Abstract
Summary
Objective
To determine if antagonizing miR 29 enhances elastin (ELN) levels in cells and tissues lacking ELN. Methods and
Results
miR-29 mimics reduced ELN levels in fibroblasts and smooth muscle cells, whereas miR-29 inhibition increased ELN levels. Antagonism of miR-29 also increased ELN levels in cells from patients haploinsufficient for ELN and in bioengineered human vessels.
Conclusion
miR-29 antagonism may promote increased ELN levels during conditions of ELN deficiencies.
Keywords: microRNA, elastin, smooth muscle, fibroblasts, synthetic vessels
Introduction
Elastin (ELN) is a major component of the extracellular matrix in arteries that is essential for normal structural integrity and function. In humans, a single ELN gene is found on chromosome 7 and mutations in one allele of the ELN gene can lead to several elastinopathies, including supravalvular aortic stenosis (SVAS) and a congenital narrowing of the ascending aorta or other vessels 1. SVAS is also a prominent component of Williams-Beuren syndrome (WBS), a frequent heterozygous deletion of a ~1.5 Mb segment at chromosome 7q11.23 that includes ELN2. Despite having one intact allele , the expression of elastin is less than 50% of normal in WBS, thus suggesting post-transcriptional modulation of the mRNA 3.
Elastin is secreted into the extracellular space as soluble tropoelastin, which is cross-linked by lysyl oxidase (LOX) and lysyl oxidase-like 1 (LOXL1) to form insoluble, mature elastin fibrils 4. Rodent studies have shown that elastogenesis begins during mid-gestation,peaks in the perinatal period, and drops sharplythereafter to low levels that persist into adulthood 1. Interestingly, tropoelastin pre-mRNA levels remain elevated in adult rat lungs despite considerably reduced steady-state mRNA levels , thus suggesting mRNA post-transcriptional regulation may be a predominant mechanism to regulate elastin mRNA and protein levels in adults 5.
In the past decade, microRNAs (miRNAs) have emerged as important regulators of gene expression robustness 6. miRNAs predominantly target the 3’UTR of mRNAs, either destabilizing the mRNA transcript or interfering with its translation into protein. Previous work has shown that miR-29 mimics down-regulate the expression of ELN, COL1A1 and COL3A1 7. Here we show that inhibition of miR-29a can dramatically increase ELN expression in human cells and miR29 inhibition upregulates ELN levels in cells from patients with ELN haploinsufficiencies and in bioengineered human blood vessels. Thus, antagonizing the actions of miR-29 may promote increased ELN levels during conditions of enhanced elastinolysis or deficiencies.
Results and Discussion
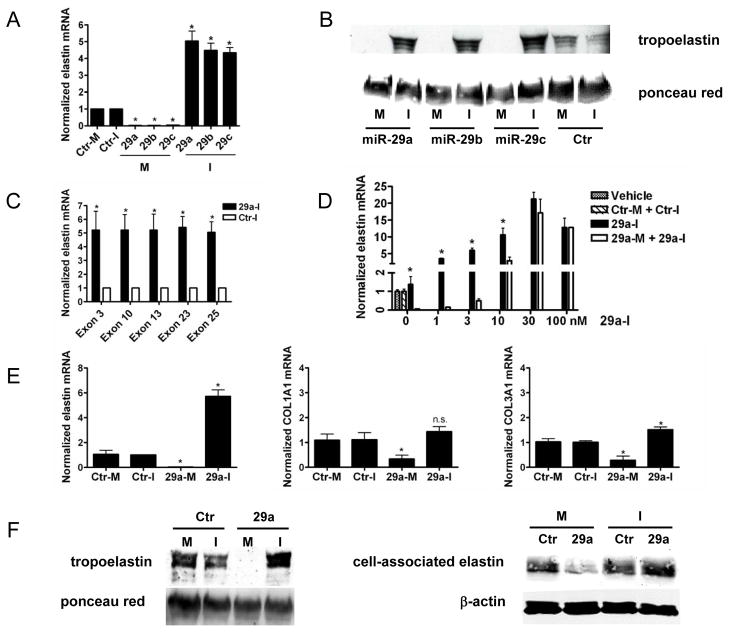
Human dermal fibroblasts (HDF) were transfected with miRNA mimics for miR-29a, -29b and -29c or inhibitors (60 nM of each) for 48 h and the levels of ELN mRNA were quantified using qRT-PCR. Fig. 1A shows that miR-29a, -29b and -29c mimics significantly reduced ELN mRNA levels (to 2-3% of control), while transfection with miR-29 inhibitors increased ELN mRNA levels (4-6 fold). Due to homology within the seed sequence of miR-29 family members 7, miR-29 inhibitors also reduced the levels of other miR-29 miRNAs (Supplemental Fig 1). Computational analyses have predicted three seed sequences (38-44, 297-303, 310-316) for miR-29 binding in the 3’UTR of the human ELN mRNA and these sequences are highly conserved among mammals. As seen in Fig 1B, treatment of HDF with mimics reduced tropoelastin protein levels (lanes 1, 3 and 5) while miR-29a, -29b and -29c inhibitors increased levels (lanes 2, 4 and 6) compared to controls (lanes 7 and 8). To further test whether the miR-29 inhibitor increases major ELN isoforms, we used exon-specific primers to detect the existence of major ELN exons. As seen in Fig.1C, ELN mRNAs containing the known exons 3, 10, 13, 23, and 25 were upregulated to a similar degree with the miR-29a inhibitor.
