Abstract
Previous PET and fMRI brain imaging studies targeting neural networks processing itch sensation have previously used histamine as the sole itch inducer. In contrast with histamine, cowhage-induced itch is mediated via proteinase activated receptors PAR2 and is transmitted through a separate spinothalamic pathway, therefore imaging the brain activation evoked by cowhage could provide further insight central processing of itch. We report for the first time a functional MRI Arterial Spin Labeling (ASL) study of neuronal processing of itch induced by cowhage analyzed in contrast with histamine-induced itch. We also explored the brain responses induced by histamine and cowhage combined in a tight sequence. The results of our analyses obtained in a group of 15 healthy volunteers suggested that cowhage and histamine co-activated a core group of brain structures, while also revealed notable differences. Core areas activated by both stimuli were found in the thalamus, primary and secondary somatosensory cortices, posterior parietal cortex, superior and middle temporal cortices, PCC, ACC, precuneus and cuneus. Cowhage induced a notably distinct and more extensive involvement of the insular cortex, claustrum, basal ganglia, putamen, thalamic nuclei and pulvinar. The differences observed between these two itch modalities were investigated to determine the impact of quantitative versus qualitative factors, and correlations between itch intensity and the patterns in brain activation were explored. Our analysis revealed that the most significant differences between cowhage and histamine itch were not affected by stimulus intensity, although a subset of regions displayed activations which were intensity-dependent. The combined application of cowhage and histamine highlighted the role of insula and claustrum in the processing of both itch modalities in the same time. The present results suggest the existence of overlapping but also distinct neuronal networks processing these two different types of itch.
Keywords: cerebral representation of itch, cowhage, histamine, Arterial Spin Labeling, functional MRI
1. Introduction
Itch is a multidimensional sensory experience that all human beings experience in the course of their lives (Yosipovitch, 2004). From a neurophysiological perspective, two distinct peripheral and spinothalamic pathways have been discovered for itch: a histaminergic pathway and a non-histaminergic pathway mediated by proteinase activated receptors 2, PAR2 (Steinhoff et al, 2003). The latter is activated exogenously by the spicules of cowhage, a tropical plant which represents a particularly valuable experimental itch inducer (Papoiu et al, 2011). Upon skin contact, cowhage spicules release mucunain, a cysteine protease that serves as a ligand for the PAR2 receptors (Reddy et al., 2008) and elicits a strong sensation of “itch without a flare” that lasts for several minutes (LaMotte et al, 2009; Sikand et al, 2009). Current reports suggest that PAR2 mediated itch may be a more appropriate model for pathological pruritus than models based on histamine, since antihistamines are not effective in clinical practice as antipruritics. PAR2 receptors have been shown to play a role in itch of atopic eczema (Steinhoff et al, 2003). While brain processing of histamine-induced itch has been investigated in several studies (Hsieh et al, 1994; Darsow et al, 2000; Drzezga et al, 2001; Mochizuki et al, 2003, 2009; Leknes et al, 2007; Schneider et al, 2008; Valet et al, 2008; Vierow et al, 2009; Pfab et al, 2010) no studies have yet investigated the brain processing of cowhage-induced itch. The peripheral afferent C-nerve fibers conveying cowhage-induced itch are separate from the pathway stimulated by histamine itch (Johanek et al, 2007; Namer et al, 2008) and the subsequent spinothalamic routes are also distinct (Davidson et al, 2010; reviewed in Davidson & Giesler, 2010). Little is known about the projections of these two itch specific pathways to their superior end-processing stations in the cerebral cortex; therefore, brain imaging offers a useful methodology to track these two distinct itch pathways towards their final destinations. In the current study, we analyzed and contrasted the cerebral representation of itch induced by these two distinct modalities, histamine and cowhage, as well as the effect of the simultaneous activation of both pathways, to gain insight into their central processing. We employed Arterial Spin Labeling (ASL), a suitable technique to capture long-term changes in cerebral perfusion that parallel the extended duration of itch (Ishiuji et al, 2009). It is of notable interest to describe the brain networks involved in the processing of itch and examine if these two forms of itch have a distinct representation in the brain, especially since cowhage itch is a promising model for chronic itch of pathological origin in humans.
2. Materials & Methods
2.1. Subjects
Fifteen healthy volunteers (7 males, 8 females, ages 19 to 42, average age 31.1 ± 6.1), all right-handed, who signed a written informed consent participated in this study. All procedures were approved by the Institutional Review Board of Wake Forest University School of Medicine and were conducted in accordance with the Declaration of Helsinki.
2.2. Study design
We employed a sequential ASL scan model consisting of two successive ASL scans of 390s (6.5 minutes) performed after itch induction. Functional MRI data was acquired in baseline conditions (at rest), and immediately after itch stimulus administration. The stimuli used were histamine, cowhage, and their combination thereof, which were applied on the right (dominant) volar aspect of the forearm. Subsequent stimuli were applied to an area situated at least 5 cm away from the area used in a previous application, to avoid application on area(s) affected by alloknesis, with the exception of the combined application when stimuli were applied next to each other, 1 cm apart. When switching from one itch stimulus to another, a pause was taken to allow the itch to completely subside (intensity ratings to return to zero).
2.3. Induction of itch
Histamine itch was induced by iontophoresis to the volar aspect of the forearm. A 1% solution of histamine dissolved in 2% methylcellulose gel (Sigma, St. Louis, USA) was delivered using a current of 200 μA through a round iontophoresis electrode, 14 mm in diameter, for 30 seconds (Perimed PF 3826 Perilont Power device; Perimed, Sweden) as we previously reported (Papoiu et al, 2011). After the itch sensation from histamine iontophoresis had completely subsided, another area at least 5 cm away, on the volar aspect of the forearm was used for cowhage application. A number of 40 – 45 cowhage spicules (provided by Dr. Ethan A. Lerner, Cutaneous Biology Research Center, Department of Dermatology, Massachusetts General Hospital, Charlestown, Massachusetts) were counted under a magnifying lens, picked-up by a microtweezer and were applied within a 4 cm2 circular area on the skin. The spicules were gently rubbed for 45 seconds onto the subjects' skin with a circular motion to facilitate contact; a cotton cloth was used to demarcate the area to prevent any stray spicules from stimulating surrounding skin. When itch sensation induced by cowhage completely subsided, another area was chosen for application of both histamine and cowhage. For the combined application of itch stimuli, histamine was administered for 30 seconds by iontophoresis as described above and immediately thereafter, cowhage was applied in the same manner to an adjacent site (1 cm away).
2.4. Psychophysical measurements
Subjects used a visual analogue scale (VAS) to rate itch intensity induced by the two stimuli alone or in combination, at the end of each fMRI series. The VAS scale ranged from 0–10 and the end-points anchoring the VAS were: 0= “no itch sensation”, 10 = “maximum unbearable itch”.
The psychophysical data describing itch response were first acquired in a dedicated screening visit performed prior to fMRI portion of the study. Itch ratings were recorded continuously (by COVAS, Medoc, TSA II) for all 3 modalities, during the first 330 seconds following itch induction. The aims of the screening session were two-fold: to test whether participants responded to all itch stimuli with a minimum peak intensity of 3.0 on a scale of 10, and to obtain a time-course (a temporal profile curve) of the intensity of itch sensation. The results of this assessment were reported separately in a PLosOne article: “Cowhage-induced itch as an experimental model for pruritus. A comparative study with histamine-induced itch.” (Papoiu et al, PLoS One 6(3):e17786) and demonstrated that cowhage itch elicited a higher average intensity in comparison with histamine and was perceived as the dominant stimulus when both stimuli were combined. When itch was induced in the fMRI setting, we collected retrospective average VAS ratings of itch, which were used for regression analysis. The average values of COVAS ratings obtained in the screening session and the VAS ratings reported at the MRI scanner by the same participants were very similar. Thus, we confirmed that continuous ratings over time acquired via computerized VAS closely mirrored post-stimulus retrospective ratings, as described previously (Koyama et al, 2004). We have used previously and adapted the VAS and COVAS for rating itch perception and have demonstrated that these scales are: 1) sensitive to manipulations which can reduce itch; 2) capable of assessing changes in itch intensity over time and 3) able to distinguish differences between acute and chronic itch (Ishiuji et al, 2008; Wang et al, 2010).
2.5. fMRI scanning parameters
All images were acquired on a 1.5 Tesla MRI scanner (GE Healthcare, Milwaukee, WI, U.S.A.) using an eight-channel MR head coil array (Medrad, Inc., Warrendale, PA, U.S.A.). Total imaging protocol consists of localizer images acquired for graphical prescription, a high-resolution anatomical T1-weighted image, and eight fMRI experiments (2 baseline, 2 histamine-induced itch, 2 cowhage itch, and 2 for the combination stimuli).
