Abstract
Agents that can potentiate the efficacy of standard chemotherapy against pancreatic cancer are of great interest. Because of their low cost and safety, patients commonly use a variety of dietary supplements, although evidence of their efficacy is often lacking. One such commonly used food supplement, Zyflamend, is a polyherbal preparation with potent anti-inflammatory activities, and preclinical efficacy against prostate and oral cancer. Whether Zyflamend has any efficacy against human pancreatic cancer alone or in combination with gemcitibine, a commonly used agent, was examined in cell cultures and in an orthotopic mouse model. In vitro, Zyflamend inhibited the proliferation of pancreatic cancer cell lines regardless of p53 status and also enhanced gemcitabine-induced apoptosis. This finding correlated with inhibition of NF-κB activation by Zyflamend and suppression of cyclin D1, c-myc, COX-2, Bcl-2, IAP, survivin, VEGF, ICAM-1, and CXCR4. In nude mice, oral administration of Zyflamend alone significantly inhibited the growth of orthotopically transplanted human pancreatic tumors, and when combined with gemcitabine, further enhanced the antitumor effects. Immunohistochemical and Western blot analyses of tumor tissue showed that the suppression of pancreatic cancer growth correlated with inhibition of proliferation index marker (Ki-67), COX-2, MMP-9, NF-κB, and VEGF. Overall, these results suggest that the concentrated multiherb product Zyflamend alone can inhibit the growth of human pancreatic tumors and, in addition, can sensitize pancreatic cancers to gemcitabine through the suppression of multiple targets linked to tumorigenesis.
Keywords: Zyflamend, pancreatic cancer, inflammation
Introduction
Pancreatic cancer (PaCa), one of the most lethal malignant diseases, has a five-year survival, even with the best treatment available today, of about 5%. More than 35,000 men and women in the United States died of pancreatic cancer in 2009 1. Gemcitabine and erlotinib are the only agents that have been approved by the FDA for treatment of this cancer. However, they produce responses in <10% of patients and are associated with multiple adverse events and the development of drug resistance. Therefore, the need to think “out of the box” for treatment options against this lethal disease is needed. There are known to be around 25,431 human genes, out of which 2995 have been linked with 153 different biochemical pathways; about 350 genes have been specifically linked with cancer development and metastases. Thus, mono-targeted or “smart” drugs and single chemical entities are unlikely to treat diseases as complex as cancer. Rather, therapies that are multi-targeted, are needed. In fact, most of the drugs recently approved by the FDA are multi-targeted and these include sunitinib, sorafenib, and vandetanib.
Activation of the transcription factor nuclear factor-kappaB (NF-κB) has been linked with tumorigenesis and chemoresistance in most tumors 2, 3 including pancreatic cancer. Evidence indicates that NF-κB is constitutively active in pancreatic cancer cells 4 but not in immortalized, nontumorigenic pancreatic ductal epithelial cells 5. NF-κB activation has been reported in animal models of pancreatic cancer 6 and in human pancreatic cancer tissue 4. NF-κB promotes pancreatic cancer growth in part by opposing apoptosis 4, 7 and mediates the induction of mitogenic gene products 8. These specific genes are over-expressed in human pancreatic cancer tissue and are inversely correlated with patient survival 9. Additionally, activation of NF-κB enhances the angiogenic potential of pancreatic cancer cells via increased expression of proangiogenic factors 10, while other NF-κB-regulated gene products promote the migration and invasion of the tumor 11. NF-κB has also been linked with gemcitabine resistance in pancreatic cancer 3. Together, these findings implicate a role of activated NF-κB in development of pancreatic cancer and suggest that agents that block this pathway might inhibit tumor growth, and may also sensitize cells to gemcitabine.
The inability of standard chemotherapy regimens to improve the prognosis of pancreatic cancer has led to reconsideration of the potential of traditional medicines. Over 63% of anticancer drugs introduced over the last 25 years are natural products or can be traced back to a natural products source 12. Butler showed that 79 drugs between 2005–2007 that were entered into clinical trials as anticancer agents were natural products or natural product analogues 13. Numerous agents have been identified from isolated nutrients, herbal products, and dietary supplements with potential to modulate physiological functions and critical biological activities. Although safe and relatively inexpensive, their effectiveness against cancer is uncertain and thus requires investigation. Zyflamend is a polyherbal formulation comprised of 10 standardized, concentrated herbal extracts (rosemary, turmeric, ginger, holy basil, green tea, hu zhang, Chinese goldthread, barberry, oregano, and baikal skullcap). It is a successful food supplement sold for support of a wide variety of ailments that commonly involve inflammation and/or pain. Each of these herbs contains unique constituents that have been reported to possess anti-inflammatory and anticancer activities through modulation of different targets 14–24. But the exact mechanisms by which Zyflamend mediates its anti-cancer effect are poorly understood. Recent published reports suggest that Zyflamend can suppress cyclooxygenase (COX)-1 and COX-2 activities in human prostate cancer cells 25–28, inhibit 5-lipooxygenase (5-LOX) and prevent 7,12-dimethylbenz[α]anthracenene (DMBA)-induced oral carcinogenesis in a hamster cheek pouch model of oral carcinoma 29. Studies from our laboratory have reported Zyflamend’s ability to suppress NF-κB cell signaling pathway and NF-κB regulated gene products 30, mechanisms that underlie the effectiveness of many traditional anticancer medicines 31, 32.
The possibility that Zyflamend may potentiate the effect of chemotherapeutic agents such as gemcitabine, makes this product all the more intriguing as a part of pancreatic cancer therapy. In the present study, we investigated whether Zyflamend alone can inhibit the growth of human pancreatic cancer tumors in cell culture and in orthotopic mouse models; and whether Zyflamend can sensitize the tumors to gemcitabine. We demonstrated that Zyflamend inhibits the in vitro proliferation of various pancreatic cancer cells, enhances gemcitabine-induced apoptosis, and potentiates the antitumor activity of gemcitabine against orthotopically implanted human pancreatic tumors through downregulation of various biomarkers of this disease.
