SUMMARY
Domestic pigeons are spectacularly diverse and exhibit variation in more traits than any other bird species [1]. In The Origin of Species, Charles Darwin repeatedly calls attention to the striking variation among domestic pigeon breeds – generated by thousands of years of artificial selection on a single species by human breeders – as a model for the process of natural divergence among wild populations and species [2]. Darwin proposed a morphology-based classification of domestic pigeon breeds [3], but the relationships among major groups of breeds and their geographic origins remain poorly understood [4, 5]. We used a large, geographically diverse sample of 361 individuals from 70 domestic pigeon breeds and two free-living populations to determine genetic relationships within this species. We found unexpected relationships among phenotypically divergent breeds that imply convergent evolution of derived traits in several breed groups. Our findings also illuminate the geographic origins of breed groups in India and the Middle East, and suggest that racing breeds have made substantial contributions to feral pigeon populations.
RESULTS AND DISCUSSION
Genetic structure of domestic pigeon breeds
Charles Darwin was a pigeon aficionado and relied heavily on the dramatic results of artificial selection in domestic pigeons to communicate his theory of natural selection in wild populations and species [2]. “Believing that it is always best to study some special group, I have, after deliberation, taken up domestic pigeons,” he writes in the Origin [2] (p. 20). Darwin notes that unique pigeon breeds are so distinct that, based on morphology alone, a taxonomist might be tempted to classify them as completely different genera [3], yet he also concludes that all breeds are simply variants within a single species, the rock pigeon Columba livia.
Pigeons were probably domesticated in the Mediterranean region at least 3000–5000 years ago, and possibly even earlier as a food source [3, 6, 7]. Their remarkable diversity can be viewed as the outcome of a massive selection experiment. Breeds show dramatic variation in craniofacial structures, color and pattern of plumage pigmentation, feather placement and structure, number and size of axial and appendicular skeletal elements, vocalizations, flight behaviors, and many other traits [1–5]. Furthermore, many of these traits are present in multiple breeds. Today, a large and dedicated pigeon hobbyist community counts thousands of breeders among its ranks worldwide. These hobbyists are the caretakers of a valuable – but largely untapped – reservoir of biological diversity.
Here, as an initial step in developing the pigeon as model for evolutionary genetics and developmental biology, we address two fundamental questions about the evolution of derived traits in this species. First, what are the genetic relationships among modern pigeon breeds? Second, does genetic evidence support the shared ancestry of breeds with similar traits, or did some traits evolve repeatedly in genetically unrelated breeds?
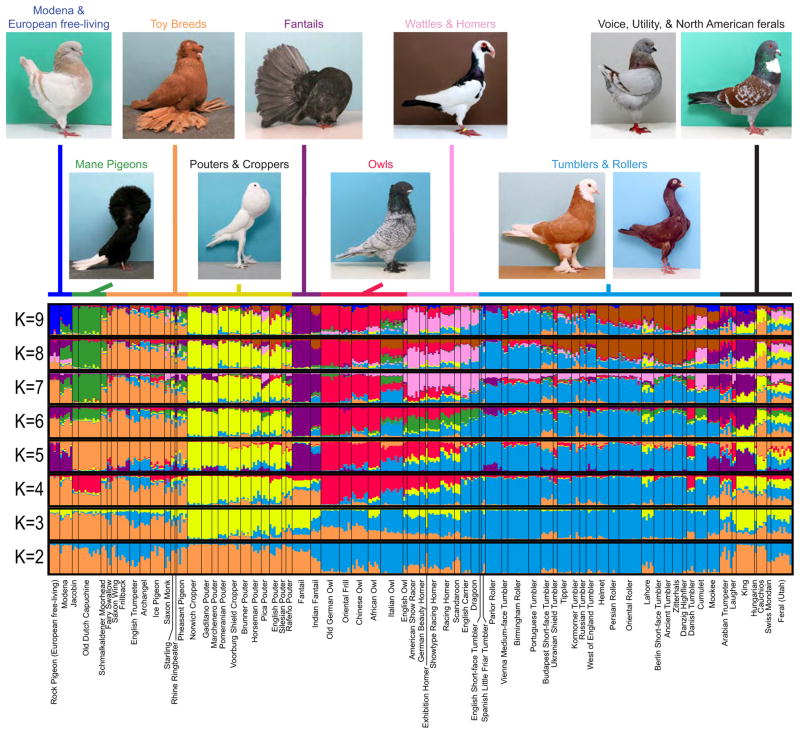
To address these questions, we studied the genetic structure and phylogenetic relationships among a large sample of domestic pigeon breeds. Our primary goal was to examine relationships among traditional breed groups, to which breeds are assigned based on phenotypic similarities and/or geographic regions of recent breed development (Fig. 1) [4, 5, 8]. First, we used 32 unlinked microsatellite markers to genotype 361 individual birds from 70 domestic breeds and two free-living populations. We next used the Bayesian clustering method in STRUCTURE software [9] to detect genetically similar individuals within the sample (Figs. 1 and S1). When two genetic clusters were assumed (K=2, where K is the number of putative clusters of genetically similar individuals; Fig. 1), the first cluster combined several breed groups with dramatically different morphologies. Principal members of this grouping included the pouters and croppers, which have a greatly enlarged, inflatable crop (an outpocketing of the esophagus); the fantails, which have supernumerary and elevated tail feathers; and mane pigeons, breeds with unusual feather manes or hoods about the head (Fig. 1).
