Abstract
17β-estradiol (estradiol or E2) is implicated as a neurodegenerative disorders. This review focuses on the mechanisms underlying E2 neuroprotection in cerebral ischemia, as well as emerging evidence from basic science and clinical studies, which suggests that there is a “critical period” for estradiol's beneficial effect in the brain. Potential mechanisms underlying the critical period are discussed, as are the neurological consequences of long-term E2 deprivation (LTED) in animals and in humans after natural menopause or surgical menopause. We also summarize the major clinical trials concerning postmenopausal hormone therapy (HT), comparing their outcomes with respect to cardiovascular and neurological disease and discussing their relevance to the critical period hypothesis. Finally, potential caveats, controversies and future directions for the field are highlighted and discussed throughout the review.
Keywords: Estrogen, Brain, Cerebral Ischemia, Stroke, Dementia, Menopause, Critical Period, Hormone Therapy
Introduction – Gender Differences in Cerebrovascular Disease
Stroke is one of the leading causes of death and disability in the U.S., and it is a sexually dimorphic disease [126]. While men have a 33% higher incidence of stroke throughout most of the lifespan, women have significantly worse stroke outcome in terms of comorbidity and mortality [3, 121, 126, 143, 178]. Women also experience several physiological periods of enhanced stroke risk, such as pregnancy, parturition (childbirth), perimenopause (45–55 years of age), and advanced age (>85 years of age) [98, 178]. Several studies have also suggested that women's risk for stroke begins to exceed men's risk for stroke following the menopausal transition [98, 118, 121, 143]. Approximately 87% of strokes are ischemic, during which a particular area of the brain is deprived of oxygenated blood for a significant period of time, and 13% of strokes are hemorrhagic, during which elevated blood pressure and/or a weakened cerebrovasculature contribute to the bursting of a blood vessel inside the brain [98]. Stroke is considered to be a neurodegenerative disease, since either subtype of stroke can cause neuronal cell death and result in profound disability. Furthermore, stroke is a known tauopathy, meaning that it leads to hyperphosphorylation of the microtubule-associated protein tau, which regulates neurotransmission by stabilizing axons [186, 204]. Since hyperphosphorylated tau proteins are more likely to form insoluble aggregates called neurofibrillary tangles (NFTs), a neuropathological hallmark of Alzheimer's disease, it is no surprise that the risk for dementia increases 4- to 12-fold following a stroke [99, 177].
Intriguingly, sexual dimorphism extends beyond stroke. In fact, gender differences exist in most disorders affecting the central nervous system (CNS), particularly the neurodegenerative conditions, with women typically having a later onset and greater severity of disease [19, 173]. Women's relative protection, later onset, and greater severity of neurodegenerative disorders can be explained, in part, by serum levels of the neuroprotective ovarian hormone, 17β-estradiol (estradiol or E2). From birth to menopause, women's ovaries produce high circulating levels of estradiol, which correlates with a low incidence of neurodegenerative disease. However, once the menopausal transition occurs, the ovaries cease to manufacture E2, and women's risk for neurodegenerative diseases, including ischemic stroke and Alzheimer's disease, increases [19]. One could dismissively attribute this correlation to senescence. However, women who enter menopause prematurely via bilateral oophorectomy (surgical removal of both ovaries) have a doubled lifetime risk for developing dementia, as well as a significantly increased risk of cognitive decline, Parkinson's disease, and mortality from neurological disorders [138, 140, 158]. Furthermore, meta-analyses of observational studies demonstrated that postmenopausal women who used oral estrogens had a 29–44% reduced risk of Alzheimer's disease [19, 80, 193].
Estrogen Neuroprotection - Evidence and Mechanisms
Animal Studies and Estrogen Neuroprotection
In further support of the correlation between high serum levels of estradiol and women's relatively low risk of neurodegenerative disease, studies in rodents have overwhelmingly demonstrated that E2 is a neuroprotective agent. Female rodents were less susceptible to ischemic stress via experimental stroke procedures, such as middle cerebral artery occlusion (MCAO) and global cerebral ischemia (GCI), than their male counterparts, and ovariectomy prior to stroke induction abolished this gender difference [19]. Serum E2 levels in intact rodents were also found to be inversely correlated with stroke infarct size [96], and pre-treatment with ICI 182,780, a competitive antagonist of both estrogen receptor isoforms alpha (ERα) and beta (ERβ), prior to stroke induction actually enhanced the size of the infarct [147]. Additionally, pre-treatment with aromatase inhibitors, which prevent the conversion of androgens to estrogens, exacerbated ischemic injury in rodent brains, and aromatase knockout (KO) mice, which are physically unable to convert testosterone into estradiol, also had larger infarct volumes after MCAO [108]. Conversely, pre-treatment with exogenous E2 decreased mortality and infarct size following MCAO and GCI in rodents [19, 43, 85, 94, 109, 145, 154, 156, 160, 194, 202–205]. A systematic review of 161 publications on estradiol and stroke performed by Gibson et al. further confirmed a dose-dependent reduction of stroke lesion volume by E2 in models of transient and permanent cerebral ischemia [59].
Aside from preventing neuronal death, exogenous E2 replacement prior to stroke was shown to attenuate behavioral deficits in ovariectomized female rats subjected to GCI [19, 94, 129]. Furthermore, exogenous E2 was shown to facilitate post-stroke recovery in mice by enhancing neurogenesis in the dentate gyrus and subventricular zone after stroke, an effect that was attenuated in estrogen receptor (ER) knockout (KO) mice and aromatase KO mice [95]. As reviewed previously by our group and others, E2 has also been demonstrated to afford protection in animal models of Parkinson's disease and Alzheimer's disease (AD) [17, 19, 108, 128]. Finally, it should be mentioned that there are dissenting studies in the literature, which found that E2 increased ischemic stroke damage in animal models [11, 28, 62, 73, 176, 199]. It is not completely clear as to why these studies yielded a different result than the majority of studies in the literature, but a recent review suggested that the difference could be due to use of E2 slow-release pellets that yielded unexpectedly high circulating E2 levels [168]. Nevertheless, as a whole, research using animal models provides strong evidence for endogenous and exogenous E2 as a neuroprotective agent.
Mechanisms of Estrogen Neuroprotection
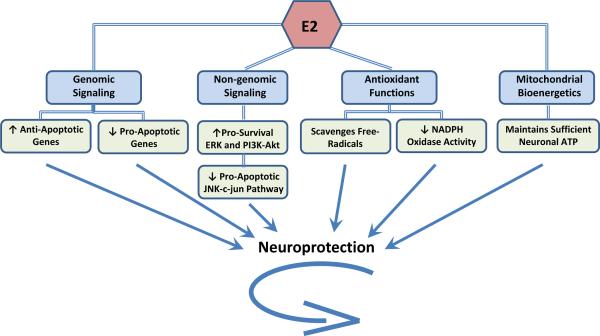
In addition to providing support for estradiol as a neuroprotective hormone, the aforementioned animal studies have also yielded mechanistic insight into how E2 protects the brain from ischemia. Figure 1 provides a schematic overview of the major mechanisms of E2 neuroprotection from cerebral ischemia. As shown in Figure 1, E2 is proposed to mediate neuroprotection via a multimodal mechanism that involves: 1) genomic signaling, 2) non-genomic signaling, 3) antioxidant actions, and 4) regulation of mitochondrial bioenergetics. The majority of these effects are suggested to involve mediation by ERs, in particular ERα. In the sections below, we will discuss evidence of a principal role for ERα in mediating E2 neuroprotection, and subsequently, we will discuss the evidence supporting each proposed mechanism of E2 neuroprotection depicted in Figure 1.
Figure 1. Mechanisms of Estradiol Neuroprotection.
This figure summarizes four major mechanisms through which 17β-estradiol (E2) protects neurons: classical genomic signaling, rapid non-genomic signaling, antioxidant functions, and mitochondrial bioenergetics. See text for further details.
ERα is a Key Mediator of E2 Neuroprotection in Cerebral Ischemia
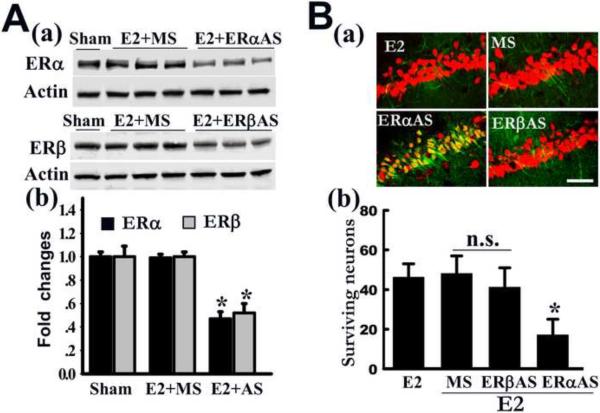
Initial evidence suggesting ER mediation of E2 neuroprotection in cerebral ischemia came from the observation that the ERα and ERβ antagonist ICI182,780 prevented E2's beneficial effects when it was given simultaneously with estradiol prior to GCI [112]. Furthermore, development of genetically modified mice led to the suggestion of ERα as the major mediator of E2 neuroprotection in experimental stroke. Ovariectomized ERα KO mice displayed a complete loss of E2 neuroprotection upon pre-treatment with E2 prior to cerebral ischemia, but ovariectomized ERβ KO mice maintained E2 neuroprotection when treated with E2 prior to stroke [45, 111]. Furthermore, as shown by Figure 2, our laboratory performed antisense (AS) oligonucleotide knockdown of either ERα or ERβ, to demonstrate that ERα, but not ERβ, mediates E2 neuroprotection against GCI [203]. As depicted in Figure 2A, bilateral intracerebroventricular injection of the ERα-AS and ERβ-AS oligonucleotides (ODNs) every 24 hours for 4 days induced a marked decrease in ERα and ERβ protein levels in the hippocampal CA1 region, respectively. Missense (MS) ODNs had no effect upon ERα or ERβ protein levels, indicating that the effects of the AS-ODNs were specific. Figure 2B shows that that ERα-AS, but not ERβ-AS ODNs, significantly attenuated the neuroprotective effect of E2 against GCI, suggesting that ERα mediates the neuroprotective actions of E2 in the hippocampal CA1 [203].
Figure 2. Evidence that ERα mediates E2-induced neuroprotection in hippocampal CA1 region following ischemia.
(A) Western blot analysis demonstrates a robust reduction in ERα or ERβ expression after pretreatment with ERα or ERβ antisense-ODNs (AS), as compared to missense ODNs (MS) controls or Sham controls (a, b). *P < 0.05 vs. MS-treatment group, respectively. (B) Effect of MS, ERα or ERβ AS on E2 neuroprotection against cerebral ischemia. Representative CA1 sections double stained with NeuN (red) and Fluoro-Jade B (green) from E2-treated animals with ERα AS, ERβ AS or MS infusion. Note that E2 protection was abolished only in the ERα AS infused rat hippocampus (a). Values are means ± SE from 6–7 animals (b). *P < 0.05 vs. E2+MS group. Scale bar, 50 μm; Magnification, 40×; n.s., No significant difference. Figure adapted from [203].
A recent study also suggested that E2 may act directly on neurons to exert neuroprotection, as E2 neuroprotection was lost in neuron-specific ERα knockout mice, but not in microglia-specific ERα knockout mice [47]. However, while this study suggests that estradiol can act directly on neurons to exert neuroprotection in cerebral ischemia, it does not completely rule out that ERs in other cell types, such as endothelial cells or astrocytes, couldn't also contribute, in part, to mediation of E2's neuroprotective effects. Along these lines, there is a significant body of evidence demonstrating that E2 can act on astrocytes to influence release of neuroprotective compounds, such as growth factors, which could provide an indirect mechanism of E2 neuroprotection [4, 6, 40]. Additionally, a recent study showed that ERα-specific knockout in astrocytes, but not neurons, resulted in the loss of E2's neuroprotective ability in an animal model of experimental autoimmune encephalomyelitis [166]. Therefore, while E2 has been implicated to act directly upon neurons to exert neuroprotection against cerebral ischemia, it is important to examine astrocyte- and endothelial-specific ER knockout animals, so as to better clarify the contribution of these non-neural cell types to E2 neuroprotection in cerebral ischemia.
It should be noted that there are a few studies which used ERβ-specific agonists to suggest a role for ERβ in neuroprotection in cerebral ischemia [29, 112]. However, when using agonists, one cannot totally exclude potential “off target” effects of the agonists that could explain the observed effects. In addition, exogenous agonist treatment studies alone do not prove a physiological role of the receptor in mediating E2 neuroprotection. As such, conclusions regarding the role of a receptor must be based on a variety of approaches, including the use of receptor knockdown and receptor knockout mice. As mentioned previously, the results of ERβ KO mouse studies did not support a major role for ERβ in mediating E2 neuroprotection in cerebral ischemia. Rather, the available evidence to date argues that ERα is the principal ER that mediates E2 neuroprotection in cerebral ischemia. Nevertheless, it should be mentioned that ERβ KO mice have been reported to show significant neurodegeneration in the cerebral cortex, beginning at six months of age and peaking at two-years of age [181]. This intriguing observation suggests that ERβ may have a role in mediating basal neuronal survival in the cerebral cortex.
GPR30 – A Putative New ER?
A third putative ER called G-Protein-Coupled ER (GPR30, also known as GPER1), has been recently identified as a potential membrane ER [51]. GPR30 is a seven transmembrane domain G-protein coupled receptor that is expressed in the hippocampus, cortex, striatum, and other brain regions [51, 68, 107]. Intriguingly, co-localization studies showed that the majority of cholinergic neurons in the forebrain co-localize with GPR30 immunoreactivity [68, 69], suggesting that it may have a regulatory role in cholinergic neurons. Evidence of a neuroprotective role for GPR30 has been primarily derived from studies using a purported selective agonist for GPR30, G-1 [14, 60]. G-1 pre-treatment has been reported to significantly attenuate glutamate-induced or oxidative stress-induced neuronal cell death in neuronal cell cultures [60, 97]. In vivo studies using the MCAO model have also shown that G-1 exerts neuroprotection against cerebral ischemia in female mice [201]. A caveat of these studies is that they only show an effect of an exogenous GPR30 agonist and do not prove a role for GPR30 in mediating neuroprotection by either endogenous or exogenous E2. Further work is needed to address the physiological role of GPR30 in mediating E2 neuroprotection through use of various approaches, including the use of mutant GPR30 mice and GPR30 knockdown.
Furthermore, it should be mentioned that work by Simpkins and colleagues showed that non-feminizing estrogens, which lack or have very low affinity for classical ERα and ERβ, can exert potent neuroprotection in animal models of cerebral ischemia [31, 50, 127, 159, 169]. They found that the most effective non-feminizing estrogens were those with large, bulky groups in the 2 and/or 4 carbon of the phenolic A ring of the steroid [159]. From a clinical viewpoint, these studies are intriguing because these novel compounds are predicted to lack negative side-effects attributed to traditional estrogens, such as stimulation of uterine and breast tissue and enhanced coagulation, since they lack any significant ability to bind the ER. However, studies are needed to actually confirm this suggestion. Further work is also needed to elucidate the precise mechanism(s) of how these analogues are neuroprotective. Along these lines, it would be interesting to assess whether non-feminizing estrogens have affinity for GPR30 and whether the neuroprotective effects of these compounds might be mediated by GPR30 signaling.
Genomic Signaling and E2 Neuroprotection
Estradiol is known to signal through both genomic and nongenomic mechanisms, and both mechanisms have been implicated to mediate E2's neuroprotective effects. With respect to genomic signaling, E2, as a lipophilic molecule, diffuses through the cell's membrane, binds to ERs in the cytosol, and translocates to the nucleus in order to regulate the transcription of genes in a matter of hours. In support of the importance of genomic signaling in E2 neuroprotection, E2 has been shown to increase transcription of pro-survival bcl-2 in vivo after MCAO and prevent transcription of pro-apoptotic bad [44]. E2 also increases bcl-2 in human NT2 neurons and rat hippocampal neurons in vitro [44, 191]. Interestingly, E2 has been shown to regulate expression of other bcl-2 family members, including enhancing expression of anti-apoptotic bcl-w while attenuating expression of pro-apoptotic bim in cortical neurons, effects shown to be important for E2 neuroprotection against beta-amyloid-induced neuronal death [198]. Furthermore, E2 has been demonstrated to prevent the translocation of cytochrome c, activation of caspase-3, and fragmentation of DNA, all events which occur during programmed cell death [34, 132].
