Abstract
Activation of type I natural killer T (iNKT) cells by CD1d-presented agonists is a potent immunotherapeutic tool. α-galactosylceramide (α-GalCer) is the prototypic agonist but its excessive potency with simultaneous production of both pro- and anti-inflammatory cytokines hampers its potential therapeutic use. In search for novel agonists, we have analyzed the structure and function of HS44, a synthetic aminocyclitolic ceramide analog designed to avoid unrestrained iNKT cell activation. HS44 is a weaker agonist compared to α-GalCer in vitro, while in vivo it induces robust IFN-γ production, and highly reduced but still functional Th2 response. The characteristic cytokine storm produced upon α-GalCer activation was not induced. Consequently, HS44 induced a very efficient iNKT cell dependent antitumoral response in B16 animal model. In addition, intranasal administration showed the capacity to induce lung inflammation and airway hyperreactivity, a cardinal asthma feature. Thus, HS44 is able to elicit both functional Th1 or Th2 responses. Structural studies show that HS44 binds to CD1d with the same conformation as α-GalCer. The TCR binds to HS44 similarly to α-GalCer but forms less contacts, thus explaining its weaker TCR affinity and, consequently, its weaker recognition by iNKT cells. The ability of this compound to activate an efficient, but not massive, tailored functional immune response makes it an attractive reagent for immune manipulation.
Keywords: CD1d, glycolipids, iNKT cells, Tumor immunity, T Cell Receptors
Introduction
Type I natural killer T cells (iNKT) are a unique subpopulation of T cells with immunomodulatory properties (1). They are characterized by the expression of a semi invariant TCR based on a constant Vα rearrangement (Vα14Jα18 in mice and Vα24Jα18 in humans) paired with a limited number of Vß chains (Vß8.2, Vß7, Vß2 in mice and Vß11 in humans), and are restricted by CD1d. This non classical antigen presenting molecule, as well as the other members of the non-polymorphic CD1 family, is specifically designed to bind hydrophobic ligands (2) and specializes in the presentation of lipid based antigens from microbial or self origin (3–5), although it may also present other antigens such as peptides (6, 7) to various T cells subsets. In the case of iNKT cells, a synthetic glycolipid derived from an α-galactosylceramide found in the marine sponge Agelas mauritianus, KRN700 (so far α-GalCer) (8), has been shown to be recognized by all iNKT cells, becoming the prototypical agonist that has been extensively used as model antigen in the structural and functional characterization of iNKT cell reactivity.
The use of α-GalCer and analogous CD1d ligands has allowed to define iNKT cells as potent immunoregulatory cells that bridge the innate and adaptive response. Constitutive high cytokine RNA levels determine an immediate cytokine release after iNKT activation (9, 10). Both Th1 and Th2 defining cytokines (e.g. IFN-γ, TNF and IL-4, IL-13, respectively) as well as other cytokines (IL-2, IL-5, IL-9, IL-10, IL-17, IL-21, TGFß, GM-CSF) are massively and concurrently produced upon α-GalCer stimulation (11), in addition to a vast array of chemokines, leading to a cytokine storm that activates a potent immune response. Secondary crosstalk regulates other immune cells as consequence of both cytokine and cell mediated contacts. The potent activation induced by α-GalCer has prompted its study as an immunomodulatory reagent in a whole range of disease models, from microbial infection, tumoral response, allergic and autoimmune diseases and as adjuvant in vaccination efforts. iNKT cells have been shown to be involved in various immune responses, both in mice and in humans, ranging from self tolerance to development of autoimmunity and responses to pathogens and tumors (12). Through their ability to produce both pro- and anti-inflammatory cytokine, depending on the nature of the agonist and the experimental model, iNKT cell activation can result in very different outcomes: for example, they can induce asthma like conditions, either alone or in combination with allergen challenge (13) or prevent this same pathological condition by stimulating a counteracting Th1 response (14, 15). Thus their activation by glycolipid agonists have shown to be beneficial or detrimental, depending on the disease model and the type of immune response induced upon the particular treatment (11, 16).
Based on these capabilities, α -GalCer has been proposed as an immunomodulatory reagent with therapeutic capabilities (17, 18). Clinical trials using α-GalCer in several tumor conditions have been performed showing its safety, but results are far from the expectations raised by animal model studies. Two main factors are held responsible for the relative failure in its therapeutic application: the massive induction of a cytokine storm with concurrent and contradictory or even antagonic functionalities and the profound anergic state induced on iNKT after their massive activation (19)
In an effort to selectively elicit either a Th1 or Th2 biased response upon antigen challenge, structural analogs of α-GalCer have been developed. Modifications include either the lipid tails responsible for CD1d binding or the sugar moiety that directly interacts with the TCR. In general, ligands that increase stability of the CD1d-ligand complex, such as those including aromatic rings in their fatty acid tail at certain positions induce a Th1 biased immune response (20), while analogs that decrease the stability of the complex, such as OCH which contains a truncated sphingosine chain, or C20:2 with a di-unsaturated acid chain, tend to induce a Th2 bias (21, 22). Nevertheless, effects on iNKT activation are difficult to predict as at least part of their functional behavior depends on the route of ligand presentation, the cellular compartments where processing and loading to CD1d (which also differs between human and mouse) (23) takes place and their efficient inclusion on lipid rafts, as well as on the presenting APC (24–26). In addition, their differential recognition by TCR depends on indirect conformational changes induced in CD1d structure (27).
Structural analogs that alter the polar head that is directly recognized by the TCR have also been synthesized. These ligands do not alter intracellular processing as they share the lipid chain with α-GalCer or previously synthesized analogs, thus their influence on iNKT activation would only derive from the stability of the binding to CD1d and the direct interaction with the semiinvariant TCR. The three dimensional structure of the CD1d/α-GalCer/TCR complex (28), as well as other ligands, have clearly established a new rigid binding mode compared with MHC recognition, based on a lock-and-key mechanism that mediates TCR interaction (3). The interaction is dominated by the invariant Vα chain that recognizes both CD1d residues and the sugar head of the ligand, while Vß exclusively interacts with CD1d α helixes. Thus the nature of the polar moiety and its molecular details are directly sensed by the dominating Vα chain and determine the affinity of the interaction, modulated by the Vß chain (29, 30). Alterations of specific positions in the sugar ring determine the affinity of the TCR interaction and therefore, the efficiency and the tone of the iNKT activation. Nevertheless, the structure-function relationship in the recognition event is still not well understood. Agonists resistant to enzymatic degradations, such as α-C-GalCer, are extremely potent inductors of the Th1 response in vivo, even if they are poorly recognized in direct recognition assays, due to the low TCR affinity (31, 32). Prolonged biodisponibility and long lasting iNKT/APC interaction seems to be more effective in secondary transactivation of NK cells, the major source of IFN-γ in the iNKT induced response, and in inducing a stronger Th1 response, with superior therapeutic effects in mouse models of cancer and microbial infections (31, 33). To add complexity to the search of therapeutic reagents, the human iNKT cells do not respond equally as mouse iNKT to glycolipid agonists, as evidenced by the weak response to OCH and α-C-GalCer (24).
Given all these considerations, the search for efficient iNKT agonists with functional differences compared to α-GalCer is an on going goal in the field, which attracts the work of many laboratories. Here we show the functional and structural properties of a new analog designed to have a weaker affinity for the TCR, while having a prolonged biodisponsibility to induce an efficient immune response. Based on a non-glycolipid architecture, in which the sugar moiety is substituted by a carba cyclitol ring and the O glycosidic linkage to the ceramide exchanged by an amino linkage resistant to glycosidase degradation, the aminocyclitol ceramide HS44 mimics the glucose configuration of the glycolipidic sugar. We have compared the functional activation of iNKT cells induced by HS44 with the one induced by α-GalCer, analyzing proliferation, cytokine production and efficiency of immune response in a tumor and asthma model. In addition, biophysical measurements and three dimensional structural characterization have been performed to define the interaction of the CD1d/HS44 complex with the semi-invariant TCR.
Materials and Methods
Mice
C57BL/6 female mice (6–8-wk-old) were either purchased from Criffa and used at the Animal Facility of the UAB, under protocols approved by the Ethic Committee on Animal and Human Experimentation, or breed in the Animal Facility of the Instituto de Salud Carlos III, Madrid, and used under protocols approved by the Ethic Committee on Animal Experimentation and Wellness. Female BALB/c Byj mice (6–8-wk-old) were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in a pathogen free mouse colony at the Keck School of Medicine, University of Southern California under protocols approved by the Institutional Animal Care and Use Committee.
