Abstract
This article provides a brief overview of earlier work of our group on the peripheral signaling of pain, summarizes more recent studies on the role of opioids in chronic neuropathic pain, and speculates on the future of gene-based therapies as novel strategies to enhance the peripheral modulation of pain. Neurophysiological and psychophysical studies have revealed features of primary afferent activity from somatic tissue that led to improved understanding of the physiology and pathophysiology of pain signaling by nociceptive and non-nociceptive fibers. The demonstration of peripheral opioid mechanisms in neuropathic pain suggests a potential role for these receptors in the modulation of pain at its initiation site. Our work has focused on characterizing this peripheral opioid analgesia in chronic neuropathic pain such that it can be exploited to develop novel and potent peripheral analgesics for its treatment. Ongoing research on virus-mediated gene transfer strategies to enhance peripheral opioid analgesia is presented.
Introduction
I am honored and humbled to receive the 2010 John J. Bonica Award from the American Society of Regional Anesthesia and Pain Medicine (ASRA)—honored by the opportunity to celebrate the accomplishments of a visionary leader in the pain field, and humbled to be included among the elite group of clinicians and scientists who have been prior recipients of the award since its inception in 1988 (see Supplemental Digital Content 1, http://links.lww.com/AAP/A40, for list of Bonica Award recipients).
John Bonica, the first chairman of the Department of Anesthesiology at the University of Washington in Seattle, was a clinician-educator, a scientist, and a leader par excellence. He led the department from 1960 to 1978 and continued to serve the institution as Professor Emeritus until his retirement in 1992. Bonica dedicated his life to educating physicians on pain management, developing and promoting the concept of multidisciplinary pain clinics, and advancing clinical and basic science research on pain. Among his major accomplishments was the founding of the International Association for the Study of Pain. I was fortunate to join the Department of Anesthesiology at the University of Washington as a resident in 1977— part of the last batch of physicians to be trained under Dr. Bonica’s chairmanship. My interests in regional anesthesia and pain were kindled during those residency years and reinforced by meeting pioneers in the field, such as Drs. Bridenbaugh, Covino, Cousins, Katz, Moore, Raj, and others at the ASRA meetings in the early 1980s.
I was introduced to pain research by Drs. James Campbell and Richard Meyer in the Department of Neurosurgery at Johns Hopkins University, and the three of us worked together for more than two decades. This review will provide a brief overview of our group’s earlier work in the peripheral signaling of pain, summarize more recent clinical and experimental studies on the role of opioids in chronic neuropathic pain, and speculate on the future of gene-based therapies as novel strategies to enhance peripheral modulation of pain.
The Peripheral Signaling of Pain
In the early 1980s, studies in our laboratory focused on the peripheral neural apparatus that responds to noxious stimuli to the skin. The characteristics of the response of single afferent fibers, teased from peripheral nerves in primates, to stimuli applied to their cutaneous receptive fields were correlated with psychophysical ratings of pain to the same stimuli in human volunteers. These studies allowed inferences to be made on the functional roles of different types of nociceptive fibers that innervate the skin and provided the neurophysiologic basis (ie, peripheral sensitization) for the hyperalgesia that results from cutaneous injuries, such as a skin burn or a surgical incision1 (reviewed in Raja et al2). We further examined which afferent fibers signaled allodynia, a common presentation of acute and chronic pain states, using models of acute burn injury and clinical neuropathic pain states, such as nerve injury, postherpetic neuralgia, and complex regional pain syndrome (CRPS).3–5 These studies led to the understanding that hyperalgesia at the site of injury (primary hyperalgesia) can be produced when nociceptors are sensitized by an “inflammatory soup” composed of prostaglandins, bradykinin, and cytokines released at the injury site.6, 7 In addition, we demonstrated that the characteristics of primary hyperalgesia and secondary hyperalgesia (increased pain sensitivity that occurs around the injury site) differ.5, 8 In studies of subjects with nerve injury-associated pain and allodynia, we presented multiple lines of evidence, using differential ischemic and local anesthetic blocks and latency measurements in response to stepped displacement stimuli to indicate that myelinated primary afferents, perhaps A-beta fibers (sensitized myelinated nociceptors or low-threshold mechanoreceptors), signal the hyperalgesia.3 Studies by Woolf et al9–11 and several other groups have demonstrated that the secondary hyperalgesia is the result of central sensitization. Additional studies we conducted in patients who had conditions, such as CRPS and postamputation pain, suggested that the input from peripheral nociceptors could be modulated by adrenergic mechanisms.12, 13
