Abstract
Cigarette smoking, either active or passive, is the most important risk factor in the development of human lung cancer. Mounting evidence indicates that cigarette smoke constituents not only contribute to tumorigenesis but also may increase the spread of cancer in the body. Nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is formed by nitrosation of nicotine and has been identified as the most potent carcinogen. NNK, an important component in cigarette smoke, may also promote tumor metastasis by regulating cell motility. Here we found that NNK can induce activation of a functionally interdependent protein kinase cascade, including c-Src, PKCι and FAK, in association with increased migration and invasion of human lung cancer cells. c-Src, PKCι and FAK are extensively co-localized in the cytoplasm. Treatment of cells with α7 nAChR specific inhibitor α-bungarotoxin (α-BTX) blocks NNK-stimulated activation of c-Src, PKCι and FAK and suppresses cell migration and invasion. Intriguingly, NNK enhances c-Src/PKCι and PKCι/FAK bindings, indicating a potential mechanism by which these kinases activate each other. Specific disruption of c-Src, PKCι or FAK expression by RNA interference significantly reduces NNK-induced cell migration and invasion. These findings suggest that NNK-induced migration and invasion may occur in a mechanism through activation of a c-Src/PKCι/FAK loop, which can contribute to metastasis and/or development of human lung cancer.
1. Introduction
Metastasis is defined as the spread of cancer from a primary tumor to distant sites of the body, which is the major cause of mortality in patients with cancer [1] . From primary tumor to secondary growth, cancer cells must invade the surrounding tissues, penetrate vessels, and travel to other sites where they arrest and resume growth [2] . During the metastatic cascade, tumor cells disrupt many physical barriers formed by epithelial and endothelial basement membranes. Active cell motility is essential during intravasation and extravasation [2] . The process of tumor invasion can be classically divided into three sequential steps: adhesion of the tumor cells to the basement membrane and extracellular matrix (ECM), disruption of the basement membrane by proteolytic digestion, migration through the modified basement membrane [2; 3] . Cell migration has been considered a required process during tumor cell metastasis [4] . Thus, mechanism of cell movement is critical to understand tumor metastasis. Cancer cells disseminate from the primary tumor either as individual cells, using amoeboid-or mesenchymal-type movement, or as cell sheets, stands and clusters using collective migration [3; 4] . Metastatic tumor cells are more motile than non-metastatic cells or most normal cells [5] .
Several clinical studies in human present an association between cigarette smoking and an increase in metastasis of lung, breast and bladder cancers [6; 7] . Cigarette smoke has also been reported to increase metastasis of lung carcinoma cells in mice [6] . However, the mechanism by which cigarette smoking promotes tumor metastasis remains enigmatic. The tobacco-related carcinogen nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a major component in cigarette smoke and derived from nicotine by opening of the pyrrolidine ring and nitrosation. Among the numerous toxic agents contained in tobacco smoke, NNK has been identified as the most potent lung carcinogen [8] .
Since enhanced PKC activity is often found in cancer cells that show marked invasive and/or metastatic potential [9] and PKC has been found to promote tumor-cell migration in a mechanism by regulating localization of cytoskeletal proteins and phosphorylation of focal adhesion kinase (FAK) [5; 10; 11; 12] , cigarette smoke constituents (i.e. NNK) may stimulate lung tumor metastasis through regulation of PKC activity. PKC is a multigene family consisting of at least 11 distinct lipid-regulated protein-serine/threonine kinases that play pivotal roles in cell growth, apoptosis, differentiation, malignant transformation and metastasis [2] . This family can be divided into three subtypes: the classic isoforms (PKCα, βI, βII, and γ), which are Ca2+ and diacylglycerol (DAG) dependent; the novel isoforms (PKC-δ, ε, η, %thetas;, and μ), which are DAG dependent but Ca2+-independent; and the atypical isoforms (PKC-ζ and λ/ι), which possess only one zinc finger and lack the characteristic C2 domain, hence they are insensitive to both Ca2+ and DAG [13; 14] . PKC isoenzymes exhibit distinct tissue distribution and play a distinct role in various cellular events including cell survival, proliferation, tumorigenesis, tumor invasion and metastasis [6; 7; 9; 15] . For example, PKCι, an atypical PKC isoform, presents predominantly in lung and brain [16] , suggesting that PKCι may play a pivotal role in lung cancer development. It has been reported that PKCι potently enhances cell survival in a mechanism involving activation of NF-κB [17; 18; 19] . PKCι is also implicated in Ras transformation and carcinogenesis [20] . Furthermore, increased expression of PKCι occurs in the melanoma lymph node associated with metastases, which may indicate a new function of PKCι in tumor metastasis [21] . FAK is a nonreceptor protein-tyrosine kinase localized prominently within focal adhesions and function as a positive regulator of both cell motility and cell survival [22] . The ability of tumor cells to migrate, invade and metastasize has been found to be associated with increased FAK expression, phosphorylation and catalytic activity [23] . FAK plays a critical role in tumor invasion and metastasis because FAK binds to the cytoplasmic domain of β1 integrin, and subsequently binds to the SH2 domain of c-Src [24] , as well as p130cas [25] and paxillin [26] . This molecular complex, known as the focal adhesion complex, facilitates activation of the c-Src/FAK signaling cascade and is critical in many cytoskeletal functions [27] . The nonreceptor tyrosine kinase c-Src is activated in most invasive cancers. c-Src becomes activated in response to integrin-mediated cell adhesion and growth factor stimuli, and is recruited to focal adhesions and cell-cell contacts [28] . Upon activation, c-Src phosphorylates multiple substrates including PKCι [18] and FAK [29; 30] , which is required for cell adhesion [31] and cell spreading [32] . The functional relationship between c-Src, PKCι and FAK represents an interdependent activation loop in cell migration and invasion signaling. Results presented here reveal that the tobacco-specific nitrosamine NNK can enhance cell migration and invasion in a mechanism through a signaling pathway involving α7 nAChR/c-Src/PKCι/FAK, which may contribute to metastasis of lung cancer.
2. Materials and Methods
2.1.Materials
Anti-c-Src, FAK, PKC ι antibodies and c-Src, FAK siRNAs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The phospho-specific FAK, c-Src and PKC ι antibodies were obtained from Cell Signaling Technology. Enolase was purchased from Sigma (St Louis, MO). Nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) was obtained from Toronto Research Chemicals (Toronto, Canada). α-BTX (α-bungarotoxin) and 4-amino-5-(4-chlorophrnyl)-7-(t-buty)pyrazolo [3,4-d] pyrimidine (PP2) were purchased from Calbiochem (La Jolla, CA). The QCM™ Chemotaxis 96-well cell migration and the QCM™ chemotaxis 24-well colorimetric cell invasion assay kits were obtained from CHEMICON INTERNATIONAL, Inc. (Temecula, CA). All reagents used were obtained from commercial sources unless otherwise stated.
