Abstract
Background and Purpose
Human albumin has been shown to exert neuroprotective effects in animal models of cerebral ischemia and humans with various intracranial pathologies. We investigated the safety and tolerability of 25% human albumin (ALB) in patients with subarachnoid hemorrhage (SAH).
Methods
The ALISAH (Albumin in Subarachnoid Hemorrhage) Pilot Clinical Trial was an open-label, dose-escalation study. We intended to study 4 different dosages of ALB of increasing magnitude (0.625 g/kg: tier 1; 1.25 g/kg: tier 2; 1.875 g/kg: tier 3; and 2.5 g/kg: tier 4). Each dosage was to be given to 20 adult patients. Treatment was administered daily for 7 days. We investigated the maximum tolerated dose of ALB based on the rate of severe-to-life-threatening heart failure and anaphylactic reaction, and functional outcome at 3 months.
Results
We treated 47 adult subjects: 20 in tier 1; 20 in tier 2; and 7 in tier 3. We found that doses ranging up to 1.25 g/kg/day × 7 days were tolerated by patients without major dose-limiting complications. We also found that outcomes trended towards better responses in those subjects enrolled in tier 2 compared to tier 1 (OR: 3.0513; CI: 0.6586 – 14.1367) and to the International Intra-operative Hypothermia for Aneurysm Surgery Trial cohort (OR: 3.1462; CI: 0.9158 – 10.8089).
Conclusions
ALB in doses ranging up to 1.25 g/Kg/day × 7 days was tolerated by patients with SAH without major complications and may be neuroprotective. Based on these results, planning of the ALISAH II, a Phase III, randomized, placebo-controlled trial to test the efficacy of ALB is underway.
Clinical Trial Registration Information: NCT00283400 (clinicaltrials.gov) http://clinicaltrials.gov/ct2/show/NCT00283400?term=subarachnoid+hemorrhage+houston&rank=1
Keywords: subarachnoid hemorrhage, albumin, neuroprotection, outcome, delayed ischemic deficit
Subarachnoid hemorrhage (SAH) is a neurological emergency that carries high morbidity and mortality. Half of the approximately 30,000 Americans that suffer SAH every year will die and a third of survivors will be left dependent1. Significant advances in the diagnosis, management, and prevention of complications in patients with SAH have occurred in the past few decades. However, improvement of clinical outcome has been modest. Newer treatments with proven neuroprotective benefits on clinical outcomes are needed. Nimodipine is the only available treatment with proven marginal efficacy2.
Administration of high doses of 25% human albumin (ALB) has been associated with improved outcomes and reduced costs in SAH patients3. Furthermore, several pre-clinical and clinical studies demonstrated the role of ALB as a neuroprotective treatment in cerebral ischemia4–9. These findings formed the rationale for the design of a phase I pilot study investigating the safety and tolerability of ALB in patients with SAH10.
Materials and Methods
The National Institutes of Health (NIH) funded the ALISAH (Albumin in Subarachnoid Hemorrhage) pilot study, initiated in May 2006 and terminated in May 2010. The study was originally planned for 3 years but mostly due to the PI’s transferring institutions and initiation of 2 non-US sites, one extra year was needed. ALISAH investigated the intravenous administration of ALB in subjects with SAH. The primary objective of this prospective dose-finding pilot study was to demonstrate the tolerability and safety of four dosages of ALB in patients with SAH. For each dosage group, 20 patients who met the eligibility criteria were to be enrolled. Maximum tolerated dosage of ALB determination was based on the rate of treatment-related severe or life-threatening heart failure10. Secondary objectives were to obtain preliminary estimates of the albumin treatment effect using incidence of neurological deterioration within 15 days of symptom onset, as well as, incidence of rebleeding, hydrocephalus, seizures, delayed cerebral ischemia (DCI) and vasospasm (both symptomatic and by transcranial Doppler ultrasound criteria) within 15 days after symptom onset. In addition, the Glasgow Outcome Scale (GOS), Barthel Index (BI), modified Rankin Scale (mRS), NIH Stroke Scale (NIHSS), and Stroke Impact Scale (SIS) at 3 months following onset of symptoms were collected to assess residual neurological deficits.
ALISAH was conducted at 6 North American sites (Online Table S1). The Central Coordinatingcenter(CCC) was at the Baylor College of Medicine. The Statistical and Data Managementcenter(SDMC) was at the Medical University of South Carolina in Charleston, SC. Each site obtained IRB/Ethics Committees approval, and the study was registered at www.clinicaltrials.gov prior to patient recruitment.
All patients presenting with SAH to the participating clinical sites were screened for study eligibility10 (Online Table S2). A cardiologist or cardiology fellow performed cardiologic evaluations and a qualified neurological surgeon or intensive care unit (ICU) physicians performed a neurological assessment upon study entry. Once informed written consent was obtained patients were enrolled into the study.
Study Intervention
Subjects were allocated in a dose escalation design into one of four dosage groups of 25% ALB: 0.625 g/kg/d × 7 days; 1.25 g/kg/d × 7 days; 1.875 g/kg/d × 7 days; and 2.5 g/kg/d × 7 days. The tolerability and safety of the previous dosage tier of ALB was evaluated by the NIH-appointed Data and Safety Monitoring Board (DSMB) prior to advancing to the next dosage. The estimated volume of infusion for a 70-kg subject ranged from 175 to 700 ml, and the delivery time was 3 h for all dosage groups. Dosage ranges of ALB were chosen based on the range administered to patients for induction of hypervolemia and hyperdynamia after SAH including our preliminary data3,10, and dosage ranges studied in animal models of focal cerebral ischemia7. The duration of treatment (7 days) was chosen on the premise that it would cover the highest risk period for DCI (peak occurrence at days 8–10). As the half-life of human albumin is around 21 days with a degradation rate of 3.7% per day and a transcapillary escape rate of 4– 5% per hour10,11, we expected that the volume repletion and other neuroprotective effects of 25% ALB would persist for 24 h or more beyond the 7 days. In addition, animal models of focal cerebral ischemia have shown significantly higher plasma oncotic pressures and serum ALB concentrations up to 3 days after treatment5.
