Abstract
Background
New HIV-1 infections are increasing in older American women largely through heterosexual transmission. Activated CD4+ T-cells and CCR5 expression are linked to HIV-1 susceptibility, but whether these parameters are altered in the cervix of older women is unknown.
Methods
Whole blood and in some instances endocervical brush samples were collected from healthy premenopausal (n=22) and postmenopausal women (n=24). Percentages of HLA-DR(DR)+CD38(38)+CD4+ T-cells, and HIV-1 chemokine coreceptor expression were determined by flow cytometry.
Results
Percentages of DR+38+CD4+ T-cells were 6-times greater in cervix (median, 6.4%) than blood (median, 1.1%; p<0.001), but did not differ within each compartment between premenopausal and postmenopausal women (p=0.2). Postmenopausal women had greater percentages of CCR5+CD4+ and CCR5+DR+38+CD4+ T-cells compared to premenopausal women in cervix (median, 70% vs. 42%, p=0.005; and 80% vs. 57%; p<0.05, respectively) and blood (medians, 22% vs. 13%, and 76% vs. 62%, respectively; p<0.001). Postmenopausal women had more CCR5 molecules on cervical DR+38+CD4+ T-cells (median, 3,176) than premenopausal women (median, 1,776; p=0.02). Age and percent CCR5+CD4+ and CCR5+DR+38+CD4+-cells were linearly related in cervix (r2=0.47, p<0.001 and r2=0.25, p=0.01, respectively) and blood (r2=0.20, p=0.001 and r2=0.31, respectively; p<0.001), but confounding of age with menopause could not be excluded. Cervical CXCR4 expression did not differ substantially between premenopausal and postmenopausal women.
Conclusions
Elevated cervical CCR5 expression in postmenopausal women may increase their risk for HIV-1 acquisition. Studies are needed to confirm whether elevated CCR5 expression confers increased HIV-1 susceptibility in postmenopausal women, and if it is related to hormonal or nonhormonal effects of aging.
BACKGROUND
Almost half of the 33 million individuals infected with HIV-1 in the world are women, and approximately 300,000 of these women reside in North America.1 Most HIV-1 seropositive women acquired HIV-1 through heterosexual sex, and the majority are of reproductive age.1 Nevertheless, epidemiologic studies suggest that HIV-1 is more readily transmitted heterosexually to older compared to younger women. Specifically, a European study evaluating HIV-1 discordant couples found that women over age 45 years had almost a 4-fold higher risk of HIV-1 acquisition compared to women less than 45 years.2 Less frequent condom use,3 lack of awareness of risk, and difficulty discussing sex with a partner4 may contribute to some of the observed increases in new HIV-1 infections in older women. Nonetheless, the finding that ovariectomized macaques are more susceptible to SIV infection than those with intact ovaries, which is reversed by exogenous estrogen5–6 supports a biologic mechanism. Although thinning of the vaginal and cervical mucosa that occurs with menopause7 has been proposed as a risk factor for retroviral transmission, whether there are other menopause- or age-related effects on the immunologic milieu of the cervix contributing to HIV-1 acquisition in older women is unknown.
Endocervical CD4+ T-cells play a pivotal role in heterosexual transmission of HIV-1. In non-human primates, the endocervix was the first tissue infected after intravaginal inoculation of SIV and the major virus-producing cells were T-cells.8–9 Furthermore, CD4+ T-cells are the first cells to become productively infected with HIV-1 in cervical tissue explants.10–11 In vivo, endocervical CD4+ T-cells are believed to be particularly vulnerable to infection because they are located within or below a single-layer columnar epithelium, whereas ectocervical and vaginal cells lie under a thicker, stratified squamous epithelium.12–13 HIV-1 requires chemokine receptors, either CCR5 or CXCR4, to enter a cell. Cell surface CCR5 expression on CD4+ T-cells is associated with increased HIV-1 susceptibility both in vitro and in vivo.11, 14–16 Co-expression of the activation markers HLA-DR(DR) and CD38(38) on CD4+ T-lymphocytes is also linked to HIV-1 susceptibility.17–18 Whether expression of CCR5 or activation markers on CD4+ T-cells is elevated in the endocervix of postmenopausal women and may contribute to HIV-1 acquisition in this group is unknown.
The present study was undertaken to evaluate whether differences in expression of HIV-1 chemokine coreceptors or activation markers on CD4+ T-cells exist between premenopausal and postmenopausal women. We hypothesized that percentages of activated CD4+ T-cells and expression of CCR5 on CD4+ T-cells are elevated in both the endocervix and blood of healthy postmenopausal compared to premenopausal women.
METHODS
Study Subjects and Clinical Specimens
Premenopausal women with normal menstrual cycles (>26 days, <32 days) and postmenopausal women with no menses for at least twelve months were recruited from the Denver metropolitan area; some were identified by co-enrollment in other studies. Criteria for enrollment included the absence of medical problems, absence of a history of genital ulcer disease, and no use of exogenous sex hormones for at least 6 months. Samples from premenopausal women were collected during the follicular phase (day 1 to 5) and confirmed by serum progesterone and estradiol concentrations (Beckman-Coulter Access II Immunoassay). Informed consent was obtained from all subjects. Studies were approved by the Colorado Multiple Institutional Review Board.
