment for elevated LDL cholesterol. HDL cholesterol has been sought as the treatment target for this residual CAD risk. Perhaps persistent small, dense LDL particles seen with HTG might explain the lack of further decrease in this residual CAD. HDL cholesterol levels may not always reflect the rate of reverse cholesterol transport. An increase in HDL formation with increased HDL2 levels may reflect an antiatherogenic state. In contrast, drugs that inhibit HDL catabolism, also with increased HDL2, may be pro-atherogenic. Direct measures of reverse cholesterol transport would more accurately reflect the function of the HDL pathway [24]. The Heart Protection Study 2, Thrive, is a similar ongoing study with niacin and statin in Europe to be completed in 2012. Results of this study will be of great interest.
HIGHLIGHTS.
In hypertriglyceridemia hepatic lipase hydrolyses LDL and HDL triglyceride and phospholipid.
In hypertriglyceridemia hepatic lipase leads to smaller and denser LDL and HDL particles.
In coronary disease small-dense LDL and decreased HDL2 are due to high hepatic lipase activity.
Drugs that decrease hepatic lipase are anti-atherogenic due to clearance of small-dense LDL.
With normal triglyceride levels and big-buoyant LDL, high hepatic lipase causes increased RCT.
Acknowledgements
We thank Dr. Helen Dichek for review of the manuscript. This work was supported by NIH grants HL 64322 (SD) and HL 30086,Project 1 (JB). We appreciate the effect that Jack Oram had on this work and HDL metabolism in general.
Abbreviations
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- HL
hepatic lipase
- LPL
lipoprotein lipase
- CETP
cholesteryl ester transfer protein
- PLTP
phospholipid transfer protein
- sdLDL
small-dense LDL
- bbLDL
big-buoyant LDL
- CAD
coronary artery disease
- FATS
Familial atherosclerosis treatment study
- HATS
HDL atherosclerosis treat study
- RCT
reverse cholesterol transport
- GWAS
genome wide association study
- HTG
hypertriglyceridemia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This paper will focus primarily on human data. Data about hepatic lipase that are adjusted for collinear variables were not included in this paper, nor were data using preheparin LPL and HL activity. This paper was presented in part at the International Atherosclerosis Society workshop on HDL in Newport, RI, June 19, 2009.
This work in this article was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Carr, MC and Brunzell, JD, Increased hepatic lipase activity and intra-abdominal fat across the transition from pre- to post-menopause. Program of the 85th Annual Meeting of The Endocrine Society, Philadelphia, PA, 2003, p374 (Abstract P 2-280) Section 3.1.
Contributor Information
JOHN D. BRUNZELL, University of Washington, Department of Medicine, Division of Metabolism, Endocrinology and Nutrition; Box 356426; 1959 NE Pacific Avenue, Seattle, Washington 98195, USA. brunzell@uw.edu.
ALBERTO ZAMBON, University of Washington, Department of Medicine, Division of Metabolism, Endocrinology and Nutrition; Box 356426; 1959 NE Pacific Avenue, Seattle, Washington 98195, USA. iodza@tin.it.
SAMIR S. DEEB, University of Washington, Department of Medicine, Division of Medical Genetics, and Department of Genome Sciences; Box 357720; 1959 NE Pacific Avenue, Seattle, Washington 98195, USA. sdeeb@uw.edu.