Patient Management during Acute Period
All subjects had vital signs monitored hourly and were assessed by ICU nursing staff and/or site investigators for any new episode of neurological or cardiovascular deterioration. In addition, subjects had daily laboratory evaluations, assessment of neurologic function (GCS, NIHSS), transcranial Doppler ultrasound, complete cardiac examinations, evaluation of intravascular volume status, and monitoring of vital signs (including weight) for 15 days, or until hospital discharge if prior to that.
All subjects were treated with maintenance fluids of 0.9% normal saline (NS) at 80–125 ml/h (total 2–3 liters per day) with the goal central venous pressure (CVP) between 5–8 mmHg. In the event that subjects required further fluid administration beyond their maintenance to maintain the desired CVP, they received 250–500 ml intermittent intravenous boluses of NS as needed. Extra ALB administration was not allowed, and the initial fluid balance goal was set at < +2000 ml/day.
Neurological deterioration after treatment was defined as a decline in more than 2 points in the GCS10,12. Investigators determined causes for such deterioration. Acute left heart failure was defined as pulmonary edema occurring during the 7 days and up to 48 hours of treatment administration10,13–15. All neurological and cardiovascular complications were managed according to a pre-specified management protocol.
Post-Discharge Follow Up
Patients returned to the clinic 90-days after enrollment. During this follow-up visit subjects underwent a head computed tomography (CT), and were assessed using the GOS, mRS, NIHSS, BI, and the SIS. Information on adverse events and concomitant medications since the time of discharge was also collected. Following the 90-day evaluation, subjects were discharged from the study.
Data Management
Data management was handled by the SDMC at the Medical University of South Carolina (MUSC).The SDMC developed the Case Report Forms with input from the CCC. The clinical site staff entered data electronically into the database via the WebDCU™ System, a user-friendly menu-driven system with built-in warnings and rules to facilitate the data collection process and ensure sufficient quality control.
Statistical Considerations and Safety Monitoring
Sample size consideration for this phase I dose escalation study was based on the feasibility of recruiting patients in a three-year study period at five sites10. The recruitment yield would be a maximum of 80 patients or 20 patients per dosage group. Statistical analyses were mainly descriptive.
Serious adverse events (SAEs) were defined as those resulting in: death from any cause, a life-threatening adverse experience, prolongation of hospitalization, persistent or significant disability, or an important medical event requiring medical or surgical intervention to prevent one of the previously mentioned outcomes. Safety guidelines for escalation to the next ALB dosage level were based on the rate of cardiovascular SAEs defined as severe or life-threatening heart failure considered to be related (probably, possibly, and definitely) to ALB treatment10,16. Upon completion of each ALB dosage level, if 2 or more subjects out of 20 experienced one of these events, then the independent Medical Safety Monitor (MSM) and DSMB could suggest termination of escalation to the next dosage level with the maximum tolerated dosage designated as the one dosage level below the current level10..
In addition we analyzed the functional clinical outcome and embarked upon comparisons between the safe dosage tiers (tiers 1 and 2) and data from the International Intra-operative Hypothermia for Aneurysm Surgery Trial (IHAST)17. Historical data from the latter was chosen because the inclusion criteria were very similar to ALISAH allowing for more comparable populations than seen in other studies. Since ALISAH was an open-label study without concurrent controls, two analytical approaches to evaluate for potential treatment effects were adopted. First, statistical comparisons were made between subjects receiving the two dosage tiers deemed to be safe based on the safety analyses (tier 1: 0.625 and tier 2: 1.25 g/kg/d × 7 days). Second, data from ALB tiers 1 and 2 were compared to the entire IHAST cohort, IHAST normothermia, and IHAST hypothermia groups. Data from ALB tier 3 were not included due to very small numbers of observations and the safety issues present in that tier. The analyses conducted here were not pre-specified but rather were conducted in a purely exploratory, post-hoc fashion. Favorable outcome was defined as a score of 0 to 1 on the GOS, reflecting good recovery at 3 months. GOS is the most commonly-used outcome measure in SAH clinical trials. For these exploratory comparisons, relative risks were generated using the generalized linear model with log link.
Results
A total of 383 subjects were screened and 47 (12.3%) enrolled in the ALISAH study. The most common reasons for exclusion were unavailable written-informed consent (17%); no aneurysm found on cerebral angiography (14%); aneurysm treatment >72 hours from symptom onset (12%); stupor or coma (11%); age >80 years (7%); ALB administration prior to screening (7%); symptoms not related to SAH (6%); and unreliable time of onset (5%). The majority of subjects were female (72.3%), Caucasian (87%), and median age was 51 years (Table 1). Most subjects were current smokers and all were fully independent prior to symptom onset. Ruptured aneurysms were mostly located in the anterior circulation and were treated within 24 hours of symptom onset.
Table 1.
Demographic and Baseline Characteristics by ALB Dosage Group.