Clinical Efficacy of Opioids for Chronic Pain
In the 1990s, the role of opioids in the management of patients with chronic non-cancer pain was highly controversial, with diametrically opposed schools of thought. Unfortunately, few controlled trials had been carried out to help resolve the issue. Based on preliminary observational studies on the efficacy of chronic opioid therapy for neuropathic pain, we conducted 2 double-blind, randomized controlled trials of the efficacy of opioids for postherpetic neuralgia and postamputation pain.14, 15 Controlled-release morphine effectively attenuated pain in subjects with postherpetic neuralgia, and patients preferred treatment with the opioid to treatment with the tricyclic antidepressant nortriptyline.14 In amputees with postamputation pain, therapy with controlled-release morphine, but not mexiletine, resulted in a decrease in intensity of pain, but that form of therapy was also associated with a higher rate of side effects and no improvement in self-reported levels of overall functional activity and pain-related interference in daily activities.15
These studies, as well as those by other investigators, indicate that opioids are effective in providing short-term relief for chronic non-cancer pain.16, 17 However, the beneficial effects of opioids on function were not studied with objective measures. In a 16-week, prospective, open-label study that used an actigraph as an objective measure of function, we observed that a reduction in pain intensity was associated with a corresponding increase in functional activity in neuropathic pain patients treated with transdermal fentanyl.18 However, the systemic use of opioids is associated with significant adverse effects, including constipation, sedation, respiratory depression, etc. Additionally, concern has been growing about the increasing abuse of prescription opioids in the community, particularly among teenagers and young adults.19, 20
Modulating the Nerve Injury-Induced Plasticity of the Peripheral Nervous System
A considerable body of evidence built over the last decade suggests that, after tissue injury and inflammation, the analgesic effect of opioids may be mediated partly by peripheral opioid receptors.21–23 Opioid receptors are synthesized in dorsal root ganglion (DRG) cells and transported to their central and peripheral terminals in the spinal cord and peripheral tissues, respectively.24, 25 We postulated that a similar peripheral opioid mechanism may play a role in chronic pain states, such as neuropathic pain that results from a partial nerve injury. The presence of peripheral opioid receptors would provide a novel target that could be activated by peripherally restricted opioids. Such a peripherally active agent is an attractive treatment strategy for several reasons:26 1) An agent that works primarily on the peripheral nociceptors will target “pain at its source”27 and act on the pain-signaling pathway in the peripheral nervous system (PNS) before the signals diverge over multiple pathways in the central nervous system (CNS). 2) Analgesic drugs restricted to the PNS are less likely to produce “off-target” CNS-mediated adverse effects, such as sedation and cognitive dysfunction.28, 29 3) PNS-acting drugs are likely to be associated with fewer drug interactions and a larger safety profile (for example, topical local anesthetics and capsaicin).30 4) Interrupting pain signals in the periphery may prevent the development of, or reverse, the CNS neuroplastic changes that underlie central sensitization.31, 32
In studies conducted during the last 5 years, we observed that the expression of opioid receptors in the periphery and spinal cord are altered after peripheral nerve injury, and that a peripheral opiate receptor-mediated antihyperalgesia occurs in experimental animals.33, 34 Using a rat model of persistent neuropathic pain (ligation of the L5 spinal nerve root, L5 SNL), we characterized the temporal and spatial expression of mu-opioid receptor (mOR) mRNA and protein in primary afferent neurons. We found that the expression of mOR mRNA and protein underwent dynamic changes in primary afferent neurons after L5 SNL. In rats with an L5 SNL, the mOR mRNA transiently decreased on day 7 and then increased significantly on day 14 in adjacent uninjured L4 DRG cells.34 We postulated that these temporal changes in mOR expression that occur after nerve injury are likely to have functional consequences on pain behaviors and opioid analgesia.
