Abstract
Cotinine, the most predominant metabolite of nicotine in mammalian species, has a pharmacological half-life that greatly exceeds its precursor. However, until recently, relatively few studies had been conducted to systematically characterize the behavioral pharmacology of cotinine. Our previous work indicated that cotinine improves prepulse inhibition of the auditory startle response in rats in pharmacological impairment models and that it improves working memory in non-human primates. Here we tested the hypothesis that cotinine improves sustained attention in rats and attenuates behavioral alterations induced by the glutamate (NMDA) antagonist MK-801. The effects of acute subcutaneous (dose range 0.03–10.0 mg/kg) and chronic oral administration (2.0 mg/kg/day in drinking water) of cotinine were evaluated in fixed and variable stimulus duration (VSD) as well as variable intertrial interval (VITI) versions of a five choice serial reaction time task (5C-SRTT). The results indicated only subtle effects of acute cotinine (administered alone) on performance of the 5C-SRTT (e.g., decreases in timeout responses). However, depending on dose, acute treatment with cotinine attenuated MK-801-related impairments in accuracy and elevations in timeout responses, and it increased the number of completed trials. Moreover, chronic cotinine attenuated MK-801-related impairments in accuracy and it reduced premature and timeout responses when the demands of the task were increased (i.e., by presenting VSDs or VITIs in addition to administering MK-801). These data suggest that cotinine may represent a prototype for compounds that have therapeutic potential for neuropsychiatric disorders (i.e., by improving sustained attention and decreasing impulsive and compulsive behaviors), especially those characterized by glutamate receptor alterations.
Keywords: Attention, schizophrenia, impulsivity, compulsivity, nicotinic
1. Introduction
The neuropharmacological and behavioral properties of the tobacco alkaloid nicotine have been investigated extensively; however, the most predominant metabolite of nicotine in humans and other mammalian species, cotinine (1-methyl-5-[3-pyridynl]-2-pyrrolidinone), has received considerably less attention. Cotinine has a long pharmacological half-life in humans with a range of ~15–20 hr (depending on the body fluid analyzed) relative to nicotine which has a range of ~30 min-3 hr [1–5]. Thus, after nicotine consumption, cotinine levels in vivo greatly exceed that of nicotine over time [6]. The comparatively lower potency of cotinine (i.e., when compared to nicotine) in a number of early experiments may have contributed to the relatively low level of interest in the compound, particularly in its neuropharmacological and behavioral effects. For example, cotinine was found to have very low potency in producing cardiovascular or respiratory effects [7], in altering electroencephalogram activation, or producing behavioral arousal [8], and it did not generalize to the discriminative stimulus properties of nicotine [9]. In nicotinic acetylcholine receptor (nAChR) binding assays, cotinine was also found to be approximately 100–1000 fold less potent than nicotine at displacing radiolabeled nAChR ligands in rodent brain preparations [10–13]. Moreover, in functional assays, cotinine was approximately 30–50 fold less potent than nicotine in evoking dopamine release in brain slices or synaptosomes [14].
A number of more recent studies, however, indicate that cotinine's pharmacological effects may be worthy of further investigation. For example, cotinine protects against toxic insults in PC12 cells with potency similar to that of nicotine [15], it suppresses the release of oxygen free radicals from neutrophils [16], and it augments PI3K-dependent anti-inflammatory pathways in human monocytes [17]. Cotinine has also been shown to selectively activate a subpopulation of α3/α6β2 nAChRs in monkey striatum, an effect that could prove useful for the future development of Parkinson's disease therapies [18]. Most recently, chronically administered cotinine was found to prevent memory loss in transgenic (Tg) 6799 Alzheimer's disease mice as well as to stimulate the Akt/GSK3β pathway and reduce Aβ aggregation in their brains [19].
Several years ago we made a relevant observation when conducting a series of experiments that were designed to determine the effects of nicotine on Delayed-Match to Sample (DMTS) performance in monkeys. When the doses of nicotine were optimized for individual monkeys (i.e., for positive effects on DMTS performance), the pro-cognitive effects lasted until the following day's testing session (i.e., 24 hr later) without any additional nicotine administration [20, 21]. Given the relatively short half life of nicotine, this observation suggested that a metabolite of nicotine (e.g., cotinine) might be contributing to the sustained pro-cognitive effects. Accordingly, we subsequently evaluated cotinine in several behavioral assays in non-human primates and rodents for potential effects on information processing and cognition. In these experiments, cotinine (in monkeys) elicited dose-dependent improvements of a DMTS task as well as a modified version of the task (DMTS-D) where randomly-presented (task-relevant) distractors were presented [22]. Cotinine also attenuated deficits in DMTS produced by the glutamate NMDA receptor antagonist ketamine [23]. In rats, cotinine was evaluated for its ability to improve prepulse inhibition (PPI) of the acoustic startle response, a property that may predict the efficacy of compounds as antipsychotic agents as well as cognitive enhancers. In this study, PPI was disrupted in Wistar rats in three pharmacologic models: dopamine receptor agonism by apomorphine, NMDA receptor antagonism by MK801, or muscarinic acetylcholine receptor antagonism by scopolamine. Cotinine (depending on the dose) improved PPI deficits in all three PPI disruption models [22].
Collectively, the results described above indicated that cotinine improves information processing and memory-related task performance in animal models. Given the reported safety profile of cotinine compared to nicotine in humans especially on blood pressure, heart rate and the electrocardiogram [24], the results indicate that cotinine might serve as a prototypical agent for the treatment of dementia and/or psychiatric illnesses such as schizophrenia. The objective of the experiments described here was to test the hypothesis that cotinine improves sustained attention in rodents and attenuates behavioral alterations induced by the glutamate (NMDA) antagonist MK-801 (i.e., studies potentially relevant to schizophrenia symptomatology and therapeutics). The effects of acute and chronic cotinine administration on the performance of a five choice serial reaction time task (5C-SRTT) were evaluated.
2. Materials and Methods
2.1. Chemicals and Reagents
(−)-Cotinine was obtained from Sigma-Aldrich (St. Louis, MO) and TRC (Toronto Research Chemicals, Inc), North York, Ontario as crystals. MK-801 was obtained from Sigma-Aldrich (St. Louis, MO). Deuterated internal standard (±)-Cotinine-D3 solution (1mg/mL in methanol) was obtained from Cerilliant (Round Rock, TX). Sodium hydroxide, sodium chloride and ammonium acetate were bought from Baker (Phillipsburg, NJ). Formic acid was from Sigma (St. Louis, MO). Acetonitrile, ethyl acetate and water were obtained from Fisher (Pittsburgh, PA) as HPLC/ACS grade.
2.2. Test Subjects
Male albino Wistar rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN, USA) approximately 2 months old were housed individually in a temperature controlled room (25°C), maintained on a 12:12h normal light-dark cycle (lights on at 6AM) with free access to water and food during the first week. From week 2 until the end of the study animals were food restricted to approximately 85% of their age-dependent, free-feeding weights based upon Harlan Laboratories growth rate curves. All procedures employed during this study were reviewed and approved by the Georgia Health Sciences University Institutional Animal Care and Use Committee and are consistent with AAALAC guidelines. Measures were taken to minimize pain and discomfort in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised 1996.
2.3 Behavioral Experiments
Study subjects were transferred (in their home cages) to the behavioral testing rooms each morning approximately 30 min before the beginning of experiments. Training and testing in the Five Choice Serial Reaction Time Task (5C-SRTT) was conducted using six ventilated, sound attenuated operant chambers (Med Associates, St. Albans, VT, USA) as we have described previously [25]. Each operant chamber consisted of 9 nose pokes/apertures, 4 of which were closed off with metal inserts, leaving every other nose poke available (2.5cm wide, 4cm deep). The apertures were arranged on a curved panel 2 cm above the floor of the chamber and were equipped with a photocell beam to detect nose pokes. There was a lamp (2.8W) on the rear wall of each aperture that could be illuminated randomly and for varying durations. Food pellets were delivered automatically to a magazine on the opposite wall to the nose pokes. A light inside the food magazine was also turned on to indicate that a pellet (45mg chow pellet, BioServ, Frenchtown, NJ, USA) had been dispensed. The food magazine was equidistant from all nose poke apertures. There was a house light that remained on for the entire session unless an error or omission occurred; the light was located towards the roof of the operant chamber above the magazine. The apparatus was controlled using MedPC software (Med Associates, St. Albans, VT, USA).
Week 1 consisted of handling animals to reduce stress and anxiety in preparation for training and testing. Week 2 consisted of handling and food restriction. Week 3 consisted of food restriction, habituation to the apparatus and preparation for training. Specifically, on days 1 and 2 of week 3 subjects were placed in the operant chambers for 15 minutes with the house light and magazine light on and 10 pellets in the magazine dispenser. On days 3–5 of week 3 animals performed a non-spatial training program. Briefly, the animals were placed in the 5C-SRTT operant chamber with the house light illuminated. A pellet was released into the food magazine which was simultaneously illuminated for a maximum of 5 sec or until the animal collected the pellet. After a 10 sec interval another pellet was released from the simultaneously illuminated food magazine. This continued until the 15 min habituation period expired.