Whole blood and endocervical brush samples were collected by a single investigator. Cervical samples were collected in a subset of subjects because speculum examinations were declined by some subjects and prohibited by some studies in which subjects were co-enrolled. During a speculum examination, first swabs were collected for subsequent evaluation by direct microscopy for clue cells, Trichomonas, and Candida and by nucleic acid amplification for C. trachomatis and N. gonorrhea. Next, cells were collected from the endocervical canal using a cytobrush.19 Herein, endocervical cells will be subsequently referred to as cervical cells. If blood was observed in the vaginal canal, it was removed with cotton swabs as described by others prior to obtaining samples.19 Subjects with a vaginal infection were excluded from analyses.
Flow Cytometry
Within one hour of collection, blood and cervical cells were stained with antibodies to CD3-PEcy5 (BD Biosciences), CD4-APC-H7 (BD Biosciences), CD38-FITC (Invitrogen Life Science), HLA-DR-APC (BD Biosciences), and CCR5-PE or CXCR4-PE (BD Biosciences with known 1:1 PE:antibody ratio). Data were acquired using a LSR II flow-cytometer (BD Immunocytometry Systems) and analyzed using FlowJo (Tree Star). QuantiBRITE beads (BD Biosciences) were used to determine the mean number of CCR5 or CXCR4 molecules (mol) on the surface of lymphocytes as previously described.20 If there were insufficient numbers of cervical cells to stain for both HIV-1 coreceptors, cells were only stained for CCR5.
CCR5 delta 32 Genotyping
DNA was extracted from peripheral blood mononuclear cells using a Qiagen Blood and Tissue Kit andpolymerase-chain-reaction was performed using the following primers: sense, 5′ TGGTGGCTGTGTTTGCGTCTC 3′; antisense, 5′ AGCGGCAGGACCAGCCCCAAG 3′ (Integrated DNA Technologies).21 Results were confirmed by running plasmid DNA for wild type CCR5 and delta 32 CCR5 (gift from Robert W. Doms, M.D., Ph.D., University of Pennsylvania) in parallel.
Statistical Analysis
All statistical analyses assumed a two-sided significance level of 0.05 and were performed using GraphPad Prism 5.04. Fisher’s exact test was used for comparisons of categorical outcomes. Continuous outcomes were compared using nonparametric t-tests (Mann-Whitney, Wilcoxon signed rank). Ordinary least squares regression (SAS, SAS Institute, Inc.) was used to evaluate the relationship between CCR5 expression and age.
RESULTS
Characteristics of Study Subjects
Twenty-four postmenopausal and 22 premenopausal women were included in this study. Consistent with study design the median age of postmenopausal women was 56 years (range, 50–56 years), which was significantly higher than that of premenopausal women (34; range, 23–49 years; p<0.001). Postmenopausal women experienced last menstrual cycle a median of 3.5 years (range, 1–17 years) prior to study. The majority of postmenopausal and premenopausal women (median, 71% and 86%, respectively) were white and there were no significant differences in racial distribution (p=0.23). There were no significant differences in percentages of premenopausal versus postmenopausal subjects heterozygous for the CCR5 delta 32 mutation (median, 23 vs. 13%, respectively; p=0.26).
Activated CD4+ T-cells in Blood and Cervix
Percentages of DR+38+CD4+ T-cells were higher in the cervix (median, 6.4%; 95%CI, 5.2–16%, n=19) than blood (median 1.1%; 95%CI, 1.1–1.7%, n=46, p<0.001). Percentages of DR+38+CD4+ T-cells did not significantly differ in postmenopausal versus premenopausal women in the blood (median, 1.2%, 95%CI, 1.1–1.8%, n=24 vs. median, 1.0%, 95%CI, 0.9–1.7%, n=22; p=0.2) or cervix (median, 4.7%, 95%CI, 0.7–12.9%, n=6 vs. median, 7.4%, 95%CI, 4.6–20.1%, n=13; p=0.2).
HIV-1 Chemokine Coreceptor Expression in Whole Blood and Cervix
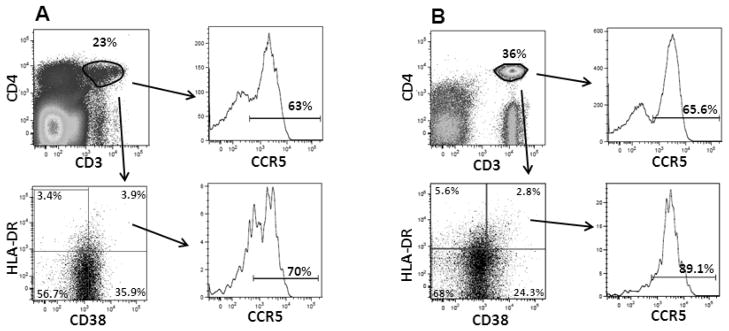
Percentages of HIV-1 chemokine receptor expressing cells as well as density of chemokine receptors were measured on CD4+ T-cells and DR+38+CD4+ T-cells in the whole blood and cervix (Table 1). Figure 1 shows representative flow cytometry plots of CCR5 on cervical and blood CD4+ and DR+38+CD4+ T-cells from one subject. Percentages of CXCR4+ cells were significantly higher than percentages of CCR5+ cells on CD4+ T-cells in blood and cervix, as well as cervical DR+38+CD4+ T-cells. There were no significant differences between the coreceptors in terms of density of expression on CD4+ T-cells in blood or cervix. On activated CD4+ T-cells, CXCR4 density was 1.7-fold higher than CCR5 density in the cervix, whereas CCR5 density was higher than CXCR4 density in the blood. Comparison between CD4+ T-cells and activated CD4+ T-cells revealed that percentages of CCR5+ cells (median, 69%) and density of CCR5 expression (median 4,166 mol/cell) was significantly higher on activated CD4+ T-cells compared to total CD4+ T-cells in blood (medians, 18% and 2,384 mol/cell, respectively; p<0.001). In the cervix, there was a trend of higher percentages of CCR5, but not density, on activated CD4+ T-cells compared to total CD4+ T-cells (p=0.068 and p=0.5). Neither percentages of CXCR4+ cells nor density of CXCR4 differed significantly between total and activated CD4+ T-cells in the blood or cervix (p≥0.4) except that percentages of CXCR4+ cells were lower on activated compared to total CD4+ T-cells in the blood (p<0.001).