Figure 1. Genetic structure of the rock pigeon (Columba livia).
Results from STRUCTURE analysis showing coefficients of genetic cluster membership of 361 individuals representing 70 domestic breeds and 2 free-living populations (European and North American, at the far left and far right of the plots, respectively) of rock pigeon. Each vertical line represents an individual bird, and proportion of membership in a genetic cluster is represented by different colors. Thin black lines separate breeds. At K=2, the tumblers, wattles, and owls are the predominant members of one cluster (blue), while other breeds comprise another cluster (orange). At K=3, the pouters and fantails (yellow) separate from the toys and other breeds, and at K=5, the fantails separate from the pouters. Pouters and fantails also share genetic similarity with the recently derived King, a breed with a complex hybrid background that probably includes contributions from Indian breeds [5]. At K=5, fantails are also united with the Modena, an ancient Italian breed, and a free-living European population. The latter two form a discrete cluster at K=9. At K=10 and greater (Fig. S1), some of the breed groups are assigned to different genetic clusters. This suggests that a number of assumed clusters beyond K=9 reveals the structure of individual breeds, rather than lending additional insights about genetically similar breed groups. Top row of photos, left to right: Modena, English Trumpeter, Fantail, Scandaroon, King, Cauchois. Bottom row: Jacobin, English Pouter, Oriental Frill, West of England Tumbler, Zitterhals (Stargard Shaker). Photos courtesy of Thomas Hellmann and are not to scale. See Fig. S1 for results from K=2–25, and Tables S1 and S2 for breed and marker information, respectively.
The second ancestral cluster consisted mainly of the tumblers (including rollers and highflyers), the most breed-rich of the major groups (≥80 breeds recognized in the USA) [4, 8]. Tumblers are generally small-bodied and were originally bred as performance flyers, with many breeds still capable of performing backward somersaults in flight. In most modern tumbler breeds, however, selection is most intense on morphological traits such as beak size and plumage. Also included in this cluster are the owl and the wattle breeds (wattles are skin thickenings emanating from the beak). These two breed groups contrast dramatically in several key traits: owls are typically diminutive in body size, have a pronounced breast or neck frill, and have among the smallest beaks of all breeds, while the wattle breeds (English Carrier, Scandaroon, and Dragoon in our analysis) are larger-bodied, lack a frill, and have among the most elaborated beak skeletons of all domestic pigeons [4, 5, 10]. The homers (homing pigeons and their relatives) are included in the second cluster as well. The Carrier, Cumulet, and owl breeds – all members of this cluster – contributed to the modern homing pigeon during its development in England and Belgium approximately 200 years ago [5]. Consistent with this recent admixture, the owls and several homer breeds continue to share partial membership in the same cluster at K=4 and beyond, and the Cumulet shares similarity with the homers and wattles at K=7. Numbers of clusters beyond K=9 reveals the structure of individual breeds, rather than lending additional insights about breed groups (Fig. S1). Notably, while allelic similarity is potentially indicative of shared ancestry, this analysis does not explicitly generate a phylogenetic hypothesis. Moreover, an alternative explanation for clustering is that large effective population sizes might result in an abundance of shared alleles.