Spatial training in the task began on day 1 of week 4. Animals began with the stimulus duration of 10 sec, each session being 100 trials or 30 min in duration. An initial pellet was delivered to the magazine to facilitate the start of each session. One of the 5 nose poke apertures was illuminated randomly for 10 sec after which the light was extinguished. The animal was then required to respond correctly by nose poking the previously illuminated aperture within 5 sec of the light being extinguished. A correct response in the previously established time frame (5 sec) resulted in a pellet being dispensed into the magazine that was simultaneously illuminated for a maximum of 5 sec or until the animal retrieved the pellet. Collection of this pellet initiated the intertrial interval, a delay of 5 sec, before the next trial began. An incorrect response, premature response, or failure to respond (omission) resulted in a 5 sec timeout, marked by the extinction of the house light for 5 sec and no food reward, after which the animal initiated the next trial by a nose poke into the magazine. Animals were trained 5 days a week until they reached stable performance levels at the 10 sec stimulus duration (stable performance criterion defined as 2 consecutive days at >80% accuracy, <20% omissions and completion of all 100 trials). Once this criterion was achieved the animals were moved to the next, more challenging stimulus duration and the same performance criterion was applied before the next progression. The following stimulus durations were employed: 5 → 2.5 →1.25 → 1.0 → 0.8 → 0.6 → 0.5 seconds. The animals were required to meet criterion for a minimum of 3 days at the 0.5 second stimulus duration prior to being moved to the testing groups. Reaching criterion at the 0.5 second stimulus duration required on average 43.8±2.7 sessions. After these training criterion were achieved, rats were tested in a protocol that used a fixed stimulus duration (0.5 sec) and intertrial interval (5.0 sec) or protocols that used the pseudorandom presentation of variable stimulus durations (VSD, 0.1, 0.25 and 0.5 sec) or variable intertrial intervals (VITI, 1.0, 5.0, and 10.0 sec).
The following parameters were measured to assess performance: % correct = [# correct /(# correct + # incorrect)]×100; % omissions = [# omissions/(# trials completed)]×100; # of premature responses (impulsivity) = the # of responses made after a trial began, but before onset of the light stimulus (i.e., during the 5 second intertrial interval); # of perseverative responses (compulsivity) = the # of nose pokes made after the correct response was made (i.e., in same aperture), but before collecting the reward. # of timeout responses = the # of nose pokes made in any aperture during a timeout period; Trials completed = (# correct + # incorrect + # omissions); Latency to correct = time elapsed from the onset of the light stimulus to making the correct nose poke response; Latency to incorrect = time elapsed from the onset of the light stimulus to making the incorrect nose poke response; Latency to reward = time elapsed from making a correct nose poke response to retrieving the food reward from the magazine.
2.4 Drug Administration
MK-801 Dose-Effect and Acute Cotinine Studies
For acute experiments where the effects of MK-801 alone were evaluated and subsequently, the ability of cotinine to attenuate the effects of MK-801 was assessed, a total of 33 rats were trained to meet the performance criterion described above. Using a crossover design, subjects (N=11–13) were initially treated with vehicle (saline) or one of three doses of MK-801 (dissolved in vehicle) s.c., 10 min before testing. After the MK-801 dose-effect analysis, cotinine-MK-801 reversal studies were conducted. In these experiments, test subjects (N=6–12) were administered vehicle or cotinine dissolved in vehicle s.c., 30 min before testing followed by either vehicle or MK-801 0.05 mg/kg (dissolved in vehicle) s.c., 10 min before testing. Cotinine doses and vehicle were administered in a pseudorandom manner to obviate any effects associated with the order of drug administration. For the studies in which the acute effects of cotinine (alone) were evaluated, a new cohort of test subjects were trained (N=12), and several doses of cotinine (again administered in a pseudorandom fashion) were dissolved in vehicle and injected s.c., 30 min before testing.
Chronic Cotinine Studies
For chronic dosing experiments, a third cohort of rats (N=12) were trained to meet the aforementioned performance criteria then randomly assigned to cotinine 2.0 mg/kg/day (N=6) or vehicle (normal drinking water, N=6). The total daily dose of cotinine was based on previous rodent autoradiographic studies in our laboratory in which cotinine-related alterations in the densities of α7 nAChRs in rat brain were detected [22]. Oral administration of cotinine in drinking water was based on the average daily fluid consumption and the weight of the animals. A concentrated solution of cotinine (10 mg/ml) was prepared in distilled water and then diluted for individual drug delivery in drinking water bottles. As an example, the rats on average drank approximately 30 ml of water every 24 hrs. For a 0.4 kg rat with a dose of 2.0 mg/kg/day: (2.0 mg/kg × 0.4 kg)/30 ml = a final concentration of 0.027 mg/ml (27 μg/ml) in the drinking water bottle. All rats readily consumed the solution without the need for sweeting agents. The water bottles were changed every 48 hours For the MK-801 reversal experiments in the chronic cotinine studies, test subjects were administered either vehicle or MK-801 0.10 mg/kg (dissolved in vehicle) s.c., 10 min before testing. In these studies, protocols that used the fixed stimulus duration, the fixed intertrial interval version of the task, as well as the presentation of VSDs and VITIs were employed.
2.5. LC-MS/MS Analyses
Plasma and Brain
Plasma and brain samples were collected from the chronically treated rats (N=6) at the end of behavioral testing. For the acute subcutaneous dosing assessment, separate groups of rats (i.e., animals not behaviorally tested, N=3–4) were utilized. Subjects were administered the respective subcutaneous dose of cotinine then anesthetized 30 min later with isofluorane. Subsequently, 3.0 ml of blood was collected via cardiac puncture into a Vacutainer® tube containing potassium EDTA. The blood was centrifuged for 15 min at 2500 × g at 4–5°C and the resulting plasma was frozen at −80°C until analyzed. Brains were removed from the same animals, washed with phosphate-buffered saline and frozen at −80°C until analyzed.
Stability of Cotinine in Drinking Water
To ensure that cotinine was stable in the subjects' drinking water in the chronic administration studies (at the concentrations that were provided to the animals), LCMS analysis of three separate samples of cotinine dissolved in distilled water (30 μg/ml) were analyzed at several time points up to 72 hours.
LC-MS/MS Experimental Details
Solutions-Stock solutions containing 10mg/ml cotinine was prepared in 90% acetonitrile/water (v/v 9/1). Working solutions were obtained by 10-fold serial dilutions from the stock solution. Internal standard was diluted into 1μg/ml dilution by serial dilutions. All standard solutions were stored in 4°C refrigerator.
Samples
As noted above, plasma and whole brains were obtained from cotinine-treated rats. Brains were homogenized in two volumes of distilled water. For samples above the linear range of standard curve, each 100μl of plasma or brain homogenate was diluted by 3ml water. Spiked samples for standard were prepared by spiking 10μl of standard cotinine solutions into each 100μl of (diluted) blank plasma or brain homogenate. All samples were stored in −4°C freezer.
Equipment- LC-MS/MS
analysis was performed by using an Agilent (Santa Clara, CA) Model 1100 binary pump HPLC system interfaced to a triple quadrupole mass spectrometer with ESI(+) source, Micromass (Milford, MA) Quattro Micro. A ZORBAX Rx-Sil, 5μm, 2.1×150 mm, HILIC column (Agilent, Santa Clara, CA) was used for separation of the analyte.
Sample Preparation
To each 100μl of sample, 10μl of 1μg/ml deuterated IS was added together with 40μl of 5M NaOH and 200μl of 2.5M NaCl, which was for protein precipitation. Then 3mL of ethyl acetate was added to the mixture for extraction. 10min of vortex followed by 10min centrifugation at 4000rpm was needed. The upper ester layer was moved into a new tube and evaporated in a centrifuge evaporator. 100μl of 90% acetonitrile/water (v/v 9/1) was added to reconstitute the analyte.
LC Conditions
Mobile phases were 10mM ammonium acetate with 0.1% formic acid (A) and acetonitrile (B). An isocratic elution of 10% A and 90% B was used at the flow rate of 0.3ml/min. Column temperature was 40°C. The method was 5 min in duration and the retention time of the analyte was 3.47~3.52 min.
MS/MS Conditions
Acquisition parameters were: capillary voltage, 3.0kV; cone voltage, 35V; source temperature, 120°C; desolvation temperature, 300°C; desolvation gas flow, 500L/h; cone gas flow, 50L/h. Ions were detected in ESI(+) mode. With the collision energy of 27eV, transitions 177–80 and 180–80 were monitored for cotinine and the deuterated IS respectively.
2.6 Statistical Analyses
For one and two factor comparisons, analysis of variance (with repeated measures when indicated) was used followed by the Student Newman Keuls method for post hoc analysis (SigmaPlot 11.2). A Friedman Repeated Measures Analysis of Variance on Ranks was utilized in one situation when the data did not meet the requirements for normality (see below). When multiple factors were analyzed, a multi-factorial analysis of variance with repeated measures (JMP 5.0 statistical software package) was used. For post hoc comparisons, an orthogonal multi-comparison t-test (Bonferroni corrected) was used to compare individual means. For each figure presented, error values denoted by ± indicates the standard error of the mean. Differences between means from experimental groups were considered significant at the p<0.05 level. Trends toward significance were considered at the p<0.10.
