SUMMARY
1. Curcumin is the active ingredient of the dietary spice turmeric and has been consumed for medicinal purposes for thousands of years. Modern science has shown that curcumin modulates various signaling molecules, including inflammatory molecules, transcription factors, enzymes, protein kinases, protein reductases, carrier proteins, cell survival proteins, drug resistance proteins, adhesion molecules, growth factors, receptors, cell-cycle regulatory proteins, chemokines, DNA, RNA, and metal ions.
2. Because of this polyphenol's potential to modulate multiple signaling molecules, it has been reported to possess pleiotropic activities. First shown to have anti-bacterial activity in 1949, curcumin has since been shown to have anti-inflammatory, anti-oxidant, pro-apoptotic, chemopreventive, chemotherapeutic, anti-proliferative, wound healing, anti-nociceptive, anti-parasitic, and anti-malarial properties as well. Animal studies have suggested that curcumin may be active against a wide range of human diseases, including diabetes, obesity, neurologic and psychiatric disorders, and cancer, as well as chronic illnesses affecting the eyes, lungs, liver, kidneys, and gastrointestinal and cardiovascular systems.
3. Although many clinical trials evaluating curcumin's safety and efficacy against human ailments have already been completed, others are still ongoing. Moreover, curcumin is used as a supplement in several countries, including India, Japan, the United States, Thailand, China, Korea, Turkey, South Africa, Nepal, and Pakistan. Although inexpensive, apparently well tolerated, and potentially active, curcumin has yet not been approved for treatment of any human disease.
4. In this article, we discuss the discovery and key biological activities of curcumin, with a particular emphasis on its activities at the molecular, cellular, animal, and human levels.
DISCOVERY OF CURCUMIN
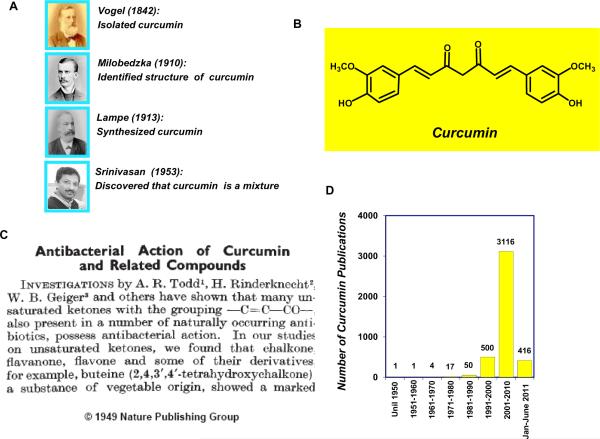
The discovery of curcumin dates to around two centuries ago when Vogel and Pelletier reported the isolation of “yellow coloring-matter” from the rhizomes of Curcuma longa (turmeric) and named it curcumin (1). Later, this substance was found to be a mixture of resin and turmeric oil. In 1842, Vogel Jr. obtained a pure preparation of curcumin but did not report its formula (2). In the decades that followed, several chemists reported possible structures of curcumin (3–5). However, it was not until 1910 that Milobedzka and Lampe identified the chemical structure of curcumin as diferuloylmethane, or 1,6-heptadiene-3,5-dione-1,7-bis (4-hydroxy-3-methoxyphenyl)-(1E, 6E) (6). Further work by the same group in 1913 resulted in the synthesis of the compound (7). Subsequently, Srinivasan separated and quantified the components of curcumin by chromatography (8) (Fig 1A and 1B).
Figure 1.
A, Key curcumin discoverers (courtesy: JP Synder, Emory University, Atlanta, GA). B, Chemical structure of curcumin. C, The first article published in Nature showing curcumin's anti-bacterial activity (image used with permission from the Nature Publishing Group). D, The number of publications on curcumin has increased remarkably over the years (source: www.ncbi.nlm.nih.gov/sites/entrez).
Although turmeric, the major source of curcumin, has been consumed as a dietary spice and a cure for human ailments for thousands of years in Asian countries, the biological characteristics of curcumin were not scientifically identified until the mid-twentieth century. In a paper published in Nature in 1949, Schraufstatter and colleagues reported that curcumin is a biologically active compound that has anti-bacterial properties (9). The authors found that curcumin was active against strains of Staphylococcus aureus, Salmonella paratyphi, Trichophyton gypseum, and Mycobacterium tuberculosis (Fig 1C). Despite those findings, only five papers were published on curcumin during the next two decades. In the 1970s, curcumin became the subject of scientific investigation, and three independent groups discovered diverse characteristics of curcumin, including cholesterol-lowering (10), anti-diabetic (11), anti-inflammatory (12), and anti-oxidant (13) activities. Later, in the 1980s, Kuttan and colleagues demonstrated the anti-cancer activity of curcumin in both in vitro and in vivo models (14). In 1995, our group was the first to demonstrate that curcumin exhibits anti-inflammatory activity by suppressing the pro-inflammatory transcription factor nuclear factor (NF)-κB; we also delineated the molecular mechanism of the inhibition (15).
The interest in curcumin research has increased dramatically over the years (Fig 1D). As of June 2011, more than 4000 articles on curcumin were listed in the National Institutes of Health PubMed database (www.ncbi.nlm.nih.gov/sites/entrez). We now know that curcumin can modulate multiple signaling pathways in either a direct or indirect manner. This polyphenol has been shown to possess activities in animal models of many human diseases. In human clinical trials, curcumin has been found to be safe and efficacious, and the U.S. Food and Drug Administration has approved curcumin as a “generally regarded as safe” compound.
Although curcumin has shown therapeutic efficacy against many human ailments, one of the major problems with curcumin is its poor bioavailability (16), which appears to be primarily due to poor absorption, rapid metabolism, and rapid systemic elimination. Therefore, efforts have been made to improve curcumin's bioavailability by improving these features. Adjuvants that can block the metabolic pathway of curcumin have been most extensively used to increase the bioavailability of this polyphenol. For instance, in humans receiving a dose of 2 g curcumin alone, serum levels have been either undetectable or very low, but concomitant administration of piperine was associated with an increase of 2000% in the bioavailability of curcumin (17). Furthermore, the effect of piperine in enhancing curcumin's bioavailability has been shown to be much greater in humans than in rats (16). Other promising approaches to increase the bioavailability of curcumin include use of nanoparticles (18), liposomes (19), micelles (20), phospholipid complexes (21), and structural analogues (22, 23).
Curcumin is now regarded as a “new drug” with great potential and is being used as a supplement in several countries. For example, in India, turmeric containing curcumin has been used in curries; in Japan, it is popularly served in tea; in Thailand, it is used in cosmetics; in China, it is used as a colorant; in Korea, it is served in drinks; in Malaysia, it is used as an antiseptic; in Pakistan, people use it as an anti-inflammatory agent to get relief from gastrointestinal discomfort; and in the United States, it is used in mustard sauce, cheese, butter, and chips, as a preservative and a coloring agent. Curcumin is marketed in several forms including capsules, tablets, ointments, energy drinks, soaps, and cosmetics.
Our laboratory and others have shown that many other nutraceuticals in addition to curcumin have therapeutic potential against inflammatory conditions. Some of these nutraceuticals are resveratrol, ursolic acid, butein, silymarin, caffeic acid phenethyl ester, anethole, berberine, capsaicin, flavopiridol, thymoquinone, gossypin, withanolides, γ-tocotrienol, zerumbone, morin, plumbagin, and celastrol. Although these nutraceuticals have been shown to exhibit anti-inflammatory activity, very little is known about their efficacy in humans. In the sections to follow, we review the biological activities of curcumin, with a special focus on its major activities at the molecular, cellular, animal, and human levels.
BIOLOGICAL ACTIVITIES OF CURCUMIN
Molecular level
At the molecular level, curcumin has been shown to modulate a wide range of signaling molecules. Curcumin may cause upregulation or downregulation depending on the target and cellular context (Table 1). These targets fall into two categories: those to which curcumin binds directly and those whose activity curcumin modulates indirectly. Included among the indirect targets are transcription factors, enzymes, inflammatory mediators, protein kinases, drug resistance proteins, adhesion molecules, growth factors, receptors, cell-cycle regulatory proteins, cell-survival proteins, chemokines, and chemokine receptors (Table 1). Direct targets include inflammatory molecules, cell-survival proteins, protein kinases, protein reductases, histone acetyltransferase, histone deacetylase, glyoxalase I, xanthine oxidase, proteasomes, HIV1 integrase, HIV1 protease, sarco/endoplasmic reticulum Ca2+ ATPase, DNA methyltransferase 1, FtsZ protofilaments, carrier proteins, and metal ions. A comprehensive review of the molecular targets of curcumin and the molecular mechanisms involved can be found in numerous articles published by us and others (24–28).
Table 1.