Materials and Methods
Materials
Zyflamend, that contains holy basil (12.8%), turmeric (14.1%), ginger (12.8%), green tea (12.8%), rosemary (19.2%), Hu zhang (10.2%), barberry (5.1%), oregano (5.1%), baikal skullcap (2.5%) and Chinese goldthread (5.1%); was kindly supplied by New Chapter, Inc. (Brattleboro, VT). It was dissolved in dimethyl sulfoxide (DMSO) as a 100 mg/ml stock solution and stored at −20 °C. The following polyclonal antibodies against p65 (recognizing the epitope within the NH2-terminal domain of human NF-κB p65) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA): intercellular adhesion molecule-1 (ICAM-1), cyclin D1, matrix metalloproteinase 9 (MMP-9), survivin, cellular inhibitor of apoptosis protein 1 (cIAP-1), procaspase-3, and procaspase-9. Also obtained from Santa Cruz Biotechnology were monoclonal antibodies against COX-2, c-myc, Bcl-2, and Bcl-xL. Antibodies against VEGF and Ki-67 were purchased from Thermo Fisher Scientific (Fremont, CA). The liquid DAB+ Substrate Chromogen System-HRP used for immunocytochemistry was obtained from Dako (Carpinteria, CA). Penicillin, streptomycin, RPMI 1640, and fetal bovine serum (FBS) were obtained from Invitrogen (Grand Island, NY). Tris, glycine, NaCl, sodium dodecyl sulfate (SDS), and bovine serum albumin (BSA) were obtained from Sigma Chemical (St. Louis, MO). Gemcitabine (Gemzar; kindly supplied by Eli Lilly, Indianapolis, IN) was stored at 4°C and dissolved in sterile phosphate-buffered saline (PBS) on the day of use.
Cell lines
The pancreatic cancer cell lines AsPC-1, BxPC-3, MIA PaCa-2, and PANC-1 were obtained from the American Type Culture Collection (Manassas, VA). All cell lines were cultured in RPMI 1640 supplemented with 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin. The above-mentioned cell lines were procured more than 6 months ago and have not been tested recently for authentication in our laboratory. The human pancreatic duct epithelial (HPDE) cells were a generous gift from Dr. Ming-Sound Tsao (University of Toronto, Ontario, Canada). These cells were cultured in keratinocyte growth medium (KGM) supplied with 5 ng/mL epidermal growth factor (EGF) and 50 μg/mL bovine pituitary extract (Lonza, Walkersville, MD). The mouse embryonic fibroblast (MEF) derived from p65 −/− C57Bl/6J mice and its wild type were kindly provided by Dr. David Baltimore (California Institute of Technology, Pasadena, CA). Cells were cultured in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Proliferation assay
The effect of Zyflamend on cell proliferation was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) uptake method as described previously 33. Cells (2,000 cells/well) were incubated with Zyflamend in triplicate in a 96-well plate and then incubated for 2, 4, or 6 days at 37°C. An MTT solution was added to each well and incubated for 2 h at 37°C. An extraction buffer (20% SDS and 50% dimethylformamide) was added, and the cells were incubated overnight at 37°C. The absorbance of the dissolved cell suspension was measured at 570 nm with an MRX Revelation 96-well multiscanner (Dynex Technologies, Chantilly, VA).
Apoptosis assay
To determine whether Zyflamend could potentiate the apoptotic effects of gemcitabine in pancreatic cancer cells, we used a LIVE/DEAD cell viability assay kit (Invitrogen), which is used to determine intracellular esterase activity and plasma membrane integrity 34. Briefly, cells (5,000/well) were incubated in chamber slides, pretreated with Zyflamend solution for 4 h, and treated with gemcitabine for 24 h. Cells were then stained with the assay reagents for 30 min at room temperature. Cell viability was determined under a fluorescence microscope by counting live (green) and dead (red) cells.
Annexin V assay
One of the early indicators of apoptosis is the rapid translocationand accumulation of the membrane phospholipid phosphatidylserinefrom the cell’s cytoplasmic interface to the extracellular surface. This loss of membrane asymmetry can be detected using the bindingproperties of annexin V. To detect apoptosis, we used annexinV antibody conjugated with the fluorescent dye fluorescein isothiocyanate(FITC). Briefly, cells (1 ×106) were treated with Zyflamend for 24 h, and then subjected to annexin V and propidium iodide (PI) staining. Cells were washed, stained with FITC-conjugated anti-annexin V antibody, and then analyzed with a flow cytometer (FACSCalibur, BD Biosciences).
Cell-cycle distribution
To determine the effect of Zyflamend on the cell cycle, cells were treated with Zyflamend for 24 h, and then were stained with PI as mentioned earlier 35.
Animals
Male athymic nu/nu mice (6–8 weeks old) were obtained from the breeding colony of the Department of Experimental Radiation Oncology at M. D. Anderson Cancer Center. The animals were housed in standard plexiglass cages (five per cage) in a room maintained at constant temperature and humidity and in a 12 h:12 h light-dark cycle. Their diet consisted of regular autoclave chow and water ad libitum. None of the mice exhibited any lesions, and all were tested pathogen-free. Before initiating the experiment, all of the mice were acclimatized to a standard rodent chow pulverized diet for 7 days. Our experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee at M.D. Anderson Cancer Center.
Orthotopic implantation of MIA PaCa-2 cells
MIA PaCa-2 cells were stably transfected with luciferase as previously described for PANC-1 cells 36. The MIA PaCa-2 cells were orthotopically implanted as described previously 34. Briefly, luciferase-transfected MIA PaCa-2 cells were harvested from subconfluent cultures after a brief exposure to 0.25% trypsin and 0.2% ethylenediaminetetraacetic acid (EDTA). Trypsinization was stopped with medium containing 10% FBS. The cells were washed once in serum-free medium and resuspended in PBS. Only suspensions consisting of single cells, with >95% viability, were used for the injections. After mice were anesthetized with ketamine-xylazine solution, a small incision was made in the left abdominal flank, and MIA PaCa-2 cells (1 ×106) in 50 μL PBS were injected into the subcapsular region of the pancreas with a 27-gauge needle and a calibrated push button-controlled dispensing device (Hamilton Syringe Co., Reno, NV). A cotton swab was held for 1 min over the site of injection to prevent leakage. The abdominal wound was closed in one layer with wound clips (Braintree Scientific, Inc., Braintree, MA).
Experimental protocol
One week after tumor implantation, mice were randomized into the following treatment groups (n = 6/group) based on the bioluminescence first measured with an in vivo imaging system (IVIS 200, Xenogen Corp., Alameda, CA): (a) untreated control (olive oil, 100 μL by gavage, daily); (b) Zyflamend (1g/kg once daily orally [p.o.]); (c) gemcitabine alone (25 mg/kg twice weekly by intraperitoneal [i.p.] injection); and (d) combination (Zyflamend, 1g/kg once daily p.o., and gemcitabine, 25 mg/kg twice weekly by i.p. injection). Tumor volumes were monitored weekly with the bioluminescence IVIS, which includes a cryogenic cooling unit, and a data acquisition computer running Living Image software (Xenogen Corp.). Before imaging, the animals were anesthetized in an acrylic chamber with 2.5% isoflurane/air mixture and injected i.p. with 40 mg/mL D-luciferin potassium salt in PBS at a dose of 150 mg/kg body weight. After 10 min of incubation with luciferin, each mouse was placed in a right lateral decubitus position and a digital grayscale animal image was acquired, followed by the acquisition and overlay of a pseudo-color image representing the spatial distribution of detected photons emerging from active luciferase within the animal. Signal intensity was quantified as the sum of all detected photons within the region of interest per second. The mice were subjected to imaging on days 0, 7, 16, 22, and 29 of treatment. Therapy was continued for 4 weeks, and the animals were euthanized 1 week later. Primary tumors in the pancreas were excised, and the final tumor volume was measured as V = 2/3πr3, where r is the mean of the three dimensions (length, width, and depth). Half of the tumor tissue was fixed in formalin and embedded in paraffin for immunohistochemistry and routine hematoxylin and eosin (H&E) staining. The other half was snap frozen in liquid nitrogen and stored at −80°C. H&E staining confirmed the presence of tumor(s) in each pancreas.
