Abstract
Exocytosis is a highly regulated, multistage process consisting of multiple functionally definable stages, including recruitment, targeting, tethering, priming, and docking of secretory vesicles with the plasma membrane, followed by calcium-triggered membrane fusion. The acrosome reaction of spermatozoa is a complex, calcium-dependent regulated exocytosis. Fusion at multiple sites between the outer acrosomal membrane and the cell membrane causes the release of the acrosomal contents and the loss of the membranes surrounding the acrosome. Not much is known about the molecules that mediate membrane docking in this particular fusion model. In neurons, the formation of the ternary RIM/Munc13/Rab3A complex has been suggested as a critical component of synaptic vesicles docking. Previously, we demonstrated that Rab3A localizes to the acrosomal region in human sperm, stimulates acrosomal exocytosis, and participates in an early stage during membrane fusion. Here, we report that RIM and Munc13 are also present in human sperm and localize to the acrosomal region. Like Rab3A, RIM and Munc13 participate in a prefusion step before the efflux of intra-acrosomal calcium. By means of a functional assay using antibodies and recombinant proteins, we show that RIM, Munc13 and Rab3A interplay during acrosomal exocytosis. Finally, we report by electron transmission microscopy that sequestering RIM and Rab3A alters the docking of the acrosomal membrane to the plasma membrane during calcium-activated acrosomal exocytosis. Our results suggest that the RIM/Munc13/Rab3 A complex participates in acrosomal exocytosis and that RIM and Rab3A have a central role in membrane docking.
Keywords: RIM, Munc13, Rab3A, acrosome reaction, membrane docking, human sperm
INTRODUCTION
Membrane fusion is an essential step for intracellular transport in eukaryotic cells. The process requires the recognition of the membrane-bound compartments that are going to fuse and the assembly of a complex fusion machinery that mediates the mixing of the two bilayers [23]. Rabs are small GTPases that play a central role in intracellular membrane recognition and fusion [14]. Rabs are activated by specific GEFs (guanine nucleotide exchange factors), which catalyze the exchange of GDP for GTP. The prevailing members of the Rab family present in secretory granules are Rab3 [31] and Rab27 [16]. In particular, Rab3A has been localized to the acrosomal granule of mammalian sperm from several species [15;36]. The exocytosis of the acrosomal granule proceeds through unusual membrane fusion events. Upon activation, a cytoplasmic calcium increase triggers the fusion of the acrosomal granule membrane with the plasma membrane at multiple points, causing the fenestration of both membranes and the release of hybrid vesicles composed of patches of acrosomal and plasma membrane [39]. According to several pieces of evidence, Rab3A is an essential factor in the cascade of reactions leading to acrosomal exocytosis. Moreover, prenylated and GTP-loaded recombinant Rab3A can trigger by itself exocytosis in permeabilized sperm [38] and in intact cells when attached to a membrane permeant peptide [21]. When in the active form, Rabs interact with several molecules that are generically called Rab effectors [11]. Among the most prominent Rab3 effectors are RIM1α and RIM2α, two proteins associated to the active zone in presynaptic membranes that bind the small GTPase through the N-terminus containing the Zn-finger domain [35]. Numerous pieces of evidence indicate that RIM1α and RIM2α isoforms govern several steps of calcium-activated exocytosis. These RIM isoforms have multiple domains and interact with numerous factors [25]. RIM1α and RIM2α bind Munc13-1 and ubMunc13-2 (other two active zone proteins) through the Zn-finger domain in neurons [1]. Interestingly, the Rab3A- and Munc13-binding sites are adjacent but separate, allowing the formation of a heterotrimeric RIM/Munc13/Rab3A complex [8]. Genetic studies in several model organisms have shown that Munc13-1 has an important role in several aspects of regulated exocytosis [2;4]. The molecular basis for this role is not completely understood, although interactions with syntaxin have been reported [3].
At present, it is unknown if RIM and Munc13, two components of the heterotrimeric RIM/Munc13/Rab3A complex, participate in acrosomal exocytosis. We have previously shown that Rab3A stimulates acrosomal exocytosis and participates in an early stage during membrane fusion [6;38]. Here, we report that, like Rab3A, RIM and Munc13 are present in human sperm and that they play a functional role in acrosomal exocytosis before the acrosomal calcium efflux. We also report that RIM and Rab3A have a critical role in the docking of membranes during acrosomal exocytosis.
MATERIALS METHODS
Reagents
Recombinant streptolysin O (SLO) was obtained from Dr Bhakdi (University of Mainz, Mainz, Germany). O-Nitrophenyl EGTA-acetoxymethyl ester (NP-EGTA-AM) was purchased from Molecular Probes (Eugene, OR, USA). Anti-RIM1/2 antibody (polyclonal rabbit antibody raised against the Zn-finger domain of RIM1/2 isoforms), anti-RIM1 (polyclonal rabbit antibody raised against the PDZ domain of RIM1), anti-Munc13 (monoclonal mouse antibody against the C2A domain of Munc 13-1), and anti-Munc13-1 (polyclonal rabbit antibody against Munc 13-1) were from Synaptic Systems (Göttingen, Germany). Anti-RIM1/2 (N-20) (polyclonal goat antibody against the N-terminus of RIM1/2 isoforms) was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-Rab3A (rabbit polyclonal, purified IgG) was from Stressgen Biotechnologies (Victoris, BC, Canada). Alexa Fluor 546-conjugated goat anti-rabbit IgG, donkey anti-mouse IgG, and donkey anti-goat antibodies were from Molecular Probes (Eugene, OR, USA). Horseradish peroxidase conjugated goat anti-rabbit IgG, donkey anti-mouse IgG, and donkey anti-goat IgG were from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). Glutathion-sepharose was from GE Healthcare (Buenos Aires, Argentina). 2-aminoethoxydiphenylborate (2APB) and acetone were from Calbiochem (Merck, Buenos Aires, Argentina). Methanol was purchased from Merck (Buenos Aires, Argentina) and FITC-Pisum sativum lectin (PSA) was from ICN (Eurolab SA, Buenos Aires, Argentina). Low melting agarose and tannic acid were from Sigma (Sigma-Aldrich, Buenos Aires, Argentina). All electron microscopy supplies were from Pelco (Ted Pella INC. California, USA). All other chemicals were reagent of analytical grade and were purchased from ICN or Sigma- Aldrich (Buenos Aires, Argentina).
Recombinant proteins
Plasmids encoding the Zn-finger domain of RIM2 (residues 82-142, RIM ZF), and Munc13-1 C2A domain (residues 3-209, Munc13 C2A) were kindly provided by Dr. Rizo (UT Southwestern Medical Center, Dallas, TX, USA). Plasmid encoding N-terminus domain of RIM1 (residues 1-399, RIM 1N) was kindly provided by Dr. Regazzi (University of Lausanne, Lausanne, Switzerland). A plasmid encoding Rab3A was generously provided by Dr. P. Stahl (Washington University, St. Louis, MO, USA). All plasmids fused to GST in pGEX-2T or pGEX-KG vectors were transformed in BL21 (DE3) cells (Stratagene, La Jolla, CA, USA) and expression was induced overnight at 22°C with 0.5 mM IPTG. Recombinant proteins were purified by affinity chromatography on glutathione–sepharose beads as previously described [29]. Purified proteins are shown in Supplementary Fig. S4. Rab3A was always used prenylated and loaded with guanosine 5′-[γ-thio] triphosphate as described in [38].
Human sperm sample preparation and acrosome reaction
Human semen samples were obtained from normal healthy donors. The informed consent and protocol for semen handling were approved by the Ethic Committee of the Medical School, Universidad Nacional de Cuyo. Semen was allowed to liquefy for 30–60 min at 37°C. We used a swim-up protocol to isolate highly motile sperm under capacitating conditions in Human Tubal Fluid media (HTF, as formulated by Irvine Scientific, Santa Ana, CA) supplemented with 0.5% bovine serum albumin (BSA) for 1 h at 37°C in an atmosphere of 5% CO2/95% air. Sperm concentrations were adjusted to 5–10 × 106 cells/ml before incubating for at least 2 h under capacitating conditions. Permeabilization was accomplished as described in [38]. Briefly, washed spermatozoa were resuspended in cold PBS containing 1.5 U/ml SLO for 15 min at 4°C. Cells were washed once with PBS and resuspended in ice-cold sucrose buffer (250 mM sucrose, 0.5 mM EGTA, 20 mM Hepes-K, pH 7) containing 2 mM dithiothreitol. For acrosome reaction assays, we added inhibitors and stimulants sequentially as indicated in the figures, and incubated for 10–15 min at 37°C after each addition. Sperm were spotted on teflon-printed slides, air dried, and fixed/permeabilized in ice-cold methanol for 1 min. Acrosomal status was evaluated by staining with FITC-coupled Pisum sativum (FITC-PSA) according to Mendoza et al., 1992 [24]. At least 200 cells were scored using a Nikon Optiphot II microscope (Nikon, Inc., Melville, NY) equipped with epifluorescence optics. Basal (no stimulation, —control) and positive (0.5 mM CaCl2, corresponding to 10 μM free Ca2+, —calcium)controls were included in all experiments. Acrosomal exocytosis indexes (A.E. index) were calculated by subtracting the number of spontaneously reacted spermatozoa from all values and expressing the results as a percentage of the acrosomal exocytosis observed in the positive control. Data were evaluated using one-way ANOVA and post hoc tests. Differences were considered significant at the p<0.05 level.