TABLE 1.
Chemokine Coreceptor on Total and Activated CD4+ T-cells in Whole Blood and Cervix
| CCR5 (95%CI) | CXCR4 (95%CI) | p value | ||
|---|---|---|---|---|
|
Whole Blood
| ||||
| CD4+ a | % | 18 (17–25) | 84 (78–85) | <0.001 |
| Mol/cell | 2,384 (2,295–2,953) | 2,487 (2,416–2,930) | 0.6 | |
| DR+38+ b | % | 69 (63–73) | 44 (42–54) | <0.001 |
| Mol/cell | 4,166 (3,721–4,923) | 2,396 (2,339–3,641) | 0.012 | |
|
| ||||
|
Cervix
| ||||
| CD4+c | % | 47 (36–56) | 75 (70–83) | <0.001 |
| Mol/cell | 2,224 (2,002–3,872) | 2,724 (2,846–6,650) | 0.3 | |
| DR+38+d | % | 59 (48–70) | 81 (69–86) | 0.016 |
| Mol/cell | 2,045 (1,757–3,584) | 3,416 (3,048–12,620) | <0.001 | |
n=45;
n=43;
CCR5 n=25, CXCR4 n=22;
CCR5+ n=22, CXCR4+ n=20
FIGURE 1.
Representative flow cytometry plots of (A) cervical cells and (B) whole blood acquired from the lymphocyte gate of the forward-scatter versus side-scatter profile. Gates for HLA-DR, CD38, and CCR5 were set with Fluorescence Minus One (FMO) controls. CD3+CD4+ cells were defined in a dot plot then evaluated in a CD38 versus HLA-DR plot. CCR5 expression was evaluated on cells from CD3+CD4+ and CD38+HLA-DR+ quadrants using a histogram. Data were analyzed and compensated using FlowJo Software (Treestar).
For women in whom simultaneous blood and cervical measurements were obtained (Table 2) percent CCR5 expression was 3-fold higher on cervical CD4+ T-cells compared to blood CD4+ T-cells. There were no differences, however, in density of CCR5 molecules or CXCR4 percentages or density between CD4+ T-cells in blood and cervix. CCR5 was highly expressed on activated CD4+ T-cells, over 50% expressing CCR5. Density of CCR5 was lower on activated cervical CD4+ T-cells compared to blood. CXCR4 was expressed by a significantly higher percentage of activated CD4+ T-cells in the cervix compared to blood, but CXCR4 density did not differ between these cells.
TABLE 2.
Comparison of HIV-1 Coreceptor Expression on CD4+ T-Cells in Paired Cervical and Whole Blood Samples
| Cervix | Whole Blood | p value | ||
|---|---|---|---|---|
| Median (95%CI) | Median (95%CI) | |||
|
CD4+ T cells
| ||||
| CCR5a | % | 50 (40–60) | 16 (13–26) | <0.001 |
| Mol/cell | 2,211 (1,920–3,865) | 2,422 (2,237–2,938) | 0.7 | |
| CXCR4b | % | 75 (70–83) | 81 (72–85) | 0.4 |
| Mol/cell | 2,724 (2,846–6,660) | 2,253 (2,162–2,793) | 0.3 | |
|
| ||||
|
DR+38+CD4+T cells
| ||||
| CCR5c | % | 62 (52–74) | 68 (64–77) | 0.1 |
| Mol/cell | 2,106 (1,649–3,106) | 4,305 (3,406–5,074) | 0.001 | |
| CXCR4d | % | 81 (69–88) | 41 (37–58) | <0.001 |
| Mol/cell | 3,416 (2,118–12,660) | 2,056 (1,958–4,190) | 0.1 | |
n=24,
n=22,
n=17,
n=16
Differences in HIV-1 Chemokine Coreceptor Expression Between Premenopausal and Postmenopausal Women
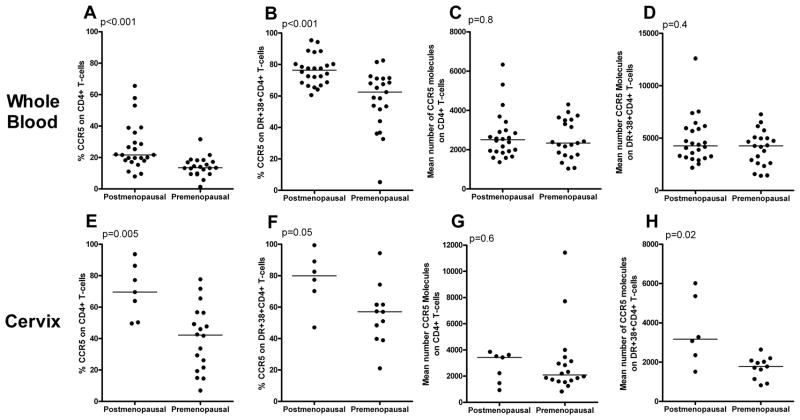
In the whole blood, there were significantly higher percentages of CCR5+CD4+ T-cells (median, 22% versus 13%) and CCR5+DR+38+CD4+ T-cells (median, 76% versus 62%) in postmenopausal compared to premenopausal women (Figure 2A, 2B). In the cervix, there were even greater differences in percentages of CCR5+ cells between postmenopausal and premenopausal women on CD4+ T-cells (median, 70% versus 42%) and DR+38+CD4+ T-cells (median, 80% versus 57%) (Figures 2E, 2F). There were no significant differences between premenopausal and postmenopausal women in the mean number of CCR5 molecules on whole blood CD4+ T-cells or activated CD4+ T-cells (Figure 2C, 2D). The concentration of CCR5 on cervical CD4+ T-cells tended to be higher in postmenopausal (median, 3,427 mol/cell) compared to premenopausal women (median, 2,091 mol/cell) (Figure 2G), and postmenopausal women had significantly more CCR5 molecules on activated cervical CD4+ T-cells (median, 3,176 mol/cell) than premenopausal women (median, 1,776 mol/cell) (Figure 2H).
