Abstract
OBJECTIVES
Frailty is a dynamic geriatric syndrome characterized by decreased reserve and increased vulnerability. Low serum 25-hydroxyvitamin D [25(OH)D] concentrations in older adults are associated with many physiological changes that portend frailty and its consequences. We aimed to assess whether serum 25(OH)D concentrations relate to transitions between the states of robustness, prefrailty, and frailty, and to mortality.
DESIGN, SETTING, and PARTICIPANTS
Adults aged≥65 years (N=1,155) enrolled in Invecchiare in Chianti (InCHIANTI), a prospective cohort study in Tuscany, Italy.
MEASUREMENTS
Serum 25(OH)D concentrations measured at baseline and frailty state (robust, prefrail, frail) assessed at baseline and at three and six years post enrollment. Vital status was also determined at three and six years post enrollment.
RESULTS
The median (interquartile range) 25(OH)D concentration was 16.0 (10.4—25.6) ng/mL (multiply by 2.496 to convert to nmol/L). Prefrail participants with 25(OH)D<20 ng/mL were 8.9% (95% Confidence Interval [CI], 2.5—15.2%) more likely to die, 3.0% (95%CI, −5.6—14.6%) more likely to become frail, and 7.7% (95%CI, −3.5—18.7%) less likely to become robust than prefrail participants with 25(OH)D≥20 ng/mL. Among prefrail participants, each 5 ng/mL decrement of continuous 25(OH)D was associated with 1.46 times higher odds of dying (95%CI, 1.18—2.07) and 1.13 higher odds of incident frailty (95%CI, 0.90—1.39) versus recovery of robustness. Transitions from robustness or frailty were not associated with 25(OH)D.
CONCLUSION
Results provide evidence that prefrailty is an “at risk” state from which older adults with high 25(OH)D are more likely to recover than to decline. However, high 25(OH)D was not associated with recovery from frailty. Thus, 25(OH)D should be investigated as a potential therapy to treat prefrailty and prevent further decline.
Keywords: Frailty, Mortality, Vitamin D
INTRODUCTION
Frailty has been defined as a geriatric syndrome resulting from declines in anatomical integrity and function across multiple physiological systems1, 2. Frailty is characterized by reduced reserve capacity and increased risk of morbidity and mortality, but is distinct from disability and comorbidity1, 2. Fried et al1. proposed an operational definition of frailty based on five criteria: unintentional weight loss, exhaustion, sedentariness, muscle weakness, and slow walking speed. Presence of three or more criteria denotes frailty, presence of one or two criteria denotes prefrailty, and absence of any criteria denotes robustness. Frailty is common and dynamic, with high rates of transitions between states3. Gill et al3. found that in 18 months, 12% of prefrail elders transitioned to robustness and 25% transitioned to frailty while 23% of frail elders transitioned to prefrailty. Identifying mutable factors that may help prevent or remediate frailty is an important step to developing interventions for frailty.
A potentially modifiable factor that has been associated with frailty is 25-hydroxyvitamin D [25(OH)D] insufficiency. Hypovitaminosis D is common in older adults due to reduced consumption of vitamin D-rich food, reduced sunlight exposure, and inefficient 25(OH)D synthesis4–6. Older adults with lower 25(OH)D levels have higher prevalence7 and incidence8 of frailty and increased risk of adverse outcomes including depressed mood, pain, falls, fractures, disability, and mortality9–14. Additionally, relationships of 25(OH)D with muscle weakness, a dimension of the frailty syndrome, and functional performance have been reported5, 15, 16. However, no study has examined whether 25(OH)D relates to transitions between frailty states.
We hypothesized that older adults with high 25(OH)D concentrations were more likely to recover to less frail states and those with low 25(OH)D concentrations were more likely to decline to more frail states or die. Additionally, we hypothesized that 25(OH)D concentrations relate to frailty transitions mainly due to changes in muscle weakness and walking speed. Therefore, we also aimed to assess the relationship between 25(OH)D and transitions between states of the five individual frailty criteria. Lastly, because low 25(OH)D leads to compensatory increases in parathyroid hormone (PTH)4, 6, which are also associated with multiple adverse outcomes5, we aimed to assess whether low 25(OH)D relates to frailty transitions independent of secondary hyperparathyroidism.
METHODS
Participants
Invecchiare in Chianti (InChianti) is a longitudinal study of adults recruited from two Italian towns (Bagno a Ripoli in Tuscany, Greve in Chianti). Residents were randomly sampled from the population registry (residents aged ≥90 years were over-sampled); 1,155 out of 1,260 sampled residents aged ≥65 years (91.6%) agreed to participate. The Italian National Research Council of Aging Ethical Committee approved the study, and all participants provided written informed consent. Details regarding design and sampling are available elsewhere17.
Data Collection
Data were collected upon enrollment (baseline) and three and six years post-enrollment. Baseline assessments occurred between 1998 and 2000; and three- and six-year visits occurred between 2001 and 2003 and between 2004 and 2006, respectively. Participants responded to in-home surveys administered by trained interviewers. Physicians and physical therapists performed medical examinations and physical function tests in the study clinic.
Frailty Assessment
Frailty was defined according to the five criteria proposed by Fried et al1: unintentional weight loss, exhaustion, sedentariness, muscle weakness, and slow walking speed. Defining frailty as presence of at least three criteria and prefrailty as presence of one or two criteria has been previously validated18. Due to differences in measurement instruments in inCHIANTI, our previously published operationalizations of the five criteria7 slightly differed from those in Fried et al1. Specifically, participants with self-reported weight loss >4.5 kg (10 lbs) in the past year for reasons other than dieting were classified as having unintentional weight loss at baseline. Participants at the follow-up visits also reported direction and amount of weight change since the previous visit.
Participants who had unintentional weight loss at the previous visit were deemed positive for unintentional weight loss at subsequent visits if they self-reported either weight loss or no weight change. Participants who responded “occasionally” or “often/always” to the statement “I felt that everything was an effort” were considered positive for exhaustion. This statement came from the Center for Epidemiological Studies-Depression (CES-D) scale, which was validated in Italian19. Participants were classified as sedentary if they self-reported either complete inactivity or spending less than one hour per week performing low-intensity activities. Slow walking speed at baseline was defined as usual walking speed in the slowest quintile within groups defined by sex and height. Walking speed was measured using a 4-meter course with photocell recordings at the start and finish. Final walking speed was the average of two walks. Slow walking speed at follow-up visits was determined using the baseline cut points. Muscle weakness at baseline was defined as grip strength in the lowest quintile within groups defined by sex and body mass index (BMI). Grip strength was measured using a handheld dynamometer (Nicholas Muscle Tester; Sammon Preston, Inc., Chicago, Il) by a standard method. Muscle weakness at follow-up visits was determined using the baseline cutpoints.