2.2. Cell migration and invasion assay
Human lung cancer cell lines (i.e. H1299, H82, H157 cells and H460 cells) were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in RPMI 1640 with 10% fetal bovine serum. For measurement of cell migration, 100μl of 5×105cells/ml were seeded into Chemotaxis 96-well cell migration chamber. Cells were treated with agonist or inhibitor as indicated. Cell migration was assessed using QCM™ Chemotaxis 96-well cell migration assay kit following the manufacturer’s instructions (Chemicon, Temecula, CA). This new technique does not require cell labeling, scraping, washing or counting. The 96-well insert and homogenous fluorescence detection format allows for large-scale screening and quantitative comparison of multiple samples. Migratory cells on the bottom of the insert membrane are dissociated from the membrane when incubated with cell detachment buffer. These cells are subsequently lysed and detected by the patented CyQuant GR dye. This green-fluorescent dye exhibits strong enhancement of fluorescence when bound to cellular nucleic acids. Samples were read with a fluorescence plate reader (SPECTRA FLUOR, TECAN INC) using 480–520 nm filter set. Cell invasion was assessed using the Chemicon cell invasion assay kit. This assay was performed in an invasion chamber, which is a 24-well tissue plate with 12 cell culture inserts. The inserts contain an 8-μm pore size polycarbonate membrane over which a thin layer of ECMatrix™ is dried. The extracellular matrix (ECM) layer occludes the membrane pores, blocking non-invasive cells from migrating through. Invasion cells migrate through the ECM layer and cling to the bottom of the polycarbonate membrane. The insert membrane with invaded cells on the bottom was placed in the wells with cell stain/dissociation solution after incubation and reincubated for 30 min at 37 °C. Absorbance was measured with a microplate reader at 560 nm. Each experiment was repeated three times, and data represent the mean ± S.D. of three determinations.
2.3. Immunofluorescent staining
Cells were washed with 1 PBS, fixed with methanol and acetone (1:1) for 5 minutes and blocked with 10% donkey serum. Then, cells were incubated with a rabbit PKCι or a rabbit c-Src and a mouse FAK primary antibody for 90 min. After washing, samples were incubated with rhodamine-conjugated anti-rabbit and FITC-conjugated anti-mouse secondary antibodies for 60 min. Cells were washed with PBS and observed under a fluorescent microscope (Zeiss). Pictures were taken and colored with the same exposure setting for each experiment. To determine subcellular regions of protein co-localization, individual red-and green-stained images derived from the same field were merged using Openlab 3.1.5 software from Improvision, Inc. (Lexington, MA). Areas of protein co-localization appear yellow.
2.4. Measurement of intracellular c-Src activity
H1299 cells were stimulated with NNK, harvested, and lysed in immunoprecipitation lysis buffer. The c-Src was immunoprecipitated from the lysates using a c-Src antibody. The complexes were washed three times with 500μl of lysis buffer and twice with c-Src kinase assay buffer (20mM HEPES, pH 7.0, 10mM MnCL2, 0.05% Triton X-100). Then, the immune complex beads were suspended in 45μl of kinase assay buffer containing 1μg of acid-treated enolase as described [18] . The kinase reaction was initiated by the addition of 2μCi of [γ32 P] ATP, and the reaction mixture was incubated at 30°C for 10 min. The reaction was stopped by the addition of 50μl of 2×SDS-PAGE sample buffer. Radiolabeled proteins were resolved by 10%SDS-PAGE, transferred to a nitrocellulose membrane, and exposed to Kodak X-Omat film at-80°C for 24h. The activity of c-Src was determined by autoradiography. The same filter was then probed by Western blot analysis using a c-Src antibody.
2.5.PKCι activity assay
H1299 cells were treated with NNK (100pM) as indicated. PKCι was immunoprecipitated using agarose-conjugated PKCι antibody. Immunoprecipitated PKCι was suspended in 50μl of kinase assay buffer containing 20mM HEPES, pH7.4, 100μM CaCl2, 10mM MgCl2, 200μg/ml Histone H1, 100μM ATP, 100μg/ml phosphatidylserine, 2μCi of [γ32 P] ATP, and 0.03%Triton X-100 as described [33] . The mixture was incubated for 30 min at 30°C. The reaction was stopped by the addition of 2×SDS sample buffer and boiling the sample for 5 minutes. The samples were separated by 10% SDS-PAGE. PKCι activity was determined by autoradiography.
2.6. Depletion of c-Src or FAK expression by RNA interference (RNAi)
Human c-Src siRNA (CUCGGCUCAUUGAAGACAA ) and human FAK siRNA (GCAUGUGGCCUGCUAUGG ) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Cells were transfected with c-Src or FAK siRNA using Lipofectamine ™-2000 according to the manufacturer’s instructions. A control siRNA (non-homologous to any known gene sequence) was used as a negative control. The levels of c-Src or FAK expression were analyzed by Western blotting using a c-Src or a FAK antibody, respectively. Specific silencing of the targeted c-Src or FAK gene was confirmed by at least three independent experiments.
Vector-based gene silence of PKCι by RNAi
PKCι DNA target sequence for siRNA design is AACTCTTGATTCATGTGTTCC which was determined by Ambion’s siRNA Target Finder according to human PKCι cDNA sequence. The PKCι specific hairpin siRNA insert was determined using a computerized insert design tool based on a target sequence following instructions from Ambion’s web site. Then, the oligonucleotide encoding PKCι specific hairpin siRNA insert was synthesized and ligated into pSilencer™ 2.1-U6 hygro vector (Ambion, Austin, TX). The pSilencer™ 2.1-U6 hygro plasmids bearing PKCι hairpin siRNA insert were transfected into H1299 cells using Lipofectamine ™-2000 according to the manufacturer’s instructions. The levels of PKCι expression were analyzed by Western blot using PKCι antibody.
2.7. Monolay wound healing assay
Cells were seeded into a six-well tissue culture dish and allowed to grow to 90% confluency in complete medium. Cell monolayers were wounded by a plastic tip (1 mm) that touched the plate as described [34] . Wounded monolayers were then washed four times with medium to remove cell debris and incubated in 0.5% FBS medium in the absence or presence of NNK (100pm) or inhibitor for various times up to 24 h. Cells were monitored under a microscope equipped with a camera (Zeiss).
