Abstract
Obesity increases circulating cell-endothelial cell interactions; an early marker of inflammation in laboratory model of sepsis, but little is known about the effect of different adipokines. Adiponectin is an anti-inflammatory adipokine secreted by adipocytes. Adiponectin deficiency is implicated in exaggerated proinflammatory phenotype in both obesity and sepsis via increased proinflammatory cytokine expression. However the effect of adiponectin deficiency on circulating cell-endothelial cell interactions in polymicrobial sepsis is unknown. Furthermore although brain dysfunction in septic patients is a known predictor of death, the pathophysiology involved is unknown. In the current study, we examined the effects of adiponectin deficiency on leukocyte (LA) and platelet adhesion (PA) in cerebral microcirculation of septic mice. Adiponectin deficient (Adipoq−/−: Adko) and background strain C57Bl/6 (wild type (WT)) mice were used. Sepsis was induced using cecal ligation and puncture (CLP). We studied LA and PA in the cerebral microcirculation using intravital fluorescent video microscopy (IVM), blood brain barrier (BBB) dysfunction using Evans Blue (EB) leakage method and E-selectin expression using dual radiolabeling technique in different WT and Adko mice with CLP. Adiponectin deficiency significantly exaggerated LA (WT-CLP:201 ± 17; Adko-CLP: ± 53 cells/mm2; P < 0.05) and PA (WT-CLP:125 ± 17; Adko-CLP:188 ± 20 cells/mm2; P < 0.05) in cerebral microcirculation, EB leakage (WT-CLP:10 ± 3.7; Adko-CLP:24 ± 4.3 ng/g × µl plasma; P < 0.05) and E-selectin expression (WT-CLP:0.06 ± 0.11; Adko-CLP:0.44 ± 0.053 ng/g; P < 0.05) in the brain tissue of the mice with CLP. Furthermore, E-selectin monoclonal antibody (mAb) treatment attenuated cell adhesion and BBB dysfunction of Adko-CLP mice. Adiponectin deficiency is associated with exaggerated leukocyte and PA in cerebral microcirculation of mice with CLP via modulation of E-selectin expression.
INTRODUCTION
Sepsis and septic shock are the tenth leading causes of death in the United States (1). Leukocyte/platelet-endothelial cell interaction is a critical rate limiting step in endothelial dysfunction in sepsis (2,3). Endothelial dysfunction is implicated in tissue damage and organ failure leading to increased mortality in sepsis and septic shock (4,5). Early organ failure in sepsis and septic shock affect lung, heart, liver, kidney, and brain. While respiratory, liver cell, renal, and heart failure are well studied in sepsis, relatively little is known about the sepsis associated brain dysfunction. Recent data indicate that brain dysfunction in sepsis precedes the onset of shock, making it one of the earliest organs to be involved in multiple organ dysfunction syndrome of sepsis (6). Brain dysfunction, especially the blood brain barrier (BBB) dysfunction in sepsis is linked to increased mortality in sepsis (7,8).
Obesity incidence has increased dramatically since the 1990s across the world (9). Evidence suggests that obesity is associated with increased mortality in critically ill patients including those with septic shock (10,11). We have shown previously using a rodent model of sepsis that that obese-septic mice had increased circulating cell-endothelial cell interactions via exaggeration of adhesion molecule expression in the cerebral microcirculation (12–14). Specifically, using cecal ligation and puncture (CLP) model, we have reported that increased BBB dysfunction in obese-septic mice occurs by increased leukocyte (LA) and platelet adhesion (PA) via modulation of P-selectin expression in the brain (12–14). However, the effect of different adipokines on the course of sepsis and septic shock is largely unknown.
Adiponectin is an anti-inflammatory adipokine secreted by adipocytes. Obesity is an adiponectin deficient state (15). Adiponectin deficiency is associated with exaggerated inflammatory response in patients with critical illness including sepsis (16,17). In a rodent model of polymicrobial sepsis, adiponectin deficiency is implicated in development of proinflammatory phenotype (18,19). Furthermore, replacement of adiponectin in adiponectin deficient mice results in attenuation of endothelial dysfunction in aortic endothelial cells, suggesting a protective role for adiponectin in tumor necrosis factor-induced inflammation (19). Adiponectin deficiency is also known to modulate tumor necrosis factor-induced inflammatory changes in the intestinal microcirculation via endothelial cell adhesion molecule expression (20). However the exact effect of adiponectin deficiency on circulating cell-endothelial cell interaction in vivo in a clinically relevant model of polymicrobial sepsis is unknown. Especially, the effect of adiponectin deficiency on cerebral microcirculation in sepsis is unknown.
We hypothesized that adiponectin deficiency may alter BBB dysfunction by increasing leukocyte and PA via adhesion molecule expression in polymicrobial sepsis. In the current study we tested the effect of adiponectin deficiency on the leukocyte/platelet-endothelial cell interaction, adhesion molecule expression and barrier dysfunction in the cerebral microcirculation in rodent sepsis. We found that adiponectin deficiency is associated with increased LA/PA and BBB dysfunction in sepsis. We further observed E-selectin modulated this increased cell adhesion in cerebral microcirculation.
METHODS AND PROCEDURES
Animals
This study was approved by the Institutional Animal Care and Use Committee of the Wake Forest University School of Medicine, Winston-Salem, NC and was performed according to the National Institutes of Health guidelines. Mice with homozygous deficiency for adiponectin (Adko: Adipoq−/−) and background strain of C57Bl/6 (wild type (WT) (n = 5–7/group for each parameter and 5–7 weeks of age) were used. All WT mice were purchased from Jackson Laboratories (Bar Harbor, ME). Breeding pairs for homozygous adiponectin deficient (Adipoq−/−: Adko) mice were obtained from Dr Lawrence Chan, Baylor College of Medicine; Houston TX and mice were bred in Assessment and Accreditation of Laboratory Animal Care approved facility at Wake Forest School of Medicine. Adipoq−/− mice were developed by Dr Chan on C57Bl/6 background. Adipoq−/− and C57Bl/6 mice are known to have identical body weights, similar fat pad weights, plasma lipid, and leptin levels (21). C57Bl/6 mice are used as a background stain for Adipoq−/− mice in literature (18,20,21).
CLP
Sepsis was induced using CLP. This model of sepsis is very well described in literature (12). Briefly, we anesthetized the mice by injecting ketamine hydrochloride (150 mg/kg) and xylazine (7.5 mg/kg) intramuscularly. A two-centimeter midline incision in the abdomen was made; the cecum was isolated, tightly ligated and perforated three times with a 20-gauge needle. A special precaution was taken to ensure that intestinal obstruction was not caused. Using a cotton swab, fecal contents were squeezed to ensure patency and small amount of feces expressed was spread around the cecum. The abdominal incision was closed in two layers (peritoneum and skin) and each mouse then received fluid resuscitation with subcutaneous administration of 1 ml of normal saline.