3. Results
3.1. MK-801 Dose-Effect Study
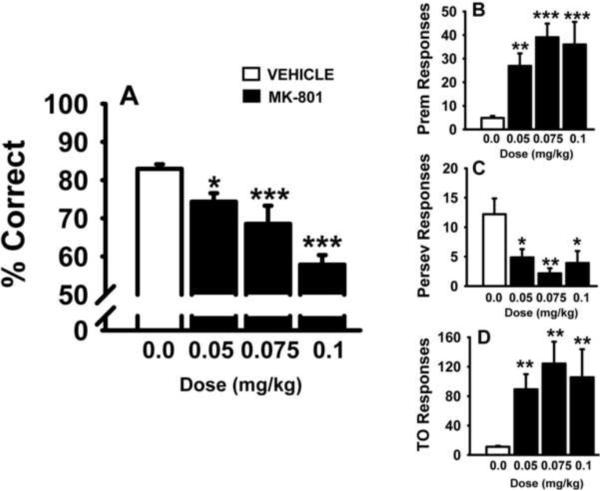
MK-801 was evaluated for dose-related effects on performance in the standard version of the 5C-SRTT (single 0.5 sec SD and 5 sec ITI). The results of these experiments are provided in Fig 1 and Table 1. As illustrated in Fig 1A, all three doses of MK-801 impaired accuracy of the task (p<0.05 versus vehicle). MK-801 also significantly increased the number of premature responses (Fig 1B), decreased the number of perseverative responses (Fig 1C), and increased the number of timeout responses (Fig 1D). The other notable observations were that all 3 doses of MK-801 decreased the number of completed trials and the highest dose of MK-801 significantly increased the number of omissions (see Table 1).
Fig 1.
Dose-related effects of the glutamate (NMDA) antagonist MK-801 on performance of the 5C-SRTT in rats. Rats were trained to meet specific performance criterion (described in the Methods section) at a stimulus duration (SD) of 0.5 sec in the 5C-SRTT. Subsequently, vehicle and several doses of MK-801 (administered s.c., 10 min before testing) were evaluated for effects on 5C-SRTT performance. A. Percent Correct (accuracy); B. Premature Responses (impulsive behavior); C. Perseverative Responses (compulsive behavior); D. Timeout Responses (compulsive behavior/cognitive inflexibility). Each bar represents the mean ± SEM for each test group. For statistical comparisons of following dependent measures, the main effects of dose are presented as follows: accuracy, F(3,56)=15.7, p<0.001; premature responses, F(3,56)=10.8, p<0.001; perseverative responses, F(3,56)=4.8, p<0.01; timeout responses, F(3,56)=6.3, p<0.001. For post hoc results, *(p<0.05); **(p<0.01); ***(p<0.001) =significantly different compared to vehicle-associated performance level. N=11–13
Table 1.
Dose-Effects of MK-801 on Latencies, % Omissions, and Trials Completed in the Standard (Single 5 sec Stimulus Duration) Version of the Five Choice Serial Reaction Time Task
| Treatment | Latency Correct (s) | Latency Incorrect (s) | Magazine Latency (s) | % Omissions | Trials Completed |
|---|---|---|---|---|---|
| Vehicle + Vehicle | 0.92±0.13 | 1.58±0.12 | 1.53±0.11 | 5.39±1.01 | 99.90±0.06 |
| Vehicle + MK-801 0.05 mg/kg | 0.70±0.03 | 1.40±0.10 | 1.24±0.10 | 8.95±2.89 | 73.53±6.79*** |
| Vehicle + MK-801 0.075 mg/kg | 0.79±0.05 | 1.53±0.16 | 1.09±0.07 | 14.55±3.87 | 58.31±7.67*** |
| Vehicle + MK-801 0.10 mg/kg | 0.91±0.08 | 1.53±0.19 | 1.53±0.14 | 41.13±7.92*** | 47.91±4.91*** |
Data are presented as the mean ± SEM.
statistically significant difference (p<0.001) from vehicle -treated group; N=11−13
3.2. Acute Cotinine Studies (effects on MK-801 impairment)
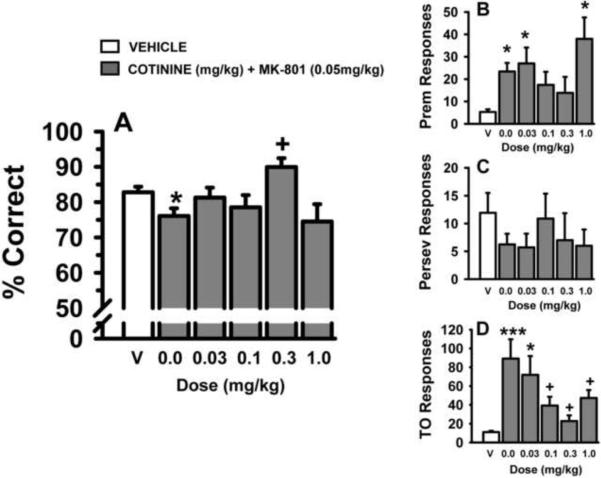
In the standard version of the 5C-SRTT (single 0.5 sec SD and 5 sec ITI), several doses of cotinine were evaluated for the ability to attenuate behavioral alterations induced by the glutamate NMDA antagonist, MK-801 (0.05 mg/kg). The results of these experiments are provided in Fig 2 and Table 2. As indicated in Fig 2A, MK-801 (0.05 mg/kg) impaired accuracy of the task and the 0.3 mg/kg dose of cotinine attenuated this impairment. Likewise, MK-801 increased the number of premature responses (Fig 2B), an effect that was not significantly altered by any of the doses of cotinine. The number of perseverative responses (Fig 2C) was not significantly affected by MK-801 or the combinations of MK-801 and cotinine in this portion of the study. MK-801 also increased the number of timeout responses (Fig 2D), an effect that was significantly attenuated by the 3 highest doses of cotinine. MK-801 also decreased the number of trials completed, and this effect was also attenuated by cotinine (see Table 2). There were no significant drug-related effects on the response latencies or the magazine latencies in this portion of the study (see Table 2).
Fig 2.
Dose-related effects of acutely administered cotinine on MK-801-related impairments in performance of the 5C-SRTT in rats. Rats were trained to meet specific performance criterion (described in the Methods section) at a stimulus durations (SD) of 0.5 sec in the 5C-SRTT. Subsequently, vehicle and several doses of cotinine (administered s.c. 30 min before testing) were evaluated for their ability to attenuate the negative effects of MK-801 (administered s.c., 10 min before testing). A. Percent Correct (accuracy); B. Premature Responses (impulsive behavior); C. Perseverative Responses (compulsive behavior); D. Timeout Responses (compulsive behavior/cognitive inflexibility). Each bar represents the mean ± SEM for each test group. For statistical comparisons of following dependent measures, the main effects of treatment are presented as follows: accuracy, F(5,66)=3.6, p=0.006; premature responses, F(5,66)=4.8, p<0.001; timeout responses, F(5,65)=6.4, p<0.001. The perseverative responses was not significantly affected by MK-801 or the combinations of MK-801 and cotinine in this portion of the study. For post hoc results, *(p<0.05); ***(p<0.001) =significantly different compared to vehicle-associated performance level. +=significantly different (p<0.05) compared to MK-801-associated performance level. N=6–12
Table 2.
Effects of Acute Cotinine and MK-801 on Latencies, % Omissions, and Trials Completed in the Standard (Single 5 sec Stimulus Duration) Version of the Five Choice Serial Reaction Time Task
| Treatment | Latency Correct (s) | Latency Incorrect (s) | Magazine Latency (s) | % Omissions | Trials Completed |
|---|---|---|---|---|---|
| Vehicle + Vehicle | 0.92±0.13 | 1.58±0.12 | 1.54±0.11 | 5.39±1.01 | 99.90±0.07 |
| Vehicle + MK-801 | 0.70±0.03 | 1.40±0.10 | 1.24±0.10 | 8.95±2.89 | 73.53±6.79*** |
| Cotinine 0.03 mg/kg + MK-801 | 0.58±0.04 | 1.11±0.16 | 1.13±0.09 | 2.18±0.096 | 85.14±6.31 |
| Cotinine 0.1 mg/kg + MK-801 | 0.65±0.04 | 1.29±0.20 | 1.29±0.12 | 2.86±1.19 | 92.13±4.01 |
| Cotinine 0.3 mg/kg + MK-801 | 0.70±0.05 | 1.41±0.36 | 1.09±0.17 | 1.34±0.21 | 99.33±0.67+ |
| Cotinine 1.0 mg/kg + MK-801 | 0.62±0.03 | 1.18±0.17 | 1.23±0.08 | 7.01±1.87 | 82.00±6.27* |
MK-801 dose = 0.05 mg/kg. Data are presented as the mean ± SEM.
p<0.05;
p<0.001, statistically significant difference from Vehicle-treated group;
statistically significant difference (p<0.05) from the Vehicle-MK-801-treated group. N=6−12
Cotinine (administered acutely) was subsequently evaluated for the ability to attenuate behavioral alterations induced by varying the stimulus durations in the 5C-SRTT (Supplemental Fig S1). As expected, accuracy in all subjects was reduced with each decrease in stimulus duration, however, there were no statistically significant cotinine-related effects on accuracy (Fig S1A), the total number of premature responses (Fig S1B), or perseverative responses (Fig S1C). There was, however, a significant effect on the number of timeout responses (Fig S1D), Chi-square= 9.62 with 4 degrees of freedom, p<0.05. Post hoc analysis indicated that the number of timeout responses associated with the 10.0 mg/kg dose of cotinine was significantly lower (p<0.05) than that associated with vehicle administration. There were no statistically significant cotinine-related effects on the mean latencies associated with correct responses, incorrect responses, or reward collection (Supplemental Table 1). Likewise, there were no significant effects on the number of omissions, or the number of trials completed.
