Abstract
The dose-limiting side-effect of taxane, platinum-complex, and other kinds of anti-cancer drugs is a chronic, distal, bilaterally symmetrical, sensory peripheral neuropathy that is often accompanied by neuropathic pain. Work with animal models of these conditions suggests that the neuropathy is a consequence of toxic effects on mitochondria in primary afferent sensory neurons. If this is true, then additional mitochondrial insult ought to make the neuropathic pain worse. This prediction was tested in rats with painful peripheral neuropathy due to the taxane agent, paclitaxel, and the platinum-complex agent, oxaliplatin. Rats with established neuropathy were given one of three mitochondrial poisons: rotenone (an inhibitor of respiratory Complex I), oligomycin (an inhibitor of ATP synthase), and auranofin (an inhibitor of the thioredoxin-thioredoxin reductase mitochondrial anti-oxidant defense system). All three toxins significantly increased the severity of paclitaxel-evoked and oxaliplatin-evoked mechano-allodynia and mechano-hyperalgesia while having no effect on the mechano-sensitivity of chemotherapy naïve rats. Chemotherapy-evoked painful peripheral neuropathy is associated with an abnormal spontaneous discharge in primary afferent A-fibers and C-fibers. Oligomycin, at the same dose that exacerbated allodynia and hyperalgesia, significantly increased the discharge frequency of spontaneously discharging A-fibers and C-fibers in both paclitaxel-treated and oxaliplatin-treated rats, but did not evoke any discharge in naïve control rats. These results implicate mitochondrial dysfunction in the production of chemotherapy-evoked neuropathic pain and suggest that drugs that have positive effects on mitochondrial function may be of use in its treatment and prevention.
Keywords: allodynia, auranofin, hyperalgesia, neuropathic pain, oligomycin, rotenone
1. Introduction
Paclitaxel, oxaliplatin and other chemotherapeutics produce chronic, bilaterally symmetrical, sensory, peripheral neuropathies that are often accompanied by neuropathic pain. Clinical descriptions of these neuropathies suggest that they produce similar neuropathic pain states [2,4,8,9,31]. It has been hypothesized that chemotherapy-evoked damage to mitochondria in primary afferent sensory axons leads to a chronic energy deficiency that is the proximate cause of the symptoms of the neuropathy [1,13,16,33,34]. Chronic deficits in peripheral nerve mitochondrial function (decreased respiration and ATP production) have been shown in rats with paclitaxel-evoked and oxaliplatin-evoked painful neuropathies [34].
If the mitotoxicity hypothesis is true, then the mitochondria in sensory axons of paclitaxel-treated and oxaliplatin-treated rats should be especially vulnerable to additional insult and such an insult should make the neuropathic pain worse. The experiments reported here tested this prediction.
2. Methods
These experiments conformed to the ethics guidelines of the International Association for the Study of Pain [35], the National Institutes of Health (USA), and the Canadian Institutes of Health Research. All experimental protocols were approved by the Animal Care Committee of the Faculty of Medicine, McGill University, in accordance with the regulations of the Canadian Council on Animal Care.
2.1. Animals
Adult male Sprague-Dawley rats (175–250 g, Harlan Inc., Indianapolis, IN; Frederick, MD breeding colony) were housed on sawdust bedding in plastic cages. Artificial lighting was provided on a fixed 12 hour light-dark cycle with food and water available ad libitum.
2.2. Chemotherapy models
Paclitaxel (Taxol®) was administered as described previously [13,24]: a stock solution (6 mg/ml in Cremophor/EL; Biolyse Pharma Corp.; St. Catherines, ON, Canada) was diluted with saline to a concentration of 2 mg/ml and injected IP at 2 mg/kg on four alternate days (D0, D2, D4 and D6) for a cumulative dose of 8 mg/kg. Control animals received matched injections of the vehicle.
For oxaliplatin (Eloxatin®; Sanofi-Aventis), a stock solution (5 mg/ml) was diluted to 2 mg/ml with 5% dextrose in distilled water and injected IP at 2 mg/kg on five consecutive days (D0–D4) for a cumulative dose of 10 mg/kg [34]. Control animals received matched vehicle injections.