REFERENCES
- [1].Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- [2].Lamarche B, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Despres JP, Prospective results from the Quebec Cardiovascular Study Associations of HDL2 and HDL3 subfractions with ischemic heart disease in men. Arterioscler Thromb Vasc Biol. 1997;17:1098–1105. doi: 10.1161/01.atv.17.6.1098. [DOI] [PubMed] [Google Scholar]
- [3].Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Despres JP, Prospective results from the Quebec Cardiovascular Study Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Circulation. 1997;95:69–75. doi: 10.1161/01.cir.95.1.69. [DOI] [PubMed] [Google Scholar]
- [4].Musunuru K, Orho-Melander M, Caulfield MP, Li S, Salameh WA, Reitz RE, Berglund G, Hedblad B, Engstrom G, Williams PT, Kathiresan S, Melander O, Krauss RM. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol. 2009;29:1975–1980. doi: 10.1161/ATVBAHA.109.190405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Deeb S, Peng R. Structure of the human lipoprotein lipase gene. Biochemistry. 1989;28:4131–4135. doi: 10.1021/bi00436a001. [DOI] [PubMed] [Google Scholar]
- [6].Brunzell J.D.a.D., S. S., editors. Familial Lipoprotein Lipase Deficiency, Apo C-II Deficiency, and Hepatic Lipase Deficiency. McGraw-Hill; New York: 2001. [Google Scholar]
- [7].Nakajima K, Kobayashi J, Mabuchi H, Nakano T, Tokita Y, Nagamine T, Imamura S, Ai M, Otokozawa S, Schaefer EF. Association of angiopoietin-like protein 3 with hepatic triglyceride lipase and lipoprotein lipase activities in human plasma. Ann Clin Biochem. 2010;47:423–431. doi: 10.1258/acb.2010.009307. [DOI] [PubMed] [Google Scholar]
- [8].Imamura S, Kobayashi J, Nakajima K, Sakasegawa S, Nohara A, Noguchi T, Kawashiri MA, Inazu A, Deeb SS, Mabuchi H, Brunzell JD. A novel method for measuring human lipoprotein lipase and hepatic lipase activities in postheparin plasma. J Lipid Res. 2008;49:1431–1437. doi: 10.1194/jlr.M700528-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zambon A, Deeb SS, Bensadoun A, Foster KE, Brunzell JD. In vivo evidence of a role for hepatic lipase in human apoB-containing lipoprotein metabolism, independent of its lipolytic activity. J Lipid Res. 2000;41:2094–2099. [PubMed] [Google Scholar]
- [10].Dichek HL, Qian K, Agrawal N. Divergent effects of the catalytic and bridging functions of hepatic lipase on atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:1696–1702. doi: 10.1161/01.ATV.0000135981.61827.9d. [DOI] [PubMed] [Google Scholar]
- [11].Deeb SS, Zambon A, Carr MC, Ayyobi AF, Brunzell JD. Hepatic lipase and dyslipidemia: interactions among genetic variants, obesity, gender, and diet. J Lipid Res. 2003;44:1279–1286. doi: 10.1194/jlr.R200017-JLR200. [DOI] [PubMed] [Google Scholar]
- [12].Despres JP, Ferland M, Moorjani S, Nadeau A, Tremblay A, Lupien PJ, Theriault G, Bouchard C. Role of hepatic-triglyceride lipase activity in the association between intra-abdominal fat and plasma HDL cholesterol in obese women. Arteriosclerosis. 1989;9:485–492. doi: 10.1161/01.atv.9.4.485. [DOI] [PubMed] [Google Scholar]
- [13].Jansen H, Verhoeven AJ, Sijbrands EJ. Hepatic lipase: a pro- or anti-atherogenic protein? J Lipid Res. 2002;43:1352–1362. doi: 10.1194/jlr.r200008-jlr200. [DOI] [PubMed] [Google Scholar]
- [14].Zambon A, Hokanson JE, Brown BG, Brunzell JD. Evidence for a new pathophysiological mechanism for coronary artery disease regression: hepatic lipase-mediated changes in LDL density. Circulation. 1999;99:1959–1964. doi: 10.1161/01.cir.99.15.1959. [DOI] [PubMed] [Google Scholar]