Cotinine (administered acutely) was also evaluated for the ability to attenuate behavioral alterations induced by varying the intertrial intervals in the 5C-SRTT (Supplemental Fig S2). Statistical analysis indicated that accuracy (Fig S2A) in all subjects was reduced in association with the shortest intertrial interval (1.0 sec), but that there were no treatment-related differences in accuracy. There were also no significant differences in the total number of premature responses (Fig S2B) between any of the doses of cotinine and vehicle. Further, there were no statistically significant cotinine-related effects on the total number of perseverative responses (Fig S2C), or timeout responses (Fig S2D). Likewise, there were no significant ITI- or cotinine-related effects on the response or reward latencies or the number of omissions (Supplemental Table 2). However, the 1.0 mg/kg dose of cotinine (in combination with the VITI) was associated with a decrease in the number of trials completed.
3.3. Chronic Cotinine Studies
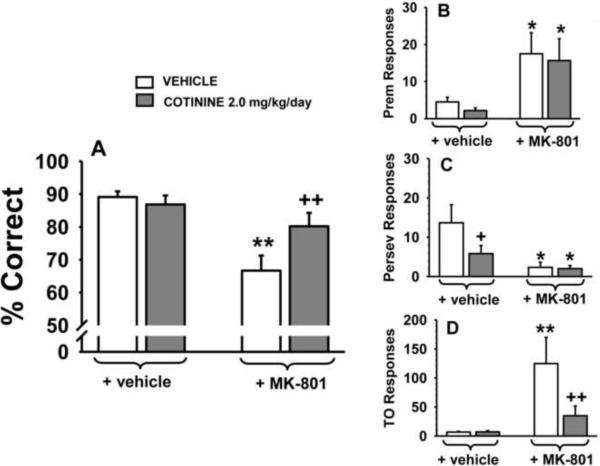
Chronic cotinine administration was also evaluated for its ability to attenuate behavioral alterations induced by the glutamate NMDA antagonist, MK-801 in the standard version of the 5C-SRTT (single 0.5 sec SD and 5 sec ITI). Separate groups of subjects (N=6) were assigned to chronic vehicle-treatment or chronic cotinine treatment and then challenged with acute MK-801 administration. Thus, in this series of experiments both within and between group differences were analyzed. In the accuracy assessment, (Fig 3A) there were significant group-related and treatment-related differences. In the within-group post hoc analyses MK-801 impaired accuracy of the task in the chronic vehicle-treated group (p=0.003), but not in the chronic cotinine-treated group (p=0.20). In addition, in the between-group comparison there was a significant difference in the response to MK-801 (denoted by the + in Fig 3A) indicating that cotinine attenuated the impairing effect of MK-801. As observed in the acute studies, MK-801 also increased the number of premature responses in the chronic studies (Fig 3B), an effect that was not significantly altered by cotinine. In the assessment of the number of perseverative responses (Fig 3C), there were two notable findings: 1) chronic cotinine reduced the number of perseverative responses (p<0.05 versus chronic vehicle rats) and 2) the number of perseverative responses was reduced over time in the vehicle-treated animals (i.e., irrespective of MK-801 treatment). MK-801 also significantly elevated the number of timeout responses (Fig 3D), in the animals chronically treated with vehicle (p<0.01 for the within group analysis), but not cotinine. This positive effect of cotinine was confirmed in the between group analysis (p<0.01 for the comparison between animals administered cotinine + vehicle versus cotinine + MK-801, see the right portion of Fig 3D). As in the acute cotinine studies, in the chronic studies, MK-801 decreased the number of trials completed (70.3±12.9 versus 100.0 in the vehicle-treated animals, see Table 3). While the within group post hoc comparison for cotinine + vehicle versus cotinine + MK-801, was not statistically significant, there was little evidence of a reversal of the MK-801 effect on the number of trials completed in this portion of the study (76.7±8.8. versus 100.0 in the vehicle-treated animals). There were also no significant drug-related effects on the response latencies or the magazine latencies in this portion of the study (data not shown).
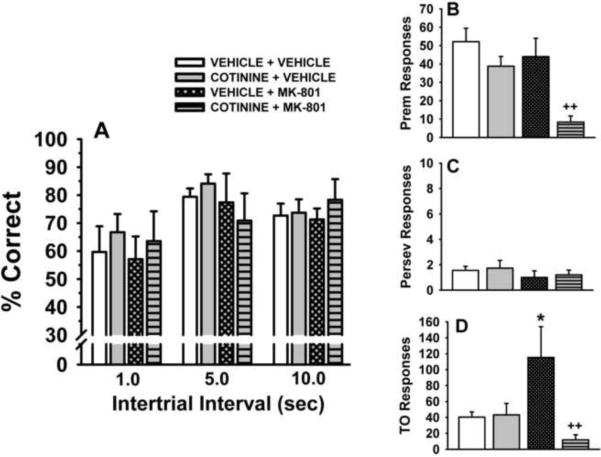
Fig 3.
Effects of chronically administered cotinine on performance of the 5C-SRTT in rats. Rats were trained to meet specific performance criterion (described in the Methods section) at a stimulus durations (SD) of 0.5 sec in the 5C-SRTT. Subsequently, six weeks of administration of vehicle and cotinine (2.0 mg/kg/day) were evaluated for their ability to influence performance of the 5C-SRTT as well as to the attenuate the negative effects of MK-801 (0.05 mg/kg administered s.c., 10 min before testing). A. Percent Correct (accuracy); B. Premature Responses (impulsive behavior); C. Perseverative Responses (compulsive behavior); D. Timeout Responses (compulsive behavior/cognitive inflexibility). Each bar represents the mean ± SEM for each test group. For statistical comparisons of the following dependent measures, results are presented as follows: accuracy assessment, group, F(1,10)=5.6, p=0.03; treatment F(1,8)=15.8, p=0.004, group × treatment interaction F(1,8)=4.0, p=0.08; premature responses, group, F(1,10)=0.24, p=0.63; treatment, F(1,10)=10.6, p=0.009, group × treatment interaction F(1,10)=0.004, p=0.95; perseverative responses, group F(1,10)=2.2, p=0.17, treatment, F(1,10)=9.3, p=0.01, group × treatment interaction F(1,10)=2.3, p=0.16; timeout responses group, F(1,10)=5.4, p=0.041, treatment, F(1,9)=6.0, p=0.036, group × treatment interaction F(1,9)=6.1, p=0.036. For post hoc results, *(p<0.05); **(p<0.01)=significant (within group) difference (i.e., before and after MK-801 challenge within the chronic vehicle-treated group or within the chronic cotinine-treated group); +(p<0.05); ++(p<0.01) =significant (between group) difference in performance (i.e., between the chronic vehicle-treated group and the chronic cotinine-treated group). N=6
Table 3.
Effects of Chronic Cotinine (with and without acute MK-801) on Latencies, Omissions, and Trials Completed in the Variable Stimulus Duration (VSD) Version of the Five Choice Serial Reaction Time Task
| Treatment | Latency Correct (s) | Latency Incorrect (s) | Magazine Latency (s) | % Omissions | Trials Completed |
|---|---|---|---|---|---|
| Vehicle + Vehicle | 0.73±0.03 | 1.55±0.08 | 1.53±0.15 | 5.33±1.76 | 100.00±0.00 |
| Vehicle + MK-801 | 0.72±0.03 | 1.49±0.06 | 1.52±0.12 | 45.00±9.73*** | 44.67±7.44*** |
| Cotinine + Vehicle | 1.06±0.23 | 1.66±0.25 | 1.18±0.10 | 4.94±0.85 | 100.00±0.00 |
| Cotinine + MK-801 | 1.04±0.13 | 1.63±0.16 | 1.18±0.08 | 63.58±8.94*** | 48.20±6.7*** |
Cotinine dose = 2.0 mg/kg/day; MK-801 dose = 0.10 mg/kg. Data are presented as the mean ± SEM.