Mortality
Mortality was ascertained via the Tuscany Region Mortality General Registry and from death certificates from the registry office of the municipality of residence. Because our focus was on transitions between frailty states, mortality was dichotomized to indicate death since the previous scheduled visit.
Biomarker Assessment
Fasting blood samples were drawn, processed, and stored at −80 degrees Celsius until analysis. Serum 25(OH)D was assessed at baseline by radioummunoassay (RIA kit; DiaSorin, Stillwater, MN). Intra- and interassay coefficients of variation (CV) were 8.1% and 10.2%, respectively. Serum intact PTH was assessed by a two-site immunoradiometric assay kit (N-tact PTHSP; DiaSorin). Intra- and interassay CVs were <3.0% and 5.5%, respectively. To account for effects of renal function on 25(OH)D levels, creatinine clearance was calculated using serum creatinine and the Cockroft-Gault formula. Serum creatinine was assessed using a standard Jaffe method (Roche Diagnostics, GmbH, Mannheim, Germany).
Baseline Covariates
Alcohol consumption (drinks/week) and calcium intake (mg/day) were assessed using the European Prospective Investigation into Cancer and Nutrition questionnaire on dietary habits19. The Mini-Mental State Examination (MMSE) scale measured cognitive function20. The CES-D scale measured depressive symptoms (one CES-D statement was used in the frailty assessment; however, baseline frailty was not an outcome in analyses). Comorbidities were determined using adjudicated measures that combined self-report, medical records, and clinical examination. Specific comorbidities considered were congestive heart failure, diabetes mellitus, myocardial infarction, peripheral arterial disease, hypertension, angina, osteoarthritis, renal disease, and chronic obstructive pulmonary disease. We also considered age, education (years of schooling), smoking (pack-years), BMI (kg/m2), sex, and blood collection season.
Statistical Analysis
Although there is currently no clear consensus on optimal 25(OH)D levels, <20 ng/mL (50 nmol/L; multiply by 2.496 to convert ng/mL to nmol/L) has been proposed to define vitamin D deficiency by the Institute of Medicine (IoM) and others21, 22. Thus, “low vitamin D” in analyses was defined as 25(OH)D<20 ng/mL. Weighted multinomial regression was used to estimate transition probabilities from frailty state at time t to frailty state or death at time t+1 by vitamin D level. Participants could transition between any two frailty states, but death was an absorbing state. We used marginal structural models (MSM) to adjust for covariates and produce standardized transition probabilities23, 24. MSMs are a type of inverse probability of exposure-weighted analysis. The weights were estimated by logistically regressing vitamin D status (high/low) on covariates to estimate the probability of observed vitamin D status, then taking the reciprocal of the probability. We used stabilized weights and truncated extreme weights based on published diagnostics25 to ensure adequate model fit. We used weighted estimating equations (WEE) to address potential selection bias from missing data26, 27. Logistic regression was used to calculate the probability of being observed by regressing observed-data indicators (observed/missing) on fully-observed covariates. Separate weights were calculated for missing 25(OH)D, covariates, and frailty status at times t and t+1. The inverse probability of being observed was multiplied by the inverse probability of vitamin D status to form a total weight used in weighted multinomial regression. Standardized transition probabilities and their differences (low 25(OH)D – high 25(OH)D) were computed; 95% confidence intervals (CI) were calculated using 500 bootstrap samples. We also estimated transition probabilities for each frailty criterion using the same general approach. Separate weights for WEE were calculated for each criterion, because not all criteria were missing for participants with missing frailty status. Next, we performed the transition analysis for frailty status and all five criteria using continuous 25(OH)D. The MSM weight was the inverse density of observed 25(OH)D. This weight was calculated by regressing log-transformed 25(OH)D (to ensure normality) on covariates. Standardized odds ratios and 95% bootstrap CIs were computed.
Lastly, we used MSM to assess whether 25(OH)D relates to frailty transitions independent of its effects on PTH. As in previous work7, we defined high PTH as PTH ≥32.4 ng/L (upper quartile in the study sample). We calculated the inverse probability of PTH category using covariates, 25(OH)D, and age-by-25(OH)D interaction (as per previous reports on age-related effects of low 25(OH)D on PTH)28. Weighted multinomial logistic models, stratified by frailty status at time t, included continuous 25(OH)D, PTH category, and their interaction. The reported odds ratio is the unmediated portion of the relationship between 25(OH)D and frailty, interpreted as the standardized odds ratio for 25(OH)D if PTH remains low (<32.4 ng/L). If we included all 25(OH)D-frailty confounders and all PTH-frailty confounders in the models, then the standardized odds ratio is interpreted as the “controlled direct effect” of 25(OH)D on frailty if PTH is set to be low for all29, 30. SAS 9.2 was used for all analyses. PROC SURVEYLOGISTIC was used to perform weighted multinomial regression. Statistical significance for differences in transition probabilities was defined as 95%CIs that exclude 0; statistical significance for odds ratios was defined as 95%CIs that exclude 1.
RESULTS
Among 1,155 eligible participants aged ≥65 years, 1,005 had measured 25(OH)D, of whom 904 had all covariates observed. Among 904 participants with complete baseline data, 595 had two observed transitions (from baseline to 3 years, and from 3 years to 6 years), 131 only had an observed transition from baseline to 3 years, and 7 only had an observed transition from 3 years to 6 years, for a total of 1,328 observed transitions among 733 unique participants. One hundred-forty one participants died before the 3-year visit, and an additional 154 participants died before the 6-year visit. Information from participants who were alive but had incomplete data was incorporated into statistical analyses via estimated weights.
Median 25(OH)D was 16.0 ng/mL (interquartile range, 10.4—25.6 ng/mL), and 64.9% of participants had 25(OH)D<20 ng/mL. Table 1 shows that participants with 25(OH)D<20 ng/mL were older and more likely to be female. Participants with 25(OH)D≥20 ng/mL had lower PTH, lower prevalence of baseline frailty and each frailty criterion except exhaustion. Participants with missing 25(OH)D measurements were older, consumed fewer alcoholic drinks, had lower MMSE and higher CES-D scores, fewer comorbid conditions, and higher prevalence of baseline frailty compared to participants with measured 25(OH)D (results not shown).