Acquisition of structural high-resolution images. High-resolution T1-weighted anatomical images were used to identify regions of activation, to classify tissues, to normalize images to a standard space, and to screen for abnormalities. T1-weighted structural scans were obtained using an inversion prepared 3D spoiled gradient echo sequence using a matrix size of 256×192, field of view (FOV) of 24×18cm, echo time (TE) of 1.9 ms, and inversion preparation time of 600 ms, flip angle of 20°, slice thickness of 1.5 mm with no gap between slices and 128 slices, giving an in-plane resolution of 0.94mm.
2.6. T1 map image acquisition of the brain tissue signals
Accurate quantitative cerebral blood flow (CBF) maps require that the T1 of the tissue be measured at each voxel. T1 maps were calculated from data acquired from a separate inversion recovery (IR) echo-planar imaging (EPI) sequence. A global inversion C-shaped frequency offset corrected inversion (C-FOCI) pulse is used to minimize underestimating the T1 due to fresh spins perfusing into the imaging slice. Twelve inversion times (TIs) were acquired logarithmically from 10 ms to 6 s with a repetition time (TR) of 10 s by changing the order of slice acquisition. After a deliberate 6-s delay, the first volume was acquired without an inversion pulse to obtain an M0 image. Thirteen imaging volumes were then acquired in a total scan time of 2 min 10 s. All other imaging acquisition parameters (FOV, matrix size, TE, flip angle, slice thickness, slice location etc.) are identical to the Q2TIPS-FAIR protocol as described in the next section. The T1 for each voxel was calculated by curve fitting the IR curve to a three-parameter decaying exponential model.
2.7. Acquisition of Cerebral Blood Perfusion Data
CBF was measured with quantitative imaging of perfusion using a single subtraction with thin slice TI1 periodic saturation (QUIPSS II TIPS, also known as Q2TIPS) with flow-sensitive alternating inversion recovery (FAIR). The Q2TIPS-FAIR sequence is a multi-slice sequence that incorporates saturation pulses to minimize the uncertainty associated with tagged blood's transit time into the imaging slice. The saturation pulses in implementation of Q2TIPS are very selective suppression (VSS) radio frequency pulses, which are applied every 25 ms between 800 ms (TI1) and 1200 ms (TI1s). The VSS pulses saturate a 2-cm slab of tissue with a 1-cm gap between the saturation slab and the first imaging slice. Our current implementation of the Q2TIPS-FAIR sequence uses a C-FOCI pulse (β = 1361, μ = 6). A C-FOCI pulse is used instead of the standard adiabatic hyperbolic secant pulse to improve perfusion sensitivity by minimizing slice imperfections. The 11 oblique slices were prescribed parallel to the anterior commissure/posterior commissure line and were acquired sequentially, inferior to superior. This provided coverage from the vertex to the ventral aspect of the thalamus and prefrontal cortex. Other imaging parameters were as follows: TE 28 ms, TI1 800 ms, TI1s 1200 ms, TI 2000 ms, TR 3000 ms, receiver bandwidth 62·5 kHz, flip angle 90°, FOV 24 cm (frequency) × 18 cm (phase), acquisition matrix 64 × 48 (11 slices, 8 mm thickness, 0 mm slice gap), and frequency encoding direction anterior/posterior. A diffusion gradient with an equivalent b value of 5·25 mm2 s–1 is added to suppress intra-arterial spins. The Q2TIPS-FAIR sequence was used to acquire 60 label/control pairs (slice selective in version/global inversion) in 6 min 30 s. These label/control pairs are pair-wise subtracted and then averaged to generate a perfusion-weighted image. The first 30 s (five label/control pairs) is used to establish steady state. During this 30 s a single-shot EPI proton density (M0) image is acquired. This M0 image serves as an internal reference to scale the perfusion-weighted images appropriately to calculate absolute quantitative CBF maps according to the general kinetic model described by Buxton et al. (1998). All the CBF measures were calibrated and calculated in ml blood/100g brain tissue/min.
2.8. Statistical Analysis of Local CBF Signal Fluctuations
The functional image analysis package FSL [Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (Center for FMRIB, University of Oxford, Oxford, U.K.)] was used for image processing and statistical analysis. The CBF data were movement corrected and spatially smoothed using a 5 mm 3D isotropic Gaussian kernel. Each CBF image was scaled by its mean global intensity (intensity normalization) to minimize variability due to global CBF changes. Next, each subject's CBF images were registered to their structural data using a seven-parameter linear 3D transformation and transformed into standard stereotaxic space (as defined by the Montreal Neurological Institute) using a 12-parameter linear 3D transformation. Standard general linear model-based analyses using fixed-effects models within subjects and random effects models between subjects were performed to: 1) identify effects due to histamine, cowhage and their combined administration in comparison to a baseline (resting) state and to contrast them against each other, and 2) to test correlation of brain activation with stimulus intensity. The patterns of activation observed during the two ASL subsequent scans following a single administration of the itch stimulus were also contrasted with each another. Analysis of brain activations induced by the same itch stimulus over time was performed by a pairwise t test of the data acquired in the two consecutive ASL scans. Since the itch induced by the same stimulus had a different magnitude during the two scans (itch intensity gradually decreased, from the first scan to the second) this approach also offered a way to ascertain patterns of activation related to itch intensity. Clusters of voxels exceeding a Z score > 2.3 and p < 0.05 were considered statistically significant.
2.9. Analysis of quantitative vs. qualitative aspects in the comparison of cowhage versus histamine itch
A couple of questions arise when attempting to compare differences in activations evoked by two forms of itch of a sensibly different intensity. Therefore, an important goal was to devise an approach that allowed a distinction between intensity related effects and effects associated with stimuli characteristics. In order to distinguish between intensity-dependent and stimulus-specific effects we: a) generated a direct comparison map (via paired t test) which depicted the significantly different areas activated by cowhage in contrast to histamine, and b) generated an “intensity-dependent field” of differences where the intensity factor was weighted in a linear regression analysis; the latter displayed areas where cowhage induced a stronger effect due to a higher intensity of the induced sensation. A contrast of these results illuminated important areas that responded differently to itch stimuli in a manner dependent or independent of the itch intensity perceived.
3. Results
3.1. Behavioral aspects of itch induced with histamine and cowhage during fMRI series
The intensity ratings of perceived itch sensation were taken at the end of each fMRI series and showed that cowhage induced a more intense itch sensation, compared to histamine (p= 0.00005) (Figure 1). The combination of both stimuli induced an itch comparable in intensity to the itch induced by cowhage alone. Analysis of the time course of perceived itch intensity from continuous ratings (Papoiu et al, 2011; previously published separately) revealed that itch intensity varied significantly across time (analyzed by ANOVA as a main effect time: p<0.0001). According to our previous report, cowhage itch intensity quickly rose to reach peak intensity 120 seconds post-application and started to taper down slowly, still maintaining a half-maximal intensity after 330 seconds. Histamine reached an intensity plateau after approximately 120 seconds which was subsequently maintained relatively constant up to the 330 sec. mark, where it intersected the temporal curve of cowhage itch intensity (i.e. at the 300–330 sec time points these itches were of a virtually instantaneous equal intensity). In figure 1 we show that the slopes of itch decreasing (in average value) during the two consecutive scans are slightly different in dynamic for the conditions studied. Histamine peaked at a lower magnitude than cowhage itch and decreased slower, while cowhage itch was more intense initially (during the first scan) and decreased faster in the second period. Retrospective average VAS ratings recorded at the end of the second ASL scan indicated itch was still perceived as substantial during the second scans (Figure 1). The average values calculated for COVAS ratings obtained in continuous recordings in the psychophysical screening session matched the VAS ratings reported at the scanner by the same participants. Since brain processing of itch was imaged as itch intensity was gradually decreasing, differences between histamine and cowhage induced itch became apparent in the time course of the brain processing of itch, when captured and contrasted in two sequential ASL scans of 390 seconds (detailed below).
Figure 1.

Figure 1A. The experimental paradigm indicating the sequence of operations: structural scan, T1 map acquisition, ASL scanning series and the order of stimuli used in the induction of itch. Figure 1B. Visual analog scale itch intensity ratings during ASL scans are indicated (average ± standard deviation), as they were reported immediately at the end of each series when itch was induced with histamine, cowhage or their combination. Each ASL scan lasted 390s (6 ½ minutes). Itch ratings are reported on a VAS scale from 0 to 10: 0 = no itch, 10= maximum unbearable itch.