Figure 1. miR-29 inhibitors effectively increase elastin mRNA and protein in HDF and VSM.
HDF cells were treated with miR-29abc mimics (M),inhibitors (I) or controls (Ctr-M or Ctr-I) and ELN RNA (A) and protein (B) examined 48 h post-treatment. PCR primers for multiple ELN exons were used to detect ELN mRNA after miR29a-I treatment (C). Increasing amount of miR29a-I were incubated with fixed amount of miR-29a mimic (60nM) and the ELN mRNA examined (D). Human VSM were treated as above and the levels of ELN, COL1A1 and COL3A1 examined (E). Soluble or cell associated ELN protein was detected in by Western blotting (F).Data are representative of 3 independent experiments using cells from three different human donors. In all cases, data are mean+/− SEM, *p<0.05 vs. control.
Since all miR-29 members are abundantly present in the cell types in this study and can potentially regulate ELN levels, we performed additional experiments using only miR-29a. To test the specificity of the miR-29a inhibitor, it was titrated (0-100 nM) in the presence or absence of 60 nM of the miR-29a mimic. As seen in Fig 1D, miR-29a inhibitor (29a-I) dose dependently increases ELN mRNA levels. In the presence of miR-29a mimic (60 nM), more miR-29a inhibitor was needed to achieve the same fold increase of ELN mRNA, but this difference disappeared between 30 and 100 nM of the inhibitor. These data together with data in Fig. 1A suggested that: 1) the miR-29a inhibitor specifically antagonizes endogenous miR-29, as well as the miR-29a mimic, in a dose-dependent manner, and 2) the miR-29a inhibitor specifically protects ELN mRNA from endogenous miR-29a dependent decay.
Computer analyses also predict several other genes with miR-29 seed sequences, including COL1A1, COL3A1 and VEGF. Overexpression of miR-29 can decrease the activity of luciferase reporter plasmids containing 3’UTRs of COL1A1, COL3A1 and ELN1, and inhibiting miR-29 in vivo can modestly upregulate COL1A1 and COL3A1 mRNA levels in the heart (7). Indeed, miR-29a mimic (60 nM) reduced ELN, COL1A1, COL3A1 and VEGF-A (not shown) mRNA levels in human vascular smooth muscle (VSM, Fig 1E). In contrast, the miR-29a inhibitor enhanced ELN expression but did not significantly change the levels of COL1A or COL3A. Additional genes involved in matrix assembly, fibrillin-1-3, emilin1, Lox1, LoxL1/2 were also tested in VSM and fibroblasts and miR-29a-I did not increase their expression levels (Supplemental Fig 2). The miR-29a inhibitor increased soluble and cell associated elastin levels in VSM (Fig 1F, left and right panels). This suggests that miR mimics may achieve concentrations that destabilize multiple transcripts having seed sequences, whereas miR inhibition may selectively upregulate physiological mRNA partners regulated by the endogenous miRNA levels.
