Abstract
Previous studies demonstrate a positive correlation between pesticide usage and Parkinson’s disease (PD), which preferentially targets dopaminergic (DAergic) neurons. In order to examine the potential relationship between two common pesticides and specific neurodegeneration, we chronically (24 hours) or acutely (30 min) exposed two Caenorhabditis elegans (C. elegans) strains to varying concentrations (LC25, LC50 or LC75) of TouchDown® (TD) as per cent active ingredient (glyphosate), or Mancozeb® (MZ) as per cent active ingredient (manganese/zinc ethylene-bis-dithiocarbamate). Furthermore, to more precisely model environmental exposure, worms were also exposed to TD for 30 min, followed by 30-min incubation with varying MZ concentrations. Previous data from out lab suggested general neuronal degeneration using the worm strain NW1229 (pan-neuronal::green fluorescent protein (GFP) construct). To determine whether distinct neuronal groups were preferentially affected, we specifically used EG1285 (GABAergic neurons::GFP construct) and BZ555 (DAergic neurons::GFP construct) worms to verify GABAergic and DAergic neurodegeneration, respectively. Results indicated a statistically significant decrease, when compared to controls (CN), in number of green pixels associated with GABAergic neurons in both chronic (*p < 0.05) and acute (*p < 0.05) treatment paradigms. Analysis of the BZ555 worms indicated a statistically significant decrease (*p < 0.05) in number of green pixels associated with DAergic neurons in both treatment paradigms (chronic and acute) when compared to CN. Taken together, our data suggest that exposure to TD and/or MZ promotes neurodegeneration in both GABAergic and DAergic neurons in the model organism C. elegans.
Keywords: C. elegans, glyphosate, mancozeb, neurodegeneration, BZ555, EG1285
INTRODUCTION
Parkinson’s disease (PD) is typically associated with degeneration of the dopaminergic (DAergic) neurons of the substantia nigra pars compacta (Weintraub et al. 2008). Thus, many studies involving environmental toxicants, such as pesticides or heavy metals, which may contribute to the etiology of PD, mainly focus on the ability of such chemicals to damage DAergic neurons (Cicchetti et al. 2005; Kim et al. 2002; Li et al. 2005). More recently, however, the importance of non-DAergic neurons, particularly those using γ-aminobutyric acid (GABA), has been recognized in conjunction with non-motor symptoms associated with the disease (Barone 2010; Langston 2006). Unfortunately, in vivo determination of neuronal populations affected by pesticides is often complicated by the complexity of human and mammalian nervous systems (Peterson et al. 2008). In vitro experiments involving mixed cell or tissue cultures are often hampered by the inability to recreate complex neural circuits. Model organisms with simple nervous systems (Danio rerio, Periplaneta americana, Drosophila melanogaster) are being used with greater frequency in toxicological studies to address these apparent discrepancies. We used the nematode Caenorhabditis elegans (C. elegans) to examine the sensitivity of two neuronal populations, specifically those containing dopamine or GABA, to two widely-used pesticides, TouchDown® (TD) and Mancozeb® (MZ).
The glyphosate-containing herbicides, of which TD is a member, are some of the most widely-used pesticides in the US (Donaldson et al. 2004; Kiely et al. 2004) and in the world (Powles et al. 1997). Although the introduction of glyphosate-resistant crops in the late 1980s resulted in an exponential increase in their use (Fernandez-Cornejo 2010), glyphosate-containing herbicides have steadily gained market shares since their introduction in the 1970s (USGS 2002). While the active ingredient in these herbicides is glyphosate (2% to 52.3%), the actual application mixture also contains what is referred to as “inert” or “inactive” ingredients (Syngenta 2010). Although the formulation of glyphosate-containing herbicides, but not glyphosate itself, are mitochondrial inhibitors (Olorunsogo et al. 1979; Peixoto 2005), and mitochondrial inhibitors are known to induce parkinsonism in animal models (Betarbet et al. 2006; Betarbet et al. 2000; Saravanan et al. 2005), few studies have examined whether TD might actually lead to neurodegeneration (Axelrad et al. 2003; Tierney et al. 2006; Tierney et al. 2007). Rather, pesticide research related to PD has typically focused on organophosphate insecticides (Arima et al. 2003; Bhatt et al. 1999; Manthripragada et al. 2010), due to their relatively long biological half-life and their prolonged presence in lipophilic tissues. Since it is well-documented that PD patients have decreased mitochondrial function (Mounsey and Teismann 2010; Zhu and Chu 2010), and it is hypothesized that glyphosate pesticide formulations induce mitochondrial inhibition (Olorunsogo et al. 1979; Peixoto 2005), we chose to examine whether C. elegans exposed to TD show neurodegeneration in DAergic neurons similar to that observed in PD.
Additionally, we were interested in whether MZ, a manganese (Mn)/zinc (Zn) ethylene-bis-dithiocarbamate fungicide closely related to maneb, would induce degeneration in either DAergic or GABAergic neurons. While evidence suggests that exposure to Mn (and other heavy metals) may be a risk factor for PD (Hozumi et al. 2011; Racette et al. 2005; Willis et al. 2010), other studies using maneb suggest that DAergic neurons are vulnerable to its exposure (Cicchetti et al. 2005; Costello et al. 2009; Domico et al. 2006; Ferraz et al. 1988; Meco et al. 1994). Maneb, however, is currently being removed from the market (Billingslea 2009) due to recent rulings by the Environmental Protection Agency, and is being replaced with MZ (Donaldson et al. 2004; Kiely et al. 2004). Thus, we wanted to determine whether MZ is also capable of inducing changes in DAergic neurons similar to what has been documented for maneb (Cicchetti et al. 2005; Domico et al. 2007; Filipov et al. 2005; Thiruchelvam et al. 2000).
In order to determine whether exposure to TD and/or MZ could lead to DAergic or GABAergic neurodegeneration, we treated two transgenic C. elegans strains either acutely or chronically with these two agrochemicals. The highest concentration used in either paradigm for TD was 10%, which is within the commercial recommendations for spot spraying broad-leaf weeds (4–10% glyphosate; Syngenta 2010). Although the recommended application concentration for MZ is 0.29 – 0.49% Mn/Zn-EBDC (Bonide 2010), is less than some of the concentrations used here, our concentrations are still well within the range of those to which humans could be exposed, namely commercial formulations of up to 37% Mn/Zn-EBDC (Bonide 2010). The third treatment paradigm involved a dual exposure of 2% TD and varying concentrations of MZ (the approximate LC25, LC50 and LC75). This study models agrochemical practices on farms, since weeds are initially killed with a herbicide then, as crops mature, fungicides could be used.
Additionally, we used BZ555 worms in which all DAergic neurons are tagged with green fluorescent protein (GFP). The advantage to this model system is that C. elegans hermaphrodites have only eight DAergic neurons: two anterior deirid (AD), four cephalic (CEP) and two posterior deirid neurons (Chase and Koelle 2007). This is contrast to rodents, which have 10,000–20,000 DAergic, or humans, which have greater than 40,000 DAergic neurons (Cooper et al. 1996). Additionally, we used the EG1285 strain that has GABAergic::GFP neurons. Of the 302 neurons in the hermaphrodite, 26 are GABAergic: 19 dorsal or ventral motor neurons (DDn, VDn) that innervate dorsal or ventral body muscles, four motor neurons that innervate the head (RME), one interneuron (RIS), one mixed anterior motor neuron/interneuron (AVL) and one mixed dorsal motor neuron/interneuron (Jorgensen 2005). Considering their tremendously simplified nervous system, we wanted to determine the vulnerability of DAergic and/or GABAergic neurons in C. elegans to exposure to either acute or chronic TD and/or MZ.
MATERIALS AND METHODS
C. elegans and Escherichia coli strains
EG1285 and BZ555 worms, as well as NA22 Escherichia coli (E. coli) and OP50 E. coli (a uracil auxotroph) used in this work were provided by the Caenorhabditis Genetics Center (CGC; University of Minnesota), which is funded by the National Institutes of Health (NIH) National Center for Research Resources (NCRR). In EG1285 worms (oxIs12[Punc-47::GFP; lin-15(+)]), a green fluorescent protein gene (gfp) is fused with unc-47, a transmembrane protein found in all GABAergic neurons and used to load GABA into synaptic vesicles (http://www.wormbase.org/db/gene/strain?name=EG1285;class=Strain). BZ555 worms express GFP at the neuronal soma and processes due to the integration of egIs[Pdat-1::GFP], resulting in the fusion of gfp with dat-1, a pre-synaptic DA reuptake protein found in all DAergic neurons (http://www.wormbase.org/db/gene/strain?name=BZ555;class=Strain).