3.2. Common features and notable differences in the processing of cowhage and histamine itch
The brain activation evoked by the stimulation of these two itch pathways overlapped to a substantial extent, while in the same time presented distinguishable particularities (Figure 2). The pattern of brain activation observed for histamine itch was in agreement with previous results reported in the literature (Hsieh et al, 1994; Darsow et al, 2000; Drzezga et al, 2001; Mochizuki et al, 2003, 2009; Leknes et al, 2007; Schneider et al, 2008); however, we also found significant activations in the thalamus and in the primary and secondary somatosensory areas which were reported before, but not consistently.
Figure 2.

The overlap of brain activations maps induced by histamine itch (in green) and by cowhage itch (in blue) illustrates the regions co-activated (in red) and distinct areas activated separately by the two itch pathways. Standard space Talairach coordinates. ACC = anterior cingulate cortex; PCC = posterior cingulate cortex; SPL = superior parietal lobule; M1 = primary motor cortex; S1 = primary somatosensory area; SMG = supramarginal gyrus; MTG= middle temporal gyrus; IPL = inferior parietal lobule; S2 = secondary somatosensory area.
The cerebral representation of cowhage itch showed a pattern that resembled histamine itch, although it displayed certain features that were clearly unique. In contrast to histamine itch activations, which were more bilateral and symmetrical, cowhage itch presented a slight contralateral (left side) emphasis. Cowhage itch also evoked a more extensive activation of the insular cortex and claustrum, predominantly on the contralateral side in basal ganglia: globus pallidum, caudate body, putamen and thalamic nuclei, pulvinar and the anterior cingulate cortex (Brodmann area 24). Cortical and subcortical areas activated by cowhage itch are presented in Table 1.
Table 1.
Brain areas significantly activated by cowhage induced itch in comparison with baseline conditions. Standard space Talairach coordinates (Z scores > 2.3, p < 0.05).
| Brain area | x | y | z | Z score |
|---|---|---|---|---|
| Supramarginal gyrus (L) | −44 | −50 | 40 | 2.92 |
| −62 | −36 | 40 | 2.99 | |
| −38 | −42 | 40 | 2.53 | |
| Supramarginal gyrus (R) | 36 | −70 | 8 | 3.59 |
| Angular gyrus (L) | −40 | −58 | 40 | 3.45 |
| −50 | −54 | 40 | 3.21 | |
| −60 | −56 | 40 | 3.12 | |
| −52 | −62 | 12 | 3.91 | |
| −56 | −56 | 12 | 3.12 | |
| Angular gyrus | ||||
| Ipsilateral (R) | 48 | −48 | 44 | 3.65 |
| 60 | −56 | 34 | 3.98 | |
| 40 | −48 | 42 | 4.12 | |
| 18 | −70 | 8 | 3.66 | |
| 16 | −50 | 6 | 2.64 | |
| Middle temporal gyrus (L) | −48 | −52 | −2 | 2.36 |
| (BA 39) | −64 | −46 | −2 | 4.25 |
| Cuneus (L) | −6 | −78 | 34 | 2.38 |
| −8 | −76 | 40 | 2.63 | |
| −18 | −72 | 12 | 4.06 | |
| −12 | −88 | 12 | 3.1 | |
| Precuneus (L) | −34 | −74 | 40 | 3.52 |
| −42 | −72 | 40 | 3.55 | |
| −44 | −126 | 40 | 3.22 | |
| −2 | −58 | 40 | 2.92 | |
| −26 | −58 | 40 | 3.01 | |
| −10 | −68 | 40 | 3.79 | |
| −10 | −76 | 40 | 2.4 | |
| −14 | −42 | 40 | 3.84 | |
| −8 | −62 | 2 | 3.37 | |
| Thalamus | −14 | −20 | −2 | 3.89 |
| Ventral Posterior Lateral nucleus |
−18 | −20 | −2 | 3.46 |
| Anterior Nucleus | −8 | −4 | 8 | 3.84 |
| Mediodorsal Nucleus | −4 | −20 | 8 | 3.65 |
| −4 | −18 | 8 | 3.48 | |
| −4 | −18 | 12 | 2.72 | |
| −6 | −18 | 12 | 2.68 | |
| Lateral posterior nucleus | −16 | −22 | 12 | 3.29 |
| −20 | −22 | −72 | 3.33 | |
| Ventral Anterior | −12 | −6 | 8 | 2.66 |
| nucleus | −12 | −4 | 8 | 2.43 |
| Thalamus (R) | ||||
| Ventrolateral nucleus | 18 | −26 | 6 | 3.24 |
| 20 | −16 | 6 | 3.21 | |
| 22 | −16 | 6 | 2.66 | |
| Ventral Posterior Lateral Nucleus |
16 | −16 | 4 | 2.79 |
| 22 | −18 | 4 | 2.81 | |
| Lateral dorsal nc. | 12 | −10 | 4 | 3.31 |
| Mediodorsal nc. | 10 | −12 | 4 | 3.44 |
| Ventral Anterior Nc. | 12 | −22 | 6 | 4.83 |
| 10 | −12 | 6 | 4.25 | |
| Pulvinar (L) | −14 | −32 | 2 | 2.95 |
| −14 | −24 | 2 | 2.72 | |
| −16 | −46 | −2 | 4.06 | |
| Mammillary bodies (R) | −12 | −16 | −2 | 3.29 |
| PCC (L) | −18 | −44 | −2 | 2.96 |
| −4 | −44 | 0 | 2.54 | |
| −6 | −20 | 0 | 2.55 | |
| −16 | −44 | 2 | 3.26 | |
| −16 | −46 | 2 | 2.99 | |
| −6 | −54 | 12 | 4.15 | |
| PCC (R) | 14 | −50 | 6 | 2.64 |
| 6 | −42 | 6 | 2.35 | |
| ACC (L) | −4 | −6 | 40 | 2.44 |
| −2 | 14 | 40 | 2.37 | |
| Paracingulate gyrus (R) | −2 | 16 | 42 | 2.98 |
| −2 | 14 | 44 | 2.99 | |
| Sup. occipital gyrus (BA 19) | −54 | −70 | 12 | 2.55 |
| Middle occipital gyrus (L) | −10 | −16 | −2 | 2.87 |
| Occipital Pole (L) | −10 | −96 | 12 | 2.31 |
| Occipital cortex (R) | 20 | −70 | 6 | 3.84 |
| 10 | −82 | 6 | 3.21 | |
| S2 (L) | −46 | −28 | 18 | 3.18 |
| −58 | −28 | 26 | 3.10 | |
| −54 | 0 | 4 | 2.95 | |
| S2 (R) | 50 | −4 | 10 | 3.02 |
| BA6 (L) | −48 | −2 | 12 | 3.32 |
| −52 | −26 | −72 | 2.90 | |
| Opercular cortex | −56 | 0 | 14 | 3.14 |
| Insula (L) | −32 | 16 | −2 | 2.52 |
| −38 | 2 | −2 | 2.7 | |
| −36 | −12 | −2 | 3.09 | |
| −36 | 4 | −2 | 2.87 | |
| −48 | 14 | −2 | 2.54 | |
| −40 | 14 | 4 | 2.71 | |
| −38 | 6 | 4 | 2.73 | |
| −34 | 12 | 2 | 2.54 | |
| −38 | 14 | 2 | 3.02 | |
| −40 | 12 | 2 | 2.69 | |
| −42 | 10 | 2 | 2.46 | |
| −42 | 8 | 2 | 2.6 | |
| −34 | −24 | 2 | 2.49 | |
| −32 | 8 | −2 | 2.52 | |
| Insula (R) | 50 | 12 | 6 | 2.47 |
| Claustrum (L) | −34 | 14 | −2 | 2.66 |
| −36 | 0 | −2 | 2.42 | |
| −36 | 2 | 0 | 2.97 | |
| −34 | 4 | 0 | 2.62 | |
| −32 | 8 | 0 | 2.41 | |
| −28 | 16 | 4 | 2.5 | |
| −26 | 18 | 2 | 3.21 | |
| −32 | 12 | 0 | 2.78 | |
| −30 | 16 | 0 | 2.67 | |
| −28 | 18 | 0 | 3.15 | |
| −30 | 18 | 0 | 3.25 | |
| −36 | 6 | 0 | 2.97 | |
| −36 | 0 | 0 | 2.89 | |
| −36 | −2 | 0 | 2.51 | |
| −30 | 18 | −4 | 2.66 | |
| −30 | 20 | −4 | 2.55 | |
| −32 | 14 | −4 | 3.48 | |
| −34 | 10 | −4 | 2.67 | |
| −34 | 8 | −4 | 2.45 | |
| −36 | −6 | −4 | 2.68 | |
| −36 | −16 | 2 | 2.56 | |
| −36 | −14 | 2 | 2.72 | |
| −36 | −20 | 2 | 2.36 | |
| −30 | 12 | 2 | 2.33 | |
| −30 | 14 | 2 | 2.88 | |
| −32 | 8 | 2 | 2.57 | |
| −32 | 10 | 2 | 2.99 | |
| −34 | 4 | 2 | 2.56 | |
| −34 | 10 | 2 | 2.78 | |
| −36 | 2 | 2 | 2.54 | |
| Claustrum (R) | 30 | 20 | 6 | 2.35 |