| TIER 1 (0.625 G/KG) |
TIER 2 (1.25 G/KG) |
TIER 3 (1.875 G/KG) |
All Groups |
||
|---|---|---|---|---|---|
| N | 20 | 20 | 7 | 47 | |
| Age | |||||
| Median (Min – Max) | 51 (25 – 79) | 51 (33 – 77) | 55 (38 – 75) | 51 (25 – 79) | |
| Gender | |||||
| FEMALE | 15 (75.0%) | 13 (65.0%) | 6 (85.7%) | 34 (72.3%) | |
| Race | |||||
| WHITE | 18 (90.0%) | 18 (90.0%) | 5 (71.4%) | 41 (87.2%) | |
| Ethnicity | |||||
| HISPANIC OR LATINO | 2 (10.0%) | 1 (5.0%) | 1 (14.3%) | 4 (8.5%) | |
| NOT HISPANIC/NOT LATINO | 17 (85.0%) | 14 (70.0%) | 3 (42.9%) | 34 (72.3%) | |
| UNKNOWN | 1 (5.0%) | 5 (25.0%) | 3 (42.9%) | 9 (19.1%) | |
| Smoking Status | |||||
| NEVER SMOKED | 5 (25.0%) | 8 (40.0%) | 2 (28.6%) | 15 (31.9%) | |
| PAST SMOKER | 0 (0.0%) | 1 (5.0%) | 2 (28.6%) | 3 (6.4%) | |
| CURRENT SMOKER | 15 (75.0%) | 11 (55.0%) | 3 (42.9%) | 29 (61.7%) | |
| Alcohol Use | |||||
| NONE | 6 (30.0%) | 7 (35.0%) | 3 (42.9%) | 16 (34.0%) | |
| < 3 DRINKS/DAY AVERAGE | 12 (60.0%) | 12 (60.0%) | 4 (57.1%) | 28 (59.6%) | |
| >= 3 DRINKS/DAY AVERAGE | 1 (5.0%) | 1 (5.0%) | 0 (0.0%) | 2 (4.3%) | |
| UNKNOWN | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.1%) | |
| Recreational Drug Use | |||||
| NONE | 17 (85.0%) | 16 (80.0%) | 7 (100.0%) | 40 (85.1%) | |
| PAST USER | 0 (0.0%) | 1 (5.0%) | 0 (0.0%) | 1 (2.1%) | |
| CURRENT USER | 2 (10.0%) | 2 (10.0%) | 0 (0.0%) | 4 (8.5%) | |
| UNKNOWN | 1 (5.0%) | 1 (5.0%) | 0 (0.0%) | 2 (4.3%) | |
| History of Hypertension (YES) | 8 (40.0%) | 9 (45.0%) | 3 (42.9%) | 20 (42.6%) | |
| Symptom Onset to Aneurysm Treatment (days) | |||||
| Median (Min – Max) | 1 (0 – 2) | 1 (0 – 2) | 1 (0 – 2) | 1 (0 – 2) | |
| Premorbid mRS | |||||
| NO SYMPTOMS AT ALL | 20 (100.0%) | 19 (95.0%) | 7 (100.0%) | 46 (97.9%) | |
| NO SIGNIFICANT DISABILITY DESPITE SYMPTOMS | 0 (0.0%) | 1 (5.0%) | 0 (0.0%) | 1 (2.1%) | |
| World Federation of Neurological Surgeons Scale | |||||
| GLASGOW COMA SCALE SCORE 15 | 9 (45.0%) | 9 (45.0%) | 3 (42.9%) | 21 (44.7%) | |
| GLASGOW SCORE 14 OR 13 W/O MOTOR DEFICIT | 6 (30.0%) | 11 (55.0%) | 3 (42.9%) | 20 (42.6%) | |
| GLASGOW SCORE 14 OR 13 W/MOTOR DEFICIT | 5 (25.0%) | 0 (0.0%) | 1 (14.3%) | 6 (12.8%) | |
| GLASGOW SCORE 12 TO 7 WITH OR W/O MOTOR DEFICIT | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| GLASGOW SCORE 6 TO 3 W/ OR W/O MOTOR DEFICIT | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Baseline Glasgow Coma Scale | |||||
| MODIFIED FISHER SCALE | |||||
| GRADE 2 | 2 (10%) | 3 (15%) | 1 (14.2%) | 6 (12.8%) | |
| GRADE 3 | 5 (25%) | 4 (20%) | 3 (42.9%) | 12(25.5%) | |
| GRADE 4 | 13 (65%) | 13 (65%) | 3 (42.9%) | 29 (61.7%) | |
| Median (Min – Max) | 14 (13 – 15) | 14 (13 – 15) | 15 (13 – 15) | 14 (6 – 15) | |
| Baseline NIHSS | |||||
| Median (Min – Max) | 2 (0 – 16) | 2 (0 – 14) | 2 (0 – 14) | 2 (0 – 16) | |
| Location of Aneurysm | |||||
| MCA | 3 (15.0%) | 2 (10.0%) | 0 (0.0%) | 5 (10.6%) | |
| ICA | 3 (15.0%) | 0 (0.0%) | 1 (14.3%) | 4 (8.5%) | |
| ACA | 0 (0.0%) | 2 (10.0%) | 1 (14.3%) | 3 (6.4%) | |
| ACOM | 4 (20.0%) | 5 (25.0%) | 0 (0.0%) | 9 (19.1%) | |
| MULTIPLE ANEURYSMS | 6 (30.0%) | 5 (25.0%) | 1 (14.3%) | 12 (25.5%) | |
| PCOM | 4 (20.0%) | 2 (10.0%) | 3 (42.9%) | 9 (19.1%) | |
| BASILAR | 0 (0.0%) | 2 (10.0%) | 0 (0.0%) | 2 (4.3%) | |
| PICA | 0 (0.0%) | 2 (10.0%) | 0 (0.0%) | 2 (4.3%) | |
| MISSING | 0 (0.0%) | 0 (0.0%) | 1 (14.3%) | 1 (2.1%) | |
| Treatment of Aneurysm | |||||
| Surgical clipping | 6 (30.0%) | 4 (20.0%) | 3 (42.9%) | 13 (27.7%) | |
| Endovascular coiling | 14 (70.0% | 16 (80.0% | 4 (57.1% | 34 (72.3%) | |
Abbreviations: mRs: modified Rankin Scale; SD: standard deviation; Min: minimum; Max: maximum; MCA: middle cerebral artery; ICA: internal carotid artery; ACA: anterior cerebral artery; ACOM: anterior communicating artery; PCOM: posterior communicating artery; PICA: posterior inferior cerebellar artery.
We enrolled 20 subjects in dosage tier 1, 20 subjects in dosage tier 2, and 7 subjects in dosage tier 3. Two subjects died while in the study: 1 in tier 1 and 1 in tier 3. The former died of septic shock secondary to gram-negative ventriculitis 2 weeks after symptom onset and was adjudicated as not related to ALB. The latter developed pulmonary edema possibly related to ALB, and aspiration pneumonia with ARDS which was the presumed cause of death.
Most subjects completed ALB treatment. In the first dosage tier 3 subjects did not complete ALB infusion: 1 withdrew consent after developing DCI on day 6; 1 skipped 1 day of treatment due to mild pulmonary edema on day 3; and 1 developed mild pulmonary edema on day 3 of treatment and medication was held. The latter was reported as a protocol violation since the clinical site did not consult with the primary coordinating center. In the second dosage tier 4 subjects did not complete ALB infusion: 1 withdrew consent after 5 days of treatment; 1 skipped 1 day of treatment due to neurological deterioration on day 2; 1 developed serious respiratory insufficiency possibly related to ALB after the first day of treatment; and 1 patient developed SAE with respiratory failure unlikely related to ALB on day 3 of treatment.
Physiologic and Laboratory Data
Daily serum albumin concentrations increased from baseline in all dosage tiers during the treatment period (Figure 1A). The changes were more prominent for tier 2 and were sustained up to 15 days after enrollment (online Table S3).