NF-κB activation in pancreatic cancer cells
To assess NF-κB activation, we isolated nuclei from pancreatic cancer cell lines and carried out electrophoretic mobility shift assays (EMSA) essentially as described previously 34. Briefly, nuclear extracts prepared from pancreatic cancer cells (1 ×106/mL) and tumor samples were incubated with 32P-end-labeled 45-mer double-stranded NF-κB oligonucleotide (4 μg of protein with 16 fmol of DNA) from the HIV long terminal repeat (5′-TTGTTACAAGGGACTTTCCGCTGGGGACTTTCCAGG GGGAGGCGTGG-3′; underline indicates NF-κB–binding sites) for 15 min at 37°C. The resulting DNA-protein complex was separated from free oligonucleotide on 6.6% native polyacrylamide gels. The dried gels were visualized, and radioactive bands were quantitated with a PhosphorImager and ImageQuant software (GE Healthcare, Piscataway, NJ).
Immunolocalization of NF-κB p65, COX-2, VEGF and MMP-9 in tumor samples
The nuclear localization of p65 and the expression of COX-2 and VEGF were examined via an immunohistochemical method described previously 37. Briefly, pancreatic cancer tumor samples were embedded in paraffin and fixed with paraformaldehyde. After being washed in PBS, the slides were blocked with protein block solution (Dako) for 20 min and then incubated overnight with rabbit polyclonal anti-human p65, mouse monoclonal anti-human VEGF, and anti-COX-2 antibodies (1:400, 1:50, and 1:75, respectively). After incubation with the antibodies, the slides were washed and then incubated with biotinylated link universal antiserum followed by horseradish peroxidase-streptavidin detection with the LSAB+ kit (Dako). The slides were rinsed, and color was developed with 3,3′-diaminobenzidine hydrochloride used as a chromogen. Finally, sections were rinsed in distilled water, counterstained with Mayer’s hematoxylin, and mounted with DPX mounting medium (Sigma) for evaluation. Pictures were captured with a Photometrics CoolSNAP CF color camera (Nikon, Lewisville, TX) and MetaMorph software version 4.6.5 (Universal Imaging, Downingtown, PA).
Ki-67 immunohistochemistry
Formalin-fixed, paraffin-embedded sections (5 μm) were stained with anti-Ki-67 (rabbit monoclonal clone SP6) antibody as described previously 34. Results were expressed as the percentage of Ki-67+ cells ±standard error per ×40 magnification. A total of ten 40×fields were examined and counted from three tumors of each of the treatment groups.
Western blot analysis
Pancreatic cancer cells were harvested and incubated on ice for 30 min in 0.5 mL of ice-cold whole-cell lysate buffer (10% NP40, 5 mol/L NaCl, 1 mol/L HEPES, 0.1 mol/L EGTA, 0.5 mol/L EDTA, 0.1 mol/L PMSF, 0.2 mol/L sodium orthovanadate, 1 mol/L NaF, 2 μg/mL aprotinin, 2 μg/mL leupeptin). The proteins were then separated by SDS-PAGE, electrotransferred to nitrocellulose membranes, blotted with each antibody, and detected by enhanced chemiluminescence reagents (GE Healthcare).
Statistics
In vitro experiments were repeated twice. The values were initially subjected to one-way analysis of variance (ANOVA), which revealed significant differences between groups, and then they were compared between groups with an unpaired Student’s t-test, which revealed significant differences between two sample means. Values from in vivo experiments were initially subjected to one-way ANOVA and then later compared among groups with an unpaired Student’s t-test.
Results
This study was designed to determine whether Zyflamend a) could inhibit the growth of human pancreatic cancer cells; b) could enhance the antitumor effects of gemcitabine in vitro; and c) could potentiate the effects of a chemotherapeutic agent in vivo in models of human pancreatic cancer. In addition, we sought to explore potential mechanism(s) by which Zyflamend might enhance the effects of the chemotherapeutic agent. We used four different human pancreatic cancer cell lines-AsPC-1, BxPC-3, MIA PaCa-2, and PANC-1-of different origins for this investigation. To monitor tumor growth in vivo, we used noninvasive imaging of the luciferase-transfected MIA PaCa-2 cell line.
Zyflamend inhibits the proliferation of pancreatic cancer cells in vitro
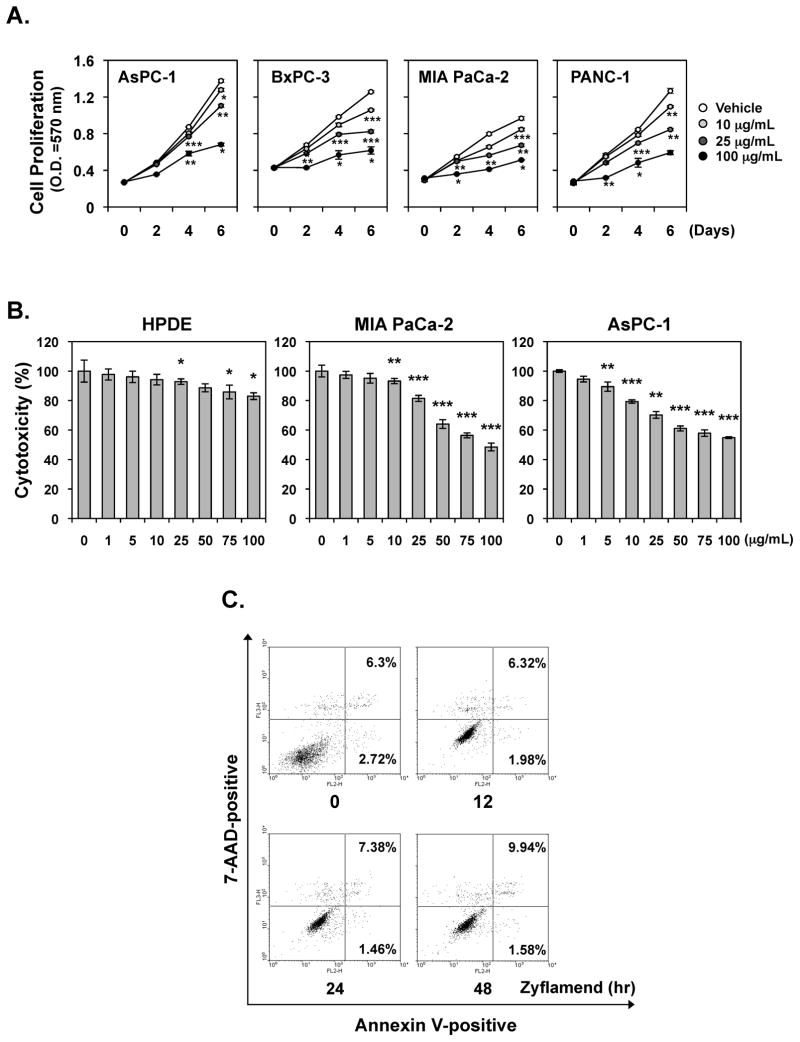
We examined whether Zyflamend could inhibit the proliferation of human pancreatic cancer cell lines AsPC-1, PANC-1, BxPC-3, and MIA PaCa-2. Cell lines were treated with different doses of Zyflamend for various time periods following which the inhibition of proliferation was determined by examining mitochondrial activity via the MTT uptake method. As shown in Fig. 1A, Zyflamend suppressed the proliferation of all four pancreatic cancer cell lines in a concentration-dependent manner, irrespective of the cell genetic background (Fig. 1A). Proliferation of pancreatic cancer cells was also significantly suppressed by 25 μg/mL dose, while 10 μg/mL had minimal effect. MIA PaCa-2 and BxPC-3 cell lines were more sensitive to Zyflamend than AsPC-1 or PANC-1 (Fig. 1A).