3. Results
3.1. NNK induces activation of c-Src, PKCι and FAK in association with increased cell migration/invasion and accelerated wound healing of human lung cancer cells
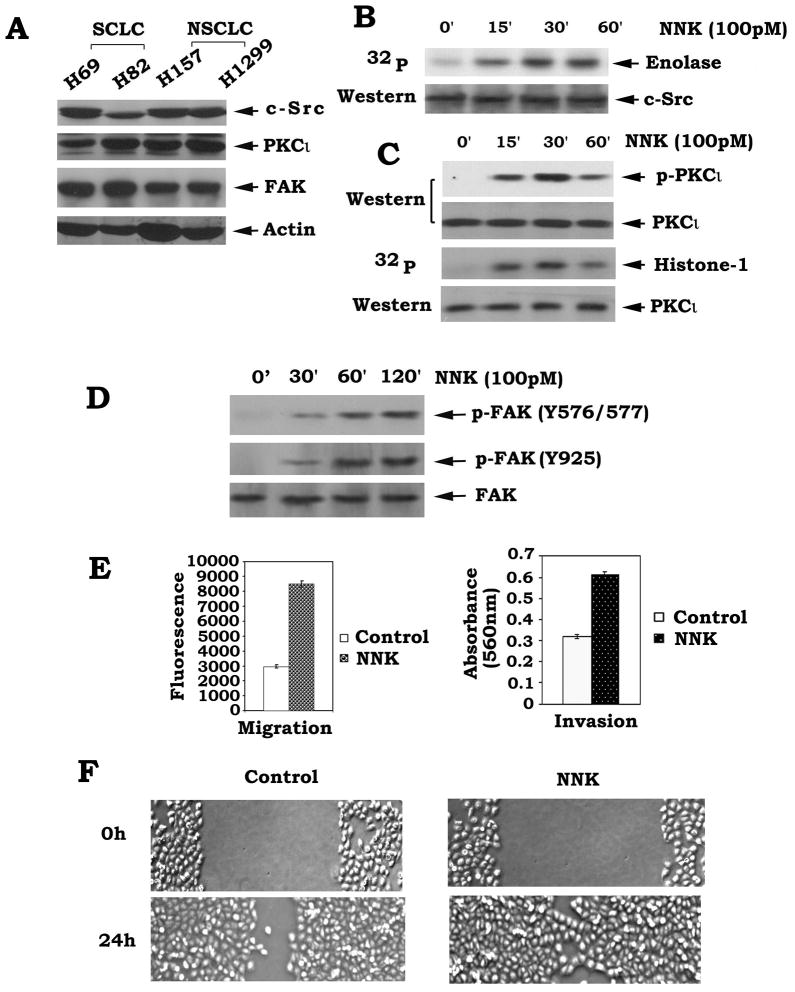
Growing evidence reveals that c-Src, PKC and FAK are closely involved in regulating tumor invasion and metastasis [6; 23; 32] . Here we found that c-Src, PKCι and FAK are widely expressed in both SCLC and NSCLC cells (Fig. 1A). To test whether cigarette smoke component NNK can activate c-Src, PKCι and FAK in human lung cancer cells, H1299 cells were treated with NNK (100pM) for various times. Results indicate that NNK not only potently activates c-Src and PKCι but also induces multi-site phosphorylation of FAK at Y576, 577 and 925 which can enhance multiple functions of FAK (Fig. 1B, C and D). Intriguingly, treatment of cells with NNK significantly enhances migration and invasion and accelerates wound healing of H1299 cells (Fig. 1E and F). Other human lung cancer cell lines (i.e. H82, H157 cells, etc.) were also tested and the similar results were obtained (data not shown). The c-Src is the physiological kinase for both PKCι and FAK that can directly phosphorylate and/or activate PKCι and FAK, which can positively regulate cell motility [18; 29; 30] . Thus, NNK-induced activation of PKCι and FAK may occur through activation of their upstream kinase c-Src leading to enhanced migration and invasion of human lung cancer cells.
Fig.1.
NNK induces activation of c-Src, PKCι and FAK in association with increased migration/invasion and accelerated wound healing of human lung cancer cells. (A) H69, H82, H157 and H1299 human lung cancer cells were lysed in detergent buffer. Expression levels of c-Src, PKCι and FAK were analyzed by Western blot. (B), (C) and (D) H1299 cells were treated with NNK for various times. Activity or phosphorylation of c-Src, PKCι and FAK was analyzed as described in “Methods”. (E) H1299 cells were treated with NNK (100pM) for 24h. Cell migration or invasion was assessed using a QCM™ Chemotaxis 96-well cell migration assay kit or a QCM™ 24-well colorimetric cell invasion kit, respectively. Each experiment was repeated three times and data represent the mean ± S.D. of three determinations. (F) H1299 cells were seeded into a six-well tissue culture dish and allowed to grow to 90% confluency in complete medium. Cell monolayers were wounded by a plastic tip (1 mm) that touched the plate. Wounded monolayers were then washed four times with medium to remove cell debris and incubated in 0.5% FBS medium in the absence or presence of NNK (100pM) for various times up to 24 h. Pictures were taken under a microscope equipped with a camera (Deiss).