The Sham-operated mice underwent laparotomy and exteriorization of cecum and fluid resuscitation as described above, without CLP. The abdomen was closed in two layers as described above.
Intravital fluorescent video microscopy (IVM)
Four hours after CLP/Sham surgery, the cerebral microcirculation of the mice was studied, using IVM as described previously (12). Since preliminary experiments revealed no differences in the inflammatory responses at 4 h vs. 6 h after CLP all subsequent experiments were performed using a postinjury period of 4 h (data not shown). The mice were anesthetized using intramuscular ketamine hydrochloride (150 mg/kg i.m.) and xylazine (7.5 mg/kg i.m.). A tracheotomy, femoral arterial and femoral venous cannulations were performed. The head of the mouse was fixed in a stereotaxic frame and a right parietal craniotomy was performed. Dura was left intact as described previously (12–14). A round cover glass was placed on the cranial window with an artificial cerebrospinal fluid filling the space between the cranial window and the cover glass. All the animals were placed on mechanical ventilator and allowed to stabilize for 30 min prior to observation of cerebral postcapillary venules (just prior to penetration into the cerebral cortex) under an upright microscope.
Circulating cell-endothelial cell interactions
In order to visualize interactions between leukocytes/platelets and venular endothelium in the mouse brain, leukocytes were labeled in vivo by injecting intravenous acridine orange (0.05% in 100 µl of phosphate buffered saline). The leukocyte-endothelial interactions were recorded prior to the infusion of platelets. Platelets were labeled ex vivo with carboxyfluorescein diacetate succinimidyl ester (90 µmol/l). This allowed separate monitoring of both cell types in each venule, since platelets were observed after significant acridine orange fluorescence was no longer detected.
The details of the platelet isolation technique are as outlined previously (12). Contamination of the platelet suspension by leukocytes was evaluated from a 25 µl sample to ensure that the leukocyte count did not exceed 0.01%. The platelets (n = 100 × 106) were infused intravenously over 5 min (yielding <5% of the total platelet count) and allowed to circulate for a period of 5 min before videotaping. Evidence suggests that these platelet isolation procedures have no significant effect on the activity or viability of isolated platelets (22).
Postcapillary venules (three to five in number) in each mouse brain were recorded to quantify leukocyte and PA, with 5–7 mice per group. Each vessel recording was analyzed for 1 min. A circulating cell (leukocyte/platelet) was considered to be adherent if it remained stationary for at least 30 consecutive seconds of the 1 min recording. The mean of the average values of leukocyte and PA determined in each mouse were then used to generate a group mean.
Platelets recordings were reanalyzed for the platelet velocity. Only the noninteracting platelets (with the endothelium/leukocyte) were used to assess platelet velocity. Estimates of pseudo-shear rate in venules were obtained using measurements of venular diameter and the maximal velocity of noninteracting and free flowing platelets (Vplt) according to the following formulation: pseudo shear rate = ((Vplt/1.6)/venular diameter) × 8/s (23). Venular wall shear rates (WSRs) are important to elucidate the effect of venular WSRs on leukocyte/PA in cerebral microcirculation of different groups of mice.
Evans blue leakage in the brain
Evans blue (EB) leakage method was used to evaluate the BBB dysfunction as described in literature (13,24). Each mouse received of 2% EB (4 ml/kg) intravenously within 15 min of injury. Each mouse was anesthetized 4 h after initial injury and blood sample was obtained from the left ventricle. The left ventricle then was perfused with normal saline at 110 mm Hg until clear fluid appeared through the right atrial incision. Whole brain tissue was harvested, weighed and homogenized and sonicated in 50% trichloroacetic acid and centrifuged at 10,000 rpm for 20 min. The supernatant obtained was read on spectrophotometer at 610 nm wavelength read against a concentration curve.
The blood sample from left ventricle also was centrifuged and 20 µl plasma was diluted in 980 µl of trichloroacetic acid and then processed and read the same fashion as the brain tissue as stated above. EB leakage was expressed as ng/g brain tissue/µl plasma activity.
In vivo quantification of adhesion molecule expression
The adhesion molecule expression in the brain tissue was quantified using dual radiolabeled monoclonal antibody (mAb) technique as described previously (25). The E-selectin binding mAb (cat. no. 553748; BD Pharmingen, San Jose, CA) was labeled with 125I and the nonbinding control mAb (cat. no. 553926; BD Pharmingen) was labeled with 131I using the iodogen method (25).
The dual radiolabeled mAb procedure was performed 4 h postCLP or Sham surgery. The left external jugular vein and carotid artery were cannulations were performed for venous and arterial access. A mixture of 10 µg of 125I–labeled E-selectin mAb and a dose (0.5–5.0 µg depending on 131I decay) of nonbinding mAb was injected slowly via the jugular venous cannula and allowed to circulate for 5 min. After obtaining a blood sample from the carotid catheter, bicarbonate-buffered saline (pH: 7.4) was exchanged isovolemically by simultaneous infusion of bicarbonate-buffered saline through the jugular vein and withdrawal of blood/buffer from the carotid arterial catheter. Subsequently, after transection of the inferior vena cava at the thoracic level, a copious flushing with bicarbonate-buffered saline through the carotid catheter was performed. Whole brain tissue was harvested and weighed.
The method for calculating E-selectin expression has been described previously (25,26). The 125I (binding mAb) and 131I (nonbinding mAb) activities in different tissues and in 50 µl samples of cell free plasma were counted in a 1480-011 Wizard 3 gamma-counter (Wallac, Turku, Finland), with automatic correction for background activity and spillover. A small aliquot (2 µl) of the radiolabeled mAb mixture was assayed in order to determine total radioactivity of injectate of each labeled mAb. The remaining radioactivity in the syringe used to inject the mixture and the tube used to mix the mAbs were subtracted from the total injected activity. The accumulated activity of each mAb in an organ was expressed as the percent of the injected activity (ng mAb) per gram of tissue. E-selectin expression was calculated by subtracting the accumulated activity per gram tissue of the nonbinding mAb (131I IgG isotype) from the activity of the binding anti-E-selectin mAb (125I 10E9.6). Previous reports suggest, that following radio-iodination mAbs retain their functional activity (26). It has also been shown that constitutive and induced expression of E-selectin are undetectable in different tissue beds of E-selectin deficient mice (27).
Experimental protocol
WT mice (C57Bl/6) and adiponectin deficient (Adko) mice were subjected to Sham or CLP surgery as described. After initial injury, mice were allowed to awaken. All the mice were reanaesthetized for cannulations, tracheotomy, craniotomy (for IVM experiments) or cannulations alone (for dual radiolabeling) or transcardiac perfusion of the normal saline (for EB leakage) and further procedures were performed for a specific read out (cell adhesion, EB leakage or E-selectin expression) at 4 h from the initial injury.