Figure 1. Zyflamend inhibits proliferation, induces apoptosis and cell cycle arrest in pancreatic cancer cells in vitro.
(A) Zyflamend inhibits the proliferation of pancreatic cancer cells. MTT assay results showed dose-dependent suppression of cell proliferation in all four pancreatic cancer cell lines tested. Points, mean of triplicate; bars, SE. (B) Cells (2000 cells/well) were seeded in triplicate onto 96-well plates, treated with the indicated concentration of Zyflamend for 72 h, and then measured cell viability by the MTT method and presented as percent cell viability. Points, mean of triplicate; bars, SE (*P < 0.05, **P < 0.01, ***P < 0.001 vs. untreated cells). (C) MIA PaCa-2 cells were incubated with the indicated concentration of Zyflamend for 24 h and then incubated with anti-annexin V antibody conjugated with fluorescein isothiocyanate and then analyzed with a flow cytometer for early apoptotic effects.
The anti-proliferative effect of Zyflamend on non-tumorigenic pancreatic ductal cell was also examined. As shown in Fig. 1B, in pancreatic cancer cell lines, MIA PaCa-2 and AsPC-1, Zyflamend suppressed viability in a dose-dependent manner but had minimal effect on human pancreatic ductal epithelial (HPDE) cells, suggesting the effect of Zyflamend was more specific to tumor cells.
Zyflamend induces apoptosis and cell cycle arrest in pancreatic cancer cells
How Zyflamend induces growth inhibition was examined. MIA PaCa-2 were treated with different doses of Zyflamend for 24 h following which the apoptosis and cell cycle arrest were determined by flow cytometry analysis. As shown in Fig. 1C, Zyflamend increased annexin V-positive cells in a dose-dependent manner from 7% to 35%.
We next examined whether Zyflamend could affect cell cycle distribution. We found that after 24 h treatment with Zyflamend, a significant accumulation of cells in G2-M phase was observed (Supplement Table 1).
Zyflamend sensitizes human pancreatic cancer cells to gemcitabine
Gemcitabine is a standard treatment for patients with pancreatic cancer. To determine whether Zyflamend enhances the apoptotic effects of gemcitabine in pancreatic cell lines, we performed the Live/Dead assay. Zyflamend (10μg/mL) and gemcitabine (200 nM) alone had minimally toxic effects, but the two together induced a high level of apoptosis in pancreatic cancer cell lines in vitro (Fig. 2). No significant difference was observed between the cell lines with respect to their sensitivity to the combination.
Figure 2. Zyflamend potentiates the apoptotic effects of gemcitabine in pancreatic cancer cells in vitro.
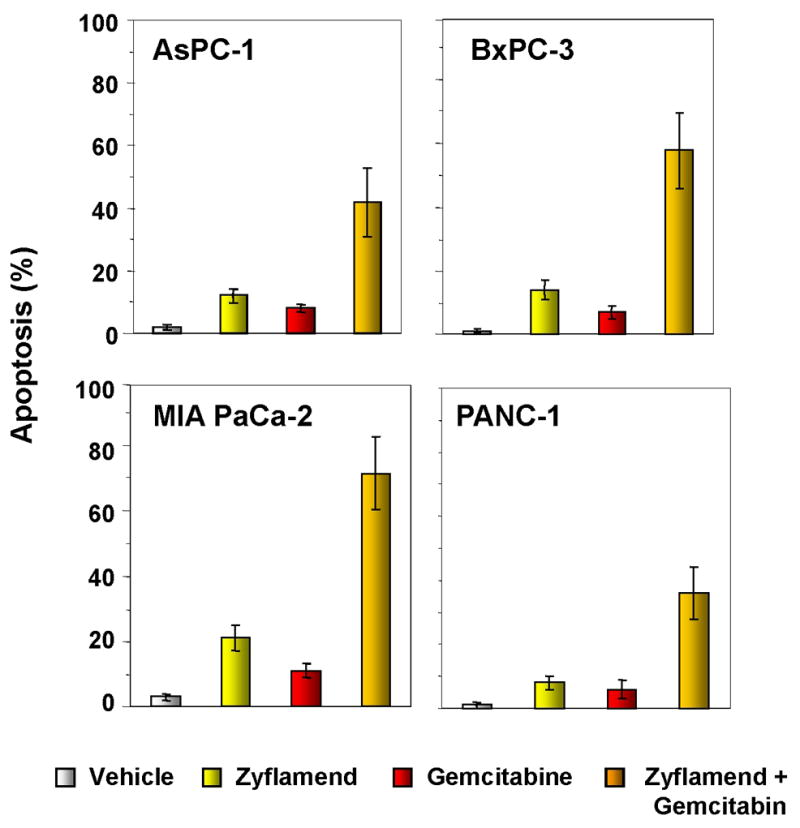
Live/Dead assay results indicated that Zyflamend potentiates gemcitabine-induced cytotoxicity. Percentages, proportions of apoptotic pancreatic cancer cells. Data are representative of two independent experiments.
