Abstract
Antisocial traits are common among alcoholics— particularly in certain subtypes. Although people with antisocial tendencies show atypical brain activation in some emotion and reward paradigms, how the brain reward systems of heavy drinkers (HD) are influenced by antisocial traits remains unclear. We used subjects’ preferred alcohol drink odors (AO), appetitive (ApCO) and non-appetitive (NApO) control odors in functional magnetic resonance imaging (fMRI) to determine if reward system responses varied as a function of antisocial trait density (ASD). In this retrospective analysis, we examined 30 HD who had participated in imaging twice: once while exposed to clamped intravenous alcohol infusion targeted to 50 mg%, and once during placebo saline infusion. Under placebo, there were positive correlations between ASD and blood oxygenation level dependent (BOLD) activation in the [AO > ApCO] contrast in the left dorsal putamen, while negative correlations were present in medial orbitofrontal cortex (OFC) and the bilateral amygdala. A similar pattern was observed in the correlation with the [AO > NApO] contrast. This inverse relationship between ASD and activation to alcohol odors in OFC and amygdala was specific to AO. However, negative correlations between ASD and the [ApCO > NApO] contrast were also present in the insula, putamen, and medial frontal cortex. These data suggest that frontal and limbic reward circuits of those with significant ASD are less responsive to reward cues in general, and particularly to alcohol cues in medial OFC and amygdala. These findings are broadly consistent with the reward deficiency syndrome hypothesis, although positive correlation in the striatum suggests regional variability.
Keywords: alcoholism, alcohol use disorder, ethanol, personality disorder, prefrontal, orbital
Introduction
Alcohol use disorders are highly comorbid with other psychiatric disorders (Kessler et al., 1997, Di Sclafani et al., 2007), and in particular with externalizing and antisocial behaviors (Mulder, 2002). The co-occurrence of antisocial personality disorder (ASPD) in subjects with alcohol use disorders (AUD) is 4–7 times as high as the general population (Compton et al., 2005, Di Sclafani et al., 2007) with the externalizing behaviors being more characteristic of a particular latent class of antisocial individuals with AUD (Moss et al., 2007). Such a subtype appears to have high practical relevance, as ASPD among alcoholics predicts poorer treatment outcome after one year (Hesselbrock, 1991). As assessed in youth, antisocial/disinhibitory behaviors are also potent longitudinal predictors of later AUD (Tarter et al., 2003, Knop et al., 2009). The robust association of antisocial/disinhibitory traits and AUD suggest that AUD can be better understood by assessing outcomes based on antisocial traits.1
Given this background, it is important to understand the extent to which such externalizing traits affect the brain’s response to stimuli associated with alcohol. Stimuli that are present during drug consumption acquire Pavlovian properties, and become conditioned stimuli (CS) for intoxication. That is, the sight, sounds, tastes, and smells of alcoholic drinks signal impending intoxication to those with extensive drinking histories. The effect of CS presentation is controversial, however, with contrary predictions supported by different studies. Withdrawal models predict that CS presentation with no drug reinforcement should produce a withdrawal-like state (Wikler, 1948, Siegel, 1975). An opposing viewpoint maintains that CS presentation elicits arousal states consistent with intoxication (Stewart et al., 1984, Rohsenow et al., 1990). Mixed findings have not fully resolved the debate (Niaura et al., 1988, Rohsenow et al., 1990), but in either case CS presentation should activate motivational mechanisms related to drug taking (Robinson and Berridge, 1993, Koob and Le Moal, 1997), particularly in heavy users. Indeed, converging evidence suggests that drug-paired CS acquire unique motivational and rewarding power (Everitt and Robbins, 2005). Aromas of alcoholic drinks are salient CS that can be delivered during functional neuroimaging studies, are thought to provoke the “somatic states” associated with alcohol’s pharmacologic actions (Verdejo-García and Bechara, 2009), as well as promote craving and activation of reward-related brain areas (e.g. Grüsser et al., 2000, Schneider et al., 2001, Bragulat et al., 2008, Kareken et al., 2010a, Kareken et al., 2010b).
However, it may or may not be the case that heavy drinkers with antisocial traits possess similar neural vulnerabilities as do heavy drinkers without such behavioral problems. For example, the preclinical literature suggests that a propensity to alcoholism includes inherited abnormalities in the brain’s mesocorticolimbic dopaminergic system (for review see Murphy et al. 2002) that lead to disordered “wanting” and ethanol seeking (Gonzales et al. 2004). Similar disordered “wanting” or “valuing” of alcohol’s pharmacologic reward could play a similar role in ASPD variants, and the brain structures thought to encode such desires, such as the ventral striatum and medial prefrontal cortex (e.g. Hare, et al., 2009, Berridge, 2007). However, other neurobehavioral mechanisms may instead be more prominent, such as those involved with behavioral regulation, planning, and the coding of visceral signals that guide behavior to avoid aversive outcomes (Verdejo-García and Bechara, 2009). To date, the literature remains unclear on this point.
In some cases, antisocial traits have been linked to greater activation in reward areas, which suggests hypersensitivity to rewards and their associated CSs. In one recent study of healthy individuals without histories of substance abuse, impulsive/antisocial traits correlated positively with amphetamine-induced DA release from the nucleus accumbens (NAcc) as measured by positron emission tomography (PET), and were associated with NAcc activation from the anticipation of monetary reward during fMRI (Buckholtz et al., 2010). Another fMRI study using monetary rewards revealed that those with ASPD showed greater activation in orbitofrontal cortex (OFC) relative to controls (Völlm et al., 2010), although the task itself did not provoke significant activation in brain reward regions. Similarly, receipt of reward provoked greater ventral striatal activation in adolescents presenting externalizing symptoms measured in fMRI (Bjork et al., 2010). These findings collectively suggest a heightened response to rewarding (monetary) outcomes in the brain’s appetitive system—and potentially by extension to a reward’s CS. However, other studies have found that antisocial traits predicted reduced activation in OFC to reward, either in ASPD and Borderline personality disorder (Völlm et al., 2007), or in boys with conduct disorder when compared to ADHD boys without conduct disorder (Rubia et al., 2009). Thus, the extent to which brain reward systems respond similarly in heavy drinkers with and without significant externalizing behaviors remains an important and unresolved question.