NP-EGTA-AM assay
Permeabilized spermatozoa were loaded with 10 μM NP-EGTA-AM for 15 min at 37 °C to chelate intra-acrosomal Ca2+. AE was then initiated by adding 0.5 mM CaCl2. After further 15 min incubation at 37 °C to allow exocytosis to proceed up to the intraacrosomal Ca2+sensitive step; sperm were then treated for 15 min at 37 °C with different protein domains or specific antibodies. All these procedures were carried out in the dark. UV flash photolysis of the chelator was induced at the end of the incubation period (hυ), and the samples were incubated for 5 min to promote exocytosis.
Immunoblot analysis
Sperm were washed in cold PBS, and proteins were extracted in sample buffer as described [32]. Twenty μg (mouse brain) or 50 μg (human sperm) of total protein were loaded on 6% polyacrylamide gels according to Laemmli [19], and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). Nonspecific reactivity was blocked by incubation for 1 h at room temperature (RT) with 5% nonfat dry milk dissolved in washing buffer (PBS, pH 7.6, 0.1% Tween 20). Blots were incubated with primary antibodies anti-RIM or anti-Munc13 (1μg/ml), in blocking solution for 1 h at RT or overnight at 4°C. Horseradish peroxidase-conjugated goat anti-rabbit IgG and donkey anti-mouse were used as secondary antibodies (0.25 μg/ml) during 60 min incubations at RT. Excess first and second antibodies were removed by washing five times for 10 min each in washing buffer. Detection was accomplished with an enhanced chemiluminescence system (ECL; Amersham Biosciences) and visualized with a FujiFilm LAS-4000 Scanner (FujiFilm, Tokyo, Japan).
Indirect immunofluorescence
Sperm were spotted on round cover slips, fixed in 2% paraformaldehyde and permeabilized in 0.1% Triton X-100 in PBS for 10 min at RT. Cells were incubated in 50 mM glycine-PBS for 30 min at RT and then blocked in 5% bovine fetal serum (BFS) in PBS containing 0.4% polyvinylpyrrolidone (PVP) (40,000 average MW) during 1h. Cells were labeled with the anti-RIM1/2 antibody, anti-RIM1 antibody, or anti-Munc13-1 antibody (60 min at RT, 20 μg/ml in 1% BFS-PBS/PVP), followed by a Alexa Fluor 546-conjugated anti-rabbit IgG (1 h at RT, 4 μg/ml in 1% BFS-PBS/PVP). Alexa Fluor 546-conjugated anti-mouse IgG was used when anti-Munc13 was the primary antibody (60 min at RT, 4 μg/ml in 1% BFS-PBS/PVP). Cover slips were washed with 0.4% PVP in PBS between incubations. Finally, cells were incubated 1 min in cold methanol and stained with FITC-PSA [24], and washed with distilled water 20 min at 4°C. Cover slips were mounted in Vectashield (Vector Labs), and examined with an Eclipse TE2000 Nikon microscope equipped with a Plan Apo 603/1.40 oil objective and a Hamamatsu (Bridgewater, NJ, USA) Orca 100 camera operated with MetaMorph 6.1 software (Universal Imaging, Downingtown, USA). Background was subtracted and brightness/contrast was adjusted to render an all-or-nothing labeling pattern using Image J. The presence of immunostaining in the acrosomal region was evaluated in at least 200 cells in three independent experiments.
Transmission electron microscopy
We processed permeabilized human sperm (5–6 x106/tube) as described for acrosome reaction assays except that 1.6 mM tannic acid was added 5 min before stopping the reaction with 2.5% glutaraldehyde in sucrose buffer. Sperm suspensions were incubated overnight at 4°C. Fixed sperm samples were washed twice in PBS and postfixed in 1% osmium tetroxide-PBS for 2 h at RT, washed three times in PBS, and dehydrated sequentially with increasing concentrations of ice-cold acetone. Cells were infiltrated in 1:1 acetone:Spurr 2 h or overnight at RT and finally embedded in fresh pure resin overnight at RT. Samples were cured 24 h at 70°C. Thin sections were cut (60–80 nm) with a diamond knife (Diatome, Washington, DC, USA) on a Leica Ultracut R ultramicrotome, collected on 200-mesh copper grids and stained with saturated uranyl acetate in methanol plus lead citrate. Grids were observed and photographed in a Zeiss 902 electron microscope at 50 kV. We included negative (not stimulated) and positive (stimulated with 0.5 mM CaCl2 in the presence of 200 μM 2APB) controls in all experiments. The distance distributions are plotted as histograms, which were compared using the Kolmogorov-Smirnof test.
RESULTS
RIM, a Rab3A effector, is present in human sperm and localizes to the acrosomal region
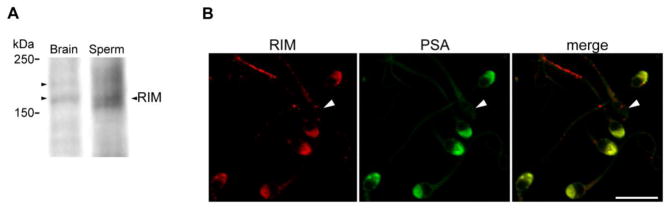
Active (in the GTP-bound form) Rab proteins bind numerous factors to regulate different cellular functions. These factors are generically called Rab effectors. RIM1 and RIM2 are well-known cytosolic Rab3 effectors, which participate in regulated exocytosis [35]. Rab3A has an important role in acrosomal exocytosis; hence, we assessed the presence of RIM isoforms in human sperm by Western blot using an antibody raised against the Zn- finger domain of RIM1/2. In mouse brain extracts, the antibody recognized two bands around the 180 kDa region (likely corresponding to RIM1, upper band, and RIM2, lower band). Proteins from whole sperm extracts were resolved by SDS-PAGE, transferred to PVDF membranes and probed with the anti-RIM1/2 antibody. The antibody recognized a predominant band of the expected molecular weight as shown in Fig. 1A. Indirect immunofluorescence images using the same antibody showed labeling predominantly in the acrosomal region in 84% of acrosome intact cells. Acrosomal labeling was blocked by preincubating of the antibody with the Zn-finger domain of RIM2 (residues 82-142) fused to GST (RIM ZF domain). Blocking experiment also showed that label on the flagellum was unspecific (data not shown). Notice that the immunostaining was not present in spontaneously reacted sperm, which have lost the acrosome and the overlying plasma membrane (Fig. 1B, arrowhead). Similar results were obtained with a different antibody that recognized the N terminus region of RIM1/2 (anti-RIM1/2, N-20, data not shown). A third antibody raised against the PDZ domain of RIM1 gave a similar acrosomal pattern by immunofluorescence (Supplementary Fig. S1). All antibodies used recognize both RIM1 and RIM2 isoforms. Nevertheless, the western blot result suggests that RIM2 might be the main isoform present in sperm.
FIGURE 1. RIM is present in sperm and localizes to the acrosomal region.