| 30 | 16 | 6 | 2.42 | |
| 16 | 18 | 6 | 2.32 | |
| 32 | 18 | 0 | 2.9 | |
| 36 | 18 | 0 | 3.14 | |
| 32 | 14 | 10 | 2.7 | |
| 38 | 12 | 10 | 2.51 | |
| 32 | 10 | 10 | 2.46 | |
| Cerebellum (L) | −6 | −68 | −2 | 3.02 |
| culmen vermis | −6 | −46 | −2 | 2.99 |
| −14 | −58 | −2 | 5.38 | |
| Culmen vermis (R) | 12 | −60 | −2 | 3.32 |
| 4 | −58 | −2 | 3.35 | |
| Lingual gyrus (L) | −12 | −72 | 2 | 2.46 |
| −12 | −70 | 2 | ||
| Lingual gyrus (R) | 10 | −66 | −2 | 2.42 |
| 10 | −64 | −2 | 2.48 | |
| Putamen (L) | −24 | −6 | −2 | 3.5 |
| −26 | 0 | −2 | 2.96 | |
| −26 | 6 | 2 | 2.63 | |
| −26 | −6 | 2 | 2.58 | |
| Putamen (R) | 26 | 2 | 6 | 2.42 |
| 24 | 2 | 6 | 2.51 | |
| 26 | 4 | 6 | 2.32 | |
| 28 | 6 | 10 | 2.63 | |
| 24 | 2 | 10 | 2.67 | |
| Parahippocampal gyrus (L) | −26 | −42 | −2 | 4.71 |
| −16 | −46 | 2 | 2.99 | |
| Juxtapositional Lobule (L) |
−14 | 0 | 42 | 3.12 |
| −14 | 4 | 42 | 4.36 | |
| S1 (L) | −20 | −28 | 48 | 2.81 |
| −22 | −28 | 52 | 3.01 | |
| −24 | −126 | −72 | 2.82 | |
| Premotor area (L) | −22 | −28 | 64 | 2.41 |
| Hippocampus (L) | −26 | −40 | 2 | 4.5 |
| −12 | −18 | 2 | 2.64 |
3.3. Quantitative vs. qualitative considerations in the analysis of cowhage versus histamine itch
We attempted to dissect the impact of pathway specific qualitative aspects (studied by direct contrast) and quantitative aspects (the relationship with itch intensity) on the differences observed in the processing of itch induced with these two modalities. Therefore, the statistical analysis of cowhage vs. histamine differences in brain activation was performed in two ways. One was to contrast data via a paired t test and the other consisted in a regression analysis introducing VAS intensity ratings as covariates of interest to assess differences which depended on stimulus intensity. Lastly, the overlay of this these two maps was generated to synthesize the results and to assess whether the areas that exhibited a differential activation did so in a manner dependent or independent on stimulus intensity.
The direct contrast between the activation maps induced by cowhage itch and histamine itch by a pairwise t test revealed a number of cortical and subcortical areas that were differently activated by cowhage itch in comparison with histamine: the insular cortex and claustrum, angular gyrus, secondary somatosensory area (S2), inferior frontal gyrus and the inferior parietal lobule, among the most prominent (Figure 3, Table 2).
Figure 3.
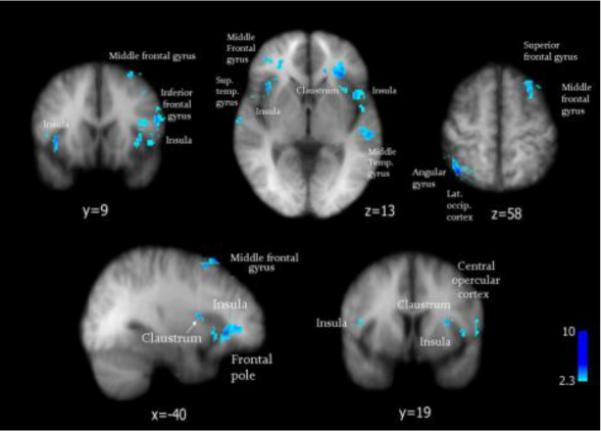
A straight pairwise t test reveals areas activated significantly distinct by cowhage in comparison with histamine itch (in blue). Standard Talairach space coordinates.
Table 2.
Brain regions activated differently by cowhage in contrast with histamine, as emerging from a pairwise t test contrast of activations evoked by the two stimuli during the first 390s. Standard space Talairach coordinates, Z scores > 2.3, p < 0.05.
| BRAIN AREA | CONTRALATERAL (L) | IPSILATERAL (R) | ||||||
|---|---|---|---|---|---|---|---|---|
| Talairach coordinates | x | y | z | Z | x | y | z | Z |
| Claustrum | −30 | 20 | −4 | 3.01 | 34 | 12 | 4 | 2.48 |
| −30 | 18 | −4 | 2.53 | 22 | −12 | 8 | 2.50 | |
| Insula | −32 | 20 | −4 | 3.33 | 38 | 16 | 4 | 3.45 |
| −34 | 16 | −4 | 2.65 | |||||
| −42 | 8 | −4 | 3.10 | |||||
| −48 | 16 | −4 | 2.51 | |||||
| −48 | 0 | −4 | 2.75 | |||||
| Middle frontal gyrus | −40 | 28 | 46 | 3.68 | ||||
| Temporal gyrus | −56 | −24 | −4 | 3.64 | ||||
| Sup. temporal gyrus | −54 | −22 | −4 | 3.66 | ||||
| Centr. opercular cortex | −52 | −2 | 4 | 4.23 | ||||
| Angular gyrus | 38 | −60 | 54 | 4.96 | ||||
| Inferior parietal lobule | 50 | −48 | 54 | 2.43 | ||||
| Inferior frontal gyrus | 56 | 12 | 8 | 2.95 | ||||
A linear regression analysis performed to identify areas where brain perfusion was increased much strongly by cowhage in comparison to histamine itch as a function of a stronger stimulus showed their cerebral representation was extensive, as shown in Figure 4. The intensity-dependent activations evoked by cowhage itch significantly involved the claustral area and the insular cortex.
Figure 4.

A regression analysis reveals activation maps where cowhage activates significantly stronger than histamine (Z > 2.3, p < 0.05), due to the higher itch intensity it evokes (p=0.0005). VAS ratings of itch intensity of both stimuli in the same subjects were used as covariates of interest in the analysis. The contrast was performed with the results from the first ASL scan for both stimuli and the corresponding VAS ratings (first 6 ½ minutes after stimulus administration). ACC = anterior cingulate cortex; PCC = posterior cingulate cortex; SPL = superior parietal lobule; S1 = primary somatosensory area; SMG = supramarginal gyrus.
Interesting observations can be drawn from the overlay of mapping results derived from these two analyses, when three main outcomes can theoretically occur: in one possibility these maps could appear so similar inasmuch as becoming identical or indistinguishable; this would translate to a situation where the main differences between cowhage vs. histamine activations would co-register with the differences driven by intensity. A second possibility would be a partial overlap, where some of the differences depend on itch intensity, while others are independent; and the third possibility would find the two maps segregated (in mathematical terms orthogonal), which is the case (most closely matching the outcome) found in our analysis.
If the first possibility would have occurred, it would have suggested that itch induced by cowhage and histamine would have been qualitatively the same itch processed at different levels of intensity; however, in our contrast of the two itches, the opposite was true (Figure 5). An overlap of the previous two maps (Figures 3 and 4, combined in Figure 5) revealed that most of the significantly different areas activated by cowhage vs. histamine (in red) were found outside the map of intensity-driven differences (in blue), which can only be interpreted that they are independent on stimulus intensity.
Figure 5.