3.2. The c-Src, PKCι and FAK are co-localized in the cytoplasm and NNK stimulates their interactions
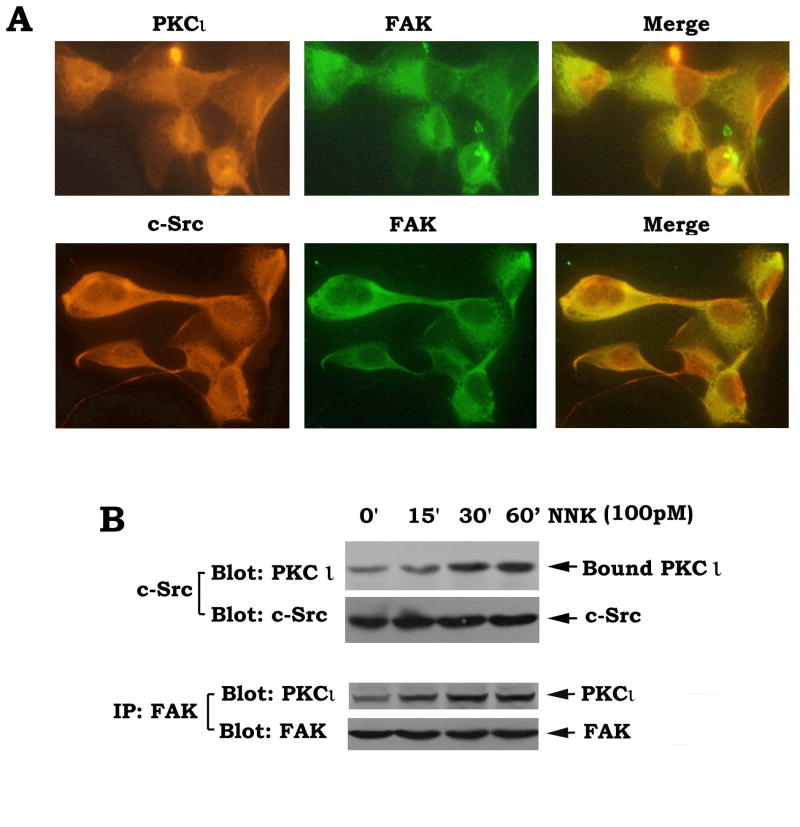
Our data show that NNK potently stimulates activation of c-Src, PKCι and FAK in association with enhanced cell migration and invasion (Fig. 1). Immunofluorescent staining reveals that these three proteins are co-localized in the cytoplasm (Fig. 2A). PKCι is insensitive to Ca2+ due to the absence of the calcium-binding domain [15] . Thus, NNK-induced activation of PKCι may occur through a calcium-independent mechanism. The c-Src has been reported to directly induce tyrosine phosphorylation of PKCι along with activation of enzyme activity [18] . Since NNK can activate c-Src (Fig. 1B), it is possible that c-Src may function as NNK-activated PKCι kinase which phosphorylates and activates PKCι. It is becoming more apparent that phosphorylation and protein-protein interactions play a major role in activation of PKCι [18] . Because the SH3 domain of c-Src could bind and interact with the regulatory domain of PKCι [18; 35] , NNK-induced activation of PKCι may occur in a mechanism involving regulation of PKCι/c-Src interaction. FAK is a major positive regulator for migration and invasion of tumor cells [22; 23] . PKCι and FAK may also have a functionally cooperative role in cell migration signaling. To test whether NNK promotes PKCι to associate with c-Src or FAK, co-immunoprecipitation experiments were performed using c-Src or FAK antibody, respectively, following treatment of H1299 cells with NNK. Results indicate that NNK stimulates increased associations between c-Src and PKCι or PKCι and FAK (Fig. 2B). It can be speculated that NNK-induced tyrosine phosphorylation of PKCι may induce some conformational change that facilitates direct interactions between c-Src and PKCι, or PKCι and FAK. These findings imply a potential mechanism of c-Src, PKCι and FAK in NNK-stimulated cell migration/invasion signaling.
Fig. 2.
NNK induces PKCι/c-Src and PKCι/FAK associations. (A) co-localization of PKCι/c-Src and PKCι/FAK in H1299 cells was detected by co-immunofluorescent staining as described in “Methods”. (B) H1299 cells were treated with NNK for various times. Co-immunoprecipitations were performed using c-Src or FAK antibody, respectively. PKCι/c-Src and PKCι/FAK associations were analyzed by Western blotting.
3.3. The Src specific inhibitor PP2 inhibits NNK-induced activation of c-Src and PKCι in association with suppression of cell migration and invasion
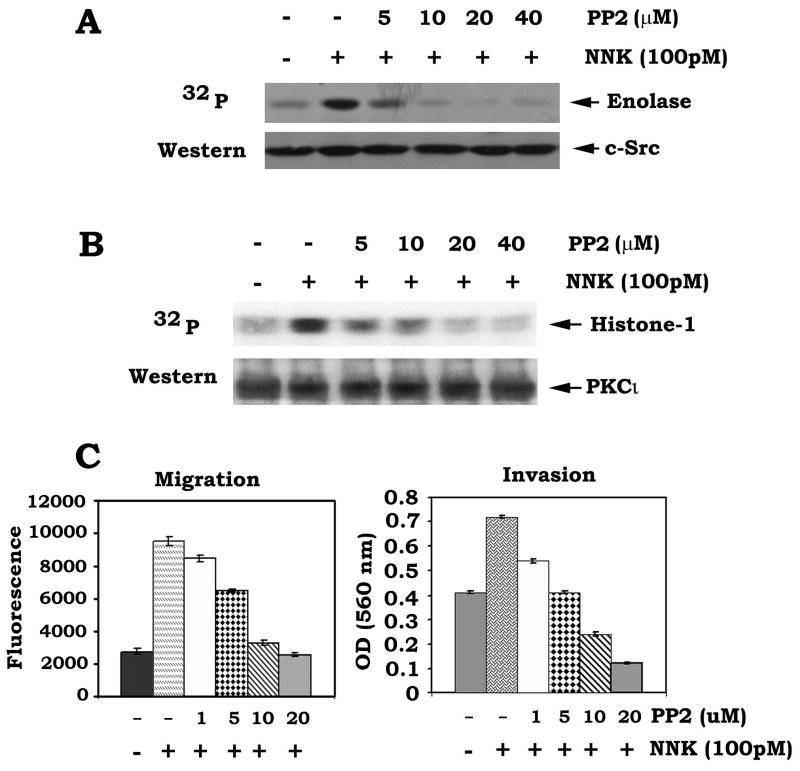
It has been reported that c-Src can directly phosphorylate and activate PKCι [18] . PP2 is a potent and selective inhibitor of the Src family of protein tyrosine kinases which can compete for the ATP binding site [36] . To further pharmacologically demonstrate whether c-Src is the NNK-activated upstream kinase of PKCι, H1299 cells were treated with NNK in the absence or presence of increasing concentrations of PP2 (0~40 μM). Results show that PP2 not only blocks NNK-stimulated c-Src activity but also potently suppresses NNK-induced PKCι activity (Fig. 3AB). To further assess whether PP2 affects NNK-stimulated cell migration and invasion, cells were treated with NNK in the absence or presence of increasing concentrations of PP2 as described above. Results reveal that treatment of lung cancer cells with PP2 blocks NNK-induced migration and invasion in a dose-dependent manner (Fig. 3C). These findings provide the pharmacological evidence that c-Src is the upstream activator of PKCι in a NNK-stimulated cell migration/invasion signaling pathway.
Fig. 3.
The Src specific inhibitor PP2 reduces c-Src and PKCι activities and suppresses cell migration and invasion induced by NNK. (A) and (B) H1299 cell were treated with NNK in the absence or presence of increasing concentrations of PP2 for 30 min. Activities of c-Src and PKCι were analyzed as described in “Methods”. (C) H1299 cells were treated with NNK in the absence or presence of increasing concentrations of PP2 for 24h. Cell migration and invasion were analyzed as the legend of figure 1E.