A. Proteins from whole sperm homogenates (Sperm, 100 × 106 cells) were resolved in 6% SDS-PAGE gels and immunoblotted with an antibody raised against the Zn-finger domain of RIM1/2 (anti-RIM1/2). Brain extracts (Brain) was used as a positive control. Molecular weight markers are indicated on the left. Arrowheads on the left of brain lane indicate RIM1, (upper band), and RIM2 (lower band). Arrowhead on the right indicates the main RIM isoform detected in sperm. Shown is a representative result from three independent experiments. B. Human sperm were fixed, permeabilized and double-stained with the anti-RIM1/2 antibody (RIM) and FITC-PSA lectin (PSA). Note that RIM labeling is absent in reacted sperm (arrowhead). Shown are representative images from three different experiments. Bars = 10 μm
RIM is necessary in an early step for acrosomal exocytosis
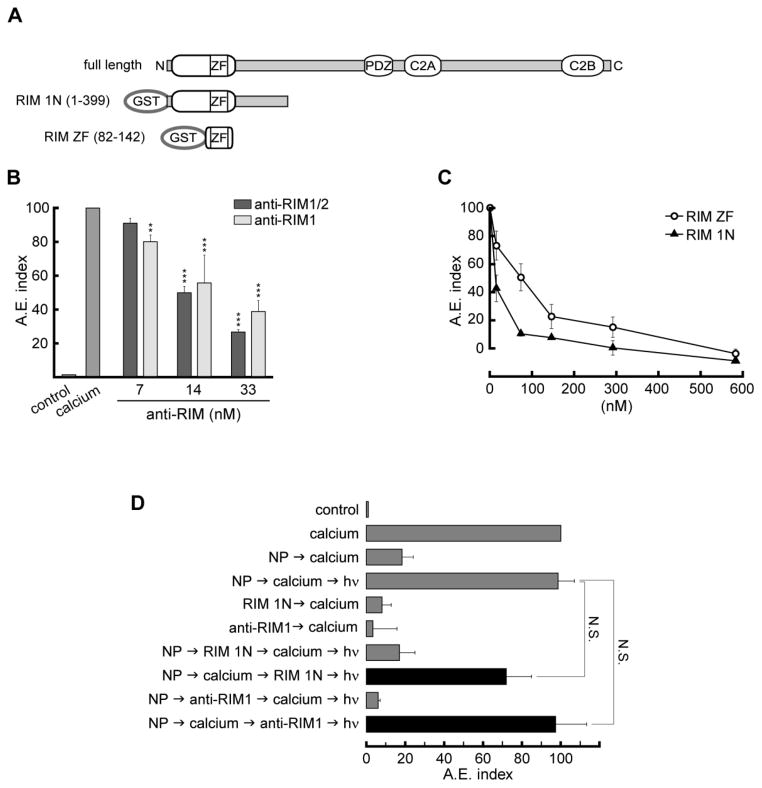
RIM was first identified as a Rab3A interacting molecule [35]. We speculated that RIM is one of the relevant Rab3A effectors in the exocytosis of acrosome. To test this hypothesis, we first assayed antibodies raised against two different domains of RIM: anti-RIM1/2 (recognizes the Zn-finger domain of RIM1/2) and anti-RIM1, (recognizes the PDZ domain of RIM1) in the acrosome reaction stimulated by calcium (Fig. 2B). The RIM1/2 antibody might cross-react with the highly homologous Zn-finger domain of the Rab-effector rabphilin, whereas the anti-PDZ antibody is specific for RIM proteins. Both anti-RIM1/2 and anti-RIM1 antibodies inhibited calcium-triggered exocytosis in a concentration-dependent manner, as shown in Fig. 2B. The inhibitory effect of the anti-RIM1/2 antibody was blocked when the antibody was preincubated with the recombinant RIM ZF domain (see Supplementary Fig. S2B). We also tested the effect of different RIM domains in the functional assay. The N-terminus domain of RIM1 (residues 1-399, RIM 1N), which binds Rab3A and Munc13, and the Zn-finger domain of RIM2 (residues 82-142, RIM ZF), which binds Munc13, added to the system as GST fusion proteins, also inhibited acrosomal exocytosis in a concentration-dependent manner (Fig. 2C), suggesting that the recombinant domains compete with the endogenous RIM protein for interactions that are essential for the acrosome reaction. These results indicate that RIM has an active role in acrosomal exocytosis. Next, we explored in which step of this process RIM was participating. By using a membrane-permeable light-sensitive calcium chelator that accumulates intraacrosomally (NP-EGTA-AM), we have been able to distinguish factors that act during the early or the late steps in the cascade [6]. When permeabilized sperm with NP-EGTA-AM loaded acrosomes are stimulated, exocytosis proceeds to a stage that requires the release of calcium from inside the acrosome. If an inhibitor affecting an early-acting factor is added at this point, when NP-EGTA-AM is photoinactivated, exocytosis will be completed, because the step sensitive to the inhibitor was accomplished before the addition of the inhibitor. On the other hand, if exocytosis is not completed after inactivating the chelator, it means that the inhibitor is affecting a factor that is required after the release of calcium from the acrosome. Using this criterion in combination with antibodies, proteins, or protein domains, we have been able to characterize early and late factors. When an anti-Rab3A antibody was tested in the NP-EGTA-AM assay, we observed that Rab3A is an early acting factor [6]. To test whether RIM is acting at the same stage as Rab3A, we assayed the RIM 1N domain and the anti-RIM1 antibody in the NP-EGTA-AM assay (Fig. 2D). Neither the blocking domain nor the sequestering antibody inhibited exocytosis when added after calcium in the presence of NP-EGTA-AM. Same results were obtained when the RIM ZF domain was assayed (Supplementary Fig. S3). These results indicate that endogenous RIM, similar to Rab3A, has an active role in acrosome reaction at a stage before the acrosomal calcium efflux.
FIGURE 2. RIM is necessary in an early step for acrosomal exocytosis.
A. Diagrams of full-length RIM (full length), RIM1α N-terminal domain (RIM 1N, residues 1-399) fused to GST, and RIM2α-ZF domain (RIM ZF, residues 82-142) fused to GST. B. Permeabilized spermatozoa were incubated for 15 min at 37°C in the presence of different concentrations either anti-RIM1/2 or anti-RIM1 antibody. Acrosomal exocytosis (AE) index was evaluated by lectin binding after an additional 15-min incubation at 37°C in the absence (control) or presence of 0.5 mM CaCl2 (calcium), see Materials and Methods for more details. Significant differences from the positive control (calcium) are indicated by ** p<0.05, and *** p<0.001 (one way ANOVA and confidence intervals of the means). C. Permeabilized spermatozoa were incubated for 15 min at 37°C in the presence of different concentrations of purified RIM ZF (open circles) or RIM 1N (closed triangle) domains fused to GST. AE was evaluated by lectin binding after an additional 15-min incubation at 37°C in the presence or absence of 0.5 mM CaCl2. D. NP-EGTA-AM assay: sperm loaded with NP-EGTA-AM (see Material and Methods) were treated for 15 min at 37 °C with either RIM 1N domain (146 nM; NP-> calcium -> RIM 1N domain -> hν, black bar) or anti-RIM1 antibody (33 nM; NP-> calcium -> RIM ZF -> hν, black bar) in the dark. UV flash photolysis of the chelator was induced at the end of the incubation period (hν), and the samples were incubated for 5 min to promote exocytosis (see Materials and Methods). RIM 1N domain and anti-RIM1 antibody did not block exocytosis when added after calcium stimulation in the presence of calcium chelator. Several controls (gray bars) were included: background AE in the absence of any stimulation (control): AE stimulated by 0.5 mM CaCl2 (calcium); inhibitory effect of NP in the dark (NP -> calcium) and recovery upon illumination (NP -> calcium -> hν); AE Inhibited by either 146 nM 1N domain (RIM 1N -> calcium) or 33 nM anti-RIM1 antibody (anti-RIM1 -> calcium); and the inhibitory effect of either RIM 1N domain (NP -> RIM 1N -> calcium -> hν) or anti-RIM1 antibody (NP -> anti-RIM1 -> calcium -> hν) when present throughout the experiment. The values were normalized as explained under Material and Methods. The data represent the mean ± SEM of at least three independent experiments. Not significant (N.S.) differences between experimental (black bars) and control conditions were tested by Dunnett’s test.
Munc13 is expressed in human sperm and localizes to the acrosomal region
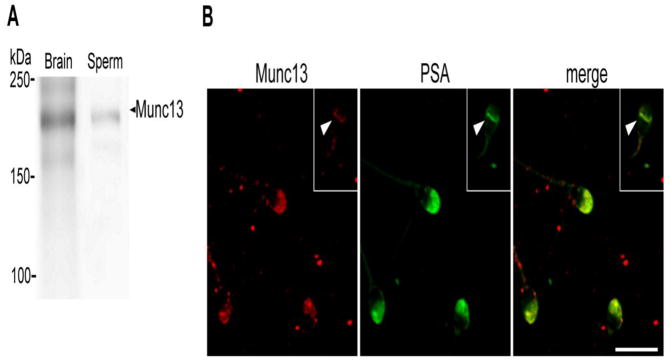
RIM1 and RIM2 can bind simultaneously active Rab3A and Munc13-1 [8]. This latter protein has a diacylglycerol (DAG) binding C1 domain and is a necessary factor for regulated exocytosis. Because DAG can trigger the acrosome reaction [34], we hypothesized that Munc13 may participate in acrosomal exocytosis; hence we investigated the presence of Munc13 by Western blot and its localization by indirect immunofluorescence. A monoclonal antibody raised against the C2A domain of Munc13-1 isoform recognized a predominant band of the expected molecular weight (around 200 kDa) in human sperm (Fig. 3A). Indirect immunofluorescence images using a polyclonal anti-Munc13-1 antibody showed labeling predominantly in the acrosomal region in 79% of acrosome intact cells (Fig. 3B). Similar results were obtained when a monoclonal mouse anti-Munc13 antibody was assayed (data not shown). Notice that the immunostaining was not present in spontaneously reacted sperm, which have lost the acrosome and the overlying plasma membrane (Fig. 3B, arrowhead in the inset). Both antibodies assayed recognized the Munc13-1 isoform. These results show that the Munc13-1 isoform is present in human sperm and localizes to the acrosomal region of the sperm head.