Table 1.
Baseline Participant Characteristics
| Characteristic | 25(OH)D 20 ng/mL (50 nmol/L) N=353 |
25(OH)D<20 ng/mL (50 nmol/L) N=652 |
P* | ||||
|---|---|---|---|---|---|---|---|
| Mean, Median, or Number | SD†, IQR‡, or % | N§ | Mean, Median, or Number | SD†, IQR‡, or % | N§ | ||
| Age (years) mean (SD) | 72.4 | (6.1) | 76.4 | (7.6) | <0.001 | ||
| Female Sex number (%) | 138 | (39.1) | 423 | (64.9) | <0.001 | ||
| Education (years) mean (SD) | 6.2 | (3.3) | 352 | 4.9 | (3.2) | <0.001 | |
| Season of blood collection number (%) | |||||||
| Spring (March-May) | 27 | (7.7) | 169 | (25.9) | <0.001 | ||
| Summer (June-August) | 55 | (15.6) | 60 | (9.2) | |||
| Fall (Sept-Nov) | 176 | (49.9) | 165 | (25.3) | |||
| Winter (Dec-Feb) | 95 | (26.9) | 258 | (39.6) | |||
| Smoking (pack-years) mean (SD) | 15.5 | (21.5) | 10.7 | (20.3) | <0.001 | ||
| Alcohol consumption (drinks/wk) mean (SD) | 10.4 | (12.3) | 351 | 6.2 | (9.5) | 646 | <0.001 |
| Body Mass Index (kg/m2) mean (SD) | 27.2 | (3.6) | 346 | 27.6 | (4.4) | 590 | 0.11 |
| Mini-Mental State Examination score mean (SD) | 25.8 | (3.6) | 23.8 | (5.1) | <0.001 | ||
| Center for Epidemiologic Studies – Depression Scale mean (SD) | 11.2 | (8.3) | 345 | 13.6 | (8.9) | 609 | <0.001 |
| Congestive heart failure number (%) | 8 | (2.3) | 48 | (7.4) | <0.001 | ||
| Peripheral arterial disease number (%) | 35 | (9.9) | 86 | (13.2) | 0.13 | ||
| Hypertension number (%) | 156 | (44.2) | 316 | (48.5) | 0.20 | ||
| Diabetes number (%) | 39 | (11.0) | 74 | (11.4) | 0.89 | ||
| Osteoarthritis number (%) | 59 | (16.7) | 137 | (21.0) | 0.10 | ||
| Renal Disease number (%) | 119 | (33.7) | 293 | (44.9) | <0.001 | ||
| Myocardial Infarction number (%) | 14 | (4.0) | 32 | (4.9) | 0.50 | ||
| Angina number (%) | 17 | (4.8) | 28 | (4.3) | 0.70 | ||
| Chronic Obstructive Pulmonary Disease number (%) | 30 | (8.5) | 56 | (8.6) | 0.96 | ||
| Calcium Intake (mg/day) median (IQR) | 820 | (642, 993) | 351 | 780 | (589, 959) | 646 | 0.03 |
| Creatinine Clearance (mL/min) median (IQRc) | 67.7 | (57.4, 83.6) | 348 | 61.6 | (47.9, 73.4) | 593 | <0.001 |
| Serum parathyroid hormone (ng/L) median (IQR) | 17.4 | (13.7, 24.5) | 26.0 | (18.4, 36.5) | <0.001 | ||
| Serum parathyroid hormone ≥32.4 ng/L number (%) | 30 | (8.5) | 225 | (34.5) | <0.001 | ||
| 25-hydroxyvitamin D (ng/mL) median (IQR) | 29.5 | (25.1, 36.4) | 12.2 | (8.4, 15.7) | |||
| Baseline Frailty Status | |||||||
| Frail | 16 | (4.8) | 337 | 84 | (14.3) | 586 | <0.001 |
| Prefrail | 109 | (32.3) | 243 | (41.5) | |||
| Robust | 212 | (62.9) | 259 | (44.2) | |||
| Baseline Frailty criteria number (%) | |||||||
| Weight loss | 13 | (3.7) | 348 | 44 | (7.0) | 626 | 0.04 |
| Exhaustion | 58 | (16.8) | 345 | 117 | (29.3) | 609 | 0.36 |
| Sedentariness | 26 | (7.4) | 191 | (19.2) | <0.001 | ||
| Slowness | 50 | (14.6) | 343 | 191 | (28.7) | 586 | <0.001 |
| Weakness | 61 | (17.6) | 347 | 163 | (26.1) | 624 | 0.002 |
Mini-Mental State Examination range: 0–30; Center for Epidemiological Studies-Depression Scale range: 0–60
P-values compare variables between 25(OH)D≥20 ng/mL (50 nmol/L) and 25(OH)D<20 ng/mL (50 nmol/L). Chi-square or Fisher’s exact test for categorical variables; ANOVA or Kruskal-Wallis test for continuous variables.
SD=standard deviation
IQR=interquartile range
Reported if N is less than number in column due to missing covariates
Standardized frailty state transition probabilities by 25(OH)D category are shown in Table 2. Robust participants in both 25(OH)D categories were most likely to stay robust three years later [low 25(OH)D: 58.5%; high 25(OH)D: 63.4%]. Prefrail participants in both 25(OH)D categories were most likely to stay prefrail [low 25(OH)D: 45.0%; high 25(OH)D: 49.3%]. However, prefrail participants with low 25(OH)D were 8.9% (95%CI, 2.5—15.2%) more likely to die, 3.0% (95%CI, −5.6—14.6%) more likely to become frail, and 7.7% (95%CI, −3.5—18.7%) less likely to become robust three years later than those with high 25(OH)D. Frail participants in both 25(OH)D categories were most likely to stay frail [low 25(OH)D: 49.9%; high 25(OH)D: 60.2%] or die [low 25(OH)D: 35.2%; high 25(OH)D: 34.7%]. Unexpectedly, frail participants with low 25(OH)D were 13.5% (95%CI, 2.1—23.4%) more likely to become prefrail than those with high 25(OH)D.
Table 2.