2.3. Behavioral assays
Assays for chemotherapy-evoked mechano-allodynia (4 g von Frey hair test) and mechano-hyperalgesia (15 g von Frey hair test) were conducted as described previously [12]. Results are presented as the percentage of trials (5 to each hind paw for each von Frey hair) that evoked a withdrawal reflex. Responses to the von Frey hair stimuli were assessed prior to chemotherapy and 3–4 weeks afterwards to confirm the presence of chemotherapy-evoked pain hypersensitivity. The effects of rotenone, oligomycin, auranofin, and their respective vehicles were assessed 3–7 days later by an observer who was blind as to drug condition. All rats were euthanized via a sodium pentobarbital overdose (150 mg/kg, IP) immediately after the behavioral tests.
2.4. Rotenone effects on behavior
There is a deficit in mitochondrial respiratory Complex I (nicotinamide adenine dinucleotide (NADH):ubiquinone oxidoreductase)-mediated respiration in peripheral nerves taken from rats with paclitaxel-evoked and oxaliplatin-evoked painful peripheral neuropathy [34]. Rotenone is a Complex I inhibitor. Rotenone (3 mg/kg, 1.0 ml/kg, IP; Sigma Chemicals, St. Louis, MO) or vehicle (corn oil) was given to paclitaxel-treated and oxaliplatin-treated rats and their respective naive (no chemotherapy) controls (n = 10 /group). Behavioral testing began 30 min after injection. The dose was chosen based on previous work showing that a continuous systemic 24 h infusion of 3 mg/kg has a mitotoxic effect [19].
2.5. Oligomycin effects on behavior
Peripheral nerve axons from paclitaxel-treated and oxaliplatin-treated rats generate significantly less ATP than normal [34]. Oligomycin, is a potent inhibitor of ATP synthase [6] and will thus exacerbate an energy deficiency due to impairment of any of the respiratory complexes (and also due to impairment of ATP synthase itself). Oligomycin (1.0 mg/kg, IP; Sigma) or vehicle (45% ethanol in distilled water) was given to paclitaxel-treated and oxaliplatin-treated rats and to naive (no chemotherapy) controls (n = 10/group). Behavioral testing began 15 min after injection. The dose was chosen based on pilot experiments where we found that although 1.0 mg/kg was lethal, no deaths occurred during the first few hours post-injection. Others have shown that in the rat decreased oxygen consumption after a single oligomycin injection of 0.5 mg/kg is first detected at 2–3 h and recovers by 6 h; no change in heart rate or blood pressure is seen 2–3 h post-injection [21]. The 0.5 mg/kg dose is approximately the LD33, but the cause of death is unknown and may not be due directly to the acute mitotoxic effect because the animals die hours after oxygen consumption normalizes [21].
2.6. Auranofin effects on behavior
Mitochondrial dysfunction is generally associated with an increase in the production of the superoxide ion and a resulting increase in oxidative stress [5]. The thioredoxin (Trx) - thioredoxin reductase (TrxR) system is one of the key mitochondrial systems for controlling protein degradation due to oxidation [15,30]. Auranofin is a potent inhibitor of mitochondrial TrxR [23,25]. Auranofin (5 mg/kg, 1.0 ml/kg, PO; Sigma) or vehicle (0.25% methylcellulose in saline) was given to paclitaxel-treated and oxaliplatin-treated rats and to naive (no chemotherapy) controls (n = 10 /group). Behavioral tests were conducted before and 60 min after drug. The dose was chosen based on prior work with auranofin in the rat [27].
2.7. Oligomycin effects on primary afferent spontaneous discharge
Paclitaxel-evoked painful peripheral neuropathy is associated with an abnormal, low-frequency, irregular, spontaneous discharge in A-fiber and C-fiber primary afferent axons [32,33]. Spontaneously active A-fibers and C-fibers in paclitaxel-treated and oxaliplatin-treated rats were assessed according to methods described previously 3–4 weeks after exposure to chemotherapy. Briefly, the animal was anesthetized with isoflurane, the sural nerve (a nearly pure cutaneous sensory nerve in the rat [28,29] was severed, and microfilaments were dissected from the distal stump. Axons were recorded in response to electrical stimulation of the nerve at the level of the ankle and individual axons were identified on the basis of their constant waveforms and invariant thresholds and latencies. Microfilaments were chosen for study if they contained 1–3 individually identifiable axons that had spontaneous discharge (at least one spike per min). Microfilaments with spontaneous discharge from axons that could not be individually identified (because their shock-evoked response was obscured by the waveforms of other fibers) were discarded. As in our prior work [32,33], we did not differentiate between A-fibers with conduction velocities in the Aδ and Aβ ranges because it is impossible to differentiate functional classes of A-fibers on this basis [7]. We purposely avoided characterizing the fibers’ responses to receptive field stimulation. To do so would require repeated application of noxious stimuli that might sensitize nociceptors. Sensitized nociceptors have an ongoing discharge that would be impossible to distinguish from paclitaxel-evoked spontaneous discharge. The average discharge frequency was computed for a 5 min epoch prior to injection and then for six additional 5 min epochs after injection of oligomycin (1.0 mg/kg, IP) or an equal volume of vehicle. Conduction velocity was computed from the latency of the response to the nerve shock and the distance between the two electrodes.