FIGURE 3. Munc13 is present in human sperm and localizes to the acrosomal region.
A. Proteins from whole sperm homogenates (Sperm, 100 × 106 cells) were resolved in 6% SDS-PAGE gels and immunoblotted with the anti-Munc13 antibody. Brain extracts (Brain) was used as a positive control. Molecular weight markers are indicated on the left and Munc13 band is indicated on the right (arrowhead). Shown is a representative result from three independent experiments. B. Human sperm were fixed, permeabilized and double-stained with the anti-Munc13-1 antibody (Munc13) and FITC-PSA lectin (PSA). Inset shows a reacted sperm. Note that Munc13 labeling is absent in the acrosome region (arrowhead). Shown are representative images from three different experiments. Bars = 10 μm
Munc13 is necessary at an early step for acrosomal exocytosis
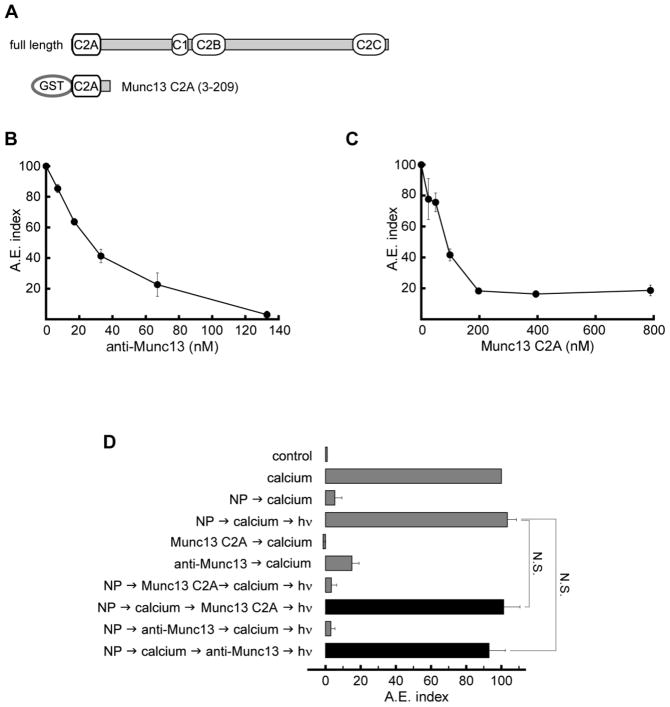
We next investigated if Munc13 has a role in acrosomal exocytosis by inhibiting Munc13 function with a specific antibody. Addition of anti-Munc13-1 antibody inhibited calcium-triggered exocytosis in a concentration-dependent manner (Fig. 4B). The inhibitory effect of the anti-Munc13-1 antibody was blocked when the antibody was preincubated with the recombinant Munc13 C2A domain (see Supplementary Fig. S2A). The Munc13 C2A domain, which binds to the Zn-finger domain of RIM, was also able to block exocytosis stimulated by calcium in a concentration-dependent manner (Fig. 4C). When the NP-EGTA-AM experiments were performed, we found that both the Munc13 C2A domain and the anti-Munc13-1 antibody inhibited before but not after the release of calcium from the acrosome (Fig. 4D). Altogether, our data indicate that Munc13, as well as RIM and Rab3A, has an active role in acrosome reaction at a stage before the acrosomal calcium efflux.
FIGURE 4. Munc13 is necessary in an early step for acrosomal exocytosis.
A. Diagram of full-length Munc13 and Munc13 C2A domain (residues 3-209) fusioned with GST. B. Permeabilized spermatozoa were incubated for 15 min at 37°C in the presence of different concentrations of anti-Munc13-1 antibody. AE was evaluated by lectin binding after an additional 15-min incubation at 37°C in the absence or presence of 0.5 mM CaCl2 as explained in Materials and Methods. C. Permeabilized spermatozoa were incubated for 15 min at 37°C in the presence of different concentrations of purified Munc13 C2A fused to GST. AE was evaluated by lectin binding after an additional 15-min incubation at 37°C in the presence or absence of 0.5 mM CaCl2. D. NP-EGTA-AM assay: sperm loaded with NP-EGTA-AM (see Material and Methods) were treated for 15 min at 37 °C with either Munc13 C2A domain (300 nM; NP -> calcium -> Munc13 C2A -> hν) or anti-Munc13-1 antibody (67 nM; NP -> calcium -> Munc13 C2A -> hν) in the dark. UV flash photolysis of the chelator was induced at the end of the incubation period (hν), and the samples were incubated for 5 min to promote exocytosis (see Materials and Methods for more details). Munc13 C2A domain and anti-Munc13-1 antibody did not block exocytosis when added after calcium stimulation in the presence of calcium chelator. Several controls (gray bars) were included: background AE in the absence of any stimulation (control); AE stimulated by 0.5mM CaCl2 (calcium); inhibitory effect of NP in the dark (NP -> calcium) and recovery upon illumination (NP -> calcium-> hν); AE inhibited by either 300 nM Munc13 C2A domain (Munc13 C2A -> calcium) or 67 nM anti-Munc13-1 antibody (anti-Munc13 -> calcium); and the inhibitory effect of either Munc13 C2A domain (NP -> Munc13 C2A -> calcium -> hν) or anti-Munc13-1 antibody (NP -> anti-Munc13 -> calcium -> hν) when present throughout the experiment. The values were normalized as explained under Material and Methods. The data represent the mean ± SEM of at least three independent experiments. Not significant (N.S.) differences between experimental (black bars) and control conditions were tested by Dunnett’s test.
RIM, Munc13, and Rab3A functionally interact during acrosomal exocytosis
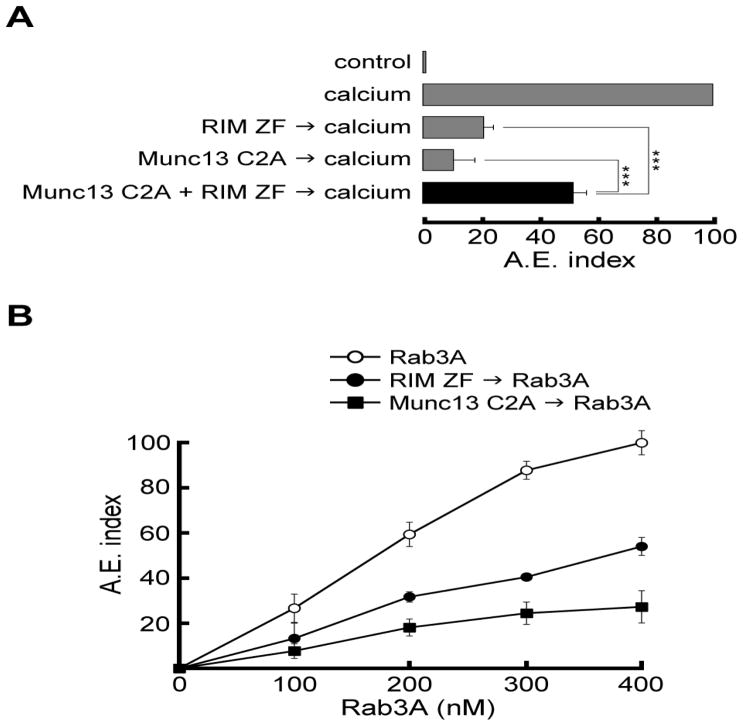
Because addition of Munc13 C2A domain to permeabilized sperm inhibited calcium-stimulated exocytosis in a concentration-dependent manner (Fig. 4C), we hypothesized that this domain bound endogenous RIM and prevented its interaction with endogenous Munc13. We predicted that the inhibitory effect of Munc13 C2A would be abolished by preincubation with recombinant RIM ZF domain. In effect, when both domains were preincubated at the same molar ratio concentration and then added to the assay, the inhibitory effect of both domains was not additive; on the contrary, secretion was partially restored (Fig. 5A).