p<0.001, statistically significant difference from Vehicle-treated group. N=6
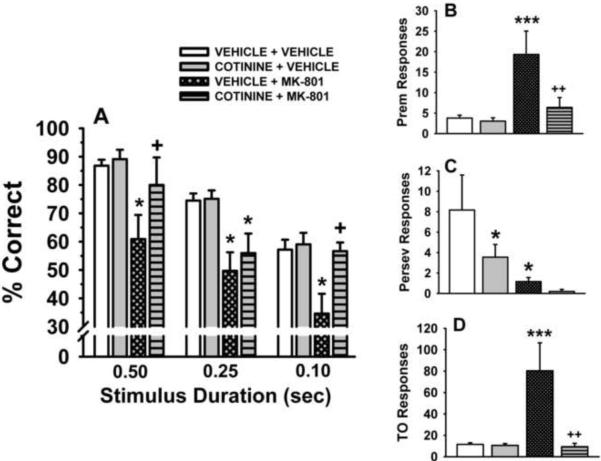
In the final two series of behavioral experiments in the chronic studies, the demands of the task were increased by the presentation of VSD trials, VITI trials, and a combination MK-801 0.1 mg/kg and VSD or VITI trials. The results of these experiments are presented in Figs 4 and 5, respectively. For the VSD accuracy assessment (Fig 4A) the following observations were made: 1) there was a stimulus duration-dependent decrease in accuracy under vehicle conditions, 2) this effect was not altered by chronic cotinine administration, 3) MK-801 significantly (p<0.05) impaired accuracy (relative to control) at all 3 SDs, and 4) chronic cotinine significantly (p<0.05) attenuated the MK-801-related impairments in accuracy at the 0.1 and 0.5 sec SDs. In the assessment of the total number of premature responses when VSDs were presented (Fig 4B), MK-801 was found to significantly elevate premature responses in the animals chronically treated with vehicle (p<0.01 for the within group analysis), but not cotinine (effect confirmed in the between group analysis). In the perseverative response assessment (Fig 4C), chronic cotinine administration significantly reduced the number of perseverative responses compared to chronic vehicle-treated animals. Interestingly, in the within group analysis, MK-801 was also associated with a significant decrease in perseverative responses in the chronic vehicle-treated animals. In the assessment of the number of timeout responses (Fig 4D), MK-801 significantly elevated timeout responses in the animals chronically treated with vehicle (p<0.01 for the within group analysis), but not cotinine. Again this effect was confirmed in the between group analysis. Two other notable observations in this portion of the study (see Table 3) were that the 0.1 mg/kg dose of MK-801 markedly increased the percentage of omissions, (p<0.001) and decreased the number of trials completed (p<0.001). Moreover, chronic exposure to cotinine did not attenuate this effect.
Fig 4.
Effects of chronically administered cotinine in a variable stimulus duration (VSD) version of the 5C-SRTT. Rats were trained to meet specific performance criterion (described in the Methods section) at a fixed stimulus duration (SD) of 0.5 sec. Subsequently, six weeks of administration of vehicle and cotinine (2.0 mg/kg/day) were evaluated for their ability to influence performance of the VSD version of the 5C-SRTT as well as to the attenuate the negative effects of MK-801 (0.1 mg/kg administered s.c., 10 min before testing). A. Percent Correct (accuracy); B. Premature Responses (impulsive behavior); C. Perseverative Responses (compulsive behavior); D. Timeout Responses (compulsive behavior/cognitive inflexibility). Each bar represents the mean ± SEM for each test group. For statistical comparisons of the following dependent measures, results are presented as follows: accuracy assessment, treatment F(3,9)=15.4, p<0.0001, stimulus duration, F(2,43)= 28.4, p<0.0001, treatment by stimulus duration interaction (F(6,43)= 0.82, p=0.56; premature responses, group F(1,10)=4.8, p=0.05, treatment, F(1,9)=7.7, p=0.022, group × treatment interaction F(1,9)=6.5, p=0.031; perseverative responses, group, F(1,10)=9.4, p=0.01, treatment, F(1,9)=1.3, p=0.29, group × treatment interaction F(1,9)=0.43, p=0.53; timeout responses, group F(1,10)=2.9, p=0.12, treatment, F(1,9)=12.2, p=0.007, group × treatment interaction F(1,9)=11.7, p=0.008. For post hoc results, *(p<0.05); ***(p<0.001) =significant (within group) difference (i.e., before and after MK-801 challenge within the chronic vehicle-treated group or within the chronic cotinine-treated group). +(p<0.05); ++(p<0.01) =significant (between group) difference in performance (i.e., between the chronic vehicle-treated group and the chronic cotinine-treated group). N=6
Fig 5.
Effects of chronically administered cotinine in a variable interval trial (VITI) version of the 5C-SRTT. Rats were trained to meet specific performance criterion (described in the Methods section) at a stimulus duration (SD) of 0.5 sec. Subsequently, six weeks of administration of vehicle and cotinine (2.0 mg/kg/day) were evaluated for their ability to influence performance of the VITI version of the 5C-SRTT as well as to the attenuate the negative effects of MK-801 (0.1 mg/kg administered s.c., 10 min before testing). A. Percent Correct (accuracy); B. Premature Responses (impulsive behavior); C. Perseverative Responses (compulsive behavior); D. Timeout Responses (compulsive behavior/cognitive inflexibility). Each bar represents the mean ± SEM for each test group. For statistical comparisons of the following dependent measures, results are presented as follows: accuracy assessment, treatment F(3,9)=0.48, p=0.70, intertrial interval, F(2,41)= 7.65, p<0.002, treatment by intertrial interval interaction (F(6,41)= 0.38, p=0.89; premature responses, group F(1,9)=11.0, p=0.009, treatment, F(1,9)=6.8, p=0.028, group x treatment interaction F(1,9)=2.3, p=0.17; perseverative responses, group F(1,9)=0.12, p=0.74, treatment, F(1,9)=2.4, p=0.16, group × treatment interaction F(1,9)=0.01, p=0.98; timeout responses group F(1,9)=4.2, p=0.07, treatment, F(1,9)=1.1, p=0.33, group × treatment interaction F(1,9)=6.4, p=0.032. For post hoc results, *(p<0.05) =significant (within group) difference (i.e., before and after MK-801 challenge within the chronic vehicle-treated group; ++(p<0.01) =significant (between group) difference in performance (i.e., between the chronic vehicle-treated group and the chronic cotinine-treated group). N=6
For the VITI accuracy assessment in the chronic studies (Fig 5A) statistical analysis indicated that accuracy was lower when the 1.0 sec ITI was presented compared to the 5.0 and 10 sec ITI (under vehicle conditions), and this effect that was not significantly altered by any of the treatment conditions. In addition, when VITIs were presented (Fig 5B), the number of premature responses was lowest in the animals chronically administered cotinine and then challenged with MK-801 (statistically different from the vehicle-vehicle as well as the vehicle-MK-801-related response). For the perseverative response assessment (Fig 5C), there were no group or treatment-related differences detected. When VITIs were presented, MK-801 significantly elevated the number of timeout responses (Fig 5D) in the animals chronically treated with vehicle (p<0.01 for the within group analysis), but not cotinine. This effect was confirmed in the between group analysis (i.e., in the comparison of vehicle + MK-801 and the cotinine + MK-801 treated groups). Finally, there were no significant ITI- or cotinine-related effects on the response or reward latencies (Table 4). The number of omissions was higher in the subjects administered the combination of chronic cotinine and MK-801 (compared to vehicle). However, the total number of trials completed was markedly reduced in the subjects administered vehicle + MK-801 (possibly confounding the ability to accurately assess the number of omissions) and this effect on the total number of trials completed was significantly attenuated in the subjects administered chronic cotinine and MK-801 (again indicating that chronic cotinine is capable of attenuating the deleterious effects of MK-801).
Table 4.
Effects of Chronic Cotinine (with and without acute MK-801) on Latencies, % Omissions, and Trials Completed in the Variable Intertrial Interval (VITI) Version of the Five Choice Serial Reaction Time Task
| Treatment | Latency Correct (s) | Latency Incorrect (s) | Magazine Latency (s) | % Omissions | Trials Completed |
|---|---|---|---|---|---|
| Vehicle + Vehicle | 0.73±0.04 | 1.94±0.24 | 1.34±0.14 | 21.29±3.14 | 56.64±10.67 |
| Vehicle + MK-801 | 0.77±0.09 | 2.05±0.23 | 1.20±0.11 | 33.27±10.22 | 25.83±4.38* |
| Cotinine + Vehicle | 0.79±0.03 | 1.73±0.22 | 1.53±0.12 | 27.62±3.52 | 53.20±7.84 |
| Cotinine + MK-801 | 0.88±0.09 | 1.83±0.24 | 1.35±0.15 | 57.70±8.07* | 66.60±7.57++ |
Cotinine dose = 2.0 mg/kg/day; MK-801 dose = 0.10 mg/kg. Data are presented as the mean ± SEM.
statistically significant difference from Vehicle-treated group.
statistically significant difference (p<0.01). from the VEH-MK-801 treated group. N=6
3.4. LC-MS/MS Analyses
The limit of quantitation (LOQ) for the LC-MS/MS method was 1.0 ng/ml from plasma and 3.0 ng/g from brain tissue, which was adequate for the detection and quantification of cotinine from treated animals. Cotinine concentrations in both plasma and brain tissue from rats administered the lowest effective dose (0.2 mg/kg s.c.) was measured with accuracy and precision of better than 15%. The linear range of the method was wide enough to accommodate either acute or chronic dosing. The calibration curves had excellent linearity (R2>0.998). The method reproducibility was consistent enough to be applied to multiple batches of samples.
Plasma and Brain Cotinine Levels
Plasma and brain levels of cotinine associated with the various dosing schedules are provided in Table 5A. For the subcutaneous route of administration, cotinine was easily detectable and well above the LOQ in both plasma and brain at the 0.1 mg/kg dose. Over the range of doses analyzed (0.1, 1.0, and 10.0 mg/kg) each 10-fold increase in dose was accompanied by an approximate 10-fold increase in cotinine level in both plasma and brain as expected. The ratio of brain to plasma for the subcutaneous route of administration ranged from approximately 0.54 to 0.62. For the oral dosing schedule, in both plasma and brain, the levels of cotinine were lower than would be expected from a plot of the data (and extrapolation) from the subcutaneous route of administration. However, this is not particularly surprising given that drug exposures occurred gradually over a 24 hr period in these subjects, whereas for the acute studies, subjects were sacrificed 30 min after the subcutaneous dose was administered (where peak levels might be considerably higher, and first pass liver metabolism would not be involved). The ratio of brain to plasma for the oral route was similar to the subcutaneous route at approximately 0.63.