Figure 1. Laboratory and physiologic variables during the treatment period.
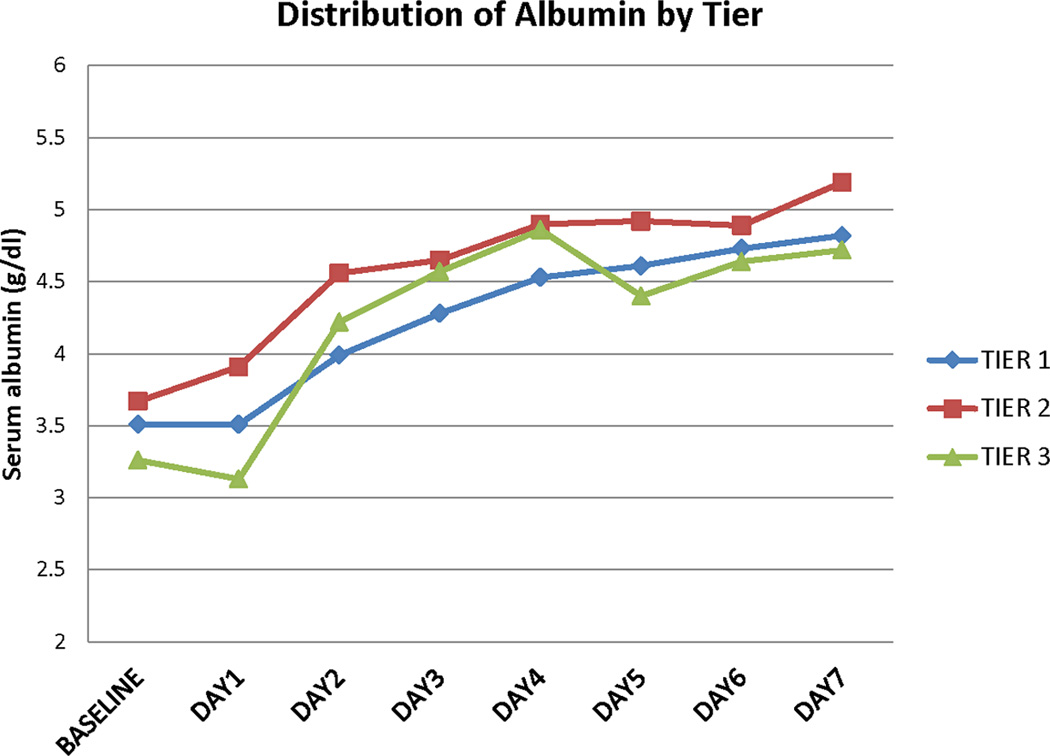
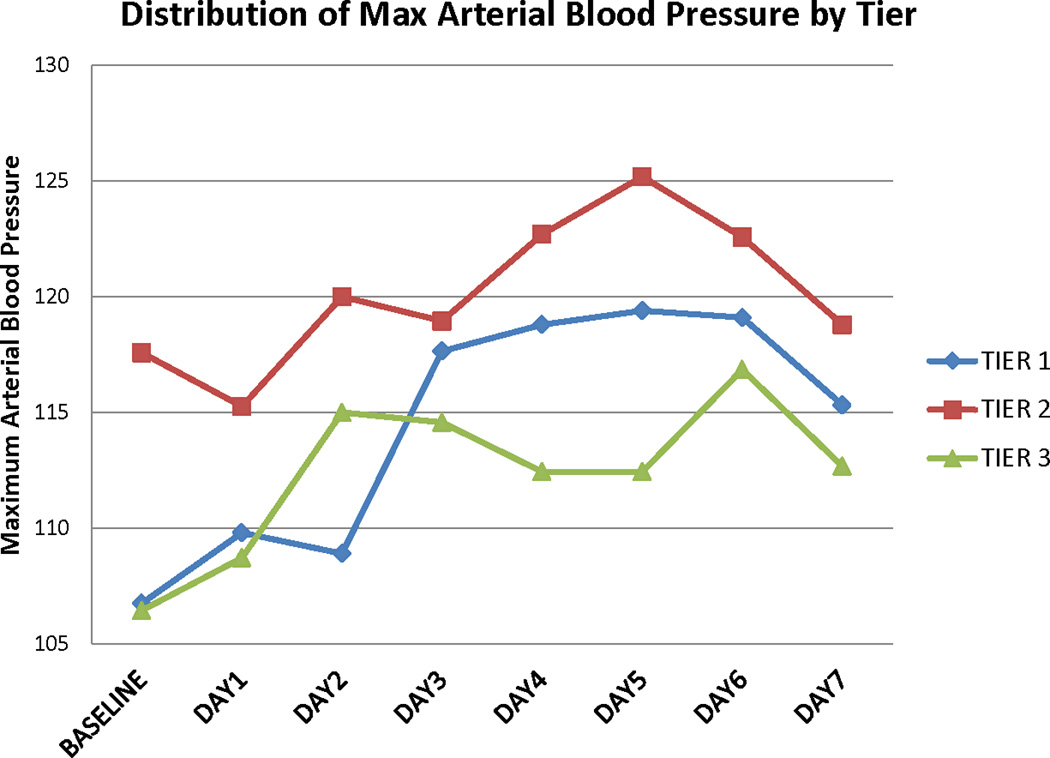
A. Serum albumin concentrations
B. Maximum mean arterial blood pressure
Daily maximum mean arterial blood pressures also increased from baseline for all treatment tiers during the treatment period (Figure 1B). These values tended to decrease to baseline after treatment was completed (online Table S4). Baseline blood pressures were higher for subjects in tier 2 compared to the other tiers. This was not related to prior known history of hypertension. We observed no differences in serum osmolality, serum creatinine, daily fluid balance, and daily weight from baseline in all the dosage tiers (Online Tables S5, S6, S7, and S8). In addition, there was no difference in CVP values. However due to concerns with higher values of fluid intake potentially leading to higher incidence of cardiac complications, the DSMB recommended reducing the fluid balance value to <+1000 ml/day after 22 subjects had been enrolled.