The overlay of the mapping results from regression analysis and from the paired t test contrasting cowhage with histamine itch, shows that most of the cowhage specific activations (in red) fall outside the map of intensity driven effects (in blue), revealing that distinct cowhage responses are mainly intensity-independent. ACC = anterior cingulate cortex; PCC = posterior cingulate cortex; SPL = superior parietal lobule; S1 = primary somatosensory area; S2 = secondary somatosensory area; BA6 = Brodmann area 6.
In an interesting twist, however, the anatomical structures of claustrum and insula appeared to contain discrete, interspersed regions of both types: discrete territories `sensitive' to stimulus intensity, and other subtle regions (activated by cowhage in a manner) `independent' of stimulus intensity. Claustral areas were strongly activated on the contralateral side (left) of stimulus application and involved both anterior, central and posterior claustrum. The ipsilateral (right hemisphere) activation of this structure was more limited to its anterior region (Figures 3, 4, 5).
3.4. Analysis of brain processing of itch modalities over time
Long-lasting changes in brain activation were captured in two sequential ASL scans of 390 seconds, for a total duration of 780 seconds (13 minutes). Differences between histamine and cowhage induced itch were apparent in the time course of the brain processing of itch. Histamine itch appeared to be processed in the same network in the first 390 seconds, as in the following 390s, although less extensively (not shown). Likely due to its lower intensity, itch induced by histamine in the second period was less represented in the same structures. In contrast with histamine itch, the processing of cowhage-induced itch dramatically shifted its representation during the second scan (minutes 6½ to 13) compared to the first 6½ minutes (Figure 6). Although cowhage itch is stronger initially and then subsides more abruptly on a steeper slope (Figure 1) [see also Papoiu et al, 2011], the decrease in perceived intensity alone does not seem to explain the substantial shift in activation towards more anterior regions of ACC, the paracingulate gyrus and the middle frontal gyrus (Figure 6b). In a contrast analysis performed between the activation induced by cowhage during these two periods, activations were significantly stronger in the ACC, paracingulate gyrus, middle and superior frontal gyri and caudate body in the second scan compared to the first, while during the first scan activations in S1, supramarginal gyrus (SMG), superior parietal lobule (SPL) and lateral occipital cortex were stronger than in the second (Figure 6c).
Figure 6.
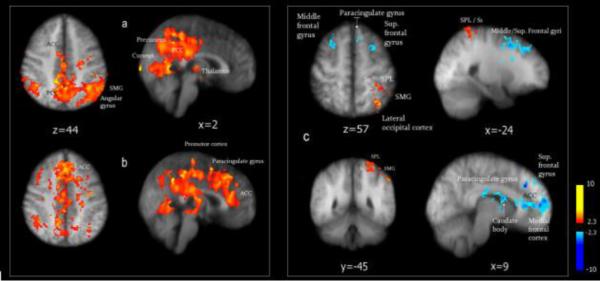
Maps of brain activation induced by cowhage itch during two consecutive ASL scans: during the first period of 390s (a), during the second period (b), and the contrast in activation between a and b, recorded in two consecutive scans is depicted in (c). In panel c) stronger activations captured in the first 390s are depicted in red, while activations stronger in the second period (compared to the first) appear in blue. A lower itch intensity during the second scan may not account for the shifts in brain activation observed over time for cowhage itch, since stronger and more extensive activations in rostral ACC, middle frontal gyrus and the paracingulate gyrus occurred during the second interval. ACC = anterior cingulate cortex. SPL = superior parietal lobule; S1 = primary somatosensory area; SMG = supramarginal gyrus.
3.5. Cerebral representation of itch during concomitant stimulation of cowhage and histamine pathways
The simultaneous stimulation of cowhage and histamine itch pathways evoked a stronger and more extensive activation of the brain areas that each stimulus activated in single application (Figure 7, Table 3). Importantly, activations were bilateral (quasi-symmetrical) and were identified in the following areas: precuneus, cuneus, inferior, superior and posterior parietal cortices, supramarginal & angular gyri, superior and middle temporal cortices, thalamus (Ventral Posterior Lateral, Ventral Posterior Medial & Mediodorsal nuclei), posterior cingulate cortex (PCC), anterior cingulate cortex (ACC), paracentral lobule and secondary somatosensory cortex (S2). Cowhage and histamine combination also activated other structures such as the amygdala, enthorinal cortex, and dentate gyrus in hippocampus, structures connected with memory and with the processing of emotional experiences (Papez circuit; Papez, 1937). The combination of both itch modalities also induced a significant deactivation of the medial prefrontal cortex, which was not observed when either of these stimuli was applied alone (Figure 7).
Figure 7.

Illustration of brain activation and deactivations induced by the concurrent stimulation of the two itch pathways using a combination of cowhage & histamine, during the first ASL scan, compared to baseline conditions. Standard space Talairach coordinates. ACC = anterior cingulate cortex; PCC = posterior cingulate cortex; SPL = superior parietal lobule; M1 = primary motor cortex; S1 = primary somatosensory area; SMG = supramarginal gyrus; MDNc = mediodorsal nucleus; Ant Nc = anterior nucleus.
Table 3.
Brain areas activated by the simultaneous stimulation of both itch pathways with cowhage and histamine during the first 390s (captured in the first ASL scan). Standard space Talairach coordinates, Z scores > 2.3, p < 0.05.
| Brain area | x | y | z | Z score |
|---|---|---|---|---|
| Amygdala (R) | 26 | −10 | −14 | 3.72 |
| 26 | 0 | −4 | 3.28 | |
| Amygdala (L) | −20 | 2 | −12 | 3.53 |
| Putamen (R) | 26 | 0 | −2 | 3.83 |
| 26 | 0 | 8 | 2.86 | |
| 26 | 0 | 2 | 4.53 | |
| 26 | 0 | 2 | 4.53 | |
| 26 | −6 | 2 | 2.69 | |
| Putamen (L) | −22 | −2 | −4 | 3.94 |
| −22 | 6 | −4 | 2.75 | |
| Enthorinal cortex (L) | −20 | −20 | −12 | 2.54 |
| Frontal Orbital, pars triangularis; BA47 | −54 | 22 | −12 | 2.46 |
| Inf. frontal gyrus (R) | 38 | 26 | −12 | 3.36 |
| Insula (R) | 38 | 10 | −8 | 4.33 |
| 48 | 0 | −4 | 3.12 | |
| 34 | 18 | −4 | 3.09 | |
| 36 | 12 | −4 | 3.31 | |
| 44 | 12 | 10 | 2.78 | |
| 44 | 8 | 10 | 2.97 | |
| 44 | −12 | 10 | 2.41 | |
| Insula (L) | −40 | −2 | 2 | 2.95 |
| −38 | 8 | 2 | 2.80 | |
| −50 | −8 | 2 | 3.67 | |
| −36 | 16 | −4 | 3.47 | |
| −46 | 12 | 10 | 3.47 | |
| −42 | −10 | 10 | 3.85 | |
| Lat. Globus Pallidus (L) | −22 | −4 | −4 | 3.48 |
| Parahipocampal gyrus (L) | −22 | −46 | −4 | 3.68 |
| Culmen vermis (L) | −10 | −50 | −4 | 2.96 |
| −10 | −58 | −4 | 2.76 | |
| Culmen vermis (R) | 6 | −56 | −4 | 3.56 |
| 6 | −52 | −4 | 3.08 | |
| Dentate gyrus (R) | 24 | −32 | −4 | 3.66 |
| Middle temp. gyrus | 44 | −30 | −4 | 5.29 |
| Claustrum (L) | −32 | 10 | 2 | 2.36 |
| −32 | 8 | 2 | 2.57 | |
| −34 | 4 | 2 | 2.50 | |
| −36 | 2 | 2 | 3.31 | |
| −36 | 0 | 2 | 3.19 | |
| −36 | −2 | 2 | 2.66 | |
| −36 | −16 | 2 | 2.93 | |
| −36 | 8 | 2 | 3.16 | |
| −36 | −20 | 2 | 2.95 | |
| −36 | −22 | 2 | 2.75 | |
| −32 | 14 | −4 | 3.66 | |
| −30 | 18 | −4 | 2.63 | |
| −26 | 22 | 10 | 3.11 | |
| −30 | 8 | 10 | 2.39 | |
| −30 | 6 | 10 | 2.35 | |
| Claustrum (R) | 34 | 16 | −4 | 3.45 |
| 34 | 4 | 10 | 3.17 | |
| 34 | 10 | 2 | 2.65 | |
| 36 | 8 | 2 | 2.71 | |
| 30 | 24 | 2 | 2.46 | |
| Pulvinar (R) | −22 | −26 | 2 | 4.32 |
| −12 | −26 | 2 | 2.77 | |
| −10 | −32 | 2 | 2.56 | |
| Mammillary bodies (L) | −12 | −20 | 2 | 2.76 |
| −10 | −18 | 2 | 2.48 | |
| PCC (R) | 6 | −50 | 14 | 3.19 |
| 6 | −44 | 14 | 3.25 | |
| 4 | −18 | 22 | 2.85 | |
| 4 | −52 | 22 | 3.11 | |
| 2 | −28 | 48 | 5.78 | |
| 2 | −44 | 24 | 6.43 | |
| 2 | −46 | 26 | 6.01 | |
| PCC (L) | −2 | −46 | 14 | 3.51 |
| −4 | −44 | 14 | 2.61 | |
| −4 | −70 | 14 | 2.50 | |
| −4 | −10 | 22 | 3.23 | |