In this regard, and unlike these prior studies, we examined heavy drinkers, who constitute individuals with extensive conditioning to alcohol cues. We previously showed that the response to alcohol odors was mediated by a family history of alcoholism, such that family history positive subjects showed a larger medial frontal response to alcohol odors than family history negative subjects under placebo— a pattern that was reversed by acute alcohol intoxication (Kareken et al. 2010a). Using this same data set, we hypothesized that antisocial symptom density (ASD) would positively correlate with activation to alcohol odors in reward-relevant areas; specifically the ventral striatum and limbic areas such as OFC, insula, and amygdala (de Wit and Richards, 2004; Newman and Wallace, 1993), which would be suggestive of a positive relationship between externalizing behaviors and stimulus-induced desire. To assess the effect of acute alcohol intoxication, subjects were imaged under both intravenous alcohol and saline.
Methods
Subjects
The subjects were recruited and assessed using the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; Bucholz et al., 1994), the Timeline Followback interview for recent drinking (TFLB; Sobell et al., 1986), and the Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993). Thirty right-handed heavy drinkers (HD) were tested (Table 1); 17 were family history positive for alcoholism (FHP; at least two first or second degree relatives with probable alcoholism on the SSAGA family history module, excluding mothers to preclude possible fetal alcohol effects) and 13 without any known family history of alcoholism (FHN). None had been treated for alcohol disorders, had evidence of Axis-I psychiatric disorders, had neurological disorders of the brain, or failed olfactory screening. Although all subjects denied using illicit drugs, one subject tested positive for cannabinoids on both the placebo and alcohol day, while another subject also tested positive for cannabinoids on the placebo day. Additionally, one other subject tested positive for amphetamines on both placebo and alcohol days, and another subject tested positive for amphetamines only on the alcohol day. Another subject’s alcohol scan had to be excluded for excessive motion. This analysis comprises heavy drinkers reported in previous studies (Kareken et al., 2010a, Kareken et al., 2010b), but: 1) includes 4 additional drinkers who fit DSM-IV criteria for alcohol dependence, and 2) excludes 14 social drinkers from Kareken, et al., 2010b, so as to focus on a more uniform sample of heavier drinkers in whom there would be greater ASD. All subjects voluntarily signed informed consent statements, which were approved by the Indiana University School of Medicine Institutional Review Board.
Table 1.
Subject Characteristics
| Mean | SD | |
|---|---|---|
| Age | 24.0 | 2.5 |
| Education | 15.4 | 1.3 |
| Relatives with Alcoholism | 1.7 | 1.8 |
| ASD | 4.6 | 4.0 |
| Drinks per week | 18.2 | 8.2 |
| Drinks per heavy drinking daya | 5.6 | 2.0 |
| Heavy drinking daysa | 1.7 | 0.7 |
| AUDIT | 12.0 | 4.0 |
| Age of first drink | 15.1 | 2.1 |
| Age of regular drinking | 18.6 | 1.5 |
ASD= Antisocial Trait Density; AUDIT= Alcohol Use Disorders Identification Test;
‘heavy drinking’= >4 drinks per day.
Procedure
Subjects participated in two imaging sessions during exposure to the aromas of the subjects’ individually preferred alcoholic drinks, as well as two sets of control odors. During each fMRI session, subjects underwent intravenous infusion of alcohol or placebo in a randomized order. To minimize expectations, subjects were told that they could receive alcohol or placebo during any imaging session (i.e., one session did not predict the other).
Assessment of externalizing behaviors
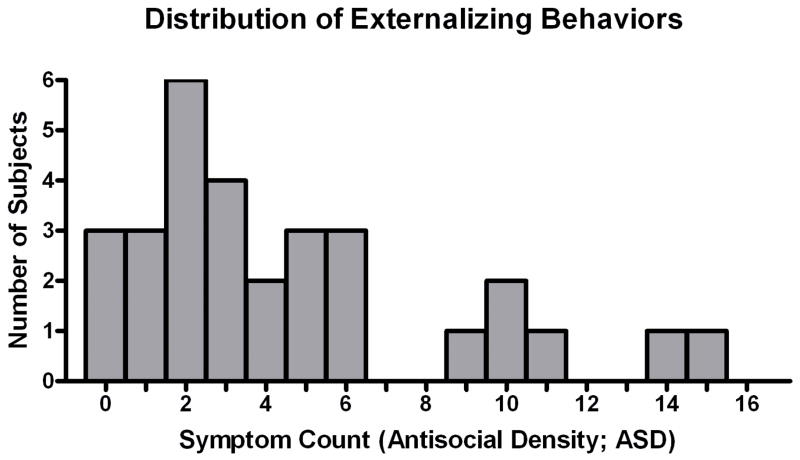
Subjects completed a computerized adaptation of the SSAGA’s portion that assesses ASD and externalizing behaviors consistent with conduct disorder and antisocial personality disorder (41 questions; see Supplemental 1). Answering affirmatively to any one item earned a point, with the total number of points summed. An additional point was earned for questions that separated both the occurrence of a given behavior (e.g., admitting to having acted in a way that could have led to arrest) as well as for the extent to which particular behaviors occurred on multiple occasions (e.g., “Did any of these things happen 3 or more times?”). Thus the possible range was 0 – 41. The distribution of score totals is shown in Figure 1.
Figure 1.
Distribution of the total number of self-reported externalizing behaviors from a modified SSAGA assessment, which defines the trait antisocial density (ASD), ranged from 0 to 15 in this heavy drinking subject sample.
Olfactory stimuli
Odorants were delivered using an air-dilution olfactometer as previously described (Kareken et al., 2004, Bragulat et al., 2008). Three classes of odorants were used: alcohol odors (AO, alcohol odors), appetitive control odors (ApCO; chocolate and grape juice; McCormick & Company, Inc., Hunt Valley, MD), and non-appetitive odors (NApO; two out of grass, leather, and Douglas fir; International Flavors & Fragrances, Union Beach, NJ) that represented stimuli not normally ingested, or evocative of ingestive behavior. The alcohol odors were the subject’s two most preferred alcoholic drinks.