3.4. The α7 nicotinic acetylcholine receptor (α7nAChR) specific inhibitor α-bungarotoxin (α-BTX) blocks NNK-induced activation of c-Src, PKCι and FAK in association with decreased migration and invasion of human lung cancer cells
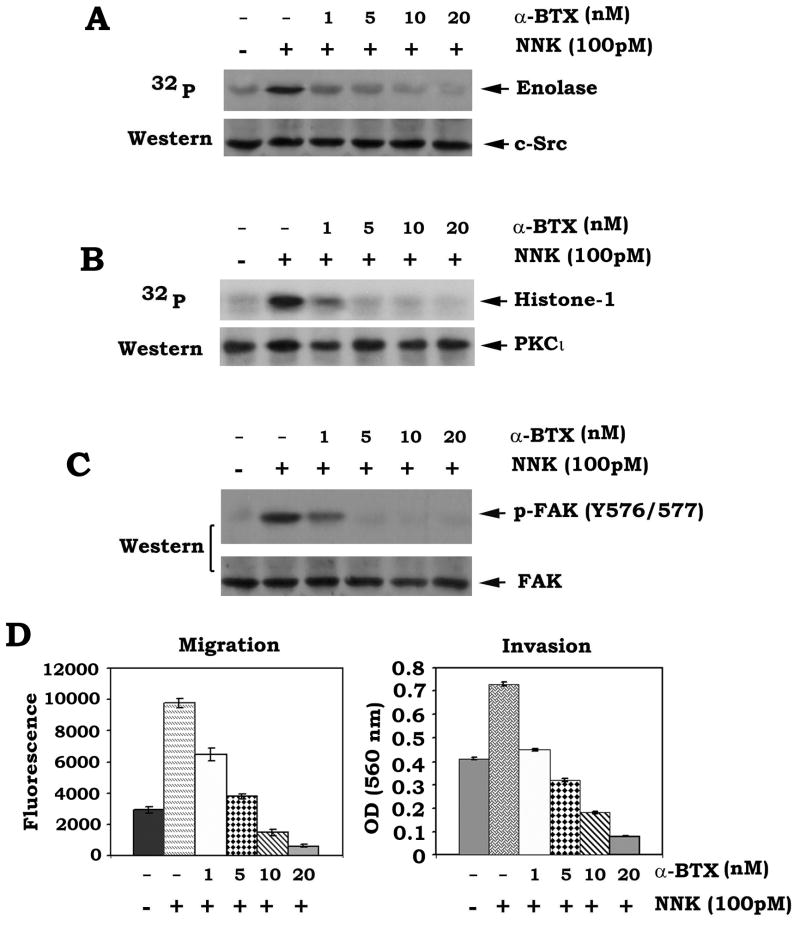
The α-bungarotoxin (α-BTX) has been identified as the site-selective antagonist for α7 nAChR [37] . The α7 nAChR plays an important role in lung cancer cell signaling and NNK is a site-selective, high-affinity agonist for the α7nAChR [1; 37; 38] . We have previously reported that NNK enhances cell proliferation of human lung cancer cells through phosphorylation of Bcl2 and c-Myc, which can be inhibited by α-BTX [38] . In addition to cell proliferation, here we found that NNK can also stimulate migration and invasion of human lung cancer cells through activation of c-Src, PKCι and FAK (Fig. 1). To further test whether α7 nAChR plays a role in NNK-induced activation of c-Src, PKCι and FAK as well as cell migration and invasion, H1299 cells were treated with NNK in the presence of increasing concentrations of α-BTX. Results show that α-BTX potently blocks NNK-induced activation of c-Src, PKCι and FAK in a dose-dependent manner (Fig. 4ABC), which is associated with suppression of cell migration and invasion (Fig. 4D). This indicates that the α7 nAChR may function as the upstream receptor in NNK-stimulated c-Src-PKCι-FAK signaling pathway.
Fig. 4.
The α7 nAChR-specific inhibitor α-bungarotoxin (α-BTX) inhibits NNK-induced activation of c-Src, PKCι and FAK leading to suppression of migration and invasion of human lung cancer cells. (A), (B) and (C) H1299 cells were treated with NNK in the absence or presence of increasing concentrations of α-BTX for 30 min. Activities of c-Src and PKCι were analyzed as described in “Methods”. Phosphorylation of FAK was detected by Western blotting. (D) H1299 cells were treated with NNK in the absence or presence of increasing concentrations of α-BTX for 24h. Cell migration and invasion were analyzed as the legend of figure 1E.
3.5. Depletion of PKCι, c-Src or FAK by RNA interference blocks NNK-induced cell migration and invasion
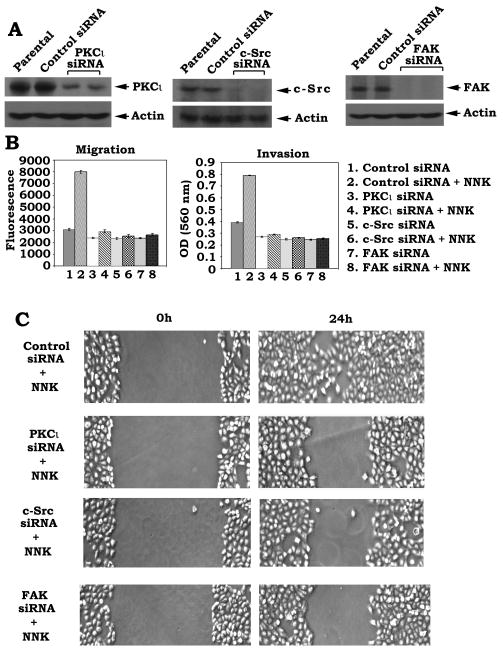
Our findings suggest that NNK-stimulated activation of c-Src, PKCι and FAK may contribute to increased migration and invasion of human lung cancer cells. To genetically demonstrate this, c-Src, PKCι or FAK was knocked down by RNA interference (RNAi) as described in “Methods”. For silence of PKCι, a vector-based stable gene silencing approach was employed for specific depletion of PKCι from human lung cancer cells. The pSilencer™ 2.1-U6 hygro plasmids bearing the PKCι hairpin siRNA insert were transfected into H1299 cells. The stable clones persistently producing PKCι siRNA were selected using hygromycin. Results indicate that cells expressing PKCι siRNA display >85% reduction of PKCι protein expression (Fig. 5A). For depletion of c-Src and FAK, c-Src siRNA and FAK siRNA purchased from Santa Cruz were used. Recent studies have demonstrated that transfection of cells with siRNA concentrations greater than 100 nM frequently produces nonspecific off-target effects, and a concentration of 20–100 nM only occasionally produces effects. One group has reported that siRNA concentrations of 10–20 nM generally do not exert nonspecific effects [39] . To minimize the nonspecific effect, a low concentration (i.e.15nM) of c-Src or FAK siRNA was used in the experiments. H1299 cells were transfected with c-Src siRNA, FAK siRNA or control siRNA. Cells expressing c-Src siRNA or FAK siRNA displayed a >98% reduction of c-Src or FAK protein expression (Fig. 5A). Functionally, depletion of either PKCι c-Src or FAK by RNAi significantly suppresses NNK-stimulated migration and invasion as well as wound healing of lung cancer cells (Fig. 5BC).