Molecular targets of curcumin
| Transcription factors | Inflammatory mediators |
| Activating transcription factor-3 ↓ | C-reactive protein ↓ |
| Activator protein-1 ↓ | Interleukin-1β ↓ |
| β-catenin ↓ | Interleukin-2 ↓ |
| CREB-binding protein ↓ | Interleukin-5 ↓ |
| C/EBP homologous protein ↓ | Interleukin-6 ↓ |
| Electrophile response element ↑ | Interleukin-8 ↓ |
| Early growth response gene-1 ↓ | Interleukin-12 ↓ |
| Hypoxia inducible factor-1α↓ | Interleukin-18 ↓ |
| Nuclear factor κ-B ↓ | Interferon-γ ↓ |
| Notch-1 ↓ | Inducible nitric oxide synthase ↓ |
| NFE2 related factor ↑ | 5-Lipoxygenase ↓ |
| p53↑ | Monocyte chemoattractant protein ↓ |
| Peroxisome-proliferator-activated receptor -γ ↑ | Migration inhibition protein ↓ |
| Specificity protein-1 ↓ | Macrophage inflammatory protein-1α↓ |
| STAT-1 ↓ | Prostate specific antigen ↓ |
| STAT-3 ↓ | |
| STAT-4 ↓ | Protein kinases |
| STAT-5 ↓ | Autophosphorylation-activated protein kinase ↓ |
| Wilms' tumor gene 1 ↓ | Ca2+, phospholipid-dependent protein kinase C ↓ |
| c-jun N-terminal kinase ↓ | |
| Enzymes | cAMP-dependent protein kinase ↓ |
| Acetylcholinesterase ↓ | CSN-associated kinase ↓ |
| Aldose reductase ↓ | EGF receptor-kinase ↓ |
| Arylamine N-acetyltransferases-1 ↓ | Extracellular receptor kinase ↓ |
| Beta-site APP-cleaving enzyme-1 ↓ | Focal adhesion kinase ↓ |
| CD13 ↓ | IL-1 receptor-associated kinase ↓ |
| DNA polymerase I ↓ | IκB kinase ↓ |
| DNA topoisomerase-II ↓ | Janus kinase ↓ |
| GTPase (microtubule assembly) ↓ | Mitogen-activated protein kinase ↓ |
| Glutathione reductase ↓ | pp60c-src tyrosine kinase ↓ |
| Glutathione-peroxidase ↓ | Phosphorylase kinase ↓ |
| Glutathione S-transferase ↑ | Protein kinase A ↓ |
| Hemeoxygenase-1 ↑ | PI3K-Akt ↓ |
| Ca2+-dependent ATPase ↓ | Protamine kinase ↓ |
| Inosine monophosphate dehydrogenase ↓ | |
| 17β-HSD3 ↓ | Drug resistance proteins |
| Ornithine decarboxylase ↓ | Multi-drug resistance protein-1 ↓ |
| Monoamine oxidase ↓ | Multi-drug resistance protein-2 ↓ |
| NADP(H):quinoneoxidoreductase -1 ↓ | |
| Phospholipase D ↓ | Adhesion molecules |
| Thioredoxinreductase 1 ↓ | Intracellular adhesion molecule-1 ↓ |
| Telomerase ↓ | Endothelial leukocyte adhesion molecule-1 ↓ |
| Ubiquitin isopeptidases ↓ | Vascular cell adhesion molecule-1 ↓ |
| Growth factors | Cell-survival proteins |
| Connective tissue growth factor ↓ | B-cell lymphoma protein-xL ↓ |
| Epidermal growth factor ↓ | Cellular FLICE-like inhibitory protein ↓ |
| Fibroblast growth factor ↓ | Inhibitory apoptosis protein ↓ |
| HER2 ↓ | X-linked IAP ↓ |
| Hepatocyte growth factor ↓ | |
| Platelet derived growth factor ↓ | Chemokines and chemokine receptor |
| Tissue factor ↓ | Chemokine ligand 1 ↓ |
| Transforming growth factor-β1 ↓ | Chemokine ligand 2 ↓ |
| Chemokine receptors 4 ↓ | |
| Receptors | Invasion and angiogenesis biomarkers |
| Androgen receptor ↓ | Matrix metalloproteinase-9 ↓ |
| Aryl hydrocarbon receptor ↓ | Urokinase-type plasminogen activator ↓ |
| Death receptor-5 ↓ | Vascular endothelial growth factor ↓ |
| EGF-receptor ↓ | |
| Endothelial protein C-receptor ↓ | Others |
| Estrogen receptor-α ↓ | cAMP response element binding protein ↓ |
| Fas ↑ | DNA fragmentation factor 40-kD subunit ↑ |
| Histamine (2)- receptor ↓ | Fibrinogen ↓ |
| Interleukin 8-receptor ↓ | Ferritin H and L ↓ |
| Inositol 1,4,5-triphosphate receptor ↓ | Heat-shock protein 70 ↑ |
| Integrin receptor ↓ | Iron regulatory protein ↓ |
| Low density lipoprotein-receptor ↑ | Prion fibril ↓ |
| Transferrin receptor 1 ↓ | |
| Cell-cycle regulatory proteins | |
| Cyclin D1 ↓ | |
| Cyclin E ↓ | |
| c-Myc ↓ | |
| p21 ↓ |
17β-HSD3,17 β-hydroxysteroid dehydrogenase 3; Akt, AKT8 virus oncogenecellular homolog; APP, amyloid precursor protein; ATP, adenosine triphosphate; cAMP, cyclicadenosine monophosphate; CD, cluster of differentiation; CSN, COP9 signalosome; EGF, epidermal growth factor; FLICE, FADD like interleukin-1- β-converting enzyme; GTP, guanosine triphosphate; HER2, human EGF receptor 2; IAP, inhibitor of apoptosis; IL, interleukin; NADP, nicotinamide adenine dinucleotide phosphate; NFE2, nuclear factor-erythroid 2; PI3K, phosphoinositide 3-kinase; STAT, signal transducers and activators of transcription protein.
One of the most important targets of curcumin is pro-inflammatory transcription factors, such as NF-κB, activator protein-1, and signal transducer and activator of transcription (STAT) proteins (29). These transcription factors regulate the expression of genes that contribute to tumorigenesis, cell survival, cell proliferation, invasion, and angiogenesis. Curcumin has been shown to negatively regulate these transcription factors (29). Protein kinases are another major target of curcumin. For instance, the polyphenol has been shown to downregulate epidermal growth factor receptor and the activity of extracellular signal-regulated kinase 1/2 (also called mitogen-activated protein kinase) in pancreatic and lung adenocarcinoma cells (30). Curcumin has also been shown to inhibit the phosphatidylinositol 3 kinase/AKT pathway in malignant glioma cells (31). The polyphenol has been shown to completely inhibit the activity of several protein kinases, including phosphorylase kinase, protein kinase C, protamine kinase, autophosphorylation-activated protein kinase, and pp60c-src tyrosine kinase (29, 32).
Cellular level
Extensive in vitro studies over the past half century have shown that curcumin is a highly pleiotropic molecule and that its pleiotropic activity comes from its ability to modulate multiple signaling molecules. In particular, curcumin has been shown in numerous in vitro models to possess anti-inflammatory, anti-oxidant, pro-apoptotic, chemopreventive, chemotherapeutic, anti-proliferative, wound healing, anti-nociceptive, anti-parasitic, and anti-malarial properties (Table 2).
Table 2.
Biological activities of curcumin as revealed by in vitro models
| Anti-inflammatory | Chemosensitization | Wound healing |
| Mouse fibroblast (36) | Colon cancer (51) | Skin fibroblasts (66) |
| Blood monocytes (37) | Leukaemia (142) | |
| Multiple myeloma (38) | Bladder cancer (143) | Anti-nociceptive |
| BNHL (144) | Colorectal cancer (145) | Ganglion neurons (67) |
| Breast cancer (146) | Glioma (52) | |
| Mouse macrophage (147) | Ovarian cancer (148) | Antiparasitic |
| Myeloid leukaemia (32) | Breast cancer (148) | African trypanosomes (68) |
| Oesophageal epithelial cancer (149) | Lung cancer (150) | |
| Prostate cancer (53) | Schistosomicidal | |
| Antioxidant | Schistosoma mansoni (69) | |
| Blood plasma (151) | Radiosensitization | |
| Blood platelets (151) | Glioma (52) | Antimalarial (70) |
| Rat macrophages (46) | Prostate cancer (53) | |
| Brain membrane (47) | Cervical carcinoma (54) | Nematocidal (71) |
| Rat liver microsomes (48) | Squamous cell carcinoma (55) | |
| Leukaemia (152) | ||
| Multiple myeloma (152) | Anti-proliferative | |
| Prostate cancer (56) | ||
| Pro-apoptotic | Biliary cancer (57) | |
| Colon cancer (153) | Pituitary tumor (58) | |
| Esophageal adenocarcinoma (154) | Oral cancer (59) | |
| Biliary cancer (57) | Uterine leiomyoma(60) | |
| Leukaemia (155) | ||
| Medulloblastoma (156) | ||
| Multiple myeloma (152) | Antimicrobial | |
| Osteosarcoma (157) | Candida albicans (61) | |
| Prostate cancer (158) | Candida glabrata (62) | |
| Breast cancer (159) | Coxsackie virus (64) |
BNHL, B cell non-Hodgkin'slymphoma
Inflammation is an integral component of many chronic diseases. The pro-inflammatory transcription factors NF-κB and signal transducer and activator of transcription 3 (STAT3) play a major role in mediating inflammatory response by modulating the production of pro-inflammatory cytokines (33, 34). Extensive research using a wide range of in vitro models over the past several years has indicated that curcumin can reduce inflammatory response by regulating the production of inflammatory molecules (35). For example, in one study, curcumin was shown to inhibit phorbol 12-myristate 13-acetate (PMA)-induced inflammation of mouse fibroblast cells (36). Curcumin has also been shown to act as an anti-inflammatory agent by inhibiting production of pro-inflammatory cytokines in PMA or lipopolysaccharide-stimulated peripheral blood monocytes and alveolar macrophages (37). Our laboratory was the first to demonstrate that curcumin is a potent inhibitor of STAT3 (38). The hydroxyphenyl unit in curcumin has been shown to be crucial to its anti-inflammatory activity (39). One study specifically identified the presence of a 4-hydroxyphenyl unit as crucial in this role; an increase in the anti-inflammatory activity was found by introducing additional small-sized alkyl or methoxy groups on the adjacent 3- and 5-positions on the phenyl ring (40).
Curcumin activity as an anti-oxidant and free-radical scavenger has been demonstrated from several in vitro studies. This activity can arise either from the hydroxyl group or the methylene group of the β-diketone (heptadiene-dione) moiety (41, 42). The importance of the phenolic hydroxyl group to curcumin's anti-oxidant activity is supported by several more studies (43–45). As shown in Table 2, curcumin has demonstrated anti-oxidant activities in blood plasma and platelets and in numerous cell lines. In one study, curcumin was shown to completely inhibit the in vitro production of superoxide anions, hydrogen peroxide, and nitrite radical production by rat macrophages (46). A recent study revealed that oxidative stimulation of G proteins in human brain membranes by the metabolic pro-oxidants homocysteine and hydrogen peroxide can be significantly depressed by curcumin (47). In another study, curcumin was shown to inhibit lipid peroxidation in a rat liver microsome preparation (48).
Curcumin has been found to be cytotoxic to a variety of tumor cells. The action of curcumin depends on the cell type, the curcumin concentration, and the length of treatment. The major mechanism by which curcumin induces cytotoxicity is the induction of apoptosis. Curcumin also has the potential to inhibit cancer development and progression by targeting multiple steps in the process of tumorigenesis. It has activity both as a blocking agent, inhibiting the initiation step of cancer, and as a suppressing agent, inhibiting malignant cell proliferation during the promotion and progression of carcinogenesis (49). In addition to its role as a chemopreventive and chemotherapeutic agent, curcumin has been shown to have the potential to help eliminate chemoresistant cells by sensitizing tumors to chemotherapy, in part by inhibiting pathways that lead to treatment resistance (50). For example, adding curcumin to either 5-fluorouracil alone or 5-fluorourcil + oxaliplatin resulted in statistically significant growth inhibition and an enhancement in apoptosis in HCT116 and HT29 colon cancer cells (51). Similarly, many in vitro studies have supported the potential chemosensitizing ability of curcumin in multiple cancers and have provided evidence for curcumin's use singly or as an adjunct to current chemotherapeutic drugs (50). In addition to its role as a potentially potent chemosensitizer, curcumin is also a promising radiosensitizer in a wide variety of cancer cells (52–55). Curcumin has also been shown to suppress the growth of numerous cancer cells, including those from cancer cells of the prostate (56), biliary (57), pituitary gland (58), oral (59), and uterine leiomyoma (60).