Stimulus training and craving
Before entering the scanner room, subjects were familiarized with the odorants by smelling each (grouped by the stimulus classes of AO, NApO, ApCO) through the olfactometer while simultaneously viewing representative images on a computer monitor. Just prior to combined odor/picture cue-exposure (baseline), and again after each of the three stimulus classes, subjects answered questions probing mood and craving. Subjects rated desire to drink alcohol by responding to four items (#11, #18, #21, #32) from the Alcohol Craving Questionnaire (ACQ; Singleton et al., 2000) on a visual analog scale (VAS; 1=strongly disagree, 7=strongly agree).
Activation paradigm
Three functional scans of olfactory stimulation per subject session were performed as previously reported (24 odor events in each of the three stimulus classes of AO, ApCO and NApO plus 42 odorless control events in alternating blocks, but with activation assessed in response to individual odorant pulses; see Kareken et al., 2010a). No images were presented during imaging, and subjects underwent olfactory stimulation with eyes closed. Subjects reported the presence (button 1) or absence (button 2) of an odorant on a 4-button response box (Current Designs, http://curdes.com), but were not asked to identify the odorants.
Odor ratings
After each imaging session, subjects were re-exposed to the odors. After smelling each odor, the subjects rated the odor’s intensity, pleasantness, and representativeness (how well the odor represented its intended source) on a 9-point VAS.
Alcohol administration
Subjects were intravenously infused with either alcohol (6% vol/vol) or saline (placebo) in counter-balanced order as previously described (Bragulat et al., 2008). Infusion pump rates were computer-controlled, with the infusion profile customized for each individual to achieve the same time course of breath alcohol concentration (BrAC) for all subjects: A linear ascension to 50 mg% in 10 min, followed by constant exposure at 50 mg% throughout approximately 45-min of functional imaging. The placebo infusion employed the same pump-rate profile as was/would be used in the individual’s alcohol session, but infused only saline. Prior to and immediately after imaging sessions, BrAC was measured.
Image acquisition and statistical analysis
Whole-brain blood oxygenation level dependent (BOLD) imaging was conducted on a Siemens (Erlangen, Germany) 3T Magnetom Trio scanner using the imaging protocol previously described (Kareken et al., 2010a, Kareken et al., 2010b). A whole-brain high resolution anatomical image volume (1.0 mm×1.0 mm×1.2 mm voxels) was first collected using a 3D magnetization prepared rapid gradient echo (MP-RAGE) sequence for anatomic registration of functional images. In three functional scans, BOLD volumes with 37 slices covering a 111-mm superior–inferior extent of the brain were acquired, using an echo planar imaging sequence that incorporated a 3D prospective acquisition correction (gradient echo, 96×96 acquisition matrix, 2.5 mm×2.5 mm×3.0 mm voxels; for 15 subjects (10 FHP, 5 FHN): 134 measurements, 3000 ms repetition time (TR), 40 ms echo time (TE), 90° flip angle, 2.5 mm slice thickness with 0.5 mm interslice gap, no acceleration; for 15 subjects (7 FHP, 8 FHN): 174 measurements, 2250 ms TR, 30 ms TE, 78° flip angle, 3.0 mm slice thickness with no inter-slice gap, GRAPPA acceleration factor 2). These minor acquisition differences were necessary given an upgrade to the Trio. Direct whole-brain voxel-wise testing of the two acquisitions showed no significant differences in BOLD activation to olfactory stimulation across all three odorant types (p < 0.05, false discovery rate corrected). ASD symptom count also did not differ before and after the upgrade (medians 4 and 3 for pre- and post-upgrade, respectively; Mann-Whitney U, p > 0.8).
Data were analyzed using SPM5 (Wellcome Department of Imaging Neuroscience, University College, London). Functional volumes were corrected for slice acquisition timing differences and rigid-body realigned to the initial volume of the first functional imaging scan to account for residual movement after prospective motion correction. Each subject’s high-resolution anatomic image was co-registered to the reference functional volume, segmented into tissue classes, and nonlinear spatial transformation parameters from this segmentation were subsequently applied to transform BOLD volumes into the Montreal Neurological Institute (MNI) space. Normalized functional image volumes were resampled to 2mm per side isotropic voxels and smoothed by a 6-mm full-width at half-maximum isotropic Gaussian kernel.
Discrete 2-s periods of odorant (or sham) valve events were modeled in a fixed-effects general linear model using SPM’s canonical hemodynamic response function (HRF) and its time and dispersion derivatives. The model also included movement parameter regressors and a 1/128 Hz high pass filter. This within subject model yielded contrast images of activation within an odorant condition (AO, NApO, and ApCO) for each subject, with each odorant set contrasted against sniffing of an odorless control event (i.e., control valve opening without odorant delivery). This permitted quantifying the extent to which the BOLD response from an odorant class was different from stimulation (auditory commands, sniffing, attentional processing, and motor response) without a chemosensory stimulus.
Of special interest was the extent to which ASD correlated with the effects of alcohol-specific reward vs. general appetitive odor cues, as reflected by the [AO > ApCO] BOLD contrast. We also analyzed the [AO > NApO] contrast to identify responses in which alcohol related aromas were the only appetitive cues present. Scans done under alcohol and placebo were analyzed separately, given alcohol-induced differences in activation observed in this sample and other studies (Gilman et al., 2008, Kareken et al., 2010a). ASD, FH, and an ASD×FH interaction term were entered into an SPM voxel-wise multivariate regression model with the [AO > ApCO] or [AO > NApO] contrasts as the dependent variable. Family history (FH) was included to account for variance that has been previously observed (Kareken et al. 2010a), although only correlations with ASD (after accounting for FH) are reported here. The criterion for statistical significance within a priori regions of interest (ROI) was set at a height threshold, pFWE < 0.05 (where a family-wise error correction for multiple comparisons was evaluated across all ROI voxels) and an extent threshold of 10 voxels. Insula, amygdala, and putamen ROI boundaries were defined anatomically (Tzourio-Mazoyer et al., 2002) and implemented in the MarsBaR toolbox for SPM (Brett et al., 2002) while dorsal and ventral medial prefrontal regions were previously-defined, as was piriform cortex (Kareken et al., 2010a). To focus on reward-related areas and limit the number of comparisons, the search volumes were constrained to these a priori regions.