Table 5.
LC-MS/MS Analytical Results
| A: Plasma and Brain Levels of Cotinine | ||||
|---|---|---|---|---|
| Dose of Cotinine | Route of Administration | N | Plasma Levels (μg/ml) | Brain Levels (μg/g tissue) |
| 0.1 mg/kg | Subcutaneous (acute) | 3 | 0.147±0.011 | 0.079±0.002 |
| 1.0mg/kg | Subcutaneous (acute) | 4 | 1.200±0.050 | 0.740±0.040 |
| 10.0 mg/kg | Subcutaneous (acute) | 4 | 12.000±0.500 | 7.400±0.600 |
| 2.0 mg/kg/24 hrs | Oral (Chronic) | 6 | 0.963±0.110 | 0.611±0.090 |
| B: Stability of Cotinine in Drinking Water | |||
|---|---|---|---|
| Time Point (hr) | # of Samples Analyzed | Cotinine Concentration (μg/ml) (Added) | Cotinine Concentration (μg/ml) (Detected) |
| 0 | 3 | 30.0 | 30.0±0.4 |
| 6 | 3 | 30.4±0.2 | |
| 12 | 3 | 30.4±0.2 | |
| 24 | 3 | 29.9±0.7 | |
| 48 | 3 | 29.7±1.1 | |
| 72 | 3 | 30.1±0.2 | |
Stability of Cotinine in Drinking Water
The stability of cotinine in distilled water at the concentrations that were provided to the animals in the chronic administration studies is evident in Table 5B. As indicated, cotinine was clearly stable for up to 72 hours with no significant change in concentration. Given that the water bottles were changed every 48 hrs (with freshly prepared cotinine solutions), these results indicate that the solutions were stable for at least 24 hours longer than required.
4. Discussion
The results of this study can be summarized as follows: 1) neither acute nor chronic administration of cotinine significantly affected accuracy in the various versions of the 5C-SRTT, 2) depending on dose, however, acute cotinine attenuated MK-801-related impairments in accuracy and it increased the number of completed trials, 3) chronically administered cotinine attenuated MK-801-related impairments in accuracy and it reduced impulsive- and compulsive-like behaviors (premature responses and timeout responses, respectively) when the demands of the task were markedly increased (i.e., by presenting VSDs or VITIs in addition to administering a higher dose of MK-801). 4) cotinine was easily detected in both plasma and brain from either the subcutaneous or oral (chronic) route of administration at each of the doses that were evaluated, 5) cotinine was found to be stable in water for up to 72 hours, indicating no apparent (stability-related) limitations to the oral dosing approach we used in drinking water.
The ability of cotinine to attenuate MK-801-related deficits in performance of the 5C-SRTT may have several therapeutic ramifications. Glutamate receptor antagonists are commonly used to produce schizophrenia-like symptoms in animals [26, 27] and further, alterations in glutamatergic neurotransmission have been described as especially relevant in schizophrenia patients who demonstrate significant levels of cognitive impairment [28]. Among the variety of cognitive deficits that have been reported in schizophrenia patients, impairments in sustained attention, as assessed by the continuous performance test (CPT), are a relatively consistent observation [29, 30]. Moreover, deficits in sustained attention appear to persist during both actively psychotic episodes and periods of remission. The rodent 5C-SRTT [31] is considered to be analogous to the human CPT [32] and attention and inhibitory response control (thought to be a form of executive functioning) in the 5C-SRTT is indexed by the accuracy measurement. Accordingly, the ability of cotinine to attenuate MK-801-related deficits in accuracy of the 5C-SRTT suggests a potential to improve attention and executive function in schizophrenia. The marked increase in premature responses in animals administered MK-801 was also notable in this study. Premature responses in the 5C-SRTT occur during the inter-trial interval before the target stimulus has been presented and are generally interpreted as a form of impulsive behavior. Perseverative responses only referred to a repeated nose poke into the same aperture after a correct response (as defined by our predetermined criteria) and were (accordingly) generally low in this study. Perseverative responses are generally interpreted as a form of compulsive-like behavior and interestingly, both cotinine and MK-801 (depending on the version of the 5C-SRTT utilized) reduced the total number perseverative responses in some cases. The basis this effect of MK-801 is unclear but may simply reflect the fact that MK-801 tended to decrease the total number of trials completed (thereby decreasing the total number of perseverative responses). The ability of chronic cotinine to attenuate the increases in total timeout responses induced my MK-801 was clear and reproducible across the different versions of the 5C-SRTT utilized. Timeout responses refer to any nose poke during a punished timeout period, and it has been hypothesized that these responses may provide an additional measure of compulsivity and/or cognitive inflexibility [27]. Collectively, the results described above could, therefore, suggest that cotinine might have the potential to improve impulsivity and compulsivity (and/or cognitive inflexibility) in schizophrenia or other neuropsychiatric disorder where glutamate anomalies are present.
The MK-801-related increase in omissions (associated with the higher, 0.1 mg/kg dose) and the decrease in the number of trials completed (associated with both the 0.05 and the 0.1 mg/kg doses) in the current study could, however, suggest that the NMDA antagonist alters motivation and/or locomotor activity (i.e., effects that would confound interpretations related to MK-801 effects on attention, impulsivity, etc). To argue against this possible conclusion, other investigators have demonstrated that changes in motor activity and motivation are dissociated from changes in accuracy and response control [31, 33, 34]. In the current study, we did not observe any alterations in the magazine latency (i.e., the latency to collect food rewards) which has been described as an index of motivation in the 5C-SRTT. It has been suggested that increases in omissions in the absence of changes in magazine latencies are most likely due to gross impairments in attention [31]. In addition, we did not observe any alterations in response latencies, another factor that could (if present) indicate gross effects of MK-801 on locomotor activity, motivation, or other non-cognitive behavior.
In addition to the potential therapeutic ramifications of this study (discussed above), there is another clinically relevant issue: tobacco use. Tobacco use in schizophrenia patients is inordinately high (i.e., at least 2–4 times that of the general population), schizophrenics extract more nicotine per cigarette than do control smokers, and they report a greater reinforcing effect of smoking compared to non-schizophrenic controls [35–37]. These observations have given rise to the notion that smoking in schizophrenia may in part represent an attempt by the afflicted individual to self-medicate some of the cognitive deficits of the disorder or the adverse reactions associated with antipsychotic therapy. There have been a wide range of potential mechanisms discussed for this self-medication hypothesis including pharmacologic interactions of nicotine with dopaminergic, glutamatergic, and cholinergic transmitter systems that might result in an improvement of disease symptoms (see also the aforementioned reviews). As noted above, however, after nicotine consumption, cotinine levels in vivo greatly exceed that of nicotine over time [6]. If the self medication hypothesis is indeed viable, the results of the experiments described here suggest that cotinine (via its relevant pharmacologic interactions, particularly with the glutamatergic system) may at least contribute to amelioration of schizophrenia-related symptoms.
The neurobiological substrate of the behavioral effects of cotinine observed in this study, particularly its ability to attenuate or reverse the effects of the glutamate NMDA antagonist, MK-801 is unclear at present. Cotinine's weak agonist actions at α7- nAChRs [38] may be important given that MK-801 has been shown to be an antagonist at α7-containing nAChRs [39]. In addition, activation of α7-nAChRs triggers action potential-independent glutamate release from axon terminals [40–46]. Moreover, in rats and mice, α7-nAChR activation enhances field stimulation-evoked glutamate currents [43, 44, 47, 48]. However, cotinine is also a low affinity ligand at heteromeric nAChRs including those composed of α3/α6β2 and α4β2-nAChR subunits [11, 12, 13, 18]. Importantly, selective ligands at α4β2-nAChRs (e.g., Sazetidine-A) have also been shown to attenuate impairments in tasks of sustained attention associated with MK-801 [49]. The ability of cotinine to desensitize nAChRs at concentrations that have little or no agonist activity could also be a contributing factor to its overall behavioral pharmacology and the effects observed in this study. Due to the complex and sometimes paradoxical effects of nicotinic ligands (i.e., strikingly similar effects of nAChR agonists and antagonists in some experimental paradigms), nAChR desensitization has been gaining more attention recently as a significant (i.e., therapeutically relevant) aspect of the pharmacology of nicotinic ligands [50, 51].