In a behavioral study of rats with L5 SNL, we examined whether activation of the peripheral mORs could effectively alleviate neuropathic pain.33 Systemic loperamide hydrochloride, a peripherally acting mOR-preferring agonist, dose-dependently reversed the mechanical allodynia after SNL. Several lines of evidence suggested that this effect of loperamide was mediated primarily through activation of peripheral mORs in local tissue. For example, the anti-allodynic effect of systemic loperamide was blocked by systemic pretreatment with naloxone hydrochloride or the peripherally acting MOR-preferring antagonist, methylnaltrexone. The anti-allodynic effects of systemic loperamide were also blocked by ipsilateral intraplantar pretreatment with methylnaltrexone and the highly selective mOR antagonist CTAP, but not by the delta-opioid receptor antagonist, naltrindole. These observations were consistent with our postulate that peripheral mORs are a potential analgesic target and that peripherally acting mOR agonists may represent a promising therapeutic approach for alleviating neuropathic pain.
Enhancing Peripheral Opioid Receptors—Virus-Mediated Gene Transfer Strategies
Gene therapy has been used to introduce a potentially therapeutic DNA or RNA sequence into cells for the treatment of experimental and clinical pain.35 Although many gene therapy strategies have been employed to deliver analgesic genes to the nervous system, viral vectors (modified viruses that have been disabled by recombination to reduce pathogenic potential) are most commonly used.36, 37
Several adeno-associated or herpes simplex (HSV) recombinant viruses have been used in experimental animals to effectively increase the expression of the mOR or the endogenous opioid ligand enkephalin (Enk) in afferent neurons.38 Such studies have generally used viral gene transfer strategies to deliver preproenkephalin cDNA to DRG or trigeminal neurons in different animal models of chronic pain.39–47 The opioid peptides are synthesized and stored in the sensory neurons, and activation of these neurons results in release of the peptides along with other endogenous peptides—a form of self-medication for pain.
As compared with adeno-associated virus, the use of recombinant HSV offers several advantages as a vector: 1) Because of the natural neurotropism of the parent wild-type virus, the viral vector can be injected subcutaneously or applied topically. The HSV vector targets primary afferent neurons that innervate the inoculation site, thus bypassing the need for surgical delivery of virus. 2) HSV can naturally establish a lifelong latent state as an intranuclear element in sensory neurons of the DRG.48 In animal models, expression of virus-delivered marker genes persists for 3 to 4 months, suggesting the possibility of long-term ef?cacy.38, 43, 49 3) Immunological studies have shown that a broad range of sensory neurons in the DRG and trigeminal ganglia, including nociceptors, are able to establish latent infections.
HSV-1–mediated expression of Enk in DRG neurons has been shown to attenuate capsaicin-induced thermal hyperalgesia in rats and primates45, 50 and to be antinociceptive in animal models of cancer pain,39 arthritis,51, 52 neuropathic pain,40 postoperative pain,53 interstitial cystitis,46 and alcohol- and nonalcohol-induced pancreatitis.44, 54 Furthermore, this Enk-induced antinociception is mediated via opioid receptors, as the analgesia is reversed by naloxone.39, 42, 46, 50
Our research team has been studying whether viral vector strategies could be used to increase the number of peripheral opioid receptors on primary afferents. Such an approach might enhance analgesia while limiting opioid-induced side effects. We used recombinant HSV constructs with the mOR cDNA inserted in the sense (SGMOR) or antisense (SGAMOR) orientation relative to the promoter to selectively increase or decrease expression of the mOR in primary afferent neurons. Using a green fluorescent protein reporter gene, we demonstrated that the HSV vectors were incorporated into small and large cells in the DRG.55 Administration of the SGMOR virus led to an increase in expression of mORs, which were transported from the DRG both anterograde to central spinal terminals and retrograde to the skin afferent endings in the periphery (Fig. 1). In contrast, the SGAMOR virus decreased expression of mORs in the skin, DRG, and spinal cord. This finding is significant because it shows not only that the receptor is being made but also that it is being transported to sites relevant for pain modulation. In addition, we demonstrated that the change in mOR expression was accompanied by a change in function, as evidenced by a shift in the dose-response curve of the peripherally acting opioid agonist loperamide, leftward for the SGMOR and rightward for the SGAMOR. This finding confirmed that HSV-1 vectors can be used to decrease or increase peripheral opioid analgesia and supports the further development of novel peripheral opioid agonists that can produce analgesia without the centrally mediated side effects.
Figure 1.