Our primary interest was the correlation between ASD and the differences between two odor classes (i.e., [AO > ApCO], [AO > NApO], and [ApCO > NApO]). Therefore, to restrict the analyses only to regions in which correlations were alcohol odor-specific, i.e. [AO > ApCO], simple correlations between ASD and the [ApCO > odorless] contrast were masked out by excluding voxels that correlated (height threshold, p < 0.005) with the appetitive control odors alone (i.e., [ApCO > odorless] contrast). This eliminated the problem of correlations with the appetitive odor class driving any apparent correlation in [AO > ApCO]. Likewise, the analyses of [AO > NApO] and [ApCO > NApO] excluded voxels where simple correlations with [NApO > odorless] contrast were present.
Results
Intravenous alcohol infusion
Mean BrACs at the end of the imaging session closely reflected the targeted value of 50 mg% (actual: 50 mg% ± 1, mean ± SD).
Craving Self-Report
Craving scores to AO were averaged across Condition as there was no alcohol present during either assessment, and baseline ratings were subtracted. AO increased self-reported craving (p < 0.05, mean difference from baseline 0.75 ± 0.77 ± SD), and increased craving relative to ApCO and NApO ts(29) > 5.5, ps < 0.001. Importantly, self-reported craving to AO did not correlate with ASD (p > 0.3).
Pleasantness Self-Report
Self-reported pleasantness to AO, ApCO, and NApO were averaged across Condition as there was no alcohol present during either assessment. Pleasantness ratings for AO did not differ from ApCO (p > 0.4). Analogous to the fMRI contrasts of interest, AO – ApCO values were calculated; these difference scores did not correlate with ASD (p > 0.6).
Intensity Self-Report
Intensity difference scores were calculated in the same manner as pleasantness. Intensity for AO did not differ from ApCO (p > 0.1), and did not correlate with ASD (p > 0.08), although a positive trend was noted.
fMRI
As previously described, alcohol administration did not affect the primary olfactory cortex (piriform) response, indicating that alcohol did not fundamentally change its BOLD response (Kareken et al., 2010a). To assess any potential effects that antisocial traits may have on basal olfactory activation, multivariate regression was performed on all subjects for activation with ASD on odors alone, i.e. [ApCO > odorless] and [NApO > odorless] contrasts. There were no positive or negative correlations with left or right piriform cortex activation in these simple contrasts suggesting that ASD did not influence odor processing.
Relationship between AO activation and ASD
Initial analyses of correlations between [AO > ApCO] and ASD, and [AO > NApO] and ASD done separately under alcohol and placebo did not reveal any significant voxels under alcohol. Thus, we focused all subsequent analyses on placebo only.
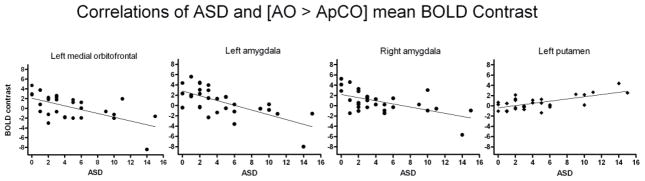
An analysis of the [AO > ApCO] contrast revealed that, under placebo, ASD was negatively correlated with activation in the medial OFC and bilateral amygdala (Figure 2A, yellow), but positively correlated with the left rostral putamen activation (Figure 2B, yellow). A complimentary analysis of the [AO > NApO] contrast showed a similar pattern of negatively and positively correlated activation (Figures 2A and B, respectively: cyan). While only the left medial OFC reached statistical significance, several areas of correlated activation overlapped with the [AO > ApCO] contrast findings (Figure 2A and B, green). All significant imaging results in a priori defined regions are summarized in Table 2. For illustrative purposes only, significant correlations between ASD and BOLD response in the putamen (positive), and OFC and bilateral amygdala (negative) are plotted in Figure 3, depicting each subject’s mean extracted BOLD response values in the functionally defined clusters of activation (voxel-wise height threshold, p < 0.001, uncorrected).
Figure 2.
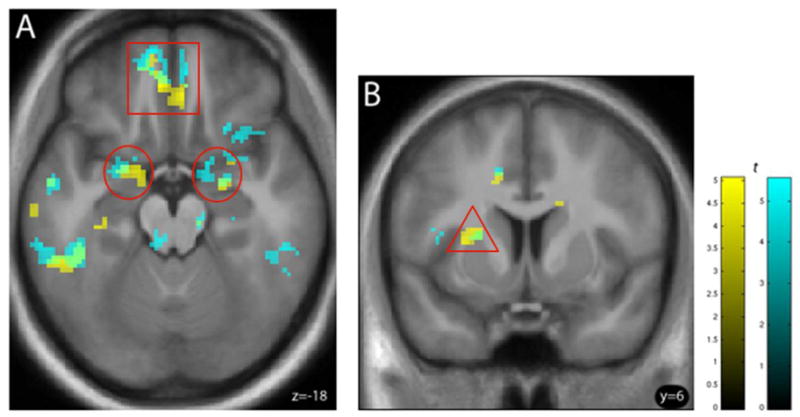
(A) Negative correlations between the [AO > ApCO] (yellow) and [AO > NApO] (cyan) contrasts and ASD in bilateral amygdala (red circles) and left medial orbitofrontal cortex (red square) and (B) positive correlation with the same contrasts and ASD in the left putamen (red triangle). Overlapping correlations are shown in green, indicating a similar pattern in both AO contrasts. Both correlations in heavy drinkers (n=30) under placebo are shown at (A) axial plane z=−18 (B) coronal plane y = 6. For illustration, voxel height threshold was p < 0.005 (uncorrected); extent threshold k > 10. See text for abbreviations.
Table 2.