Curcumin possesses anti-microbial activities as well (61–64). For example, in a recent study of 14 Candida strains, curcumin displayed anti-fungal properties against all tested strains (62). In another study, curcumin was shown to improve the activity of common azole and polyene anti-fungals (65). In some cell culture systems, curcumin has been shown to possess anti-viral activities (63, 64). Other common activities of curcumin as demonstrated in in vitro cell culture models are wound healing in skin fibroblasts (66), anti-nociceptive activity in ganglion neurons (67), anti-parasitic activity against African trypanosomes (68), schistosomicidal activity against Schistosoma mansoni adult worms (69), anti-malarial activity (70), and nematocidal activity (71).
Animal level
Research carried out in animals during the past half century provides strong evidence for the beneficial role of curcumin against various diseases (41, 72, 73); the conditions in which curcumin appears to be active are listed in Table 3. Most of these studies used rodents, although some used rabbits. For example, curcumin was shown to significantly reduce intestinal inflammation in multidrug resistance gene-deficient mice, which spontaneously develop colitis (74). In another study, curcumin was shown to attenuate colitis in the dinitrobenzene sulfonic acid-induced murine model of colitis (75). In a study investigating the protective effect of curcumin on trinitrobenzene sulfonic acid-induced colitis in mice, treatment with curcumin was associated with significant decreases in diarrhea and in disruption of the colonic architecture in mice (76). Finally, in a rat model, curcumin administration was associated with a significant reduction in chronic inflammation and inflammatory biomarkers (77).
Table 3.
Biological activities of curcumin as revealed by animal models
| Disease | Animal model | Disease | Animal model |
|---|---|---|---|
| Inflammatory condition | Cancer treatment | ||
| Intestinal inflammation | Mouse (74) | Hepatocellular | Mouse (118) |
| Inflammatory bowel disease | Mouse (75, 76) | Breast | Mouse (119) |
| Chronic inflammation | Rat (77) | Ovarian | Mouse (120) |
| Bladder | Rat (121) | ||
| Diabetes | Cholangiocarcinoma | Hamster (113) | |
| Diabetes | Mouse (78) | ||
| Type 2 diabetes | Rat (79) | Psychiatric disorders | |
| Type 2 diabetes | Mouse (80) | Depression | Mouse (122) |
| Rat (123) | |||
| Obesity | Mouse (81) | ||
| Eye disorders | |||
| Neurological disorders | Cataract | Rat (160) | |
| Alzheimer's disease | Mouse (82, 83) | Diabetic retinopathy | Rat (161) |
| Parkinson's disease | Rat (85) | Corneal neovascularization | Rabbit (162) |
| Epilepsy | Rat (89, 90) | ||
| Diabetic encephalopathy | Rat (91) | Lung disorders | |
| Encephalomyelitis | Mouse (92) | Acute lung injury | Rat (163) |
| Intracerebral hemorrhage | Mouse (93) | COPD | Mouse (164) |
| Spinal cord injury | Rat (94) | Emphysema | Mouse (165) |
| Cerebral malaria | Mouse (95) | ||
| Convulsions | Mouse (96) | Liver disorders | |
| Brain ischemia | Rat (97) | Liver injury | Rat (166) |
| Liver fluke infection | Hamster (167) | ||
| Cancer prevention | |||
| Colon | Mouse (99) | Gastro intestinal disorders | |
| Esophageal | Rat (100) | Enterocolitis | Rat (168) |
| Lung | Mouse (101) | Gastric ulcer | Rat (169) |
| Kidney | Mouse (102) | Helicobacter pylori infection | Mouse (170) |
| Stomach | Rat (103) | Colitis | Mouse (75) |
| Liver | Rat (104) | ||
| Mouth | Hamster (105) | Renal disorders | |
| Breast | Mouse (106) | Polycystic kidney disease | Mouse (171) |
| Bladder | Mouse (107) | Ischemic reperfusion injury | Rat (172) |
| Leukaemia | Mouse (106) | ||
| Skin | Mouse (108) | Cardiovascular disorders | |
| Small intestine | Mouse (109) | Hypertension | Rat (173) |
| Pancreatic | Mouse (110) | Atherosclerosis | Mouse (174) |
| Brain | Mouse (111) | Ischemia reperfusion injury | Rabbit (175) |
| Prostate | Mouse (112) | ||
| Fibrosis | Hamster (176) | ||
| Cancer treatment | Rat (177) | ||
| Lymphoma | Mouse (14) | Mouse (178) | |
| Melanoma | Mouse (114) | ||
| Prostate | Mouse (115) | Woundhealing | Rat (179) |
| Pancreatic | Mouse (116) | Guinea pig (180) | |
| Colorectal | Mouse (117) | Mouse (181) | |
| Aging | Rat (182) | ||
| Mouse (183) | |||
| D. melanogaster (184) | |||
| Miscellaneous conditions | |||
| Asthma | Mouse (185) | ||
| Endrometriosis | Rat (186) | ||
| Mercury toxicity | Rat (187) | ||
| Fatigue | Mouse (124, 125) | ||
| Neuropathic pain | Mouse (127) | ||
| Cognitive deficit | Rat (91) | ||
| Coagulopathy | Rat (188) | ||
| Memory enhancer | Rat (189) | ||
| Morphine addiction | Mouse (190) | ||
| Muscle wasting | Mouse (191) |
COPD, chronic obstructive pulmonary disease.
Curcumin has also been shown to improve the symptoms associated with diabetes. For example, in a streptozotocin-induced diabetic mouse model, curcumin (60 mg/kg body weight) was shown to act as an anti-diabetic agent and to maintain the normal structure of the kidney (78). The effect of curcumin on the progression of insulin resistance and type 2 diabetes mellitus (T2DM) was investigated in another study. Insulin resistance and T2DM were induced in male Sprague Dawley rats by high-fat diet feeding for 60 and for 75 days. Curcumin was administered in the last 15 days of high-fat diet feeding after induction of insulin resistance and T2DM. Curcumin showed an anti-hyperglycemic effect and improved insulin sensitivity; these actions were attributed in part to its anti-inflammatory properties and anti-lipolytic effects. The authors concluded that curcumin could be a beneficial adjuvant therapy in T2DM (79). In T2DM mice, curcumin appeared to be a potent glucose-lowering agent, but it had no effect in non-diabetic mice (80).
Obesity is a major risk factor for the development of T2DM, and curcumin's potential to prevent obesity was investigated in a mouse model. Mice were fed a high-fat diet (22% fat) supplemented with 500 mg curcumin/kg for 12 weeks. Supplementing the high-fat diet of mice with curcumin did not affect food intake, but it did reduce body weight gain, adiposity, and microvessel density in adipose tissue, which coincided with reduced expression of vascular endothelial growth factor and its receptor-2, peroxisome proliferator-activated receptor-γ, and CCAAT/enhancer-binding protein-α. These findings suggest that dietary curcumin may have the potential to prevent obesity (81).
Curcumin has also been shown to affect various neurological disorders. In one study, curcumin treatment for 7 days was shown to reduce plaque formation and amyloid beta accumulation in a mouse model of Alzheimer's disease (82). In another mouse model, curcumin was shown to cross the blood-brain barrier, reduce amyloid levels and plaque burden, and exhibit significant activity against Alzheimer's disease (83). One of the pathological hallmarks of another prominent neurological disorder, Parkinson's disease, is the presence of intracellular inclusions called Lewy bodies that consist of aggregates of the presynaptic soluble protein α-synuclein (84). Drug therapy for Parkinson's disease includes replacing or mimicking dopamine in the brain. Whether curcumin can be neuroprotective against a 6-hydroxydopamine model of Parkinson's disease was investigated in a rat model. Rats pretreated with curcumin showed a clear protection in dopamine levels in the striata of rat brain. Curcumin's ability to exhibit neuroprotection against 6-hydroxydopamine was related to its anti-oxidant capability and ability to penetrate into the brain (85). Another study evaluated the protective role of curcumin against dopaminergic neurotoxicity induced by MPTP or the 1-methyl-4-phenylpyridnium ion (MPP+) in C57BL/6N mice (86). Curcumin was shown to substantially improve behavioral deficits and enhance neuron survival in the substantia nigra in the MPTP-induced Parkinson's disease mouse model. Curcumin treatment was also associated with a significant inhibition of MPTP/MPP+-induced phosphorylation of c-Jun N-terminal kinase 1/2 and c-Jun. In addition, several other studies using animal models have shown that curcumin has the potential to be active against Parkinson's disease (87, 88).
Epilepsy is another chronic neurological disorder in which curcumin has shown promise. A recent study examined the effect of curcumin on pentylenetetrazole-induced seizure in a rat model. Rats pretreated with curcumin had less severe seizures and less cognitive impairment than those not pretreated (89). Curcumin exhibited anti-epileptic effects in another rat model in which seizure was induced by kainic acid treatment (90). Other neurological disorders in which curcumin has shown promise in animal models are diabetic encephalopathy (91), encephalomyelitis (92), intracerebral hemorrhage (93), spinal cord injury (94), cerebral malaria (95), convulsions (96), and brain ischemia (97).
During the past two decades, our laboratory and others have demonstrated curcumin's potential as both a chemopreventive and a chemotherapeutic agent against cancer in rodent models. The chemopreventive efficacy of curcumin for colon cancer is particularly well established (98, 99). Other common cancers in which curcumin has shown protective effects in rodent models include esophageal (100), lung (101), kidney (102), stomach (103), liver (104), mouth (105), breast (106), bladder (107), leukemia (106), skin (108), small intestine (109), pancreatic (110), brain (111), and prostate (112) cancers. Accumulating evidence over the past several years has indicated that curcumin can be used for the treatment of established cancers as well. Most of these studies have used orthotopic or xenotransplant models and have employed curcumin either alone or in combination with existing therapies. Curcumin has shown potential for treatment of the following transplanted human cancers: cholangiocarcinoma (113), lymphoma (14), and melanoma (114), and prostate (115), pancreatic (116), colorectal (117), hepatocellular (118), breast (119), ovarian (120), and bladder (121) cancers.
Mounting evidence over the past several years has indicated curcumin's efficacy in various animal models of psychiatric disorders. For example, in a mouse model of depression, curcumin exhibited anti-depressant activity that was potentiated by the concomitant administration of fluoxetine, venlafaxine, or bupropion (122). When curcumin (20 and 40 mg/kg, intraperitoneally) was administered along with the bioavailability-enhancing agent piperine in these mice, enhancement of the anti-depressant action and increased brain penetration of curcumin were observed (122). Curcumin is also known to reverse olfactory bulbectomy-induced major depression in a rat model (123).