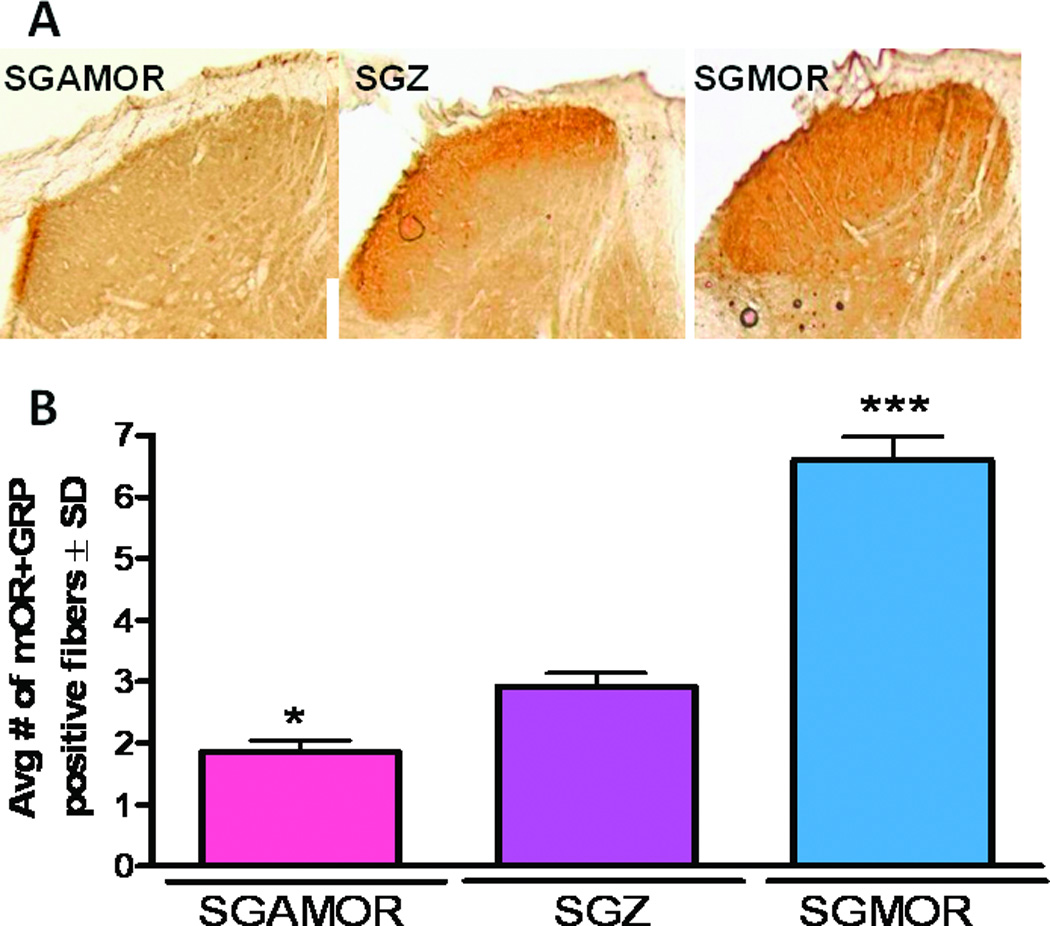
Immunoreactive µ-opioid receptor (MOR) in the ipsilateral dorsal horn of the lumbar spinal cord (A) and skin (B) at 4 weeks after topical hind paw infection with herpes simplex virus encoding the gene for the µOR in antisense (SGAMOR) or sense (SGMOR) direction relative to the cytomegalovirus promoter, or the viral control encoding [beta]-galactosidase (SGZ). A). MOR immunoreactivity is decreased in the ipsilateral L3 dorsal horn after SGAMOR infection (left panel) when compared with infection with the control SGZ virus (middle panel) while inoculation of the SGMOR viral vector results in increased MOR immunoreactivity in ipsilateral L3 dorsal horn (right panel). B) Quantitative changes in expression of MOR and overlapping immunoreactivity (-ir) with GFP in dorsal hind paw skin at 4 weeks after topical hind paw infection with herpes simplex virus. Compared with control SGZ infection, infection with SGAMOR decreases the number of MOR-ir afferent terminals in the epidermis of the medial dorsal hind paw skin. In contrast, infection with SGMOR increased the number of MOR-ir afferent terminals. Data are presented as average ± SD. *, *** P < 0.05, 0.001 compared with SGZ. (Adapted from Zhang et al 2008)55
Potential Future Therapies
The potential role of the peripheral opioid receptor as a useful target for clinical pain states needs to be tested. The only FDA-approved opioid agonist drug that has a predominant peripheral effect is loperamide. This drug is presently available only as an oral formulation and is approved for the treatment of acute diarrhea and chronic irritable bowel syndrome, not as an analgesic. A few peripherally restricted kappa-agonists are undergoing early phase clinical trials.56
Gene therapy for pain is based on the postulate that the delivery of peptides that target specific, well established sites in the pain pathway or that enhance receptors known to modulate the pain signaling process is likely to selectively interrupt the transmission of nociceptive information. A broad range of gene products has been studied, including vectors designed to produce inhibitory neurotransmitters such as the delta opioid agonist peptide Enk, the mu opioid agonist peptide endomorphin-2, and glutamic acid decarboxylase, which enhances the release of gamma amino butyric acid. Other vectors have been developed to release anti-inflammatory peptides, e.g., interleukin (IL)-4, IL-10, and a tumor necrosis factor α receptor. Many of these viral vectors have been shown to be effective in several preclinical models of chronic pain--inflammatory pain, visceral pain, neuropathic pain, and cancer pain.
Gene transfer strategies for the treatment of pain in humans are being studied presently, and initial signals are promising. The effects of treatment with a replication-defective HSV-based vector that expresses human preproenkephalin have been reported recently.36 In this multicenter, dose-escalation phase I clinical trial of the vector in 10 subjects with intractable focal cancer pain, no adverse effects occurred. Patients reported pain relief with the middle and high doses tested during the 4-week observation period. Whether similar effects can be obtained with the strategy of enhancing the peripheral opioid receptor population remains to be examined.
Summary
