Abstract
The blood-brain barrier (BBB) disruption and brain edema are important pathophysiologies of early brain injury after subarachnoid hemorrhage (SAH). This study is to evaluate whether Rho kinase (Rock) enhances BBB permeability via disruption of tight junction proteins during early brain injury. Adult male rats were assigned to five groups; sham-operated, SAH treated with saline, a Rock inhibitor hydroxyfasudil (HF) (10mg/kg) treatment at 0.5 hours after SAH, HF treatment at 0.5 and 6 hours (10mg/kg, each) after SAH, and another Rock inhibitor Y27632 (10mg/kg) treatment at 0.5 hrs after SAH. The perforation model of SAH was performed and neurological score and brain water content were evaluated 24 and 72 hours after surgery. Evans blue extravasation, Rock activity assay, and Western blotting analyses were evaluated 24 hours after surgery. Treatment of HF significantly improved neurological scores 24 hours after SAH. Single treatment with HF and Y27632, and two treatments with HF reduced brain water content in the ipsilateral hemisphere. HF reduced Evans blue extravasation in the ipsilateral hemisphere after SAH. Rock activity increased 24 hours after SAH, and HF reversed the activity. SAH significantly decreased the levels of tight junction proteins, occludin and zonula occludens-1 (ZO-1), and HF preserved the levels of occluding and ZO-1 in ipsilateral hemisphere. In conclusion, HF attenuated BBB permeability after SAH, possibly by protection of tight junction proteins.
Keywords: Subarachnoid hemorrhage, Early brain injury, Rho kinase inhibitor, Hydroxyfasudil, Brain edema, Tight junction
1. Introduction
Subarachnoid hemorrhage (SAH), accounting for only 5% of strokes but with a case fatality rate of around 50%, is a severe condition caused in approximately 85% patients by rupture of an intracranial aneurysm (van Gijn, et al., 2007). During early brain injury (EBI) period, a ruptured aneurysm brings on many physiological derangements such as increasing intracranial pressure, decreased cerebral blood flow, and global cerebral ischemia, which initiate secondary injuries. Of these, BBB disruption and brain edema is an important pathophysiological event of EBI after SAH (Ostrowski, et al., 2006). Because brain edema has been described as an independent risk factor for mortality and poor outcome (Claassen. et al., 2002), decreasing of BBB disruption should be considered a key target for EBI following SAH. Endothelial cells (ECs) are interconnected by tight junctions (TJs), which is one of the components of junctional complex in the BBB (Ballabh, et al. 2004). TJs are comprised of integral membrane proteins, such as occludin, and membrane-associated proteins, such as zonula occludens-1 (ZO-1) (Aijaz, et al., 2006). Degrading occludin and ZO-1 facilitates capillary leakage and hence increases BBB permeability.
Rho kinase (Rock) is a serine-threonine kinase activated by GTP-bound RhoA (Chrissobolis and Sobey, 2006). Increasing RhoA/Rock pathway activity regulates actin microfilament dynamics (Chrissobolis and Sobey, 2006). Rock activation in oxygen-glucose deprivation accounts for endothelial barrier breakdown (Allen, et al., 2010) and Rock opens the BBB by mediating TJ proteins (Stamatovic, et al., 2003; Persidsky, et al., 2006). In this study we tested two hypotheses. (A) Rock inhibitor, Hydroxyfasudil (HF), improves the outcomes after SAH; (B) the Rock inhibition decreases BBB permeability via preservation of TJ proteins.
2. Material and Methods
2.1. Animals and Drugs
This experiment was a controlled in vivo laboratory study taking place in an animal research laboratory utilizing adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighting 260 to 340 g. All protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University. One hundred and seventy-five (175) animals were assigned to following groups: Sham (n=27), Vehicle (SAH treated with saline; n=83), HF (SAH treated with HF; n=53), Y27632 (SAH treated with Y27632; n=12). SAH animals received one of the following treatments intraperitoneally; vehicle (1.5ml of 0.9% saline), HF (10mg/kg, Toronto Research Chemicals, Ontario, Canada) or Y27632 (10mg/kg, Ascent Scientific LLC, Princeton, NJ) with single treatment at 0.5 hours after SAH, or two treatments with vehicle (1.5ml of 0.9% saline, each) or HF (10mg/kg, each) at 0.5 and 6 hours after SAH. The dosage of HF and Y27632 were selected from previous literature that reported reduced infarct size in cerebral ischemia animal models (Shin, et al., 2007). HF or Y27632 were dissolved in 0.9% saline with a total volume of 1.5ml when they were administered. Animals were sacrificed at 24 hours after procedure except for animals of Sham, single treatment with Vehicle and HF which were randomly assigned to be sacrificed at 24 or 72 hours after SAH. The severity of the SAH was evaluated in a blinded manner as previously described (Sugawara, et al., 2008). Briefly, after removing the brain, a picture of the basement of the brain was taken and pictures were divided into six segments. Each part was sub-scored (0 to 3) according to occurrences of blood in the subarachnoid space and a total score was calculated as the sum of all sub-scores. The animals received a total score ranging from 0 (no SAH) to 18 (most severe SAH). Animals with SAH score of 7 or less were excluded from the study for low SAH grade (Sugawara, et al., 2008).
