Abstract
Pregnancy and lactation produce a plethora of hormonal changes in females that promote maternal care of offspring. Males in the biparental marmoset species, (Callithrix jacchus), demonstrate high levels of parenting behaviour and express enhanced circulating reproductive hormones. Furthermore, these hormonal changes are influenced by paternal experience. In order to determine if the paternally experienced male marmoset has altered neurocrine hypothalamic release, as the maternal females does, we examined the release of several reproductive neurocrines, dopamine (DA), oxytocin (OT) and vasopressin (AVP) and prolactin (PRL), in cultured explants of the hypothalamus of paternally experienced male marmosets compared with naïve, paternally inexperienced males. DA levels secreted from the isolated hypothalamus were significantly lower in the experienced males while OT and PRL levels were significantly higher than levels found in inexperienced males. PRL levels decreased rapidly in the hypothalamic media suggesting PRL production occurs elsewhere. AVP levels did not change. Stimulation of the cultured explants with oestradiol significantly decreased DA levels in the inexperienced males but did not alter the other neurocrines suggesting a direct effect of oestradiol on DA suppression in the hypothalamus. While other factors such as age and rearing experience with siblings may play a role in hypothalamic neurocrine levels, these results demonstrate that paternal experience may impact the secretion of neurocrines in a male biparental primate.
Keywords: paternal experience, prolactin, OT, oestradiol, dopamine, vasopressin, hypothalamus
Introduction
Understanding the role of neuroendocrine regulation of parenting behaviors has implications for optimizing the health outcomes of offspring. Neural pathways and the regulation of maternal care has been studied extensively in rodents (1–3).Indeed, long-term changes in the brain occur with parenting experience illustrating the neuroplasticity of the brain (4). Parenting behaviors are important for fathers of biparental species, including humans, where fathers help support the survival and promote positive physical and mental health in offspring (5). The common marmoset, Callithrix jacchus, is one of the few nonhuman primates that show bi-parental care where the father’s early care of infants is critical to their survival (6). Father marmosets begin carrying infants on the first day of their birth and show paternal behaviors such as cleaning the infant(s), stimulating urination, protecting and food sharing (7).
Neural and endocrine differences in male marmosets have been associated with paternal experience. Both first-time and experienced marmoset fathers have an increased density of dendritic spines on pyramidal neurons in prefrontal cortex when compared with nonfathers (8). This area of the brain has been implicated in goal directed behaviors. Additionally, fathers have increased AVP V1a receptors in the prefrontal cortex during direct parenting but the receptors decline as the infant age increases, indicating a relationship with direct care (8). Oxytocin (OT) has been shown to produce an effect on marmoset fathers behavior towards offspring. Intracerebroventricular infusion of OT into marmoset fathers reduces their refusal of food sharing with their young offspring (9). Physical changes associated with the paternal experience have been observed in the biparental marmosets and tamarins, Saguinus oedipus, where in both species experienced fathers show weight gain during their mate’s pregnancy suggesting a metabolic process to prepare for infant care (10). Additionally, expectant fathers show increased urinary prolactin (PRL), oestrogens, glucocorticoids and testosterone in a predictable pattern across the gestational period (11). Experienced fathers have more pronounced hormonal changes than first-time fathers, indicating an experience effect. Physical and hormonal changes that occur in marmoset fathers are not seen in nonfathers. Behaviorally, inexperienced males show significantly less motivation to respond to infants than experienced fathers (12). PRL levels also significantly increase in the male following parturition while testosterone levels decline during the infant care period (13) and testosterone levels vary related to paternal experience in the Wied’s marmoset, Callithrix kuhlii (14). Lowered testosterone during the intensive infant care period occurs in humans as well and is thought to reduce aggressive behaviors (15). Marmoset fathers show a direct relationship of immediate increased PRL in response to carrying infants (16,17) and decreased testosterone and increased oestrogens are observed in response to infant odor stimuli (18,19).
The peripheral changes of parenting hormones found in male marmosets with paternal experience should be associated with altered brain hormones or “neurocrines” known to be regulating maternal care. PRL has been demonstrated to exert its effect in the hypothalamus in promoting maternal care behaviours in rodents (2,3,20). Brain PRL receptors are found in key target areas for maternal behavior (21). PRL regulates nest building and pup retrieval by acting in the medial preoptic area (MPOA) of the rat brain (3). Further direct evidence of PRL’s role in parental behavior has been found in birds (22) and in fish (23). PRL is under direct control by dopamine (DA) produced in the hypothalamic arcuate nucleus (24). The dopaminergic system that provides the major input to the anterior pituitary’s PRL producing lactotrophs is the tuberoinfundibular dopaminergic system (TIDA). Moreover, pituitary PRL feeds back on the TIDA neurons to regulate its own secretion via receptors expressed on the arcuate neurons (25). During lactation in the female, an impaired negative feedback of PRL on DA occurs through the suckling stimulus from the neonate and allows for prolonged high secretion of PRL (2). Given PRL’s role in regulating hypothalamic neurons associated with maternal and paternal care, we predict that PRL will be found in the hypothalamus of paternally experienced marmoset fathers.
Other hypothalamic neurocrines regulate parental behavior. OT and AVP may regulate parental behavior in both males and females of biparental species. OT has been reported to facilitate maternal-infant bonding in many mammals (e.g.: 26,27,28). OT antagonists disrupt maternal-infant bonds (29) and pregnancy and lactation increase OT receptor binding in central brain areas (8). In rodents both central OT and AVP receptor binding have been found in several brain regions regulating paternal behavior (30). OT and AVP antagonists injected intracerebroventricularly reduce paternal care behaviors in prairie voles, Microtus ochrogaster (31). Infusions of AVP in the prairie vole lateral septum increase paternal behaviors such as licking/grooming, crouching/huddling over, contacting and retrieving pups (32). A relationship between prairie vole paternal experience and AVP immunoreactive fiber density has been demonstrated in the lateral septum. AVP has been found to be involved in maternal memory in the rat (33) and marmoset fathers have increasd AVP V1a receptors in the prefrontal cortex during direct parenting (8). If OT and AVP are regulating aspects of paternal care, we would predict that these neurocrines would have increased synthesis in the hypothalamus of paternally experienced marmosets.
Oestrogen has been reported to influence the release or suppression of several neurocrines that act in the hypothalamus. Increased oestrogen will increase the production of prolactin receptors in the choroid plexus and thereby facilitate PRL’s entry into the brain (34,35). Additionally, oestrogen depresses the activity of TIDA neurons and therefore stimulates PRL secretion (36). If oestrogens are involved in regulating DA release in the primate brain, we would expect to see a suppression of DA with oestradiol stimulation.