Zyflamend inhibits constitutive NF-κB activation and proteins associated with inflammation, proliferation, invasion, and angiogenesis in pancreatic cancer cells
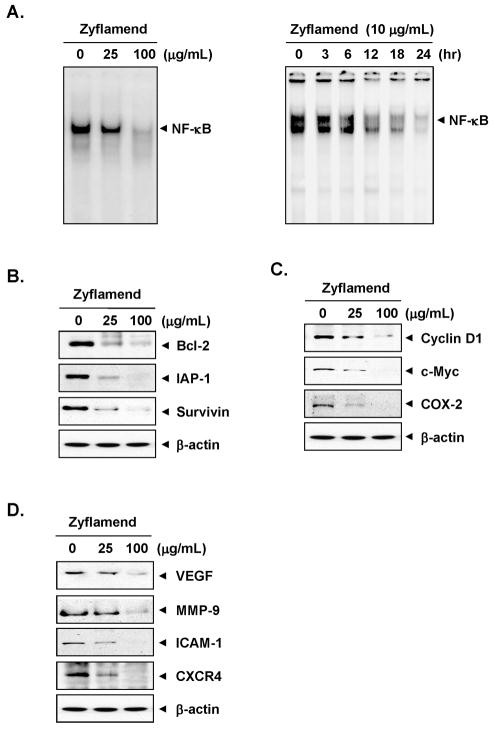
Because NF-κB has been linked with both proliferation and chemoresistance, we next examined whether Zyflamend could inhibit constitutive NF-κB activation in MIA PaCa-2 cell lines. Our results showed that this multi-herb product inhibited constitutive NF-κB activation (Fig. 3A, left panel).
Figure 3. Zyflamend inhibits constitutive activation of NF-κB and various biomarkers in vitro.
(A, left), MIA PaCa-2 (1 × 106) cells were treated with Zyflamend (25 and 100 μg/mL) for 4 h, nuclear extracts were prepared and then assayed for NF-κB activation by EMSA; (Left) MIA PaCa-2 cells were incubated with Zyflamend (10 μg/mL) for indicated time, nuclear extracts were prepared and then assayed for NF-κB activation by EMSA. (B–D), Western blot analysis showed that Zyflamend inhibited constitutive expression of NF-κB-regulated gene products that regulate apoptosis (B), proliferation (C), and metastasis and angiogenesis (D) in pancreatic cancer cells. The MIA PaCa-2 (1 × 106) cells were treated with Zyflamend (25 and 100 μg/mL) for 24 h. Whole-cell lysates were prepared and assayed for NF-κB-regulated gene products by Western blotting. Data represent two independent experiments.
The dose of Zyflamend employed to examine its effect with gemcitabine, was 10 μg/mL. Whether this dose can suppress constitutive NF-κB activation, was examined. For this, MIA PaCa-2 cells were exposed to 10 μg/mL Zyflamend for different times and then examined for NF-κB. We found that Zyflamend suppressed constitutive NF-κB activation in a time-dependent manner in these cells (Fig. 3A, right panel).
Next, we investigated the effects of Zyflamend on the constitutive expression of antiapoptotic proteins Bcl-2, IAP-1, and survivin. The expression of all these proteins was downregulated by Zyflamend in a concentration-dependent manner (Fig. 3B). The same was true for the constitutive expression of proliferation-associated proteins cyclin D1, c-myc, and COX-2 (Fig. 3C). Likewise, Zyflamend downregulated the expression of proteins linked to invasion, adhesion, and angiogenesis--MMP-9, ICAM-1, VEGF, and CXCR4--in a concentration-dependent manner (Fig. 3D).
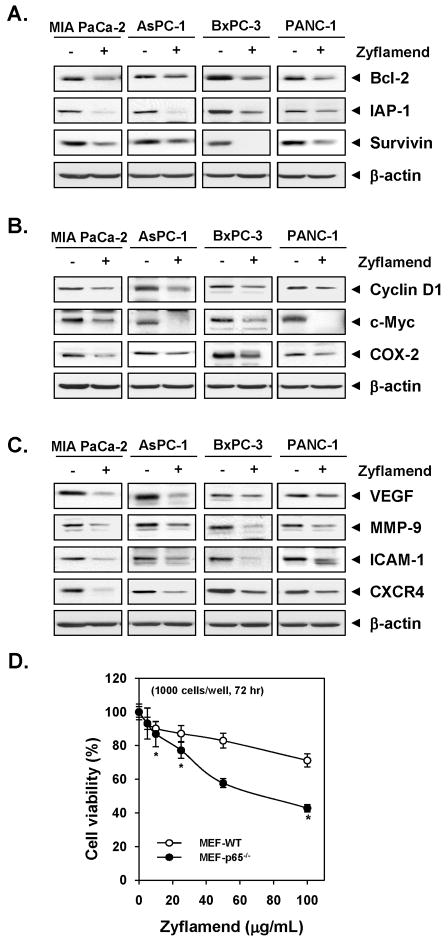
Zyflamend downmodulates NF-κB-regulated gene products involve in inflammation, proliferation, invasion, and angiogenesis in all pancreatic cancer cell lines
Up to this point, our all studies were carried out with MIA PaCa-2 cells. Whether Zyflamend downregulates expression of inflammation, proliferation, invasion, and angiogenesis-associated gene products in other pancreatic cancer cells, was examined. Cells were treated with 100μg/mL Zyflamend for 24 h and then examined for gene expression by Western blot analysis. Zyflamend downregulated the expression of antiapoptotic proteins (Bcl-2, IAP-1 and survivin) in all four different pancreatic cancer cell lines (Fig. 4A). The expression proteins associated with cell proliferation (cyclin D1, c-myc, and COX-2), invasion, and angiogenesis (MMP-9, ICAM-1, VEGF, and CXCR4), was also suppressed by Zyflamend in all four different pancreatic cancer cell lines (Fig. 4B and C).
Figure 4. Zyflamend downmodulates expression of proteins associated with inflammation, proliferation, invasion, and angiogenesis in pancreatic cancer cells.
(A–C) Cells (1 × 106) cells were treated with Zyflamend (100 μg/mL) for 24 h, whole-cell lysates were prepared and subjected to Western blotting, using antibodies as indicated. Data represent two independent experiments. (D) The wild-type and p65−/− deficient (1 × 103) cells were treated with indicated concentrations of Zyflamend for 72 h, and then measured cell viability by the MTT method. Results are presented as percent cell viability. Points, mean of triplicate; bars, SE (*P < 0.05 vs. wild-type cells).
Deletion of NF-κB enhances the effect of Zyflamend on cell proliferation
Whether the effect of Zyflamend is mediated through inhibition of NF-κB was examined by using p65 −/− cells. Results showed that p65 −/− cells that lack NF-κB were more sensitive to Zyflamend as compared to wild type cells (Fig. 4D). Overall, these results indicated that NF-κB activation plays an essential role in tumor cell proliferation.