2.2. Surgery
The endovascular perforation model of SAH was performed as previously described (Bederson, et al., 1995; Duris, et al., in-press). Briefly, rats were anesthetized, intubated and kept on artificial ventilation during surgery with 3% Isoflurane in 70%/30% medical-air/oxygen. Body temperature was monitored by rectal probe and normo-thermia was maintained by a heating lamp. A sharpened 4-0 nylon suture was introduced into the left internal carotid artery (ICA) until resistance was felt (approximately 18 mm from the common carotid bifurcation). The suture was then pushed further to perforate the bifurcation of the anterior and middle cerebral arteries until resistance was overcome and withdrawn immediately after perforation. In sham operated animals the suture was inserted into the left ICA, however no perforation was performed. After suture removal the incision was closed, and rats were individually housed in heated cages until recovery.
2.3. Physiological parameters
Physiological parameters were measured via the right femoral artery which was cannulated for continuous measurement of mean arterial blood pressure, heart rate and for blood sampling. The pH, arterial blood gases, and serum glucose were measured 15 minutes before, immediately after and 30 minutes after SAH. After that, animals were allowed to wake up from anesthesia.
2.4. Neurological Score
At 24 or 72 hours after procedure, neurological scores were evaluated before sacrifice in a blinded fashion using a modification of Garcia scoring system (Garcia, et al., 1995; Sugawara, et al., 2008). The evaluation consists of six tests that can be scored 0 to 3 or 1 to 3. The following were evaluated: spontaneous activity, symmetrical movements of limbs, forelimbs outstretching, climbing a wall of a wire cage, axillary touch response and vibrissae touch response. The best score is 18 and the worst score is 2.
2.5. Brain Water Content (Brain Edema)
Brains were removed 24 or 72 hours after surgery and separated into left hemisphere, right hemisphere, cerebellum and brain stem as previously described (Suzuki, et al., 2010). Each part was weighed immediately after removal (wet weight) and after drying in 100 °C for 72 hours (dry weight). The percentage of water content was calculated as [(wet weight- dry weight)/wet weight] × 100%. The number of animals used in each group was Vehicle (n=26), HF (n=26), Y27632 (n=10), and Sham (n=27).
2.6. BBB Disruption
At 24 hours after surgery, Evans blue dye (2%; 5 ml/kg) was injected over 2 minutes into the right femoral vein and allowed to circulate for 60 minutes as previously described (Suzuki, et al., 2010). Under deep anesthesia, rats were killed by intracardial perfusion with phosphate-buffered saline (PBS), and brains were removed and divided into the same regions as the brain water content study. Brain specimens were weighed, homogenized in 1 ml of PBS, and centrifuged at 15,000g for 30 mins. Then, 0.6 ml of the resultant supernatant was added to an equal volume of trichloroacetic acid. After overnight incubation at 4°C and centrifugation at 15,000g at 4°C for 30 mins, the supernatant was taken for spectrophotometric quantification of extravasated Evans blue dye at 615 nm.
2.7. Rock Activity and Western Blot Analyses
Animals were euthanized at 24 hours after SAH. Brains were removed and stored at −80 °C immediately until analysis. Protein extraction from left hemisphere was obtained by homogenizing in RIPA buffer (Santa Cruz Biotechnology, Inc, Santa Cruz, CA) supplemented with protease and phosphatase inhibitor cocktail (Sigma-Aldrich, Saint Louis, MO) followed by centrifuging at 14,000g at 4 °C for 20 min. The supernatant was used as whole cell protein extract and stored at −80 °C until use. The protein concentration was determined by using a detergent compatible assay (Bio-Rad, DC protein assay, CA).
The supernatants were used for assessing Rock activities using the Rock Activity Assay Kit (Cell Biolabs, Inc, San Diego, CA) as indicated by the manufacturer’s protocol. Briefly, aliquots of supernatants containing 20 μg of proteins was added in 96-well plates pre-coated with a recombinant myosin phosphatase target subunit 1 (MYPT1), the phosphorylation of which was specifically mediated by ROCK at Thr696, for 60 minutes at 30 °C. Subsequently, 100μl of horseradish peroxidase conjugated anti-phospho-MYPT1 (Thr696) antibody was added, and incubated for 1 hour at room temperature. The colored product was developed by incubating with 100μl of substrate reagent. The reaction was stopped by adding 100μl of stop solution containing sulfuric acid, and the absorbance was read at 450 nm in an automated plate reader.