Curcumin has also been investigated for its potential to reduce cancer-related symptoms such as fatigue, neuropathic pain, and cognitive deficit. For example, curcumin's ability to reduce immunologically induced fatigue was investigated in a mouse model (124). Reduction of chronic fatigue in these mice was associated with a marked decrease in serum tumor necrosis factor-α levels (124). In another mouse model, curcumin was found to reduce fatigue in association with decreases in the levels of interleukin-β, interleukin-6, and tumor necrosis factor-α in the soleus muscles (125). Another study explored the effect of curcumin against glutamate excitotoxicity, mainly focusing on the neuroprotective effects of curcumin on the expression of brain-derived neurotrophic factor (BDNF), which is involved in the development of depression (126). Exposure of rat cortical neurons to 10 μM glutamate for 24 h caused a significant decrease in the BDNF level, accompanied by reduced cell viability and enhanced cell apoptosis. Pretreatment of neurons with curcumin prevented the declines in BDNF expression and cell viability in a dose- and time-dependent manner. The study concluded that the neuroprotective effects of curcumin might be mediated through the BDNF signaling pathway (126). An investigation into the role of curcumin in reducing neuropathic pain in mice with streptozotocin-induced diabetes found that treating mice with insulin in combination with curcumin significantly reduced diabetic neuropathic pain that was associated with a reduction in tumor necrosis factor-α level (127). Curcumin has also been shown to improve cognitive function in animal models. One study investigated the effect of curcumin on cognitive function and inflammation in diabetic rats. These rats exhibited cognitive deficits in association with enhancements in serum tumor necrosis factor-α levels, which were significantly attenuated after chronic treatment with curcumin (60 mg/kg) (91).
In addition to the activities discussed above, curcumin has shown potential activity against numerous other disorders and diseases, including those of eyes, lungs, liver, kidneys, and gastrointestinal and cardiovascular systems, as well as conditions such as fibrosis, wound healing problems, aging, asthma, endometriosis, and muscle wasting. The potential of curcumin to enhance memory and ameliorate morphine addiction has also been reported.
Curcumin analogues have also shown potential against animal models of human diseases. One study examined the effect of bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione, a bisdemethoxycurcumin analog (BDMC-A) on 1,2-dimethylhydrazine (DMH)-induced colon carcinogenesis in male Wistar rats (128). The study also compared the efficacy of BDMC-A with that of curcumin. Both BDMC-A and curcumin were equipotent in inhibiting the DMH-induced colon tumor incidence and normalizing histological changes. The study concluded that the presence of a terminal phenolic group and the conjugated double bonds in the central seven-carbon chain could be responsible for the agents' beneficial effects (128). In another study, curcumin and demethoxycurcumin were shown to reduce lead-induced memory deficits in male Wistar rats (129). In addition, tetrahydrocurcumin, another analogue of curcumin, has been shown to reduce the development of preneoplastic aberrant crypt foci initiated by 1,2-dimethylhydrazine dihydrochloride in the colons of mice (99). THC has also been shown to ameliorate oxidative stress-induced renal injury in mice (130). The anti-diabetic activity of THC in streptozotocin-nicotinamide-induced diabetes in rats has been investigated (131), and in one study, THC was found to possess more potent anti-diabetic activity than curcumin in type 2 diabetic rats (132).
Human level
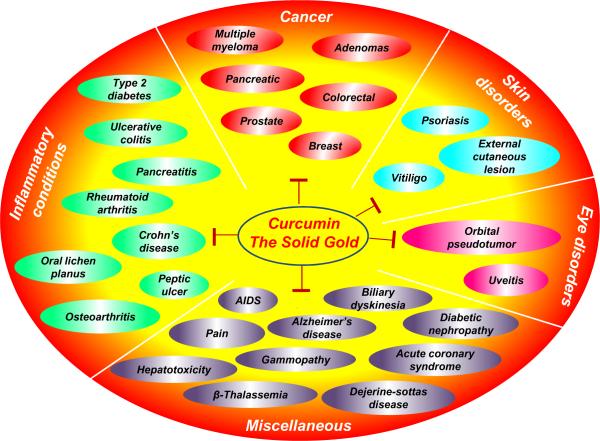
Preclinical data obtained over the past several years have provided a strong foundation for testing curcumin's potential in human subjects. To date, approximately 50 clinical trials using human subjects have been completed. Although still in the initial phases, most of these trials have suggested that curcumin is safe and effective in a number of human diseases (Fig 2). The most promising effects of curcumin have been observed with cancer; inflammatory conditions; skin, eye, and neurological disorders; diabetic nephropathy; and pain.
Figure 2.
The variety of human disorders against which curcumin's potential has been revealed by numerous clinical trials.
In one of the early studies, curcumin was found to produce remarkable symptomatic relief in 62 patients with external cancerous lesions. The effect continued for several months in many patients, and an adverse reaction was noticed in only one patient (133).
A phase II clinical trial from our group evaluated the efficacy of oral curcumin in 25 patients with advanced pancreatic cancer. Patients received 8 g curcumin daily until disease progression, with disease restaging done every 2 months. Circulating curcumin was detectable in both glucuronide and sulfate conjugate forms, albeit at low steady-state levels, suggesting poor oral bioavailability. Two patients showed clinical biological response to curcumin; nevertheless, one additional patient showed a brief but marked tumor regression by 73%, and one patient had disease stability for >18 months. None of the patients showed toxic effects from curcumin. We concluded that oral curcumin is well tolerated and has biological activity in some patients with pancreatic cancer (134).
In some clinical trials, curcumin has been found to be useful in combination with existing drugs for pancreatic cancer patients. For example, one study evaluated the activity and feasibility of gemcitabine and curcumin combinations in patients with advanced pancreatic cancer. Seventeen patients who enrolled in the study were given 8 g curcumin by mouth daily, concurrently with gemcitabine (1000 mg/m2, intravenously, three times a week for 4 weeks). Five patients discontinued curcumin after a few days to 2 weeks because of intractable abdominal fullness or pain. In two other patients, the dose of curcumin was reduced to 4 g/day because of abdominal complaints. One of 11 evaluable patients had a partial response, 4 had stable disease, and 6 had tumor progression. Time to tumor progression was 1–12 months (median, 2.5 months), and overall survival was 1–24 months (median, 5 months). It was concluded that low compliance for curcumin at a dose of 8 g/day, when taken with systemic gemcitabine, may prevent the use of high doses of oral curcumin needed to achieve a systemic effect (135).
A phase I/II study also evaluated the safety and efficacy of a combination therapy of curcumin with gemcitabine for pancreatic cancer. The study enrolled 21 patients with gemcitabine-resistant disease; they received 8 g oral curcumin daily in combination with gemcitabine. The primary endpoint was safety for the phase I portion of the trial and the feasibility of oral curcumin for phase II. The phase I portion of the study revealed the absence of limiting toxicities, and 8 g/day oral curcumin was selected as the recommended dose for the phase II portion. Curcumin was found to be well tolerated in this phase as well, and the plasma curcumin concentration ranged from 29 to 412 ng/ml in the five patients tested. It was concluded that combination therapy using 8 g oral curcumin daily with gemcitabine is safe and feasible for patients with pancreatic cancer (136). Other common cancers in which curcumin has shown efficacy either alone or in combination with existing drugs include prostate cancer, breast cancer, multiple myeloma, and adenoma.
The role of curcumin in improving body weight was evident from a recent study of patients with colorectal cancer. Curcumin administration (360 mg/day for 10–30 days) in these patients significantly improved body weight; the effect of curcumin was associated with a significant decrease in serum tumor necrosis factor-α levels (137).
Curcumin has shown promise against cardiac conditions as well. For example, one study evaluated the efficacy of curcumin in reducing lipid content in patients with acute coronary syndrome. Seventy-five patients with acute coronary syndrome participated in the study, and curcumin was administered at three different doses: low (15 mg/day, three times a day), moderate (30 mg/day, three times a day), and high (60 mg/day, three times a day). The effect of curcumin administration on total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglyceride levels was investigated. Interestingly, curcumin was found to be more effective at low doses than at high doses in reducing total cholesterol and low-density lipoprotein cholesterol levels in these patients (138). Usharani et al. recently evaluated the potential of a standardized preparation of curcuminoids (NCB-02) against different oxidative stress and inflammatory markers in patients with T2DM. Seventy-two patients with T2DM were randomized to receive NCB-02 (two capsules containing 150 mg of curcumin, twice a day), atorvastatin (10 mg, once a day), or placebo for 8 weeks. Of the 72 patients, 67 completed the study. Curcumin treatment significantly improved endothelial function and reduced oxidative stress and inflammatory markers in diabetic patients (139).
Curcumin has also been shown to have potential against abnormal eye conditions, most importantly uveitis, an inflammation of the uvea, which is characterized by symptoms such as red eye, infected conjunctiva, pain, and decreased vision. In a clinical trial from Italy, curcumin was administered orally (600 mg, twice a day) for 12–18 months to 106 patients with uveitis. Curcumin was well tolerated and reduced eye discomfort in more than 80% of patients after a few weeks of treatment (140).
One study investigated the effect of oral curcumin in combination with piperine on pain and the markers of oxidative stress in patients with tropical pancreatitis. Twenty patients were randomized to receive 500 mg of curcumin with 5 mg of piperine, 3 times a day, or placebo for 6 weeks. The effects on the pattern of pain and on the malondialdehyde and glutathione content in red blood cells were assessed. Curcumin therapy in combination with piperine was correlated with a significant reduction in the erythrocyte malondialdehyde content and a significant increase in glutathione levels in patients with tropical pancreatitis (141).
Thus, curcumin's potential as a therapeutic agent against many human diseases is clear. At the time of this writing, a search on clinicaltrials.gov revealed that more than 30 clinical trials are further evaluating curcumin's potential. Considering the promise curcumin holds against various diseases, we expect that completion of these clinical trials will provide further credence to the already established positive effects of curcumin.
CONCLUSIONS AND FUTURE PERSPECTIVES
The medicinal properties of turmeric, the source of curcumin, have been known for thousands of years to ancient people, but advancements in modern science have provided a scientific basis for the practice of using curcumin therapy against numerous human diseases. This polyphenol has been shown to target multiple signaling molecules and has shown activities at the cellular and organism levels that provide a basis for its use against multi-factorial human diseases. Although most of the currently available mono-targeted therapies are associated with numerous side effects, curcumin has been found to be safe for human use at gram doses. However, most of the known activities of curcumin are based only on in vitro and in vivo studies. Curcumin has yet not been approved for treatment of any human disease. Therefore, more extensive and well-controlled human studies are required to demonstrate this polyphenol's safety and efficacy. Future research should be focused on bringing this fascinating molecule to the forefront of therapeutic agents for the treatment of human diseases.
ACKNOWLEDGEMENTS
We thank Kathryn B. Carnes and John H. McCool of the MD Anderson Department of Scientific Publications for carefully editing the manuscript and providing valuable comments. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a core grant from the National Institutes of Health (CA16672), a program project grant from the National Institutes of Health (NIH CA124787-01A2), and a grant from the Center for Targeted Therapy at the MD Anderson Cancer Center. WK was supported by the Korea Science and Engineering Foundation (KOSEF) from the Korean government (MEST) (No. 2011-0063466).