Spleen Cell cultures
Splenocytes from C57BL/6 mice were obtained by smashing the spleen and removing red blood cells with lysing buffer (Sigma). 5×105 cells were incubated with indicated amounts of KRN7000 (α–GalCer) (a gift of G. González-Aseguinolaza, UNAV), OCH (A. Stout, NIH Tetramer Facility), or HS44 aminocyclitol ceramide in RPMI-1640 supplemented with, 10% FCS, 50 μM 2-mercaptoethanol, 2 mM L-glutamine, 1% methanol, in 96-well plates. For IL-4 and IFN-γ determinations, cell-free supernatants of day 4 were collected and cytokines levels quantified by ELISA (eBioscience) following manufacturer's instructions. At least 3 different experiments were performed. Statistical significance observed between the different analogs was analyzed using the Student t test and differences were considered significant at *P < 0,05 and **P<0,01.
Aminocyclitol compounds were resuspended in 100% methanol or 100% DMSO at 1 mg/mL and a use stock at 100 μg/mL was prepared. Compounds were heated at 56°C for 10 min and sonicated, before being diluted in complete culture media to a final concentration of 1% vehicle in the stimulation cellular assay.
Flow cytometry analysis
Briefly, spleen cultures stimulated with HS44 or OCH at 1μg/mL or 100ng/ml (α-GalCer), were preincubated with anti-CD16 (clone 2.4G2). After washing, cells were resuspended in staining media with FITC-conjugated anti-mouse TCRß (clone H57-597, BD-Pharmingen) and PE-conjugated PBS57-CD1d tetramer (A. Stout, NIH Tetramer Facility) for 30 min on ice. Samples were analyzed on a flow cytometer (FACSCalibur, BD Biosciences) and the data were processed using CellQuest software (BD Biosciences).
NKT cells enrichment and in vitro culture
iNKT cell lines were prepared as described before (34) with some modifications. Briefly, iNKT cells were negatively selected from splenocytes of wild type BALB/c mice, using a cocktail of PE conjugated mAbs against CD8 (clone eBio35-17.2), CD11c (clone N418), CD62L (clone MEL-14) and CD19 (clone eBio1D3). All antibodies were purchased from eBioscience. The samples were then stained with anti-PE micro beads mAb (Miltenyi Biotech,) prior to be enriched using magnetic cell sorting. The percentage of iNKT cells was assessed by flow cytometry (15–20%) using a FACS Canto II 8 color flow cytometry (BD Biosciences), gated on the CD3+ α-GC/CD1d tetramer+ αβTCR+ cells. According to the percentage of purification, negatively enriched iNKT cells were then put in culture with RPMI supplemented with 10% calf serum (equivalent to 2×104 NKT cells/well) in presence or absence of increasing concentration of α-GalCer, HS44 or OCH.
Quantitative real-time PCR
Total RNA was extracted from in vitro culture cells using the RNeasy mini kit (Qiagen) and cDNAs were generated with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer's recommendations. Quantification of mRNA levels was carried out by quantitative real-time PCR on a CFX96 thermal cycler (Biorad) with predesigned Taqman gene expression assays for (Actin-β: Mm0060732_m1, IL-4: Mm00445259_m1, IFN-γ: Mm00801778_m1, IL-2: Mm99999222_m1, IL-10: Mm00439616_m1, IL-5: Mm99999063_m1, IL-17A: Mm00439619_m1; (Applied Biosystems) according to the manufacturer's instructions.
Determination of in vivo cytokines
Aminocyclitol analog and α-GalCer in 200 μL of PBS, 2%DMSO, were injected intraperitoneally to C57BL/6 mice. Sera were collected at 2 hours and 21 hours and cytokines quantified by cytometric bead assay (Th1-Th2-Th17 CBA kit, BD Biosciences) according to manufacturer's instructions.
Induction of AHR and measurement of airway responsiveness
For measurement of AHR, mice were immunized intranasally with 1μg of α-GalCer or HS44 glycolipids in 50 μL of PBS. AHR responses were assessed 24h later by methacholine-induced airflow obstruction in conscious mice placed in a whole-body plethysmograph (Buxco Electronics) as described before (13). In some experiments, we assessed AHR by invasive measurement of airway resistance, in which anesthetized and tracheostomized mice were mechanically ventilated using a modified version of a described method (34). Aerosolized methacholine was administered in increasing concentrations of methacholine and we continuously computed RL and Cdyn by fitting flow, volume, and pressure to an equation of motion.
Collection of BAL fluid and lung histology
After the measurement of AHR a lethal dose of phenobarbital (450 mg per kg body weight) was administered intraperitoneally to mice, the trachea was cannulated, the lung was then lavaged twice with 1 mL of PBS supplemented with 2 % fetal calf serum and the fluid pooled as previously described (13, 34). After the BAL was performed, lungs were removed, washed with PBS, fixed in 10% formalin and stained with PAS and H&E. The relative number of different types of leukocyte (lung cell differential) was determined from slide preparations of BAL fluid stained with H&E.
Determination of B16 Melanoma Lung Metastases
C57BL/6 mice were intravenously challenged with syngeneic B16F10 melanoma, resuspended in RPMI. 500.000 cells in 200 μL were administered 3 days after administration of indicated dosis of iNKT agonists. 2 weeks after challenge, mice were killed, lungs removed and the number of metastatic nodules were visually counted.
Protein production
The expression and purification of fully glycosylated mouse CD1d/b2m heterodimer was carried out using the baculovirus expression system as reported previously (35) (36).
Vα14-Vβ8.2 TCR Refolding
TCRα and TCRβ chains were expressed in E. coli inclusion bodies and refolded together by step-wise dialysis according to established methods (35)). After final dialysis, refolded TCR was bound to DEAE sepharose beads and eluted with 100mM NaCl in 10mM Tris-HCl, pH 8 and further purified to homogeneity by anion-exchange and size exclusion chromatography as reported previously (35)).
Glycolipid loading and ternary complex formation
Mouse CD1d was loaded overnight with 3–6 molar excess of HS44 (1mg/mL in DMSO) in presence of 0.05% Tween-20 and 100 mM Tris-Cl pH7.0. HS44 loaded CD1d was purified by size exclusion chromatography first, then incubated with equimolar amount of TCR for 30 min. Finally the formed mCD1d-HS44-TCR ternary complex was purified by a second size exclusion chromatography. The complex was concentrated to 3.4 mg/mL in 10mM Hepes pH 7.5, 30mM NaCl for subsequent crystallization.
Surface plasmon resonance studies
SPR studies were performed using a Biacore 3000 (Biacore) according to the methods described previously (37). Biotin labeled mCD1d was loaded with α-GalCer and HS44 (1mg/mL in DMSO) overnight, and ≈ 200 RU of mCD1d-glycolipds complex was immobilized on a streptavidin sensor chip (Biacore). The TCR was diluted in running buffer (10 mM Hepes, 150 mM NaCl, and 3 mM EDTA, pH 7.4) and a series of increasing concentrations (0.02–1.25 μM) of the TCR in duplicate were passed over the mCD1d-glycolipids complex at 25 °C with a flow rate of 30 μL/min. The experiments were repeated twice with a different TCR preparation. Kinetic parameters were calculated after substracting the respone of TCR to empty mCD1d molecules using a simple Langmuir 1:1 model in the BIA evaluation software version 4.1.
Crystallization and structure determination
Crystals of mCD1d-HS44-TCR complexes were grown at 22.3C by sitting drop vapor diffusion while mixing 0.5 μL protein with 0.5μL precipitate (16% polyethylene glycol 3350, 8% v/v Tacsimate pH 5.0). Crystals were flash-cooled at 100 K in mother liquor containing 20% glycerol. Diffraction data were collected at the Stanford Synchrotron Radiation Lightsource (SSRL) beamline 9.2 and processed with the iMosflm software (38). The mCD1d-HS44-TCR crystal belongs to space group C2221 with cell parameters a=78.78 Å; b=191.66 Å; c=150.92 Å. The asymmetric unit contains one mCD1d-glycolipid-TCR molecule with an estimated solvent content of 57.68%. The crystal structure was determined by molecular replacement using MOLREP as part of the CCP4 suite (39, 40) using the protein coordinates from the mCD1d-iGB3 structure [PDB code 2Q7Y] (41), followed by the Vα14Vβ8.2 TCR (from PDB code 3QUZ) as the search model. After the MR solution was obtained containing both mCD1d and TCR, the model was rebuilt into a-weighted 2Fo–Fc and Fo–Fc difference electron density maps using the program COOT (42). The final refinement steps were performed using the TLS procedure in REFMAC (43) with five anisotropic domains (α1-α2 domain of CD1d, including carbohydrates and lipid, α3-domain, β2m, variable domain and constant domain of TCR). The mCD1d- HS4 -TCR structure was refined to 2.8 Å to an Rcryst and Rfree of 22.7% and 26.8% respectively. The quality of the model was examined with the program Molprobity (44 ). Data collection and refinement statistics are presented in Table 1. Coordinates and structure factors have been deposited in the Protein Data Bank under accession code 3RTQ (http://www.rcsb.org/pdb/home/home.do).
Table I.