Summary of correlations between ASD scores and odor-induced activation under placebo
| Cluster size | PFWE | Z | MNI coordinates | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| [AO > ApCO] contrast | ||||||
| ASD: Positively correlated | ||||||
| Left dorsal anterior putamen | 19 | 0.040 | 3.76 | −24 | 8 | 12 |
| ASD: Negatively correlated | ||||||
| Left medial OFC | 41 | 0.004 | 4.32 | −6 | 40 | −18 |
| Left amygdala | 22 | 0.004 | 4.01 | −22 | 0 | −20 |
| Right amygdala | 13 | 0.016 | 3.64 | 24 | −6 | −16 |
| [AO > NApO] contrast | ||||||
| ASD: Negatively correlated | ||||||
| Left rostral medial OFC | 23 | 0.011 | 3.95 | −10 | 56 | −18 |
| [ApCO > NApO] contrast | ||||||
| ASD: Negatively correlated | ||||||
| Right medial prefrontal cortex | 18 | 0.001 | 4.60 | 10 | 56 | 4 |
| Right dorsal middle insula | 144 | 0.008 | 4.30 | 34 | −4 | 14 |
| Right dorsal anterior putamen | 29 | 0.021 | 3.93 | 20 | 18 | 2 |
Alcohol-specific activation was derived from the [AO > ApCO] and [AO > NApO] contrasts, while the [ApCO > NApO] contrast reflects activation to appetitive odors as compared to non-appetitive odors. Family wise error correction (pFWE) was performed using the appropriate a priori region of interest. Z-scores and MNI coordinates reflect peak voxel. AO = Alcohol Odors; ApCO = Appetitive Control Odors; NApO = Non-Appetitive Control Odors; ASD = Antisocial Density; OFC = Orbitofrontal Cortex.
Figure 3.
For illustrative purposes, mean [AO > ApCO] BOLD contrast values extracted from functionally-defined clusters were plotted as a function of ASD scores. BOLD response in heavy drinkers (n=30) was negatively correlated with ASD within left medial orbitofrontal cortex and bilateral amygdala (closed circles), and positively correlated in left putamen (diamonds). See text for abbreviations.
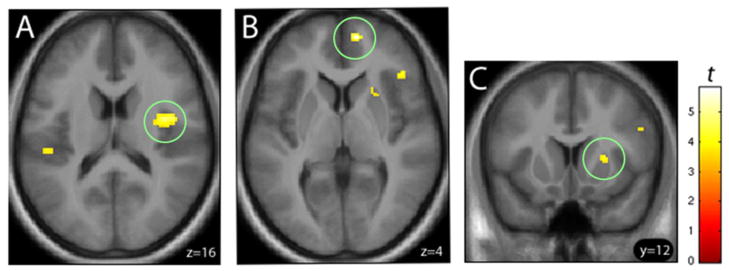
To address the possibility that ASD relates to a more generalized response to non-alcohol-related appetitive odors, we analyzed correlations between ASD and the [ApCO > NApO] contrast. No positive correlations emerged, although negative correlations were present in right dorsal middle insula, right medial prefrontal, and right dorsal anterior putamen, as shown in Figure 4 (A, B, and C, respectively) and Table 2. However, there were no significant correlations between ASD and the [ApCO > NApO] contrast in the medial OFC or amygdala, as with alcohol odors. Thus, although there did appear to be a robust relationship between greater ASD and lower activation from both alcohol odors (in both [AO > ApCO] and [AO > NApO]) and non-alcohol appetitive odors ([ApCO > NApO]) in several relevant loci, the results in medial OFC and amygdala appeared to be specific to alcohol odors, with the OFC finding being the most consistent.
Figure 4.
Heavy drinking subjects (n=30) showed negative correlations between ASD and the [ApCO > NApO] BOLD response in the (A) right dorsal middle insula (z=16), (B) right medial prefrontal cortex (z=4), and (C) right dorsal anterior putamen (y=12). Voxel height threshold, p < 0.001 (uncorrected); extent threshold k > 10. See text for abbreviations.
To assess any relationship that craving might have with activation to alcohol odors, the same type of random effects multivariate regression analysis was employed with self-reported Craving as the primary covariate of interest along with FH and Craving×FH terms in the [AO > ApCO] contrast. No voxels survived family-wise error correction using our regions of interest as search volumes.
To confirm that our results would be consistent if drug positive subjects were excluded, the same random effects analyses of [AO > ApCO] under placebo were performed with drug positive subjects excluded (n = 27). Similarly to results from the complete sample, activation in the [AO > ApCO] contrast negatively correlated with left medial OFC. The reduction in power resulted in the loss of some of the weaker correlations reported in the full sample, but the most pronounced finding in left medial OFC remained evident.
Discussion
Contrary to our hypothesis, ASD negatively correlated with left medial OFC activation, and there was no relationship in the ventral striatum. Although the ventral striatum might be expected to be a prime site mediating responses to alcohol-related stimuli in heavy drinkers, a ROI analysis of these data as originally presented did not pass the threshold of detection (Kareken et al. 2010a). A recent study using alcohol tastes did find correlations of ventral striatal ROI and various risk factors, but this study used a very large sample (n=326), which greatly enhanced detection of subtler correlations (Claus et al. 2011). Our hypothesis that higher ASD would correlate with greater alcohol cue responses was not supported; on the contrary, the converse occurred, lending weight to the idea that subjects with high ASD were “reward-deficient.” Thus, to the extent that drug cues are provocative of “somatic states” (Verdejo-García and Bechara, 2009)— unconditioned responses induced by the drug’s pharmacology— our findings might suggest an association between an under-responsive limbic system and sociopathic behaviors. In turn, this could suggest alternate mechanisms that lead to abuse in such individuals, including a more predominant role of impulsive behavior and difficulty anticipating the adverse outcomes of poor decisions via under-weighting aversive emotional states (Benning et al., 2005, Fairchild et al., 2008). In turn, this would also suggest that alcoholism risk in those with significant histories of antisocial behaviors may be less closely tied with brain systems involved in alcohol valuation, seeking, and wanting.
In particular, OFC appears to be critical in updating the incentive value of stimuli (Rolls et al., 1989, Critchley and Rolls, 1996, O’Doherty et al., 2000, Kringelbach et al., 2003, Hare et al., 2008). A recent meta-analysis of 142 reward imaging studies found that the medial OFC is an area sensitive not just to salience, but also to valence such that it responds preferentially to positive rewards (Liu et al., 2011). The current data are consistent with previous findings suggesting that antisocial traits mediate reduced OFC activation to rewarding stimuli (Völlm et al., 2007, Rubia et al., 2009), although contrary results do exist (Völlm et al., 2010). Comparisons with previous findings should be made with caution, however, as these studies tested neither heavy drinkers, per se, nor alcohol cues.