| −4 | −54 | 22 | 2.57 | |
| Angular gyrus (L) | −46 | −64 | 22 | 2.95 |
| SMG (L) | −58 | −36 | 22 | 3.07 |
| −60 | −24 | 22 | 2.79 | |
| −52 | −4 | 10 | 3.32 | |
| −58 | −4 | 10 | 3.00 | |
| −48 | −16 | 22 | 2.86 | |
| −34 | −18 | 22 | 3.20 | |
| S2 (R) | 46 | 2 | 22 | 4.48 |
| 46 | −14 | 22 | 3.58 | |
| 54 | −26 | 22 | 2.42 | |
| 46 | −20 | 10 | 2.53 | |
| 58 | −44 | 22 | 3.31 | |
| S1 (L) | −52 | 2 | 22 | 2.85 |
| Angular gyrus (L) | 52 | −54 | 22 | 3.49 |
| 46 | −52 | 22 | 3.60 | |
| Lat. occip. cortex (L) | −40 | −64 | 22 | 2.95 |
| IPL (L) | −44 | −70 | 22 | 3.01 |
| Cuneus (L) | −18 | −78 | 22 | 4.78 |
| (R) | 6 | −78 | 22 | 2.74 |
| 2 | −80 | 38 | 2.58 | |
| 2 | −74 | 18 | 2.55 | |
| ACC (R) | 4 | 4 | 26 | 2.50 |
| 4 | −14 | 26 | 3.11 | |
| ACC (L) | −4 | −2 | 28 | 2.89 |
| −4 | −16 | 28 | 2.35 | |
| Paracingulate gyrus (R) | 6 | 16 | 44 | 3.36 |
| /BA 24 | 6 | 22 | 44 | 3.15 |
| PMA/BA6 (R) | 6 | −30 | 68 | 2.87 |
| SPL (L) | −18 | −58 | 54 | 3.08 |
| Medial frontal gyrus (L) | 2 | 16 | 48 | 3.54 |
| Sup. frontal gyrus | 2 | 14 | 60 | 2.77 |
| Precuneus(L) | 2 | −68 | 66 | 4.59 |
| 2 | −42 | 16 | 2.63 | |
| −4 | −66 | 22 | 4.22 | |
| −12 | −52 | 14 | 3.37 | |
| −22 | −56 | 14 | 2.80 | |
| Precuneus (R) | 16 | −54 | 14 | 3.11 |
| Lingual gyrus (R) | 1 | −80 | 6 | 2.31 |
| 1 | −78 | 2 | 2.84 | |
| 1 | −56 | 6 | 3.06 | |
| Inf. frontal gyrus (I) | −60 | −10 | 10 | 3.22 |
| Thalamus | ||||
| Anterior nucleus | −6 | −8 | 10 | 3.25 |
| Mediodorsal (L) | −6 | −10 | 10 | 3.60 |
| nucleus | −6 | −18 | 10 | 2.43 |
| −10 | −16 | 10 | 3.09 | |
| Ventral Lateral | −12 | −8 | 10 | 2.45 |
| nucleus (L) | −12 | −12 | 10 | 2.51 |
| Ventral Posterior Lateral nucleus (L) | −18 | −18 | 2 | 3.12 |
| −20 | −22 | 2 | 2.98 |
The involvement of the insular and claustral areas in itch processing appeared even more poignant when both itch pathways were stimulated in the same time. A good illustration of the role played by the claustrum and the insular cortex was revealed in an contrast analysis of brain activation in two consecutive ASL scans capturing itch induced simultaneously with the two modalities: these areas were extensively activated bilaterally, with the ipsilateral insula almost entirely `lighted-up' (Figure 8). Strikingly, no other area displayed a significant change in activation between the first period to the next, during the processing of a dual-mode itch. In summary, the results of different types of analysis showed that claustrum and the insular cortex are activated by cowhage in a fashion sensitive to itch intensity, that these areas are significantly activated by cowhage than by histamine itch and that a stronger involvement of these regions is more evident when these two pathways are stimulated in the same time.
Figure 8.

A contrast between activations induced in two consecutive ASL scans by the simultaneous activation of both itch pathways, emphasizes the involvement of insula and claustrum in the processing of dual-mode itch (the combination of cowhage and histamine) at two different intensities. The images show a significantly stronger activation is captured in the first period (6 and ½ minutes), in comparison with the second interval (from minutes 6 ½ to 13). Standard space Talairach coordinates. ACC = anterior cingulate cortex.
4. Discussion
4.1. General observations
The peripheral C nerve fibers as well as the corresponding spinothalamic pathways transmitting ascending information on cowhage and histamine itch are distinct and their separation is maintained at spinal and thalamic levels (Johanek et al, 2007 Namer et al, 2008; Davidson et al, 2008, 2009; reviewed by Davidson & Giesler, 2010). Prior neurophysiological information to describe the projection of the “third neuron” carrying ascending information on cowhage or histamine itch to the cerebral cortex is sparse. In this study we image for the first time the brain processing of cowhage-induced itch, analyzed in contrast with histamine itch, by Arterial Spin Labeling fMRI. ASL was successfully used in the neuroimaging of pain (Owen et al, 2008) and was also demonstrated as a suitable methodology to investigate the cerebral processing of pruritus (Ishiuji et al, 2009). ASL offers advantages over BOLD, in terms of superior signal sensitivity when imaging responses evoked by a prolonged sensory experience such as experimental pruritus.
The present results show that the brain processing of cowhage and histamine itch in healthy subjects is similar, but not identical. The observed patterns of brain activation induced with either histamine or cowhage are complex and reflect the multidimensionality of itch experience, involving sensory, cognitive and emotional-affective aspects. Although both forms of itch activated the thalamus, S1 and S2, precuneus, cuneus, PCC, ACC, inferior & posterior parietal cortices, superior and middle temporal gyri, cowhage itch induced an asymmetric, distinct and more extensive activation of the posterior parietal cortex, insular cortex and claustrum, putamen, thalamic nuclei and pulvinar. Cowhage itch was recognizable by an asymmetric fingerprint: a pronounced effect on contralateral insula and claustrum, which may be qualitatively specific to cowhage-induced itch. A comparison of psychophysical characteristics of cowhage and histamine when both stimuli were applied by way of cowhage spicules reported that cowhage itch was still differentiated as more `stinging, sharp, or prickling' than histamine (Kosteletzky et al, 2009). Visual analog scale ratings recorded in this study, as well as previous psychophysical experiments showed that cowhage induced itch had a stronger amplitude, lasted longer that histamine induced itch, and had a more variable or transient character, presenting burning or stinging overtones (LaMotte et al, 2009; Sikand et al, 2009; Kosteletzky et al, 2009; Papoiu et al, 2011). These features combined could explain the differential changes in the pattern of brain activation observed over time that appear to distinguish it from histamine itch. Synthesizing the results of our regression and contrast analyses, the map of activations driven by a stronger cowhage itch did not overlap with the areas where cowhage responses significantly differ from histamine's, which leads to the conclusion that the significant differences in brain processing of the two itches appear pathway specific.
4.2. Cowhage and histamine itch activate thalamic nuclei and cortical projection stations S1 and S2
Classical relay stations in the thalamus and somatosensory areas S1 and S2 were activated during the processing of histamine and cowhage itches. Activation of the thalamus by histamine itch (Darsow et al, 2000), or by histamine and allergen-induced itch (Leknes et al, 2007) was reported previously, however, activations in somatosensory areas were not found consistently in previous studies. Cowhage itch appears to induce a stronger and more extensive activation in S1 and S2 than histamine. Histamine and cowhage itch appear to activate several nuclei in the thalamus. The expected relay station in the thalamus for a sensory pathway conducting information that originates in the right forearm would be the (contralateral) VPL nucleus and indeed we observed a significant activation of thalamus in a region consistent with the contralateral VPL, by both itch modalities. Strong activations were also noted in areas which would be consistent with the ventral anterior nucleus, anterior nucleus, lateral posterior nucleus, and the mediodorsal nucleus. Comparing maps of activations induced by cowhage versus histamine, it appears that cowhage activates more extensively the thalamus than histamine itch, although differences do not reach statistical significance.