Adverse Events
There were 171 AEs in 32 subjects. The only expected and observed AEs related to ALB were those related to volume overload leading to acute heart failure or pulmonary edema. There were 17 SAEs reported in 7 subjects (15%) but only 3 adjudicated as related to ALB (Table 2). In the second dosage tier 1 subject experienced pulmonary edema which was possibly related to ALB. In the third dosage tier 2 subjects experienced pulmonary edema with 1 possibly and 1 definitely related to ALB. Because of the 2 SAEs related to ALB in dosage tier 3, the study was terminated after 47 patients had been enrolled and completed treatment.
Table 2.
Cardiovascular and Respiratory Serious Adverse Events
| ALB Dosage Tier | Serious Adverse Event | Relationship to ALB* |
|---|---|---|
| 1 |
|
|
| 2 |
|
|
| 3 |
|
|
Abbreviations: ALB: 25% human albumin; ARDS: Acute Respiratory Distress Syndrome.
After adjudication by Medical Safety Monitor and review by Data and Safety Monitoring Board.
Functional Outcomes
The summary functional outcome scores for all the tiers and historical IHAST treatment groups are presented in Table 3. We also present the distribution of GOS, mRs, and BI scores at 3 months in Figure 2. There was a consistent dose response relationship in that those subjects in tier 2 did better overall than those in tier 1 on all functional outcome measures, and those in tier 3 did worse overall. In addition, the overall incidence of DCI secondary to symptomatic vasospasm was low (17%). However, the proportion was lower for subjects in tier 2 (15%) compared to those in tier 3 (20%). We also reviewed head CT scans at 90 days to investigate new cerebral infarctions that were not present at the baseline study. We found a total of 7 new cerebral infarctions (3 in dosage tier 1, 3 in dosage tier 2, and 1 in dosage tier 3).
Table 3.
Outcome summary by treatment group for ALISAH and IHAST
| STUDY AND TREATMENT GROUPS |
mRs ≤ 1 | mRs ≤ 2 | Barthel Index 95–100 |
Glasgow Outcome Scale 0 – 1 |
|
|---|---|---|---|---|---|
| -ALISAH | |||||
| TIER 1 (0.625 G/KG) | 11 (55%) | 15 (70%) | 15 (75%) | 13 (65%) | |
| TIER 2 (1.25 G/KG) | 15 (75%) | 18 (90%) | 19 (95%) | 17 (85%) | |
| TIER 3 (1.875 G/KG) | 3 (43%) | 3 (43%) | 5 (71%) | 3 (43%) | |