C. elegans maintenance and treatment
C. elegans were maintained at 20°C and synchronized according to standard protocols (Brenner 1974), which are also consistent with previously published protocols (Negga et al. 2011). In order to maximize reproduction and growth, gravid worms were grown on 8P plates (51.3 mM NaCl, 25.0 g bactoagar/L, 20.0 g bactopeptone/L, 1 mM CaCl2, 0.5 mM potassium phosphate buffer (pH 6), 0.013 mM cholesterol (in 95% ethanol), 1 mM MgSO4) with a lawn of NA22 E. coli (grown in 16 g tryptone/L, 10 g yeast extract/L, 85.5 mM NaCl). They were then synchronized by exposure to a solution of sodium hypochlorite (4.0 mM) and sodium hydroxide (0.5 mM), and monitored using an ACCU-SCOPE light microscope (4X) until worms released eggs. Approximately 18 h following isolation and purification of eggs, 5000 synchronous L2 staged worms per treatment group were exposed to the LC25, LC50 and LC75 concentrations of TD (Syngenta AG, Wilmington, DE), MZ (Bonide Products, Inc., Oriskany, NY), or a sequential treatment, which reflects the actual agricultural paradigm, of the two (TD/MZ; Table 1). In the combination treatment, all the worms were exposed to 2% TD (application concentration recommended by the manufacturer) for 30 min (acute), followed by the LC25, LC50 and LC75 concentrations of MZ for 30 min (acute).
Table 1.
Comparison of lethal concentrations among paradigms
| Pesticides | Active Ingredient |
Recommended Application Concentration |
^Acute LC25, LC50, LC75 |
^Chronic LC25, LC50, LC75 |
^Dual LC25, LC50, LC75 |
|---|---|---|---|---|---|
| TouchDown® (TD) | Glyphosate | 0.6–10% | 3.0%, 7.0%, 10.0% | 2.7%, 5.5%, 9.8% | *2% |
| Mancozeb® (MZ) | (Mn)/zinc (Zn) ethylene-bis-dithiocarbamate | 0.29–0.49% | 0.7%, 7.5%, 30% | 0.1%, 1.0%, 1.5% | 10%, 12%,20% |
2% is the typically available application concentration
All the experiments have a value of (N ≥ 4) and (n ≥ 12).
Table summarizes relevant pesticide application concentrations, and compares the lethal concentrations used for the various study paradigms.
For acute pesticide treatments, worms were exposed to the respective pesticide for 30 min, washed at least 3X with sterile dH2O, then placed on fresh nematode growth media (NGM; 51.3 mM NaCl, 17.0 g bactoagar/L, 2.5g bactopeptone/L, 1 mM CaCl2, 1 mM MgSO4, 0.5 mM potassium phosphate buffer (pH 6.0), 12.9 mM cholesterol in 95% ethanol, 1.25 mL nystatin/L, 0.2 g streptomycin/L) plates, with a lawn of OP50 E. coli (grown in 25 g Luria broth/L, 200 mg streptomycin/L). Following a 24-h incubation period at 20°C, treatment groups were counted and compared to controls under a dissecting microscope. Finally, pictures were taken using a digital camera attached to a fluorescent microscope.
For chronic treatments, synchronous L2 staged worms were exposed to the respective pesticide for 30 min, then, without additional washes, placed on clean NGM plates with the respective pesticide still on the worms. After a 24-h incubation period (corresponding to an equivalent human exposure of 10.5 years) at 20°C, the worms were counted, and the treated were compared to controls under a dissecting microscope and photographed.
In agricultural settings, concentrated TD and MZ are diluted with water to the appropriate application concentrations. As such, for all treatments paradigms (acute, chronic or dual), control worms were treated with sterile dH2O.
Fluorescence Microscopy