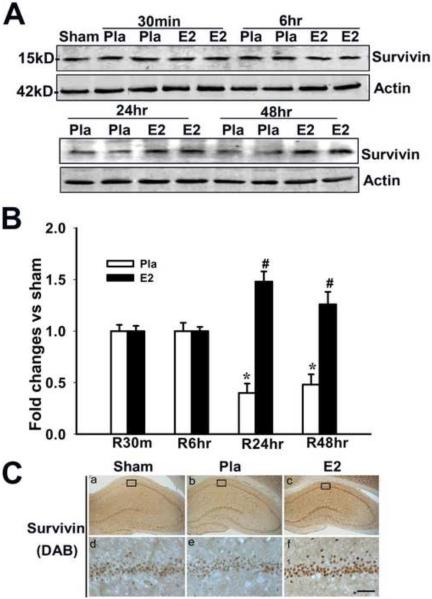
Additionally, strongly as shown in Figure 3, work by our laboratory showed that E2 strongly enhances expression of the anti-apoptotic, pro-survival factor, survivin in the CA1 hippocampal region 24–48h following GCI, which facilitates neuronal survival [204]. Recent work has also shown that E2 induces survivin expression in the peri-contusional area of the cerebral cortex following traumatic brain injury (TBI), which is thought to contribute to its neuroprotective effects in TBI [7]. Estradiol is also able to enhance expression of growth factors in the brain, such as transforming growth factor-β1 (TGFβ1) and brain-derived neurotrophic factor (BDNF) [41, 52, 103], as well as growth factor receptors, such as the insulin-like growth factor 1 (IGF-1) receptor [26, 54]. Importantly, the growth factor BDNF is well known to exert neuroprotective and neurotrophic effects in the cortex and hippocampus and to be important for synaptic plasticity, learning, and memory [9, 10, 162, 163, 194]. Finally, work by the Garcia-Segura laboratory has shown that there is significant cross-talk between the E2 and IGF-1 receptor signaling pathways and further suggested that this cross-talk plays a role in mediating E2's neuroprotective effects [26, 54].
Figure 3. Estradiol Induces Expression of Survivin in CA1 Hippocampal Neurons 24 and 48 Hours After Global Cerebral Ischemia.
(A, B) 17β-Estradiol enhances expression of the anti-apoptotic protein survivin in hippocampus CA1 after global cerebral ischemia. Values are mean ± SEM of determinations from five to six individual rats expressed as fold change versus sham control. Pla, Placebo; R, reperfusion. *P < 0.05 versus sham control; #P < 0.05 versus the Pla group at the same time point. (C) DAB and confocal analysis shows that survivin is induced in NeuN-positive neurons by E2 in hippocampus CA1 at 24 h after global cerebral ischemia. Results are representative of staining observed in five individual animals per group (magnifications, 40×). Scale bars, 50μm. Figure adapted from [204].
Nongenomic Signaling and E2 Neuroprotection
Recent work has also implicated a role for nongenomic signaling in mediating E2 neuroprotection. In addition to nuclear localization of ERs, both ERα and ERβ have been demonstrated to be at the plasma membrane of neurons in various brain regions, including the cortex and hippocampus, and at other neuronal extranuclear sites, such as dendrites and dendritic spines [41, 53, 86, 89, 102, 114, 142, 180, 190]. Extranuclear ERs are thought to play a key role in mediating rapid signaling effects of E2 in neurons to regulate kinase signaling pathways, calcium signaling, and activation/inactivation of key cellular proteins [190]. The non-genomic signaling by E2 may also cross-talk to the nucleus to exert genomic regulation, as will be discussed later. Currently, it is not entirely clear how ERα and ERβ are targeted to the membrane and to other extranuclear sites. Palmitoylation of ERs, and ER interaction with the scaffold protein, caveolin-1 have been suggested to play a role in trafficking of ERs to the membrane [15, 30]. A recent study also provided evidence that heat shock protein-27 (HSP27) can bind to ERα, thereby promoting its palmitoylation and delivery to the cell membrane [133]. The majority of the studies on mechanisms of ER trafficking to the membrane were conducted in non-neuronal cells; however, it is assumed that the same mechanisms apply to neurons as well.
A large body of evidence supports a role for E2 and extranuclear ERs in the rapid activation/phosphorylation of neuronal extracellular signal-related kinases (ERK) and the phosphotidylinositol-3-kinase (PI3K)-Akt pathway in neurons [23, 42, 81, 82, 90, 105, 106, 149, 163, 185, 188, 191]. Once activated, ERK can phosphorylate/regulate over 100 downstream targets, including transcription factors, cytoskeleton proteins and kinases, and nuclear kinases [131]. As such, ERK activation has been implicated in the regulation of many key cellular functions such as survival, adhesion, metabolism and proliferation [131]. Akt, also known as protein kinase B, is a kinase that can promote cell survival by inhibiting the pro-apoptotic JNK-c-jun signaling pathway and by inhibiting the activity of pro-apoptotic bad and glycogen synthase kinase-3β (GSK-3β) [182]. Since JNK and GSK-3β are kinases known to phosphorylate the microtubule associated protein tau, and hyperphosphorylated tau is one of the hallmarks of Alzheimer's disease neuropathology, this may be one of the mechanisms through which E2 can protect the brain from AD [61].
There is significant evidence that ERK activation is important for E2 neuroprotection. For instance, in vitro studies have shown that administration of a MEK inhibitor, which prevents E2-induced ERK activation, blocks the neuroprotective effects of E2 in neurons [66, 91, 123, 191]. In addition, E2 enhances ERK activation in the hippocampal CA1 region following GCI, and E2's neuroprotective effects in GCI are blocked by administration of a MEK inhibitor [84]. However, it is important to note that while E2's activation of ERK1/2 has been proposed to be neuroprotective in cerebral ischemia, evidence also suggests that ERK activation may play a pro-apoptotic role. Along these lines, MEK inhibitors have been shown to attenuate ischemic damage following GCI and MCAO, which suggests that ERK activation may actually contribute to neurodegeneration following cerebral ischemia [79, 119, 183]. In contrast, it has also been hypothesized that enhanced ERK1/2 activation may initiate a neuroprotective signaling cascade, which eventually culminates in the down-regulation of ERK itself, thereby preventing prolonged ERK activation. The apparent paradox of ERK activation having both positive and negative effects in cerebral ischemia could be feasibly resolved by considering several factors, such as the neural cell type in which ERK is activated (neuron, glia, or endothelial cell), the pattern/duration of ERK activation (acute, biphasic, or chronic), and/or the subcellular localization of activated ERK (nucleus versus cytoplasm). For a more authoritative and thorough discussion of this complex subject, the reader is referred to Sawe et al.'s review concerning activated ERK's dual role in cerebral ischemia [148].
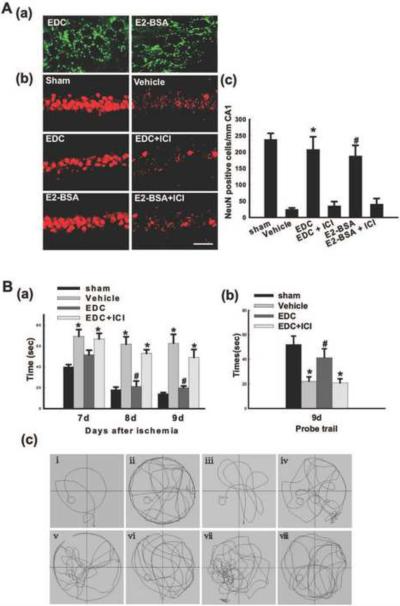
To further demonstrate the role of extranuclear ERs in estradiol's neuroprotective actions, we employed E2 conjugates (E2 dendrimer, EDC; E2-BSA), which can interact with extranuclear ERs and exert rapid nongenomic signaling but cannot interact with nuclear ERs due to an inability to enter the nucleus [72, 194]. Using FITC-tagged EDC and E2-BSA, we first examined localization of the E2 conjugates in the hippocampal CA1 region at 60 minutes following intracerebroventricular (icv) injection in the lateral ventricles of ovariectomized female rats. As shown in Figure 4A (a), FITC-EDC and FITC-E2-BSA conjugates are heavily localized in the CA1 after icv injection, displaying a punctate extranuclear/membrane pattern of localization. Figure 4A (b–c) shows the neuroprotective ability of EDC and E2-BSA administered icv 60 min prior to GCI. Examination of NeuN-positive cells at 7d after GCI revealed that Vehicle-treated animals that underwent 10 minutes of GCI displayed a significant loss of NeuN-positive cells in the hippocampal CA1 region as compared to Sham controls. Intriguingly, the cell-impermeable E2 conjugates, EDC and E2-BSA, both exerted robust neuroprotection against GCI, an effect that appeared to be mediated by ERs, since pretreatment with the ER antagonist ICI182,780 completely abolished the neuroprotective effect of both EDC and E2-BSA.
Figure 4.
(A-a) Extranuclear localization of EDC and E2-BSA in neurons in hippocampal CA1 region of rats. FITC-tagged EDC and E2-BSA were injected into the lateral ventricles. 1h later, the rats underwent transcardial perfusion, and their brains were cut into 25mm coronal brain sections with a cryostat. Confocal analysis showed that EDC and E2-BSA entered neurons in the hippocampal CA1 region but were incapable of penetrating into the nucleus. (A–b, c) EDC and E2-BSA2 protect neurons of the hippocampus CA1 region from injury induced by GCI. NeuN immunostaining of representative hippocampus sections from sham, vehicle, EDC, EDC+ICI, E2-BSA and E2-BSA+ICI-treated female ovariectomized rats subjected to 10min GCI followed by 7d reperfusion. Global ischemia induced significant neuronal cell loss in the CA1 pyramidal cell layer. EDC or E2-BSA treatment afforded nearly complete protection from global cerebral ischemia-induced neuronal cell loss. ICI182,780 (ICI) abrogated the neuroprotection induced by EDC or E2-BSA. NeuN-positive neurons per 1 mm length of CA1 region were counted as surviving neurons. F (5, 30) = 105, *P < 0.001 vs. vehicle and EDC+ICI groups; F (5, 30) = 105, #P < 0.001 vs. vehicle and E2-BSA+ICI groups. Magnification, 40×; Scale bar, 50μm. (B) Effects of EDC on spatial learning and memory ability in the Morris water maze. (a) Latency to find the submerged platform. F (11, 48) = 77, *P < 0.001 vs. sham; F (11, 48) = 77, #P < 0.001 vs. Vehicle and EDC+ICI. (b) Time spent in the quadrant, which initially contains the platform. F (3, 16) = 37, *P < 0.001 vs. sham; F (3, 16) = 37, #P < 0.01 vs. Vehicle and EDC+ICI. (c) Representative sample paths from the maze trials (i–iv) and the probe trials (v–viii) at 9 days after reperfusion. (i, v: sham; ii, vi: vehicle; iii, vii: EDC; iv, viii: EDC+ICI). R: reperfusion. ICI: ICI182,780. Reprinted, with permission, from PLoSOne [194].
We next examined the effect of EDC upon functional outcome following GCI by analyzing EDC's effect on spatial learning and memory in rats 7–9 days after reperfusion using the Morris water maze (Figure 4B, a–c). As shown in Figure 4B (a), Vehicle-treated animals that underwent GCI showed significantly higher latencies in finding the submerged platform on days 7–9 post stroke as compared to sham control rats. In contrast, EDC-treated rats had significantly decreased latencies to find the submerged platform on day 7–9 as compared to the Vehicle group, an effect that was significantly reversed by the ER antagonist ICI182,780. Furthermore, Figure 4B (b) shows that Vehicle-treated animals spent significantly less time in the quadrant where the submerged platform was located as compared to sham animals on Day 9. In contrast, EDC-treated rats spent significantly greater amount of time in the quadrant where the submerged platform was located as compared to Vehicle group, and this effect was significantly reversed by the ER antagonist, ICI182,780, suggesting ER mediation of the cognitive enhancing/preservation effect of EDC. Finally, representative sample paths for the various groups and treatments for the maze and probe trials at 9 days reperfusion are provided in Figure 4B (c), which illustrates that EDC-treated animals in the probe trials spent significantly more time in the quadrant where the platform was located.
Further studies demonstrated that EDC and E2-BSA rapidly enhanced ERK and Akt activation in the hippocampal CA1 region after cerebral ischemia, and that inhibition of either ERK or Akt activation by specific inhibitors abolished the neuroprotective effects of the E2 conjugates [194]. While we observed that acute E2 conjugate treatment in vivo both enhanced and prolonged ERK activation in the hippocampal CA1 region following GCI, this finding does not support a role for E2 in the aforementioned proposed model of prolonged ERK activation facilitating its own inactivation. However, it is important to note that our study was limited to 24 hours after GCI. As such, examination of more distant time points post ischemia-reperfusion may be required to determine if prolonged ERK activation by E2 leads to subsequent down-regulation of ERK more than one day following GCI. Interestingly, our studies also revealed that EDC (and E2-BSA) enhanced phosphorylation of the transcription factor, CREB in a rapid fashion following reperfusion, and that this effect was both ERK- and Akt-dependent [194]. Among the best known CREB transcriptional targets is the growth factor, BDNF, and subsequent studies by our group demonstrated that EDC strongly increased BDNF expression in the hippocampal CA1 region following GCI [194]. These findings raise the possibility that extranuclear ER-mediated nongenomic signaling may involve cross-talk to the nucleus via kinase-induced activation of transcription factors, with subsequent transcription factor-induced changes in expression of pro-survival and/or pro-death genes.
Another potential example of E2-induced nongenomic cross-talk to the nucleus involves E2 regulation of the JNK-c-Jun signaling pathway. Along these lines, work by our lab has shown that E2 and EDC exert a prolonged attenuation of phosphorylation of JNK at Thr183/Tyr185 in the hippocampal CA1 region after cerebral ischemia, phosphorylation sites known to be critical for JNK activation [194, 203]. JNK is known to phosphorylate many cellular proteins, including several implicated in apoptosis, and it can translocate to the nucleus to activate c-Jun and AP-1-mediated gene transcription, which leads to upregulation of pro-death genes [41]. Previous work by our group and others has shown that administration of a JNK inhibitor or knockout of JNK results in profound protection of the brain against cerebral ischemia [47, 53, 204], further demonstrating a key pro-apoptotic role of JNK in ischemic neuronal cell death. Thus, the ability of E2/EDC to markedly attenuate JNK activation after cerebral ischemia likely contributes significantly to its neuroprotective actions. Taken as a whole, the above studies suggest that in addition to classical nuclear ERs, extranuclear ERs play a critical role in mediating E2 neuroprotection in cerebral ischemia via modulation of rapid kinase signaling pathway activation. These studies also raise the intriguing possibility that extranuclear ER-initiated nongenomic signaling can cross-talk to the nucleus by effecting kinase signaling modulation of nuclear transcription factors, with subsequent regulation of transcription.
Oxidative Stress, Mitochondrial Bioenergetics and E2 Neuroprotection
An emerging and important additional mechanistic layer to E2 neuroprotection is estradiol's regulation of oxidative stress and mitochondrial bioenergetics in the brain. Oxidative stress and mitochondrial dysfunction have been implicated to play a key role in promoting neuronal damage and demise in cerebral ischemia and other neurological disorders, such as Alzheimer's disease [32, 49, 122, 134]. With respect to mitochondrial dysfunction, a growing body of evidence indicates that E2 can have beneficial effects upon mitochondria and preserve mitochondrial function. These effects include regulation/preservation of ATP generation, ROS production, mitochondrial apoptotic factors, and antioxidant mechanisms. With respect to mitochondrial bioenergetics, E2 has been demonstrated to protect the brain by enhancing regional cerebral blood flow in vivo and facilitating the utilization of glucose as the primary source of energy for the brain by up-regulating glucose transporters in neurons [21, 195]. Furthermore, E2 has been shown to promote oxidative phosphorylation of energy substrates by enhancing activity of electron transport chain complexes I and II in order to maintain sufficient energy supply for neurons [67, 196]. It is intriguing that these aforementioned mechanisms of E2 neuroprotection also implicate E2 with protection of the brain from Alzheimer's disease, due to theories of neuronal free radical damage and cerebral hypometabolism as critical precursors to AD onset [171, 195–197]. E2's effects on mitochondria have been extensively reviewed previously, and the reader is referred to these excellent reviews [22, 165, 170].