2.8 Statistics
Statistical analyses were carried out with InStat version 3 (GraphPad Software, Inc., La Jolla, CA). Multiple comparisons within an experiment were analyzed with Bonferroni-corrected t-tests or Dunnett’s t-test for multiple comparisons to control. p < 0.05 was considered significant.
3. Results
3.1. Paclitaxel- and oxaliplatin-evoked neuropathic pain
The paclitaxel-treated and oxaliplatin-treated rats had statistically significant (p < 0.01) mechano-allodynia and mechano-hyperalgesia prior to administration of rotenone, oligomycin, or auranofin as shown by comparison to their pre-chemotherapy baselines (not shown) and by comparison to the naïve (no chemotherapy) control groups (Figs. 1 and 2).
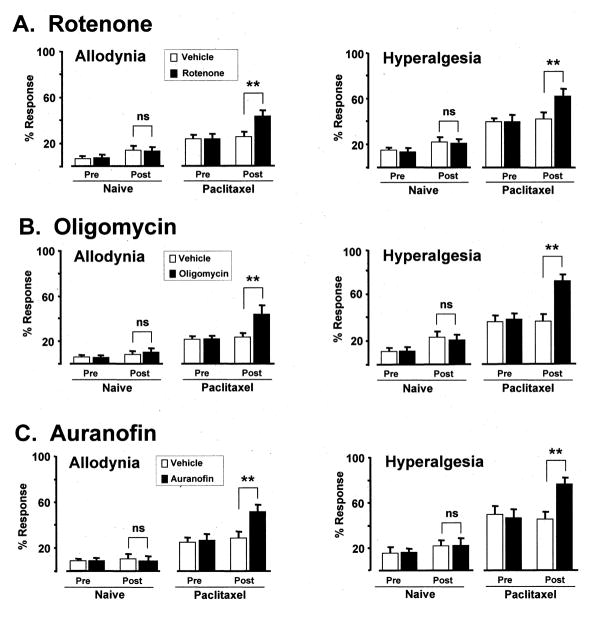
Fig. 1.
Effects of (A) rotenone, (B) oligomycin and (C) auranofin in paclitaxel-treated rats and naïve (no chemotherapy) controls. First column: mechano-allodynia (mean ± SEM response frequency to 4 g von Frey hair); second column: mechano-hyperalgesia (15 g von Frey hair). Pre/Post: before and after injection of mitotoxin or vehicle. ns: statistically non-significant; ** p < 0.01 compared to vehicle-injected group (Bonferroni-corrected t-tests).
Fig. 2.
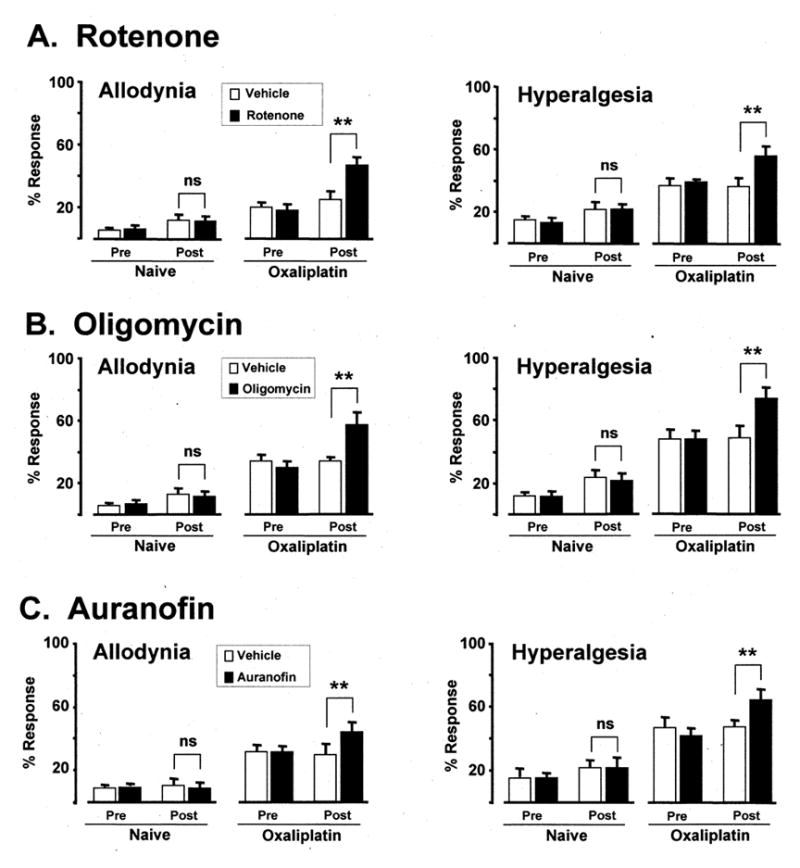
Effects of (A) rotenone, (B) oligomycin and (C) auranofin in oxaliplatin-treated rats and naïve (no chemotherapy) controls. First column: mechano-allodynia (mean ± SEM response frequency to 4 g von Frey hair); second column: mechano-hyperalgesia (15 g von Frey hair). Pre/Post: before and after injection of mitotoxin or vehicle. ns: statistically non-significant; ** p < 0.01 compared to vehicle-injected group (Bonferroni-corrected t-tests).
3.2. Rotenone effects on behavior
Rotenone significantly (p < 0.01) worsened paclitaxel-evoked (Fig. 1) and oxaliplatin-evoked (Fig. 2) mechano-allodynia and mechano-hyperalgesia. For paclitaxel, mechano-allodynia increased (relative to the vehicle-injected group) by 69.0% and mechano-hyperalgesia increased by 50.0%. For oxaliplatin, mechano-allodynia increased by 88.0% and mechano-hyperalgesia by 51.4%. Rotenone had no significant effect on mechano-sensitivity in the naïve control group.
3.3. Oligomycin effects on behavior