Data collection and refinement statistics.
| Data collection statistics | mCD1d-HS44-TCR |
|---|---|
| Space group | C2221 |
| Cell dimension | |
| a, b, c, (Å) | 78.78, 191.66, 150.92 |
| 〈, ®, © (°) | 90.00, 90.00, 90.00 |
| Resolution range (Å) [outer shell] | 40.0–2.80 [2.90–2.80] |
| No. reflections | 28431 |
| Rmerge (%) | 12.1 [56.1] |
| Multiplicity | 3.6 [3.7] |
| Average I/⏔I | 11.7 [2.2] |
| Completeness (%) | 99.5 [100.0] |
| Refinement statistics | |
| No. atoms | 6589 |
| Protein | 6313 |
| Ligand | 59 |
| Carbohydrate | 91 |
| Waters | 126 |
| R/Rfree | 71/88 |
| Ramachandran plot (%) | |
| Favored | 976 |
| Allowed | 1000 |
| R.m.s. deviations | |
| Bonds (Å) | 7 |
| Angles (°) | 1065 |
| B-factors (Å 2) | |
| Protein | 420 |
| Ligand | 302 |
| Carbohydrate | 626 |
| Waters | 340 |
Results
In vitro activation of iNKT cells by HS44
We have previously shown that NKT cells are activated in in vitro cell cultures by a novel series of α-GalCer synthetic analogs in which the sugar ring is substituted by a cyclitol with different conformations of hydroxyl groups and the O glycosidic linkage is replaced by a N (45). Among them, HS44, an aminocyclitol that more closely mimics glucose configuration (see figure 6 for structure), was the most active reagent of a series of glucose-like analogs. To more precisely define its capacity to activate iNKT cells, we performed a series of in vitro and in vivo experiments, analyzing the functional consequences of its recognition.
FIGURE 6. TCR binding kinetics and HS44 ternary complex crystal structure and, stereo view of HS44 electron density and HS44 binding to mCD1d.
(A) A representative sensorgram of the TCR binding to mCD1d-HS44 complex is shown (from three independent experiments). Each colored curve depicts a different concentration of injected TCR. (B) Crystal structure of the mCD1d-HS44-TCR ternary complex is shown. HS44 shown as yellow sticks, between mCD1d (gray) and TCR (α chain in cyan; TCR β chain in orange). Chemical structures of HS44 and α-GalCer for comparison are depicted with the galactose and aminocyclitol head groups highlighted in red. (C) Representation of the final 2Fo-Fc map drawn around glycolipid HS44 from the ternary mCD1d-HS44-TCR complex. The 2FoFc electron density map is contoured for HS44 at 1 σ and drawn as a blue mesh in a side view. Hydrophobic mCD1d residues interacting with the lipid backbone and charged residues contacting the polar moieties of the glycolipid aminocyclitol are depicted. (D) HS44 and α-GalCer are shown superimposed in the mCD1d binding groove. The aminocyclitol head of HS44 (yellow) and galactose head of α-GalCer (green) are exposed similarly for TCR recognition. Binding to CD1d is very similar for both ligands. H-bonds between mCD1d and HS44 are highlighted in blue dash lines and involve the CD1d residues D80, D153 and T156. Left panel represents a top view onto the CD1d binding groove with the semi-transparent molecular surface of CD1d shown to visualize the ligand binding inside the groove. α-GalCer is shown in green; HS44 is shown in yellow; mCD1d heavy chain and β2m are shown in gray. Oxygen depicted in red and nitrogen in blue.
First we checked its capacity to induce iNKT cell proliferation in an in vitro culture system, as a measurement of its capacity to specifically activate this immune cell population. We cultured single spleen cells suspensions in the presence of HS44 and compared the expansion of iNKT cells induced upon HS44 recognition with Th1 and Th2 prototypical iNKT agonists α-GalCer and OCH, respectively. As shown in Figure 1, HS44 induces expansion of CD1d-α-GalCer tetramer labeled lymphocytes, specifically defining type I iNKT cells, in a weaker manner than either α-GalCer or OCH. In the case of α-GalCer, we used a 100ng/mL concentration at which maximum stimulation is achieved. Analogs that are not recognized by iNKT cells (such as HS58 which is identical to HS44 except for a ß orientation of the N-linkage between polar aminocylitol head and ceramide) do not induce any expansion of iNKTs.
FIGURE 1. Expansion of iNKT cells upon treatment with HS44.
Spleen cells from B6 mice were cultured in the presence or absence of 1μg/ml of HS44 or OCH or 100ng/ml of α-GalCer for 4 days. Flow cytometry analysis of splenocytes labeled with CD1d-PBS57-PE tetramer and anti-TCR-FITC. iNKT percentages (double positive cells) were calculated among electronically gated lymphocyte population. A representative of 2 different experiments is shown.
HS44 induces iNKT cells cytokine expression
The ability to produce both Th1 and Th2 cytokines is a hallmark of iNKT cell activation. To analyze the iNKT cell response towards HS44, we measured the production of IFN-γ and IL-4 in an in vitro spleen cell culture assay in which splenocytes were cultured in the presence or absence of increasing amounts of HS44 and compared with the production in response to α-GalCer and OCH. As shown in Figure 2A, HS44 induces secretion of both IFN-γ and IL-4, although in a weaker fashion that α-GalCer or the Th2 inducer OCH, coincidental with previous report (45). The induction of IFN-γ at the plateau level was far from the level induced by either α-GalCer or OCH (8–10 times lower) and the response was saturated at higher concentrations (approximately 300 ng/mL vs. 33 ng/mL concentration for α-GalCer). In the case of IL-4, the difference was not so dramatic, accounting for a 3 fold difference. Thus the ratio of IFN-γ/IL-4 production is lower than when activating with α-GalCer and drops with decreasing HS44 concentrations (from 2,5 to 1) as occurs with OCH stimulation (IFN-γ/IL-4 ratio drops from 10 to 2,5 at 10 ng/ml), as opposed to the stable ratio of ~8 for α-GalCer, suggesting a certain Th2 bias in the HS44 activation of iNKT cells.
FIGURE 2. Expression of cytokines by HS44 treated iNKT cells.
A) Spleen cells from B6 mice were cultured in the presence or absence of increasing concentrations α-GalCer, HS44 or OCH. At day 4, supernatants were collected and IFN-γ and IL-4 were measured by ELISA. Data are the mean ± s.e.m. of 4 well cultures for α-GalCer and OCH and 8 well cultures for HS44, from 3 different mice. A representative of 2 separate experiments is shown. B) iNKT cells were negatively enriched from the spleens of BALB/c mice. Enriched iNKT cells were cultured in the presence or absence of increasing concentration of α-GalCer, HS44 or OCH. 24 hours and 48 hours later, the expression of IL-4, IFN-γ, IL-2, IL-10, IL-5 and IL-17A cytokines were assessed by real-time PCR. Data are the mean ± s.e.m., representative of three separate experiments.
We further assessed the mRNA cytokine profile of HS44 treated iNKT cells by real-time PCR and compared the results to those obtained from NKT cells activated with the two glycolipids, α-GalCer and OCH. To avoid activation of iNKT cells, splenocytes from BALB/c mice were negatively enriched for iNKT cell as described in the material and methods. The iNKT enriched fraction was then cultured in presence or absence of increasing concentrations of HS44, α-GalCer or OCH. The expression of IL-4, IFN-γ, IL-2, IL-10, IL-5, IL-17A cytokines was then assessed by real-time PCR after 24 or 48 hours of in vitro stimulation (Figure 2B).
As expected, in the presence of α-GalCer or OCH iNKT cells expressed significant higher levels of IL-4, IFN-γ, IL-2, IL-10 and IL-17A mRNA after 24h of stimulation. After 48 hours of stimulation IL-4 and IL-2 RNA expression levels were even significantly higher (up to 6 fold increased) and also IL-5 was induced. In the presence of HS44, iNKT cells also significantly increased cytokine RNA expression, although expressed IL-4, IFN-γ and IL-2 RNA levels were lower as compared to α-GalCer and OCH treated cells (about 3 fold difference at the highest concentration tested after 24 hours of stimulation), thus correlating with protein quantification. Contrary to α-GalCer and OCH, HS44 activated iNKT cells did not show increased IL-2 induction at 48h and IL-4 mRNA expression returned to constitutive levels. Induction of IL-4 mRNA at 24 hours was much higher than IFN-γ (about 5 fold difference) and was sustained at lower HS44 concentrations (up to 30 ng/mL vs. 250 ng/mL for IFN-γ), again suggesting a certain Th2 bias at initial times. Moreover, the level of IFN-γ and IL-4 expression was significantly decreased after 48h of culture, suggesting that activation by HS44 is not long lasting in comparison to the other two glycolipids. On the other hand, cytokines that were less relevantly induced by prototypic agonists (IL-5, IL-10 and IL-17) are similarly induced by HS44, indicating a certain basal level of cytokine induction after iNKT activation independently of agonist strength.