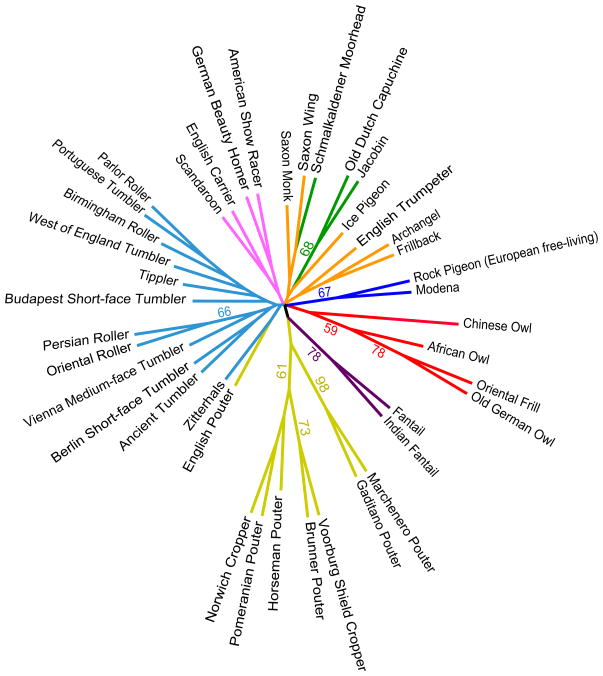
We next used multilocus genotype data from a subset of breeds (those with >50% membership in a cluster at K=9) to calculate genetic distances among breeds and to generate a neighbor-joining tree (Fig. 2). Among the major groups, only subsets of the pouter, fantail, mane, tumbler, Modena and free-living European, and owl branches of the tree have strong statistical support (Fig. 2). Nevertheless, at the breed level we observed substantial genetic differentiation, suggesting that in many cases, hybridization among breeds has been limited (mean pairwise FST = 0.204 for all breeds, maximum FST = 0.446; potentially more reliable differentiation estimates considering the modest sample sizes for some breeds [11]: mean Dest = 0.156, maximum Dest = 0.421; Tables S4 & S5). As a comparison, mean pairwise differentiation among African and Eurasian human populations with historically limited gene flow is lower (mean FST = 0.106, maximum FST = 0.240 for the comparison between Pygmy and Chinese populations using a dense genome-wide SNP set) [12].
Figure 2. Consensus neighbor-joining tree of 40 domestic breeds and one free-living population of rock pigeon.
The tree was constructed using pairwise Cavalli-Sforza chord genetic distances and includes the subset of breeds with >50% membership in one genetic cluster at K=9. Branch colors match cluster colors in Figure 1, except all tumbler breeds are represented with light blue for clarity. A notable incongruence between the STRUCTURE analysis and tree is the grouping of the English Pouter with a tumbler rather than with the other pouters; however, this grouping is not well supported. Percent bootstrap support on branches (≥50%) is based on 1000 iterations, and branch lengths are proportional to bootstrap values.
Taken together, our analysis shows both expected and unexpected genetic affinities among breeds. Like other domesticated animals such as dogs and chickens, pigeons probably have a reticular rather than hierarchical evolutionary history, which is reflected in the complex genetic structure of many breeds and a star-shaped phylogeny. These findings probably result from hybridization that has occurred throughout the domestication history of the pigeon; this practice continues among some modern breeders as well, often with the goal of transferring a new color into an established breed, or “improving” an existing trait. Unlike the stringent regulations for registering purebred dogs, in which modern breeds are effectively closed breeding populations separated by large genetic distances [13, 14], no barriers exist to mixed ancestry or parentage of pigeons (average FST=0.33 between dog breeds [13] compared to 0.24 for pigeons). On the other hand, little genetic variation divides dog breeds into subgroups [14], and like our tree (Fig. 2), neighbor-joining trees of dogs show limited structuring of the internal branches [13, 14].