| -IHAST | |||||
| HYPOTHERMIA | 333 (67%) | - | 416 (89%) | 329 (66%) | |
| NORMOTHERMIA | 318 (63%) | - | 403 (86%) | 314 (63%) | |
Abbreviations: mRs: modified Rankin Scale score; BI: Barthel Index; GOS: Glasgow Outcome Scale.
Figure 2. Distribution of functional outcomes at 90 days by treatment tier.
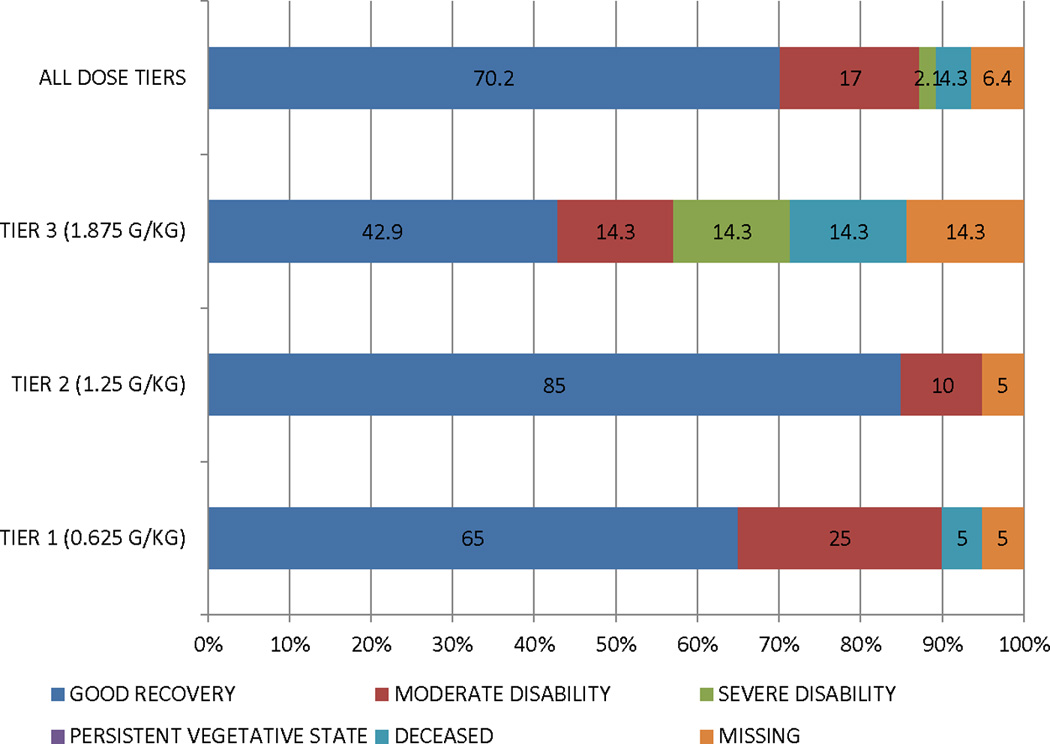
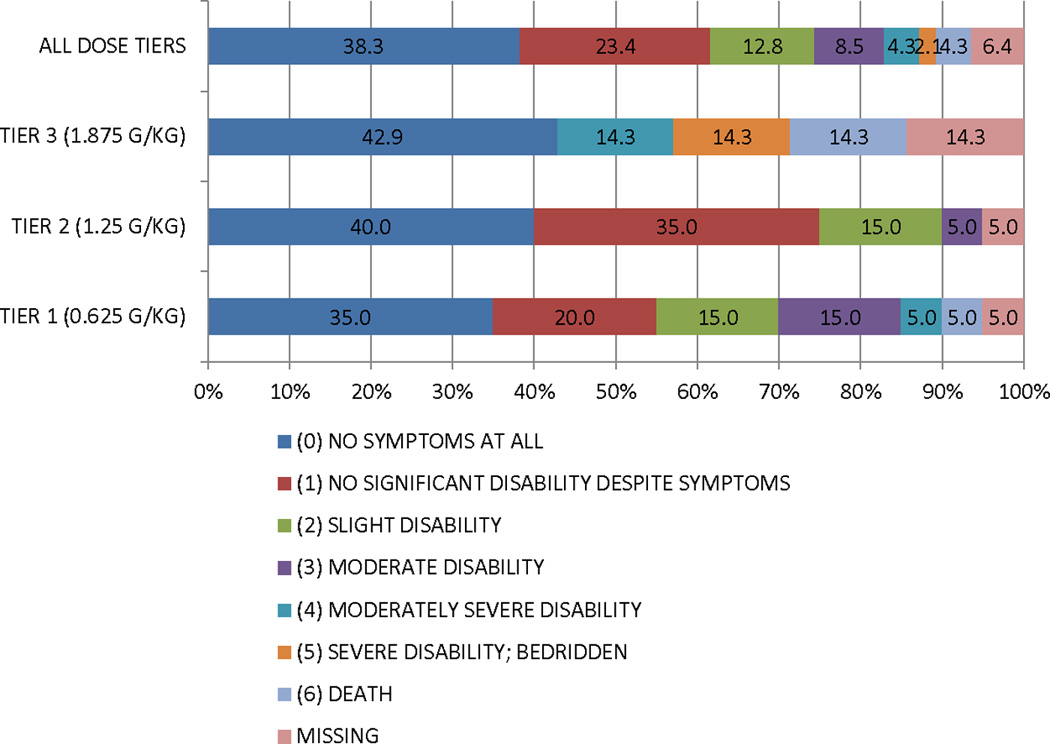
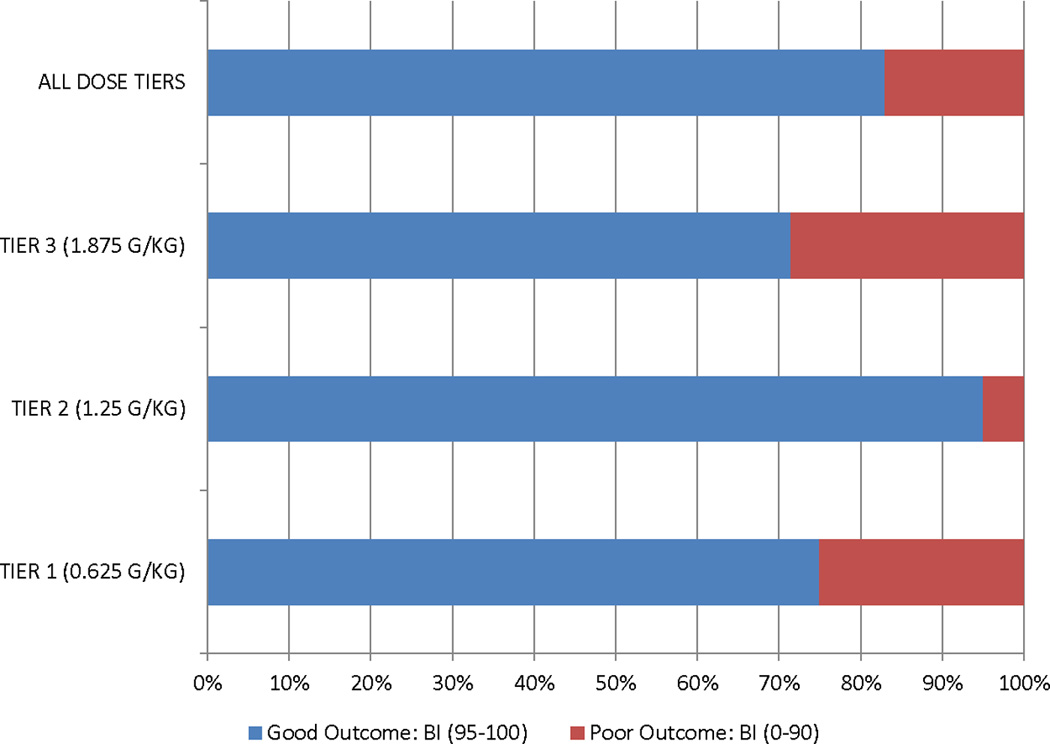
A. Glasgow Outcome Scale
B. Modified Rankin Scale
C. Barthel Index
Subjects in ALB dosage tiers 1 and 2 were compared with subjects in the IHAST study (Total N= 1000; hypothermia group = 499; normothermia group = 501)17. Subjects in tier 2 had better outcomes compared to those of tier 1 suggesting a dose response (Table 4). In addition, when compared to IHAST subjects, outcomes for tier 2 subjects were better.
Table 4.
Glasgow Outcome Scale comparisons between dosage tiers and IHAST data.
| Comparison | Odds Ratio for GOS=1 |
95% Confidence interval |
|---|---|---|
| ALISAH Tier 2 vs ALISAH Tier 1 | 3.0513 | 0.6586, 14.1367 |
| ALISAH Tier 2 vs IHAST all | 3.1462 | 0.9158, 10.8089 |
| ALISAH Tier 1 vs IHAST all | 1.0311 | 0.4077, 2.6079 |
| ALISAH Tier 3 vs IHAST all | 0.4164 | 0.0927, 1.8709 |
| ALISAH Tier 2 vs IHAST normothermia | 3.3747 | 0.9760, 11.6694 |
| ALISAH Tier 2 vs IHAST hypothermia | 2.9281 | 0.8463, 10.1310 |
Abbreviations: GOS: Glasgow Outcome Scale; ALISAH: Albumin in Subarachnoid Hemorrhage; IHAST: Intraoperative Hypothermia for Aneurysm Surgery Trial.
Discussion
We have shown that large doses of ALB up to 1.25 g/Kg/day × 7 days are safe in patients with aneurysmal SAH. The safety stopping rule of at least 2 events of severe-to-life-threatening heart failure in dosage tiers 1 and 2 was not met. Dosages higher than 1.25 g/Kg/day were associated with significant cardiovascular complications including 2 SAEs related to ALB. The latter resulted in early termination of the study. It is important to note that in all instances pulmonary edema was easily treated and resolved. We have also demonstrated that our treatment protocol was feasible and successfully implemented in several international centers. Our data are supported by findings from ALB studies in patients with acute ischemic stroke8,9.