Following the respective treatments, EG1285 worms from at least four separate synchronizations were washed with sterile dH2O from plates and 10 µL of worms were placed on 4% agarose pads on microscope slides. Fluorescence observations were performed with an epifluorescence microscope (Leitz & Wetzlar, Halco Instruments, Inc) equipped with a 50-W AC mercury source lamp (E. Leitz, Rockleigh, NJ) and various objective lenses (Leitz & Wetzlar, Halco Instruments, Inc). The microscope was coupled to a digital camera (Micrometrics, MilesCo Scientific, Princeton, MN) operated by Micrometrics software (Micrometrics SE Premium, v2.7) for image acquisitions with consistent camera settings of exposure time, saturation, light and color. All images are presented at a total magnification of 400X. In Adobe Photoshop® 6.0.1 (Adobe Systems, Inc, San Jose, CA), the wand tool (with a pre-assigned tolerance level of 18) was used for isolation of green pixels in the photomicrographs. This allowed for selection of all pixels in a defined region that met the minimum criteria. The histogram function in the program was used to assess the number of green pixels. Data was entered into Graph Pad Prism® (version 4.00 GraphPad Software, San Diego, CA). The same procedure was used for studies with BZ555 worms.
Statistical Analysis
Fluorescence data from photomicrograph analysis are presented as mean green pixel number ± SD. Data are from at least four separate synchronizations (N ≥ 4), with at least eight to ten worms per intra-experimental replication (n ≥ 8), for each treatment paradigm. Differences in pixel number were determined by one-way analysis of variance (ANOVA), followed by a post-hoc Bonferroni test. Data was considered to be statistically significantly different from controls when *p < 0.05.
RESULTS
Decreased GFP Pixel Number in DAergic Neurons Following Acute (30-min) Treatment
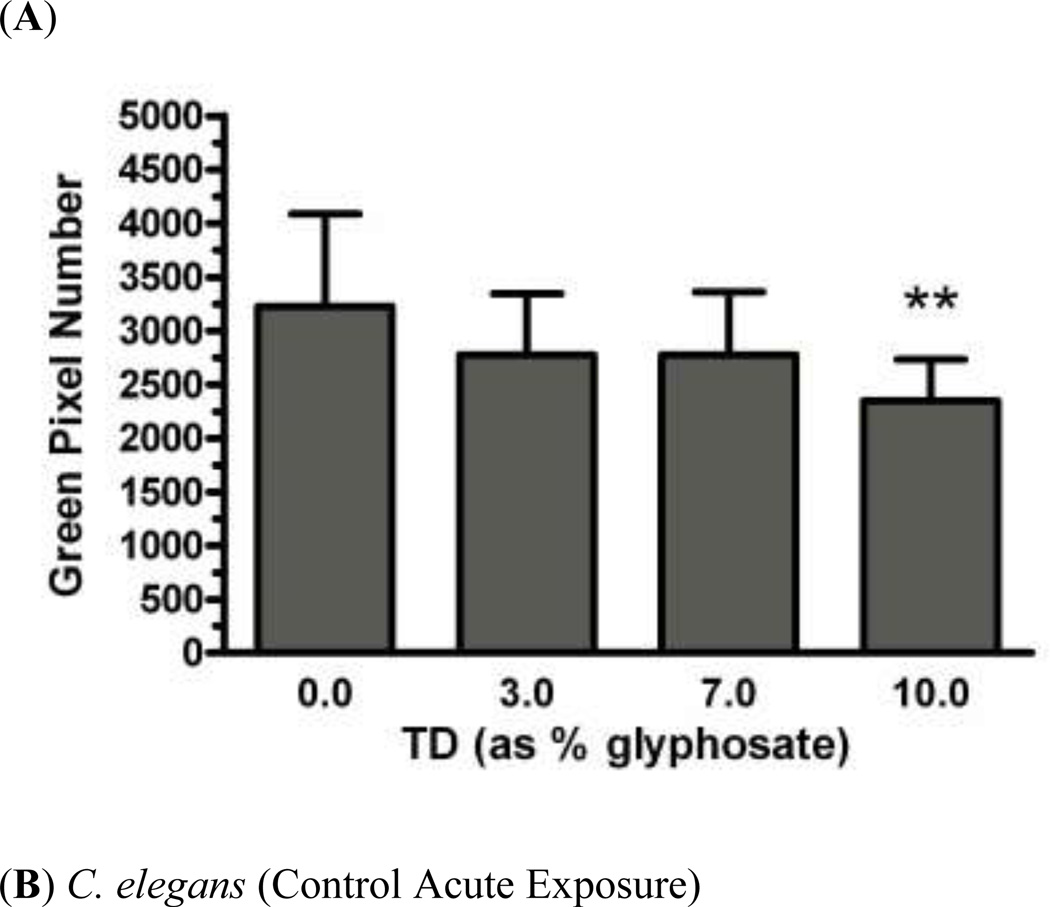
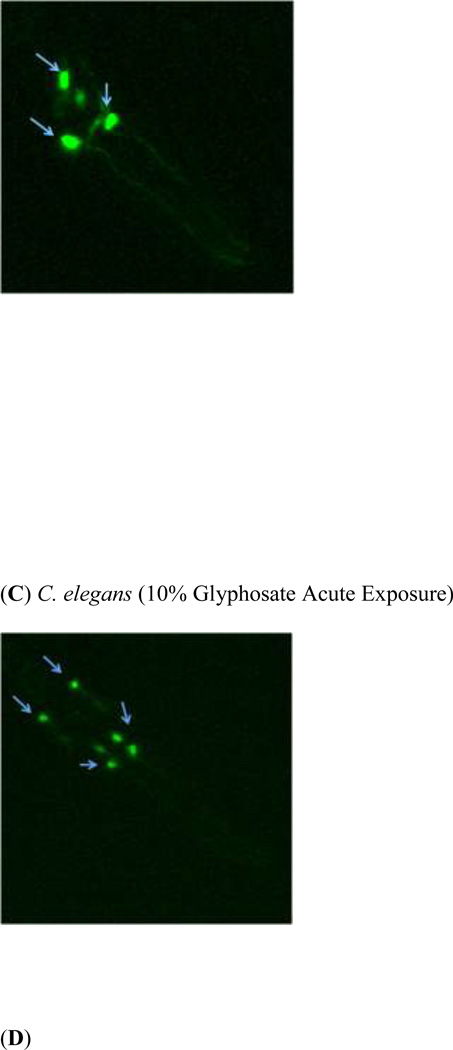
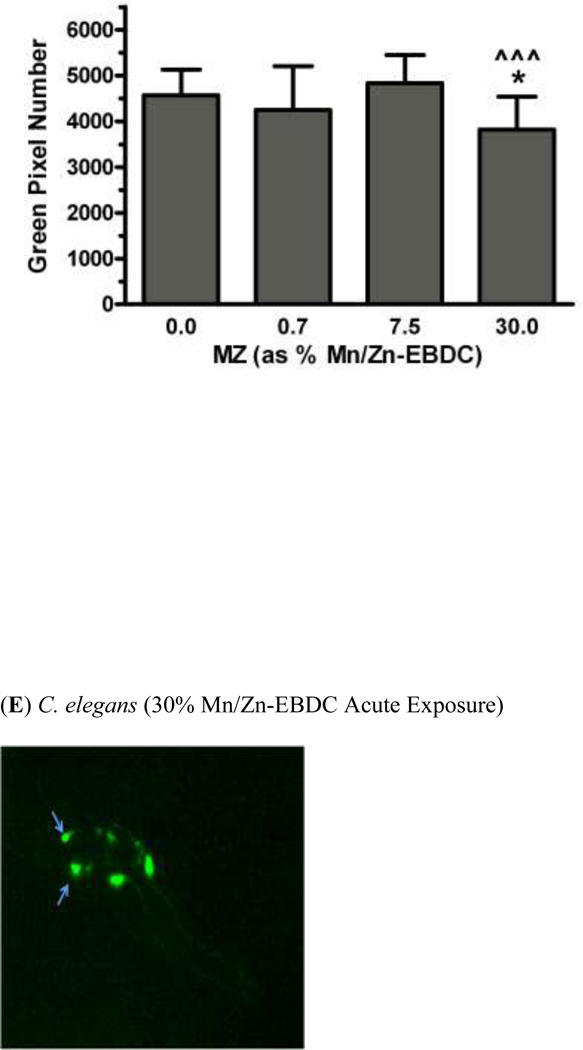
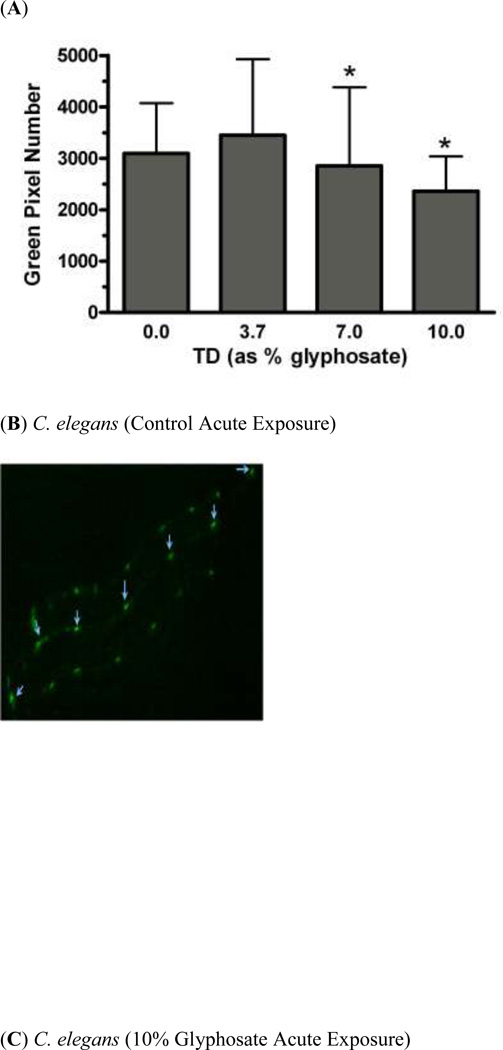
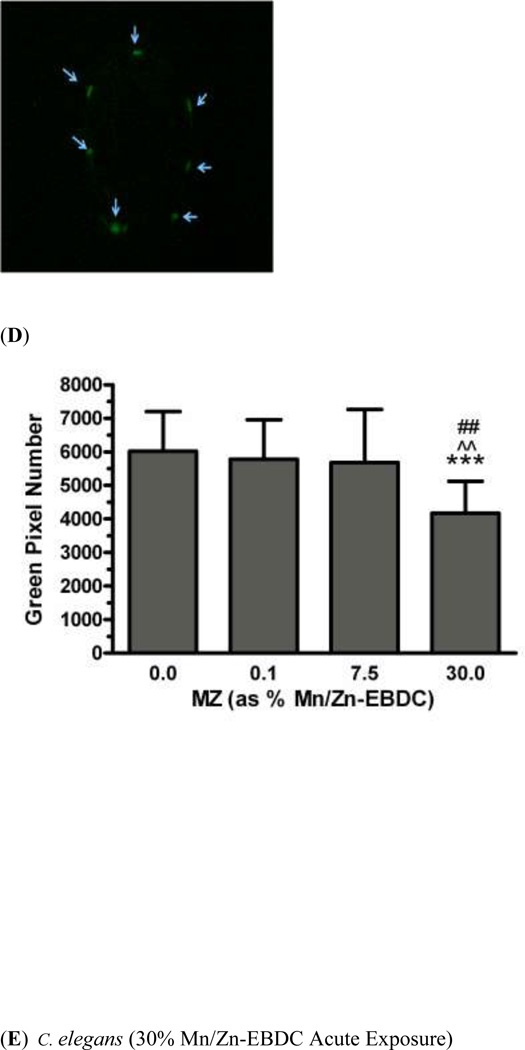
In order to determine whether acute exposure to TD or MZ leads to neurotoxicity in DAergic neurons in C. elegans, BZ555 worms were acutely treated with varying concentrations of either TD or MZ (Figure 1). Number of green pixels was analyzed in the nerve ring, which contains GFP-tagged DAergic neurons (Rand and Nonet 1997). Analysis indicated that acute treatments with 10% glyphosate (LC75; Figure 1a) resulted in a statistically significant decrease in pixel number compared to controls (*p < 0.01). Computer analysis of photomicrograph indicated a decrease in the soma of DAergic neurons of treated worms (Figure 1c) when compared to controls (Figure 1b). Similarly, following acute treatment with MZ (Figure 1d), a statistically significant reduction in green pixel number was observed at 30% Mn/Zn-EBDC (LC75) compared to either controls (*p < 0.05) or worms treated with 7.5% Mn/Zn-EBDC (LC50; ^^^p < 0.001). This decrease in pixel number corresponded to a shrinkage of the soma of DAergic neurons (Figure 1e) compared to those in control worms (Figure 1b). These data confirmed what was detected during visual inspection of the same photomicrographs.
Fig 1.
Acute treatments of BZ555 nematodes. Following analysis of photomicrographs from worms acutely exposed to TD or MZ, the number of green pixels was determined as described in the methods. Data in (A) and (D) are presented as mean pixel number ± SD of the DAergic neurons in the nerve ring. *p < 0.05 and **p < 0.01 compared to nerve ring of controls and ^^^ p < 0.001 compared to the nerve ring of 0.7% Mn/Zn-EBDC. The blue arrows point to soma in the region of the nerve ring. Blue arrows in photomicrographs of worms treated with 10% (C) glyphosate and 30% (E) Mn/Zn-EBDC emphasize the decreased sizes of the soma compared to the soma of CN worms (B).
