Abstract
Background and Aims
Deceased donor liver transplantation (DDLT) rates for candidates with hepatocellular carcinoma (HCC) have significantly increased in the MELD era because of the extra priority given to these candidates. We examined the incidence and pre-DDLT radiological and donor factors associated with post-DDLT HCC recurrence in the MELD era.
Methods
Outcomes of HCC candidates age ≥18 years that underwent DDLT between 2/28/02-6/30/08 (N=94) were reviewed. Primary outcome was biopsy-proven post-LT HCC recurrence at any site. Kaplan-Meier analysis was used to calculate the cumulative incidence and Cox regression was used to identify the predictors of post-LT HCC recurrence.
Results
Median age of the 94 candidates who met the study criteria was 54 years, 64% had hepatitis C, median lab MELD was 13 and median pre-LT AFP was 47 ng/dl. Based upon pre-DDLT imaging, 94% candidates met Milan criteria. The median waiting time to transplant was 47 days and 27% received pre-DDLT loco-regional therapy. Seventeen (18%) developed HCC recurrence after 2.1 median years with a cumulative incidence of 6.8%, 12% and 19% at 1, 2 and 3-years post-DDLT. The pre-DDLT number of lesions (P=0.015), largest lesion diameter (P=0.008), and higher donor age (P=0.002) were the significant predictors of HCC recurrence after adjusting for pre-LT loco-regional therapy and waiting time. Post-LT HCC recurrence (P<0.0001) and higher donor age (P=0.029) were associated with lower post-LT survival.
Conclusions
Post-LT HCC recurrence is higher in our MELD era cohort than the reported rate of 8% at 4-year in Mazzaferro et al. study. The risk of HCC recurrence was significantly associated with the number of lesions and size of the largest lesion at the time of DDLT as well as with older donor age. Risk stratification using a predictive model for post-LT HCC recurrence based on pre-LT imaging and donor factors may help guide candidate selection and tailoring of HCC surveillance strategies after LT.
Keywords: Hepatocellular Carcinoma, Deceased Donor Liver transplantation, Model for End Stage Liver Disease
Introduction
Surgical resection is not a viable option for majority of candidates with end stage liver disease and early stage hepatocellular carcinoma (HCC) candidates because of the risk of hepatic decompensation due to insufficient functional hepatic synthetic reserve after the removal of hepatic parenchyma. Liver transplantation offers the best chance of cure and long term survival for majority of patients with end stage liver disease and early stage hepatocellular carcinoma (HCC) (1). Mazzaferro et al. prospectively performed deceased donor liver transplantation (DDLT) in 48 patients with cirrhosis and small HCC defined as single lesions 2 to 5 cm or three lesions each less than 3 cm in maximal diameter and with no evidence of portal vein thrombosis or extrahepatic invasion (Milan-criteria) (1). The 4-year recurrence free survival in their cohort was 83% with an overall recurrence rate of 8% at 4 years (1). Since then several other studies reported either similar or superior post-transplant outcomes of early stage HCC meeting Milan-criteria compared to nonmalignant indications (1–4).
Since progression beyond the Milan-criteria was equated with unsuitability for DDLT, which may result in death, the policymakers incorporated the priority MELD score for HCC candidates meeting Milan criteria in the MELD-based allocation adopted in February 2002. Based upon the tumor doubling time for small HCC, initially, it was estimated that HCC candidates with stage T1 (1-lesion < 2 cm), and stage T2 (1-lesion 2–5 cm or 2–3 lesions all <3 cm) tumors would have mortality risk of 15% (equivalent to MELD score of 24) and 30% (equivalent to MELD score of 29), respectively (5, 6).