REFERENCES
- 1.Vogel Pelletier. Journal de Pharmacie. 1815;I:289. [Google Scholar]
- 2.Vogel A., Jr. Journal de Pharma. et de Chemie. 1842;3:20. [Google Scholar]
- 3.Ivanow-Gajewsky Ibid. 1870;3:624. [Google Scholar]
- 4.Ivanow-Gajewsky Ber. Deut. Chem. Ges. 1872;5:1103. [Google Scholar]
- 5.Daube Ber. Deut. Chem. Ges. 1870;3:609. [Google Scholar]
- 6.Milobedzka J, Kostanecki S, Lampe V. Zur Kenntnis des Curcumins. Ber. Deut. Chem. Ges. 1910;43:2163–70. [Google Scholar]
- 7.Lampe V, Milobedzka J. Studien uber Curcumin. Ber. Deut. Chem. Ges. 1913;46:2235–7. [Google Scholar]
- 8.Srinivasan KR. A chromatographic study of the curcuminoids in Curcuma longa, L. J Pharm Pharmacol. 1953;5:448–57. doi: 10.1111/j.2042-7158.1953.tb14007.x. [DOI] [PubMed] [Google Scholar]
- 9.Schraufstatter E, Bernt H. Antibacterial action of curcumin and related compounds. Nature. 1949;164:456. doi: 10.1038/164456a0. [DOI] [PubMed] [Google Scholar]
- 10.Patil TN, Srinivasan M. Hypocholesteremic effect of curcumin in induced hypercholesteremic rats. Indian J Exp Biol. 1971;9:167–9. [PubMed] [Google Scholar]
- 11.Srinivasan M. Effect of curcumin on blood sugar as seen in a diabetic subject. Indian J Med Sci. 1972;26:269–70. [PubMed] [Google Scholar]
- 12.Srimal RC, Dhawan BN. Pharmacology of diferuloyl methane (curcumin), a nonsteroidal anti-inflammatory agent. J Pharm Pharmacol. 1973;25:447–52. doi: 10.1111/j.2042-7158.1973.tb09131.x. [DOI] [PubMed] [Google Scholar]
- 13.Sharma OP. Antioxidant activity of curcumin and related compounds. Biochem Pharmacol. 1976;25:1811–2. doi: 10.1016/0006-2952(76)90421-4. [DOI] [PubMed] [Google Scholar]
- 14.Kuttan R, Bhanumathy P, Nirmala K, George MC. Potential anticancer activity of turmeric (Curcuma longa) Cancer Lett. 1985;29:197–202. doi: 10.1016/0304-3835(85)90159-4. [DOI] [PubMed] [Google Scholar]
- 15.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 16.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 17.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 18.Tiyaboonchai W, Tungpradit W, Plianbangchang P. Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int J Pharm. 2007;337:299–306. doi: 10.1016/j.ijpharm.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104:1322–31. doi: 10.1002/cncr.21300. [DOI] [PubMed] [Google Scholar]
- 20.Suresh D, Srinivasan K. Studies on the in vitro absorption of spice principles--curcumin, capsaicin and piperine in rat intestines. Food Chem Toxicol. 2007;45:1437–42. doi: 10.1016/j.fct.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Liu A, Lou H, Zhao L, Fan P. Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. J Pharm Biomed Anal. 2006;40:720–7. doi: 10.1016/j.jpba.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 22.Preetha A, Banerjee R, Huilgol N. Tensiometric profiles and their modulation by cholesterol: implications in cervical cancer. Cancer Invest. 2007;25:172–81. doi: 10.1080/07357900701209053. [DOI] [PubMed] [Google Scholar]
- 23.Ohori H, Yamakoshi H, Tomizawa M, et al. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol Cancer Ther. 2006;5:2563–71. doi: 10.1158/1535-7163.MCT-06-0174. [DOI] [PubMed] [Google Scholar]
- 24.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Lin JK. Molecular targets of curcumin. Adv Exp Med Biol. 2007;595:227–43. doi: 10.1007/978-0-387-46401-5_10. [DOI] [PubMed] [Google Scholar]
- 27.Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr. 2010;103:1545–57. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 28.Gupta SC, Prasad S, Kim JH, et al. Multitargeting by curcumin as revealed by molecular interaction studies. Natural product Reports. 2011 doi: 10.1039/c1np00051a. DOI: 10.1039/C1031NP00051A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shishodia S, Singh T, Chaturvedi MM. Modulation of transcription factors by curcumin. Adv Exp Med Biol. 2007;595:127–48. doi: 10.1007/978-0-387-46401-5_4. [DOI] [PubMed] [Google Scholar]
- 30.Lev-Ari S, Starr A, Vexler A, et al. Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, down-regulation of COX-2 and EGFR and inhibition of Erk1/2 activity. Anticancer Res. 2006;26:4423–30. [PubMed] [Google Scholar]
- 31.Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 33.Gupta SC, Kim JH, Kannappan R, Reuter S, Dougherty PM, Aggarwal BB. Role of nuclear factor-{kappa}B-mediated inflammatory pathways in cancer-related symptoms and their regulation by nutritional agents. Exp Biol Med (Maywood) 2011;236:658–71. doi: 10.1258/ebm.2011.011028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 35.Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–34. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang TS, Lee SC, Lin JK. Suppression of c-Jun/AP-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1991;88:5292–6. doi: 10.1073/pnas.88.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39:41–7. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- 38.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–71. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 39.Mukhopadhyay A, Basu N, Ghatak N, Gujral PK. Anti-inflammatory and irritant activities of curcumin analogues in rats. Agents Actions. 1982;12:508–15. doi: 10.1007/BF01965935. [DOI] [PubMed] [Google Scholar]
- 40.Nurfina AN, Reksohadiprodjo MS, Timmerman H, Jenie UA, Sugiyanto D, van der Goot H. Synthesis of some symmetrical curcumin derivatives and their antiinflammatory activity. Eur J Med Chem. 1997;32:321–8. [Google Scholar]
- 41.Anand P, Thomas SG, Kunnumakkara AB, et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590–611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Ligeret H, Barthelemy S, Zini R, Tillement JP, Labidalle S, Morin D. Effects of curcumin and curcumin derivatives on mitochondrial permeability transition pore. Free Radic Biol Med. 2004;36:919–29. doi: 10.1016/j.freeradbiomed.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 43.Priyadarsini KI, Maity DK, Naik GH, et al. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic Biol Med. 2003;35:475–84. doi: 10.1016/s0891-5849(03)00325-3. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki M, Nakamura T, Iyoki S, et al. Elucidation of anti-allergic activities of curcumin-related compounds with a special reference to their anti-oxidative activities. Biol Pharm Bull. 2005;28:1438–43. doi: 10.1248/bpb.28.1438. [DOI] [PubMed] [Google Scholar]
- 45.Chen WF, Deng SL, Zhou B, Yang L, Liu ZL. Curcumin and its analogues as potent inhibitors of low density lipoprotein oxidation: H-atom abstraction from the phenolic groups and possible involvement of the 4-hydroxy-3-methoxyphenyl groups. Free Radic Biol Med. 2006;40:526–35. doi: 10.1016/j.freeradbiomed.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Joe B, Lokesh BR. Role of capsaicin, curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim Biophys Acta. 1994;1224:255–63. doi: 10.1016/0167-4889(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 47.Jefremov V, Zilmer M, Zilmer K, Bogdanovic N, Karelson E. Antioxidative effects of plant polyphenols: from protection of G protein signaling to prevention of age-related pathologies. Ann N Y Acad Sci. 2007;1095:449–57. doi: 10.1196/annals.1397.048. [DOI] [PubMed] [Google Scholar]
- 48.Reddy AC, Lokesh BR. Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol Cell Biochem. 1992;111:117–24. doi: 10.1007/BF00229582. [DOI] [PubMed] [Google Scholar]
- 49.Duvoix A, Blasius R, Delhalle S, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223:181–90. doi: 10.1016/j.canlet.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 50.Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62:919–30. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- 51.Patel BB, Sengupta R, Qazi S, et al. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122:267–73. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- 52.Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J Neurochem. 2007;102:522–38. doi: 10.1111/j.1471-4159.2007.04633.x. [DOI] [PubMed] [Google Scholar]
- 53.Li M, Zhang Z, Hill DL, Wang H, Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–96. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 54.Javvadi P, Segan AT, Tuttle SW, Koumenis C. The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Mol Pharmacol. 2008;73:1491–501. doi: 10.1124/mol.107.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khafif A, Hurst R, Kyker K, Fliss DM, Gil Z, Medina JE. Curcumin: a new radio-sensitizer of squamous cell carcinoma cells. Otolaryngol Head Neck Surg. 2005;132:317–21. doi: 10.1016/j.otohns.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 56.Teiten MH, Gaascht F, Cronauer M, Henry E, Dicato M, Diederich M. Anti-proliferative potential of curcumin in androgen-dependent prostate cancer cells occurs through modulation of the Wingless signaling pathway. Int J Oncol. 2011;38:603–11. doi: 10.3892/ijo.2011.905. [DOI] [PubMed] [Google Scholar]
- 57.Prakobwong S, Gupta SC, Kim JH, et al. Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinogenesis. 2011;32:1372–80. doi: 10.1093/carcin/bgr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schaaf C, Shan B, Buchfelder M, et al. Curcumin acts as anti-tumorigenic and hormone-suppressive agent in murine and human pituitary tumour cells in vitro and in vivo. Endocr Relat Cancer. 2009;16:1339–50. doi: 10.1677/ERC-09-0129. [DOI] [PubMed] [Google Scholar]
- 59.Chen JW, Tang YL, Liu H, et al. Anti-proliferative and anti-metastatic effects of curcumin on oral cancer cells. Hua Xi Kou Qiang Yi Xue Za Zhi. 2011;29:83–6. [PubMed] [Google Scholar]
- 60.Malik M, Mendoza M, Payson M, Catherino WH. Curcumin, a nutritional supplement with antineoplastic activity, enhances leiomyoma cell apoptosis and decreases fibronectin expression. Fertil Steril. 2009;91:2177–84. doi: 10.1016/j.fertnstert.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 61.Dovigo LN, Pavarina AC, Ribeiro AP, et al. Investigation of the Photodynamic Effects of Curcumin Against Candida albicans. Photochem Photobiol. 2011;87:895–903. doi: 10.1111/j.1751-1097.2011.00937.x. [DOI] [PubMed] [Google Scholar]