FIGURE 5. RIM, Munc13, and Rab3A interplay in acrosomal exocytosis.
A. Permeabilized spermatozoa were incubated for 15 min at 37 °C with either Munc13 C2A domain (300 nM), RIM ZF domain (300 nM), or Munc13 C2A + RIM ZF domains (preincubated before adding to the assay). AE was evaluated by lectin binding after an additional 15-min incubation at 37°C in the absence (control) or presence of 0.5 mM CaCl2 (calcium). Significant differences are indicated by ***, p<0.001 (one way ANOVA and Dunnett’ s test). B. Permeabilized sperm were incubated for 15 min at 37 °C in absence or presence of RIM ZF domain (300 nM, closed circles), and Munc13 C2A domain (300 nM, closed squares). AE was initiated by adding increasing concentrations of Rab3A (open circles). Notice that both RIM ZF and Munc13 C2A domains inhibited exocytosis even at 400 nM Rab3A concentration. The values in A and B were normalized as explained under Material and Methods. The data represent the mean ± SEM of at least three independent experiments.
We have previously documented that Rab3A triggers acrosomal exocytosis [38]. We predict that Rab3A would promote secretion by interacting directly or indirectly with endogenous RIM and Munc13. Hence, the inhibitory recombinant domains of these proteins should block Rab3A-triggered exocytosis and an excess of the active GTPase should not be able to overcome this effect. As shown in Fig. 5B, when both domains were assayed in the presence of increasing concentrations of Rab3A, acrosomal exocytosis was not fully restored. Munc13 C2A and RIM ZF domains inhibited exocytosis even at 400 nM Rab3A concentration. Altogether, these results suggest that Munc13 and RIM act downstream of Rab3A and that the interplay between these three proteins is essential for human sperm acrosomal exocytosis.
RIM and Rab3A are required for membrane docking during acrosomal exocytosis
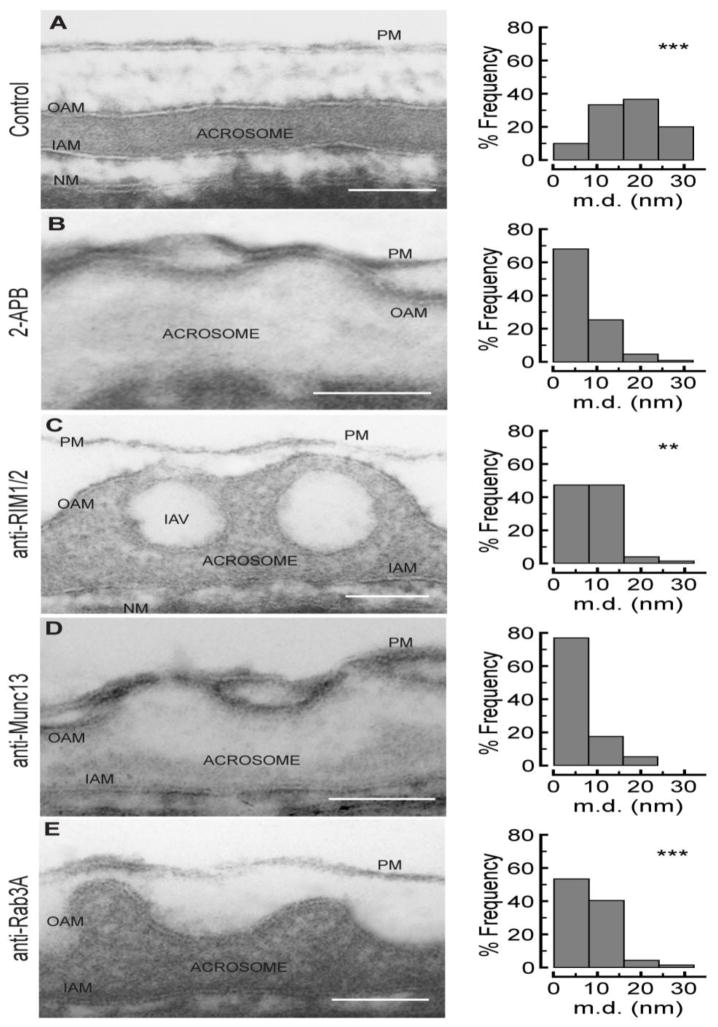
Using transmission electron microscopy, we have previously reported that when the acrosome exocytosis is arrested at the stage where an efflux of intraacrosomal calcium is needed, SNARE proteins are assembled in loose trans complexes, acrosomes are profusely swollen with deep invaginations of the outer acrosomal membrane, and the protruding edges of these invaginations are tightly apposed or docked to the plasma membrane [39]. RIM proteins are active zone scaffolding molecules that—among others—mediate vesicle docking [27]. Therefore, we wonder whether the docking process would be affected by depleting RIM, Munc13, or Rab3A by specific antibodies. As a positive control, exocytosis was inhibited by 2APB, an IP3-sensitive calcium channel inhibitor. As shown in Fig. 6, in not stimulated cells the acrosome showed no swelling, the outer acrosomal membrane was parallel to the plasma membrane and there were no appositions (Fig. 6A, control). This topology changed dramatically in cells stimulated in the presence of 2APB (Fig. 6B, 2APB, positive control). In this case, the acrosome was profusely swollen and most distances between both membranes, measured at the edge of outer acrosomal membrane invaginations, were in the 0–8 nm range. A similar distance distribution was observed when acrosomal exocytosis was stimulated in the presence of the anti-Munc13-1 antibody (Fig. 6D). In cells preincubated with the anti-RIM1/2 (Fig. 6C) or the anti-Rab3A (Fig. 6E) antibodies the swelling process was normal; however, docking was perturbed. The membrane distance distribution was significatively altered and displaced to the 8–16 nm range. Altogether, these results indicate that when exocytosis is stimulated in cells where the Rab3A or RIM function is perturbed, acrosome exocytosis cannot proceed to the docking stage. In contrast, when Munc13 is inhibited, fusion is stopped at a post docking stage.
FIGURE 6. RIM and RAB3A inhibition alters membrane docking in acrosomal exocytosis.
Permeabilized sperm were stimulated in absence (A, control) or presence of 0.5 mM CaCl2 and 200 μM 2-APB (B, 2APB), 133 nM anti-RIM1/2 (C, anti-RIM1/2), 133 nM anti-Munc13-1 (D, anti-Munc13) or 66 nM anti-Rab3A antibodies (E, anti-Rab3A). After 15 min of incubation in each condition, 1.6 mM tannic acid was added and the reaction proceeded by 5 additional minutes. The reaction was stopped with 2.5% glutaraldehyde. Samples were dehydrated and plastic embedded sections as described in Materials and Methods. Electronmicrograph in A illustrates a non stimulated cell where the outer acrosomal membrane (OAM) and plasma membrane (PM) are parallel. B illustrates a stimulated cell where the acrosome is swollen and the OAM and PM are in close apposition. C is a representative image showing that anti-RIM antibody disrupts membrane apposition in stimulated sperms. D is a representative image showing that anti-Munc13-1 antibody did not affect membrane apposition in stimulated sperm. E is a representative image showing that anti-Rab3A antibody disrupts membrane apposition in stimulated sperms (IAV: intraacrosomal vesicle; IAM: inner acrosomal membrane; NM: nuclear membrane). A quantification of frequency of membrane distances for each condition is shown on the right of each panel. The distance between the outer acrosomal membrane and the plasma membrane was measured at the edge of acrosomal invaginations as described in [39]. Distances were measured only in images where the membrane unit was clearly distinguished. Number (N) of apposition counted: not stimulated cells, N=33; stimulated cells, N= 211; anti-RIM1/2, N= 79; anti-Munc13, N= 57; anti-Rab3A, N= 69. The distance distributions are plotted as histograms, which were compared with the 2-APB condition by using the Kolmogorov-Smirnof test. Significant differences are indicated for each histogram (***, p< 0.001; **, p< 0.01). Bars = 100 nm.
