Abstract
The pharmacological stressor yohimbine increases ongoing alcohol self-administration and reinstates alcohol seeking in rats. This effect is attenuated by systemic injections of a corticotropin-releasing factor (CRF) antagonist. The brain sites involved in CRF's role in yohimbine-induced alcohol taking and seeking are unknown. We report that injections of the CRF receptor antagonist d-Phe CRF into the median raphe nucleus (MRN) attenuated yohimbine-induced reinstatement of alcohol seeking but had no effect on yohimbine-induced increases in alcohol intake during ongoing self-administration. Results indicate an important role of MRN CRF receptors in yohimbine-induced reinstatement of alcohol seeking but not yohimbine-induced increases in alcohol intake.
Keywords: CRF receptors, d-Phe CRF, Median raphe nucleus, Alcohol self-administration, Reinstatement, Relapse, Stress
Yohimbine is an alpha-2 adrenoceptor antagonist that provokes stress- and anxiety-like responses in both humans and laboratory animals. In recently detoxified alcoholics, yohimbine induces alcohol craving (Umhau et al., 2011). In laboratory rats, yohimbine reinstates alcohol seeking after extinction of the alcohol-reinforced responding and increases ongoing alcohol self-administration (Cippitelli et al., 2010; Le et al., 2005; Richards et al., 2008). There is evidence that activation of central corticotropin-releasing factor (CRF) stress systems plays a role in yohimbine-induced reinstatement of alcohol seeking and yohimbine-induced increases in ongoing alcohol self-administration. Systemic injections of the CRF1 receptor antagonist antalarmin reversed yohimbine-induced alcohol seeking and taking but had no effect on yohimbine-induced corticosterone secretion, a measure of hypothalamic-pituitary-adrenal (HPA) axis activation (Marinelli et al., 2007). This dissociation suggests that yohimbine’s effects on drug seeking are mediated by extrahypothalamic CRF systems.
We previously found that blockade of CRF receptors in the median raphe nucleus (MRN), a cell body region of brain serotonin neurons, decreased intermittent footshock-induced reinstatement of alcohol seeking (Le et al., 2002). Here we assessed whether MRN CRF receptors also contribute to yohimbine-induced reinstatement of alcohol seeking and yohimbine-induced increases in ongoing alcohol self-administration.
The experimental procedures followed the “Principles of laboratory animal care” (NIH publication no. 85-23, 1996) and were approved by the local animal care and use committee. These procedures are described in detail in a supplementary online materials section (SOM). Seventeen rats were excluded because of inaccurate cannula placements outside the MRN, low alcohol intake during training (less then 0.4 g/kg/session), or failure to reach the extinction criterion (a mean of less than 12 active lever presses per 60 min for 3 sessions) after 15 extinction sessions.
In Exp. 1, we determined the effect of MRN injections of vehicle or d-Phe CRF on yohimbine-induced increases in ongoing alcohol self-administration under a fixed ratio 3 (FR3) reinforcement schedule. Thirty rats were given a choice between water and increasing alcohol concentrations in Richter tubes (Dyets Inc., Bethlehem PA) for 3 weeks and were then trained to lever-press for oral alcohol solutions (0.19 ml of 12% w/v, 1-h daily sessions); during training, the schedule requirement was gradually increased from FR1 to FR3 over 3 weeks. They were then implanted with guide cannulae aimed at the MRN, received 1 week of recovery, and then were given 7 additional daily 1-h self administration sessions prior to testing. We used a 2-way mixed factorial design with the between-subjects factor of d-Phe CRF dose (vehicle [saline], 25, or 50 ng per 0.5 μl) and the within-subjects factor of yohimbine dose (vehicle [water], 1.25 mg/kg, i.p., counterbalanced). Forty-five min prior to the test sessions (performed 2-3 days apart), the rats were injected with saline or d-Phe CRF into the MRN and 15 min later were injected systemically with water or yohimbine. On the days between drug tests, the rats received regular alcohol self-administration sessions. We used the non-selective peptide CRF receptor antagonist d-Phe CRF rather than antalarmin (the selective CRF1 receptor antagonist used in our previous study (Marinelli et al., 2007)), because of technical difficulties related to using this drug, which is difficult to dissolve, for intracranial injections.