Although medial OFC has been implicated in reward processing in antisocial populations, other behavioral deficits related to its function that are relevant to addiction pathologies have also been observed in Cluster B personality disorder (e.g. ASPD, borderline) populations (Newman et al., 1987, Shapiro et al., 1988, Daugherty and Quay, 1991). Optimal performance in these reward dominance tasks requires competent reversal learning (recognizing when a reward signal has turned to a punishment or non-reward signal), which appears to require intact medial OFC (O’Doherty et al., 2001, Tsuchida et al., 2010). Therefore, medial OFC appears to be critically involved in updating incentive value and reward discrimination; traits which are impaired in antisocial and addicted populations. Furthermore, the similar behavior of bilateral amygdala seen here suggests the possibility that a circuit common to both is deficient. The amygdala has long been implicated in affective processing (Sterzer et al., 2005, Marsh et al., 2008; for meta-analysis see Marsh and Blair, 2008) and decision-making (e.g. Crowley et al., 2010), and is likely part of an important valuation circuit that includes the medial OFC (Padoa-Schioppa and Assad, 2006; Schoenbaum and Roesch, 2005).
The dorsal putamen has been implicated in reinforcement learning (for review see Packard and Knowlton, 2002), updating of incentive value (Muranishi et al., 2011), and valuation of immediate rewards (Wittmann et al., 2007). Although dorsal striatum is usually associated with motor processing/planning/habit learning, and ventral striatum is generally associated with reward/valuation (Delgado, 2007), these roles are not always so straightforward. It has been hypothesized that reward signal shifts from ventral/medial striatal sites of goal-directed reward-seeking behavior to dorsolateral striatum with extended learning and increased automatic habit formation (O’Doherty et al., 2004, Voorn et al., 2004, Everitt and Robbins, 2005, See et al., 2007, Takahashi et al., 2007). This idea is supported by human PET studies showing dopamine release in dorsal, but not ventral striatum in response to feeding (Small et al., 2001, Small et al., 2003). In these studies, subjects had considerable experience with the appetitive reward to which they were exposed, similar to the alcohol odors in the current study.
The insula appears to be a critical part of the neurocircuitry that maintains addiction (Naqvi et al., 2007), as well as a key integration site for sensory stimuli (Shelley and Trimble, 2004) that is widely involved in appetitive and consummatory behaviors (Wang et al., 2004, Uher et al., 2006). The role of the insula in monitoring interoceptive states and loss signal (Bjork et al., 2008, Baliki et al., 2009) makes it a candidate area for modulating reward. Although we did not detect correlations with insula activation using alcohol cues, there appeared to be decreased sensitivity to appetitive cues more generally, as illustrated by the robust negative correlation in the right insula in the [ApCO > NApO] contrast. This could represent a more general reward deficiency and insensitivity to the somatic conditioned responses elicited by such stimuli.
Although it is well-established that antisocial traits are commonplace in addiction, how such traits lead to addiction is not entirely clear. The current data indicate that the presence of significant ASD may alter the OFC response to alcohol-paired stimuli: this suggests an altered processing of stimulus incentive value (Kable and Glimcher, 2007, Hare et al., 2008). Indeed, reduced activation in reward areas have been widely implicated in addiction (Tupala and Tiihonen, 2004, Volkow et al., 2006, Volkow et al., 2007) and provide an attractive mechanistic hypothesis consistent with a “reward deficiency syndrome”. This concept, which posits that addictive drugs supplant natural reinforcers due to underactive reward areas, is supported by data from genetic and neuroimaging studies (Blum et al., 1995, Volkow et al., 2004), and parallels findings in selected lines of alcohol-preferring rodents in which there is reduced synaptic dopamine in brain reward regions even prior to alcohol exposure (Murphy et al., 2002). A competing set of ideas, broadly referred to as “impulsivity hypotheses”, feature exaggerated reward-seeking combined with reduced inhibition. This concept predicts greater activation to reward-related cues among AUDs (Goldstein and Volkow, 2002, de Wit and Richards, 2004). This viewpoint enjoys support from neuroimaging studies as well; for instance in AUD/heavy drinkers alcohol-related stimuli provoke reward-relevant brain responses compared to controls (Tapert et al., 2003, Lingford-Hughes et al., 2006) and compared to non-alcohol cues (Kareken et al., 2010a). Similarly, alcoholic patients in treatment have ventral striatal and medial frontal responses to visual alcohol cues that subside after treatment with naltrexone (Myrick et al., 2008). The current data are more consistent with a reward deficiency syndrome (RDS), at least in the specific case of responses to alcohol reward cues in medial OFC and amygdala; in the broader sense, decreased activation was related to higher ASD in the insula, PFC, and right dorsal putamen to other appetitive odors. The concept of a reward deficiency syndrome does not make predictions specific to the abused substance in question (i.e., it poses a more generalized deficit in the processing of, and responses to, rewards and their cues). However, we speculate that RDS need not be interpreted as a binary phenomenon; rather, RDS might conceivably scale proportionately with ASD such that greater degrees of ASD confers progressively less activation in reward areas (mainly medial OFC in this sample), and that this kind of linear relationship is best observed in a population to whom a particular reward (in this case, alcohol) is most frequently used (abused). Future research would, however, be required to explore this idea further. For clarity, we do note that in analyses comprising largely the same subjects, a more dorsal medial frontal region was found to respond differentially to AO as a function of a family history of alcoholism (Kareken, et al., 2010a).
More likely than not, however, the marked heterogeneity of risk factors (family history, ASPD, impulsivity/novelty-seeking, anxiety and depression, e.g. Nurnberger et al., 2004) that have been associated with AUDs and their risk confer different vulnerabilities in different brain regions. In the case of the current findings, those with significant ASD may process reward cues differently in brain areas known to mediate the incentive value of rewards, or perhaps even reflect a broader deficit in limbic-frontal signaling by affectively charged cues (Marsh et al., 2008, Sterzer, et al., 2005). Although we did not find any explicit relationship between craving scores and ASD, differences in neural processing may not be reflected in self-reported craving. If rewards are processed differently as a function of ASD, that might have implications for treatment. In particular, therapies that concentrate on cue-induced relapse (Payne et al., 1992, Price et al., 2010) may work differently in those with significant ASD, even if a formal diagnosis of ASPD does not apply.