HS44 selectively induces Th1 cytokine production in vivo
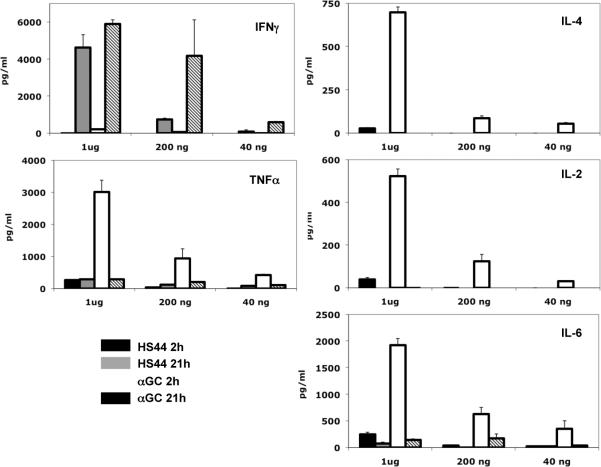
To study the physiological activity of HS44 in the activation of iNKT cells and its immediate downstream effects we intraperitoneally administered different amounts of HS44 and α-GalCer to B6 mice and analyzed the serum cytokine levels at 2 different time points, 2h and 21 h, coincidental with the times in which maximum levels of IL-4 and IFN-γ are reached. By means of a CBA assay, we measured the levels of IFN-γ, TNF, IL-4, IL-2, IL-5, IL-10 and IL-17. In vivo HS44 administration induced a potent production of IFN-γ at 21h at high dose (1ug), similar to the amount induced by α-GalCer at the same dose (less than 2 fold difference), in clear contrast to the comparatively weak production found in in vitro stimulation assays (Figure 3A). HS44 differential activity was mainly found in the dose response profile, as while α-GalCer maintained the maximum stimulatory capacity at 200ng, HS44 dropped to levels equivalent to an α-GalCer dose of 40 ng, which accounts for a 5 fold higher potency than the analog. The other Th1 cytokine measured in the assay, TNF, was very poorly induced, although production at 21h was consistently higher than at 2 hours time, while α-GalCer induced an strong response at 2h that was not sustained over time.
FIGURE 3. Serum cytokine production after HS44 administration.
Indicated amounts of HS44 or α-GalCer were i.p. administered to B6 mice. 2h and 21h later, blood samples were collected and cytokine levels were determined by CBA assay. Data indicate the mean of three different mice. A representative of three different experiments is shown.
On the contrary, IL-4 was only poorly induced and only at high doses in vivo, very differently from α-GalCer (or OCH, unpublished observation), which correlates more with the poor mRNA induction at longer times in vitro (Figure 2B). ELISA analysis of IFN-γ and IL-4 levels corroborated these results, although a general underestimation of about 50% of serum IL-4 by CBA assay could be noted. In these ELISA quantifications, IL-4 levels induced by HS44 at 1 ug dose were higher and IL-4 could also be detected at 200ng dose of HS44 (unpublished observation), not altering above conclusion. Similarly, IL-2 and IL-6 levels in serum were also poorly increased, in contrast to the high amounts found in animals treated with a high dose (1 μg) of α-GalCer. IL-10 and IL-17, on the contrary, were very poorly induced by either α-GalCer or HS44, in concordance with the weak mRNA induction in in vitro experiments (the amounts detected were too close to background as to allow reliable comparisons). Thus, in sharp contrast to in vitro stimulation, HS44 induces a strong production of IFN-γ in treated animal, with similar efficiency as α-GalCer although with lower potency, while systemic induction of IL-4 was much lower than expected, as were other cytokines typically produced in response to α-GalCer treatment. The cytokine storm characteristic of the overt superactivation induced by α-GalCer was thus restricted to specific high IFN-γ production.
Antitumoral activity induced by activation of iNKT with HS44
Because HS44 induced a stronger than anticipated in vivo production of prototypic Th1 cytokine IFN-γ, we reasoned that it might be effective in disease models that require a Th1-type immune response for their control. The metasases in the lung of the B16F10 melanoma tumor cell line is one of such models in which iNKT activation prevents the establishment of lung metastases after challenge with the tumor, through the sequential production of IFN-γ by iNKT and NK cells (46).
To investigate the potential role of HS44 to induce an effective antitumoral Th1 response, we intravenously administered different doses of either HS44 or α-GalCer to B6 mice, 3 days before challenge with syngeneic B16F10 melanoma cells. Two weeks later, mice were sacrificed, lungs extracted and lung metastatic nodules quantified. As expected, α-GalCer administration prevented establishment of metastases up to a 10ng dose per animal (Figure 4A). HS44 was also highly efficient in preventing establishment of metastasis at 200 ng and 100 ng per mice dose and even at 10 ng still showed a relevant control of the numbers of metastasis (less than 50% of maximum quantifiable). Thus HS44 was only slightly less potent than α-GalCer, as the small differences found in antitumoral effect when comparing same doses of both treatments, were not statistically significant. Of note, we used a more stringent assay than previously published, administering iNKT agonists 3 days earlier than tumor cells and challenging with 500000 B16 cells instead of the 2 days difference and 200000 cells used in other studies (33). Thus, despite the low level of iNKT activation in vitro, HS44 was able to mount a very strong Th1 response exemplified by efficient tumor suppression that effectively prevented the establishment of metastases. The antitumoral response activated by HS44 not only parallels the high induction of IFN-γ but in fact is significantly more potent as it takes place at doses (10ng) in which systemic Th1 cytokines production is minimal.
FIGURE 4. HS44 antitumoral activity controls establishment of melanoma metastases.
C57BL/6 mice were intravenously treated with indicated amounts of either HS44 or α-GalCer or with vehicle 3 days before i.v. challenge with 5×105 B16 melanoma cells. 2 weeks later, the lungs were extracted for metastases quantification. A representative experiment out of two, with five mice per condition is shown. Horizontal continous lines show statistical differences between relevant treatments, with * p<0,05, ** p< 0,01. Discontinous lines indicate relevant treatments without statistically significant differences. Photographic images from a representative mice and treatment are shown
Activation of Pulmonary iNKT Cells with HS44 Induces Airway Hyperreactivity (AHR)
Despite the low induction of systemic IL-4 in B6 mice, the in vitro experiments both in B6 and BALB/c mice indicated that HS44 mediated activation of iNKT cells was able to induce both RNA cytokine expression and secretion, at least at early time points. Therefore we resorted to determine the capacity of HS44 to induce a physiologically relevant Th2 response in a mouse strain prone to produce Th2 immune responses and in a characteristic disease model. We analyzed the specific role of HS44 in the development of AHR, and compared it to glycolipids such as α-GalCer, by administering intranasally to naïve BALB/c mice the iNKT agonists. BALB/c mice challenged with HS44 or α-GalCer developed severe AHR by 24h measured as PenH (Figure 5A), although the AHR level obtained from HS44 treated group was significantly lower when compared to α-GalCer treated group. AHR was also confirmed by direct measurement of airway resistance (RL) and dynamic compliance (Cdyn; a measure of the elasticity of the lung) in anesthetized, tracheotomized, intubated, and mechanically ventilated wild-type BALB/c (Figure 5B). Although RL showed a lower induction of hyperreactivity, lung elasticity was equally reduced by HS44 and α-GalCer. The difference in the level of AHR between HS44 and α-GalCer sensitized mice was also correlated with the total cells infiltrate obtained from BAL (Figure 5C). We examined the lung histology of mice from Figure 5A by hematoxylin and eosin to determine cellular infiltration and by periodic acid Schiff (PAS) staining to determine mucus production. As expected, lung tissues of α-GalCer sensitized mice showed extensive cellular infiltration surrounding the airways and thickened membrane and mucus production (Figure 5D). The lung histology of HS44 sensitized mice showed a lower airway inflammation in comparison to what was observed in the α-GalCer sensitized mice, but still a very significant cellular infiltration, with a similar mucus production. Overall, these results suggest that intranasal administration of HS44 in mice induces moderate AHR characterized by moderate airway inflammation and mucus production.
FIGURE 5. Intranasal administration of HS44 induces airway inflammation and airway hyperreactivity (AHR).
(A, B) A group of BALB / c mice (n=4) were treated i.n. with 1 μg of HS44 or α-GalCer (Positive control) or PBS (Negative control) on day 0. 24 hours later, the mice were assessed for the development of airway hyperreactivity by measuring PenH (A) or lung resistance (RL) and dynamic compliance (Cdyn) (B). Data are the mean ± s.e.m., representative of three separate experiments with * P < 0.05 and ** P < 0.005. (C) Bronchoalveolar lavage (BAL) fluid from the mice in panel A was analyzed 24h after AHR measurement. Results show the total number of cells in BAL fluid. Total, total cell number; Mac, monocyte / macrophage; Eos, eosinophils; Lym, lymphocytes; PMN, neutrophils (* P < 0.05, ** P< 0.01). (D) Lung histology. Lung tissue of mice from panel A were stained with hematoxylin and eosin (H&E) (upper panel) or with periodic acid Schiff (PAS) (lower panel) and analyzed for the presence of inflammation and mucus respectively.