Although the outcomes including time to DDLT, 5-month dropout rates from the waitlist and DDLT rates for HCC candidates with stage T1 (MELD=24) and stage T2 (MELD=29) tumor improved significantly compared to pre-MELD era, the dropout rates for non-HCC candidates with similar MELD scores (24 and 29) were significantly higher and their DDLT rates were significantly lower than HCC candidates (3). These observations suggested a disproportionately higher advantage of priority MELD score to HCC candidates relative to non-HCC candidates and led to downward revisions in priority scores for HCC candidates (4, 6).
Despite these revisions in the priority score, a significantly higher proportion of HCC candidates are getting deceased donor DDLT in the MELD era, compared to pre-MELD era (4, 7). However, the time lag between radiological imaging and DDLT despite palliation with loco-regional therapies may still result in HCC progression. Moreover, Organ Procurement and Transplantation Network regions with shorter waiting time may transplant HCC candidates with aggressive tumor biology at a faster rate than other regions. All these factors may increase the risk of HCC recurrence after DDLT in the MELD era.
We hypothesized that the incidence of HCC recurrence after DDLT may be higher than the historically reported recurrence rate of 8% at four years after DDLT (1). Furthermore, there are additional recipient and donor factors may contribute to HCC recurrence. In this study, we aimed to determine the incidence and recipient and donor specific predictors of post-DDLT HCC recurrence in the MELD era.
Methods and Material
Patient population and Data Collection
Medical records of all adult patients (age ≥ 18 years) who received DDLT between February 28, 2002 and June 30, 2008 for HCC at the University of Michigan were reviewed. The study was approved by University of Michigan Institutional Review Board. Candidates listed as status 1, received living donor liver transplant, repeat DDLT, multi-organ transplant, or found to have incidental HCC on explants were excluded. Patients were followed till December 31st, 2008.
Data collected included demographics (age, gender, race/ethnicity), date of listing, date of transplant, date of HCC recurrence, date of last follow-up and death, diagnosis, history of smoking, alcohol, pre-DDLT history of hypertension, diabetes, laboratory MELD score at the time of listing and transplant, alpha fetoprotein (AFP) within 6 months of listing and DDLT, pre-DDLT radiology data including number of lesions, size of each lesion (cm), total size of all lesions(cm), size of largest lesion(cm), portal venous thrombosis, meeting Milan-criteria pre-DDLT, pre-DDLT biopsy, history of loco-regional treatment. We also collected data on donor factors such as donor age, gender and cold ischemia time, explant factors such as number of lesion on explants, size of each lesion(cm), total size of all lesions(cm), size of largest lesion(cm), Milan-criteria on explants, microvascular and macrovascular invasion, post-DDLT recipient factors such as immunosuppression, history of rejection, date of recurrence of HCC, site of recurrence, status at the end of follow-up, and history of graft failure.
Pre-DDLT imaging and loco-regional therapy
Pre-DDLT imaging consisted of MRI with gadolinium or helical CT of the liver for tumor staging and diagnosis. Per center protocol, all subjects also underwent a chest CT to rule out extrahepatic spread and serum AFP testing. Every candidate with HCC is presented at the multidisciplinary liver tumor board and the decision is made regarding the type of pre-DDLT loco-regional therapy based upon tumor location, liver disease severity, and projected waiting time. Imaging was then repeated at 3 to 4 month intervals to assess for tumor progression and/or response to loco-regional therapy. The imaging study closest to the DDLT was used for the analysis.
Immunosuppression
Immunosuppression consisted of tacrolimus, mycophenolate, and prednisone. Most patients were tapered to a single drug by one year. Basiliximab induction was used in subjects with renal insufficiency at LT or early acute kidney injury.
Post-DDLT HCC surveillance and HCC recurrence
DDLT recipients with HCC on explant underwent surveillance at 3 month, 6 month and yearly thereafter for 3 years with AFP and imaging with CT scan of chest, abdomen and pelvis and bone scan. Any suspicious lesion was further confirmed by directed biopsy. Patients with known or suspected HCC recurrence had their immunosuppression minimized and were offered palliative surgical or radiation therapy for bulky or symptomatic metastases.
