Abstract
We report the cloning of KIN17 cDNA, 1414 bp long with an ORF of 391 residues showing a zinc finger and nuclear localization signals. By recloning the cDNA into an appropriate vector, we produced kin17 protein in E. coli, purified it partially and shown that kin17 protein binds to double-stranded DNA. The KIN17 gene was localized by cytogenetic mapping in mouse chromosome 2, band A. Genomic sequences homologous to KIN17 cDNA were detected also in rat and human DNAs. KIN17 mRNA is highly expressed in rodent transformed AtT-20 neuroendocrine cells whereas it can be detected only in the total RNA of mouse embryos and various normal adult tissues by reverse transcription and PCR amplification. The mouse nuclear kin17 protein was identified by a local small structural similarity with E.coli recA protein. Kin17 and recA have only 39 amino acid residues in a region that might be involved in DNA-binding.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
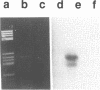
- Angulo J. F., Moreau P. L., Maunoury R., Laporte J., Hill A. M., Bertolotti R., Devoret R. KIN, a mammalian nuclear protein immunologically related to E. coli RecA protein. Mutat Res. 1989 Mar;217(2):123–134. doi: 10.1016/0921-8777(89)90064-5. [DOI] [PubMed] [Google Scholar]
- Angulo J. F., Rouer E., Benarous R., Devoret R. Identification of a mouse cDNA fragment whose expressed polypeptide reacts with anti-recA antibodies. Biochimie. 1991 Feb-Mar;73(2-3):251–256. doi: 10.1016/0300-9084(91)90210-r. [DOI] [PubMed] [Google Scholar]
- Benarous R., Chow C. M., RajBhandary U. L. Cytoplasmic leucyl-tRNA synthetase of Neurospora crassa is not specified by the leu-5 locus. Genetics. 1988 Aug;119(4):805–814. doi: 10.1093/genetics/119.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descombes P., Chojkier M., Lichtsteiner S., Falvey E., Schibler U. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev. 1990 Sep;4(9):1541–1551. doi: 10.1101/gad.4.9.1541. [DOI] [PubMed] [Google Scholar]
- Dessen P., Fondrat C., Valencien C., Mugnier C. BISANCE: a French service for access to biomolecular sequence databases. Comput Appl Biosci. 1990 Oct;6(4):355–356. doi: 10.1093/bioinformatics/6.4.355. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Davis R. W. Identification and isolation of the gene encoding the small subunit of ribonucleotide reductase from Saccharomyces cerevisiae: DNA damage-inducible gene required for mitotic viability. Mol Cell Biol. 1987 Aug;7(8):2783–2793. doi: 10.1128/mcb.7.8.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Cannizzaro L. A., Battini R., Huebner K., Baserga R. The gene encoding human vimentin is located on the short arm of chromosome 10. Am J Hum Genet. 1987 Oct;41(4):616–626. [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Chick W. L., Oie H. K., Sims H. L., King D. L., Weir G. C., Lauris V. Continuous, clonal, insulin- and somatostatin-secreting cell lines established from a transplantable rat islet cell tumor. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3519–3523. doi: 10.1073/pnas.77.6.