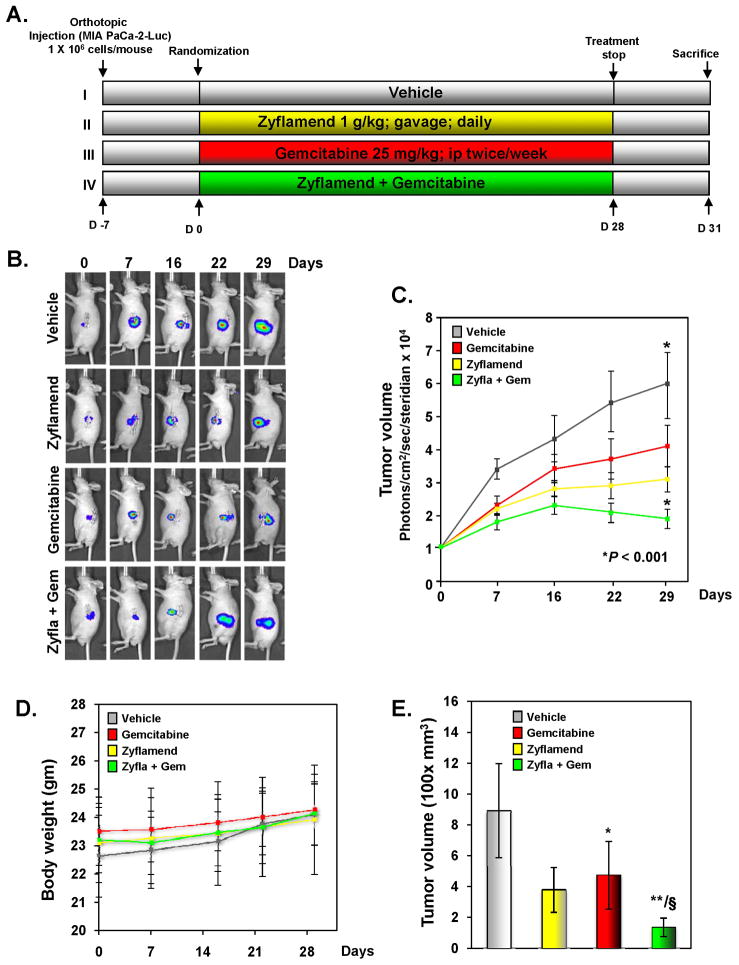
Zyflamend potentiates the antitumor activity of gemcitabine in an orthotopic pancreatic tumor model in nude mice
Based on our in vitro results, we designed studies to determine the effects of Zyflamend on gemcitabine in orthotopically implanted human pancreatic tumors in nude mice (Fig. 5A).
Figure 5. Zyflamend potentiates the effect of gemcitabine in blocking the growth of pancreatic cancer in nude mice.
(A), Schematic of experimental protocol described in Materials and Methods. Group I was given olive oil (100 μL, p.o., daily) and PBS (100 μL, i.p., twice weekly), group II was given Zyflamend (1g/kg in 100 μL olive oil, p.o., daily), group III was given gemcitabine (25 mg/kg in 100 μL PBS, i.p., twice weekly), and group IV was given Zyflamend (1g/kg in 100 μL olive oil, p.o., daily) and gemcitabine (25 mg/kg in 100 μL PBS, i.p., twice weekly). (B), Bioluminescence IVIS images of orthotopically implanted pancreatic tumors in live, anesthetized mice. (C), Measurements of photons per second depicting tumor volume at various time points using live IVIS imaging at the indicated times (n = 6). Points, mean; bars, SE (*P < 0.05 vs. vehicle). (D), Body weight changes of mice was measured at indicated times (n = 6). Points, mean; bars, SE. (E), Tumor volumes in mice measured on the last day of the experiment at autopsy with vernier calipers and calculated using the formula V = 2/3πr3 (n = 6). Columns, mean; bars, SE (*P < 0.05, **P < 0.01 vs. vehicle; §P < 0.05 vs. Zyflamend alone).
Luciferase-transfected MIA PaCa-2 cells were implanted in the tails of the pancreas in nude mice. One week later, based on the IVIS imaging, the mice were randomized into four groups, at which point treatment with Zyflamend or gemcitabine alone or Zyflamend plus gemcitabine was started; treatment was continued for 4 weeks. The animals were euthanized 38 days after tumor cell injection and 31 days from the date of treatment (Fig. 5A). To determine tumor development and the effects of Zyflamend and gemcitabine, we assessed the tumors with the bioluminescence IVIS on days 7, 16, 22, and 29 after the start of treatment. The bioluminescence imaging results (Fig. 5B and C) indicated a gradual increase in tumor volume in the control group compared to the three treatment groups. The imaging results showed that the tumor volume in the combination treatment group was significantly lower than in the group treated with Zyflamend or gemcitabine alone as well as in the vehicle-treated control group (P < 0.001 versus gemcitabine; P < 0.001 versus vehicle). On the 42nd day, we euthanized the mice and measured the tumor volume with vernier calipers. Additionally, there was no significant change in average animal weight between the vehicle group and Zyflamend-treated groups (Fig. 5D), suggesting lack of toxicity. The results were in concordance with those from bioluminescence imaging and showed that the combination treatment reduced the tumor volume more than in the other three groups (Fig. 5E). The reduction of tumor volumes in combination treatment group was statistically significant when compared with Zyflamend-treated group (P < 0.05). The imaging result also revealed that Zyflamend alone was at least as active as gemcitabine alone in inducing tumor regression.
Zyflamend inhibits proliferative index in orthotopic pancreatic tumors
We next examined the expression of the cell proliferation marker Ki-67 in tumor tissues from the four groups. The results in Fig. 6A show that Zyflamend plus gemcitabine significantly downregulated the expression of Ki-67 in tumor tissues compared with the control group (P < 0.001 versus vehicle). The results also showed that Zyflamend alone significantly suppressed the expression of Ki-67 (P < 0.005 versus vehicle; Fig. 6B). The decrease of proliferation index Ki-67 in combination group was statistically significant when compared with gemcitabine alone group (P = 0.001)
Figure 6. Zyflamend inhibits the expression of various biomarkers and further enhances the effect of gemcitabine in pancreatic cancer.
(A) Immunohistochemical analysis of NF-κB-regulated genes COX-2, VEGF, and MMP-9 in pancreatic tumor tissues from mice. Percentages indicate positive staining for the given biomarker. Samples from three animals in each group were analyzed, and representative data are shown. (B), Mechanism of action of Zyflamend against pancreatic tumors.
Zyflamend inhibits NF-κB, COX2, VEGF, and MMP-9 in orthotopic pancreatic tumors
Immunohistochemical analysis showed that Zyflamend alone suppressed various biomarkers in orthotopically grown human pancreatic tumor samples (Fig. 6C). Zyflamend also further suppressed NF-κB activation in gemcitabine-treated tissues. Immunohistochemical analysis further demonstrated that Zyflamend suppressed the expression of COX-2 (Fig. 6C, second panel), VEGF (Fig. 6C, second panel), and MMP-9 (Fig. 6C, bottom panel) in human pancreatic tumor tissues, and this effect was enhanced in tissues treated with gemcitabine.