The main physiologic effects of ALB treatment were elevation in the serum albumin concentration and mean arterial blood pressure. The latter improved to baseline values after treatment completion. Serum albumin values remained elevated 7 days after treatment suggesting that any potential beneficial effects of ALB may remain throughout the critical period of DCI risk. We observed no changes in serum osmolality or renal function. In addition, we found that fluid intake increased in direct relationship to higher dosage tiers. However, there was no correlation between higher fluid intake or fluid balances and cardiovascular complications. This suggests that factors other than pure intravascular volume augmentation may play a role. We speculate that diastolic cardiac dysfunction may be a contributing factor rather than systolic abnormalities due to the fact that left ventricular ejection fractions were within normal limits in all our subjects. Note, however that the sample size was small and therefore our findings will have to be validated in a larger cohort of SAH subjects.
The data also suggest that high dosage of ALB up to 1.25 g/Kg/d × 7 days are not only safe but also may be neuroprotective. This is supported by data from ischemic stroke subjects treated with ALB8,9,18 and retrospective data from SAH patients3. ALB has several potential mechanisms that could explain its neuroprotective effects. The increase in serum albumin concentrations is expected to increase the serum oncotic pressure which in turn will mobilize interstitial fluid and improve organ function including the brain19. ALB also possesses antioxidant and scavenger properties in part by its potential to replete thiol stores20–22, and it can modulate apoptosis23. In addition, ALB administration may also improve microcirculatory blood flow, increase organ blood flow, decrease leukocyte rolling and adherence, and reduce the inflammatory response24.
The potential reduction DCI secondary to symptomatic vasospasm may be explained by the interaction of ALB with the albumin-specific binding sites of the microvasculature endothelium.25–27. By binding to the endothelial glycocalyx, albumin maintains the normal permeability of microvessel walls26, 28, 29. It has also been suggested that albumin may be a factor in mediating the effect of blood coagulation on vascular tone and capillary permeability30. Moreover, serum albumin reacts with nitric oxide to form a stable S-nitrosothiol that has endothelium-derived relaxing factor-like properties31.
Our study has several limitations. ALISAH is an early phase design and we do not have concurrent controls. In addition, the study was neither randomized, nor powered to test for efficacy effects. Therefore, caution is advised in the interpretation of our data and comparison with the IHAST study. Moreover, we did not obtain head CT scans following aneurysmal treatment. This limits our ability to determine whether the cerebral infarctions found at 90 days were due to treatment modalities or ischemic complications from SAH. Lastly, the severity of radiological infarctions cannot be ascertained since we did not measure infarction volume.
Conclusion
The ALISAH Pilot Study has demonstrated that large ALB dosages up to 1.25 g/Kg/d × 7 days are safe and the treatment protocol is feasible. These dosages have been found to be neuroprotective in animal models of cerebral ischemia. Pulmonary edema, the main systemic complication related to ALB, was easily managed in the ICU setting. Despite the limited sample size, the ALISAH data also provide preliminary evidence that high-dose ALB may be neuroprotective after aneurysmal SAH. Based on these encouraging results, initial planning of ALISAH II, a large Phase III multicenter, randomized, placebo-controlled clinical trial to evaluate the efficacy of ALB in subjects with SAH is underway.
Supplementary Material
Acknowledgments
Sources of funding: This study was supported by NIH Pilot Clinical Trial Grant NS049135 (P.I.: JI Suarez). ALISAH operated under IND approval from the FDA: BB-IND # 13022
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke of the National Institutes of Health. Study intervention was provided at no cost by Grifols International (Barcelona, Spain).
References
- 1.Bederson JB, Connolly ES, Batjer HH, Dacey RG, Dion JE, Diringer MN, et al. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage: a statement for healthcare professionals from a special writing group of the stroke council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 2.Rinkel GJ, Feigin VL, Algra A, Vermeulen M, van Gijn J. Calcium antagonists for aneurismal subarachnoid hemorrhage. Cochrane Database Syst Rev. 2000;4:CD000277. doi: 10.1002/14651858.CD000277. [DOI] [PubMed] [Google Scholar]
- 3.Suarez JI, Shannon L, Zaidat OO, Suri MF, Singh G, Lynch G, et al. Effect of human albumin administration on clinical outcome and hospital cost in patients with subarachnoid hemorrhage. J Neurosurg. 2004;100:585–590. doi: 10.3171/jns.2004.100.4.0585. [DOI] [PubMed] [Google Scholar]
- 4.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- 5.Belayev L, Busto R, Zhao W, Clemens JA, Ginsberg MD. Effect of delayed albumin hemodilution on infarction volume and brain edema after transient middle cerebral artery occlusion in rats. J Neurosurg. 1997;87:595–601. doi: 10.3171/jns.1997.87.4.0595. [DOI] [PubMed] [Google Scholar]
- 6.Belayev L, Zhao W, Pattany PM, Weaver RG, Huh PW, Lin B. Diffusion-weighted magnetic resonance imaging confirms marked neuroprotection efficacy of albumin therapy in focal cerebral ischemia. Stroke. 1998;29:2587–2599. doi: 10.1161/01.str.29.12.2587. [DOI] [PubMed] [Google Scholar]
- 7.Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001;32:553–560. doi: 10.1161/01.str.32.2.553. [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg MD, Hill MD, Palesch YY, Ryckborst KJ, Tamariz D. The ALIAS Pilot Trial: a dose escalation and safety study of albumin therapy for acute ischemic stroke-I: physiological responses and safety results. Stroke. 2006;37:2100–2106. doi: 10.1161/01.STR.0000231388.72646.05. [DOI] [PubMed] [Google Scholar]