In a separate group of Adko mice, the mice were subjected to CLP and were treated with E-selectin mAb (cat. no. 553748; BD Pharmingen 2 mg/kg body weight) intravenously within 15 min of injury. These mice were then reanesthetized and underwent tracheotomy, femoral arterial and venous cannulations, and craniotomy for IVM experiments or transcardiac perfusion for EB leakage.
Statistical analysis
The data were analyzed using a one-way ANOVA with Fisher’s (post hoc) test in all statistical comparisons. All values are reported as means ± s.e. Statistical significance was set at P < 0.05.
RESULTS
Mean arterial pressure, venular pseudo wall shear rates, arterial pH/PaCO2, and body weight
Venular WSRs indicate the background blood flow in that particular blood vessel and in some studies low WSRs are correlated to the leukocyte adhesion in various models of inflammation (28). Mean arterial pressures (MAP) and venular WSRs in different groups of mice are shown in Table 1. There was a significant decrease of MAP in WT-CLP and Adko-CLP mice compared to their respective Sham counterparts. Difference between the MAP of WT-CLP vs. Adko-CLP mice was not statistically significant. Similarly, the WSR in cerebral postcapillary venules of WT and Adko-CLP mice were significantly lower than in their respective Sham counterparts. Furthermore, the WSR in the cerebral postcapillary venules of Adko-CLP mice were significantly lower than those of the WT-CLP mice, supporting decreased cerebral blood flow in Adko-CLP mice.
Table 1.
Mean arterial blood pressure (MAP), venular wall shear rate (WSR), arterial pH, PaCO2, and body weight in different experimental groups
| Strain | Group | MAP (mm Hg) | WSR (s−1) | Arterial pH | Arterial PaCO2 | Body weight (g) |
|---|---|---|---|---|---|---|
| WT | Sham | 63.33 ± 3.373 | 602 ± 47.49 | 7.40 ± 0.039 | 24.06 ± 2.60 | 22.66 ± 0.49 |
| CLP | 45 ± 0.51* | 271.14 ± 23.49* | 7.34 ± 0.041 | 21.70 ± 2.29 | 24.33 ± 0.61 | |
| Adko | Sham | 61.80 ± 1.59 | 570.72 ± 29.39 | 7.30 ± 0.037 | 24.38 ± 0.39 | 24.15 ± 0.84 |
| CLP | 41± 4.72* | 151 ± 12.74*,** | 7.32 ± 0.038 | 23.33 ± 0.71 | 22.71 ± 0.77 | |
| CLP + E-sel mAb | 49.60 ± 1.60*** | 312.68 ± 55.27*** | 7.33 ± 0.010 | 23.65 ± 0.56 | 22.20 ± 1.02 |
Mean arterial blood pressure (MAP), venular wall shear rate (WSR), arterial blood pH, PaCO2, and body weight in different experimental groups are depicted here. Values are expressed as mean ± s.e.m.
E-sel mAb, E-selectin monoclonal antibody.
P < 0.05 vs. corresponding Sham;
P < 0.05 vs. WT-CLP;
P < 0.05 vs. Adko-CLP.
Body weights, arterial PaCO2, and pH of all the groups of mice were not significantly different from each other as depicted in Table 1. Cerebral hemodynamic is extremely dependent on arterial PaCO2 and pH. In order to be able to evaluate the effect of underlying injury of sepsis without the confounding effects of the same on cerebral microcirculation, all the mice were mechanically ventilated to equalize pH and PaCO2. As shown in the table, pH, PaCO2 in all the groups were not significantly different from each other.
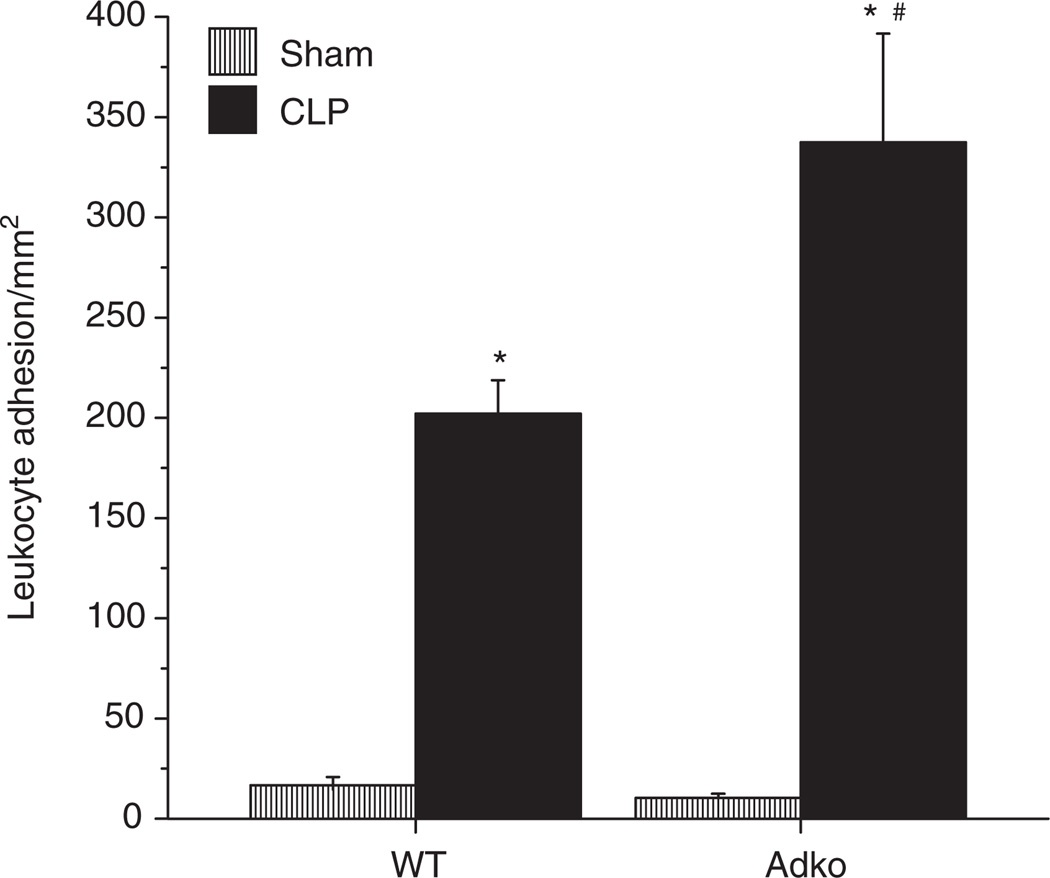
Adiponectin deficiency increases CLP-induced leukocyte adhesion in the cerebral microcirculation
Figure 1 depicts the LA responses in cerebral postcapillary venules of WT and Adko mice with Sham and CLP surgeries. LA in WT and Adko mice with Sham surgery were not significantly different from each other. In both, WT and Adko mice, there was increased number of adherent leukocytes in cerebral microcirculation of postCLP mice compared to their respective Sham counterparts. Moreover, Adko-CLP mice had significantly enhanced LA in cerebral postcapillary venules compared to WT-CLP mice.