- 62.Neelofar K, Shreaz S, Rimple B, Muralidhar S, Nikhat M, Khan LA. Curcumin as a promising anticandidal of clinical interest. Can J Microbiol. 2011;57:204–10. doi: 10.1139/W10-117. [DOI] [PubMed] [Google Scholar]
- 63.Zandi K, Ramedani E, Mohammadi K, et al. Evaluation of antiviral activities of curcumin derivatives against HSV-1 in Vero cell line. Nat Prod Commun. 2010;5:1935–8. [PubMed] [Google Scholar]
- 64.Si X, Wang Y, Wong J, Zhang J, McManus BM, Luo H. Dysregulation of the ubiquitinproteasome system by curcumin suppresses coxsackievirus B3 replication. J Virol. 2007;81:3142–50. doi: 10.1128/JVI.02028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma M, Manoharlal R, Negi AS, Prasad R. Synergistic anticandidal activity of pure polyphenol curcumin I in combination with azoles and polyenes generates reactive oxygen species leading to apoptosis. FEMS Yeast Res. 2010;10:570–8. doi: 10.1111/j.1567-1364.2010.00637.x. [DOI] [PubMed] [Google Scholar]
- 66.Demirovic D, Rattan SI. Curcumin induces stress response and hormetically modulates wound healing ability of human skin fibroblasts undergoing ageing in vitro. Biogerontology. 2011;12:437–44. doi: 10.1007/s10522-011-9326-7. [DOI] [PubMed] [Google Scholar]
- 67.Yeon KY, Kim SA, Kim YH, et al. Curcumin produces an antihyperalgesic effect via antagonism of TRPV1. J Dent Res. 2010;89:170–4. doi: 10.1177/0022034509356169. [DOI] [PubMed] [Google Scholar]
- 68.Nose M, Koide T, Ogihara Y, Yabu Y, Ohta N. Trypanocidal effects of curcumin in vitro. Biol Pharm Bull. 1998;21:643–5. doi: 10.1248/bpb.21.643. [DOI] [PubMed] [Google Scholar]
- 69.Magalhaes LG, Machado CB, Morais ER, et al. In vitro schistosomicidal activity of curcumin against Schistosoma mansoni adult worms. Parasitol Res. 2009;104:1197–201. doi: 10.1007/s00436-008-1311-y. [DOI] [PubMed] [Google Scholar]
- 70.Ji HF, Shen L. Interactions of curcumin with the PfATP6 model and the implications for its antimalarial mechanism. Bioorg Med Chem Lett. 2009;19:2453–5. doi: 10.1016/j.bmcl.2009.03.060. [DOI] [PubMed] [Google Scholar]
- 71.Kiuchi F, Goto Y, Sugimoto N, Akao N, Kondo K, Tsuda Y. Nematocidal activity of turmeric: synergistic action of curcuminoids. Chem Pharm Bull (Tokyo) 1993;41:1640–3. doi: 10.1248/cpb.41.1640. [DOI] [PubMed] [Google Scholar]
- 72.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 74.Nones K, Knoch B, Dommels YE, et al. Multidrug resistance gene deficient (mdr1a−/−) mice have an altered caecal microbiota that precedes the onset of intestinal inflammation. J Appl Microbiol. 2009;107:557–66. doi: 10.1111/j.1365-2672.2009.04225.x. [DOI] [PubMed] [Google Scholar]
- 75.Salh B, Assi K, Templeman V, et al. Curcumin attenuates DNB-induced murine colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G235–43. doi: 10.1152/ajpgi.00449.2002. [DOI] [PubMed] [Google Scholar]
- 76.Ukil A, Maity S, Karmakar S, Datta N, Vedasiromoni JR, Das PK. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br J Pharmacol. 2003;139:209–18. doi: 10.1038/sj.bjp.0705241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Banerjee M, Tripathi LM, Srivastava VM, Puri A, Shukla R. Modulation of inflammatory mediators by ibuprofen and curcumin treatment during chronic inflammation in rat. Immunopharmacol Immunotoxicol. 2003;25:213–24. doi: 10.1081/iph-120020471. [DOI] [PubMed] [Google Scholar]
- 78.Sawatpanich T, Petpiboolthai H, Punyarachun B, Anupunpisit V. Effect of curcumin on vascular endothelial growth factor expression in diabetic mice kidney induced by streptozotocin. J Med Assoc Thai. 2010;93(Suppl 2):S1–8. [PubMed] [Google Scholar]
- 79.El-Moselhy MA, Taye A, Sharkawi SS, El-Sisi SF, Ahmed AF. The antihyperglycemic effect of curcumin in high fat diet fed rats. Role of TNF-alpha and free fatty acids. Food Chem Toxicol. 2011;49:1129–40. doi: 10.1016/j.fct.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 80.Seo KI, Choi MS, Jung UJ, et al. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol Nutr Food Res. 2008;52:995–1004. doi: 10.1002/mnfr.200700184. [DOI] [PubMed] [Google Scholar]
- 81.Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr. 2009;139:919–25. doi: 10.3945/jn.108.100966. [DOI] [PubMed] [Google Scholar]
- 82.Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem. 2007;102:1095–104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- 83.Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 84.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 85.Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson's disease. Free Radic Res. 2005;39:1119–25. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]
- 86.Yu S, Zheng W, Xin N, et al. Curcumin prevents dopaminergic neuronal death through inhibition of the c-Jun N-terminal kinase pathway. Rejuvenation Res. 2010;13:55–64. doi: 10.1089/rej.2009.0908. [DOI] [PubMed] [Google Scholar]
- 87.Jagatha B, Mythri RB, Vali S, Bharath MM. Curcumin treatment alleviates the effects of glutathione depletion in vitro and in vivo: therapeutic implications for Parkinson's disease explained via in silico studies. Free Radic Biol Med. 2008;44:907–17. doi: 10.1016/j.freeradbiomed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 88.Rajeswari A, Sabesan M. Inhibition of monoamine oxidase-B by the polyphenolic compound, curcumin and its metabolite tetrahydrocurcumin, in a model of Parkinson's disease induced by MPTP neurodegeneration in mice. Inflammopharmacology. 2008;16:96–9. doi: 10.1007/s10787-007-1614-0. [DOI] [PubMed] [Google Scholar]
- 89.Mehla J, Reeta KH, Gupta P, Gupta YK. Protective effect of curcumin against seizures and cognitive impairment in a pentylenetetrazole-kindled epileptic rat model. Life Sci. 2010;87:596–603. doi: 10.1016/j.lfs.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 90.Gupta YK, Briyal S, Sharma M. Protective effect of curcumin against kainic acid induced seizures and oxidative stress in rats. Indian J Physiol Pharmacol. 2009;53:39–46. [PubMed] [Google Scholar]
- 91.Kuhad A, Chopra K. Curcumin attenuates diabetic encephalopathy in rats: behavioral and biochemical evidences. Eur J Pharmacol. 2007;576:34–42. doi: 10.1016/j.ejphar.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 92.Verbeek R, van Tol EA, van Noort JM. Oral flavonoids delay recovery from experimental autoimmune encephalomyelitis in SJL mice. Biochem Pharmacol. 2005;70:220–8. doi: 10.1016/j.bcp.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 93.King MD, McCracken DJ, Wade FM, Meiler SE, Alleyne CH, Dhandapani KM. Attenuation of hematoma size and neurological injury with curcumin following intracerebral hemorrhage in mice. J Neurosurg. 2011;115:116–23. doi: 10.3171/2011.2.JNS10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sahin Kavakli H, Koca C, Alici O. Antioxidant effects of curcumin in spinal cord injury in rats. Ulus Travma Acil Cerrahi Derg. 2011;17:14–8. [PubMed] [Google Scholar]
- 95.Waknine-Grinberg JH, McQuillan JA, Hunt N, Ginsburg H, Golenser J. Modulation of cerebral malaria by fasudil and other immune-modifying compounds. Exp Parasitol. 2010;125:141–6. doi: 10.1016/j.exppara.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 96.Bharal N, Sahaya K, Jain S, Mediratta PK, Sharma KK. Curcumin has anticonvulsant activity on increasing current electroshock seizures in mice. Phytother Res. 2008;22:1660–4. doi: 10.1002/ptr.2551. [DOI] [PubMed] [Google Scholar]
- 97.Shukla PK, Khanna VK, Ali MM, Khan MY, Srimal RC. Anti-ischemic effect of curcumin in rat brain. Neurochem Res. 2008;33:1036–43. doi: 10.1007/s11064-007-9547-y. [DOI] [PubMed] [Google Scholar]
- 98.Rao CV, Simi B, Reddy BS. Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci formation in the rat colon. Carcinogenesis. 1993;14:2219–25. doi: 10.1093/carcin/14.11.2219. [DOI] [PubMed] [Google Scholar]
- 99.Kim JM, Araki S, Kim DJ, et al. Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis. 1998;19:81–5. doi: 10.1093/carcin/19.1.81. [DOI] [PubMed] [Google Scholar]
- 100.Ushida J, Sugie S, Kawabata K, et al. Chemopreventive effect of curcumin on N-nitrosomethylbenzylamine-induced esophageal carcinogenesis in rats. Jpn J Cancer Res. 2000;91:893–8. doi: 10.1111/j.1349-7006.2000.tb01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hecht SS, Kenney PM, Wang M, et al. Evaluation of butylated hydroxyanisole, myoinositol, curcumin, esculetin, resveratrol and lycopene as inhibitors of benzo[a]pyrene plus 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Lett. 1999;137:123–30. doi: 10.1016/s0304-3835(98)00326-7. [DOI] [PubMed] [Google Scholar]
- 102.Okazaki Y, Iqbal M, Okada S. Suppressive effects of dietary curcumin on the increased activity of renal ornithine decarboxylase in mice treated with a renal carcinogen, ferric nitrilotriacetate. Biochim Biophys Acta. 2005;1740:357–66. doi: 10.1016/j.bbadis.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 103.Ikezaki S, Nishikawa A, Furukawa F, et al. Chemopreventive effects of curcumin on glandular stomach carcinogenesis induced by N-methyl-N'-nitro-N-nitrosoguanidine and sodium chloride in rats. Anticancer Res. 2001;21:3407–11. [PubMed] [Google Scholar]
- 104.Chuang SE, Cheng AL, Lin JK, Kuo ML. Inhibition by curcumin of diethylnitrosamine-induced hepatic hyperplasia, inflammation, cellular gene products and cell-cycle-related proteins in rats. Food Chem Toxicol. 2000;38:991–5. doi: 10.1016/s0278-6915(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 105.Azuine MA, Bhide SV. Protective single/combined treatment with betel leaf and turmeric against methyl (acetoxymethyl) nitrosamine-induced hamster oral carcinogenesis. Int J Cancer. 1992;51:412–5. doi: 10.1002/ijc.2910510313. [DOI] [PubMed] [Google Scholar]