The experiments described in this report have been conducted for more than 25 years. These studies have revealed features of primary afferent signaling from somatic tissue, which, in turn, has improved understanding of the physiology and pathophysiology of pain signaling by nociceptive and non-nociceptive fibers. The demonstration of peripheral opioid mechanisms suggests a potential role for these receptors in the modulation of pain signals before they enter the spinal cord and higher CNS sites. Our work has focused on characterizing this peripheral opioid analgesia in chronic neuropathic pain such that it can be exploited to develop novel and potent peripheral analgesics for its treatment. Research on virus-mediated gene transfer strategies to enhance peripheral opioid analgesia for the treatment of neuropathic pain is ongoing.
Supplementary Material
Acknowledgments
The secretarial assistance of Ms. Folashade Domingo and the editorial assistance of Ms. Claire Levine are gratefully acknowledged. The work described here was conducted by several colleagues and post-doctoral fellows with whom the author has had the pleasure to collaborate over the last three decades: Jennifer Haythornthwaite, Mitchell Max, Zahid Ali, Shefali Agarwal, Jasenka Borzan, Michael Clark, Karen Davis, Robert Edwards, Yun Guan, Donald Manning, Heikki Mansikka, Marco Pappagallo, Esther Pogatzki, Matthias Ringkamp, Amit Sharma, Yoram Shir, Sarah Sweitzer, Yuan-Xiang Tao, Rolf-Detlef Treede, and Christopher Wu. I am grateful to the following mentors who have played a critical role in my research career: Drs. James Campbell, Robert Epstein, Raymond Fink, Patrice Guyenet, and Richard Meyer. Finally, I am grateful for the financial support for the research from the NIH (NS-26363), Allergan, and Medtronic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meyer RA, Campbell JN. Myelinated nociceptive afferents account for the hyperalgesia that follows a burn to the hand. Science. 1981;4515:1527–1529. doi: 10.1126/science.7280675. [DOI] [PubMed] [Google Scholar]
- 2.Raja SN, Meyer RA, Campbell JN. Peripheral mechanisms of somatic pain. Anesthesiology. 1988;4:571–590. doi: 10.1097/00000542-198804000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;1:89–94. doi: 10.1016/0304-3959(88)90027-9. [DOI] [PubMed] [Google Scholar]
- 4.Davis KD, Treede RD, Raja SN, Meyer RA, Campbell JN. Topical application of clonidine relieves hyperalgesia in patients with sympathetically maintained pain. Pain. 1991;3:309–317. doi: 10.1016/0304-3959(91)90221-I. [DOI] [PubMed] [Google Scholar]
- 5.Raja SN, Campbell JN, Meyer RA. Evidence for different mechanisms of primary and secondary hyperalgesia following heat injury to the glabrous skin. Brain. 1984:1179–1188. doi: 10.1093/brain/107.4.1179. [DOI] [PubMed] [Google Scholar]
- 6.Manning DC, Raja SN, Meyer RA, Campbell JN. Pain and hyperalgesia after intradermal injection of bradykinin in humans. Clin Pharmacol Ther. 1991;6:721–729. doi: 10.1038/clpt.1991.212. [DOI] [PubMed] [Google Scholar]
- 7.Raja SN, Campbell JN, Meyer RA, Colman RW. Role of kinins in pain and hyperalgesia: psychophysical studies in a patient with kininogen deficiency. Clin Sci (Lond) 1992;3:337–341. doi: 10.1042/cs0830337. [DOI] [PubMed] [Google Scholar]
- 8.Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;4:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- 9.Ma QP, Woolf CJ. Progressive tactile hypersensitivity: an inflammation-induced incremental increase in the excitability of the spinal cord. Pain. 1996;1:97–106. doi: 10.1016/0304-3959(96)03105-3. [DOI] [PubMed] [Google Scholar]