Histamine and cowhage itch evoked a strong activation of precuneus, along with PCC and the posterior parietal cortex (the angular and supramarginal gyri), a feature remarkably contrasting with the deactivation of these areas observed in the processing of pain (Veldhuijzen et al, 2009; Owen et al, 2010). Moreover, these regions seem constantly activated during itch processing, in the two successive fMRI series. These differences can add a new layer of understanding to the dialectic relationship between pain and itch processing. It is known that pain has an inhibitory effect on the itch sensation, while pharmacological analgesia is often accompanied by the occurrence of itch.
An extensive activation of the precuneus during itch processing was found in the three sub-regions of this structure which have distinct sensory, cognitive and emotional-affective specializations. Taken together this suggests precuneus may serve as an associative “hub” for processing itch-related information, in its various phenomenological aspects. Precuneus is considered a part of the `default network', a part of interceptive cortex, predominantly activated in “awake” states. Its activation was reported in neuroimaging studies of itch using different modalities (Mochizuki et al, 2009) and in atopic dermatitis (Ishiuji et al, 2009), strengthening the idea that precuneus has a role in itch processing.
4.3. The role of claustrum in the processing of cowhage itch
In several analyses performed to assess differences in the brain processing of cowhage vs. histamine itch, claustrum was consistently activated and overall, emerged as a significant region of interest, being prominently involved in the time course of cowhage itch and of cowhage-histamine itch. Claustrum is a shallow, largely under-investigated formation, embedded between external capsule and the extreme capsule in higher primates, thoroughly connected to almost all regions of the cortex (Edelstein & Denaro, 2004; Crick & Kock, 2005). Contralateral activations in the claustrum were observed earlier, evoked by both painful and itch stimuli delivered by microdialysis (Herde et al, 2007), but a significant role of claustrum in the processing of itch has not been clearly emphasized previously. Although its functions are far from being elucidated in humans, several lines of evidence indicate that claustrum is a multisensory detector for processing fast inputs that require alertness and a quick response to sudden changes in the environment (Remedios et al, 2010). It was proposed that short response latencies within the claustrum may reflect a major driving input from thalamic structures and early sensory cortices. Since thalamus was extensively activated by cowhage itch (and to a lesser extent by histamine itch) this functional connection could be highly operative in the context of processing itch information exhibiting transient shifts in amplitude and quality (pricking, burning). Thus, it appears plausible that claustrum is a “sensitive” structure able to detect subtle nuances of the sensory experience elicited by cowhage. Claustrum was suggested to play a role in gating or thresholding nociceptive information (Persinger et al, 1997) and also to receive inputs via ascending pathways that may bypass thalamus and convey information to the cingulate cortex (Sloniewski et al, 1995). Claustrocingulate pathways were implicated in the emotional responses to noxious stimuli (Neafsey et al, 1993), which raises the question whether the emotional and affective aspects of the itch sensation may be conveyed through the same channels in humans.
Thanks to the widespread connectivity with the cortex (Sherk, 1996) the claustrum could send alerting signals to several cortical areas at the same time (Remedios et al, 2010). Connectivity studies of the subdivisions within claustrum in non-human primates (Pearson et al, 1982) linked the anterior part of claustrum to prefrontal cortex (Brodmann areas 8 and 9) and area 4 of the precentral gyrus, possibly revealing its role in planning of motor activity (i.e. scratching) and the posterior regions with S1 (receiving sensory input from the primary somatosensory cortex). Claustrum may also function in synergy with the neighboring insular cortex to which it is intimately connected. Interestingly, when a dual input of cowhage and histamine itch was transmitted to the cortical stations and processed in the same time, a robust involvement of the insular and claustral regions was observed bilaterally. Furthermore, a recent neuroimaging report in patients with severe pruritus diagnosed with familial Creutzfeldt-Jacob disease, using diffusion weighted imaging identified the right claustrum among the affected areas displaying a significantly lower perfusion, alongside periaqueductal gray and midbrain (Cohen et al, 2011).
4.4.
The implication of the insular cortex in the processing of itch is noteworthy due to its involvement in high-level cognitive awareness (interoception), attentional tuning and formation of addictive behaviors. The anterior insular cortex has been described as an integral part of salience network (Menon and Uddin, 2010). Insula plays an important role in the evaluation of nociceptive stimulus intensity; it is therefore likely insula plays a role in the evaluation of itch intensity as well, as previously suggested (Drzezga et al, 2001). Cowhage itch has significantly more extensive representation in this region in comparison with histamine induced itch and most of the areas activated by cowhage in the insular cortex were dependent on stimulus intensity. The insula could play a larger role, due to its ample connectivity to the anterior cingulate cortex, in planning of motor activity (i.e. scratching) and can reflect the urge to scratch in the given circumstances, a desire inexorably linked to the sensation of itch. The itch-scratch cycle is known to display characteristics of addictive behavior and therefore the involvement of the insular cortex in this process, also reported earlier (Yosipovitch et al, 2008) deserves further investigation.
4.5.
In conclusion, our analyses converge to suggest a complex role of insula and claustrum in assessing both qualitative and quantitative aspects of itch (Figures 3–8). Although the processing of cowhage and histamine itch involves the same main relays in the thalamus and somatosensory cortices, a differential processing of these two forms of itch is also manifest in other areas. Particular sensory qualities of cowhage itch (pricking, stinging, burning overtones) seem to evoke emotional-affective and cognitive associations powerful enough to register in the brain in a meaningful manner to differentiate it from histamine itch. It is difficult to dissect at this stage whether the shift observed in processing cowhage itch over time reflects purely sensory aspects variable in quality, or different cognitive-evaluative processes. More studies are required to further clarify this distinction. Due to the involvement of cowhage-sensitive PAR2 pathway in chronic pruritic diseases such as atopic dermatitis, the comparative analysis of cowhage in contrast to histamine itch could offer a new tool for understanding the central processing of pathological pruritus.
4.6 Limitations
1) In our study, the order of the application of stimuli was histamine, cowhage and their combination, with ample time in between sessions for itch sensation to completely subside. This sequence was preferred due to initial concerns that cowhage could produce an itch for a substantially longer period of time than histamine. However, our final psychophysical data indicated that was not the case. Since the order of itch stimuli was not randomized, cognitive factors such as fatigue, expectation and perceived novelty may have slightly influenced both the perception of itch as well as the observed patterns of brain activation, therefore a putative order effect cannot be completely eliminated. 2) The two itch stimuli were applied using different delivery modalities cowhage via native spicules, and histamine via iontophoresis. The neuroimaging experiments were performed after itch sensation was induced, in such a way that the delivery route would have a minimal impact on the evoked responses; however, a link between these factors cannot be excluded.