DISCUSSION
The acrosome reaction is a regulated exocytotic process leading to a massive fusion between the outer acrosomal membrane and the cell membrane. We have previously documented that Rab3A is present in human sperm cells and that it acts as a positive regulator of acrosome content secretion. In fact, GTP-loaded recombinant Rab3A can trigger exocytosis by itself in human sperm [38]. However, the exact mechanism by which Rab3 participates in exocytosis is still elusive. In most fusion-dependent transport steps, Rabs promote fusion by inducing the formation of multiprotein complexes on the membranes that are going to fuse [10]. When in the active form, Rab3 interact directly with several molecules such as rabphilin [28] and RIM [9;35], and indirectly with proteins that are crucial for regulated exocytosis, such as Munc13 [8;13]. Since RIM and Munc13 have not been described in human spermatozoa, we first assessed their presence by Western blot. Predominant bands of the expected molecular weight of RIM and Munc13 (around 180 and 200 kDa, respectively) were detected using commercial antibodies (Fig. 1A and 3A). Immunostaining of fixed and permeabilized cells showed that, like Rab3A, RIM and Munc13 localize to the acrosomal region of the sperm head (Fig. 1B and 3B). We hypothesized that perturbing the function of either RIM or Munc13 would inhibit acrosome reaction activated by calcium. In fact, antibodies against either RIM (anti-RIM1/2 and anti-RIM1) or Munc13 (anti-Munc13-1) inhibited the acrosome reaction (Fig. 2B and 4B). The RIM1/2 antibody may cross-react with the highly homologous Zn-finger domain of the rabphilin, a known Rab effector. However, the effect of the RIM1 antibody, which was raised against a PDZ domain that is not present in rabphilin, confirms that RIM is involved in acrosomal exocytosis. Similar results were obtained when GST-fused protein domains corresponding to active regions of these proteins were introduced into SLO-permeabilized sperm (Fig. 2C and 4C). Altogether, these results suggest that RIM and Munc13 participate in the signal transduction pathway of the acrosome reaction. By using a membrane-permeable, light-sensitive calcium chelator that accumulates intraacrosomally (NP-EGTA-AM), we have determined that Rab3A is an early acting factor [6]. RIM 1N domain and anti-RIM1 antibody did not block exocytosis when added after calcium stimulation in the presence of NP-EGTA-AM (Fig. 2D), consistent with the idea that RIM is acting in an early stage of acrosome reaction. Similar results were obtained when the Munc13 C2A domain and the anti-Munc13 antibody (Fig. 4C and 4D, respectively) were assayed. These results indicate that, like Rab3A, RIM and Munc13 have active roles in acrosome reaction at a stage before the acrosomal calcium efflux, which is consistent with the idea of the formation of a tripartite Rab3/RIM/Munc13 complex in an early stage of membrane fusion.
In this report, we also showed that RIM, Munc13, and Rab3A functionally interact during acrosomal exocytosis (Fig. 5). First, we demonstrated that Munc13 interacts with the endogenous RIM during calcium-activated exocytosis. The inhibitory effect of Munc13 C2A was abolished by preincubating of this domain with the RIM ZF domain (Fig. 5A), indicating a Munc13/RIM interaction. A recent work showed that RIM protein activates vesicle priming through the interaction with Munc13 [7]. Second, we demonstrated that RIM and Munc13 interact with Rab3A during acrosomal exocytosis. We previously documented that Rab3A triggers acrosomal exocytosis [38]. In this work, we tested the hypothesis that Rab3A-triggered acrosomal exocytosis would be inhibited by both RIM ZF and Munc13 C2A domains. When both domains were assayed in the presence of increasing concentrations of Rab3A, acrosomal exocytosis was not restored. RIM ZF and Munc13 C2A domains inhibited exocytosis even at 400 nM Rab3A concentration (Fig. 5B). Again, these results are consistent with the idea of the formation of a Rab3/RIM/Munc13 complex during acrosomal exocytosis in human sperm.
Tethering and docking are two terms used to refer to secretory vesicles in close proximity to the plasma membrane. However, in general, they denote two mechanistically different processes. Tethering defines the initial recognition and binding of membranes that are going to fuse. It is a SNARE-independent process, triggered by Rabs and accomplished by the recruitment of tethering factors. Docking in contrast, involves the formation of the trans-SNARE complexes that will directly participate in the membrane fusion process. Since SNAREs are relatively small membrane proteins, they cannot interact at distances larger than 8 nm. It is then reasonable to think that tethering plays an important role to facilitate docking by holding the membranes together and promoting trans-SNARE interactions. In a previous study, we have shown that botulinal neurotoxin C and tetanus toxin, two SNARE-specific proteases, inhibited acrosomal docking measured at the edge of outer acrosomal membrane invaginations [39]. In this report we show that when the function of Rab3A or RIM -two proteins that participate in tethering in other secretory process- was blocked by specific antibodies, a significantly decreased in the frequency of tight apposition between membranes was observed (Fig. 6C and 6E). The effect was not as dramatic as with the neurotoxins, suggesting that docking may still occur, but with much less efficiency. In our previous report, we did not observed an effect of GDI, a protein that binds GDP-bound Rab3A. We speculate that active Rab3A on the membranes may not be affected by GDI explaining the resistance of docking/tethering to this protein. In contrast, the anti-Rab3A antibody would inactivate both GDP- and GTP- bound Rab3A.
It is interesting that anti-Munc13 antibody did not affect docking (Fig. 6D). This observation suggests that the interaction between Rab3A and RIM is sufficient for membrane apposition. In the presence of the anti-Munc13-1 antibody, docking was normal, but exocytosis was blocked (Fig. 4B), suggesting a post-docking function for Munc13. Recent findings of Dr. Rizo’s group support this hypothesis. Using NMR and fluorescence experiments they showed that the Munc13-1 MUN domain, which plays a central role in vesicle priming, dramatically accelerates the transition from the syntaxin-1–Munc18-1 complex to the SNARE complex [20;22]. This activity depends on weak interactions of the Munc13-1 MUN domain with the syntaxin-1 SNARE motif. These results illustrate how weak protein-protein interactions can play a crucial role in membrane fusion by promoting transitions between high-affinity macromolecular assemblies.
In pig sperm, ultrastructural and biochemical data indicate that capacitation induces a close apposition of the plasma membrane with the outer acrosomal membranes and the formation of SNARE complexes[33]. In guinea pig, during capacitation, some acrosomal proteins are exposed at the plasma membrane, suggesting that some transient fusion pores may open connecting the acrosome with the plasma membrane before stimulation of the acrosome reaction [18]. We propose that in capacitated human sperm, SNAREs are in cis complexes that must disassemble to interact in trans, a process that requires Rab3A and RIM. The observations in the three animal models seem to be contradictory, and may reflect specie-specific differences. However, they may be still compatible. All three studies use experimental strategies addressing different aspects of the secretory process. The capacitation-related changes observed and the mechanism triggered by calcium may be part of the acrosome reaction. Membrane docking at the tip of the acrosome in pig may not be sufficient for the acrosome reaction, and the process may still require the assembly of SNARE complexes during the acrosomal exocytosis. The opening of transient pores in guinea pig sperm during capacitation does not preclude the requirement of the tethering/docking machinery upon stimulation of acrosome secretion.
Initial studies showed that RIMs acts as a Rab3 effector [5;35]. It has been reported that RIM2α regulates insulin secretion through interaction with Rab3A, Munc13-1, EpacII and calcium channels in pancreatic beta cells [37], and that Rab3A is essential for the functioning of Munc13-1 during vesicle docking in PC12 cells [13]. Previous results from our laboratory and in this report suggest that all these proteins participate in acrosomal exocytosis. The recruitment of presynaptic high voltage calcium channels –N- and P/Q calcium channels- to the active zone during neuronal secretion is mediated by RIM [12;17]. Although N- and P/Q-calcium channels have not been identified in human sperm, our previous results indicate that IP3-sensitive calcium channels are necessary for synaptotagmin activation [6; 26]. The possibility that the tethering machinery concentrates calcium channels to the membrane docking areas is very attractive.
Based on previous results in neurons [30], and our results, we propose a working model for Rab3A, RIM, and Munc13 function in acrosomal exocytosis (Fig. 7). Nevertheless, further work is needed to unveil the molecular mechanisms by which RIM, Munc13, and Rab3A regulate membrane docking and fusion in human sperm.
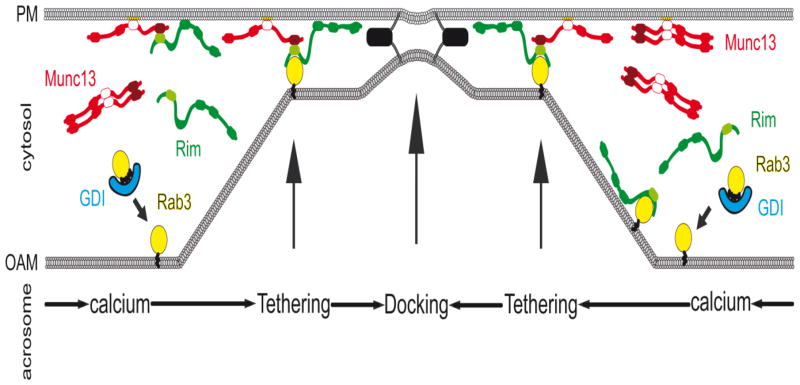
FIGURE 7. Working model for RIM, Munc13, and Rab3 function in membrane docking.