Standardized Frailty Transition Probabilities, by 25(OH)D Status
| Status at Time t | Standardized* Probabilities (%) and Differences (%) for Status at Time t+1 (95%CI) | |||
|---|---|---|---|---|
| Robust | Prefrail | Frail | Dead | |
| Robust | ||||
| Low† 25(OH)D (N‡=360) | 58.5 (54.0, 65.9) | 34.6 (28.0, 39.5) | 4.6 (1.8, 7.3) | 2.4 (0.9, 4.0) |
| High† 25(OH)D (N=323) | 63.4 (57.8, 73.0) | 28.2 (20.0, 34.0) | 3.2 (0.3, 7.2) | 5.2 (1.8, 9.6) |
| Low 25(OH)D – High 25(OH)D | −4.9 (−14.9, 3.9) | 6.4 (−1.8, 14.7) | 1.4 (−3.2, 5.5) | −2.8 (−7.4, 1.0) |
| Prefrail | ||||
| Low 25(OH)D (N=335) | 19.3 (16.1, 26.7) | 45.0 (37.9, 50.3) | 21.5 (15.6, 26.3) | 14.1 (9.2, 19.2) |
| High 25(OH)D (N=171) | 27.0 (19.6, 38.0) | 49.3 (39.7, 59.3) | 18.5 (8.4, 25.9) | 5.2 (1.5, 9.6) |
| Low 25(OH)D – High 25(OH)D | −7.7 (−18.7, 3.5) | −4.3 (−17.2, 6.8) | 3.0 (−5.6, 14.6) | 8.9 (2.5, 15.2) |
| Frail | ||||
| Low 25(OH)D (N=112) | 0.0 -- | 14.8 (6.6, 24.2) | 49.9 (33.2, 66.6) | 35.2 (20.2, 52.2) |
| High 25(OH)D (N=27) | 3.8 (0.0, 17.6) | 1.3 (0.0, 6.6) | 60.2 (28.4, 87.8) | 34.7 (7.9, 65.0) |
| Low 25(OH)D – High 25(OH)D | −3.8 (−17.6, 0.0) | 13.5 (2.1, 23.4) | −10.3 (−41.7, 25.8) | 0.5 (−31.4, 33.5) |
Standardized for age (years), sex, education (years), season of blood collection, smoking (pack-years), alcohol consumption (drinks/wk), body mass index (kg/m2), Mini-Mental State Examination (range: 0–30), Center for Epidemiologic Studies – Depression Scale (range: 0–60), comorbidities (congestive heart failure, peripheral arterial disease, hypertension, diabetes, osteoarthritis, renal disease, myocardial infarction, angina, chronic obstructive pulmonary disease), calcium intake (mg/day), and creatinine clearance (mL/min)
Low 25(OH)D: 25(OH)D<20 ng/mL (50 nmol/L); High 25(OH)D: 25(OH)D≥20 ng/mL (50 nmol/L).
N indicates number of transitions.
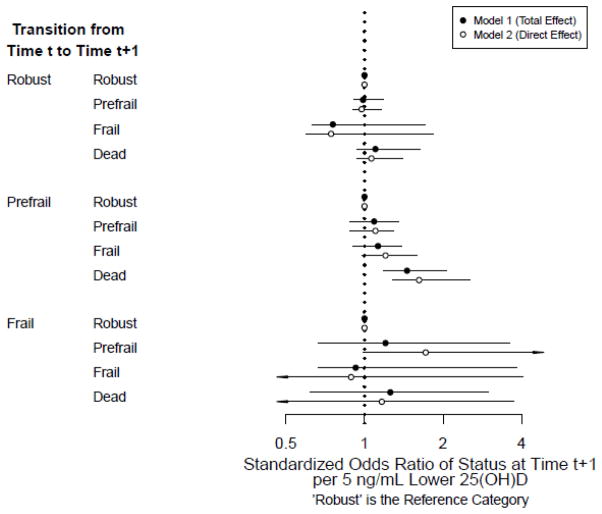
Figure 1 shows the standardized transition odds ratios of frailty state for continuous 25(OH)D using two models. Model 1 includes potential confounders, whereas Model 2 additionally includes PTH as a potential mediator. Among prefrail participants, each 5 ng/mL decrement of 25(OH)D was associated with a 46% greater odds of dying versus transitioning to robustness (Model 1 standardized odds ratio [sOR], 1.46; 95%CI, 1.18—2.07); a finding that was strengthened after including PTH (Model 2 sOR, 1.62; 95%CI, 1.28—2.54). Positive, though not statistically significant, associations were also found between low 25(OH)D and mortality for robust and frail participants. Also, after including PTH, borderline significant associations were found between 5 ng/mL decrements of 25(OH)D and transitions from prefrailty to frailty (Model 2 sOR, 1.20; 95%CI, 0.98—1.59) and transitions from frailty to prefrailty (Model 2 sOR, 1.72; 95%CI, 0.99—13.39).
Figure 1.
Standardized Odds Ratios of Frailty Transitions, per 5 ng/mL Lower 25(OH)D
Standardized odds ratios and 95% confidence intervals per 5 ng/mL lower 25(OH)D. Arrowheads indicate confidence intervals that extend beyond the figure limits.
Standardized for age (years), sex, education (years), season of blood collection, smoking (pack-years), alcohol consumption (drinks/wk), body mass index (kg/m2), Mini-Mental State Examination, Center for Epidemiologic Studies – Depression Scale, comorbidities (congestive heart failure, peripheral arterial disease, hypertension, diabetes, osteoarthritis, renal disease, myocardial infarction, angina, chronic obstructive pulmonary disease), calcium intake (mg/day), and creatinine clearance (mL/min)
Model 1: Relationship between 25(OH)D and frailty status without including parathyroid hormone (PTH) in the model as a potential mediator
Model 2: Relationship between 25(OH)D and frailty status including parathyroid hormone in the model as a potential mediator; interpreted as the association between 25(OH)D and frailty status if PTH were set to low (<32.4 ng/L).
Standardized transition probabilities for each frailty criterion by 25(OH)D category are shown in Table 3. Participants without exhaustion who had low 25(OH)D were 8.5% (95%CI, 2.4—15.6%) less likely to remain without exhaustion than those with high 25(OH)D. Also, participants with exhaustion who had low 25(OH)D were 12.5% (95%CI, 0.3—32.2%) less likely to recover from exhaustion and 9.3% (95%CI, −0.5—19.7%) more likely to die than those with high 25(OH)D. Participants without slowness who had high 25(OH)D were 19.3% (95%CI, 0.5—46.7%) more likely to remain without slowness than those with low 25(OH)D, and participants with low 25(OH)D were 17.8% (95%CI, −2.0—48.6%) more likely to die. Also, participants with slowness who had low 25(OH)D were 19.3% (95%CI, 4.4—37.8%) more likely to die than those with high 25(OH)D. Participants without weakness who had low 25(OH)D were 15.7% (95%CI, 3.2—34.1%) less likely to remain without weakness and 12.3% (95%CI, 0.5—33.9%) more likely to die than those with high 25(OH)D. There were no statistically significant differences in transitions for participants with weakness. We also found no statistically significant differences in transitions between sedentariness or weight loss states by 25(OH)D category.