While the etiology of maternal behavior has been well studied, little is known about the neuroendocrine control of paternal behavior and how it is activated in lieu of pregnancy-induced hormonal signals. Previous studies with marmosets have focused on paternal care regulation by examining male behaviours and circulating hormones. No studies have examined hypothalamic secretion of the “parental hormones” and possible interactions due to oestrogen stimulation. Based upon the physical and behavioral manifestations in the common marmoset fathers, it is reasonable to expect alterations in neurocrine hypothalamic secretion due to paternal experience.
To determine if the hormonal changes that have been seen peripherally are also found in the brain of parental male marmosets, we studied the secretion of the hypothalamic neurocrines, DA, OT and AVP from hypothalamic explants and levels of PRL. Given the neuroendocrine changes associated with both fatherhood and direct care of infants in the marmoset, we hypothesized that hypothalamic explants from experienced fathers would show differences in neurocrine release when compared to explants from naïve, parentally inexperienced males. Furthermore, we predicted that these changes would be long-term, lasting beyond the infant dependent age as seen with maternal care and thus used males with no dependent offspring at the time of the study. In addition, we stimulated the hypothalamic explants with oestradiol to test its influence on the release or suppression of these hypothalamic neurocrines. Male marmosets have high circulating levels of oestradiol (as high as cycling female marmosets during the follicular and periovulatory period) that are influenced by parenting experience and PRL levels (19). Infant stimuli, such as odor of their own infant, can stimulate a significant increase in oestrogens in fathers when their infants are dependent upon them (19).
Materials and Methods
The ten male marmosets selected for this study were housed at the Wisconsin National Primate Research Center, University of Wisconsin, Madison, WI, USA. Five of the males were paternally experienced and housed with their mate and any offspring remaining. The experienced males had at least two sets of offspring prior to the study and were between 8.7 to 11.2 years of age. None of the experienced males had experienced infant births recently (8 months – 36 months). The other five males had not fathered offspring and were between the ages of 2.9 to 5.8 years. However, all the inexperienced males had previous experience with younger siblings (Table 1) All males were housed with a female who was prevented from becoming pregnant with the use of a prostaglandin F2α analogue (cloprostenol sodium, 37). There were no differences in weight between the experienced and the inexperienced males at the time of the study (t = 2.0, P = 0.08). While all males in this study could be exposed to olfactory, auditory or visual cues from infants in other cages, males do not respond to cues of infants other than their own (19). Table 1 provides detailed information on the males and their sibling experience and pairing conditions.
Table 1.
Age, parental experience and housing conditions for male common marmosets at the time of the study.
| Male | Age | Paternal experience |
Infant sets borna |
Younger sibling sets bornb |
Time from pairing with mate (months) |
Offspring sex/age living with male |
|---|---|---|---|---|---|---|
| 711 | 9.3 | Experienced | 4 | 0 | 14 | 0 |
| 583 | 11 | Experienced | 6 | 1 | 26 | 0 |
| 911 | 7.8 | Experienced | 5 | 2 | 18 | 0 |
| 833 | 8.7 | Experienced | 5 | 2 | 13 | male: 36 months old |
| 573 | 11.2 | Experienced | 10 | 0 | 9 | male/ female: 9 months |
| 1251 | 4.2 | Naïve | 0 | 3 | 33 | 0 |
| 1247 | 4.3 | Naïve | 0 | 1 | 29 | 0 |
| 1257 | 4.5 | Naïve | 0 | 5 | 17 | 0 |
| 1457 | 2.9 | Naïve | 0 | 4 | 8 | 0 |
| 1117 | 5.8 | Naïve | 0 | 1 | 14 | 0 |
Infant sets born refers to the number of infant sets (twins or triplets) that were born to the father.
Younger sibling sets refers to the number of times infants (twins or triplets) were born while the males were subadults in their family unit.
Marmosets were on a 12L: 12D cycle with lights on at 06:30 and temperature was maintained at 27°C with ~50% humidity. All test subjects were fed their allotment of standardized daily diet (Mazuri Callithrichid High Fiber Diet 5MI6, Purina Mills, St. Louis, MO at 64 kcal/day/animal) plus an additional daily enrichment food and tap water was available ad libitum. The marmosets were housed in 0.6×0.91×1.83 m cages equipped with a metal nest-box, wooden dowels and other tactile items. The Animal Care and Use Committee of the Graduate School at the University of Wisconsin, Madison, WI, approved this study.
Tissue collection
All tissue collection and blood sampling took place between 09:00 and 11:00. Immediately prior to euthanasia at least 1 ml blood sample was taken to use for assessing circulating hormones. The procedure required males to be restrained in a restraint tube that they were habituated to and a femoral vein blood sample taken and processed for serum samples. Once the blood sample was acquired, the males were anesthetized with 1–2% isoflurane (0.6L/min oxygen) and then euthanized by exposure to 100% CO2 until respiration ceased. The hypothalamus-delimited laterally by the hypothalamic fissures, the anterior by a cut 2 mm anterior to the anterior aspect of the optic chiasm, posterior by the rostral portion of the mamillary bodies - was carefully removed by a horizontal cut 3 mm in depth. The hypothalami were bisected down midline and the hemihypothalami were placed in 10 ml culture media until perifusion was initiated two to three hours following removal from the brain.
Hypothalamic perifusion
The culture media was a modified Kreb-Ringer bicarbonate buffer containing 2.2 mM CaCl2, 154 mM NaCl, 5.6 mM KCl, 1.0 mM MgCl2, 6.0 mM NaHCO3, 10mM glucose, 2 mM HEPES, 0.1% BSA, and 0.006% bacitracin, pH = 7.40. Hypothalamic explants were transported to an Endotronics Accusyst S cell perifusion system and were exposed to a continuous flow of media (37°C) at a flow rate of 1ml/20 minutes for a period of 24 hours using the perifusion system described for marmosets (38). Each male provided one hemi-hypothalamus that was used for media and oestradiol challenge while the other half was used for media and cholesterol challenge at the same time post collection and during the same perfusion run. The explants were treated for 1 hour with media then followed by 2 hours of treatment with 1.0 ng/ml 17-β oestradiol or 1.0 ng/ml cholesterol. Samples were fractioned at 20-minute intervals and frozen at −80° C until assay.
Assays
Samples were thawed and an aliquot was taken for DA, PRL, OT and AVP for the individual assays. For most samples all assays were started on the same day.