- 106.Huang MT, Lou YR, Xie JG, et al. Effect of dietary curcumin and dibenzoylmethane on formation of 7,12-dimethylbenz[a]anthracene-induced mammary tumors and lymphomas/leukemias in Sencar mice. Carcinogenesis. 1998;19:1697–700. doi: 10.1093/carcin/19.9.1697. [DOI] [PubMed] [Google Scholar]
- 107.Sindhwani P, Hampton JA, Baig MM, Keck R, Selman SH. Curcumin prevents intravesical tumor implantation of the MBT-2 tumor cell line in C3H mice. J Urol. 2001;166:1498–501. [PubMed] [Google Scholar]
- 108.Limtrakul P, Lipigorngoson S, Namwong O, Apisariyakul A, Dunn FW. Inhibitory effect of dietary curcumin on skin carcinogenesis in mice. Cancer Lett. 1997;116:197–203. doi: 10.1016/s0304-3835(97)00187-0. [DOI] [PubMed] [Google Scholar]
- 109.Huang MT, Lou YR, Ma W, Newmark HL, Reuhl KR, Conney AH. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994;54:5841–7. [PubMed] [Google Scholar]
- 110.Swamy MV, Citineni B, Patlolla JM, Mohammed A, Zhang Y, Rao CV. Prevention and treatment of pancreatic cancer by curcumin in combination with omega-3 fatty acids. Nutr Cancer. 2008;60(Suppl 1):81–9. doi: 10.1080/01635580802416703. [DOI] [PubMed] [Google Scholar]
- 111.Purkayastha S, Berliner A, Fernando SS, et al. Curcumin Blocks Brain Tumor Formation. Brain Res. 2009 doi: 10.1016/j.brainres.2009.01.066. [DOI] [PubMed] [Google Scholar]
- 112.Narayanan NK, Nargi D, Randolph C, Narayanan BA. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer. 2009;125:1–8. doi: 10.1002/ijc.24336. [DOI] [PubMed] [Google Scholar]
- 113.Prakobwong S, Khoontawad J, Yongvanit P, et al. Curcumin decreases cholangiocarcinogenesis in hamsters by suppressing inflammation-mediated molecular events related to multistep carcinogenesis. Int J Cancer. 2011;129:88–100. doi: 10.1002/ijc.25656. [DOI] [PubMed] [Google Scholar]
- 114.Odot J, Albert P, Carlier A, Tarpin M, Devy J, Madoulet C. In vitro and in vivo anti-tumoral effect of curcumin against melanoma cells. Int J Cancer. 2004;111:381–7. doi: 10.1002/ijc.20160. [DOI] [PubMed] [Google Scholar]
- 115.Dorai T, Cao YC, Dorai B, Buttyan R, Katz AE. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47:293–303. doi: 10.1002/pros.1074. [DOI] [PubMed] [Google Scholar]
- 116.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–61. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 117.Li L, Ahmed B, Mehta K, Kurzrock R. Liposomal curcumin with and without oxaliplatin: effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol Cancer Ther. 2007;6:1276–82. doi: 10.1158/1535-7163.MCT-06-0556. [DOI] [PubMed] [Google Scholar]
- 118.Yoysungnoen P, Wirachwong P, Bhattarakosol P, Niimi H, Patumraj S. Antiangiogenic activity of curcumin in hepatocellular carcinoma cells implanted nude mice. Clin Hemorheol Microcirc. 2005;33:127–35. [PubMed] [Google Scholar]
- 119.Aggarwal BB, Shishodia S, Takada Y, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11:7490–8. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 120.Lin YG, Kunnumakkara AB, Nair A, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13:3423–30. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 121.Tian B, Wang Z, Zhao Y, et al. Effects of curcumin on bladder cancer cells and development of urothelial tumors in a rat bladder carcinogenesis model. Cancer Lett. 2008;264:299–308. doi: 10.1016/j.canlet.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 122.Kulkarni SK, Bhutani MK, Bishnoi M. Antidepressant activity of curcumin: involvement of serotonin and dopamine system. Psychopharmacology (Berl) 2008;201:435–42. doi: 10.1007/s00213-008-1300-y. [DOI] [PubMed] [Google Scholar]
- 123.Xu Y, Ku BS, Yao HY, et al. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol Biochem Behav. 2005;82:200–6. doi: 10.1016/j.pbb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 124.Gupta A, Vij G, Sharma S, Tirkey N, Rishi P, Chopra K. Curcumin, a polyphenolic antioxidant, attenuates chronic fatigue syndrome in murine water immersion stress model. Immunobiology. 2009;214:33–9. doi: 10.1016/j.imbio.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 125.Davis JM, Murphy EA, Carmichael MD, et al. Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2168–73. doi: 10.1152/ajpregu.00858.2006. [DOI] [PubMed] [Google Scholar]
- 126.Wang R, Li YB, Li YH, Xu Y, Wu HL, Li XJ. Curcumin protects against glutamate excitotoxicity in rat cerebral cortical neurons by increasing brain-derived neurotrophic factor level and activating TrkB. Brain Res. 2008;1210:84–91. doi: 10.1016/j.brainres.2008.01.104. [DOI] [PubMed] [Google Scholar]
- 127.Sharma S, Kulkarni SK, Agrewala JN, Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol. 2006;536:256–61. doi: 10.1016/j.ejphar.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 128.Devasena T, Rajasekaran KN, Gunasekaran G, Viswanathan P, Menon VP. Anticarcinogenic effect of bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione a curcumin analog on DMH-induced colon cancer model. Pharmacol Res. 2003;47:133–40. doi: 10.1016/s1043-6618(02)00283-9. [DOI] [PubMed] [Google Scholar]
- 129.Dairam A, Limson JL, Watkins GM, Antunes E, Daya S. Curcuminoids, curcumin, and demethoxycurcumin reduce lead-induced memory deficits in male Wistar rats. J Agric Food Chem. 2007;55:1039–44. doi: 10.1021/jf063446t. [DOI] [PubMed] [Google Scholar]
- 130.Okada K, Wangpoengtrakul C, Tanaka T, Toyokuni S, Uchida K, Osawa T. Curcumin and especially tetrahydrocurcumin ameliorate oxidative stress-induced renal injury in mice. J Nutr. 2001;131:2090–5. doi: 10.1093/jn/131.8.2090. [DOI] [PubMed] [Google Scholar]
- 131.Pari L, Murugan P. Antihyperlipidemic effect of curcumin and tetrahydrocurcumin in experimental type 2 diabetic rats. Ren Fail. 2007;29:881–9. doi: 10.1080/08860220701540326. [DOI] [PubMed] [Google Scholar]
- 132.Murugan P, Pari L. Antioxidant effect of tetrahydrocurcumin in streptozotocinnicotinamide induced diabetic rats. Life Sci. 2006;79:1720–8. doi: 10.1016/j.lfs.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 133.Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori. 1987;73:29–31. doi: 10.1177/030089168707300105. [DOI] [PubMed] [Google Scholar]
- 134.Dhillon N, Aggarwal BB, Newman RA, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–9. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 135.Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer. 2010;62:1137–41. doi: 10.1080/01635581.2010.513802. [DOI] [PubMed] [Google Scholar]
- 136.Kanai M, Yoshimura K, Asada M, et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol. 2011;68:157–64. doi: 10.1007/s00280-010-1470-2. [DOI] [PubMed] [Google Scholar]
- 137.He ZY, Shi CB, Wen H, Li FL, Wang BL, Wang J. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest. 29:208–13. doi: 10.3109/07357907.2010.550592. [DOI] [PubMed] [Google Scholar]
- 138.Alwi I, Santoso T, Suyono S, et al. The effect of curcumin on lipid level in patients with acute coronary syndrome. Acta Med Indones. 2008;40:201–10. [PubMed] [Google Scholar]
- 139.Usharani P, Mateen AA, Naidu MU, Raju YS, Chandra N. Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: a randomized, parallel-group, placebo-controlled, 8-week study. Drugs R D. 2008;9:243–50. doi: 10.2165/00126839-200809040-00004. [DOI] [PubMed] [Google Scholar]
- 140.Allegri P, Mastromarino A, Neri P. Management of chronic anterior uveitis relapses: efficacy of oral phospholipidic curcumin treatment. Long-term follow-up. Clin Ophthalmol. 2010;4:1201–6. doi: 10.2147/OPTH.S13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Durgaprasad S, Pai CG, Vasanthkumar, Alvres JF, Namitha S. A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J Med Res. 2005;122:315–8. [PubMed] [Google Scholar]
- 142.Everett PC, Meyers JA, Makkinje A, Rabbi M, Lerner A. Preclinical assessment of curcumin as a potential therapy for B-CLL. Am J Hematol. 2007;82:23–30. doi: 10.1002/ajh.20757. [DOI] [PubMed] [Google Scholar]
- 143.Kamat AM, Sethi G, Aggarwal BB. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-kappaB and nuclear factor-kappaB-regulated gene products in IFN-alpha-sensitive and IFN-alpha-resistant human bladder cancer cells. Mol Cancer Ther. 2007;6:1022–30. doi: 10.1158/1535-7163.MCT-06-0545. [DOI] [PubMed] [Google Scholar]
- 144.Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–13. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 145.Howells LM, Mitra A, Manson MM. Comparison of oxaliplatin- and curcumin-mediated antiproliferative effects in colorectal cell lines. Int J Cancer. 2007;121:175–83. doi: 10.1002/ijc.22645. [DOI] [PubMed] [Google Scholar]
- 146.Bachmeier B, Nerlich AG, Iancu CM, et al. The chemopreventive polyphenol Curcumin prevents hematogenous breast cancer metastases in immunodeficient mice. Cell Physiol Biochem. 2007;19:137–52. doi: 10.1159/000099202. [DOI] [PubMed] [Google Scholar]
- 147.Liang G, Zhou H, Wang Y, et al. Inhibition of LPS-induced production of inflammatory factors in the macrophages by mono-carbonyl analogues of curcumin. J Cell Mol Med. 2009;13:3370–9. doi: 10.1111/j.1582-4934.2009.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chirnomas D, Taniguchi T, de la Vega M, et al. Chemosensitization to cisplatin by inhibitors of the Fanconi anemia/BRCA pathway. Mol Cancer Ther. 2006;5:952–61. doi: 10.1158/1535-7163.MCT-05-0493. [DOI] [PubMed] [Google Scholar]
- 149.Rafiee P, Nelson VM, Manley S, et al. Effect of curcumin on acidic pH-induced expression of IL-6 and IL-8 in human esophageal epithelial cells (HET-1A): role of PKC, MAPKs, and NF-kappaB. Am J Physiol Gastrointest Liver Physiol. 2009;296:G388–98. doi: 10.1152/ajpgi.90428.2008. [DOI] [PubMed] [Google Scholar]
- 150.Chanvorachote P, Pongrakhananon V, Wannachaiyasit S, Luanpitpong S, Rojanasakul Y, Nimmannit U. Curcumin sensitizes lung cancer cells to cisplatin-induced apoptosis through superoxide anion-mediated Bcl-2 degradation. Cancer Invest. 2009;27:624–35. doi: 10.1080/07357900802653472. [DOI] [PubMed] [Google Scholar]