The supernatants were also used for performing Western blot analyses as previously described (Suzuki, et al., 2010). Equal amounts of protein (40 μg) were loaded on a SDS-PAGE gel. After being electrophoresed and transferred to a nitrocellulose membrane, the membrane was then blocked and incubated with the primary antibody overnight at 4 °C. The primary antibody was goat polyclonal anti-ZO-1 (1:200) and goat polyclonal anti-occludin (1:200). Nitrocellulose membranes were incubated with secondary antibodies (1:2000) for 1 hour at room temperature. Immunoblots were then probed with an ECL Plus chemiluminescence reagent kit (Amersham Bioscience, Arlington Heights, IL). Blot bands were quantified by densitometry with Image J software (Image J 1.40g, NIH). β-actin (1:2000, all antibodies from Santa Cruz Biotechnology) was blotted on the same membrane as a loading control.
2.8. Statistical analyses
Data were expressed as mean ± standard deviation. Mortality was analyzed by Fischer exact test. SAH grading and neurologic scores analyzed using Kruskal-Wallis analysis of variance (ANOVA) on ranks followed by Dunn’s method or Mann-Whitney U-test. The data in physiological parameters were analyzed using Two-way repeated measures ANOVA. All other values were analyzed by one-way ANOVA followed by Tukey post hoc analysis. A p value of < 0.05 was considered statistically significant.
3. Result
3.1. Mortality rate in each group
We performed 175 surgeries, 27 rats having mild SAH (17 rats sacrificed at 24 hours and 10 rats at 72 hours) and 26 rats died because of severe SAH were excluded from assessment of neurological deficits and brain edema. However, these dead rats were included in mortality statistics. The 24 hours mortality rates in each groups were as follows: Sham group 0.0% (0 of 22 rats), Vehicle group with single treatment 26.3% (10 of 38 rats), HF group with single treatment 21.2% (7 of 33 rats), Y27632 group 9.1% (1 of 11 rats), Vehicle with two treatments 18.2% (2 of 11 rats), and HF with two treatments 0% (0 of 11 rats). There were no difference between Vehicle, HF and Y27623 groups in the mortality rates (p = NS). However, both Vehicle and HF groups with single treatment showed statistically significant higher mortality than Sham group (p <0.01 and p <0.05, each). The 72 hours mortality rates were 50.0% in Vehicle group (6 of 12 rats) and 0% in Sham and HF group (0 of 5 rats, each), which was no significant difference between Vehicle and HF group. After all exclusions, we enrolled 106 rats for 24 hours evaluation and 16 rats for 72 hours evaluation, respectively.
3.2. Physiological Parameters and Grading Score of SAH
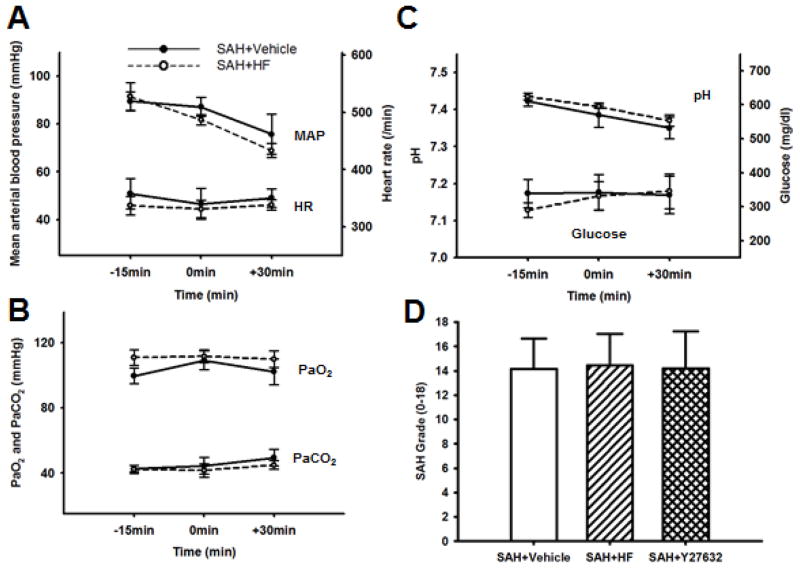
Physiological parameters were monitored before, during, and after surgery (Figures 1A through 1C). No statistical differences were observed between Vehicle and HF group with regard to mean arterial blood pressure, heart rate, arterial blood gases (paO2, paCO2, pH levels), and glucose levels at 15 min before, immediately after puncture and 30 min after SAH (p = NS). Overall SAH grading score (Figure 1D) was similar between Vehicle (13.95 ± 2.58), HF (14.45 ± 2.58), and Y27632 (14.20 ± 3.05) groups (p = NS).