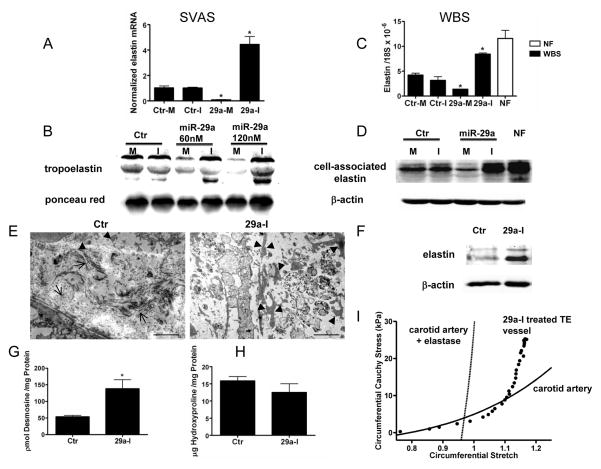
Next, we tested the ability of miR-29a inhibitor to upregulate ELN expression in pulmonary VSM cells isolated from a patient with SVAS. A GTAT insertion in exon 9 of the ELN gene caused a frameshift and a premature stop codon in exon 10. qPCR experiments using primers recognizing ELN exon 18 showed that SVAS cells had low levels of ELN mRNA (about 10% of normal VSM) but equal amounts of endogenous miR-29a compared to normal VSM (Supplementary Fig. 3A left panel). Transfection of SVAS cells with miR-29a inhibitor upregulated ELN expression 3.5 fold (Fig 2A). The expression of COL1A1 and COL3A1 mRNAs were moderately higher in SVAS when compared to normal cells (0.68 ± 0.24 vs 0.23 ± 0.09 for COL1A1; 17.5 ± 2.2 vs 9.6 ± 4.6 for COL3A1), and miR-29a inhibitor (60 nM) increased COL1A1 (1.7 fold) but not COL3A1 mRNA levels. miR-29a inhibition increased tropoelastin levels in SVAS cells dose-dependently and in contrast miR 29 mimic decreased tropoelastin (Fig 2B).
Figure 2. miR 29 inhibition increases ELN levels in haploinsufficient cells and in synthetic humanized vessels.
Cells from SVAS (A,B) or WBS (C, D) were treated with miR-29a mimic (M) or inhibitor (I) and the levels of ELN mRNA and protein examined. Data are mean ± SEM, * p<0.05 vs control inhibitor in 3 different experiments. Representative TEM showing abundant collagen bundles either perpendicular or parallel to the plane (arrows) with little ELN(arrowheads) in control vessels compared with miR-29a inhibition (E). Scale bar inset denotes 2 μM. Vessel ELN (F) and desmosine (G, n=4 per group), but not hydroxyproline (H) levels, were elevated in miR-29a inhibitor treated vessels. (I) miR-29 inhibitor treated bioengineered vessel (filled circle) was subjected to distending pressure up to 60 mmHg at the estimated bioreactor axial stretch (1.18), biaxial mechanical tests were performed on the vessel in comparison to a normal mouse carotid artery before (solid line) and after (dashed) exposure to elastase.
Next, we examined if miR-29 regulates ELN in dermal fibroblasts isolated from two donors with WBS. Comparative Genomic Hybridization verified that these cells had a typical ~1.5 Mb microdeletion at 7q11.23 encoding 25 genes including ELN (supplement Fig 4;2. These ELN-haplodeficient cells express only 26-36% of ELN mRNA levels but comparable levels of miR-29a (Supplemental Fig 3A right panel, B left) compared to age-matched normal fibroblasts (NF) from the same bank. Treatment of WBS cells with miR-29 inhibitor upregulated ELN mRNA levels close to those seen in normal cells (Fig.2C. Suppl Fig 3B middle panel). In contrast, the levels of COL1A1 were comparable in WBS cells and miR-29 inhibition increased COL1A1 levels. Western-blot analysis showed ELN protein was increased by 40% in WBS cells 48 h after miR-29 inhibitor treatment (Fig.2D).
Bioengineered human blood vessels are collagen replete, but ELN deficient 8 and this paucity of ELN may compromise their mechanical properties. To examine if miR-29a inhibition can enhance ELN levels and therefore improve vascular mechanics, polyglycolic acid (PGA) scaffolds were seeded with early passage human VSM as described 8 and vessels were grown in a bioreactor for 6 weeks in the absence or presence of miR-29a inhibitor. As seen in Fig 2E (left panel), there was little ELN (via transmission electron microscopy) in the control vessels, whereas miR-29a inhibition increased the appearance of ELN “islands” (Fig 2E, right panel) and its detection by Western blotting (Fig 2F). This ELN was crosslinked as quantified by desmosine content (Fig 2G) and levels of hydroxyproline were comparable between the vessels (Fig 2H). The number of VSM in the vessels were not different between groups (1434 ± 437 vs 1797 ± 453 DAPI positive/mm2 for treated versus control vessels, respectively). Finally, biaxial mechanical tests revealed that miR-29a inhibition increased the distensibility of the bioengineered vessels at low pressures (Fig 2I); this response is similar to that of native vessels and suggests the ELN conferred some structural benefit to the vessel.