Statistical Analysis
The quantitative variables and categorical variables were expressed as median and range, and counts and percentage, respectively. The primary outcome was HCC recurrence defined as biopsy proven HCC in liver, bone or any other extra-hepatic site. The continuous variables and categorical variables between HCC recurrence and non-HCC recurrence group were compared using Mann-Whitney test and the Chi-square or Fisher exact test.
Kaplan-Meier analysis was used to calculate the probability of post-DDLT HCC recurrence and overall patient survival. Log rank test was used to compare the overall post-DDLT survival probabilities among DDLT recipients for HCC (n=94) and DDLT recipients of non-HCC indications (n=221) at our institution during the study period. Multivariable Cox regression analysis (backward selection-likelihood ratios) was used to evaluate the predictors of post-DDLT HCC recurrence.
The accuracy of HCC recurrence risk prediction based upon the independent predictors and pre-DDLT Milan criteria in predicting HCC recurrence was assessed using index of concordance (IOC), the goodness of fit for survival models. It estimates the probability of concordance between predicted and observed outcomes in pairs of candidates (16, 17). It is the percentage of pairs of candidates in which at least one had an event (death) of interest and for which the observed mortality and predicted mortality are concordant. IOC of 1 is perfect separation of patients with different outcomes whereas IOC of 0.5 is no predictive discrimination by the model (16). The IOC for time dependent model is equivalent to ROC (C-statistics) for logistic regression model.
The effect of post-DDLT HCC recurrence on post-DDLT survival was assessed using time-dependent Cox regression. P-value <0.05 was considered as significant.
Results
Description of Cohort and Patient Characteristics
Three hundred and fifteen patients underwent DDLT at our institution during the study period. Two hundred and twenty one patients were transplanted for non-HCC indications and 94 underwent DDLT for the diagnosis of HCC.
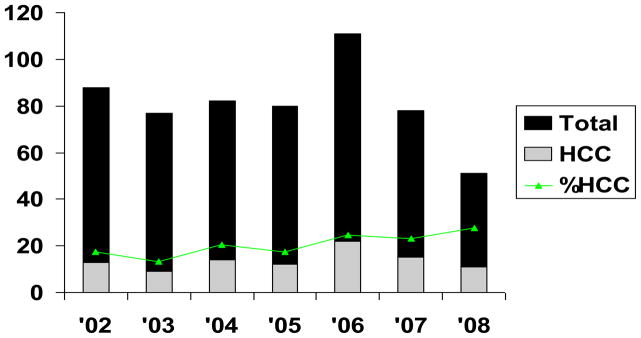
The median age at DDLT of 94 recipients for HCC was 54 years, 71% were males and 69% had hepatitis C. Median lab MELD score at DDLT was 13 and the median AFP within six months of DDLT was 47 ng/ml. The median time to DDLT was 47 days. The demographic and pre-DDLT radiological characteristics of patients with and without post-DDLT HCC recurrence are shown in Table 1. Figure 1 shows the proportion of HCC candidates transplanted per year during the study period at our institution.
Table 1.
Patient characteristics and pre-DDLT radiological donor factors of patients with and without post-DDLT HCC.