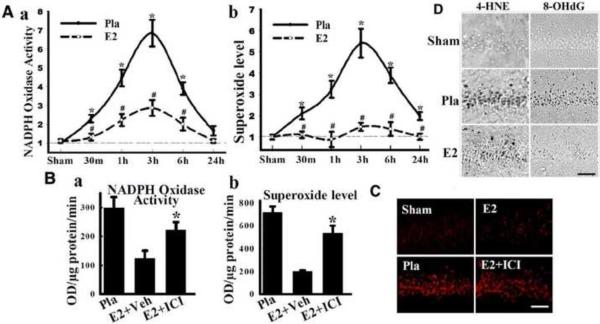
In addition to mitochondria-generated ROS, recent evidence suggests that the plasma membrane, via NADPH oxidase, may play an additional critical role in ROS generation in neurons following cerebral ischemia. The NADPH oxidase enzyme is composed of key subunits from the NOX family, whose primary job is to transport electrons across biological membranes in order to reduce molecular oxygen to superoxide (O2−). O2− is a highly reactive free radical anion that is the precursor of most ROS, including the highly toxic and damaging hydroxyl ion and peroxynitrite [5, 161]. The NOX family is composed of five isoforms (NOX1–NOX5). The activation of NOX2, the most studied and best characterized NOX isoform, involves interaction between the subunits p22phox, p67phox, p40phox and p47phox subunits [8, 33, 179]. In addition, the GTPase, Rac1 has been shown to be critical for NOX2 activation [8, 33]. Work by our laboratory has shown that NADPH oxidase activation and O2− production increases rapidly in the hippocampal CA1 region following GCI in both male and female rats, with an elevation occurring as early as 30 minutes after reperfusion and peak levels observed at 3 hours after reperfusion (Figure 5A & 5B) [203]. Further work demonstrated that NOX2 is predominantly localized to neurons in the hippocampus following GCI [203], but NOX2 is also known to be expressed in microglia at later time-points following cerebral ischemia [44]. The importance of NADPH oxidase activation to neuronal damage following cerebral ischemia was suggested by the fact that inhibition or knockout of the NOX2 NADPH oxidase enzyme significantly reduced infarct damage [18, 56, 174, 200, 203]. Intriguingly, further work by our laboratory showed that low dose E2 replacement profoundly attenuated NADPH oxidase activation and O2− production in the hippocampal CA1 region following reperfusion (Figure 5A), an effect blocked by the ER antagonist ICI180,782 (Figure 5B) [203].
Figure 5. E2 attenuates NADPH oxidase activity, superoxide production and oxidative damage in hippocampal CA1 after global cerebral ischemia.
(A) Time course of NADPH oxidase activity (a) and superoxide production (b) in hippocampal CA1 region from sham, placebo (Pla) and E2-treated rats. Values are means ±SE of 4–5 rats in each group and expressed as fold changed vs. sham+Pla group. *P < 0.05 vs. sham; #P < 0.05 vs. Pla at the same time point. There was no difference between Pla+sham and E2+sham groups. (B) Effects of ICI182,780 on NADPH oxidase activity (a) and superoxide production (b) in CA1 at 3h after reperfusion. *P < 0.05 vs. vehicle group. (C) Representative pictures of oxidized HEt staining taken from medial CA1 region 3 h after reperfusion. Strong oxidized HEt staining was detected in Pla and E2+ICI treated brains after ischemia, while weak oxidized HEt signals were detected in sham and E2 groups (a). E2 significantly attenuated superoxide generation compared to Pla, which was reversed by ICI182,780 (b). *P < 0.01, vs. sham and E2. (D) Effect of E2 on oxidative damage markers for lipid peroxidation (4-HNE) and DNA damage (8-OHdG) 1 day after ischemia. Note that E2 strongly decreased 4-HNE and 8-OHdG staining. Scale bar in all sections = 50 μm; Magnification, 40×. HEt: hydroethidine; ICI: ICI182,780. Adapted from [203].
O2− production was also assessed using the in situ oxidized hydroethidine (HEt) method, in which HEt, a marker of O2− production, is selectively taken up by cells and oxidized by O2− into ethidium, which provides a red fluorescence signal. As shown in Figure 5C, assessment of oxidized HEt signal in the CA1 region at 3 hours after reperfusion revealed a robust induction of O2− in the ischemic group, as compared to Sham controls. E2 markedly attenuated the induction of O2− in the CA1, an effect blocked by the ER antagonist, ICI182,780. Examination of oxidative damage markers revealed that, in agreement with reduction of NADPH oxidase activity and O2− by E2 following GCI, E2 markedly attenuated oxidative damage in the CA1 region 24 hours after GCI, as measured by immunostaining for 4-HNE, a marker of lipid oxidative damage, as well as 8-OHdG, a marker of DNA oxidative damage (Figure 5D). Further work implicated a critical role for extranuclear ERα in E2's antioxidant effects, as evidenced by the fact that EDC was fully capable of attenuating NADPH oxidase activation and O2− production after GCI and that antisense oligonucleotide knockdown of ERα, but not ERβ, blocked the antioxidant effects of E2 [203].
Additional studies showed that E2 inhibited activation of the GTPase Rac1 in an Akt-dependent manner following cerebral ischemia, which is critical for NOX2 NADPH oxidase activation [203]. The finding that E2's antioxidant effects involve mediation by extranuclear ERα and rapid kinase activation is in agreement with studies in other systems such as bone, where E2 suppression of oxidative stress did not require ERα binding to DNA response elements but instead, resulted from the activation of kinases [2]. While the above studies suggest that extranuclear ERα plays a key role in mediating the antioxidant effects of E2, it should be mentioned that there is also evidence that estrogens, in an ER-independent manner, can act as direct hydroxyl radical scavengers, converting captured nonphenolic quinols back into their respective parent estrogens without the production of reactive oxygen species as a byproduct [130]. Thus, E2's antioxidant effects may be a sum of both ER-dependent and ER-independent effects.
In summary, as illustrated in Figure 1, the neuroprotective effects of E2 in cerebral ischemia are thought to involve both genomic and nongenomic signaling, be principally mediated by ERα, and achieved via attenuation of oxidative stress, preservation of mitochondrial bioenergetics, and regulation of the balance of pro-survival and pro-death factors. In the following sections of the review, we examine the cardiovascular and neural consequences of long-term E2 deprivation, which occur after menopause and surgical menopause, and we review the literature on hormone therapy (HT) and the critical window/critical period hypothesis.
Reproductive Senescence, Surgical Menopause and the CNS
Natural Menopause
The menopausal transition is a natural part of reproductive senescence in women. Menopause itself is defined by the complete absence of menstrual cycles for one year, and women experience menopause at a median age of 51 years in developed countries [87, 140]. Menopause is physiologically characterized by gradual ovarian follicle depletion, cessation of menstruation, and a dramatic decrease in serum levels of ovarian-derived estradiol [87]. However, many undesirable side effects result from this reproductive senescence. Common symptoms of E2 deficiency during the menopausal transition include vasomotor “hot flushes,” sweating, vaginal dryness, vaginal atrophy, dyspareunia, urinary incontinence, irritability, depression, and insomnia, all of which range in severity among women [87, 189]. These bothersome symptoms are likely to be noticed by menopausal women and are subsequently brought to the attention of a physician as a primary complaint approximately 10% of the time [189]. However, more serious side effects can result from a prolonged period of E2 deficiency, or long-term E2 deprivation (LTED), and these side effects often remain undetected. This includes a significant loss of bone mineral density, which increases the risk of osteoporosis and bone fractures, and an increased risk of serious cardiovascular diseases, such as atherosclerosis and myocardial infarction [87, 125, 158]. Intriguingly, an increased risk for cardiovascular disease corresponds to an increased risk for cerebrovascular disease, including ischemic stroke, and underlying cerebrovascular disease has been observed in human cases of Alzheimer's disease (AD) [120]. This suggests that LTED via menopause could promote AD development through the increased risk of cardiovascular and cerebrovascular disease. In fact, scientific evidence supports that LTED may enhance women's chances of developing mild cognitive impairment (MCI), which can further progress to vascular dementia or AD [38, 78, 115, 141]. Unfortunately, since postmenopausal women are unable to detect the underlying increased risks for osteoporosis, cardiovascular disease, and cerebrovascular disease, these problems are often clinically silent until significant disease progression has occurred.
Surgical Menopause
Surgical menopause is an ambiguous umbrella term that pertains to surgical removal of either the uterus alone (hysterectomy), removal of one or both ovaries (unilateral or bilateral oophorectomy, respectively), or some combination of the two events [140]. While the natural menopausal transition typically occurs over a period of years, surgical menopause is extremely abrupt. Rather than a gradual cessation of menstrual cycles, removal of the uterus and/or the ovaries leads to instantaneous amenorrhea. Therefore, since menopause is defined by the absence of menstrual cycles, these women immediately enter into menopause following surgery, regardless of whether the ovaries are left intact. However, it is important to note that in the subset of women who undergo hysterectomy but retain one or both of their ovaries, the remaining ovary(ies) will continue to produce E2 until follicular depletion occurs at the age of natural menopause, even though these women will not have menses [140]. This means that these hysterectomized women will not experience the symptoms of E2 deficiency until they reach the age of natural menopause [140]. Conversely, in the case of bilateral oophorectomy, instead of the steady decline in serum E2 values seen in naturally menopausal and hysterectomized women, serum E2 values instantly and dramatically plummet once the hormone-producing ovaries are removed. In these women, typical menopausal symptoms resulting from E2 deficiency manifest quickly after surgery.
Importantly, women who experience surgical menopause through bilateral oophorectomy are, on average, much younger than women who experience natural menopause [78]. Consequently, these prematurely menopausal women will experience a much longer period of E2 deprivation than traditionally menopausal women, along with the detrimental side effects. As such, many studies have focused on the health and quality of life of women who experience premature menopause (< 40 years of age) or early menopause (40–45 years of age). Both subsets of these women were found to have increased morbidity and mortality when compared to women who entered menopause at the median age of 51, with the risk of disease increasing as the age of entry into menopause decreases [78, 158]. Furthermore, studies have shown that women who enter menopause prematurely due to bilateral oophorectomy before the age of 45 and do not receive supplemental E2 have a 5-fold increased risk for mortality directly related to neurological disorders, such as depression, parkinsonism, and dementia [78, 125, 137, 141]. Understandably, these researchers caution against premature removal of both ovaries unless medically necessary [78, 125, 137, 141]. These striking statistics highlight the detrimental neurological consequences of LTED, which will be discussed in further detail in a later section, and the corresponding importance of replacing estradiol in E2-deficient menopausal women, especially those who enter menopause prematurely.
Clinical Trials with HT
Observational Studies with HT and Prevention of Dementia
Serum E2 levels have been shown to be directly correlated with cognitive functioning in women, with high serum levels of E2 significantly enhancing verbal working memory [144, 150]. Furthermore, human hippocampal volumes have been demonstrated to be larger in postmenopausal women undergoing current treatment with hormone therapy (HT, any variety of estrogens ± progestogens) when compared to women who had used HT in the past, women who had never used HT, and men [100]. Cognitive impairment is more prevalent in postmenopausal women who have low serum levels of E2, and several studies demonstrated that postmenopausal women who received some form of estrogens through HT performed better on neuropsychological tests of cognition [150]. In fact, many promising observational studies showed a decreased risk or delayed onset of cognitive impairment and neurodegenerative diseases in women who used estrogens after menopause [19, 173]. A meta-analysis of 12 case-control and prospective cohort studies performed in the 1990s demonstrated a 29–44% reduction in the risk of Alzheimer's disease development in postmenopausal women who received hormone therapy versus those who never took hormones [80, 124, 193]. Specifically, ten of these 12 observational studies found a reduced risk of AD in users of estrogens versus nonusers [19]. However, in most studies that initiated hormone therapy after symptoms of AD appeared, estrogens were an ineffective treatment for ameliorating cognitive decline, except in a few of the earliest cases [19, 77, 117]. Considering the positive cognitive effects of estrogens documented by these observational studies, the detrimental effects of LTED, and the possible preventative role of estrogens in postmenopausal dementia, it is no surprise that the next step was to conduct large, prospective clinical trials with HT.
The WHI Study
Since laboratory research in animals and observational studies in humans both suggested that estradiol was a neuroprotective hormone that could attenuate the increased risk of stroke and dementia associated with menopause, a large clinical trial of hormone therapy was conducted in postmenopausal women. This study, the Women's Health Initiative (WHI), was a multi-center, randomized, placebo-controlled trial that enrolled over 16,000 postmenopausal women and looked at the effect of oral HT on the incidence of stroke. However, the WHI was stopped prematurely because instead of seeing the expected decrease in the number of stroke cases in the HT-treated women, conjugated equine estrogens (CEEs) plus medroxyprogesterone acetate (MPA) seemed to increase the risk for ischemic stroke in postmenopausal women (1.8% strokes and a hazard ratio (HR) of 1.44 in CEE plus MPA users versus 1.3% in placebo) [184]. Furthermore, the Women's Health Initiative Memory Study (WHIMS), which enrolled a subset of the same women from the WHI, saw an increased risk of dementia for women over the age of 65 who were treated with oral CEEs plus MPA (66% dementia, HR 2.05) versus placebo (34% dementia) [157]. Finally, the WHIMS study also examined the effect of oral CEEs alone on cognition in the same women, and they found that the relative risk for having a significant decrease in cognition, measured by Modified Mini-Mental Status Examination score, was 1.47 in women treated with CEEs versus those treated with placebo [48]. Taken together, these findings warned against correcting the E2 deficiency in postmenopausal women through HT, citing possible severe neurological repercussions.
However, several concerns were raised about the WHI studies with regard to statistical analysis, study design, and interpretations. A very basic criticism was the choice of statistics used to interpret the findings of the WHI, the hazard ratio or relative risk. First of all, the 99% confidence interval for the WHI's hazard ratio for stroke included 1 (0.86–2.31), which is not statistically significant [35]. Additionally, upon re-calculating the WHI data, the absolute risk for ischemic stroke was only 0.08%, which is highly unlikely to be statistically significant [35]. Other experts also highlighted the fact that oral hormone use is common via oral contraceptives, and oral estrogens/progestogens are already known to incur an increased risk for blood clots and subsequent ischemic strokes, especially in women who smoke or are over the age of 35 [71, 88]. Therefore, a different route of hormone administration, such as transdermal application, could bypass the first-pass effect and make the formation of more reactive and harmful metabolites less likely in these older women [71, 88, 110]. Another suggestion was that 17β-estradiol (E2) should have been used instead of Premarin (conjugated equine estrogens or CEEs) since all previous positive laboratory results demonstrating the neuroprotective effects of estrogens used E2, not CEEs [71, 88]. They also posited that the progestogen used, Provera (medroxyprogesterone acetate, MPA) should have been administered in a cyclic fashion (regularly increasing and decreasing doses) to mimic the normal menstrual cycle, even if this might have reinstated menses as a side effect [35, 71]. The final, and quite possibly the most important, criticism of the WHI was the average age of the menopausal women enrolled, 63.3 years. Since the median age of onset of natural menopause is 51, these women were more than a decade past the onset of menopause and had already been E2-deficient for an average of 12 years before the WHI began. Therefore, the negative findings of the WHI may not be applicable to women who are currently experiencing or have recently completed the menopausal transition [71, 146].