Oligomycin significantly (p < 0.01) worsened mechano-allodynia and mechano-hyperalgesia in both paclitaxel-treated (Fig. 1) and oxaliplatin-treated rats (Fig. 2). For paclitaxel, mechano-allodynia increased by 85.2% and mechano-hyperalgesia increased by 94.4%. For oxaliplatin, mechano-allodynia increased by 67.6% and mechano-hyperalgesia by 74.0%. Oligomycin had no significant effect on mechano-sensitivity in the naïve control animals.
3.4. Auranofin effects on behavior
Auranofin significantly (p < 0.01) worsened mechano-allodynia and mechano-hyperalgesia in both paclitaxel-treated (Fig. 1) and oxaliplatin-treated rats (Fig. 2). For paclitaxel, mechano-allodynia increased by 80.0% and mechano-hyperalgesia increased by 68.8%. For oxaliplatin, mechano-allodynia increased by 37.5% and mechano-hyperalgesia by 36.2%. Auranofin had no significant effect on mechano-sensitivity in the naïve control animals.
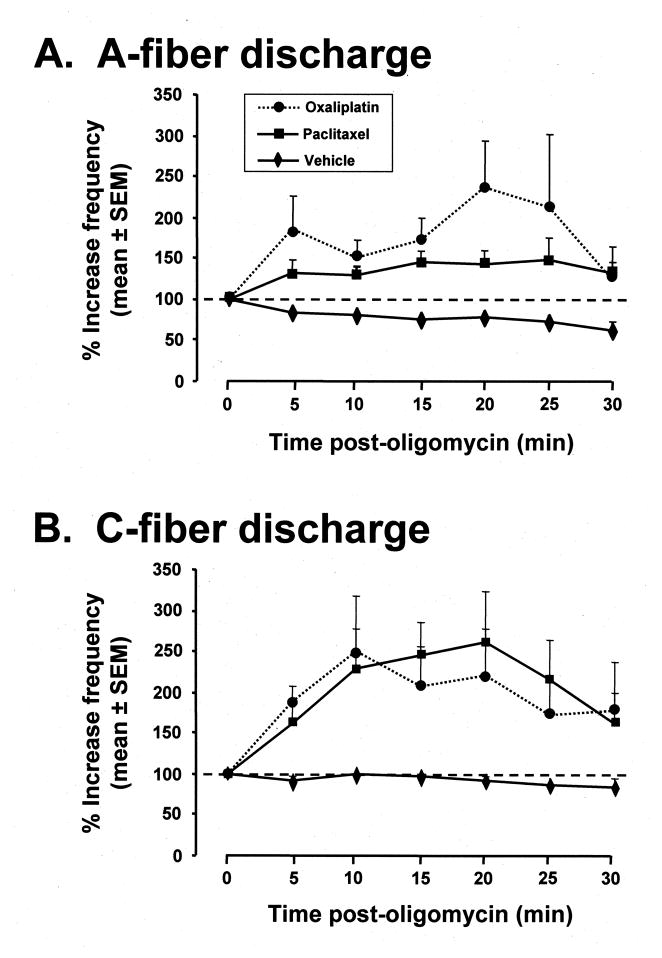
3.5. Oligomycin effect on chemotherapy-evoked spontaneous discharge
Prior to injection, spontaneously discharging A-fibers and C-fibers in paclitaxel-treated and oxaliplatin-treated rats had low-frequency irregular discharge like that described previously [32,33]: (mean ± SEM) A-fibers: 1.1 ± 0.4 Hz (range 0.03–4.23 Hz) for paclitaxel; 1.1 ± 0.4 Hz (range 0.03–3.43 Hz) for oxaliplatin; C-fibers: 0.8 ± 0.1 Hz (range 0.03–1.33 Hz) for paclitaxel; 0.6 ± 0.1 Hz (range 0.02–1.57 Hz) for oxaliplatin.
Oligomycin significantly (p < 0.05) increased discharge frequency in both A-fibers and C-fibers in both paclitaxel-treated and oxaliplatin-treated rats (Fig. 3). For both fiber types and both chemotherapeutics, the increase in discharge frequency began within 5 min, peaked in 15–20 min, and returned towards the pre-injection level at 30 min. Mean (± SEM) peak oligomycin-evoked increases in discharge frequency for A-fibers were 148 ± 28% in paclitaxel-treated rats and 236 ± 58% for oxaliplatin-treated rats; for C-fibers the peak increases were 260 ± 63% in paclitaxel-treated rats and 249 ± 68% for oxaliplatin-treated rats.
Fig. 3.