Figure 1.
CLP induced leukocyte adhesion in the cerebral microcirculation was significantly increased in adiponectin deficient mice. Intravital fluorescent microscopy showed that WT and Adko mice subjected to CLP had significantly increased leukocyte adhesion when compared to Sham operated controls (*P < 0.05). Adiponectin deficiency significantly exaggerated leukocyte adhesion vs. WT-CLP in the cerebral microcirculation (#P < 0.05). Adko, adiponectin knockout; CLP, cecal ligation and puncture; WT, wild type.
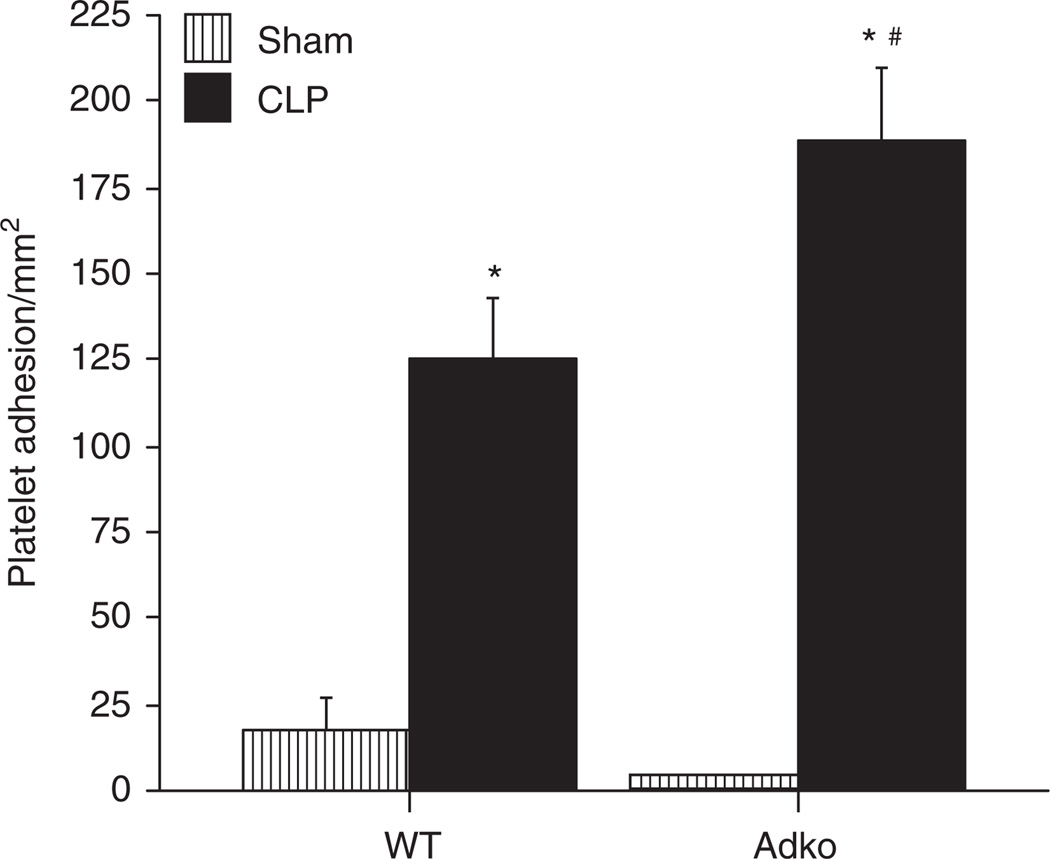
Adiponectin deficiency increases PA in cerebral microcirculation in sepsis
The PA responses in cerebral postcapillary venules of WT mice subjected to Sham and CLP and Adko-CLP mice are shown in Figure 2. Similar to LA response, PA in WT and Adko mice with Sham surgery were not significantly different from each other. In both, WT and Adko mice, there was increased number of adherent platelets in CLP mice compared to their respective Sham counterparts. Moreover, Adko-CLP mice had significantly enhanced PA in cerebral postcapillary venules compared to WT-CLP mice.
Figure 2.
CLP induced platelet adhesion in the cerebral microcirculation was significantly increased in adiponectin deficient mice. Intravital fluorescent microscopy showed that WT and Adko mice subjected to CLP had significantly increased platelet adhesion when compared to Sham operated controls (*P < 0.05). Adiponectin deficiency further exaggerated platelet adhesion response vs. WT-CLP in the cerebral microcirculation significantly (#P < 0.05). Adko, adiponectin knockout; CLP, cecal ligation and puncture; WT, wild type.
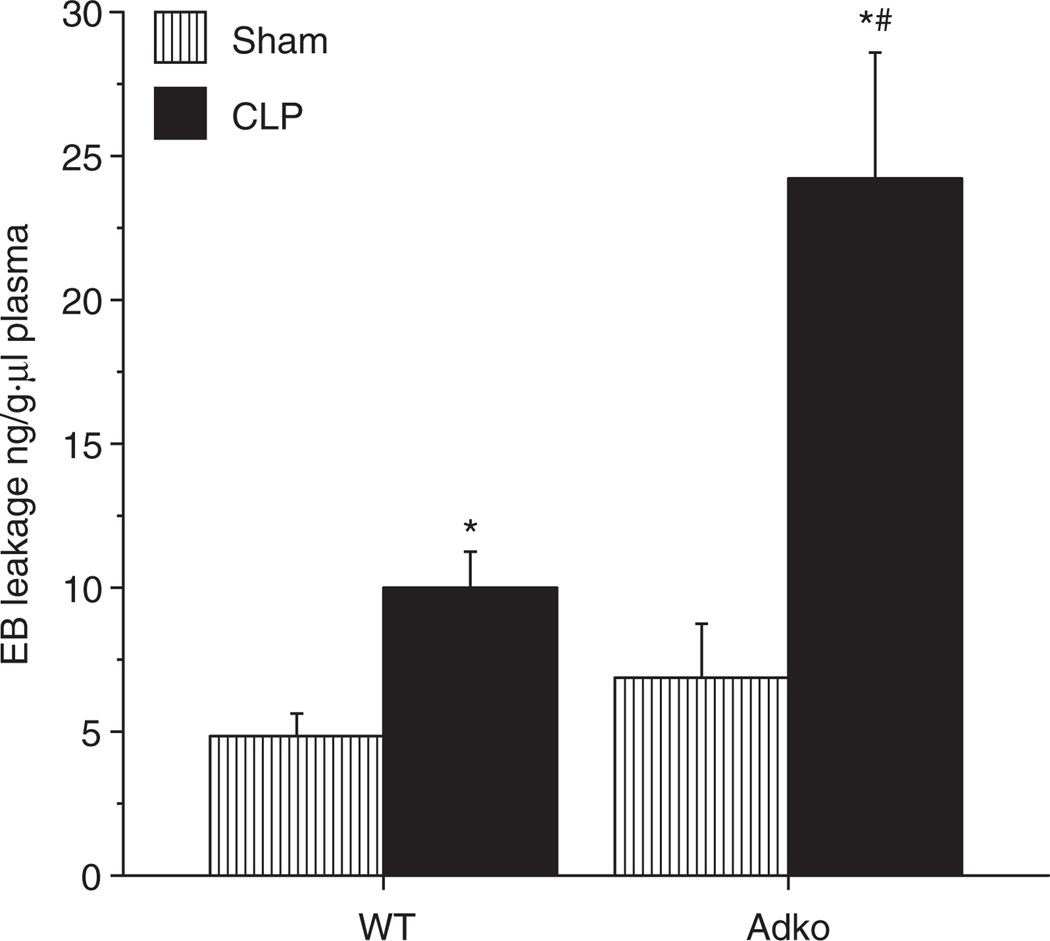
Adiponectin deficiency exaggerates BBB dysfunction in sepsis
BBB dysfunction is implicated in leukoencephalopathy and poor outcomes in septic patients (7). EB leakage method is used for the assessment of BBB dysfunction in sepsis (13,24). We demonstrated BBB dysfunction (using EB leakage) method in WT and Adko mice subjected to Sham and CLP surgeries and results are depicted in Figure 3. EB leakage was increased in both, WT and Adko mice with CLP compared to their Sham counterparts. Moreover, EB leakage was significantly increased in Adko-CLP compared to WT-CLP mice.