Convergent evolution of traits
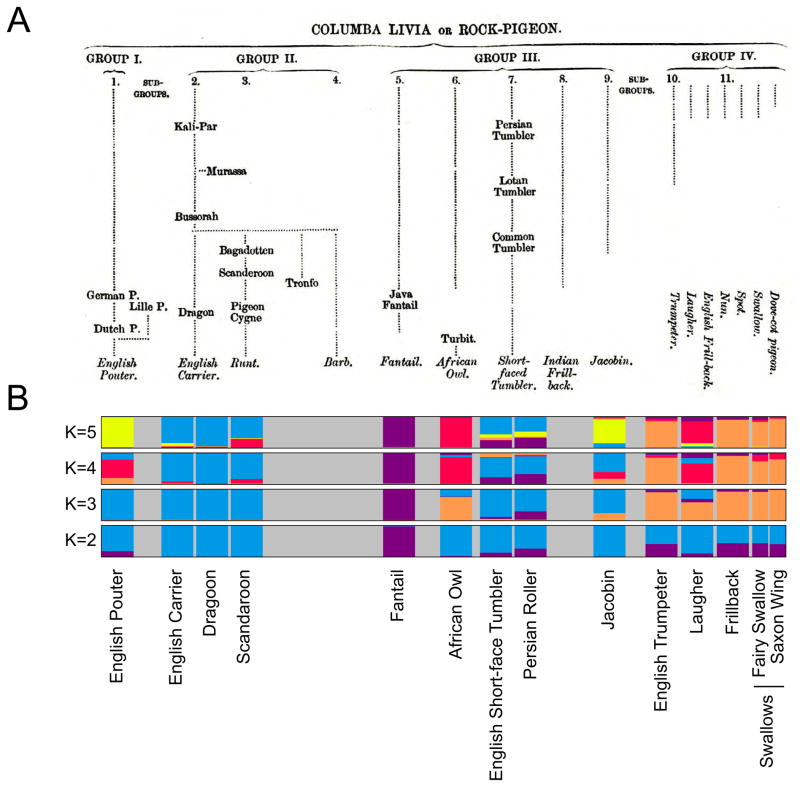
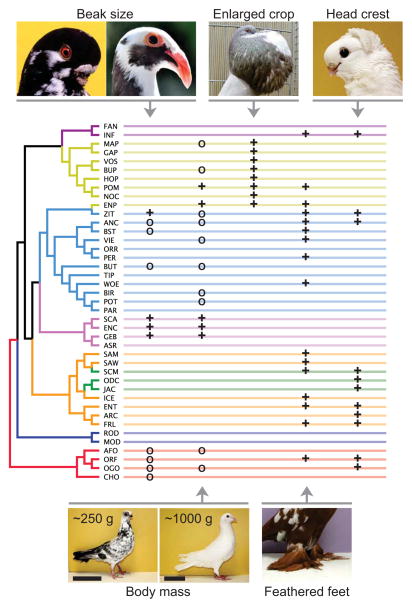
Darwin classified 32 pigeon breeds into four major groups based primarily on morphological traits, especially beak size (Fig. 3A). We repeated our STRUCTURE analysis with 13 breeds from Darwin’s study that were available to us and found that his morphological classification is broadly congruent with our genetic results (Fig. 3B). Beak size is only one of many traits that pigeon breeders have selected over the past several centuries, or in some cases, millennia. Feathered feet, head crests, and a multitude of color variants appear in many lineages [8] and must have evolved more than once (Fig. 4). Together, these findings suggest that traits do often, but not always, track the ancestry of breeds. This theme of repeated evolution is widespread in genetic studies of other natural and domesticated species as well [15–18].
Figure 3. Comparison of Darwin’s morphology-based classification and genetic structure analysis of domestic pigeon breeds.