Fig. 5.
Specific knockdown of PKCι, c-Src or FAK by RNA interference results in suppression of NNK-induced cell migration/invasion and wound healing. (A) PKCι, c-Src or FAK was silenced by RNAi as described in “Methods”. Expression levels of PKCι, c-Src or FAK were analyzed by Western Blot. (B) H1299 cells expressing PKCι, c-Src or FAK siRNA were treated with NNK (100pM) for 24h. Cell migration and invasion were analyzed as the legend of figure 1E. (C) H1299 cells expressing PKCι, c-Src or FAK siRNA were treated with NNK (100pM) for 24h. Wound healing was analyzed as the legend of figure 1F.
4. Discussion
Cigarette smoking has been found to enhance dissemination of tumor cells in the body of patients with various types of cancer [6; 7] . Thus, tobacco-related agents concerning human health are much more important than experimental tumor promoters in influencing metastatic abilities of tumor cells [6] . NNK is formed by nitrosation of nicotine and has been identified as a potent pulmonary carcinogen contained in cigarette smoke, independent of the route and type of administration [40; 41] . The amount of NNK in one stick of cigarette ranges between 16 and 369 ng [42] . The concentration of NNK (i.e. 100 pM) used in our in vitro studies is clinically relevant and of translational value because exposure to NNK via cigarette smoking can result in detectable plasma NNK concentrations ranging from pM to nM [43] .
Previous studies reveal that cigarette smoking-induced tumor cell invasion and metastasis may result, at least in part, from activation of PKC signal transduction pathway [6] . The c-Src, PKCι and FAK are major regulators of cell migration and invasion [30; 31; 32; 44] . Here we found that NNK not only activates c-Src but also PKCι and FAK, which leads to enhanced migration and invasion of human lung cancer H1299 cells (Fig. 1). Since activated c-Src has been reported to phosphorylate FAK at Y576 and Y577 leading to activation of FAK [29; 30] , NNK-induced FAK phosphorylayion at Y576 and Y577 may occur through activation of c-Src (Fig. 1B and D). Intriguingly, c-Src has also reported to function as a physiological PKCι kinase that directly phosphorylates and activates PKCι [18] . This helps explain why inhibition of c-Src by treatment of cells with PP2 (i.e. c-Src specific inhibitor) not only blocks NNK-stimulated c-Src activation but also potently suppresses NNK-induced PKCι activity (Fig. 3). Recent report indicates that c-Src directly interacts with FAK and this association facilitates activation of both c-Src and FAK (45). We found that PKCι is associated with both c-Src and FAK, which can be enhanced by NNK treatment (Fig. 2). The enhanced interactions between c-Src, FAK and PKCι may facilitate their activation. Based our findings, we propose that NNK-enhanced migration and invasion of human lung cancer cells may occur through activation of a signaling cascade involving c-Src, FAK and PKCι (Fig. 6). We have previously demonstrated that NNK can induce proliferation of SCLC H69 and H82 cells but not NSCLC H1299 cells [38] . Thus, proliferation should not affect NNK-stimulated migration/invasion in human lung cancer H1299 cells.
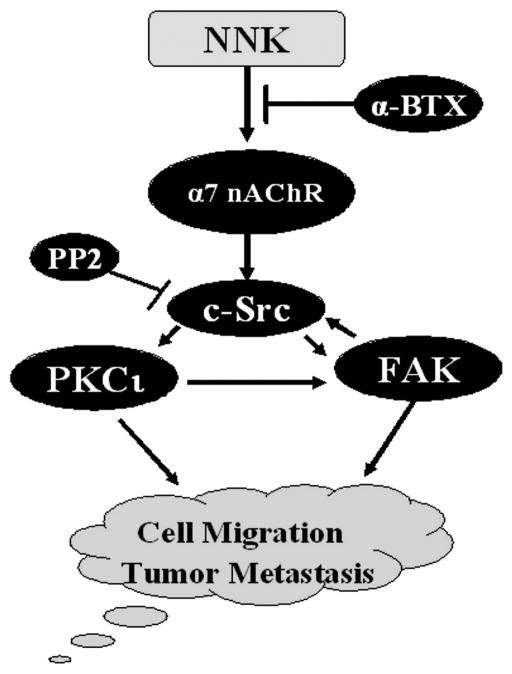
Fig. 6. Proposed model.
NNK-induced migration and invasion of human lung cancer cells occurs through activation of the α7 nAChR/c-Src/PKCι/FAK signaling transduction pathway.
The nAChRs are cationic channels whose opening is controlled by acetylcholine and nicotinic receptor agonists. The α7 nAChR is expressed in normal human small airway epithelial cells, SCLC and NSCLC cells [8; 37; 45] . NNK binds to and activates the α7 nAChR with a high affinity in lung cancer cells [8; 37] . The α-BTX has been identified as the site-selective antagonist for α7nAChRs [37; 46; 47] . Because α-BTX potently blocks NNK-induced activation of c-Src, PKCι and FAK in association with inhibition of cell migration and invasion (Fig. 4), this indicates that NNK-induced cell migration and invasion may occur through activation of the signal transduction pathway involving α7 nAChR/c-Src/PKCι/FAK in lung cancer cells (Fig. 6). Importantly, α-BTX may have potential clinical relevance in strategies designed to restrain tumor invasion and metastasis through this novel mechanism in patients with lung cancer.
Because NNK-activated c-Src, FAK and PKCι positively regulate migration and invasion of human lung cancer cells, specific targeting of c-Src, FAK or PKCι may represent an attractive and novel therapeutic approach in restraining metastasis of lung cancer. Intriguingly, specific knockdown of c-Src, FAK or PKCι expression by RNA interference significantly blocks NNK-induced migration and invasion as well wound healing (Fig. 5). This indicates that c-Src, FAK are PKCι are required targets in NNK-induced migration and invasion of human lung cancer cells. Additionally, a recent report has demonstrated that NNK can increase adhesive ability of lung cancer cells [48] . This may be another potential mechanism by which NNK enhances migration and invasion of lung cancer cells.