- [15].Johansson J, Nilsson-Ehle P, Carlson LA, Hamsten A. The association of lipoprotein and hepatic lipase activities with high density lipoprotein subclass levels in men with myocardial infarction at a young age. Atherosclerosis. 1991;86:111–122. doi: 10.1016/0021-9150(91)90207-j. [DOI] [PubMed] [Google Scholar]
- [16].Katzel LI, Coon PJ, Busby MJ, Gottlieb SO, Krauss RM, Goldberg AP. Reduced HDL2 cholesterol subspecies and elevated postheparin hepatic lipase activity in older men with abdominal obesity and asymptomatic myocardial ischemia. Arterioscler Thromb. 1992;12:814–823. doi: 10.1161/01.atv.12.7.814. [DOI] [PubMed] [Google Scholar]
- [17].Juo SH, Han Z, Smith JD, Colangelo L, Liu K. Promoter polymorphisms of hepatic lipase gene influence HDL(2) but not HDL(3) in African American men: CARDIA study. J Lipid Res. 2001;42:258–264. [PubMed] [Google Scholar]
- [18].Carr MC, Hokanson JE, Zambon A, Deeb SS, Barrett PH, Purnell JQ, Brunzell JD. The contribution of intraabdominal fat to gender differences in hepatic lipase activity and low/high density lipoprotein heterogeneity. J Clin Endocrinol Metab. 2001;86:2831–2837. doi: 10.1210/jcem.86.6.7586. [DOI] [PubMed] [Google Scholar]
- [19].Cheung MC, Sibley SD, Palmer JP, Oram JF, Brunzell JD. Lipoprotein lipase and hepatic lipase: their relationship with HDL subspecies Lp(A-I) and Lp(A-I,A-II) J Lipid Res. 2003;44:1552–1558. doi: 10.1194/jlr.M300091-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brewer HB., Jr. The Evolving Role of HDL in the Treatment of High-Risk Patients with Cardiovascular Disease. J Clin Endocrinol Metab. 96:1246–1257. doi: 10.1210/jc.2010-0163. [DOI] [PubMed] [Google Scholar]
- [21].Zambon A, Deeb SS, Pauletto P, Crepaldi G, Brunzell JD. Hepatic lipase: a marker for cardiovascular disease risk and response to therapy. Curr Opin Lipidol. 2003;14:179–189. doi: 10.1097/00041433-200304000-00010. [DOI] [PubMed] [Google Scholar]
- [22].Collet X, Tall AR, Serajuddin H, Guendouzi K, Royer L, Oliveira H, Barbaras R, Jiang XC, Francone OL. Remodeling of HDL by CETP in vivo and by CETP and hepatic lipase in vitro results in enhanced uptake of HDL CE by cells expressing scavenger receptor B-I. J Lipid Res. 1999;40:1185–1193. [PubMed] [Google Scholar]
- [23].Vergeer M, Korporaal SJ, Franssen R, Meurs I, Out R, Hovingh GK, Hoekstra M, Sierts JA, Dallinga-Thie GM, Motazacker MM, Holleboom AG, Van Berkel TJ, Kastelein JJ, Van Eck M, Kuivenhoven JA. Genetic variant of the scavenger receptor BI in humans. N Engl J Med. 2011;364:136–145. doi: 10.1056/NEJMoa0907687. [DOI] [PubMed] [Google Scholar]
- [24].Francis GA. The complexity of HDL. Biochim Biophys Acta. 2010;1801:1286–1293. doi: 10.1016/j.bbalip.2010.08.009. [DOI] [PubMed] [Google Scholar]
- [25].Edmondson AC, Brown RJ, Kathiresan S, Cupples LA, Demissie S, Manning AK, Jensen MK, Rimm EB, Wang J, Rodrigues A, Bamba V, Khetarpal SA, Wolfe ML, Derohannessian S, Li M, Reilly MP, Aberle J, Evans D, Hegele RA, Rader DJ. Loss-of-function variants in endothelial lipase are a cause of elevated HDL cholesterol in humans. J Clin Invest. 2009;119:1042–1050. doi: 10.1172/JCI37176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vergeer M, Boekholdt SM, Sandhu MS, Ricketts SL, Wareham NJ, Brown MJ, de Faire U, Leander K, Gigante B, Kavousi M, Hofman A, Uitterlinden AG, van Duijn CM, Witteman JC, Jukema JW, Schadt EE, van der Schoot E, Kastelein JJ, Khaw KT, Dullaart RP, van Tol A, Trip MD, Dallinga-Thie GM. Genetic variation at the phospholipid transfer protein locus affects its activity and high-density lipoprotein size and is a novel marker of cardiovascular disease susceptibility. Circulation. 2010;122:470–477. doi: 10.1161/CIRCULATIONAHA.109.912519. [DOI] [PubMed] [Google Scholar]