(A) Darwin classified 32 breeds into four groups: (I) the pouters and croppers, which have enlarged crops (also see Figs. 1 & 4); (II) wattle breeds, many of which have elaborated beaks, and the large-bodied runts; (III) an “artificial” grouping diagnosed by a relatively short beak; and (IV) breeds that resemble the ancestral rock pigeon “in all important points of structure, especially in the beak” [3] (p. 154). (B) Mean coefficients of genetic cluster membership for 14 domestic breeds represented in Darwin’s classification and our genetic analysis. When two clusters are assumed (K=2), fantails are separated from all other breeds. At K=3, the breeds in Darwin’s Group IV and the African Owl (Group II) share a high coefficient of membership in a new cluster. At K=4, the African Owl, Laugher, and (to a lesser extent) English Pouter share membership in a new cluster that includes members of three different morphological Groups. At K=5, the English Pouter and Jacobin form a cluster. While some genetic clusters span more than one morphological Group, others are consistent within a Group. For example, the wattle breeds (Group II), tumblers (Group III), and most of Group IV remain united with breeds of similar morphology at K=2–5. Taken together, these results confirm that morphology is a good general predictor of genetic similarity in domestic pigeons, yet they also show that breeds that share allelic similarity can be morphologically distinct. Darwin, too, recognized that breeds united in form were not necessarily united in ancestry and, conversely, that anatomically dissimilar breeds might be related. For example, he classified the short-beaked Barb (not in our genetic data set) with the long-beaked breeds of Group II. Darwin’s tree reproduced from [3] (darwin-online.org.uk).
Figure 4. Distribution of several derived traits across groups of domestic pigeons.
Phylogenetic tree in Fig. 2 was converted to a cladogram format with equal branch lengths (far left). For beak size column, “+” indicates a substantial increase in size relative to the ancestral condition, and “O” indicates a decrease [4, 8]. For body mass, “+” indicates breeds with a maximum over 550 g, “O” indicates those under 340 g [4, 8]. Although a four-fold difference in body mass is depicted here, extremes in body mass among all known breeds differ by more than an order of magnitude. For crop, feathered feet, and head crest, “+” indicates fixed or variable presence of the trait (substantial departure from the ancestral condition [4, 8]). All traits shown were selected in multiple groups except an enlarged crop, which is confined to the pouters and croppers. A possible exception is the Cauchois (not included in the tree; see Fig. 1), a non-pouter breed with an enlarged and inflatable crop, thought to have been developed centuries ago from a cross between a pouter and large-bodied mondain breed [5, 34]. Our STRUCTURE analysis supports this hypothesis, with the Cauchois sharing 37.8–89.7% membership in the genetic cluster containing the pouters at K=2–9 (Fig. 1). Breeds shown (clockwise from upper left): African Owl*, Scandaroon, Norwich Cropper, Old German Owl, West of England Tumbler*, White Carneau, Budapest Short-face Tumbler (*photos courtesy of Thomas Hellmann). Scale bars = 10 cm.
Geographic origins of breeds
Modern breeds are frequently described as having origins in England, Germany, Belgium or elsewhere in Europe, but their progenitors were probably brought there from afar by traders or colonialists [3–5, 19, 20]. While we may never definitively know the sites of pigeon domestication, genetic data combined with historical records may provide new clues about the geographic origins of some of the major breed groups.
Most historical accounts trace the origins of the wattle breeds, owls, and tumblers to the Middle and Near East hundreds of years ago, with ancient breeds transported to Europe and India for further development by hybridization or selection [3, 5, 20–22]. Our genetic analyses are consistent with this common geographic origin, as these three groups share substantial membership in the same genetic cluster at K=2–3, and two of the three wattle breeds (English Carrier and Dragoon) retain high membership coefficients in the tumbler cluster through K=5 (Fig. 1).