DA
At first thaw, 150 µl of perfusate was aliquotted for HPLC-ECD (high pressure liquid chromatography – electrochemical detection) into polypropylene inserts for HPLC vials. To this, 50 µl of internal standard, DHBA(3,4-Dihydroxybenzylamine), was added at a 1:10,000 concentration. Samples were loaded onto a −5° C autosampler (ESA #542) and injected at 50 µl using 100 µl partial loop onto a 4.6 × 250 mm C18 column (Ultrasphere, Beckman Coulter). Our system consisted of ESA (Chelmsford, MA) isocratic pumps with Colochem III electricalchemical detector. The mobile phase consisted of 10.5% acetonitrile in phosphate buffer, pH 3.0 containing 1.7 mM 1-octanesulfonic acid. The voltage was set as following: guard at −350 mV, E1 at −150 mV and E2 at +250 mV. Standards for catecholamines were serially diluted from 45.55 ng to 0.0445 ng and the limit of detection was 44.5 pg. Inter- and intraassay coefficient of variation (CV) for our control at 37.5 pg in quadruplet was 4.62% and 1.37%, respectively, n = 5. Our DHBA internal standard resulted in an interassay CV of 5.57% and an intraassay CV of 0.43%.
PRL
We analyzed media and serum PRL according to the method reported in Ziegler et al. (13). This assay uses recombinant monkey PRL (cynomolgus, AFP1059) and anti-monkey PRL (AFP291891) from the National Hormone & Peptide Program. The assay has been fully validated and biological validation was performed to indicate the assay is measuring marmoset PRL (13). Serial dilutions of marmoset serum were parallel to the standard curve, t = 1.19, P > 0.05 and accuracy was 96.77 ± 1.29%.. Hypothalamic samples were run in singles at 200 µl of media. Inter- and intraassay CVs were 7.27% and 1.56%, respectively. Blood samples collected at the time of euthanasia were analyzed for PRL by using 50 µl of the sample in duplicate.
OT and AVP
Perfusates were aliquotted as 400 µl of samples into glass culture tubes and 600 µl of water were added to the sample. Samples were purified and concentrated by solid phase extraction using SepPak (#WAT023590, Waters, MA). Columns were conditioned with 1 ml methanol, 1ml purified water and then sample was added and vacuumed through the column. The column was washed with 10% acetonitrile, 1% trifluroacetic acid and allowed to dry. The sample was eluted with 80% acetonitrile and dried in a 62° C waterbath under air. Samples were reconstituted in assay buffer and half were plated for OT and half for AVP. Samples were quantified using the Assay Design EIA (Ann Arbor, MI) following the kit instructions. The OT assay was validated using accuracy and parallelism. Serial dilutions of pooled marmoset perfusate was parallel to the standards (t=0.88, P>0.05, n=7) and accuracy was 94.86% (n=7). Recoveries for OT were 84.81±4.78 (n=12). Sensitivity of the OT assay is 1.56 pg. Added AVP into the OT assay did not show cross-reactivity. We tested the specificity of the antibody used or this assay. A marmoset sample processed through HPLC separation and fractionation indicated that OT binding in the EIA assay only occurred in the fraction where OT standard elutes indicating specificity. Inter and intra- assay CVs were 19.35% and 4.54%, respectively for OT. For the AVP assay serial dilutions of pooled marmoset perfusate were parallel to the standards (t=0.85, P>0.05, n=7) and accuracy was 103.02±1.40%. Recoveries for AVP were 93.32±8.01% and sensitivity of the AVP assay was 0.24 pg. Added OT into the AVP assay did not show cross-reactivity. Inter- and intraassay CVs were 14.27% and 2.44% for AVP.
Analyses