Biophysical Characterization of HS44 recognition of iTCR
To analyze the structural basis that may help explain the presented functional data, we analyzed the biophysical interaction between the HS44-CD1d complex and a TCR representative of iNKT cells (derived from iNKT clone 2C12 (47)). We measured the equilibrium binding constants of the refolded Vα14Vß8.2 TCR toward α-GalCer and HS44 bound to mouse CD1d by surface plasmon resonance (SPR) (Figure 6A). With an equilibrium binding constant (KD) of 155 nM, the TCR binding affinity toward HS44 is considerably weaker than that of α-GalCer (11.2 nM from ref. (37)). The kinetic parameters reveal that the TCR binds HS44 and α-GalCer with a similar association rate (Ka=1.26 × 105 M−1s−1 and 1.30 × 105 M−1s−1). However, the dissociation of HS44 (ka=19.5 × 10−3 s−1) is 10–15 times faster than α GalCer (Kd=1.45 × 10−3 s−1). In summary, the kinetic data indicate that the initial binding of the TCR to mCD1d-HS44 and mCD1d-α-GalCer complexes is very similar. This suggests that HS44 and α-GalCer bind similarly to CD1d before TCR engagement. As a result, the chemical differences between HS44 and α-GalCer are mostly affecting the dissociation rate, and thus the stability of the CD1d-glycolipid-TCR complexes, which translates in a less potent activation of iNKT, compared to α-GalCer.
CD1d-HS44-TCR structure
To reveal the structural basis for HS44 antigen recognition by mouse iNKT TCRs and to understand how the replacement of the galactose of α-GalCer with an aminocyclitol group still retain relatively high TCR binding affinity, we have determined the crystal structure of the ternary complex with the HS44 antigen, at a resolution of 2.8 Å (Figure 6B and Table 1). The crystal structure exhibits very clear electron density for the glycolipid ligand suggesting an ordered orientation upon TCR engagement (Figure 6C). As expected, HS44 binding to CD1d, as well as TCR binding to CD1d-HS44 is overall very similar to α-GalCer (48). HS44 is bound to mCD1d with its phytosphingosine chain inserted in the F′ pocket and the long chain fatty acid in the A′ pocket. The aminocyclitol head is exposed similarly at the CD1d binding groove for TCR recognition (Figure 6). Both HS44 and α-GalCer bind very similar to mCD1d in the ternary complex (Figure 6D and 7). H bonds between both ligands and CD1d residues are conserved indicating that the chemical differences between the polar moieties of HS44 and α-GalCer (N vs. O-glycosidic linkage, aminocyclitol vs. galactose, respectively) do not alter the lipid binding to CD1d but rather affect TCR interaction. Significantly, the aminocyclitol defining N that links the cyclitol with the ceramide in substitution of glycosidic O, retains the H bond with T156.
FIGURE 7. Comparison of TCR binding to α-GalCer and HS44.
Interaction of the TCR α-chain (cyan) with α-GalCer from PDB ID 3H6 (green) and HS44 (yellow). There are 4 H-bonds between α-GalCer and TCR compared to only 3 direct H-bonds (dashed blue lines) between HS44 and TCR, while HS44 forms also one water-mediated H bond with the TCR (N30). Lower panels depict the conserved contacts between TCR α and ß chains and CD1 residues, mainly through CDR3α and CDR2ß, as well as one salt bridge involving E96 of CDR3ß and K148 of CD1d.
The iNKT cell TCR docks parallel to the CD1d-binding cleft, with the a chain above the F' pocket (Figure 6 and 7). The TCR a chain exclusively contacts the aminocyclitol ligand through its CDR1 and CDR3 regions, while TCR α and ß chains together form conserved contacts with CD1d residues, mainly through CDR3α, CDR2ß and CDR3ß (Figure 7).
TCR–lipid interaction
To compare TCR recognition of HS44 with α-GalCer, the interactions between TCR and both lipids were analyzed (Figure 7 and Table 2). In the α-GalCer complex, the 2' hydroxyl of the galactose ring head forms an H bond with Gly96α of CDR3α, whereas the 3' and 4' hydroxyl forms H bond to Asn30α of CDR1α. However, note that the H bond contact between the 4'OH of HS44 and N30-α of TCR is lost, due to the structural differences between the aminocyclitol ring, which is more similar to glucose (equatorial configuration of the HS44 hydroxyl vs. the opposite axial orientation in α-GalCer), and the galactose. Instead, a water molecule mediates an H bond between the 3'-and 4'-OH of HS44 and N30α of TCR (Figure 7, top right), while the rest of the contacts are retained. In total, α-GalCer forms four H bonds with the TCR, while HS44 forms only three direct H bonds with TCR, in addition to the water-mediated H bond, leading to a reduced interaction with the TCR. In conclusion, and in agreement with other α-GalCer analog structures (49), the loss of H-bonds between the TCR and the antigen leads to a faster TCR dissociation and overall reduced binding affinity, thus explaining the generally weaker potency of HS44 compared to α-GalCer.
Table II.
Molecular contacts in the mCD1d-HS44-iNKT TCR complexi
| CDR | TCR | mCD1d | Bonds | Notes |
|---|---|---|---|---|
| CDR1〈 | Pro28 | Ser76 | VDW | |
| CDR3〈 | Asp94 OD1 | Arg79 NH1, Arg79 NH2 | H bond | |
| Asp94 OD2 | Arg79 NH1, | Salt bridge | ||
| Arg79 NH2 | H bond | |||
| Asp94 | Arg79 | VDW | ||
| Arg95 NE | Asp80 OD1 | H bond | ||
| Asp80 OD2 | H bond | Distance: 3.48 Å | ||
| Arg79 NH1 | Salt bridge | |||
| Arg95 NH1 | Asp80 OD1 | H bond | ||
| Arg79 NH1 | H bond | |||
| Arg79 NE | Salt bridge | |||
| Ser76 O | H bond | |||
| Arg95 NH2 | Arg79 NH1 | H bond | Distance: 3.32 Å | |
| Asp80 OD1 | Salt bridge | |||
| Arg79 NH2 | Salt bridge | |||
| Arg95 | Arg79, Asp80, Ser76 | VDW | ||
| Gly96 O | Alal52 O | H bond | Distance: 3.32 Å | |
| Gly96 | Asp153, Ala152 | VDW | ||
| Ser97 | Val149, Ala152 | VDW | ||
| Leu99 O | Arg79 NH1 | H bond | Distance: 3.34 Å | |
| Leu99 | Val149, Asp80, Glu83 | VDW | ||
| Arg103 NH2 | Glu83 OEl, Glu83 OE2 | salt bridge | ||
| Arg103 | Arg79, Glu83 | VDW | ||
| CDR2® | Asn30 | Leu145 | VDW | |
| Tyr48 OH | Glu83 OEl, Glu83 OE2, Lys86 NZ | H bond | ||
| Tyr48 | Glu83, Lys86 | VDW | ||
| Tyr50 OH | Glu83 OE1 | H bond | ||
| Tyr50 | Met87, Glu83, Lys86 | VDW | ||
| Glu56 OE1 | Arg21 NH1 | H bond | ||
| Arg21 NH2 | Salt bridge | |||
| Lys86 NZ | Salt bridge | |||
| Arg21, Lys86 | VDW | |||
| CDR3® | Glu96 OE1 | Lys148 NZ | salt bridge | |
| Glu96 OE2 | Lys148 NZ | salt bridge | ||
| Glu96 | Lys148, Ala152 | VDW |
| CDR | TCR | HS44 | Bonds | Notes |
|---|---|---|---|---|
| CDR1〈 | Pro28 | C-1, C-6 | VDW | |
| Asn30 | 3'-O, C-3 | VDW | ||
| Asn30 ND2 | 3'-O | H bond | ||
| CDR3〈 | Arg95 | C-2, 2'-O and 3'-OH on sphinganine moiety | VDW | |
| Arg95 NE | 3'-OH on sphinganine moiety | H bond | Distance: 3.34 Å | |
| Gly96 N | 2'-O | H bond | ||
| Gly96 | C-2, 2'-O | VDW |
The molecular interactions within the complex were analyzed using the program CONTACT (CCP4, 1994). In consistent with the analysis in Pellicci et al., 2009, the cutofffs used were 4 Å (van der Waals interactions), 3.3 Å (hydrogen bonds) and 4.5 Å (salt bridges). Additional significant interactions with distances comparable with the cutoffs applied are also listed with the corresponding distance indicated in the Notes section
Discussion
iNKT cells are innate type of lymphocytes that recognize glycolipid ligands restricted by CD1d, inducing their immediate activation which implies a rapid and massive production of cytokines and the downstream transactivation of DC, T cells, B cells. Due to this capacity to be activated in early phases of the immune response, iNKT are potent immunoregulatory cells that have been shown to participate in antimicrobial, antitumoral and autoimmune responses (11, 12). Because of this capacity to control the immune response by their direct activation with pharmaceutical deliverable agonists, they have been proposed as immunotherapeutic targets of first interest. α-GalCer, the prototypical ligand recognized by all iNKT cells, has been the subject of an intense investigation in animal models of human diseases, showing its efficacy in the activation of efficient antimicrobial and antitumoral responses and in the amelioration or potentiation of autoimmune diseases, depending on the disease model and paucity of treatment (16, 18). It is a clinical trial phase I therapeutic, but its effects are far from the expectations derived from animal model studies.