Effects of oligomycin on chemotherapy-evoked abnormal spontaneous discharge in primary afferent axons in paclitaxel-treated and oxaliplatin-treated rats. Baseline discharge frequency (dashed line) was computed from the discharge rate seen in a 5 min epoch prior to injection and is set at 100%. (A) Effects on A-fibers after injection of oligomycin (paclitaxel-treated and oxaliplatin-treated rats, 10 fibers each) or vehicle (10 fibers; 5 each from paclitaxel-treated and oxaliplatin-treated rats) at time zero. (B) Effects on C-fibers after injection of oligomycin (paclitaxel-treated and oxaliplatin-treated rats, 7 fibers each) or vehicle injection (10 fibers; 5 each from paclitaxel-treated and oxaliplatin-treated rats). The oligomycin-evoked changes are statistically significant (p < 0.05) for both the paclitaxel-treated and oxaliplatin-treated A-fibers and C-fibers (ANOVA on the area-under-the-curve values followed by Dunnett’s t-test). The mitochondrial poisons, rotenone, oligomycin, and auranofin, significantly increase chemotherapy-evoked neuropathic pain at doses that have no effect on the responses of normal rats.
Vehicle injections had no significant effect on A-fiber and C-fiber spontaneous discharge in either paclitaxel-treated or oxaliplatin-treated rats. For the A-fibers there was a trend for decreasing discharge frequency over the 30 min observation period (Fig. 3).
We recorded from a total of 30 microfilaments in these experiments, 20 of which were examined after oligomycin injection and 10 after vehicle injection. In addition to the individually-identifiable spontaneously discharging axons, each microfilament contained dozens of axons that did not have spontaneous discharge. If oligomycin or vehicle injection activated any of these silent fibers, their discharge would have been easily seen. This occurred only once; an A-fiber from an oxaliplatin-treated rat began to discharge (0.04 Hz) within 10 min of oligomycin injection and had a peak discharge of 0.06 Hz 25 min after the injection (the data from this fiber are not included in Fig. 3).
4. Discussion
4.1. Methodological considerations
The interpretation of the result of any pharmacological intervention is always constrained by the possibility that the test drug might work via an off-target mechanism of action. It is thus noteworthy that we obtained very similar results with three different agents, each of which has a distinctly different toxic effect on mitochondria.