Evidence for a Critical Period of E2 Replacement
The Critical Period Hypothesis and Healthy Cell Bias
Soon after the WHI results were published, Sherwin, Maki and others proposed the “critical period hypothesis,” which states that a precise window of opportunity exists for beneficial hormone therapy following menopause [55, 74, 104, 151–153]. This theory suggests that if hormone therapy is initiated soon after natural or surgical menopause, it may prevent cognitive decline [55, 74, 104, 151, 152]. However, if hormone therapy is initiated after a significant period of time has elapsed following menopause, outside the window of opportunity, then the beneficial effects of estradiol could be significantly attenuated [151, 152]. Brinton expanded upon this concept with the “healthy cell bias of E2 benefit,” which suggested that E2 only yields neurological benefit if it is applied to healthy neurons [20–22]. Since the health of neurons tends to deteriorate with aging, the benefits of E2 exposure in postmenopausal women wanes as the time since onset of menopause increases, until E2 eventually becomes detrimental and able to exacerbate neurological injury [20–22]. The “critical period hypothesis” and the “healthy cell bias” both could plausibly explain the surprisingly negative results of the WHI, which initiated hormone therapy in late postmenopausal women. In the sections below, we will review the basic science and clinical evidence for the critical period hypothesis.
Supporting Animal Studies
Abundant animal studies have been conducted that provide support for the critical period hypothesis, as well as mechanistic evidence for the neurological consequences of long-term E2 deprivation (LTED) (See Figure 6 for summary.) [16, 38, 57, 116]. In fact, long-term ovariectomy, a model of surgical menopause, in macaques led to a significant decline in hippocampal response to estrogens [67]. Middle-aged female rats also demonstrated improvement in working memory, a hippocampal-dependent task, when treated with E2 immediately but not 5 months after bilateral ovariectomy [39]. Chronic E2 treatment was also found to enhance attention performance in middle-aged ovariectomized rats, and LTED before E2 treatment was found to significantly attenuate this effect [13]. Likewise, E2 replacement beginning up to 15 months post ovariectomy in young adult rats was found to increase long-term potentiation and dendritic spine density at hippocampal CA3-CA1 synapses, two processes which are critical for learning and memory [164]. However, if E2 treatment was delayed 19 months after ovariectomy, E2's beneficial effect on hippocampal synaptic physiology was lost, suggesting that a critical period exists for E2 replacement [164]. Furthermore, researchers saw that 5 months of E2 deprivation abolished E2's ability to acutely regulate intrinsic membrane excitability in CA1 hippocampal neurons of rats [192]. In fact, the number of action potentials evoked and firing duration per each acute injection of E2 decreased in LTED neurons compared to control [192].
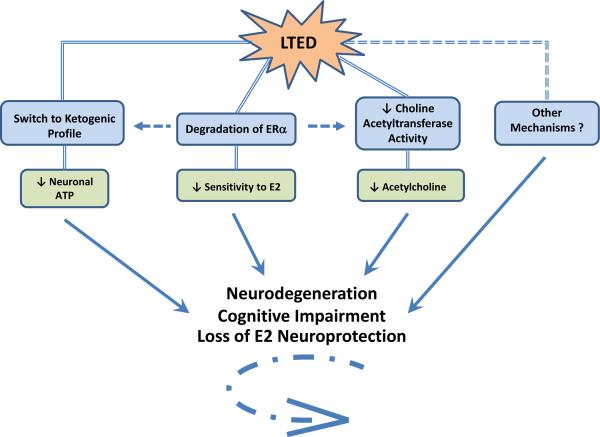
Figure 6. Neurological Consequences of Long-Term Estradiol Deprivation.
This figure summarizes the mechanisms currently thought to underlie the three major neurological consequences of long-term E2 deprivation (LTED): neurodegeneration, cognitive impairment, and loss of E2 neuroprotection. Brinton and others have shown that E2 maintains favorable mitochondrial bioenergetics in neurons, facilitating their use of glucose as the primary source of energy through oxidative phosphorylation [195]. However, during LTED, E2 is not able to serve its purpose, and neurons begin to switch their preferred energy source from glucose to ketones [195]. Ketones are a much less efficient source of energy for neurons, and their usage leads to an overall decreased amount of available ATP. Secondly, our lab recently demonstrated, for the first time, that the unbound portion of E2's cognate receptor, ERα, is degraded in the hippocampus during LTED [202]. This leads to hippocampal-specific insensitivity to E2 and loss of E2 neuroprotection against cerebral ischemia. Finally, E2's ability to enhance attentional processes, long-term potentiation, dendritic spine density, and the activity of choline acetyltransferase, the rate-limiting enzyme for synthesis of the neurotransmitter acetylcholine (ACh), is lost during LTED [12]. A lack of ACh hinders learning and memory and is closely linked with cognitive impairment and Alzheimer's disease. Additionally, there may be some cross-talk between these major events that occur during LTED, as evidenced by connecting arrows. See text for more details. E2, 17β-estradiol; ERα, Estrogen Receptor Alpha; ATP, Adenosine Triphosphate.
Additionally, E2 was able to increase choline acetyltransferase activity, which is responsible for synthesizing the neurotransmitter acetylcholine, in both the hippocampus and pre-frontal cortex, two neural areas that are critical for memory [12]. However, the same study showed that 5 months after ovariectomy E2 could no longer enhance choline acetyltransferase activity in the hippocampus [12]. Intriguingly, donepezil, an acetylcholinesterase inhibitor commonly used to treat Alzheimer's disease, was demonstrated to restore E2's enhancement of cognition after LTED via bilateral ovariectomy at 3 months of age in middle-aged (12–17 months), but not aged (22–27 months), female rats [58]. These last two studies are especially fascinating because in addition to providing support for the critical period hypothesis, they further suggest that maintaining a proper balance of acetylcholine in the basal forebrain may restore E2's ability to enhance task-specific cognitive performance.
Furthermore, Brinton and colleagues provided evidence that LTED promotes a switch to ketogenic profile in the brain [195]. Specifically, they observed that wild-type mice and transgenic mice that model Alzheimer's disease both had significant decreases in activity of mitochondrial complexes responsible for oxidative phosphorylation and significant increases in enzymes required for fatty acid formation and ketone body metabolism [67, 195]. Since these phenomena are thought to underlie Alzheimer's disease in humans, this finding could mechanistically explain the increased risk of dementia seen in postmenopausal women [195]. They also observed that LTED induced formation of mitochondrial β-amyloid and expression of mitochondrial β-amyloid-binding-alcohol-dehydrogenase, further connecting LTED with AD development [196]. Importantly, this phenotype was prevented by replacement of E2 in ovariectomized mice, which suggests that HT may alleviate the increased risk of AD development in postmenopausal women if treatment is initiated close to the onset of menopause [196].
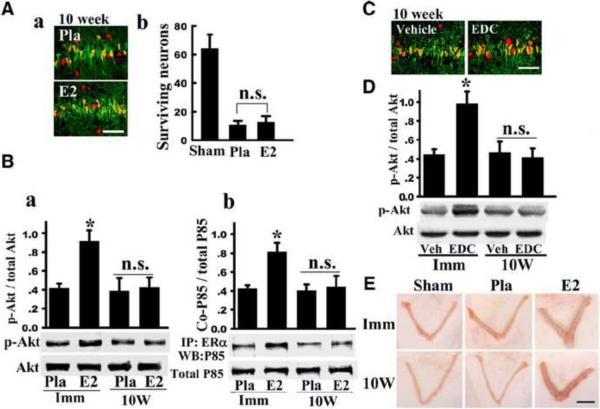
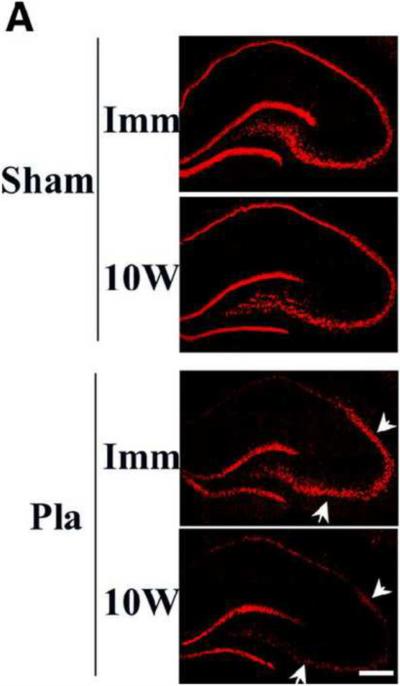
In further support of the critical period hypothesis, E2 was also found to exert profound neuroprotection against ischemic damage if it was replaced immediately but not 10 weeks after ovariectomy [172, 203]. For instance, in a recent study, we examined whether a period of LTED (10 weeks, “10W”) would lead to diminishment of E2 signaling and neuroprotective actions in the rat hippocampal CA1 region following GCI. Additionally, the trophic effect of E2 upon the uterus was examined to determine whether changes in E2 sensitivity following long-term E2 deprivation were brain-specific. As shown in Figure 7A, the ability of E2 to exert neuroprotection in the hippocampal CA1 region was lost in LTED (10W) animals as determined by NeuN and Fluoro Jade B staining and counting of number of surviving neurons [203]. Additionally, E2's ability to suppress NADPH oxidase activation and O2− production in the CA1 region at 3 hours after reperfusion was lost in LTED (10W) animals [203].
Figure 7. Loss of nongenomic signaling and neuroprotective actions of E2 in the hippocampus following long-term E2 deprivation.
(A) NeuN (red) and Fluoro-Jade B (green) staining results in hippocampal CA1 of long-term E2 deprived animals treated with E2 or Placebo (Pla). Note that E2 loses its ability to protect CA1 neurons against ischemic damage after long-term E2 deprivation. (B) Effect of long term E2 deprivation on nongenomic signaling by E2. Hippocampal CA1 protein lysate samples at 3 h reperfusion from immediate (Imm) and 10-week later (10W) E2-replaced rats were analyzed by Western blot for p-Akt and Akt levels (a). (b) Samples were IP with anti-ERα antibody and blotted with anti-P85 subunit of PI3K. An equal amount of protein was also Western blotted with anti-P85 to examine total protein expression. *P < 0.05 vs. Pla and 10-week E2 groups. (C) NeuN (red) and Fluoro-Jade B (green) staining reveals that EDC neuroprotection is lost in 10W animals. (D) EDC loses ability to activate Akt after 10-week E2 deprivation. *P < 0.05 vs. vehicle and 10-week EDC groups. n.s: no significant difference. (E) In contrast to loss of E2 neural actions after long-term E2 deprivation, the trophic effect of E2 on the uterus is not lost following 10-week E2 deprivation. Scale bar, 1 cm; 1×. Reprinted, with permission, from Journal of Neuroscience [203].
Interestingly, E2's nongenomic signaling ability was also lost in LTED animals, as evidenced by loss of E2's ability to enhance Akt phosphorylation in the CA1 region of LTED animals at 3 hours after reperfusion (Figure 7B). Also, as shown in Figure 7B, E2 enhanced ERα and PI3K P85 subunit interaction in the hippocampal CA1 of animals treated with E2 immediately after ovariectomy (Imm), but this interaction was lost in LTED animals (10W). Furthermore, examining NeuN and Fluoro Jade B staining and counting the number of surviving neurons in the CA1 revealed that the neuroprotective effect of EDC is lost in 10W animals (Figure 7C), as well as its ability to enhance Akt phosphorylation (Figure 7D). However, in contrast to the loss of E2 sensitivity observed in the hippocampus CA1 of LTED animals, the well known uterotrophic effect of E2 upon the uterus was not lost in LTED animals, indicating that loss of E2 sensitivity after LTED is tissue-specific (Figure 7E).
Furthermore, additional work by our group showed that the loss of sensitivity to E2 neuroprotection following LTED was correlated with a significant decrease in ERα, but not ERβ, in the hippocampal CA1 region following LTED [203], which could explain the decreased hippocampal sensitivity to E2's neuroprotective actions following LTED. The decrease of ERα in the hippocampal CA1 region after LTED was tissue-specific, as there was no significant decrease observed in the uterus. In follow-up studies, we sought to determine the mechanism underlying the decrease in ERα and to determine whether aging leads to a similar loss of hippocampal ERα and E2 sensitivity. The results of the study revealed that ERα in the rat hippocampal CA1 region, but not the uterus, undergoes enhanced interaction with the E3 ubiquitin ligase, CHIP (carboxyl terminus of Hsc70-interacting protein), which leads to ubiquitination and proteasomal degradation of ERα following LTED [202]. E2 treatment initiated before but not after LTED, prevented the enhanced ERα-CHIP interaction and ERα ubiquitination/degradation, and was fully neuroprotective against global cerebral ischemia (GCI). In addition, administration of a proteasomal inhibitor or CHIP antisense oligonucleotides reversed the LTED-induced down-regulation of ERα.
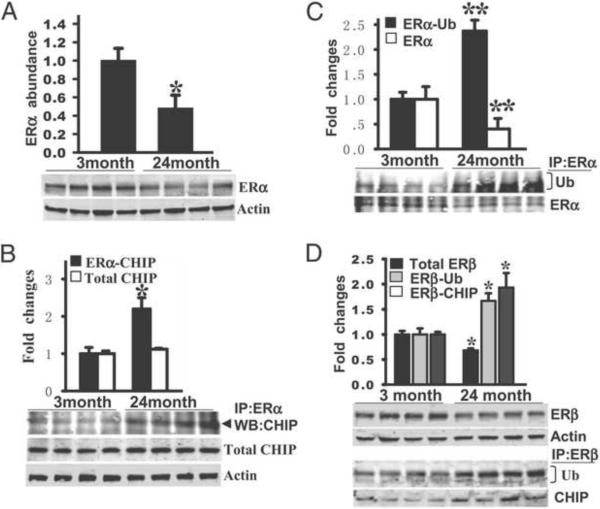
Further work showed that these observations extended to natural aging, since aged (24-month-old) female rats had decreased ERα in the hippocampal CA1 region compared to young (3-month-old) female rats (Figure 8A), enhanced CHIP interaction with ERα (Figure 8B), and ubiquitination and degradation of hippocampal ERα (Figure 8C) [202]. ERβ also demonstrated enhanced interaction with CHIP, ubiquitination and degradation in the hippocampal CA1 region of 24-month-old female rats (Figure 8D). Importantly, these events in aged rats were correlated with loss of E2 neuroprotection against GCI. Interestingly, E2-treated aged (24 month old) rats actually had a 16% increase in mortality (e.g. worse outcome), as compared to placebo controls [202]. This is consistent with the WHI results in aged humans, where neurological outcome was worse after E2 treatment. However, of significant importance, E2 administration to young (3-month-old) and middle-aged (10-month-old) rats in our study yielded strong neuroprotection of the hippocampus against cerebral ischemia [202]. These findings provide support for the critical period and healthy cell bias hypothesis for E2 neuroprotection.
Figure 8. Decreased expression of ER, and increased expression of ER ubiquitination and bindings with CHIP protein in hippocampal CA1 in aged (24 month) rats compared to young (3 month) rats.
(A) Hippocampal homogenates from young and aged F344 rats were subjected to Western blot analysis to test the expression profile of ERα protein (a, b). (B, C) Hippocampal CA1 protein samples were immunoprecipitated (IP) with anti-ERα antibody and probed with anti-CHIP, anti-ubiquitin (Ub) or anti-ERα antibody. CHIP protein expression did not change in young versus aged rats. (D) ERβ protein expression and its bindings with ubiquitin and CHIP were examined by Western blotting and co-IP analyses, respectively. All the data are shown as means ± SE from independent animals (n = 5–7) and expressed as fold vs. group of young animals. * and ** denote P < 0.05 and P < 0.001 respectively, compared to group of young animals. Adapted from [202].