Two-way ANOVAs were performed for each compound to examine the source of variation by experience condition and time. Since samples were collected every 20 minutes, the media were analyzed at 20, 40 and 60 minute time points in both the experienced (n=5) and the inexperienced (n=5) males. Where significant interactions were observed one-way ANOVAs were performed. To test the effect of oestradiol treatment on the hormones we compared the values from media, oestradiol stimulation and cholesterol stimulation by repeated measures ANOVA. Circulating serum PRL levels were compared between the experienced and inexperienced males by unpaired t-tests. All comparisons were two-tailed and significant differences were less than a probability of 0.05. Age, number of infant sets raised and number of past sibling experience were correlated to the neurocrine values by using Spearman rank.
Results
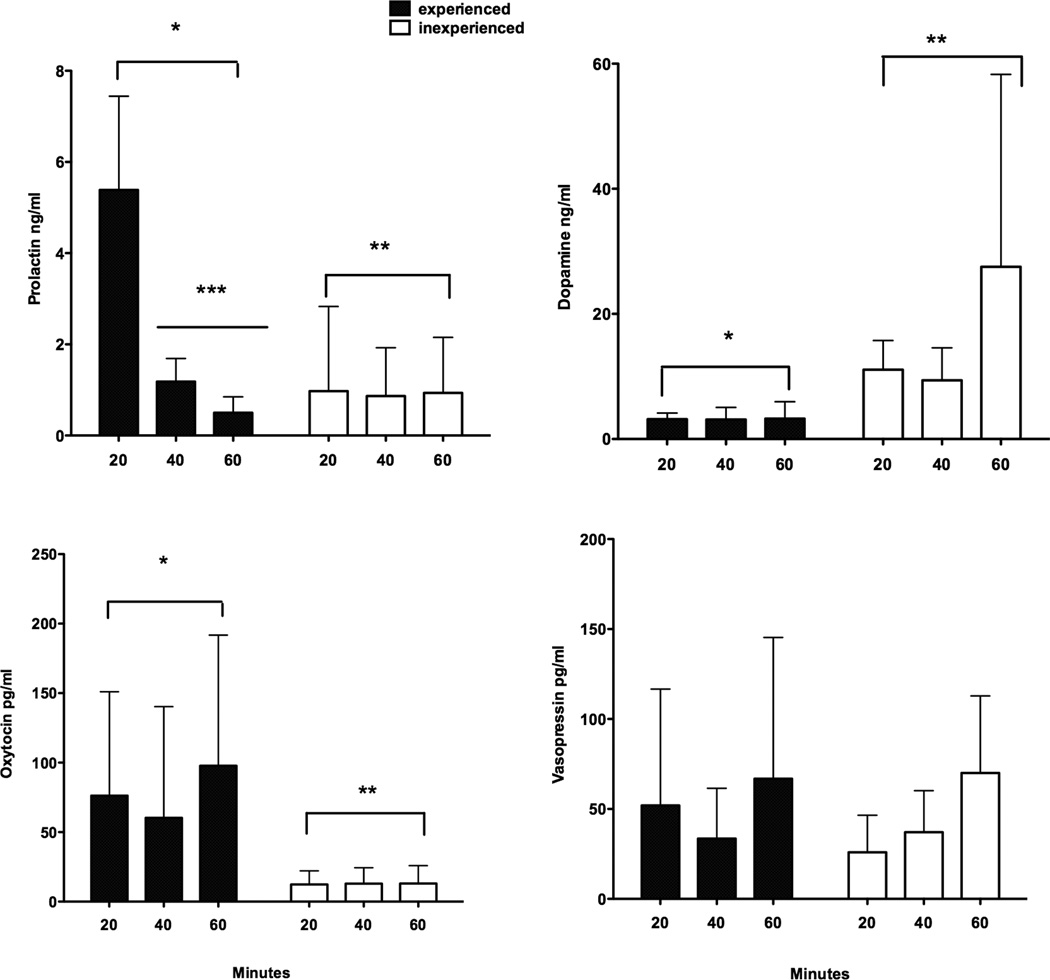
Figure 1 shows the results of experimental condition across time for PRL, DA, OT and AVP found in hypothalamic media. There was a significant interaction between the experimental condition and time for PRL (F = 9.58, P = 0.009, df = 2,24). Over the 60 minutes of incubation the two groups behaved differently as PRL levels declined over time in the experienced males (F = 23.08, P = 0.005, df = 2,4) while no changes over time were found for the inexperienced males (F = 0.01, P = 0.88, df = 2,4). PRL levels were significantly different by condition (F=8.69, P=0.007, df=2,24) and by time (F=10.14, P=0.0006, df=2,24). Post hoc Tukey’s indicated that the 20-minute sample for experienced males was significantly different from the 40 and 60-minute samples for experienced males (P < 0.05). DA did not show a significant interaction (F = 1.50, P = 0.244, df = 2,24) but DA was significantly higher for inexperienced than experienced males (F = 7.37, P = 0.01, df = 2,24) but not by time (F = 1.52, P = 0.24, df = 2,24). OT was significantly higher for the experienced males (F = 5.47, P = 0.05, df = 1,8) but not by time (F = 0.38, P = 0.69, df = 2,16). AVP was not significantly different by condition (F = 0.11, P = 0.75, df = 1,8) or time (F = 1.57, P = 0.24, df = 2,16).
Figure 1.
Comparisons of mean ± s.e.m. levels of DA, PRL, OT and AVP in parentally experienced and inexperienced male marmosets from hypothalamic explants cultured in media for 60 minutes. * Indicates significant differences by condition from experienced *and inexperienced **. In the experienced condition PRL at 20 minutes was significantly differencent from the other times ***. PRL levels (upper left panel) were significantly different by experience and by time for experienced males. DA levels (upper right) were significantly higher for experienced males. OT levels (lower left panel) were significantly higher for the experienced males. AVP levels (lower right panel) were not different by experienced condition or time.
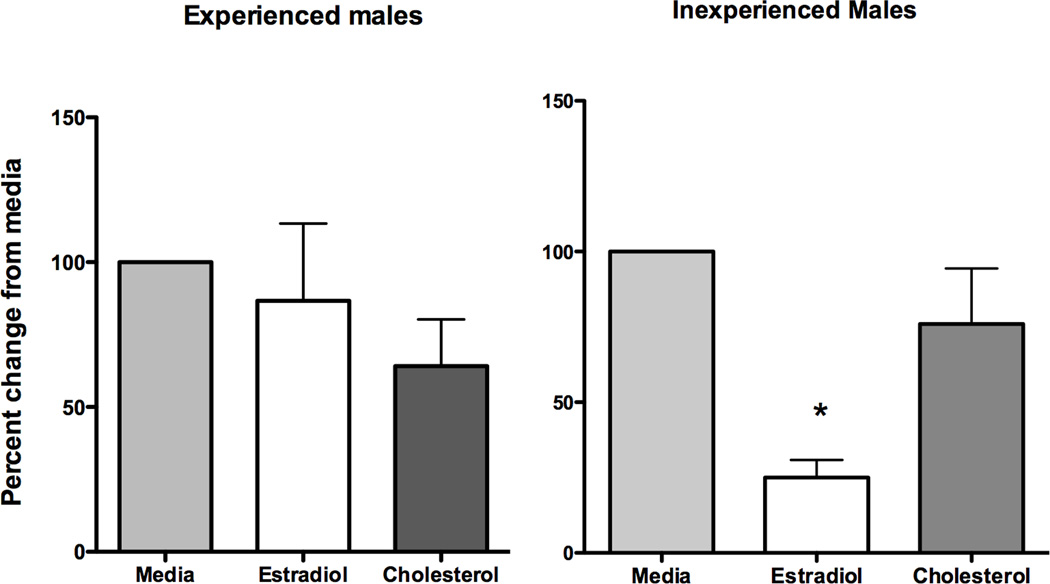
Oestradiol treatment of the hypothalamic explants indicated a significant percent decrease in DA levels but only for the inexperienced males (F = 9.9, P = 0.007) where DA levels were significantly lower with oestradiol stimulation than in the media and cholesterol condition (P < 0.05), Figure 2. Oestradiol stimulation did not change the already low levels of DA found in experienced male hypothalamic perfusates. Oestradiol stimulation also did not significantly alter the hormone levels of PRL, OT and AVP from the media or the cholesterol control.
Figure 2.
Mean ± s.e.m. percent change in DA levels from initial media levels following stimulation of hypothalamic tissue with either oestradiol (1 ng/ml) or cholesterol (1 ng/ml) for paternally experienced and inexperienced males. Oestradiol but not cholesterol significantly lowered DA secretion for inexperienced males where as no difference was found for the experienced males.