FIGURE 2.
Percentages of CCR5 and number of CCR5 molecules on CD4+ T-cells and DR+38+CD4+ T-cells in the whole blood (A-D) and cervix (E-H) from healthy postmenopausal and premenopausal women. Horizontal lines indicate median values.
In the blood, percentages of CXCR4+CD4+ T-cells were slightly, but significantly, higher in postmenopausal (median, 88%; 95%CI, 81–90%) compared to premenopausal women (median, 81%; 95%CI, 72–85%; p=0.04), but there were no differences in CXCR4 expression in the activated subset (median, 43% vs. 44%, respectively, p=0.8). Postmenopausal women tended to have a higher percentage of cervical CXCR4+CD4+ T-cells than premenopausal women (median, 87% versus 67%; p=0.2). Percentages of CXCR4+DR+38+CD4+ T-cells in the cervix and density of CXCR4 on CD4+ T-cells from the blood or cervix did not differ between postmenopausal and premenopausal women (data not shown).
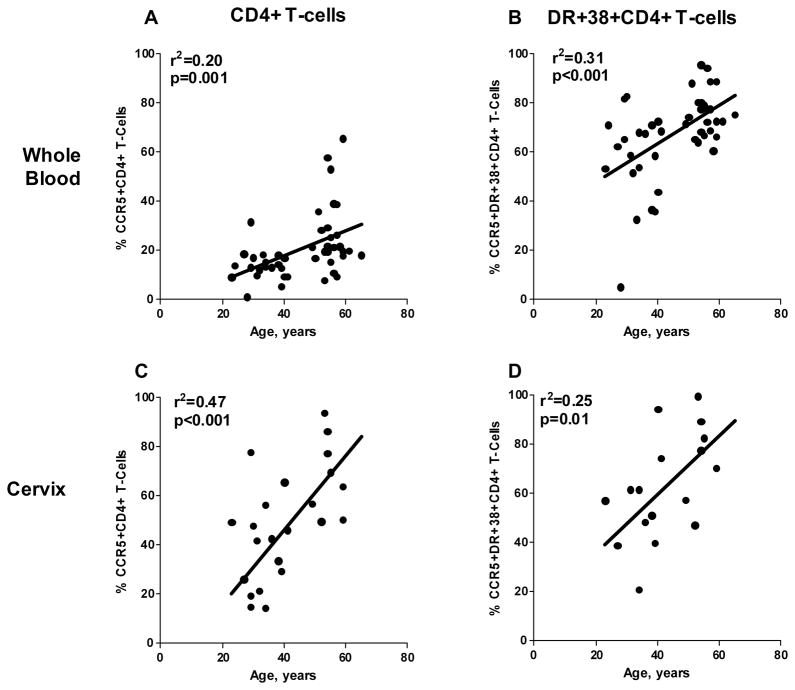
Relationship of Age to HIV-1 Coreceptor Expression
To further investigate differences in HIV-1 coreceptor expression between premenopausal and postmenopausal women, data were analyzed to determine the relationship between age and CCR5 or CXCR4 expression. There was a linear relationship between age and percentages of CCR5+CD4+ T-cells and CCR5+DR+38+CD4+ T-cells in both whole blood and cervix (Figure 3). Using an adjusted model, it could not be determined if the linear relationship between percent CCR5 expression and age was due to menopause or the aging process. There were no significant linear relationship between age and percentages of CXCR4+ cells or density of CXCR4 expression on total or activated CD4+ T-cells in blood or cervix (data not shown).
FIGURE 3.
Age and CCR5 expression on CD4+ and activated CD4+ T-cells had a significant linear relationship in the whole blood (A, B) and cervix (C,D). Lines generated by ordinary least squares regression.
DISCUSSION
This is the first study to investigate expression of the activation markers HLA-DR and CD38, and HIV-1 chemokine coreceptors on blood and cervical CD4+ T-cells in premenopausal and postmenopausal women. CD4+ T-cells in the cervix were found to have several characteristics that would support HIV-1 transmission including elevated immune activation compared to blood and high levels of expression of both CCR5 (median, 47%) and CXCR4 (median, 75%). Percentages of activated cells and CXCR4 expression did not differ substantially between premenopausal and postmenopausal women in either blood or the cervix. However, postmenopausal women had significantly higher expression of CCR5 in both blood and cervix. These findings suggest that postmenopausal women may be at greater biologic risk of R5 HIV-1 acquisition than premenopausal women.