- 151.Kolodziejczyk J, Olas B, Saluk-Juszczak J, Wachowicz B. Antioxidative properties of curcumin in the protection of blood platelets against oxidative stress in vitro. Platelets. 2011;22:270–6. doi: 10.3109/09537104.2010.547637. [DOI] [PubMed] [Google Scholar]
- 152.Alaikov T, Konstantinov SM, Tzanova T, Dinev K, Topashka-Ancheva M, Berger MR. Antineoplastic and anticlastogenic properties of curcumin. Ann N Y Acad Sci. 2007;1095:355–70. doi: 10.1196/annals.1397.039. [DOI] [PubMed] [Google Scholar]
- 153.Wang JB, Qi LL, Zheng SD, Wu TX. Curcumin induces apoptosis through the mitochondria-mediated apoptotic pathway in HT-29 cells. J Zhejiang Univ Sci B. 2009;10:93–102. doi: 10.1631/jzus.B0820238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hartojo W, Silvers AL, Thomas DG, et al. Curcumin promotes apoptosis, increases chemosensitivity, and inhibits nuclear factor kappaB in esophageal adenocarcinoma. Transl Oncol. 2010;3:99–108. doi: 10.1593/tlo.09235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rao J, Xu DR, Zheng FM, et al. Curcumin reduces expression of Bcl-2, leading to apoptosis in daunorubicin-insensitive CD34+ acute myeloid leukemia cell lines and primary sorted CD34+ acute myeloid leukemia cells. J Transl Med. 2011;9:71. doi: 10.1186/1479-5876-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee SJ, Krauthauser C, Maduskuie V, Fawcett PT, Olson JM, Rajasekaran SA. Curcumin-induced HDAC inhibition and attenuation of medulloblastoma growth in vitro and in vivo. BMC Cancer. 2011;11:144. doi: 10.1186/1471-2407-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Walters DK, Muff R, Langsam B, Born W, Fuchs B. Cytotoxic effects of curcumin on osteosarcoma cell lines. Invest New Drugs. 2008;26:289–97. doi: 10.1007/s10637-007-9099-7. [DOI] [PubMed] [Google Scholar]
- 158.Piantino CB, Salvadori FA, Ayres PP, et al. An evaluation of the anti-neoplastic activity of curcumin in prostate cancer cell lines. Int Braz J Urol. 2009;35:354–60. doi: 10.1590/s1677-55382009000300012. discussion 61. [DOI] [PubMed] [Google Scholar]
- 159.Chen YR, Tan TH. Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene. 1998;17:173–8. doi: 10.1038/sj.onc.1201941. [DOI] [PubMed] [Google Scholar]
- 160.Manikandan R, Beulaja M, Thiagarajan R, Arumugam M. Effect of curcumin on the modulation of alphaA- and alphaB-crystallin and heat shock protein 70 in selenium-induced cataractogenesis in Wistar rat pups. Mol Vis. 2011;17:388–94. [PMC free article] [PubMed] [Google Scholar]
- 161.Gupta SK, Kumar B, Nag TC, et al. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J Ocul Pharmacol Ther. 2011;27:123–30. doi: 10.1089/jop.2010.0123. [DOI] [PubMed] [Google Scholar]
- 162.Kim JS, Choi JS, Chung SK. The effect of curcumin on corneal neovascularization in rabbit eyes. Curr Eye Res. 2010;35:274–80. doi: 10.3109/02713680903528345. [DOI] [PubMed] [Google Scholar]
- 163.Liu K, Shen L, Wang J, et al. The Preventative Role of Curcumin on the Lung Inflammatory Response Induced by Cardiopulmonary Bypass in Rats. J Surg Res. 2010 doi: 10.1016/j.jss.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 164.Moghaddam SJ, Barta P, Mirabolfathinejad SG, et al. Curcumin inhibits COPD-like airway inflammation and lung cancer progression in mice. Carcinogenesis. 2009;30:1949–56. doi: 10.1093/carcin/bgp229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Suzuki M, Betsuyaku T, Ito Y, et al. Curcumin attenuates elastase- and cigarette smoke-induced pulmonary emphysema in mice. Am J Physiol Lung Cell Mol Physiol. 2009;296:L614–23. doi: 10.1152/ajplung.90443.2008. [DOI] [PubMed] [Google Scholar]
- 166.Cerny D, Lekic N, Vanova K, et al. Hepatoprotective effect of curcumin in lipopolysaccharide/-galactosamine model of liver injury in rats: Relationship to HO-1/CO antioxidant system. Fitoterapia. 2011;82:786–91. doi: 10.1016/j.fitote.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 167.Charoensuk L, Pinlaor P, Prakobwong S, et al. Curcumin induces a nuclear factor-erythroid 2-related factor 2-driven response against oxidative and nitrative stress after praziquantel treatment in liver fluke-infected hamsters. Int J Parasitol. 2011;41:615–26. doi: 10.1016/j.ijpara.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 168.Jia SH, Wei H, Yu JL, Wei XD, Zhang XP, Li JC. Protective effects of curcumin on neonatal rats with necrotizing enterocolitis. Zhongguo Dang Dai Er Ke Za Zhi. 2010;12:132–6. [PubMed] [Google Scholar]
- 169.Tuorkey M, Karolin K. Anti-ulcer activity of curcumin on experimental gastric ulcer in rats and its effect on oxidative stress/antioxidant, IL-6 and enzyme activities. Biomed Environ Sci. 2009;22:488–95. doi: 10.1016/S0895-3988(10)60006-2. [DOI] [PubMed] [Google Scholar]
- 170.De R, Kundu P, Swarnakar S, et al. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Agents Chemother. 2009;53:1592–7. doi: 10.1128/AAC.01242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Leonhard WN, van der Wal A, Novalic Z, et al. Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: in vivo evidence from a Pkd1-deletion model. Am J Physiol Renal Physiol. 2011;300:F1193–202. doi: 10.1152/ajprenal.00419.2010. [DOI] [PubMed] [Google Scholar]
- 172.Shoskes DA. Effect of bioflavonoids quercetin and curcumin on ischemic renal injury: a new class of renoprotective agents. Transplantation. 1998;66:147–52. doi: 10.1097/00007890-199807270-00001. [DOI] [PubMed] [Google Scholar]
- 173.Nakmareong S, Kukongviriyapan U, Pakdeechote P, et al. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with L-NAME-induced hypertension. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:519–29. doi: 10.1007/s00210-011-0624-z. [DOI] [PubMed] [Google Scholar]
- 174.Yuan HY, Kuang SY, Zheng X, et al. Curcumin inhibits cellular cholesterol accumulation by regulating SREBP-1/caveolin-1 signaling pathway in vascular smooth muscle cells. Acta Pharmacol Sin. 2008;29:555–63. doi: 10.1111/j.1745-7254.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 175.Yeh CH, Chen TP, Wu YC, Lin YM, Jing Lin P. Inhibition of NFkappaB activation with curcumin attenuates plasma inflammatory cytokines surge and cardiomyocytic apoptosis following cardiac ischemia/reperfusion. J Surg Res. 2005;125:109–16. doi: 10.1016/j.jss.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 176.Pinlaor S, Prakobwong S, Hiraku Y, Pinlaor P, Laothong U, Yongvanit P. Reduction of periductal fibrosis in liver fluke-infected hamsters after long-term curcumin treatment. Eur J Pharmacol. 2010;638:134–41. doi: 10.1016/j.ejphar.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 177.Bruck R, Weiss S, Traister A, et al. Induced hypothyroidism accelerates the regression of liver fibrosis in rats. J Gastroenterol Hepatol. 2007;22:2189–94. doi: 10.1111/j.1440-1746.2006.04777.x. [DOI] [PubMed] [Google Scholar]
- 178.Egan ME, Pearson M, Weiner SA, et al. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 2004;304:600–2. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]
- 179.Singer AJ, Taira BR, Lin F, et al. Curcumin reduces injury progression in a rat comb burn model. J Burn Care Res. 2011;32:135–42. doi: 10.1097/BCR.0b013e318203337b. [DOI] [PubMed] [Google Scholar]
- 180.Sidhu GS, Singh AK, Thaloor D, et al. Enhancement of wound healing by curcumin in animals. Wound Repair Regen. 1998;6:167–77. doi: 10.1046/j.1524-475x.1998.60211.x. [DOI] [PubMed] [Google Scholar]
- 181.Jagetia GC, Rajanikant GK. Effect of curcumin on radiation-impaired healing of excisional wounds in mice. J Wound Care. 2004;13:107–9. doi: 10.12968/jowc.2004.13.3.26589. [DOI] [PubMed] [Google Scholar]
- 182.Papiez MA, Kaja M, Gebarowska A. Age-dependent different action of curcumin in thyroid of rat. Folia Histochem Cytobiol. 2008;46:205–11. doi: 10.2478/v10042-008-0031-6. [DOI] [PubMed] [Google Scholar]
- 183.Kumar A, Prakash A, Dogra S. Protective effect of curcumin (Curcuma longa) against D-galactose-induced senescence in mice. J Asian Nat Prod Res. 2011;13:42–55. doi: 10.1080/10286020.2010.544253. [DOI] [PubMed] [Google Scholar]
- 184.Lee KS, Lee BS, Semnani S, et al. Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Res. 2010;13:561–70. doi: 10.1089/rej.2010.1031. [DOI] [PubMed] [Google Scholar]
- 185.Karaman M, Arikan Ayyildiz Z, Firinci F, et al. Effects of curcumin on lung histopathology and fungal burden in a mouse model of chronic asthma and oropharyngeal candidiasis. Arch Med Res. 2011;42:79–87. doi: 10.1016/j.arcmed.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 186.Zhang Y, Cao H, Hu YY, Wang H, Zhang CJ. Inhibitory effect of curcumin on angiogenesis in ectopic endometrium of rats with experimental endometriosis. Int J Mol Med. 2011;27:87–94. doi: 10.3892/ijmm.2010.552. [DOI] [PubMed] [Google Scholar]
- 187.Agarwal R, Goel SK, Behari JR. Detoxification and antioxidant effects of curcumin in rats experimentally exposed to mercury. J Appl Toxicol. 2010;30:457–68. doi: 10.1002/jat.1517. [DOI] [PubMed] [Google Scholar]
- 188.Chen HW, Kuo HT, Chai CY, Ou JL, Yang RC. Pretreatment of curcumin attenuates coagulopathy and renal injury in LPS-induced endotoxemia. J Endotoxin Res. 2007;13:15–23. doi: 10.1177/0968051907078605. [DOI] [PubMed] [Google Scholar]
- 189.Tang H, Lu D, Pan R, Qin X, Xiong H, Dong J. Curcumin improves spatial memory impairment induced by human immunodeficiency virus type 1 glycoprotein 120 V3 loop peptide in rats. Life Sci. 2009;85:1–10. doi: 10.1016/j.lfs.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 190.Matsushita Y, Ueda H. Curcumin blocks chronic morphine analgesic tolerance and brain-derived neurotrophic factor upregulation. Neuroreport. 2009;20:63–8. doi: 10.1097/WNR.0b013e328314decb. [DOI] [PubMed] [Google Scholar]
- 191.Siddiqui RA, Hassan S, Harvey KA, et al. Attenuation of proteolysis and muscle wasting by curcumin c3 complex in MAC16 colon tumour-bearing mice. Br J Nutr. 2009;102:967–75. doi: 10.1017/S0007114509345250. [DOI] [PubMed] [Google Scholar]