There are limitations to consider in the data reported here. Although the size of the sample was reasonable (n= 30), the subjects were not highly antisocial. Nonetheless, these may be regarded as representative of young heavy drinking subjects not explicitly diagnosed as ASPD or incarcerated. It should also be noted that the corrected statistics in the placebo session do not take into account analyses from the alcohol infusion session. The analyses reported here include both positive and negative correlations in 6 (bilateral) search volumes in three contrasts of interest. Thus, Type I error may be somewhat higher than typical, and the findings presented here should be considered as provocative results requiring replication.
In summary, we found that density of externalizing behaviors modulated the brain’s response to the aromas of alcoholic beverages in this sample of heavy drinkers. In particular, we had previously reported that FHP subjects have a significantly greater medial prefrontal response to alcoholic drink odors (Kareken et al., 2010a). Here we show here that ASD is inversely correlated to this response in these heavy drinkers in ventromedial orbitofrontal cortex— a region implicated in reward valuation. Given the important role that OFC is known to have in salience attribution and in learning to alter behavior in response to reward cues, we suggest that heavy drinkers with significant externalizing behaviors may respond to and process alcohol cues differently than those drinkers with minimal externalizing behaviors. How this orbital reactivity interacts with other brain regions, and affects long-term outcome, bears monitoring in future research.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support of Michele Beal and Courtney Robbins (Department of Radiology and Imaging Sciences), and Stephen Warrenburg of International Flavors & Fragrances.
Role of the Funding Sources
Supported by R01 AA014605 (DAK), R01 AA017661 (DAK), R21 AA018020 (DAK), and the Indiana Alcohol Research Center P60 AA007611, for design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Supported by M01 RR000750 (General Clinical Research Center at Indiana University School of Medicine) for conduct of the study, and collection and management of the data. Supported by T32 AA007462 (BGO) for analysis, interpretation of the data; and preparation, review, and approval of the manuscript.
Footnotes
Abbreviations: AO = Alcohol Odor, ApCO = Appetitive Odor, ASD = AntiSocial Density, HD = Heavy Drinkers, NApO = Non-Appetitive Odor.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baliki MN, Geha PY, Apkarian AV. Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol. 2009;101:875–887. doi: 10.1152/jn.91100.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Iacono WG. Psychopathy, startle blink modulation, and electrodermal reactivity in twin men. Psychophysiology. 2005;42:753–762. doi: 10.1111/j.1469-8986.2005.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Chen G, Smith AR, Hommer DW. Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. J Child Psychol Psychiatry. 2010;51:827–837. doi: 10.1111/j.1469-7610.2009.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Comings DE. Dopamine D2 receptor gene variants: association and linkage studies in impulsive-addictive-compulsive behaviour. Pharmacogenetics. 1995;5:121–141. doi: 10.1097/00008571-199506000-00001. [DOI] [PubMed] [Google Scholar]
- Bragulat V, Dzemidzic M, Talavage T, Davidson D, O’Connor SJ, Kareken DA. Alcohol sensitizes cerebral responses to the odors of alcoholic drinks: an fMRI study. Alcohol Clin Exp Res. 2008;32:1124–1134. doi: 10.1111/j.1530-0277.2008.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox [Abstract] Neuroimage. 2002;16 [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Cole D, Kessler RM, Zald DH. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci. 2010;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Conway KP, Stinson FS, Colliver JD, Grant BF. Prevalence, correlates, and comorbidity of DSM-IV antisocial personality syndromes and alcohol and specific drug use disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2005;66:677–685. doi: 10.4088/jcp.v66n0602. [DOI] [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Rolls ET. Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. J Neurophysiol. 1996;75:1673–1686. doi: 10.1152/jn.1996.75.4.1673. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Dalwani MS, Mikulich-Gilbertson SK, Du YP, Lejuez CW, Raymond KM, Banich MT. Risky decisions and their consequences: neural processing by boys with Antisocial Substance Disorder. PLoS One. 2010;5:e12835. doi: 10.1371/journal.pone.0012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty TK, Quay HC. Response perseveration and delayed responding in childhood behavior disorders. J Child Psychol Psychiatry. 1991;32:453–461. doi: 10.1111/j.1469-7610.1991.tb00323.x. [DOI] [PubMed] [Google Scholar]
- de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. In: Bevins RA, Bardo MT, editors. Motivational Factors in the Etiology of Drug Abuse, Nebraska Symposium on Motivation. Vol. 50. Lincoln, NE: University of Nebraska Press; 2004. pp. 19–55. [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Ann N Y Acad Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Finn P, Fein G. Psychiatric comorbidity in long-term abstinent alcoholic individuals. Alcohol Clin Exp Res. 2007;31:795–803. doi: 10.1111/j.1530-0277.2007.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Van Goozen SH, Stollery SJ, Goodyer IM. Fear conditioning and affective modulation of the startle reflex in male adolescents with early-onset or adolescence-onset conduct disorder and healthy control subjects. Biol Psychiatry. 2008;63:279–285. doi: 10.1016/j.biopsych.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28:4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Grüsser SM, Heinz A, Flor H. Standardized stimuli to assess drug craving and drug memory in addicts. J Neural Transm. 2000;107:715–720. doi: 10.1007/s007020070072. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock MN. Gender comparison of antisocial personality disorder and depression in alcoholism. J Subst Abuse. 1991;3:205–219. doi: 10.1016/s0899-3289(05)80037-9. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, O’Connor SJ. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage. 2010a;50:267–276. doi: 10.1016/j.neuroimage.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Claus ED, Sabri M, Dzemidzic M, Kosobud AE, Radnovich AJ, Hector D, Ramchandani VA, O’Connor SJ, Lowe M, Li TK. Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: preliminary findings. Alcohol Clin Exp Res. 2004;28:550–557. doi: 10.1097/01.alc.0000122764.60626.af. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Liang T, Wetherill L, Dzemidzic M, Bragulat V, Cox C, Talavage T, O’Connor SJ, Foroud T. A Polymorphism in GABRA2 Is Associated With the Medial Frontal Response to Alcohol Cues in an fMRI Study. Alcohol Clin Exp Res. 2010b doi: 10.1111/j.1530-0277.2010.