| HCC Recurrence
|
P-Value | ||
|---|---|---|---|
| Yes (N=17) | No (N=77) | ||
| Age at LT | 55 (48–70) | 54 (23–72) | 0.21 |
|
| |||
| Males | 12 (71%) | 55 (71%) | 0.95 |
|
| |||
| Caucasians | 14 (82%) | 59 (77%) | 0.86 |
| African-Americans | 1 (6%) | 11 (14%) | |
| Others | 2 (12%) | 7 (9%) | |
|
| |||
| Hepatitis B | 1 (6%) | 5 (7%) | 0.63 |
| Hepatitis C | 12 (70%) | 52 (68%) | |
| Alcoholic Cirrhosis | 2 (12%) | 9 (12%) | |
| Non-alcoholic liver disease | 2 (12%) | 6 (8%) | |
| Others | 0 | 5 (7%) | |
|
| |||
| History of Smoking | 10 (59%) | 41 (53%) | 0.79 |
|
| |||
| History of Alcohol | 12(71%) | 38 (49%) | 0.18 |
|
| |||
| Pre-DDLT Diabetes | 4 (24%) | 19 (25%) | 0.92 |
|
| |||
| Pre-DDLT hypertension | 4 (24%) | 12 (16%) | 0.48 |
|
| |||
| Pre-DDLT AFP (ng/dl) | 23 (1–20100) | 21 (1–1550) | 0.76 |
|
| |||
| Lab MELD at listing | 13 (6–19) | 13 (7–31) | 0.20 |
|
| |||
| Waiting time (days) | 48 (2–2806) | 46 (2–3461) | 0.96 |
|
| |||
| Pre-LT Radiological Features | |||
|
| |||
| Within Milan | 14 (82%) | 74 (97%) | 0.04 |
| • T1 | 1 (6%) | 5 (7%) | |
| • T2 | 13 (82%) | 69 (91%) | |
| Outside Milan | 3 (12%) | 3(2%) | |
| Portal Vein Thrombus | 1 (6%) | 1 (1%) | 0.33 |
|
| |||
| Number of lesions | 1 (1–4) | 1 (1–4) | 0.35 |
| Largest lesion Diameter (cm) | 3.1 (1.8–6.1) | 2.8 (1.2–5.6) | 0.15 |
| Total Size all lesion (cm) | 4.3 (1.9–6.4) | 3.2 (1.2–8.7) | 0.06 |
|
| |||
| Loco-regional treatment | 7(41%) | 18 (23%) | 0.14 |
Figure 1.
Proportion of Patients Transplanted with HCC during the study period (2002–2008)
Pre-DDLT Radiological Tumor Characteristics
Based upon pre-DDLT imaging, 88 (94%) patients met Milan-criteria. The proportion of patients outside pre-DDLT Milan criteria was higher in post-DDLT HCC recurrence group compared to the patients without post-DDLT HCC recurrence (12% vs. 2%, P=0.041). Pre-DDLT biopsy of the lesion is not necessary to make the diagnosis of HCC. However, 43% patients had directed liver biopsy of the lesion before DDLT that confirmed the HCC in our cohort. The proportion of pre-DDLT liver biopsy for HCC confirmation was similar in patients with and without HCC recurrence (47% vs. 42%, P=0.17).
Overall, pre-DDLT loco-regional treatment of HCC was given to 25 (27%) patients including 5 patients who were downsized as they were outside the Milan-criteria. Sixteen (80%) had radiofrequency ablation. Eight (32%) had trans-arterial chemoembolization, and 1 (4%) patient had resection. The proportion of patients underwent pre-DDLT loco-regional therapy was similar in patients with and without post-LT HCC recurrence (Table 1)
Donor Characteristics
The median donor age was 38 years for the entire cohort, 50% were male donors, and median cold ischemia time was 7.5 hours. The donor risk index could not be calculated because of the lack of availability of donor data (height, race, cause of death and regional versus local) on all of the donors. The median donor age for the patients with HCC recurrence was higher than those who did not have HCC recurrence (49 years vs. 36 years; P=0.008). The distribution of donor gender (P=0.95) and cold ischemia time (P =0.3) were similar in both groups.
Explant Tumor Characteristics
Sixty (64%) patients met Milan-criteria based upon the explant data. Six (6%) patients did not have HCC, 5 (5%) had stage T1 disease, 55 (59%) had stage T2 disease and 28 (30%) were beyond stage T2. Microvascular invasion was seen in eighteen (19%) patients (stage T1, n=2; stage T2, n=12 and > T2, n=4). The explant characteristics, post-LT patient immunosuppression and outcomes of patients with and without post-DDLT HCC are shown in Table 2.