Figure 3.
Adiponectin deficiency significantly increased CLP-induced blood brain barrier dysfunction in mice. Evans Blue Dye leakage in WT and Adko mice CLP was significantly increased compared to Sham operated mice (*P < 0.05). CLP-induced dye leakage was significantly increased further in Adko-CLP vs. WT-CLP mice (#P < 0.05). Adko, adiponectin knockout; CLP, cecal ligation and puncture; WT, wild type.
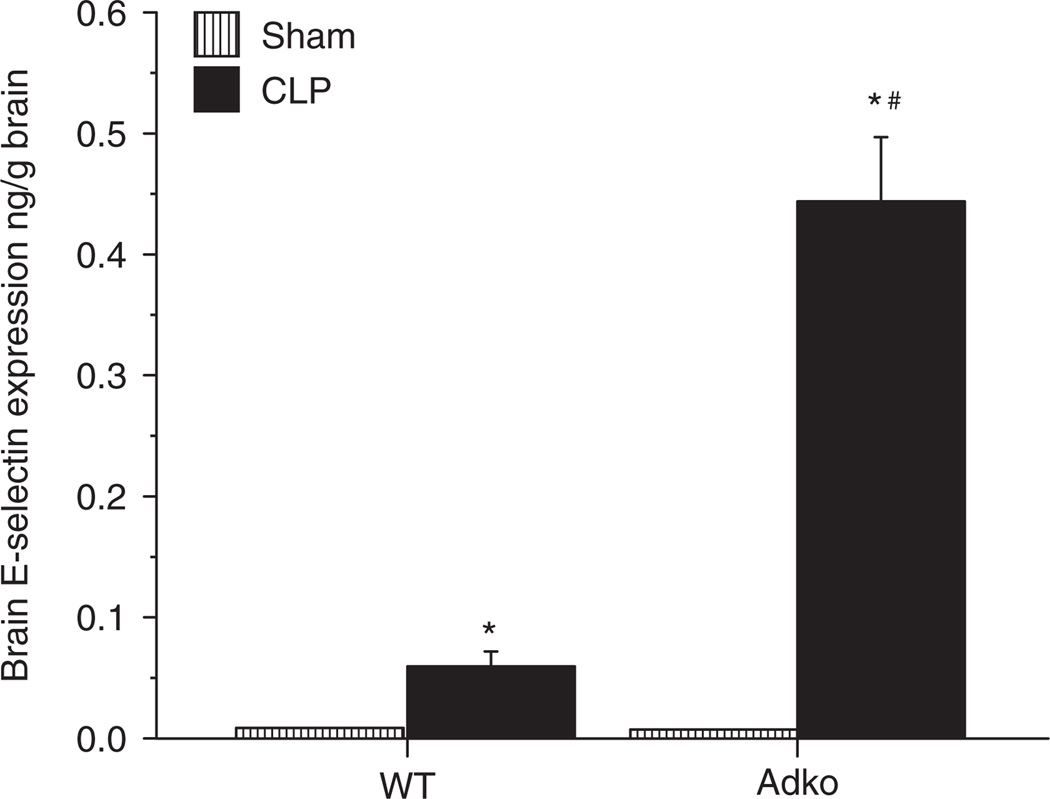
Adiponectin deficiency is associated with increased E-selectin expression in the brain tissue after sepsis
CLP-induced sepsis is associated with increased E-selectin and P-selectin expression (29). To study the effect of adiponectin deficiency on the adhesion molecule expression in the brain in mice with sepsis, we measured the expression of E-selectin and P-selectin in the brain tissue of WT and Adko mice with Sham and CLP. Figure 4 demonstrates the E-selectin expression in the brain tissue of WT and Adko mice subjected to Sham and CLP. In both, WT and Adko strains, there was a significant increase in the E-selectin expression of brain tissue of postCLP mice compared to their respective Sham counterparts. While there was no significant difference between WT and Adko mice with Sham surgery, there was a significant increase in the brain E-selectin expression in Adko-CLP mice compared with WT-CLP mice. However, while there was a significant increase in the P-selectin in the brain tissue of the WT-CLP mice compared to the WT-Sham mice (consistent with our previous work), there was no further increase in the brain P-selectin expression of the Adko-CLP mice compared to either Sham or WT-CLP mice (data not shown).
Figure 4.
CLP induced E-selectin expression in the brain is significantly increased in adiponectin deficient mice E-selectin expression measured with dual radiolabeling technique showed that WT and Adko mice with CLP had significantly increased E-selectin expression in the brain when compared to Sham operated controls (*P < 0.05). Adko-CLP mice showed significantly increased E-selectin expression compared to WT-CLP (#P < 0.05). Adko, adiponectin knockout; CLP, cecal ligation and puncture; WT, wild type.