An additional concern is that systemic administration of these toxins will affect mitochondria in all tissues, and thus might influence behavioral measurements in many ways. To prevent such a confound, our strategy was to test the drugs’ effects at an early time when systemic effects were likely to be minimal (30 min for IP rotenone, 15 min for IP oligomycin, and 60 min for PO auranofin). This strategy appears to have been successful. None of the toxins had any effect on mechano-sensitivity in the naive control (no chemotherapy) animals. Moreover, if the systemic activity of the toxins affected motor performance, one might expect to see a depressive effect that would result in a decrease in response frequency, whereas the effect that we saw was an increase in response frequency. It thus seems unlikely that our results are due to a toxin-evoked systemic action that compromised the behavioral assays.
4.2. Comparison to prior studies
The exacerbation of pain produced by rotenone and oligomycin is consistent with prior reports demonstrating a chronic functional deficit in respiratory Complex I of the electron transport system (ETS) and a deficit in ATP production in peripheral nerves (sciatic) taken from rats with established paclitaxel-evoked and oxaliplatin-evoked neuropathy [34].
An increase in the leakage of electrons from the ETS is an expected consequence of mitochondrial dysfunction [20] and this suggests that oxidative and nitrative stresses are factors in chemotherapy-evoked neuropathy. Recent evidence supports the idea that paclitaxel-evoked painful peripheral neuropathy is accompanied by an increase in free radical levels [10,22]. Auranofin, an inhibitor of TrxR, reduces mitochondrial anti-oxidant defense and we show here that it increases paclitaxel-evoked and oxaliplatin-evoked mechano-allodynia and mechano-hyperalgesia. The increases in allodynia and hyperalgesia evoked by auranofin in paclitaxel-treated rats was about double that evoked in oxaliplatin-treated rats, suggesting that the role of free radicals may be greater for paclitaxel-evoked mitotoxicity. Two types of TrxR are known, one is specific for the cytoplasm and the other is specific for the mitochondrial matrix [5]. The effects seen here may have come from either site of action. However, we have seen (unpublished observations) significant change in the level of TrxR activity in the mitochondrial fraction, but not in the cytosolic fraction, of sciatic nerves from paclitaxel-treated rats. This suggests that the effect reported here is the result of inhibition of mitochondrial TrxR. In addition to an anti-oxidant role, the Trx-TrxR system plays a key role in redox signaling [30]; we can not exclude the possibility that altered redox protein regulation is a factor in our result.
4.3. Spontaneous discharge and pain
Paclitaxel-evoked neuropathy is associated with abnormal spontaneous discharge of low-frequency and irregular pattern in A-fiber and C-fiber sensory afferents [32,33] and we found the same in oxaliplatin-treated rats. It is highly probable that this discharge is associated with the neuropathic pain symptoms, although the exact mechanism is unknown. Oligomycin increased the frequency of this abnormal discharge in both fiber types in both paclitaxel-treated and oxaliplatin-treated rats, while it did not evoke discharge in chemotherapy-naive animals. The rapid latency to onset of oligomycin’s effect on discharge is congruent with the idea that the increase in the discharge causes the exacerbation of allodynia and hyperalgesia.
4.4. Spontaneous discharge and mitochondria
The link between mitochondrial dysfunction and spontaneous discharge is unknown, but the rapid onset of the oligomycin effect reported here suggests that there is a tight link between the discharge and the availability of ATP. It has been estimated [11] that about 50% of a neuron’s energy budget is used to maintain membrane polarization via operation of the sodium-potassium pump (Na+/K+ ATPase), and the percentage is likely substantially higher if one considers the energy budget of just the neuron’s axon. For chemotherapy-evoked spontaneous discharge, we speculate that a spontaneous impulse arises when a localized pumping insufficiency due mitochondrial impairment leads to a Na+ leak-dependent depolarization that crosses the threshold for impulse initiation. Such depolarizations would arise at loci corresponding to fortuitous aggregations of functionally impaired mitochondria that were unable to provide sufficient energy to run the Na+/K+ pump. One would expect that such a process would occur sporadically at variable locations because the position of mitochondria within the axon is in constant flux. Such a process would lead to an irregular discharge pattern, which is what is observed. One would also expect that increasing the mitochondrial impairment would increase the frequency of super-threshold depolarizations without necessarily altering the irregular pattern of discharge, which is what we observed following oligomycin injection. Sporadic depolarizations might occur in the peripheral nerve axon or in the sensory terminal arbor, and they might occur in either intact fibers or in fibers whose sensory terminal arbors (intraepidermal nerve fibers; IENFs) have degenerated. Paclitaxel-evoked painful peripheral neuropathy is accompanied by a partial, but significant, loss of IENFs [1,3,16,26], and we have found a nearly identical loss in oxaliplatin-treated rats (unpublished observations). The onset of paclitaxel-evoked IENF degeneration corresponds to the onset of hyperalgeisa and allodynia, and the time of peak IENF loss corresponds to the time of peak pain severity [1,3]. Oxaliplatin-evoked and vincristine-evoked neuropathic pain is modulated by intradermal injections of rotenone and other compounds that effect mitochondrial function [17,18]. This suggests that spontaneous discharge might be due to impaired mitochondrial function at the distal-most tips of the afferent axons, either in the sensory terminal arbors of intact fibers, or in the subepidermal parent axon of those fibers whose IENFs have degenerated.