Table 3.
Standardized Transition Probabilities of Individual Frailty Criteria, by 25(OH)D Status
| Criterion | Status at Time t | Standardized* Probabilities (%) and Differences (%) for Status at Time t+1 (95%CI) | ||
|---|---|---|---|---|
| Criterion Absent | Criterion Present | Dead | ||
| Exhaustion: | ||||
| Criterion Absent | ||||
| Low† 25(OH)D (N‡=675) | 72.1 (67.9, 76.6) | 18.0 (14.9, 21.9) | 9.9 (6.9, 12.5) | |
| High† 25(OH)D (N=499) | 80.6 (75.8, 87.1) | 13.4 (8.2, 18.4) | 5.9 (2.8, 9.0) | |
| Low 25(OH)D – High 25(OH)D | −8.5 (−15.6, −2.4) | 4.6 (−0.6, 10.3) | 4.0 (−0.5, 7.9) | |
| Criterion Present | ||||
| Low 25(OH)D (N=205) | 43.1 (32.9, 50.8) | 40.2 (32.5, 51.4) | 16.7 (10.4, 24.5) | |
| High 25(OH)D (N=86) | 55.6 (44.7, 71.1) | 37.0 (23.0, 49.0) | 7.4 (0.8, 15.6) | |
| Low 25(OH)D – High 25(OH)D | −12.5 (−32.2, −0.31) | 3.2 (−10.2, 20.7) | 9.3 (−0.5, 19.7) | |
| Sedentariness: | ||||
| Criterion Absent | ||||
| Low 25(OH)D (N=699) | 72.4 (66.3, 87.6) | 21.8 (9.8, 26.8) | 5.8 (2.6, 9.0) | |
| High 25(OH)D (N=552) | 77.0 (72.8, 83.8) | 18.1 (12.1, 21.6) | 4.9 (2.6, 7.4) | |
| Low 25(OH)D – High 25(OH)D | −4.6 (−14.3, 10.4) | 3.7 (−8.2, 12.3) | 0.9 (−3.1, 5.3) | |
| Criterion Present | ||||
| Low 25(OH)D (N=265) | 13.8 (7.5, 24.1) | 44.9 (23.4, 63.8) | 41.3 (16.7, 69.0) | |
| High 25(OH)D (N=57) | 17.9 (7.2, 36.7) | 59.9 (39.4, 77.3) | 22.2 (5.8, 38.8) | |
| Low 25(OH)D – High 25(OH)D | −4.1 (−24.6, 11.7) | −15.0 (−43.2, 17.2) | 19.1 (−14.7, 53.2) | |
| Slowness | ||||
| Criterion Absent | ||||
| Low 25(OH)D (N=571) | 63.0 (37.6, 80.4) | 14.9 (8.6, 20.8) | 22.1 (3.1, 53.2) | |
| High 25(OH)D (N=450) | 82.3 (77.9, 88.2) | 13.4 (8.3, 17.1) | 4.3 (1.7, 7.5) | |
| Low 25(OH)D – High 25(OH)D | −19.3 (−46.7, −0.5) | 1.5 (−5.6, 10.0) | 17.8 (−2.0, 48.6) | |
| Criterion Present | ||||
| Low 25(OH)D (N=191) | 13.8 (8.3, 21.1) | 48.4 (37.5, 58.3) | 37.8 (26.3, 49.0) | |
| High 25(OH)D (N=67) | 20.1 (11.5, 37.8) | 61.3 (46.1, 75.0) | 18.5 (6.0, 30.3) | |
| Low 25(OH)D – High 25(OH)D | −6.3 (−23.1, 4.9) | −12.9 (−31.1, 4.3) | 19.3 (4.4, 37.8) | |
| Weakness | ||||
| Criterion Absent | ||||
| Low 25(OH)D (N=694) | 72.1 (55.1, 84.4) | 10.1 (5.6, 14.4) | 17.7 (7.0, 39.3) | |
| High 25(OH)D (N=458) | 87.8 (84.3, 92.5) | 6.7 (3.7, 9.4) | 5.4 (2.4, 8.4) | |
| Low 25(OH)D – High 25(OH)D | −15.7 (−34.1, −3.2) | 3.4 (−2.1, 8.5) | 12.3 (0.5, 33.9) | |
| Criterion Present | ||||
| Low 25(OH)D (N=157) | 42.0 (32.6, 55.1) | 32.7 (23.1, 42.2) | 25.3 (15.4, 33.2) | |
| High 25(OH)D (N=80) | 41.9 (30.6, 60.0) | 39.3 (24.7, 52.6) | 18.8 (5.8, 28.5) | |
| Low 25(OH)D – High 25(OH)D | 0.1 (−21.1, 17.2) | −6.6 (−24.1, 10.4) | 6.5 (−7.0, 22.5) | |
| Weight Loss | ||||
| Criterion Absent | ||||
| Low 25(OH)D (N=842) | 82.7 (77.4, 91.4) | 7.9 (3.7, 10.9) | 9.4 (4.8, 13.0) | |
| High 25(OH)D (N=563) | 86.0 (82.8, 91.0) | 7.7 (3.6, 10.7) | 6.3 (3.4, 8.9) | |
| Low 25(OH)D – High 25(OH)D | −3.3 (−11.0, 5.4) | 0.2 (−4.8, 5.5) | 3.1 (−2.3, 7.8) | |
| Criterion Present | ||||
| Low 25(OH)D (N=98) | 5.9 (0.9, 12.9) | 78.8 (65.0, 90.8) | 15.2 (5.9, 25.4) | |
| High 25(OH)D (N=33) | 10.6 (0.0, 31.6) | 66.5 (40.3, 92.4) | 22.9 (0.0, 54.2) | |
| Low 25(OH)D – High 25(OH)D | −4.7 (−25.7, 8.5) | 12.3 (−14.9, 42.8) | −7.7 (−40.6, 17.3) | |
Standardized for age (years), sex, education (years), season of blood collection, smoking (pack-years), alcohol consumption (drinks/wk), body mass index (kg/m2), Mini-Mental State Examination (range: 0–30), Center for Epidemiologic Studies Depression Scale (range: 0–60), comorbidities (congestive heart failure, peripheral arterial disease, hypertension, diabetes, osteoarthritis, renal disease, myocardial infarction, angina, chronic obstructive pulmonary disease), calcium intake (mg/day), and creatinine clearance (mL/min)
Low 25(OH)D: 25(OH)D<20 ng/mL (50 nmol/L); High 25(OH)D: 25(OH)D 20 ng/mL (50 nmol/L).