We propose two possible alternatives for the formation of a ternary RIM/Munc13/Rab3 complex during membrane docking in acrosomal exocytosis stimulated by calcium. On the left half of the figure, RIM converts Munc13 homodimer into RIM/Munc13 heterodimer on the plasma membrane (PM); this dimer is able to bind active Rab3A on the outer acrosomal membrane (OAM). On the right half of the figure, RIM binds active Rab3A, and then, the Zn-finger domain of RIM activates Munc13 by disrupting the homodimer. Both models converge at the formation of a RIM/Munc13/Rab3 complex that drives membrane docking. After this step, SNARE proteins (black boxes) are assembled in loose trans complexes.
Supplementary Material
A. Human sperm were fixed, permeabilized and double-stained with the anti-RIM1 antibody (antibody raised against the PDZ domain) and FITC-PSA lectin (PSA). Note that RIM labeling is absent in reacted sperm (arrowhead). Shown are representative images from three different experiments. B. Indirect immunofluorescence (IIF) in absence of primary antibody incubation. Representative images of the incubation of secondary antibody alone (Alexa Fluor 546) used in all IIFs show that sperm head are not stained. Bars = 10 μm.
A. Permeabilized spermatozoa were treated at 37 °C for 10 min with 67 nM anti-Munc13 antibody preincubated with 134 nM Munc13 C2A (Anti-Munc13 + Munc13 C2A -> calcium, black bar). Acrosomal exocytosis was initiated with 0.5 mM CaCl2 and the incubation continued for an additional 15 min. Controls include (gray bars): background AE in the absence of any stimulation (control); AE stimulated by 0.5 mM CaCl2 (calcium); treatment with 67 nm of anti-Munc13 antibody (anti-Munc13 -> calcium). B. Permeabilized spermatozoa were treated at 37 °C for 10 min with 33 nM anti-RIM1/2 antibody premixed with 67nM of recombinant RIM ZF domain. (Anti-RIM1/2 + RIM ZF-> calcium, black bar). Acrosomal exocytosis was initiated with 0.5 mM CaCl2 and the incubation continued for an additional 15 min. Controls include (gray bars): background AE in the absence of any stimulation (control); AE stimulated by 0.5 mM CaCl2 (calcium); treatment with 33 nm of anti-RIM1/2 antibody (anti-RIM1/2 -> calcium). Note that preincubated antibody did not inhibit acrosomal exocitosis. Differences between experimental (black bars) and control condition were tested by Dunnett’s test. Significant differences are indicated by ***, p< 0.001.
Sperm were treated for 15 min at 37 °C with RIM ZF domain (670 nM; NP-> calcium -> RIM ZF domain -> hν, black bar) in the dark. UV flash photolysis of the chelator was induced at the end of the incubation period (hν), and the samples were incubated for 5 min to promote exocytosis (see Materials and Methods). RIM ZF domain did not block exocytosis when added after calcium stimulation in the presence of calcium chelator.
Several controls (gray bars) were included: background AE in the absence of any stimulation (control); AE stimulated by 0.5 mM CaCl2 (calcium); inhibitory effect of NP in the dark (NP -> calcium) and recovery upon illumination (NP -> calcium -> hν); AE inhibited by 670 nM RIM ZF domain (RIM ZF -> calcium); and the inhibitory effect of RIM ZF domain (NP -> RIM ZF -> calcium -> hν) when present throughout the experiment. The values were normalized as explained under Material and Methods. The data represent the mean ± SEM of at least three independent experiments. Not significant (N.S.) differences between experimental (black bars) and control conditions were tested by Dunnett’s test.
Purified GST-proteins of RIM 1N (residues 1-399), RIM ZF (residues 82-142), Munc13 C2A (residues 3-209), and Rab3A (full lenght) were resolved in 10% SDS-PAGE gels and stained with Coomassie Brilliant Blue R-250. Each line was loaded with 1μl of each recombinant protein. Molecular mass markers are indicated to the left of each panel.
HIGHTLIGTHS.
RIM and Munc13 are present in human sperm and localize to the acrosomal region.
RIM and Munc13 are necessary for acrosomal exocytosis.
RIM and Munc13 participate before the acrosomal calcium efflux.
RIM, Munc13 and Rab3A interplay in human sperm acrosomal exocytosis.
RIM and Rab3A has a critical role in membrane docking.
Acknowledgments
The authors thank M. Furlán and A. Medero for technical assistance; Dr. Claudia N. Tomes for critical reading of the manuscript, and Dr. J. Rizo, Dr. R. Regazzi, and Dr. P. Stahl, for plasmids. O.D.B. and M.N.Z. are thankful to CONICET, Argentina for fellowships. This work was supported by NIH Research Grant # R01 TW007571, funded by the Fogarty International Center, and Universidad Nacional de Cuyo grant to M.A.M., and grants from ANPCyT and CONICET, Argentina to L.S.M.
Abbreviations
- AE
acrosomal exocytosis
- 2APB
2-aminoethoxydiphenylborate
- BFS
bovine fetal serum
- NP-EGTA-AM
O-nitrophenyl EGTA–acetoxymethyl ester
- FITC
fluorescein isothiocyanate
- PAGE
polyacrylamide gel electrophoresis
- PBS
phosphate buffered saline
- PSA
Pisum sativum agglutinin
- PVDF
polyvinylidene difluoride
- PVP
polyvinylpyrrolidone
- RIM
Rab-interacting protein
- RT
room temperature
- SEM
standard error of the mean
- SLO
streptolysin-O
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Andrews-Zwilling YS, Kawabe H, Reim K, Varoqueaux F, Brose N. Binding to Rab3A-interacting molecule RIM regulates the presynaptic recruitment of Munc13-1 and ubMunc13-2. J Biol Chem. 2006;281:19720–19731. doi: 10.1074/jbc.M601421200. [DOI] [PubMed] [Google Scholar]
- 2.Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K. Drosophila UNC-13 is essential for synaptic transmission. Nat Neurosci. 1999;2:965–971. doi: 10.1038/14764. [DOI] [PubMed] [Google Scholar]
- 3.Betz A, Okamoto M, Benseler F, Brose N. Direct interaction of the rat unc-13 homologue Munc13-1 with the N terminus of syntaxin. J Biol Chem. 1997;272:2520–2526. doi: 10.1074/jbc.272.4.2520. [DOI] [PubMed] [Google Scholar]
- 4.Brose N, Rosenmund C, Rettig J. Regulation of transmitter release by Unc-13 and its homologues. Curr Opin Neurobiol. 2000;10:303–311. doi: 10.1016/s0959-4388(00)00105-7. [DOI] [PubMed] [Google Scholar]
- 5.Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- 6.De Blas GA, Roggero CM, Tomes CN, Mayorga LS. Dynamics of SNARE assembly and disassembly during sperm acrosomal exocytosis. PLoS Biol. 2005;3:e323. doi: 10.1371/journal.pbio.0030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng L, Kaeser PS, Xu W, Sudhof TC. RIM proteins activate vesicle priming by reversing autoinhibitory homodimerization of Munc13. Neuron. 2011;69:317–331. doi: 10.1016/j.neuron.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dulubova I, Lou X, Lu J, Huryeva I, Alam A, Schneggenburger R, Sudhof TC, Rizo J. A Munc13/RIM/Rab3 tripartite complex: from priming to plasticity? EMBO J. 2005;24:2839–2850. doi: 10.1038/sj.emboj.7600753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuda M. Assay and functional interactions of Rim2 with Rab3. Methods Enzymol. 2005;403:457–468. doi: 10.1016/S0076-6879(05)03040-5. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65:2801–2813. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han Y, Kaeser PS, Sudhof TC, Schneggenburger R. RIM determines Ca(2)+ channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69:304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang CC, Yang DM, Lin CC, Kao LS. Involvement of Rab3A in vesicle priming during exocytosis: interaction with Munc13-1 and Munc18-1. Traffic. 2011;12:1356–1370. doi: 10.1111/j.1600-0854.2011.01237.x. [DOI] [PubMed] [Google Scholar]
- 14.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iida H, Yoshinaga Y, Tanaka S, Toshimori K, Mori T. Identification of Rab3A GTPase as an acrosome-associated small GTP-binding protein in rat sperm. Dev Biol. 1999;211:144–155. doi: 10.1006/dbio.1999.9302. [DOI] [PubMed] [Google Scholar]