Table 2.
Explant and post-DDLT characteristics of patients with and without post-DDLT HCC recurrence
| HCC Recurrence
|
P-Value | ||
|---|---|---|---|
| Yes (N=17) | No (N=77) | ||
| Explant Characteristics | |||
| Within Milan | 7(44%) | 53 (75%) | 0.033 |
| T1 Stage | 0 | 5(7%) | 0.017 |
| T2 Stage | 7(44%) | 48 (67%) | |
| > T2 Stage (outside Milan) | 9 (56%) | 19 (26%) | |
| No HCC | 1 (6%) | 5 (7%) | 1.0 |
| Lesions | 2(0–7) | 1 (0–7) | 0.2 |
| Largest Diameter | 3.1 (1.5–7) | 2.85 (1–6.5) | 0.31 |
| Total size | 5.8 (0–10.9) | 3.4 (0–11) | 0.02 |
| Micro invasion | 3(18%) | 15 (20%) | 1.0 |
|
| |||
| Tacrolimus | 14(82%) | 64 (83%) | 1.0 |
| Cyclosporine | 3(18%) | 13(17%) | |
|
| |||
| Rejection | 1(6%) | 5(7%) | 1.0 |
|
| |||
| No. of deaths | 10(59%) | 11 (14%) | <0.0001 |
|
| |||
| Follow up time (years) | 2.0 (0.2–6.4) | 2.3 (0.1–6.6) | 0.39 |
Incidence of HCC Recurrence after DDLT
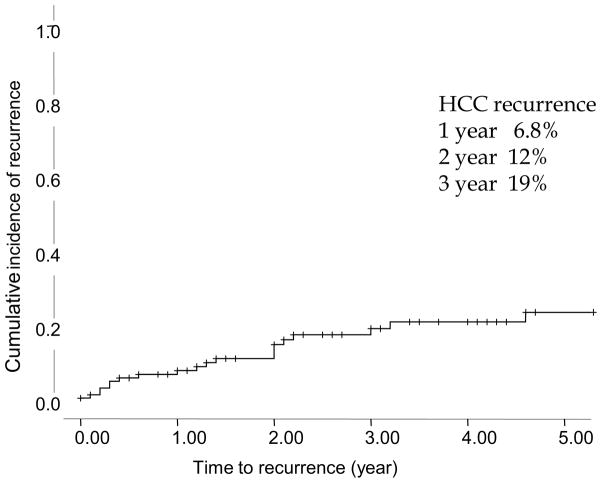
HCC recurred in 17 (18%) patients after DDLT. The incidence rate of post-DDLT HCC recurrence was 7.34 per 100 patient-years. The cumulative probability of HCC recurrence after DDLT at 1-, 2- and 3-year was 6.8%, 12% and 19% (Figure 2).
Figure 2.
Cumulative incidence of post-DDLT HCC recurrence
Site of HCC recurrence
Almost all patients with HCC recurrence were asymptomatic. They were diagnosed with post-LT HCC recurrence on surveillance imaging which was subsequently biopsied. Only two patients presented with the symptoms of cord compression. Six patients had only one site of HCC recurrence (adrenal mass=3, lung nodule=1, abdominal soft tissue mass=2) whereas eleven patients presented with multiple sites of HCC recurrence including abdomen, thorax, bones and spinal cord. Of these 11 patients, five had adrenal mass, four had pulmonary nodule while two had abdominal soft tissue masses on surveillance imaging, seven patients had spinal cord involvement on imaging study, and only six patients had HCC recurrence in their allograft. None of them had brain metastases.