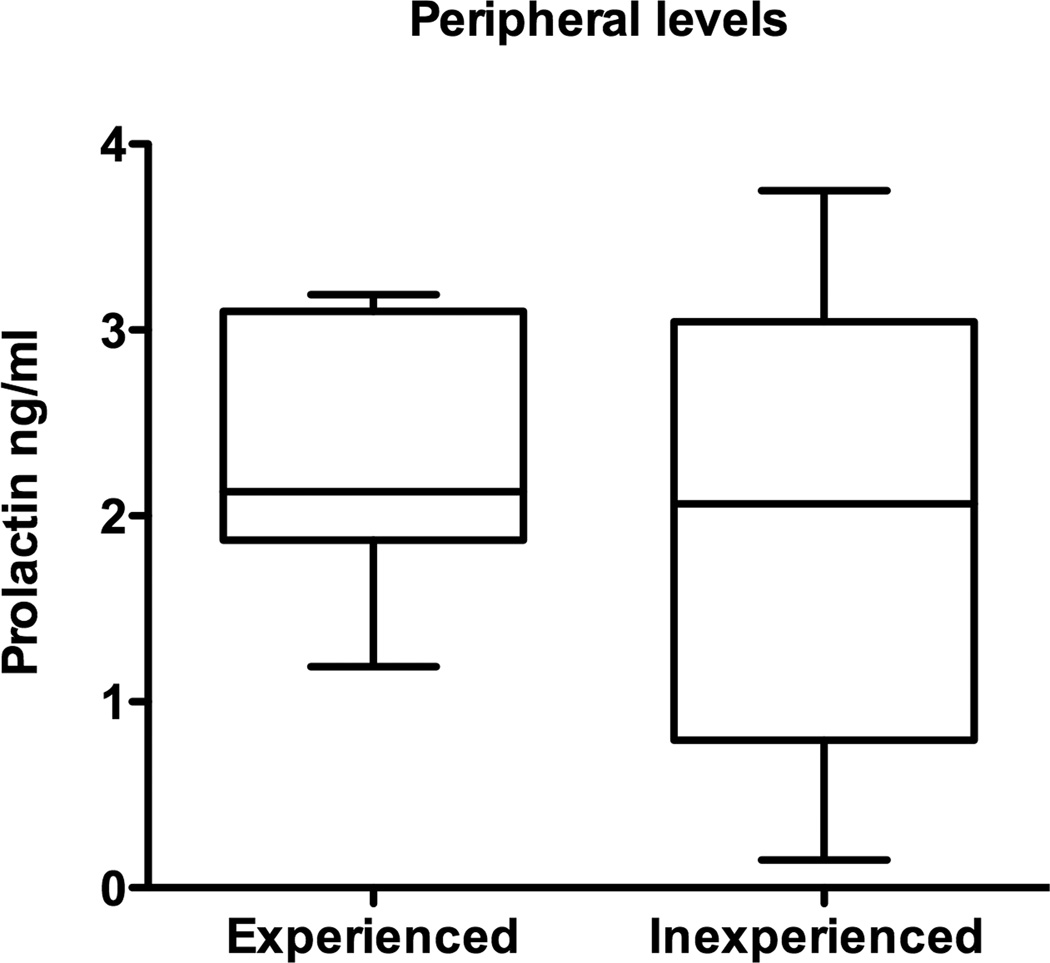
Since PRL would not be expected in large amounts in the hypothalamus, we measured circulating levels of PRL in blood collected at the time of hypothalamic collection and compared the levels from experienced males and inexperienced males. No differences in the levels of circulating PRL were found between the experienced males and the inexperienced: experienced males = 2.344 ± 0.28 ng/ml (mean ± sem), inexperienced = 2.02 ± 0.44 ng/ml (Figure 3).
Figure 3.
Median and range of peripheral serum levels of PRL taken from experienced and inexperienced males prior to hypothalamic removal. No significant differences in PRL levels were found due to experience condition.
We also tested the correlation of the neurocrines with age, paternal experience and sibling experience in the combined 60-minute media from the perfusates, Table 2. Age was significantly correlated with media levels of PRL and DA but not OT or AVP. There were no significant correlations of number of own infant sets raised with media PRL, DA, OT or AVP. However, the number of previous siblings males had participated in rearing did show a significant correlation with DA levels and nearly significant negative correlation with PRL but no correlation with OT or AVP.
Table 2.
Age and number of previous sibling experience correlation with neurocrine secretion in the media for 10 male marmosets.
| Factor | Media Neurocrine |
R (Spearman Rank) | P value |
|---|---|---|---|
| Age | PRL | 0.77 | 0.01 |
| Age | DA | −0.70 | 0.03 |
| Age | OT | 0.39 | 0.26 |
| Age | AVP | 0.22 | 0.54 |
| Number sibling experience | PRL | −0.61 | 0.07 |
| Number sibling experience | DA | 0.70 | 0.03 |
| Number sibling experience | OT | −0.48 | 0.17 |
| Number sibling experience | AVP | −0.15 | 0.68 |
Discussion
This study presents the first evidence of differential neurosecretion from the hypothalamus in a male bi-parental primate. Here we find significant differences in hypothalamic secretion from several neurocrines in tissue explants. DA levels were significantly lower in the paternally experienced compared to the naïve, inexperienced males; OT levels were significantly higher in the experienced fathers as was PRL. While our sample size was small and other factors may be involved, the results of this study suggest that differences in hypothalamic secretion of DA and OT may be related to paternal experience in male marmosets. Additionally, prolactin entering the hypothalamus may also be altered by parenting experience. The study also clearly demonstrated suppression of DA release by oestradiol in the isolated hypothalamus.
Media levels of DA were very low in the experienced males while they were significantly higher in the inexperienced males. Additionally, DA levels increased over time in the nonfathers, such that the 60-minute interval had the highest levels of DA. Thus during the study, the hypothalamic cells were viable and had the ability to increase their secretion over time. This suggests that some inhibition of DA release changed over the 60-minute period to allow a higher release of DA. We found that DA levels were significantly reduced when oestradiol was introduced to the hypothalamic cells of inexperienced males but not experienced males. The DA levels of experienced males were already low and therefore, this may explain why they did not respond to the oestradiol.
The source of the DA secretion in the hypothalamic explants is unknown. DA is produced in many areas of the hypothalamus. The TIDA system is located in the mediobasal hypothalamic arcuate nucleus and adjacent periventricular nucleus. These nuclei project to the external layer of the median eminence where they secrete into the hypophysial portal blood system to the anterior pituitary where DA inhibits PRL release. The diencephalic DA neurons are located in the dorsal regions of the hypothalamus, ventral thalamus and the regions adjacent to the third ventricle and additional DA neurons are located in the posterior regions of the dorsal hypothalamus and the periventricular gray of the central thalamus which projects to the spinal cord (39). Autoreceptors are present on DA neurons with the exception of TIDA neurons (40). It is likely that the DA secretion originated from the TIDA neurons since the stimulation with oestradiol reduced the DA secretion. In rodents oestradiol regulates DA by acting on the TIDA neurons and inhibiting DA release (35,41,42,43).