N indicates number of transitions
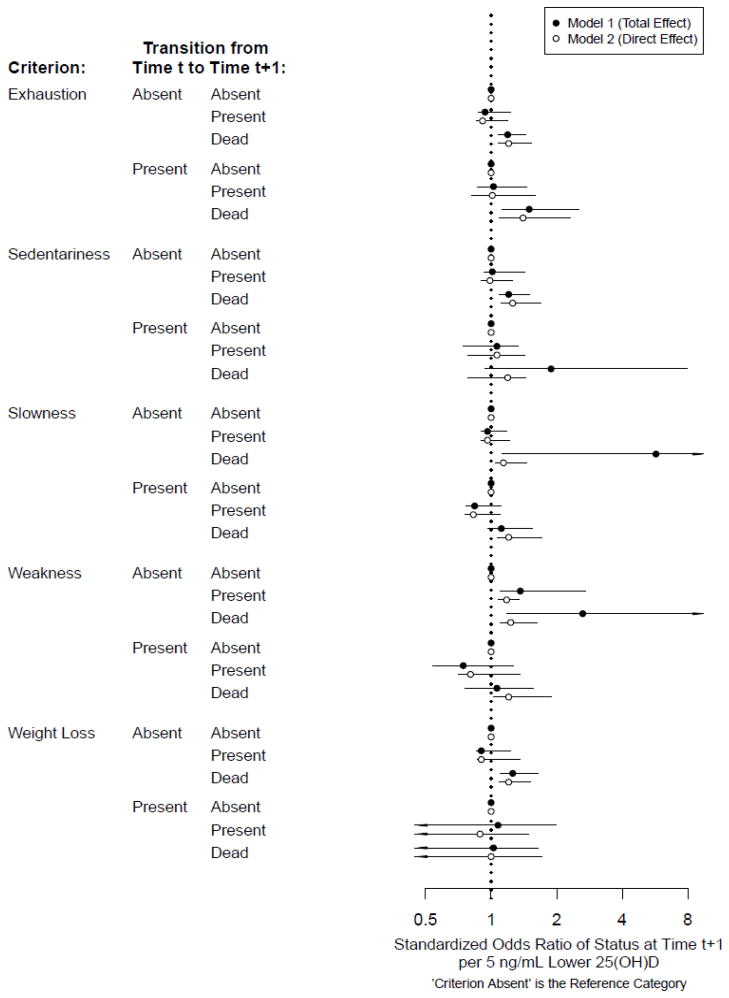
Figure 2 shows the standardized odds ratios for individual criteria as a function of continuous 25(OH)D using two models. Once again, Model 1 includes potential confounders, whereas Model 2 additionally includes PTH as a potential mediator. For participants without exhaustion, each decrement of 5 ng/mL of 25(OH)D was associated with higher standardized odds of death (Model 1 sOR, 1.19; 95%CI, 1.08—1.44); for participants with exhaustion, 5 ng/mL decrements of 25(OH)D were associated with 50% higher odds of dying than recovering from exhaustion (Model 1 sOR, 1.50; 95%CI, 1.13—2.54). Accounting for PTH had a negligible impact on results. Unlike findings with dichotomous 25(OH)D, among participants who were not sedentary, each 5 ng/mL decrement of 25(OH)D was associated with higher odds of death before (Model 1 sOR, 1.20; 95%CI, 1.09—1.50) and after (Model 2 sOR, 1.26; 95%CI, 1.11—1.70) accounting for PTH. Among participants without slowness, each 5 ng/mL decrement of 25(OH)D was associated with an almost 6-fold higher odds of death (Model 1 sOR, 5.71; 95%CI, 1.13—26.74), a finding that was consistent with results using dichotomous 25(OH)D. This association was greatly attenuated yet still statistically significant after accounting for PTH (Model 2 sOR, 1.14; 95%CI, 1.05—1.46). Among participants with slowness, each 5 ng/mL decrement of 25(OH)D was associated with marginally significantly higher odds of death versus recovery from slowness (Model 1 sOR, 1.11; 95%CI, 0.96—1.55), an association that became statistically significant after accounting for PTH (Model 2 sOR, 1.20; 95%CI, 1.06—1.72). Among participants without weakness, each 5 ng/mL decrement of 25(OH)D was associated with greater odds of developing weakness (Model 1 sOR, 1.36; 95%CI, 1.10—2.72) or dying (Model 1 sOR, 2.63; 95%CI, 1.18—10.40). After accounting for PTH, the sOR of mortality became attenuated by almost half (Model 2 sOR, 1.23; 95%CI, 1.10—1.63), whereas the sOR of weakness remained of similar magnitude (Model 2 sOR, 1.18; 95%CI, 1.08—1.35). Among participants without weight loss, each 5 ng/mL decrement of 25(OH)D was associated with greater odds of mortality both before (Model 1 sOR, 1.26; 95%CI, 1.10—1.65) and after (Model 2 sOR, 1.20; 95%CI, 1.09—1.51) accounting for PTH.
Figure 2.
Standardized Odds Ratios of Transitions of Individual Frailty Criteria, per 5 ng/mL Lower 5(OH)D
Standardized odds ratios and 95% confidence intervals per 5 ng/mL lower 25(OH)D. Arrowheads indicate confidence intervals that extend beyond the figure limits.
Standardized for age (years), sex, education (years), season of blood collection, smoking (pack-years), alcohol consumption (drinks/wk), body mass index (kg/m2), Mini-Mental State Examination (range: 0–30), Center for Epidemiologic Studies – Depression Scale (range: 0–60), comorbidities (congestive heart failure, peripheral arterial disease, hypertension, diabetes, osteoarthritis, renal disease, myocardial infarction, angina, chronic obstructive pulmonary disease), calcium intake (mg/day), and creatinine clearance (mL/min)
Model 1: Relationship between 25(OH)D and frailty status without including parathyroid hormone (PTH) in the model as a potential mediator
Model 2: Relationship between 25(OH)D and frailty status including parathyroid hormone in the model as a potential mediator; interpreted as the association between 25(OH)D and frailty status if PTH were set to low (<32.4 ng/L).