Decreased GFP Pixel Number in DAergic Neurons Following Chronic (24-h) Treatment
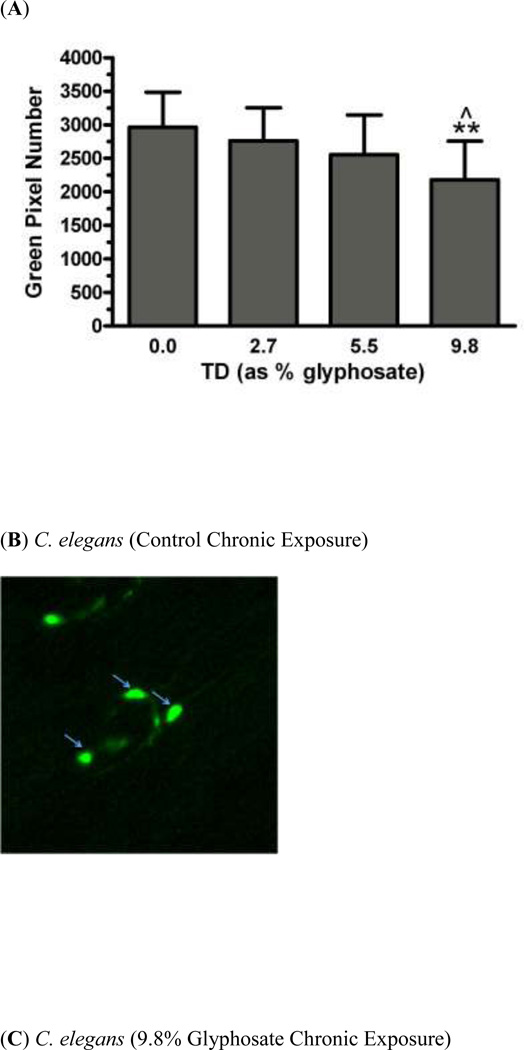
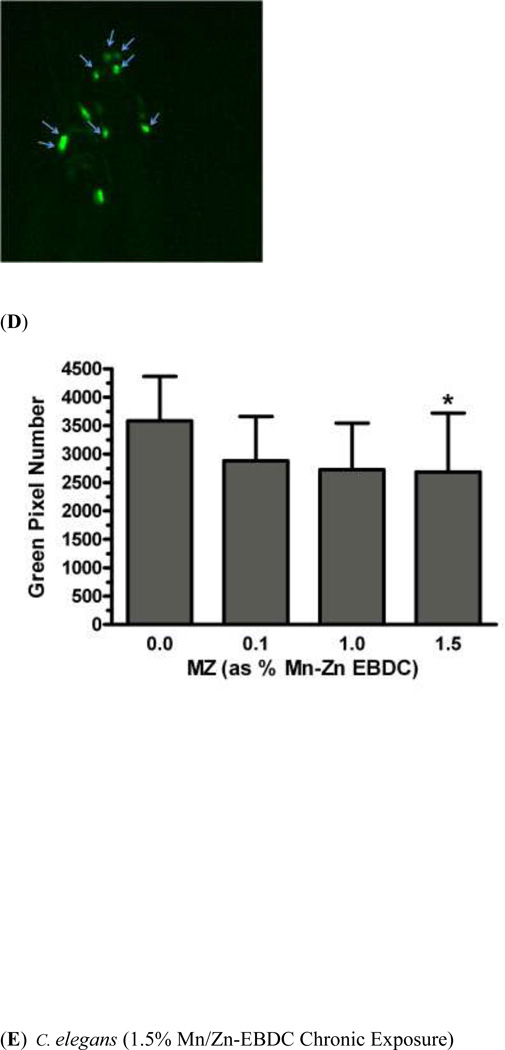
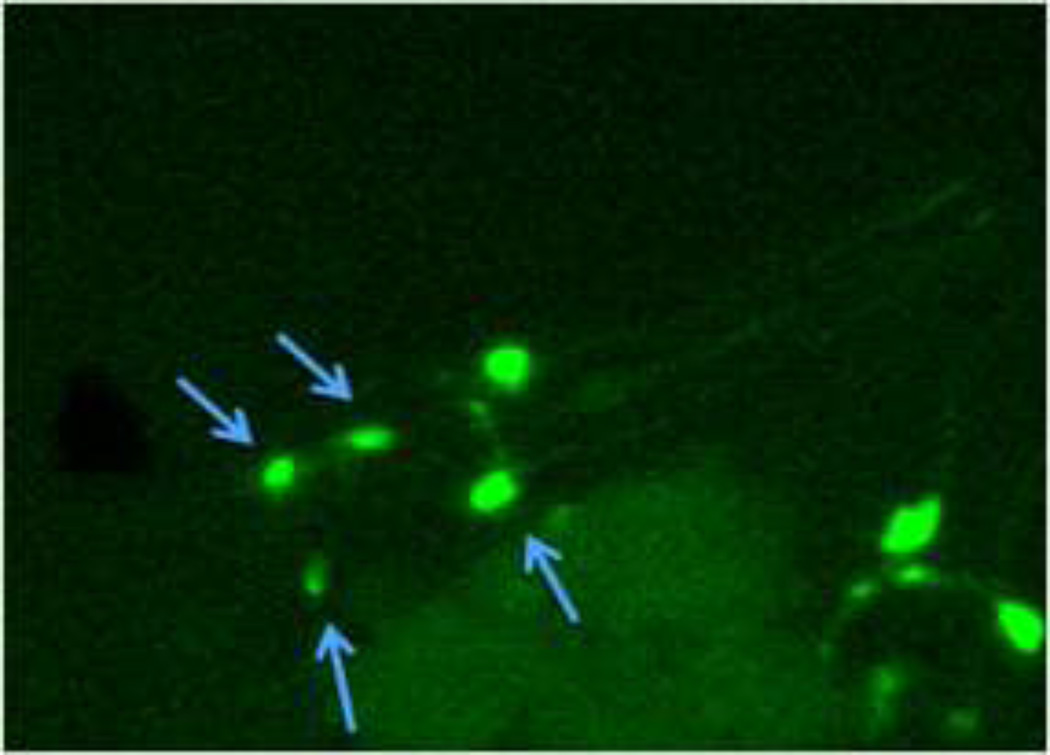
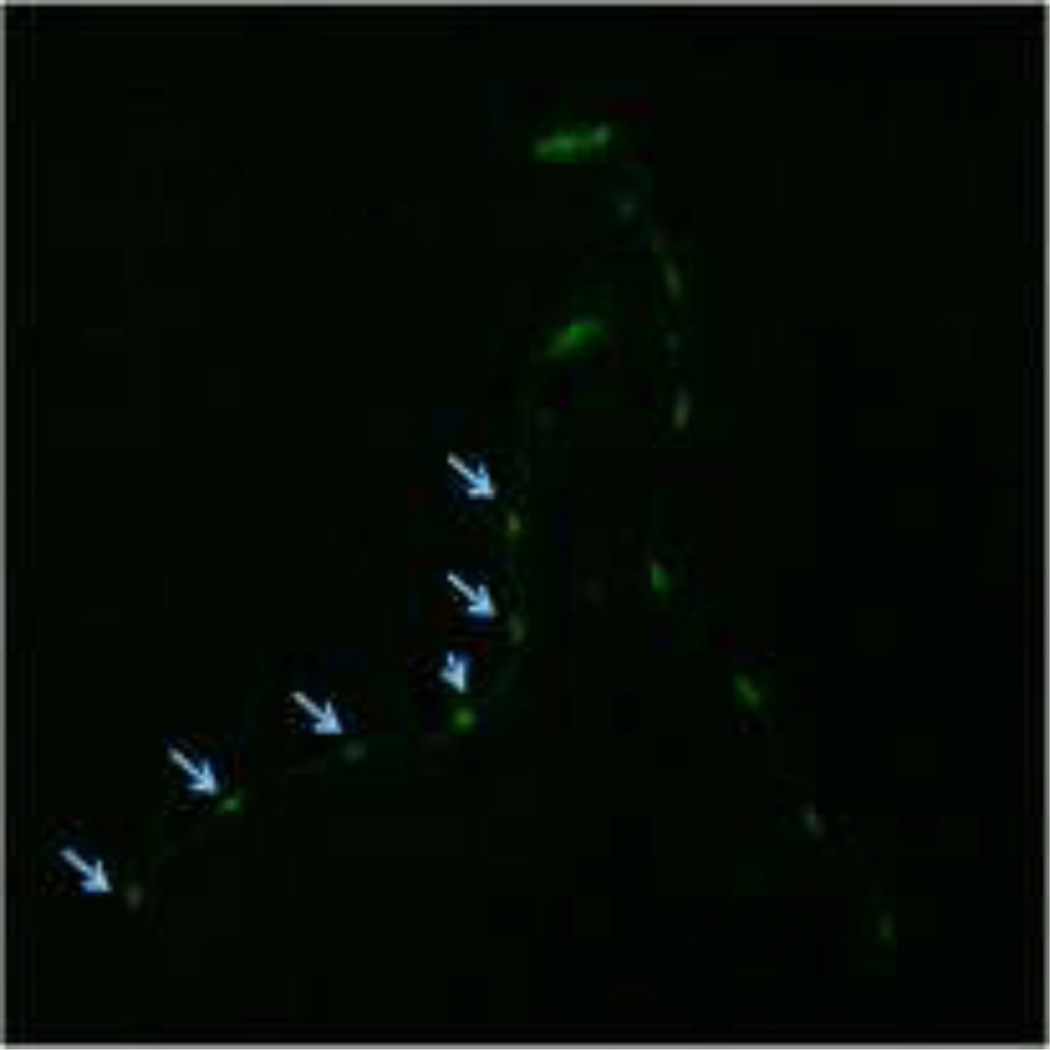
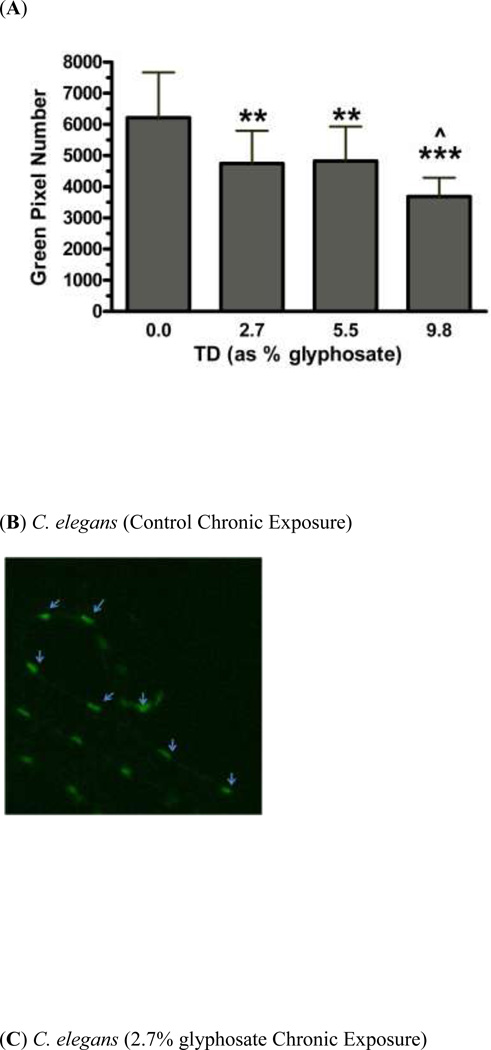
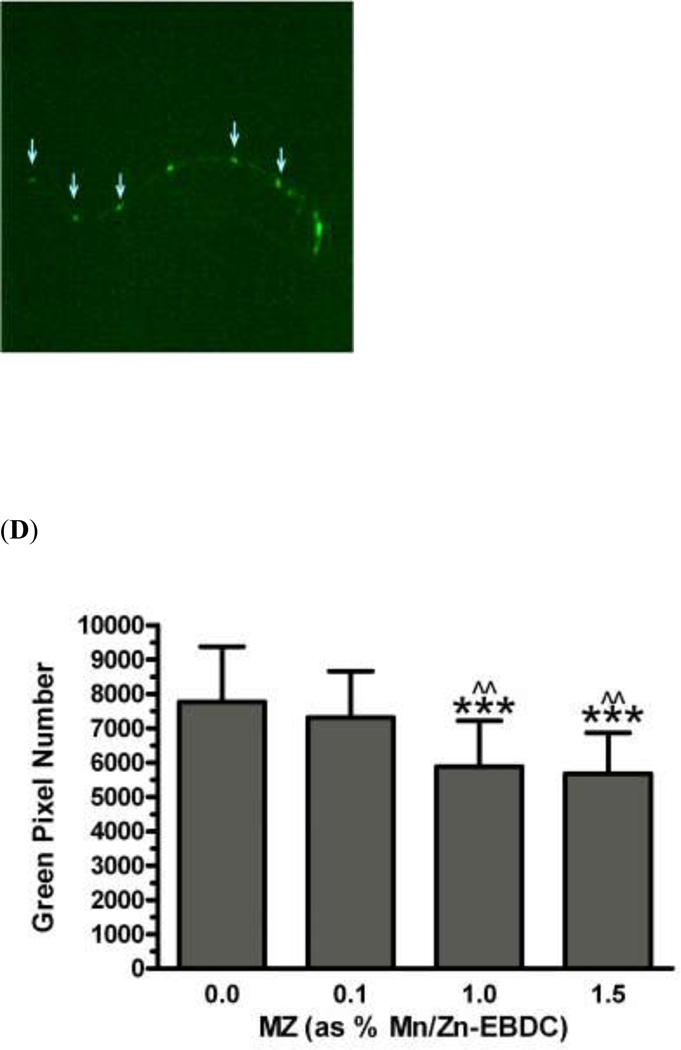
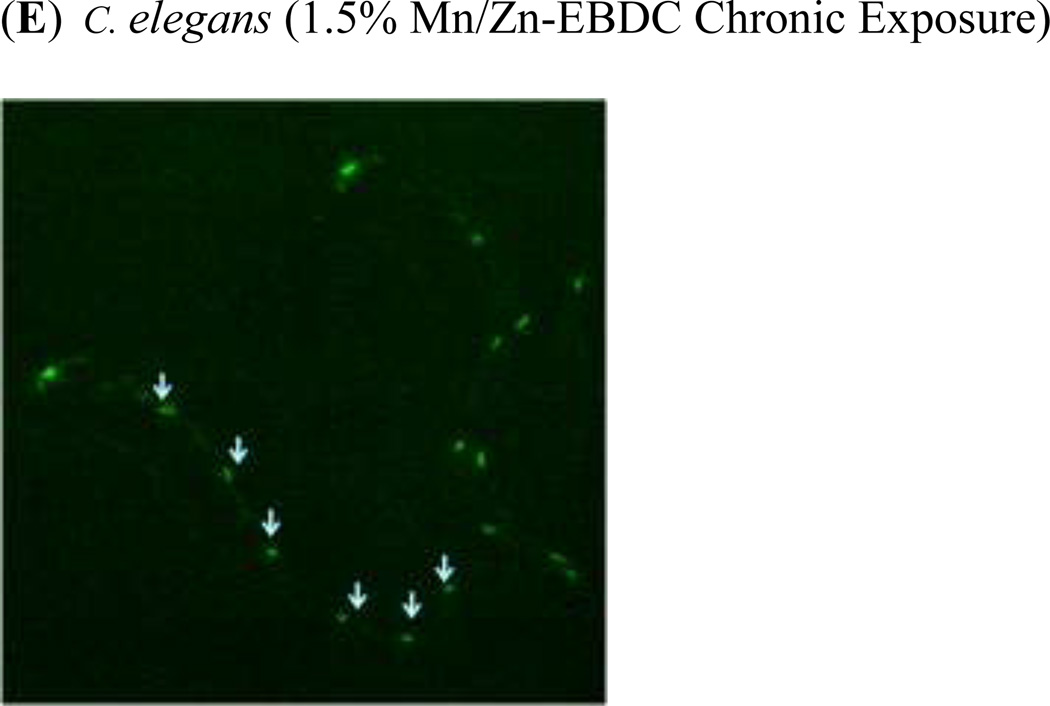
In order to determine whether chronic exposure to TD or MZ might lead to DAergic neurodegeneration, BZ555 worms were chronically treated with either TD or MZ (Figure 2). Analysis of green pixel number associated with DAergic neurons showed a statistically significant decrease (Figure 2a) in 9.8% glyphosate treatment (LC75) compared to controls (**p < 0.01) or 2.7% glyphosate (^p < 0.05). As with the acute treatments, the reduction in pixel number corresponded with an apparent shrinkage of the soma of neurons in the worms treated with 9.8% glyphosate (Figure 2c) compared to control worms (Figure 2b). Chronic treatment with MZ (Figure 2d) showed a statistically significant reduction in green pixel number, accompanied by decreased somal size (Figure 2e), at 1.5% Mn/Zn-EBDC (LC75) compared to control (*p < 0.05). The computer analysis again reinforced what was observed visually following examination of the photomicrographs.
Fig 2.
Chronic treatments of BZ555 nematodes. Following analysis of photomicrographs of worms chronically exposed to TD or MZ, the number of green pixels was determined as described in the methods. Data in (A) and (D) are presented as mean pixel number ± SD of the DAergic neurons in the nerve ring. *p < 0.05 and **p < 0.01 compared to nerve ring of controls and ^p < 0.05 compared to nerve ring of 2.7% glyphosate. The blue arrows point to soma in the region of the nerve ring. Blue arrows in photomicrographs of worms treated with 9.8% (C) glyphosate and 1.5% (E) Mn/Zn-EBDC emphasizes the decreased sizes of the soma compared to the soma of CN worms (B).
GFP Pixel Number in DAergic Neurons Following Dual Treatment