Figure 1.
Physiological parameters during surgical procedure (A, B, C). Mean arterial blood pressure (MAP) and heart rate (HR) (A), pO2 and pCO2 (B), pH and blood serum glucose (C) 15 minutes before to 30 minutes after SAH in Vehicle and HF group (n=4, each). There is no difference between the groups. Demonstrating equivalent SAH severity between Vehicle (n=43), HF (n=42), and Y27632 (n=10) groups (D). Values are mean ± SD.
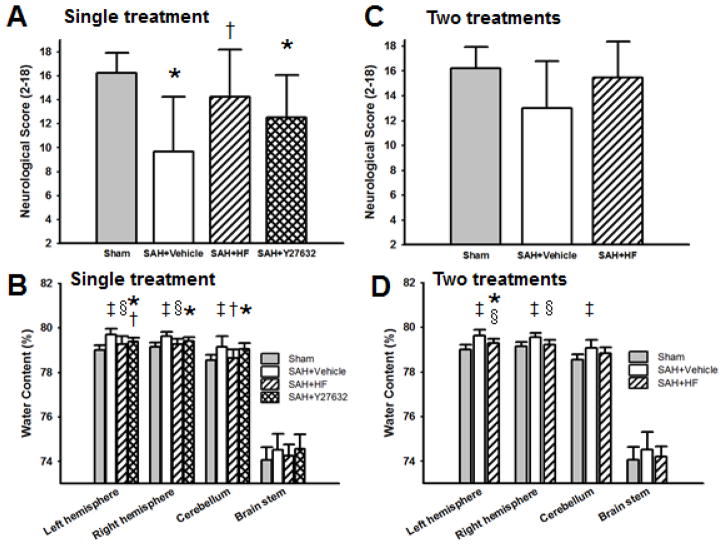
3.3. Neurological score and brain water content with single treatment at 24 hours after surgery
For the 24 hours outcome study, we used Sham, Vehicle, HF, and Y27632 groups with single treatment. There was no significant difference in SAH grade between Vehicle (14.27 ± 2.78), HF (14.32 ± 2.43), and Y27632 (14.20 ± 3.05) groups (p = NS). Neurological score (Figure 2A) was significantly lower in Vehicle group compared to Sham (p <0.05) and HF (p <0.05) groups but not statistically significance compared with Y27632 group. Brain water content for single treatment (Figure 2B) was significantly higher in the left hemisphere with Vehicle compared to Sham (p <0.01), HF (p <0.01), and Y27632 (p <0.05); in the right hemisphere with Vehicle compared to Sham (p <0.01) and HF (p <0.01); in the cerebellum with Vehicle compared to Sham (p <0.01) and HF (p <0.05).
Figure 2.
Effect of HF and Y27632 treatment on neurological score (A, C) and on BWC (B, D) 24 hrs after surgery. Single treatment result (A, B) and two treatments result (C, D). Modified Garcia score was used as a neurological score in Sham (n=27), Vehicle (n=34), HF (n=31), and Y27632 groups (n=10) in single treatment; Vehicle (n=9) and HF groups (n=11) in two treatments. HF, but not Y27632, ameliorated neurological score after SAH in single treatment group (A). Both HF and Y27632 attenuated BWC in the left hemisphere and HF also attenuated it in the right hemisphere and cerebellum after SAH (B). Neurological score with two treatments was no difference between groups (C). HF with two treatments ameliorated BWC in both hemispheres after SAH (D)Values are mean ± SD. * p< 0.05 vs Sham, †p< 0.05 vs Vehicle, ‡p< 0.01 vs Sham, §p< 0.01 vs Vehicle group.
3.4. Neurological score and brain water content with two treatments at 24 hours after surgery
Two treatments were used for Sham, Vehicle, and HF groups for the 24 hours outcome study. There was no significant difference in SAH grade between Vehicle (13.67 ± 1.11) and HF (14.82 ± 3.06) groups (p = NS). Neurological score with two treatments was no difference between groups (p = NS, Figure 2C). However brain water content for two treatments (Figure 2D) was significantly higher in the left hemisphere with Vehicle compared to Sham (p <0.01) and HF (p <0.01); in the right hemisphere with Vehicle compared to Sham (p <0.01) and HF (p <0.01); in the cerebellum with Vehicle compared to Sham (p< 0.01).