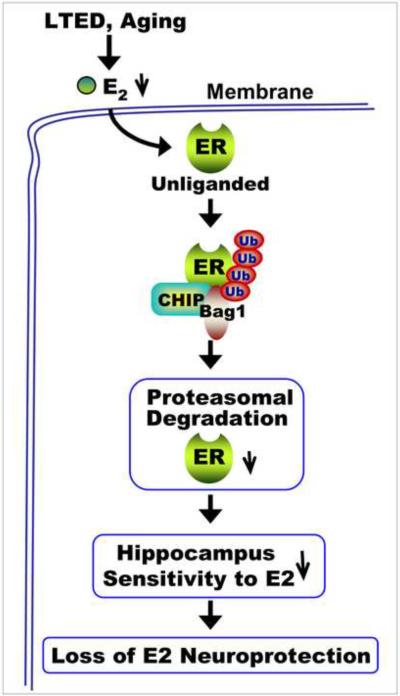
As illustrated in Figure 9, our results suggest that in periods of low circulating E2 levels, such as following long-term E2 deprivation induced by surgical menopause or natural aging, the unliganded ER in the hippocampus CA1 region displays increased binding to the ubiquitin ligase, CHIP, leading to its ubiquitination. There is also enhanced interaction of ER and CHIP with the proteasome-associated co-chaperone, Bag1, which is thought to promote delivery of ubiquitinated proteins to the proteasome for degradation. The proteasomal degradation of ER is proposed to underlie decreased sensitivity of the hippocampus to E2 and the corresponding loss of E2 neuroprotection that was observed after LTED and in aged rats. Taken as a whole, this study provides support for a “critical period” for E2 neuroprotection of the hippocampus, and provides important insight into the mechanism underlying the critical period.
Figure 9. Long-Term Estradiol Deprivation Leads to Brain-Specific Degradation of Unliganded Estrogen Receptor Alpha.
CHIP-mediated ubiquitination and proteasomal degradation of estrogen receptors (ER) in the hippocampal CA1 region occurs following long-term E2 deprivation (LTED) or natural aging. Long-term E2 deprivation or natural aging leads to an unliganded ER. The unliganded ER displays enhanced binding with the E3 ubiquitin ligase, CHIP, and the co-chaperone Bag1, leading to enhanced ubiquitination and proteasomal degradation of ER. The enhanced proteasomal degradation of ER leads to a decreased sensitivity of the hippocampal CA1 region to E2 and a correlated loss of E2 neuroprotection. Reprinted, with permission, from PNAS [202].
Finally, an additional novel observation derived from our studies was dramatically enhanced hypersensitivity of the hippocampal CA3/CA4 region to ischemic injury and neuronal cell death following long-term E2 deprivation (Figure 10) [203]. Compared to the CA1 region, the CA3/CA4 region is normally resistant to ischemic insult and not significantly damaged during GCI. However, our work showed that after LTED, the CA3/CA4 region becomes extensively damaged by the same ischemic insult that caused little to no damage in animals that have not experienced LTED. This is intriguing because long-term ovariectomy (surgical menopause) in humans has been correlated with an increased risk of cognitive decline, dementia and mortality from neurological disorders [138–141], although the mechanisms underlying these effects have remained unclear. Our study may provide a mechanistic explanation for this increased risk by demonstrating a hypersensitivity of the hippocampal CA3/CA4 to injury after prolonged hypoestrogenicity. Sham animals showed no loss of CA3/CA4 neurons after LTED, suggesting that the ability of the CA3/CA4 to withstand stress following prolonged hypoestrogenicity is severely compromised, and that E2 deprivation itself, per se, does not cause loss of CA3/CA4 neurons. Of significant interest, E2 treatment begun after prolonged hypoestrogenicity did not prevent the induction of the CA3/CA4 hypersensitivity, which may be due to a loss of E2 sensitivity of this region, as was shown in the CA1 region. These findings suggest that LTED can increase sensitivity of the hippocampus to ischemic stress, leading to enhanced damage of hippocampal neurons. The mechanism(s) underlying this hippocampal hypersensitivity to ischemic stress following LTED is unknown and is an area of active investigation by our laboratory.
Figure 10. Long-term E2 deprivation leads to hypersensitivity of CA3/CA4 neurons to ischemic cell death following 10 min of global cerebral ischemia, and E2 replacement is incapable of exerting neuroprotection or reversing the CA3/CA4 hypersensitivity to ischemic damage.
Typical microscopy images showing NeuN staining in hippocampus from Sham or Placebo (Pla)-treated Imm (immediate) or 10W (10 week) ovariectomized rats. Note the increased sensitivity of the CA3/CA4 region in Pla 10W animals to neuronal cell death, as evidenced by loss of NeuN staining. Magnification is 5× and the scale bar = 200 μm. Adapted from [203].
Altogether, the aforementioned animal studies provide support for the critical period hypothesis and could mechanistically explain why the WHI found that hormone therapy was ineffective in preventing stroke and dementia when given to women who were more than a decade past the onset of menopause. They also provide a potential mechanistic understanding to some of the negative neurological outcomes associated with LTED (surgical menopause) in humans, such as increased risk of cognitive decline, dementia, and mortality due to neurodegenerative disorders.
Supporting Clinical Studies / Clinical Trials Attempting to Address Criticisms of the WHI
Following the results of the WHI, a number of clinical trials were and are still being conducted in an attempt to test the critical period hypothesis and address the aforementioned concerns regarding the WHI study (See Table 1 for summary). The REMEMBER pilot study provided support for the critical period hypothesis by assessing cognitive function in 428 women aged 60 or older who initiated systemic hormone therapy within five years of natural or surgical menopause (hysterectomy plus bilateral oophorectomy) [101]. Although, they recommended using a larger sample size in the future, the REMEMBER researchers found that women who initiated HT early performed significantly better on cognitive tests than women who initiated HT late or never used HT at all, suggesting that timing of E2 replacement, with regard to menopause, is critical for neurological benefit [101]. Along these lines, a larger population-based study was conducted to further assess the effect of HT timing on prevalence of dementia in postmenopausal women. Whitmer et al. found that, at least in middle-aged women using the Kaiser Permanente Medical Care Program of Northern California (KPNC) between 1964 and 1973, early menopausal HT was associated with a 26% decreased risk of developing dementia [187]. However, in the same set of women, late menopausal HT was associated with a 48% increased risk of dementia [187], suggesting that a critical window exists for beneficial E2 replacement after menopause, with respect to prevention of dementia.
Table 1.
| Study Type / # of Subjects | Women Studied | Type of HT | Outcome | |
|---|---|---|---|---|
| WHI [184] | RCT: 16,608 | Late Menopausal | Oral CEEs +/− MPA | ↑ Stroke |
| WHIMS [36, 48, 157] | RCT – Subsets of WHI: 7,340 total | Late Menopausal | Oral CEEs +/− MPA | CEEs + MPA ↑ dementia. CEEs alone ↓ cognition. |
| WHIMS-MRI [36, 37] | RCT - Subset of WHIMS: 1,403 | Late Menopausal | Oral CEEs +/− MPA | NO ↑ in volume of ischemic brain lesions, but ↓ average volume of hippocampus and frontal lobe. |
| WHISCA [36, 136] | RCT - Subset of WHI/WHIMS: 2,302 | Late Menopausal | Oral CEEs +/− MPA | CEEs + MPA ↓ verbal memory. CEEs alone ↓ spatial rotational ability. |
| HERS and HERS II [63–65, 83] | RCT: 2,763 | Late Menopausal | Oral CEEs + MPA | HT ↑ VTE risk and does NOT ↓ CHD or ↑ cognition in women with existing CAD. |
| WHI 10-year Follow-Up [93] | RCT: 10,739 | Early and Late Menopausal | Oral CEEs Alone (No Progestogen) | NO ↑ risk of CHD, DVT, stroke or mortality. Early HT ↓ risk of CHD and MI. |
| REMEMBER Pilot [101] | Cohort: 428 | >60 Years of Age | Multiple | Early HT ↑ cognition. Late HT ↓ cognition. |
| KPNC [187] | Cohort: 5,504 | Early and Late Menopausal | Multiple | Early HT ↓ dementia. Late HT ↓ dementia. |
| UK GPRD [135] | Nested Case-Control: 75,668 | 50–79 Years of Age | Multiple | Low-dose transdermal HT does NOT ↑ stroke, but High-dose and oral HT ↑ stroke. |
| ESTHER [25] | Case Control: 881 | Early and Late Menopausal | Oral CEEs and Transdermal E2 | Oral HT and norpregnanes ↑ risk of VTE. |
| E3N [24] | Cohort: 80,308 | Early and Late Menopausal | Oral CEEs and Transdermal E2 | Transdermal HT does NOT ↑ risk of VTE. |
| WISE [155] | Cohort: 654 | Early and Late Menopausal; Naturally and Surgically Menopausal | Multiple | Early HT ↓ CAD in natural menopause only. |
| KEEPS [70, 113] | RCT | Early Menopausal | Oral CEEs and Transdermal E2 | Ongoing |
| ELITE [75, 76, 110] | RCT | Early and Late Menopausal | Oral and Transdermal E2 | Ongoing |
Dumas et al. conducted a small, randomized, placebo-controlled trial of oral E2 (17β-estradiol) to test the effect of E2 on cognition in younger (50–62) versus older (70–81) postmenopausal women [46]. Intriguingly, they showed that pre-treatment with oral E2 attenuated anti-cholinergic drug-induced deficits of episodic memory in the younger postmenopausal women but, conversely, further hindered the older postmenopausal women [46]. This critical observation provides supports that E2 may yield neurological benefit if administered within 10 years of menopause but may also exacerbate cognitive deficits if administered more than 20 years after the onset of menopause. Additionally, the Women's Ischemia Syndrome Evaluation study (WISE) examined the timing of hormone therapy with respect to cardiovascular disease in postmenopausal women having coronary angiography performed during evaluation for ischemia. They found that naturally menopausal women who initiated HT before the age of 55 had less severe coronary artery disease than never users, and this effect was not observed in naturally menopausal women who initiated HT at or after the age of 55 [155], another result consistent with the critical period hypothesis. Furthermore, WISE researchers suggested that these conclusions were only valid in the event that the HT user was healthy with little or no pre-existing coronary artery atherosclerosis, which is also consistent with the healthy cell bias of E2 replacement [155].
Along these lines, Rocca's recent meta-analyses of clinical studies concerning HT and cognitive aging provided clinical evidence supporting the critical period hypothesis, since the majority of included clinical studies demonstrated neuroprotection in women who underwent treatment with estrogens perimenopausally (50–60 years of age) [139, 140]. Conversely, these meta-analyses also revealed that studies on women who initiated hormone therapy in late menopause (65–79 years of age) demonstrated an increased risk of dementia and cognitive decline [139, 140]. Considering this data, Rocca suggested that the neuroprotective effects of estrogens depend on age at initiation of HT, type of menopause (natural versus surgical) and stage of menopause, and he further recommended that women who experience premature menopause, either naturally or surgically via bilateral oophorectomy, should be treated as ideal candidates for HT until around the age of 51 [140].
It is also important to note that recently, a 10-year follow-up of the WHI's “estrogen-alone trial,” which treated hysterectomized postmenopausal women with unopposed oral CEEs for a median of 5.9 years, was published. Intriguingly, the study showed no increase in the risk of cardiovascular disease, stroke, hip fracture, colon cancer, or death and a decreased risk for breast cancer in these women either during or after treatment with oral CEEs [93]. Furthermore, upon examination of results by age group, oral CEEs appeared to provide significant benefit to women aged 50–59. These hormone-treated perimenopausal women experienced a significantly decreased risk of coronary heart disease (approximately 41% decrease) (HR 0.59, 95% CI 0.38–0.90), myocardial infarction (approximately 46% decrease) (HR 0.54, 95% CI 0.34–0.86), and total mortality (HR 0.73, 95% CI 0.53–1.00) versus those treated with placebo [93]. These benefits were not observed in older women aged 60 – 69 or 70 – 79. Perimenopausal hysterectomized women treated with unopposed oral CEEs also had relatively neutral risks for deep vein thrombosis, pulmonary embolism, stroke, invasive breast cancer, hip fracture, and colon cancer compared to placebo-treated women [93]. Most importantly, the WHI ten-year follow-up study supports the critical period hypothesis, because the global index of chronic diseases was decreased in women aged 50–59 (HR 0.85, 95% CI 0.70–1.03), neutral in women aged 60–69 (HR 1.00, 95% CI 0.89–1.13) and elevated in women aged 70–79 (HR 1.15, 95% CI 1.01–1.32) [93]. Therefore, the WHI's estrogen alone trial suggests that HT may provide benefit in perimenopausal women aged 50–59, but this benefit may wane significantly as the time since the onset of menopause increases, until the risks of HT significantly outweigh the benefits.
Finally, there are several ongoing studies concerning the critical period hypothesis, the results of which the scientific community is eagerly awaiting. First is the KRONOS Early Estrogen Prevention Study (KEEPS), which is an ongoing multi-center, placebo-controlled, randomized clinical trial for HT that is restricted to women who are within 3 years of menopause onset [113]. In addition to testing the critical period hypothesis, KEEPS will also vary HT regimens to include either low-dose CEEs or transdermal 17β-estradiol (E2) opposed by oral, cyclic, micronized progesterone and measure cardiovascular disease progression via carotid artery intimal-medial thickness and coronary artery calcium build-up in postmenopausal women [113]. Second is the Early Versus Late Intervention with Estradiol trial (ELITE), which is an ongoing clinical trial that aims to measure the progression of atherosclerosis in postmenopausal women. These researchers plan to focus on the timing of postmenopausal HT initiation, using oral E2 instead of CEEs, in women who are either less than 6 years (early) or greater than 10 years (late) from the onset of menopause [76, 110]. It is hoped that the results of these two trials will provide more clarity with regard to the timing of HT initiation after menopause and its benefits.
While all of the aforementioned clinical trials focused on the timing of HT in postmenopausal women, other clinical trials attempted to address the remaining concerns of the WHI, such as the type of estrogen/progestogen included and the route of HT administration. The ESTHER study was a multi-center case-control study that measured occurrence of venous thromboembolism (VTE), a pro-thrombotic disease that could contribute to ischemic strokes, in women using either oral or transdermal HT. They found that users of oral estrogens were 4-times more likely to have a VTE than HT nonusers (OR 4.2; 95% CI 1.5–11.6), whereas transdermal E2 users did not demonstrate any increased risk for VTE, compared to nonusers (OR 0.9; 95% CI 0.4–2.1) [25]. These data were confirmed in the E3N study, which followed a huge prospective cohort of menopausal French women for an average of 10 years, documenting HT use and the incidence of first idiopathic venous thromboembolism. The E3N study found that past HT users did not incur a significantly increased risk of VTE compared to never-users [24]. They also demonstrated that oral CEEs were associated with an increased risk of VTE, but this increased risk was not observed when transdermal E2 was used instead of oral CEEs [24]. Finally, both the ESTHER study and the E3N determined that oral norpregnanes, but not progesterone, pregnanes, or nortestosterones were associated with an increased risk of VTE [24, 25]. Together, these results could explain why the WHI, which used oral CEEs in late postmenopausal women, saw an increased risk of ischemic stroke and dementia.
Transdermal Estradiol and NeuroSERMs: Menopausal Miracle Drugs?
Previous work has shown that orally administered estrogens lead to a hepatic concentration of E2 that is 4 to 5 fold higher than serum levels of E2 due to the first-pass effect, which is the absorption and transport of oral drugs from the gut to the liver [110]. This supraphysiological dose of E2 subsequently leads to hepatic elevation of harmful metabolites such as inflammatory C-reactive protein, serum Amyloid A, prothrombin fragments, and atherosclerotic plaque-disrupting enzymes [110]. Considering this effect, it is no surprise that oral CEEs are associated with increased risks for VTE and ischemic stroke. However, since transdermal E2 bypasses the gut and liver entirely, transdermal HT seems to be a logical, intuitive, and safer option for menopausal women [27, 92, 167, 175]. In fact, as mentioned earlier, the ESTHER study observed a 4-fold increased risk of VTE in oral HT users versus nonusers but no increased risk of VTE in transdermal HT users [25]. Furthermore, a recent nested case control study using the UK's General Practice Research Database demonstrated that low-dose (50 micrograms or less), but not high dose, E2 patches did not increase the risk of stroke in postmenopausal women (rate ratio 0.81, 95% CI 0.62–1.05) [135]. The same study also observed that current transdermal E2 use did not carry an increased risk of stroke in current users versus nonusers (adjusted rate ratio 0.95, 95% CI 0.75–1.20) [135]. Conversely, in alignment with the WHI, the same study showed that users of oral estrogens did demonstrate an increased risk of stroke versus nonusers (rate ratio 1.28, 95% CI 1.15–1.42), equating to 0.8 additional strokes per 1000 women per year [135, 167]. It should be mentioned that this observational study included over 400 general practices in the UK and over 75,000 women, avoiding both recall bias and selection bias in the process [167].