Applicability of α-GalCer as an immunomodulatory reagent that may be useful as immunotherapeutic tool is hampered by two intrinsic characteristics: its extraordinary potency, based on its capacity to activate all iNKT cells inducing their full activation characterized by a cytokine storm with counteracting Th1, Th2 and even Th17 activities, and the induction of a drastic and prolonged phase of anergy consequence of this excessive activation. The superantigen-like characteristics of α-GalCer recognition translates in the unpredictable consequences of its administration, in such a way that the activation pattern of the immune response depends on variables that are mechanistically poorly defined, such as dose, timing, repetitiveness, environment, via of administration, etc. (17). An empirical rather than rational approach, based on the experience of previous experiments but not on the full understanding of the complex set of molecular mechanisms involved in activation of the immune system after α-GalCer recognition by iNKT cells, guides its use both in animal assays as well as in clinical trials. In addition, it is the target of catabolic degradation by glycosidades that limits its biodisponibility, and presumably limits its efficacy in long term treatment of chronic diseases, as cancer or autoimmunity. Because of these limitations, there is a great interest in obtaining CD1d ligands that modulate iNKT cell response in a more predictable and specific manner manner. Therefore, we resorted to search for new reagents that may overcome some of these problems and be of potential use in immunotherapy. We developed a series of analogs of the α-GalCer, based on the substitution of the sugar O-glycosidic linked moiety of the glycolipid by a cyclitol head linked by an amino linkage to the ceramide, generally termed aminocyclitols, able to be recognized by NKT cells (45).
The aminocyclitol ligands we have generated have the same lipid backbone as α-GalCer, therefore, their differential recognition and activation of iNKT will not be determined by differing CD1d loading kinetics or distinct intracellular processing and presentation, factors that alter iNKT activation in a poorly predictable manner still under investigation (24, 27, 31). Instead, their modulatory effects will be solely determined by the polar head group, which varies between the different ligands, with the common influence on in vivo bioactivity derived from the resistance to glycosidase degradation imposed by their molecular architecture, that is, the cyclitol nature of the ring and its N linkage to the ceramide. The HS44 analog here studied has a cyclitol conformation that mimics the glucose ring, with the opposite 4′OH orientation relative to galactose sugar, except for the substitution of the C5 methanol group, which does not participate in neither TCR recognition nor CD1d binding, by an OH. As α-GlcCer is a less potent activator of iNKT cells, it was expected that HS44 would be a less potent agonist (8). In vitro studies showed that to be the case, being HS44 able to be recognized by and activate iNKT cells in a weaker fashion than α-GalCer. Proliferation studies with whole spleen cell cultures and cytokine expression, both at protein and mRNA level, showed that both Th1 and Th2 biasing prototypical ligands, α-GalCer and OCH, were more potent activators. Particularly, secretion of both Th1 and Th2 defining cytokines, IFN-γ and IL-4, is drastically diminished upon activation with HS44, showing both less efficacy and less potency in in vitro experiments. Analysis of a larger panel of cytokines by mRNA profiling show that α-GalCer, OCH and HS44 have almost identical dose-dependent profiles for IL-10, IL-5 and IL-17 at 24h and 48h post-activation. On the contrary, IFN-γ, IL-4 and IL-2 showed very different profiles depending on the agonist. While α-GalCer and OCH show similar responses, except for the lower induction of IFN-γ by OCH at lower doses at 48 h (correlating with its known Th2 biasing response) (21), HS44 showed a drastic decrease of efficacy in inducing cytokine mRNA, specially at 48 h, suggesting that activation of iNKT is less potent and short lasting, comparing with full agonists.
One of the structural characteristics of HS44 that may have a major impact on its biological effects is the substitution of the O atom of the glycosidic linkage by a N (amino linkage), thus impeding the degradative action of intracellular and extracellular glycosidases, presumably allowing for a prolonged half life in vivo and a prolonged activation of iNKT cells. This has been suggested to be a major determinant of the reactivity of α-GalCer analogs that are poorly recognized in in vitro assays, due to a weak TCR recognition (31, 32), but that are potent agonists in vivo, inducing a potent Th1 response (33, 50). Administration of HS44 to model animals proved this to be the case. Opposite to what could be expected from in vitro experiments, HS44 induced an efficient IFN-γ response close in magnitude to α-GalCer, although with lower potency. This cytokine response was heavily biased, as none of the other cytokines induced by α-GalCer treatment that were tested, was highly induced by HS44: only at high doses (1 μg per animal) minor levels of TNF, IL-4, IL-2 and IL-6 were found in the serum of treated animals. Consequently with this highly specific Th1 cytokine induction, HS44 proved to be also highly efficient in inducing a potent antitumoral response. The B16 melanoma tumor model is a characteristic Th1 immune response model in which control of the establishment of metastases in the lung is totally dependent on the activation of a potent Th1 response characterized by a high production of IFN-γ, mainly by activation of NK cells secondary to iNKT activation (46). In this model, HS44 proved to be almost as efficient as α-GalCer in preventing the establishment of metastases with a very similar potency, despite being a much weaker in vitro agonist. In conclusion, the antitumor effect was far more efficient than systemic cytokine production as metastatic growth was prevented at agonist doses in which serum IFN-γ levels were minimal. This indicates that either extremely high levels of IFN-γ are not necessary for controlling metastasis implantation or that other secondary effector mechanisms, presumably more efficiently induced by HS44, also participate in a relevant manner in the antiumoral response.
It could be argued that inducing exclusively a Th1 response may not be as useful in the natural context of disease treatment as a more complete activation of the immune response but biased to the specific effect required (51). In fact, efficient IFN-γ production by iNKT cells requires full IL-4 production capabilities at early stages of activation (52). We demonstrated that HS44 was able to induce Th2 immune responses in vivo as intranasal administration of HS44 resulted in induction of moderate AHR, a complex inflammatory disorder caused by Th2-driven inflammatory responses (53–55). Several groups have previously shown that intranasal administration of glycolipids such as α-GalCer induces severe AHR by activating iNKT cells to produce significant levels of IL-4 and IL-13 (56). Although administration of HS44 resulted in a significantly lower airway function in comparison to the group treated with α-GalCer, the lung inflammation, mucus production as well as lymphocyte infiltration in the lungs were comparable.
All these biological assays show that HS44 is an iNKT agonist, able to mount efficient Th1 and Th2 immune responses with capacity to generate a very efficient anti-tumoral response and to induce autoimmune effects characteristics of allergic responses, in specific mice models.
The lower potency of HS44 in comparison with α-GalCer is the consequence of a lower interaction with the Va14Ja18 TCR characteristic of iNKT. Surface plasma resonance experiments show that the KD of the TCR interaction with the HS44-CD1d complex is 155nM, 14 times higher than for α-GalCer complex. This reduced affinity is exclusively due to a 13 fold faster dissociation kinetics of the TCR. This is in total agreement with recent measurements of another TCR interaction that showed that the affinity for α-GalCer is 10 times higher than for 4′deoxy α-GalCer and the affinity of OCH also is 10 times higher than for the equivalent glucose analog (that differs in the orientation of the 4′OH as HS44 does), both of them due to a faster dissociation kinetics of the trimolecular complex (49). In addition, a 6' galactose modified analog of α-GalCer, BnNHGSL-1', of which the head group is pulled toward the CD1 plane in the crystal structure, due to additional interactions with CD1d, also loses its 4' OH H-bond interaction with the TCR, resulting in an almost identical TCR binding kinetics compared to HS44 (57). Therefore, we can conclude that the introduction of a N amino linkage of the polar cyclitol head to the ceramide and the substitution of the CH2-OH in the C5′ position by an OH have minor, if any, effects on the interaction with the TCR and that the reduced affinity would mostly be the consequence of the altered conformation of the 4′OH. Also in concordance with above study, there is an strict correlation between TCR affinity and in vitro cytokine response. On the contrary the proliferative response of activated iNKT cells is only partially affected by the diminished affinity, as previously published (32, 49), decreasing in a moderate manner compared to the large differences in interaction strengh, indicating that proliferation does not directly correlates with affinity. Similarly, sustained in vivo production as well as secondary cell activation are only partially affected by lower affinity with the TCR and more dependent on the continuity of agonist presentation. Thus, the resistance to glycosidase degradation and therefore, its prolonged bioavailability, is a major factor for in vivo functionality more relevant than intrinsic affinity of the analog or the in vitro iNKT activation profile, specially in inducing antitumoral responses, which is more dependent on secondary activation of effector cells.