The hypothalamic cultured media from experienced males contained significantly higher PRL levels than levels found for inexperienced males, but the source of hypothalamic PRL observed in culture media is unknown. The decline in PRL levels in experienced fathers over the 20-minute intervals indicates an extrahypothalamic source of this PRL. There is some evidence that prolactin is secreted in the rat brain (44,45,46) and is found in the cerebellum, thalamus, brainstem, hippocampus, cerebral cortex and caudate (47). Altering PRL regulation with hypophysectomy or stress-induced PRL release does not alter extrahypothalamic PRL concentrations in the brain. However, it is also likely that PRL’s source is from the lactotrophes of the anterior pituitary via the choroid plexus. Interestingly, the differences we observed in PRL levels found in the hypothalamus due to experiential condition were not found in circulating PRL levels. Circulating PRL levels should reflect pituitary release of PRL (24). Reproductively experienced female rats have lower serum PRL concentrations compared with inexperienced rats where PRL feedback on the DA neurons in the arcuate nucleus may be upregulated (48). While the source of PRL is unknown for our experienced males, it is unlikely that improper dissection caused the PRL levels in the brain since it was condition dependent.
While PRL secretion from the hypothalamic explants was significantly higher in parentally experienced males, it was also higher by age. However, in our study circulating PRL levels were not higher in experienced males who were also older. While there is some evidence that human males increase circulating levels of prolactin with age (49), these are modest changes and other studies do not demonstrate age increases in circulating PRL in men (50). PRL levels from the hypothalamic explants did correlate with both the male’s age and experience. We cannot resolve whether age or experience have the observed effect of increasing brain PRL. Also, we know of no other studies providing insight into hypothalamic PRL differences due to age or parental experience.
The number of previous sibling sets a male had experienced correlated with the media secretion of PRL and DA. In marmosets, helping to carry and care for younger siblings provides an opportunity to learn the process of parenting and therefore could influence their parental care behaviours and potentially their neuroendocrine responses. In the present study PRL levels had a negative correlation with sibling experience while DA had a positive correlation. This is opposite the predicted outcome if sibling experience were a factor in PRL and DA levels in the hypothalamus. Additionally, different levels of sibling experience, where the inexperienced males had more sibling experience while in their families than the experienced males, confounded our study. While it is unlikely that in the present study sibling experience was a factor in our results, it cannot be ruled out.
Reproductive experience may increase central responsiveness to PRL without showing increasing levels of circulating PRL in rats. PRL stimulates maternal care by acting on neurons in the medial preoptic area (3). Reproductive experience in female rats, as defined by pregnancy and lactation, increases central PRL responsiveness and is likely involved in the retention of maternal behaviour and changes in PRL secretion (51). Parentally experienced female rats show lower circulating PRL than age-matched virgin rats but have higher PRL receptor mRNA and increased receptor expression in the mPOA and the arcuate nucleus indicating the increased central responsiveness to PRL that is not reflected in the peripheral measures (51). While we do not have receptor data or localized nuclear areas of activation in PRL for this species yet, similar changes are possible in our parentally experienced males. This would explain our results.
There is evidence that both OT and AVP are involved in regulating marmoset paternal care behaviors. Father marmosets infused with OT in the intercerebroventricular area and tested for food sharing with their offspring decreased the number of refusals to share thus increasing paternal tolerance to marmoset offspring during food sharing bouts (9). Food sharing bouts occur during the infant weaning phase when the father is highly involved in this process (9). Infant cues are known to increase OT receptors in specific brain areas of prairie voles (53) and can have lasting effects on social memory. Therefore the higher levels of OT in the hypothalamic media of experienced fathers in the present study may be due to a sustained release of OT. While AVP has been shown to be a component of prairie vole paternal care (53) and has been shown to be involved in experienced marmoset fathers increase in AVP V1a receptors in the brain, AVP may play a more immediate role than a long-term role since prefrontal cortex receptors decline once an infant age increases and fathers have reduced their infant care behaviors (8). Therefore, it is likely that we would not see AVP differences in the hypothalamus by experience in our study.
The changes found here for the experienced males are not a direct priming effect from infant sensory stimuli since none of the males were caring for dependent infants at the time of the study. These differences could be due to long-term changes in the male parental brain, as occurs in females (54). Multi-tasking and improved spatial memory have been demonstrated for mother rats (55,56). The neuroplasticity and re-patterning of the brain have provided many benefits to experienced mothers. For fathers of bi-parental species, these benefits might also be available with parental experience. For instance, in bi-parental California mouse (Peromyscus californicus) fathers will navigate a dry-land maze faster than nonparental males and are quicker to inspect novel stimuli (57). Common marmoset parents have been shown to learn which trees contain food containers (a foraging tree) significantly better than nonparents (for reference to unpublished work see 58). This suggests that in bi-parental species, neuroplasticity and altered brain hormones may be occurring in both sexes.
Besides the possible paternal experience effects on the differences found in hypothalamic explants on neurocrine secretion, this study shows for the first time that male marmosets have PRL in their hypothalamus. Additionally, the males only showed low PRL when DA levels were elevated suggesting an interaction between the two. Oestradiol stimulation of the hypothalamus significantly lowered DA indicating oestradiol’s direct regulation of DA in a primate brain. Further studies on PRL receptor density and PRL activation of specific DA and OT neurons are needed to establish PRL’s role in paternal care in the male marmoset. Since male marmosets do not undergo pregnancy and lactation it would be interesting to determine the specific cues that stimulate the neuroendocrine changes found with parenting behaviors for this species.
Acknowledgements
This work was supported by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, HD057684 to T.E. Ziegler, ARRA Summer Salary Supplement, and in part by the Wisconsin National Primate Research Center, RR000167 and the Institute for Clinical and Translational Research grant IUL1RR025011. We thank Dan Wittwer, Frtiz Wegner, Allison Bernard, Lara Bowers and Maisie Buntin for their help in hormonal determinations. We thank Robert Bridges for helpful comments to a previous version of the manuscript.