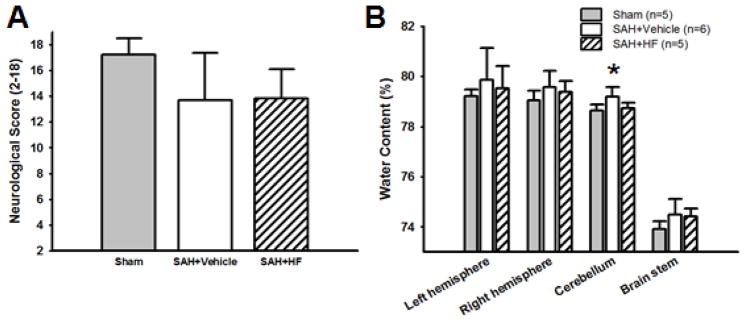
3.5. Neurological score and brain water content with single treatment at 72 hours after surgery
For the 72 hours outcome study we used Sham, Vehicle, and HF groups. There was no significant difference in SAH grade between Vehicle (11.67 ± 2.16) and HF (12.60 ± 2.30) groups (p = NS). Neurological score was not significant difference between groups (Figure 3A). Brain water content in cerebellum in Vehicle group was significantly higher than in Sham group (p <0.05), however there were no significant differences between groups in brain water content in other regions (p = NS, Figure 3B).
Figure 3.
Effect of HF with single treatment on neurological score (A) and on BWC (B) 72 hrs after surgery. N=5 for Sham and HF groups, and n=6 for Vehicle group. Neurological score was not significant difference between groups (A). BWC in cerebellum in Vehicle group was significantly higher than in Sham group (B). Values are mean ± SD. * p< 0.01 vs Sham group.
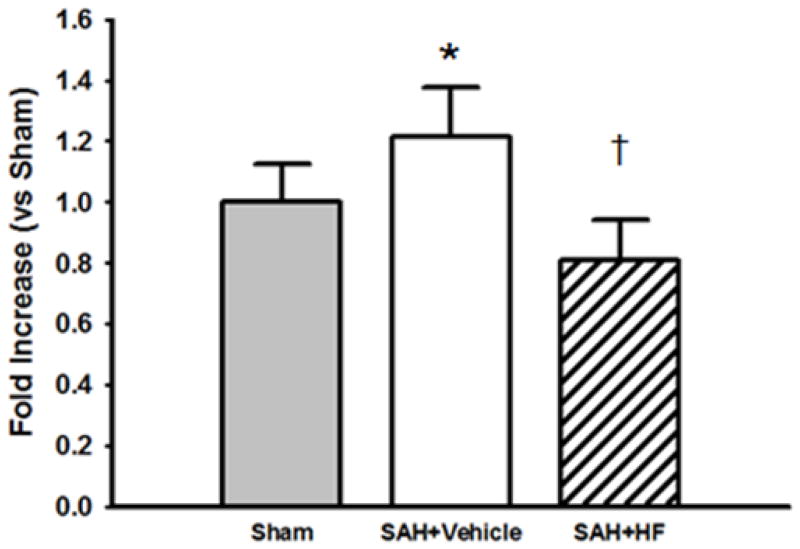
3.6. Rho-kinase Activity Assay
Rock activity assay was used for quantification of Rock levels in ipsilateral hemispheres at 24 hours after surgery in Sham, Vehicle, and HF groups. The activity level of Rock (Figure 4) was significantly higher in Vehicle group compared to both Sham (p <0.05) and HF (p <0.01) groups.
Figure 4.
Rock activity assay in the presence and absence of HF after SAH 24 hrs after SAH in the ipsilateral hemispheres with single treatment. N=6 rats per each group. The activity level of Rock was significantly higher in Vehicle group compared to both Sham and HF groups. Values are mean ± SD. * p< 0.05 vs Sham, †p<0.01 vs Vehicle group.