Discussion
Zyflamend is a polyherbal preparation taken by many people to prevent as well as treat various chronic diseases that typically involve inflammation. Although pharmacologically safe and affordable, the ability of Zyflamend to inhibit pancreatic cancer has not previously been established. Whether this agent can affect the activity of standard chemotherapy used by cancer patients is also not clear. In the present study we used pancreatic cancer models to examine the activity of Zyflamend alone and in combination with chemotherapeutic agent. We selected this cancer because pancreatic cancer as it is one of most lethal types of malignant disease with an estimated 5-year survival rate of around 4% despite the best treatment available today.
We found that Zyflamend alone suppressed the proliferation of various pancreatic cancer cell lines including PANC-1, MIA PaCa-2, AsPC-1, and BxPC-3 cells. This is the first report to describe the antiproliferative effects of this agent against PaCa cells. How Zyflamend mediates an antiproliferative effect also was investigated in detail. We found that Zyflamend suppressed the expression of antiapoptic (Bcl-2, IAP-1, and survivin) and cell proliferative proteins (cyclin D1, c-myc, and COX-2). Consistent with these effects, Zyflamend also suppressed the constitutive activation of NF-κB in pancreatic tumor cell lines. These results on suppression of NF-κB by Zyflamend are in agreement with a previous report on other tumor cell lines 30 and with reports that berberine, resveratrol, epigallocatechins, gingerol, curcumin and ursolic acid, all present within Zyflamend, are linked with NF-κB suppression 14, 17, 19, 24, 38.
We also found that Zyflamend can enhance the apoptotic effect of gemcitabine in various pancreatic cancer cell lines in vitro. We found that this effect may be mediated through the down-regulation of cell survival proteins such as Bcl-2, IAP-1, and survivin. Moreover, constitutive activation of NF-κB expressed in human pancreatic cancer cell lines has been linked with chemoresistance 3, and the suppression of NF-κB activation can also sensitize cells to chemotherapeutic agents. These results are consistent with those previously reported for curcumin 34 and resveratrol 39.
Interestingly, oral administration of Zyflamend alone inhibited the growth of human pancreatic tumors in an orthotopic nude mouse model. Tumor growth was inhibited by almost 50% by 1g/kg Zyflamend alone, and this level of inhibition was comparable to that produced by gemcitabine alone. Zyflamend was very well tolerated by the animals. When the two agents were used in combination, they were even more effective.
How Zyflamend exhibited its effects in vivo was also investigated in several ways. First, Ki-67 expression in tumor tissues, a measure of proliferation, was inhibited by Zyflamend. Likewise, Zyflamend alone inhibited constitutive activation of NF-κB. Zyflamend significantly down-regulated the expression of proinflammatory enzyme COX-2, suppressed the expression of invasion biomarker MMP-9, and inhibited the angiogenic biomarker VEGF in tissues. All of these effects were further enhanced by gemcitabine as indicated by immunohistochemical analysis. These data indicate for the first time the mechanisms by which Zyflamend may exert its effects against human pancreatic cancer.
Our results are in agreement with a previous report describing Zyflamend’s anticancer activities against oral cancer in a hamster cheek pouch model 29. Yang’s studies showed that reduction of leukotriene (LT) B4 generated by 5-LOX played a major role in the action of Zyflamend. Recently the effect of oral administration of Zyflamend (780 mg capsule 3 ×daily) was examined in men (23 subjects) with high-grade prostatic intraepithelial neoplasia 26–28, 40. Zyflamend was found to be safe, and after 18 months, 48% of the subjects showed a 2–50% decrease in prostate-specific antigen, and a reduction in serum C-reactive protein as well as a reduction in expression of NF-κB. We used 1g/kg dose in mice, which corresponds to 7 gram/day in man, thus, this dose is achievable in humans.
Overall, our results show for the first time that Zyflamend alone can inhibit the growth of human pancreatic tumors in mice and it can further potentiate the antitumor activity of gemcitabine by inhibiting various biomarkers of the disease, leading to the inhibition of proliferation. However, further studies are necessary to confirm our findings in patients with pancreatic cancer. Considering that Zyflamend can modulate multiple targets, it merits further exploration.
Supplementary Material
Acknowledgments
We thank Walter Pagel for carefully proof-reading the manuscript and providing valuable comments. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a grant from New Chapter, a core grant from the National Institutes of Health (CA-16 672), a program project grant from National Institutes of Health (NIH CA-124787-01A2), and a grant from the Center for Targeted Therapy of the Univ. of Texas M.D. Anderson Cancer Center.
Footnotes
No potential conflicts of interest were disclosed.
References
- 1.http://www.cancer.gov/cancertopics/types/pancreatic
- 2.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Arlt A, Gehrz A, Muerkoster S, Vorndamm J, Kruse ML, Folsch UR, Schafer H. Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243–51. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 4.Liptay S, Weber CK, Ludwig L, Wagner M, Adler G, Schmid RM. Mitogenic and antiapoptotic role of constitutive NF-kappaB/Rel activity in pancreatic cancer. Int J Cancer. 2003;105:735–46. doi: 10.1002/ijc.11081. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–27. [PubMed] [Google Scholar]
- 6.Fujioka S, Sclabas GM, Schmidt C, Frederick WA, Dong QG, Abbruzzese JL, Evans DB, Baker C, Chiao PJ. Function of nuclear factor kappaB in pancreatic cancer metastasis. Clin Cancer Res. 2003;9:346–54. [PubMed] [Google Scholar]
- 7.Greten FR, Weber CK, Greten TF, Schneider G, Wagner M, Adler G, Schmid RM. Stat3 and NF-kappaB activation prevents apoptosis in pancreatic carcinogenesis. Gastroenterology. 2002;123:2052–63. doi: 10.1053/gast.2002.37075. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–42. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kornmann M, Ishiwata T, Itakura J, Tangvoranuntakul P, Beger HG, Korc M. Increased cyclin D1 in human pancreatic cancer is associated with decreased postoperative survival. Oncology. 1998;55:363–9. doi: 10.1159/000011879. [DOI] [PubMed] [Google Scholar]
- 10.Xiong HQ, Abbruzzese JL, Lin E, Wang L, Zheng L, Xie K. NF-kappaB activity blockade impairs the angiogenic potential of human pancreatic cancer cells. Int J Cancer. 2004;108:181–8. doi: 10.1002/ijc.11562. [DOI] [PubMed] [Google Scholar]