- [27].Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–219. [PubMed] [Google Scholar]
- [28].Varbo A, Nordestgaard BG, Tybjaerg-Hansen A, Schnohr P, Jensen GB, Benn M. Nonfasting triglycerides, cholesterol, and ischemic stroke in the general population. Ann Neurol. 69:628–634. doi: 10.1002/ana.22384. [DOI] [PubMed] [Google Scholar]
- [29].Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Almeras N, Bergeron J, Gaudet D, Tremblay G, Prud’homme D, Nadeau A, Despres JP. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102:179–184. doi: 10.1161/01.cir.102.2.179. [DOI] [PubMed] [Google Scholar]
- [30].Brunzell JD. Clinical practice. Hypertriglyceridemia. N Engl J Med. 2007;357:1009–1017. doi: 10.1056/NEJMcp070061. [DOI] [PubMed] [Google Scholar]
- [31].Carr MC, Brunzell JD. Abdominal obesity and dyslipidemia in the metabolic syndrome: importance of type 2 diabetes and familial combined hyperlipidemia in coronary artery disease risk. J Clin Endocrinol Metab. 2004;89:2601–2607. doi: 10.1210/jc.2004-0432. [DOI] [PubMed] [Google Scholar]
- [32].Zambon A, Brown BG, Deeb SS, Brunzell JD. Genetics of apolipoprotein B and apolipoprotein AI and premature coronary artery disease. J Intern Med. 2006;259:473–480. doi: 10.1111/j.1365-2796.2006.01645.x. [DOI] [PubMed] [Google Scholar]
- [33].Despres JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990;10:497–511. doi: 10.1161/01.atv.10.4.497. [DOI] [PubMed] [Google Scholar]
- [34].Purnell JQ, Kahn SE, Albers JJ, Nevin DN, Brunzell JD, Schwartz RS. Effect of weight loss with reduction of intra-abdominal fat on lipid metabolism in older men. J Clin Endocrinol Metab. 2000;85:977–982. doi: 10.1210/jcem.85.3.6402. [DOI] [PubMed] [Google Scholar]
- [35].Carr MC, Knopp RH, Brunzell JD, Wheeler BS, Zhu X, Lakshmanan M, Rosen AS, Anderson PW. Effect of raloxifene on serum triglycerides in women with a history of hypertriglyceridemia while on oral estrogen therapy. Diabetes Care. 2005;28:1555–1561. doi: 10.2337/diacare.28.7.1555. [DOI] [PubMed] [Google Scholar]
- [36].Blackburn P, Lemieux I, Lamarche B, Bergeron J, Perron P, Tremblay G, Gaudet D, Despres JP. Hypertriglyceridemic waist: a simple clinical phenotype associated with coronary artery disease in women. Metabolism. 2011 doi: 10.1016/j.metabol.2011.05.017. [DOI] [PubMed] [Google Scholar]
- [37].Carr MC, Kim KH, Zambon A, Mitchell ES, Woods NF, Casazza CP, Purnell JQ, Hokanson JE, Brunzell JD, Schwartz RS. Changes in LDL density across the menopausal transition. J Investig Med. 2000;48:245–250. [PubMed] [Google Scholar]
- [38].Hokanson JE, Kamboh MI, Scarboro S, Eckel RH, Hamman RF. Effects of the hepatic lipase gene and physical activity on coronary heart disease risk. Am J Epidemiol. 2003;158:836–843. doi: 10.1093/aje/kwg230. [DOI] [PubMed] [Google Scholar]
- [39].Grarup N, Andreasen CH, Andersen MK, Albrechtsen A, Sandbaek A, Lauritzen T, Borch-Johnsen K, Jorgensen T, Schmitz O, Hansen T, Pedersen O. The −250G>A promoter variant in hepatic lipase associates with elevated fasting serum high-density lipoprotein cholesterol modulated by interaction with physical activity in a study of 16,156 Danish subjects. J Clin Endocrinol Metab. 2008;93:2294–2299. doi: 10.1210/jc.2007-2815. [DOI] [PubMed] [Google Scholar]