Predictors of HCC Recurrence based upon pre-DDLT Patient, Radiological and Donor Characteristics
Since the aim of this study was to determine the predictors of HCC recurrence based upon pre-DDLT radiological tumor characteristics and donor factors, only pre-DDLT patient characteristics, pre-DDLT radiological and donor factors were used as covariates in the Cox regression model. In the covariate-adjusted model, after adjusting for age at DDLT, waiting time, loco-regional therapy and AFP, pre-DDLT number of lesions (HR=2.18 per lesion, P=0.015), pre-DDLT largest lesion diameter (HR=2.25 per cm, P=0.008), and donor age (HR=1.06 per year, P=0.002) were the independent predictors of HCC recurrence (Table 2).
HCC-Recurrence Risk Index was constructed based upon the significant predictors obtained from multivariate analysis (Table 3). The components included pre-DDLT number of lesion, diameter of the largest lesion and donor age. The IOC (95% confidence interval) of HCC-recurrence risk index was 0.76 (0.63–0.88) whereas the IOC (95% confidence interval) of pre-DDLT Milan-criteria in our cohort was only 0.55 (0.46–0.63).
Table 3.
Multivariable Analysis: Predictors of post-DDLT HCC recurrence based upon pre-DDLT and donor characteristics
| Variables | HR (95% Confidence Interval) | P-Value |
|---|---|---|
| No. of lesions | 2.18 (1.16–4.13) | 0.015 |
| Largest lesion diameter (cm) | 2.25 (1.23–4.10) | 0.008 |
| Donor Age (per year) | 1.06 (1.02–1.10) | 0.002 |
Abbreviations: DDLT Liver Transplantation;
Overall Post-DDLT Patient Survival
A total of 21 deaths occurred after a median follow up of 2.2 years (0.02–6.6) in the study cohort. Ten patients in the HCC recurrence group and 11 patients in non- HCC recurrence group died. The risk of death increased with increase in donor age (HR=1.035, P=0.029) and presence of HCC recurrence (HR= 43.4, P<0.0001).
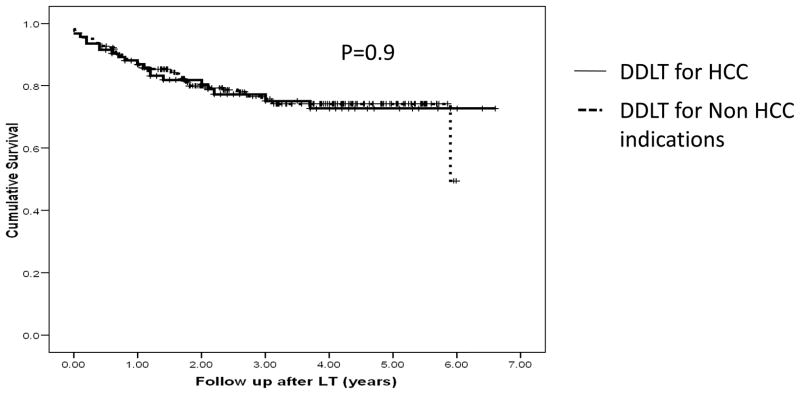
However, the overall post-transplant patient survival among DDLT recipients for HCC and non-HCC indications during the study period was similar (Figure 3).
Figure 3.
Post-DDLT survival among DDLT recipients for HCC (N=94) and non-HCC indications (N=221). P=0.9 (Log-rank test)
Discussion
In the MELD era, post-DDLT HCC recurrence rate was higher compared to the historical rate of 8% at four years in Mazzaferro’s study(1). The number of lesions, size of the largest lesion and the donor age were the significant predictors of post-DDLT HCC recurrence. Every centi-meter increase in the size of largest lesion was associated with a 2.25 fold increased risk of HCC recurrence after DDLT. AFP, use of loco-regional therapy before DDLT and waiting time did not predict the post-DDLT recurrence in our cohort.