DISCUSSION
Among prefrail participants, lower 25(OH)D was related to significantly increased mortality risk and marginally significantly increased risk of transitioning to frailty, independent of its effects on PTH. This result is important because it provides evidence that prefrailty is an “at-risk” state amenable to remediation. In contrast, we found little evidence that 25(OH)D relates to transitions from robustness or frailty. In models with continuous 25(OH)D, weakness onset was the only frailty criterion associated with low 25(OH)D, suggesting that incident weakness likely explains the association of low 25(OH)D with the transition from prefrailty to frailty. The association of 25(OH)D levels with incident weakness changed little after including PTH, suggesting a mechanism independent of PTH.
Previous observational studies have found associations between low 25(OH)D and prevalent frailty in Italian men7 and incident frailty in Dutch elders8. Additionally, low 25(OH)D is associated with prevalence15 and incidence5 of muscle weakness among older European adults. Although one published study did not find an association between low 25(OH)D and incident muscle weakness, study participants were disabled older women, leading to speculation that participants’ strength was already below a threshold that could be affected by vitamin D31. Elevated PTH is also associated with declining muscle strength5, a potential mechanism through which 25(OH)D affects muscle. Further, some randomized controlled trials32, 33, but not all34, 35, have found that vitamin D supplementation can improve muscle function.
Observational research has also linked low 25(OH)D with increased mortality risk among older Italian adults, older American women13, 14 and the American population36. High PTH is also associated with mortality37. Additionally, a meta-analysis of randomized controlled trials suggested that vitamin D supplementation can reduce the risk of mortality in older adults38. Vitamin D supplementation dosing studies found that 25(OH)D increases by 0.24—0.48 ng/mL per 1 μg of vitamin D per day (1 μg = 40 IU). Thus, these data suggest that 25(OH)D can be increased by 5 ng/mL by increasing vitamin D intake by 417—833 IU/day39, an amount found in many commercially available multivitamins and supplements.
Low 25(OH)D may increase risks of frailty and mortality through multiple pathways. Effects of vitamin D on bone are well known22; however, low 25(OH)D also has effects on muscle that may explain the association with weakness found here. First, low 25(OH)D may lead to decreased muscle strength and function by inhibiting the effects of vitamin D metabolites on muscle cell metabolism40. The active metabolite 1,25-dihydroxyvitamin D [1,25(OH)2D] mediates gene transcription by binding to the vitamin D receptor located on skeletal muscle cells and enhancing muscle cell calcium uptake, phosphate transport, and differentiation into mature muscle fibers41, 42. Additionally, 1,25(OH)2D enhances calcium uptake by acting directly on muscle cell membranes41, 42. 1,25(OH)2D is also an immunomodulator and regulator of cell proliferation, which may explain the increased risks of infectious and chronic diseases found in vitamin D deficient individuals22. When 25(OH)D concentrations are low, 1,25(OH)2D absorption decreases, inhibiting its effects on muscle, and PTH rises, which decreases the number of type 2 muscle fibers and inhibits phosphate transport. Additionally, PTH induces production of inflammatory cytokines, which are associated with declining physical function, multiple age-related diseases, frailty and mortality43–45. Pain and muscle weakness are prominent clinical features of vitamin D deficiency46. Results from this study among participants without weakness suggested that the relationship between 25(OH)D and new weakness was mostly direct, whereas about half of the association between 25(OH)D and mortality appeared to be mediated by PTH.
This study’s strengths include use of a large longitudinal population-based cohort and availability of serum 25(OH)D, PTH, and many measured potential confounders. Also, this work overcomes the limitation of previous research that cross-sectionally examined 25(OH)D and frailty by ensuring that 25(OH)D was measured prior to frailty. Further, this study is novel in that it considered potential recovery from frailty and individual frailty criteria, and it assessed mortality risk stratified by frailty status. Lastly, 25(OH)D was analyzed both as a categorical measure using the IoM cutpoint of 20 ng/mL21 and as a continuous measure. Despite these strengths, some limitations must be noted. First, because InCHIANTI is an observational cohort, we cannot rule out the possibility of unmeasured confounders. Second, this lstudy had missing data, but it was rigorously handled in analysis to mitigate selection bias. Also, because visits were spaced far apart, we likely missed some transitions; therefore, estimated associations are likely conservative. For example, low 25(OH)D was more strongly associated with death than frailty, perhaps due to short sojourns in the frailty state prior to death. Lastly, although we used the updated IoM cut-off for low 25(OH)D, the IoM report has been criticized47, 48. The Endocrine Society agreed that 25(OH)D below 20 ng/mL is indeed deficient, but recommended maintaining 25(OH)D above 30 ng/mL for optimal benefits49. Unfortunately, assessing transitions from three frailty groups for three 25(OH)D categories produced too much data sparseness to reliably estimate transition probabilities (especially due to small numbers of frail participants); therefore, this work should be replicated in a larger, frailer sample.
In conclusion, low 25(OH)D was common and was associated with increased risk of frailty and death among prefrail participants. In contrast, prefrail participants with high vitamin D were more likely to transition to robustness than frailty. These results suggest that 25(OH)D may delay progression to frailty among those who are at risk for frailty and that future research should investigate 25(OH)D not only as a potential preventive therapy for frailty and mortality, but also as a treatment for prefrailty.
Acknowledgments
Funding Sources: The InCHIANTI study was supported by the Italian Ministry of Health and in part by the U.S. National Institute on Aging Intramural Research Program (contracts 263 MD 916413 and 263 MD 821336). This research was partly supported by an unrestricted grant from Procter and Gamble, SrL, Italy. Dr. Shardell was supported by grant K25 AG034216, Dr. D’Adamo was supported by grant T32 AG00262, Dr. Alley was supported by grant K12HD04389, Dr. Miller was supported by grant K23AG027746, Dr. Hicks was supported by grants K12HD055931 and R21HD057274, and Dr. Semba was supported by grants R01 HL094507 and R01 AG027012 from the National Institutes of Health. Drs. Shardell and Alley were also supported by the Geriatrics and Gerontology Education and Research (GGEAR) Program at the University of Maryland School of Medicine.
Sponsor’s role: The sponsor was not involved in data analysis, interpretation, or manuscript preparation.
Footnotes
Presented at: Gerontological Society of America, November 20, 2009, Atlanta, Georgia
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author contributions: Dr. Shardell designed the study and conducted statistical analysis. All authors assisted with interpretation of data and critical revision of the manuscript. Drs. Bandinelli and Ferrucci obtained funding for data collection. All authors approved the final version.