Acknowledgements
This work was supported by NIAMS (NIH) grant 5R01AR055902.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edleman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40:383–396. doi: 10.1002/mrm.1910400308. [DOI] [PubMed] [Google Scholar]
- Cohen OS, Chapman J, Lee H, Nitsan Z, Appel S, Hoffman C, Rosenmann H, Korczyn AD, Prohovnik Pruritus in familial Creutzfeldt-Jakob disease: a common symptom associated with central nervous system pathology. J Neurol. 2011;258:89–95. doi: 10.1007/s00415-010-5694-1. [DOI] [PubMed] [Google Scholar]
- Crick FC, Koch C. What is the function of the claustrum? Philos Trans R Soc Lond B Biol Sci. 2005;360:1271–1279. doi: 10.1098/rstb.2005.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow U, Drzezga A, Frisch M, Munz F, Weilke F, Bartenstein P, Schwaiger M, Ring J. Processing of histamine-induced itch in the human cerebral cortex: a correlation analysis with dermal reactions. J Invest Dermatol. 2000;115:1029–1033. doi: 10.1046/j.1523-1747.2000.00193.x. [DOI] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:1007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr. Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat Neurosci. 2009;12:544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Giesler GJ., Jr. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550–558. doi: 10.1016/j.tins.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzezga A, Darsow U, Treede RD, Siebner H, Frisch M, Munz F, Weilke F, Ring J, Schwaiger M, Bartenstein P. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of 15O-H2O positron emission tomography studies. Pain. 2001;92(1–2):295–305. doi: 10.1016/s0304-3959(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Edelstein LR, Denaro FJ. The claustrum: a historical review of its anatomy, physiology, cytochemistry and functional significance. Cell Mol Biol (Noisy-le-grand) 2004;50:675–702. [PubMed] [Google Scholar]
- Hsieh JC, Hägermark O, Ståhle-Bäckdahl M, Ericson K, Eriksson L, Stone-Elander S, Ingvar M. Urge to scratch represented in the human cerebral cortex during itch. J Neurophysiol. 1994;72:3004–3008. doi: 10.1152/jn.1994.72.6.3004. [DOI] [PubMed] [Google Scholar]
- Herde L, Forster C, Strupf M, Handwerker HO. Itch induced by a novel method leads to limbic deactivations a functional MRI study. J Neurophysiol. 2007;98:2347–2356. doi: 10.1152/jn.00475.2007. [DOI] [PubMed] [Google Scholar]
- Ishiuji Y, Coghill RC, Patel TS, Dawn A, Fountain J, Oshiro Y, Yosipovitch G. Repetitive scratching and noxious heat do not inhibit histamine-induced itch in atopic dermatitis. Br J Dermatol. 2008;158:78–83. doi: 10.1111/j.1365-2133.2007.08281.x. [DOI] [PubMed] [Google Scholar]
- Ishiuji Y, Coghill RC, Patel TS, Oshiro Y, Kraft RA, Yosipovitch G. Distinct patterns of brain activity evoked by histamine-induced itch reveal an association with itch intensity and disease severity in atopic dermatitis. Br J Dermatol. 2009;161:1072–1080. doi: 10.1111/j.1365-2133.2009.09308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27:7490–7. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosteletzky F, Namer B, Forster C, Handwerker HO. Impact of scratching on itch and sympathetic reflexes induced by cowhage (Mucuna pruriens) and histamine. Acta Derm Venereol. 2009;89:271–277. doi: 10.2340/00015555-0624. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Koyama T, Kroncke AP, Coghill RC. Effects of stimulus duration on heat induced pain: the relationship between real-time and post-stimulus pain ratings. Pain. 2004;107:256–266. doi: 10.1016/j.pain.2003.11.007. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Shimada SG, Green BG, Zelterman D. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J Neurophysiol. 2009;101:1430–1443. doi: 10.1152/jn.91268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes SG, Bantick S, Willis CM, Wilkinson JD, Wise RG, Tracey I. Itch and motivation to scratch: an investigation of the central and peripheral correlates of allergen- and histamine-induced itch in humans. J Neurophysiol. 2007;97:415–422. doi: 10.1152/jn.00070.2006. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H, Tashiro M, Kano M, Sakurada Y, Itoh M, Yanai K. Imaging of central itch modulation in the human brain using positron emission tomography. Pain. 2003;105:339–346. doi: 10.1016/s0304-3959(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Inui K, Tanabe HC, Akiyama LF, Otsuru N, Yamashiro K, Sasaki A, Nakata H, Sadato N, Kakigi R. A Combined MEG –fMRI Study Time Course of Activity in Itch-Related Brain Region. J Neurophysiol. 2009;102:2657–2666. doi: 10.1152/jn.00460.2009. [DOI] [PubMed] [Google Scholar]
- Namer B, Carr R, Johanek LM. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey EJ, Terreberry RR, Hurley KM, Ruit KG, Frysztak RJ. Anterior cingulate cortex in rodents: connections, visceral control functions and implications for emotion. In: Vogt BA, M Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus. Birkhauser; Boston, MA: 1993. pp. 206–223. [Google Scholar]
- Owen DG, Bureau Y, Thomas AW, Prato FS, St Lawrence KS. Quantification of pain-induced changes in cerebral blood flow by perfusion. MRI Pain. 2008;136:85–96. doi: 10.1016/j.pain.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Owen DG, Clarke CF, Ganapathy S, Prato FS, St Lawrence KS. Using perfusion MRI to measure the dynamic changes in neural activation associated with tonic muscular pain. Pain. 2010;148:375–386. doi: 10.1016/j.pain.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. J Neuropsychiatry Clin Neurosci. 1937 1995 Winter;7(1):103–12. doi: 10.1176/jnp.7.1.103. [DOI] [PubMed] [Google Scholar]
- Papoiu ADP, Tey HL, Coghill RC, Wang H, Yosipovitch G. Cowhage-induced itch as an experimental model for pruritus. A comparative study with histamine-induced itch. PLoS One. 2011;6(3):e17786. doi: 10.1371/journal.pone.0017786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RC, Brodal P, Gatter KC, Powell TP. The organization of the connections between the cortex and the claustrum in the monkey. Brain Res. 1982;234:435–441. doi: 10.1016/0006-8993(82)90883-6. [DOI] [PubMed] [Google Scholar]
- Persinger MA, Peredery O, Bureau YR, Cook LL. Emergent properties following brain injury: the claustrum as a major component of a pathway that influences nociceptive thresholds to foot shock in rats. Percept Mot Skills. 1997;85:387–98. doi: 10.2466/pms.1997.85.2.387. [DOI] [PubMed] [Google Scholar]
- Pfab F, Valet M, Sprenger T, Huss-Marp J, Athanasiadis GI, Baurecht HJ, Konstantinow A, Zimmer C, Behrendt H, Ring J, Tölle TR, Darsow U. Temperature modulated histamine-itch in lesional and nonlesional skin in atopic eczema - a combined psychophysical and neuroimaging study. Allergy. 2010;65:84–94. doi: 10.1111/j.1398-9995.2009.02163.x. [DOI] [PubMed] [Google Scholar]
- Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remedios R, Logothetis NK, Kayser C. Unimodal responses prevail within the multisensory claustrum. J Neurosci. 2010;30:12902–12907. doi: 10.1523/JNEUROSCI.2937-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G, Ständer S, Burgmer M, Driesch G, Heuft G, Weckesser M. Significant differences in central imaging of histamine-induced itch between atopic dermatitis and healthy subjects. Eur J Pain. 2008;12:834–841. doi: 10.1016/j.ejpain.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Sherk H. The claustrum and the cerebral cortex. In: Jones EG, Peters A, editors. Cerebral Cortex, Vol. 5 Sensorimotor Areas and Aspects of Cortical Connectivity. Plenum Publ.; New York: 1986. [Google Scholar]
- Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Słoniewski P, Moryś J, Pilgrim C. Stimulation of glucose utilization in the rat claustrum by pain. Folia Neuropathol. 1995;33(3):163–8. [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, Luger TA, Schmelz M. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valet M, Pfab F, Sprenger T, Wöller A, Zimmer C, Behrendt H, Ring J, Darsow U, Tölle TR. Cerebral processing of histamine-induced itch using short-term alternating temperature modulation - an fMRI study. J Invest Dermatol. 2008;128:426–433. doi: 10.1038/sj.jid.5701002. [DOI] [PubMed] [Google Scholar]
- Veldhuijzen DS, Nemenov MI, Keaser M, Zhuo J, Gullapalli RP, Greenspan JD. Differential brain activation associated with laser-evoked burning and pricking pain: An event-related fMRI study. Pain. 2009;141:104–113. doi: 10.1016/j.pain.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierow V, Fukuoka M, Ikoma A, Dörfler A, Handwerker HO, Forster C. Cerebral Representation of the Relief of Itch by Scratching. J Neurophysiol. 2009;102:3216–3224. doi: 10.1152/jn.00207.2009. [DOI] [PubMed] [Google Scholar]
- Wang H, Papoiu AD, Coghill RC, Patel T, Wang N, Yosipovitch G. Ethnic differences in pain, itch and thermal detection in response to topical capsaicin: African Americans display a notably limited hyperalgesia and neurogenic inflammation. Br J Dermatol. 2010;162:1023–9. doi: 10.1111/j.1365-2133.2009.09628.x. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G. Itch: Basic Mechanisms and Therapy. Marcel Dekker Inc.; New York - Basel: 2004. Epidemiology of Itching in Skin and Systemic Disease; pp. 183–191. [Google Scholar]
- Yosipovitch G, Ishiuji Y, Patel TS, Hicks MI, Oshiro Y, Kraft RA, Winnicki E, Coghill RC. The Brain Processing of Scratching. J Invest Dermatol. 2008;128:1806–1811. doi: 10.1038/jid.2008.3. [DOI] [PubMed] [Google Scholar]