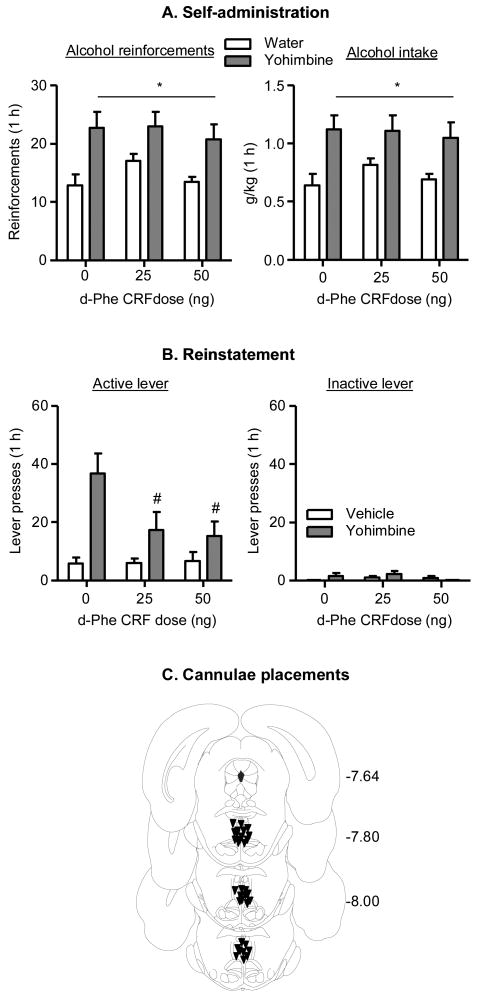
Yohimbine increased alcohol self-administration but this effect was not attenuated by MRN injections of d-Phe CRF (Fig. 1A). The statistical analyses on the number of alcohol deliveries under the FR3 reinforcement schedule and alcohol intake (g/kg) showed significant effects of yohimbine dose (F(1,45)=18.9 and F(1,45)=17.7, respectively, p values<0.05) but not d-Phe CRF dose or an interaction between yohimbine dose and d-Phe CRF dose.
Figure 1. Median raphe nucleus (MRN) injections of d-Phe CRF decreased yohimbine-induced reinstatement of alcohol seeking but had no effect on yohimbine-induced increases in alcohol self-administration.
(A) Alcohol self-administration. Data are mean±sem number of alcohol reinforcements consumed (deliveries, left) and alcohol intake (g/kg, right). * Main effect of yohimbine dose, p<0.05. n=6-9 rats per group. (B) Reinstatement of alcohol seeking. Data are mean mean±sem number of responses on the previously active lever (left) and on the inactive levers (right). # Different from the d-Phe CRF vehicle in the yohimbine condition, p<0.05; n= 6-7 rats per group. (C) Approximate placements of the injector tips in the MRN; numbers refer to distance in mm from bregma (Paxinos and Watson, 2005).
In Exp. 2, we determined the effects of MRN injections of vehicle or d-Phe CRF on yohimbine-induced reinstatement of alcohol seeking. Thirty rats were trained to self-administer alcohol under the same conditions described in Exp. 1. Next, the rats underwent 1-h extinction sessions over 15 days during which lever-presses were not reinforced with alcohol delivery. They were then implanted with guide cannulae aimed at the MRN, recovered for 1 week, and were given 7 additional daily 1-h extinction sessions prior to testing. We used a 2-way mixed factorial design with the between-subjects factor of d-Phe CRF dose (saline, 25, or 50 ng per 0.5 μl) and the within-subjects factor of Yohimbine dose (water, 1.25 mg/kg, counterbalanced). The rats received the injections of d-Phe CRF and yohimbine as described above in Exp. 1, with 2-3 extinction sessions on the days between testing. Yohimbine caused reinstatement of lever-presses after extinction and this effect was attenuated by MRN injections of d-Phe CRF (Fig. 1B). The analysis of active lever responding showed a significant interaction between yohimbine dose and d-Phe CRF dose (F(2,17)=5.78, p<0.05). Post-hoc (Newman Keuls test) differences are shown in Fig. 1B.
The results of our study indicate a role of MRN CRF receptors in yohimbine-induced reinstatement of alcohol seeking. These findings extend our previous report on the role of MRN CRF receptors in intermittent-footshock induced reinstatement of alcohol seeking (Le et al., 2002) and suggest a general role for CRF in this brain area in stress-induced relapse to drug seeking.