The fantail breeds probably originated in India and have undergone less outcrossing than many other breeds [5]. In our STRUCTURE analysis, the Fantail (and the Indian Fantail to a lesser extent) shows a surprising affinity with the pouters at K=2–3, and these two groups share a major branch on the neighbor-joining tree (Figs. 1 & 2); these two groups are among the most morphologically extreme of all domestic pigeons, and among the most different from each other. European breeders have developed pouters for several hundred years [23, 24], and Dutch traders might have originally brought them to Europe from India [5]. Together, historical accounts and genetic similarity between fantails and pouters support the hypothesis of common geographic origin in India.
Ancestry of feral pigeon populations
Domestic rock pigeons were first brought to North America approximately 400 years ago and feral populations were probably established shortly thereafter [25, 26]. Likewise, some Eurasian and North African feral populations are probably nearly as old as the most ancient domestication events. In addition to the domestic breeds in our study, we also included a feral pigeon population (Salt Lake City, Utah). Escaped individuals from nearly any domestic breed have the potential to contribute to the feral gene pool, and feral birds showed highly heterogeneous membership across clusters at most values of K (Fig. 1). However, we expected that the Racing Homer would be a major contributor to the feral gene pool. Pigeon racing is an enormously popular and high-stakes hobby worldwide. While many birds in homing competitions are elite racers that reliably navigate hundreds of miles to their home lofts, some breeders report that up to 20% of their birds that start a race do not return. As predicted, pairwise Dest for the racing homer to feral comparison was among the lowest 0.1% of all pairwise comparisons (Dest =0.006), and pairwise FST was the lowest for any pairwise comparison (FST = 0.049). Therefore, feral pigeons and Racing Homers show very little genetic differentiation, and wayward Racing Homers probably make a substantial contribution to the genetic profile of this local feral population.
We also included samples of free-living rock pigeons (the existence of “pure” wild populations uncontaminated by domestics or ferals is questionable [27]) from Scotland to test for genetic similarities with domestic breeds, and with our North American feral sample. Consistent with previous studies [25, 28], European and North American free-living populations are highly differentiated (Dest =0.162). The European sample groups with the Modena, a former-racing breed that was developed in Italy up to 2000 years ago [5] (Figs. 1 & 2). This suggests that either Modenas were developed from European free-living populations, or that, as in North America, wayward racers contributed to the local feral population, perhaps for centuries. Studies of additional feral populations will reveal whether strong affinities with racing breeds occur locally and sporadically or, as we suspect, almost everywhere.
The domestic pigeon as a model for avian genetics and diversity
Darwin enthusiastically promoted domestic pigeons as a proxy for understanding natural selection in wild populations and species, and pigeons thus hold a unique station in the history of evolutionary biology. More recently, domesticated animals have emerged as important models for rapid evolutionary change [29]. Feathered feet, head ornamentation, skeletal differences, plumage color variation, and other traits prized by breeders offer numerous opportunities to examine the genetic and developmental bases of morphological novelty in birds. These and other traits evolved repeatedly in many breeds, and a challenge arising from this study is to determine whether this distribution of traits resulted from selection on standing variation (either by hybridization between breeds or repeated selection on variants in wild populations), from de novo mutation in independent lineages, or both. In the first case, we would expect certain regions of the pigeon genome to share histories and haplotypes that reflect the transfer of valued traits between breeds. This hypothesis will be testable when we have more detailed information about genomic diversity in this species. Pigeons are also easily bred in the lab and morphologically distinct breeds are interfertile [2, 3, 30]. Therefore, hybrid crosses should be a fruitful method to map the genetic architecture of derived traits, many of which are known to have a relatively simple genetic basis [4, 30].
The extreme range of variation in domestic pigeons mirrors, if not exceeds, the diversity among wild species of columbids (pigeons and doves) and other birds. Domestic pigeons and wild bird species vary in many of the same traits, so domestic pigeons provide an entry point to the genetic basis of avian evolutionary diversity in general [1, 31]. Changes in the same genes, and even in some cases the same mutations, have recently been shown to underlie similar phenotypes in both wild and domesticated populations [32, 33]. The genetic history of pigeons is a critical framework for the analysis of the genetic control of many novel traits in this fascinating avian species.
Supplementary Material
HIGHLIGHTS.