References
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 3.Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 4.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 5.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 6.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Shardell M, Hicks GE, Miller RR, et al. Association of low vitamin D levels with the frailty syndrome in men and women. J Gerontol A Biol Sci Med Sci. 2009;64:69–75. doi: 10.1093/gerona/gln007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puts MT, Visser M, Twisk JW, et al. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf) 2005;63:403–411. doi: 10.1111/j.1365-2265.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- 9.Hicks GE, Shardell M, Miller RR, et al. Associations between vitamin D status and pain in older adults: The Invecchiare in Chianti study. J Am Geriatr Soc. 2008;56:785–791. doi: 10.1111/j.1532-5415.2008.01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milaneschi Y, Shardell M, Corsi AM, et al. Serum 25-hydroxyvitamin D and depressive symptoms in older women and men. J Clin Endocrinol Metab. 2010;95:3225–3233. doi: 10.1210/jc.2010-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Fracture prevention with vitamin D supplementation: A meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 12.Snijder MB, van Schoor NM, Pluijm SM, et al. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J Clin Endocrinol Metab. 2006;91:2980–2985. doi: 10.1210/jc.2006-0510. [DOI] [PubMed] [Google Scholar]
- 13.Semba RD, Houston DK, Bandinelli S, et al. Relationship of 25-hydroxyvitamin D with all-cause and cardiovascular disease mortality in older community-dwelling adults. Eur J Clin Nutr. 2010;64:203–209. doi: 10.1038/ejcn.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semba RD, Houston DK, Ferrucci L, et al. Low serum 25-hydroxyvitamin D concentrations are associated with greater all-cause mortality in older community-dwelling women. Nutr Res. 2009;29:525–530. doi: 10.1016/j.nutres.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houston DK, Cesari M, Ferrucci L, et al. Association between vitamin D status and physical performance: The InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62:440–446. doi: 10.1093/gerona/62.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 17.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 18.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: Characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 19.Fava GA. Assessing depressive symptoms across cultures: Italian validation of the CES-D self-rating scale. J Clin Psychol. 1983;39:249–251. doi: 10.1002/1097-4679(198303)39:2<249::aid-jclp2270390218>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 23.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14:680–686. doi: 10.1097/01.EDE.0000081989.82616.7d. [DOI] [PubMed] [Google Scholar]
- 25.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robins JM, Rotnitzky A, Zhao LP. Estimation of regression coefficients when some regressors are not always observed. J Am Stat Assoc. 1994;89:846–866. [Google Scholar]
- 27.Robins JM, Rotnitzky A, Zhao LP. Analysis of semiparametric regression models for repeated outcomes in the presence of missing data. J Am Stat Assoc. 1995;90:106–121. [Google Scholar]
- 28.Maggio D, Cherubini A, Lauretani F, et al. 25(OH)D Serum levels decline with age earlier in women than in men and less efficiently prevent compensatory hyperparathyroidism in older adults. J Gerontol A Biol Sci Med Sci. 2005;60:1414–1419. doi: 10.1093/gerona/60.11.1414. [DOI] [PubMed] [Google Scholar]
- 29.VanderWeele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology. 2009;20:18–26. doi: 10.1097/EDE.0b013e31818f69ce. [DOI] [PubMed] [Google Scholar]
- 30.Vanderweele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172:1339–1348. doi: 10.1093/aje/kwq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verreault R, Semba RD, Volpato S, et al. Low serum vitamin d does not predict new disability or loss of muscle strength in older women. J Am Geriatr Soc. 2002;50:912–917. doi: 10.1046/j.1532-5415.2002.50219.x. [DOI] [PubMed] [Google Scholar]
- 32.Bischoff HA, Stahelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: A randomized controlled trial. J Bone Miner Res. 2003;18:343–351. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 33.Dhesi JK, Jackson SH, Bearne LM, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33:589–595. doi: 10.1093/ageing/afh209. [DOI] [PubMed] [Google Scholar]
- 34.Kenny AM, Biskup B, Robbins B, et al. Effects of vitamin D supplementation on strength, physical function, and health perception in older, community-dwelling men. J Am Geriatr Soc. 2003;51:1762–1767. doi: 10.1046/j.1532-5415.2003.51561.x. [DOI] [PubMed] [Google Scholar]
- 35.Latham NK, Anderson CS, Lee A, et al. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: The Frailty Interventions Trial in Elderly Subjects (FITNESS) J Am Geriatr Soc. 2003;51:291–299. doi: 10.1046/j.1532-5415.2003.51101.x. [DOI] [PubMed] [Google Scholar]
- 36.Melamed ML, Michos ED, Post W, et al. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bjorkman MP, Sorva AJ, Tilvis RS. Elevated serum parathyroid hormone predicts impaired survival prognosis in a general aged population. Eur J Endocrinol. 2008;158:749–753. doi: 10.1530/EJE-07-0849. [DOI] [PubMed] [Google Scholar]
- 38.Autier P, Gandini S. Vitamin D supplementation and total mortality: A meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 39.Heaney RP. The case for improving vitamin D status. J Steroid Biochem Mol Biol. 2007;103:635–641. doi: 10.1016/j.jsbmb.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Wassner SJ, Li JB, Sperduto A, et al. Vitamin D Deficiency, hypocalcemia, and increased skeletal muscle degradation in rats. J Clin Invest. 1983;72:102–112. doi: 10.1172/JCI110947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mosekilde L. Vitamin D and the elderly. Clin Endocrinol (Oxf) 2005;62:265–281. doi: 10.1111/j.1365-2265.2005.02226.x. [DOI] [PubMed] [Google Scholar]
- 42.Janssen HC, Samson MM, Verhaar HJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75:611–615. doi: 10.1093/ajcn/75.4.611. [DOI] [PubMed] [Google Scholar]
- 43.Taaffe DR, Harris TB, Ferrucci L, et al. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55:M709–715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 44.Schaap LA, Pluijm SM, Deeg DJ, et al. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526 e529–517. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 45.De Martinis M, Franceschi C, Monti D, et al. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res. 2011;26:455–457. doi: 10.1002/jbmr.328. [DOI] [PubMed] [Google Scholar]
- 48.Holick MF. The D-batable Institute of Medicine report: A D-lightful perspective. Endocr Pract. 2011;17:143–149. doi: 10.4158/ep.17.1.143. [DOI] [PubMed] [Google Scholar]
- 49.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]