3.7. Quantification of BBB permeability and tight junction proteins
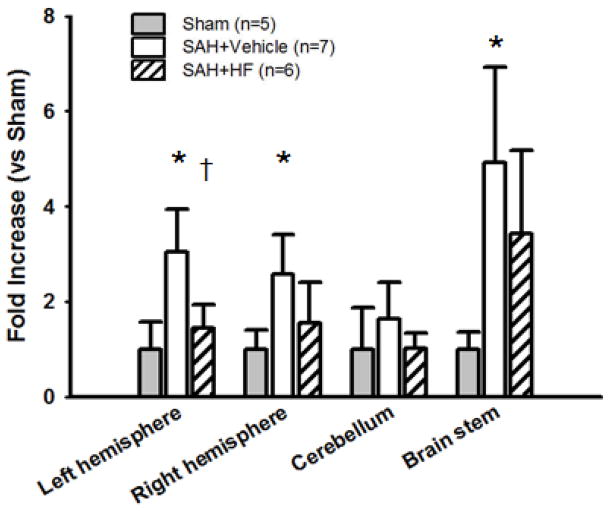
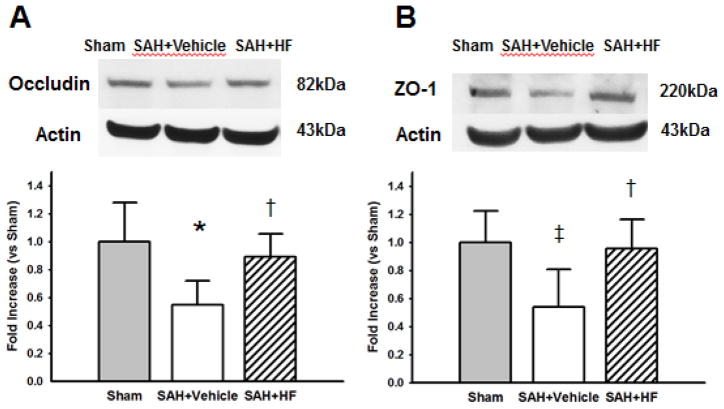
The levels of Evans blue dye extravasation (Figure 5) in Vehicle group was significantly higher in left hemisphere compared to Sham (p <0.01) and HF (p <0.01) groups; Vehicle group in right hemisphere compared to Sham (p <0.01) group; and Vehicle group in brain stem compared to Sham (p <0.01) group. Western Blot analyses were evaluated for quantification of occludin and ZO-1 in left hemispheres at 24 hours after surgery. The levels of occludin (Figure 6A) was significantly lower in Vehicle group compared to Sham (p <0.01) and HF (p <0.05) groups. ZO-1 level was also significantly lower in Vehicle group compared to Sham (p <0.05) and HF (p <0.05) groups.
Figure 5.
Evans blue extravasation after SAH in single treatment groups. HF group showed significantly attenuation in Evans blue extravasation compard with Vehicle in left hemisphere. Values are mean ± SD. * p< 0.01 vs Sham, †p< 0.01 vs Vehicle group.
Figure 6.
Representative Western blots and quantitative analysis after SAH. Occludin (A) and zona occludens-1 (ZO-1) (B) in the ipsilateral hemisphere with single treatment were evaluated 24 hours after SAH. Expression levels of each proteins in Western blot are expressed as a ratio of Actin levels for normalization. The levels of occludin in HF group was significantly restored compared to Vehicle groups (A). ZO-1 level in HF group was also significantly restored compared to Vehicle group (B). N=6 rats per group. Values are mean ± SD. * p< 0.01 vs Sham, †p< 0.05 vs Vehicle, ‡p< 0.05 vs Vehicle group.
4. Discussions
Here we showed that, (1) Rock was activated at 24 hours after SAH and HF decreased the activity, (2) single treatment of HF ameliorated neurological impairment and brain edema at 24 hours, (3) BBB leakage was reduced by HF via preservation of TJ proteins, Occludin and Zo-1, in SAH. To the best of our knowledge, this is the first report to describe the protective effects of Rock on early brain injury in SAH. SAH grade, at each time point, was comparable between groups. Basic physiological parameters were also comparable during operation between Vehicle and HF group. All cases of mortality suffered severe SAH. The mortality rate was not statistically significant improved by treatment with HF and Y27632 compared to Vehicle.
Rock is strongly activated in endothelial cells of peripheral region of brain ischemia after 24 hours (Yagita, et al., 2007). Activation of RhoA/Rock was determined to increase myosin light chain phosphorylation, the generation of F-actin stress fibers, and endothelial hyperpermeability (van NieuwAmerongen, et al., 2007). Rock inhibitor prevents monocyte infiltration across endothelial cells (Honing, et al., 2004). Abnormal Rock activity contributes abnormal smooth muscle cell contractions observed in cerebral vasospasm (Liao, et al., 2007). Although Rock expression increases on day 5 in the basilar artery of SAH rat models (Miyagi, et al., 2000), the profiles of Rock activation in the brain during early brain injury period remained to be elucidated. Our quantification demonstrated a 1.21-fold increase in Rock activity after SAH in 24 hours, and HF treatment restored this disorder. HF (isoquinolinesulfonic acid derivative) and Y27632 (pyridine derivative) are structurally unrelated selective Rock inhibitors (Uehata, et al., 1997). In our study both HF and Y27632 attenuated brain water content in ipsilateral hemisphere, however only HF showed statistically significant amelioration in neurological dysfunction 24 hours after SAH. Rock exists as two isomers, Rock-I and Rock-II, and Y27632 inhibits both Rock isomers stronger than HF (Yano, et al., 2008). Targeting both Rock isoforms by siRNA severely disrupted barrier integrity, whereas targeting one isoform was dispensable for forming a proper barrier in endothelial cells (van NieuwAmerongen, et al., 2007). Although HF and Y27632 showed similar tendency for amelioration of brain water content, this suggests that HF is more feasible drug than Y27632 for SAH. Therefore for the rest of study we chose HF.