Dual exposure to TD and MZ was used in order to determine neurotoxicity in DAergic neurons in C. elegans. BZ555 worms were acutely (30-min) treated with TD (2% glyphosate) followed by acute (30-min) treatment of the approximate LC25, LC50 or LC75 of MZ (as Mn/Zn-EBDC). Visual inspection was confirmed by analysis of green pixel number in the nerve ring showing no statistically significant differences compared to control worms (data not shown).
Decreased GFP Pixel Number in GABAergic Neurons Following Acute (30-min) Treatment
In order to determine whether acute exposure to TD or MZ might be neurotoxic to GABAergic neurons in C. elegans, EG1285 worms were acutely treated with varying concentrations of either TD or MZ (Figure 3). The number of green pixels was analyzed in GABAergic neurons of the ventral nerve cord. Pixel analysis of acute treatments with TD (Figure 3a) demonstrated a statistically significant decrease in pixel number at 7% glyphosate (LC50) and 10% glyphosate (LC75) compared to controls (*p < 0.05). Compared to control worms (Figure 3b), the somas of GABAergic neurons from worms treated with 10% glyphosate were much smaller (Figure 3c). Acute treatment with MZ (Figure 3d) lead to a significant reduction in green pixel number when comparing 30% Mn/Zn-EBDC (LC75) with controls (***p < 0.001), 0.1% Mn/Zn-EBDC (^^p < 0.01) or with 7.5% Mn/Zn-EBDC (##p < 0.01). The somas of the GABAergic neurons from treated worms (30% Mn/Zn-EBDC) were observed to be smaller (Figure 3e), when compared to worms treated only with water (Figure 3b).
Fig 3.
Acute treatments of EG1285 nematodes. Following analysis of photomicrographs of worms chronically exposed to TD or MZ, the number of green pixels was determined as described in the methods. Data in (A) and (D) are presented as mean pixel number ± SD of GABAergic neurons in the ventral nerve cord. *p < 0.05, ***p < 0.001 compared to ventral nerve cord of controls, ^^p < 0.01 compared to ventral nerve cord of 0.1% Mn/Zn-EBDC and ##p < 0.01 compared to ventral nerve cord of 7.5% Mn/Zn-EBDC. The blue arrows point to soma along the ventral nerve cord. Blue arrows in photomicrographs of worms treated with 10% (C) glyphosate and 0.5% (E) Mn/Zn-EBDC emphasize the decreased sizes of the soma compared to the soma of CN worms (A).