- [40].Goldberg AP, Busby-Whitehead MJ, Katzel LI, Krauss RM, Lumpkin M, Hagberg JM. Cardiovascular fitness, body composition, and lipoprotein lipid metabolism in older men. J Gerontol A Biol Sci Med Sci. 2000;55:M342–349. doi: 10.1093/gerona/55.6.m342. [DOI] [PubMed] [Google Scholar]
- [41].Bergeron J, Couillard C, Despres JP, Gagnon J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C, Results from the HERITAGE Family Study Race differences in the response of postheparin plasma lipoprotein lipase and hepatic lipase activities to endurance exercise training in men. Atherosclerosis. 2001;159:399–406. doi: 10.1016/s0021-9150(01)00515-9. [DOI] [PubMed] [Google Scholar]
- [42].Teran-Garcia M, Santoro N, Rankinen T, Bergeron J, Rice T, Leon AS, Rao DC, Skinner JS, Bergman RN, Despres JP, Bouchard C. Hepatic lipase gene variant −514C>T is associated with lipoprotein and insulin sensitivity response to regular exercise: the HERITAGE Family Study. Diabetes. 2005;54:2251–2255. doi: 10.2337/diabetes.54.7.2251. [DOI] [PubMed] [Google Scholar]
- [43].Ahmad T, Chasman DI, Buring JE, Lee IM, Ridker PM, Everett BM. Physical activity modifies the effect of LPL, LIPC, and CETP polymorphisms on HDL-C levels and the risk of myocardial infarction in women of European ancestry. Circ Cardiovasc Genet. 2011;4:74–80. doi: 10.1161/CIRCGENETICS.110.957290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].St-Pierre J, Miller-Felix I, Paradis ME, Bergeron J, Lamarche B, Despres JP, Gaudet D, Vohl MC. Visceral obesity attenuates the effect of the hepatic lipase −514C>T polymorphism on plasma HDL-cholesterol levels in French-Canadian men. Mol Genet Metab. 2003;78:31–36. doi: 10.1016/s1096-7192(02)00223-8. [DOI] [PubMed] [Google Scholar]
- [45].Zhang C, Lopez-Ridaura R, Rimm EB, Li T, Hunter DJ, Hu FB. Genetic variation in the hepatic lipase gene and the risk of coronary heart disease among US diabetic men: potential interaction with obesity. Diabetologia. 2006;49:1552–1559. doi: 10.1007/s00125-006-0235-2. [DOI] [PubMed] [Google Scholar]
- [46].Schwartz RS, Brunzell JD. Increase of adipose tissue lipoprotein lipase activity with weight loss. J Clin Invest. 1981;67:1425–1430. doi: 10.1172/JCI110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Eckel RH, Yost TJ. Weight reduction increases adipose tissue lipoprotein lipase responsiveness in obese women. J Clin Invest. 1987;80:992–997. doi: 10.1172/JCI113193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- [49].Brown G, Albers JJ, Fisher LD, Schaefer SM, Lin JT, Kaplan C, Zhao XQ, Bisson BD, Fitzpatrick VF, Dodge HT. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- [50].Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- [51].Dugi KA, Brandauer K, Schmidt N, Nau B, Schneider JG, Mentz S, Keiper T, Schaefer JR, Meissner C, Kather H, Bahner ML, Fiehn W, Kreuzer J. Low hepatic lipase activity is a novel risk factor for coronary artery disease. Circulation. 2001;104:3057–3062. doi: 10.1161/hc5001.100795. [DOI] [PubMed] [Google Scholar]
- [52].Jansen H, Hop W, van Tol A, Bruschke AV, Birkenhager JC. Hepatic lipase and lipoprotein lipase are not major determinants of the low density lipoprotein subclass pattern in human subjects with coronary heart disease. Atherosclerosis. 1994;107:45–54. doi: 10.1016/0021-9150(94)90140-6. [DOI] [PubMed] [Google Scholar]