- 10.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;5944:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 11.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;(3 Suppl):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali Z, Raja SN, Wesselmann U, Fuchs PN, Meyer RA, Campbell JN. Intradermal injection of norepinephrine evokes pain in patients with sympathetically maintained pain. Pain. 2000;2:161–168. doi: 10.1016/S0304-3959(00)00327-4. [DOI] [PubMed] [Google Scholar]
- 13.Lin EE, Horasek S, Agarwal S, Wu CL, Raja SN. Local administration of norepinephrine in the stump evokes dose-dependent pain in amputees. Clin J Pain. 2006;5:482–486. doi: 10.1097/01.ajp.0000202980.51786.ae. [DOI] [PubMed] [Google Scholar]
- 14.Raja SN, Haythornthwaite JA, Pappagallo M, et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2002;7:1015–1021. doi: 10.1212/wnl.59.7.1015. [DOI] [PubMed] [Google Scholar]
- 15.Wu CL, Agarwal S, Tella PK, et al. Morphine versus mexiletine for treatment of postamputation pain: a randomized, placebo-controlled, crossover trial. Anesthesiology. 2008;2:289–296. doi: 10.1097/ALN.0b013e31817f4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowbotham MC, Twilling L, Davies PS, Reisner L, Taylor K, Mohr D. Oral opioid therapy for chronic peripheral and central neuropathic pain. N Engl J Med. 2003;13:1223–1232. doi: 10.1056/NEJMoa021420. [DOI] [PubMed] [Google Scholar]
- 17.Watson CP, Babul N. Efficacy of oxycodone in neuropathic pain: a randomized trial in postherpetic neuralgia. Neurology. 1998;6:1837–1841. doi: 10.1212/wnl.50.6.1837. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal S, Polydefkis M, Block B, Haythornthwaite J, Raja SN. Transdermal fentanyl reduces pain and improves functional activity in neuropathic pain states. Pain Med. 2007;7:554–562. doi: 10.1111/j.1526-4637.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- 19.DuPont RL. Prescription drug abuse: an epidemic dilemma. J Psychoactive Drugs. 2010;2:127–132. doi: 10.1080/02791072.2010.10400685. [DOI] [PubMed] [Google Scholar]
- 20.Riggs P. Non-medical use and abuse of commonly prescribed medications. Curr Med Res Opin. 2008;3:869–877. doi: 10.1185/030079908X273435. [DOI] [PubMed] [Google Scholar]
- 21.Lewanowitsch T, Miller JH, Irvine RJ. Reversal of morphine, methadone and heroin induced effects in mice by naloxone methiodide. Life Sci. 2006;7:682–688. doi: 10.1016/j.lfs.2005.05.062. [DOI] [PubMed] [Google Scholar]
- 22.Obara I, Przewlocki R, Przewlocka B. Local peripheral effects of mu-opioid receptor agonists in neuropathic pain in rats. Neurosci Lett. 2004;1–2:85–89. doi: 10.1016/j.neulet.2004.01.056. [DOI] [PubMed] [Google Scholar]
- 23.Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat Med. 2003;8:1003–1008. doi: 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- 24.Coggeshall RE, Zhou S, Carlton SM. Opioid receptors on peripheral sensory axons. Brain Res. 1997;1–2:126–132. doi: 10.1016/s0006-8993(97)00446-0. [DOI] [PubMed] [Google Scholar]
- 25.Truong W, Cheng C, Xu QG, Li XQ, Zochodne DW. Mu opioid receptors and analgesia at the site of a peripheral nerve injury. Ann Neurol. 2003;3:366–375. doi: 10.1002/ana.10465. [DOI] [PubMed] [Google Scholar]