As the terminal elimination half-life of HF was only 0.88 hours in rats (Satoh, et al., 2001), we tested the effect of two treatments. There was no more adverse effect and the mortality rate in HF group with two treatments was prevented (0%) when compared with single treatment (21.2%). However, the results of neurological scores and brain water content with two treatments did not show prominent amelioration compared with one treatment. One of the possibilities is that since all animals treated with two HF survived, including the severe SAH, this survival rate contaminated the results of brain water content and neurological outcome because even severe SAH animals survived and compared. We also observed that there was no difference between Vehicle and HF group with single treatment in neurological score and brain water content at 72 hours after surgery, even though the trend remained, suggesting that it is because BBB damage partially recovers at 72 hours in rat perforation model of SAH (Schöller, et al., 2007) and the advantage of single HF treatment may not last up to 72 hours.
Evans blue stain has an ability to bind to serum albumin (Manaenko, et al., 2011). Since plasma albumin does not pass the BBB under normal physiologic conditions, spectrophotometric determination of Evans blue stain accumulation is an easy and reliable way to estimate BBB permeability and vasogenic edema (Manaenko, et al., 2011). We observed that SAH aggravated Evans blue extravasation, and HF treatment significantly reduced the aggravation in ipsilateral hemisphere. BBB dysfunction may amplify inflammation, leading to further parenchymal damage and edema formation, which contributes to ischemic secondary injuries (Ostrowski, et al., 2006). Occludin is a transmembrane protein and its cytoplasmic tails are linked to ZO-1 which anchors the junction to the actin cytoskeleton (Aijaz, et al., 2006). Recent studies reported that Rock opened the BBB by degrading TJ proteins. Rock activation mediated occludin phosphorylation resulting in diminished barrier tightness (Persidsky, et al., 2006). Rock inhibition prevented ZO-1 redistribution and increased endothelial permeability (Stamatovic, et al., 2003). In our study, SAH degraded occludin and ZO-1, and HF restored these proteins after surgery.
HF is an active metabolite of non-selective Rock inhibitor, Fasudil, which has been marketed since 1995 in Japan for the treatment of vasospasm after SAH (Liao, et al., 2007). Here we focused on the association between selective Rock inhibition and protection of TJ proteins in endothelial cells which play a significant role in BBB permeability, a major contributor to early brain injury after SAH. However there are also other mechanisms of Rock inhibition which could have a beneficial effect after SAH. Rock inhibition increases phosphatidylinositol 3 kinase/protein kinase Akt activity, which leading to release the endothelial nitric oxide synthase in endothelial cells (Wolfrum, et al., 2004).
Rock inhibition may have the potential to reduce early brain injury especially BBB rupture and brain edema after SAH.
Highlights.
Rock was activated at 24 hours after SAH and hydroxyfasudil (HF) treatment decreased Rock activity.
HF ameliorates neurological impairment and brain edema after SAH.
HF treatment reduced Evans blue extravasation in the ipsilateral hemisphere after SAH.
HF prevented BBB disruption via preservation of Occludin and Zo-1 after SAH.
Acknowledgments
This study is partially supported by a grant from NIH NINDS 54685 to JH.