In summary, our studies have identified a novel NNK-stimulated cell migration and invasion signal transduction pathway involving α7 nAChR/c-Src/FAK/PKCι (Fig. 6). NNK-induced tyrosine phosphorylation of c-Src, FAK and PKCι may facilitate their interactions leading to activation of c-Src, FAK and PKC. Because our findings demonstrate that c-Src, FAK and PKCι are essential targets in NNK-induced migration and invasion of human lung cancer cells, novel therapeutic strategies for prevention of metastasis and/or treatment of metastatic tumors could be developed by specifically targeting c-Src, FAK or PKCι in human lung cancer or other malignancies.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 CA112183 and R01CA136534, by Flight Attendant Medical Research Institute Clinical Innovator Awards, by Developmental Research Program Award of Emory SPORE in Head/Neck Cancer, and by Winship’s Inaugural Kennedy Seed Grant.
Footnotes
Conflict of Interest
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fidler IJ. Cancer metastasis. Br Med Bull. 1991;47:157–177. doi: 10.1093/oxfordjournals.bmb.a072453. [DOI] [PubMed] [Google Scholar]
- 2.Gomez DE, Skilton G, Alonso DF, Kazanietz MG. The role of protein kinase C and novel phorbol ester receptors in tumor cell invasion and metastasis (Review) Oncol Rep. 1999;6:1363–1370. doi: 10.3892/or.6.6.1363. [DOI] [PubMed] [Google Scholar]
- 3.Liotta LA. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986;46:1–7. [PubMed] [Google Scholar]
- 4.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 5.Bredin CG, Liu Z, Hauzenberger D, Klominek J. Growth-factor-dependent migration of human lung-cancer cells. Int J Cancer. 1999;82:338–345. doi: 10.1002/(sici)1097-0215(19990730)82:3<338::aid-ijc6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Gopalakrishna R, Chen ZH, Gundimeda U. Tobacco smoke tumor promoters, catechol and hydroquinone, induce oxidative regulation of protein kinase C and influence invasion and metastasis of lung carcinoma cells. Proc Natl Acad Sci U S A. 1994;91:12233–12237. doi: 10.1073/pnas.91.25.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murin S, Inciardi J. Cigarette smoking and the risk of pulmonary metastasis from breast cancer. Chest. 2001;119:1635–1640. doi: 10.1378/chest.119.6.1635. [DOI] [PubMed] [Google Scholar]
- 8.Schuller HM, Plummer HK, 3rd, Jull BA. Receptor-mediated effects of nicotine and its nitrosated derivative NNK on pulmonary neuroendocrine cells. Anat Rec A Discov Mol Cell Evol Biol. 2003;270:51–58. doi: 10.1002/ar.a.10019. [DOI] [PubMed] [Google Scholar]
- 9.Dumont JA, Bitonti AJ. Modulation of human melanoma cell metastasis and adhesion may involve integrin phosphorylation mediated through protein kinase C. Biochem Biophys Res Commun. 1994;204:264–272. doi: 10.1006/bbrc.1994.2454. [DOI] [PubMed] [Google Scholar]
- 10.Herbert JM, Maffrand JP. Tumor cell adherence to cultured capillary endothelial cells is promoted by activators of protein kinase C. Biochem Pharmacol. 1991;42:163–170. doi: 10.1016/0006-2952(91)90695-2. [DOI] [PubMed] [Google Scholar]
- 11.Quigley RL, Shafer SH, Williams CL. Regulation of integrin-mediated adhesion by muscarinic acetylcholine receptors and protein kinase C in small cell lung carcinoma. Chest. 1998;114:839–846. doi: 10.1378/chest.114.3.839. [DOI] [PubMed] [Google Scholar]
- 12.Lewis JM, Cheresh DA, Schwartz MA. Protein kinase C regulates alpha v beta 5-dependent cytoskeletal associations and focal adhesion kinase phosphorylation. J Cell Biol. 1996;134:1323–1332. doi: 10.1083/jcb.134.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 14.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332( Pt 2):281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spitaler M, Villunger A, Grunicke H, Uberall F. Unique structural and functional properties of the ATP-binding domain of atypical protein kinase C-iota. J Biol Chem. 2000;275:33289–33296. doi: 10.1074/jbc.M002742200. [DOI] [PubMed] [Google Scholar]
- 16.Selbie LA, Schmitz-Peiffer C, Sheng Y, Biden TJ. Molecular cloning and characterization of PKC iota, an atypical isoform of protein kinase C derived from insulin-secreting cells. J Biol Chem. 1993;268:24296–24302. [PubMed] [Google Scholar]
- 17.Murray NR, Fields AP. Atypical protein kinase C iota protects human leukemia cells against drug-induced apoptosis. J Biol Chem. 1997;272:27521–27524. doi: 10.1074/jbc.272.44.27521. [DOI] [PubMed] [Google Scholar]
- 18.Wooten MW, Vandenplas ML, Seibenhener ML, Geetha T, Diaz-Meco MT. Nerve growth factor stimulates multisite tyrosine phosphorylation and activation of the atypical protein kinase C’s via a src kinase pathway. Mol Cell Biol. 2001;21:8414–8427. doi: 10.1128/MCB.21.24.8414-8427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lallena MJ, Diaz-Meco MT, Bren G, Paya CV, Moscat J. Activation of IkappaB kinase beta by protein kinase C isoforms. Mol Cell Biol. 1999;19:2180–2188. doi: 10.1128/mcb.19.3.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray NR, Jamieson L, Yu W, Zhang J, Gokmen-Polar Y, Sier D, Anastasiadis P, Gatalica Z, Thompson EA, Fields AP. Protein kinase Ciota is required for Ras transformation and colon carcinogenesis in vivo. J Cell Biol. 2004;164:797–802. doi: 10.1083/jcb.200311011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selzer E, Okamoto I, Lucas T, Kodym R, Pehamberger H, Jansen B. Protein kinase C isoforms in normal and transformed cells of the melanocytic lineage. Melanoma Res. 2002;12:201–209. doi: 10.1097/00008390-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Hanks SK, Ryzhova L, Shin NY, Brabek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci. 2003;8:d982–996. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- 23.Gabarra-Niecko V, Schaller MD, Dunty JM. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev. 2003;22:359–374. doi: 10.1023/a:1023725029589. [DOI] [PubMed] [Google Scholar]