Decreased GFP Pixel Number in GABAergic Neurons Following Chronic (24-h) Treatment
In order to assess the effect of chronic exposure to TD or MZ on GABAergic neurons, EG1285 worms were chronically treated with either TD or MZ (Figure 4). Analysis of the green pixels in the ventral nerve cord showed a statistically significant decrease in green pixel number at 2.7% (LC25), 5.5% glyphosate (LC50; **p < 0.01) and 9.8% (LC75; ***p < 0.001) compared to controls (Figure 4a). In addition a significant reduction in green pixel number was seen at 9.8% glyphosate compared to 5.5% glyphosate (^p < 0.05). Compared to control worms (Figure 4b), this decrease in pixel numbers was accompanied by a concomitant shrinkage of the somas for all concentrations (data only shown for 2.7% glyphosate in Figure 4c). Following chronic MZ treatment (Figure 4d), a statistically significant reduction in green pixel number was observed at 1.0% (LC50) and 1.5% Mn/Zn-EBDC (LC75; ***p < 0.001) in comparison to controls. Also a significant reduction in green pixel number was observed at 1.0% and 1.5% Mn/Zn-EBDC (^^p < 0.01) compared to 0.1% Mn/Zn-EBDC (LC25). Similar to the chronic treatment with TD, the somas of GABAergic neurons from worms treated with Mn/Zn-EBDC were smaller (data shown only for 1.5% Mn/Zn-EBDC in Figure 4e) than controls (Figure 4b).
Fig 4.
Chronic treatments of EG1285 nematodes. Following analysis of photomicrographs of worms chronically exposed to TD or MZ, the number of green pixels was determined as described in the methods. Data in (A) and (D) are presented as mean pixel number ± SD of GABAergic neurons in the ventral nerve cord. **p < 0.01 and ***p < 0.001 compared to ventral nerve cord of controls, ^p < 0.05 compared to 2.7% glyphosate and ^^p < 0.01 compared to 0.1% Mn/Zn-EBDC. The blue arrows point to soma along the ventral nerve cord. Blue arrows in photomicrographs of worms treated with 2.7% (C) glyphosate and 1.5% (E) Mn/Zn EBDC emphasize the decreased sizes of the soma compared to the soma of CN worms (B).
GFP Pixel Number in GABAergic Neurons Following Dual Treatment
The dual treatment paradigm exposure to TD and MZ was used in order to determine neurotoxicity in GABAeric neurons in C. elegans. EG1285 worms were acutely treated with TD (2% glyphosate) followed by acute treatment with varying concentrations of MZ. Analysis of number of green pixels in the ventral nerve cord showed no significant reduction in green pixel number at any concentrations (data not shown). This was confirmed by visual inspection of the neurons as well, as no difference in soma size or projections was observed.
DISCUSSION
Although there is a well-documented genetic contribution to early onset PD (Bekris et al. 2010), multiple studies strongly suggest that the interplay of environmental toxicants and potential susceptibility genes play a much greater role in the later onset of the disease (Brown et al. 2006; Tanner 2010). In general, numerous pesticides are recognized as playing a role in neurodegeneration in studies involving rodents (Bahrami et al. 2009; Binukumar and Gill 2010; Jiang et al. 2010; Slotkin et al. 2009). This degeneration may or may not strictly involve the DAergic system, which contributes to the cardinal symptomatology associated with PD. Interestingly, few studies exist in the literature related to glyphosate-containing herbicides even though they are some of the most ubiquitously applied pesticides in the world (Woodburn 2000). While it is recognized that glyphosate is relatively non-toxic, with an oral LD50 = 4320 mg/kg in rats (Birch 1993), several reports suggest that the actual formulations of glyphosate-containing herbicides, such as RoundUp® or TD, may be mitochondrial inhibitors (Olorunsogo et al. 1979; Peixoto 2005). In light of data potentially delineating differences between the active ingredient and commercial formulations, we sought to determine what effect the actual formulations to which humans are exposed had on DAergic and GABAergic neurons in C. elegans.
Previous work from our lab (Negga et al. 2011) demonstrated that neurons in C. elegans are vulnerable to treatment with varying concentrations of commercially available TD or MZ, which has been linked previously to DAergic degeneration (Domico et al. 2007; Domico et al. 2006) and mitochondrial inhibition (Zhang et al. 2003). Those studies (Negga et al. 2011) were limited in their specificity by the fact that all neurons in the worm strain used (NW1229) were tagged with GFP. Although this provided evidence of general neurodegeneration, it did not allow in-depth analysis of the effect of TD and/or MZ on specific neuronal populations. In the current study, we used strains that had GFP selectively attached to proteins found in either DAergic (BZ555) or GABAergic (EG1285) neuronal populations. Both strains have been used to examine sensory neuron cilia formation (Senti and Swoboda 2008), neuronal degeneration (Samara et al. 2010), and DAergic neurodegeneration (Benedetto et al. 2010). By comparing data from our first study (Negga et al. 2011) with the present information, it is possible to better assess the vulnerability of discrete neuronal populations.
It is important to note that the pesticide concentrations used in the present investigation, while at the LC25, LC50 and LC75 for the worms (Table 1) are within the environmentally relevant concentrations available and applied. For example, the recommended application concentration for TD for fields with weeds over 15 cm tall is 0.6 – 0.9% glyphosate, although the concentration can be as high as 4 – 10% glyphosate for spot spraying (Syngenta 2010). Typically, however, glyphosate products for use by the general public are sold with 2% glyphosate, a general use concentration that does not require dilution. On the other hand, the recommended application concentration for MZ is 0.29 – 0.49% Mn/Zn-EBDC (Bonide 2010). When considering the concentrations for the dual treatment, we considered the following: (1) in a growing season, herbicides (in this case TD) would be applied prior to any fungicides (Randall et al. 2008a); (2) because of the time difference during a growing season when herbicides or fungicides were used do not generally overlap, the pesticides should not be applied as a true mixture (which would also violate the application instructions provided for agricultural workers; Randall et al. 2008b)); (3) since the most common concentration of TD used is 2% glyphosate, this was the concentration used in the dual treatment studies (Negga et al, 2011).
When BZ555 worms were treated acutely with either TD (Figure 1A) or MZ (Figure 1D), changes in green pixel numbers and neuronal morphology were only observable at the LC75 for each pesticide concentration (10% glyphosate or 30% Mn/Zn-EBDC, respectively). This was in contrast to previous data (Negga et al, 2011), in which morphological and pixel changes in the nerve ring and head region were also observed at the LC25 and LC50 for TD concentrations. In addition to DAergic neurons, which were the only neurons labeled in the BZ555 strain, the NW1229 worms also had glutamatergic (Glu) interneurons, serotonergic (5-HT) amphid and interneurons, and cholinergic (ACh) ring interneurons and motor neurons labeled. As the previous data demonstrated noticeable neurodegeneration at lower concentrations of TD or MZ, the current data suggest that other populations (Glu, 5-HT and ACh) are more vulnerable than DAergic neurons following an acute exposure to TD when considered in light of our previous data (Table 2). This is similar to the acute treatment with MZ, in which statistically significant changes in pixel number and morphology were only noted at the LC75 for MZ in BZ555 worms, rather than the lower concentrations (Figure 1D).
Table 2.
Summary of Vulnerability of Neuronal Populations
| Treatment Group |
DAergic Sensitivity (BZ555) |
GABAergic Sensitivity (EG1285) |
Other Neuronal Populations (Based on Negga et al, 2001) |
|---|---|---|---|
| Acute TD | No | Yes | Yes (head) |
| Acute MZ | No | Yes | Yes (head) |
| Chronic TD | Yes | Yes | No |
| Chronic MZ | No | Yes | Yes (head) |
| Dual Treatment | No | No | Likely |
Differences in data between the current study and Negga et al. 2011 indicate that GABAergic neurons C. elegans may be more sensitive to TD or MZ exposure than DAergic neurons, regardless of single exposure paradigm.
When BZ555 worms were treated chronically with MZ (Figure 2D), DAergic neurons were affected only at the highest concentration of Mn/Zn-EBDC (1.5%), suggesting that previous data (Negga et al. 2011) indicating morphological changes more likely reflected degeneration of Glu, 5-HT and ACh neurons (Table 2). The data from MZ-treated worms (BZ555) were in contrast to the data from worms chronically treated with TD (Figure 2A). Here the TD data closely paralleled that generated from NW1229 worms, suggesting that in a chronic paradigm, DAergic neurons are more vulnerable than other neuronal populations in C. elegans.