- 26.Joseph EK, Levine JD. Mu and delta opioid receptors on nociceptors attenuate mechanical hyperalgesia in rat. Neuroscience. 2010;1:344–350. doi: 10.1016/j.neuroscience.2010.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;1:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Argoff CE. Topical agents for the treatment of chronic pain. Curr Pain Headache Rep. 2006;1:11–19. doi: 10.1007/s11916-006-0004-4. [DOI] [PubMed] [Google Scholar]
- 29.Smith HS. Peripherally-acting opioids. Pain Physician. 2008;(2 Suppl):S121–S132. [PubMed] [Google Scholar]
- 30.Derry S, Lloyd R, Moore RA, McQuay HJ. Topical capsaicin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2009;4:CD007393. doi: 10.1002/14651858.CD007393.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;1:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jarvis MF, Boyce-Rustay JM. Neuropathic pain: models and mechanisms. Curr Pharm Des. 2009;15:1711–1716. doi: 10.2174/138161209788186272. [DOI] [PubMed] [Google Scholar]
- 33.Guan Y, Johanek LM, Hartke TV, et al. Peripherally acting mu-opioid receptor agonist attenuates neuropathic pain in rats after L5 spinal nerve injury. Pain. 2008;2:318–329. doi: 10.1016/j.pain.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee CY, Perez FM, Wang W, et al. Dynamic temporal and spatial regulation of mu opioid receptor expression in primary afferent neurons following spinal nerve injury. Eur J Pain. 2011;7:669–675. doi: 10.1016/j.ejpain.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davidson BL, Breakefield XO. Viral vectors for gene delivery to the nervous system. Nat Rev Neurosci. 2003;5:353–364. doi: 10.1038/nrn1104. [DOI] [PubMed] [Google Scholar]
- 36.Fink DJ, Wechuck J, Mata M, et al. Gene therapy for pain: Results of a phase I clinical trial. Ann Neurol. 2011;2:207–212. doi: 10.1002/ana.22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goss JR, Goins WF, Glorioso JC. Gene therapy applications for the treatment of neuropathic pain. Expert Rev Neurother. 2007;5:487–506. doi: 10.1586/14737175.7.5.487. [DOI] [PubMed] [Google Scholar]
- 38.Wilson SP, Yeomans DC. Genetic therapy for pain management. Curr Rev Pain. 2000;6:445–450. doi: 10.1007/s11916-000-0068-5. [DOI] [PubMed] [Google Scholar]
- 39.Goss JR, Harley CF, Mata M, et al. Herpes vector-mediated expression of proenkephalin reduces bone cancer pain. Ann Neurol. 2002;5:662–665. doi: 10.1002/ana.10343. [DOI] [PubMed] [Google Scholar]
- 40.Hao S, Mata M, Goins W, Glorioso JC, Fink DJ. Transgene-mediated enkephalin release enhances the effect of morphine and evades tolerance to produce a sustained antiallodynic effect in neuropathic pain. Pain. 2003;1–2:135–142. doi: 10.1016/s0304-3959(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 41.Hao S, Hu J, Fink DJ. Transgene-mediated enkephalin expression attenuates signs of naloxone-precipitated morphine withdrawal in rats with neuropathic pain. Behav Brain Res. 2009;1:84–89. doi: 10.1016/j.bbr.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meunier A, Latremoliere A, Mauborgne A, et al. Attenuation of pain-related behavior in a rat model of trigeminal neuropathic pain by viral-driven enkephalin overproduction in trigeminal ganglion neurons. Mol Ther. 2005;4:608–616. doi: 10.1016/j.ymthe.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Wilson SP, Yeomans DC, Bender MA, Lu Y, Goins WF, Glorioso JC. Antihyperalgesic effects of infection with a preproenkephalin-encoding herpes virus. Proc Natl Acad Sci U S A. 1999;6:3211–3216. doi: 10.1073/pnas.96.6.