References
- 1.Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nature Rev, Neurosci. 2008;9:11–25. doi: 10.1038/nrn2280. [DOI] [PubMed] [Google Scholar]
- 2.Gratan DR. Behavioral significance of PRL signaling in the central nervous system during pregnancy and lactation. Reproduction. 2002;123:497–506. doi: 10.1530/rep.0.1230497. [DOI] [PubMed] [Google Scholar]
- 3.Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE. Central PRL infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc Natl Acad Sci USA. 1990;87:8003–8007. doi: 10.1073/pnas.87.20.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinsley CH, Meyer EA. The construction of the maternal brain: theoretical comment on Kim et al. Behav Neurosci. 2010;124:710–704. doi: 10.1037/a0021057. [DOI] [PubMed] [Google Scholar]
- 5.Leckman, Leckman JF, Feldman R, Swain JE, Eicher V, Thompson N, Mayes LC, et al. Primary parental preoccupation: circuits, genes, and the crucial role of the environment. J Neural Transm. 2004;111:753–771. doi: 10.1007/s00702-003-0067-x. 2004. [DOI] [PubMed] [Google Scholar]
- 6.Koenig A. Group size, composition and reproductive success in wild common marmosets (Callithrix jacchus) Amer J Primatol. 1995;35:311–317. doi: 10.1002/ajp.1350350407. [DOI] [PubMed] [Google Scholar]
- 7.Brown GR, Almond RE, Bates NJ. Adult-infant food transfer in common marmosets: an experimental study. Amer J Primatol. 2005;65:301–312. doi: 10.1002/ajp.20117. [DOI] [PubMed] [Google Scholar]
- 8.Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and AVP V1a receptors in the primate prefrontal cortex. Nature Neuroscience. 2006;9:1094–1095. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- 9.Saito A, Nakamura K. OT changes primate paternal tolerance to offspring in food transfer. J Comp Physiol A. 2011;197:329–337. doi: 10.1007/s00359-010-0617-2. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler TE, Prudom SL, Zahed SR, Parlow AF, Wegner F. Pregnancy weight gain: marmoset and tamarin dads show it too. Biol Lett. 2006;2:181–183. doi: 10.1098/rsbl.2005.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziegler TE, Washabaugh KF, Snowdon CT. Responsiveness of expectant male cotton-top tamarins, Saguinus Oedipus, to mate’s pregnancy. Horm Behav. 2004;45:84–92. doi: 10.1016/j.yhbeh.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Zahed SR, Prudom SL, Snowdon CT, Ziegler TE. Male parenting and response to infant stimuli in the common marmoset (Callithrix jacchus) Am J Primatol. 2008;70:84–92. doi: 10.1002/ajp.20460. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler TE, Prudom SL, Zahed SR, Parlow AF, Wegner FH. PRL’s meditative role in male parenting in parentally experienced marmosets (Callithrix jacchus) Horm Behav. 2009;56:436–443. doi: 10.1016/j.yhbeh.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunes S, Fite JE, French JA. Variation in steroid hormones associated with infant behaviour and experience in male marmosets (Callithrix kuhlii) Anim Behav. 2000;60:857–865. doi: 10.1006/anbe.2000.1524. [DOI] [PubMed] [Google Scholar]
- 15.Gray PB, Parkin JC, Samms-Vaughan ME. Hormonal correlates of human paternal interactions: a hospital-based investigation in urban Jamaica. Horm Behav. 2007;52:499–507. doi: 10.1016/j.yhbeh.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Dixson AF, George L. PRL and parental behaviour in a male New World primate. Nature. 1982;299:551–553.. doi: 10.1038/299551a0. [DOI] [PubMed] [Google Scholar]
- 17.Mota MT, Sousa MB. Prolactin levels of fathers and helpers related to alloparental care in common marmosets, Callithrix jacchus. Folia Primatol. 2000;2:22–26. doi: 10.1159/000021727. [DOI] [PubMed] [Google Scholar]
- 18.Prudom SL, Broz CA, Schultz-Darken N, Ferris CT, Snowdon CT, Ziegler TE. Exposure to infant scent lowers serum testosterone in father common marmosets (Callithrix jacchus) Biol Lett. 2008;4:603–605. doi: 10.1098/rsbl.2008.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziegler TE, Peterson LJ, Sosa ME, Barnard AM. Differential endocrine responses to infant odors in common marmoset (Callithrix jacchus) fathers. Horm Behav. 2011;59:265–270. doi: 10.1016/j.yhbeh.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bridges RS, DiBiase R, Loundes DD, Doherty PC. Prolactin stimulation of maternal behavior in female rats. Science. 1985;227:782–784. doi: 10.1126/science.3969568. [DOI] [PubMed] [Google Scholar]
- 21.Pi XJ, Grattan DR. Distribution of prolactin receptor immunoreactivity in the brain of estrogen-treated, ovariectomized rats. J Comp Neurol. 1998;394:462–474. doi: 10.1002/(sici)1096-9861(19980518)394:4<462::aid-cne5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Buntin JD, Tesch D. Effects of intracranial prolactin adminstration on maintenance of incubation readiness, ingestive behavior, and gonadal condition in ring doves. Horm Behav. 1985;19:188–203. doi: 10.1016/0018-506x(85)90018-2. [DOI] [PubMed] [Google Scholar]
- 23.Bender N, Taborsky M, Power DM. The role of prolactin in the regulation of brood care in the cooperatively breeding fish Neolamprologus pulcher. J Exp Zool A Ecol Genet Physiol. 2008;309:515–524. doi: 10.1002/jez.482. [DOI] [PubMed] [Google Scholar]
- 24.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 25.Grattan DR. The actions of prolactin in the brain during pregnancy and lactation. Prog Brain Res. 2001;133:153–171. doi: 10.1016/s0079-6123(01)33012-1. [DOI] [PubMed] [Google Scholar]
- 26.Kendrick KM, Keverne EB, Baldwin BA. Intracerebroventricular OT stimulates maternal behaviour in the sheep. Neuroendocrinolgy. 1987;46:56–61. doi: 10.1159/000124796. [DOI] [PubMed] [Google Scholar]