An alternative interpretation of our data is that d-Phe CRF's effect on yohimbine-induced reinstatement is mediated by drug diffusion into the nearby dorsal raphe nucleus (DRN) or the cerebral aqueduct. This interpretation is unlikely because the cannulae were implanted on an angle to avoid drug diffusion into the DRN or the cerebral aqueduct; additionally, in our previous studies we found that the DRN does not play a role in reinstatement of alcohol seeking (Le et al., 2002).
A question raised by our data is how does MRN CRF activity modulate stress-induced reinstatement of alcohol seeking? We speculate that this modulation occurs by stress-induced activation of local CRF that inhibit MRN serotonergic neurons that control reinstatement of alcohol seeking. Several indirect lines of evidence support this speculation. First, both CRF1 and CRF2 receptors are found in this brain area (Van Pett et al., 2000). Second, while the effect of CRF on neuronal activity in the MRN is unknown, in the anterior/medial dorsal raphe nucleus (DRN), the predominant effect of low to moderate ICV doses of CRF (0.1-1 μg) or low local doses (3-10 ng) is neuronal inhibition (Kirby et al., 2000). Third, systemic injections of fluoxetine or fenfluramine, which increases serotonin levels in the brain, decreased intermittent footshock-induced reinstatement of alcohol seeking (Le et al., 2006; Le et al., 1999). Fourth, local injections of the 5-HT1a receptor agonist 8-OH-DPAT (which inhibit serotonin cell firing and release) into the MRN (but not the DRN) or CRF mimicked the effect of intermittent footshock on reinstatement of alcohol seeking (Le et al., 2002). Fifth, yohimbine has agonist-like activity at the 5-HT1a receptor and it reduces serotonin cell firing and 5-HT release in the forebrain (Millan et al., 2000). Finally, yohimbine injections increase mRNA expression of CRF in several brain areas (Funk et al., 2006). Together, we speculate that antagonism of CRF receptors in the MRN counteracts the inhibitory effect of intermittent footshock or yohimbine on MRN serotonin transmission, leading to attenuation of stress-induced reinstatement of alcohol seeking.
Finally, a surprising finding in our study was the selective effect of blockade of MRN CRF receptors on yohimbine-induced reinstatement of alcohol seeking but not on yohimbine-induced increases in alcohol self-administration. The reasons for this dissociation are unknown and such dissociation suggests that the mechanisms underlying yohimbine's effects on reinstatement versus ongoing self-administration are different. This possibility is supported by our previous finding that the alpha-2 adrenoceptor agonist clonidine decreased yohimbine-induced reinstatement but not yohimbine-induced increases in alcohol self-administration (Le et al., 2009). As systemic injections of a CRF1 antagonist blocks both yohimbine-induced reinstatement of alcohol seeking and yohimbine-induced increases in alcohol self-administration, a question for future research is the brain sites involved in the effect of blockade of CRF1 receptors on yohimbine-induced increases in alcohol intake.
Supplementary Material
Acknowledgments
This work was supported by a grant from the NIAAA (AA13108) to A.D. Lê.
Footnotes
CONFLICT OF INTEREST
All authors declare that they have no conflict of interest.
Authors Contribution: ADL, DF, and YS participated in the design of the experiments. The experiments were conducted by DF, KC and ZL. DF, KC and YS performed the statistical analysis and prepared the figures. ADL, DF and YS interpreted the data and drafted the manuscript. All authors contributed to critically reviewing the manuscript and approved the final submitted version.
References
- Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, Thorsell A, Heilig M. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacology. 2010;208:417–426. doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Fletcher PJ. The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology (Berl) 2009;204:477–488. doi: 10.1007/s00213-009-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. Effects of dexfenfluramine and 5-HT3 receptor antagonists on stress-induced reinstatement of alcohol seeking in rats. Psychopharmacology. 2006;186:82–92. doi: 10.1007/s00213-006-0346-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J Neurosci. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, Nicolas JP, Coge F, Galizzi JP, Boutin JA, Rivet JM, Dekeyne A, Gobert A. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse. 2000;35:79–95. doi: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. Elsevier Academic Press; Amsterdam: 2005. [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology. 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George TD, Heilig M. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology. 2011;60:303–311. doi: 10.1038/npp.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.