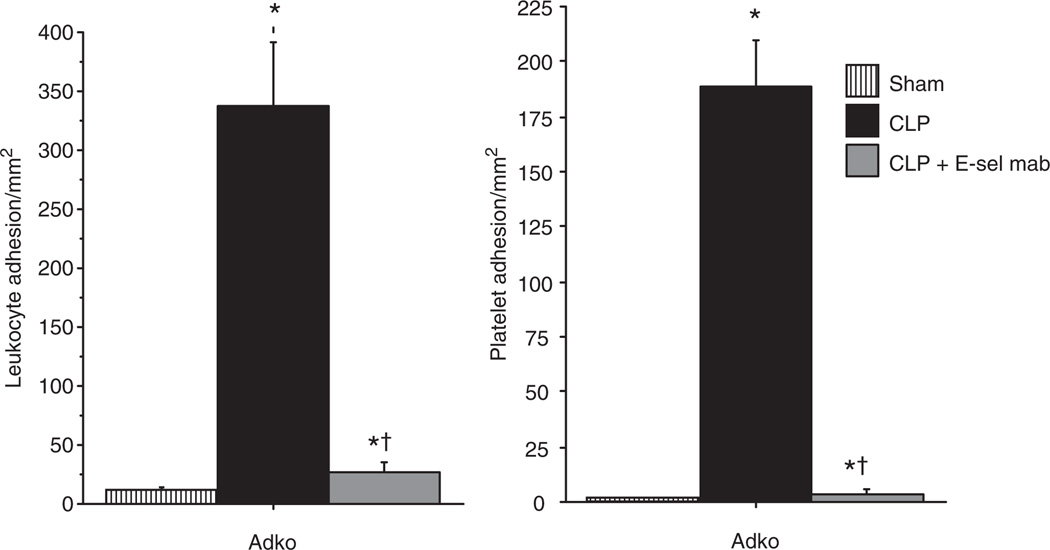
E-selectin monoclonal antibody treatment attenuates leukocyte and PA and BBB dysfunction in the cerebral microcirculation of adiponectin deficient mice with sepsis
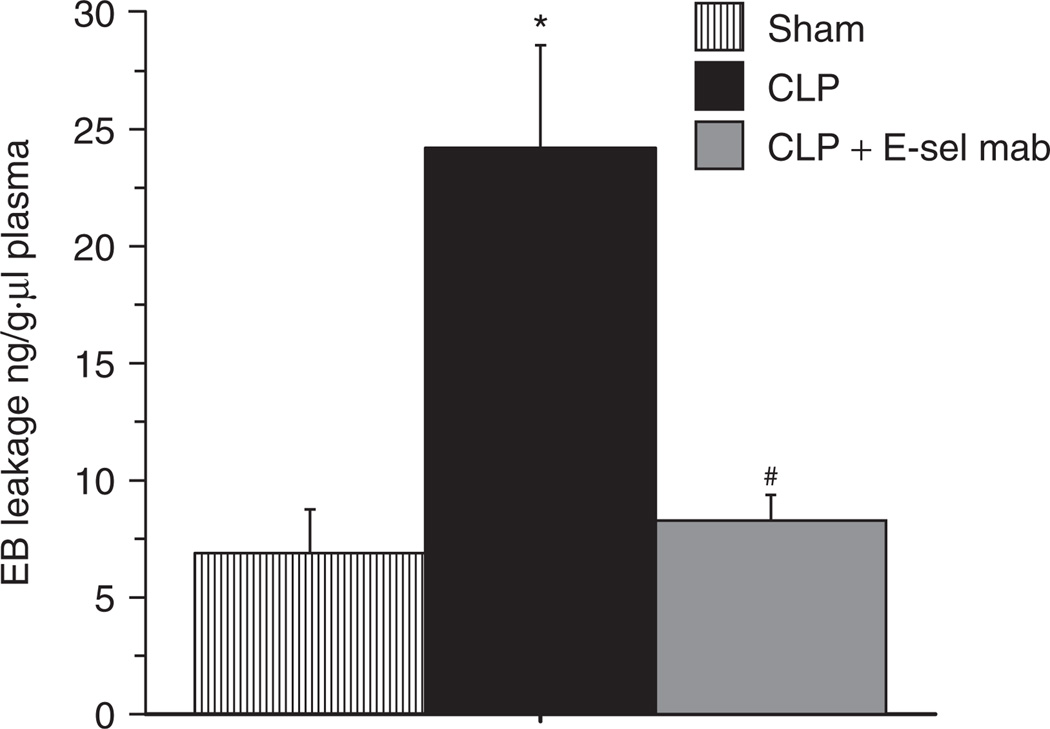
We further studied the effect of adiponectin deficiency on modulation of E-selectin in sepsis, by treatment of adiponectin deficient mice with E-selectin mAb. As shown in Figure 5, there was a marked reduction of LA and PA in the cerebral microcirculation of Adko-CLP mice with E-selectin mAb treatment compared to the untreated mice. Moreover, E-selectin mAb treatment in the Adko-CLP mice showed significantly attenuated EB leakage, indicating decreased BBB dysfunction in the brain as shown in Figure 6. Together, the results from Figures 4–6 indicate that adiponectin modulates the BBB dysfunction via LA and PA by an E-selectin dependent mechanism in the cerebral microcirculation during sepsis.
Figure 5.
E-selectin monoclonal antibody treatment decreased leukocyte and platelet adhesion in the brain of adiponectin deficient mice with CLP. E-selectin monoclonal antibody treatment in Adko-CLP mice lead to significantly decreased leukocyte and platelet adhesion in the brain when compared to Adko-CLP mice without treatment. However, the leukocyte and platelet adhesion in Adko-CLP+E-sel mAb group remained significantly higher than the Adko-Sham group (*P < 0.05 vs. Adko-Sham; †P < 0.05 vs. Adko-CLP). CLP, cecal ligation and puncture.
Figure 6.
E-selectin monoclonal antibody treatment decreased Evans Blue dye leakage in the brain of adiponectin deficient mice with CLP. E-selectin monoclonal antibody treatment in Adko-CLP mice lead to significantly decreased Evans Blue dye leakage in the brain when compared to Adko-CLP mice without treatment (*P < 0.05 vs. Adko-Sham; #P < 0.05 vs. Adko-CLP). There was no significant difference in the EB leakage of Adko-Sham and Adko-CLP + E-sel mAb treatment groups. Adko, adiponectin knockout; CLP, cecal ligation and puncture; E-sel mab, E-selectin monoclonal antibody.
DISCUSSION
Adiponectin is an anti-inflammatory adipokine secreted by adipose tissue. Obesity, a known adiponectin deficient state (15), is associated with increased mortality and morbidity in septic patients (11). We and others have demonstrated that obesity exaggerates microvascular dysfunction in different microvascular beds in rodent model of CLP (12,30). The effect of adipokines individually or in combination in sepsis-induced inflammation needs to be characterized further. Recently published report suggests that adiponectin deficiency and insulin resistance seen in lean septic patients is comparable to that seen in the morbid obesity states (16). Adiponectin deficiency is associated with microcirculatory disturbances in tumor necrosis factor-induced intestinal inflammation and insulin resistance (20), increased levels of proinflammatory cytokine expression and increased mortality in mice with CLP (18,19). Despite these observations, how adiponectin deficiency affects the microcirculation in clinically relevant polymicrobial sepsis is unknown.