The primary outcome in our study was post-LT HCC recurrence, which is one of major cause of graft failure and mortality in these patients. The HCC-RRI based upon pre-LT number of lesion, size of largest lesion and donor age performed better than Milan criteria (gold standard for listing HCC candidates) in post-DDLT HCC risk prediction. However, the accuracy of HCC-RRI needs to be investigated in the larger independent dataset(s). Although the pre-DDLT tumor size and pre-DDLT number of lesions are the integral components of the Milan criteria(1), our study used them as continuous variables as opposed to categorical variables (pre-set cut offs).
In our study, the donor age was associated with HCC recurrence. This was a novel finding. While the biological plausibility of donor age in the development of post-DDLT HCC recurrence needs to be investigated further, we speculate that older donor livers have a lower tolerance for preservation and greater susceptibility to cold ischemia. In addition, age-related immune changes in liver response, steatosis, iron content, preexistent fibrosis, and telomeres or replicative senescence may be some of the key factors that determine the increased susceptibility of the older liver to HCC recurrence. Alternatively, there might be a selection bias (accepting older donor allograft for HCC candidates with tumor size approaching the upper limit of Milan-criteria), however, the interaction between donor age and tumor size was not significant.
Implementation of Milan-criteria as the basis of selecting candidates with HCC for DDLT has improved the post transplant survival among these patients. Given the good post-DDLT outcomes, there has been a significant amount of debate in expanding the Milan-criteria. Strategies for expansion of HCC-eligibility include the University of California-San Francisco (UC-SF) crteria based upon the total tumor volume and expanding Milan-criteria to Milan-7(7, 8). Most of the published studies that support an expansion of the limits are based upon the post-DDLT survival and an analysis of explanted livers, information that is not available prior to surgery. In some studies, the radiological staging was not available or not uniformly performed (7, 9, 10). Moreover, the patients in the expanded population are analyzed together with patients within the conventional Milan-criteria (11). This may have introduced confounding and resulted in a dilution of the potentially poor outcome cohort with those individuals having a good outcome.
The modification or acceptance of a new pre-DDLT listing criteria for HCC candidates should be based upon a critical balance between of risk of progression of the HCC on the waiting list and the acceptable post-transplant life expectancy of these patients while minimizing the negative impact for non-HCC candidates. However, the lack of pre-DDLT predictive data to identify patients at higher risk of progression (and thus dropout) and aggressive listing and transplantation of HCC candidates may result in excessive post-DDLT HCC recurrence. The results from the Adult to Adult Living Donor Liver transplantation retrospective cohort analysis showed higher recurrence among living donor DDLT recipients compared to deceased donor DDLT recipients (13). The increased risk of post-DDLT HCC recurrence among living donor DDLT recipient could not be explained based upon their data analysis, although the living donor DDLT recipients had higher tumor stage compared to deceased donor DDLT recipients (12).
Some studies including national data from the Scientific Registry of Transplant Recipients (SRTR) have modeled post-LT survival as their primary outcome as opposed to HCC recurrence because HCC recurrence after LT is not captured by SRTR, while some other studies used recurrence free survival as primary outcome (1, 11, 13, 14). The predictors of HCC recurrence may be different than the predictors of post-LT survival because LT recipients may die due to causes other than HCC recurrence. Our study shows that once the HCC recurrence is detected, the risk of death increased several fold. Similar to Ioannou et al. our study did not find any relationship between post-LT survival and waiting time as well pre-DDLT loco-regional therapy. In addition, our study did not find any association between pre-DDLT AFP and post-DDLT survival as previously described because of small sample size (13).
The major limitations of our study include its retrospective design and associated problems including bias and confounding due to patient selection and unmeasurable patient characteristics, small number of events and the lack of a comparison arm from the pre-MELD era. Despite these limitations, this hypothesis generating study has evaluated the incidence of post-DDLT HCC recurrence and identified the important recipient and donor risk factors associated with post-DDLT HCC recurrence.