Collectively, these data suggest that antagonizing miR 29 may increase functional ELN levels in conditions of ELN haploinsufficiency or during enhanced elastinolysis secondary to aneurysms, emphysema or aging.
Materials and Methods
Cell culture and miRNA treatment
Human dermal fibroblasts (HDF), isolated from human foreskin or adult skin were purchased from Coriell. WBS and age-matched normal HDF were purchased from NICHD Brain and Tissue Bank for Developmental Disorders (University of Maryland, Baltimore, MD). Human VSMC and SVAS pulmonary VSMC were explanted by outgrowth as described 9. Cells were treated with miRIDIAN miR-29 mimic, hairpin inhibitors or corresponding controls (Dharmacon, Chicago, IL) at 60 nM for 15 h unless specified otherwise, then were grown in complete media for 48h before harvest for RNA or protein studies.
Western blot
To measure tropoelastin expression in the media, cells were grown in 0.5% FBS DMEM media for the final 24h and media proteins were precipitated by 10% trichloroacetic acid (TCA) and resolved on 10% SDS-PAGE. To measure elastin protein associated with cell membrane, equal number of cells were lysed in 6M urea-containing sample loading buffer and loaded onto 10% SDS-PAGE. Western membranes were probed with a polyclonal antibody recognizing human elastin. Ponceau red staining (for media proteins) or β-actin levels (in cell lysates) were used to normalize protein loading.
Culture and analyses of Tissue Engineered Vessels
Engineered arteries 1 mm in diameter and 3 cm in length were grown as previously described 10. Biaxial tests were performed using a custom device as described before 11.
Desmosine and Hydroxyproline analysis
Desmosine and hydroxyproline, demonstrating cross-linked elastin and collagen respectively, were measured as described 12.
Statistical Analysis
Comparisons between two groups were by unpaired student t test. Statistical analyses were performed using Prism 4 software (GraphPad). P values were two-tailed and values <0.05 were considered to indicate statistical significance.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Barbara Pober for all her help and advice with this project. B. This work was supported by grants from the Kiev Foundation (to W.C.S, FJG and FT) and R01 HL64793, R01 HL61371, R01 HL081190, HL096670 from the National Institutes of Health (to WCS). C. There are no conflicts of interest.
While this work was in review, a recent paper has documented that anti-miR 29a reduces the extent of aneurysms (Boon et al, Circ Res. 2011 Oct 28;109(10):1115-9). A
References
- 1.Mecham RP. Methods in elastic tissue biology: elastin isolation and purification. Methods. 2008;45:32–41. doi: 10.1016/j.ymeth.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pober BR, Johnson M, Urban Z. Mechanisms and treatment of cardiovascular disease in Williams-Beuren syndrome. J Clin Invest. 2008;118:1606–1615. doi: 10.1172/JCI35309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urban Z, Riazi S, Seidl TL, Katahira J, Smoot LB, Chitayat D, Boyd CD, Hinek A. Connection between elastin haploinsufficiency and increased cell proliferation in patients with supravalvular aortic stenosis and Williams-Beuren syndrome. Am J Hum Genet. 2002;71:30–44. doi: 10.1086/341035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 5.Swee MH, Parks WC, Pierce RA. Developmental regulation of elastin production. Expression of tropoelastin pre-mRNA persists after down-regulation of steady-state mRNA levels. J Biol Chem. 1995;270:14899–14906. doi: 10.1074/jbc.270.25.14899. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahl SL, Rhim C, Song YC, Niklason LE. Mechanical properties and compositions of tissue engineered and native arteries. Ann Biomed Eng. 2007;35:348–355. doi: 10.1007/s10439-006-9226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang P, Manes TD, Pober JS, Tellides G. Human vascular smooth muscle cells lack essential costimulatory molecules to activate allogeneic memory T cells. Arterioscler Thromb Vasc Biol. 2010;30:1795–1801. doi: 10.1161/ATVBAHA.109.200758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 11.Ferruzzi J, Collins MJ, Yeh AT, Humphrey JD. Mechanical assessment of elastin integrity in fibrillin-1-deficient carotid arteries: implications for Marfan syndrome. Cardiovasc Res. 2011;92(2):287–95. doi: 10.1093/cvr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starcher B, Conrad M. A role for neutrophil elastase in the progression of solar elastosis. Connect Tissue Res. 1995;31:133–140. doi: 10.3109/03008209509028401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.