Abbreviations
- SAH
subarachnoid hemorrhage
- EBI
early brain injury
- BBB
blood-brain barrier
- ECs
endothelial cells
- TJs
tight junctions
- ZO-1
zonula occludens-1
- Rock
Rho kinase
- HF
Hydroxyfasudil
- ICA
internal carotid artery
- PBS
phosphate-buffered saline
- ANOVA
analysis of variance
- BWC
brain water content
- MAP
mean arterial blood pressure
- HR
heart rate
Footnotes
Conflict of Interest
Authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol. 2006;248:261–298. doi: 10.1016/S0074-7696(06)48005-0. [DOI] [PubMed] [Google Scholar]
- Allen C, Srivastava K, Bayraktutan U. Small GTPase RhoA and its effector rho kinase mediate oxygen glucose deprivation-evoked in vitro cerebral barrier dysfunction. Stroke. 2010;41:2056–2063. doi: 10.1161/STROKEAHA.109.574939. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neuro Biol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26:1086–1092. doi: 10.1161/01.str.26.6.1086. [DOI] [PubMed] [Google Scholar]
- Chrissobolis S, Sobey CG. Recent evidence for an involvement of rho-kinase in cerebral vascular disease. Stroke. 2006;37:2174–2180. doi: 10.1161/01.STR.0000231647.41578.df. [DOI] [PubMed] [Google Scholar]
- Claassen J, Carhuapoma JR, Kreiter KT, Du EY, Connolly ES, Mayer SA. Global cerebral edema after subarachnoid hemorrhage: frequency, predictors, and impact on outcome. Stroke. 2002;33:1225–1232. doi: 10.1161/01.str.0000015624.29071.1f. [DOI] [PubMed] [Google Scholar]
- Duris K, Manaenko A, Suzuki H, Rolland WB, Krafft PR, Zhang JH. Alpha7 nicotinic acetylcholine receptor agonist PNU-282987 attenuates early brain injury in a perforation model of subarachnoid hemorrhage in rats. Stroke. 2011;42:3530–3536. doi: 10.1161/STROKEAHA.111.619965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- Honing H, van den Berg TK, van der Pol SM, Dijkstra CD, van der Kammen RA, Collard JG, de Vries HE. RhoA activation promotes transendothelial migration of monocytes via ROCK. J Leukoc Biol. 2004;75:523–528. doi: 10.1189/jlb.0203054. [DOI] [PubMed] [Google Scholar]
- Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol. 2007;50:17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaenko A, Chen H, Kammer J, Zhang JH, Tang J. Comparison Evans Blue injection routes: Intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. J Neurosci Methods. 2011;195:206–210. doi: 10.1016/j.jneumeth.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi Y, Carpenter RC, Meguro T, Parent AD, Zhang JH. Upregulation of rho A and rho kinase messenger RNAs in the basilar artery of a rat model of subarachnoid hemorrhage. J Neurosurg. 2000;93:471–476. doi: 10.3171/jns.2000.93.3.0471. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Colohan AR, Zhang JH. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28:399–414. doi: 10.1179/016164106X115008. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, Shimokawa H, Kaibuchi K, Ikezu T. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE) Blood. 2006;107:4770–4780. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Utsunomiya T, Tsurui K, Kobayashi T, Ikegaki I, Sasaki Y, Asano T. Pharmacological profile of hydroxyfasudil as a selective rho kinase inhibitor on ischemic brain damage. Life Sci. 2001;69:1441–1453. doi: 10.1016/s0024-3205(01)01229-2. [DOI] [PubMed] [Google Scholar]
- Schöller K, Trinkl A, Klopotowski M, Thal SC, Plesnila N, Trabold R, Hamann GF, Schmid-Elsaesser R, Zausinger S. Characterization of microvascular basal lamina damage and blood-brain barrier dysfunction following subarachnoid hemorrhage in rats. Brain Res. 2007;1142:237–246. doi: 10.1016/j.brainres.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Shin HK, Salomone S, Potts EM, Lee SW, Millican E, Noma K, Huang PL, Boas DA, Liao JK, Moskowitz MA, Ayata C. Rho-kinase inhibition acutely augments blood flow in focal cerebral ischemia via endothelial mechanisms. J Cereb Blood Flow Metab. 2007;27:998–1009. doi: 10.1038/sj.jcbfm.9600406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci. 2003;116:4615–4628. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Ayer R, Sugawara T, Chen W, Sozen T, Hasegawa Y, Kanamaru K, Zhang JH. Protective effects of recombinant osteopontin on early brain injury after subarachnoid hemorrhage in rats. Crit Care Med. 2010;38:612–618. doi: 10.1097/CCM.0b013e3181c027ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vanGijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- van NieuwAmerongen GP, Beckers CM, Achekar ID, Zeeman S, Musters RJ, van Hinsbergh VW. Involvement of Rho kinase in endothelial barrier maintenance. Arterioscler Thromb Vasc Biol. 2007;27:2332–2339. doi: 10.1161/ATVBAHA.107.152322. [DOI] [PubMed] [Google Scholar]
- Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24:1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Yagita Y, Kitagawa K, Sasaki T, Terasaki Y, Todo K, Omura-Matsuoka E, Kaibuchi K, Hori M. Rho-kinase activation in endothelial cells contributes to expansion of infarction after focal cerebral ischemia. J Neurosci Res. 2007;85:2460–2469. doi: 10.1002/jnr.21375. [DOI] [PubMed] [Google Scholar]
- Yano K, Kawasaki K, Hattori T, Tawara S, Toshima Y, Ikegaki I, Sasaki Y, Satoh S, Asano X, Seto M. Demonstration of elevation and localization of Rho-kinase activity in the brain of a rat model of cerebral infarction. Eur J Pharmacol. 2008;594:77–83. doi: 10.1016/j.ejphar.2008.07.045. [DOI] [PubMed] [Google Scholar]