The final series of experiments were designed to make an assessment of the levels of cotinine achieved in plasma and in the brain after peripheral administration in this study. Steady state plasma concentrations of cotinine reported in human smokers typically range between 200–300 ng/ml [3,4,52], although levels as high as 900 ng/ml have been observed [6]. As indicated in Table 5, the plasma levels detected in our rat studies ranged between 147 and 1200 ng/ml depending on the dose. Thus, in the acute studies where cotinine was evaluated for its ability to attenuate the negative effects of MK-801, the 0.1 and 0.3 mg/kg doses (which were active depending on the outcome measure analyzed) generated cotinine levels in plasma that would be relevant to those observed in most smokers. The levels associated with chronic administration in our rats studies (963 ng/ml) would reflect the highest plasma levels that have been reported in smokers. The efficiency of cotinine at crossing the blood-brain barrier (BBB) has been debated. Notably, one small clinical study using positron emission tomography with [11C]-cotinine found little evidence cotinine penetration through the BBB [53]. Later, cotinine was detected in the brain of experimental animals and in the cerebrospinal fluid of smokers [54, 55] and it was suggested that cotinine detected in the brain was most likely of peripheral origin, since no evidence for nicotine C-oxidation in the brain has been found [55, 56]. In a rat study [57] the brain-to-plasma ratio of cotinine (0.26) after peripheral administration was found to be slightly less than one-third that of nicotine (0.65) reported in another study [58]. It should be noted however, that to date, relatively few studies have been specifically designed to measure cotinine levels in the mammalian brain after peripheral administration of cotinine (as opposed to nicotine). Our study indicates that cotinine readily penetrates the brain from subcutaneous and oral administration with brain to plasma ratios as high as 0.62. In addition, our water stability studies indicate that the oral dosing protocol used for the chronic studies (in drinking water) is a viable approach, and the plasma and brain levels achieved were not limited by degradation of cotinine in drinking water.
There are some potential limitations to this study that should be discussed. As noted above, we did not observe robust effects of cotinine (administered alone either acutely or chronically) in the various versions of the 5C-SRTT. This observation was somewhat perplexing given our previous findings of cotinine-related improvements in performance of a distractor-based (attention) version of a delayed match to sample task in monkeys [22]. Interestingly, while the chronic effects of cotinine on cognitive function in humans have not been evaluated to date, in one acute study in nonsmokers, subtle impairments in verbal recall and reaction times were observed in association with cotinine [59]. In is also important to note, that in our acute rodent studies, only a single dose of cotinine was associated with a statistically significant attenuation of the effects of MK-801 on accuracy (although 3 doses were associated with a reduction in timeout responses). Therefore, the lack of a clear dose-related effect on accuracy may challenge the strength of conclusions related to the acute effects of cotinine on sustained attention (specifically in an NMDA antagonist-impairment model). While more robust effects on accuracy and several other dependent measures (e.g., impulsivity, cognitive flexibility) were associated with chronically administered cotinine, again, this was observed only in the context of attenuating the negative effects of a pharmacological antagonist (MK-801). Pharmacologic impairment models (particularly it the context of cognitive function and modeling complex illnesses such as schizophrenia) have been challenged for their limited level of face and construct validity, as well as the number of false positives generated [reviewed 60, 61]. Finally, while several nicotinic-based compounds have been observed to improve performance of the 5C-SRTT (and other attention-based assays), to date, no nicotinic ligand has clearly been proven to be effective in clinical trials for schizophrenia-related cognitive or attention-related deficits.
In conclusion, the results of this study indicate that cotinine (depending on the dose and length of administration) effectively attenuated MK-801-related impairments in accuracy of the 5C-SRTT and it reduced impulsive- and compulsive-like behaviors when the demands of the task were increased. As noted in the Introduction, we have previously observed that cotinine could also attenuate impairments in prepulse inhibition of the auditory startle response in rats induced by MK-801 as well as deficits in delayed match to sample performance in monkeys induced by the NMDA antagonist, ketamine [23]. Thus, collectively, the results of these rodent and non-human primate studies suggest that cotinine may have the ability to address several key cognitive symptoms that are often observed in schizophrenia: deficits of information processing and sustained attention, impairments in working/short-term memory, as well as increases in impulsivity and compulsivity. Given the superior safety profile of cotinine compared to nicotine in humans [24], the cotinine structure might serve as a platform for novel drug development especially for neuropsychiatric disorders where cognitive function is impaired.
Supplementary Material
Acknowledgments
The authors would like to thank Ms. Ashley Davis for her administrative assistance in preparing this article and Ms Samantha Warner and Ms. Kristy Bouchard for technical assistance. This work was supported by the National Institute of Aging/National Institutes of Health (AG032140).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers hat apply to the journal pertain.
References
- [1].Benowitz NL, Kuyt F, Jacob P., 3rd Circadian blood nicotine concentrations during cigarette smoking. Clin Pharmacol Ther. 1982 Dec;32(6):758–64. doi: 10.1038/clpt.1982.233. [DOI] [PubMed] [Google Scholar]
- [2].Feyerabend C, Ings RM, Russel MA. Nicotine pharmacokinetics and its application to intake from smoking. Br J Clin Pharmacol. 1985 Feb;19(2):239–47. doi: 10.1111/j.1365-2125.1985.tb02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jarvis MJ, Russell MA, Benowitz NL, Feyerabend C. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health. 1988 Jun;78(6):696–8. doi: 10.2105/ajph.78.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Benowitz NL, Jacob P., 3rd Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994 Nov;56(5):483–93. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- [5].Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- [6].Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005 Mar;57(1):79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- [7].Borzelleca JF, Bowman ER, McKennis H., Jr Studies on the respiratory and cardiovascular effects of (−)-cotinine. J Pharmacol Exp Ther. 1962 Sep;137:313–8. [PubMed] [Google Scholar]
- [8].Yamamoto KI, Domino EF. Nicotine-induced EEG and behavioral arousal. Int J Neuropharmacol. 1965 Nov;4(6):359–73. doi: 10.1016/0028-3908(65)90016-x. [DOI] [PubMed] [Google Scholar]
- [9].Rosecrans JA. Nicotine as a discriminative stimulus to behavior: its characterization and relevance to smoking behavior. NIDA Res Monogr. 1979 Jan;(23):58–69. [PubMed] [Google Scholar]
- [10].Abood LG, Reynolds DT, Booth H, Bidlack JM. Sites and mechanisms for nicotine's action in the brain. Neurosci Biobehav Rev. 1981 Winter;5(4):479–86. doi: 10.1016/0149-7634(81)90018-x. [DOI] [PubMed] [Google Scholar]
- [11].Sloan JW, Todd GD, Martin WR. Nature of nicotine binding to rat brain P2 fraction. Pharmacol Biochem Behav. 1984 Jun;20(6):899–909. doi: 10.1016/0091-3057(84)90015-7. [DOI] [PubMed] [Google Scholar]
- [12].Anderson DJ, Arneric SP. Nicotinic receptor binding of [3H]cytisine, [3H]nicotine and [3H]methylcarbamylcholine in rat brain. Eur J Pharmacol. 1994 Mar 3;253(3):261–7. doi: 10.1016/0014-2999(94)90200-3. [DOI] [PubMed] [Google Scholar]
- [13].Vainio PJ, Tuominen RK. Cotinine binding to nicotinic acetylcholine receptors in bovine chromaffin cell and rat brain membranes. Nicotine Tob Res. 2001 May;3(2):177–82. doi: 10.1080/14622200110043095. [DOI] [PubMed] [Google Scholar]
- [14].Dwoskin LP, Teng L, Buxton ST, Crooks PA. (S)-(−)-cotinine, the major brain metabolite of nicotine, stimulates nicotinic receptors to evoke [3H]dopamine release from rat striatal slices in a calcium-dependent manner. Journal of Pharmacology Experimental Therapeutics. 1999;288:905–11. [PubMed] [Google Scholar]
- [15].Buccafusco JJ, Terry AV., Jr The potential role of cotinine in the cognitive and neuroprotective actions of nicotine. Life Sci. 2003 May 16;72(26):2931–42. doi: 10.1016/s0024-3205(03)00226-1. [DOI] [PubMed] [Google Scholar]
- [16].Srivastava ED, Hallett MB, Rhodes J. Effect of nicotine and cotinine on the production of oxygen free radicals by neutrophils in smokers and non-smokers. Hum Toxicol. 1989 Nov;8(6):461–3. doi: 10.1177/096032718900800605. [DOI] [PubMed] [Google Scholar]
- [17].Rehani K, Scott DA, Renaud D, Hamza H, Williams LR, Wang H, et al. Cotinine-induced convergence of the cholinergic and PI3 kinase-dependent anti-inflammatory pathways in innate immune cells. Biochim Biophys Acta. 2008 Mar;1783(3):375–82. doi: 10.1016/j.bbamcr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- [18].O'Leary KT, Loughlin SE, Chen Y, Leslie FM. Nicotinic acetylcholine receptor subunit mRNA expression in adult and developing rat medullary catecholamine neurons. J Comp Neurol. 2008 Oct 20;510(6):655–72. doi: 10.1002/cne.21833. [DOI] [PubMed] [Google Scholar]