The structure of the trimolecular complex explains these effects on affinity. The HS44-CD1d-TCR complex is highly similar to the α-GalCer complex, with differences affecting only at the contacts between the polar ring of HS44 with the CDR1α and CDR3α segments of the TCR, with minor rearrangements of few lateral chains involved in the interaction. HS44 adopts an almost idential orientation compared with α-GalCer, although slightly closer to the CD1d plane, maintaining the three hydrogen bonds at the entrance of binding groove that anchor the glycolipid to CD1d, including that of the glycosidic O that is maintained in the substituting polar N. In contrast, the recently solved structure of α-GlcCer analogs shows a repositioning of the sugar along the A′-pocket and flatting against the CD1d plane (49). Thus, the TCR interaction with HS44 follows the rigid docking characteristic of α-GalCer-CD1d interaction, without the need for an induced fit of the ligand, indicated by the almost identical TCR association rate (Ka), compared to α-GalCer.
In summary, we have shown the functional and structural properties of an α-GalCer analog that may be useful in therapeutic applications. HS44 is characterized by a lower intrinsic capacity to activate iNKT cells, due to lower affinity with the semi-invariant TCR, which may be an advantageous property for its use as a modulatory agent (18, 58). The lower potency may not only be beneficial as to the induction of an efficient immune response, but also may signify a lower capacity to transactivate DCs presenting self antigens and license cross-priming (59), thus reducing the possibility of stimulating an autoimmune response either if used as a therapeutic reagent or as an adjuvant, a risk associated with the exacerbated activity of α-GalCer. In addition, DC loaded with weaker agonists are less susceptible to iNKT killing, providing a prolonged life span for iNKT activation and sustained in vivo activation (60). The large number of studies aimed to dissect the potential applicability of α-GalCer indicates that ex vivo approaches, such as loading of autologous DC or expansion of iNKT, seem to be the only effective methodologies applicable to unsure effective immune responses, at least in cancer treatments. The possibility of obtaining an agonist that would directly activate iNKT in a useful and safe manner as to unsure effective and tailored immune responses in a classic pharmacological format, without the need of extensive and specialized ex vivo manipulation, is obviously of great interest and it is open to immune modulators as the one here described.
Acknowledgements
Gloria Gonzalez Aseguinolaza for providing α-GalCer. NIH Tetramer Core facility for providing OCH and PBS57-CD1d tetramer. Stanford Synchrotron Radiation Lightsource (SSRL) beamline 9.2 for remote data collection. YH thanks MICINN for a Juan de la Cierva fellowship. DMZ is recipient of an investigator award from CRI.
Footnotes
Author Contributions JK, performed in vitro cytokine mRNA profiling and airway hiperreactivity experiments; OA designed and analyzed in vitro cytokine mRNA profiling and airway hiperreactivity experiments; EDY, EG performed biophysical and crystal structure experiments; DMZ designed and analyzed crystal structure experiments; CMB performed and analyzed in vitro spleen culture experiments; EAP, PL, ARC performed and designed B16 tumor experiments; YH, AL contributed material; ARC designed in vitro spleen culture experiments, designed and performed in vivo cytokine determinations, analyzed the data; AL, ARC devised the project; OA, DMZ, ARC wrote the manuscript.
Support from MICINN (Project CTQ2008-01426/BQU), to AL. From the Ministerio de Educación, Ciencia y Tecnología (SAF2010-15649) and La Fundació La Marató de TV3 - Cancer to PL. From the National Institutes of Health Public Health Service Grant R01 AI066020 to OA. From the NIH grant AI074952 to DMZ.
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Zeng Z, Castano AR, Segelke BW, Stura EA, Peterson PA, Wilson IA. Crystal structure of mouse CD1: An MHC-like fold with a large hydrophobic binding groove. Science. 1997;277:339. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- 3.Zajonc DM, Kronenberg M. Carbohydrate specificity of the recognition of diverse glycolipids by natural killer T cells. Immunol Rev. 2009;230:188. doi: 10.1111/j.1600-065X.2009.00802.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 5.Gapin L. iNKT cell autoreactivity: what is `self' and how is it recognized? Nat Rev Immunol. 2010;10:272. doi: 10.1038/nri2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castano AR, Tangri S, Miller JE, Holcombe HR, Jackson MR, Huse WD, Kronenberg M, Peterson PA. Peptide binding and presentation by mouse CD1. Science. 1995;269:223. doi: 10.1126/science.7542403. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Teige A, Mondoc E, Ibrahim S, Holmdahl R, Issazadeh-Navikas S. Endogenous collagen peptide activation of CD1d-restricted NKT cells ameliorates tissue-specific inflammation in mice. J Clin Invest. 2011;121:249. doi: 10.1172/JCI43964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda JL, Gapin L, Baron JL, Sidobre S, Stetson DB, Mohrs M, Locksley RM, Kronenberg M. Mouse V alpha 14i natural killer T cells are resistant to cytokine polarization in vivo. Proc Natl Acad Sci U S A. 2003;100:8395. doi: 10.1073/pnas.1332805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the `Swiss-Army knife' of the immune system. Curr Opin Immunol. 2008;20:358. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 13.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda H, Takeda K, Koya T, Okamoto M, Shiraishi Y, Miyahara N, Dakhama A, Matsuda JL, Gapin L, Gelfand EW. Plasticity of invariant NKT cell regulation of allergic airway disease is dependent on IFN-gamma production. J Immunol. 2011;185:253. doi: 10.4049/jimmunol.0902301. [DOI] [PubMed] [Google Scholar]
- 15.Chang YJ, Kim HY, Albacker LA, Lee HH, Baumgarth N, Akira S, Savage PB, Endo S, Yamamura T, Maaskant J, Kitano N, Singh A, Bhatt A, Besra GS, Elzen P. van den, Appelmelk B, Franck RW, Chen G, DeKruyff RH, Shimamura M, Illarionov P, Umetsu DT. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Invest. 2011;121:57. doi: 10.1172/JCI44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Kaer L. alpha-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005;5:31. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 18.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 19.Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang YJ, Huang JR, Tsai YC, Hung JT, Wu D, Fujio M, Wong CH, Yu AL. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc Natl Acad Sci U S A. 2007;104:10299. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 22.Forestier C, Takaki T, Molano A, Im JS, Baine I, Jerud ES, Illarionov P, Ndonye R, Howell AR, Santamaria P, Besra GS, Dilorenzo TP, Porcelli SA. Improved outcomes in NOD mice treated with a novel Th2 cytokine-biasing NKT cell activator. J Immunol. 2007;178:1415. doi: 10.4049/jimmunol.178.3.1415. [DOI] [PubMed] [Google Scholar]
- 23.Cohen NR, Garg S, Brenner MB. Antigen Presentation by CD1 Lipids, T Cells, and NKT Cells in Microbial Immunity. Adv Immunol. 2009;102:1. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 24.Im JS, Arora P, Bricard G, Molano A, Venkataswamy MM, Baine I, Jerud ES, Goldberg MF, Baena A, Yu KO, Ndonye RM, Howell AR, Yuan W, Cresswell P, Chang YT, Illarionov PA, Besra GS, Porcelli SA. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009;30:888. doi: 10.1016/j.immuni.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai L, Sagiv Y, Liu Y, Freigang S, Yu KO, Teyton L, Porcelli SA, Savage PB, Bendelac A. Lysosomal recycling terminates CD1d-mediated presentation of short and polyunsaturated variants of the NKT cell lipid antigen alphaGalCer. Proc Natl Acad Sci U S A. 2009;106:10254. doi: 10.1073/pnas.0901228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bezbradica JS, Stanic AK, Matsuki N, Bour-Jordan H, Bluestone JA, Thomas JW, Unutmaz D, Van Kaer L, Joyce S. Distinct roles of dendritic cells and B cells in Va14Ja18 natural T cell activation in vivo. J Immunol. 2005;174:4696. doi: 10.4049/jimmunol.174.8.4696. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy C, Shepherd D, Fleire S, Stronge VS, Koch M, Illarionov PA, Bossi G, Salio M, Denkberg G, Reddington F, Tarlton A, Reddy BG, Schmidt RR, Reiter Y, Griffiths GM, van der Merwe PA, Besra GS, Jones EY, Batista FD, Cerundolo V. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2007;204:1131. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 29.Mallevaey T, Clarke AJ, Scott-Browne JP, Young MH, Roisman LC, Pellicci DG, Patel O, Vivian JP, Matsuda JL, McCluskey J, Godfrey DI, Marrack P, Rossjohn J, Gapin L. A Molecular Basis for NKT Cell Recognition of CD1d-Self-Antigen. Immunity. 2011;34:315. doi: 10.1016/j.immuni.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matulis G, Sanderson JP, Lissin NM, Asparuhova MB, Bommineni GR, Schumperli D, Schmidt RR, Villiger PM, Jakobsen BK, Gadola SD. Innate-like control of human iNKT cell autoreactivity via the hypervariable CDR3beta loop. PLoS Biol. 2010;8:e1000402. doi: 10.1371/journal.pbio.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan BA, Nagarajan NA, Wingender G, Wang J, Scott I, Tsuji M, Franck RW, Porcelli SA, Zajonc DM, Kronenberg M. Mechanisms for glycolipid antigen-driven cytokine polarization by Valpha14i NKT cells. J Immunol. 2010;184:141. doi: 10.4049/jimmunol.0902880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel O, Cameron G, Pellicci DG, Liu Z, Byun HS, Beddoe T, McCluskey J, Franck RW, Castano AR, Harrak Y, Llebaria A, Bittman R, Porcelli SA, Godfrey DI, Rossjohn J. NKT TCR Recognition of CD1d-{alpha}-C-Galactosylceramide. J Immunol. 2011;187:4705. doi: 10.4049/jimmunol.1100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J Exp Med. 2003;198:1631. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lombardi V, Stock P, Singh AK, Kerzerho J, Yang W, Sullivan BA, Li X, Shiratsuchi T, Hnatiuk NE, Howell AR, Yu KO, Porcelli SA, Tsuji M, Kronenberg M, Wilson SB, Akbari O. A CD1d-dependent antagonist inhibits the activation of invariant NKT cells and prevents development of allergen-induced airway hyperreactivity. J Immunol. 2010;184:2107. doi: 10.4049/jimmunol.0901208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Girardi E, Wang J, Yu ED, Painter GF, Kronenberg M, Zajonc DM. The Valpha14 invariant natural killer T cell TCR forces microbial glycolipids and CD1d into a conserved binding mode. J Exp Med. 2010;207:2383. doi: 10.1084/jem.20101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zajonc DM, Cantu C, 3rd, Mattner J, Zhou D, Savage PB, Bendelac A, Wilson IA, Teyton L. Structure and function of a potent agonist for the semiinvariant natural killer T cell receptor. Nat Immunol. 2005;6:810. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Li Y, Kinjo Y, Mac TT, Gibson D, Painter GF, Kronenberg M, Zajonc DM. Lipid binding orientation within CD1d affects recognition of Borrelia burgorferi antigens by NKT cells. Proc Natl Acad Sci U S A. 2010;107:1535. doi: 10.1073/pnas.0909479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leslie AG. The integration of macromolecular diffraction data. Acta Crystallogr D Biol Crystallogr. 2006;62:48. doi: 10.1107/S0907444905039107. [DOI] [PubMed] [Google Scholar]
- 39.Vagin AA, Teplyakov A. MOLREP: an automated programm for molecular replacement. J. Appl. Cryst. 1997;30:1022. [Google Scholar]
- 40.CCP4, C. C. P., Number 4 The CCP4 Suite: Programs for Protein Crystallography. Acta Crystallogr. 1994;D50:760. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 41.Zajonc DM, Savage PB, Bendelac A, Wilson IA, Teyton L. Crystal structures of mouse CD1d-iGb3 complex and its cognate Valpha14 T cell receptor suggest a model for dual recognition of foreign and self glycolipids. J Mol Biol. 2008;377:1104. doi: 10.1016/j.jmb.2008.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 43.Winn MD, Isupov MN, Murshudov GN. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr D Biol Crystallogr. 2001;57:122. doi: 10.1107/s0907444900014736. [DOI] [PubMed] [Google Scholar]
- 44.Lovell SC, Davis IW, Arendall WB, 3rd, de Bakker PI, Word JM, Prisant MG, Richardson JS, Richardson DC. Structure validation by Calpha geometry: phi,psi and Cbeta deviation. Proteins. 2003;50:437. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 45.Harrak Y, Barra CM, Bedia C, Delgado A, Castano AR, Llebaria A. Aminocyclitol-substituted phytoceramides and their effects on iNKT cell stimulation. ChemMedChem. 2009;4:1608. doi: 10.1002/cmdc.200900193. [DOI] [PubMed] [Google Scholar]
- 46.Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, Yagita H, Godfrey DI. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 2002;99:1259. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 47.Gui M, Li J, Wen LJ, Hardy RR, Hayakawa K. TCR beta chain influences but does not solely control autoreactivity of V alpha 14J281T cells. J Immunol. 2001;167:6239. doi: 10.4049/jimmunol.167.11.6239. [DOI] [PubMed] [Google Scholar]
- 48.Pellicci DG, Patel O, Kjer-Nielsen L, Pang SS, Sullivan LC, Kyparissoudis K, Brooks AG, Reid HH, Gras S, Lucet IS, Koh R, Smyth MJ, Mallevaey T, Matsuda JL, Gapin L, McCluskey J, Godfrey DI, Rossjohn J. Differential recognition of CD1d-alpha-galactosyl ceramide by the V beta 8.2 and V beta 7 semi-invariant NKT T cell receptors. Immunity. 2009;31:47. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wun KS, Cameron G, Patel O, Pang SS, Pellicci DG, Sullivan LC, Keshipeddy S, Young MH, Uldrich AP, Thakur MS, Richardson SK, Howell AR, Illarionov PA, Brooks AG, Besra GS, McCluskey J, Gapin L, Porcelli SA, Godfrey DI, Rossjohn J. A molecular basis for the exquisite CD1d-restricted antigen specificity and functional responses of natural killer T cells. Immunity. 2011;34:327. doi: 10.1016/j.immuni.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tashiro T, Sekine-Kondo E, Shigeura T, Nakagawa R, Inoue S, Omori-Miyake M, Chiba T, Hongo N, Fujii S, Shimizu K, Yoshiga Y, Sumida T, Mori K, Watarai H, Taniguchi M. Induction of Th1-biased cytokine production by alpha-carba-GalCer, a neoglycolipid ligand for NKT cells. Int Immunol. 2010;22:319. doi: 10.1093/intimm/dxq012. [DOI] [PubMed] [Google Scholar]
- 51.Gillessen S, Naumov YN, Nieuwenhuis EE, Exley MA, Lee FS, Mach N, Luster AD, Blumberg RS, Taniguchi M, Balk SP, Strominger JL, Dranoff G, Wilson SB. CD1d-restricted T cells regulate dendritic cell function and antitumor immunity in a granulocyte-macrophage colony-stimulating factor-dependent fashion. Proc Natl Acad Sci U S A. 2003;100:8874. doi: 10.1073/pnas.1033098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Togashi Y, Chamoto K, Wakita D, Tsutsumi N, Iwakura Y, Matsubara N, Kitamura H, Nishimura T. Natural killer T cells from interleukin-4-deficient mice are defective in early interferon-gamma production in response to alpha-galactosylceramide. Cancer Sci. 2007;98:721. doi: 10.1111/j.1349-7006.2007.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest. 2003;111:291. doi: 10.1172/JCI17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Renauld JC. New insights into the role of cytokines in asthma. J Clin Pathol. 2001;54:577. doi: 10.1136/jcp.54.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 56.Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci U S A. 2006;103:2782. doi: 10.1073/pnas.0510282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aspeslagh S, Li Y, Yu ED, Pauwels N, Trappeniers M, Girardi E, Decruy T, Beneden K. Van, Venken K, Drennan M, Leybaert L, Wang J, Franck RW, Van Calenbergh S, Zajonc DM, Elewaut D. Galactose-modified iNKT cell agonists stabilized by an induced fit of CD1d prevent tumour metastasis. Embo J. 2011;30:2294. doi: 10.1038/emboj.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bricard G, Venkataswamy MM, Yu KO, Im JS, Ndonye RM, Howell AR, Veerapen N, Illarionov PA, Besra GS, Li Q, Chang YT, Porcelli SA. Alpha-galactosylceramide analogs with weak agonist activity for human iNKT cells define new candidate anti-inflammatory agents. PLoS ONE. 2010;5:e14374. doi: 10.1371/journal.pone.0014374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Semmling V, Lukacs-Kornek V, Thaiss CA, Quast T, Hochheiser K, Panzer U, Rossjohn J, Perlmutter P, Cao J, Godfrey DI, Savage PB, Knolle PA, Kolanus W, Forster I, Kurts C. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat Immunol. 2010;11:313. doi: 10.1038/ni.1848. [DOI] [PubMed] [Google Scholar]
- 60.Silk JD, Salio M, Reddy BG, Shepherd D, Gileadi U, Brown J, Masri SH, Polzella P, Ritter G, Besra GS, Jones EY, Schmidt RR, Cerundolo V. Cutting edge: nonglycosidic CD1d lipid ligands activate human and murine invariant NKT cells. J Immunol. 2008;180:6452. doi: 10.4049/jimmunol.180.10.6452. [DOI] [PubMed] [Google Scholar]