Activated CD4+ T-cells have been linked to increased risk of HIV-1 infection,17–18 although mechanisms underlying this association are not fully understood. In the present study, we observed that percentages of DR+38+CD4+ T-cells were six-times higher in the cervix than in the blood of healthy HIV-1 seronegative women, consistent with two other small studies.22–23 Reasons for higher proportions of activated CD4+ T-cells in the cervix are unknown, although, higher percentages of memory T-cell populations are found in the cervix than blood.24–25 It has been proposed that elevated immune activation in the cervix is a result of antigenic stimulation.26 A strength of this study was that women were screened and excluded if they were found to have a vaginal infection, which can increase DR and 38 expression in the cervix.27 Nevertheless, antigens, from vaginal flora are present in all women, and may contribute to levels of immune activation and CCR5 expression. This is the first study to measure CCR5 and CXCR4 on DR+38+CD4+ T-cells in the cervix. A prior study evaluating 12 premenopausal women demonstrated higher percentages of CCR5+CD4+ cells in the cervix compared to blood.22 In addition, a study of Rhesus macaques demonstrated over 4-fold higher CCR5 expression in vaginal compared to blood CD4+ T-cells.26 Our results build on these prior studies, showing that the majority of activated CD4+ T-cells in the cervix expressed CCR5 and CXCR4. These data suggest that activated cervical CD4+ T-cells may be highly susceptible to HIV-1 infection due to high levels of expression of both HIV-1 coreceptors, which may account for the association of activated CD4+ T-cells with HIV-1 transmission.
The relationship between menopause and percentages of activated blood and cervical CD4+ T-cells was evaluated for the first time in this study. Declines in ovarian sex hormone production28 and aging29 have been linked to increases in proinflammatory cytokines including, TNF-α and IL-6. Interestingly, we found no significant differences in percentages activated CD4+ T-cells between premenopausal and postmenopausal women in either the blood or the cervix. These findings are consistent with those of a previous study that reported no age-related differences in percentages of DR+38+CD4+ T-cells in blood of either healthy HIV-1 seronegative individuals or HIV-1-infected individuals,30 and further extends these observations to the female genital tract.
An important determinant of HIV-1 susceptibility is CCR5 expression on CD4+ T-cells. Levels of CCR5 expression correlate with cellular susceptibility, in vitro.31–33 Furthermore, individuals heterozygous for the CCR5 delta 32 mutation, which is associated with lower levels of CCR5 expression, may be less susceptible to HIV-1 acquisition.15 In the present study, substantially higher percentages of CCR5+CD4+ T-cells and CCR5+DR+38+CD4+ T-cells were observed in postmenopausal compared to premenopausal women in both blood and the cervix. Density of CCR5 molecules was also elevated on activated cervical CD4+ T-cells of postmenopausal women. The relative importance of percentages of CCR5+ cells and density of CCR5 molecules in HIV-1 transmission and replication is somewhat controversial. Several in vitro studies suggest concentrations of CCR5 molecules are the most important determinant of HIV-1 susceptibility,31–33 and one group reported that concentrations of CCR5 molecules on peripheral blood CD4+ T-cells correlate with viral load.34 Nonetheless, data from our lab35 suggest that CCR5 percentages and density are both important determinants; in lymph node cells from untreated R5-tropic HIV-1-infected individuals, percentages of CCR5+ cells and numbers of CCR5 molecules per cell predicted the amount of HIV-1 RNA levels within subsets of cells defined by DR and 38 expression. Extrapolation of existing data on the role of CCR5 in HIV-1 transmission and chronic infection suggests that elevated percentages of CCR5+ target cells and density of CCR5 in postmenopausal women may increase their vulnerability to HIV-1 acquisition as well as contribute to higher levels of virus replication following HIV-1 infection.
Mechanisms underlying differences in percentages of CCR5+CD4+ T-cells in postmenopausal and premenopausal women are unclear. Estrogen and progesterone receptors have been demonstrated on T-cells28, 36 and the CCR5 promoter contains hormone response elements, supporting transcriptional control of CCR5 by sex hormones.37 In oophorectomized mice38 receiving exogenous estrogen (blood levels 150–200 pg/mL) and in women receiving oral contraceptives,39 CCR5 expression on CD4+ T-cells was increased, opposite to the effect on CCR5 expression in women with physiologically low estrogen observed in the current study. Nevertheless, the effect of sex hormones on immune modulators may change over the lifespan40 and therefore it is difficult to directly extrapolate these studies to hormonal effects in menopause.
Alternatively, immune changes related to aging could account for heightened CCR5 expression in postmenopausal women. Prior studies have shown CCR5 RNA is higher in blood of older compared to younger mice41 and blood CD4+ T-cells of older compared to younger men and women.40 Although one group found that the percentage of CCR5+CD4+ T-cells was not significantly different between older and younger HIV-1-infected individuals,30 these results should be viewed with caution because some specimens were shipped overnight prior to analysis, a process known to downregulate CCR5 expression. Importantly, if age rather than sex hormones underlies the increased CCR5 expression observed in the present study, older women and men may be at increased risk of HIV-1 acquisition.