- 16.Izumi T, Gomi H, Kasai K, Mizutani S, Torii S. The roles of Rab27 and its effectors in the regulated secretory pathways. Cell Struct Funct. 2003;28:465–474. doi: 10.1247/csf.28.465. [DOI] [PubMed] [Google Scholar]
- 17.Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Sudhof TC. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KS, Foster JA, Kvasnicka KW, Gerton GL. Transitional states of acrosomal exocytosis and proteolytic processing of the acrosomal matrix in guinea pig sperm. Mol Reprod Dev. 2011;78:930–941. doi: 10.1002/mrd.21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Ma C, Guan R, Xu Y, Tomchick DR, Rizo J. The crystal structure of a Munc13 C-terminal module exhibits a remarkable similarity to vesicle tethering factors. Structure. 2011;19:1443–1455. doi: 10.1016/j.str.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez CI, Belmonte SA, De Blas GA, Mayorga LS. Membrane-permeant Rab3A triggers acrosomal exocytosis in living human sperm. FASEB J. 2007;21:4121–4130. doi: 10.1096/fj.06-7716com. [DOI] [PubMed] [Google Scholar]
- 22.Ma C, Li W, Xu Y, Rizo J. Munc13 mediates the transition from the closed syntaxin-Munc18 complex to the SNARE complex. Nat Struct Mol Biol. 2011;18:542–549. doi: 10.1038/nsmb.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 24.Mendoza C, Carreras A, Moos J, Tesarik J. Distinction between true acrosome reaction and degenerative acrosome loss by a one-step staining method using Pisum sativum agglutinin. J Reprod Fertil. 1992;95:755–763. doi: 10.1530/jrf.0.0950755. [DOI] [PubMed] [Google Scholar]
- 25.Mittelstaedt T, Alvarez-Baron E, Schoch S. RIM proteins and their role in synapse function. Biol Chem. 2010;391:599–606. doi: 10.1515/BC.2010.064. [DOI] [PubMed] [Google Scholar]
- 26.Roggero CM, De Blas GA, Dai H, Tomes CN, Rizo J, Mayorga LS. Complexin/synaptotagmin interplay controls acrosomal exocytosis. J Biol Chem. 2007;282:26335–26343. doi: 10.1074/jbc.M700854200. [DOI] [PubMed] [Google Scholar]
- 27.Schoch S, Mittelstaedt T, Kaeser PS, Padgett D, Feldmann N, Chevaleyre V, Castillo PE, Hammer RE, Han W, Schmitz F, Lin W, Sudhof TC. Redundant functions of RIM1alpha and RIM2alpha in Ca(2+)-triggered neurotransmitter release. EMBO J. 2006;25:5852–5863. doi: 10.1038/sj.emboj.7601425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shirataki H, Kaibuchi K, Sakoda T, Kishida S, Yamaguchi T, Wada K, Miyazaki M, Takai Y. Rabphilin-3A, a putative target protein for smg p25A/rab3A p25 small GTP-binding protein related to synaptotagmin. Mol Cell Biol. 1993;13:2061–2068. doi: 10.1128/mcb.13.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 30.Sudhof TC, Rizo J. Synaptic vesicle exocytosis. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takai Y, Sasaki T, Shirataki H, Nakanishi H. Rab3A small GTP-binding protein in Ca(2+)-dependent exocytosis. Genes Cells. 1996;1:615–632. doi: 10.1046/j.1365-2443.1996.00257.x. [DOI] [PubMed] [Google Scholar]
- 32.Tomes CN, Carballada R, Moses DF, Katz DF, Saling PM. Treatment of human spermatozoa with seminal plasma inhibits protein tyrosine phosphorylation. Mol Hum Reprod. 1998;4:17–25. doi: 10.1093/molehr/4.1.17. [DOI] [PubMed] [Google Scholar]
- 33.Tsai PS, Garcia-Gil N, van HT, Gadella BM. How pig sperm prepares to fertilize: stable acrosome docking to the plasma membrane. PLoS One. 2010;5:e11204. doi: 10.1371/journal.pone.0011204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vazquez JM, Roldan ER. Diacylglycerol species as messengers and substrates for phosphatidylcholine re-synthesis during Ca2+-dependent exocytosis in boar spermatozoa. Mol Reprod Dev. 1997;48:95–105. doi: 10.1002/(SICI)1098-2795(199709)48:1<95::AID-MRD12>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Okamoto M, Schmitz F, Hofmann K, Sudhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- 36.Ward CR, Faundes D, Foster JA. The monomeric GTP binding protein, rab3a, is associated with the acrosome in mouse sperm. Mol Reprod Dev. 1999;53:413–421. doi: 10.1002/(SICI)1098-2795(199908)53:4<413::AID-MRD7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 37.Yasuda T, Shibasaki T, Minami K, Takahashi H, Mizoguchi A, Uriu Y, Numata T, Mori Y, Miyazaki J, Miki T, Seino S. Rim2alpha determines docking and priming states in insulin granule exocytosis. Cell Metab. 2010;12:117–129. doi: 10.1016/j.cmet.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 38.Yunes R, Michaut M, Tomes C, Mayorga LS. Rab3A triggers the acrosome reaction in permeabilized human spermatozoa. Biol Reprod. 2000;62:1084–1089. doi: 10.1095/biolreprod62.4.1084. [DOI] [PubMed] [Google Scholar]
- 39.Zanetti N, Mayorga LS. Acrosomal swelling and membrane docking are required for hybrid vesicle formation during the human sperm acrosome reaction. Biol Reprod. 2009;81:396–405. doi: 10.1095/biolreprod.109.076166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Human sperm were fixed, permeabilized and double-stained with the anti-RIM1 antibody (antibody raised against the PDZ domain) and FITC-PSA lectin (PSA). Note that RIM labeling is absent in reacted sperm (arrowhead). Shown are representative images from three different experiments. B. Indirect immunofluorescence (IIF) in absence of primary antibody incubation. Representative images of the incubation of secondary antibody alone (Alexa Fluor 546) used in all IIFs show that sperm head are not stained. Bars = 10 μm.
A. Permeabilized spermatozoa were treated at 37 °C for 10 min with 67 nM anti-Munc13 antibody preincubated with 134 nM Munc13 C2A (Anti-Munc13 + Munc13 C2A -> calcium, black bar). Acrosomal exocytosis was initiated with 0.5 mM CaCl2 and the incubation continued for an additional 15 min. Controls include (gray bars): background AE in the absence of any stimulation (control); AE stimulated by 0.5 mM CaCl2 (calcium); treatment with 67 nm of anti-Munc13 antibody (anti-Munc13 -> calcium). B. Permeabilized spermatozoa were treated at 37 °C for 10 min with 33 nM anti-RIM1/2 antibody premixed with 67nM of recombinant RIM ZF domain. (Anti-RIM1/2 + RIM ZF-> calcium, black bar). Acrosomal exocytosis was initiated with 0.5 mM CaCl2 and the incubation continued for an additional 15 min. Controls include (gray bars): background AE in the absence of any stimulation (control); AE stimulated by 0.5 mM CaCl2 (calcium); treatment with 33 nm of anti-RIM1/2 antibody (anti-RIM1/2 -> calcium). Note that preincubated antibody did not inhibit acrosomal exocitosis. Differences between experimental (black bars) and control condition were tested by Dunnett’s test. Significant differences are indicated by ***, p< 0.001.
Sperm were treated for 15 min at 37 °C with RIM ZF domain (670 nM; NP-> calcium -> RIM ZF domain -> hν, black bar) in the dark. UV flash photolysis of the chelator was induced at the end of the incubation period (hν), and the samples were incubated for 5 min to promote exocytosis (see Materials and Methods). RIM ZF domain did not block exocytosis when added after calcium stimulation in the presence of calcium chelator.
Several controls (gray bars) were included: background AE in the absence of any stimulation (control); AE stimulated by 0.5 mM CaCl2 (calcium); inhibitory effect of NP in the dark (NP -> calcium) and recovery upon illumination (NP -> calcium -> hν); AE inhibited by 670 nM RIM ZF domain (RIM ZF -> calcium); and the inhibitory effect of RIM ZF domain (NP -> RIM ZF -> calcium -> hν) when present throughout the experiment. The values were normalized as explained under Material and Methods. The data represent the mean ± SEM of at least three independent experiments. Not significant (N.S.) differences between experimental (black bars) and control conditions were tested by Dunnett’s test.
Purified GST-proteins of RIM 1N (residues 1-399), RIM ZF (residues 82-142), Munc13 C2A (residues 3-209), and Rab3A (full lenght) were resolved in 10% SDS-PAGE gels and stained with Coomassie Brilliant Blue R-250. Each line was loaded with 1μl of each recombinant protein. Molecular mass markers are indicated to the left of each panel.