- [19].Echeverria V, Zeitlin R, Burgess S, Patel S, Barman A, Thakur G, et al. Cotinine Reduces Amyloid-β Aggregation and Improves Memory in Alzheimer's Disease Mice. J Alzheimers Dis. 2011 Jan;24(4):817–35. doi: 10.3233/JAD-2011-102136. [DOI] [PubMed] [Google Scholar]
- [20].Buccafusco JJ, Jackson WJ. Beneficial effects of nicotine administered prior to a delayed matching-to-sample task in young and aged monkeys. Neurobiol Aging. 1991 May-Jun;12(3):233–8. doi: 10.1016/0197-4580(91)90102-p. [DOI] [PubMed] [Google Scholar]
- [21].Terry AV, Jr, Buccafusco JJ, Jackson WJ. Scopolamine reversal of nicotine enhanced delayed matching-to-sample performance in monkeys. Pharmacol Biochem Behav. 1993 Aug;45(4):925–9. doi: 10.1016/0091-3057(93)90141-f. [DOI] [PubMed] [Google Scholar]
- [22].Terry AV, Jr, Hernandez CM, Hohnadel EJ, Bouchard KP, Buccafusco JJ. Cotinine, a neuroactive metabolite of nicotine: potential for treating disorders of impaired cognition. CNS Drug Rev. 2005 Autumn;11(3):229–52. doi: 10.1111/j.1527-3458.2005.tb00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Buccafusco JJ, Terry AV., Jr A reversible model of the cognitive impairment associated with schizophrenia in monkeys: potential therapeutic effects of two nicotinic acetylcholine receptor agonists. Biochemical Pharmacology. 2009;78:852–62. doi: 10.1016/j.bcp.2009.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hatsukami DK, Grillo M, Pentel PR, Oncken C, Bliss R. Safety of cotinine in humans: physiologic, subjective, and cognitive effects. Pharmacol Biochem Behav. 1997 Aug;57(4):643–50. doi: 10.1016/s0091-3057(97)80001-9. [DOI] [PubMed] [Google Scholar]
- [25].Middlemore-Risher ML, Buccafusco JJ, Terry AV., Jr Repeated exposures to low-level chlorpyrifos results in impairments in sustained attention and increased impulsivity in rats. Neurotoxicology and Teratology. 2010;32:415–24. doi: 10.1016/j.ntt.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rung JP, Carlsson A, Rydén Markinhuhta K, Carlsson ML. (+)-MK-801 induced social withdrawal in rats; a model for negative symptoms of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29(5):827–32. doi: 10.1016/j.pnpbp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- [27].Amitai N, Markou A. Disruption of performance in the five-choice serial reaction time task induced by administration of N-methyl-D-aspartate receptor antagonists: relevance to cognitive dysfunction in schizophrenia. Biol Psychiatry. 2010 Jul 1;68(1):5–16. doi: 10.1016/j.biopsych.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia, Annu. Rev. Pharmacol. Toxicol. 2002;42:165–79. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- [29].Nuechlerlein KH, Dawson M. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophrenia Bulletin. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- [30].Cornblatt BA, Kielp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophrenia Bulletin. 1994;20:3l–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- [31].Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–80. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- [32].Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Jr., Beck LH. A Continuous Performance Test of brain damage. J Consult Psychol. 1956;20:343–50. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- [33].Carli M, Samanin R. Serotonin2 receptor agonists and serotonergic anorectic drugs affect rats' performance differently in a five-choice serial reaction time task. Psychopharmacology (Berl) 1992;106(2):228–34. doi: 10.1007/BF02801977. [DOI] [PubMed] [Google Scholar]
- [34].Mirjana C, Baviera M, Invernizzi RW, Balducci C. The serotonin 5-HT2A receptors antagonist M100907 prevents impairment in attentional performance by NMDA receptor blockade in the rat prefrontal cortex. Neuropsychopharmacology. 2004 Sep;29(9):1637–47. doi: 10.1038/sj.npp.1300479. [DOI] [PubMed] [Google Scholar]
- [35].Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29(6):1021–34. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- [36].Leonard S, Mexal S, Freedman R. Smoking, Genetics and Schizophrenia: Evidence for Self Medication. J Dual Diagn. 2007 Nov 1;3(3–4):43–59. doi: 10.1300/J374v03n03_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].D'Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2011 Feb 1; doi: 10.1016/j.neuropharm.2011.01.044. PMID: 21288470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Briggs CA, McKenna DG. Activation and inhibition of the human alpha7 nicotinic acetylcholine receptor by agonists. Neuropharmacology. 1998 Sep;37(9):1095–102. doi: 10.1016/s0028-3908(98)00110-5. [DOI] [PubMed] [Google Scholar]
- [39].Briggs CA, McKenna DG. Effect of MK-801 at the human alpha 7 nicotinic acetylcholine receptor. Neuropharmacology. 1996 Apr;35(4):407–14. doi: 10.1016/0028-3908(96)00006-8. [DOI] [PubMed] [Google Scholar]
- [40].Alkondon M, Rocha ES, Maelicke A, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat brain. V. alpha-Bungarotoxin-sensitive nicotinic receptors in olfactory bulb neurons and presynaptic modulation of glutamate release. J Pharmacol Exp Ther. 1996 Sep;278(3):1460–71. [PubMed] [Google Scholar]
- [41].Girod R, Barazangi N, McGehee D, Role LW. Facilitation of glutamatergic neurotransmission by presynaptic nicotinic acetylcholine receptors. Neuropharmacology. 2000 Oct;39(13):2715–25. doi: 10.1016/s0028-3908(00)00145-3. [DOI] [PubMed] [Google Scholar]
- [42].Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996 Oct 24;383(6602):713–6. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- [43].Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001 Jul 19;31(1):131–41. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- [44].Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000 Aug;27(2):349–57. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- [45].Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002 Mar 14;33(6):905–19. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- [46].McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995 Sep 22;269(5231):1692–6. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- [47].Aramakis VB, Metherate R. Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex. J Neurosci. 1998 Oct 15;18(20):8485–95. doi: 10.1523/JNEUROSCI.18-20-08485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Santos MD, Alkondon M, Pereira EF, Aracava Y, Eisenberg HM, Maelicke A, et al. The nicotinic allosteric potentiating ligand galantamine facilitates synaptic transmission in the mammalian central nervous system. Mol Pharmacol. 2002 May;61(5):1222–34. doi: 10.1124/mol.61.5.1222. [DOI] [PubMed] [Google Scholar]
- [49].Rezvani AH, Cauley M, Sexton H, Xiao Y, Brown ML, Paige MA, McDowell BE, Kellar KJ, Levin ED. Sazetidine-A, a selective α4β2 nicotinic acetylcholine receptor ligand: effects on dizocilpine and scopolamine-induced attentional impairments in female Sprague-Dawley rats. Psychopharmacology (Berl) 2011 Jun;215(4):621–30. doi: 10.1007/s00213-010-2161-8. [DOI] [PubMed] [Google Scholar]
- [50].Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008 Apr;84(4):329–42. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Buccafusco JJ, Beach JW, Terry AV., Jr Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exp Ther. 2009 Feb;328(2):364–70. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Williams JM, Ziedonis DM, Abanyie F, Steinberg ML, Foulds J, Benowitz NL. Increased nicotine and cotinine levels in smokers with schizophrenia and schizoaffective disorder is not a metabolic effect. Schizophr Res. 2005 Nov 15;79(2–3):323–35. doi: 10.1016/j.schres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- [53].Halldin C, Någren K, Swahn CG, Långström B, Nybäck H. (S)- and (R)-[11C]nicotine and the metabolite (R/S)-[11C]cotinine. Preparation, metabolite studies and in vivo distribution in the human brain using PET. Int J Rad Appl Instrum B. 1992 Nov;19(8):871–80. doi: 10.1016/0883-2897(92)90173-v. [DOI] [PubMed] [Google Scholar]
- [54].Paulson GW, Olson BL. Can smoking be detected from cerebrospinal fluid? Clin Neuropharmacol. 1995 Aug;18(4):375–6. doi: 10.1097/00002826-199508000-00010. [DOI] [PubMed] [Google Scholar]
- [55].Crooks PA, Li M, Dwoskin LP. Metabolites of nicotine in rat brain after peripheral nicotine administration. Cotinine, nornicotine, and norcotinine. Drug Metab Dispos. 1997 Jan;25(1):47–54. [PubMed] [Google Scholar]
- [56].Hansson E, Schmitterlöw CG. Metabolism of nicotine in various tissues. In: von Euler US, editor. Tobacco Alkaloids and Related Compounds. Pergamon Press; Oxford: 1965. pp. 87–97. [Google Scholar]
- [57].Riah O, Courrière P, Dousset JC, Todeschi N, Labat C. Nicotine is more efficient than cotinine at passing the blood-brain barrier in rats. Cell Mol Neurobiol. 1998 Jun;18(3):311–8. doi: 10.1023/a:1022501131709. [DOI] [PubMed] [Google Scholar]
- [58].Reavill C, Walther B, Stolerman IP, Testa B. Behavioural and pharmacokinetic studies on nicotine, cytisine and lobeline. Neuropharmacology. 1990 Jul;29(7):619–24. doi: 10.1016/0028-3908(90)90022-j. [DOI] [PubMed] [Google Scholar]
- [59].Herzig KE, Callaway E, Halliday R, Naylor H, Benowitz NL. Effects of cotinine on information processing in nonsmokers. Psychopharmacology (Berl) 1998 Jan;135(2):127–32. doi: 10.1007/s002130050493. [DOI] [PubMed] [Google Scholar]
- [60].Marcotte ER, Pearson DM, Srivastava LK. Animal models of schizophrenia: a critical review. J Psychiatry Neurosci. 2001 Nov;26(5):395–410. [PMC free article] [PubMed] [Google Scholar]
- [61].Decker MW. In: Chapter 16 Cognition Models and Drug Discovery, Animal Models of Cognitive Impairment. Levin ED, Buccafusco JJ, editors. CRC Press; Boca Raton (FL): 2006. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.