5. Conclusion
The mitotoxicity hypothesis predicts that mitochondrial insult due to chemotherapy should combine with an additional mitochondrial insult and worsen the symptoms of the neuropathy. The experiments reported here confirmed this prediction – three mitochondrial poisons all significantly worsened chemotherapy-evoked mechano-allodynia and mechano-hyperalgesia. The opposite prediction – ameliorating mitochondrial dysfunction will improve the pain – has also received experimental support [10,14,16,33]. These results suggest that pharmacological interventions aimed at neuronal mitochondria may prevent and reverse chemotherapy-evoked neuropathic pain.
Acknowledgments
This work was supported by research grants to GJB from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, U.S.A. (R01-NS0522550), the Neuropathy Association, the Louise and Alan Edwards Foundation, and the Canada Research Chairs Program. The authors declare that they have no conflicts of interest with respect to this report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bennett GJ, Liu GK, Xiao WH, Jin HW, Siau C. Terminal arbor degeneration (TAD): a novel lesion produced by the antineoplastic agent, paclitaxel. Eur J Neurosci. 2011;176:447–54. doi: 10.1111/j.1460-9568.2011.07652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binder A, Stengel M, Maag R, Wasner G, Schoch R, Moosig F, Schommer B, Baron R. Pain in oxaliplatin-induced neuropathy – Sensitization in the peripheral and central nociceptive system. Eur J Cancer. 2007;43:2658–63. doi: 10.1016/j.ejca.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Boyette-Davis J, Xin W, Zhang H, Dougherty PM. Intraepidermal nerve fiber loss corresponds to the development of Taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2011;152:308–13. doi: 10.1016/j.pain.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cata JP, Weng HR, Lee BN, Reubgen JM, Dougherty PM. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiol. 2006;72:151–69. [PubMed] [Google Scholar]
- 5.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Rad Biol Med. 2010;48:749–62. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devenish RJ, Prescott M, Boyle GM, Nagley P. The oligomycin axis of mitochondrial ATP synthase: OSCP and the proton channel. J Bioenerg Biomembr. 2000;32:507–15. doi: 10.1023/a:1005621125812. [DOI] [PubMed] [Google Scholar]
- 7.Djouhri L, Lawson SN. A-beta-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev. 2004;46:131–45. doi: 10.1016/j.brainresrev.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–42. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Dougherty PM, Cata JP, Burton AW, Vu K, Weng HR. Dysfunction in multiple primary afferent fiber subtypes revealed by quantitative sensory testing in patients with chronic vincristine-induced pain. J Pain Sym Manage. 2007;33:166–79. doi: 10.1016/j.jpainsymman.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Doyle T, Chen Z, Salvemini D. Peroxynitrite decomposition catalyst blocks paclitaxel-induced neuropathic pain: microarray analysis of spinal cord gene expression. J Pain. 2011;12(Suppl 2):37. [Google Scholar]
- 11.Erecinska M, Silver IA. Ions and energy in mammalian brain. Prog Neurobiol. 1994;43:37–71. doi: 10.1016/0301-0082(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 12.Flatters SJL, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109:150–61. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Flatters SJL, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: Evidence for mitochondrial dysfunction. Pain. 2006;122:247–57. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flatters SJL, Xiao WH, Bennett GJ. Acetyl-L-carnitine prevents and reduces paclitaxel-induced painful peripheral neuropathy. Neurosci Lett. 2006;397:219–23. doi: 10.1016/j.neulet.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem Biophys Res Commun. 2010;396:120–4. doi: 10.1016/j.bbrc.2010.03.083. [DOI] [PubMed] [Google Scholar]