- [53].Jansen H, Verhoeven AJ, Weeks L, Kastelein JJ, Halley DJ, van den Ouweland A, Jukema JW, Seidell JC, Birkenhager JC. Common C-to-T substitution at position −480 of the hepatic lipase promoter associated with a lowered lipase activity in coronary artery disease patients. Arterioscler Thromb Vasc Biol. 1997;17:2837–2842. doi: 10.1161/01.atv.17.11.2837. [DOI] [PubMed] [Google Scholar]
- [54].Auwerx JA, Marzetta CA, Hokanson JE, Brunzell JD. Large buoyant LDL-like particles in hepatic lipase deficiency. Arteriosclerosis. 1989;9:319–325. doi: 10.1161/01.atv.9.3.319. [DOI] [PubMed] [Google Scholar]
- [55].Annema W, Tietge UJ. Role of hepatic lipase and endothelial lipase in high-density lipoprotein-mediated reverse cholesterol transport. Curr Atheroscler Rep. 2011;13:257–265. doi: 10.1007/s11883-011-0175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Johannsen TH, Kamstrup PR, Andersen RV, Jensen GB, Sillesen H, Tybjaerg-Hansen A, Nordestgaard BG. Hepatic lipase, genetically elevated high-density lipoprotein, and risk of ischemic cardiovascular disease. J Clin Endocrinol Metab. 2009;94:1264–1273. doi: 10.1210/jc.2008-1342. [DOI] [PubMed] [Google Scholar]
- [57].McCaskie PA, Cadby G, Hung J, McQuillan BM, Chapman CM, Carter KW, Thompson PL, Palmer LJ, Beilby JP. The C-480T hepatic lipase polymorphism is associated with HDL-C but not with risk of coronary heart disease. Clin Genet. 2006;70:114–121. doi: 10.1111/j.1399-0004.2006.00659.x. [DOI] [PubMed] [Google Scholar]
- [58].Jansen H. Hepatic lipase: friend or foe and under what circumstances? Curr Atheroscler Rep. 2004;6:343–347. doi: 10.1007/s11883-004-0044-3. [DOI] [PubMed] [Google Scholar]
- [59].Faghihnia N, Tsimikas S, Miller ER, Witztum JL, Krauss RM. Changes in lipoprotein(a), oxidized phospholipids, and LDL subclasses with a low-fat high-carbohydrate diet. J Lipid Res. 2010;51:3324–3330. doi: 10.1194/jlr.M005769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zambon A, Brown BG, Hokanson JE, Motulsky AG, Brunzell JD. Genetically determined apo B levels and peak LDL density predict angiographic response to intensive lipid-lowering therapy. J Intern Med. 2006;259:401–409. doi: 10.1111/j.1365-2796.2006.01626.x. [DOI] [PubMed] [Google Scholar]
- [61].van Acker BA, Botma GJ, Zwinderman AH, Kuivenhoven JA, Dallinga-Thie GM, Sijbrands EJ, Boer JM, Seidell JC, Jukema JW, Kastelein JJ, Jansen H, Verhoeven AJ. High HDL cholesterol does not protect against coronary artery disease when associated with combined cholesteryl ester transfer protein and hepatic lipase gene variants. Atherosclerosis. 2008;200:161–167. doi: 10.1016/j.atherosclerosis.2007.11.019. [DOI] [PubMed] [Google Scholar]
- [62].Muntner P, Lee F, Astor BC. Association of high-density lipoprotein cholesterol with coronary heart disease risk across categories of low-density lipoprotein cholesterol: the atherosclerosis risk in communities study. Am J Med Sci. 341:173–180. doi: 10.1097/MAJ.0b013e3181f97e4a. [DOI] [PubMed] [Google Scholar]
- [63].Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, Blechacz B, Bassler D, Wei X, Sharman A, Whitt I, Alves da Silva S, Khalid Z, Nordmann AJ, Zhou Q, Walter SD, Vale N, Bhatnagar N, O’Regan C, Mills EJ, Bucher HC, Montori VM, Guyatt GH. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. Bmj. 2009;338:b92. doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- [65].The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol: baseline characteristics of study participants. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: impact on Global Health outcomes (AIM-HIGH) trial. Am Heart J. 2011;161:538–543. doi: 10.1016/j.ahj.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol. 2008;101:20B–26B. doi: 10.1016/j.amjcard.2008.02.029. [DOI] [PubMed] [Google Scholar]