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang H, McNearney TA, Chu R, et al. Enkephalin-encoding herpes simplex virus-1 decreases inflammation and hotplate sensitivity in a chronic pancreatitis model. Mol Pain. 2008:8. doi: 10.1186/1744-8069-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeomans DC, Lu Y, Laurito CE, et al. Recombinant herpes vector-mediated analgesia in a primate model of hyperalgesia. Mol Ther. 2006;3:589–597. doi: 10.1016/j.ymthe.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 46.Yokoyama H, Sasaki K, Franks ME, et al. Gene therapy for bladder overactivity and nociception with herpes simplex virus vectors expressing preproenkephalin. Hum Gene Ther. 2009;1:63–71. doi: 10.1089/hum.2008.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshimura N, Franks ME, Sasaki K, et al. Gene therapy of bladder pain with herpes simplex virus (HSV) vectors expressing preproenkephalin (PPE) Urology. 2001;6 Suppl 1:116. doi: 10.1016/s0090-4295(01)01060-3. [DOI] [PubMed] [Google Scholar]
- 48.Glorioso JC, Fink DJ. Herpes vector-mediated gene transfer in treatment of diseases of the nervous system. Annu Rev Microbiol. 2004:253–271. doi: 10.1146/annurev.micro.58.030603.123709. [DOI] [PubMed] [Google Scholar]
- 49.Palmer JA, Branston RH, Lilley CE, et al. Development and optimization of herpes simplex virus vectors for multiple long-term gene delivery to the peripheral nervous system. J Virol. 2000;12:5604–5618. doi: 10.1128/jvi.74.12.5604-5618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson SP, Yeomans DC. Virally mediated delivery of enkephalin and other neuropeptide transgenes in experimental pain models. Ann N Y Acad Sci. 2002:515–521. doi: 10.1111/j.1749-6632.2002.tb04516.x. [DOI] [PubMed] [Google Scholar]
- 51.Braz J, Beaufour C, Coutaux A, et al. Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons. J Neurosci. 2001;20:7881–7888. doi: 10.1523/JNEUROSCI.21-20-07881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu Y, McNearney TA, Wilson SP, Yeomans DC, Westlund KN. Joint capsule treatment with enkephalin-encoding HSV-1 recombinant vector reduces inflammatory damage and behavioural sequelae in rat CFA monoarthritis. Eur J Neurosci. 2008;5:1153–1165. doi: 10.1111/j.1460-9568.2008.06076.x. [DOI] [PubMed] [Google Scholar]
- 53.Cabanero D, Celerier E, Garcia-Nogales P, et al. The pro-nociceptive effects of remifentanil or surgical injury in mice are associated with a decrease in delta-opioid receptor mRNA levels: Prevention of the nociceptive response by on-site delivery of enkephalins. Pain. 2009;1–2:88–96. doi: 10.1016/j.pain.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 54.Lu Y, McNearney TA, Lin W, Wilson SP, Yeomans DC, Westlund KN. Treatment of inflamed pancreas with enkephalin encoding HSV-1 recombinant vector reduces inflammatory damage and behavioral sequelae. Mol Ther. 2007;10:1812–1819. doi: 10.1038/sj.mt.6300228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang G, Mohammad H, Peper BD, Raja S, Wilson SP, Sweitzer SM. Enhanced peripheral analgesia using virally mediated gene transfer of the mu-opioid receptor in mice. Anesthesiology. 2008;2:305–313. doi: 10.1097/01.anes.0000299836.61785.79. [DOI] [PubMed] [Google Scholar]
- 56.Vadivelu N, Mitra S, Hines RL. Peripheral opioid receptor agonists for analgesia: a comprehensive review. J Opioid Manag. 2011;1:55–68. doi: 10.5055/jom.2011.0049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