It is also worth mentioning that transdermal E2 has been commonly prescribed for postmenopausal women in Europe for decades, but oral estrogens remain the mainstay of treatment in the U.S. [27]. Transdermal E2 should provide all the benefits of hormone therapy without elevating cardiovascular or neurological disease risk, in accordance with supporting laboratory and clinical E2 cardioprotection and neuroprotection studies. However, while it is true that physicians should consider transdermal E2 as a safer option for postmenopausal HT, with respect to the heart and brain, it is important that clinicians keep in mind other potential risks of steroid hormone treatment, such as the development of cancer [195]. Along these lines, much effort is still needed in the area of drug discovery for safer postmenopausal HT. The ultimate goal is to create a compound that will maintain the neuroprotective and cardioprotective effects of E2 but circumvent the serious potential risks associated with long-term steroid hormone therapy. Simpkins and Brinton suggest a class of compounds known as neuroSERMs (selective estrogen receptor modifiers), which can selectively traverse the blood-brain barrier and exert neuroprotection without acting on peripheral tissues or causing unwanted estrogenic side-effects [159]. Intriguingly, this idea is quite plausible, with the potential to benefit millions through prevention of cerebrovascular disease and associated cognitive decline.
Summary and Conclusion
In summary, 17β-estradiol is an endogenous steroid hormone produced primarily by the ovaries, which has the ability to attenuate damage from cerebral ischemia and delay onset of cognitive decline in both animals and humans [19]. Furthermore, high levels of serum E2 can partially explain women's relative protection from heart attacks, strokes, and neurodegenerative diseases until late in life [19, 98, 118, 121, 143, 173]. Unfortunately, during natural reproductive senescence and surgical menopause induced by bilateral oophorectomy, women experience a dramatic reduction in circulating E2 levels. This phenomenon leads to vasomotor and psychological menopausal symptoms and a subclinical, but significant, increased risk of osteoporosis, cardiovascular disease, cerebrovascular disease, and dementia [38, 78, 115, 125, 141, 158]. To avert bothersome symptoms and prevent an increased risk of disease in postmenopausal women, the logical solution is to compensate for the ovaries' inability to produce E2 after menopause with hormone therapy. Despite overwhelming evidence of the neuroprotective and cardioprotective functions of estradiol and the dire consequences of long-term E2 deprivation, the Women's Health Initiative determined that oral HT (CEEs with or without MPA) led to a significantly increased risk of stroke and dementia [48, 157, 184]. However, the route of drug administration, type of estrogen (CEEs vs. E2) and progestogen utilized, and the average age of WHI participants were heavily criticized [35, 71, 88, 110].
In the wake of the controversy spurred by the WHI, the “critical period hypothesis” and the “healthy cell bias of E2 replacement” were proposed [20, 22, 55, 74, 104, 151–153]. Both of these hypotheses suggested that the timing of hormone therapy, in regard to the onset of menopause, is crucial. When administered in the perimenopausal period, while women are theoretically still healthy, E2 could provide neurological, and cardiovascular benefit [20, 22, 151, 152]. However, if hormone therapy is delayed after menopause and a period of long-term E2 deprivation, such as 10 years, is allowed to occur before treatment, estradiol may become detrimental and pose undue harm to these less healthy women via an exaggerated risk of venous thromboembolism, ischemic stroke and dementia [20, 22, 146]. Intriguingly, both of these hypotheses are well supported by studies in animals and humans, although the scientific community is still awaiting the results of key clinical trials, such as ELITE and KEEPS, in order to confirm whether perimenopausal HT is safer than postmenopausal HT in humans [38, 116, 139, 140]. Furthermore, both the Endocrine Society's scientific statement concerning menopausal HT and the North American Menopause Society's current clinical guidelines acknowledge the “critical period hypothesis” and its supporting studies by recommending initiation of HT as close to the onset of menopause as possible and using the lowest effective dose of hormones [1, 146]. This knowledge, along with the hypothesized safety and efficacy of low-dose transdermal E2 and neuroSERMs, provide hope that future E2-based hormone therapy will alleviate the bothersome symptoms of menopause, attenuate postmenopausal risk of cardiovascular and cerebrovascular disease, and avert cognitive decline in the aging woman. Finally, as shown in Box 1, there are many unresolved issues that require further study for continued advancement of the field and resolution of existing controversies. Addressing these complex issues from both basic science and clinical perspectives should ensure continued progress and provide much-needed answers for the field.
Box 1. Outstanding Questions in the Field.
This figure lists current outstanding questions in the fields of E2 neuroprotection and hormone therapy (HT). Each of these questions deserves more study both in the laboratory and in the clinical arena. LTED, Long-Term 17β-Estradiol Deprivation.
Box 1
-
■
What genes are altered in the event of LTED, and to what extent do genetics play a role in LTED and its neurological consequences?
-
■
What is the most safe and effective option for HT in perimenopausal women?
-
■
How long should we treat perimenopausal women with HT to avert increased risk of disease and cognitive decline?
-
■
How can we attenuate cardiovascular and neurological disease risk in women who have already experienced LTED?
>Estrogen is neuroprotective against cerebral ischemia. >extranuclear and nuclear estrogen receptors mediate neuroprotection. >long-term estrogen deprivation increases risk for dementia/neurological disorders. >a critical period exists for estrogen neuroprotective effects.
Acknowledgements
The authors' research presented in this review was supported by a research grant to DWB from the NINDS (NS050730), National Institutes of Health, United States of America, and a Scientist Development Research Grant to QZ from the American Heart Association.
ABBREVIATIONS
- AD
Alzheimer's disease
- Aβ
Beta-Amyloid
- CEEs
Conjugated Equine Estrogens
- CNS
Central Nervous System
- E2
17β-Estradiol
- EDC
Estrogen Dendrimer Conjugate
- ER
Estrogen Receptor
- ERα
Estrogen Receptor Alpha
- ERβ
Estrogen Receptor Beta
- FCI
Focal Cerebral Ischemia
- GCI
Global Cerebral Ischemia
- HT
Hormone Therapy
- ICV
Intracerebroventricular
- LTED
Long-Term Estrogen Deprivation
- KO
Knockout
- MCAO
Middle Cerebral Artery Occlusion
- MCI
Mild Cognitive Impairment
- NADPH Oxidase
Nicotinamide Adenine Dinucleotide Phosphate Oxidase
- NFTs
Neurofibrillary Tangles of hyperphosphorylated tau
- PHF
Paired Helical Filaments
- ROS
Reactive Oxygen Species
- TBI
Traumatic Brain Injury
- WHI
Women's Health Initiative
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17:242–255. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- [2].Almeida M, Martin-Millan M, Ambrogini E, Bradsher R, 3rd, Han L, Chen XD, Roberson PK, Weinstein RS, O'Brien CA, Jilka RL, Manolagas SC. Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA-binding-independent actions of the ERalpha. J. Bone Miner. Res. 2010;25:769–781. doi: 10.1359/jbmr.091017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40:1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- [4].Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: Implications for neuroprotection. Biochim. Biophys. Acta. 2010;1800:1106–1112. doi: 10.1016/j.bbagen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [5].Arnold S, Beyer C. Neuroprotection by estrogen in the brain: the mitochondrial compartment as presumed therapeutic target. J. Neurochem. 2009;110:1–11. doi: 10.1111/j.1471-4159.2009.06133.x. [DOI] [PubMed] [Google Scholar]
- [6].Azcoitia I, Santos-Galindo M, Arevalo MA, Garcia-Segura LM. Role of astroglia in the neuroplastic and neuroprotective actions of estradiol. Eur. J. Neurosci. 2010;32:1995–2002. doi: 10.1111/j.1460-9568.2010.07516.x. [DOI] [PubMed] [Google Scholar]
- [7].Bao YJ, Li LZ, Li XG, Wang YJ. 17Beta-estradiol differentially protects cortical pericontusional zone from programmed cell death after traumatic cerebral contusion at distinct stages via non-genomic and genomic pathways. Mol. Cell. Neurosci. 2011 doi: 10.1016/j.mcn.2011.07.004. [DOI] [PubMed] [Google Scholar]
- [8].Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- [9].Bekinschtein P, Cammarota M, Izquierdo I, Medina JH. BDNF and memory formation and storage. The Neurosci. 2008;14:147–156. doi: 10.1177/1073858407305850. [DOI] [PubMed] [Google Scholar]
- [10].Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH. BDNF is essential to promote persistence of long-term memory storage. Proc. Natl. Acad. Sci. U S A. 2008;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bingham D, Macrae IM, Carswell HV. Detrimental effects of 17beta-oestradiol after permanent middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 2005;25:414–420. doi: 10.1038/sj.jcbfm.9600031. [DOI] [PubMed] [Google Scholar]
- [12].Bohacek J, Bearl AM, Daniel JM. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol. 2008;20:1023–1027. doi: 10.1111/j.1365-2826.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- [13].Bohacek J, Daniel JM. The beneficial effects of estradiol on attentional processes are dependent on timing of treatment initiation following ovariectomy in middle-aged rats. Psychoneuroendocrinology. 2010;35:694–705. doi: 10.1016/j.psyneuen.2009.10.010. [DOI] [PubMed] [Google Scholar]
- [14].Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat. Chem. Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- [15].Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. J. Neurosci. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Boulware MI, Kent BA, Frick KM. The Impact of Age-Related Ovarian Hormone Loss on Cognitive and Neural Function. Curr. Top. Behav. Neurosci. 2011 doi: 10.1007/7854_2011_122. [DOI] [PubMed] [Google Scholar]
- [17].Bourque M, Dluzen DE, Di Paolo T. Neuroprotective actions of sex steroids in Parkinson's disease. Front. Neuroendocrinol. 2009;30:142–157. doi: 10.1016/j.yfrne.2009.04.014. [DOI] [PubMed] [Google Scholar]
- [18].Brait VH, Jackman KA, Walduck AK, Selemidis S, Diep H, Mast AE, Guida E, Broughton BR, Drummond GR, Sobey CG. Mechanisms contributing to cerebral infarct size after stroke: gender, reperfusion, T lymphocytes, and Nox2-derived superoxide. J. Cereb. Blood Flow Metab. 2010;30:1306–1317. doi: 10.1038/jcbfm.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- [21].Brinton RD. Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer's disease. Adv Drug Deliv Rev. 2008;60:1504–1511. doi: 10.1016/j.addr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bryant DN, Bosch MA, Ronnekleiv OK, Dorsa DM. 17-Beta estradiol rapidly enhances extracellular signal-regulated kinase 2 phosphorylation in the rat brain. Neuroscience. 2005;133:343–352. doi: 10.1016/j.neuroscience.2005.02.024. [DOI] [PubMed] [Google Scholar]
- [24].Canonico M, Fournier A, Carcaillon L, Olie V, Plu-Bureau G, Oger E, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F, Scarabin PY. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30:340–345. doi: 10.1161/ATVBAHA.109.196022. [DOI] [PubMed] [Google Scholar]
- [25].Canonico M, Oger E, Plu-Bureau G, Conard J, Meyer G, Levesque H, Trillot N, Barrellier MT, Wahl D, Emmerich J, Scarabin PY. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840–845. doi: 10.1161/CIRCULATIONAHA.106.642280. [DOI] [PubMed] [Google Scholar]
- [26].Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogens and insulin-like growth factor-I in the brain: implications for neuroprotection. Brain Res. Brain Res. Rev. 2001;37:320–334. doi: 10.1016/s0165-0173(01)00137-0. [DOI] [PubMed] [Google Scholar]
- [27].Carroll N. A review of transdermal nonpatch estrogen therapy for the management of menopausal symptoms. J Womens Health (Larchmt) 2010;19:47–55. doi: 10.1089/jwh.2008.1206. [DOI] [PubMed] [Google Scholar]
- [28].Carswell HV, Bingham D, Wallace K, Nilsen M, Graham DI, Dominiczak AF, Macrae IM. Differential effects of 17beta-estradiol upon stroke damage in stroke prone and normotensive rats. J. Cereb. Blood Flow Metab. 2004;24:298–304. doi: 10.1097/01.WCB.0000112322.75217.FD. [DOI] [PubMed] [Google Scholar]
- [29].Carswell HV, Macrae IM, Gallagher L, Harrop E, Horsburgh KJ. Neuroprotection by a selective estrogen receptor beta agonist in a mouse model of global ischemia. Am J Physiol Heart Circ Physiol. 2004;287:H1501–1504. doi: 10.1152/ajpheart.00227.2004. [DOI] [PubMed] [Google Scholar]
- [30].Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- [31].Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- [32].Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid. Redox Signaling. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- [34].Choi YC, Lee JH, Hong KW, Lee KS. 17 Beta-estradiol prevents focal cerebral ischemic damages via activation of Akt and CREB in association with reduced PTEN phosphorylation in rats. Fundam Clin Pharmacol. 2004;18:547–557. doi: 10.1111/j.1472-8206.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- [35].Clark JH. A critique of Women's Health Initiative Studies (2002-2006) Nucl Recept Signal. 2006;4:e023. doi: 10.1621/nrs.04023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Coker LH, Espeland MA, Rapp SR, Legault C, Resnick SM, Hogan P, Gaussoin S, Dailey M, Shumaker SA. Postmenopausal hormone therapy and cognitive outcomes: the Women's Health Initiative Memory Study (WHIMS) J Steroid Biochem Mol Biol. 2010;118:304–310. doi: 10.1016/j.jsbmb.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Coker LH, Hogan PE, Bryan NR, Kuller LH, Margolis KL, Bettermann K, Wallace RB, Lao Z, Freeman R, Stefanick ML, Shumaker SA. Postmenopausal hormone therapy and subclinical cerebrovascular disease: the WHIMS-MRI Study. Neurology. 2009;72:125–134. doi: 10.1212/01.wnl.0000339036.88842.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Daniel JM, Bohacek J. The critical period hypothesis of estrogen effects on cognition: Insights from basic research. Biochim Biophys Acta. 2010;1800:1068–1076. doi: 10.1016/j.bbagen.2010.01.007. [DOI] [PubMed] [Google Scholar]
- [39].Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- [40].Dhandapani KM, Brann DW. Role of astrocytes in estrogen-mediated neuroprotection. Exp. Gerontol. 2007;42:70–75. doi: 10.1016/j.exger.2006.06.032. [DOI] [PubMed] [Google Scholar]
- [41].Dhandapani KM, Wade FM, Mahesh VB, Brann DW. Astrocyte-derived transforming growth factor-{beta} mediates the neuroprotective effects of 17{beta}-estradiol: involvement of nonclassical genomic signaling pathways. Endocrinology. 2005;146:2749–2759. doi: 10.1210/en.2005-0014. [DOI] [PubMed] [Google Scholar]
- [42].Dominguez R, Liu R, Baudry M. 17-Beta-estradiol-mediated activation of extracellular-signal regulated kinase, phosphatidylinositol 3-kinase/protein kinase B-Akt and N-methyl-D-aspartate receptor phosphorylation in cortical synaptoneurosomes. J. Neurochem. 2007;101:232–240. doi: 10.1111/j.1471-4159.2006.04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- [44].Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J Neurosci. 1999;19:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. Estradiol interacts with the cholinergic system to affect verbal memory in postmenopausal women: evidence for the critical period hypothesis. Horm Behav. 2008;53:159–169. doi: 10.1016/j.yhbeh.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Elzer JG, Muhammad S, Wintermantel TM, Regnier-Vigouroux A, Ludwig J, Schutz G, Schwaninger M. Neuronal estrogen receptor-alpha mediates neuroprotection by 17beta-estradiol. J Cereb Blood Flow Metab. 2010;30:935–942. doi: 10.1038/jcbfm.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- [49].Facecchia K, Fochesato LA, Ray SD, Stohs SJ, Pandey S. Oxidative toxicity in neurodegenerative diseases: role of mitochondrial dysfunction and therapeutic strategies. J. Toxicol. 2011;2011:683728. doi: 10.1155/2011/683728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Fujimura M, Morita-Fujimura Y, Kawase M, Copin JC, Calagui B, Epstein CJ, Chan PH. Manganese superoxide dismutase mediates the early release of mitochondrial cytochrome C and subsequent DNA fragmentation after permanent focal cerebral ischemia in mice. J. Neurosci. 1999;19:3414–3422. doi: 10.1523/JNEUROSCI.19-09-03414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem. Biophys. Res. Comm. 2006;346:904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- [52].Galbiati M, Magnaghi V, Martini L, Melcangi RC. Hypothalamic transforming growth factor beta1 and basic fibroblast growth factor mRNA expression is modified during the rat oestrous cycle. J. Neuroendocrinol. 2001;13:483–489. doi: 10.1046/j.1365-2826.2001.00659.x. [DOI] [PubMed] [Google Scholar]