CXCR4 levels have not been associated with HIV-1 transmission, although they are linked to HIV-1 susceptibility in cell lines.42 In the present study, percentages of CXCR4+ were higher than CCR5+ cells on total and activated cervical CD4+ T-cells. It has been hypothesized that lower density of CXCR4 compared to CCR5 may account for preferential R5 virus transmission.42 Nevertheless, CXCR4 density was similar to or higher than CCR5 density on cervical lymphocytes in the present study. Thus, these findings support the hypothesis that there are multiple factors contributing to preferential R5 virus transmission13 and that coreceptor expression is not the only restriction factor. Intriguingly, recent studies suggest that seminal plasma induces increased CCR5 expression on CD4+ T-cells and thereby may promote R5 over X4-tropic HIV-1 transmission.43
One limitation of our study is that flow cytometry provides relative percentages, not absolute numbers of cells. HIV-1 susceptibility likely relates to the absolute number of available target cells in cervical tissue, not just relative percentage within CD4+ T-cells. Absolute numbers of blood CD4+ T-cells do decline after age 65 years,44 but women included here were younger. Further, it is unclear whether absolute CD4+ T-cell count from the blood translates to absolute numbers in mucosal tissue. Future research should be directed at evaluating absolute numbers of CCR5+ cells within cervical tissues of premenopausal compared to postmenopausal women. Another shortcoming was that the present study only evaluated CCR5 expression in the endocervix. Nevertheless, there are other sites in the female genital tract where CCR5+ T-cells are present and HIV-1 transmission may occur including the vagina,26, 45 the ectocervix,45 and the endometrium.46 Further studies are needed to determine whether differences between premenopausal and postmenopausal women in CCR5 expression on CD4+ T-cells are also found in other areas of the female genital tract. Final limitations of the present study are that our observations are phenotypic and the sample size of cervical data from postmenopausal women is small. Further studies are needed to confirm our observations and demonstrate whether these differences in CCR5 expression result in true differences in how readily HIV-1 is transmitted to postmenopausal women. Importantly, a recent study demonstrated enhanced HIV-1 replication in ectocervical explants obtained from postmenopausal compared to those from premenopausal women, supporting the hypothesis that elevated CCR5 expression in postmenopausal women may result in differential HIV-1 susceptibility.47
Currently an estimated 50 million American women are postmenopausal48 and approximately 21 million more women will reach menopause in the next 10 years.49 These data suggest increasing numbers of postmenopausal women will be exposed to HIV-1 infection in the next decade. Indeed, new HIV-1 infections are already increasing in older women compared to younger women in the United States; from 1999 to 2004, numbers of new diagnoses increased by 28% in women over 40 years old whereas they decreased by 13% in women between the ages of 13 and 39.4 Importantly, our study suggests that postmenopausal women are likely to be at greater biologic risk of HIV-1 acquisition than reproductive-age women. One of the major priorities of the National HIV/AIDS Strategy is to lower the annual number of new infections by 2015.50 The present study underscores the urgent need to better understand the impact of sex hormones and aging on HIV-1 acquisition, and mechanisms underlying differential risk in order to design appropriate public health messages and effective prevention measures for this older population.
Acknowledgments
We thank Kristina Carroll, Lauren Tobin, and Chelsea Bergman for their technical assistance.
FUNDING
This work was supported by T. Franklin Williams Scholars Program (Atlantic Philanthropies Inc. (USA), Infectious Disease Society of America, the John A. Hartford Foundation, and the Association of Specialty Professors); National Institutes of Health Awards: K08 AI080285, AG027678, and Colorado Clinical Translational Sciences Institute RR-025780; and University of Colorado Denver (UCD) Center for Women’s Health Research Junior Faculty Development Award and UCD Women’s Health Research Pilot Project Grant.
Footnotes
Potential Conflicts of Interest: None
Part of data reported here has been presented at 18th Conference on Retroviruses and Opportunistic Infections, Abstract # U-134 Boston, MA; 2011
References
- 1.Diagnoses of HIV Infection and AIDS in the United States and Dependent Areas. [Accessed April 27, April 27, 2011];CDC HIV Surveillance Report. 2011 April 27;21 http://www.cdc.gov/hiv/surveillance/resources/reports/2009report/index.htm.
- 2.Comparison of female to male and male to female transmission of HIV in 563 stable couples. European Study Group on Heterosexual Transmission of HIV. BMJ. 1992 Mar 28;304(6830):809–813. doi: 10.1136/bmj.304.6830.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindau ST, Leitsch SA, Lundberg KL, Jerome J. Older women’s attitudes, behavior, and communication about sex and HIV: a community-based study. J Womens Health (Larchmt) 2006 Jul–Aug;15(6):747–753. doi: 10.1089/jwh.2006.15.747. [DOI] [PubMed] [Google Scholar]