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B., Kelly R. B. Secretory granules of an anterior pituitary cell line, AtT-20, contain only mature forms of corticotropin and beta-lipotropin. Proc Natl Acad Sci U S A. 1981 Jan;78(1):318–322. doi: 10.1073/pnas.78.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashitani A., Tabata S., Ogawa T., Ogawa H., Shibata M., Hotta Y. ATP-independent strand transfer protein from murine spermatocytes, spermatids, and spermatozoa. Exp Cell Res. 1990 Feb;186(2):317–323. doi: 10.1016/0014-4827(90)90311-w. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Bootsma D. Molecular genetics of eukaryotic DNA excision repair. Cancer Cells. 1990 Oct;2(10):311–320. [PubMed] [Google Scholar]
- Horellou P., Guibert B., Leviel V., Mallet J. Retroviral transfer of a human tyrosine hydroxylase cDNA in various cell lines: regulated release of dopamine in mouse anterior pituitary AtT-20 cells. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7233–7237. doi: 10.1073/pnas.86.18.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii T., Ogawa T., Ogawa H. Organization of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):313–317. doi: 10.1073/pnas.77.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd H. K., Roberts C. W., Roberts J. W. Identification of the gene for the yeast ribonucleotide reductase small subunit and its inducibility by methyl methanesulfonate. Mol Cell Biol. 1987 Oct;7(10):3673–3677. doi: 10.1128/mcb.7.10.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M., Makino O., Shibata T. Epitopes and active sites of the RecA protein. J Biol Chem. 1990 May 25;265(15):8948–8956. [PubMed] [Google Scholar]
- Ikeda M., Makino O., Shibata T. Probing the activation stages of the RecA protein by monoclonal IgGs during the pairing of homologous DNA molecules. J Biol Chem. 1990 May 25;265(15):8957–8965. [PubMed] [Google Scholar]
- Klug A., Rhodes D. Zinc fingers: a novel protein fold for nucleic acid recognition. Cold Spring Harb Symp Quant Biol. 1987;52:473–482. doi: 10.1101/sqb.1987.052.01.054. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivi G. G., Bittner M. L., Rowold E., Jr, Wong E. Y., Glenn K. C., Rose K. S., Tiemeier D. C. Purification of recA-based fusion proteins by immunoadsorbent chromatography. Characterization of a major antigenic determinant of Escherichia coli recA protein. J Biol Chem. 1985 Aug 25;260(18):10263–10267. [PubMed] [Google Scholar]
- Mattei M. G., Lilienbaum A., Lin L. Z., Mattei J. F., Paulin D. Chromosomal localization of the mouse gene coding for vimentin. Genet Res. 1989 Jun;53(3):183–185. doi: 10.1017/s0016672300028147. [DOI] [PubMed] [Google Scholar]
- Mattei M. G., Philip N., Passage E., Moisan J. P., Mandel J. L., Mattei J. F. DNA probe localization at 18p113 band by in situ hybridization and identification of a small supernumerary chromosome. Hum Genet. 1985;69(3):268–271. doi: 10.1007/BF00293038. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. Nuclear location signal-mediated protein transport. Biochim Biophys Acta. 1989 Aug 14;1008(3):263–280. doi: 10.1016/0167-4781(89)90016-x. [DOI] [PubMed] [Google Scholar]
- Roca A. I., Cox M. M. The RecA protein: structure and function. Crit Rev Biochem Mol Biol. 1990;25(6):415–456. doi: 10.3109/10409239009090617. [DOI] [PubMed] [Google Scholar]