LA and PA are the rate limiting steps in the endothelial dysfunction and microcirculation changes in sepsis (2,3). We showed previously that LA/ PA in cerebral microcirculation and BBB dysfunction of mice subjected to CLP are significantly increased compared to the Sham operated mice (13,14). Here we show for the first time to our knowledge, that enhanced LA and PA in the cerebral microcirculation occur in Adko-CLP mice compared to the WT-CLP mice. The mechanisms responsible for this could be complex. One possibility is decreased WSR in the postcapillary venule. Low venular WSR or low blood flow state is shown to increase LA in other models of inflammation (28). We studied the WSRs in our study to elucidate this effect further and WSRs of our groups of mice are shown in Table 1. The lowest venular WSR we report in this study is in Adko-CLP mice (151 ± 12.74−1). Decreased WSR by itself could be a factor for increased leukocyte adhesion in Adko-CLP group. However, we reported previously that in nonseptic mice, the venular WSRs required to induce LA and PA in cerebral microcirculation are lower (51–100/s) (31,13) than the lowest observed group in this study (Adko-CLP: 151 ± 12.74−1), making it less likely to be a major factor.
A major contributor to LA/PA could be changes in selectin expression. We and others reported previously that the adhesion molecule expression is increased in different tissues, including brain in mice subjected to CLP (12,29). In the current study, we measured adhesion molecule (E- and P-selectin) expression in the brain tissue of WT and adiponectin deficient mice with CLP. We showed that there was a significant enhancement of E-selectin expression in the brain tissue of Adko-CLP mice compared to WT-CLP mice. Furthermore Adko-CLP mice, when treated with E-selectin monoclonal antibody, showed a marked reduction of cell adhesion and EB leakage compared to untreated counterparts. These two observations together support the notion that E-selectin plays a major role in CLP-induced cell adhesion and BBB dysfunction in adiponectin deficient mice.
We also studied P-selectin expression in WT and Adko mice with CLP. Consistent with our earlier reports in WT mice, we showed significant increase in P-selectin in the WT-CLP compared to the WT-Sham mice (12,13,32)). However, we did not observe upregulation of P-selectin expression in the brain tissue of Adko-CLP mice compared to either Sham or WT-CLP mice. Differential expression of E-selectin and P-selectin are described in literature (33,34). Our data suggest that adiponectin deficiency modulates E-selectin expression but not P-selectin expression in mouse brain with CLP. The exact mechanism responsible needs further evaluation.
Sepsis-induced BBB dysfunction is described in literature (24) and linked to poor outcomes in septic patients (7). In our previous work, we have shown that the BBB dysfunction is exaggerated in obese-septic compared to lean-septic mice (31). In the current study we demonstrate that the Adko-CLP mice had significantly increased BBB dysfunction compared to the WT-CLP mice. Our current data suggest that adiponectin deficiency seen in obesity may be responsible for at least part of the exaggeration of BBB dysfunction seen in obese-septic mice. We also show that the BBB dysfunction is attenuated in Adko-CLP mice with E-selectin monoclonal antibody treatment compared to untreated group, along with the attenuation in LA/PA. Literature suggests that while adiponectin does not cross the BBB, it decreases proinflammatory cytokine expression by the brain endothelial cells (35).Adiponectin deficiency is known to increase the expression of the same proinflammatory cytokines such as tumor necrosis factor and IL-6 in polymicrobial sepsis (18) that are known to modulate adhesion molecule expression (36). These results together suggest that the adiponectin deficiency exacerbates the LA/PA and subsequent BBB dysfunction via modulation of E-selectin expression which in turn is known to be upregulated by adiponectin deficiency (20).
Adiponectin deficiency is implicated in obesity-induced exaggeration of inflammation (37). In the current study, we show that the sepsis-induced inflammation in adiponectin deficient mice is exaggerated via modulation of E-selectin, an adhesion molecule expressed predominantly by endothelial cells. In the current study, we studied adiponectin deficiency in lean mice, while the impact of adiponectin deficiency in obese mice needs to be studied further. Additionally, while we studied acute inflammation in adiponectin deficient mice, this finding may have an impact in chronic inflammatory states such as Alzheimer’s disease. The impact of adiponectin deficiency on brain dysfunction in chronic inflammatory states needs to be explored further.
In conclusion, adiponectin deficiency may contribute brain dysfunction during sepsis by enhancing LA and PA and in turn BBB dysfunction in cerebral microcirculation. E-selectin expression plays a dominant role in this process, but a role for P-selectin expression is not apparent in our model. These processes may contribute to exaggerated inflammatory phenotype seen in obesity.
ACKNOWLEDGMENTS
We thank Professor Lawrence Chan, Baylor College of Medicine, Houston, TX, for his gift of Adipoq−/− mice. This work was supported by the grant NIGMS: K08GM086470.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Miniño AM, Heron MP, Murphy SL, Kochanek KD. Centers for Disease Control and Prevention National Center for Health Statistics National Vital Statistics System. Deaths: final data for 2004. Natl Vital Stat Rep. 2007;55:118–119. [PubMed] [Google Scholar]
- 2.Jung U, Norman KE, Scharffetter-Kochanek K, Beaudet AL, Ley K. Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J Clin Invest. 1998;102:1526–1533. doi: 10.1172/JCI119893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lush CW, Kvietys PR. Microvascular dysfunction in sepsis. Microcirculation. 2000;7:83–101. doi: 10.1038/sj.mn.7300096. [DOI] [PubMed] [Google Scholar]
- 4.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 5.Marshall JC, Cook DJ, Christou NV et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Taccone FS, Su F, Pierrakos C et al. Cerebral microcirculation is impaired during sepsis: an experimental study. Crit Care. 2010;14:R140. doi: 10.1186/cc9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharshar T, Carlier R, Bernard F, et al. Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med. 2007;33:798–806. doi: 10.1007/s00134-007-0598-y. [DOI] [PubMed] [Google Scholar]
- 8.Sprung CL, Peduzzi PN, Shatney CH, et al. Impact of encephalopathy on mortality in the sepsis syndrome. The Veterans Administration Systemic Sepsis Cooperative Study Group. Crit Care Med. 1990;18:801–806. doi: 10.1097/00003246-199008000-00001. [DOI] [PubMed] [Google Scholar]