In general, GABAergic neuronal degeneration was more evident in EG1285 worms (Figure 3 and 4) at lower concentrations of pesticides than that observed in NW1229 worms. Data from our current study confirmed that, for acute TD and chronic MZ treatments, the neurons that are apparently the most vulnerable are those of the GABAergic system. In addition to GABAergic neurons in the ventral nerve cord of C. elegans, there are also ACh ventral motor neurons, and 5-HT motor neurons (Rand and Nonet 1997). As no measureable degeneration was observed in NW1229 worms treated either acutely with MZ or chronically with TD, our interpretation is that, because of the multiple populations tagged in NW1229 worms, the GABAergic degeneration observed in the current study was overshadowed by the presence of the other neuronal populations. Thus, by focusing solely on the GABAergic neurons in this study, we were able to increase the specificity and sensitivity of our evaluation of these neurons (Table 2).
In complex in vivo model systems, it is often difficult to examine the effect of various toxicants on the nervous system. In the case of C. elegans, with its 302 neurons (Chalfie and White 1988; Thomas and Lockery 1999), it is much easier to determine the fate of distinct populations than in mammals, which have millions of neurons. This is evident in the current study where we were able to determine the differential vulnerability of various neuronal populations in the nematode following exposure to environmental toxicants. While a large body of work involving pesticide exposure has focused on DA, greater recognition of the role of other neurotransmitters has become the focus of more recent studies (Lester et al. 2010; Wang et al. 2010; Yamamoto and Soghomonian 2009). In the context of the expanding interest of neurotransmitters other than DA involved in PD, it is timely that our research has shown a potentially increased vulnerability of GABAergic neurons to a widely-used glyphosate-containing herbicide (TD), and a popular Mn-containing fungicide, MZ.
Interestingly, however, our dual treatment paradigm, based on current commercial application procedures continues to demonstrate a decreased, rather than increased, neuronal vulnerability (see also Negga et al, 2011). In light of the visual differences between treated and control neuronal populations in NW1229 worms, and the measured loss of green pixel number, we had anticipated that the dual treatment would lead to greater degeneration in both the BZ555 and EG1285 worms. This was obviously not the case, and requires further mechanistic investigations.
CONCLUSION
This work is significant because it represents one of the first studies in the literature to systematically identify potentially vulnerable neuronal populations in C. elegans following exposure to the glyphosate-containing herbicide TD, a representative of a ubiquitous herbicide class, and a commonly- and highly-used fungicide (MZ) similar to maneb, which has already been linked to DAergic neurodegeneration. It also demonstrates that chronic, but perhaps not acute, exposure to TD leads to DAergic degeneration in C. elegans. Furthermore, it highlights the sensitivity of GABAergic neurons to these pesticides, whether an acute or chronic exposure paradigm is used, suggesting that future work with to these two pesticide classes examine GABAergic degeneration as a potentially early marker of neuronal vulnerability.
Acknowledgments
FUNDING
This work was supported by the National Institute of Environmental Health Sciences [R15 ES015628 to VF] and by the Appalachian College Association Colonel Lee B. Ledford Endowment Fund [to RN, AJ and VF].
References Cited
- Arima H, Sobue K, So M, Morishima T, Ando H, Katsuya H. Transient and reversible parkinsonism after acute organophosphate poisoning. J Toxicol Clin Toxicol. 2003;41:67–70. doi: 10.1081/clt-120018273. [DOI] [PubMed] [Google Scholar]
- Axelrad JC, Howard CV, McLean WG. The effects of acute pesticide exposure on neuroblastoma cells chronically exposed to diazinon. Toxicology. 2003;185:67–78. doi: 10.1016/s0300-483x(02)00592-9. [DOI] [PubMed] [Google Scholar]
- Bahrami F, Yousefpour M, Mehrani H, Golmanesh L, Sadraee SH, Khoshbaten A, Asgari A. Type of cell death and the role of acetylcholinesterase activity in neurotoxicity induced by paraoxon in cultured rat hippocampal neurons. Acta Biol Hung. 2009;60:1–13. doi: 10.1556/ABiol.60.2009.1.1. [DOI] [PubMed] [Google Scholar]
- Barone P. Neurotransmission in Parkinson's disease: beyond dopamine. Eur J Neurol. 2010;17:364–376. doi: 10.1111/j.1468-1331.2009.02900.x. [DOI] [PubMed] [Google Scholar]
- Bekris LM, Mata IF, Zabetian CP. The genetics of Parkinson disease. J Geriatr Psychiatry Neurol. 2010;23:228–242. doi: 10.1177/0891988710383572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetto A, Au C, Avila DS, Milatovic D, Aschner M. Extracellular dopamine potentiates mn-induced oxidative stress, lifespan reduction, and dopaminergic neurodegeneration in a BLI-3-dependent manner in Caenorhabditis elegans. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Canet-Aviles RM, Sherer TB, Mastroberardino PG, McLendon C, Kim JH, Lund S, Na HM, Taylor G, Bence NF, Kopito R, Seo BB, Yagi T, Yagi A, Klinefelter G, Cookson MR, Greenamyre JT. Intersecting pathways to neurodegeneration in Parkinson's disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis. 2006;22:404–420. doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer T, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nature Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bhatt MH, Elias MA, Mankodi AK. Acute and reversible parkinsonism due to organophosphate pesticide intoxication: five cases. Neurology. 1999;52:1467–1471. doi: 10.1212/wnl.52.7.1467. [DOI] [PubMed] [Google Scholar]
- Billingslea J. In: Maneb: Notice of Receipt of a Request to Voluntarily Cancel Pesticide Registrations of Certain Products. U. E. P. Agency, editor. Environmental Protection Agency; 2009. [Google Scholar]
- Binukumar BK, Gill KD. Cellular and molecular mechanisms of dichlorvos neurotoxicity: cholinergic, nonchlolinergic, cell signaling, gene expression and therapeutic aspects. Indian J Exp Biol. 2010;48:697–709. [PubMed] [Google Scholar]
- Birch M. Toxicological investigation of CP 67573-3. In: O. o. P. US Environmental Protection Agency, Pesticides, and Toxic Substances, editor. Washington, DC: Office of Pesticide Programs, US Government Printing Office; 1993. Unpublished Report no. 4-70-90 (1970) by Monsanto Corporation, prepared by Younger Laboratories, Inc. [Google Scholar]
- Bonide . In: Mancozeb Flowable with Zinc Concentrate. I. Bonide Products, editor. Oriskany, NY: Bonide Products, Inc; 2010. [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TP, Rumsby PC, Capleton AC, Rushton L, Levy LS. Pesticides and Parkinson's disease--is there a link? Environ Health Perspect. 2006;114:156–164. doi: 10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, White J. The Nervous System. In: Wood WB, editor. The Nematode Caeorhabditis Elegans. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1988. pp. 337–392. [Google Scholar]
- Chase DL, Koelle MR. T. C. e. R. Community, editor. Biogenic amine neurotransmitters in C. elegans. Worm Book. 2007 doi: 10.1895/wormbook.1.132.1. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Cicchetti F, Lapointe N, Roberge-Tremblay A, Saint-Pierre M, Jimenez L, Ficke BW, Gross RE. Systemic exposure to paraquat and maneb models early Parkinson's disease in young adult rats. Neurobiol Dis. 2005;20:360–371. doi: 10.1016/j.nbd.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Cooper J, Bloom F, Roth R. Dopamine. In: Cooper J, Bloom F, Roth R, editors. The Neurochemical Basis on Neuropharmacology. New York: Oxford University Press; 1996. pp. 293–351. [Google Scholar]
- Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol. 2009;169:919–926. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domico LM, Cooper KR, Bernard LP, Zeevalk GD. Reactive oxygen species generation by the ethylene-bis-dithiocarbamate (EBDC) fungicide mancozeb and its contribution to neuronal toxicity in mesencephalic cells. Neurotoxicology. 2007;28:1079–1091. doi: 10.1016/j.neuro.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domico LM, Zeevalk GD, Bernard LP, Cooper KR. Acute neurotoxic effects of mancozeb and maneb in mesencephalic neuronal cultures are associated with mitochondrial dysfunction. Neurotoxicology. 2006;27:816–825. doi: 10.1016/j.neuro.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Donaldson D, Kiely T, Grube A. In: Pesticides IndustrySales and Usage: 2000 and 2001Market Estimates. U. S. E. P. Agency, editor. United States Environmental Protection Agency; 2004. [Google Scholar]
- Fernandez-Cornejo J. Rapid Growth in Adoption of Genetically Engineered Crops Continues in the US. In: U. S. D. o. Agriculture, editor. United States Department of Agriculture; 2010. http://www.ers.usda.gov/Data/BiotechCrops/ [Google Scholar]
- Ferraz HB, Bertolucci PH, Pereira JS, Lima JG, Andrade LA. Chronic exposure to the fungicide maneb may produce symptoms and signs of CNS manganese intoxication. Neurology. 1988;38:550–553. doi: 10.1212/wnl.38.4.550. [DOI] [PubMed] [Google Scholar]
- Filipov NM, Sistrunk SC, Coban A, Norwood AB. Peripheral LPS enhances dopaminergic toxicity of the fungicide maneb in a strain-dependent manner in mice. Brain, Behavior, and Immunity. 2005;19:e20. [Google Scholar]
- Hozumi I, Hasegawa T, Honda A, Ozawa K, Hayashi Y, Hashimoto K, Yamada M, Koumura A, Sakurai T, Kimura A, Tanaka Y, Satoh M, Inuzuka T. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J Neurol Sci. 2011 doi: 10.1016/j.jns.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Jiang W, Duysen EG, Hansen H, Shlyakhtenko L, Schopfer LM, Lockridge O. Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents. Toxicol Sci. 2010;115:183–193. doi: 10.1093/toxsci/kfq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen EM, editor. GABA. 2005 http://www.wormbook.org, http://www.wormbook.org.