- 4.Espinoza L, Hall HI, Hardnett F, Selik RM, Ling Q, Lee LM. Characteristics of persons with heterosexually acquired HIV infection, United States 1999–2004. Am J Public Health. 2007 Jan;97(1):144–149. doi: 10.2105/AJPH.2005.077461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SM, Baskin GB, Marx PA. Estrogen protects against vaginal transmission of simian immunodeficiency virus. J Infect Dis. 2000 Sep;182(3):708–715. doi: 10.1086/315776. [DOI] [PubMed] [Google Scholar]
- 6.Smith SM, Mefford M, Sodora D, et al. Topical estrogen protects against SIV vaginal transmission without evidence of systemic effect. Aids. 2004 Aug 20;18(12):1637–1643. doi: 10.1097/01.aids.0000131393.76221.cc. [DOI] [PubMed] [Google Scholar]
- 7.Linsk NL. HIV among older adults: age-specific issues in prevention and treatment. AIDS Read. 2000 Jul;10(7):430–440. [PubMed] [Google Scholar]
- 8.Miller CJ, Li Q, Abel K, et al. Propagation and Dissemination of Infection after Vaginal Transmission of Simian Immunodeficiency Virus. J Virol. 2005 July 15;79(14):9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Schuler T, Zupancic M, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999 Nov 12;286(5443):1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 10.Gupta P, Collins KB, Ratner D, et al. Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol. 2002 Oct;76(19):9868–9876. doi: 10.1128/JVI.76.19.9868-9876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hladik F, Sakchalathorn P, Ballweber L, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007 Feb;26(2):257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirbod T, Kaldensjo T, Broliden K. In Situ Distribution of HIV-Binding CCR5 and C-Type Lectin Receptors in the Human Endocervical Mucosa. PLoS One. 2011;6(9):e25551. doi: 10.1371/journal.pone.0025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grivel J-C, Shattock RJ, Margolis LB. Selective transmission of R5 HIV-1 variants: where is the gatekeeper? Journal of Translational Medicine. 2010;9(Suppl 1):S1–17. doi: 10.1186/1479-5876-9-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogan CM, Hammer SM. Host determinants in HIV infection and disease. Part 2: genetic factors and implications for antiretroviral therapeutics. Ann Intern Med. 2001 May 15;134(10):978–996. doi: 10.7326/0003-4819-134-10-200105150-00012. [DOI] [PubMed] [Google Scholar]
- 15.Marmor M, Sheppard HW, Donnell D, et al. Homozygous and heterozygous CCR5-Delta32 genotypes are associated with resistance to HIV infection. J Acquir Immune Defic Syndr. 2001 Aug 15;27(5):472–481. doi: 10.1097/00126334-200108150-00009. [DOI] [PubMed] [Google Scholar]
- 16.Reeves JD, Gallo SA, Ahmad N, et al. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc Natl Acad Sci U S A. 2002 Dec 10;99(25):16249–16254. doi: 10.1073/pnas.252469399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Begaud E, Chartier L, Marechal V, et al. Reduced CD4 T cell activation and in vitro susceptibility to HIV-1 infection in exposed uninfected Central Africans. Retrovirology. 2006;3:35. doi: 10.1186/1742-4690-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koning FA, Otto SA, Hazenberg MD, et al. Low-level CD4+ T cell activation is associated with low susceptibility to HIV-1 infection. J Immunol. 2005 Nov 1;175(9):6117–6122. doi: 10.4049/jimmunol.175.9.6117. [DOI] [PubMed] [Google Scholar]
- 19.Reichelderfer PS, Coombs RW, Wright DJ, et al. Effect of menstrual cycle on HIV-1 levels in the peripheral blood and genital tract. WHS 001 Study Team. Aids. 2000 Sep 29;14(14):2101–2107. doi: 10.1097/00002030-200009290-00005. [DOI] [PubMed] [Google Scholar]
- 20.Meditz AL, Schlichtemeier R, Folkvord JM, et al. SDF-1alpha is a potent inducer of HIV-1-Specific CD8+ T-cell chemotaxis, but migration of CD8+ T cells is impaired at high viral loads. AIDS Res Hum Retroviruses. 2008 Jul;24(7):977–985. doi: 10.1089/aid.2007.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Roda Husman AM, Koot M, Cornelissen M, et al. Association between CCR5 genotype and the clinical course of HIV-1 infection. Ann Intern Med. 1997 Nov 15;127(10):882–890. doi: 10.7326/0003-4819-127-10-199711150-00004. [DOI] [PubMed] [Google Scholar]
- 22.Prakash M, Kapembwa MS, Gotch F, Patterson S. Higher levels of activation markers and chemokine receptors on T lymphocytes in the cervix than peripheral blood of normal healthy women. J Reprod Immunol. 2001 Oct-Nov;52(1–2):101–111. doi: 10.1016/s0165-0378(01)00114-0. [DOI] [PubMed] [Google Scholar]
- 23.Quayle AJ, Kourtis AP, Cu-Uvin S, et al. T-lymphocyte profile and total and virus-specific immunoglobulin concentrations in the cervix of HIV-1-infected women. J Acquir Immune Defic Syndr. 2007 Mar 1;44(3):292–298. doi: 10.1097/QAI.0b013e31802c5b3a. [DOI] [PubMed] [Google Scholar]
- 24.Saba E, Grivel JC, Vanpouille C, et al. HIV-1 sexual transmission: early events of HIV-1 infection of human cervico-vaginal tissue in an optimized ex vivo model. Mucosal Immunol. 2010 May;3(3):280–290. doi: 10.1038/mi.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hladik F, Lentz G, Delpit E, McElroy A, McElrath MJ. Coexpression of CCR5 and IL-2 in human genital but not blood T cells: implications for the ontogeny of the CCR5+ Th1 phenotype. J Immunol. 1999 Aug 15;163(4):2306–2313. [PubMed] [Google Scholar]
- 26.Veazey RS, Marx PA, Lackner AA. Vaginal CD4+ T Cells Express High Levels of CCR5 and Are Rapidly Depleted in Simian Immunodeficiency Virus Infection. Journal of Infectious Diseases. 2003 March 1;187(5):769–776. doi: 10.1086/368386. [DOI] [PubMed] [Google Scholar]