- [53].Garcia-Ovejero D, Azcoitia I, Doncarlos LL, Melcangi RC, Garcia-Segura LM. Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res. Brain Res. Rev. 2005;48:273–286. doi: 10.1016/j.brainresrev.2004.12.018. [DOI] [PubMed] [Google Scholar]
- [54].Garcia-Segura LM, Arevalo MA, Azcoitia I. Interactions of estradiol and insulin-like growth factor-I signalling in the nervous system: new advances. Prog. Brain Res. 2010;181:251–272. doi: 10.1016/S0079-6123(08)81014-X. [DOI] [PubMed] [Google Scholar]
- [55].Genazzani AR, Pluchino N, Luisi S, Luisi M. Estrogen, cognition and female ageing. Hum. Reprod. Update. 2007;13:175–187. doi: 10.1093/humupd/dml042. [DOI] [PubMed] [Google Scholar]
- [56].Genovese T, Mazzon E, Paterniti I, Esposito E, Bramanti P, Cuzzocrea S. Modulation of NADPH oxidase activation in cerebral ischemia/reperfusion injury in rats. Brain Res. 2011;1372:92–102. doi: 10.1016/j.brainres.2010.11.088. [DOI] [PubMed] [Google Scholar]
- [57].Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr. Rev. 2010;31:224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gibbs RB, Mauk R, Nelson D, Johnson DA. Donepezil treatment restores the ability of estradiol to enhance cognitive performance in aged rats: evidence for the cholinergic basis of the critical period hypothesis. Horm. Behav. 2009;56:73–83. doi: 10.1016/j.yhbeh.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gibson CL, Gray LJ, Murphy SP, Bath PM. Estrogens and experimental ischemic stroke: a systematic review. J. Cereb. Blood Flow Metab. 2006;26:1103–1113. doi: 10.1038/sj.jcbfm.9600270. [DOI] [PubMed] [Google Scholar]
- [60].Gingerich S, Kim GL, Chalmers JA, Koletar MM, Wang X, Wang Y, Belsham DD. Estrogen receptor alpha and G-protein coupled receptor 30 mediate the neuroprotective effects of 17beta-estradiol in novel murine hippocampal cell models. Neuroscience. 2010;170:54–66. doi: 10.1016/j.neuroscience.2010.06.076. [DOI] [PubMed] [Google Scholar]
- [61].Goodenough S, Schleusner D, Pietrzik C, Skutella T, Behl C. Glycogen synthase kinase 3beta links neuroprotection by 17beta-estradiol to key Alzheimer processes. Neuroscience. 2005;132:581–589. doi: 10.1016/j.neuroscience.2004.12.029. [DOI] [PubMed] [Google Scholar]
- [62].Gordon KB, Macrae IM, Carswell HV. Effects of 17beta-oestradiol on cerebral ischaemic damage and lipid peroxidation. Brain Res. 2005;1036:155–162. doi: 10.1016/j.brainres.2004.12.052. [DOI] [PubMed] [Google Scholar]
- [63].Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- [64].Grady D, Wenger NK, Herrington D, Khan S, Furberg C, Hunninghake D, Vittinghoff E, Hulley S. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. The Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2000;132:689–696. doi: 10.7326/0003-4819-132-9-200005020-00002. [DOI] [PubMed] [Google Scholar]
- [65].Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett-Connor E. Effect of postmenopausal hormone therapy on cognitive function: the Heart and Estrogen/progestin Replacement Study. Am J Med. 2002;113:543–548. doi: 10.1016/s0002-9343(02)01270-6. [DOI] [PubMed] [Google Scholar]
- [66].Guerra B, Diaz M, Alonso R, Marin R. Plasma membrane oestrogen receptor mediates neuroprotection against beta-amyloid toxicity through activation of Raf-1/MEK/ERK cascade in septal-derived cholinergic SN56 cells. J. Neurochem. 2004;91:99–109. doi: 10.1111/j.1471-4159.2004.02695.x. [DOI] [PubMed] [Google Scholar]
- [67].Hamilton RT, Rettberg JR, Mao Z, To J, Zhao L, Appt SE, Register TC, Kaplan JR, Brinton RD. Hippocampal responsiveness to 17beta-estradiol and equol after long-term ovariectomy: implication for a therapeutic window of opportunity. Brain Res. 2011;1379:11–22. doi: 10.1016/j.brainres.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hammond R, Gibbs RB. GPR30 is positioned to mediate estrogen effects on basal forebrain cholinergic neurons and cognitive performance. Brain Res. 2011;1379:53–60. doi: 10.1016/j.brainres.2010.11.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hammond R, Nelson D, Gibbs RB. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology. 2011;36:182–192. doi: 10.1016/j.psyneuen.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Miller VM, Naftolin F, Santoro N. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:3–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- [71].Harman SM, Brinton EA, Clarkson T, Heward CB, Hecht HS, Karas RH, Judelson DR, Naftolin F. Is the WHI relevant to HRT started in the perimenopause? Endocrine. 2004;24:195–202. doi: 10.1385/ENDO:24:3:195. [DOI] [PubMed] [Google Scholar]
- [72].Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol. Endocrinol. 2006;20:491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- [73].Harukuni I, Hurn PD, Crain BJ. Deleterious effect of beta-estradiol in a rat model of transient forebrain ischemia. Brain Res. 2001;900:137–142. doi: 10.1016/s0006-8993(01)02278-8. [DOI] [PubMed] [Google Scholar]
- [74].Henderson VW. Alzheimer's disease and other neurological disorders. Climacteric. 2007;10(Suppl 2):92–96. doi: 10.1080/13697130701534097. [DOI] [PubMed] [Google Scholar]
- [75].Henderson VW. Estrogens, episodic memory, and Alzheimer's disease: a critical update. Semin Reprod Med. 2009;27:283–293. doi: 10.1055/s-0029-1216281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer's disease risk and therapy. Prog. Brain Res. 2010;182:77–96. doi: 10.1016/S0079-6123(10)82003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Henderson VW, Paganini-Hill A, Miller BL, Elble RJ, Reyes PF, Shoupe D, McCleary CA, Klein RA, Hake AM, Farlow MR. Estrogen for Alzheimer's disease in women: randomized, double-blind, placebo-controlled trial. Neurology. 2000;54:295–301. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- [78].Henderson VW, Sherwin BB. Surgical versus natural menopause: cognitive issues. Menopause. 2007;14:572–579. doi: 10.1097/gme.0b013e31803df49c. [DOI] [PubMed] [Google Scholar]
- [79].Henriksson M, Stenman E, Vikman P, Edvinsson L. MEK1/2 inhibition attenuates vascular ETA and ETB receptor alterations after cerebral ischaemia. Exp Brain Res. 2007;178:470–476. doi: 10.1007/s00221-006-0753-7. [DOI] [PubMed] [Google Scholar]
- [80].Hogervorst E, Williams J, Budge M, Riedel W, Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience. 2000;101:485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- [81].Honda K, Sawada H, Kihara T, Urushitani M, Nakamizo T, Akaike A, Shimohama S. Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. J. Neurosci. Res. 2000;60:321–327. doi: 10.1002/(SICI)1097-4547(20000501)60:3<321::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- [82].Honda K, Shimohama S, Sawada H, Kihara T, Nakamizo T, Shibasaki H, Akaike A. Nongenomic antiapoptotic signal transduction by estrogen in cultured cortical neurons. J. Neurosci. Res. 2001;64:466–475. doi: 10.1002/jnr.1098. [DOI] [PubMed] [Google Scholar]
- [83].Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- [84].Jover-Mengual T, Zukin RS, Etgen AM. MAPK signaling is critical to estradiol protection of CA1 neurons in global ischemia. Endocrinology. 2007;148:1131–1143. doi: 10.1210/en.2006-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Jover T, Tanaka H, Calderone A, Oguro K, Bennett MV, Etgen AM, Zukin RS. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J. Neurosci. 2002;22:2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kalita K, Szymczak S, Kaczmarek L. Non-nuclear estrogen receptor beta and alpha in the hippocampus of male and female rats. Hippocampus. 2005;15:404–412. doi: 10.1002/hipo.20066. [DOI] [PubMed] [Google Scholar]
- [87].Kato I, Toniolo P, Akhmedkhanov A, Koenig KL, Shore R, Zeleniuch-Jacquotte A. Prospective study of factors influencing the onset of natural menopause. J. Clin. Epidemiol. 1998;51:1271–1276. doi: 10.1016/s0895-4356(98)00119-x. [DOI] [PubMed] [Google Scholar]
- [88].Klaiber EL, Vogel W, Rako S. A critique of the Women's Health Initiative hormone therapy study. Fertil. Steril. 2005;84:1589–1601. doi: 10.1016/j.fertnstert.2005.08.010. [DOI] [PubMed] [Google Scholar]
- [89].Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc. Natl. Acad. Sci. U S A. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. Eur. J. Pharm. 2000;400:205–209. doi: 10.1016/s0014-2999(00)00425-8. [DOI] [PubMed] [Google Scholar]
- [91].Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Neuroprotection by estrogen via extracellular signal-regulated kinase against quinolinic acid-induced cell death in the rat hippocampus. Eur. J. Neurosci. 2001;13:472–476. doi: 10.1046/j.0953-816x.2000.01409.x. [DOI] [PubMed] [Google Scholar]
- [92].L'Hermite M, Simoncini T, Fuller S, Genazzani AR. Could transdermal estradiol + progesterone be a safer postmenopausal HRT? A review. Maturitas. 2008;60:185–201. doi: 10.1016/j.maturitas.2008.07.007. [DOI] [PubMed] [Google Scholar]
- [93].LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, Margolis KL, Stefanick ML, Brzyski R, Curb JD, Howard BV, Lewis CE, Wactawski-Wende J. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM. Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids. 2009;74:555–561. doi: 10.1016/j.steroids.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Li J, Siegel M, Yuan M, Zeng Z, Finnucan L, Persky R, Hurn PD, McCullough LD. Estrogen enhances neurogenesis and behavioral recovery after stroke. J. Cereb. Blood Flow Metab. 2011;31:413–425. doi: 10.1038/jcbfm.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Liao S, Chen W, Kuo J, Chen C. Association of serum estrogen level and ischemic neuroprotection in female rats. Neurosci. Lett. 2001;297:159–162. doi: 10.1016/s0304-3940(00)01704-3. [DOI] [PubMed] [Google Scholar]
- [97].Liu SB, Han J, Zhang N, Tian Z, Li XB, Zhao MG. Neuroprotective effects of estrogen against oxidative toxicity through activation of GPR30 receptor. Clin. Exp. Pharm. Phys. 2011 doi: 10.1111/j.1440-1681.2011.05549.x. [DOI] [PubMed] [Google Scholar]
- [98].Lloyd-Jones D, Adams R, Carnethon M, Simone G. De, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- [99].Loeb C, Gandolfo C, Croce R, Conti M. Dementia associated with lacunar infarction. Stroke. 1992;23:1225–1229. doi: 10.1161/01.str.23.9.1225. [DOI] [PubMed] [Google Scholar]
- [100].Lord C, Buss C, Lupien SJ, Pruessner JC. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: a possible window of opportunity effect. Neurobiol. Aging. 2008;29:95–101. doi: 10.1016/j.neurobiolaging.2006.09.001. [DOI] [PubMed] [Google Scholar]
- [101].MacLennan AH, Henderson VW, Paine BJ, Mathias J, Ramsay EN, Ryan P, Stocks NP, Taylor AW. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause. 2006;13:28–36. doi: 10.1097/01.gme.0000191204.38664.61. [DOI] [PubMed] [Google Scholar]
- [102].Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol. Endocrinol. 2008;22:2116–2127. doi: 10.1210/me.2008-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Mahesh VB, Dhandapani KM, Brann DW. Role of astrocytes in reproduction and neuroprotection. Mol. Cell. Endocrinol. 2006;246:1–9. doi: 10.1016/j.mce.2005.11.017. [DOI] [PubMed] [Google Scholar]
- [104].Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006;138:1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- [105].Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J. Neurosci. 2006;26:9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Manthey D, Heck S, Engert S, Behl C. Estrogen induces a rapid secretion of amyloid beta precursor protein via the mitogen-activated protein kinase pathway. Eur. J. Biochem. 2001;268:4285–4291. doi: 10.1046/j.1432-1327.2001.02346.x. [DOI] [PubMed] [Google Scholar]
- [107].Matsuda K, Sakamoto H, Mori H, Hosokawa K, Kawamura A, Itose M, Nishi M, Prossnitz ER, Kawata M. Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation. Neurosci. Lett. 2008;441:94–99. doi: 10.1016/j.neulet.2008.05.108. [DOI] [PubMed] [Google Scholar]
- [108].McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J. Neurosci. 2003;23:8701–8705. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J. Cereb. Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- [110].Menon DV, Vongpatanasin W. Effects of transdermal estrogen replacement therapy on cardiovascular risk factors. Treat. Endocrinol. 2006;5:37–51. doi: 10.2165/00024677-200605010-00005. [DOI] [PubMed] [Google Scholar]
- [111].Merchenthaler I, Dellovade TL, Shughrue PJ. Neuroprotection by estrogen in animal models of global and focal ischemia. Ann. N.Y. Acad. Sci. 2003;1007:89–100. doi: 10.1196/annals.1286.009. [DOI] [PubMed] [Google Scholar]
- [112].Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146:3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- [113].Miller VM, Black DM, Brinton EA, Budoff MJ, Cedars MI, Hodis HN, Lobo RA, Manson JE, Merriam GR, Naftolin F, Santoro N, Taylor HS, Harman SM. Using basic science to design a clinical trial: baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) J. Cardiovasc. Transl. Res. 2009;2:228–239. doi: 10.1007/s12265-009-9104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J. Comp. Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- [115].Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J. Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Moura PJ, Petersen SL. Estradiol acts through nuclear- and membrane-initiated mechanisms to maintain a balance between GABAergic and glutamatergic signaling in the brain: implications for hormone replacement therapy. Rev. Neurosci. 2010;21:363–380. doi: 10.1515/revneuro.2010.21.5.363. [DOI] [PubMed] [Google Scholar]
- [117].Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer's Disease Cooperative Study. JAMA. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- [118].Murphy SJ, McCullough LD, Smith JM. Stroke in the female: role of biological sex and estrogen. ILAR J. 2004;45:147–159. doi: 10.1093/ilar.45.2.147. [DOI] [PubMed] [Google Scholar]
- [119].Namura S, Iihara K, Takami S, Nagata I, Kikuchi H, Matsushita K, Moskowitz MA, Bonventre JV, Alessandrini A. Intravenous administration of MEK inhibitor U0126 affords brain protection against forebrain ischemia and focal cerebral ischemia. Proc Natl Acad Sci U S A. 2001;98:11569–11574. doi: 10.1073/pnas.181213498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Nation DA, Hong S, Jak AJ, Delano-Wood L, Mills PJ, Bondi MW, Dimsdale JE. Stress, exercise, and Alzheimer's disease: a neurovascular pathway. Med. Hypotheses. 2011;76:847–854. doi: 10.1016/j.mehy.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Niewada M, Kobayashi A, Sandercock PA, Kaminski B, Czlonkowska A. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology. 2005;24:123–128. doi: 10.1159/000082999. [DOI] [PubMed] [Google Scholar]
- [122].Niizuma K, Endo H, Chan PH. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J. Neurochem. 2009;109(Suppl 1):133–138. doi: 10.1111/j.1471-4159.2009.05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Numakawa Y, Matsumoto T, Yokomaku D, Taguchi T, Niki E, Hatanaka H, Kunugi H, Numakawa T. 17beta-estradiol protects cortical neurons against oxidative stress-induced cell death through reduction in the activity of mitogen-activated protein kinase and in the accumulation of intracellular calcium. Endocrinology. 2007;148:627–637. doi: 10.1210/en.2006-1210. [DOI] [PubMed] [Google Scholar]