- [67].Lamon-Fava S, Diffenderfer MR, Barrett PH, Buchsbaum A, Nyaku M, Horvath KV, Asztalos BF, Otokozawa S, Ai M, Matthan NR, Lichtenstein AH, Dolnikowski GG, Schaefer EJ. Extended-release niacin alters the metabolism of plasma apolipoprotein (Apo) A-I and ApoB-containing lipoproteins. Arterioscler Thromb Vasc Biol. 2008;28:1672–1678. doi: 10.1161/ATVBAHA.108.164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Haas MJ, Alamir AR, Sultan S, Chehade JM, Wong NC, Mooradian AD. Nicotinic acid induces apolipoprotein A-I gene expression in HepG2 and Caco-2 cell lines. Metabolism. 2011 doi: 10.1016/j.metabol.2011.05.005. [DOI] [PubMed] [Google Scholar]
- [69].Morgan JM, Capuzzi DM, Baksh RI, Intenzo C, Carey CM, Reese D, Walker K. Effects of extended-release niacin on lipoprotein subclass distribution. Am J Cardiol. 2003;91:1432–1436. doi: 10.1016/s0002-9149(03)00394-1. [DOI] [PubMed] [Google Scholar]
- [70].Shepherd J, Packard CJ, Patsch JR, Gotto AM, Jr., Taunton OD. Effects of nicotinic acid therapy on plasma high density lipoprotein subfraction distribution and composition and on apolipoprotein A metabolism. J Clin Invest. 1979;63:858–867. doi: 10.1172/JCI109385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- [72].Sivapalaratnam S, Boekholdt SM, Trip MD, Sandhu MS, Luben R, Kastelein JJ, Wareham NJ, Khaw KT. Family history of premature coronary heart disease and risk prediction in the EPIC-Norfolk prospective population study. Heart. 2010;96:1985–1989. doi: 10.1136/hrt.2010.210740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Samani NJ, Deloukas P, Erdmann J, Hengstenberg C, Kuulasmaa K, McGinnis R, Schunkert H, Soranzo N, Thompson J, Tiret L, Ziegler A. Large scale association analysis of novel genetic loci for coronary artery disease. Arterioscler Thromb Vasc Biol. 2009;29:774–780. doi: 10.1161/ATVBAHA.108.181388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lettre G, Palmer CD, Young T, Ejebe KG, Allayee H, Benjamin EJ, Bennett F, Bowden DW, Chakravarti A, Dreisbach A, Farlow DN, Folsom AR, Fornage M, Forrester T, Fox E, Haiman CA, Hartiala J, Harris TB, Hazen SL, Heckbert SR, Henderson BE, Hirschhorn JN, Keating BJ, Kritchevsky SB, Larkin E, Li M, Rudock ME, McKenzie CA, Meigs JB, Meng YA, Mosley TH, Newman AB, Newton-Cheh CH, Paltoo DN, Papanicolaou GJ, Patterson N, Post WS, Psaty BM, Qasim AN, Qu L, Rader DJ, Redline S, Reilly MP, Reiner AP, Rich SS, Rotter JI, Liu Y, Shrader P, Siscovick DS, Tang WH, Taylor HA, Tracy RP, Vasan RS, Waters KM, Wilks R, Wilson JG, Fabsitz RR, Gabriel SB, Kathiresan S, Boerwinkle E. Genome-wide association study of coronary heart disease and its risk factors in 8,090 African Americans: the NHLBI CARe Project. PLoS Genet. 7:e1001300. doi: 10.1371/journal.pgen.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin Cho Y, Jin Go M, Jin Kim Y, Lee JY, Park T, Kim K, Sim X, TweeY R, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Hua Zhao J, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RY, Wright AF, Witteman JC, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJ, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BW, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O’Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Aulchenko YS, Ripatti S, Lindqvist I, Boomsma D, Heid IM, Pramstaller PP, Penninx BW, Janssens AC, Wilson JF, Spector T, Martin NG, Pedersen NL, Kyvik KO, Kaprio J, Hofman A, Freimer NB, Jarvelin MR, Gyllensten U, Campbell H, Rudan I, Johansson A, Marroni F, Hayward C, Vitart V, Jonasson I, Pattaro C, Wright A, Hastie N, Pichler I, Hicks AA, Falchi M, Willemsen G, Hottenga JJ, de Geus EJ, Montgomery GW, Whitfield J, Magnusson P, Saharinen J, Perola M, Silander K, Isaacs A, Sijbrands EJ, Uitterlinden AG, Witteman JC, Oostra BA, Elliott P, Ruokonen A, Sabatti C, Gieger C, Meitinger T, Kronenberg F, Doring A, Wichmann HE, Smit JH, McCarthy MI, van Duijn CM, Peltonen L. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat Genet. 2009;41:47–55. doi: 10.1038/ng.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Musunuru K, Kathiresan S. Genetics of coronary artery disease. Annu Rev Genomics Hum Genet. 2010;11:91–108. doi: 10.1146/annurev-genom-082509-141637. [DOI] [PubMed] [Google Scholar]
- [78].Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M, Gieger C, Absher D, Aherrahrou Z, Allayee H, Altshuler D, Anand SS, Andersen K, Anderson JL, Ardissino D, Ball SG, Balmforth AJ, Barnes TA, Becker DM, Becker LC, Berger K, Bis JC, Boekholdt SM, Boerwinkle E, Braund PS, Brown MJ, Burnett MS, Buysschaert I, Carlquist JF, Chen L, Cichon S, Codd V, Davies RW, Dedoussis G, Dehghan A, Demissie S, Devaney JM, Diemert P, Do R, Doering A, Eifert S, Mokhtari NE, Ellis SG, Elosua R, Engert JC, Epstein SE, de Faire U, Fischer M, Folsom AR, Freyer J, Gigante B, Girelli D, Gretarsdottir S, Gudnason V, Gulcher JR, Halperin E, Hammond N, Hazen SL, Hofman A, Horne BD, Illig T, Iribarren C, Jones GT, Jukema JW, Kaiser MA, Kaplan LM, Kastelein JJ, Khaw KT, Knowles JW, Kolovou G, Kong A, Laaksonen R, Lambrechts D, Leander K, Lettre G, Li M, Lieb W, Loley C, Lotery AJ, Mannucci PM, Maouche S, Martinelli N, McKeown PP, Meisinger C, Meitinger T, Melander O, Merlini PA, Mooser V, Morgan T, Muhleisen TW, Muhlestein JB, Munzel T, Musunuru K, Nahrstaedt J, Nelson CP, Nothen MM, Olivieri O, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ogawa N, Imai Y, Morita H, Nagai R. Genome-wide association study of coronary artery disease. Int J Hypertens. 2010:790539. doi: 10.4061/2010/790539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sivapalaratnam S, Motazacker MM, Maiwald S, Hovingh GK, Kastelein JJ, Levi M, Trip MD, Dallinga-Thie GM. Genome-wide association studies in atherosclerosis. Curr Atheroscler Rep. 13:225–232. doi: 10.1007/s11883-011-0173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Johansen CT, Kathiresan S, Hegele RA. Genetic determinants of plasma triglycerides. J Lipid Res. 2011;52:189–206. doi: 10.1194/jlr.R009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Pollex RL, Hegele RA. Genomic copy number variation and its potential role in lipoprotein and metabolic phenotypes. Curr Opin Lipidol. 2007;18:174–180. doi: 10.1097/MOL.0b013e32802e6c12. [DOI] [PubMed] [Google Scholar]
- [83].Lanktree M, Hegele RA. Copy number variation in metabolic phenotypes. Cytogenet Genome Res. 2008;123:169–175. doi: 10.1159/000184705. [DOI] [PubMed] [Google Scholar]
- [84].Zambon A, Brown BG, Deeb SS, Brunzell JD. Hepatic lipase as a focal point for the development and treatment of coronary artery disease. J Investig Med. 2001;49:112–118. doi: 10.2310/6650.2001.34107. [DOI] [PubMed] [Google Scholar]
- [85].Zambon A, Austin MA, Brown BG, Hokanson JE, Brunzell JD. Effect of hepatic lipase on LDL in normal men and those with coronary artery disease. Arterioscler Thromb. 1993;13:147–153. doi: 10.1161/01.atv.13.2.147. [DOI] [PubMed] [Google Scholar]