In conclusion, the incidence of post-DDLT HCC recurrence may have increased in the MELD era compared to the reported rate of 8% in the landmark Mazzaferro series (1). The post-DDLT HCC recurrence risk increases with the increase in number of lesions and the size of the largest lesion as well as the advance donor age. Although the model based upon pre-DDLT largest lesion diameter, number of lesions and donor age predicted HCC recurrence better than pre-DDLT Milan criteria in our cohort, this finding needs to be confirmed in larger datasets. Risk stratification for HCC recurrence based upon recipient and donor risk factors may help guide candidate selection and inform development of evidence-based post DDLT HCC surveillance strategies.
Acknowledgments
Dr. Pratima Sharma was supported by American Society of Transplantation/Roche clinical science faculty development grant for 2008 and Michigan Institute for Clinical and Health Research NIH-CTSA, UL1RR024986, Collaborative Type 2 (Bedside to community) grant. Dr. Sharma is also supported by National Institutes of Health grant KO8 DK-088946. This research was presented, in part, as a free communication at the American Transplant Congress, 2010, held in San Diego, California.
Abbreviations
- DDLT
Deceased Donor Liver transplantation
- HCC
Hepatocellular Carcinoma
- IOC
Index of Concordance
- MELD
Model for End Stage Liver Disease
References
- 1.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 2.Freeman RB, Wiesner RH, Edwards E, Harper A, Merion R, Wolfe R. Results of the first year of the new liver allocation plan. Liver Transpl. 2004;10:7–15. doi: 10.1002/lt.20024. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P, Balan V, Hernandez JL, Harper AM, Edwards EB, Rodriguez-Luna H, Byrne T, et al. Liver transplantation for hepatocellular carcinoma: the MELD impact. Liver Transpl. 2004;10:36–41. doi: 10.1002/lt.20012. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Harper AM, Hernandez JL, Heffron T, Mulligan DC, Wiesner RH, Balan V. Reduced priority MELD score for hepatocellular carcinoma does not adversely impact candidate survival awaiting liver transplantation. Am J Transplant. 2006;6:1957–1962. doi: 10.1111/j.1600-6143.2006.01411.x. [DOI] [PubMed] [Google Scholar]
- 5.Barbara L, Benzi G, Gaiani S, Fusconi F, Zironi G, Siringo S, Rigamonti A, et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology. 1992;16:132–137. doi: 10.1002/hep.1840160122. [DOI] [PubMed] [Google Scholar]
- 6.UNOS/OPTN. 3.6 Organ Distribution: Allocation of Livers. Policies. 2002 http://www.unos.org/PoliciesandBylaws/policies/pdfs/policy_8.pdf.
- 7.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 8.Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 9.Toso C, Trotter J, Wei A, Bigam DL, Shah S, Lancaster J, Grant DR, et al. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008;14:1107–1115. doi: 10.1002/lt.21484. [DOI] [PubMed] [Google Scholar]
- 10.Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8:765–774. doi: 10.1053/jlts.2002.34892. [DOI] [PubMed] [Google Scholar]
- 11.Toso C, Asthana S, Bigam DL, Shapiro AM, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009;49:832–838. doi: 10.1002/hep.22693. [DOI] [PubMed] [Google Scholar]
- 12.Fisher RA, Kulik LM, Freise CE, Lok AS, Shearon TH, Brown RS, Jr, Ghobrial RM, et al. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant. 2007;7:1601–1608. doi: 10.1111/j.1600-6143.2007.01802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ioannou GN, Perkins JD, Carithers RL., Jr Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134:1342–1351. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Wright F, Steinberg T, et al. Predictors of long-term outcome following liver transplantation for hepatocellular carcinoma: a single-center experience. Transpl Int. 2007;20:747–753. doi: 10.1111/j.1432-2277.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 15.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 16.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Kremers WK. Fixed and Time-Dependent Covariates and Possible Ties in Predictor and Time. Mayo Clinic Foundation; 2007. Concordance for Survival Time Data. [Google Scholar]