- 11.Yebra M, Filardo EJ, Bayna EM, Kawahara E, Becker JC, Cheresh DA. Induction of carcinoma cell migration on vitronectin by NF-kappa B-dependent gene expression. Mol Biol Cell. 1995;6:841–50. doi: 10.1091/mbc.6.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–77. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 13.Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep. 2008;25:475–516. doi: 10.1039/b514294f. [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 15.Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, Howells L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–20. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 16.Paschka AG, Butler R, Young CY. Induction of apoptosis in prostate cancer cell lines by the green tea component, (−)-epigallocatechin-3-gallate. Cancer Lett. 1998;130:1–7. doi: 10.1016/s0304-3835(98)00084-6. [DOI] [PubMed] [Google Scholar]
- 17.Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;63:4375–83. [PubMed] [Google Scholar]
- 18.Atsumi T, Murakami Y, Shibuya K, Tonosaki K, Fujisawa S. Induction of cytotoxicity and apoptosis and inhibition of cyclooxygenase-2 gene expression, by curcumin and its analog, alpha-diisoeugenol. Anticancer Res. 2005;25:4029–36. [PubMed] [Google Scholar]
- 19.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509–19. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda K, Hibiya Y, Mutoh M, Koshiji M, Akao S, Fujiwara H. Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J Ethnopharmacol. 1999;66:227–33. doi: 10.1016/s0378-8741(98)00162-7. [DOI] [PubMed] [Google Scholar]
- 21.Kelm MA, Nair MG, Strasburg GM, DeWitt DL. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine. 2000;7:7–13. doi: 10.1016/S0944-7113(00)80015-X. [DOI] [PubMed] [Google Scholar]
- 22.Subbaramaiah K, Chung WJ, Michaluart P, Telang N, Tanabe T, Inoue H, Jang M, Pezzuto JM, Dannenberg AJ. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem. 1998;273:21875–82. doi: 10.1074/jbc.273.34.21875. [DOI] [PubMed] [Google Scholar]
- 23.Kim DS, Kim HR, Woo ER, Hong ST, Chae HJ, Chae SW. Inhibitory effects of rosmarinic acid on adriamycin-induced apoptosis in H9c2 cardiac muscle cells by inhibiting reactive oxygen species and the activations of c-Jun N-terminal kinase and extracellular signal-regulated kinase. Biochem Pharmacol. 2005;70:1066–78. doi: 10.1016/j.bcp.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 24.Kim SO, Kundu JK, Shin YK, Park JH, Cho MH, Kim TY, Surh YJ. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-kappaB in phorbol ester-stimulated mouse skin. Oncogene. 2005;24:2558–67. doi: 10.1038/sj.onc.1208446. [DOI] [PubMed] [Google Scholar]
- 25.Bemis DL, Capodice JL, Anastasiadis AG, Katz AE, Buttyan R. Zyflamend, a unique herbal preparation with nonselective COX inhibitory activity, induces apoptosis of prostate cancer cells that lack COX-2 expression. Nutr Cancer. 2005;52:202–12. doi: 10.1207/s15327914nc5202_10. [DOI] [PubMed] [Google Scholar]
- 26.Yang P, Cartwright C, Chan D, Vijjeswarapu M, Ding J, Newman RA. Zyflamend-mediated inhibition of human prostate cancer PC3 cell proliferation: effects on 12-LOX and Rb protein phosphorylation. Cancer Biol Ther. 2007;6:228–36. doi: 10.4161/cbt.6.2.3624. [DOI] [PubMed] [Google Scholar]
- 27.Rafailov S, Cammack S, Stone BA, Katz AE. The role of Zyflamend, an herbal anti-inflammatory, as a potential chemopreventive agent against prostate cancer: a case report. Integr Cancer Ther. 2007;6:74–6. doi: 10.1177/1534735406298843. [DOI] [PubMed] [Google Scholar]
- 28.Nelson MA. Inhibition of lipoxygenase activity: implications for the treatment and chemoprevention of prostate cancer. Cancer Biol Ther. 2007;6:237. doi: 10.4161/cbt.6.2.4120. [DOI] [PubMed] [Google Scholar]
- 29.Yang P, Sun Z, Chan D, Cartwright CA, Vijjeswarapu M, Ding J, Chen X, Newman RA. Zyflamend reduces LTB4 formation and prevents oral carcinogenesis in a 7,12-dimethylbenz[alpha]anthracene (DMBA)-induced hamster cheek pouch model. Carcinogenesis. 2008;29:2182–9. doi: 10.1093/carcin/bgn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandur SK, Ahn KS, Ichikawa H, Sethi G, Shishodia S, Newman RA, Aggarwal BB. Zyflamend, a polyherbal preparation, inhibits invasion, suppresses osteoclastogenesis, and potentiates apoptosis through down-regulation of NF-kappa B activation and NF-kappa B-regulated gene products. Nutr Cancer. 2007;57:78–87. doi: 10.1080/01635580701268295. [DOI] [PubMed] [Google Scholar]
- 31.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27:2712–25. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gullett NP, Ruhul Amin AR, Bayraktar S, Pezzuto JM, Shin DM, Khuri FR, Aggarwal BB, Surh YJ, Kucuk O. Cancer prevention with natural compounds. Semin Oncol. 2010;37:258–81. doi: 10.1053/j.seminoncol.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–10. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 34.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–61. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 35.Pandey MK, Sung B, Aggarwal BB. Betulinic acid suppresses STAT3 activation pathway through induction of protein tyrosine phosphatase SHP-1 in human multiple myeloma cells. Int J Cancer. 2010;127:282–92. doi: 10.1002/ijc.25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khanbolooki S, Nawrocki ST, Arumugam T, Andtbacka R, Pino MS, Kurzrock R, Logsdon CD, Abbruzzese JL, McConkey DJ. Nuclear factor-kappaB maintains TRAIL resistance in human pancreatic cancer cells. Mol Cancer Ther. 2006;5:2251–60. doi: 10.1158/1535-7163.MCT-06-0075. [DOI] [PubMed] [Google Scholar]
- 37.Kunnumakkara AB, Diagaradjane P, Anand P, Harikumar KB, Deorukhkar A, Gelovani J, Guha S, Krishnan S, Aggarwal BB. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int J Cancer. 2009;125:2187–97. doi: 10.1002/ijc.24593. [DOI] [PubMed] [Google Scholar]
- 38.Pandey MK, Sung B, Kunnumakkara AB, Sethi G, Chaturvedi MM, Aggarwal BB. Berberine modifies cysteine 179 of IkappaBalpha kinase, suppresses nuclear factor-kappaB-regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 2008;68:5370–9. doi: 10.1158/0008-5472.CAN-08-0511. [DOI] [PubMed] [Google Scholar]
- 39.Harikumar KB, Kunnumakkara AB, Sethi G, Diagaradjane P, Anand P, Pandey MK, Gelovani J, Krishnan S, Guha S, Aggarwal BB. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int J Cancer. 2010;127:257–68. doi: 10.1002/ijc.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Capodice JL, Gorroochurn P, Cammack AS, Eric G, McKiernan JM, Benson MC, Stone BA, Katz AE. Zyflamend in men with high-grade prostatic intraepithelial neoplasia: results of a phase I clinical trial. J Soc Integr Oncol. 2009;7:43–51. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.