Relationships of domestic pigeon breeds are revealed using genetic data
Distinct pigeon breeds have converged with respect to numerous traits
Escaped racing pigeons have probably contributed substantially to feral populations
Acknowledgments
We thank Kyle Christensen and members of the Utah Pigeon Club, National Pigeon Association, and Bund Deutscher Rassegflügelzüchter for their spirited collaboration; Elena Boer, Terry Dial, Jennifer Koop, Matt Miller, and Jessica Waite for collection assistance; Jon Seger and Kyle Christensen for comments on drafts of the manuscript; and Thomas Hellmann for photos used in Figures 1 & 4. This work was supported by NIH T32GM007464 (SAS, EJO), NSF DGE0841233 (SAS), University of Utah BioURP and UROP programs (EEM, MWG), NIH/NHGRI K99HG005846 (JX), a Burroughs Wellcome Fund Career Award in the Biomedical Sciences (MDS), and a gift from Onorio Catenacci.
Footnotes
Supplemental data include Supplemental Experimental Procedures, 1 figure, and 4 tables.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- 1.Price TD. Domesticated birds as a model for the genetics of speciation by sexual selection. Genetica. 2002;116:311–327. [PubMed] [Google Scholar]
- 2.Darwin C. On the Origin of Species by Means of Natural Selection. London: John Murray; 1859. [Google Scholar]
- 3.Darwin CR. The Variation of Animals and Plants Under Domestication. Vol. 1. London: John Murray; 1868. [Google Scholar]
- 4.Levi WM. Encyclopedia of Pigeon Breeds. Sumter, S.C: Levi Publishing Co, Inc; 1965. [Google Scholar]
- 5.Levi WM. The Pigeon. 2. Sumpter, S.C: Levi Publishing Co., Inc; 1986. Revised Edition. [Google Scholar]
- 6.Sossinka R. Domestication in birds. Avian Biology. 1982;6:373–403. [Google Scholar]
- 7.Driscoll CA, Macdonald DW, O’Brien SJ. From wild animals to domestic pets, an evolutionary view of domestication. Proc Natl Acad Sci U S A. 2009;106(Suppl 1):9971–9978. doi: 10.1073/pnas.0901586106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National_Pigeon_Association. National Pigeon Association Book of Standards. Goodlettsville, TN: Purebred Pigeon Publishing; 2010. [Google Scholar]
- 9.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young NM, Fondon JW., III Artificial Selection, Developmental Constraints and Craniofacial Variation in the Feral and Domesticated Pigeon (Columba livia) Integrative and Comparative Biology. 2009;49:e188. [Google Scholar]
- 11.Jost L. G(ST) and its relatives do not measure differentiation. Mol Ecol. 2008;17:4015–4026. doi: 10.1111/j.1365-294x.2008.03887.x. [DOI] [PubMed] [Google Scholar]
- 12.Xing J, Watkins WS, Witherspoon DJ, Zhang Y, Guthery SL, Thara R, Mowry BJ, Bulayeva K, Weiss RB, Jorde LB. Fine-scaled human genetic structure revealed by SNP microarrays. Genome Res. 2009;19:815–825. doi: 10.1101/gr.085589.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker HG, Kim LV, Sutter NB, Carlson S, Lorentzen TD, Malek TB, Johnson GS, DeFrance HB, Ostrander EA, Kruglyak L. Genetic structure of the purebred domestic dog. Science. 2004;304:1160–1164. doi: 10.1126/science.1097406. [DOI] [PubMed] [Google Scholar]
- 14.Vaysse A, Ratnakumar A, Derrien T, Axelsson E, Rosengren Pielberg G, Sigurdsson S, Fall T, Seppala EH, Hansen MS, Lawley CT, Karlsson EK, Bannasch D, Vila C, Lohi H, Galibert F, Fredholm M, Haggstrom J, Hedhammar A, Andre C, Lindblad-Toh K, Hitte C, Webster MT. Identification of genomic regions associated with phenotypic variation between dog breeds using selection mapping. PLoS Genet. 2011;7:e1002316. doi: 10.1371/journal.pgen.1002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aldenhoven JT, Miller MA, Corneli PS, Shapiro MD. Phylogeography of ninespine sticklebacks (Pungitius pungitius) in North America: glacial refugia and the origins of adaptive traits. Mol Ecol. 2010;19:4061–4076. doi: 10.1111/j.1365-294X.2010.04801.x. [DOI] [PubMed] [Google Scholar]