- 27.Higham JP, Barr CS, Hoffman CL, Mandalaywala TM, Parker KJ, Maestripieri D. Mu-opiod receptor (OPRM1) variation, oxytocin levels and maternal attachment in free-ranging rhesus macaques Macaca mulatta. Behav Neurosci. 2011;125:131–136. doi: 10.1037/a0022695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both female and males. J Neuroendocrin. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen CA, Boccia ML. OT antagonism alters rat dams’oral grooming and upright posturing over pups. Physiol Behav. 2003;80:233–241. doi: 10.1016/j.physbeh.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaiors in the monogamous prairie vole (microtus ochrogaster) Front Behav Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bales KL, Kim AJ, Lewis-Reese AD, Carter CS. Both OT and AVP may influence alloparental behavior in male prairie voles. Horm Behav. 2007;45:354–361. doi: 10.1016/j.yhbeh.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Ferris CF, De Viries GJ. Role of septal AVP innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc Natl Acad Sci. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nephew BC, Bridges RS. Arginine vasopressin V1a receptor antagonist impairs maternal memory in rats. Physiol Behav. 2008;95:182–186. doi: 10.1016/j.physbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Augustine RA, Kokay IC, Andrews Z, Ladyman SR, Grattan DR. Quantitation of prolactin receptor mRNA in the maternal rat brain during pregnancy and lactation. J mol Endocrinol. 2003;31:221–232. doi: 10.1677/jme.0.0310221. [DOI] [PubMed] [Google Scholar]
- 35.Pi X, Zhang B, Li J, Voogt JL. Promoter usage and estrogen regulation of prolactin receptor gene in the brain of the female rat. Neuroendocrinology. 2003;77:187–197. doi: 10.1159/000069510. [DOI] [PubMed] [Google Scholar]
- 36.DeMaria JE, Livingstone JD, freeman ME. Ovarian steroids influence the activity of neuroendocrine dopaminergic neurons. Brain Res. 2000;879:139–147. doi: 10.1016/s0006-8993(00)02763-3. [DOI] [PubMed] [Google Scholar]
- 37.Summers PM, Wennink CJ, Hodges JK. Cloprostenol-induced luteolyisis in the marmoset monkey (Callithrix jacchus) 1985;73:133–138. doi: 10.1530/jrf.0.0730133. [DOI] [PubMed] [Google Scholar]
- 38.Woller MJ, Tannenbaum PL, Schultz-Darken NJ, Eshelman BD, Abbott DH. Pulsatile gonadotropin-releasing hormone release from hypothalamic explants of male marmoset monkeys compared with male rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R70–R80. doi: 10.1152/ajpregu.00193.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore KE, Lookingl KJ. Neurospychopharmacology: The Fifth Generation of Progress. 2000 [Google Scholar]
- 40.Hentschel K, Fleckenstein AE, Toney TW, Lawson DM, Moore KE, Lookingland KJ. Prolactin regulation of tuberoinfundibular dopaminergic neurons: immunoneutralization studies. Brain Res. 2000;852:28–36. doi: 10.1016/s0006-8993(99)02182-4. [DOI] [PubMed] [Google Scholar]
- 41.Cramer CR, Parker OM, Porter JC. Estrogen inhibition of DA release into hypophysial portal blood. Endocrinology. 1979;104:419–422. doi: 10.1210/endo-104-2-419. [DOI] [PubMed] [Google Scholar]
- 42.Andrews ZB. Neuroendocrine regulation of PRL secretion during late pregnancy: easing the transition into lactation. Journal Neuroendocrinol. 2005;17:466–473. doi: 10.1111/j.1365-2826.2005.01327.x. [DOI] [PubMed] [Google Scholar]
- 43.Morel GR, Caron RW, Console GM, Soaje M, Sosa YE, Rodriguez SS, Jahn GA, Goya G. Estrogen inhibits tuberinfundibular dopaminergic neurons but does not cause irreversible damage. Brain Res Bull. 2009;80:347–352. doi: 10.1016/j.brainresbull.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuxe K, Hokfelt T, Eneroth P, Gustafsson JA, Skett P. PRL-like immunoreactivity: localization in nerve terminals of rat hypothalamus. Science. 1977;196:899–900. doi: 10.1126/science.323973. [DOI] [PubMed] [Google Scholar]
- 45.DeVito WJ. Distribution of immunoreactive PRL in the male and female rat brain: effects of hypophysectomy and intraventricular administration of colchicines. Neuroendocrinology. 1988;47:284–289. doi: 10.1159/000124926. [DOI] [PubMed] [Google Scholar]
- 46.Harlan RE, Shivers BD, Fox SR, Kaplove KA, Schachter BS, Pfaff SW. Distribution and partial characterization of immunoreactive PRL in the rat brain. Neuroendocrinology. 1989;49:7–22. doi: 10.1159/000125085. [DOI] [PubMed] [Google Scholar]
- 47.Emanuele NV, Metcalfe L, Wallock L, Tentler J, Hagen TC, Beer CT, Martinson C, Gout PW, Kirsteins L, Lawrence AM. Extrahypothalamic brain prolactin: characterization and evidence for independence from pituitary prolactin. Brian Res. 1987;421:255–262. doi: 10.1016/0006-8993(87)91295-9. [DOI] [PubMed] [Google Scholar]
- 48.Anderson GM, Grattan DR, van den Ancker W, Bridges RS. Reproductive experience increases PRL responsiveness in the medial preoptic area and arcuate nucleus of female rats. Endocrinology. 2006;147:4688–4694. doi: 10.1210/en.2006-0600. [DOI] [PubMed] [Google Scholar]
- 49.Vekemans M, Robyn C. Influence of age on serum prolactin levels in women and men. Br Med J. 1975;4:738–739. doi: 10.1136/bmj.4.5999.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaji T, Shimamoto K, Ishibashi M, Kosaka K, Orimo H. Effect of age and sex on circulating and pituitary prolactin levels in human. Europ J Endocrine. 1976;83:711–719. doi: 10.1530/acta.0.0830711. [DOI] [PubMed] [Google Scholar]
- 51.Anderson GM, Grattan DR, van den Ancker W, Bridges RS. Reproductive experience increases PRL responsiveness in the medial preoptic area and arcuate nucleus of female rats. Endocrinology. 2006;147:4688–4694. doi: 10.1210/en.2006-0600. [DOI] [PubMed] [Google Scholar]
- 52.Francis DD, Champagne FA, Liu D, Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann New York Acad Sci. 1999;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- 53.Ross HE, Young LJ. OT and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinsley CH, Meyer EA. The construction of the maternal brain: theoretical comment on Kime et al 2010. Behav Neuroscience. 2010;124:710–714. doi: 10.1037/a0021057. [DOI] [PubMed] [Google Scholar]
- 55.Kinsley CM, Modonia, Gifford GW, Tureski K, Griffin GR, Lowry C, Williams J, Collins J, McLearie H, Lambert KG. Motherhood improves learning and memory. Nature. 1999;402:137–138. doi: 10.1038/45957. [DOI] [PubMed] [Google Scholar]
- 56.Gatewood JD, Morgan MD, Eaton M, MCNamara IM, Stevens LF, Macbeths AH, Meyer EAA, Lomas LM, Kozub F, Lambert KG, Kinsley GH. Motherhood mitigates aging-related decrements in learning and memory and positively affects brain again in the rat. Brain Res Bull. 2005;66:91–98. doi: 10.1016/j.brainresbull.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 57.Leuner B, Glasper ER, Gould E. Parenting and plasticity. Trends Neurosci. 2010;33:465–473. doi: 10.1016/j.tins.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kinsley CH, Lambert KG. The maternal brain. Scientific American. 2006;294:72–79. doi: 10.1038/scientificamerican0106-72. [DOI] [PubMed] [Google Scholar]