- 9.de Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 10.Yaegashi M, Jean R, Zuriqat M, Noack S, Homel P. Outcome of morbid obesity in the intensive care unit. J Intensive Care Med. 2005;20:147–154. doi: 10.1177/0885066605275314. [DOI] [PubMed] [Google Scholar]
- 11.Bercault N, Boulain T, Kuteifan K et al. Obesity-related excess mortality rate in an adult intensive care unit: A risk-adjusted matched cohort study. Crit Care Med. 2004;32:998–1003. doi: 10.1097/01.ccm.0000119422.93413.08. [DOI] [PubMed] [Google Scholar]
- 12.Vachharajani V, Russell JM, Scott KL et al. Obesity exacerbates sepsis-induced inflammation and microvascular dysfunction in mouse brain. Microcirculation. 2005;12:183–194. doi: 10.1080/10739680590904982. [DOI] [PubMed] [Google Scholar]
- 13.Vachharajani V, Vital S, Russell J, Scott LK, Granger DN. Glucocorticoids inhibit the cerebral microvascular dysfunction associated with sepsis in obese mice. Microcirculation. 2006;13:477–487. doi: 10.1080/10739680600777599. [DOI] [PubMed] [Google Scholar]
- 14.Vachharajani V, Vital S, Russell J, Granger DN. Hypertonic saline and the cerebral microcirculation in obese septic mice. Microcirculation. 2007;14:223–231. doi: 10.1080/10739680601139153. [DOI] [PubMed] [Google Scholar]
- 15.Arita Y, Kihara S, Ouchi N et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 16.Hillenbrand A, Knippschild U, Weiss M et al. Sepsis induced changes of adipokines and cytokines - septic patients compared to morbidly obese patients. BMC Surg. 2010;10:26. doi: 10.1186/1471-2482-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkatesh B, Hickman I, Nisbet J, Cohen J, Prins J. Changes in serum adiponectin concentrations in critical illness: a preliminary investigation. Crit Care. 2009;13:R105. doi: 10.1186/cc7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uji Y, Yamamoto H, Tsuchihashi H et al. Adiponectin deficiency is associated with severe polymicrobial sepsis, high inflammatory cytokine levels, and high mortality. Surgery. 2009;145:550–557. doi: 10.1016/j.surg.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Teoh H, Quan A, Bang KW, et al. Adiponectin deficiency promotes endothelial activation and profoundly exacerbates sepsis-related mortality. Am J Physiol Endocrinol Metab. 2008;295:E658–E664. doi: 10.1152/ajpendo.90384.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouedraogo R, Gong Y, Berzins B, et al. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J Clin Invest. 2007;117:1718–1726. doi: 10.1172/JCI29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma K, Cabrero A, Saha PK, et al. Increased beta -oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem. 2002;277:34658–34661. doi: 10.1074/jbc.C200362200. [DOI] [PubMed] [Google Scholar]
- 22.Tailor A, Granger DN. Hypercholesterolemia promotes P-selectin-dependent platelet-endothelial cell adhesion in postcapillary venules. Arterioscler Thromb Vasc Biol. 2003;23:675–680. doi: 10.1161/01.ATV.0000056742.97580.79. [DOI] [PubMed] [Google Scholar]
- 23.Tsujikawa A, Kiryu J, Yamashiro K, et al. Interactions between blood cells and retinal endothelium in endotoxic sepsis. Hypertension. 2000;36:250–258. doi: 10.1161/01.hyp.36.2.250. [DOI] [PubMed] [Google Scholar]
- 24.Esen F, Erdem T, Aktan D, et al. Effect of magnesium sulfate administration on blood-brain barrier in a rat model of intraperitoneal sepsis: a randomized controlled experimental study. Crit Care. 2005;9:R18–R23. doi: 10.1186/cc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eppihimer MJ, Russell J, Anderson DC, Wolitzky BA, Granger DN. Endothelial cell adhesion molecule expression in gene-targeted mice. Am J Physiol. 1997;273:H1903–H1908. doi: 10.1152/ajpheart.1997.273.4.H1903. [DOI] [PubMed] [Google Scholar]
- 26.Panés J, Perry MA, Anderson DC, et al. Regional differences in constitutive and induced ICAM-1 expression in vivo. Am J Physiol. 1995;269:H1955–H1964. doi: 10.1152/ajpheart.1995.269.6.H1955. [DOI] [PubMed] [Google Scholar]
- 27.Eppihimer MJ, Wolitzky B, Anderson DC, Labow MA, Granger DN. Heterogeneity of expression of E- and P-selectins in vivo. Circ Res. 1996;79:560–569. doi: 10.1161/01.res.79.3.560. [DOI] [PubMed] [Google Scholar]
- 28.Russell J, Cooper D, Tailor A, Stokes KY, Granger DN. Low venular shear rates promote leukocyte-dependent recruitment of adherent platelets. Am J Physiol Gastrointest Liver Physiol. 2003;284:G123–G129. doi: 10.1152/ajpgi.00303.2002. [DOI] [PubMed] [Google Scholar]
- 29.Bauer P, Lush CW, Kvietys PR, Russell JM, Granger DN. Role of endotoxin in the expression of endothelial selectins after cecal ligation and perforation. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1140–R1147. doi: 10.1152/ajpregu.2000.278.5.R1140. [DOI] [PubMed] [Google Scholar]
- 30.Singer G, Stokes KY, Terao S, Granger DN. Sepsis-induced intestinal microvascular and inflammatory responses in obese mice. Shock. 2009;31:275–279. doi: 10.1097/SHK.0b013e3181834ab3. [DOI] [PubMed] [Google Scholar]
- 31.Vachharajani V, Vital S, Russell J. Modulation of circulating cell-endothelial cell interaction by erythropoietin in lean and obese mice with cecal ligation and puncture. Pathophysiology. 2010;17:9–18. doi: 10.1016/j.pathophys.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Vachharajani V, Wang SW, Mishra N, et al. Curcumin modulates leukocyte and platelet adhesion in murine sepsis. Microcirculation. 2010;17:407–416. doi: 10.1111/j.1549-8719.2010.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hickey MJ, Kanwar S, McCafferty DM, et al. Varying roles of E-selectin and P-selectin in different microvascular beds in response to antigen. J Immunol. 1999;162:1137–1143. [PubMed] [Google Scholar]
- 34.Wood K, Russell J, Hebbel RP, Granger DN. Differential expression of E- and P-selectin in the microvasculature of sickle cell transgenic mice. Microcirculation. 2004;11:377–385. doi: 10.1080/10739680490437559. [DOI] [PubMed] [Google Scholar]
- 35.Spranger J, Verma S, Göhring I, et al. Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes. 2006;55:141–147. [PubMed] [Google Scholar]
- 36.Bhatwadekar AD, Glenn JV, Curtis TM, et al. Retinal endothelial cell apoptosis stimulates recruitment of endothelial progenitor cells. Invest Ophthalmol Vis Sci. 2009;50:4967–4973. doi: 10.1167/iovs.09-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trevaskis JL, Gawronska-Kozak B, Sutton GM, et al. Role of adiponectin and inflammation in insulin resistance of Mc3r and Mc4r knockout mice. Obesity (Silver Spring) 2007;15:2664–2672. doi: 10.1038/oby.2007.318. [DOI] [PMC free article] [PubMed] [Google Scholar]