- [124].Paganini-Hill A. Oestrogen replacement therapy and Alzheimer's disease. Br. J. Obstet. Gynaecol. 1996;103(Suppl 13):80–86. [PubMed] [Google Scholar]
- [125].Parker WH, Jacoby V, Shoupe D, Rocca W. Effect of bilateral oophorectomy on women's long-term health. Womens Health (Lond Engl) 2009;5:565–576. doi: 10.2217/whe.09.42. [DOI] [PubMed] [Google Scholar]
- [126].Persky RW, Turtzo LC, McCullough LD. Stroke in women: disparities and outcomes. Curr. Cardiol. Rep. 2010;12:6–13. doi: 10.1007/s11886-009-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Peters O, Back T, Lindauer U, Busch C, Megow D, Dreier J, Dirnagl U. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab. 1998;18:196–205. doi: 10.1097/00004647-199802000-00011. [DOI] [PubMed] [Google Scholar]
- [128].Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front. Neuroendocrinol. 2009;30:239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Plamondon H, Morin A, Charron C. Chronic 17beta-estradiol pretreatment and ischemia-induced hippocampal degeneration and memory impairments: a 6-month survival study. Horm. Behav. 2006;50:361–369. doi: 10.1016/j.yhbeh.2006.04.006. [DOI] [PubMed] [Google Scholar]
- [130].Prokai L, Prokai-Tatrai K, Perjesi P, Zharikova AD, Perez EJ, Liu R, Simpkins JW. Quinol-based cyclic antioxidant mechanism in estrogen neuroprotection. Proc. Natl. Acad. Sci. U S A. 2003;100:11741–11746. doi: 10.1073/pnas.2032621100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Ramos JW. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int. J. Biochem. Cell Biol. 2008;40:2707–2719. doi: 10.1016/j.biocel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- [132].Rau SW, Dubal DB, Bottner M, Gerhold LM, Wise PM. Estradiol attenuates programmed cell death after stroke-like injury. J. Neurosci. 2003;23:11420–11426. doi: 10.1523/JNEUROSCI.23-36-11420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Razandi M, Pedram A, Levin ER. Heat shock protein 27 is required for sex steroid receptor trafficking to and functioning at the plasma membrane. Mol. Cell. Bio. 2010;30:3249–3261. doi: 10.1128/MCB.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Readnower RD, Sauerbeck AD, Sullivan PG. Mitochondria, Amyloid beta, and Alzheimer's Disease. Int. J. Alzheimer's Dis. 2011;2011:104545. doi: 10.4061/2011/104545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Renoux C, Dell'aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj.c2519. [DOI] [PubMed] [Google Scholar]
- [136].Resnick SM, Coker LH, Maki PM, Rapp SR, Espeland MA, Shumaker SA. The Women's Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clin Trials. 2004;1:440–450. doi: 10.1191/1740774504cn040oa. [DOI] [PubMed] [Google Scholar]
- [137].Rivera CM, Grossardt BR, Rhodes DJ, Rocca WA. Increased mortality for neurological and mental diseases following early bilateral oophorectomy. Neuroepidemiology. 2009;33:32–40. doi: 10.1159/000211951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ., 3rd Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- [139].Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen, and cognitive aging: the timing hypothesis. Neurodegener. Dis. 2010;7:163–166. doi: 10.1159/000289229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. 2011;1379:188–198. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Rocca WA, Shuster LT, Grossardt BR, Maraganore DM, Gostout BS, Geda YE, Melton LJ., 3rd Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health (Lond Engl) 2009;5:39–48. doi: 10.2217/17455057.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Roepke TA, Ronnekleiv OK, Kelly MJ. Physiological consequences of membrane-initiated estrogen signaling in the brain. Front. Biosci. 2011;16:1560–1573. doi: 10.2741/3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke. 2003;34:1581–1585. doi: 10.1161/01.STR.0000078562.82918.F6. [DOI] [PubMed] [Google Scholar]
- [144].Rosenberg L, Park S. Verbal and spatial functions across the menstrual cycle in healthy young women. Psychoneuroendocrinology. 2002;27:835–841. doi: 10.1016/s0306-4530(01)00083-x. [DOI] [PubMed] [Google Scholar]
- [145].Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD. 17beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999;30:1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- [146].Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, Burger HG, Colditz GA, Davis SR, Gambacciani M, Gower BA, Henderson VW, Jarjour WN, Karas RH, Kleerekoper M, Lobo RA, Manson JE, Marsden J, Martin KA, Martin L, Pinkerton JV, Rubinow DR, Teede H, Thiboutot DM, Utian WH. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J. Clin. Endocrinol. Metab. 2010;95:s1–s66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Sawada M, Alkayed NJ, Goto S, Crain BJ, Traystman RJ, Shaivitz A, Nelson RJ, Hurn PD. Estrogen receptor antagonist ICI182,780 exacerbates ischemic injury in female mouse. J. Cereb. Blood Flow Metab. 2000;20:112–118. doi: 10.1097/00004647-200001000-00015. [DOI] [PubMed] [Google Scholar]
- [148].Sawe N, Steinberg G, Zhao H. Dual roles of the MAPK/ERK1/2 cell signaling pathway after stroke. J Neurosci Res. 2008;86:1659–1669. doi: 10.1002/jnr.21604. [DOI] [PubMed] [Google Scholar]
- [149].Setalo G, Jr., Singh M, Nethrapalli IS, Toran-Allerand CD. Protein kinase C activity is necessary for estrogen-induced Erk phosphorylation in neocortical explants. Neurochem. Res. 2005;30:779–790. doi: 10.1007/s11064-005-6871-y. [DOI] [PubMed] [Google Scholar]
- [150].Sherwin BB. Estrogen and cognitive functioning in women. Endocr. Rev. 2003;24:133–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- [151].Sherwin BB. The clinical relevance of the relationship between estrogen and cognition in women. J. Steroid Biochem. Mol. Biol. 2007;106:151–156. doi: 10.1016/j.jsbmb.2007.05.016. [DOI] [PubMed] [Google Scholar]
- [152].Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? J. Neuroendocrinol. 2007;19:77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- [153].Sherwin BB. Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol. 2009;5:620–627. doi: 10.1038/nrendo.2009.193. [DOI] [PubMed] [Google Scholar]
- [154].Shi J, Panickar KS, Yang SH, Rabbani O, Day AL, Simpkins JW. Estrogen attenuates over-expression of beta-amyloid precursor protein messager RNA in an animal model of focal ischemia. Brain Res. 1998;810:87–92. doi: 10.1016/s0006-8993(98)00888-9. [DOI] [PubMed] [Google Scholar]
- [155].Shufelt CL, Johnson BD, Berga SL, Braunstein GD, Reis SE, Bittner V, Yang Y, Pepine CJ, Sharaf BL, Sopko G, Kelsey SF, Merz CN. Timing of hormone therapy, type of menopause, and coronary disease in women: data from the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation. Menopause. 2011 doi: 10.1097/gme.0b013e3182113672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Shughrue PJ, Merchenthaler I. Estrogen prevents the loss of CA1 hippocampal neurons in gerbils after ischemic injury. Neuroscience. 2003;116:851–861. doi: 10.1016/s0306-4522(02)00790-x. [DOI] [PubMed] [Google Scholar]
- [157].Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- [158].Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Simpkins JW, Perez E, Wang X, Yang S, Wen Y, Singh M. The Potential for Estrogens in Preventing Alzheimer's Disease. Ther. Adv. Neurol. Disord. 2009;2:31–49. doi: 10.1177/1756285608100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J. Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- [161].Simpkins JW, Yi KD, Yang SH, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Biochim. Biophys. Acta. 2010;1800:1113–1120. doi: 10.1016/j.bbagen.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J. Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Singh M, Setalo G, Jr., Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J. Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Smith CC, Vedder LC, Nelson AR, Bredemann TM, McMahon LL. Duration of estrogen deprivation, not chronological age, prevents estrogen's ability to enhance hippocampal synaptic physiology. Proc. Natl. Acad. Sci. U S A. 2010;107:19543–19548. doi: 10.1073/pnas.1009307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid. Redox Signaling. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- [166].Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, Cao Y, Bondar G, Lam J, Ao Y, Sandoval F, Suriany S, Sofroniew MV, Voskuhl RR. Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc. Natl. Acad. Sci. U S A. 2011;108:8867–8872. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Speroff L. Transdermal hormone therapy and the risk of stroke and venous thrombosis. Climacteric. 2010;13:429–432. doi: 10.3109/13697137.2010.507111. [DOI] [PubMed] [Google Scholar]
- [168].Strom JO, Theodorsson A, Theodorsson E. Dose-related neuroprotective versus neurodamaging effects of estrogens in rat cerebral ischemia: a systematic analysis. J. Cereb. Blood Flow Metab. 2009;29:1359–1372. doi: 10.1038/jcbfm.2009.66. [DOI] [PubMed] [Google Scholar]
- [169].Sugawara T, Noshita N, Lewen A, Gasche Y, Ferrand-Drake M, Fujimura M, Morita-Fujimura Y, Chan PH. Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation. J. Neurosci. 2002;22:209–217. doi: 10.1523/JNEUROSCI.22-01-00209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, Yenari MA, Swanson RA. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann. Neurol. 2008;64:654–663. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, Song W. Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression. Proc. Natl. Acad. Sci. U S A. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc. Natl. Acad. Sci. U S A. 2007;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- [174].Tang XN, Cairns B, Cairns N, Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 2008;154:556–562. doi: 10.1016/j.neuroscience.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Taylor HS, Manson JE. Update in hormone therapy use in menopause. J. Clin. Endocrinol. Metab. 2011;96:255–264. doi: 10.1210/jc.2010-0536. [DOI] [PubMed] [Google Scholar]
- [176].Theodorsson A, Theodorsson E. Estradiol increases brain lesions in the cortex and lateral striatum after transient occlusion of the middle cerebral artery in rats: no effect of ischemia on galanin in the stroke area but decreased levels in the hippocampus. Peptides. 2005;26:2257–2264. doi: 10.1016/j.peptides.2005.04.013. [DOI] [PubMed] [Google Scholar]
- [177].Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O'Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann. Neurol. 2008;64:168–176. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Turtzo LC, McCullough LD. Sex-specific responses to stroke. Future Neurol. 2010;5:47–59. doi: 10.2217/fnl.09.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- [180].Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endo. Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- [181].Wang L, Andersson S, Warner M, Gustafsson JA. Morphological abnormalities in the brains of estrogen receptor beta knockout mice. Proc. Natl. Acad. Sci. U S A. 2001;98:2792–2796. doi: 10.1073/pnas.041617498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Wang R, Zhang QG, Han D, Xu J, Lu Q, Zhang GY. Inhibition of MLK3-MKK4/7-JNK1/2 pathway by Akt1 in exogenous estrogen-induced neuroprotection against transient global cerebral ischemia by a non-genomic mechanism in male rats. J. Neurochem. 2006;99:1543–1554. doi: 10.1111/j.1471-4159.2006.04201.x. [DOI] [PubMed] [Google Scholar]
- [183].Wang X, Wang H, Xu L, Rozanski DJ, Sugawara T, Chan PH, Trzaskos JM, Feuerstein GZ. Significant neuroprotection against ischemic brain injury by inhibition of the MEK1 protein kinase in mice: exploration of potential mechanism associated with apoptosis. J Pharmacol Exp Ther. 2003;304:172–178. doi: 10.1124/jpet.102.040246. [DOI] [PubMed] [Google Scholar]
- [184].Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- [185].Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- [186].Wen Y, Yang S, Liu R, Brun-Zinkernagel AM, Koulen P, Simpkins JW. Transient cerebral ischemia induces aberrant neuronal cell cycle re-entry and Alzheimer's disease-like tauopathy in female rats. J. Biol. Chem. 2004;279:22684–22692. doi: 10.1074/jbc.M311768200. [DOI] [PubMed] [Google Scholar]
- [187].Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann. Neurol. 2011;69:163–169. doi: 10.1002/ana.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Wilson ME, Liu Y, Wise PM. Estradiol enhances Akt activation in cortical explant cultures following neuronal injury. Brain Res. 2002;102:48–54. doi: 10.1016/s0169-328x(02)00181-x. [DOI] [PubMed] [Google Scholar]
- [189].Woods NF, Mitchell ES. Symptoms during the perimenopause: prevalence, severity, trajectory, and significance in women's lives. Am. J. Med. 2005;118(Suppl 12B):14–24. doi: 10.1016/j.amjmed.2005.09.031. [DOI] [PubMed] [Google Scholar]
- [190].Wu TW, Chen S, Brinton RD. Membrane estrogen receptors mediate calcium signaling and MAP kinase activation in individual hippocampal neurons. Brain Res. 2011;1379:34–43. doi: 10.1016/j.brainres.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Wu TW, Wang JM, Chen S, Brinton RD. 17Beta-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- [192].Wu WW, Adelman JP, Maylie J. Ovarian hormone deficiency reduces intrinsic excitability and abolishes acute estrogen sensitivity in hippocampal CA1 pyramidal neurons. J. Neurosci. 2011;31:2638–2648. doi: 10.1523/JNEUROSCI.6081-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- [194].Yang LC, Zhang QG, Zhou CF, Yang F, Zhang YD, Wang RM, Brann DW. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS One. 2010;5:e9851. doi: 10.1371/journal.pone.0009851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Yao J, Hamilton RT, Cadenas E, Brinton RD. Decline in mitochondrial bioenergetics and shift to ketogenic profile in brain during reproductive senescence. Biochim. Biophys. Acta. 2010;1800:1121–1126. doi: 10.1016/j.bbagen.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Yao J, Irwin R, Chen S, Hamilton R, Cadenas E, Brinton RD. Ovarian hormone loss induces bioenergetic deficits and mitochondrial beta-amyloid. Neurobiol. Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U S A. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Yao M, Nguyen TV, Pike CJ. Estrogen regulates Bcl-w and Bim expression: role in protection against beta-amyloid peptide-induced neuronal death. J. Neurosci. 2007;27:1422–1433. doi: 10.1523/JNEUROSCI.2382-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Yong Y, Xie HJ, Zhang YF, Yang QD, Liao DF, Yang HL, Yan PK, Liu ZJ. 17beta-estradiol potentiates ischemia-reperfusion injury in diabetic ovariectomized female rats. Brain Res. 2005;1054:192–199. doi: 10.1016/j.brainres.2005.05.069. [DOI] [PubMed] [Google Scholar]
- [200].Yoshioka H, Niizuma K, Katsu M, Okami N, Sakata H, Kim GS, Narasimhan P, Chan PH. NADPH oxidase mediates striatal neuronal injury after transient global cerebral ischemia. J. Cereb. Blood Flow Metab. 2011;31:868–880. doi: 10.1038/jcbfm.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Zhang B, Subramanian S, Dziennis S, Jia J, Uchida M, Akiyoshi K, Migliati E, Lewis AD, Vandenbark AA, Offner H, Hurn PD. Estradiol and G1 reduce infarct size and improve immunosuppression after experimental stroke. J. Immuno. 2010;184:4087–4094. doi: 10.4049/jimmunol.0902339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Zhang QG, Han D, Wang RM, Dong Y, Yang F, Vadlamudi RK, Brann DW. From the Cover: C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-{alpha} and the critical period hypothesis of estrogen neuroprotection. Proc. Natl. Acad. Sci. U S A. 2011;108:E617–624. doi: 10.1073/pnas.1104391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Zhang QG, Raz L, Wang R, Han D, De Sevilla L, Yang F, Vadlamudi RK, Brann DW. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. J. Neurosci. 2009;29:13823–13836. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Zhang QG, Wang R, Khan M, Mahesh V, Brann DW. Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J. Neurosci. 2008;28:8430–8441. doi: 10.1523/JNEUROSCI.2752-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Zhang YQ, Shi J, Rajakumar G, Day AL, Simpkins JW. Effects of gender and estradiol treatment on focal brain ischemia. Brain Res. 1998;784:321–324. doi: 10.1016/s0006-8993(97)00502-7. [DOI] [PubMed] [Google Scholar]