- 16.Jin HW, Flatters SJL, Xiao WH, Mulhern HL, Bennett GJ. Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-L-carnitine: Effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Exptl Neurol. 2008;210:229–37. doi: 10.1016/j.expneurol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph EK, Levine JD. Mitochondrial electron transport in models of neuropathic and inflammatory pain. Pain. 2006;121:105–14. doi: 10.1016/j.pain.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Joseph EK, Levine JD. Comparison of oxaliplatin- and cisplatin-induced painful peripheral neuropathy in the rat. J Pain. 2009;10:534–41. doi: 10.1016/j.jpain.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koopman WJ, Nijtmans LG, Dieteren CE, Roestenberg P, Valsecchi F, Smeitink JA, Willems PH. Mammalian mitochondrial complex I: biogenesis, regulation, and reactive oxygen species generation. Antioxid Redox Signal. 2010;12:1431–70. doi: 10.1089/ars.2009.2743. [DOI] [PubMed] [Google Scholar]
- 20.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47:333–43. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Kramar R, Hohenegger M, Srour SN, Khanakah G. Oligomycin toxicity in intact rats. Agents and Actions. 1994;15:660–3. doi: 10.1007/BF01966788. [DOI] [PubMed] [Google Scholar]
- 22.Nassini R, Gees M, Harrison S, De Siena G, Materazzi S, Moretto N, Failli P, Preti D, Marchetti N, Cavazzini A, Mancini F, Pedretti P, Nilius B, Patacchini R, Geppetti P. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain. 2011;152:1621–31. doi: 10.1016/j.pain.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 23.Omata Y, Folan M, Shaw M, Messer RL, Lockwood PE, Hobbs D, Bouillaguet S, Sano H, Lewis JB, Wataha JC. Sublethal concentrations of diverse gold compounds inhibit mammalian cytosolic thioredoxin reductase (TrxR1) Toxicol In Vitro. 2006;20:882–90. doi: 10.1016/j.tiv.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Polomano R, Clark U, Mannes AJ, Bennett GJ. A painful peripheral neuropathy in rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- 25.Rigobello MP, Bindoli A. Mitochondrial thioredoxin reductase purification, inhibitor studies, and role in cell signaling. Methods Enzymol. 2010;474:109–22. doi: 10.1016/S0076-6879(10)74007-6. [DOI] [PubMed] [Google Scholar]
- 26.Siau C, Xiao WH, Bennett GJ. Paclitaxel- and vincristine–evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exptl Neurol. 2006;201:507–14. doi: 10.1016/j.expneurol.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith RJ, Sly LM. Type II collagen-induced arthritis in the diabetic-resistant biobreeding rat: inflammatory and histopathological features of joint pathology and effects of anti-inflammatory and antirheumatic drugs on this chronic arthritic process. J Pharmacol Exptl Therap. 1996;277:1801–13. [PubMed] [Google Scholar]
- 28.Swett JE, Torigoe Y, Elie VR, Bourassa CM, Miller PG. Sensory neurons of the rat sciatic nerve. Exptl Neurol. 1991;114:82–103. doi: 10.1016/0014-4886(91)90087-s. [DOI] [PubMed] [Google Scholar]
- 29.Swett JE, Wikholm RP, Blanks RHI, Swett AL, Conley LC. Motoneurons of the rat sciatic nerve. Exptl Neurol. 1986;93:227–52. doi: 10.1016/0014-4886(86)90161-5. [DOI] [PubMed] [Google Scholar]
- 30.Ugarte N, Petropoulos I, Friguet B. Oxidized mitochondrial protein degradation and repair in aging and oxidative stress. Antioxid Redox Signal. 2010;13:539–49. doi: 10.1089/ars.2009.2998. [DOI] [PubMed] [Google Scholar]
- 31.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Periph Nerv Syst. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 32.Xiao WH, Bennett GJ. Chemotherapy-evoked neuropathic pain: Abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-L-carnitine. Pain. 2008;135:262–70. doi: 10.1016/j.pain.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao WH, Zheng FY, Bennett GJ, Bordet T, Pruss RM. Olesoxime (cholest-4-en-3-one, oxime): Analgesic and neuroprotective effects in a rat model of painful peripheral neuropathy produced by the chemotherapeutic agent, paclitaxel. Pain. 2009;147:202–9. doi: 10.1016/j.pain.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng H, Xiao WH, Bennett GJ. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exptl Neurol. 2011;232:154–61. doi: 10.1016/j.expneurol.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]