- Kiely T, Donaldson D, Grube A. In: Pesticides Industry Sales and Usage: 2000 and 2001 Market Estimates. U. S. E. P. Agency, editor. Office of Prevention and Toxic Substances; 2004. [Google Scholar]
- Kim Y, Kim JM, Kim JW, Yoo CI, Lee CR, Lee JH, Kim HK, Yang SO, Chung HK, Lee DS, Jeon B. Dopamine transporter density is decreased in parkinsonian patients with a history of manganese exposure: what does it mean? Mov Disord. 2002;17:568–575. doi: 10.1002/mds.10089. [DOI] [PubMed] [Google Scholar]
- Langston JW. The Parkinson's complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- Lester DB, Rogers TD, Blaha CD. Acetylcholine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16:137–162. doi: 10.1111/j.1755-5949.2010.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yin J, Cheng CM, Sun JL, Li Z, Wu YL. Paraquat induces selective dopaminergic nigrostriatal degeneration in aging C57BL/6 mice. Chin Med J (Engl) 2005;118:1357–1361. [PubMed] [Google Scholar]
- Manthripragada AD, Costello S, Cockburn MG, Bronstein JM, Ritz B. Paraoxonase 1, agricultural organophosphate exposure, and Parkinson disease. Epidemiology. 2010;21:87–94. doi: 10.1097/EDE.0b013e3181c15ec6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meco G, Bonifati V, Vanacore N, Fabrizio E. Parkinsonism after chronic exposure to the fungicide maneb (manganese ethylene-bis-dithiocarbamate) Scand.J. Work Environ. Health. 1994;20:301–305. doi: 10.5271/sjweh.1394. [DOI] [PubMed] [Google Scholar]
- Mounsey RB, Teismann P. Mitochondrial dysfunction in Parkinson's disease: pathogenesis and neuroprotection. Parkinsons Dis. 2010;2011 doi: 10.4061/2011/617472. 617472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negga R, Rudd DA, Davis NS, Justice AN, Hatfield HE, Valente AL, Fields AS, Fitsanakis VA. Exposure to Mn/Zn Ethylene-bis-Dithiocarbamate and Glyphosate Pesticides Leads to Neurodegeneration in Caenorhabditis elegans. Neurotoxicology. 2011 doi: 10.1016/j.neuro.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olorunsogo OO, Bababunmi EA, Bassir O. Effect of glyphosate on rat liver mitochondria in vivo. Bull Environ Contam Toxicol. 1979;22:357–364. doi: 10.1007/BF02026955. [DOI] [PubMed] [Google Scholar]
- Peixoto F. Comparative effects of the Roundup and glyphosate on mitochondrial oxidative phosphorylation. Chemosphere. 2005;61:1115. doi: 10.1016/j.chemosphere.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Nass R, Boyd WA, Freedman JH, Dong K, Narahashi T. Use of non-mammalian alternative models for neurotoxicological study. Neurotoxicology. 2008;29:546–555. doi: 10.1016/j.neuro.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles S, Preston C, Bryan I, Jutsun A. Herbicde Resistance: Impact and Management. Adv Agron. 1997;58:57–93. [Google Scholar]
- Racette BA, Tabbal SD, Jennings D, Good L, Perlmutter JS, Evanoff B. Prevalence of parkinsonism and relationship to exposure in a large sample of Alabama welders. Neurology. 2005;64:230–235. doi: 10.1212/01.WNL.0000149511.19487.44. [DOI] [PubMed] [Google Scholar]
- Rand JB, Nonet ML. Appendix 2: Neurotransmitter Assignments for Specific Neurons. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Plainview, New York: Cold Spring Harbor Laboratory Press; 1997. [Google Scholar]
- Randall C, Hock W, Crow E, Hudak-Wise C, Kasai J. National Pesticide Applicator Certification Core Manual. Washington, DC: National Association of State Departments of Agriculture Research Foundation; 2008a. Pesticide Application Procedures; pp. 163–176. [Google Scholar]
- Randall C, Hock W, Crow E, Hudak-Wise C, Kasai J. National Pesticide Applicator Certification Core Manual. Washington, DC: National Association of State Departments of Agriculture Research Foundation; 2008b. Planning the Pesticide Application; pp. 149–162. [Google Scholar]
- Samara C, Rohde CB, Gilleland CL, Norton S, Haggarty SJ, Yanik MF. Large-scale in vivo femtosecond laser neurosurgery screen reveals small-molecule enhancer of regeneration. Proc Natl Acad Sci U S A. 2010;107:18342–18347. doi: 10.1073/pnas.1005372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan KS, Sindhu KM, Mohanakumar KP. Acute intranigral infusion of rotenone in rats causes progressive biochemical lesions in the striatum similar to Parkinson's disease. Brain Res. 2005;1049:147–155. doi: 10.1016/j.brainres.2005.04.051. [DOI] [PubMed] [Google Scholar]
- Senti G, Swoboda P. Distinct isoforms of the RFX transcription factor DAF-19 regulate ciliogenesis and maintenance of synaptic activity. Mol Biol Cell. 2008;19:5517–5528. doi: 10.1091/mbc.E08-04-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Levin ED, Seidler FJ. Developmental neurotoxicity of parathion: progressive effects on serotonergic systems in adolescence and adulthood. Neurotoxicol Teratol. 2009;31:11–17. doi: 10.1016/j.ntt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syngenta. TouchDown Hi Tech. In: Syngenta, editor. Product Label. Greensboro, NC: Syngenta Crop Protection; 2010. [Google Scholar]
- Tanner CM. Advances in environmental epidemiology. Mov Disord. 2010;25 Suppl 1:S58–S62. doi: 10.1002/mds.22721. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Brockel BJ, Richfield EK, Baggs RB, Cory-Slechta DA. Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: environmental risk factors for Parkinson's disease? Brain Res. 2000;873:225–234. doi: 10.1016/s0006-8993(00)02496-3. [DOI] [PubMed] [Google Scholar]
- Thomas JH, Lockery S. Neurobiology. In: I. A. Hope, editor. C. elegans: A Practical Approach. New York: Oxford Univeristy Press; 1999. [Google Scholar]
- Tierney KB, Ross PS, Jarrard HE, Delaney KR, Kennedy CJ. Changes in juvenile coho salmon electro-olfactogram during and after short-term exposure to current-use pesticides. Environ Toxicol Chem. 2006;25:2809–2817. doi: 10.1897/05-629r1.1. [DOI] [PubMed] [Google Scholar]
- Tierney KB, Singh CR, Ross PS, Kennedy CJ. Relating olfactory neurotoxicity to altered olfactory-mediated behaviors in rainbow trout exposed to three currently-used pesticides. Aquat Toxicol. 2007;81:55–64. doi: 10.1016/j.aquatox.2006.11.006. [DOI] [PubMed] [Google Scholar]
- U. S. D. o. t. Interior and U. S. G. Survey, editor. USGS. Pesticide National Synthesis Project: Glyphosate Usage. United States Geological Survey; 2002. [Google Scholar]
- Wang Y, Zhang QJ, Liu J, Ali U, Gui ZH, Hui YP, Chen L, Wang T. Changes in firing rate and pattern of GABAergic neurons in subregions of the substantia nigra pars reticulata in rat models of Parkinson's disease. Brain Res. 2010;1324:54–63. doi: 10.1016/j.brainres.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Comella CL, Horn S. Parkinson's disease--Part 1: Pathophysiology, symptoms, burden, diagnosis, and assessment. Am J Manag Care. 2008;14:S40–S48. [PubMed] [Google Scholar]
- Willis AW, Evanoff BA, Lian M, Galarza A, Wegrzyn A, Schootman M, Racette BA. Metal emissions and urban incident Parkinson disease: a community health study of Medicare beneficiaries by using geographic information systems. Am J Epidemiol. 2010;172:1357–1363. doi: 10.1093/aje/kwq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodburn AT. Glyphosate: Production, pricing and use worldwide. Pest Management Science. 2000;56:309–312. [Google Scholar]
- Yamamoto N, Soghomonian JJ. Metabotropic glutamate mGluR5 receptor blockade opposes abnormal involuntary movements and the increases in glutamic acid decarboxylase mRNA levels induced by l-DOPA in striatal neurons of 6-hydroxydopamine-lesioned rats. Neuroscience. 2009;163:1171–1180. doi: 10.1016/j.neuroscience.2009.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fitsanakis VA, Gu G, Jing D, Ao M, Amarnath V, Montine TJ. Manganese ethylene-bis-dithiocarbamate and selective dopaminergic neurodegeneration in rat: a link through mitochondrial dysfunction. J. Neurochem. 2003;84:336–346. doi: 10.1046/j.1471-4159.2003.01525.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Chu CT. Mitochondrial dysfunction in Parkinson's disease. J Alzheimers Dis. 2010;20 Suppl 2:S325–S334. doi: 10.3233/JAD-2010-100363. [DOI] [PubMed] [Google Scholar]