- 27.Nkengasong JN, Kestens L, Ghys PD, et al. Human immunodeficiency virus Type 1 (HIV-1) plasma virus load and markers of immune activation among HIV-infected female sex workers with sexually transmitted diseases in Abidjan, Cote d’Ivoire. J Infect Dis. 2001 May 1;183(9):1405–1408. doi: 10.1086/319855. [DOI] [PubMed] [Google Scholar]
- 28.Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in Proinflammatory Cytokine Activity after Menopause. Endocr Rev. 2002 February 1;23(1):90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 29.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001 May;8(3):131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Kalayjian RC, Landay A, Pollard RB, et al. Age-Related Immune Dysfunction in Health and in Human Immunodeficiency Virus (HIV) Disease: Association of Age and HIV Infection with Naive CD8+ Cell Depletion, Reduced Expression of CD28 on CD8+ Cells, and Reduced Thymic Volumes. Journal of Infectious Diseases. 2003 June 15;187(12):1924–1933. doi: 10.1086/375372. [DOI] [PubMed] [Google Scholar]
- 31.Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1999 Apr 27;96(9):5215–5220. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998 Apr;72(4):2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeves JD, Gallo SA, Ahmad N, et al. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proceedings of the National Academy of Sciences. 2002 December 10;99(25):16249–16254. doi: 10.1073/pnas.252469399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reynes J, Portales P, Segondy M, et al. CD4 T cell surface CCR5 density as a host factor in HIV-1 disease progression. Aids. 2001 Sep 7;15(13):1627–1634. doi: 10.1097/00002030-200109070-00004. [DOI] [PubMed] [Google Scholar]
- 35.Meditz AL, Haas MK, Folkvord JM, et al. HLA-DR+CD38+CD4+ T Lymphocytes Have Elevated CCR5 Expression and Produce the Majority of R5-tropic HIV-1 RNA in Vivo. J Virol. 2011 October;85(19) doi: 10.1128/JVI.02529-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asin SN, Heimberg AM, Eszterhas SK, Rollenhagen C, Howell AL. Estradiol and progesterone regulate HIV type 1 replication in peripheral blood cells. AIDS Res Hum Retroviruses. 2008 May;24(5):701–716. doi: 10.1089/aid.2007.0108. [DOI] [PubMed] [Google Scholar]
- 37.Moriuchi H, Moriuchi M, Fauci AS. Nuclear factor-kappa B potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J Immunol. 1997 Apr 1;158(7):3483–3491. [PubMed] [Google Scholar]
- 38.Mo R, Chen J, Grolleau-Julius A, Murphy HS, Richardson BC, Yung RL. Estrogen regulates CCR gene expression and function in T lymphocytes. J Immunol. 2005 May 15;174(10):6023–6029. doi: 10.4049/jimmunol.174.10.6023. [DOI] [PubMed] [Google Scholar]
- 39.Prakash M, Kapembwa MS, Gotch F, Patterson S. Oral contraceptive use induces upregulation of the CCR5 chemokine receptor on CD4(+) T cells in the cervical epithelium of healthy women. J Reprod Immunol. 2002 Mar;54(1–2):117–131. doi: 10.1016/s0165-0378(01)00125-5. [DOI] [PubMed] [Google Scholar]
- 40.Yung RL, Mo R. Aging is associated with increased human T cell CC chemokine receptor gene expression. J Interferon Cytokine Res. 2003 Oct;23(10):575–582. doi: 10.1089/107999003322485071. [DOI] [PubMed] [Google Scholar]
- 41.Mo R, Chen J, Han Y, et al. T cell chemokine receptor expression in aging. J Immunol. 2003 Jan 15;170(2):895–904. doi: 10.4049/jimmunol.170.2.895. [DOI] [PubMed] [Google Scholar]
- 42.Fiser AL, Vincent T, Brieu N, et al. High CD4+ T-Cell Surface CXCR4 Density as a Risk Factor for R5 to X4 Switch in the Course of HIV-1 Infection. J Acquir Immune Defic Syndr. 2010 Sep 21; doi: 10.1097/QAI.0b013e3181f25bab. [DOI] [PubMed] [Google Scholar]
- 43.Balandya E, Sheth S, Sanders K, Wieland-Alter W, Lahey T. Semen Protects CD4+ Target Cells from HIV Infection but Promotes the Preferential Transmission of R5 Tropic HIV. The Journal of Immunology. 2010 December 15;185(12):7596–7604. doi: 10.4049/jimmunol.1002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huppert FA, Solomou W, O’Connor S, Morgan K, Sussams P, Brayne C. Aging and lymphocyte subpopulations: whole-blood analysis of immune markers in a large population sample of healthy elderly individuals. Exp Gerontol. 1998 Sep;33(6):593–600. doi: 10.1016/s0531-5565(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 45.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005 Dec;73(6):1253–1263. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 46.Kaldensjo T, Petersson P, Tolf A, Morgan G, Broliden K, Hirbod T. Detection of intraepithelial and stromal Langerin and CCR5 positive cells in the human endometrium: potential targets for HIV infection. PLoS One. 2011;6(6):e21344. doi: 10.1371/journal.pone.0021344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rollenhagen C, Asin SN. Enhanced HIV-1 replication in ex vivo ectocervical tissues from post-menopausal women correlates with increased inflammatory responses. Mucosal Immunol. 2011 Aug 31; doi: 10.1038/mi.2011.34. [DOI] [PubMed] [Google Scholar]
- 48.The 2011 Statistical Abstract. U.S. Census Bureau; [Accessed August 3, 2011]. http://www.census.gov/compendia/statab/cats/population.html. [Google Scholar]
- 49.North American Menopause Society. [Accessed August 5, 2011];2011 2011 http://www.menopause.org/meetings/exprospectus.pdf.
- 50. [Accessed July 22, 2011];National HIV/AIDS Strategy. 2010 http://www.whitehouse.gov/files/documents/nhas-implementation.pdf.