- 9.Palesch YY, Hill MD, Ryckborst KJ, Tamariz D, Ginsberg MD. The ALIAS Pilot Trial: a dose-escalation and safety study of albumin therapy for acute ischemic stroke-II: neurologic outcome and efficacy analysis. Stroke. 2006;37:2107–2114. doi: 10.1161/01.STR.0000231389.34701.b5. [DOI] [PubMed] [Google Scholar]
- 10.Suarez JI, Martin RH. Treatment of subarachnoid hemorrhage with human albumin: ALISAH Study. Rationale and design. Neurocrit Care. 2010;13:263–277. doi: 10.1007/s12028-010-9392-8. [DOI] [PubMed] [Google Scholar]
- 11.Peters T. The albumin molecule: its structure and chemical properties. In: Peters T, editor. All about albumin: biochemistry, genetics, and clinical applications. San Diego: Academic Press; 1996. pp. 9–75. [Google Scholar]
- 12.Haley EC, Kassell NF, Torner JC. A randomized controlled trial of high-dose intravenous nicardipine in aneurysmal subarachnoid hemorrhage. J Neurosurg. 1993;78:537–547. doi: 10.3171/jns.1993.78.4.0537. [DOI] [PubMed] [Google Scholar]
- 13.Bellone A, Monari A, Cortellano F, Vettorello M, Arlati S, Coen D. Myocardial infarction rate in acute pulmonary edema: noninvasive pressure support ventilation versus continuous positive airway pressure. Crit Care Med. 2004;32:1860–1865. doi: 10.1097/01.ccm.0000139694.47326.b6. [DOI] [PubMed] [Google Scholar]
- 14.Mehta S, Jay G, Woolard R, Hipona R, Connolly E, Cimini D, Drinkwine J, Hill N. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med. 1997;25:620–628. doi: 10.1097/00003246-199704000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Masip J, Roque M, Sanchez B, Fernandez R, Subirana M, Exposito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema. Systematic review and meta-analysis. JAMA. 2005;294:3124–3130. doi: 10.1001/jama.294.24.3124. [DOI] [PubMed] [Google Scholar]
- 16.Lennihan L, Mayer SA, Fink ME, Beckford A, Paik M, Zhang H, et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage: a randomized controlled trial. Stroke. 2000;31:383–391. doi: 10.1161/01.str.31.2.383. [DOI] [PubMed] [Google Scholar]
- 17.Todd MM, Hindman BJ, Clarke WR, Torner JC for the Intraoperative Hypothermia for Aneurysm Surgery Trial (IHAST) Investigators. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Enlg J Med. 2005;352:135–145. doi: 10.1056/NEJMoa040975. [DOI] [PubMed] [Google Scholar]
- 18.Hill MD, Martin RH, Palesch YY, Tamariz D, Waldman BD, Ryckborst KJ, et al. The Albumin in Acute Stroke Part 1 Trial: An Exploratory Efficacy Analysis. Stroke. 2011 doi: 10.1161/STROKEAHA.110.610980. published online May 5 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubois MJ, Orellana-Jimenez C, Melot C, De Backer D, Berre J, Leeman M, et al. Albumin administration improves organ function in critically ill hypoalbuminemic patients: a prospective, randomized, controlled pilot study. Crit Care Med. 2006;34:2536–2540. doi: 10.1097/01.CCM.0000239119.57544.0C. [DOI] [PubMed] [Google Scholar]
- 20.Quinlan GJ, Margarson MP, Mumby S, Evans TW, Gutteridge JM. Aministration of albumin to patients with sepsis syndrome: a possible beneficial role in plasma thiol repletion. Clin Sci (Lond) 1998;95:459–465. [PubMed] [Google Scholar]
- 21.Lang JD, Jr, Figueroa M, Chumley P, Aslan M, Hurt J, Tarpey MM, et al. Albumin and hydroxyethyl starch modulate oxidative inlfmmatory injury to vascular endothelium. Anesthesiology. 2004;100:51–58. doi: 10.1097/00000542-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Quinlan GJ, Mumby S, Martin GS, Bernard GR, Gutteridge JM, Evans TW. Albumin influences total plasma antioxidant capacity favorably in patients with acute lung injury. Crit Care Med. 2004;32:755–759. doi: 10.1097/01.ccm.0000114574.18641.5d. [DOI] [PubMed] [Google Scholar]
- 23.Zoellner H, Hofler M, Beckmann R, Hufnagl P, Vanyek E, Bielek E, et al. Serum albumin is a specific inhibitor of apoptosis in human endothelial cells. J Cell Sci. 1996;109(Pt 10):2571–2580. doi: 10.1242/jcs.109.10.2571. [DOI] [PubMed] [Google Scholar]
- 24.Horstick G, Lauterbach M, Kempf T, Bhakdi S, Heimann A, Horstick M, et al. Early albumin infusion improves global and local hemodynamics and reduces inflammatory responses in hemorrhagic shock. Crit Care Med. 2002;30:851–855. doi: 10.1097/00003246-200204000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Ghitescu L, Fixman A, Simionercu N. Specific binding sites for albumin restricted to plasmalemmal vesicles of continuous capillary endiothelium: receptor-mediated transcytosis. J Cell Biol. 1986;102:1304–1311. doi: 10.1083/jcb.102.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnitzer JE, Oh P. Antibodies to SPARC inhibit albumin binding to SPARC, gp60, and microvascular endothelium. Am J Physiol. 1992;263:H1872–H1879. doi: 10.1152/ajpheart.1992.263.6.H1872. [DOI] [PubMed] [Google Scholar]
- 27.Schnitzer JE. gp60 is an albumin-binding glycoprotein expressed by continuous endothelium ilvolved in albumin transcytosis. Am J Physiol. 1992;262:H246–H254. doi: 10.1152/ajpheart.1992.262.1.H246. [DOI] [PubMed] [Google Scholar]
- 28.He P, Curry FE. Albumin modulation of capillary permeability: role of endothelial cell [Ca2]i. Am J Physiol. 1993;263:H74–H82. doi: 10.1152/ajpheart.1993.265.1.H74. [DOI] [PubMed] [Google Scholar]
- 29.Schnitzer JE, Sung A, Hovart R, Bravo J. Preferential interaction of albumin-binding proteins, gp30 and gp18, with conformationally modified albumins. Presence in many cells, and tissues with a possible role in catabolism. J Biol Chem. 1992;267:24544–24553. [PubMed] [Google Scholar]
- 30.Reinhart WH, Nagy C. Albumin affects erythrocyte aggregation and sedicmentation. Eur. J Clin Invest. 1995;25:523–528. doi: 10.1111/j.1365-2362.1995.tb01739.x. [DOI] [PubMed] [Google Scholar]
- 31.Keaney JFJ, Simon DI, Stamler JS, Jaraki O, Sharfstein J, Vita JA, Loscalzo J. NO forms an adduct with serum albumin that has endothelium-derived relaxing facotr-like properties. J Clin Invest. 1993;91:1582–1589. doi: 10.1172/JCI116364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