- Sancar A., Stachelek C., Konigsberg W., Rupp W. D. Sequences of the recA gene and protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2611–2615. doi: 10.1073/pnas.77.5.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Simpson N. E. Report of the committee on the genetic constitution of chromosomes 9 and 10. Cytogenet Cell Genet. 1989;51(1-4):202–225. doi: 10.1159/000132792. [DOI] [PubMed] [Google Scholar]
- Struhl K. Helix-turn-helix, zinc-finger, and leucine-zipper motifs for eukaryotic transcriptional regulatory proteins. Trends Biochem Sci. 1989 Apr;14(4):137–140. doi: 10.1016/0968-0004(89)90145-X. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
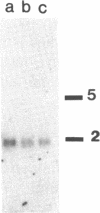
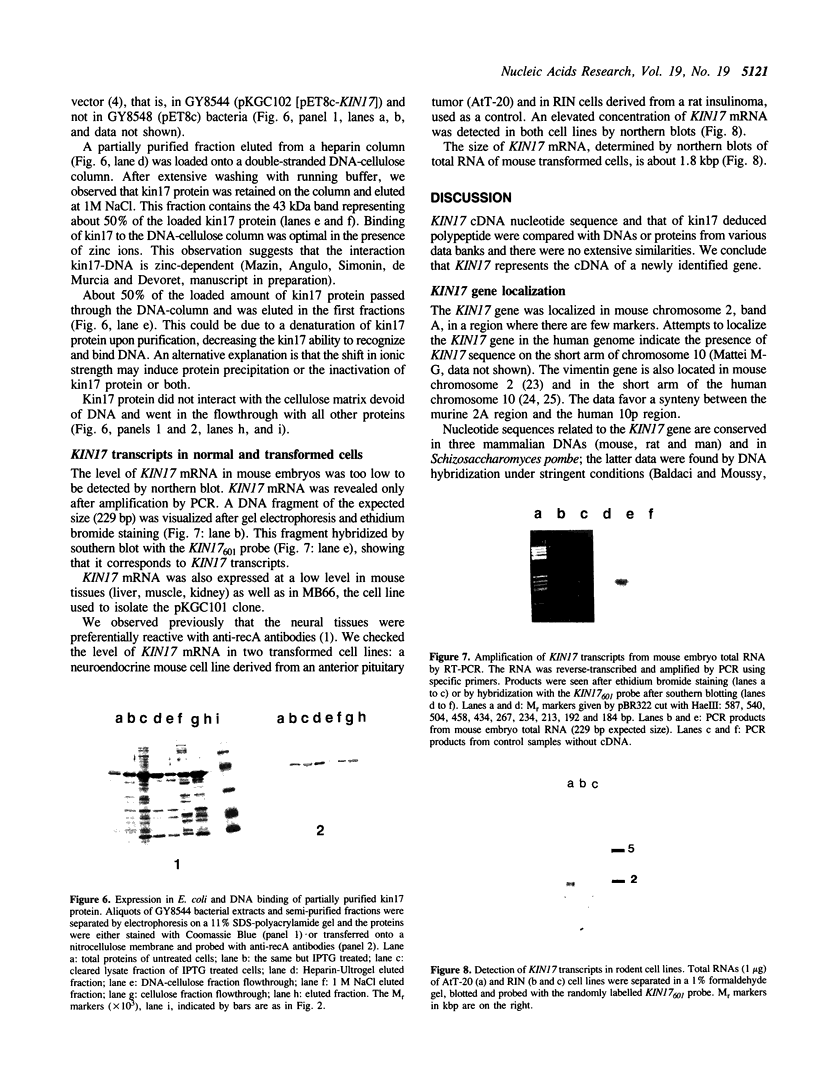
- Tagawa M., Sakamoto T., Shigemoto K., Matsubara H., Tamura Y., Ito T., Nakamura I., Okitsu A., Imai K., Taniguchi M. Expression of novel DNA-binding protein with zinc finger structure in various tumor cells. J Biol Chem. 1990 Nov 15;265(32):20021–20026. [PubMed] [Google Scholar]
- Tanaka K., Miura N., Satokata I., Miyamoto I., Yoshida M. C., Satoh Y., Kondo S., Yasui A., Okayama H., Okada Y. Analysis of a human DNA excision repair gene involved in group A xeroderma pigmentosum and containing a zinc-finger domain. Nature. 1990 Nov 1;348(6296):73–76. doi: 10.1038/348073a0. [DOI] [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. Molecular cloning and biological characterization of a human gene, ERCC2, that corrects the nucleotide excision repair defect in CHO UV5 cells. Mol Cell Biol. 1988 Mar;8(3):1137–1146. doi: 10.1128/mcb.8.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger C., Hollenberg S. M., Ong E. S., Harmon J. M., Brower S. T., Cidlowski J., Thompson E. B., Rosenfeld M. G., Evans R. M. Identification of human glucocorticoid receptor complementary DNA clones by epitope selection. Science. 1985 May 10;228(4700):740–742. doi: 10.1126/science.2581314. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Cook M., Boncinelli E., Krumlauf R. Segmental expression of Hox-2 homoeobox-containing genes in the developing mouse hindbrain. Nature. 1989 Oct 5;341(6241):405–409. doi: 10.1038/341405a0. [DOI] [PubMed] [Google Scholar]
- van Duin M., de Wit J., Odijk H., Westerveld A., Yasui A., Koken M. H., Hoeijmakers J. H., Bootsma D. Molecular characterization of the human excision repair gene ERCC-1: cDNA cloning and amino acid homology with the yeast DNA repair gene RAD10. Cell. 1986 Mar 28;44(6):913–923. doi: 10.1016/0092-8674(86)90014-0. [DOI] [PubMed] [Google Scholar]
- van Duin M., van Den Tol J., Hoeijmakers J. H., Bootsma D., Rupp I. P., Reynolds P., Prakash L., Prakash S. Conserved pattern of antisense overlapping transcription in the homologous human ERCC-1 and yeast RAD10 DNA repair gene regions. Mol Cell Biol. 1989 Apr;9(4):1794–1798. doi: 10.1128/mcb.9.4.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin M., van den Tol J., Warmerdam P., Odijk H., Meijer D., Westerveld A., Bootsma D., Hoeijmakers J. H. Evolution and mutagenesis of the mammalian excision repair gene ERCC-1. Nucleic Acids Res. 1988 Jun 24;16(12):5305–5322. doi: 10.1093/nar/16.12.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]