- 24.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 25.Nakamoto T, Sakai R, Ozawa K, Yazaki Y, Hirai H. Direct binding of C-terminal region of p130Cas to SH2 and SH3 domains of Src kinase. J Biol Chem. 1996;271:8959–8965. doi: 10.1074/jbc.271.15.8959. [DOI] [PubMed] [Google Scholar]
- 26.Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol. 2000;2:E231–236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- 27.Lin EH, Hui AY, Meens JA, Tremblay EA, Schaefer E, Elliott BE. Disruption of Ca2+-dependent cell-matrix adhesion enhances c-Src kinase activity, but causes dissociation of the c-Src/FAK complex and dephosphorylation of tyrosine-577 of FAK in carcinoma cells. Exp Cell Res. 2004;293:1–13. doi: 10.1016/j.yexcr.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Jones RJ, Brunton VG, Frame MC. Adhesion-linked kinases in cancer; emphasis on src, focal adhesion kinase and PI 3-kinase. Eur J Cancer. 2000;36:1595–1606. doi: 10.1016/s0959-8049(00)00153-2. [DOI] [PubMed] [Google Scholar]
- 29.Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calalb MB, Zhang X, Polte TR, Hanks SK. Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem Biophys Res Commun. 1996;228:662–668. doi: 10.1006/bbrc.1996.1714. [DOI] [PubMed] [Google Scholar]
- 31.Verbeek BS, Vroom TM, Rijksen G. Overexpression of c-Src enhances cell-matrix adhesion and cell migration in PDGF-stimulated NIH3T3 fibroblasts. Exp Cell Res. 1999;248:531–537. doi: 10.1006/excr.1999.4416. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan KB, Swedlow JR, Morgan DO, Varmus HE. c-Src enhances the spreading of src-/-fibroblasts on fibronectin by a kinase-independent mechanism. Genes Dev. 1995;9:1505–1517. doi: 10.1101/gad.9.12.1505. [DOI] [PubMed] [Google Scholar]
- 33.Jamieson L, Carpenter L, Biden TJ, Fields AP. Protein kinase Ciota activity is necessary for Bcr-Abl-mediated resistance to drug-induced apoptosis. J Biol Chem. 1999;274:3927–3930. doi: 10.1074/jbc.274.7.3927. [DOI] [PubMed] [Google Scholar]
- 34.Xu L, Deng X. Suppression of cancer cell migration and invasion by protein phosphatase 2A through dephosphorylation of mu-and m-calpains. J Biol Chem. 2006;281:35567–35575. doi: 10.1074/jbc.M607702200. [DOI] [PubMed] [Google Scholar]
- 35.Seibenhener ML, Roehm J, White WO, Neidigh KB, Vandenplas ML, Wooten MW. Identification of Src as a novel atypical protein kinase C-interacting protein. Mol Cell Biol Res Commun. 1999;2:28–31. doi: 10.1006/mcbr.1999.0140. [DOI] [PubMed] [Google Scholar]
- 36.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck-and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 37.Jull BA, Plummer HK, 3rd, Schuller HM. Nicotinic receptor-mediated activation by the tobacco-specific nitrosamine NNK of a Raf-1/MAP kinase pathway, resulting in phosphorylation of c-myc in human small cell lung carcinoma cells and pulmonary neuroendocrine cells. J Cancer Res Clin Oncol. 2001;127:707–717. doi: 10.1007/s004320100289. [DOI] [PubMed] [Google Scholar]
- 38.Jin Z, Gao F, Flagg T, Deng X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone promotes functional cooperation of Bcl2 and c-Myc through phosphorylation in regulating cell survival and proliferation. J Biol Chem. 2004;279:40209–40219. doi: 10.1074/jbc.M404056200. [DOI] [PubMed] [Google Scholar]
- 39.Semizarov D, Frost L, Sarthy A, Kroeger P, Halbert DN, Fesik SW. Specificity of short interfering RNA determined through gene expression signatures. Proc Natl Acad Sci U S A. 2003;100:6347–6352. doi: 10.1073/pnas.1131959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maser E, Stinner B, Atalla A. Carbonyl reduction of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) by cytosolic enzymes in human liver and lung. Cancer Lett. 2000;148:135–144. doi: 10.1016/s0304-3835(99)00323-7. [DOI] [PubMed] [Google Scholar]
- 41.Schuller HM. Mechanisms of smoking-related lung and pancreatic adenocarcinoma development. Nat Rev Cancer. 2002;2:455–463. doi: 10.1038/nrc824. [DOI] [PubMed] [Google Scholar]
- 42.Brunnemann KD, Mitacek EJ, Liu Y, Limsila T, Suttajit M. Assessment of major carcinogenic tobacco-specific N-nitrosamines in Thai cigarettes. Cancer Detect Prev. 1996;20:114–121. [PubMed] [Google Scholar]
- 43.Maser E, Friebertshauser J, Volker B. Purification, characterization and NNK carbonyl reductase activities of 11beta-hydroxysteroid dehydrogenase type 1 from human liver: enzyme cooperativity and significance in the detoxification of a tobacco-derived carcinogen. Chem Biol Interact. 2003;143–144:435–448. doi: 10.1016/s0009-2797(02)00180-1. [DOI] [PubMed] [Google Scholar]
- 44.Hauck CR, Hsia DA, Schlaepfer DD. The focal adhesion kinase--a regulator of cell migration and invasion. IUBMB Life. 2002;53:115–119. doi: 10.1080/15216540211470. [DOI] [PubMed] [Google Scholar]
- 45.Jin Z, Xin M, Deng X. Survival function of protein kinase C{iota} as a novel nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-activated bad kinase. J Biol Chem. 2005;280:16045–16052. doi: 10.1074/jbc.M413488200. [DOI] [PubMed] [Google Scholar]
- 46.Lopez MG, Montiel C, Herrero CJ, Garcia-Palomero E, Mayorgas I, Hernandez-Guijo JM, Villarroya M, Olivares R, Gandia L, McIntosh JM, Olivera BM, Garcia AG. Unmasking the functions of the chromaffin cell alpha7 nicotinic receptor by using short pulses of acetylcholine and selective blockers. Proc Natl Acad Sci U S A. 1998;95:14184–14189. doi: 10.1073/pnas.95.24.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salvaterra PM, Mahler HR. Nicotinic acetylcholine receptor from rat brain. Solubilization, partial purification, and characterization. J Biol Chem. 1976;251:6327–6334. [PubMed] [Google Scholar]
- 48.Hung YH, Hung WC. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) enhances invasiveness of lung cancer cells by up-regulating contactin-1 via the alpha7 nicotinic acetylcholine receptor/ERK signaling pathway. Chem Biol Interact. 2009;179:154–159. doi: 10.1016/j.cbi.2008.10.042. [DOI] [PubMed] [Google Scholar]