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Knop J, Penick EC, Nickel EJ, Mortensen EL, Sullivan MA, Murtaza S, Jensen P, Manzardo AM, Gabrielli WF., Jr Childhood ADHD and conduct disorder as independent predictors of male alcohol dependence at age 40. J Stud Alcohol Drugs. 2009;70:169–177. doi: 10.15288/jsad.2009.70.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Lingford-Hughes AR, Daglish MR, Stevenson BJ, Feeney A, Pandit SA, Wilson SJ, Myles J, Grasby PM, Nutt DJ. Imaging alcohol cue exposure in alcohol dependence using a PET 15O-H2O paradigm: results from a pilot study. Addict Biol. 2006;11:107–115. doi: 10.1111/j.1369-1600.2006.00001.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Blair RJ. Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neurosci Biobehav Rev. 2008;32:454–465. doi: 10.1016/j.neubiorev.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJ. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY. Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend. 2007;91:149–158. doi: 10.1016/j.drugalcdep.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder RT. Alcoholism and personality. Aust N Z J Psychiatry. 2002;36:44–52. doi: 10.1046/j.1440-1614.2002.00958.x. [DOI] [PubMed] [Google Scholar]
- Muranishi M, Inokawa H, Yamada H, Ueda Y, Matsumoto N, Nakagawa M, Kimura M. Inactivation of the putamen selectively impairs reward history-based action selection. Exp Brain Res. 2011;209:235–246. doi: 10.1007/s00221-011-2545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JP, Wallace JF. Diverse pathways to deficient self-regulation: Implications for disinhibitory psychopathology in children. Clinical Psychology Review. 1993;13:699–720. [Google Scholar]
- Newman JP, Patterson CM, Kosson DS. Response perseveration in psychopaths. J Abnorm Psychol. 1987;96:145–148. doi: 10.1037//0021-843x.96.2.145. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Wiegand R, Bucholz K, O’Connor S, Meyer ET, Reich T, Rice J, Schuckit M, King L, Petti T, Bierut L, Hinrichs AL, Kuperman S, Hesselbrock V, Porjesz B. A family study of alcohol dependence: coaggregation of multiple disorders in relatives of alcohol-dependent probands. Arch Gen Psychiatry. 2004;61:1246–1256. doi: 10.1001/archpsyc.61.12.1246. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, Renner B, Ahne G. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 2000;11:893–897. doi: 10.1097/00001756-200003200-00046. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Rychtarik RG, Rappaport NB, Smith PO, Etscheidt M, Brown TA, Johnson CA. Reactivity to alcohol-relevant beverage and imaginal cues in alcoholics. Addict Behav. 1992;17:209–217. doi: 10.1016/0306-4603(92)90026-r. [DOI] [PubMed] [Google Scholar]
- Price KL, Saladin ME, Baker NL, Tolliver BK, DeSantis SM, McRae-Clark AL, Brady KT. Extinction of drug cue reactivity in methamphetamine-dependent individuals. Behav Res Ther. 2010;48:860–865. doi: 10.1016/j.brat.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Niaura RS, Childress AR, Abrams DB, Monti PM. Cue reactivity in addictive behaviors: theoretical and treatment implications. Int J Addict. 1990;25:957–993. doi: 10.3109/10826089109071030. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Sienkiewicz ZJ, Yaxley S. Hunger Modulates the Responses to Gustatory Stimuli of Single Neurons in the Caudolateral Orbitofrontal Cortex of the Macaque Monkey. Eur J Neurosci. 1989;1:53–60. doi: 10.1111/j.1460-9568.1989.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Halari R, Matsukura F, Mohammad M, Taylor E, Brammer MJ. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am J Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Honig K, Maier W, Gaebel W, Zilles K. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Shapiro SK, Quay HC, Hogan AE, Schwartz KP. Response perseveration and delayed responding in undersocialized aggressive conduct disorder. J Abnorm Psychol. 1988;97:371–373. doi: 10.1037//0021-843x.97.3.371. [DOI] [PubMed] [Google Scholar]
- Shelley BP, Trimble MR. The insular lobe of Reil--its anatamico-functional, behavioural and neuropsychiatric attributes in humans--a review. World J Biol Psychiatry. 2004;5:176–200. doi: 10.1080/15622970410029933. [DOI] [PubMed] [Google Scholar]
- Siegel S. Evidence from rats that morphine tolerance is a learned response. J Comp Physiol Psychol. 1975;89:498–506. doi: 10.1037/h0077058. [DOI] [PubMed] [Google Scholar]
- Singleton EG, Tiffany ST, Henningfield JE. Intramural Research Program. National Institute on Drug Abuse; Baltimore, MD: 2000. Alcohol Craving Questionnaire (ACQ-NOW): Background, Scoring, and Administration. [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addict Behav. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry. 2005;57:7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Takahashi Y, Roesch MR, Stalnaker TA, Schoenbaum G. Cocaine exposure shifts the balance of associative encoding from ventral to dorsolateral striatum. Front Integr Neurosci. 2007;1 doi: 10.3389/neuro.07.011.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Tsuchida A, Doll BB, Fellows LK. Beyond reversal: a critical role for human orbitofrontal cortex in flexible learning from probabilistic feedback. J Neurosci. 2010;30:16868–16875. doi: 10.1523/JNEUROSCI.1958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupala E, Tiihonen J. Dopamine and alcoholism: neurobiological basis of ethanol abuse. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1221–1247. doi: 10.1016/j.pnpbp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res. 2006;169:111–119. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2009;56 (Suppl 1):48–62. doi: 10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein R, Alexoff D, Thanos PK. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völlm B, Richardson P, McKie S, Elliott R, Dolan M, Deakin B. Neuronal correlates of reward and loss in Cluster B personality disorders: a functional magnetic resonance imaging study. Psychiatry Res. 2007;156:151–167. doi: 10.1016/j.pscychresns.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Völlm B, Richardson P, McKie S, Reniers R, Elliott R, Anderson IM, Williams S, Dolan M, Deakin B. Neuronal correlates and serotonergic modulation of behavioural inhibition and reward in healthy and antisocial individuals. J Psychiatr Res. 2010;44:123–131. doi: 10.1016/j.jpsychires.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M, Zhu W, Wong CT, Pappas NR, Geliebter A, Fowler JS. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21:1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Wikler A. Recent progress in research on the neurophysiologic basis of morphine addiction. Am J Psychiatry. 1948;105:329–338. doi: 10.1176/ajp.105.5.329. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Leland DS, Paulus MP. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp Brain Res. 2007;179:643–653. doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.