- 16.Colosimo PF, Hosemann KE, Balabhadra S, Villarreal G, Jr, Dickson M, Grimwood J, Schmutz J, Myers RM, Schluter D, Kingsley DM. Widespread parallel evolution in sticklebacks by repeated fixation of Ectodysplasin alleles. Science. 2005;307:1928–1933. doi: 10.1126/science.1107239. [DOI] [PubMed] [Google Scholar]
- 17.Arendt J, Reznick D. Convergence and parallelism reconsidered: what have we learned about the genetics of adaptation? Trends Ecol Evol. 2008;23:26–32. doi: 10.1016/j.tree.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Hovav R, Chaudhary B, Udall JA, Flagel L, Wendel JF. Parallel Domestication, Convergent Evolution and Duplicated Gene Recruitment in Allopolyploid Cotton. Genetics. 2008;179:1725–1733. doi: 10.1534/genetics.108.089656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyell JC. Fancy Pigeons. London: A. Bradley; 1881. [Google Scholar]
- 20.Tegetmeier WB. Pigeons: Their Structure, Varieties, Habits, and Management. London: George Routledge and Sons; 1868. [Google Scholar]
- 21.Alla-oodeen The art of training pigeons in the East. The Zoologist (London) 1888;12:209–219. 252–258. [Google Scholar]
- 22.Fazl A. The art of training pigeons in the East [annotated translation from Ain-i-Akbari, 1590] The Zoologist (London) 1888;12:167–174. [Google Scholar]
- 23.Aldrovandi U. Ornithologiae. Frankfurt: Nicolai Bassaei; 1610. [Google Scholar]
- 24.Ray J. The Ornithology of Francis Willughby. London: John Martin; 1676. [Google Scholar]
- 25.Johnston RF. Geographic variation of size in feral pigeons. The Auk. 1994;111:398–404. [Google Scholar]
- 26.Schorger AW. Introduction of the domestic pigeon. The Auk. 1952;69:462–463. [Google Scholar]
- 27.Goodwin D. Pigeons and Doves of the World. 3. Ithaca, NY: Comstock Publishing Associates; 1983. [Google Scholar]
- 28.Johnston RF, Siegel-Causey D, Johnson SG. European Populations of the Rock Dove Columba livia and Genotypic Extinction. American Midland Naturalist. 1988;120:1–10. [Google Scholar]
- 29.Akey JM, Ruhe AL, Akey DT, Wong AK, Connelly CF, Madeoy J, Nicholas TJ, Neff MW. Tracking footprints of artificial selection in the dog genome. Proc Natl Acad Sci U S A. 2010;107:1160–1165. doi: 10.1073/pnas.0909918107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sell A. Breeding and Inheritance in Pigeons. Hengersberg, Germany: Schober Verlags-GmbH; 1994. [Google Scholar]
- 31.Baptista LF, Gomez Martinez JE, Horblit HM. Darwin’s pigeons and the evolution of the columbiforms: recapitulation of ancient genes. Acta Zoológica Mexicana. 2009;25:719–741. [Google Scholar]
- 32.Arnaud N, Lawrenson T, Ostergaard L, Sablowski R. The same regulatory point mutation changed seed-dispersal structures in evolution and domestication. Curr Biol. 2011;21:1215–1219. doi: 10.1016/j.cub.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Hoekstra HE. Genetics, development and evolution of adaptive pigmentation in vertebrates. Heredity. 2006;97:222–234. doi: 10.1038/sj.hdy.6800861. [DOI] [PubMed] [Google Scholar]
- 34.Buffon GLL. Histoire Naturelle. Vol. 43. Paris: Imprimerie de F. Dufart; 1774. Le Pigeon; pp. 154–311. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.